Abstract
Objective:
Delirium is a complex neurocognitive syndrome suspected to be bidirectionally linked to dementia. Circadian rhythm disturbances likely contribute to dementia pathogenesis, but whether these disturbances are related to delirium risk and progression to all-cause dementia is unknown.
Methods:
We analyzed continuous actigraphy data from 53,417 middle-aged or older UK Biobank participants during a median 5 years of follow-up. Four measures were used to characterize the 24-hour daily rest–activity rhythms (RARs): normalized amplitude, acrophase representing the peak activity time, interdaily stability, and intradaily variability (IV) for fragmentation of the rhythm. Cox proportional hazards models examined whether RARs predicted incident delirium (n = 551) and progression to dementia (n = 61).
Results:
Suppressed 24-hour amplitude, lowest (Q1) versus highest (Q4) quartile (hazard ratio [HR]Q1 vs Q4 = 1.94, 95% confidence interval [CI] = 1.53–2.46, p < 0.001), and more fragmented (higher IV: HRQ4 vs Q1 = 1.49, 95% CI = 1.18–1.88, p < 0.001) rhythms predicted higher delirium risk, after adjusting for age, sex, education, cognitive performance, sleep duration/disturbances, and comorbidities. In those free from dementia, each hour of delayed acrophase was associated with delirium risk (HR = 1.13, 95% CI = 1.04–1.23, p = 0.003). Suppressed 24-hour amplitude was associated with increased risk of progression from delirium to new onset dementia (HR = 1.31, 95% CI = 1.03–1.67, p = 0.03 for each 1-standard deviation decrease).
Interpretation:
Twenty-four-hour daily RAR suppression, fragmentation, and potentially delayed acrophase were associated with delirium risk. Subsequent progression to dementia was more likely in delirium cases with suppressed rhythms. The presence of RAR disturbances before delirium and prior to progression to dementia suggests that these disturbances may predict higher risk and be involved in early disease pathogenesis.
Delirium is a distressing neurological complication in older adults after major illness or surgery. It is characterized by acute changes in attention, awareness, and cognition.1 The incidence dramatically increases with aging and is linked to subsequent dementia, which suggests delirium and dementia may have shared pathophysiological risk factors.2 Circadian rhythms are ~24-hour “body clock” cycles ubiquitous to physiological processes implicated in delirium and dementia (eg, brain function, metabolism, and immune modulation)3 that are synchronized to the day/night cycle primarily by light and enable the organism to anticipate predictable daily changes in the environment.4 Rest–activity rhythms (RARs) of motor activity refer to a person’s regular daily 24-hour pattern of being active and resting; they are commonly used to estimate circadian rhythms or disturbances, because the circadian timing system influences these RARs, and RAR perturbations can also disrupt the underlying circadian rhythms. RARs are estimated via actigraphy, an objective measurement of motor/physical rest and activity over a defined period of time using a noninvasive accelerometer.5
Circadian disturbances are thought to have a bidirectional link to dementia.6,7 At the same time, there is increasing realization that many defining features and common risk factors for delirium and dementia, such as cognitive performance, executive function, and behaviors such as sleep–wake cycle and motor activity levels, are also under circadian control.3 Emerging evidence has shown that actigraphy-derived RAR degrades with age and predicts cognitive decline, incident mild cognitive impairment (MCI), and Alzheimer disease (AD).8–10 In addition, disrupted RARs were found to be associated with amyloid/tau pathology even in cognitively intact humans.9,11 The very same pathology has recently been implicated in delirium.12,13 Tan et al recently found similar RAR disruption and advanced (earlier) timing of peak activity (acrophase) in a small study for delirium.14 Thus, there is a suspected overlap in the etiology of delirium and dementia.2 Nonetheless, the pathophysiological mechanisms remain unknown, and the relevance of daily rhythm disruption in delirium has yet to be determined from large-scale cohorts with extended follow-up.
To address these gaps in the literature and better understand the relationship between daily rhythm regulation, delirium, and progression to dementia, we examined daily RARs via estimations of the daily rhythm of motor activity that was recorded for up to 7 days between 2013 and 2015, in >53,000 middle-aged or older adults from the UK Biobank. Participants’ health was followed via electronic health records documenting hospitalization records for up to 7.5 years. We tested 2 hypotheses: (1) participants with more RARs disturbances at baseline (ie, suppressed amplitude, advanced peak activity time, reduced stability, and increased fragmentation) will have a higher risk for developing new onset delirium during hospitalizations and (2) these RARs disturbances can predict those who progress to incident MCI and/or all-cause dementia within 3 years of their first delirium episode.
Subjects and Methods
Study Population and Data Source
Between 2013 and 2015, 103,711 community-based participants were recruited for actigraphy-based monitoring from the UK Biobank, a longitudinal population-based cohort of individuals across the UK aged between 43.5 and 79.1 years (mean age = 63.6, standard deviation [SD] = 7.6 years, 54% female). Participants completed extensive questionnaires on demographics, lifestyle choices, and medical conditions at initial enrollment (2.8-9.7 years before actigraphy) and agreed to provide access to their health care records; these are collected in the UK Biobank’s Hospital Inpatient Data, an electronic health record that includes diagnoses, hospitalization, and operations for each participant from the UK’s centralized National Health Service (NHS), where the vast majority of health care is accessed in the UK.15 Follow-up for participants was until the occurrence of delirium, death, withdrawal, or the latest available data, up to 7.5 (median = 5) years. The demographic and clinical characteristics of participants at baseline (with/without delirium) are shown in Table 1.
TABLE 1.
Demographics, Lifestyle, and Clinical Comorbidities
| Developed Delirium, n = 551, Mean (SD) or n (%)a | Did Not Develop Delirium, n = 52,866, Mean (SD) or n (%) | p | |
|---|---|---|---|
| Demographicsb | |||
| Age at actigraphy, yr | 69.5 (5.4) | 63.5 (7.7) | <0.001 |
| Sex | |||
| Males | 60.6% | 45.0% | <0.001 |
| Females | 39.4% | 55.0% | |
| Education/socioeconomic status | |||
| College attendance | 37.9% | 40.6% | 0.18 |
| Townsend deprivation index | −1.5 (2.9) | −1.7 (2.8) | 0.04 |
| Ethnic background | 0.29 | ||
| European | 97.4% | 96.6% | |
| Non-European | 2.6% | 3.4% | |
| Rest–activity rhythmicity characteristics | |||
| Amplitude, normalized units | 0.44 (0.1) | 0.47 (0.1) | <0.001 |
| Acrophase, h | 13.8 (1.4) | 13.9 (1.2) | 0.43 |
| Interdaily stability, a.u. | 0.52 (0.1) | 0.53 (0.1) | 0.60 |
| Intradaily variability, a.u. | 0.97 (0.3) | 0.91 (0.2) | <0.001 |
| Sleep | |||
| Total nighttime duration | 0.02 | ||
| Short [<7 h] | 69% | 68% | |
| Moderate [7–8 h] | 24% | 28% | |
| Long [>8 h] | 6% | 4% | |
| Number of awakenings | 5.3 (1.8) | 5.2 (1.9) | 0.15 |
| Physical activity | |||
| Total daily activity count [× 105] | 2.8 (1.2) | 3.5 (1.3) | <0.001 |
| Cognition | |||
| Mean reaction time, msb | 581 (121) | 550 (107) | <0.001 |
| Comorbidities | |||
| BMI, kg/m2b | 27.9 (5.0) | 27.0 (4.7) | <0.001 |
| Cardiovascular disease [present] | 53% | 30% | <0.001 |
| Morbidity burden | <0.001 | ||
| None [0 comorbidities] | 21% | 33% | |
| Moderate [1–3] | 55% | 57% | |
| Significant [4+] | 24% | 10% |
Note: Cardiovascular disease means the presence of any of the following: hypertension, high cholesterol, smoking, diabetes, ischemic heart disease, and peripheral vascular disease.
