Abstract
Personality traits have been associated with risk of dementia and Alzheimer’s disease neuropathology, including amyloid and tau. This study examines whether personality traits are concurrently related to plasma glial fibrillary acidic protein (GFAP), a marker of astrogliosis, and neurofilament light (NfL), a marker of neuronal injury. Cognitively unimpaired participants from the Baltimore Longitudinal Study on Aging (N=786; age: 22-95) were assayed for plasma GFAP and NfL and completed the Revised NEO Personality Inventory, which measures five domains and 30 facets of personality. Neuroticism (particularly vulnerability to stress, anxiety, and depression) was associated with higher GFAP and NfL. Conscientiousness was associated with lower GFAP. Extraversion (particularly positive emotions, assertiveness, and activity) was related to lower GFAP and NfL. These associations were independent of demographic, behavioral, and health covariates and not moderated by age, sex, or APOE genotype. The personality correlates of astrogliosis and neuronal injury tend to be similar, are found in individuals without cognitive impairment, and point to potential neurobiological underpinnings of the association between personality traits and neurodegenerative diseases.
Keywords: Personality, glial fibrillary acidic protein, GFAP, neurofilament light, NfL, plasma biomarkers, astrogliosis, neurodegeneration, Alzheimer’s disease
1. Introduction
Personality traits are fundamental measures of individual differences in emotional, cognitive, and behavioral patterns that characterize people over time. Most personality traits can be hierarchically organized under five major traits (neuroticism, extraversion, openness, agreeableness, and conscientiousness), known as the five-factor model or big five (Costa and McCrae, 1992). Individuals who score higher on neuroticism (the tendency to experience more negative emotions, such as sadness and anxiety) tend to have poor cognitive health, whereas individuals who score higher on conscientiousness (a tendency to be organized, reliable, motivated, and disciplined) tend to have better cognitive health. Indeed, higher neuroticism and lower conscientiousness are associated with worse performance on standard neuropsychological tests in older adults (Caselli et al., 2016; Luchetti et al., 2021; Sutin et al., 2019a), poor subjective memory (Aschwanden et al., 2020; Smit et al., 2021), and worse informant-rated cognitive functioning in everyday life (Sutin et al., 2019b). Several prospective studies further indicate that personality traits assessed in unimpaired individuals predict the risk of Alzheimer’s disease (AD) and related dementias years or decades later; neuroticism and conscientiousness tend to have the most robust and consistent associations with risk of dementia (Aschwanden et al., 2021; Duberstein et al., 2011; Duchek et al., 2020; Johansson et al., 2014; Kaup et al., 2019; Terracciano et al., 2021a; Wilson et al., 2007). At least for neuroticism, comparable associations are found for Alzheimer’s Disease (AD) and Vascular dementia, but not frontotemporal dementia (Terracciano et al., 2021a). The associations are also similar for other neurodegenerative conditions, like Parkinson’s disease (Terracciano et al., 2021b). In contrast, the associations between the other five-factor model personality traits (extraversion, openness, and agreeableness) and risk of dementia tend to be less consistent across studies (Aschwanden et al., 2021).
There are likely multiple interacting pathways underlying the associations between personality and cognitive health. Since early in life, high neuroticism and low conscientiousness are associated with more emotional distress (Kotov et al., 2010), maladaptive coping skills (Connor-Smith and Flachsbart, 2007), poor job and relationship outcomes (Barrick and Mount, 1991), sleep problems (Sutin et al., 2020), and engagement in health-risk behaviors such as smoking, physical inactivity, and poor dietary habits (Hill and Roberts, 2016; Mõttus et al., 2013). In turn, these stressors can undermine health over time. Indeed, personality traits have been associated with inflammatory and metabolic dysfunction (Luchetti et al., 2014; Tanios et al., 2022; Wagner et al., 2019), even at the level of mitochondrial DNA (Oppong et al., 2022). Individuals who score high on neuroticism and low on conscientiousness are thus more likely to experience emotional and physiological stressors that expose the brain to repeated micro insults, disrupt homeostasis, and feed vicious cycles that increase the vulnerability of neurons and glial cells to neurodegenerative processes. To support this conceptual model, more research is needed on crucial biological markers that may play a role in the association between personality and dementia. Recent studies have made progress in identifying the neurobiological bases of these associations, including the role of the amyloid (A), tau (T), and neurodegeneration (N) components of the ATN framework (Jack et al., 2016). While there are some inconsistencies across studies and markers of brain health (Baena et al., 2021; Booth et al., 2014; Byun et al., 2020; Yao et al., 2022), a meta-analysis found that neuroticism and conscientiousness were related to amyloid and tau accumulation, the hallmarks of AD neuropathology (Terracciano et al., 2022). The meta-analysis included research based on positron emission tomography (PET), cerebrospinal fluid (CSF), or post-mortem studies (Terracciano et al., 2022). To our knowledge, no study has reported the association between personality and more novel and accessible blood-based biomarkers of neurodegeneration.
To identify potential biological underpinnings of the associations between personality and neurodegenerative conditions and further understand the role of psychological traits in the pathophysiology of AD and related dementias (ADRD), this study tests whether personality traits are related to glial fibrillary acidic protein (GFAP) and neurofilament light (NfL) measured from plasma. The markers assessed in plasma have been shown to correlate substantially with the corresponding markers assessed in CSF and with PET measures of β amyloid (Palmqvist et al., 2022; Preische et al., 2019). Astrocytes are glial cells and the most abundant cells in the brain; astrocytes support neurons and are involved in the blood brain barrier and reactive astrogliosis (Escartin et al., 2021). GFAP is the most widely used marker of reactive astrogliosis, that is the morphological and functional changes in astrocytes that occur after brain injuries or during neurodegeneration. While not a specific AD marker, GFAP levels are elevated across the full spectrum of AD (Benedet et al., 2021; Chouliaras et al., 2022; Cicognola et al., 2021), including in the early stage of the disease (Benedet et al., 2021; Chatterjee et al., 2021). GFAP is also related to amyloid pathology (Benedet et al., 2021; Chatterjee et al., 2022) and predicts conversion to AD (Cicognola et al., 2021). NfL is a cytoskeletal protein expressed by neurons. NfL is considered a marker of neuro-axonal damage (Bridel et al., 2019) because it is altered in neurological disorders, including in patients with AD and other dementias (Ashton et al., 2021; Bridel et al., 2019; Chouliaras et al., 2022; Preische et al., 2019). There is some mixed evidence that NfL is related to amyloid pathology in pre-symptomatic stages (Chatterjee et al., 2022; Hu et al., 2019; Verberk et al., 2020). Finding a significant association between personality traits and markers of reactive astrogliosis and neuronal injury in cognitively normal adults would further support the hypothesis that personality and neurodegenerative biology are closely linked, perhaps through stress responses that drive neuroinflammation (Luchetti et al., 2014), even in the absence of clinically meaningful cognitive impairment.
