Abstract
Background:
Biologic pathways underlying the association between outdoor air pollution and breast cancer risk are poorly understood. Breast tissue composition may reflect cumulative exposure to breast cancer risk factors and has been associated with breast cancer risk among patients with benign breast disease. Herein, we evaluated whether fine particulate matter (PM2.5) was associated with the histologic composition of normal breast tissue.
Methods:
Machine-learning algorithms were applied to digitized hematoxylin and eosin-stained biopsies of normal breast tissue to quantify the epithelium, stroma, adipose and total tissue area from 3,977 individuals aged 18–75 years from a primarily Midwestern United States population who donated breast tissue samples to the Susan G. Komen Tissue Bank (2009-2019). Annual levels of PM2.5 were assigned to each woman’s residential address based on year of tissue donation. We applied predictive k-means to assign participants to clusters with similar PM2.5 chemical composition and used linear regression to examine the cross-sectional associations between a 5-μg/m3 increase in PM2.5 and square root-transformed proportions of epithelium, stroma, adipose, and epithelium-to-stroma proportion [ESP], overall and by PM2.5 cluster.
Results:
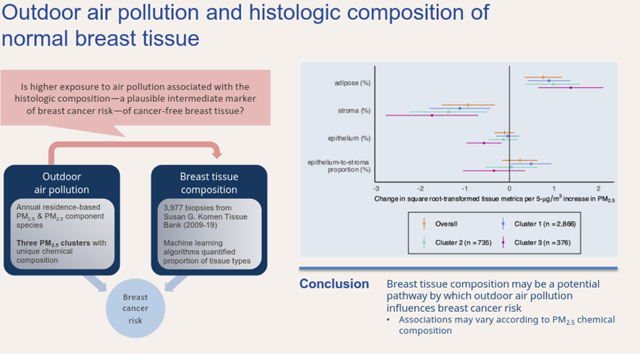
Higher residential PM2.5 was associated with lower proportion of breast stromal tissue [β=−0.93,95% confidence interval: (−1.52, −0.33)], but was not related to the proportion of epithelium [β=−0.11 (0.34, 0.11)]. Although PM2.5 was not associated with ESP overall [β=0.24 (−0.16, 0.64)], the association significantly differed by PM2.5 chemical composition (p-interaction=0.04), with a positive association evident only among an urban, Midwestern cluster with higher concentrations of nitrate (NO3−) and ammonium (NH4+) [β=0.49 (0.03, 0.95)].
Conclusions:
Our findings are consistent with a possible role of PM2.5 in breast cancer etiology and suggest that changes in breast tissue composition may be a potential pathway by which outdoor air pollution impacts breast cancer risk. This study further underscores the importance of considering heterogeneity in PM2.5 composition and its impact on breast carcinogenesis.
Keywords: Air pollution, Particulate matter, Breast cancer, K-means clustering
Graphical Abstract
1. Background
Existing evidence suggests that exposure to ambient air pollution is associated with an increased risk of breast cancer. For example, nitrogen dioxide (NO2) and polycyclic aromatic hydrocarbons (PAHs), markers of traffic-related air pollution, have consistently been associated with elevated breast cancer risk (Cheng et al. 2020; Datzmann et al. 2018; Gabet et al. 2021; Niehoff et al. 2019; White et al. 2018). Studies of outdoor exposure to particulate matter less than 2.5 microns in diameter (PM2.5) and breast cancer published prior to 2018 have reported largely null results (Andersen et al. 2017a; Andersen et al. 2017b; Hart et al. 2016; Reding et al. 2015). However, subsequent studies that have considered geographic heterogeneity in the composition of PM2.5 have observed evidence of a positive association (Villeneuve et al. 2018; White et al. 2021; White et al. 2019a). For example, higher exposure to PM2.5 in two large, United States (US)-based prospective cohort studies was associated with a higher risk of breast cancer, with findings varying according to geographic region, suggesting that varying PM2.5 chemical composition is an important consideration when evaluating the role of PM2.5 in breast cancer (White et al. 2021; White et al. 2019a).
Studies of air pollution and breast density further support a role of air pollution in breast cancer etiology (DuPre et al. 2017; Huynh et al. 2015; Yaghjyan et al. 2017). Increased mammographic breast density—a measure of the relative proportion of fibroglandular to adipose tissue in the breast—is a well-established breast cancer risk factor (Nazari and Mukherjee 2018). Among individuals who underwent mammography screening within the US Breast Cancer Surveillance Consortium, those with dense breasts were more likely to have higher residential exposure to PM2.5 (Yaghjyan et al. 2017) and PAHs (White et al. 2019c). Additionally, our group previously found that residential PM2.5 may impact normal breast tissue characteristics through its association with reduced involution of terminal duct lobular units (TDLUs) (Niehoff et al. 2020), the epithelial structures where most breast cancers arise (Russo et al. 2000), and the reduction of which in benign breast biopsies has been associated with increased breast cancer risk (Figueroa et al. 2014; Milanese et al. 2006). Together these findings support the continued investigation of breast tissue composition to inform biologic pathways underlying the association between air pollution and breast cancer.
Emerging data suggest that the relative amount of fibroglandular tissue that is epithelium versus stroma may be an important contributing factor to breast cancer development (Abubakar et al. 2021; Vellal et al. 2021). Using novel machine learning-based methods, Abubakar et al. (2021) and Vellal et al. (2021) showed that among independent study populations of individuals with benign breast disease (BBD), the relative proportion or ratio of epithelium-to-stroma was associated with subsequent invasive breast cancer risk, suggesting that the interplay between epithelial and stromal tissue may create conditions that protect against or favor carcinogenesis (Abubakar et al. 2021; Vellal et al. 2021). Abubakar et al. (2023) also demonstrated that these same histologic features within the normal breast were related to established breast cancer risk factors, including obesity and breastfeeding. Hence, quantitative changes in breast tissue composition might constitute plausible intermediate markers of breast cancer risk that can be used to interrogate the potential biologic pathways underlying the associations between environmental exposures and breast cancer. In the present study, we evaluated how residential outdoor PM2.5 exposure is related to quantitative tissue composition metrics of the normal breast, with considerations of how associations vary according to PM2.5 chemical composition.
2. Methods
2.1. Study Population
A total of 4,722 individuals enrolled in and donated breast tissue to the Susan G. Komen Tissue Bank—a biorepository of breast tissue donated by healthy volunteers—between 2009–2019 (https://komentissuebank.iu.edu/). For participants who donated multiple samples, we considered the earliest instance of tissue donation. We excluded participants without available digitized images of hematoxylin and eosin-stained biopsies (n = 345), with a personal history of breast cancer (n = 163), who were pregnant or breastfeeding at the time of donation (n = 57), who did not have information on menopause status (n = 13) or who were 75 years of age or older (n = 43). We also excluded participants for whom we were unable to assign annual PM2.5 estimates (n = 66) either because they did not provide a donation address, or their donation address was not within the contiguous US. We also excluded participants who were missing covariate information used for adjustment in this study (n = 65). Thus, the final sample included in our analysis consisted of 3,977 participants.
At the time of tissue donation, enrollees completed a questionnaire that ascertained information about demographics, medical history, lifestyle factors, and reproductive history. The protocol was approved by the Indiana University Institutional Review Board. All participants provided written informed consent prior to enrollment.