Includes all delirium cases, including nonoperative and postoperative delirium.
Data come from baseline UK Biobank recruitment prior to actigraphy.
Abbreviation: a.u. = arbitrary units; BMI = body mass index; SD = standard deviation.
Standard Protocol Approvals, Registrations, and Patient Consents
The UK Biobank received National Research Ethics Approval, and participants gave written informed consent. This study was conducted under the terms of UK Biobank access number 33883 and Mass General Brigham Institutional Review Board approval (#2020P002097).
Assessment of RARs
A triaxial accelerometer device (AX3; Axivity, Newcastle, UK) was worn continuously for up to 7 days. Data collection and quality checks were performed based on prior work with actigraphy in older adults8,10,11 and established criteria from the UK Biobank.16 We excluded 11,018 records (10.6%) due to flagged cases with data problems, poor calibration, and large gaps/likely off-wrist periods.
We examined 4 properties of circadian RARs17,18: (1) 24-hour amplitude, the difference in the magnitude of activity between the active and rest phases; this was normalized by the subject’s SD to account for individual variations in the actigraphy signal, and enable between-participant comparison; (2) acrophase of the 24-hour component that presents the peak activity time in hours extracted from motor activity recordings based on parametric cosinor fitting that extracts the 24-hour component from the raw actigraphy signal, and nonparametric assessment for (3) interdaily stability (IS) for assessment of the stability of the rhythm (ie, larger IS for more stable rhythms)19; and (4) intradaily variability (IV) for assessment of fragmentation of the rhythm (ie, smaller IV for less fragmentation).19 In addition to the cosinor 24-hour amplitude, we also derived the nonparametric relative amplitude (RA)—the difference in activity between the most active 10-hour period and the least active 5-hour period in the average 24-hour pattern, normalized by their sum. The UK Biobank actigraphy protocol was for 7 days. We used the first 6 available days of data to include at least 1 weekend day and excluded those with <6 days (n = 3,634; see Fig 1). We kept this consistent for both parametric (cosinor) and nonparametric analyses. We used MATLAB (vR2020a; MathWorks, Natick, MA) programs to implement parametric cosinor fitting for normalized amplitude/acrophase and nonparametric analyses for IS/IV.
FIGURE 1:
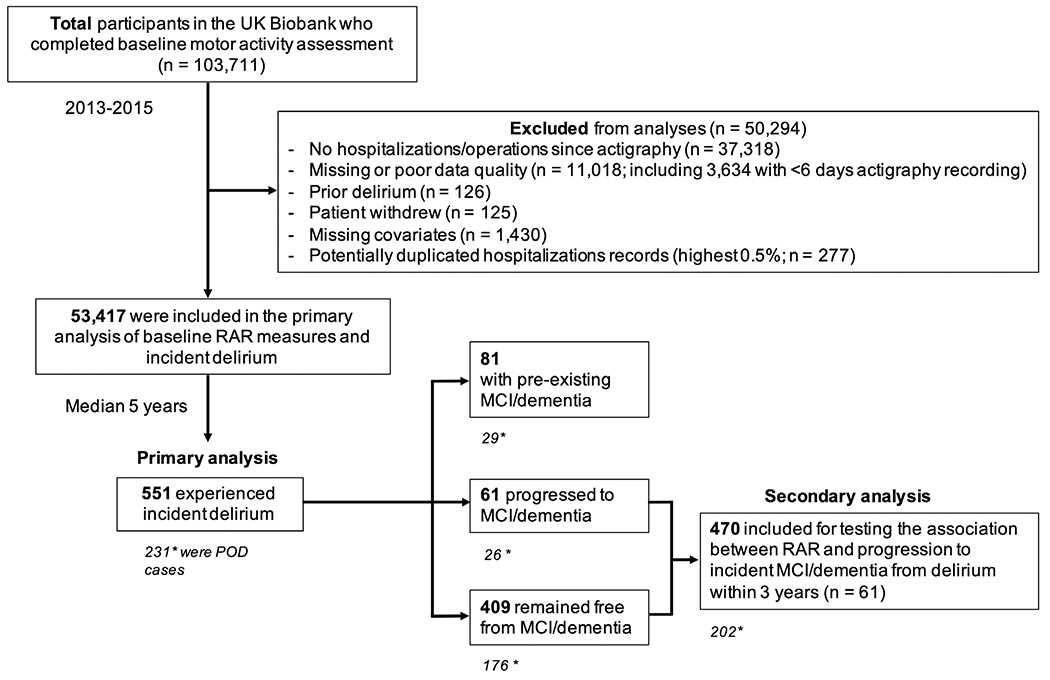
Flowchart of participants in the study. The primary analysis included 551 for all cases of delirium, of which 231 were postoperative delirium (POD) cases (denoted by asterisk). Eighty-one cases had pre-existing mild cognitive impairment (MCI)/dementia, 61 progressed to MCI/dementia, and 409 remained free from MCI/dementia (see also Table 2). Four hundred seventy (*202 were POD cases) without MCI/dementia were included in the secondary analysis (see also Table 4). RAR = rest–activity rhythm.
Assessment of Delirium, MCI, and Dementia Diagnoses
The UK Biobank has released hospitalization records linked to study participants during the follow-up period within the UK’s NHS. Incident delirium diagnosis was derived as the first date of occurrence of the International Classification of Diseases, 10th Revision (ICD-10) code F05, included in hospital admission health records during follow-up, in keeping with similar studies using these data.20 In addition, we obtained incident MCI diagnoses (F06.7) and dementia diagnoses using the UK Biobank’s algorithmically defined “date of all-cause dementia” (field 42,018). Thus, our main outcome was “all delirium cases.” Separately, we also analyzed operation/procedure coding from the UK Biobank and matched dates of operations within 3 days before incident delirium; these were then considered as postoperative delirium (POD), our prespecified delirium subset outcome group of interest. Given the overlapping link between circadian disturbances and dementia, for exploratory analysis, we further identified the following outcome groups by comparing the dates of first occurrence (delirium, MCI/dementia): “delirium with pre-existing MCI/dementia,” “delirium progressing to MCI/dementia,” and “delirium remaining free from MCI/dementia” (see Fig 1).