Based on past evidence (Aschwanden et al., 2021; Terracciano et al., 2022), we expected higher neuroticism and lower conscientiousness to be associated with markers of reactive astrogliosis (higher GFAP) and neuronal injury (higher NfL). While meta-analyses suggest that extraversion and openness have a small protective effect against dementia (Aschwanden et al., 2021), the findings tend to be inconsistent, and thus we had no strong hypotheses for these two traits or for agreeableness, which tends to be unrelated to dementia risk. We further test the facets of each major trait for a more in-depth understanding of which aspects of personality are more strongly related to the different biomarkers. Supplemental analyses tested whether the association between neuroticism and conscientiousness and the biomarkers were moderated by age, sex, or apolipoprotein E (APOE) ε4 carrier status. These moderation analyses explore whether the personality associations are stronger among older, female, and APOE ε4 carrier individuals who are at greater risk for neurodegeneration or ADRD.
2. Methods
2.1. Participants.
The Baltimore Longitudinal Study of Aging (BLSA; ClinicalTrials.gov: NCT00233272) is an ongoing longitudinal study that started in 1958 (Ferrucci, 2008). Participants are community-dwelling adults who are generally healthy at enrollment (e.g., free of severe cardiac or pulmonary disease). Participants receive health and functional screenings during study visits, as described in detail elsewhere (Ferrucci, 2008).
The plasma biomarkers were available for 818 individuals; 20 individuals (about 2%) were not included in the analyses because personality data were missing; an additional 12 individuals (about 1.5%) were excluded because they had dementia or another cognitive impairment at the time of blood collection (cognitive status was determined at a consensus case conference based on neuropsychological battery and clinical examination, including informant- and participant-structured interviews). The remaining sample (n = 786) included in the analyses was 46.1% female and 32.3% non-White (24.5% Black). Table 1 presents descriptive statistics for the included participants.
Table 1.
Descriptive statistics
| Variable | Mean [or N (%)] | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age, yr. | 66.53 | 14.89 | 22.4 | 94.7 |
| Education, yr. | 17.07 | 2.37 | 8 | 21 |
| Sex (Women) | 362 (46.1%) | - | 0 | 1 |
| Black | 193 (24.5%) | - | 0 | 1 |
| White | 532 (67.7%) | - | 0 | 1 |
| Other race | 61 (7.8%) | - | 0 | 1 |
| GFAP | 165.39 | 101.57 | 31.59 | 1621.35 |
| GFAP-Winsorized | 163.69 | 86.67 | 31.59 | 521.89 |
| NfL | 21.26 | 12.25 | 2.98 | 112.43 |
| NfL-Winsorized | 21.11 | 11.57 | 2.98 | 59.76 |
| Neuroticism | 45.13 | 9.19 | 17 | 78 |
| Extraversion | 51.34 | 10.04 | 19 | 82 |
| Openness | 53.55 | 10.59 | 20 | 85 |
| Agreeableness | 52.57 | 9.89 | 9 | 79 |
| Conscientiousness | 52.99 | 10.00 | 11 | 81 |
N = 786. Age and education are in years. Personality traits are in T-scores, which are simple linear transformation based on combined gender norms that have a mean of 50 and SD of 10, see NEO-PI-R manual (Costa and McCrae, 1992).
Local institutional review boards approved the BLSA study protocols. All participants provided written informed consent at each visit. The BLSA complies with the NIH ethical standards and with the Helsinki Declaration of 1975, as revised in 2008. Deidentified BLSA data is available on request (http://blsa.nih.gov).
2.2. Measures.
Personality traits were assessed with the self-report version of the Revised NEO Personality Inventory (NEO-PI R)(Costa and McCrae, 1992), a reliable measure of personality. The questionnaire included 240 items answered on a 5-point Likert scale, from “strongly disagree” to “strongly agree”. The NEO-PI-R assesses 30 facets, six for each of the five factors. The five-factor scales have high internal consistency (Cronbach alpha ≥ .85) and high test-retest correlations (rtt = 0.78–0.85) over ten years in the BLSA (Terracciano et al., 2006). The NEO-PI-R provides valid assessment across age groups and clinical and non-clinical samples (Bagby et al., 1999; Costa and McCrae, 1992; Islam et al., 2019; Terracciano et al., 2006; Terracciano et al., 2018).
Personality was assessed at the same visit as the biomarker collection for most participants (n=723), or we used the assessment from the previous (n=51; time gap ranged from −19 to −1 years; M = −4.74, SD = 4.02) or following (n=12; time gap ranged from 1 to 4 years; M = 1.78, SD = 0.88) visit.
2.3. Plasma biomarkers.
Fasting venous blood was collected and processed using standardized methods. Samples were kept at −80°C until the biomarkers were measured in EDTA plasma using the Single Molecule Array (Simoa) Neurology 4-Plex E (N4PE) assay on the Simoa HD-X instrument (Quanterix Corporation). Assays were run in duplicate, and values were averaged. Intra-assay coefficients of variation were 5.0 for GFAP and 5.1 for NfL (Duggan et al., 2022; Peng et al., 2022). To address the right-skewed distributions of the plasma biomarkers, we winsorized (replaced with the closest highest value in the distribution) 3 outliers for GFAP and 5 for NfL (the Winsorized values were identified by visual inspection and were > 3 SD above the mean). The resulting variables had acceptable skewness and kurtosis (< 1.5) and were used in the analyses. Sensitivity analyses that used log-transformation produced similar results (Supplementary Table S1).
2.4. Covariates/Moderators.
Participant-reported age, sex (male/female), race (white/other), education (years), and the time between personality assessment and biomarker assay were included as covariates. CKD-EPI criteria were used for the estimated glomerular filtration rate (eGFR)-creatinine (Levey et al., 2009). APOE ε4 carrier status was coded as 0 for no ε4 allele and 1 for one or two ε4 alleles. Physical activity was assessed with questions on household chores, household updating/maintenance/repair, gardening and yardwork, walking and climbing stairs, exercise, and recreation. Responses were translated into metabolic equivalent of task (MET) minutes per day and coded as 0=Inactive (<50 MET-min/d), 1=Low (50-250 MET-min/d), 2=Moderate (250-500 MET-min/d), and 3=Very active (≥500 MET-min/d). Body mass index (BMI) was calculated from staff assessed weight and height. Smoking was coded as current (vs. other) and former (vs. other) smokers. Disease burden was the sum of the following conditions: depression, hypertension, diabetes, stroke, anemia, cancer, congestive heart failure, ischemic heart disease, and chronic obstructive pulmonary disease.
2.5. Statistical analyses.
We calculated descriptive statistics for study variables as means and standard deviations (SD) or proportions. We computed z-scores for all variables in the regression analyses. The main results are presented as standardized Beta (β) coefficients with 95% confidence intervals (CI) from linear regression analyses with personality traits entered as predictors of the two biomarkers. Model 1 included age and sex as covariates. Model 2 further included the time gap between personality assessment and the blood collection, eGFR, years of education, race, physical activity, BMI, smoking, and disease burden. We ran separate models for each trait to avoid spurious results due to suppression effects. We further tested whether the associations of neuroticism and conscientiousness with the two biomarkers were moderated by age, sex, or APOE ε4 carrier status by testing interaction terms. Because of the a priori hypotheses based on published evidence, and to avoid inflating type I error, we set significance at p < .05 for neuroticism and conscientiousness and their facets. For the other traits, we report the nominal p-value but mainly discuss findings with p < .01. SPSS version 27 was used for the analyses.
