Abstract
The prognosis of multiple extramedullary plasmacytomas (MEP) and plasma cell leukemia (PCL) is extremely poor, with the median overall survival (OS) of only 8 months with standard chemotherapy. Innovative treatment approaches incorporating various strategies are required to improve outcome. From November 2019 to September 2021, a total of 12 newly diagnosed MEP or PCL patients were enrolled in our department. An intensive chemotherapy treatment as VRD-PDCE consisted of bortezomib, lenalidomide, dexamethasone plus cisplatin, pegylated liposomal doxorubicin, cyclophosphamide and etoposide was first proposed. Disease activity and toxicity were evaluated after each cycle. Of the patients receiving therapy achieved a rapid and sustained response, and the overall response rate (ORR) was up to 75%. Nine patients achieved partial response (PR) or better, the response was the best response and the median time to best response was 4 cycles. Median overall survival (OS) and progression-free survival (PFS) were 24 (5–30) months and 18 (2–23) months. The toxicities were acceptable and there was no treatment related mortality. Our intensive treatment showed encouraging results in terms of disease control and improving survival, VRD-PDCE may be a novel regimen which is feasible and generally well-tolerated in MEP or PCL patients.
Keywords: Multiple extramedullary plasmacytomas, Plasma cell leukemia, Multiple myeloma, Lenalidomide, Pegylated liposomal doxorubicin
Introduction
Multiple myeloma (MM) is a malignant proliferation of clonal plasma cells characterized by hypercalcemia, anemia, renal insufficiency, and osteolytic bone lesions, which accounts for 1% of all cancers and approximately 10% of all hematologic malignancies [1–3]. Plasma cells are usually restricted to the bone marrow, but also migrate into perivascular spaces and direct extend from bony lesions. Multiple extramedullary plasmacytomas (MEP) is a rare monoclonal plasmacytic proliferation, which occurs in extraskeletal sites in patients with MM either at diagnosis or during course of the disease [4]. Plasma cell leukemia (PCL) is MM patients with circulating plasma cells to account for 20% of peripheral blood leukocytes and/or an absolute circulating plasma cell count of 2.0 × 109/L [5].
In the past two decades, myeloma treatment has resulted in significant improvement in prognosis, with the median survival of the MM patients has greatly increased up to more than 10 years [6]. However, newer challenges are emerging, such as the treatment of refractory MEP and PCL. Markers of poor prognosis are frequently observed in these extramedullary forms of plasma cell cancers, and the survival is significantly inferior compared to MM patients [7]. Patients with PCL are exceedingly resistant, rapid progressive and fatal, the overall survival (OS) from diagnosis ranges from 7 to 14 months [8–10]. Recent reports have suggested that the incidence of these disorders is increased, and multi-agent combination chemotherapy regimen VTD-PACE (bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide and etoposide) is particularly useful [11, 12].
Currently, novel anti-plasma cell agents appear to be effective against these aggressive cancers. Lenalidomide has been reported to be efficacious in MEP or secondary PCL [13, 14]. Pegylated liposomal doxorubicin (PLD) prolongs plasma half-life, and allows for greater accumulation within tumor sites and less accumulation within the heart [15]. PLD combined with bortezomib is associated with a higher rate of ≥ very good partial response (VGPR) and ultimately improvement in time to progression compared with bortezomib alone in patients with relapsed refractory MM [16].
However, lenalidomide and PLD applied simultaneously are infrequently in multiple combination treatment of MEP or PCL. Herein, we first proposed VRD-PDCE (bortezomib, dexamethasone, lenalidomide, cisplatin, PLD, cyclophosphamide and etoposide) as a novel chemotherapy regimen in the setting of MEP or PCL patients, which achieved a rapid and sustained response. Although some additional toxicity was noted, VRD-PDCE was generally well tolerated in patients.
Materials and Methods
Patients Eligibility
This study was a retrospective analysis of patients treated with the regimen. From November 2019 to September 2021, 12 newly diagnosed patients with progressive or symptomatic MEP or PCL according to the updated diagnostic criteria of International Myeloma Working Group (IMWG) were enrolled in this study [17]. The median age of patients was 55 years (range 47–68 years). The inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0–4, platelets ≥ 100 × 109/L, hemoglobin ≥ 78 g/L, absolute neutrophils ≥ 1.0 × 109/L, as well as adequate kidney and liver function (creatinine clearance ≥ 30 mL/min and bilirubin concentration < 2 mg/dL). Additional exclusion criteria included clinically significant cardiac disease, left ventricular ejection fraction (LVEF) less than institutional normal limits, clinically relevant and active infection, and grade 2 or above peripheral neuropathy (PN). Chromosome karyotype analysis and fluorescence in situ hybridization (FISH) detection were performed in all patients for risk stratification and assessment prognosis. This study was conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonization for Good Clinical Practice. The protocol was approved by institutional review boards and independent ethics committees. The ethics committee number was KY2019-324. Patients provided written informed consent before onset of treatment.
