Abstract
Immunosuppressive therapy (IST) with anti-thymocyte globulin (ATG) and Cyclosporine (CSA) in aplastic anaemia (AA) results in improvement of blood counts between 3 and 6 months for the majority of patients. Infection is the most lethal complication in aplastic anemia and may arise due to several factors. We performed this study to determine the prevalence and predictors of specific infection types before and after IST. Six hundred and seventy-seven (546 adults; 434 males) transplant ineligible patients received ATG and CSA between 1995 and 2017. All patients who were transplant ineligible and received IST in this period were included. Infections before IST was seen in 209 (30.9%) and in 430 (63.5%) patients post IST. There were 700 infective episodes in the six months post-IST, including 216 bacterial, 78 fungal, 33 viral, and 373 culture-negative febrile episodes. Infections were highest (98, 77.8%) in very severe aplastic anaemia as compared to Severe AA (SAA) and Non-Severe AA (NSAA) (p < 0.001). Infections were also significantly higher in those who did not respond to ATG (71.1% vs. 56.8%, p = 0.003). At six months post-IST were 545 (80.5%) alive, and there were 54 (7.9%) deaths due to infection. Significant predictors of mortality were paediatric AA, very severe aplastic anaemia, pre or post ATG infections, and lack of response to ATG. Mortality was highest in those with combined bacterial and fungal infections post IST (p < 0.001). We conclude that infections are a common complication (63.5%) of IST. Mortality was highest when both bacterial and fungal infections were present. Routine use of growth factors and prophylactic antifungal and antibacterial agents was not part of our protocol, despite which 80.5% of the cohort was alive at the end of six months.
Keywords: Aplastic Anemia, Infections, Immunosupressive therapy (IST)
Introduction
Aplastic anemia has evolved from being a universally fatal disease to a treatable condition over the last half a century. The introduction of hematopoietic stem cell transplantation (HCT) [1] and immunosuppressive therapy (IST) [2] with equine anti-thymocyte globulin (ATG), Cyclosporine, and most recently, eltrombopag [3] has improved survival. Disease-related persistent neutropenia is associated with bacterial and invasive fungal infections [4, 5], but post-IST lymphocytolysis further complicates these [6, 7] until further recovery facilitates infection clearance [8]. The infection susceptibility and pathogen profile are different from cancer therapy-induced neutropenia, owing to the unique immunosuppression patterns [5] and the off target effects of chemotherapy which cause breach of mucosal barrier.
We sought to study infective complications of aplastic anemia in the immediate post-IST setting in an era of changing antibiotics and drug resistance patterns. Therefore, this study was undertaken to characterize a) the prevalence and profile of bacterial, viral, and fungal infection, b) predictors for infection and survival, and c) survival rates and hazard of death from infection in patients undergoing IST with ATG/ALG and CSA at six months post-therapy.
Methods
Patient Population
We performed this study at a hematology referral service in a teaching hospital in south India with an annual turnover of approximately 25,000 patients. A retrospective chart (outpatient and inpatient) based review was conducted in all patients with acquired aplastic anemia who were transplant ineligible and hence were treated with ATG/ALG and CSA for the first time between 1995 and 2017 (22 years). Patients who required a second IST with ATG/ALG were excluded from this analysis.
Classification of Aplastic Anemia
The diagnosis of aplastic anemia was made based on standard criteria [9] and the severity was classified as per the modified Camitta criteria [10, 11]. Patients under twenty years of age were screened for Fanconi anemia by stress cytogenetics after culturing the patient and control sample with mitomycin C [12]. In some patients where there were phenotypic abnormalities or borderline scores on stress cytogenetics, skin biopsy for FANC D2 by western blot was performed. Next generation sequencing (NGS) for inherited bone marrow failure (IBMFS) syndromes was not available during the study period (Figs. 1, 2, 3 and 4).
Fig. 1.
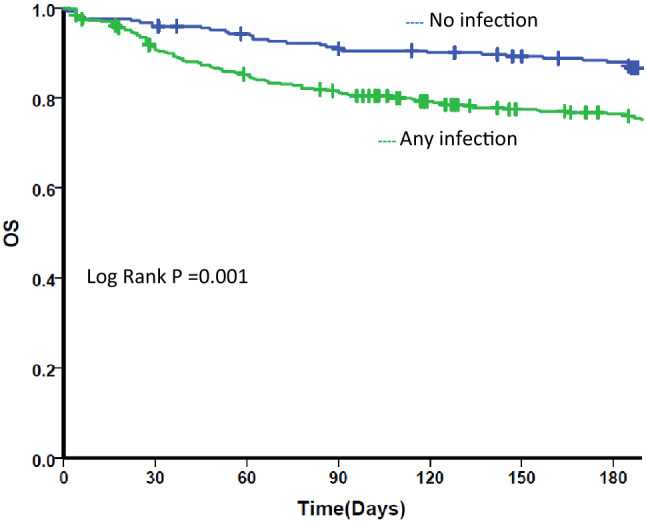
Kaplan Meier survival curve for any infection. OS Overall survival. Kaplan Meier survival curve for all patients by post IST infectious status. Blue line: those who did not have any infection post IST. Green line: those who had any infection post IST
Fig. 2.