3. Results
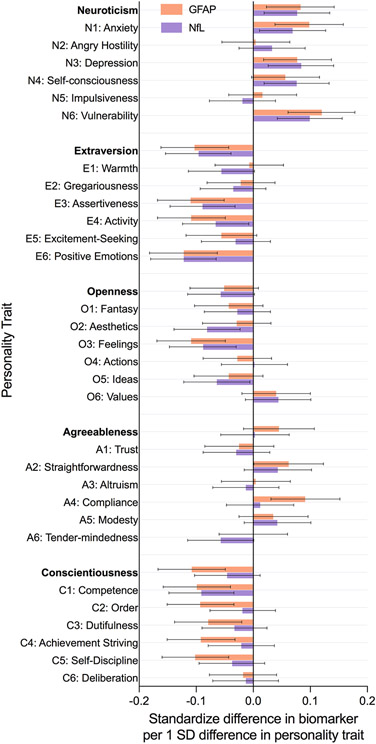
Accounting for age and sex (Model 1, Table 2), participants who scored higher on neuroticism (β = .08, 95%CI = .02, .14, p = .007) and lower on conscientiousness (β = −.11, 95%CI = −.17, −.05, p < .001) had higher levels of GFAP. Neuroticism was associated with higher levels of NfL (β = .08, 95%CI = .02, .13, p = .009), but not conscientiousness (β = −.05, 95%CI = −.10, .01, p = .119)(Table 3). Higher extraversion was significantly associated with lower GFAP (β = −.10, 95%CI = −.16 −.04, p = .001) and NfL (β = −.10, 95%CI = −.15 −.04, p = .001). Openness and agreeableness were unrelated to the biomarkers. Overall, the pattern of results was similar across GFAP and NfL, with slightly larger effects for GFAP (Figure 1). While the results were similar in model 2, the effect sizes were reduced (particularly for NfL) after accounting for eGFR, years of education, race, physical activity, BMI, smoking, and disease burden. Sensitivity analyses that excluded 63 participants with the personality assessment not at the same visit as the blood collection produced similar results: GFAP was related to higher neuroticism (β = .08, 95%CI = .02, .15, p = .009) and lower conscientiousness (β = −.12, 95%CI = −.17, −.05, p < .001); NfL was related to higher neuroticism (β = .09, 95%CI = .03, .15, p = .004) but not conscientiousness (β = −.05, 95%CI = −.11, .01, p = .088).
Table 2.
Standardized beta regression coefficients from regression models with personality traits predicting glial fibrillary acidic protein (GFAP).
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait | β | L95%CI | U95%CI | p | β | L95%CI | U95%CI | p |
| Neuroticism | 0.083 | 0.023 | 0.142 | 0.007 | 0.079 | 0.018 | 0.140 | 0.012 |
| Extraversion | −0.103 | −0.162 | −0.043 | 0.001 | −0.085 | −0.147 | −0.024 | 0.007 |
| Openness | −0.051 | −0.111 | 0.009 | 0.097 | −0.047 | −0.111 | 0.016 | 0.146 |
| Agreeableness | 0.045 | −0.017 | 0.107 | 0.151 | 0.041 | −0.022 | 0.106 | 0.199 |
| Conscientiousness | −0.108 | −0.167 | −0.049 | <0.001 | −0.098 | −0.160 | −0.038 | 0.002 |
| N1: Anxiety | 0.098 | 0.038 | 0.158 | 0.001 | 0.093 | 0.031 | 0.154 | 0.003 |
| N2: Angry Hostility | 0.004 | −0.055 | 0.064 | 0.883 | 0.007 | −0.054 | 0.069 | 0.811 |
| N3: Depression | 0.077 | 0.018 | 0.137 | 0.010 | 0.075 | 0.014 | 0.137 | 0.016 |
| N4: Self-consciousness | 0.056 | −0.003 | 0.116 | 0.063 | 0.039 | −0.022 | 0.100 | 0.208 |
| N5: Impulsiveness | 0.016 | −0.043 | 0.076 | 0.590 | 0.039 | −0.023 | 0.101 | 0.222 |
| N6: Vulnerability | 0.120 | 0.061 | 0.178 | <0.001 | 0.103 | 0.042 | 0.164 | 0.001 |
| E1: Warmth | −0.007 | −0.067 | 0.053 | 0.825 | 0.012 | −0.049 | 0.074 | 0.693 |
| E2: Gregariousness | −0.022 | −0.081 | 0.038 | 0.475 | −0.012 | −0.072 | 0.048 | 0.692 |
| E3: Assertiveness | −0.110 | −0.168 | −0.051 | <0.001 | −0.088 | −0.149 | −0.026 | 0.005 |
| E4: Activity | −0.109 | −0.168 | −0.049 | <0.001 | −0.118 | −0.183 | −0.057 | <0.001 |
| E5: Excitement-Seeking | −0.056 | −0.118 | 0.006 | 0.079 | −0.043 | −0.108 | 0.021 | 0.183 |
| E6: Positive Emotions | −0.122 | −0.182 | −0.063 | <0.001 | −0.110 | −0.172 | −0.048 | 0.001 |
| O1: Fantasy | −0.043 | −0.103 | 0.017 | 0.163 | −0.039 | −0.102 | 0.024 | 0.221 |
| O2: Aesthetics | −0.029 | −0.089 | 0.031 | 0.349 | −0.025 | −0.087 | 0.037 | 0.427 |
| O3: Feelings | −0.109 | −0.169 | −0.049 | <0.001 | −0.102 | −0.164 | −0.040 | 0.001 |
| O4: Actions | −0.028 | −0.088 | 0.032 | 0.363 | −0.028 | −0.090 | 0.033 | 0.364 |
| O5: Ideas | −0.043 | −0.104 | 0.017 | 0.160 | −0.040 | −0.104 | 0.023 | 0.212 |
| O6: Values | 0.040 | −0.020 | 0.100 | 0.188 | 0.047 | −0.016 | 0.111 | 0.141 |
| A1: Trust | −0.025 | −0.085 | 0.036 | 0.418 | −0.022 | −0.085 | 0.040 | 0.481 |
| A2: Straightforwardness | 0.062 | 0.001 | 0.123 | 0.048 | 0.048 | −0.014 | 0.111 | 0.129 |
| A3: Altruism | 0.004 | −0.056 | 0.065 | 0.887 | 0.015 | −0.047 | 0.077 | 0.632 |
| A4: Compliance | 0.091 | 0.031 | 0.152 | 0.003 | 0.082 | 0.020 | 0.146 | 0.010 |
| A5: Modesty | 0.035 | −0.025 | 0.096 | 0.252 | 0.024 | −0.039 | 0.088 | 0.446 |
| A6: Tender-mindedness | 0.000 | −0.060 | 0.060 | 0.990 | 0.009 | −0.053 | 0.072 | 0.767 |
| C1: Competence | −0.099 | −0.158 | −0.040 | 0.001 | −0.076 | −0.137 | −0.015 | 0.014 |
| C2: Order | −0.093 | −0.151 | −0.034 | 0.002 | −0.091 | −0.150 | −0.031 | 0.003 |
| C3: Dutifulness | −0.079 | −0.138 | −0.020 | 0.008 | −0.078 | −0.139 | −0.017 | 0.012 |
| C4: Achievement Striving | −0.092 | −0.151 | −0.032 | 0.003 | −0.086 | −0.149 | −0.025 | 0.006 |
| C5: Self-Discipline | −0.102 | −0.160 | −0.043 | 0.001 | −0.097 | −0.159 | −0.037 | 0.002 |
| C6: Deliberation | −0.018 | −0.077 | 0.041 | 0.556 | −0.008 | −0.069 | 0.053 | 0.795 |
Notes: Model 1 N = 786; Model 2 N = 761. Model 1 include age and sex as covariates. Model 2 further include the time gap between personality assessment and the blood collection, eGFR, years of education, race (other vs. white), smoking, physical activity, body mass index, and disease burden. L95%CI and U95%CI are the lower and upper bound of the 95% Confidence interval. p < .05 and related β are bolded.