Using the first date of diagnosis and the date of actigraphy assessment, we derived time to event (first occurrence of delirium) for our primary analysis and time from delirium to incident MCI/dementia progression for our secondary analysis. A total of 53,417 participants (mean ± SD age at baseline = 58 ± 8 years, range = 40–72 years, female: 52.7%) had available actigraphy and covariates (see below), free from delirium at baseline. Unlike chronic diseases such as diabetes or dementia, delirium requires a precipitating factor, for example, after an illness or operation in a cognitively vulnerable person. Only when a person is hospitalized/had an operation are there the conditions for delirium to occur and also be diagnosed/recorded in medical records. To ensure all participants were exposed to a precipitating factor at least once, we excluded those with no hospitalization or operations after actigraphy (see Fig 1).
Assessment of Covariates
Participants’ medical histories were made available by the UK Biobank through a combination of self-reports during nurse-led interviews at initial enrollment and medical records at the time of actigraphy. Covariates were grouped into 5 categories: demographics (age, sex, ethnicity, education, and deprivation), objective sleep characteristics (total nighttime duration [total sleep time] and the number of awakenings), physical activity, cognitive performance, and comorbidities (body mass index [BMI], presence of cardiovascular disease or risks [CVD], and morbidity burden).
Age at actigraphy was calculated in years based on the date of birth. Sex (male/female) and ethnicity (European/non--European, given that the majority was of British or “White” European descent [91%]) were self-reported. Education was college level or not. Townsend deprivation index (TDI) was a score based on national geographic census data immediately before participant enrollment. Sleep duration and awakenings were derived from actigraphy consistent with our prior studies.8,10 Physical activity was assessed by the mean total daily activity count, also from actigraphy. Cognitive performance was estimated at initial enrollment using a raw processing speed test involving the mean reaction time to correctly identify card matches. BMI at initial enrollment was calculated as weight in kilograms divided by height in meters squared. CVD was based on the presence of hypertension, high cholesterol, smoking, diabetes, ischemic heart disease, and peripheral vascular disease. We also used a previously described morbidity burden21 based on the summed presence of any cancers or respiratory, neurological, gastrointestinal, renal, hematological, endocrine, musculoskeletal, connective tissue, or infectious diseases/disorders, and classified as none (0)/moderate (1–3)/high (4 or more conditions) at the time of actigraphy.
Statistical Analysis
Descriptive characteristics are presented in Table 1, as means with SDs for quantitative variables (normally distributed) or as medians with interquartile range (non-normally distributed). Categorical variables are presented as percentages. Our primary analysis examined the associations between RAR measures, delirium incidence, and progression to MCI/dementia using Cox proportional hazard models, reported as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). In the core models, the normalized amplitude of the 24-hour component, and acrophase of the 24-hour component, IS, or IV were included separately as predictors alongside demographics (Model A). Adjusted models were subsequently used to control for the other 4 categories of covariates (Models B–E). Those with RAR disturbances may also have had unmeasured factors that increased the chance for closer follow-up, including hospitalizations or surgeries that are the necessary triggers for delirium. Thus, all models include the number of hospitalizations during follow-up to minimize misclassification and surveillance bias, as well as the time lag between initial enrollment variables (sex, TDI, BMI, and reaction time) and the time of actigraphy.
We first included POD cases only for sensitivity analysis, followed by our exploratory delirium subset outcome groups defined above (delirium with pre-existing MCI/dementia, delirium progressing to MCI/dementia, and delirium remaining free from MCI/dementia). In secondary analysis, we examined the association between RAR measures and risk for progression from delirium to new onset MCI/dementia using Cox proportional hazard models adjusted for age at delirium diagnosis, sex, education, ethnicity, morbidity burden, baseline mean reaction times, and the time lag between actigraphy and delirium diagnosis. Finally, we tested our primary models by subgroup of interest (age [<65 years/≥65 years], sex, sleep duration [<7 hours/7–8 hours/>8 hours], physical activity [above/below median mean daily activity counts], cognitive performance [above/below median reaction times], and morbidity burden [none/moderate/significant]). The proportional hazards assumption was assessed using the global χ2 test in the R package cox.zph (survival) incorporating methods described by Grambsch and Therneau.22 All statistical analyses were performed using JMP Pro (v16, SAS Institute, Cary, NC). Probability value < 0.05 was used for statistical significance.
Results
Participant Characteristics
Baseline demographics, daily rhythmicity characteristics (amplitude, acrophase, IS, and IV), sleep (total nighttime duration and number of awakenings), physical activity, cognition, and comorbidities are shown in Table 1. During a median 5-year follow-up (range = 2 months to 7.7 years, SD = 1.0 year), 551 participants developed their first episode of delirium during hospitalization after actigraphy assessment. In univariate analysis, compared to those with no incident delirium (see Table 1), participants who developed incident delirium were more likely to be older (69.5 vs 63.5 years, p < 0.001) and male (60.6% vs 45.0%, p < 0.001), and trended toward coming from areas of greater material deprivation per TDI (−1.5 vs −1.7, p = 0.04). Ethnic background and college education rates were similar between the two groups. The delirium group was likelier to be short (<7 hours) or long (>8 hours) sleepers compared to normal (7–8 h) sleepers (p = 0.02) but had a similar number of nighttime awakenings (5.3 vs 5.2), were less active (p < 0.001), and had slower reaction times (581 vs 550 milliseconds, p < 0.001), higher BMI (27.9 vs 27.0 kg/m2, p < 0.001) and higher prevalence of CVD (53% vs 30%, p < 0.001), and a higher proportion had significant morbidity burden (24 vs 10%, p < 0.001).
RARs and Risk for Incident Delirium
Lower normalized 24-hour amplitude was associated with a higher risk for delirium, with an HR of 1.28 (95% CI = 1.18–1.39, p < 0.001; Table 2) per 1-SD decrease. When the cohort was separated into quartiles, there was a graded decrease in risk across quartiles (Fig 2A), corresponding to a nearly 2-fold higher cumulative incidence of developing delirium for participants in the lowest (Q1) versus the highest quartile (Q4; see Fig 2B; [HR]Q1 vs Q4 = 1.94, 95% CI = 1.53–2.46, p < 0.001) after adjusting for demographic covariates. This association remained significant after further adjustments for covariates, including sleep, mean physical activity levels, cognition, and medical comorbidities (Table 3).
TABLE 2.