Treatment Regimen
Although autologous transplantation was the standard therapy for PCL and MEP patients, which was also a way to achieve cure and long-term survival, because of economic factors and fear of recurrence after transplantation, these patients resolutely refused transplantation. Therefore, an intensive chemotherapy was the first choice for them. VRD-PDCE consisted of bortezomib (1.0 mg/m2 subcutaneously d1, 4, 8, 11), lenalidomide (25 mg orally d1-21), dexamethasone (40 mg orally d1-4) plus cisplatin (10 mg/m2 continuous intravenously d1-4), pegylated liposomal doxorubicin (30 mg/m2 intravenously d4), cyclophosphamide (400 mg/m2 continuous intravenously d1-4) and etoposide (40 mg/m2 continuous intravenously d1-4). The cycle was repeatedly every 28 days, but dosage and interval of drugs were modifiable according to hematological toxicity and non-hematological toxicity. If patients developed grade 3 or above hematological or non-hematological toxicity, such as neutropenia, anemia, thrombocytopenia, gastrointestinal symptoms, peripheral neuropathy and so on, treatment would be delayed until the toxicity relieved to grade 2 or below, then the treatment would be continued with a reduction of 25% in drugs dosage, and lenalidomide was further adjusted to 25 mg taken orally every other day [18]. If patients had severe infectious complications, treatment was postponed until full recovery [19]. Older patients usually had basic diseases and poor tolerance to chemotherapy, they were prone to hematological and non-hematological toxicity, such as infection, intestinal obstruction and other serious complications. Therefore, for elderly patients, the occurrence of adverse events should be closely monitored during the treatment, and if necessary, treatment should be suspended in time to prevent from worsening. The VRD-PDCE treatment was continued until the occurrence of disease progression, unacceptable toxicities, patients refused or death in order to deepen the response for patients who attained response. Bortezomib, lenalidomide, dexamethasone (VRD) biannually and lenalidomide, dexamethasone (RD) monthly was as the maintenance regimens for patients who subsequently refused the intensive therapy.
Assessment of Efficacy and Safety
The response were assessed before the next cycle of treatment, and the last assessment was carried out after the last treatment. Primary endpoints were response rate, progression-free survival (PFS) and OS. Response was categorized by the International Myeloma Working Group response criteria [20, 21]. The overall response rate (ORR) was defined as a rate of response partial response (PR) and better. Assessments included measurements of plasma cell count in bone marrow and peripheral blood, 24-h urine monoclonal protein, serum free light chain assay, immunofixation, β2-microglobulin, leucocytes, hemoglobin, platelets, albumin, creatinine, lactate dehydrogenase (LDH) and inspection of target organ damage. Because of economic factors, patients with MEP chose magnetic resonance imaging (MRI) or computerized tomography (CT) for disease diagnosis and response evaluation instead of positron emission tomography-computed tomography (PET-CT). Toxicity of the regimen were evaluated after each cycle, the last evaluation was carried out 2 months after the last intervention, and the adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical Analysis
Baseline characteristics, response rate and toxicity were analyzed using descriptive statistics (median, range and proportions). PFS was defined as the interval from the start day of VRD-PDCE regimen to the date of progression or death from any causes and OS was calculated from the start day of VRD-PDCE regimen to the date of death from any causes or the final follow-up. Treatment-related mortality was defined as death attributed to treatment given within 2 months before death. The Kaplan–Meier method was used to estimate PFS and OS.
Results
Clinical Characteristics of Patients
According to the study recruitment chart (Fig. 1), a total of 12 patients met the enrollment criteria and voluntarily participated in this study, basic characteristics of patients were detailed in Table 1. Median age at initiation was 55 years, five patients with multiple extramedullary infiltration, others presented with PCL. Majority of patients were in Durie-Salmon (D-S) stage 3 and International Staging System (ISS)/revised ISS (R-ISS) stage 3. For the ECOG performance status, except for one patient, others were no more than 2. Three patients were complex karyotypes with varied structural and numerical abnormalities, in addition, accompanied with p53 deletion. The translocation of (4;14), (14;16), (14;20) and (17p) deletion were detected abnormalities by FISH analysis. All of patients were at high risk in cytogenetics. Laboratory examinations, organ functions and bone marrow features were detailed in Table 2. Cardiac function was normal in all patients.
