Abstract
The COVID-19 pandemic has strongly impacted healthcare settings. We assess changes in blood culture practices and results during the COVID-19 era. All blood culture vials processed between January 1, 2017, and December 31, 2020, by 3 clinical laboratories were included. A baseline period from January 1, 2017 to December 31, 2019, was compared to the year 2020. COVID-19 “waves” were defined as follows: “wave 1” from March 16 to May 10, 2020, and “wave 2” from October 29 to December 14, 2020. A mean of 143.5 and 158.6 vials per day were processed in 2019 and 2020 respectively. Up to 300 and 220 vials per day were processed during waves 1 and 2. Among positive vials, a higher rate of contaminant was noticed during wave 1 (55.9% vs 45.0%; P < 0.0001) and interwave (46.0% vs 38.6%; P < 0.0001) in comparison to previous years. The prevalence of contaminants returned to the baseline level during wave 2. Streptococcus pneumonia prevalence fell in 2020 in comparison to the baseline (0.4% vs 1.4%; P < 0.0001). The COVID-19 pandemic was associated with an increase in the number of blood culture vials processed, the rate of contaminants, and a fall in the number of pneumococcal bloodstream infections.
Keywords: SARS-CoV-2, contaminant, vials, Streptococcus pneumoniae, wave
1. Introduction
Outbreaks of respiratory viruses, including influenza and SARS-CoV-2, are associated with high pressure on health systems i.e., increase in the number of emergency consultations, hospital admission in medicine and intensive care units [1,2]. As these patients can present symptoms compatible with bloodstream infections (BSI), blood cultures (BC) are usually sampled. Consequently, an increased number of blood culture vials are processed by clinical laboratories during winter and automated blood culture instruments could be overwhelmed [3,4]. Considering COVID-19 pandemic, an increase in blood culture contaminants was reported during the first wave of the pandemic in comparison to the pre-epidemic period [5,6].
Furthermore, as a consequence of COVID-19 control policies and bacterial superinfections of viral infection, the distribution of bacterial species might change over the year regarding respiratory virus seasons [7]. However, the SARS-CoV-2 pandemic had strongly impacted the circulation of other respiratory viruses with an almost disappearance of traditional influenza viruses during flu season [8].
In the present study, we assess changes in blood culture practices and results during the COVID-19 era.
2. Methods
2.1. Study design
Two French hospital laboratories participated in this retrospective study. All blood culture vials sampled between January 1, 2017, and December 31, 2020 were included. A baseline period from January 1, 2017 to December 31, 2019, was compared to the year 2020. COVID-19 “waves” were defined according to French national epidemiological data, i.e. with “wave 1” from March 16 to May 10, 2020, and “wave 2” from October 29 to December 14, 2020 [9]. The national daily number of hospital admission for COVID-19 were issued from national surveys [10].
All local data were extracted from laboratory software Glims v8 (MIPS, Gent, Belgium). Each center provided the overall number of blood culture vials processed per day, and the number of vials positive for each micro-organisms. The 7-day moving average of the number of vials processed was then calculated for baseline and epidemic periods. The distribution of micro-organisms recovered from blood culture was calculated for 4 periods of 2020 and their respective baselines: (1) prepandemic from January 1st, to March 15th; (2) COVID wave 1, from March 16th to May 10th; (3) interwave, from May 11st to October 28th; (4) COVID wave 2, from October 29th to December 14th. A single strain of micro-organisms was included per patient and period.
Statistical analyses were performed using the R-Software (R-foundation, Vienna, Austria) (R [11]). A P-value lower than 0.05 using the chi-square test was considered significant.