Risk for Delirium Outcomes Related to Baseline 24-Hour Rest–Activity Rhythm Features
| 24-Hour Rest–Activity Rhythm Features (per 1-SD change) | ||||
|---|---|---|---|---|
| Outcomes | Amplitudea | Acrophaseb | Interdaily Stabilitya | Intradaily Variabilityc |
| All delirium cases, n = 551/53,417 | 1.28 (1.18–1.39)*** | 1.07 (0.98–1.15) | 1.17 (1.01–1.35)* | 1.22 (1.11–1.33)*** |
| POD cases, n = 231/53,097 | 1.18 (1.04–1.36)** | 1.04 (0.88–1.22) | 1.01 (0.86–1.18) | 1.21 (1.05–1.40)** |
| Delirium with pre-existing MCI/dementia, n = 81/52,947 | 1.54 (1.27–1.31)*** | 0.90 (0.77–1.05) | 1.01 (0.80–1.27) | 1.39 (1.13–1.71)** |
| Delirium progressing to MCI/dementia, n = 61/52,927d | 1.70 (1.41–2.07)*** | 0.88 (0.75–1.02) | 1.01 (0.80–1.27) | 1.22 (1.05–1.41)** |
| Delirium remaining free from MCI/dementia, n = 409/53,275d | 1.18 (1.07–1.31)** | 1.13 (1.04–1.23)** | 1.10 (0.98–1.23) | 1.17 (1.05–1.30)** |
Note: Data are hazard ratios (95% confidence intervals) from Cox regression Model A adjusting for age, sex, education, Townsend deprivation index, ethnic background, and number of hospitalizations. “All delirium cases” represent all delirium cases regardless of setting (operative/nonoperative) or whether participants had a delirium superimposed on dementia diagnosis or pre-existing diagnosis for MCI/dementia. POD represents all cases within 3 days of an operation.
Per 1-SD decrease.
Per 1-hour increase.
Per 1-SD increase.
New MCI/dementia diagnoses within 3 years after delirium.
p < 0.05.
p < 0.01.
p < 0.001.
Abbreviations: MCI = mild cognitive impairment; POD = postoperative delirium; SD = standard deviation.
FIGURE 2:
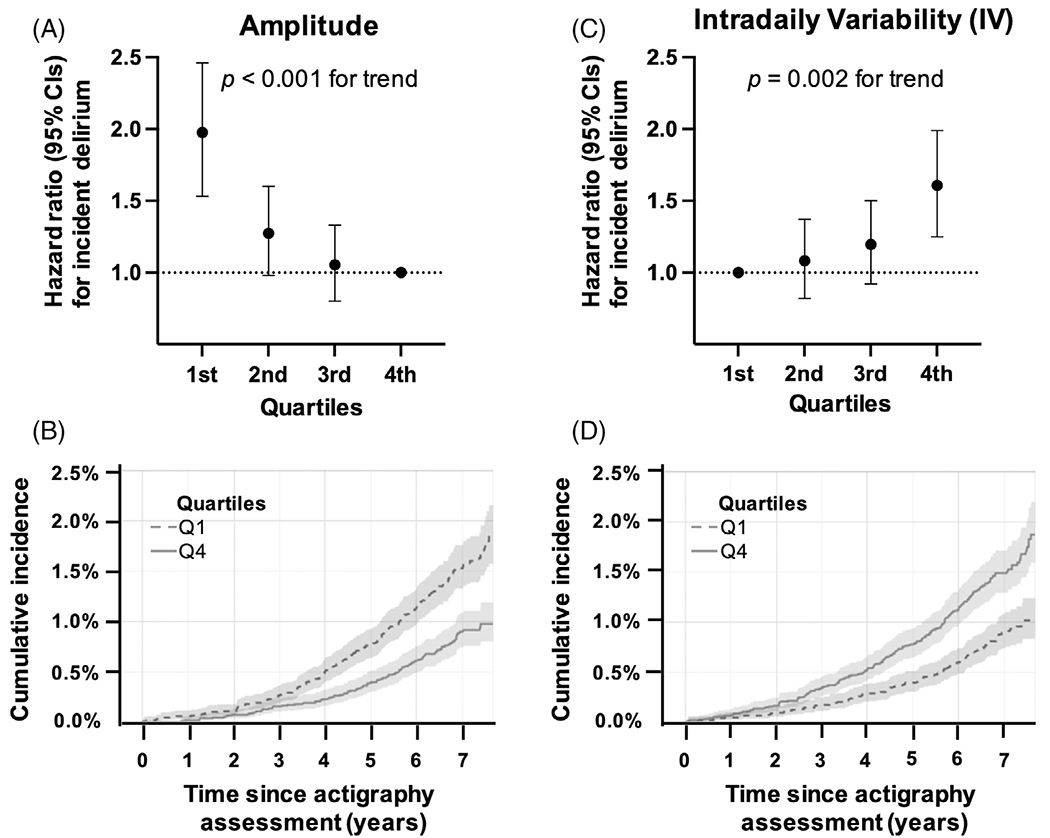
Rest–activity rhythm amplitude, fragmentation, and risk for delirium. Plots show the hazard ratio for (A) amplitude quartiles with the 4th (Q4) as the reference level and (B) intradaily variability (IV) quartiles with the 1st (Q1) as the reference level for the first occurrence of delirium adjusted for age, sex, education, ethnicity, and Townsend deprivation index. (C, D) The cumulative incidence for delirium since actigraphy assessment for the 1st and 4th quartiles of (C) amplitude and (D) IV. CI = confidence interval.
TABLE 3.
Adjusted Risk for All Delirium Cases by Baseline 24-Hour Rest–Activity Rhythm Quartiles
| 24-Hour Rest–Activity Rhythm Measures | ||||
|---|---|---|---|---|
| Models | Amplitude, Q1 vs Q4 | Acrophase, Q4 vs Q1 | Interdaily Stability, Q1 vs Q4 | Intradaily Variability, Q4 vs. Q1 |
| Model A | 1.94 (1.53–2.46)*** | 1.18 (0.95–1.47) | 1.30 (1.03–1.63)* | 1.49 (1.18–1.88)*** |
| Model B | 1.70 (1.34–2.15)*** | 1.05 (0.82–1.31) | 1.25 (0.99–1.58) | 1.49 (1.17–1.87)** |
| Model C | 1.49 (1.18–1.89)** | 1.17 (0.95–1.48) | 1.15 (0.91–1.46) | 1.20 (0.94–1.53) |
| Model D | 1.65 (1.30–2.09)*** | 1.18 (0.94–1.47) | 1.26 (0.99–1.59) | 1.49 (1.17–1.88)*** |
| Model E | 1.73 (1.36–2.17)*** | 1.16 (0.93–1.45) | 1.21 (0.96–1.52) | 1.44 (1.14–1.82)** |
Note: Data are hazard ratio (95% confidence interval) from the following Cox regression models.
Model A: adjusted for age, sex, education, Townsend deprivation index, ethnic background, and number of hospitalizations.
Model B: adjusted for Model A plus total nighttime sleep duration, number of awakenings.
Model C: adjusted for Model A plus mean activity.
Model D: adjusted for Model A plus mean reaction time.
Model E: adjusted for Model A plus body mass index, cardiovascular disease, morbidity burden.
p < 0.05.
p < 0.01.
p < 0.001.
Abbreviation: Q1 = quartile 1 (lowest); Q4 = quartile 4 (highest).
Higher IV, a measure of RAR fragmentation, was also associated with a higher risk for delirium (HR = 1.22, 95% CI = 1.11–1.33, p < 0.001; see Table 2) per 1-SD increase. There was a graded increase in risk (see Fig 2C) across quartiles corresponding to a 1.5-fold higher cumulative incidence for participants in Q4 vs Q1 (see Fig 2D; HR4Q4 vs Q1 = 1.49, 95% CI = 1.18–1.88, p < 0.001) in participants after adjusting for demographic covariates. This association remained significant after further adjustments for covariates but was nonsignificant in Model C with mean physical activity levels (see Table 3).