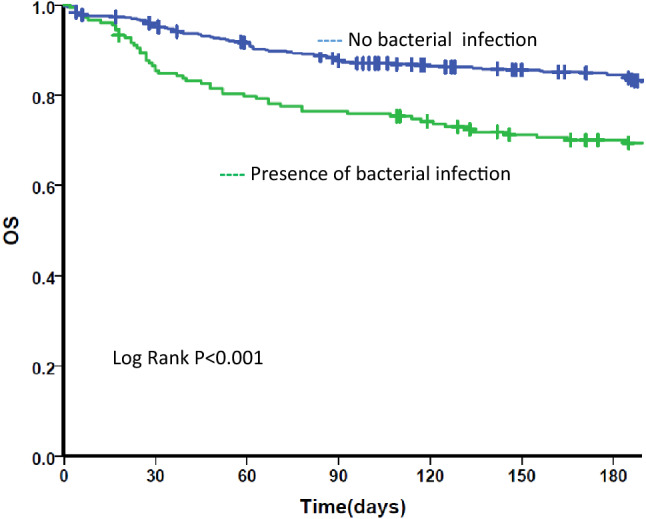
Kaplan Meier survival curve for bacterial infection. OS Overall survival. Kaplan Meier survival curve for all patients by post IST bacterial infection status. Blue line: those who did not have any bacterial infection post IST. Green line: those who had any bacterial infection post IST
Fig. 3.
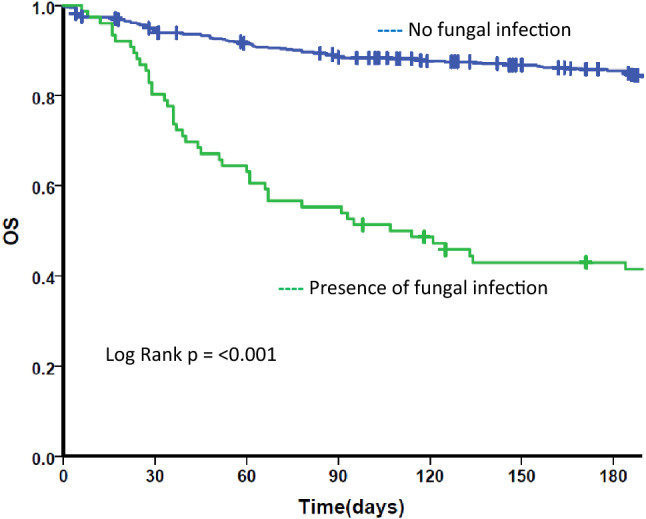
Kaplan Meier survival curve for fungal infection. OS Overall survival. Kaplan Meier survival curve for all patients by post IST fungal infectious status. Blue line: those who did not have any fungal infection post IST. Green line: those who had fungal infection post IST
Fig. 4.
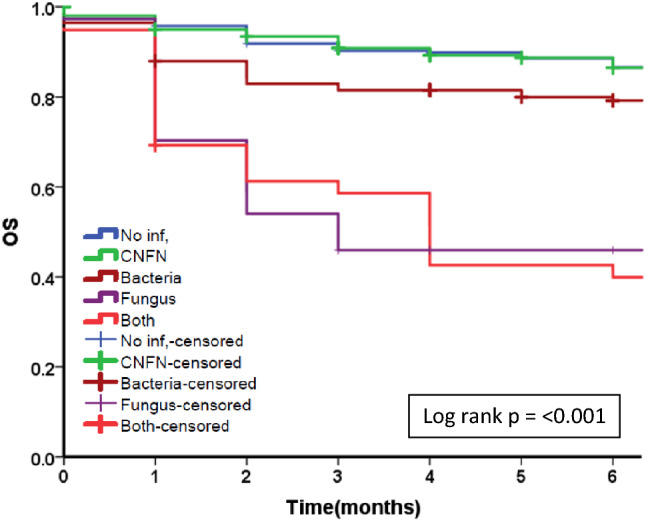
Kaplan Meier survival curve for attributable infection. OS Overall survival. Kaplan Meier survival curve for attributable infection. Blue line: No infection, Green line: Culture Negative Febrile Neutropenia (CNFN), Brown line: bacterial infection, Violet line: fungal infection, Orange: both bacterial and fungal infections
Immunosuppression Protocol and Response Criteria
A standard immunosuppression protocol was followed. From 1995 to 2004, this involved ALG (Pasteur Merieux, France) at 15 mg per kilogram daily for five days. After 2004, ATG (ATGAM, Pharmacia Upjohn, USA) was used at 40 mg per kilogram daily for four or five days. Dexamethasone 8 mg intravenous was given daily just prior to ATG administration. Oral prednisolone at 1 mg per kilogram body weight was then initiated at completion of ATG for two weeks duration followed by taper over one week as serum sickness prophylaxis, and Cyclosporine (5 mg/kg twice daily) was started once prednisolone was tapered. Cyclosporine levels were not routinely checked and was continued for up to 6 months followed by taper if complete response was achieved. Response was classified according to NIH criteria [13] as Complete Response (CR) if there was transfusion independence with Hb ≥ 11 g/dl, Neutrophils > 1.5 × 109/l and Platelets > 100 × 109/l, Partial response as transfusion independence with Hb ≥ 8 g/dl, Neutrophils > 0.5 × 109/l and Platelets > 20 × 109/l and no response if there is transfusion dependence. Red blood cells were transfused when haemoglobin decreased to less than 7 g/dl and platelets if there was bleeding or fever when it was less than 20,000/ccmm.
