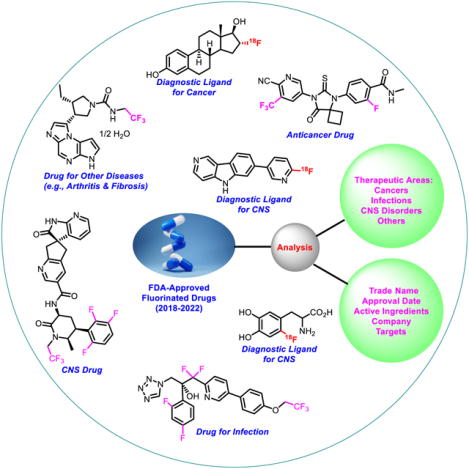
Abstract
The objective of this review is to provide an update on the fluorine-containing drugs approved by U.S. Food and Drug Administration in the span of past five years (2018–2022). The agency accepted a total of fifty-eight fluorinated entities to diagnose, mitigate and treat a plethora of diseases. Among them, thirty drugs are for therapy of various types of cancers, twelve for infectious diseases, eleven for CNS disorders, and six for some other diseases. These are categorized and briefly discussed based on their therapeutic areas. In addition, this review gives a glimpse about their trade name, date of approval, active ingredients, company developers, indications, and drug mechanisms. We anticipate that this review may inspire the drug discovery and medicinal chemistry community in both industrial and academic settings to explore the fluorinated molecules leading to the discovery of new drugs in the near future.
Graphical Abstract
1. Introduction
Fludrocortisone [1, 2] as the first fluorine-containing drug was introduced to the market for commercial use in 1954 (Fig. 1). Shortly thereafter, 5-fluorouracil [3, 4] was another fluorinated drug brought to the clinic for the treatment of cancer in 1957 (Fig. 1). Introduction of these two drugs drew the attention of pharmaceutical industry toward the biological applications of fluorine in drug discovery and development. In 1972, Ili Lilly and Company discovered a blockbuster antidepressant drug Prozac (Fluoxetine) and marketed it for medical use in 1986 (Fig. 1). Extensive literature survey has revealed that more than 50% blockbuster drugs are the molecules having fluorine atom(s) or trifluoromethyl group [5]. Notably, among these medicines, Lipitor is considered the most profitable one ever launched to the market [6]. Over the past two decades, fluorine chemistry has demonstrated its potential applications in diverse domains, such as food, health, and energy industries. In addition, fluorine-containing compounds have been exploited for the advancement of technologies due to their unique electronic, physicochemical, and biological properties [7–12].
Fig. 1.
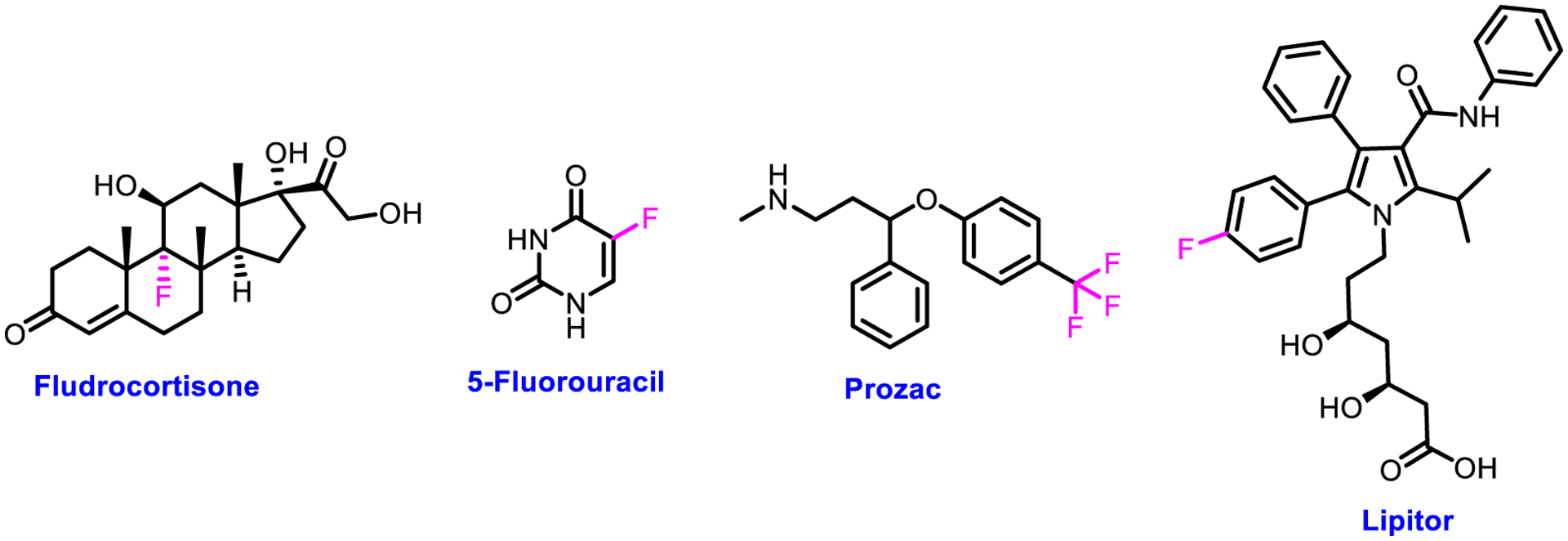
Structures of selected representative drugs Fludrocortisone, 5-Fluorouracil, Prozac, and Lipitor.
One of the exciting contributions of fluorine in the innovation of technology is the use of its isotope 18F containing molecules in positron emission tomography (PET) in potential diagnosis, drug discovery and development [13–22]. It is well known that fluorine is extensively utilized in magnetic resonance imaging (MRI) as a diagnostic tool for early monitoring the different illnesses [23–26]. Furthermore, fluorine has shown its potential applications in peptide/protein engineering [27–30]. In fact, most important application of fluorine lies in the drug design to discover new entities. Judicious installation of fluorine or groups of fluorine atoms (e.g., CF3, CHF2, OCF3) in a drug candidate may lead to improved pharmacological and pharmacokinetic profiles with increased potency, decrease in pKa, higher permeability, decrease in clearance, and conformational constraint [31–34]. The prevalence of fluorine in pharmaceuticals attracts the attention of synthetic community and encourages developing new synthetic methodologies to access structurally diverse fluorinated molecules. Consequently, numerous novel methods for the synthesis of unique fluorinated compounds have been reported in literature [35–45] in past few years. Recently, some review articles nicely covered FDA-approved fluorinated drugs year-wise until 2021 [46–50]. However, no update is available on FDA-approved fluorinated drugs in 2022. Moreover, there is no report available in the literature that presents a concise information about fluorinated drugs approved by FDA based on their therapeutic areas in a single document in the past five years (2018–2022). Therefore, there is a need of a concise and informative summary on fluorine-containing therapies to provide the latest update for the drug discovery and medicinal chemistry research community. The bar graph displays the total numbers of FDA-approved drugs along with fluorinated drugs spanning from 2018 to 2022 (Fig. 2A).
Fig. 2.
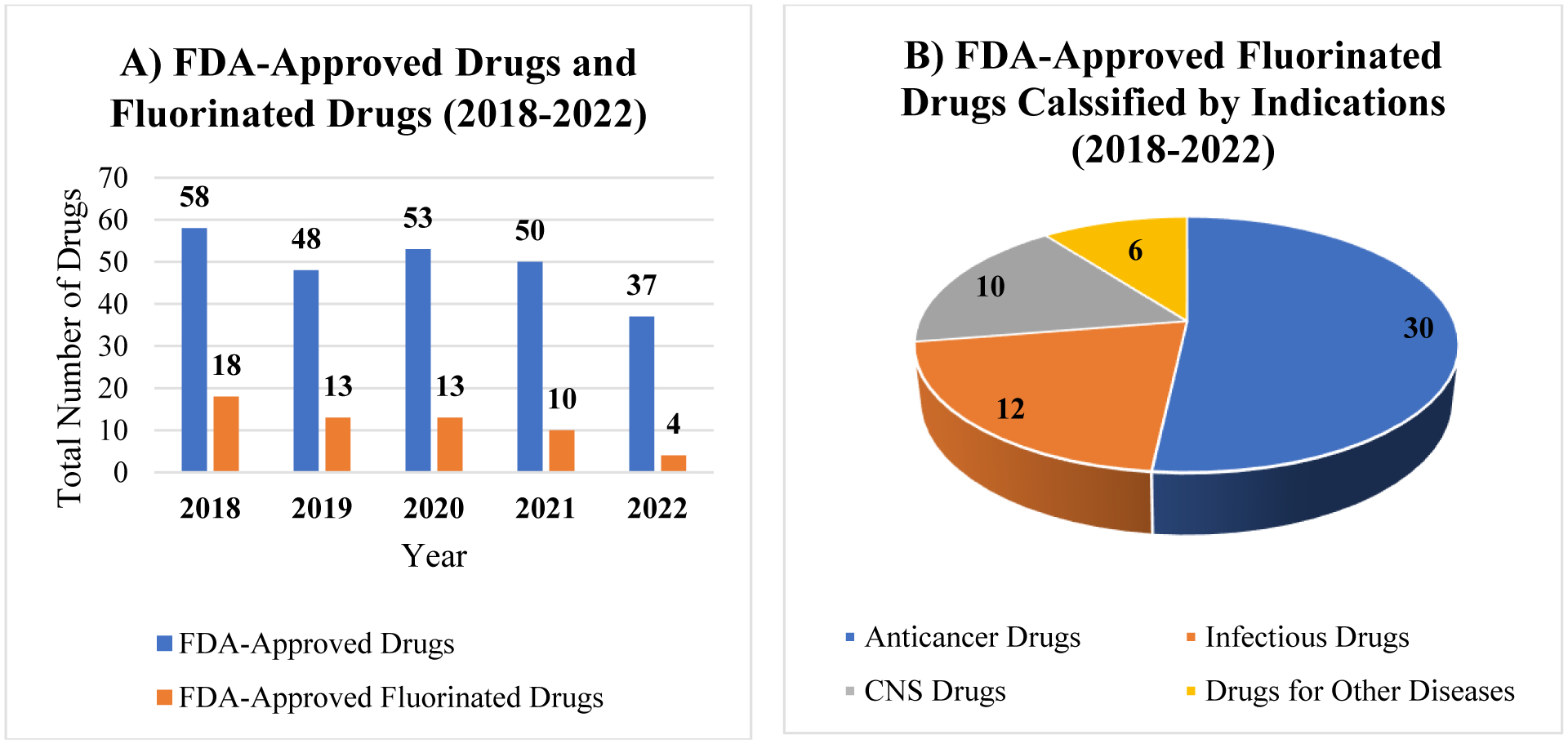
A) Bar graph illustration of U.S. FDA-approved total numbers of drugs and fluorinated drugs from 2018 to 2022; B) Pie chart presents the numbers of fluorinated drugs approved by FDA from 2018 to 2022 classified by indications such as cancers, CNS disorders, infections, and some other diseases. The source of the data is the U.S. FDA Web site [51].
The Center for Drug Evaluation and Research (CDER) of the U.S. FDA approved a total of 247 drugs including small and macromolecules from 2018 to 2022. Fifty-eight out of them are fluorinated small molecules for various therapeutic purposes, such as cancers, infections, CNS disorders and some other diseases (Fig. 2B). This review presents an overview with highlights on the fluorinated drugs for diagnosis, mitigation, and the treatment of various types of cancers, infectious diseases, CNS disorders and some other diseases. It also provides the details about these approved fluorinated drugs, such as trade name, approval date, active ingredients, company developers, indications, and drug mechanisms.
2. U.S. FDA-Approved Fluorinated Anti-Cancer Drugs (2018–2022)
Cancer is a major threat to the human health across the globe and responsible for large number of deaths each year. According to the World Health Organization (WHO)’s classification of cancers, five most common cancer types are lung, breast, liver, stomach and colorectal among the hundreds of types of cancers [52]. Very recently, the American Cancer Society has estimated around 2 million new cancer cases and more than half million cancer deaths are likely to occur in 2023 [53]. Discovering an anti-cancer drug is a great challenge because each kind of cancer requires different diagnosis and target-based treatment strategy. Nevertheless, significant growth was witnessed in the development of drugs for the treatment of cancers [54] after the first anticancer remedy in the 1940s using nitrogen mustards and antifolate drugs [55]. The CDER of U.S. FDA agency approved a total of thirty fluorine-containing medicines 1–30 for the treatment of various cancers from 2018 to 2022 (Fig. 3; Table 1). The agency approved Erleada 1, Orgovyx 23 and Pylarify 26 for the treatment or diagnosis of prostate cancer. Erleada 1 and Orgovyx 23 act as androgen receptor inhibitor and GnRH receptor antagonist, respectively. While Pylarify 26 is an advanced diagnostic imaging agent, containing 18F isotope of fluorine has been extensively utilized in developing PET imaging ligands [56–58].