2.2. Exposure assessment
We estimated annual levels of PM2.5 at each participant’s residential address corresponding to year of breast tissue donation using the US Environmental Protection Agency’s Air Quality Time Series Project (EQUATES), a Bayesian space-time downscaling model that combines modeled air pollution levels from the Community Multiscale Air Quality (CMAQ) Modeling System (version 5.3.2) with fixed site air pollution measurements from the National Air Monitoring Stations (US Environmental Protection Agency 2021). Annual average PM2.5 concentrations, created by combining daily averages estimated at the 12 × 12 km grid level, were linked to participant’s geocoded residential addresses corresponding to the respective year of tissue donation.
We also considered 2009 annual average concentrations of PM2.5 component species sulfate (SO42−), nitrate (NO3−), ammonium (NH4+), elemental carbon (EC), and organic carbon (OC), which were estimated using CMAQ v5.3.2. These five components account for approximately 80% of PM2.5 total mass on average in the US, and the relative composition of PM2.5 components was stable across the 11year study period (Bell et al. 2007).
2.3. Outcome assessment
The procedure for breast tissue collection involved the removal of up to four tissue cores from the left or right upper outer quadrant of the breast with a standard 9-gauge needle. One core was fixed in 10% buffered formalin or Paxgene, sectioned, and stained with hematoxylin and eosin (H&E). The stained tissue sections were then digitized at 20X magnification. Additional details are described by Figueroa et al. (2014). Next, machine-learning algorithms were applied to quantify histologic tissue composition. Briefly, a 85-datapoint tissue classifier script was trained by two pathologists with expertise in digital pathology and applied to digitized H&E-stained slide images to identify and quantify (in mm2) the area on each slide comprised of epithelium, stroma, and adipose tissue (Abubakar et al. 2023). The pathologists who performed the algorithm training and image analysis were blinded to all participant characteristics and used Halo Client computational pathology software (Indica Labs, Albuquerque, NM). Previous reproducibility analyses, in which another pathologist independently developed a script to analyze a subset of the images, showed excellent agreement for all three tissue types (Spearman’s rho ≥ 0.95). Additional details regarding the algorithm, training, and reproducibility are described in Abubakar et al. (2021); (2023).
Percent epithelium, stroma, and adipose tissue area were calculated by dividing the absolute value of each histologic metric by total tissue area on the slide and multiplying by 100. We calculated an integrated measure of tissue characteristics, percent epithelial-to-stroma proportion (ESP), which was defined by dividing the epithelial area by total fibroglandular tissue area (i.e., epithelium and stroma) and multiplying by 100.
2.4. Statistical analysis
As in Niehoff et al. (2020), we identified groups of individuals with similar PM2.5 component profiles using K-means clustering, an unsupervised algorithm that assigns observations in a dataset into a pre-specified number of groups (k), such that within-cluster variation is minimized and between-cluster variation is maximized (Gibson et al. 2019). To determine the k-means clusters, we used the ratio of each component to PM2.5 total mass. We identified the optimal number of clusters using two methods: 1) plotting the total within-cluster sum of squares according to number of clusters and determining at which point the plot “bends” (i.e., the “elbow method”) and 2) by determining the number of clusters with the highest Rand Index, a measure of the robustness of the clustering algorithm to variability within the population (Hubert and Arabie 1985). Both methods indicated the optimal number of clusters as three.
We evaluated the distribution of participant characteristics, geographic variability, and annual PM2.5 exposure overall and by PM2.5 component clusters. We used linear regression to model coefficients (β) and 95% confidence intervals (CI) for the association between a 5-μg/m3 increase in annual PM2.5 total mass concentrations and each tissue composition metric. We applied a square root transformation to tissue composition metrics (i.e., %ESP, %epithelium, %stroma, and %adipose tissue) in order to improve approximate homoskedasticity and normality of model residuals. Thus, the effect estimates can be interpreted as the association between a 5-μg/m3 increase in annual PM2.5 total mass concentrations and the square root-transformed proportion of epithelium, stroma, adipose, epithelial-to-stromal proportion. We also explored associations between concentrations of each PM2.5 component species and each tissue metric.
To identify potential confounders, we created a directed acyclic graph (DAG) based on previous literature (Greenland et al. 1999). Our adjustment set included the following variables: age at donation (years), smoking status (never, former, current), education (high school graduate or less, vocational/technical/associate degree, college degree, graduate/professional degree), self-identified race/ethnicity (Asian, Hispanic, non-Hispanic Black, non-Hispanic white, other [Native American/Alaskan,Native Hawaiian, Pacific Islander, other, or unknown]), body mass index (BMI, kg/m2), US census region (Midwest, Northeast, South, or West) and donation year. For models of epithelium-to-stroma proportion (ESP), we additionally adjusted for the percent adipose tissue on the slide.
Comparing models with and without cross-product terms and using a likelihood-ratio test, we considered whether associations varied by PM2.5 component cluster assignment and menopause status. Individuals were classified as postmenopausal (n = 1,367) if they reported any of the following: did not have a menstrual period in the 12 months prior to tissue donation, had a bilateral oophorectomy, had a hysterectomy without a bilateral oophorectomy and were ≥ 55 years of age, or had a uterine ablation and were ≥ 55 years of age.
In sensitivity analyses, we explored overall and cluster-specific associations with average 2009 PM2.5 total mass concentrations assigned to all participants rather annual concentration based on participants’ donation year. We also explored whether associations differed according to BMI categories and parity using the same methods described in the paragraph above and considered additional adjustment for area-level factors (i.e., census tract-level income and urbanicity; n = 1 missing) and for reproductive history (i.e., parity and breastfeeding; n = 169 missing). Lastly, to rule out any potential influence of cigarette smoking in observed associations, we restricted the analysis to participants who reported never smoking cigarettes. All analyses were carried out using R version 4.2.0 R Foundation for Statistical Computing, Vienna, Austria).
3. Results
The demographic characteristics of participants at the time of tissue donation are summarized in Table 1. Participants were, on average, about 44 years of age. Approximately 18% and 68% were non-Hispanic Black and non-Hispanic White, respectively. The majority of participants had at least a college degree (58%), were overweight or obese (69%), had never smoked cigarettes (73%), and were premenopausal (66%). While the residential locations of participants represent all four US census regions of the contiguous US, most resided in the Midwest (84%) which is reflective of the location of most tissue collection events. The distribution of each tissue metric is presented in Supplemental Table 1. Briefly, breast tissue samples were largely comprised of adipose tissue (81%), followed by stroma (17%) and epithelium (1%). ESP had a moderate to low correlation with individual tissue metrics (r = 0.14 to 0.57; Supplemental Table 1).
Table 1.