Diagnosis and Management of Infective Episodes
All bacterial infections had been adequately treated (i.e., Blood culture sterile and afebrile for 48 h after stopping antibiotics) at the time of initiation of IST. For fungal infections at least two weeks of antifungal therapy with clinical and or radiological signs of resolution of infection was mandatory before starting IST.
No prophylactic antibacterial, antiviral or antifungal therapy was used as a routine. If a patient developed fever, they were initiated on dual broad-spectrum antibiotics (an aminoglycoside and a third-generation cephalosporin) after drawing blood cultures. Carbapenems were used only if there was any evidence of severe sepsis or supporting microbiology [14] Gram-positive bacterial cover with teicoplanin was added if an indwelling central venous catheter was present, or fever persisted after 48 h of antibiotics therapy, or clinical evidence of skin and soft tissue infection was present. A chest X-ray, urine analysis, and malarial parasites screening were performed in all cases. A High-resolution CT(HRCT) scan of the chest was done if X-ray infiltrates suggested fungal infection or a persistent fever for more than three days with negative bacterial cultures. Serial galactomannan level (since 2016) was done if the HRCT was suggestive of invasive fungal pneumonia [15], and it was used as a surrogate serologic marker of invasive aspergillus infection. Invasive fungal infections were classified into proven, probable, and possible per international guidelines [16].Confirmation of etiology of pneumonia by bronchoscopy and bronchoalveolar lavage was limited by thrombocytopenia. If there were skin lesions suggestive of herpes simplex or herpes zoster then a Tzank smear was done. If there were associated upper respiratory or flu like symptoms then a multiplex viral PCR for respiratory viruses throat swab was sent. All patients had central venous catheters (peripherally inserted central catheter or internal jugular central venous catheter) inserted for ATG therapy, usually removed after partial recovery of counts and increasing transfusion independence. If there were any cutaneous or joint symptoms in the first month post ATG with or without fever it was considered to be Serum sickness and treated accordingly with dexamethasone until symptoms resolve followed by a rapid taper. In our institution, growth factors were used only in neutropenic episodes with severe sepsis for seven days [11] and is not routinely employed due to a clear lack of benefit [17].
Statistical Analysis
The descriptive data were reported as means with standard deviation (medians and interquartile range for non-parametric distributions) or frequencies with percentages as appropriate. Continuous data were compared with the T-test or Mann–Whitney U test as appropriate. Proportions were compared using the Pearson chi-square test or Fisher exact test. The relationships of clinical features to the therapy outcome were analysed by logistic regression, and their 95% confidence intervals were calculated.
The Kaplan–Meier method was used to estimate overall survival, and comparisons were based on the log-rank test. Analysis of risk factors for survival was calculated using Cox regression proportional hazards method. Variables used in the survival analysis were age (pediatric ≤ 15/adult > 15), sex, the severity of aplastic anemia, presence of PNH clone, infection (both pre and post-treatment), multidrug-resistant infection, serum sickness, year of treatment (≤ 2005/ ≥ 2006), and response to ATG (complete/ incomplete). For all tests, a 2-sided p value of 0.05 or less was considered statistically significant. Data were analysed using the IBM SPSS statistics version 24. This study was approved by the Institutional Review Board and hospital ethics committee [IRB Min. No. 12943].
Results
Patient and Disease Characteristics
Baseline characteristics have been described in Table 1. A total of 677 patients received immunosuppressive therapy with horse ATG/ALG and Cyclosporine between 1995 and December 2017. There were no significant differences in the baseline characteristics between adult and pediatric population except that the number of Very Severe Aplastic Anemia (VSAA) was significantly higher in the pediatric (34, 26%) than the adult (92, 16.8%) cohort (OR, 95% CI, p 0.048).
Table 1.
Baseline characteristics of patients undergoing treatment with IST
| Age | |
|---|---|
| Paediatric ≤ 15 years | 131 (19.4%) |
| Adult > 15 years | 546 (80.6%) |
| 16–30 years | 187 (27.6%) |
| 31–50 years | 203 (30%) |
| > 50 years | 156 (23%) |
| Total | 677 |
| Median age (in years) | 33 (1–83) |
| Sex | |
| Male | 434 (64.1%) |
| Female | 243 (35.9%) |
| Severity | |
| Non-severe (NSAA) | 166 (24.5%) |
| Severe (SAA) | 385 (56.9%) |
| Very severe (VSAA) | 126 (18.6%) |
| PNH clone | |
| Present | 49 (7.2%) |
| Absent | 417 (61.6%) |
| Unknown | 211 (31.2%) |
| Previous treatment | |
| Treatment naive | 443 (65.5%) |
| Stanazolol/danazol | 33 (4.9%) |
| Prednisolone | 85 (12.5%) |
| Cyclosporine | 114 (16.8%) |
| Others | 2 (0.3%) |
| Immunosuppressive therapy | |
| ATG (Anti Thymocyte Globulin) | 424 (62.6%) |
| ALG (Anti Lymphocyte Globulin) | 253 (37.4%) |
| Diabetes—pre-existing/therapy-induced | |
| Yes | 128 (18.9%) |
| No | 549 (18.9%) |
| Serum sickness | |
| Yes | 171 (25.3%) |
| No | 506 (74.7%) |
| Time from diagnosis to starting ATG/ALG | |
| Median (Range) | 1 month (0–189 months) |
| Mean | 4 months |
Infections Prior to IST
Two hundred and eight patients (30.9%) had infections before IST. There were 256 infective episodes, of which 116 were bacterial infections, five were fungal, two were malarial, and the rest were culture-negative febrile neutropenia (133). The most common infection was Klebsiella species (21, 18%) followed by Pseudomonas species (19, 16.3%), Coagulase Negative Staphylococcus (CONS) (18, 15.5%), Escherichia coli (17, 14.6%) and Staphylococcus aureus (13, 11.2%) and Non Fermenting Gram-negative Bacilli (NFGNB) (13, 11.2%). There were five fungal infections, of which two had candidemia, one had Mucormycosis of the paranasal sinus, and two patients had probable fungal pneumonia. Most of the infections were seen one month before IST therapy.