Fig. 3.
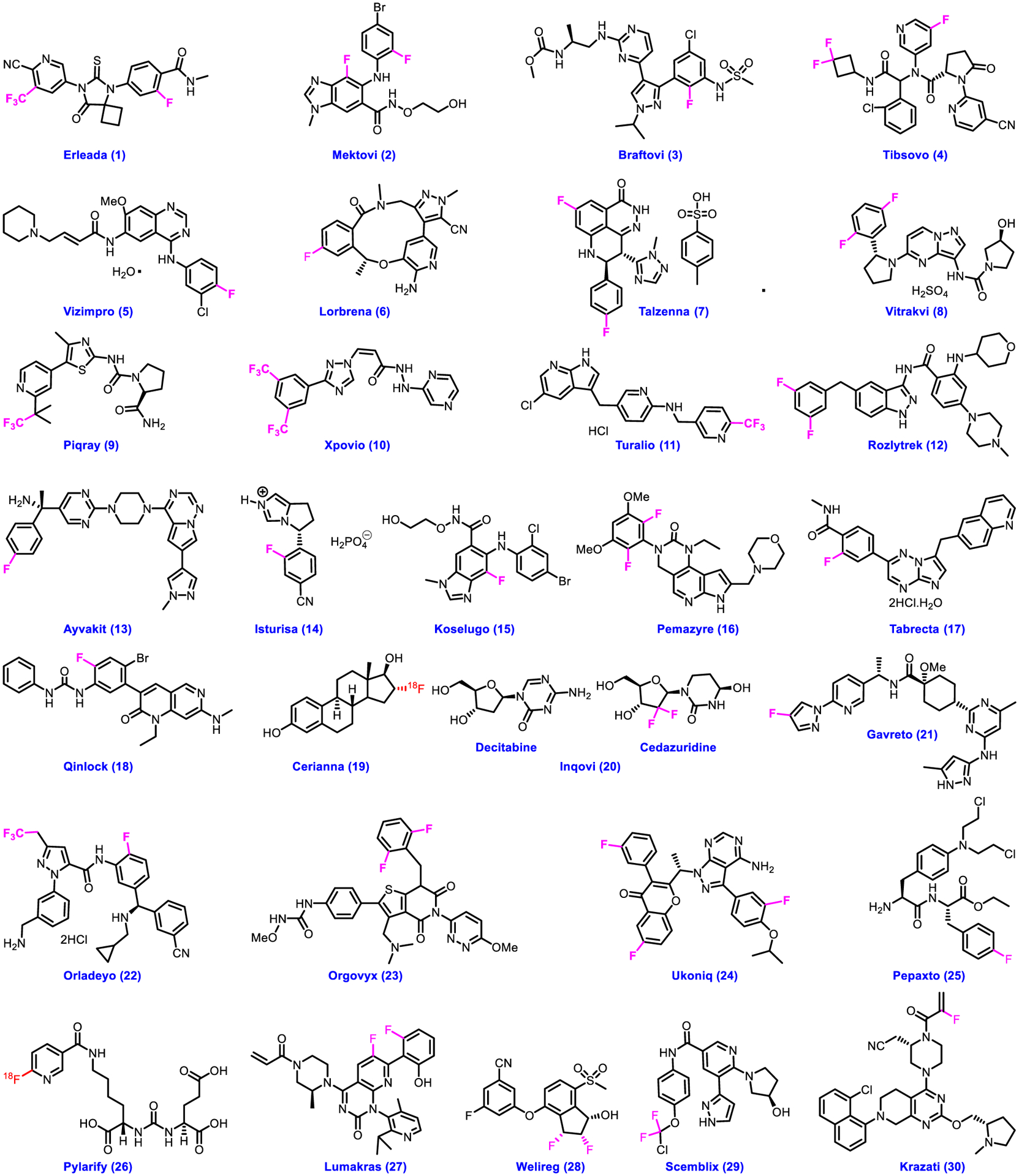
Chemical structures of U.S. FDA-approved drugs for various types of cancers from 2018 to 2022.
Table 1.
U.S. FDA-Approved Fluorinated Anti-Cancer Drugs (2018–2022).
| Drug | Approval Date | Composition | Company | Indication | Drug Mechanism |
|---|---|---|---|---|---|
| Erleada | 2/14/2018 | Apalutamide | Johnson & Johnson | Prostate cancer | Androgen receptor inhibitor |
| Mektovi | 6/27/2018 | Binimetinib | Array BioPharma | BRAF V600E- or V600K- positive metastatic melanoma (in combination with Braftovi) | MEK1/2 inhibitor |
| Braftovi | 6/27/2018 | Encorafenib | Array BioPharma | BRAF V600E- or V600K- positive metastatic melanoma (in combination with Mektovi) | BRAF inhibitor |
| Tibsovo | 7/20/2018 | Ivosidenib | Agios Pharmaceuticals | Acute myeloid leukemia | IDH1 inhibitor |
| Vizimpro | 9/27/2018 | Dacomitinib | Pfizer | Non-small-cell lung cancer | Irreversible EGFR inhibitor |
| Lorbrena | 11/2/2018 | Lorlatinib | Pfizer | ALK-positive metastatic nonsmall-cell lung cancer | ALK inhibitor |
| Talzenna | 10/16/2018 | Talazoparib | Pfizer | Breast cancer with germline BRCA mutations | PARP inhibitor |
| Vitrakvi | 11/26/2018 | Larotrectinib | Loxo Oncology | NTRK gene fusion-positive solid tumors | TRK inhibitor |
| Piqray | 5/24/2019 | Alpelisib | Novartis | Breast cancer | PI3Ka inhibitor |
| Xpovio | 7/3/2019 | Selinexor | Karyopharm Therapeutics | Multiple myeloma | Nuclear export inhibitor |
| Turalio | 8/2/2019 | Pexidartinib | Daiichi Sankyo | Symptomatic tenosynovial giant-cell tumors | CSF1R, KIT, and FLT3-ITD inhibitor |
| Rozlytrek | 8/15/2019 | Entrectinib | Roche | Non-small-cell lung cancer whose tumors are ROS1 positive | ROS1 and NTRK inhibitor |
| Ayvakit | 1/9/2020 | Avapritinib | Blueprint Medicines | Gastrointestinal-stromal tumor | PDGFRA and KIT inhibitor |
| Isturisa | 3/6/2020 | Osilodrostat | Novartis | Cushing’s disease in adults | Cortisol synthesis inhibitor |
| Koselugo | 4/10/2020 | Selumetinib | AstraZeneca | Neurofibromatosis type 1, a genetic disorder that causes tumors to grow on nerves | MEK1/2 inhibitor |
| Pemazyre | 4/17/2020 | Pemigatinib | Incyte | Cholangiocarcinoma, a rare form of cancer that forms in bile ducts | FGFR inhibitor |
| Tabrecta | 5/6/2020 | Capmatinib | Novartis | Non-small-cell lung cancer | MET inhibitor |
| Qinlock | 5/15/2020 | Ripretinib | Deciphera Pharmaceuticals | Gastrointestinal-stromal tumors | KIT and PDGFRA kinase inhibitor |
| Cerianna | 5/20/2020 | Fluoroestrdiol F18 | Zionexa and Siemens Medical Solutions | Diagnostic imaging agent for certain patients with breast cancer | Radiodiagnostic |
| Inqovi | 7/7/2020 | Decitabine and cedazuridine | Taiho Oncology | Myelodysplastic syndromes | Nucleoside metabolic inhibitor and cytidine deaminase inhibitor |
| Gavreto | 9/4/2020 | Pralsetinib | Roche/Blueprint Medicines | Non-small-cell lung cancer | RET fusion inhibitor |
| Orladeyo | 12/4/2020 | Berotralstat | BioCryst Pharmaceuticals | Prevention of hereditary angioedema | Plasma kallikrein inhibitor |
| Orgovyx | 12/18/2020 | Relugolix | Myovant Sciences | Prostate cancer | GnRH receptor antagonist |
| Ukoniq | 2/5/2021 | Umbralisib | TG Therapeutics | Marginal zone lymphoma or follicular lymphoma | Kinase inhibitor |
| Pepaxto | 2/26/2021 | Melphalan flufenamide | Oncopeptides | Relapsed or refractory multiple myeloma | DNA alkylation |
| Pylarify | 5/26/2021 | Piflufolastat F-18 | Progenics Pharmaceuticals | Identification of lesions positive for prostate-specific membrane antigen in people with prostate cancer | Binds to malignant prostate cancer cells |
| Lumakras | 5/28/2021 | Sotorasib | Amgen | Non-small-cell lung cancer | KRAS G12C inhibitor |
| Welireg | 8/13/2021 | Belzutifan | Merck & Co. | Von Hippel-Lindau disease | Hypoxiainducible factor- 2α inhibitor |
| Scemblix | 10/29/2021 | Asciminib | Novartis | Philadelphia chromosome- positive chronic myeloid leukemia | Kinase inhibitor |
| Krazati | 12/12/2022 | Adagrasib | Mirati Therapeutics | KRAS G12C-mutated nonsmall-cell lung cancer | Irreversible inhibitor of KRAS G12C |
The source of the data is the U.S. FDA Web site [51].
It binds to malignant prostate cancer cells to identify tumor in prostate. Melanoma is a type of skin cancer for which FDA approved a couple of medications, such as Mektovi 2 and Braftovi 3. Mektovi 2 in combination with Braftovi 3 treats melanoma having specific gene BRAF V600E or V600K mutation as a MEK1/2 inhibitor. Interestingly, Braftovi 3 with Mektovi 2 can also be used to treat melanoma but as a BRAF inhibitor. In addition, FDA approved Tibsovo 4 and Scemblix 29 to treat acute myeloid and philadelphia chromosome–positive chronic myeloid leukemia as an IDH1 inhibitor and kinase inhibitor, respectively. We noted that pharmaceutical companies made remarkable progress in the development of new therapies for lung cancers in the past five years. The CDER of U.S. FDA approved a total of seven fluorine-containing drugs for lung cancers. Vizimpro 5, Tabrecta 17, Gavreto 21 and Lumakras 27 received approval for the treatment of nonsmall-cell lung cancer as an irreversible EGFR inhibitor, MET inhibitor, RET fusion inhibitor, and KRAS G12C inhibitor, respectively. Lorbrena 6 is a drug used to treat ALK-positive metastatic non-small-cell lung cancer as an ALK inhibitor. Additionally, FDA approved Rozlytrek 12 as an ROS1 and NTRK inhibitor to treat non-small-cell lung cancer whose tumors are ROS1 positive. Further, Krazati 30 was approved as an effective medication of KRAS G12C–mutated non-small-cell lung cancer, which acts as an irreversible inhibitor of KRAS G12C. Fluorine-containing pharmaceuticals continued to be proven valuable in the drug discovery and development. The CDER of U.S. FDA approved three medications having fluorine for the breast cancer. FDA approved Talzenna (7) and Piqray 9 to treat breast cancer with germline BRCA mutations and breast cancer as a PARP inhibitor and PI3Ka inhibitor, respectively. Cerianna 19 also received approval as a diagnostic imaging agent for patients with breast cancer. FDA accepted Vitrakvi 8, as a TRK inhibitor for the treatment of NTRK gene fusion–positive solid tumors. Xpovio 10 and Pepaxto 25 were approved for multiple myeloma and relapsed or refractory multiple myeloma, by inhibiting nuclear export and DNA alkylation, respectively. FDA approved Turalio 11 for symptomatic tenosynovial giant-cell tumors as a CSF1R, KIT, and FLT3-ITD inhibitor. FDA also approved Inqovi 20, a combination of decitabine and cedazuridine for the treatment of myelodysplastic syndromes as a nucleoside metabolic inhibitor and a cytidine deaminase inhibitor. FDA approved Ayvakit 13 and Qinlock 18 to treat gastrointestinal-stromal tumors as KIT and PDGFRA kinase inhibitors, respectively.
As a cortisol synthesis inhibitor, Isturisa 14 received approval for the treatment of Cushing’s disease in adults. FDA approved Koselugo 15 to treat neurofibromatosis type 1, a genetic disorder that causes tumors to grow on nerves as a MEK1/2 inhibitor. As an FGFR inhibitor, Pemazyre 16 was approved as a remedy for cholangiocarcinoma, a rare form of cancer that forms in bile ducts. Orladeyo 22, Ukoniq 24 and Welireg 28 received approval for prevention of hereditary angioedema, marginal zone lymphoma or follicular lymphoma and Von Hippel-Lindau disease as a plasma kallikrein inhibitor, kinase inhibitor, and hypoxia-inducible factor-2α inhibitor, respectively.