Participant characteristics at breast tissue donation, overall and by PM2.5 component cluster, Komen Tissue Bank, 2009–2019
| Overall (n = 3,977) |
Cluster 1 (n = 2,866) |
Cluster 2 (n = 735) |
Cluster 3 (n = 376) |
|
|---|---|---|---|---|
| Census region, n (%) | ||||
| Midwest | 3327 (83.7%) | 2865 (100.0%) | 461 (62.7%) | 1 (0.3%) |
| Northeast | 111 (2.8%) | 1 (0.0%) | 29 (3.9%) | 81 (21.5%) |
| South | 260 (6.5%) | 0 (0%) | 245 (33.3%) | 15 (4.0%) |
| West | 279 (7.0%) | 0 (0%) | 0 (0%) | 279 (74.2%) |
| PM2.5 (ug/m3) | ||||
| Median [IQR] | 11.5 [5.6, 14.5] | 11.5 [6.8, 14.5] | 11.0 [5.6, 14.1] | 9.71 [6.1, 12.5] |
| Age at donation | ||||
| Mean (SD) | 43.8 (14.2) | 43.4 (14.0) | 44.4 (14.7) | 45.5 (14.5) |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic Black | 728 (18.3%) | 622 (21.7%) | 77 (10.5%) | 29 (7.7%) |
| Non-Hispanic white | 2718 (68.3%) | 1996 (69.6%) | 557 (75.8%) | 165 (43.9%) |
| Hispanic | 336 (8.4%) | 174 (6.1%) | 74 (10.1%) | 88 (23.4%) |
| Other | 195 (4.9%) | 74 (2.6%) | 27 (3.7%) | 94 (25.0%) |
| Education, n (%) | ||||
| High school or less | 658 (16.5%) | 470 (16.4%) | 147 (20.0%) | 41 (10.9%) |
| Some college | 1001 (25.2%) | 680 (23.7%) | 218 (29.7%) | 103 (27.4%) |
| Bachelors | 1306 (32.8%) | 982 (34.3%) | 203 (27.6%) | 121 (32.2%) |
| Graduate degree or higher | 1012 (25.4%) | 734 (25.6%) | 167 (22.7%) | 111 (29.5%) |
| Mean (SD) | 29.7 (7.7) | 29.8 (7.8) | 30.0 (7.6) | 28.7 (7.2) |
| < 18.5 | 44 (1.1%) | 26 (0.9%) | 10 (1.4%) | 8 (2.1%) |
| 18.5 to < 25.0 | 1208 (30.4%) | 874 (30.5%) | 198 (26.9%) | 136 (36.2%) |
| 25.0 to < 30 | 1069 (26.9%) | 792 (27.6%) | 193 (26.3%) | 84 (22.3%) |
| ≥ 30 | 1656 (41.6%) | 1174 (41.0%) | 334 (45.4%) | 148 (39.4%) |
| Smoking status, n (%) | ||||
| Never | 2930 (73.7%) | 2106 (73.5%) | 542 (73.7%) | 282 (75.0%) |
| Former | 817 (20.5%) | 595 (20.8%) | 144 (19.6%) | 78 (20.7%) |
| Current | 230 (5.8%) | 165 (5.8%) | 49 (6.7%) | 16 (4.3%) |
| Menopause status, n (%) | ||||
| Premenopausal | 2610 (65.6%) | 1936 (67.6%) | 438 (59.6%) | 236 (62.8%) |
| Postmenopausal | 1367 (34.4%) | 930 (32.4%) | 297 (40.4%) | 140 (37.2%) |
| Urbanicity, n (%) | ||||
| Urban | 3574 (89.9%) | 2651 (92.5%) | 550 (74.8%) | 373 (99.2%) |
| Suburban | 387 (9.7%) | 204 (7.1%) | 181 (24.6%) | 2 (0.5%) |
| Rural | 16 (0.4%) | 11 (0.4%) | 4 (0.5%) | 1 (0.3%) |
| Percent fat on the slide, n (%) | ||||
| 0 to ≤25% | 250 (6.3%) | 190 (6.6%) | 30 (4.1%) | 30 (8.0%) |
| > 25 to ≤50% | 502 (12.6%) | 393 (13.7%) | 67 (9.1%) | 42 (11.2%) |
| > 50 to ≤75% | 887 (22.3%) | 679 (23.7%) | 136 (18.5%) | 72 (19.1%) |
| > 75–100% | 2338 (58.8%) | 1604 (56.0%) | 502 (68.3%) | 232 (61.7%) |
| Year of donation, n (%) | ||||
| 2009 | 353 (8.9%) | 319 (11.1%) | 34 (4.6%) | 0 (0%) |
| 2010 | 360 (9.1%) | 329 (11.5%) | 31 (4.2%) | 0 (0%) |
| 2011 | 483 (12.1%) | 321 (11.2%) | 159 (21.6%) | 3 (0.8%) |
| 2012 | 997 (25.1%) | 837 (29.2%) | 156 (21.2%) | 4 (1.1%) |
| 2013 | 387 (9.7%) | 193 (6.7%) | 12 (1.6%) | 182 (48.4%) |
| 2014 | 406 (10.2%) | 385 (13.4%) | 21 (2.9%) | 0 (0%) |
| 2015 | 231 (5.8%) | 76 (2.7%) | 136 (18.5%) | 19 (5.1%) |
| 2016 | 400 (10.1%) | 251 (8.8%) | 148 (20.1%) | 1 (0.3%) |
| 2017 | 193 (4.9%) | 84 (2.9%) | 27 (3.7%) | 82 (21.8%) |
| 2018 | 128 (3.2%) | 38 (1.3%) | 5 (0.7%) | 85 (22.6%) |
| 2019 | 39 (1.0%) | 33 (1.2%) | 6 (0.8%) | 0 (0%) |
The average annual residential level of PM2.5 was 11.5 μg/m2 (interquartile range [IQR] = 5.59, 14.5). We identified three clusters of participants with similar profiles of PM2.5 chemical composition (Figure 1). The relative contributions of each component species to PM2.5 total mass within each cluster are described in Figure 2. Clusters 1 (n = 2,966) and 2 (n = 376) had similar levels of residential exposure to PM2.5 total mass (Cluster 1: mean [IQR] = 11.5-μg/m3 [6.75, 14.5]; Cluster 2: 11.0-μg/m3 [5.59, 14.1]), while participants in Cluster 3 (n = 735) had the lowest PM2.5 exposure levels overall (9.7-μg/m3 [6.05,12.5]) and a greater amount of variability in individuals’ level of exposure to each component species. While clusters 1 and 2 were similar in regard to concentrations of OC, EC, and SO42−, cluster 1 had higher relative concentrations of NO3− and NH4+ (Figure 2).
Figure 1.