Post-IST Infections (Table 2)
Table 2.
Microbiological characteristics, resistance patterns and sites of infetction of 700 episodes of infection after IST
| Infection | Number (%) |
|---|---|
| Total infectious episodes | 700 |
| Culture negative febrile illness | 373 (53.2%) |
| Bacterial | 216 (30.9%) |
| Median time to infection | 19 days (range 1 to > 180) |
| Gram-negative | 119 (55%) |
| E. Coli | 36 (16.6%) |
| Klebsiella pneumoniae | 26 (12%) |
| Pseudomonas aeruginosa | 21 (9.7%) |
| NFGNB | 10 (4.6%) |
| Salmonella | 6 (2.7%) |
| Aeromonas | 6 (2.7%) |
| Others | 14 (6.4%) |
| Gram positive | 57 (26.3) |
| Staphylococcus aureus | 17 (7.8%) |
| Coagulase negative Staph aureus | 23 (10.6%) |
| Enterococci | 11 (5.0%) |
| Others | 6 (2.7%) |
| Polymicrobial | 5 (2.3%) |
| Tuberculosis (M. tuberculosis and M. fortuitum) | 3 (1.3%) |
| Malaria | 4 (1.8%) |
| Unknown | 28 (12.9%) |
| Fungal | 78 (11.1%) |
| Median time to infection | 22 days [range: 1 to > 180 days] |
| Proven | 38 (48.7%) |
| Probable | 17 (21.8%) |
| Possible | 23 (29.5%) |
| Viral | 33 (4.8%) |
| Median time to infection | 10.5 days [range 1 to > 180] |
| Herpes | 26 (78.7%) |
| Influenza | 2 (6.1%) |
| Varicella | 2 (6.1%) |
| Others | 3 (9.1%) |
| Drug resistant bacterial infection | 30 (13.8%) |
| Extended spectrum beta lactamase | 12 (5.5%) |
| Carbapenem resistant organism | 14 (6.4%) |
| Methicillin resistant Staphylococcus aureus | 3 (1.3%) |
| Vancomycin resistant Enterococcus | 1 (0.4%) |
| Sites of bacterial infection | |
| Bloodstream infection | 120 (55.5%) |
| Skin and soft tissue | 37 (17.1%) |
| Pneumonia | 25 (11.5%) |
| Urinary tract infection | 19 (8.7%) |
| Gastrointestinal | 8 (3.7%) |
| Osteomyelitis | 3 (1.3%) |
| Others | 4 (1.8%) |
| Sites of fungal infection | |
| Invasive fungal pneumonia | 54 (69.2%) |
| Fungal sinusitis | 8 (10.2%) |
| Bloodstream (Candidemia)* | 3 (3.8%) |
| Fungal pneumonia and sinusitis | 8 (10.2%) |
| Others | 5 (6.6%) |
*All patients with candidemia succumbed to infection. E. coli Escherichia coli, NFGNB-Non Fermenting Gram Negative Bacilli, M. tuberculosis Mycobacterium tuberculosis, M. fortuitum Mycobacterium fortuitum
The microbiological characteristics, resistance patterns and sites of infection of infections post IST are described in Table 2.
We observed a total of 700 febrile episodes in 430 (63.5%) patients after administration of ATG. Culture-negative febrile episodes (373) were seen in 270 (39.8%) patients. Bacterial infections were seen in 179 (26.4%) patients with 216 bacterial episodes. Fungal infections were seen in 75 (11%) patients with 78 fungal infective episodes, and 33 (4.8%) patients had a single viral infection each.
Bacterial Infections
Bacterial infections were seen in 179 (26.4%) patients. There were 216 episodes, with 149 patients having a single episode, 26 patients had two episodes, two patients had three episodes, and one patient had four and five bacterial infections each. The majority of the bacterial infections [69.4%] were seen within the first month of therapy and the median time to infection was 19 days [range 1 to > 180]. Gram-negative infections (119, 55%) were more common than gram-positive (57, 26.3%). E. coli was the most common bacterial infection [36 (16.6%)]. Drug resistant infections were seen in 30 (13.8%) of bacterial infections.
Viral Infections
Viral infections were seen in thirty-three patients (33, 4.8%). The majority of the viral infections (21, 63%) were seen within the first month after ATG therapy, and the median time to infection was 10.5 days [range 1 to > 180]. The most common viral infection was herpes.