From the structure-activity/property relationship perspective, meticulous deployment of fluorine or groups of fluorine atoms (e.g., CF3, CHF2, OCF3) in a drug candidate may result in enhanced pharmacological and pharmacokinetic profiles with increased potency, decrease in pKa, higher permeability, decrease in clearance, and conformational constraint [31–34]. Over the past two decades, it has become a common practice using fluorine, CF3, and OCF3 at the suitable positions of aromatic/heteroaromatic rings to avoid metabolic liabilities [31–34]. The effect of the presence of fluorine or groups of fluorine atoms in drug candidates can be explained based on literature precedents. As we can see (Fig. 3), fluorine atoms and/or CF3 group are positioned directly on aromatic/heteroaromatic rings in drugs 1-8, 10-19, 22-24 and 27. The presence of fluorine atoms or CF3 group on aromatic ring may improve metabolic stability (decease in clearance) [59–63], diminishing the basicity of neighboring basic group [64–70], increasing the membrane permeability [71–75] and enhancing the binding affinity to the target proteins through non-bonding ligand-protein interactions involving fluorine (improving potency) [76–80]. Similarly, the presence of geminal difluoro group either on cyclic or aliphatic system, such as those in drugs 4, 20, 29 (Fig. 3) can be rationalized. Literature reveals that geminal difluoro group may improve the potency and metabolic stability of these drugs [81–83]. The role of CF3 group on aliphatic system like in drugs 9 and 12 may be resulting in increased potency and decreased liver microsomal clearance as per some resembling reports in literature [81, 83]. In case of 28, two vicinal fluorine atoms increased the potency and metabolic stability as compared to other pharmacophores [84]. Vinylic fluorine in 30 may improve the metabolic stability while maintaining the potency [85].
3. U.S. FDA-Approved Fluorinated Drugs for Infectious Diseases (2018–2022)
The main cause of the infectious diseases is entering the pathogenic microorganisms, such as viruses, bacteria, parasites, or fungi into the body. Being contagious in nature, infectious diseases spread rapidly within the society and sometimes cause pandemic. A wide variety of infectious diseases includes malaria, coronavirus, flavivirus, HBV, HIV/AIDS, influenza, HPV, RSV, smallpox and many more. Particularly, novel effective broad spectrum antiviral drugs are urgently needed, attracting tremendous research efforts in the field [86–91]. During the recent global pandemic, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was added to list of devastating infectious diseases [92, 93]. We came across that the CDER of U.S. FDA approved a total of twelve fluorine-containing drugs 31–42 for the treatment of various types of infections over the past five years (Fig. 4; Table 2). Each of these drugs has been briefly discussed based on their therapeutic targets in this section. Four drugs 31, 36, 39 and 42 were approved for the treatment of HIV infection. Biktarvy 31 has three active ingredients, such as Bictegravir Sodium, Emtricitabine, and Tenofovir alafenamide fumarate used for therapy of HIV infection as integrase strand transfer inhibitor, and two HIV nucleoside analog reverse transcriptase inhibitors. However, Pifeltro 36 acts as an antiviral, reverse transcriptase inhibitor. Cabenuva 39 is a combination of Cabotegravir and Rilpivirine (fluorinated). Sunlenca 42 received approval to treat HIV infections in adults that cannot be successfully managed with other previously available treatments. It is important to note that both Cabenuva 39 and Sunlenca 42 are HIV-1 antiretroviral agents. FDA approved Akynzeo 32 as a remedy of nausea. It is a combination of Netupitant and Palonosetron hydrochloride and found to be NK1 receptor antagonist and 5-HT3 receptor antagonist. As an orthopoxvirus VP37 envelope wrapping protein inhibitor, TPOXX 33 was approved for the treatment of smallpox. Krintafel 34 is an 8-aminoquinoline antiparasitic being used to prevent the relapse of malaria caused by parasites. FDA approved Xerava 35 to treat complicated intra-abdominal infections caused by bacteria. It is an antibiotic that binds 30S ribosomal subunit to block protein synthesis.
Figure 4.
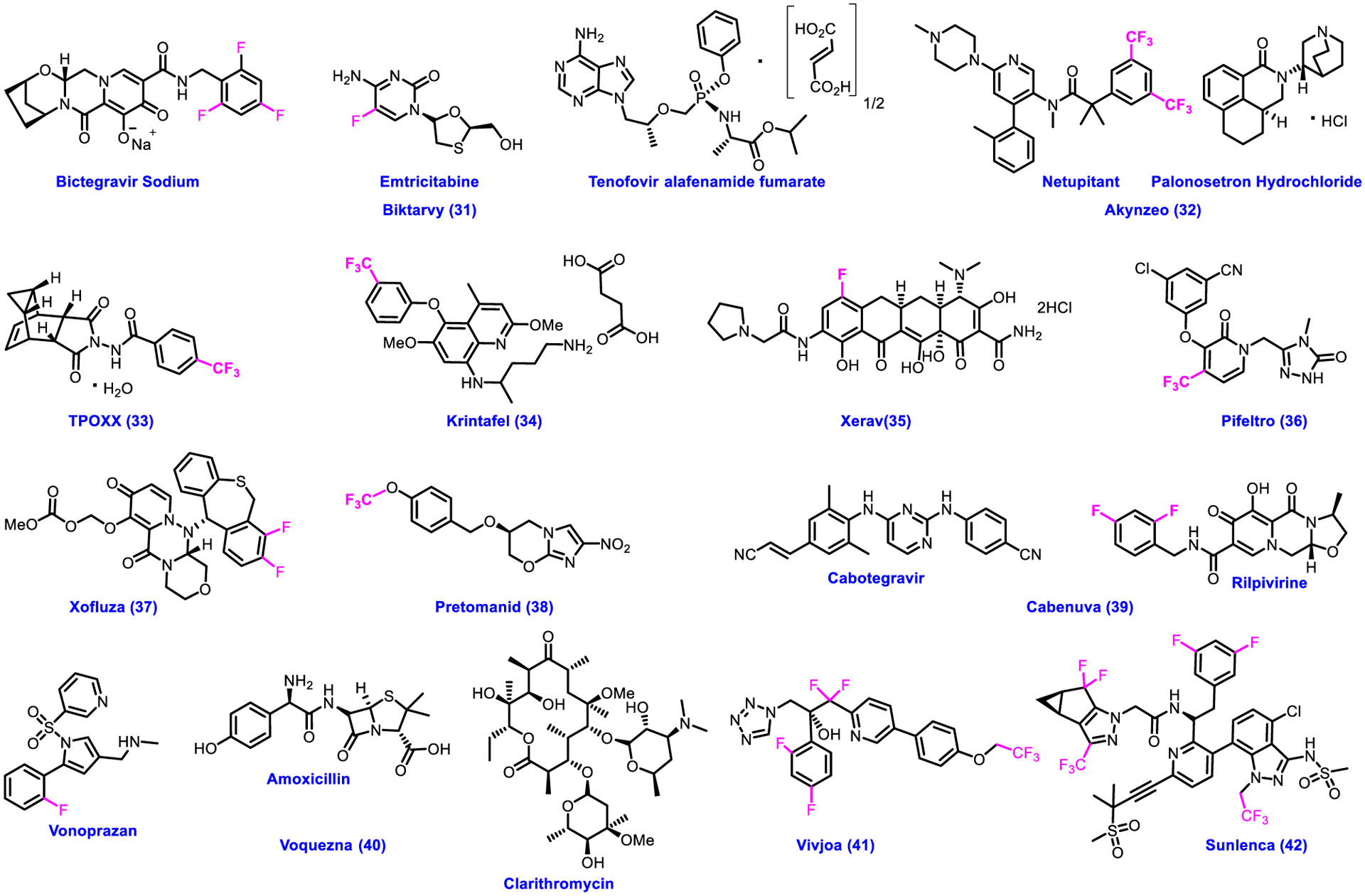
Chemical structures of U.S. FDA-approved drugs for infectious diseases from 2018 to 2022.
Table 2.
U.S. FDA-Approved Fluorinated Drugs for Infectious Diseases (2018–2022).
| Drug | Approval Date | Composition | Company | Indication | Drug Mechanism |
|---|---|---|---|---|---|
| Biktarvy | 2/7/2018 | Bictegravir Sodium, Emtricitabine and Tenofovir alafenamide fumarate | Gilead Sciences | HIV infection | Integrase strand transfer inhibitor, two HIV nucleoside analog reverse transcriptase inhibitors |
| Akynzeo | 4/19/2018 | Netupitant and palonosetron | Eisai | Nausea | NK1 receptor antagonist and 5-HT3 receptor antagonist |
| TPOXX | 7/13/2018 | Tecovirimat | SigaTechnologies | Smallpox | Orthopoxvirus VP37 envelope wrapping protein inhibitor |
| Krintafel | 7/20/2018 | Tafenoquine | GlaxoSmithKline | Malaria relapse prevention | 8-aminoquinoline antiparasitic |
| Xerava | 8/27/2018 | Eravacycline | Tetraphase Pharmaceuticals | Complicated intraabdominal infections | Antibiotic, binds 30S ribosomal subunit to block protein synthesis |
| Pifeltro | 8/30/2018 | Doravirine | Merck & Co. | HIV-1 infection | Antiviral, reverse transcriptase inhibitor |
| Xofluza | 10/24/2018 | Baloxavir marboxil | Shionogi, Genentech | Influenza | Antiviral inhibitor of influenza polymerase acidic protein |
| Pretomanid | 8/14/2019 | Pretomanid | Global Alliance for TB Drug Development | Treatmentresistant tuberculosis | Nitroimidazooxazine antimycobacterial |
| Cabenuva | 1/21/2021 | Cabotegravir and Rilpivirine | ViiV Healthcare | HIV | HIV-1 antiretrovirals |
| Voquezna | 5/3/2022 | Vonoprazan, Amoxicillin, and Carithromycin | Phathom Pharmaceuticals | Helicobacter Pylori Infection | Proton pump inhibitor and antimicrobials |
| Vivjoa | 4/26/2022 | Oteseconazole | Mycovia Pharmaceuticals | Recurrent vulvovaginal candidiasis | 14α-Demethylase inhibitor |
| Sunlenca | 12/22/2022 | Lenacapavir | Gilead Sciences | HIV infections in adults that cannot be successfully treated with other available treatments | HIV-1 antiretroviral agent |
The source of the data is the U.S. FDA Web site [51].
Xofluza 37 is an antiviral inhibitor of influenza polymerase acidic protein, which is used to treat influenza (flu). Pretomanid 38 is a nitroimidazooxazine antimycobacterial drug to treat lung tuberculosis (TB). It is used to treat TB when other available drugs do not work. Voquezna 40 is composed of three components, such as Vonoprazan, Amoxicillin, and Carithromycin. It acts as a proton pump inhibitor and antimicrobials to treat helicobacter pylori infection. Vivjoa 41 was approved as a 14α-demethylase inhibitor to treat the recurrent vulvovaginal candidiasis. Structurally, fluorine atoms and/or CF3 group are directly attached to the aromatic rings in drugs 31-37 and 39-42 (Fig. 4). As we discussed early, fluorine or CF3 group may be playing an essential role in improving metabolic stability (decease in clearance) [59–63], decreasing pKa [64–70], increasing the membrane permeability [71–75] and enhancing the binding affinity to the target proteins (improving potency) [76–80]. Interestingly, the OCF3 group attached to the aromatic ring in 38 (Fig. 4) may increase the metabolic stability because demethylation of OCF3 is less readily when compared with that of OMe [94].
4. U.S. FDA-Approved Fluorinated CNS Drugs (2018–2022)
Central nervous system disorders include a wide range of diseases, such as schizophrenia, Alzheimer’s disease (AD), Parkinson’s disease (PD), depression, migraines, insomnia, and many more that are major health issues across the globe in the present time. As per a recent report, CNS disorders has contributed 11.6% of global disability adjusted life years (DALYs) and 16.5% (2nd leading cause) of deaths from all causes [95]. Highly sophisticated protection system of brain makes the CNS research one of most challenging in the drug discovery and development. Excitingly, in the past five years, the CDER of the U.S. FDA approved a total of ten fluorinecontaining drugs 43–52 as therapeutics for the CNS disorders (Fig. 5; Table 3). Mayzent 43 was approved as a selective sphingosine 1-phosphate receptor modulator for the treatment of adults with relapsing forms of multiple sclerosis (RMS), such as clinically isolated syndrome, active secondary progressive disease, and relapsing-remitting disease. Fluorodopa F-18 44 was approved as a diagnostic agent used in positron emission tomography to detect Parkinsonian Syndromes (PS) in adults [96–99]. Tauvid 49 was another approved drug for use as a diagnostic agent in positron emission tomography to identify the aggregated neurofibrillary tangles (NFTs) in the brain of patients with suspected AD [100–102].
Fig. 5.