Table 3.
Standardized beta regression coefficients from regression models with personality traits predicting neurofilament light (NfL).
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait | β | L95%CI | U95%CI | p | β | L95%CI | U95%CI | p |
| Neuroticism | 0.077 | 0.019 | 0.134 | 0.009 | 0.060 | 0.003 | 0.117 | 0.040 |
| Extraversion | −0.096 | −0.154 | −0.039 | 0.001 | −0.059 | −0.117 | −0.002 | 0.042 |
| Openness | −0.057 | −0.115 | 0.002 | 0.056 | −0.040 | −0.100 | 0.019 | 0.178 |
| Agreeableness | 0.003 | −0.057 | 0.063 | 0.922 | −0.013 | −0.073 | 0.046 | 0.658 |
| Conscientiousness | −0.046 | −0.103 | 0.012 | 0.119 | −0.026 | −0.084 | 0.031 | 0.362 |
| N1: Anxiety | 0.069 | 0.011 | 0.127 | 0.020 | 0.052 | −0.006 | 0.109 | 0.078 |
| N2: Angry Hostility | 0.033 | −0.025 | 0.091 | 0.260 | 0.031 | −0.026 | 0.088 | 0.285 |
| N3: Depression | 0.084 | 0.026 | 0.141 | 0.004 | 0.077 | 0.021 | 0.134 | 0.008 |
| N4: Self-consciousness | 0.076 | 0.019 | 0.133 | 0.009 | 0.047 | −0.010 | 0.104 | 0.103 |
| N5: Impulsiveness | −0.019 | −0.077 | 0.039 | 0.516 | −0.002 | −0.060 | 0.056 | 0.941 |
| N6: Vulnerability | 0.099 | 0.042 | 0.156 | 0.001 | 0.060 | 0.003 | 0.117 | 0.039 |
| E1: Warmth | −0.056 | −0.114 | 0.002 | 0.056 | −0.036 | −0.094 | 0.021 | 0.210 |
| E2: Gregariousness | −0.035 | −0.093 | 0.022 | 0.227 | −0.028 | −0.084 | 0.028 | 0.328 |
| E3: Assertiveness | −0.089 | −0.146 | −0.032 | 0.002 | −0.037 | −0.095 | 0.020 | 0.203 |
| E4: Activity | −0.066 | −0.124 | −0.008 | 0.025 | −0.056 | −0.115 | 0.003 | 0.061 |
| E5: Excitement-Seeking | −0.031 | −0.091 | 0.030 | 0.322 | −0.005 | −0.065 | 0.055 | 0.874 |
| E6: Positive Emotions | −0.122 | −0.180 | −0.065 | <0.001 | −0.087 | −0.144 | −0.029 | 0.003 |
| O1: Fantasy | −0.028 | −0.086 | 0.030 | 0.345 | −0.015 | −0.073 | 0.043 | 0.616 |
| O2: Aesthetics | −0.081 | −0.139 | −0.023 | 0.006 | −0.057 | −0.114 | 0.000 | 0.050 |
| O3: Feelings | −0.088 | −0.147 | −0.030 | 0.003 | −0.078 | −0.136 | −0.020 | 0.009 |
| O4: Actions | 0.002 | −0.056 | 0.060 | 0.953 | 0.020 | −0.037 | 0.078 | 0.490 |
| O5: Ideas | −0.064 | −0.122 | −0.006 | 0.032 | −0.052 | −0.111 | 0.007 | 0.086 |
| O6: Values | 0.044 | −0.014 | 0.101 | 0.140 | 0.034 | −0.025 | 0.093 | 0.259 |
| A1: Trust | −0.030 | −0.088 | 0.029 | 0.320 | −0.042 | −0.101 | 0.015 | 0.148 |
| A2: Straightforwardness | 0.043 | −0.016 | 0.102 | 0.152 | 0.021 | −0.038 | 0.080 | 0.481 |
| A3: Altruism | −0.013 | −0.071 | 0.045 | 0.662 | −0.007 | −0.065 | 0.050 | 0.797 |
| A4: Compliance | 0.012 | −0.047 | 0.071 | 0.699 | −0.017 | −0.076 | 0.041 | 0.557 |
| A5: Modesty | 0.042 | −0.016 | 0.101 | 0.153 | 0.026 | −0.032 | 0.086 | 0.374 |
| A6: Tender-mindedness | −0.057 | −0.115 | 0.001 | 0.056 | −0.038 | −0.097 | 0.020 | 0.193 |
| C1: Competence | −0.091 | −0.148 | −0.034 | 0.002 | −0.061 | −0.118 | −0.005 | 0.034 |
| C2: Order | −0.019 | −0.076 | 0.039 | 0.526 | −0.008 | −0.065 | 0.048 | 0.768 |
| C3: Dutifulness | −0.033 | −0.090 | 0.024 | 0.254 | −0.044 | −0.101 | 0.013 | 0.127 |
| C4: Achievement Striving | −0.021 | −0.079 | 0.037 | 0.482 | 0.009 | −0.049 | 0.067 | 0.760 |
| C5: Self-Discipline | −0.037 | −0.095 | 0.020 | 0.199 | −0.020 | −0.077 | 0.037 | 0.485 |
| C6: Deliberation | −0.013 | −0.071 | 0.044 | 0.645 | −0.003 | −0.060 | 0.053 | 0.910 |
Notes: Model 1 N = 786; Model 2 N = 761. Model 1 include age and sex. Model 2 further include the time gap between personality assessment and the blood collection, eGFR, years of education, race (other vs. white), smoking, physical activity, body mass index, and disease burden. L95%CI and U95%CI are the lower and upper bound of the 95% Confidence interval. p < .05 and related β are bolded.
Figure 1.
Clustered bar chart of the sex and age-adjusted associations between personality traits and measures of neurodegeneration.