2.2. Laboratory analysis
Blood culture vials were incubated in Bactec FX (Becton Dickinson, Franklin Lakes, NJ) or BACT/ALERT Virtuo (BioMérieux, Marcy l’étoile, France) systems. All blood culture vials were incubated for 5 days before being considered negatives [12]. Micro-organisms were identified using MALDI-TOF mass spectrometry with the MicroFlex LT (Bruker Daltonic, Bremen, Germany) as recommended by the manufacturer. According to French guidelines, the presence of one of the following microorganisms in a single BC bottle or BC set was a priori considered as contaminant: coagulase-negative staphylococci (CoNS), with the exception of Staphylococcus lugdunensis, Cutibacterium spp., Bacillus spp. other than Bacillus anthracis, Corynebacterium spp. Other than Corynebacterium diphteriae, Aerococcus-like organisms, Micrococcus spp., viridans group streptococci other than Streptococcus pneumoniae, and Neisseria spp. other than Neisseria gonorrhoeae or Neisseria meningitidis [12].
3. Results
3.1. Number of vials processed
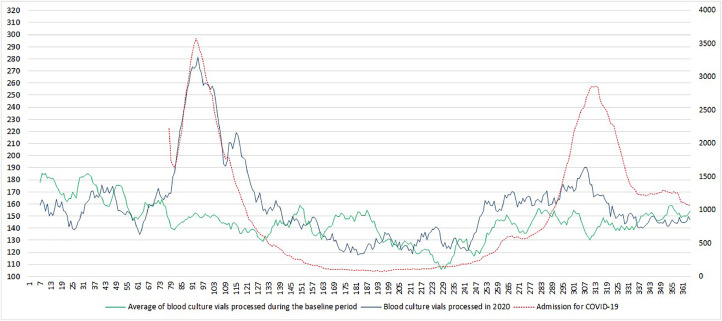
Overall, 217,788 blood culture vials were processed by the clinical laboratories during the study period. The mean number of vials processed per day was 149.6, 144.9, 143.5, and 158.6 in 2017, 2018, 2019, and 2020 respectively. The number of vials processed during the baseline period was the highest over the 70 first days, reaching more than 200 vials sampled on day 4 (Fig. 1 ). It then gradually decreases with a dip below 100 vials processed per day around days 225 to 230 (August). In contrast, the number of vials processed in 2020, was marked by 2 peaks. It dramatically increased around day 70 reaching a peak of up to 300 vials on days 94 and 97. The number of vials processed then returned to the baseline level around day 130. A slight increase occurs then on day 240, peaking at 220 vials processed on day 303, before it returned to the baseline level on day 320. Notably, the second peak of 2020 was in a similar range to the single peak of the baseline (days 1–70). The number of vials processed in 2020 followed a similar trend to the number of hospital admission during wave 1, but not wave 2.
Fig. 1.
Seven days moving average of blood culture vials processed and hospital admission for COVID-19.
3.2. Distribution of bacterial species
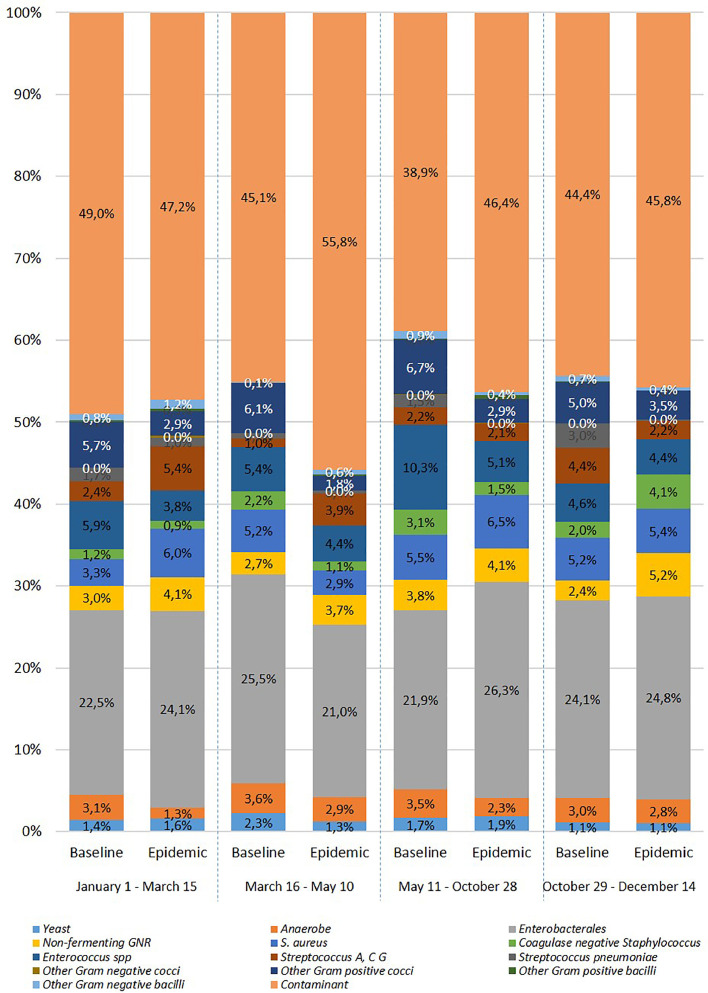
Overall, 12,042 nonduplicate positive vials were included: 3,193 in 2020 and 8,849 during the baseline period. The distribution of the micro-organisms recovered from blood culture was significantly different in 2020 in comparison to the baseline period (P < 0.0001) (Fig. 2 ). The overall rate of contaminant was significantly higher during COVID-19 wave1 (5.5% vs 2.6%; P < 0.001), interwave (2.6% vs 1.6%; P < 0.001), and COVID-19 wave2 (2.9% vs 2.0%; P < 0.001) while it was similar during the prepandemic period (2.5% vs 2.3%; P = 0.25). Among positive-vials, the rate of micro-organisms considered as contaminant was similar during the prepandemic (47.2% vs 49.1%; P = 0.42) and COVID wave 2 (45.7% vs 44.4%; P = 0.66) periods in comparison to the baseline. Conversely, a higher rate of contaminant was noticed during COVID-19 wave 1 (55.9% vs 45.0%; P < 0.0001) and interwave (46.0% vs 38.6%; P < 0.0001).