Fig. 1.
Recruitment chart of patients
Table 1.
Basic characteristics of patients with MEP or PCL
| Patient number | Age, years | Gender | ECOG, score | IFE† | D-S | ISS/R-ISS | Disease status | Cytogenetics |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 | Female | 2 | λ | III | III/III | PCL |
P53 deletion Complex karyotype |
| 2 | 49 | Male | 4 | IgA‡-λ | II | III/III | Spinal infiltration | t(4;14) |
| 3 | 50 | Female | 1 | IgG-λ | II | II/II | PCL | t(14;16) |
| 4 | 51 | Male | 1 | IgG-λ | II | II/II | PCL | t(14;20) |
| 5 | 52 | Female | 0 | IgG-λ | II | II/II | Chest wall infiltration | del(17p) |
| 6 | 54 | Male | 1 | IgA-λ | III | III/III | Chest wall infiltration | t(4;14) |
| 7 | 56 | Male | 1 | IgG-κ | III | III/III | PCL | t(14;20) |
| 8 | 58 | Female | 0 | IgG-λ | III | III/III | Liver infiltration | P53 deletion Complex karyotype |
| 9 | 60 | Male | 1 | IgA-λ | III | III/III | PCL | t(14;16) |
| 10 | 63 | Male | 2 | IgG-λ | III | III/III | Pleural infiltration | del(17p) |
| 11 | 65 | Male | 1 | IgG-κ | III | III/III | PCL | t(4;14) |
| 12 | 68 | Female | 2 | IgG-λ | III | III/III | PCL |
P53 deletion Complex karyotype |
IFE†: immunofixation electrophoresis; Ig‡: immunoglobulin
Table 2.
Laboratory examinations, organ functions and bone marrow features of patients with MEP or PCL
| Patient Number | WBC (109/L) | Hb (g/L) | Cr (μmol/L) | Ca (mmol/L) | Alb (g/L) | LDH (U/L) | β2-M (mg/L) | LVEF (%) | Myeloma cell(%) | Plasma cells or infiltration(%) | Plasma cell count(109/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.2 | 84 | 180 | 2.46 | 28 | 320 | 9.7 | 63 | 58 | 25 | 2.3 |
| 2 | 8.5 | 106 | 117 | 2.27 | 32 | 310 | 5.6 | 60 | 24 | 75 | – |
| 3 | 16.5 | 90 | 109 | 2.57 | 28 | 195 | 4.7 | 62 | 38 | 19 | 3.1 |
| 4 | 12.1 | 88 | 116 | 2.32 | 30 | 202 | 5.0 | 63 | 45 | 22 | 2.7 |
| 5 | 4.9 | 108 | 79 | 2.28 | 36 | 135 | 3.6 | 59 | 25 | 20 | – |
| 6 | 6.3 | 105 | 90 | 2.80 | 33 | 342 | 5.7 | 55 | 28 | 25 | – |
| 7 | 10.1 | 82 | 176 | 2.18 | 29 | 256 | 6.0 | 58 | 56 | 32 | 3.2 |
| 8 | 5.7 | 89 | 112 | 2.90 | 30 | 249 | 8.8 | 61 | 20 | 50 | – |
| 9 | 7.4 | 84 | 196 | 2.26 | 31 | 311 | 7.2 | 57 | 70 | 42 | 3.1 |
| 10 | 4.1 | 78 | 216 | 2.30 | 27 | 323 | 9.7 | 55 | 88 | 50 | – |
| 11 | 6.8 | 83 | 129 | 2.41 | 29 | 254 | 7.4 | 56 | 62 | 35 | 2.4 |
| 12 | 9.6 | 78 | 151 | 2.38 | 31 | 267 | 8.3 | 59 | 58 | 28 | 2.7 |
WBC white blood cell, Hb hemoglobin, Cr creatinine, Ca calcium, ALB albumin, LDH lactate dehydrogenase, β2-M β2-microglobulin; Myeloma cell: in bone marrow; plasma cells: in the classification white cell in peripheral blood; infiltration: infiltrative organs
Response of Therapy
Treatment response was summarized in Table 3. The median number received by patients was four cycles (range 2–6). The ORR was the best response of patients to the treatment of VRD-PDCE regimen, and the median number of cycles to achieve the best response was also 4 cycles. In this study, ORR was as high as 75%, while the ORR of our previous patients who received VTD-PACE was only 50%. Six patients achieved PR after two cycles of treatment, with an ORR of 50%. Nine patients achieved PR or better after four cycles of treatment, with an ORR of 75%. Notably, patient 2 were bed-ridden and paraplegic at admission due to spinal infiltration, but after two cycles of treatment, muscle strength restored to level 2 and lower limbs could move in parallelly on the bed. After four cycles of treatment, muscle strength restored to level 3 and lower limbs could overcome the gravity and lift off the bed surface. For patient 10, there was a large amount of hydrothorax on both sides of thorax and accompanied by obvious dyspnea at admission. The pathological report indicated that the puncture of right pleura