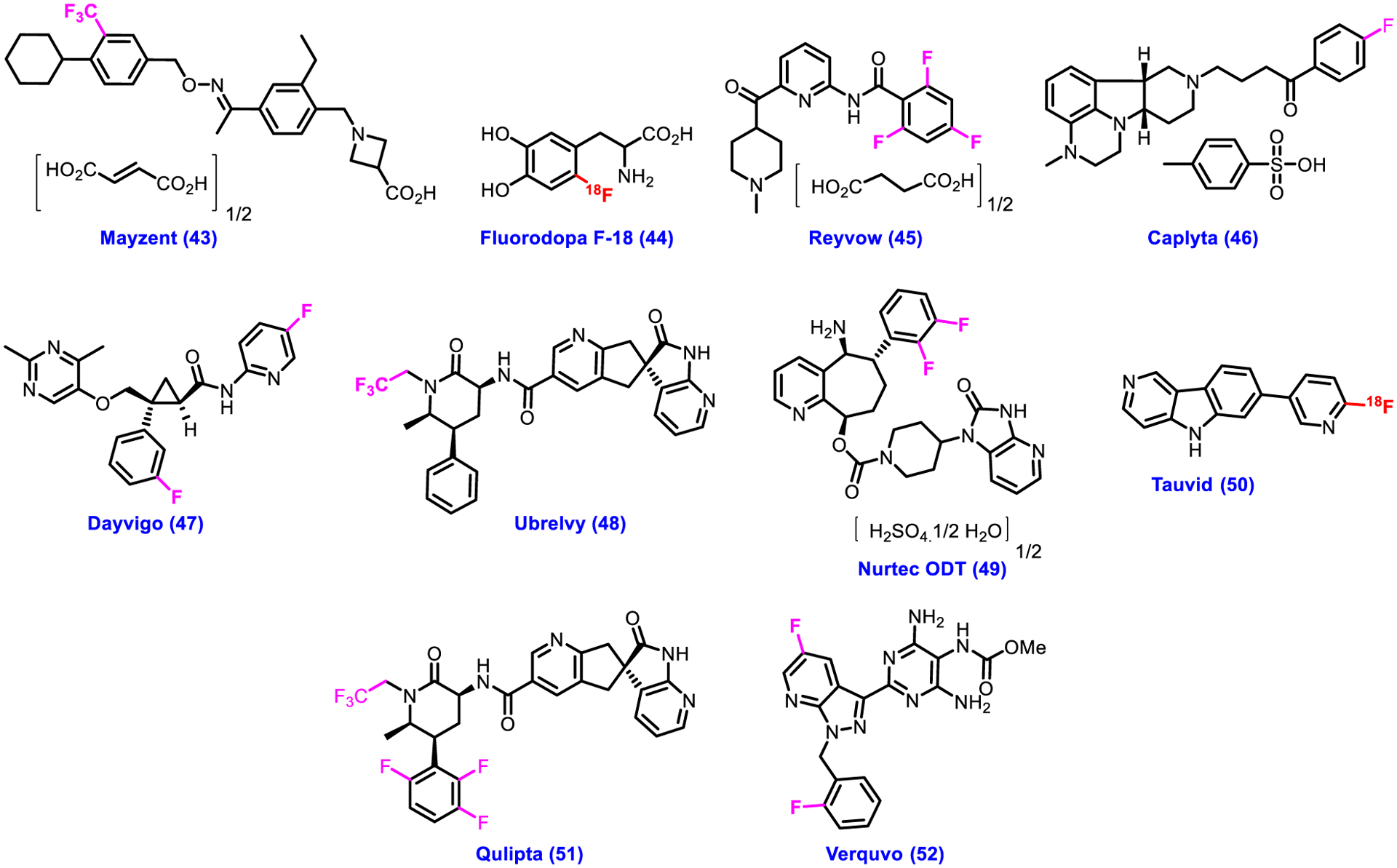
Chemical structures of U.S. FDA-approved CNS drugs from 2018 to 2022.
Table 3.
U.S. FDA-Approved Fluorinated Drugs for CNS Disorders (2018–2022).
| Drug | Approval Date | Composition | Company | Indication | Drug Mechanism |
|---|---|---|---|---|---|
| Mayzent | 3/26/2019 | Siponimod | Novartis | Relapsing forms of multiple sclerosis | Selective sphingosine 1- phosphate receptor modulator |
| Fluorodopa F-18 | 10/10/2019 | Fluorodopa F- 18 | none | Diagnostic for Parkinson’s disease | Positron-emission tomography |
| Reyvow | 10/11/20219 | Lasmiditan | Eli Lilly and Company | Acute migraines | 5-HT1F receptor agonist |
| Caplyta | 12/20/2019 | Lumateperone tosylate | Intra-Cellular Therapies | Schizophrenia | Unknown |
| Dayvigo | 12/20/2019 | Lemborexant | Eisai | Insomnia | Orexin receptor antagonist |
| Ubrelvy | 12/23/2019 | Ubrogepant | Allergan | Acute migraines | CGRP receptor antagonist |
| Nurtec ODT | 2/27/2020 | Rimegepant | Biohaven Pharmaceutical | Migraine | Calcitonin gene-related peptide receptor antagonist |
| Tauvid | 5/28/2020 | Flortaucipir F18 | Eli Lilly and Company | Alzheimer’s disease diagnostic | Radiodiagnostic |
| Qulipta | 9/28/2021 | Atogepant | AbbVie | Episodic migraines | Calcitonin gene-related peptide receptor antagonist |
| Verquvo | 1/19/2021 | Vericiguat | Merck & Co | Chronic heart failure | Soluble guanylate cyclase stimulator |
The source of the data is the U.S. FDA Web site [51].
FDA also accepted Reyvow 45, Ubrelvy 48, Nurtec ODT 49, and Qulipta 51 for migraines as 5-HT1F receptor agonist, CGRP receptor antagonist, calcitonin gene-related peptide receptor antagonist, and calcitonin gene–related peptide receptor antagonist, respectively.
Caplyta 46 is a drug used to treat schizophrenia. Dayvigo 47 was approved for the treatment of insomnia as orexin receptor antagonist. Verquvo 52 is a soluble guanylate cyclase stimulator that alleviates the risk of cardiovascular death and hospitalization. The role of fluorine or CF3 group in drugs 43–52 (Fig. 5) can be explained as per the discussion in sections 2 and 3 above.
5. U.S. FDA-Approved Fluorinated Drugs for Other Diseases (2018–2022)
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutation in the cystic fibrosis trans-membrane conductance regulator (CFTR) gene [103, 104]. It is most commonly found at least 1 out 3500 births in Caucasia population [105], 1 out of 3000 births in north Europeans, and 1 out of 30000 in Asian Americans [106]. Immune thrombocytopenia (ITP) is a kind of platelet disorder that is caused due to the destruction of platelets by immune system. Low platelet counts lead to easy bleeding and bruising. Endometriosis is a chronic with most common symptoms, such as infertility and pelvic pain [107, 108]. It is a gynecological disorder occurring in around 10% of women of reproductive age [109]. The prime cause of this debilitating disease is the presence of endometrial tissues outside the uterus where the implanted cells secrete multiple cytokines and prostaglandin E2 that obtain an inflammatory response [107, 108]. Rheumatoid arthritis (RA) is a chronic disease affecting joints as well as extra-articular or organs, including kidney, eye, digestive system, heart, lung, skin, and nerve system [110, 111]. Antineutrophil cytoplasm antibody-associated vasculitides are small-vessel vasculitides that include eosinophilic granulomatosis with polyangiitis, granulomatosis with polyangiitis, and microscopic polyangiitis [112–114]. In addition to drugs to combat cancers, infectious diseases, and CNS disorders, the CDER of U.S. FDA approved six drugs 53–58 having fluorine for the treatment of some other diseases as described above (Fig. 6; Table 4). Symdeko 53 and Trikafta 57 were approved to treat cystic fibrosis (CF). Symdeko 53 is a combination of Tezacaftor and Ivacaftor, which functions as a CFTR corrector and a CFTR potentiator [115]. Trikafta 57 has three active ingredients, such as Tezacaftor, Ivacaftor and Elexacaftor that acts as CCFTR corrector, CFTR potentiator, and CFTR corrector [116]. As a SYK inhibitor, Tavalisse 54 was approved to treat chronic immune thrombocytopenia. Orilissa 55 is a drug for moderate to severe pain associated with endometriosis, which acts as a GnRH receptor antagonist [117]. To treat adults with moderately to severely active rheumatoid arthritis, Rinvoq 56 was approved as a JAK inhibitor. Tavneos 58 is a C5a receptor antagonist utilized to treat severe active antineutrophil cytoplasmic autoantibody–associated vasculitis [118]. The critical role of fluorine atoms and/or CF3 group in drugs 53–58 (Fig. 6) can also be manifested as discussed earlier from the structure-activity/property relationship perspective.
Fig. 6.
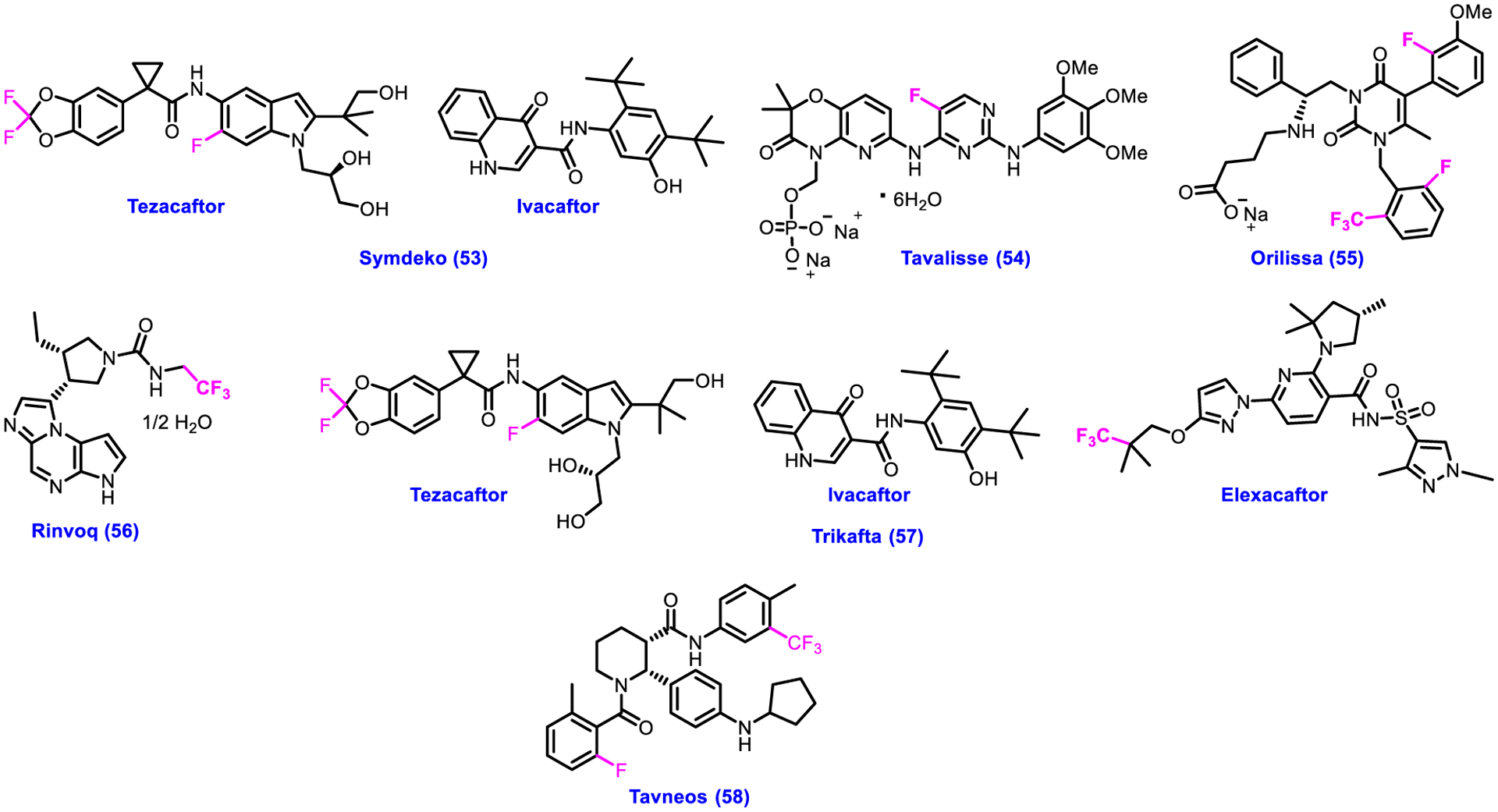
Chemical structures of U.S. FDA-approved drugs for other diseases from 2018 to 2022.
Table 4.