Fungal Infections
Seventy-five patients had a total of seventy-eight episodes of fungal infections. The distribution of infections is described in Table 2. Invasive fungal pneumonia was the most common site of infection (54, 69.2%), followed by fungal sinusitis (8, 10.2%) and bloodstream infection (3, 3.8%). Eight patients had both fungal pneumonia and fungal sinusitis. Other sites of infection were skin and soft tissue infections, urinary tract infections, and laryngeal candidiasis. The majority of the fungal infections [63%] were observed in the first month following ATG therapy, and the median time to infection was 22 days [range 1 to > 180 days]. The most common fungal infection was pneumonia with angio-invasive Aspergillus, and Aspergillus flavus, fumigatus, and tereus were all equally observed. The next most common was Candidal infection, where tropicalis species were more common than krusei and glabrata. All patients with candidemia succumbed to the illness.
Unique Infections
Certain infections unique to our cohort include gastroenteritis with Vibrio cholera, NON 01/NON0139 type (2 patients), Salmonella typhoid infection in six patients, and shigella diarrhoea in two patients. These patients recovered from the illness except one with Salmonella osteomyelitis, who later succumbed to invasive fungal pneumonia. During the 2010 H1N1 epidemic, any patient with fever and upper respiratory tract symptoms were swabbed for the H1N1 PCR and treated with oseltamivir. One patient developed acute respiratory distress syndrome with H1N1 and was successfully treated. Atypical mycobacterial infection was seen in one patient with three blood cultures growing mycobacterium fortuitum. Six-month of therapy with amikacin, clarithromycin, and levofloxacin was given. Two patients had probable pulmonary tuberculosis (typical radiological features but not confirmed microbiologically), treated with four drug anti-tuberculosis therapy.
Risk Factors for Post-IST Infections (Table 3)
Table 3.
Risk factors for infections following IST
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| Risk (95% CI) | p value | Risk (95% CI) | p value | |
| AGE (years) | NS | |||
| ≤ 15 | 1.1 (0.79–1.75) | 0.010 | ||
| > 15 | 1.0 | |||
| Sex | NS | |||
| Male | 0.9 (0.67–1.30) | 0.714 | ||
| Female | 1.0 | |||
| Diagnosis | ||||
| VSAA | 4.4 (2.64–7.43) | 0.000 | 4.5 (2.44–8.30) | 0.000 |
| SAA | 2.0 (1.31–3.14) | 0.001 | 1.8 (1.12–2.96) | 0.014 |
| NSAA | 1.0 | |||
| PNH Clone | NS | |||
| Present | 1.2 (0.67–2.26) | 0.484 | ||
| Absent | ||||
| Diabetes mellitus (Yes) | 0.6 (0.45–1.06) | 0.098 | NS | |
| Therapy used | NS | |||
| ALG | 0.3 (0.25–0.52) | 0.000 | ||
| ATG | ||||
| Serum sickness (Yes) | 0.6 (0.42–0.92) | 0.019 | NS | |
| Current disease status | 0.004 | 1.84 (1.16–2.92) | 0.009 | |
| Complete response | 1.0 | |||
| No response | 1.8 (1.22–2.77) | |||
Very Severe Aplastic Anemia and lack of response to IST were significantly associated with infections post IST on multivariate analysis. Other parameters like pedaitric age group, male sex, presence of serum sickness and use of ALG were significant only on univariate analysis
NS not significant
The total number of infections, specifically fungal [13 (16.6%) vs 65 (83.3%), p < 0.01] were significantly higher in the last decade, i.e., 2017 to 2006 compared to the years earlier than 2006. Risk factors for infection included severity of aplastic anemia [VSAA—77.8%; SAA—62.3% and NSAA—55.4% (p = 0.000)] and no response to ATG [71.1% in those with no response, 59.1% in those with partial response, and 56.8% in those with complete response (p = 0.003)].
Outcome Characteristics
Response at six months to ATG and Cyclosporine was seen in 397 (58.6%), including a complete response (CR) in 22.9% (155) and a partial response (PR) in 35.7% (242). At six months post-IST, 545 (80.5%) were alive, and at last, follow-up [median of 20 months (0–315)], 486 (71.7%) were alive. At the end of six months, bacterial infections were the cause of death in 34 (25.8%), fungal infections in 20 patients (15.2%), and uncontrolled bleeding in thirteen (10 with intracranial hemorrhage, 2 with diffuse alveolar hemorrhage, and one with gastrointestinal bleed). The cause of death was not known in sixty-five cases as they were not in the hospital at the time of death but were most often infection related as per unofficial communication with the next of kin (Table 4).
Table 4.