Acrophase (time of peak activity) was not related to incident delirium (for each hour delay, HR = 1.07, 95% CI = 0.98–1.15, p = 0.18; see Table 2). Lower IS (regularity of RARs) was associated with an increased risk for delirium in the core model (HR = 1.17, 95% CI = 1.01–1.35, p = 0.04; see Table 2) but was no longer significant with the inclusion of further covariates (see Table 3). We also separately tested the effects of sleep duration on delirium risk adjusting for demographics and reported here as follows: short sleepers (<7 hours) were at increased risk (HR = 1.28, 95% CI = 1.06–1.55, p = 0.01) compared to normal sleepers (7–8 hours) adjusted for demographics, but long sleepers (>8 hours) had marginally increased risk that was not significant (HR = 1.37, 95% CI = 0.96–1.95, p = 0.08).
RARs in Delirium Subsets
In POD cases only (n = 231, 42%), an increase in risk was observed for lower amplitude (HR = 1.18, 95% CI = 1.04–1.36 per 1-SD decrease, p = 0.01) and higher IV (HR = 1.21, 95% CI = 1.05–1.40 for 1-SD increase, p = 0.007; see Table 2), whereas no associations were seen between IS or acrophase and POD. In exploratory subsets, those with delirium with a pre-existing diagnosis of MCI/dementia (n = 81) or delirium progressing to MCI/dementia (n = 61) were also associated with lower amplitude and higher IV (see Table 2). After the exclusion of all known MCI/dementia before/after delirium, that is, delirium remaining free from MCI/dementia (n = 409), participants with delayed acrophase (HR = 1.13, 95% CI = 1.04–1.23, p = 0.003 for each 1-hour increase) in addition to lower amplitude (HR = 1.18, 95% CI = 1.07–1.31 for each 1-SD decrease, p < 0.001) and higher IV (HR = 1.17, 95% CI = 1.05–1.30 for each 1-SD increase, p = 0.007; see Table 2), were at increased risk for delirium. Results were consistent across all outcome groups using the nonparametric RA (Table S1).
Incident Delirium Risk by Subgroup of Interest
We further examined the amplitude- and IV-associated risk for delirium by subgroup (Figure 3, adjusted for demographics). We found that lower amplitude and higher IV (per 1 SD) were equally predictive by age (<65/≥65 years), sex, sleep duration (<7 hours/7–8 hours/>8 hours), cognition (faster/slower reaction times), or morbidity burden (low/moderate/significant). In addition, no significant interactions were found for amplitude or IV with age, sex, sleep duration, cognition, or morbidity burden (see Fig 3; see p values for interaction). However, the association between lower amplitude and delirium risk was higher in those with lower mean activity levels (below median; HR = 1.34, 95% CI = 1.18–1.62, p < 0.001) and was attenuated in those with higher mean activity levels (above median; HR = 1.05, 95% CI = 0.88–1.24, p = 0.61), with p = 0.001 for interaction.
FIGURE 3:
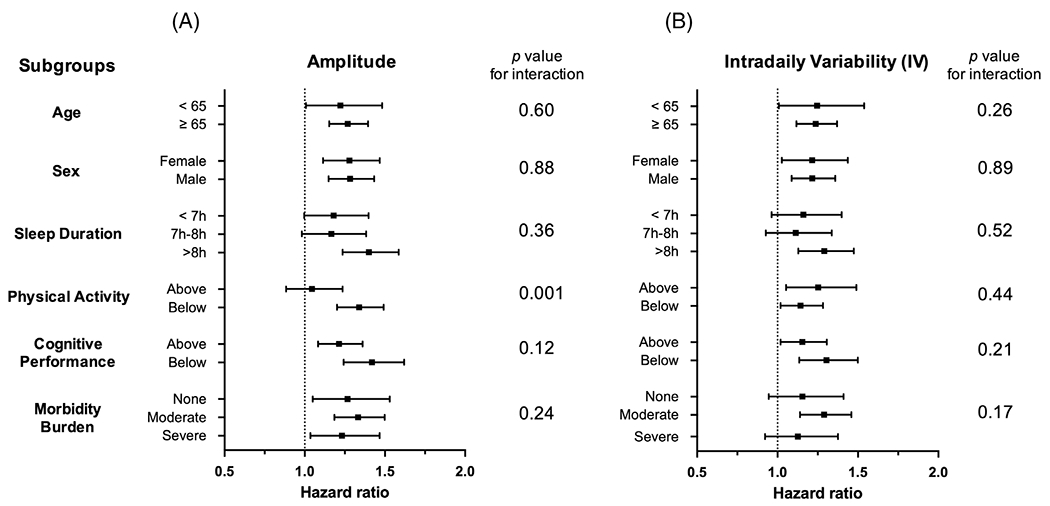
Subgroup analysis of delirium risk by 24-hour amplitude and intradaily variability. Forrest plot of hazard ratios with 95% confidence intervals for a 1-standard deviation (SD) decrease in (A) amplitude and 1-SD increase in (B) intradaily variability predicting incident delirium based on subgroups of patients by age, sex, sleep duration, physical activity level, cognitive performance (reaction time), and morbidity burden. “Above” and “Below” are relative to the median value for the corresponding groups.
RARs and Risk for Progression to Incident All-Cause Dementia after Delirium
In secondary analysis, we explored the association between RAR features and risk for progression to all-cause dementia after the first episode of delirium. Within 551 incident delirium cases, 470 (85%) participants were free from a diagnosis of MCI and/or dementia (mean age = 74 years, females 39%), and 61 (13.0%) participants developed incident MCI/dementia within 3 years of delirium (see Fig 1). Table 4 shows results for our 4 RAR features and risk for progression to incident all-cause dementia from delirium (non-dementia related and postoperative). Lower normalized 24-hour amplitude of RAR (per 1-SD decrease) was associated with a higher risk for progression to MCI/all-cause dementia in dementia-free (non-dementia related; HR = 1.31, 95% CI = 1.03–1.67, p = 0.03) and postoperative delirium (HR = 1.55, 95% CI = 1.04–2.33, p = 0.03). Age, sex, education, baseline cognition, morbidity burden, and mean activity did not significantly predict progression within this subcohort.
TABLE 4.
Adjusted Risk of Progression to All-Cause Dementia after Delirium Related to 24-Hour Rest–Activity Rhythm Features
| 24-Hour Rest–Activity Rhythm Measuresa | ||||
|---|---|---|---|---|
| Progression to All-Cause Dementia from Delirium | Amplitudeb | Acrophasec | Interdaily Stabilityb | Intradaily Variabilityd |
| All cases, excluding prior MCI/dementia, n = 61/470 | 1.31 (1.03–1.67)* | 0.84 (0.69–1.04) | 1.07 (0.82–1.38) | 1.12 (0.86–1.43) |
| Postoperative, n = 26/202 | 1.55 (1.04–2.33)* | 0.84 (0.69–1.04) | 1.05 (0.72–1.57) | 1.04 (0.70–1.51) |
Note: Data are hazard ratios (95% confidence intervals) from Cox regression models adjusted for age at delirium, sex, education, Townsend Deprivation Index, ethnic background, mean reaction time, morbidity burden, and number of hospitalizations. Time lag between actigraphy and first occurrence of delirium (mean 4.7, SD = 1.7 years).