U.S. FDA-Approved Fluorinated Drugs for Other Diseases (2018–2022).
| Drug | Approval Date | Composition | Company | Indication | Drug Mechanism |
|---|---|---|---|---|---|
| Symdeko | 2/13/2018 | Tezacaftor and Ivacaftor | Vertex Pharmaceuticals | Cystic Fibrosis | CFTR corrector and CFTR potentiator |
| Tavalisse | 4/17/2018 | Fostamatinib | Rigel Pharmaceuticals | Chronic immune thrombocytopenia | SYK inhibitor |
| Orilissa | 7/23/2018 | Elagolix sodium | AbbVie | Endometriosis | GnRH receptor antagonist |
| Rinvoq | 8/16/2019 | Upadacitinib | AbbVie | Rheumatoid arthritis | JAK inhibitor |
| Trikafta | 10/21/2019 | Tezacaftor, Ivacaftor and Elexacaftor | Vertex Pharmaceuticals | Cystic Fibrosis | CCFTR corrector, CFTR potentiator and CFTR corrector |
| Tavneos | 10/7/2021 | Avacopan | ChemoCentryx | Severe active antineutrophil cytoplasmic autoantibody- associated vasculitis | C5a receptor antagonist |
The source of the data is the U.S. FDA Web site [51].
6. Summary and Conclusion
This review has highlighted fluorinated drugs approved by the CDER of U.S. FDA over the past five years (2018–2022). It is noteworthy that 58 out of 247 representing nearly a quarter of the total are fluorine-containing drugs. Each of these drugs has been summarized and briefly discussed based on their therapeutic application areas, such as cancers, infectious diseases, CNS disorders and some other diseases. Despite the great challenge in finding the anticancer drugs given that each kind of cancer requires different specific diagnosis and target-based treatment strategies, FDA approved thirty fluorinated drugs 1–30 to diagnose, mitigate and treat various types of cancers including prostate, melanoma, acute myeloid, philadelphia chromosome–positive chronic myeloid leukemia, lung, breast cancer and so on in the last five years. Among them, Cerianna 19 and Pylarify 26 were approved as diagnostic imaging agents for their use in positron emission tomography to detect breast and prostate cancer, respectively. Twelve fluorine-containing drugs 31–42 received approval to treat a big array of infectious diseases including HIV, nausea, smallpox, influenza and many more. CNS research is one of the most challenging in the drug discovery and development due to highly sophisticated protection system of brain. Nevertheless, in the past five years, FDA approved ten fluorinated drugs 43–52 as novel therapeutic agents for CNS disorders, such as schizophrenia, insomnia, migraines, and Parkinson’s disease. In addition, Fluorodopa F-18 44 and Tauvid 50 were approved for the diagnosis of Parkinson’s disease and Alzheimer’s disease. Last but not least, FDA approved six fluorinated drugs 53–58 for other diseases, such as cystic fibrosis, chronic immune thrombocytopenia, endometriosis, rheumatoid arthritis, and severe active antineutrophil cytoplasmic autoantibody–associated vasculitis. In summary, the unique and successful applications of the fluorine and/or fluorine-containing groups may inspire the drug discovery and medicinal chemistry community in both industrial and academic settings to explore the fluorinated molecules in the early rational drug design process.
7. Future Perspectives
The prevalence of fluorine or groups of fluorine atoms or its isotope in the FDA-approved drugs manifests its significant role in the pharmaceutical research in terms of diagnosis and therapeutics. Judicious deployment of fluorine or groups of fluorine atoms in a molecule under investigation may lead to enhanced pharmacological and pharmacokinetic profiles with improved potency, decrease in pKa, higher permeability, decrease in clearance, and conformational constraint [31–34]. Inspired by the outstanding performance, sustainability, and a high pace of approval of fluorinated drugs in the past decade, pharmaceutical companies continued to explore fluorine-containing entities in an expectation to discover new medications to combat deadly diseases. It is noteworthy that FDA granted approval to four fluorine-containing drugs 59–62 within merely three months of 2023 (Fig. 7). FDA approved Rykindo 59 and Jaypirca 60 for the treatment of schizophrenia and mantle cell lymphoma, respectively. Subsequently, Skyclarys 61 received approval for the therapy of Friedrich’s Ataxia. In addition, FDA granted approval to Joenja 62 for the treatment of activated phosphoinositide 3-kinase delta syndrome. The approval of these four drugs within such a short period of this year warrants that fluorine or group of fluorine atoms play a pivotal role in the pharmaceutical industry, indicating a great potential in the future trends of drug discovery and development.
Fig. 7.
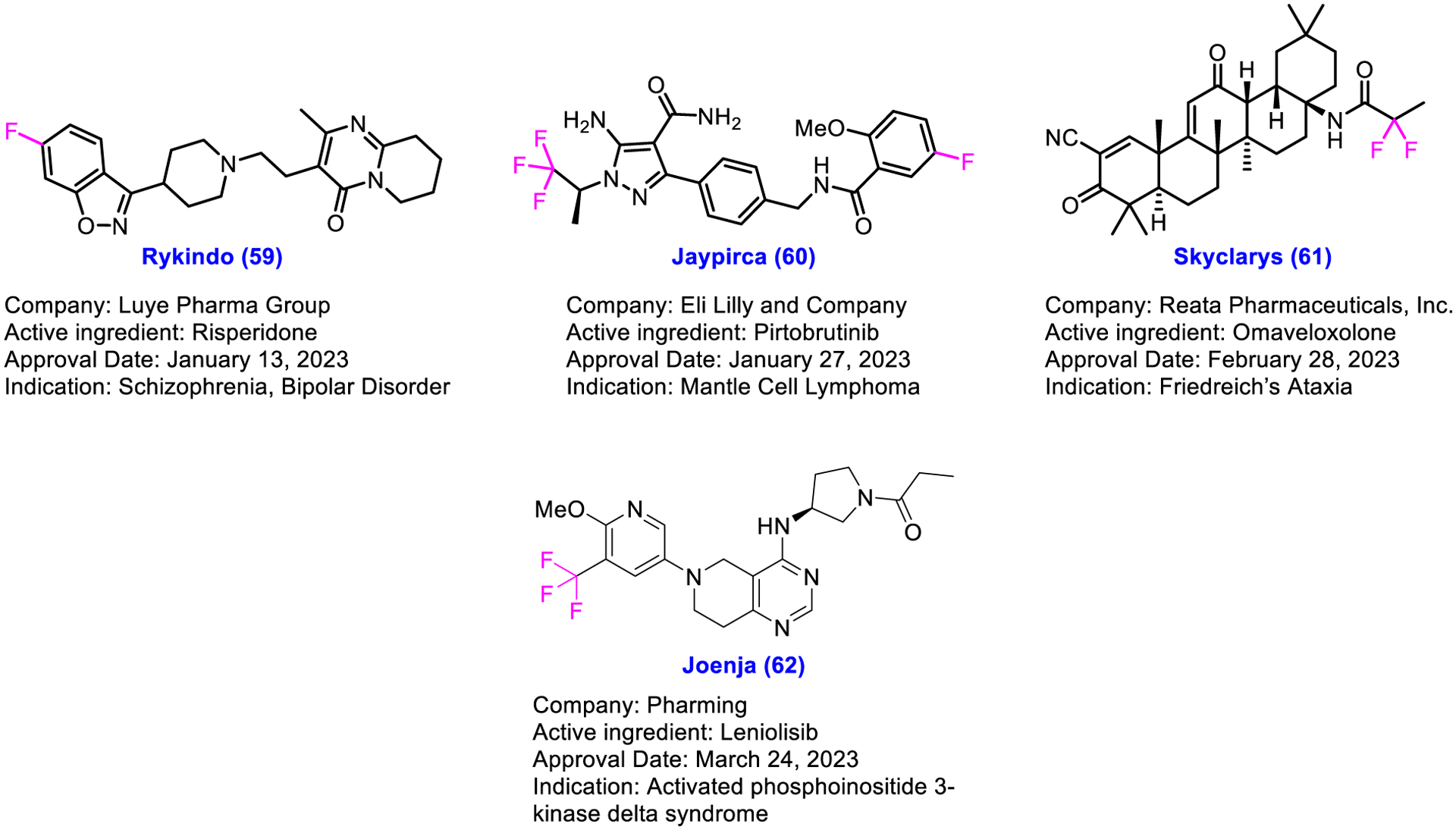
Chemical structures of U.S. FDA-approved fluorinated drugs in the first quarter of 2023.
During the literature survey, excitingly we came across a number of fluorinated investigational new drugs (INDs) under different phases of clinical trials for the treatment of various human diseases [125]. Some of these INDs with their name (application number), indication, drug mechanism, and phases have been described. There are several fluorinated INDs under clinical trials for the treatment of various types of cancers that are discussed along one by one. BAY 2965501 (NCT05614102) is the first-in-class diacylglycerol kinase zeta inhibitor (DGKzi), which is being evaluated in phase I for the treatment of advanced solid tumor. BI 907828 (NCT05372367) is another MDM2 inhibitor in phase I studies to treat solid tumor [119]. MRTX1133 (NCT05737706) is a potent, selective, and non-covalent KRAS inhibitor [120]. It is in phase I/II evaluation for solid tumor (advanced solid tumor, non-small cell lung cancer, colorectal cancer, and pancreatic adenocarcinoma). CFT1946 (NCT05668585) is under phase I/II study to characterize the safety, tolerability, and preliminary efficacy as monotherapy and in combination with trametinib in patients with BRAF V600 mutant solid tumors. MRTX 1719 (NCT05245500) is in phase I/II evaluation in solid tumors with MTAP deletion. GB 1211 (NCT05240131) is a galectin-3 inhibitor being tested in phase I for the treatment of non-small cell lung cancer. AZD9833 (NCT04818632) is under investigation in phase I for the treatment of breast cancer. Additionally, BLU-945 (NCT04862780) is another IND in the phase I/II, in the patients with EGFR mutant non-small cell lung cancer. Ziftomenib (NCT04067336) is an inhibitor of menin-MLL(KMT2A), which is in phase I/II clinical studies to treat patients with relapsed or refractory acute myeloid leukemia. Mezigdomide (NCT03989414) in under investigation in the phase I/II clinical trial in participants with relapsed or refractory multiple myeloma and newly diagnosed multiple myeloma. AZD4573 (NCT05140382) is in phase II for multicenter study to assess AZD4573 efficacy and safety as monotherapy or in combination with anti-cancer agents in patients with relapsed/refractory peripheral t-cell lymphoma or classical hodgkin lymphoma. Nirogacestat (NCT05348356) is in phase II clinical trial to learn its effectiveness in ovarian granulosa cell tumors (OvGCTs). Nirogacestat is a γ-secretase inhibitor (GSI), which is hypothesized to decrease the growth and activity of ovarian granulosa tumors. Inavolisib (NCT05306041) is a PI3K Inhibitor, which is under evaluation in phase II for the treatment of HER2-positive breast cancer [121]. Paltusotine (NCT05361668) is in phase II to evaluate the safety, PK, and dose response in subjects with carcinoid syndrome. Emraclidine (CVL-231) (NCT05443724) is a selective M4 receptor PAM, which is currently in phase II clinical trial to evaluate safety and tolerability in adult patients with Schizophrenia [122]. In addition, as a novel GlyT1 inhibitor, BI 425809 (NCT05211947) is under phase III studies to evaluate the long-term safety in the patients with Schizophrenia [123]. MK-0616 (NCT05070390) is a macrocyclic peptide that binds to PCSK9 and inhibits the interaction of PCSK9 with LDL receptors. It is in phase I being tested in participants with moderate renal impairment. Zunsemetinib (NCT05511519) is an investigational oral mitogen-activated protein kinase-activated protein kinase 2 (MK2) inhibitor. Currently, it is in phase II study to investigate the efficacy, safety, tolerability, and PK/PD of ATI-450 versus placebo in patients with moderate to severe psoriatic arthritis. Asundexian (NCT05686070) is an FXIa Inhibitor [124]. It is under investigation in phase III to learn more about asundexian for prevention of ischemic stroke in male and female patients aged 18 years and older who already had such a stroke due to a blood clot that formed outside the heart and travelled to the brain. In the view of above, we anticipate that some of these fluorine containing INDs in different phases of clinical trials will reach to FDA-approval for clinic use.
Collectively, we believe that this concise review may further attract the attention of the drug discovery and medicinal chemistry community in both industrial and academic settings towards the intelligent application of fluorine and fluorine-containing groups in rational drug design to bring more and better medications to the clinic to improve the health and enhance the life expectancy of human beings.
Highlights.
Small molecule FDA-approved fluorinated drugs in the past five years have been reviewed.
The therapeutic areas of these drugs such as cancers, infections and CNS disorders are classified.
Drug action targets and mechanisms of fluorinated drugs are provided.
The attempts to rationalize the presence of fluorination in approved drugs have been made.