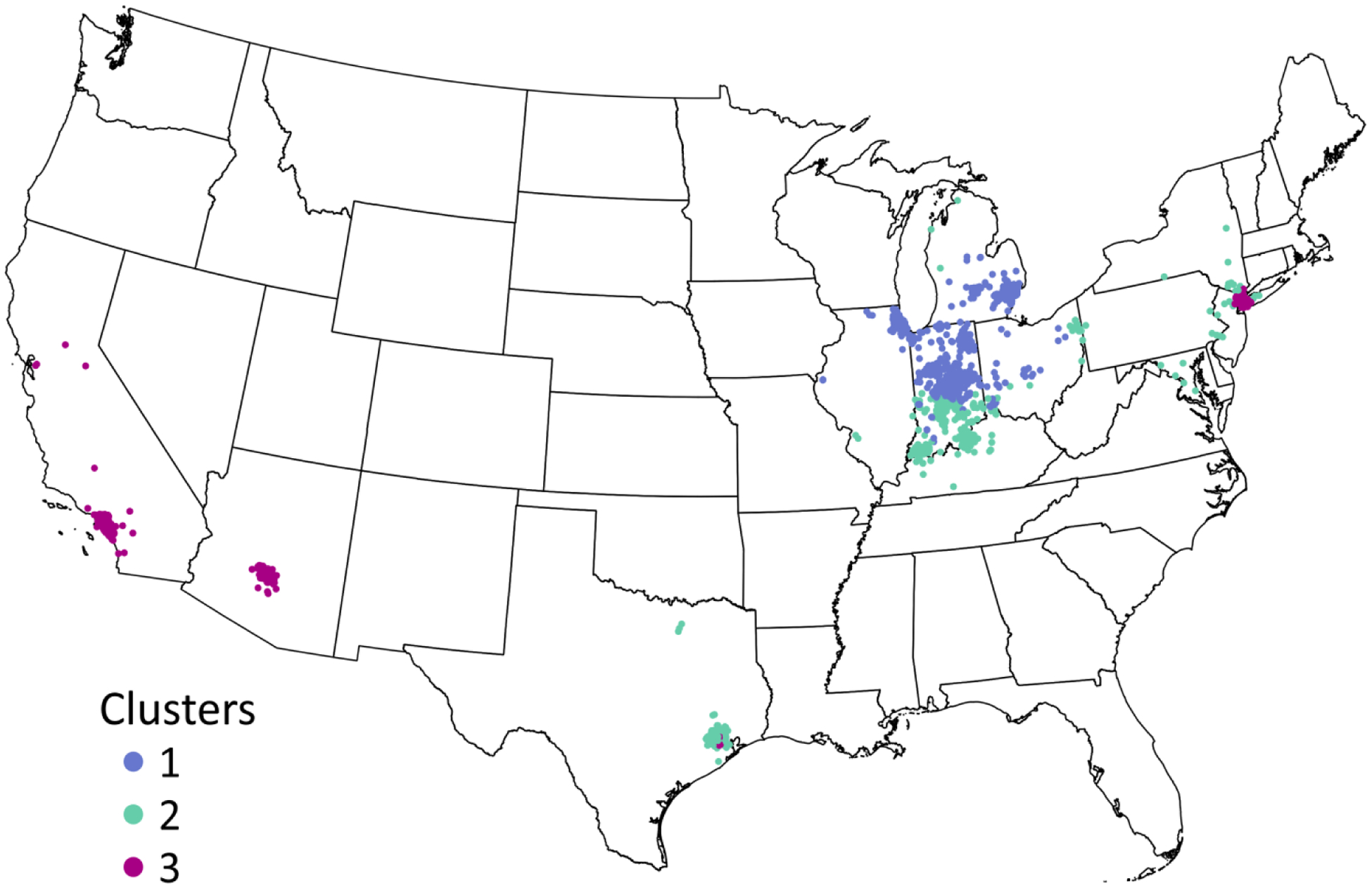
Map of participant residential locations by PM2.5 component cluster group, Komen Tissue Bank, 2009–2019 (N = 3,977). Locations of residences in states in which fewer than 5 participants reside are suppressed.
Figure 2.
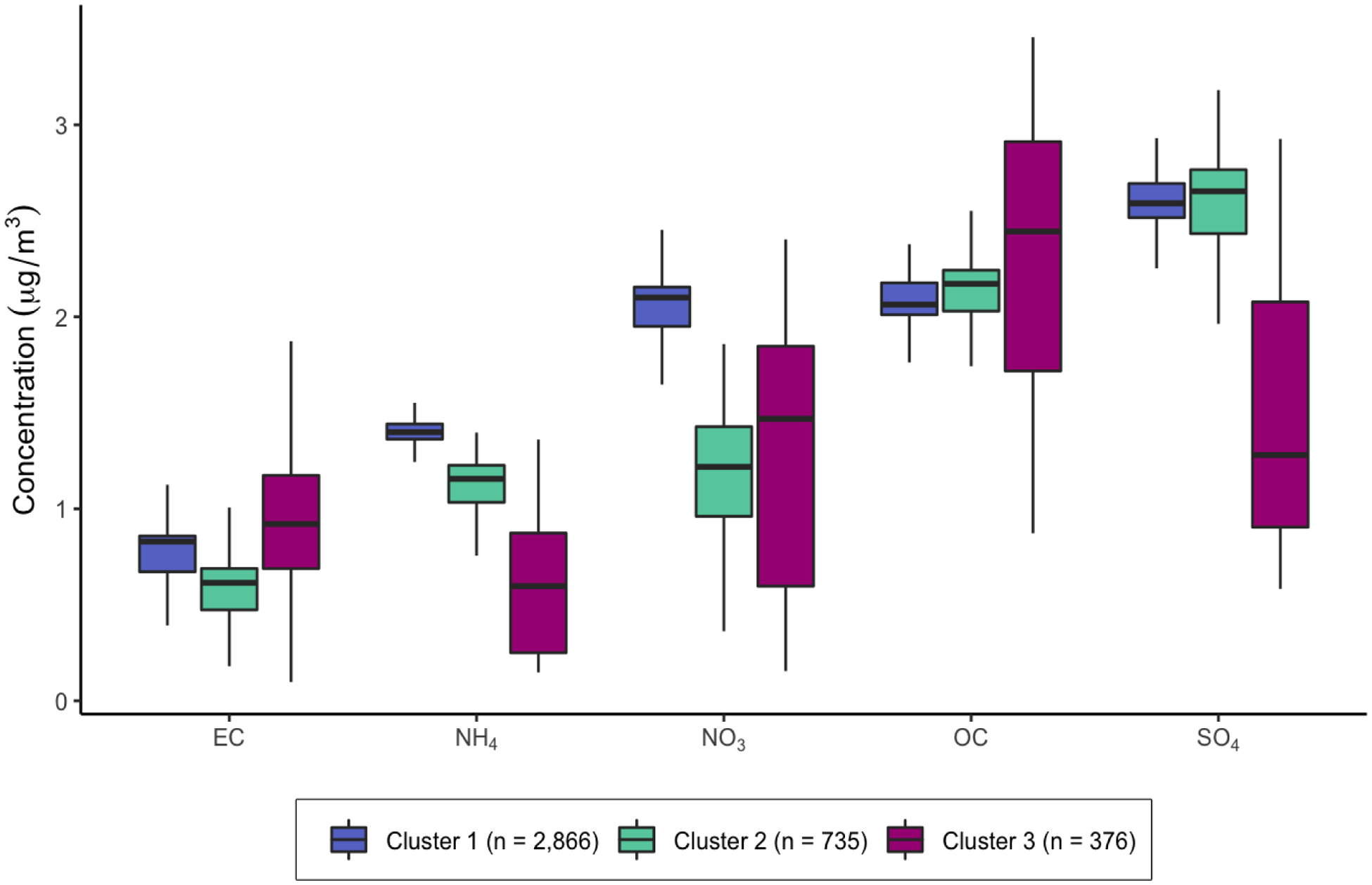
Distribution of PM2.5 component species by cluster group.
When considering tissue types individually, among all participants, a 5-μg/m3 increase in PM2.5 was associated with a lower proportion of stroma tissue (β = −0.95, 95% confidence interval (CI): −1.54–0.35) and, given that stroma and adipose proportion were perfectly negatively correlated (Pearson’s r = 1.0; Supplemental Table 1), we also observed a positive association between PM2.5 and adipose tissue proportion (β = 0.77, 95% CI: 0.35, 1.19). Neither association meaningfully differed by menopause status or cluster group (Table 2).
Table 2.