Excludes delirium superimposed on dementia or pre-existing MCI/dementia.
Per 1-SD decrease.
Per 1-hour increase.
Per 1-SD increase.
p < 0.05.
Abbreviation: MCI = mild cognitive impairment; SD = standard deviation.
Discussion
This is the first large-scale prospective study examining the link between RARs and delirium. The key finding is that suppressed (lower 24-hour amplitude) and more fragmented (higher IV) rhythm predicted a higher risk of delirium. Additionally, a suppressed rhythm predicted progression from delirium to incident MCI and/or all-cause dementia. These results were consistent for POD and further exploratory delirium subsets with an additional association between delayed acrophase and delirium, but only in those remaining free from MCI/dementia. We believe this study presents compelling evidence that certain circadian disturbances are linked to delirium vulnerability and progression to dementia, which merits further exploration.
Although previous studies have demonstrated disruption to the daily rhythms of various physiological outputs such as motor activity (using accelerometry14), hormonal levels (eg, melatonin23 or cortisol24), or temperature25 during delirium itself and critical illness, few have examined daily disturbances before stressors that precipitate delirium. Health maintenance depends on regular alignment between circadian rhythms and actual behavioral/environmental rhythms.26 An accepted hallmark of healthy physiology and resistance to stressors is a robust circadian system.27 For example, circadian dysregulation has been associated with cardiometabolic disease26,28 and brain disorders.3 Thus, both RAR disturbances and delirium are suspected to be indicators of physiological frailty, as well as possible precipitating and accelerating factors in cognitive and physical decline.29–31
One emerging factor that may account for our observations is the discovery of RAR abnormalities in the preclinical phase of AD,8,9,32 which can span more than a decade.33 We recently showed the same rhythm disturbances (lower normalized amplitude and higher IV) predicted incident AD and progression to AD from MCI; worsening cognition also correlated with decreasing amplitude and increasing IV.8 At the same time, RAR disturbances were found to be associated with preclinical amyloid/tau pathology.9,11 It is generally accepted that dementia is a significant risk factor for delirium,2 and those with preclinical dementia may already be vulnerable to the stressors precipitating delirium. This hypothesis is backed by evidence linking delirium vulnerability to AD-related amyloid and tau pathology burden.13,34 Our results were independent of baseline cognitive reaction time and consistent when MCI and dementia cases were excluded; these findings may begin to bridge the gap in knowledge of the relationship between circadian disturbances and delirium. Nonetheless, the exact time course of the relationship and specific mechanisms, particularly in the context of AD-related pathology and cognitive impairment, remains unclear.
Why some delirium sufferers appear to be more at risk of developing dementia remains unclear.30 Lower 24-hour amplitude (1 SD) predicted a 31% increased risk over 3 years independent of age at delirium diagnosis, sex, cognition, and socioeconomic status. Interestingly, higher IV fragmentation was not significantly related to dementia progression (or other RAR measures) despite recent links to dementia diagnosis and pathology.8,9 We are not aware of other similar studies using RAR to understand the progression from delirium to dementia for comparison. This is likely due to the paucity of large-scale delirium-specific cohorts with detailed longitudinal follow-up.35 The Vantaa 85+ study of 553 individuals aged ≥85 years showed that traditional neuropathological correlates of dementia (eg, amyloid/tau, vascular lesions, or APOE-e4 allele status) were strongly associated with subjects who had developed dementia but not in a small subset of 58 subjects who had progressed from delirium.36 A more recent study that followed 115 delirium subjects over 2 years did not report incident dementia but confirmed that higher baseline cognition was protective against delirium; surprisingly, they also found that older adults with high baseline cognitive function and delirium showed the most significant cognitive decline.31 Taken together, it is feasible that dementia following delirium represents different etiological pathways to the development of dementia—pathways where noncognitive factors, such as the suppressed amplitude of RARs, may play a more prominent role.
We did not find significant associations between acrophase and our prespecified main outcomes for delirium and POD. However, there is a potential association between delayed acrophase and delirium, but only in those who remained free from MCI/dementia. In one of the few studies of actigraphy-derived RARs before delirium, Tan et al confirmed lower 24-hour amplitude before hospitalization for cardiac surgery in 25 delirium cases compared to 18 nondelirium patients.14 However, they also reported a 1.5-hour advanced (earlier) acrophase (peak time of RAR rhythm) in delirium patients. Both results should be interpreted cautiously and require larger scale confirmation studies. Several factors may contribute to these inconsistent results. Their study was small and tailored to the immediate period before cardiac surgery, known to have one of the highest rates of delirium.37 In contrast, our study is larger but had to account for a time lag between actigraphy and hospitalization and included all delirium and operative cases.
Additionally, the relationship between dementia and acrophase is also unclear. For example, two landmark studies on women32 and men38 reported that delayed acrophase was associated with cognitive decline in women. In contrast, advanced acrophase was associated with greater cognitive decline in men. This was not replicated in our recent (much older) community cohort,8 where we did not see sex differences in our results. Even if delirium and dementia are on the same spectrum of neurodegeneration in relation to circadian disruption, it is possible that (1) circadian amplitude and fragmentation changes manifest in earlier stages of neurodegeneration than acrophase changes; (2) the reliability in acrophase estimation is more affected or “masked” by the scheduled daily behavioral cycle (e.g., daily work-related or study-related schedules) than by other measures, and (3) multiple peaks may occur in perturbed daily activity rhythms in people with neurodegeneration; thus, it is hard to estimate the acrophase of such a nonsinusoidal oscillation reliably. For now, we can only conclude that advancing both delirium care and dementia prevention may benefit from considering a patient’s life (rest–activity) rhythms before hospitalization. Further work is required to determine the most critical rhythm features and their optimal timing of assessment.
Another major factor is sleep disruption, which is under circadian control, and increasingly linked to delirium.37,39 It is possible that circadian disturbances can lead to sleep disruption that, in turn, uniquely contributes to neurodegenerative diseases, including delirium.40 Leung et al showed that patients who developed delirium had significantly longer awakenings after nighttime sleep onset before major surgery,41 which makes sleep a potentially modifiable target for intervention.42 Although a standard sleep assessment in this population-based cohort was lacking at the actigraphy time, we applied validated algorithms to estimate nighttime sleep duration and the number of awakenings, but 24-hour amplitude and IV results remained significant. How sleep and circadian function interact to affect delirium risk will require future studies incorporating both sleep and circadian measures to dissect these complex pathways.
In subset analyses, the RAR–delirium association was consistent for delirium remaining free from MCI/dementia and POD, which has two important implications: (1) given the known relationship between RAR disturbances and dementia, it further suggested that nondementia pathways may underlie some of the links between RAR disturbances and delirium risk; and (2) POD is the most common surgical complication in older adults,43 and is most likely to be intervenable during the preoperative period (compared to acute illness delirium). These results should be interpreted with caution, given the small sample sizes from a healthy cohort; larger, dedicated delirium cohorts, particularly postoperative, are needed to investigate these preliminary findings further.