Results for the facets were generally consistent with the factors, with some notable differences. Among the facets of neuroticism, vulnerability was the facet with the strongest association with both GFAP (β = .12, 95%CI = .06, .18, p < .001) and NfL (β = .10, 95%CI = .04, .16, p = .001)(Table 2 and 3, Model 1). The neuroticism facets of anxiety and depression were also related to higher GFAP (anxiety: β = .10, 95%CI = .04, .16, p = .001; depression: (β = .08, 95%CI = .02, .14, p = .01) and NfL (anxiety: β = .07, 95%CI = .01, .13, p = .02; depression: (β = .08, 95%CI = .03, .14, p = .009). The extraversion associations with lower GFAP and NfL were driven by the facets of positive emotions (GFAP: β = −.12, 95%CI = −.18 −.06, p < .001; NfL: β = −.12, 95%CI = −.18 −.07, p < .001), assertiveness (GFAP: β = −.11, 95%CI = −.17 −.05, p < .001; NfL: β = −.09, 95%CI = −.15 −.03, p = .002), and activity (GFAP: β = −.11, 95%CI = −.17 −.05, p < .001; NfL: β = −.07, 95%CI = −.12 −.01, p = .025). The extraversion facets that tap sociability were unrelated to either marker. The facet openness to feelings was related to both low GFAP and NfL. The facets of agreeableness were largely unrelated to both markers. Most facets of conscientiousness were related to GFAP, whereas only competence (β = −.09, 95%CI = −.15 −.03, p = .002) was related to NfL.
Age, sex, and APOE ε4 risk variant did not moderate the associations between neuroticism and conscientiousness and either biomarker (all p > .05).
4. Discussion
This study provides novel evidence for the personality correlates of astrogliosis (GFAP) and neuronal injury (NfL) markers assessed in plasma in a cognitively unimpaired sample. Consistent with the broader literature on personality and dementia (Aschwanden et al., 2021), individuals who scored higher on neuroticism and lower on conscientiousness had elevated levels of GFAP. For NfL, the expected association was found for neuroticism, but it did not reach statistical significance for conscientiousness. Thus, neuroticism was similarly related to astrogliosis and neuronal injury markers, while conscientiousness may be more relevant to reactive astrocytes and astrocyte-mediated neuroinflammation. The results were robust when accounting for demographic, behavioral, and health covariates and were not moderated by age, sex, and the APOE variant. These findings complement previous work on amyloid and tau (Terracciano et al., 2022), which found higher neuroticism and lower conscientiousness to be associated with more brain amyloid and tau deposition. In addition, individuals who scored higher on extraversion also had lower levels of GFAP and NfL, while no associations were found for openness and agreeableness. This work expands knowledge on plausible neuropathological processes that underlie the association between personality and risk of dementia (Aschwanden et al., 2021).
One major contribution of this study was the in-depth assessment of personality. The assessments of facets provide an opportunity to get a more granular understanding of which aspects of the five broader factors are driving the associations. For neuroticism, the largest effects were found for the facet labeled “vulnerability”, which assesses the ability to cope with stress and remain calm in difficult or threatening situations. The people who reported the most emotional vulnerability (e.g., those who may panic or feel helpless when facing emergencies, problems, or stressful conditions) had the highest levels of GFAP and NfL. Similar effects were also found for anxiety and depression, two facets of personality that overlap with common neuropsychiatric symptoms, as well as anxiety and depressive disorders. This finding is consistent with clinical evidence that elevated GFAP and NfL are associated with anxiety and depressive symptomatology (Lange et al., 2022; Steinacker et al., 2021). The association between extraversion and the biomarkers was not due to the sociability component of extraversion. Instead, it was the cheerful (positive emotions), forceful (assertiveness), and energetic (activity) dispositions of those high on extraversion that seemed to be protective against neurodegeneration. This finding seems consistent with evidence that individuals who report greater well-being, such as more happiness or purpose in life, tend to have better cognitive health and lower risk of dementia (Sutin et al., 2021; Zhu et al., 2023). People who score higher on the personality facet of activity tend to have more energy, vigorous movement, and a faster tempo compared to individuals who score lower on this facet; the finding that the personality facet activity is related to lower levels of GFAP and NfL, especially in older adults, is consistent with the evidence on the neuroprotective effects of physical activity (Maugeri et al., 2021; Raffin et al., 2021). Openness to feelings (high scorers have more differentiated and deeper emotional states and feelings, while low scorers are characterized by alexithymia) was the facet of openness with the strongest inverse association to GFAP and NfL. Facets of agreeableness were unrelated to the biomarkers. There was little differentiation among the facets of conscientiousness in its association with GFAP. However, only competence (a sense that one is efficient and capable) was related to NfL.
The current study design cannot determine causality. The observed associations could be due to multiple and non-mutually exclusive interpretations, including (a) neurodegeneration could cause change in personality, (b) personality could modulate risk of neurodegeneration, (c) bidirectional or reciprocal influences between personality and neurodegeneration, (d) other variables (e.g., genes, trauma) influence both personality and neurodegeneration, and (e) confounding factors leading to spurious associations. Next, we discuss the evidence related to the first two interpretations. The neurodegenerative processes leading to higher GFAP and NfL may impact personality, especially after the onset of cognitive impairment. Indeed, about a dozen studies of knowledgeable informants have reported large changes in personality among people with dementia (Islam et al., 2019; Siegler et al., 1991). Astrogliosis and neuronal injury may also interact with personality to manifest in neuropsychiatric symptoms. However, the current study was based on healthy individuals and excluded cognitively impaired participants. Thus, to explain the current findings, neurodegeneration would need to cause change in personality before causing cognitive impairment. But, substantial changes in personality are generally not apparent before the onset of MCI or dementia: A long-term longitudinal study found no evidence of personality change in the preclinical phase for people who developed AD (Terracciano et al., 2017). An alternative interpretation of the observed association is that personality traits modulate the trajectory of brain health over the lifespan by, for example, supporting brain maintenance (Nyberg et al., 2012) and resistance (Arenaza-Urquijo and Vemuri, 2018) against the risk of astrogliosis and neuronal injury. Early in life, these traits are related to educational achievement, and higher education can protect health and cognitive function in later life (Crimmins et al., 2018; Dumfart and Neubauer, 2016; Seblova et al., 2021). Personality traits also shape behaviors and lifestyles, including health risk behaviors that may influence brain health, such as physical activity and cigarette smoking (Hampson et al., 2006; Kekäläinen et al., 2022). However, in this study, the observed associations were mostly independent of the effect of the educational, behavioral, and health covariates, especially for GFAP (Model 2). Personality traits are related to other constructs that may contribute to brain health, including stress reactivity, coping skills, social connection (e.g., loneliness)(Buecker et al., 2020), and well-being (e.g., purpose in life)(Sutin et al., 2021; Zhu et al., 2023), as well as key biological pathways such as inflammation and neurotrophic factors (Hill and Roberts, 2016; Luchetti et al., 2014; Terracciano et al., 2011). High neuroticism and low conscientiousness are also major risk factors for mental health conditions (Bucher et al., 2019; Kotov et al., 2010), which can have a detrimental impact on neurodegenerative processes. Thus, these enduring personality dispositions that emerge early in life are likely to engage multiple psychosocial and neurobiological mechanisms that may regulate neurodegenerative processes; the reverse (i.e., neuropathology inducing changes in personality) may occur later, along with the onset of clinical impairment.