Model coefficients and 95% confidence intervals for the change in breast tissue composition (square root-transformed continuous proportions of epithelium, stroma, and adipose, or proportion epithelium relative to epithelium + stroma) per 5-ug/m3 increase in PM2.5
| β (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| PM2.5 total massa | N | Epithelium (%) | Stroma (%) | Adipose (%) | ESP (%)b |
| Overall | 3,977 | −0.12 (−0.34, 0.11) |
−0.95 (−1.54, −0.35) |
0.77 (0.35, 1.19) |
0.23 (−0.17, 0.63) |
| Component Cluster | |||||
| Cluster 1 | 2,866 | −0.05 (−0.31, 0.21) |
−1.15 (−1.83, −0.46) |
0.91 (0.43, 1.39) |
0.48 (0.02, 0.94) |
| Cluster 2 | 735 | −0.14 (−0.47, 0.19) |
−1.38 (−2.25, −0.52) |
0.99 (0.38, 1.6) |
0.04 (−0.54, 0.62) |
| Cluster 3 | 376 | −0.58 (−0.98, −0.19) |
−1.84 (−2.88, −0.8) |
1.45 (0.72, 2.19) |
−0.32 (−1.02, 0.37) |
| p for interaction | 0.04 | 0.43 | 0.38 | 0.04 | |
| Menopause Status | |||||
| Premenopausal | 2,610 | −0.15 (−0.38, 0.09) |
−1.11 (−1.74, −0.47) |
0.89 (0.45, 1.34) |
0.2 (−0.22, 0.62) |
| Postmenopausal | 1,367 | −0.1 (−0.36, 0.15) |
−0.71 (−1.39, −0.03) |
0.59 (0.11, 1.07) |
0.24 (−0.21, 0.7) |
| p for interaction | 0.65 | 0.14 | 0.11 | 0.81 | |
Note: ESP, epithelium-to-stroma proportion. Models are adjusted for age at donation, race/ethnicity, education, body mass index (continuous), smoking status, donation year, census region, %adipose tissue area (%ESP models only).
PM2.5 total mass concentrations (μg/m3) estimated by the EPA EQUATES model and assigned according to year of tissue donation
n = 5 missing ESP
Overall, we observed a positive but statistically non-significant positive association between a 5-μg/m3 increase PM2.5 and square root-transformed %ESP (β = 0.23, 95% CI: −0.17, 0.63; Table 2). The association did not differ by menopause status (p-interaction = 0.81), but there was significant heterogeneity when considering the association by PM2.5 component clusters (p-interaction = 0.04). Higher residential levels of PM2.5 were associated with greater %ESP in an urban, Midwestern cluster with higher relative concentrations of NO3− and NH4+ (Cluster 1: β = 0.48, 95% CI: 0.02, 0.94). Among participants living in urban areas outside of the Midwest with lower relative concentrations of SO42- and NH4+, we observed an inverse but imprecise association with %ESP (Cluster 3: β = −0.32, 95% CI: −1.02, 0.37), whereas the association was negligible among a more suburban cluster comprised of individuals living primarily in the Midwest and South (Cluster 2: β = 0.04, 95% CI: −0.54, 0.62; Table 2). Regarding the proportion of epithelial tissue, we observed an inverse association with PM2.5 within cluster 3 (β = −0.58, 95% CI: −0.98, −0.19) and null associations within cluster 1 (β = −0.05, 95% CI: −0.31, 0.21) and cluster 2 (β = −0.14, 95% CI: −0.47, 0.19; p-interaction = 0.04; Table 2).
The associations between a 1-ug/m3 increase in PM2.5 component species and the square roottransformed proportion each tissue metric are presented in Supplemental Table 2. The magnitude and direction of associations for each tissue metric were not consistent across PM2.5 component species. Compared to associations with total PM2.5 mass, we observed similar associations for NH4+ and OC with % epithelium and SO42− and OC with % stroma.
The associations stratified according to BMI categories and parity are presented in Supplemental Table 2. Across categories of BMI (i.e., < 25, 25-< 30, and ≥ 30 kg/m2), the direction of associations between PM2.5 and each tissue metric were consistent overall. There was a significant interaction between PM2.5 and BMI in relation to the proportion of epithelial and stromal tissue area (p-interaction = 0.04 and 0.05, respectively) but not in relation to the proportion of adipose tissue area or %ESP (p-interaction = 0.30 and 0.89, respectively). Among participants with a BMI < 25 kg/m3 and ≥ 30 kg/m3, the magnitude of the association between PM2.5 and % stroma was more pronounced compared to the overall association (< 25 kg/m2: β = −1.08, 95% CI: −1.76, −0.39 and ≥ 30 kg/m2: β = −1.26, 95% CI: −1.99, 0.53), and among overweight participants (BMI > 30), the association was smaller in magnitude and not statistically significant (β = −0.53, 95% CI: −1.21, 0.15). Although there was a significant interaction observed in relation to the proportion of epithelial tissue, none of the associations within strata of BMI were statistically significant. Findings did not substantially differ according to parity, nor did they meaningfully change when adjusting for area-level factors or reproductive history (Supplemental Table 2). Further, we observed similar results when assigning exposure based on 2009 PM2.5 concentrations and when restricting the analysis to never smokers (Supplemental Table 2).
4. Discussion
In this cross-sectional study of breast cancer-free individuals who donated breast tissue, we observed that participants with higher residential levels of fine particulate matter were more likely to have breast tissue characterized by a lower proportion of stromal tissue. Further, among those with residential PM2.5 with greater relative concentrations of nitrogenous compounds, PM2.5 levels were positively associated with epithelium-to-stroma proportion. Elevated ESP has been suggested as an early marker of breast cancer risk among BBD patients. Thus, our findings relating PM2.5 to histologic composition of the normal breast may inform early carcinogenic mechanisms by which air pollution influences breast cancer risk. Our findings also highlight the continued need to consider the variability in PM2.5 chemical composition in relation to breast cancer-related outcomes.
PM2.5 is a heterogenous mixture comprised of diverse chemical components, including those with both carcinogenic and endocrine disrupting properties. In addition to the five components species considered in our analysis, PM2.5 contains compounds with demonstrated estrogenic effects, including metals and PAHs (Choe et al. 2003; Santodonato 1997). While these compounds account for a relatively small proportion of PM2.5, they can exert adverse effects even at low levels of exposure. Experimental evidence suggests that tissue organization and the cross talk between epithelial and stromal tissue—important for maintaining constraints on epithelial proliferation (Maffini et al. 2004; Rønnov-Jessen et al. 1996)—is sensitive to exogenous compounds with endocrine disrupting properties (Burks et al. 2017; Macon and Fenton 2013).