Whereas no interactions for amplitude or IV were seen with age, sex, sleep duration, cognition, or morbidity burden, we found a significant interaction between suppressed 24-hour amplitude and mean activity levels. Our Model C results showed that the effect of 24-hour amplitude on delirium was attenuated by mean physical activity level but remained significant. We derived mean activity levels from the same actigraphy data, and they may have some collinearity with amplitude. Surprisingly, the effects of amplitude on delirium risk were mainly in those with lower activity. There may be a few potential factors. (1) Low physical activity has been linked to cognitive decline,44 microinfarcts, and disruption of white matter integrity,45 which may overlap with delirium etiology,46 but could not be accounted for in this study. (2) Low physical activity may also represent sedentary episodes of wakefulness versus napping and can influence the interpretation of these results. In an older community cohort, we recently showed that longer duration and more frequent daytime napping/sedentary episodes were bidirectionally linked to cognitive decline, MCI, and AD.10 Although this study did not set out to score/distinguish sedentary episodes when activity is low, a known challenge using actigraphy data alone, these periods may contribute to both low activity and overall suppressed amplitude, particularly in those with pre-existing neurodegeneration and cognitive vulnerability to delirium. (3) Conversely, higher mean physical activity may be indicative of more regular and intense exercise, which both can be protective from the adverse cerebrovascular impact of circadian dysfunction and also directly impacts circadian regulation through light exposure/feeding or sleep/wake times,47 to promote more robust amplitude and attenuate its association with delirium risk. (4) Finally, given the above factors, <30% of delirium cases occurred in half of all participants with higher activity. This may have reduced the power to show the subgroup effects of amplitude on delirium risk while maintaining overall statistical significance in the full cohort. Overall, caution should be taken when interpreting subgroup analyses. Future confirmatory studies are needed to test whether targeting a combination of circadian regulation and objective physical activity levels will be most important to delirium risk mitigation.
Strengths and Limitations
This study is unique for the large sample size of objectively measured daily activity and prospective design with nearly 8 years of follow-up. Despite this, several limitations must be acknowledged. UK Biobank participants are mostly Caucasian of European descent and may have healthier behaviors than the general UK population. In addition, those who volunteered for additional testing (eg, actigraphy) from the UK Biobank are known to be even healthier21; this may underestimate the associations, as selection bias will include participants who may have healthier or more regular daily activity patterns, fewer comorbidities, and lower rates of delirium/dementia. However, despite the potential for selection bias and low participation rate overall (~5%), risk factor associations in the UK Biobank are generalizable.48
We controlled for a wide range of confounders and stratified by subgroups. Nonetheless, there is likely to be residual confounding in the described relationships, given the complex nature of circadian regulation and the heterogeneity of delirium. We used RAR as a proxy measure for circadian organization, recognizing that RAR is influenced by other factors such as homeostatic sleep pressure dynamics, family/work/school time constraints, and emotional, cognitive, environmental, and medical/medication factors. We ascertained data on multiple comorbidities as best as possible from health care records at the time of the RAR assessment. Nonetheless, we made the assumption that certain variables (ie, TDI and education level) remained the same during follow-up. Unfortunately, we did not have up-to-date BMI or cognitive testing; we instead controlled for the time lag between baseline and actigraphy recruitment. Our multivariable-adjusted models accounted for covariates that could be on the causal pathway between RAR and delirium. For example, BMI could increase and cognition decline faster, and there could be more hospitalizations, in those with low 24-hour amplitude or higher IV fragmentation, influencing delirium outcomes. Therefore, the adjusted HRs presented may underestimate the true strength of the relationships examined.
To minimize participant burden, the UK Biobank did not conduct more extensive cognitive (e.g., MiniMental State Examination) or independent community living (e.g., Instrumental Activities of Daily Living) testing. Therefore, residual confounding might have occurred due to baseline cognitive/independent living ability, which could overestimate the association. In addition, RAR was assessed only once and could have changed over time. We cannot exclude the possibility that some with delirium had undiagnosed MCI/dementia that was only later diagnosed after delirium. Reverse causation must also be acknowledged, given that delirium does not exclusively occur during hospitalization (e.g., illnesses that occur at home, in care settings, after dialysis center visits, or after ambulatory/day surgery that does not require hospitalization will not have been captured unless the delirium led to an escalation of care, i.e., an extended observation period that constituted a hospitalization event). Although these episodes are likely uncommon before actigraphy in our healthy cohort (e.g., 1.4% delirium incidence in ambulatory patients >75 years old),49 their neurological consequences may have preceded changes in RAR. For these reasons, the associations between RAR, delirium, and progression to dementia must be interpreted cautiously and in the context of the most severe cases of delirium during hospitalization. Carefully designed longitudinal studies monitoring 24-hour RARs and cognition before major elective surgery and in the recovery phase after delirium would help to confirm our observed links. Finally, clinical data in the UK Biobank cohort were limited to ICD coding. Others have used this approach for delirium20,50 and various diseases51–53 within this cohort and are highly specific (up to 96%) for delirium,54 but the sensitivity is modest (53–64% in recent studies).54,55 Thus, we are likely missing cases, particularly milder or hypoactive forms.
Our novel findings are a valuable starting point for research that bridges circadian rhythms and delirium. We have shown that suppressed 24-hour amplitude and higher fragmentation (IV) in the daily rhythm of motor activity are associated with risk for delirium during hospitalization in middle-aged and older adults. Suppressed amplitude also uniquely predicted the risk of progression from delirium to MCI and/or all-cause dementia, suggestive of a common pathway between delirium and dementia that warrants further investigation. Future work is needed to replicate findings alongside better measures of RARs and cognitive trajectory, ideally both before major illnesses/surgery and after delirium, with the goals of both preventing delirium and delaying the progression to dementia.
Supplementary Material
Acknowledgments
This research has been conducted using the UK Biobank resource under application number 33883. L.G. is funded by NIH grant R03AG067985 (NIA) and the Foundation for Anesthesia Education and Research. P.L. is funded by the BrightFocus Foundation Alzheimer’s Disease Research Program (A2020886S). C.G. is funded by an Alzheimer’s Association Research Fellowship Grant (AARFD-22-928372). K.H. is funded by NIH grants RF1AG059867 and RF1AG064312 (both NIA). F.A.J.L.S. is funded by NIH grant R01HL153969 (NHLBI). The organizations mentioned above had no direct input on this work.
Footnotes
Potential Conflicts of Interest
F.A.J.L.S. served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham. F.A.J.L.S. interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare per their conflict of interest policies. F.A.J.L.S.’s consultancies are not related to the current work. M.K.R. has received consulting fees and speaker fees from NovoNordisk and consulting fees from Cell Catapult.