Fig. 2.
Distribution of micro-organisms recovered from positive blood culture.
The rate of Streptococcus pneumoniae was significantly lower in 2020 in comparison to the 3 previous years (0.4% vs 1.4%; P < 0.0001). The number of patients with S. pneumoniae bacteremia was 42, 35, and 44 in 2017, 2018, and 2019 respectively, and fell to 11 in 2020. However, while the rate of S. pneumoniae positive vials was not significantly different during the prepandemic (1.0% vs 1.7%, P = 0.22), and the COVID wave 1 (0.4% vs 0.7%, P = 0.41) periods in comparison to the baseline, it was significantly lower during interwave (0.1% vs 1.5%; P < 0.001), and COVID wave 2 (0.2% vs 3.0%, P < 0.001).
4. Discussion
The originality of our work was to highlight several changes that occur during the early stage of the pandemic, i.e., number of vials processed and the rate of contaminants among positive blood cultures, and later, i.e., fall of S. pneumoniae bacteremia. Furthermore, we show that the trend in the number of vials processed follows the number of hospital admission during COVID wave 1 but not COVID wave 2.
Blood cultures are usually performed in patients with severe febrile illness in order to recover an etiologic micro-organism and guide appropriate antibiotics. Related to the influenza season an increased number of blood culture vials are processed during the winter [4]. As previously reported, we found a strong increase in the number of blood cultures vials sampled during wave 1 [13]. However, our results show this increase follows the number of hospital admission for COVID-19. During wave 1, COVID-19 was the main cause of hospital admission for febrile respiratory infections. The increase in blood culture vials sampled might be related to at least 2 findings. First, according to national guidelines and due to limited resources for performing SARS-CoV-2 RT-PCR, the etiological diagnosis of SARS-CoV-2 infection was only performed in patients with viral-like pneumonia after exclusion of other etiology. Then knowledge of COVID-19 and bacterial superinfections were limited during wave 1. The increase in blood culture vials processed during wave 1 is likely attributable to over-ordering in a population with a low rate of true bacteremia [13,14]. However, an increase in hospital-acquired bloodstream infections was reported during the COVID-19 pandemic in severely hill patients [15,16]. At the Assistance Publique – Hôpitaux de Paris, about 2 third of the bloodstream infections could be considered hospital-acquired based on the time interval between patient admission and blood culture sampling [15]. In comparison to wave 1, wave 2 was characterized by a less sudden increase in the number of patients admitted to hospital settings and a better understanding of the disease. Indeed, viral and bacterial co-infection remains rare in patients with COVID-19 (Haedo et al., n.d.; [17], [18], [21]). As a consequence, the number of blood culture vials sampled remains similar to the baseline level during the flu season. Furthermore, SARS-CoV-2 RT-PCR was systematically performed in all patients presenting in the emergency department during wave 2 making the diagnosis of COVID-19 more rapid.