Risk factors for all-cause mortality following IST
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| Risk (95% CI) | p value | Risk (95% CI) | p value | |
| Age (years) | ||||
| ≤ 15 | 1.9 (1.38–2.61) | < 0.001 | 1.9 (1.20–3.13) | 0.006 |
| > 15 | 1.0 | |||
| Sex | NS | |||
| Male | 1.0 (0.75–1.36) | 0.965 | ||
| Female | 1.0 | |||
| Diagnosis | ||||
| VSAA | 2.8 (1.86- 4.45) | < 0.001 | 3.3 (1.49–7.57) | 0.003 |
| SAA | 1.5 (1.01–2.24) | 0.045 | 1.7 (0.84–3.76) | 0.132 |
| NSAA | 1.0 | |||
| PNH clone | NS | |||
| Present | 0.4 (0.14–0.89) | 0.027 | ||
| Absent | ||||
| Infection post-IST (Yes) | 2.1 (1.60–2.85) | < 0.001 | 3.3 (2.09–5.44) | < 0.001 |
| Infection pre IST (Yes) | 2.1 (1.59–2.91) | < 0.001 | NS | |
| MDR infection (Yes) | 2.1 (1.34–3.46) | 0.002 | NS | |
| Diabetes mellitus (Yes) | 0.6 (0.37–1.06) | 0.083 | NS | |
| Therapy used | ||||
| ALG | 3.1 (2.32–4.19) | < 0.001 | 3.5 (2.14–5.83) | < 0.001 |
| ATG | 1.0 | |||
| Serum sickness (Yes) | 1.4 (1.01–1.87) | 0.039 | NS | |
| Current disease status | < 0.001 | 38.9 (5.36–282.19) | < 0.001 | |
| Complete response | 1.0 | |||
| No response | 28.6 (9.13–90.07) | |||
NS not significant
All-cause mortality in the pediatric population was significantly higher (p = 0.006). Specifically, 53 (40.4%) of the pediatric cohort died compared to 51 (27.2%) of those between 16 and 30 age, 50 (24.6%) of those between 31 and 50 years and 37 (23.7%) of those older than 50 years. This can be attributed to higher VSAA (26% vs 16.8%, p = 0.048) in the pediatric cohort. The presence of PNH clone at diagnosis had lower mortality, 5 (10.2%) vs 112 (26.8%) (p = 0.027), though PNH clone was present comparably in all classes of severity (p = 0.409). Use of ALG (119, 47.03% vs 72, 16.9%) was associated with higher mortality than ATGAM [p = < 0.001], though there were more infections in the ATG group
Discussion
Infections are a leading cause of mortality in patients with aplastic anemia [18, 19]. The prevalence of infections following IST and ranges between 32% [20] and 81% [21], with a previous study from India diagnosing febrile neutropenia in 57% [22] post-IST. In our series, the prevalence of documented infections was 42.3%. In a study from the Delhi [23], Seven patients (22.58%) of 31 patients developed infection within 1 month of post ATG. In five patients there was no aetiology, one had a bacterial pneumonia and one had pseudomonas sepsis. Majority of infected patients were in the non-response group. In another single centre experience from western India [22], febrile neutropenia was the most common (affecting 57.1% patients) adverse event where infection, pneumonia and sepsis were the leading causes of death. In a recent study [24], gram negative sepsis and fungal infections were common and was the cause of mortality in all four of the patients who died, post ATG.
The use of prophylactic broad-spectrum antibiotics to prevent febrile neutropenic episodes in aplastic anemia is recommended [11], especially in those with VSAA [25]. However, because of the high prevalence of multidrug-resistant organisms in the Indian subcontinent, prophylactic antibiotic use may be ineffective [26]. Similarly, pre-emptive acyclovir and secondary prophylaxis has been recommended but in clinical practice, this is not followed since infections due to herpes viruses are not a cause of severe morbidity or mortality.
Majority of the infections occur during the first month following treatment with IST corresponding to the severity of neutropenia, at which time some guideline advises growth factors to mitigate this risk [27]. The use of growth factors with ATG therapy is controversial with no proven decrease in infection-related mortality and clonal transformation [28, 29].
Bacterial infections occurred mainly due to Gram-negative organisms which is different from the West [18, 21] but similar to studies from east Asia where again grame negative infections are common [20, 22, 30]. This predominance of gram-negative organisms in different hospitalized patient populations is well described across India [29–31] including patients undergoing chemotherapy for cancer [31] Higher community prevalence of multidrug resistant organisms and recurrent prior admissions for febrile or hemorrhagic complications may explain this high incidence [32, 33]. Fluoroquinolone prophylaxis in neutropenia is a practice across Europe and North America with a proven decrease in bloodstream infections [34]; however, widespread resistance to this drug precludes its use in a country like India [35]. The advent of organisms like NDM-1 producing Klebsiella pneumoniae [34] in the country is reflected in our study, with a significant number of MDR infections in recent years than in the earlier decade. Presence of MDR organisms in neutropenic patients post ATG therapy results in critical illness and severe sepsis since the recovery of neutrophil count is not imminent, hence proving to be dreaded and often fatal complication.
Invasive fungal disease [IFD] infections continue to remain a concern with an increasing incidence of IFD in our cohort similar to other studies across India [22, 24]. IFD has decreased over the last two decades across the world due to the addition of numerous antifungal drugs and novel diagnostic strategies [18]. Since India is endemic to malarial infection, it was not surprising to see two patients with vivax malaria pre ATG and four patients post-ATG, one of whom had infection with falciparum species and the rest vivax species. They were all diagnosed while staying in the community and were subsequently admitted. None had severe disease or significant organ dysfunction.
All infections were higher in those who had no reponse to IST [430 (63.5%) vs 247 (36.5%), p = 0.003] as was seen in the AIIMS paper [23]. This was also seen in another series of 100 children with acquired aplastic anemia treated with IST [36]. Increased infections are also universally associated with a higher risk of mortality [37]. In an elderly cohort bleeding and infection was significantly associated with mortality (p = 0.02) [38]
Severity of aplastic anemia was also a significant predictor of infections, post IST. This was also described from a center in Thailand where the “only risk factor that correlates with high probability of infection was ANC < 500/mm(HR 2.29, 95% CI 1.03–7.72, p = 0.043) [20]. In another study five out the twelve patients who died, had infections and “prolonged and severe neutropenia” [21]. Our large series corroborates the evidence that lack of response to infection and disease severity are significant predictors on infection and in turn mortality.