Perspectives for the future of fluorinated drug development are discussed.
Acknowledgements
This work is partly supported by the grant R01 DA038446 from the National Institutes of Health, the John D. Stobo, M.D. Distinguished Chair Endowment Fund (JZ), and Edith & Robert Zinn Chair in Drug Discovery Endowment Fund (JZ).
Declaration of competing interest
SA and JZ are partially supported by the pharmaceutical industry MapLight Therapeutics, Inc. through an industry-funded collaborative research project, which might be relevant, but not directly related to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Fried J, Sabo EF, Synthesis of 17α-hydroxycorticosterone and its 9α-halo derivatives from 11-epi-17α-hydroxycorticosterone, J. Am. Chem. Soc, 75 (1953) 2273–2274. [Google Scholar]
- [2].Fried J, Sabo EF, 9α-Fluoro derivatives of cortisone and hydrocortisone, J. Am. Chem. Soc, 76 (1954) 1455–1456. [Google Scholar]
- [3].Heidelberger C, Chaudhuri N, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer R, Pleven E, Scheiner J, Fluorinated pyrimidines, a new class of tumour-inhibitory compounds, Nature, 179 (1957) 663–666. [DOI] [PubMed] [Google Scholar]
- [4].Longley DB, Harkin DP, Johnston PG, 5-fluorouracil: mechanisms of action and clinical strategies, Nat. Rev. Cancer, 3 (2003) 330–338. [DOI] [PubMed] [Google Scholar]
- [5].O’Hagan D, Fluorine in health care: Organofluorine containing blockbuster drugs, J. Fluorine Chem, 131 (2010) 1071–1081. [Google Scholar]
- [6].Tobert JA, Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors, Nat. Rev. Drug Discov, 2 (2003) 517–526. [DOI] [PubMed] [Google Scholar]
- [7].Roesky HW, Sharpless KB, Efficient Preparations of Fluorine Compounds, 1st ed. ed., Wiley, Chichester, 2012. [Google Scholar]
- [8].Gouverneur V, Muller K, Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, 1st ed. ed., Imperial College Press, London, 2012. [Google Scholar]
- [9].Godhaviya PK, Studies of Fluorine: History, Application, water Fluoridation, Organo Fluorine, 1st ed. ed., CreateSpace Independent Publishing Platform, 2015. [Google Scholar]
- [10].Groult H, Leroux F, Tressaud A, Modern Synthesis Processes and Reactivity of Fluorinated Compounds: Progress in Fluorine Science, 1st ed. ed., Elsevier, Amsterdam, 2016. [Google Scholar]
- [11].Haufe G, Leroux F, Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals: Progress in Fluorine Science Series, 1st ed. ed., Academic Press, 2018. [Google Scholar]
- [12].Fang C, Jing Y, Zong Y, Lin Z, Preparation and characterization of fluorine-containing acrylic latex PSAs using a reactive surfactant, J. Fluorine Chem, 192 (2016) 113–119. [Google Scholar]
- [13].Liu H, Liu S, Miao Z, Deng Z, Shen B, Hong X, Cheng Z, Development of 18F-labeled picolinamide probes for PET imaging of malignant melanoma, J. Med. Chem, 56 (2013) 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hong H, Zhang L, Xie F, Zhuang R, Jiang D, Liu H, Li J, Yang H, Zhang X, Nie L, Li Z, Rapid one-step (18)F-radiolabeling of biomolecules in aqueous media by organophosphine fluoride acceptors, Nat. Commun, 10 (2019) 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mabuchi S, Komura N, Sasano T, Shimura K, Yokoi E, Kozasa K, Kuroda H, Takahashi R, Kawano M, Matsumoto Y, Kato H, Hatazawa J, Kimura T, Pretreatment tumor-related leukocytosis misleads positron emission tomography-computed tomography during lymph node staging in gynecological malignancies, Nat. Commun, 11 (2020) 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Syvanen S, Fang XT, Faresjo R, Rokka J, Lannfelt L, Olberg DE, Eriksson J, Sehlin D, Fluorine-18-Labeled Antibody Ligands for PET Imaging of Amyloid-beta in Brain, ACS Chem. Neurosci, 11 (2020) 4460–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hoffmann C, Evcuman S, Neumaier F, Zlatopolskiy BD, Humpert S, Bier D, Holschbach M, Schulze A, Endepols H, Neumaier B, [(18)F]ALX5406: A Brain-Penetrating Prodrug for GlyT1-Specific PET Imaging, ACS. Chem. Neurosci, 12 (2021) 3335–3346. [DOI] [PubMed] [Google Scholar]
- [18].Fu Y, Helbert H, Simeth NA, Crespi S, Spoelstra GB, van Dijl JM, van Oosten M, Nazario LR, van der Born D, Luurtsema G, Szymanski W, Elsinga PH, Feringa BL, Ultrafast Photoclick Reaction for Selective (18)F-Positron Emission Tomography Tracer Synthesis in Flow, J. Am. Chem. Soc, 143 (2021) 10041–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li J, Valkenburgh JV, Fang J, Zhang D, Chen Y, Chen Q, Jia G, Chen AZ, Zhang X, Chen K, Development of a novel radiofluorinated riboflavin probe for riboflavin receptor-targeting PET imaging, Pharmacol. Res, 183 (2022) 106395. [DOI] [PubMed] [Google Scholar]
- [20].Van Valkenburgh J, Duro MVV, Burnham E, Chen Q, Wang S, Tran J, Kerman BE, Hwang SH, Liu X, Sta Maria NS, Zanderigo F, Croteau E, Rapoport SI, Cunnane SC, Jacobs RE, Yassine HN, Chen K, Radiosynthesis of 20-[(18)F]fluoroarachidonic acid for PET-MR imaging: Biological evaluation in ApoE4-TR mice, Prostaglandins Leukot. Essent. Fat. Acids, 186 (2022) 102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang J, Takahashi K, Shoup TM, Gong L, Li Y, El Fakhri G, Zhang Z, Brownell AL, Organomediated cleavage of benzoyl group enables an efficient synthesis of 1-(6-nitropyridin-2yl)thiourea and its application for developing (18)F-labeled PET tracers, Bioorg. Chem, 124 (2022) 105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang J, Moon SH, Cleary MB, Shoup TM, El Fakhri G, Zhang Z, Brownell AL, Detailed radiosynthesis of [(18) F]mG4P027 as a positron emission tomography radiotracer for mGluR4, J. Labelled Comp. Radiopharm, 66 (2023) 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tirotta I, Dichiarante V, Pigliacelli C, Cavallo G, Terraneo G, Bombelli FB, Metrangolo P, Resnati G, (19)F magnetic resonance imaging (MRI): from design of materials to clinical applications, Chem. Rev, 115 (2015) 1106–1129. [DOI] [PubMed] [Google Scholar]
- [24].Ahrens ET, Zhong J, In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection, NMR Biomed, 26 (2013) 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chapelin F, Gedaly R, Sweeney Z, Gossett LJ, Prognostic Value of Fluorine-19 MRI Oximetry Monitoring in cancer, Mol. Imaging Biol, 24 (2022) 208–219. [DOI] [PubMed] [Google Scholar]
- [26].Hequet E, Henoumont C, Muller RN, Laurent S, Fluorinated MRI contrast agents and their versatile applications in the biomedical field, Future Med. Chem, 11 (2019) 1157–1175. [DOI] [PubMed] [Google Scholar]
- [27].Yoder NC, Kumar K, Fluorinated amino acids in protein design and engineering, Chem. Soc. Rev, 31 (2002) 335–341. [DOI] [PubMed] [Google Scholar]
- [28].Salwiczek M, Nyakatura EK, Gerling UI, Ye S, Koksch B, Fluorinated amino acids: compatibility with native protein structures and effects on protein-protein interactions, Chem. Soc. Rev, 41 (2012) 2135–2171. [DOI] [PubMed] [Google Scholar]
- [29].Marsh EN, Suzuki Y, Using (19)F NMR to probe biological interactions of proteins and peptides, ACS Chem. Biol, 9 (2014) 1242–1250. [DOI] [PubMed] [Google Scholar]
- [30].Marsh EN, Fluorinated proteins: from design and synthesis to structure and stability, Acc. Chem. Res, 47 (2014) 2878–2886. [DOI] [PubMed] [Google Scholar]
- [31].Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA, Applications of Fluorine in Medicinal Chemistry, J. Med. Chem, 58 (2015) 8315–8359. [DOI] [PubMed] [Google Scholar]
- [32].Muller K, Faeh C, Diederich F, Fluorine in pharmaceuticals: looking beyond intuition, Science, 317 (2007) 1881–1886. [DOI] [PubMed] [Google Scholar]
- [33].Bohm HJ, Banner D, Bendels S, Kansy M, Kuhn B, Muller K, Obst-Sander U, Stahl M, Fluorine in medicinal chemistry, Chembiochem, 5 (2004) 637–643. [DOI] [PubMed] [Google Scholar]
- [34].Shah P, Westwell AD, The role of fluorine in medicinal chemistry, J. Enzyme Inhib. Med. Chem, 22 (2007) 527–540. [DOI] [PubMed] [Google Scholar]
- [35].Kim DW, Ahn DS, Oh YH, Lee S, Kil HS, Oh SJ, Lee SJ, Kim JS, Ryu JS, Moon DH, Chi DY, A new class of SN2 reactions catalyzed by protic solvents: Facile fluorination for isotopic labeling of diagnostic molecules, J. Am. Chem. Soc, 128 (2006) 16394–16397. [DOI] [PubMed] [Google Scholar]
- [36].Wu BB, Xu J, Bian KJ, Gao Q, Wang XS, Enantioselective Synthesis of Secondary beta-Trifluoromethyl Alcohols via Catalytic Asymmetric Reductive Trifluoroalkylation and Diastereoselective Reduction, J. Am. Chem. Soc, 144 (2022) 6543–6550. [DOI] [PubMed] [Google Scholar]
- [37].Wright SE, Bandar JS, A Base-Promoted Reductive Coupling Platform for the Divergent Defluorofunctionalization of Trifluoromethylarenes, J. Am. Chem. Soc, 144 (2022) 13032–13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Feng G, Ku CK, Zhao J, Wang Q, Copper-Catalyzed Three-Component Aminofluorination of Alkenes and 1,3-Dienes: Direct Entry to Diverse beta-Fluoroalkylamines, J. Am. Chem. Soc, 144 (2022) 20463–20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wei Z, Wen L, Zhu K, Wang Q, Zhao Y, Hu J, Regioselective Aromatic Perfluoro-tert-butylation Using Perfluoro-tert-butyl Phenyl Sulfone and Arynes, J. Am. Chem. Soc, 144 (2022) 22281–22288. [DOI] [PubMed] [Google Scholar]
- [40].Li Y, Xie F, Liu Y, Yang X, Li X, Regio- and Diastereoselective Access to Fused Isoxazolidines via Ru(II)-Catalyzed C-H Activation of Nitrones and Coupling with Perfluoroalkylolefins, Org. Lett, 20 (2018) 437–440. [DOI] [PubMed] [Google Scholar]
- [41].Mandal A, Jang J, Yang B, Kim H, Shin K, Palladium-Catalyzed Electrooxidative Hydrofluorination of Aryl-Substituted Alkenes with a Nucleophilic Fluorine Source, Org. Lett, 25 (2023) 195–199. [DOI] [PubMed] [Google Scholar]
- [42].Granados A, Dhungana RK, Sharique M, Majhi J, Molander GA, From Styrenes to Fluorinated Benzyl Bromides: A Photoinduced Difunctionalization via Atom Transfer Radical Addition, Org. Lett, 24 (2022) 4750–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matsuo B, Majhi J, Granados A, Sharique M, Martin RT, Gutierrez O, Molander GA, Transition metal-free photochemical C-F activation for the preparation of difluorinated-oxindole derivatives, Chem. Sci, 14 (2023) 2379–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Intermaggio NE, Millet A, Davis DL, MacMillan DWC, Deoxytrifluoromethylation of Alcohols, J. Am. Chem. Soc, 144 (2022) 11961–11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao X, MacMillan DWC, Metallaphotoredox Perfluoroalkylation of Organobromides, J. Am. Chem. Soc, 142 (2020) 19480–19486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mei H, Han J, Fustero S, Medio-Simon M, Sedgwick DM, Santi C, Ruzziconi R, Soloshonok VA, Fluorine-Containing Drugs Approved by the FDA in 2018, Chem. Eur. J, 25 (2019) 11797–11819. [DOI] [PubMed] [Google Scholar]
- [47].Mei H, Remete AM, Zou Y, Moriwaki H, Fustero S, Kiss L, Soloshonok VA, Han J, Fluorine-containing drugs approved by the FDA in 2019, Chin. Chem. Lett, 31 (2020) 2401–2413. [Google Scholar]
- [48].Yu Y, Liu A, Dhawan G, Mei H, Zhang W, Izawa K, Soloshonok VA, Han J, Fluorine-containing pharmaceuticals approved by the FDA in 2020: Synthesis and biological activity, Chin. Chem. Lett, 32 (2021) 3342–3354. [Google Scholar]
- [49].Inoue M, Sumii Y, Shibata N, Contribution of Organofluorine Compounds to Pharmaceuticals, ACS Omega, 5 (2020) 10633–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He J, Li Z, Dhawan G, Zhang W, Sorochinsky AE, Butler G, Soloshonok VA, Han J, Fluorine-containing drugs approved by the FDA in 2021, Chin. Chem. Lett, 34 (2023) 107578. [Google Scholar]
- [51].Drugs@fda: Fda-approved drugs: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=reportsSearch.process (accessed January 10, 2023), in.