We found a high rate of contaminants during wave 1 of the pandemic as previously reported [5,13,19]. However, most of these previous studies were performed during the first weeks of the pandemic or included the overall first year of the pandemic without any distinction between COVID wave and interwave. Here, we highlight the rate of contaminant return to baseline during wave 2. Esquer Garriguos et al. reported a peak in the rate of contaminants in May 2020, and then a trend to decline at the end of their study in august 2020 [19]. In the present study, the rate of contaminant returned to the baseline level at the end of 2020. The increase in contaminant rate might be related to lower adherence to optimal phlebotomy technique due to an increase in workload and staffing shortages and an increase in blood culture sampling. However, mask wear were previously associated with of blood cultures contamination rates [20]. Conversely, the rate of pneumococcal bacteremia fell in the later period of the study (interwave and wave 2). A fall in the incidence of invasive infection due to S. pneumoniae, Haemophilus influenzae and, Neisseria meningitidis was reported by national references laboratories of 26 countries in 2020 since the beginning of the COVID-19 pandemic [7]. The authors attributed this finding to the COVID-19 control policies and public information campaigns that reduced the transmission of respiratory pathogens. However, we found here a delay of the decrease of pneumococcal invasive diseases after the beginning of the COVID-19 pandemic. These differences could be related either to the lower number of centers in the present study, or the under-reporting of pneumococcal invasive diseases when clinical laboratories were overwhelmed by COVID-19 during wave 1.
In conclusion, we report several changes in blood culture sampling and processing during the first year of the COVID-19 pandemic: an increase in the number of blood culture vials processed and in the rate of contaminants during the first weeks of the pandemic and then a fell in the number of pneumococcal bloodstream infections. These changes might be related to a better knowledge of COVID-19, better resources management, and COVID-19 control policies. Subsequent surveys are needed to assess these trends.
Authors contributions
EF: Conceptualization, Methodology, Investigation, Writing – Original draft, Writing – review & editing; VC: Conceptualization, Methodology, Investigation, Writing – Original draft, Writing – review & editing; GP: Conceptualization, Methodology, Writing – review & editing; EC: Writing – review & editing; MV: Writing – review & editing.
Funding
None.
Declaration of Competing Interest
The author have no competing interest to disclose.
References
- 1.Marson FAL. COVID-19 –6 million cases worldwide and an overview of the diagnosis in Brazil: a tragedy to be announced. Diagn Microbiol Infect Dis. 2020;98 doi: 10.1016/J.DIAGMICROBIO.2020.115113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trucchi C, Paganino C, Orsi A, Amicizia D, Tisa V, Piazza MF, et al. Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults ≥50 years-old. BMC Health Serv Res. 2019;19 doi: 10.1186/S12913-019-4412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalán P, Alonso R, Alcalá L, Marín M, Moure Z, Pescador P, et al. The challenge of COVID-19 for a Clinical Microbiology Department. Diagn Microbiol Infect Dis. 2021;101 doi: 10.1016/j.diagmicrobio.2021.115426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ponfilly GP, Lourtet-Hascoet J, Porcheret H, Cambau E, Le Monnier A, Jacquier H, et al. Seasonal variations in blood culture numbers and time to positivity and potential impact of reducing incubation periods. Eur J Clin Microbiol Infect Dis. 2021;40:2087–2093. doi: 10.1007/s10096-021-04248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohki R, Fukui Y, Morishita N, Iwata K. Increase of blood culture contamination during COVID-19 pandemic. A retrospective descriptive study. Am J Infect Control. 2021;49:1359–1361. doi: 10.1016/J.AJIC.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacchetti B, Travis J, Steed LL, Webb G. Effects of COVID-19 on blood culture contamination at a tertiary care Academic Medical Center. Microbiol Spectr. 2022;10:22–31. doi: 10.1128/spectrum.00277-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the invasive respiratory infection surveillance initiative: a prospective analysis of surveillance data. Lancet Digit Heal. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farfour E, Pascreau T, Jolly E, Zia-Chahabi S, Mazaux L, Vasse M. Spring is coming, where are the respiratory syncytial virus and influenza viruses? J Clin Virol. 2021;139 doi: 10.1016/j.jcv.2021.104824. [DOI] [PubMed] [Google Scholar]
- 9.Santé Publique France. Coronavirus: chiffres clés et évolution de la COVID-19 en France et dans le Monde. 2022a.https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde#block-266151. Accessed September 29, 2022.