4.1. Strengths and limitations
The current study had several strengths, including the relatively novel assessment of GFAP and NfL in plasma, the detailed assessment of all five major personality traits and 30 facets, and the well-characterized sample of participants who underwent rigorous testing, including clinical diagnoses of MCI and dementia. There are also limitations that should be considered. We tested several traits and facets in this study and some results could be due to chance. For example, the association of Extraversion with GFAP and NfL found in this study was surprising given the inconsistent associations between extraversion and health (Hill and Roberts, 2016), and particularly with markers of brain health (Terracciano et al., 2022), cognitve function (Caselli et al., 2016; Sutin et al., 2019a), and risk of dementia (Aschwanden et al., 2021). The sample included a substantial portion of African Americans, but the BLSA participants tend to have a high level of education, which may limit the generalizability of the findings. More research is clearly needed to test whether these associations are replicable, especially in samples with a broader range of education and economic status, from the US and other countries. While we discuss the findings in the context of neurodegenerative diseases like AD, both GFAP and NfL are not specific markers of AD. Another limitation was that covariates like physical activity were assessed with few items. While we found that age, sex, and APOE did not moderate the reported associations, future studies should test other potential moderators, including whether the associations change with the transition from amyloid negative to positive, or with the onset of cognitive impairment.
4.2. Conclusions
This study found that neuroticism and conscientiousness are related to markers of reactive astrogliosis and neuronal injury. The associations were similar across markers, but conscientiousness may be more relevant to reactive astrocytes and astrocyte-mediated neuroinflammation, whereas neuroticism was similarly related to astrocytes and neuronal injury. The associations were evident in a sample of cognitively healthy adults, some of whom are in the preclinical stages of AD and related neurodegenerative conditions. The findings complement other research on amyloid and tau (Terracciano et al., 2022) and point to potential neurobiological underpinnings to the broader epidemiological and clinical evidence linking personality traits to neurodegenerative diseases (Aschwanden et al., 2021).
Supplementary Material
Highlights.
Personality traits are related to measures of astrogliosis and neuronal damage.
High neuroticism and low extraversion are associated with higher GFAP and NfL.
High conscientiousness is associated with lower GFAP.
The associations are independent of other risk factors for neurodegeneration.
The associations are evident in the absence of cognitive impairment.
Acknowledgments
The authors are grateful to the BLSA study participants.
Funding:
The BLSA studies are supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. The research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (grant numbers R01AG068093 and R01AG053297). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CRediT author statement
Antonio Terracciano: Conceptualization, Data analysis, Writing-Original draft preparation, Funding acquisition
Keenan Walker: Conceptualization, Writing- Reviewing and Editing, Visualization
Yang An: Conceptualization, Data curation, Writing- Reviewing and Editing
Martina Luchetti: Conceptualization, Writing - Review & Editing
Yannick Stephan: Conceptualization, Writing - Review & Editing
Abhay R. Moghekar: Conceptualization, Writing - Review & Editing, Investigation
Angelina R. Sutin: Conceptualization, Writing - Review & Editing, Funding acquisition
Luigi Ferrucci: Conceptualization, Writing - Review & Editing, Supervision, Funding acquisition
Susan M. Resnick: Conceptualization, Writing - Review & Editing, Supervision, Funding acquisition
Competing interests: Authors declare that they have no potential conflict of interest.
Data and materials availability: The anonymized BLSA data is publicly accessible upon request at https://www.blsa.nih.gov.
References
- Arenaza-Urquijo EM, Vemuri P, 2018. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology 90(15), 695–703. 10.1212/WNL.0000000000005303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, Terracciano A, 2021. Is personality associated with dementia risk? A meta-analytic investigation. Ageing Research Reviews 67, 101269. 10.1016/j.arr.2021.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschwanden D, Sutin AR, Luchetti M, Allemand M, Stephan Y, Terracciano A, 2020. A Systematic Review and Meta-Analysis of the Association between Personality and Cognitive Failures / Complaints. Social and personality psychology compass 14(11), e12565. 10.1111/spc3.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, Benedet AL, Pascoal TA, Lleó A, Parnetti L, 2021. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nature communications 12(1), 1–12. 10.1038/s41467-021-23620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena A, Bocanegra Y, Torres V, Vila-Castelar C, Guzmán-Vélez E, Fox-Fuller JT, Gatchel JR, Sánchez J, Pluim CF, Ramirez-Gómez L, Martínez J, Pineda D, Lopera F, Quiroz YT, 2021. Neuroticism Is Associated with Tau Pathology in Cognitively Unimpaired Individuals with Autosomal Dominant Alzheimer's Disease. Journal of Alzheimer's Disease 82(4), 1809–1822. 10.3233/jad-210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Costa PT Jr., McCrae RR, Livesley WJ, Kennedy SH, Levitan RD, Levitt AJ, Joffe RT, Young LT, 1999. Replicating the five factor model of personality in a psychiatric sample. Pers Indiv Differ 27(6), 1135–1139. 10.1016/S0191-8869(99)00055-0. [DOI] [Google Scholar]
- Barrick MR, Mount MK, 1991. The Big Five personality dimensions and job performance: A meta-analysis. Personnel Psychology 44, 1–26. 10.1111/j.1744-6570.1991.tb00688.x. [DOI] [Google Scholar]
- Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F, Karikari TK, Hourregue C, Cognat E, Dumurgier J, Stevenson J, Rahmouni N, Pallen V, Poltronetti NM, Salvadó G, et al. , 2021. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA neurology 78(12), 1471–1483. 10.1001/jamaneurol.2021.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T, Mottus R, Corley J, Gow AJ, Henderson RD, Maniega SM, Murray C, Royle NA, Sprooten E, Hernandez MC, Bastin ME, Penke L, Starr JM, Wardlaw JM, Deary IJ, 2014. Personality, health, and brain integrity: the Lothian birth cohort study 1936. Health Psychology 33(12), 1477–1486. 10.1037/hea0000012. [DOI] [PubMed] [Google Scholar]
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, et al. , 2019. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA neurology 76(9), 1035–1048. 10.1001/jamaneurol.2019.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher MA, Suzuki T, Samuel DB, 2019. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clinical Psychology Review 70, 51–63. 10.1016/j.cpr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Buecker S, Maes M, Denissen JJ, Luhmann M, 2020. Loneliness and the big five personality traits: a meta–analysis. European Journal of Personality 34(1), 8–28. 10.1002/per.2229. [DOI] [Google Scholar]