was a plasma cell-derived tumor, invading the striated muscle, please combine with other relevant clinical examinations to comprehensively analyze whether it was a plasma cell myeloma involving the pleura or an extraosseous solitary plasma cell tumor. Immunohistochemistry: CD138 (+), CD38 (+), CD20 (−), CD3 (−), mum-1 (+), bcl-2 (+), kappa and lamda are light chain restricted, CD10 (−), CD30 (−), bcl-6 (−), c-myc (+), Ki67 (about 40%). In situ hybridization: EBER (−). The pathological report of pleural effusion showed that small round tumor cells were found in the examination tissue, and more nuclear heterotypic cells were found in the sediment embedded section. Combined with clinical examinations and pathological reports, we analyzed that pleural effusion was due to pleural infiltration by myeloma. However, hydrothorax reduced more than half after two cycles of treatment and completely disappeared after four cycles of treatment, as well as the symptom of dyspnea. Only two patients had progressive disease (PD) despite the initial therapeutic effect was significant. Since the intensive regimen showed a relatively good response, PR seemed to be achieved for most patients after four cycles of treatment, so we maintained this regimen for patients.
Table 3.
Response and overall cycles of patients with MEP or PCL
| Patient number | Overall Cycles | Responses after 2 cycles | Responses after 4 cycles | Best response |
|---|---|---|---|---|
| 1 | 2 | PD | – | PD |
| 2 | 6 | PR | PR | VGPR |
| 3 | 6 | SD | PR | PR |
| 4 | 4 | SD | PR | PR |
| 5 | 6 | PR | VGPR | CR |
| 6 | 6 | PR | PR | VGPR |
| 7 | 4 | SD | PR | PR |
| 8 | 2 | PD | – | PD |
| 9 | 4 | PR | PR | PR |
| 10 | 6 | PR | VGPR | VGPR |
| 11 | 4 | PR | PR | PR |
| 12 | 2 | SD | – | SD |
Adverse Events
Major toxicity profiles were detailed in Table 4. Although neuropathy was observed in all patients, most of them remained stable in grade 1 or 2 after the intervention of neurotrophic drugs. In terms of gastrointestinal toxicity, three patients experienced diarrhea, two patients experienced grade 2 constipation and ventosity which resulted in incomplete intestinal obstruction, and others experienced nausea. After the intervention of antidiarrheal, laxative, enema and other drugs, gastrointestinal symptoms were mild. Seven patients (58%) delayed treatment because of hematological toxicity and severe infection. Five patients (42%) were able to receive treatment regularly at pre-designated interval of 28 days. For patients at two cycles, one patient with the median delays of 2 weeks due to severe pneumonia and sepsis. For patients at four cycles, one patient with the median delays of seven days due to grade 3 thrombocytopenia. The median delays of 10 days in five patients at six cycles due to grade 3 or 4 thrombocytopenia and neutropenia, after suspending therapy and supporting symptomatic treatment, hematological toxicity usually relieved to grade 2 in a short time. Subcutaneous injection of granulocyte colony-stimulating factor (G-CSF) and recombinant human thrombogenin could reduce the degree and incidence of infection and bleeding, shorten the duration of myelosuppression, reduce the proportion and volume of platelet transfusion, and contribute to recover the count of neutrophil and platelet. Transfusion of red blood cells was also necessary to improve anemia. Thrombogenin was applied at platelets < 75 × 109/L, with a dose of 300 µ/kg/d, subcutaneously for 14 days, when platelets ≥ 100 × 109/L or higher than before medication 50 × 109/L, it should be stopped in time [22]. No cardiovascular toxicity was developed in follow-up. The regimen was generally well tolerable, and none of patients required termination of treatment due to adverse events.