- [52].Uttley L, Indave BI, Hyde C, White V, Lokuhetty D, Cree I, Invited commentary-WHO Classification of Tumours: How should tumors be classified? Expert consensus, systematic reviews or both?, Int. J. Cancer, 146 (2020) 3516–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics, 2023, CA. Cancer. J. Clin, 73 (2023) 17–48. [DOI] [PubMed] [Google Scholar]
- [54].Chabner BA, Roberts TG Jr., Timeline: Chemotherapy and the war on cancer, Nat. Rev. Cancer, 5 (2005) 65–72. [DOI] [PubMed] [Google Scholar]
- [55].Rhoads CP, Nitrogen mustards in the treatment of neoplastic disease; official statement, J. Am. Med. Assoc, 131 (1946) 656–658. [DOI] [PubMed] [Google Scholar]
- [56].Alam MR, Singh SB, Thapaliya S, Shrestha S, Deo S, Khanal K, A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer, Cureus, 14 (2022) e29369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Keam SJ, Piflufolastat F 18: Diagnostic First Approval, Mol. Diagn. Ther, 25 (2021) 647–656. [DOI] [PubMed] [Google Scholar]
- [58].Rowe SP, Buck A, Bundschuh RA, Lapa C, Serfling SE, Derlin T, Higuchi T, Gorin MA, Pomper MG, Werner RA, [18F]DCFPyL PET/CT for Imaging of Prostate Cancer, Nuklearmedizin, 61 (2022) 240–246. [DOI] [PubMed] [Google Scholar]
- [59].Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE, (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes, J. Med. Chem, 48 (2005) 141–151. [DOI] [PubMed] [Google Scholar]
- [60].Thornberry NA, Weber AE, Discovery of JANUVIA (Sitagliptin), a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes, Curr. Top. Med. Chem, 7 (2007) 557–568. [DOI] [PubMed] [Google Scholar]
- [61].Kim D, Kowalchick JE, Edmondson SD, Mastracchio A, Xu J, Eiermann GJ, Leiting B, Wu JK, Pryor KD, Patel RA, He H, Lyons KA, Thornberry NA, Weber AE, Triazolopiperazine-amides as dipeptidyl peptidase IV inhibitors: close analogs of JANUVIA (sitagliptin phosphate), Bioorg. Med. Chem. Lett, 17 (2007) 3373–3377. [DOI] [PubMed] [Google Scholar]
- [62].Clader JW, The discovery of ezetimibe: a view from outside the receptor, J. Med. Chem, 47 (2004) 1–9. [DOI] [PubMed] [Google Scholar]
- [63].Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR Jr., In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461, J. Pharmacol. Exp. Ther, 283 (1997) 157–163. [PubMed] [Google Scholar]
- [64].Qiu J, Stevenson SH, O’Beirne MJ, Silverman RB, 2,6-Difluorophenol as a bioisostere of a carboxylic acid: bioisosteric analogues of gamma-aminobutyric acid, J. Med. Chem, 42 (1999) 329–332. [DOI] [PubMed] [Google Scholar]
- [65].Nicolaou I, Zika C, Demopoulos VJ, [1-(3,5-difluoro-4-hydroxyphenyl)-1H-pyrrol-3-yl]phenylmethanone as a bioisostere of a carboxylic acid aldose reductase inhibitor, J. Med. Chem, 47 (2004) 2706–2709. [DOI] [PubMed] [Google Scholar]
- [66].Bey E, Marchais-Oberwinkler S, Kruchten P, Frotscher M, Werth R, Oster A, Algul O, Neugebauer A, Hartmann RW, Design, synthesis and biological evaluation of bis(hydroxyphenyl) azoles as potent and selective non-steroidal inhibitors of 17-betahydroxysteroid dehydrogenase type 1 (17beta-HSD1) for the treatment of estrogen-dependent diseases, Bioorg. Med. Chem, 16 (2008) 6423–6435. [DOI] [PubMed] [Google Scholar]
- [67].Bey E, Marchais-Oberwinkler S, Werth R, Negri M, Al-Soud YA, Kruchten P, Oster A, Frotscher M, Birk B, Hartmann RW, Design, synthesis, biological evaluation and pharmacokinetics of bis(hydroxyphenyl) substituted azoles, thiophenes, benzenes, and aza-benzenes as potent and selective nonsteroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 1 (17beta-HSD1), J. Med. Chem, 51 (2008) 6725–6739. [DOI] [PubMed] [Google Scholar]
- [68].Bey E, Marchais-Oberwinkler S, Negri M, Kruchten P, Oster A, Klein T, Spadaro A, Werth R, Frotscher M, Birk B, Hartmann RW, New insights into the SAR and binding modes of bis(hydroxyphenyl)thiophenes and -benzenes: influence of additional substituents on 17-betahydroxysteroid dehydrogenase type 1 (17beta-HSD1) inhibitory activity and selectivity, J. Med. Chem, 52 (2009) 6724–6743. [DOI] [PubMed] [Google Scholar]
- [69].Alexiou P, Demopoulos VJ, A diverse series of substituted benzenesulfonamides as aldose reductase inhibitors with antioxidant activity: design, synthesis, and in vitro activity, J. Med. Chem, 53 (2010) 7756–7766. [DOI] [PubMed] [Google Scholar]
- [70].Kotsampasakou E, Demopoulos VJ, Synthesis of derivatives of the keto-pyrroly-ldifluorophenol scaffold: some structural aspects for aldose reductase inhibitory activity and selectivity, Bioorg. Med. Chem, 21 (2013) 869–873. [DOI] [PubMed] [Google Scholar]
- [71].Pinto DJ, Orwat MJ, Wang S, Fevig JM, Quan ML, Amparo E, Cacciola J, Rossi KA, Alexander RS, Smallwood AM, Luettgen JM, Liang L, Aungst BJ, Wright MR, Knabb RM, Wong PC, Wexler RR, Lam PY, Discovery of 1-[3-(aminomethyl)phenyl]-N-3-fluoro-2’-(methylsulfonyl)-[1,1’-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC423), a highly potent, selective, and orally bioavailable inhibitor of blood coagulation factor Xa, J. Med. Chem, 44 (2001) 566–578. [DOI] [PubMed] [Google Scholar]
- [72].Quan ML, Lam PY, Han Q, Pinto DJ, He MY, Li R, Ellis CD, Clark CG, Teleha CA, Sun JH, Alexander RS, Bai S, Luettgen JM, Knabb RM, Wong PC, Wexler RR, Discovery of 1-(3’-aminobenzisoxazol-5’-yl)-3-trifluoromethyl-N-[2-fluoro-4- [(2’dimethylaminomethyl)imidazol-1-yl]phenyl]-1H-pyrazole-5-carboxyamide hydrochloride (razaxaban), a highly potent, selective, and orally bioavailable factor Xa inhibitor, J. Med. Chem, 48 (2005) 1729–1744. [DOI] [PubMed] [Google Scholar]
- [73].Kuduk SD, Di Marco CN, Chang RK, Wood MR, Schirripa KM, Kim JJ, Wai JM, DiPardo RM, Murphy KL, Ransom RW, Harrell CM, Reiss DR, Holahan MA, Cook J, Hess JF, Sain N, Urban MO, Tang C, Prueksaritanont T, Pettibone DJ, Bock MG, Development of orally bioavailable and CNS penetrant biphenylaminocyclopropane carboxamide bradykinin B1 receptor antagonists, J. Med. Chem, 50 (2007) 272–282. [DOI] [PubMed] [Google Scholar]
- [74].Ettorre A, D’Andrea P, Mauro S, Porcelloni M, Rossi C, Altamura M, Catalioto RM, Giuliani S, Maggi CA, Fattori D, hNK2 receptor antagonists. The use of intramolecular hydrogen bonding to increase solubility and membrane permeability, Bioorg. Med. Chem. Lett, 21 (2011) 1807–1809. [DOI] [PubMed] [Google Scholar]
- [75].Kaller MR, Harried SS, Albrecht B, Amarante P, Babu-Khan S, Bartberger MD, Brown J, Brown R, Chen K, Cheng Y, Citron M, Croghan MD, Graceffa R, Hickman D, Judd T, Kriemen C, La D, Li V, Lopez P, Luo Y, Masse C, Monenschein H, Nguyen T, Pennington LD, Miguel TS, Sickmier EA, Wahl RC, Weiss MM, Wen PH, Williamson T, Wood S, Xue M, Yang B, Zhang J, Patel V, Zhong W, Hitchcock S, A Potent and Orally Efficacious, Hydroxyethylamine-Based Inhibitor of beta-Secretase, ACS Med. Chem. Lett, 3 (2012) 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Olsen JA, Banner DW, Seiler P, Obst Sander U, D’Arcy A, Stihle M, Muller K, Diederich F, A fluorine scan of thrombin inhibitors to map the fluorophilicity/fluorophobicity of an enzyme active site: evidence for C-F…C=O interactions, Angew. Chem. Int. Ed. Engl, 42 (2003) 2507–2511. [DOI] [PubMed] [Google Scholar]
- [77].Garcia-Saez I, DeBonis S, Lopez R, Trucco F, Rousseau B, Thuery P, Kozielski F, Structure of human Eg5 in complex with a new monastrol-based inhibitor bound in the R configuration, J. Biol. Chem, 282 (2007) 9740–9747. [DOI] [PubMed] [Google Scholar]
- [78].Lee YK, Parks DJ, Lu T, Thieu TV, Markotan T, Pan W, McComsey DF, Milkiewicz KL, Crysler CS, Ninan N, Abad MC, Giardino EC, Maryanoff BE, Damiano BP, Player MR, 7-fluoroindazoles as potent and selective inhibitors of factor Xa, J. Med. Chem, 51 (2008) 282–297. [DOI] [PubMed] [Google Scholar]
- [79].Kaan HY, Ulaganathan V, Rath O, Prokopcova H, Dallinger D, Kappe CO, Kozielski F, Structural basis for inhibition of Eg5 by dihydropyrimidines: stereoselectivity of antimitotic inhibitors enastron, dimethylenastron and fluorastrol, J. Med. Chem, 53 (2010) 5676–5683. [DOI] [PubMed] [Google Scholar]
- [80].Anilkumar GN, Lesburg CA, Selyutin O, Rosenblum SB, Zeng Q, Jiang Y, Chan TY, Pu H, Vaccaro H, Wang L, Bennett F, Chen KX, Duca J, Gavalas S, Huang Y, Pinto P, Sannigrahi M, Velazquez F, Venkatraman S, Vibulbhan B, Agrawal S, Butkiewicz N, Feld B, Ferrari E, He Z, Jiang CK, Palermo RE, McMonagle P, Huang HC, Shih NY, Njoroge G, Kozlowski JA, Novel HCV I NS5B polymerase inhibitors: discovery of indole 2-carboxylic acids with C3-heterocycles, Bioorg. Med. Chem. Lett, 21 (2011) 5336–5341. [DOI] [PubMed] [Google Scholar]
- [81].Isabel E, Mellon C, Boyd MJ, Chauret N, Deschenes D, Desmarais S, Falgueyret JP, Gauthier JY, Khougaz K, Lau CK, Leger S, Levorse DA, Li CS, Masse F, Percival MD, Roy B, Scheigetz J, Therien M, Truong VL, Wesolowski G, Young RN, Zamboni R, Black WC, Difluoroethylamines as an amide isostere in inhibitors of cathepsin K, Bioorg. Med. Chem. Lett, 21 (2011) 920–923. [DOI] [PubMed] [Google Scholar]
- [82].Kerekes AD, Esposite SJ, Doll RJ, Tagat JR, Yu T, Xiao Y, Zhang Y, Prelusky DB, Tevar S, Gray K, Terracina GA, Lee S, Jones J, Liu M, Basso AD, Smith EB, Aurora kinase inhibitors based on the imidazo[1,2-a]pyrazine core: fluorine and deuterium incorporation improve oral absorption and exposure, J. Med. Chem, 54 (2011) 201–210. [DOI] [PubMed] [Google Scholar]
- [83].Miller MM, Liu Y, Jiang J, Johnson JA, Kamau M, Nirschl DS, Wang Y, Harikrishnan L, Taylor DS, Chen AY, Yin X, Seethala R, Peterson TL, Zvyaga T, Zhang J, Huang CS, Wexler RR, Poss MA, Lawrence RM, Adam LP, Salvati ME, Identification of a potent and metabolically stable series of fluorinated diphenylpyridylethanamine-based cholesteryl ester transfer protein inhibitors, Bioorg. Med. Chem. Lett, 22 (2012) 6503–6508. [DOI] [PubMed] [Google Scholar]
- [84].Xu R, Wang K, Rizzi JP, Huang H, Grina JA, Schlachter ST, Wang B, Wehn PM, Yang H, Dixon DD, Czerwinski RM, Du X, Ged EL, Han G, Tan H, Wong T, Xie S, Josey JA, Wallace EM, 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a Hypoxia-Inducible Factor 2alpha (HIF-2alpha) Inhibitor for the Treatment of Clear Cell Renal Cell Carcinoma, J. Med. Chem, 62 (2019) 6876–6893. [DOI] [PubMed] [Google Scholar]