References
- 1.Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primer 2020;6:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong TG, Vasunilashorn SM, Libermann T, et al. Delirium and Alzheimer disease: a proposed model for shared pathophysiology. Int J Geriatr Psychiatry 2019;34:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 2019;20:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol 2007;72:579–597. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Cole R, Alessi C, et al. The role of Actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26:342–392. [DOI] [PubMed] [Google Scholar]
- 6.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016;354:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu K, Harper DG, Shea SA, et al. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep 2013;3:2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Gao L, Gaba A, et al. Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults. Lancet Healthy Longevity 2020;1:e96–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musiek ES, Bhimasani M, Zangrilli MA, et al. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol 2018;75:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Gao L, Yu L, et al. Daytime napping and Alzheimer’s dementia: a potential bidirectional relationship. Alzheimers Assoc: Alzheimer’s Dement J, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Li P, Gaba A, et al. Fractal motor activity regulation and sex differences in preclinical Alzheimer’s disease pathology. Alzheimer’s Dementia 2021;13:e12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham EL, McGuinness B, McAuley DF, et al. CSF Beta-amyloid 1–42 concentration predicts delirium following elective arthroplasty surgery in an observational cohort study. Ann Surg 2019;269:1200–1205. [DOI] [PubMed] [Google Scholar]
- 13.Liang F, Baldyga K, Quan Q, et al. Preoperative plasma tau-PT217 and tau-PT181 are associated with postoperative delirium. Ann Surg 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan C, Saito N, Miyawaki I, Shiotani H. Preoperative circadian physical activity rhythm and postoperative delirium in cardiovascular surgery patients. Chronobiol Int 2020;37:1059–1066. [DOI] [PubMed] [Google Scholar]
- 15.Grosios K, Gahan PB, Burbidge J. Overview of healthcare in the UK. EPMA J 2010;1:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SE, van Hees VT, Mazzotti DR, et al. Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat Commun 2019;10:1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model 2014;11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinorrhythmometry. Chronobiologia 1979;6:305–323. [PubMed] [Google Scholar]
- 19.Gonçalves BSB, Cavalcanti PRA, Tavares GR, et al. Nonparametric methods in actigraphy: an update. Sleep Sci 2014;7:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman K, Jones L, Pilling LC, et al. Vitamin D levels and risk of delirium: a mendelian randomization study in the UK biobank. Neurology 2019;92:e1387–e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L, Gaba A, Li P, et al. Heart rate response and recovery during exercise predict future delirium risk-a prospective cohort study in middle- to older-aged adults. J Sport Health Sci 2021;21:S2095–S2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 23.Ángeles-Castellanos M, Ramírez-Gonzalez F, Ubaldo-Reyes L, et al. Loss of melatonin daily rhythmicity is asociated with delirium development in hospitalized older adults. Sleep Sci 2016;9:285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witlox J, Adamis D, Koenderman L, et al. Preoperative cerebrospinal fluid cortisol and the risk of postoperative delirium: a prospective study of older hip fracture patients. Dement Geriatr Cogn Disord 2020;49:604–610. [DOI] [PubMed] [Google Scholar]
- 25.van der Kooi AW, Kappen TH, Raijmakers RJ, et al. Temperature variability during delirium in ICU patients: an observational study. PLoS One 2013;8:e78923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chellappa SL, Vujovic N, Williams JS, Scheer FAJL. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab 2019;30:767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu K, Meijer JH, Shea SA, et al. Fractal patterns of neural activity exist within the Suprachiasmatic nucleus and require extrinsic network interactions. PLoS One 2012;7:e48927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Lim ASP, Wong PM, et al. Fragmentation of rest/activity patterns in community-based elderly individuals predicts incident heart failure. Nat Sci Sleep 2020;12:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Lim ASP, Gao L, et al. More random motor activity fluctuations predict incident frailty, disability, and mortality. Sci Transl Med 2019;11:eaax1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leighton SP, Herron JW, Jackson E, et al. Delirium and the risk of developing dementia: a cohort study of 12 949 patients. J Neurol Neurosurg Psychiatry 2022;93:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsui A, Searle SD, Bowden H, et al. The effect of baseline cognition and delirium on long-term cognitive impairment and mortality: a prospective population-based study. Lancet Healthy Longevity 2022;3:e232–e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011;70:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villemagne VL, Pike KE, Darby D, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia 2008;46:1688–1697. [DOI] [PubMed] [Google Scholar]
- 34.Xie Z, Swain CA, Ward SAP, et al. Preoperative cerebrospinal fluid β-amyloid/tau ratio and postoperative delirium. Ann Clin Transl Neurol 2014;1:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selbæk G, Neerland BE. Cognitive decline and dementia: does delirium matter? Lancet Healthy Longevity 2022;3:e217–e218. [DOI] [PubMed] [Google Scholar]
- 36.Davis DHJ, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain J Neurol 2012;135:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibala R, Mekonnen J, Gitlin J, et al. A polysomnography study examining the association between sleep and postoperative delirium in older hospitalized cardiac surgical patients. J Sleep Res 2021;30:e13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers-Soeder TS, Blackwell T, Yaffe K, et al. Rest-activity rhythms and cognitive decline in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc 2018;66:2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulsa MC, Xi Z, Li P, et al. Association of poor sleep burden in middle age and older adults with risk for delirium during hospitalization. J Gerontol, Ser A 2022;77:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Li Y-W, Wang L, et al. Promoting sleep and circadian health may prevent postoperative delirium: a systematic review and meta-analysis of randomized clinical trials. Sleep Med Rev 2019;48:101207. [DOI] [PubMed] [Google Scholar]
- 41.Leung JM, Sands LP, Newman S, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med 2015;11:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology 2021;135:1132–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Geriatrics Society expert panel on postoperative delirium in older adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015;220:136–148.e1. [DOI] [PubMed] [Google Scholar]
- 44.Buchman AS, Boyle PA, Yu L, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchman AS, Dawe RJ, Yu L, et al. Brain pathology is related to total daily physical activity in older adults. Neurology 2018;90:e1911–e1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis DHJ, Muniz-Terrera G, Keage HAD, et al. Association of delirium with cognitive decline in late life: a Neuropathologic study of 3 population-based cohort studies. JAMA Psychiat 2017;74:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hower IM, Harper SA, Buford TW. Circadian rhythms, exercise, and cardiovascular health. J. Circadian Rhythms 2018;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batty GD, Gale CR, Kivimäki M, et al. Comparison of risk factor associations in UK biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ 2020;368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aya AGM, Pouchain P-H, Thomas H, et al. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth 2019;53:35–38. [DOI] [PubMed] [Google Scholar]
- 50.Pilling LC, Jones LC, Masoli JAH, et al. Low vitamin D levels and risk of incident delirium in 351,000 older UK Biobank participants. J Am Geriatr Soc 2021;69:365–372. 10.1111/jgs.16853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 2020;41:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyse CA, Morales CAC, Graham N, et al. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann Med 2017;49:411–420. [DOI] [PubMed] [Google Scholar]
- 53.Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol, Ser A 2020;75:2224–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sepulveda E, Franco JG, Trzepacz PT, et al. Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: diagnostic accuracy study. BMC Psychiatry 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casey P, Cross W, Mart MW-S, et al. Hospital discharge data underreports delirium occurrence: results from a point prevalence survey of delirium in a major Australian health service. Intern Med J 2019;49:338–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