Table 4.
Major toxicities associated with treatment
| Patient number | PN (grade) | Gastrointestinal symptom (grade) | Neutropenia (grade) | Anemia (grade) | Thrombocytopenia(grade) | Infection (grade) | Fatigue (grade) | Dizziness (grade) | Lethargy (grade) | Cardiovascular (grade) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 (nausea) | 1 | 1 | 1 | – | 1 | 1 | 1 | – |
| 2 | 2 | 2 (nausea) | 3 | 2 | 2 | – | 1 | 1 | 1 | – |
| 3 | 2 | 2 (diarrhea) | 4 | 2 | 2 | – | 2 | 2 | 1 | – |
| 4 | 2 | 1 (nausea) | 2 | 1 | 2 | – | 1 | 1 | 1 | – |
| 5 | 2 | 2 (diarrhea) | 3 | 2 | 2 | – | 2 | 1 | 1 | – |
| 6 | 3 | 2 (constipation, ventosity) | 2 | 2 | 3 | – | 2 | 2 | 1 | – |
| 7 | 2 | 1 (nausea) | 2 | 2 | 2 | – | 1 | 1 | 1 | – |
| 8 | 1 | 1 (nausea) | 1 | 1 | 1 | – | 1 | 1 | 1 | – |
| 9 | 2 | 2 (diarrhea) | 2 | 2 | 3 | – | 2 | 1 | 1 | – |
| 10 | 3 | 2 (constipation, ventosity) | 3 | 2 | 4 | – | 2 | 1 | 1 | – |
| 11 | 2 | 2 (nausea) | 2 | 1 | 2 | – | 1 | 1 | 1 | – |
| 12 | 1 | 1 (nausea) | 1 | 1 | 1 | 5 (pneumonia, sepsis) | 1 | 1 | 1 | – |
Survival
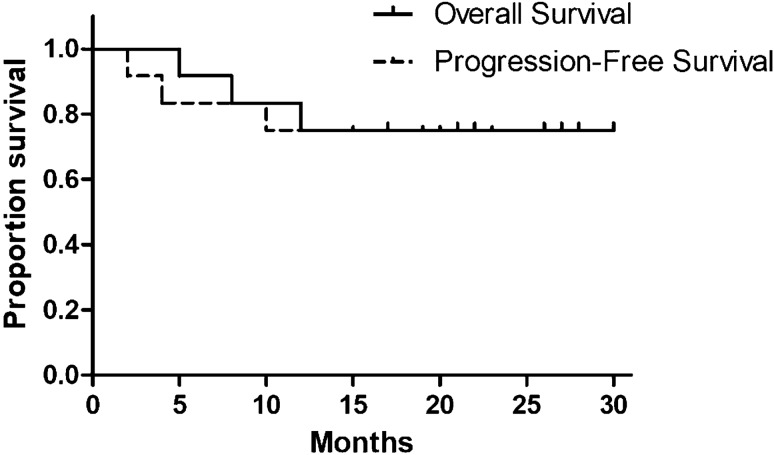
The median follow-up for the whole group was 30 months, while that for living patients was ongoing. As summarized in Table 5 and Fig. 2, median OS was 24 (5–30) months and median PFS was 18 (2–23) months. However, our previous patients who received VTD-PACE regimen, the median OS and PFS was only 8 and 4 months, respectively. At the time of analysis all cases, two patients died from disease progression and one patient died from pulmonary infection, whereas cytogenetics analysis of the three patients were all complex karyotypes with P53 deletions. Other patients are still alive in good general condition and high quality of life. The estimated 1- and 2-year survival was 83 and 50%. Patients with PR or better showed a significant improvement in OS and PFS. Median OS and PFS for patients with PR or better were 27 vs. 20 months and 8 vs. 4 months for those with SD or PD.
Table 5.
OS, PFS and prognosis of patients received VRD-PDCE regimen
| Patient number | OS (month) | PFS (month) | Prognosis |
|---|---|---|---|
| 1 | 8 | 4 | Death from disease progression |
| 2 | 28 | 21 | Still alive |
| 3 | 27 | 20 | Still alive |
| 4 | 26 | 19 | Still alive |
| 5 | 30 | 23 | Still alive |
| 6 | 28 | 22 | Still alive |
| 7 | 22 | 17 | Still alive |
| 8 | 5 | 2 | Death from disease progression |
| 9 | 21 | 17 | Still alive |
| 10 | 30 | 23 | Still alive |
| 11 | 17 | 15 | Still alive |
| 12 | 12 | 10 | Death from pulmonary infection |
Fig. 2.