- [85].Fell JB, Fischer JP, Baer BR, Blake JF, Bouhana K, Briere DM, Brown KD, Burgess LE, Burns AC, Burkard MR, Chiang H, Chicarelli MJ, Cook AW, Gaudino JJ, Hallin J, Hanson L, Hartley DP, Hicken EJ, Hingorani GP, Hinklin RJ, Mejia MJ, Olson P, Otten JN, Rhodes SP, Rodriguez ME, Savechenkov P, Smith DJ, Sudhakar N, Sullivan FX, Tang TP, Vigers GP, Wollenberg L, Christensen JG, Marx MA, Identification of the Clinical Development Candidate MRTX849, a Covalent KRAS(G12C) Inhibitor for the Treatment of Cancer, J. Med. Chem, 63 (2020) 6679–6693. [DOI] [PubMed] [Google Scholar]
- [86].Xu J, Berastegui-Cabrera J, Ye N, Carretero-Ledesma M, Pachon-Diaz J, Chen H, Pachon-Ibanez ME, Sanchez-Cespedes J, Zhou J, Discovery of Novel Substituted N-(4-Amino-2-chlorophenyl)-5-chloro-2-hydroxybenzamide Analogues as Potent Human Adenovirus Inhibitors, J. Med. Chem, 63 (2020) 12830–12852. [DOI] [PubMed] [Google Scholar]
- [87].Xu J, Xue Y, Zhou R, Shi PY, Li H, Zhou J, Drug repurposing approach to combating coronavirus: Potential drugs and drug targets, Med. Res. Rev, 41 (2021) 1375–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Xu J, Xue Y, Bolinger AA, Li J, Zhou M, Chen H, Li H, Zhou J, Therapeutic potential of salicylamide derivatives for combating viral infections, Med. Res. Rev, (2023) 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Samrat SK, Xu J, Li Z, Zhou J, Li H, Antiviral Agents against Flavivirus Protease: Prospect and Future Direction, Pathogens, 11 (2022) 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li Z, Xu J, Lang Y, Fan X, Kuo L, D’Brant L, Hu S, Samrat SK, Trudeau N, Tharappel AM, Rugenstein N, Koetzner CA, Zhang J, Chen H, Kramer LD, Butler D, Zhang QY, Zhou J, Li H, JMX0207, a Niclosamide Derivative with Improved Pharmacokinetics, Suppresses Zika Virus Infection Both In Vitro and In Vivo, ACS Infect. Dis, 6 (2020) 2616–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Samrat SK, Xu J, Xie X, Gianti E, Chen H, Zou J, Pattis JG, Elokely K, Lee H, Li Z, Klein ML, Shi PY, Zhou J, Li H, Allosteric inhibitors of the main protease of SARS-CoV-2, Antiviral Res, 205 (2022) 105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020, https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020, in, 2020.
- [93].Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF, Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses, Trends Micro., 24 (2016) 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Xu S, Zhu B, Teffera Y, Pan DE, Caldwell CG, Doss G, Stearns RA, Evans DC, Beconi MG, Metabolic activation of fluoropyrrolidine dipeptidyl peptidase-IV inhibitors by rat liver microsomes, Drug. Metab. Dispos, 33 (2005) 121–130. [DOI] [PubMed] [Google Scholar]
- [95].Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C, The global burden of neurological disorders: translating evidence into policy, Lancet Neurol, 19 (2020) 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhou LL, Wang SY, Tian YY, Jiang T, Chen YC, Huang T, Zhang YD, Wang F, Contralateral Posterior Putaminal (18)F-Fluorodopa Uptake in Mild Stage Parkinson’s Disease: A PET/CT Study, Curr Neurovasc Res, 18 (2021) 465–469. [DOI] [PubMed] [Google Scholar]
- [97].Thobois S, Ribeiro MJ, Lohmann E, Durr A, Pollak P, Rascol O, Guillouet S, Chapoy E, Costes N, Agid Y, Remy P, Brice A, Broussolle E, French G Parkinson’s Disease Genetics Study, Young-onset Parkinson disease with and without parkin gene mutations: a fluorodopa F 18 positron emission tomography study, Arch Neurol, 60 (2003) 713–718. [DOI] [PubMed] [Google Scholar]
- [98].Eisenberg DP, Lopez G, Gregory MD, Berman KF, Sidransky E, Comparison of Transcranial Sonography and [(18) F]-Fluorodopa PET Imaging in GBA1 Mutation Carriers, Mov Disord, 37 (2022) 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Borghammer P, Kumakura Y, Cumming P, Fluorodopa F 18 positron emission tomography and the progression of Parkinson disease, Arch. Neurol, 62 (2005) 1480; author reply 1480–1481. [DOI] [PubMed] [Google Scholar]
- [100].Cogswell PM, Wiste HJ, Senjem ML, Gunter JL, Weigand SD, Schwarz CG, Arani A, Therneau TM, Lowe VJ, Knopman DS, Botha H, Graff-Radford J, Jones DT, Kantarci K, Vemuri P, Boeve BF, Mielke MM, Petersen RC, Jack CR Jr., Associations of quantitative susceptibility mapping with Alzheimer’s disease clinical and imaging markers, Neuroimage, 224 (2021) 117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jie C, Treyer V, Schibli R, Mu L, Tauvid: The First FDA-Approved PET Tracer for Imaging Tau Pathology in Alzheimer’s Disease, Pharmaceuticals (Basel), 14 (2021) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mohammadi Z, Alizadeh H, Marton J, Cumming P, The Sensitivity of Tau Tracers for the Discrimination of Alzheimer’s Disease Patients and Healthy Controls by PET, Biomolecules, 13 (2023) 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Guggino WB, Banks-Schlegel SP, Macromolecular interactions and ion transport in cystic fibrosis, Am. J. Respir. Crit. Care Med, 170 (2004) 815–820. [DOI] [PubMed] [Google Scholar]
- [104].Johnson LG, Boyles SE, Wilson J, Boucher RC, Normalization of raised sodium absorption and raised calcium-mediated chloride secretion by adenovirus-mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells, J. Clin. Invest, 95 (1995) 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mehta G, Macek M Jr., Mehta A, European Registry Working G, Cystic fibrosis across Europe: EuroCareCF analysis of demographic data from 35 countries, J. Cyst. Fibros, 9 Suppl 2 (2010) S5–S21. [DOI] [PubMed] [Google Scholar]
- [106].Klimova B, Kuca K, Novotny M, Maresova P, Cystic Fibrosis Revisited - a Review Study, Med. Chem, 13 (2017) 102–109. [DOI] [PubMed] [Google Scholar]
- [107].Bulun SE, Endometriosis N. Engl. J. Med, 360 (2009) 268–279. [DOI] [PubMed] [Google Scholar]
- [108].Giudice LC, Clinical practice. Endometriosis, N. Engl. J. Med, 362 (2010) 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Fuldeore MJ, Soliman AM, Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women, Gynecol. Obstet. Invest, 82 (2017) 453–461. [DOI] [PubMed] [Google Scholar]
- [110].Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F, Giacomelli R, Cipriani P, Beyond the joints, the extra-articular manifestations in rheumatoid arthritis, Autoimmun Rev., 20 (2021) 102735. [DOI] [PubMed] [Google Scholar]
- [111].Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R, Extra-articular Manifestations in Rheumatoid Arthritis, Maedica. (Bucur) 5 (2010) 286–291. [PMC free article] [PubMed] [Google Scholar]
- [112].Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA, 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides, Arthritis Rheum, 65 (2013) 1–11. [DOI] [PubMed] [Google Scholar]
- [113].Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, et al. , The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis), Arthritis Rheum, 33 (1990) 1094–1100. [DOI] [PubMed] [Google Scholar]
- [114].Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, Calabrese LH, Fries JF, Lie JT, Lightfoot RW Jr., et al. , The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis, Arthritis Rheum, 33 (1990) 1101–1107. [DOI] [PubMed] [Google Scholar]
- [115].Tezacaftor/Ivacaftor (Symdeko) for cystic fibrosis, Med. Lett. Drugs Ther, 60 (2018) 174–176. [PubMed] [Google Scholar]
- [116].Elexacaftor/tezacaftor/ivacaftor (Trikafta) for cystic fibrosis, Med. Lett. Drugs. Ther, 62 (2020) 5–7. [PubMed] [Google Scholar]
- [117].Elagolix (Orilissa)--an oral GnRH antagonist for endometriosis pain, Med. Lett. Drugs. Ther, 60 (2018) 158–160. [PubMed] [Google Scholar]
- [118].Jayne DRW, Merkel PA, Schall TJ, Bekker P, Group AS, Avacopan for the Treatment of ANCA-Associated Vasculitis, N. Engl. J. Med, 384 (2021) 599–609. [DOI] [PubMed] [Google Scholar]
- [119].Hao X, Bahia R, Cseh O, Bozek D, Blake S, Rinnenthal J, Weyer-Czernilofsky U, Rudolph D, Luchman HA, BI-907828, a novel potent MDM2 inhibitor, inhibits GBM brain tumor stem cells in vitro and prolongs survival in orthotopic xenograft mouse models, Neuro. Oncol, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wang X, Allen S, Blake JF, Bowcut V, Briere DM, Calinisan A, Dahlke JR, Fell JB, Fischer JP, Gunn RJ, Hallin J, Laguer J, Lawson JD, Medwid J, Newhouse B, Nguyen P, O’Leary JM, Olson P, Pajk S, Rahbaek L, Rodriguez M, Smith CR, Tang TP, Thomas NC, Vanderpool D, Vigers GP, Christensen JG, Marx MA, Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS(G12D) Inhibitor, J. Med. Chem, 65 (2022) 3123–3133. [DOI] [PubMed] [Google Scholar]
- [121].Hanan EJ, Braun MG, Heald RA, MacLeod C, Chan C, Clausen S, Edgar KA, Eigenbrot C, Elliott R, Endres N, Friedman LS, Gogol E, Gu XH, Thibodeau RH, Jackson PS, Kiefer JR, Knight JD, Nannini M, Narukulla R, Pace A, Pang J, Purkey HE, Salphati L, Sampath D, Schmidt S, Sideris S, Song K, Sujatha-Bhaskar S, Ultsch M, Wallweber H, Xin J, Yeap S, Young A, Zhong Y, Staben ST, Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3Kalpha, J. Med. Chem, 65 (2022) 16589–16621. [DOI] [PubMed] [Google Scholar]
- [122].Krystal JH, Kane JM, Correll CU, Walling DP, Leoni M, Duvvuri S, Patel S, Chang I, Iredale P, Frohlich L, Versavel S, Perry P, Sanchez R, Renger J, Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: a two-part, randomised, double-blind, placebo-controlled, phase 1b trial, Lancet, 400 (2022) 2210–2220. [DOI] [PubMed] [Google Scholar]
- [123].Fleischhacker WW, Podhorna J, Groschl M, Hake S, Zhao Y, Huang S, Keefe RSE, Desch M, Brenner R, Walling DP, Mantero-Atienza E, Nakagome K, Pollentier S, Efficacy and safety of the novel glycine transporter inhibitor BI 425809 once daily in patients with schizophrenia: a double-blind, randomised, placebo-controlled phase 2 study, Lancet Psychiatry, 8 (2021) 191–201. [DOI] [PubMed] [Google Scholar]
- [124].Piccini JP, Caso V, Connolly SJ, Fox KAA, Oldgren J, Jones WS, Gorog DA, Durdil V, Viethen T, Neumann C, Mundl H, Patel MR, Investigators P-A, Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study, Lancet, 399 (2022) 1383–1390. [DOI] [PubMed] [Google Scholar]
- [125].Clinical Trials Database. National Library of Medicine (US): https://Clinicaltrials.Gov/ (April 23, 2023), in.