- 10.Santé Publique France. GEODES : Géo données en Santé publique 2022b. Available from: https://geodes.santepubliquefrance.fr/#view=map2&c=indicator.
- 11.Core Team R. R Found Stat Comput; Vienna, Austria: 2020. R: a language and environment for statistical computing.https://www.r-project.org Available from: [Google Scholar]
- 12.Société Française de Microbiologie.Hémoculture. REMIC. 2019;6(1):137–152. [Google Scholar]
- 13.Sepulveda J, Westblade LF, Whittier S, Satlin MJ, Greendyke WG, Aaron JG, et al. Bacteremia and blood culture utilization during COVID-19 Surge in New York City. J Clin Microbiol. 2020;58:20–26. doi: 10.1128/JCM.00875-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langford BJ, So M, Leung V, Raybardhan S, Lo J, Kan T, et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: living rapid review update and meta-regression. Clin Microbiol Infect. 2022;28:491–501. doi: 10.1016/J.CMI.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amarsy R, Trystram D, Cambau E, Monteil C, Fournier S, Oliary J, et al. Surging bloodstream infections and antimicrobial resistance during the first wave of COVID-19: a study in a large multihospital institution in the Paris region. Int J Infect Dis. 2022;114:90–96. doi: 10.1016/J.IJID.2021.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cataldo MA, Tetaj N, Selleri M, Marchioni L, Capone A, Caraffa E, et al. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming “collateral effect. J Glob Antimicrob Resist. 2020;23:290–291. doi: 10.1016/J.JGAR.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muggeo A, Alauzet C, Hartard C, Goury A, Schvoerer E, Andreoletti L, et al. Co-detection of SARS-CoV-2 and other respiratory pathogens: Lessons from the field to face the second wave. J Clin Virol. 2020;133 doi: 10.1016/j.jcv.2020.104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thelen JM, Buenen AGN, van Apeldoorn M, Wertheim HF, Hermans MHA, Wever PC. Community-acquired bacteraemia in COVID-19 in comparison to influenza A and influenza B: a retrospective cohort study. BMC Infect Dis. 2021;21:199. doi: 10.1186/s12879-021-05902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esquer Garrigos Z, Wingler MJB, Svoronos PA, Vijayvargiya P, Goodman-Meza D, O’horo JC, et al. Increased rates of blood culture contamination during the coronavirus disease 2019 pandemic. Infect Control Hosp Epidemiol. 2021;1:1719–1721. doi: 10.1017/ICE.2021.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders AM, Agger WA, Gray AM, Fischer CM, Kamprud EA. Use of hair nets and face masks to decrease blood culture contamination rates. Diagn Microbiol Infect Dis. 2019;95:15–19. doi: 10.1016/j.diagmicrobio.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Haedo MF, Melendi SE, Mauri ML, Ujeda C, Leis R. Usefulness of blood cultures in Covid-19 pneumonia n.d. Medicina (B Aires) 2020;(Suppl 6):44–47. [PubMed] [Google Scholar]