Since this was a retrospective analysis, data was unavailable in some cases before 2004, especially regarding the cause of death as patients who were still transfusion dependant after three months tended to go back to their local hospitals for transfusion support and were subsequently lost to follow up. The unavailability of next-generation sequencing (NGS) to detect inherited bone marrow failure syndrome (IBMFS) in all paediatric cases may have impacted the adverse outcomes in this population. Diagnosis of bacterial and fungal infections have changed rapidly over the period of the study, with the availability of High-resolution computed tomography (HRCT) thorax (Invasive fungal pneumonia), Galactomannan Elisa, Carba Xpert PCR, BioFire PCR, MALDI-TOF and other novel testing strategies. The absence of these in earlier patients may have affected the diagnosis leading to many more being termed as “culture negative febrile neutropenia”. This could be a limitation of the study, however, the large numbers included give us valuable information on the infection profile and outcomes.
Conclusion
We conclude that infections are common complications (63.5%) of IST with ATG/ALG and CSA. Routine use of growth factors, prophylactic antifungal, and antibacterial agents was not part of the regular protocol, despite which 80.5% of the cohort was alive at the end of six months, hence we do not recommend prophylactic antimicrobial use. Those with both bacterial and fungal infections had the maximum infection attributable mortality. Rising drug resistance and newer diagnostic techniques to diagnose IBMFS may decrease IST usage. We hope that newer drugs like Eltrombopag and diagnostic tests with a rapid turnaround time to detect invasive fungal infections and drug-resistant bacterial infections will positively impact clinical outcomes in this high-risk population.
Acknowledgements
There are no further acknowledgements.
Author contribution
Study conception: SL, BG. Data collection: SL, SS, AK, UK, AD, FNA. Data analysis and interpretation: SL, BG, KL. Drafting of manuscript: SL, BG. Critical revision: SS, AK, UK, AD, FNA, AA, VM. Final approval: SL, SS, UK, AD, AK, FNA, VM, AA, KL, BG.
Funding
Internal resources of the Christian Medical College Vellore.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Storb R, Thomas ED, Weiden PL, Buckner CD, Clift RA, Fefer A, et al. Aplastic anemia treated by allogeneic bone marrow transplantation: a report on 49 new cases from Seattle. Blood. 1976;48(6):817–841. doi: 10.1182/blood.V48.6.817.817. [DOI] [PubMed] [Google Scholar]
- 2.Frickhofen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Herrmann F, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German Aplastic Anemia Study Group. N Engl J Med. 1991;324(19):1297–304. doi: 10.1056/NEJM199105093241901. [DOI] [PubMed] [Google Scholar]
- 3.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger M. Approach to management of fever and infection in patients with primary bone marrow failure and hemoglobinopathies. Hematol Oncol Clin N Am. 1993;7(4):865–885. doi: 10.1016/S0889-8588(18)30225-9. [DOI] [PubMed] [Google Scholar]
- 5.Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46(3):269–276. doi: 10.1053/j.seminhematol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Young N, Griffith P, Brittain E, Elfenbein G, Gardner F, Huang A, et al. A multicenter trial of antithymocyte globulin in aplastic anemia and related diseases. Blood. 1988;72(6):1861–1869. doi: 10.1182/blood.V72.6.1861.1861. [DOI] [PubMed] [Google Scholar]
- 7.Vrochides D, Hassanain M, Metrakos P, Tchervenkov J, Barkun J, Chaudhury P, et al. Prolonged lymphopenia following anti-thymocyte globulin induction is associated with decreased long-term graft survival in liver transplant recipients. Hippokratia. 2012;16(1):66–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger M, Elattar I, Marshall D, Steinberg SM, Redner RL, Young NS, et al. Patterns of infection in patients with aplastic anemia and the emergence of Aspergillus as a major cause of death. Medicine (Baltimore) 1992;71(1):24–43. doi: 10.1097/00005792-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Samarasinghe S, Webb DK. How I manage aplastic anaemia in children. Br J Haematol. 2012;157(1):26–40. doi: 10.1111/j.1365-2141.2012.09058.x. [DOI] [PubMed] [Google Scholar]
- 10.Camitta BM, Thomas ED, Nathan DG, Gale RP, Kopecky KJ, Rappeport JM, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53(3):504–514. doi: 10.1182/blood.V53.3.504.504. [DOI] [PubMed] [Google Scholar]
- 11.Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuffel DG, Lindor NM, Litzow MR, Zinsmeister AR, Dewald GW. Mitomycin C chromosome stress test to identify hypersensitivity to bifunctional alkylating agents in patients with Fanconi anemia or aplastic anemia. Mayo Clin Proc. 1997;72(6):579–580. doi: 10.4065/72.6.579. [DOI] [PubMed] [Google Scholar]
- 13.Scheinberg P, Wu CO, Nunez O, Scheinberg P, Boss C, Sloand EM, et al. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94(3):348–354. doi: 10.3324/haematol.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74. [PubMed]
- 15.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh JC, Ganser A, Fau-Stadler M, Stadler M. Hematopoietic growth factors in the treatment of acquired bone marrow failure states (0037-1963 (Print)). [DOI] [PubMed]
- 18.Valdez JM, Scheinberg P, Nunez O, Wu CO, Young NS, Walsh TJ. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52(6):726–735. doi: 10.1093/cid/ciq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92(1):11–18. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 20.Lertpongpiroon R, Rattarittamrong E, Rattanathammethee T, Chai-Adisaksopha C, Tantiworawit A, Salee P, et al. Infections in patients with aplastic Anemia in Chiang Mai University. BMC Hematol. 2018;18:35. doi: 10.1186/s12878-018-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres HA, Bodey GP, Rolston KV, Kantarjian HM, Raad II, Kontoyiannis DP. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98(1):86–93. doi: 10.1002/cncr.11478. [DOI] [PubMed] [Google Scholar]
- 22.Shah S, Jain P, Shah K, Patel K, Parikh S, Patel A, et al. Immunosuppressive therapy for aplastic anemia: a single-center experience from western India. Ann Hematol. 2019;98(1):41–46. doi: 10.1007/s00277-018-3487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ClinicoHaematological Profileand Management of Aplastic Anaemia AIIMS Experience Mahapatra.pdf.