OS and PFS curves of patients received VRD-PDCE regimen
Discussion
MM is one of the most common hematologic malignancies, with an incidence of five cases per 100 000/year. MEP comprises 3–5% of plasma cell neoplasms and occurs in 7–17% of MM diagnosis, PCL occurs in 2–4% of MM diagnoses [23–25]. Although the introduction of new drugs, MEP and PCL are still a complication associated with poor prognosis, the median survival is only 8 months [10, 26, 27]. MEP and PCL represent a very aggressive and advanced stage disease, which cells can escape from bone marrow, and become resistant to more intensive chemotherapy [14]. It is confirmed that the accumulation of abnormalities such as TP53 trigger the extramedullary features of secondary PCL [28]. These secondary events may lead to dysregulation of some other oncogenes, such as RAS and MYC, which contribute to plasma cells escape from bone marrow [29]. However, there is still no consensus in treatment options for MEP and PCL, and efficacy of different regimens need to be further verified [30, 31].
In the last several decades, many combination regimens were developed to improve survival of MEP and PCL [32–34]. Previous studies have indicated that conventional chemotherapeutics VTD-PACE regimen can improve outcomes, but patients have not durable responses and further salvage treatments are required [35]. Lenalidomide was efficacious in the context of secondary PCL. In a recent report, two cases of secondary PCL treated with lenalidomide showed a significant response, in which one case even maintained progression-free status after lenalidomide maintenance at 12 months from diagnosis [36]. Recent studies have demonstrated that PLD could reduce cardiotoxicity, enhance clinical benefit and prolong PFS via improving the depth of PR or CR (complete response). The risk of developing progression reduced 45%, prolonged time to progress by three months, and achieved an early benefit in OS by PLD [16, 37–39].
Here we first presented a modified regimen VRD-PDCE in which thalidomide and doxorubicin were replaced by lenalidomide and PLD. The clinical trials were conducted in 12 patients with MEP or PCL in our department, all of them with high risk and poor prognosis (Table 1). The intensive VRD-PDCE regimen showed a relatively good response, nine patients achieved a best response of PR or better. After four cycles of therapy, the tumor load of most patients was significantly reduced than that before treatment, and the best response was achieved (Table 3). Our results suggested that the replacement of lenalidomide and PLD may result in a rapid reduction of disease burden, and improve the depth and duration of response. Notably, additional toxicities of the therapy did not compromise overall safety. The non-hematologic toxicities were generally well tolerated by symptomatic intervention therapy. The main hematologic toxicities were usually alleviated by dose reduction, prolonged interval, G-CSF administration, recombinant human thrombogenin and blood products transfusion. As is well-known, doxorubicin was toxic for the cardiovascular system, even resulted in heart failure, but no cardiac dysfunction was observed in our study (Table 4). At the end of follow-up period, nine patients are still alive and generally in good condition, three patients died from disease progression and infectious complication. Median OS and PFS for the whole group were 24 (5–30) months and 18 (2–23) months. Patients with a good quality of response showed a longer OS and PFS, with the median OS and PFS of 27 months vs. 20 months for the patients with PR or better (Table 5, Fig. 2).
Therefore, our results suggested that the novel combination therapy is feasible, generally well-tolerated and shows promising response in MEP an PCL patients. Despite some limitations such as small sample size and short follow up, significant results were also observed in our study. New studies will be worthy to conduct in the future in larger patient series and a longer follow up.
Conclusions
In conclusion, although MEP and PCL are progressive disease, the intensive chemotherapy regimen VRD-PDCE is very efficacious for these patients. Moreover, the toxicity of VRD-PDCE is manageable and tolerable. Therefore, the novel chemotherapy regimen is worthy clinical application in patients with MEP and PCL. However, our study is a single center, retrospective and non-randomized study, the conclusions will be further confirmed in the next multi-center and large-scale clinical studies.
Acknowledgements
This work was supported by the Second Affiliated Hospital of Harbin Medical University.