Among individuals with benign breast disease, researchers have observed that the proportion or ratio of epithelium-to-stroma was more strongly associated with subsequent invasive breast cancer risk than the proportion of epithelium alone. Vellal et al. (2021) found that a 10-fold increase in the ratio of epithelium to stroma was associated with approximately 30% higher odds of developing breast cancer (OR = 1.29, 95% CI: 1.05, 1.59). Similarly, Abubakar et al. (2021) found that women in the highest quartile of histologic-ESP had two times the odds of developing breast cancer relative to women in the lowest quartile, suggesting that high ESP is a feature of breast tissue composition that is conducive for epithelial proliferation and tumor initiation. Further, an increasing proportion of stromal tissue was associated with reduced breast cancer risk, particularly in the context of non-proliferative BBD (Abubakar et al. 2021), suggesting that the stroma has a protective effect against breast carcinogenesis during normal homeostasis and that stromal depletion may be one mechanism leading to increased ESP. Future work is needed to replicate these findings among individuals without a history of breast disease. While our results suggest that PM2.5 exposure is associated with differential histology of the breast, it is not yet clear whether a certain threshold in the proportion of stromal tissue or ESP is most influential for future breast cancer risk. However, we do note that the magnitude of the associations observed here between PM2.5 and ESP is comparable to that observed for established breast cancer risk factors such as elevated BMI and breastfeeding history (Abubakar et al. 2023). Thus, our findings of decreased proportion of stromal tissue and greater ESP among individuals with higher residential levels of PM2.5 suggests that a plausible pathway by which PM2.5 influences breast cancer risk is through contributing to stromal depletion and a concomitant increase in ESP.
Our findings build upon previous work conducted in a subset of participants in the Komen Tissue Bank in which increasing concentrations of residential PM2.5 exposure was associated with higher TLDU count. Given that TLDU involution is reflective of a reduction in the amount of epithelial tissue on BBD biopsies (Abubakar et al. 2021), higher TDLU count may reflect a greater amount of epithelium that is susceptible to carcinogenesis. In this previous work, Niehoff et al. (2020) examined tissue samples from a subset of Komen Tissue Bank who enrolled between 2009–2011. Given the labor-intensive nature of TDLU assessment, TDLU involution metrics were not available for all donors in our sample of participants enrolled up to 2019, and we were thus unable to replicate these previous findings. Nonetheless, our analysis utilizing machine learning-based methods more amenable to larger scale studies provides additional support that fine particulate matter air pollution may influence breast histologic characteristics in this larger population. This was particularly evident among individuals living in urban centers in the Midwest for whom ammonium and nitrate particles contributed to a greater proportion of residential PM2.5 exposure. We note, however, that the present analysis and the study by Niehoff et al. (2020) are cross-sectional. Future research is needed to prospectively the examine the association between air pollution exposures and breast tissue composition.
Although our study is the first to examine quantitative histologic breast tissue composition in relation to particulate matter air pollution, our findings can be discussed in the context of previous work investigating PM2.5 and mammographic density. Positive associations have been observed with categorical measures of breast density in relation to ambient PM2.5 (Yaghjyan et al. 2017) and specific air toxics that are constituents of PM2.5 (White et al. 2019c). Among participants 40 years or older in the Breast Cancer Surveillance Consortium (n = 279,967, 2001–2009), Yaghjyan et al. (2017) reported that compared to individuals with scattered fibroglandular breasts, those with heterogeneously dense breast had higher exposure to PM2.5 (fourth vs. first quartile odds ratio (OR) = 1.19, 95% CI: 1.16, 1.23). In the same study population, White et al. (2019c) found residential exposure to the airborne heavy metals arsenic, cobalt, lead, manganese, and nickel, both singly and in combination, was associated with greater breast density. Unlike mammographic density, the histologic tissue metrics used in our analysis distinguish between relative amounts of epithelial and stromal tissue. Thus, our findings complement the literature related to breast density and provide additional insight into potential biologic pathways through which air pollution may influence breast tissue composition and subsequent breast cancer risk. Notably, Abubakar et al. (2021) found that ESP and mammographic density were independent and jointly associated with breast cancer incidence. A potential direction for future research would be to examine the association between air pollution and combined measures of breast density and ESP.
In contrast to findings for NO2, studies for PM2.5 and breast cancer risk have been more inconsistent with few considering the role of PM2.5 components (Andersen et al. 2017b; White et al. 2019a) or geographic heterogeneity in PM2.5 (White et al. 2021; White et al. 2019a). Findings from two large, prospective US-wide cohorts highlight the importance of geographic heterogeneity in PM2.5 in relation to breast cancer. In the Black Women’s Health Study, increased risk of negative estrogen receptor status (ER-) breast cancer and premenopausal breast cancer was found only among participants in the Midwest (White et al. 2021). In the Sister Study, a cohort of women with a sister with breast cancer who themselves are cancer-free at baseline, White et al. (2019a) observed that higher residential levels of PM2.5 were associated with greater incidence of ductal carcinoma in situ (DCIS) breast cancer among participants in the Midwest and a greater incidence of invasive breast cancer among participants in the Western US. Similarly, in the Nurses’ Health Study, a prospective cohort of female US nurses established in 1976, DuPre et al. (2017) found no overall association between PM2.5 and a continuous measure of mammographic density although a positive association was suggested among women living in the Northeast (β = 3.9% per 10-μg/m3 increase in PM2.5, 95% CI: −0.02, 7.9).
The relative distribution of PM2.5 constituent species are known to vary by geographic regions due to different PM2.5 sources (Bell et al. 2007) and thus could contribute to geographic heterogeneity in breast cancer risk. In our study, we identified a Midwest-based cluster (cluster 1) capturing participants living in urban areas among whom we observed a positive association between PM2.5 total mass and%ESP. The profile of PM2.5 component species may be indicative of PM2.5 sources giving rise to the higher proportions of ammonium and nitrate observed within this cluster. For example, in addition to fossil fuel combustion, agricultural activity is a major source of ammonia emissions and the subsequent formation of nitrate and ammonium particles (Kundu and Stone 2014). However, the associations between levels of each individual PM2.5 component species and %ESP does not support the hypothesis that is any single component is driving the association with PM2.5 total mass. While the five components considered in our analysis together comprise the majority of PM2.5 total mass, it is possible that the PM2.5 component clusters we identified are proxies of unmeasured components or other air pollutants relevant to effects on the breast. For example, compared to other census regions, the Midwest generally has higher levels of industrial emissions. Monitoring data suggests that PM2.5 in the Midwest is comprised of higher levels of heavy metals, including cadmium, vanadium, arsenic, and cobalt (Keller et al. 2017), and higher levels of exposure to these toxic air pollutants have been associated with increased breast density (White et al.2019c) and breast cancer incidence (Andersen et al. 2017b; White et al. 2019b).
Contrary to our hypothesis, residential PM2.5 was inversely, but imprecisely, associated with %ESP and the proportion of epithelial tissue among participants assigned to cluster 3. PM2.5 exposure within this cluster can be characterized as having lower proportion of sulfate particles which may indicate fewer emissions from fossil fuel combustion including those from coal-burning power plants (Bell et al. 2007). However, one of the most defining features of cluster 3 was that it was comprised of a limited number of individuals living outside of the Midwestern US, including participants in both Southern California and New York. Given that areas on the West and East coast have different air pollution profiles (Bell et al. 2007), cluster 3 is characterized by greater heterogeneity in the levels of each PM2.5 component species compared to the other clusters, as shown in Figure 2. Thus, it is unclear whether cluster 3 represents a meaningful profile of PM2.5 chemical composition and whether any true associations may be obscured given the heterogeneity within the cluster.