- Byun MS, Jung JH, Sohn BK, Yi D, Lee JH, Jeon SY, Lee Y, Jung GJ, Lee JY, Kim YK, Shin SA, Sohn CH, Kang KM, Lee DY, 2020. Neuroticism, conscientiousness, and in vivo Alzheimer pathologies measured by amyloid PET and MRI. Psychiatry and clinical neurosciences 74(5), 303–310. 10.1111/pcn.12983. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Henslin BR, Johnson TA, Woodruff BK, Hoffman-Snyder C, Geda YE, 2016. Impact of Personality on Cognitive Aging: A Prospective Cohort Study. JINS 22(7), 765–776. 10.1017/s1355617716000527. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Pedrini S, Ashton NJ, Tegg M, Goozee K, Singh AK, Karikari TK, Simrén J, Vanmechelen E, Armstrong NJ, Hone E, Asih PR, Taddei K, Doré V, Villemagne VL, et al. , 2022. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer's disease. Alzheimer's & Dementia 18(6), 1141–1154. 10.1002/alz.12447. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Pedrini S, Stoops E, Goozee K, Villemagne VL, Asih PR, Verberk IMW, Dave P, Taddei K, Sohrabi HR, Zetterberg H, Blennow K, Teunissen CE, Vanderstichele HM, Martins RN, 2021. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Translational psychiatry 11(1), 27. 10.1038/s41398-020-01137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L, Thomas A, Malpetti M, Donaghy P, Kane J, Mak E, Savulich G, Prats-Sedano MA, Heslegrave AJ, Zetterberg H, Su L, Rowe JB, O'Brien JT, 2022. Differential levels of plasma biomarkers of neurodegeneration in Lewy body dementia, Alzheimer's disease, frontotemporal dementia and progressive supranuclear palsy. Journal of neurology, neurosurgery, and psychiatry 93(6), 651–658. 10.1136/jnnp-2021-327788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicognola C, Janelidze S, Hertze J, Zetterberg H, Blennow K, Mattsson-Carlgren N, Hansson O, 2021. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer's research & therapy 13(1), 68. 10.1186/s13195-021-00804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor-Smith JK, Flachsbart C, 2007. Relations between personality and coping: a meta-analysis. Journal of personality and social psychology 93(6), 1080. 10.1037/0022-3514.93.6.1080. [DOI] [PubMed] [Google Scholar]
- Costa PT Jr., McCrae RR, 1992. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Crimmins EM, Saito Y, Kim JK, Zhang YS, Sasson I, Hayward MD, 2018. Educational differences in the prevalence of dementia and life expectancy with dementia: Changes from 2000 to 2010. The Journals of Gerontology: Series B 73(suppl_1), S20–S28. 10.1093/geronb/gbx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, Jerant AF, Franks P, 2011. Personality and risk for Alzheimer's disease in adults 72 years of age and older: a 6-year follow-up. Psychology and aging 26(2), 351–362. 10.1037/a0021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Aschenbrenner AJ, Fagan AM, Benzinger TL, Morris JC, Balota DA, 2020. The Relation Between Personality and Biomarkers in Sensitivity and Conversion to Alzheimer-Type Dementia. Journal of the International Neuropsychological Society 26, 596–606. 10.1017/S1355617719001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan MR, Peng Z, An Y, Triolo MHK, Shafer AT, Davatzikos C, Erus G, Karikkineth A, Lewis A, Moghekar A, 2022. Herpes Viruses in the Baltimore Longitudinal Study of Aging: Associations With Brain Volumes, Cognitive Performance, and Plasma Biomarkers. Neurology 99(18), e2014–e2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumfart B, Neubauer AC, 2016. Conscientiousness is the most powerful noncognitive predictor of school achievement in adolescents. Journal of individual differences 37(1), 8. 10.1027/1614-0001/a000182. [DOI] [Google Scholar]
- Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, et al. , 2021. Reactive astrocyte nomenclature, definitions, and future directions. Nature neuroscience 24(3), 312–325. 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, 2008. The Baltimore Longitudinal Study of Aging (BLSA): A 50-year-long journey and plans for the future. The Journals of Gerontology: Series A 63, 1416–1419. 10.1093/gerona/63.12.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, Goldberg LR, Vogt TM, Dubanoski JP, 2006. Forty years on: Teachers' assessments of children's personality traits predict self-reported health behaviors and outcomes at midlife. Health Psychology 25(1), 57–64. 10.1037/0278-6133.25.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PL, Roberts BW, 2016. Personality and health: Reviewing recent research and setting a directive for the future. Handbook of the Psychology of Aging, 205–218. 10.1016/B978-0-12-411469-2.00011-X. [DOI] [Google Scholar]
- Hu H, Chen KL, Ou YN, Cao XP, Chen SD, Cui M, Dong Q, Tan L, Yu JT, 2019. Neurofilament light chain plasma concentration predicts neurodegeneration and clinical progression in nondemented elderly adults. Aging 11(17), 6904–6914. 10.18632/aging.102220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M, Mazumder M, Schwabe-Warf D, Stephan Y, Sutin AR, Terracciano A, 2019. Personality Changes With Dementia From the Informant Perspective: New Data and Meta-Analysis. Journal of the American Medical Directors Association 20(2), 131–137. 10.1016/j.jamda.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B, 2016. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87(5), 539–547. 10.1212/wnl.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Duberstein PR, Hallstrom T, Waern M, Ostling S, Skoog I, 2014. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology 83(17), 1538–1544. 10.1212/WNL.0000000000000907. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Harmell AL, Yaffe K, 2019. Conscientiousness is associated with lower risk of dementia among black and white older adults. Neuroepidemiology 52(1-2), 86–92. 10.1159/000492821. [DOI] [PubMed] [Google Scholar]
- Kekäläinen T, Luchetti M, Aschwanden D, Sutin AR, Terracciano A, 2022. Individual and country-level factors associated with self-reported and accelerometer-based physical activity in old age: a cross-national analysis of European countries. European Journal of Ageing 19(4), 1529–1542. 10.1007/s10433-022-00737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D, 2010. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological bulletin 136(5), 768–821. 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Lange R, Lippa S, Brickell T, Gill J, French L, 2022. Serum Tau, NFL, GFAP and UHCL-1 are Associated with the Chronic Deterioration of Neurobehavioral Symptoms following Traumatic Brain Injury. Journal of Neurotrauma 40, 482–492. 10.1089/neu.2022.0249. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, 2009. A new equation to estimate glomerular filtration rate. Annals of internal medicine 150(9), 604–612. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti M, Barkley JM, Stephan Y, Terracciano A, Sutin AR, 2014. Five-factor model personality traits and inflammatory markers: new data and a meta-analysis. Psychoneuroendocrinology 50, 181–193. 10.1016/j.psyneuen.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti M, Terracciano A, Stephan Y, Aschwanden D, Sutin AR, 2021. Personality Traits and Memory: A Multilevel Analysis Across 27 Countries From the Survey of Health, Ageing and Retirement in Europe. Psychological science 32(7), 1047–1057. 10.1177/0956797621993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugeri G, D'Agata V, Magrì B, Roggio F, Castorina A, Ravalli S, Di Rosa M, Musumeci G, 2021. Neuroprotective effects of physical activity via the adaptation of astrocytes. Cells 10(6), 1542. 10.3390/cells10061542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mõttus R, McNeill G, Jia X, Craig LC, Starr JM, Deary IJ, 2013. The associations between personality, diet and body mass index in older people. Health Psychology 32(4), 353. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L, 2012. Memory aging and brain maintenance. Trends in cognitive sciences 16(5), 292–305. 