Funding
This work was supported by the [Postdoctoral Science Foundation of China] under Grant [2015M580270]; [Postdoctoral Science Foundation of Heilongjiang Province] under Grant [LRB156483]; and [Young and middle-aged Science Foundation of Harbin Medical University] under Grant [KYCX2018-15].
Declaration
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dimopoulos M, Spencer A, Attal M, Prince M, Harousseau J, Dmoszynska A, Miguel JS, Hellmann A, Facon T, Facon R, Facon A, Masliak Z, Olesnyckyj M, Yu ZN, Patin J, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray MT, Thun MJ. Cancer statistics. Ca-Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Merlini MV, Kumar S, Hillengass J, Hillengass E, Richardson P. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 4.Wu TQ, Chen HF, Hou J, Li ZY, Tang JQ, Fu WJ, Yuan ZG, Shen HS. Extramedullary plasmacytoma in the presence of multiple myeloma: clinical correlates and prognostic relevance. Onco Targets Ther. 2012;5:329–334. doi: 10.2147/OTT.S35348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiménez-Zepeda VH, Domínguez VJ. Plasma cell leukemia: a rare condition. Ann Hematol. 2006;85:263–267. doi: 10.1007/s00277-005-0054-4. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SK, Rajkumar SV, Dispenzieri A. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sher T, Miller KC, Deeb G, Lee K, Chanan-Khan A. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol. 2010;150:418–427. doi: 10.1111/j.1365-2141.2010.08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsingh G, Mehan P, Luo JQ, Vij R, Morgensztern D, Santana-Davila R, Price-Troska T, Van Wier SA, Chng WJ, Ketterling RP, Gertz MA, Henderson K. Primary plasma cell leukemia: a surveillance, epidemiology, and end results database analysis between 1973 and 2004. Cancer. 2009;115:5734–5739. doi: 10.1002/cncr.24700. [DOI] [PubMed] [Google Scholar]
- 9.Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA, Chng WJ, Ketterling RP, Gertz MA, Henderson K. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22:1044–1052. doi: 10.1038/leu.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcı A-Sanz R, Orfa OA, Gonza Lez M, Tabernero MD, San Miguel JF (1999) Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood 93:1032–1037. [PubMed]
- 11.Barlogie B, Anaissie E, Rhee FV, Haessler J, Hollmig K, Pineda-Roman M, Cottler-Fox M, Mohiuddin A, Alsayed Y, Tricot G. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 12.Rhee FV, Szymonifka J, Anaissie E, Nair B, Waheed S, Alsayed Y, Petty N, John D.Jr S, Hoering A, Crowley J, Barlogie B (2010)Total therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of vtd maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapy. Blood 116:1220–1227. [DOI] [PMC free article] [PubMed]
- 13.Davies F, Baz R. Lenalidomide mode of action: linking bench and clinical findings. Blood Rev. 2010;24:S13–S19. doi: 10.1016/S0268-960X(10)70004-7. [DOI] [PubMed] [Google Scholar]
- 14.Musto P, Pagano L, Petrucci MT, Morabito F, Caravita T, Raimondo FD, Baldini L, Tosi P, Bringhen S, Offidani M. Primary plasma cell leukemia in the era of new drugs: has something changed? Crit Rev Oncol Hematol. 2012;82:141–149. doi: 10.1016/j.critrevonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form. Drug Saf. 2001;24:903–920. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 16.Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, Miguel JS, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan ZL, Rackoff W, Harousseau J. Randomized phase iii study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers Z, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Anderson BGM, Miguel JSF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma[J] Lancet Oncol. 2014;15(12):e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 18.Safaee R, Ahmadzadeh A, Sharifian R, Emami A, Yekaninejad MS, Jalili MH, Valizadeh A. Combination of cyclophosphamide, etoposide, carboplatin and dexamethasone as a salvage regimen for refractory multiple myeloma patients: a comparison with a historical control group. Hematol Rep. 2012;4:e14. doi: 10.4081/hr.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SS, Lee JJ. Efficacy and safety of melphalan, cyclophosphamide and dexamethasone (mcd) as a salvage treatment for patients with relapsed/refractory multiple myeloma. Chonnam Med J. 2019;55:25–30. doi: 10.4068/cmj.2019.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos M, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Avet-Loiseau H. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez DLC, Kyle RA, Durie BGM, Ludwig H, Usmani S, Vesole DH, Hajek R, Miguel JFS, Sezer O, Sonneveld P. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the international myeloma working group. Leukemia. 2013;27:780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumor clinical chemotherapy professional committee of China Anti Cancer Association, tumor support treatment professional committee of China Anti Cancer Association (2020) Expert diagnosis and treatment consensus of chemotherapy-related thrombocytopenia in China (2019). Chin J Medical Front 2(1):51–58.