The findings of this study should be interpreted considering the strengths and limitations of our exposure assessment methods. The use of high-quality air pollution data estimated using the ESCAPE model—a technique integrating robust modeling procedures with air pollution measurements—is an important strength of our study. Also, using modelled estimates of PM2.5 component species allowed us to consider the impact of heterogeneity in PM2.5 chemical composition. Although we assigned 2009 levels of component species to all participants regardless of donation year, the relative composition of PM2.5 was stable during the study period. In addition, we estimated annual concentrations of PM2.5 based on participants’ home residence, which does not capture indoor air pollution nor time spent away from the home that could impact their exposure. We expect resulting misclassification of exposure, however, to be non-differential with respect to breast tissue composition. Importantly, we did not have information on how long participants had lived at their address reported at the time of tissue donation or information on past residences. Thus, we were not able to evaluate long-term air pollution exposure or exposure during potentially sensitive periods in which changes in breast composition may be vulnerable to air pollution, for example, during fetal development, puberty, or pregnancy (Terry et al. 2019). Current breast tissue composition may more strongly reflect the development and remodeling of the breast during these periods rather than the influence of more recent exposures. Yet recent exposures may still have an important impact; observational studies have demonstrated breast density declines after discontinuation of hormone replacement therapy or tamoxifen use (Chow et al. 2000; Cuzick et al. 2004; Rutter 2001), highlighting the potential modifiability of this breast cancer risk factor even later in life.
Our study leveraged objective and novel quantitative histologic tissue composition metrics among a unique resource of tissue samples from individuals without a personal history of breast cancer. Given that the procedure for collection of breast tissue cores was non-targeted, we cannot rule out the possibility of non-representative sampling of breast tissue and the influence of random error in our effect estimates. However, analyzing tissue component measures as a percentage of the total tissue area helps address this limitation. Additionally, we recognize that applying a square root transformation to the outcome measurements can add difficulty in interpreting the effect estimates. However, regardless of any transformation applied to the tissue metrics, the magnitude of the effect estimates can change depending on the distribution of the tissue metrics in a given population. Thus, we emphasize the direction rather than the magnitude of the observed associations. Given regional differences in obesity (Myers et al. 2015), which is reflected by differences between clusters in the percent adipose tissue in breast tissue samples among our study population, we cannot rule out residual confounding by BMI or adiposity. We sought to minimize this concern by controlling for a continuous measure of BMI and in models examining %ESP, controlling for the proportion of adipose tissue on the slide. We also found limited evidence that associations between PM2.5 and tissue composition meaningfully differed by BMI categories. Lastly, the generalizability of our findings may be limited given that participants donated to the Komen Tissue Bank on a volunteer basis and most resided in the Midwestern US, which limited our ability to explore PM2.5 component profiles outside of the Midwest.
In conclusion, we observed that higher residential exposure to fine particulate matter with greater relative proportions of nitrogenous compounds was associated with breast histologic characteristics that are indicative of a higher underlying breast cancer risk. Thus, our findings support the possible role of PM2.5 in breast cancer etiology and suggest that breast tissue composition may be a potential pathway by which outdoor air pollution impacts breast cancer risk.
Supplementary Material
Highlights.
We evaluated air pollution and markers of breast cancer risk in cancer-free tissue
PM2.5 levels were positively associated with epithelium-to-stroma proportion
PM2.5 levels were inversely associated with proportion of stromal tissue area
Associations with PM2.5 varied according to its chemical composition
Breast histology is a potential pathway by which PM2.5 may influence breast cancer
Funding:
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences Z01-ES103332.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data statement: Requests for Komen Tissue Bank data, including the data used in this manuscript, can be requested through the study website (https://komentissuebank.iu.edu/researchers/).
https://www.elsevier.com/authors/policies-and-guidelines/credit-author-statement
Jennifer L. Ish: Formal analysis, Writing – Original draft preparation. Mustapha Abubakar: Investigation, Writing – Review & editing. Shaoqi Fan: Data curation, Methodologic support. Rena R. Jones: Writing – Review & editing. Nicole M. Niehoff: Conceptualization, Formal analysis, Writing – Review & editing. Jill E. Henry: Resources. Gretchen L. Gierach: Writing – Review & editing. Alexandra J. White: Conceptualization, Supervision, Writing – Review & editing, Funding Acquisition.
References
- Abubakar M; Fan S; Bowles EA; Widemann L; Duggan MA; Pfeiffer RM; Falk RT; Lawrence S; Richert-Boe K; Glass AG; Kimes TM; Figueroa JD; Rohan TE; Gierach GL Relation of Quantitative Histologic and Radiologic Breast Tissue Composition Metrics With Invasive Breast Cancer Risk. JNCI Cancer Spectr 2021;5:pkab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar M; Klein A; Fan S; Lawrence S; Mutreja K; Henry JE; Pfeiffer RM; Duggan MA; Gierach GL Host, reproductive, and lifestyle factors in relation to quantitative histologic metrics of the normal breast. PREPRINT (Version 1) available at Research Square 2023; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ZJ; Ravnskjær L; Andersen KK; Loft S; Brandt J; Becker T; Ketzel M; Hertel O; Lynge E; Bräuner EV Long-term Exposure to Fine Particulate Matter and Breast Cancer Incidence in the Danish Nurse Cohort Study. Cancer Epidemiol Biomarkers Prev 2017a;26:428–430 [DOI] [PubMed] [Google Scholar]
- Andersen ZJ; Stafoggia M; Weinmayr G; Pedersen M; Galassi C; Jørgensen JT; Oudin A;Forsberg B; Olsson D; Oftedal B; Aasvang GM; Aamodt G; Pyko A; Pershagen G; Korek M; De Faire U; Pedersen NL; Östenson C-G; Fratiglioni L; Eriksen KT; Tjønneland A;Peeters PH; Bueno-de-Mesquita B; Plusquin M; Key TJ; Jaensch A; Nagel G; Lang A;Wang M; Tsai M-Y; Fournier A; Boutron-Ruault M-C; Baglietto L; Grioni S; Marcon A;Krogh V; Ricceri F; Sacerdote C; Migliore E; Tamayo-Uria I; Amiano P; Dorronsoro M;Vermeulen R; Sokhi R; Keuken M; de Hoogh K; Beelen R; Vineis P; Cesaroni G;Brunekreef B; Hoek G; Raaschou-Nielsen O Long-Term Exposure to Ambient Air Pollution and Incidence of Postmenopausal Breast Cancer in 15 European Cohorts within the ESCAPE Project. Environmental Health Perspectives 2017b;125:107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML; Dominici F; Ebisu K; Zeger SL; Samet JM Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environmental Health Perspectives 2007;115:989–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks H; Pashos N; Martin E; McLachlan J; Bunnell B; Burow M Endocrine disruptors and the tumor microenvironment: A new paradigm in breast cancer biology. Molecular and Cellular Endocrinology 2017;457:13–19 [DOI] [PubMed] [Google Scholar]