- 24.Dutta B, Dolai TK, Mandal PK, Baul S, De R, Senthil K, et al. Response to immunosuppressive therapy in acquired aplastic anaemia: experience of a Tertiary Care Centre from Eastern India. Indian J Hematol Blood Transfus. 2021;37(2):197–203. doi: 10.1007/s12288-019-01158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iftikhar R, Ahmad P, de Latour R, Dufour C, Risitano A, Chaudhri N, et al. Special issues related to the diagnosis and management of acquired aplastic anemia in countries with restricted resources, a report on behalf of the Eastern Mediterranean blood and marrow transplantation (EMBMT) group and severe aplastic anemia working party of the European Society for blood and marrow transplantation (SAAWP of EBMT) Bone Marrow Transpl. 2021;56(10):2518–2532. doi: 10.1038/s41409-021-01332-8. [DOI] [PubMed] [Google Scholar]
- 26.Monserrat-Martinez A, Gambin Y, Sierecki E. Thinking outside the bug: molecular targets and strategies to overcome antibiotic resistance. Int J Mol Sci. 2019;20(6):1255. doi: 10.3390/ijms20061255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transpl. 2013;48(2):174–177. doi: 10.1038/bmt.2012.222. [DOI] [PubMed] [Google Scholar]
- 28.Socie G, Mary JY, Schrezenmeier H, Marsh J, Bacigalupo A, Locasciulli A, et al. Granulocyte-stimulating factor and severe aplastic anemia: a survey by the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2007;109(7):2794–2796. doi: 10.1182/blood-2006-07-034272. [DOI] [PubMed] [Google Scholar]
- 29.Teramura M, Kimura A, Iwase S, Yonemura Y, Nakao S, Urabe A, et al. Treatment of severe aplastic anemia with antithymocyte globulin and cyclosporin A with or without G-CSF in adults: a multicenter randomized study in Japan. Blood. 2007;110(6):1756–1761. doi: 10.1182/blood-2006-11-050526. [DOI] [PubMed] [Google Scholar]
- 30.Chuansumrit A, Hathirat P, Isarangkura P. Acquired aplastic anemia in children: a review of 100 patients. Southeast Asian J Trop Med Public Health. 1990;21(2):313–320. [PubMed] [Google Scholar]
- 31.Philip C, George B, Ganapule A, Korula A, Jain P, Alex AA, et al. Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol. 2015;170(1):110–117. doi: 10.1111/bjh.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thacker N, Pereira N, Banavali SD, Narula G, Vora T, Chinnaswamy G, et al. Alarming prevalence of community-acquired multidrug-resistant organisms colonization in children with cancer and implications for therapy: a prospective study. Indian J Cancer. 2014;51(4):442–446. doi: 10.4103/0019-509X.175310. [DOI] [PubMed] [Google Scholar]
- 33.Bhat PV, Sarkar A. Emergence and control of multidrug resistant organisms in small cities in India: a wake up call. Indian J Med Sci. 2011;65(8):337–343. doi: 10.4103/0019-5359.107771. [DOI] [PubMed] [Google Scholar]
- 34.Mikulska M, Averbuch D, Tissot F, Cordonnier C, Akova M, Calandra T, et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J Infect. 2018;76(1):20–37. doi: 10.1016/j.jinf.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh S, Chakraborty M, Samanta S, Sinha N, Saha S, Chattopadhyay A, et al. Analysis of blood stream infections, antibiograms and clinical outcomes in haematological patients with febrile neutropenia: data from a tertiary care haematology institute in India. Ann Hematol. 2021;100(2):395–403. doi: 10.1007/s00277-020-04324-8. [DOI] [PubMed] [Google Scholar]
- 36.Chuansumrit A, Hathirat P, Fau-Isarangkura P, Isarangkura P. Acquired aplastic anemia in children: a review of 100 patients. (0125–1562 (Print)). [PubMed]
- 37.Tichelli A, Socié G, Henry-Amar M, Marsh J, Passweg J, Schrezenmeier H, et al. Effectiveness of immunosuppressive therapy in older patients with aplastic anemia. European Group for Blood and Marrow Transplantation Severe Aplastic Anaemia Working Party. Ann Intern Med. 1999;130(3):193–201. doi: 10.7326/0003-4819-130-3-199902020-00004. [DOI] [PubMed] [Google Scholar]
- 38.Marsh JC, Bacigalupo A, Schrezenmeier H, Tichelli A, Risitano AM, Passweg JR, et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119(23):5391–5396. doi: 10.1182/blood-2012-02-407684. [DOI] [PubMed] [Google Scholar]