- 23.Oriol A. Multiple myeloma with extramedullary disease. Adv Ther. 2011;28:1–6. doi: 10.1007/s12325-011-0079-0. [DOI] [PubMed] [Google Scholar]
- 24.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–330. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez-Zepeda VH, Dominguez-Martinez VJ. Plasma cell leukemia: a highly aggressive monoclonal gammopathy with a very poor prognosis. Int J Hematol. 2009;89:259–268. doi: 10.1007/s12185-009-0288-3. [DOI] [PubMed] [Google Scholar]
- 26.Sun WJ, Zhang JJ, Na A, Shen M, Li X. Clinical analysis of 40 multiple myeloma patients with extramedullary plasmacytoma of the head. J Int Med Res. 2016;44:1462–1473. doi: 10.1177/0300060516664820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha CH, Park CJ, Huh JR, Chi HS, Suh CW, Kang YK. Significantly better prognosis for patients with primary plasma cell leukemia than for patients with secondary plasma cell leukemia. Acta Haematol. 2007;118:178–182. doi: 10.1159/000109470. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Zepeda VH, Neme-Yunes Y, Braggio E. Chromosome abnormalities defined by conventional cytogenetics in plasma cell leukemia: what have we learned about its biology? Eur J Haematol. 2011;87:20–27. doi: 10.1111/j.1600-0609.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 29.Musto P, Pietrantuono G, Guariglia R, Villani O, Martorelli MC, Auria F D’, Zonno A,Lerose R. Salvage therapy with lenalidomide and dexamethasone in relapsed primary plasma cell leukemia. Leuk. Res. 2008;32:1637–1638. [DOI] [PubMed]
- 30.Leake P, Coard KC, Plummer JM. Extramedullary plasmacytoma of the pancreas as an uncommon cause of obstructive jaundice: a case report. J Med Case Rep. 2009;6:8785–8789. doi: 10.4076/1752-1947-3-8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atiq M, Ali SA, Dang S, Krishna SG, Anaisse E, Olden KW, Aduli F. Pancreatic plasmacytoma presenting as variceal hemorrhage: life threatening complication of a rare entity. JOP. 2009;10:187–188. [PubMed] [Google Scholar]
- 32.Engelhardt M, Terpos E, Kleber M, Gay F, Palumbo A. European myeloma network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99:232–242. doi: 10.3324/haematol.2013.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, Voorhees P, Leleu X, Johnsen HE, Streetly M, Ludwig H, Mellqvist U-H, Chng W-J, Pilarski L, Einsele H, Hou J, Turesson I, Zamagni E, Chim J, Mazumder A, Westin J, Lu J, Reiman T, Kristinsson S, Joshua D, Roussel M, O'Gorman P, Terpos E, Dimopoulos M, Moreau P, Anderson K, Palumbo A, Kumar S, Rajkumar V, Durie B, Richardson PG. Management of relapsed multiple myeloma: recommendations of the international myeloma working group. Leukemia. 2016;30:1005–1017. doi: 10.1038/leu.2015.356. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification and management. Am J Hematol. 2014;89:999–1009. doi: 10.1002/ajh.23810. [DOI] [PubMed] [Google Scholar]
- 35.Griffin PT, Ho VQ, Fulp W, Nishihori T, Baz RC. A comparison of salvage infusional chemotherapy regimens for recurrent/refractory multiple myeloma. Cancer. 2015;121:3622–3630. doi: 10.1002/cncr.29533. [DOI] [PubMed] [Google Scholar]
- 36.Gozzetti A, Musto P, Defina M, Auria FD’, Papini G, Steduto T, D’Arena G, Bocchia M. Efficacy of bortezomib, lenalidomide and dexamethasone (vrd) in secondary plasma cell leukaemia. Br. J. Haematol. 2012;157:497–498. [DOI] [PubMed]
- 37.Skubitz KM, Blaes AH, Konety SH, Francis GS. Cardiac safety profile of patients receiving high cumulative doses of pegylated-liposomal doxorubicin: use of left ventricular ejection fraction is of unproven value. Cancer Chemother Pharmacol. 2017;80:787–798. doi: 10.1007/s00280-017-3420-8. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau J-L, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 39.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]