- Cheng I; Tseng C; Wu J; Yang J; Conroy SM; Shariff-Marco S; Li L; Hertz A; Gomez SL; Le Marchand L; Whittemore AS; Stram DO; Ritz B; Wu AH Association between ambient air pollution and breast cancer risk: The multiethnic cohort study. International Journal of Cancer 2020;146:699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S-Y; Kim S-J; Kim H-G; Lee JH; Choi Y; Lee H; Kim Y Evaluation of estrogenicity of major heavy metals. Science of The Total Environment 2003;312:15–21 [DOI] [PubMed] [Google Scholar]
- Chow CK; Venzon D; Jones EC; Premkumar A; O’Shaughnessy J; Zujewski J Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev 2000;9:917–921 [PubMed] [Google Scholar]
- Cuzick J; Warwick J; Pinney E; Warren RML; Duffy SW Tamoxifen and Breast Density in Women at Increased Risk of Breast Cancer. JNCI Journal of the National Cancer Institute 2004;96:621–628 [DOI] [PubMed] [Google Scholar]
- Datzmann T; Markevych I; Trautmann F; Heinrich J; Schmitt J; Tesch F Outdoor air pollution, green space, and cancer incidence in Saxony: a semi-individual cohort study. BMC Public Health 2018;18:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPre NC; Hart JE; Bertrand KA; Kraft P; Laden F; Tamimi RM Residential particulate matter and distance to roadways in relation to mammographic density: results from the Nurses’ Health Studies. Breast Cancer Res 2017;19:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JD; Pfeiffer RM; Patel DA; Linville L; Brinton LA; Gierach GL; Yang XR; Papathomas D; Visscher D; Mies C; Degnim AC; Anderson WF; Hewitt S; Khodr ZG; Clare SE;Storniolo AM; Sherman ME Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Inst 2014;106:dju286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabet S; Lemarchand C; Guénel P; Slama R Breast Cancer Risk in Association with Atmospheric Pollution Exposure: A Meta-Analysis of Effect Estimates Followed by a Health Impact Assessment. Environmental Health Perspectives 2021;129:57012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EA; Nunez Y; Abuawad A; Zota AR; Renzetti S; Devick KL; Gennings C; Goldsmith J; Coull BA; Kioumourtzoglou M-A An overview of methods to address distinct research questions on environmental mixtures: an application to persistent organic pollutants and leukocyte telomere length. Environmental Health 2019;18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S; Pearl J; Robins JM Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48 [PubMed] [Google Scholar]
- Hart JE; Bertrand KA; DuPre N; James P; Vieira VM; Tamimi RM; Laden F Long-term Particulate Matter Exposures during Adulthood and Risk of Breast Cancer Incidence in the Nurses’ Health Study II Prospective Cohort. Cancer Epidemiol Biomarkers Prev 2016;25:1274–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert L; Arabie P Comparing partitions. Journal of Classification 1985;2:193–218 [Google Scholar]
- Huynh S; von Euler-Chelpin M; Raaschou-Nielsen O; Hertel O; Tjønneland A; Lynge E; Vejborg I;Andersen ZJ Long-term exposure to air pollution and mammographic density in the Danish Diet, Cancer and Health cohort. Environmental Health 2015;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JP; Drton M; Larson T; Kaufman JD; Sandler DP; Szpiro AA Covariate-adaptive clutering of exposures for air pollution epidemiology cohorts. Ann Appl Stat 2017;11:93–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S; Stone EA Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ Sci Process Impacts 2014;16:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon MB; Fenton SE Endocrine Disruptors and the Breast: Early Life Effects and Later Life Disease. J Mammary Gland Biol Neoplasia 2013;18:43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini MV; Soto AM; Calabro JM; Ucci AA; Sonnenschein C The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci 2004;117:1495–1502 [DOI] [PubMed] [Google Scholar]
- Milanese TR; Hartmann LC; Sellers TA; Frost MH; Vierkant RA; Maloney SD; Pankratz VS;Degnim AC; Vachon CM; Reynolds CA; Thompson RA; Melton LJ; Goode EL; Visscher DW Age-Related Lobular Involution and Risk of Breast Cancer. JNCI: Journal of the National Cancer Institute 2006;98:1600–1607 [DOI] [PubMed] [Google Scholar]
- Myers CA; Slack T; Martin CK; Broyles ST; Heymsfield SB Regional disparities in obesity prevalence in the United States: A spatial regime analysis. Obesity 2015;23:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari SS; Mukherjee P An overview of mammographic density and its association with breast cancer. Breast Cancer 2018;25:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehoff NM; Gammon MD; Keil AP; Nichols HB; Engel LS; Sandler DP; White AJ Airborne mammary carcinogens and breast cancer risk in the Sister Study. Environment International 2019;130:104897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehoff NM; Keil AP; Jones RR; Fan S; Gierach GL; White AJ Outdoor air pollution and terminal duct lobular involution of the normal breast. Breast Cancer Res 2020;22:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reding KW; Young MT; Szpiro AA; Han CJ; DeRoo LA; Weinberg C; Kaufman JD; Sandler DP Breast Cancer Risk in Relation to Ambient Air Pollution Exposure at Residences in the Sister Study Cohort. Cancer Epidemiol Biomarkers Prev 2015;24:1907–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnov-Jessen L; Petersen OW; Bissell MJ Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996;76:69–125 [DOI] [PubMed] [Google Scholar]
- Russo J; Hu YF; Yang X; Russo IH Chapter 1: Developmental, Cellular, and Molecular Basis of Human Breast Cancer. JNCI Monographs 2000;2000:17–37 [DOI] [PubMed] [Google Scholar]
- Rutter CM Changes in Breast Density Associated With Initiation, Discontinuation, and Continuing Use of Hormone Replacement Therapy. JAMA 2001;285:171. [DOI] [PubMed] [Google Scholar]
- Santodonato J Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: Relationship to carcinogenicity. Chemosphere 1997;34:835–848 [DOI] [PubMed] [Google Scholar]
- Terry MB; Michels KB; Brody JG; Byrne C; Chen S; Jerry DJ; Malecki KMC; Martin MB; Miller RL; Neuhausen SL; Silk K; Trentham-Dietz A; Program, o.b.o.B.C.t.E.R. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res 2019;21:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. EQUATES CMAQv5.3.2 output (version 1.0) [data file and documentation]. 2021;
- Vellal AD; Sirinukunwattan K; Kensler KH; Baker GM; Stancu AL; Pyle ME; Collins LC; Schnitt SJ; Connolly JL; Veta M; Eliassen AH; Tamimi RM; Heng YJ Deep Learning Image Analysis of Benign Breast Disease to Identify Subsequent Risk of Breast Cancer. JNCI Cancer Spectr 2021;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ; Goldberg MS; Crouse DL; To T; Weichenthal SA; Wall C; Miller AB Residential exposure to fine particulate matter air pollution and incident breast cancer in a cohort of Canadian women. Environmental Epidemiology 2018;2:e021 [Google Scholar]
- White AJ; Bradshaw PT; Hamra GB Air pollution and Breast Cancer: A Review. Curr Epidemiol Rep 2018;5:92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ; Gregoire AM; Niehoff NM; Bertrand KA; Palmer JR; Coogan PF; Bethea TN Air pollution and breast cancer risk in the Black Women’s Health Study. Environmental Research 2021;194:110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ; Keller JP; Zhao S; Carroll R; Kaufman JD; Sandler DP Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A U.S.-Wide Cohort. Environmental Health Perspectives 2019a;127:107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ; O’Brien KM; Niehoff NM; Carroll R; Sandler DP Metallic Air Pollutants and Breast Cancer Risk in a Nationwide Cohort Study. Epidemiology 2019b;30:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ; Weinberg CR; O’Meara ES; Sandler DP; Sprague BL Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res 2019c;21:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghjyan L; Arao R; Brokamp C; O’Meara ES; Sprague BL; Ghita G; Ryan P Association between air pollution and mammographic breast density in the Breast Cancer Surveilance Consortium. Breast Cancer Res 2017;19:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