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Oppong RF, Terracciano A, Picard M, Qian Y, Butler TJ, Tanaka T, Moore AZ, Simonsick EM, Opsahl-Ong K, Coletta C, Sutin AR, Gorospe M, Resnick SM, Cucca F, Scholz SW, et al. , 2022. Personality traits are consistently associated with blood mitochondrial DNA copy number estimated from genome sequences in two genetic cohort studies. eLife 11 10.7554/eLife.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Stomrud E, Cullen N, Janelidze S, Manuilova E, Jethwa A, Bittner T, Eichenlaub U, Suridjan I, Kollmorgen G, Riepe M, von Arnim CAF, Tumani H, Hager K, Heidenreich F, et al. , 2022. An accurate fully automated panel of plasma biomarkers for Alzheimer's disease. Alzheimer's & Dementia 10.1002/alz.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Duggan MR, Dark HE, Daya GN, An Y, Davatzikos C, Erus G, Lewis A, Moghekar AR, Walker KA, 2022. Association of liver disease with brain volume loss, cognitive decline, and plasma neurodegenerative disease biomarkers. Neurobiology of aging 120, 34–42. 10.1016/j.neurobiolaging.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Gräber S, Kuder-Buletta E, LaFougere C, Laske C, Vöglein J, Levin J, Masters CL, Martins R, Schofield PR, et al. , 2019. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nature medicine 25(2), 277–283. 10.1038/s41591-018-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin J, Rolland Y, Aggarwal G, Nguyen AD, Morley JE, Li Y, Bateman RJ, Vellas B, Barreto P.d.S., 2021. Associations between physical activity, blood-based biomarkers of neurodegeneration, and cognition in healthy older adults: the MAPT study. The Journals of Gerontology: Series A 76(8), 1382–1390. 10.1093/gerona/glab094. [DOI] [PubMed] [Google Scholar]
- Seblova D, Fischer M, Fors S, Johnell K, Karlsson M, Nilsson T, Svensson AC, Lövdén M, Lager A, 2021. Does prolonged education causally affect dementia risk when adult socioeconomic status is not altered? A Swedish natural experiment in 1.3 million individuals. American journal of epidemiology 190(5), 817–826. 10.1093/aje/kwaa255. [DOI] [PubMed] [Google Scholar]
- Siegler IC, Welsh KA, Dawson DV, Fillenbaum GG, Earl NL, Kaplan EB, Clark CM, 1991. Ratings of personality change in patients being evaluated for memory disorders. Alzheimer disease and associated disorders 5, 240–250. 10.1097/00002093-199100540-00003. [DOI] [PubMed] [Google Scholar]
- Smit D, Koerts J, Bangma DF, Fuermaier ABM, Tucha L, Tucha O, 2021. Look who is complaining: Psychological factors predicting subjective cognitive complaints in a large community sample of older adults. Applied neuropsychology. Adult, 1–15. 10.1080/23279095.2021.2007387. [DOI] [PubMed] [Google Scholar]
- Steinacker P, Al Shweiki MR, Oeckl P, Graf H, Ludolph AC, Schönfeldt-Lecuona C, Otto M, 2021. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. Journal of Psychiatric Research 144, 54–58. 10.1016/j.jpsychires.2021.09.012. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Gamaldo AA, Stephan Y, Strickhouser JE, Terracciano A, 2020. Personality Traits and the Subjective and Objective Experience of Sleep. International journal of behavioral medicine 27(4), 481–485. 10.1007/s12529-019-09828-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Luchetti M, Terracciano A, 2021. Sense of purpose in life and healthier cognitive aging. Trends in cognitive sciences 25(11), 917–919. 10.1016/j.tics.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Damian RI, Luchetti M, Strickhouser JE, Terracciano A, 2019a. Five-factor model personality traits and verbal fluency in 10 cohorts. Psychology and aging 34(3), 362–373. 10.1037/pag0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Terracciano A, 2019b. Self-Reported Personality Traits and Informant-Rated Cognition: A 10-Year Prospective Study. Journal of Alzheimer's Disease 72(1), 181–190. 10.3233/jad-190555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanios V, Terracciano A, Luchetti M, Stephan Y, Sutin AR, 2022. Personality traits at age 16 and risk of metabolic syndrome at age 46. Journal of psychosomatic research 155, 110744. 10.1016/j.jpsychores.2022.110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, An Y, Sutin AR, Thambisetty M, Resnick SM, 2017. Personality Change in the Preclinical Phase of Alzheimer Disease. JAMA psychiatry 74(12), 1259–1265. doi: 10.1001/jamapsychiatry.2017.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Aschwanden D, Passamonti L, Toschi N, Stephan Y, Luchetti M, Lee JH, Sesker A, O'Súilleabháin PS, Sutin AR, 2021a. Is neuroticism differentially associated with risk of Alzheimer's disease, vascular dementia, and frontotemporal dementia? Journal of Psychiatric Research 138, 34–40. 10.1016/j.jpsychires.2021.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Aschwanden D, Stephan Y, Cerasa A, Passamonti L, Toschi N, Sutin AR, 2021b. Neuroticism and Risk of Parkinson's Disease: A Meta-Analysis. Movement disorders : official journal of the Movement Disorder Society 36(8), 1863–1870. 10.1002/mds.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Bilgel M, Aschwanden D, Luchetti M, Stephan Y, Moghekar AR, Wong DF, Ferrucci L, Sutin AR, Resnick SM, 2022. Personality associations with amyloid and tau: Results from the Baltimore Longitudinal Study of Aging and meta-analysis. Biological Psychiatry 91(4), 359–369. 10.1016/j.biopsych.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Costa PT Jr., McCrae RR, 2006. Personality plasticity after age 30. Personality and Social Psychology Bulletin 32, 999–1009. 10.1177/0146167206288599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, Meirelles O, Sutin AR, Zonderman AB, Uda M, Crisponi L, Schlessinger D, 2011. Neuroticism, depressive symptoms, and serum BDNF. Psychosomatic medicine 73(8), 638–642. 10.1097/PSY.0b013e3182306a4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Stephan Y, Luchetti M, Sutin AR, 2018. Cognitive impairment, dementia, and personality stability among older adults. Assessment 25(3), 336–347. 10.1177/1073191117691844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk IMW, Thijssen E, Koelewijn J, Mauroo K, Vanbrabant J, de Wilde A, Zwan MD, Verfaillie SCJ, Ossenkoppele R, Barkhof F, van Berckel BNM, Scheltens P, van der Flier WM, Stoops E, Vanderstichele HM, et al. , 2020. Combination of plasma amyloid beta((1-42/1-40)) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimer's research & therapy 12(1), 118. 10.1186/s13195-020-00682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EN, Ajdacic-Gross V, Strippoli MF, Gholam-Rezaee M, Glaus J, Vandeleur C, Castelao E, Vollenweider P, Preisig M, von Känel R, 2019. Associations of Personality Traits With Chronic Low-Grade Inflammation in a Swiss Community Sample. Frontiers in psychiatry 10, 819. 10.3389/fpsyt.2019.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA, 2007. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of general psychiatry 64(10), 1204–1212. 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- Yao J, Wu H, Ma Y, Xie W, Lian X, Chen X, 2022. The negative affectivity dimension of Type D personality associated with increased risk for acute ischemic stroke and white matter hyperintensity. Journal of psychosomatic research 160, 110973. 10.1016/j.jpsychores.2022.110973. [DOI] [PubMed] [Google Scholar]
- Zhu X, Luchetti M, Aschwanden D, Sesker AA, Stephan Y, Sutin AR, Terracciano A, 2023. Multidimensional Assessment of Subjective Well-being and Risk of Dementia: Findings from the UK Biobank Study. Journal of Happiness Studies 24, 629–650. 10.1007/s10902-022-00613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.