Abstract
The ongoing Coronavirus disease 2019 (COVID-19) pandemic illustrates the need for sensitive and reliable tools to diagnose and monitor diseases. Traditional diagnostic approaches rely on centralized laboratory tests that result in long wait times to results and reduce the number of tests that can be given. Point-of-care tests (POCTs) are a group of technologies that miniaturize clinical assays into portable form factors that can be run both in clinical areas ––in place of traditional tests–– and outside of traditional clinical settings ––to enable new testing paradigms. Hallmark examples of POCTs are the pregnancy test lateral flow assay and the blood glucose meter. Other uses for POCTs include diagnostic assays for diseases like COVID-19, HIV, and malaria but despite some successes, there are still unsolved challenges for fully translating these lower cost and more versatile solutions. To overcome these challenges, researchers have exploited innovations in colloid and interface science to develop various designs of POCTs for clinical applications. Herein, we provide a review of recent advancements in lateral flow assays, other paper based POCTs, protein microarray assays, microbead flow assays, and nucleic acid amplification assays. Features that are desirable to integrate into future POCTs, including simplified sample collection, end-to-end connectivity, and machine learning, are also discussed in this review.
Keywords: Point-of-care tests, in vitro diagnostics, infectious disease, biosensors, COVID-19
Graphical abstract
1. Introduction
Point-of-care tests (POCTs) are designed to detect the presence of biomarkers with inexpensive and easy-to-use formats for disease diagnosis and monitoring at the clinical bedside, at-home, or in global health settings. Recent infectious disease outbreaks, such as COVID-19, Ebola, and Zika virus illustrate the necessity for rapid and reliable diagnostics to effectively manage health crises. POCTs are important at the population level, for disease tracking and containment, and at the individual level, providing patients and clinicians quick information to inform treatment decisions. Conventionally, disease pathogens are detected and quantified by measuring specific nucleic acid signatures, antigens, host antibodies generated against the pathogen, or a combination of these three classes of biomarkers using multi-step tests run by trained professionals in centralized clinical laboratories. To detect nucleic acids, polymerase chain reaction (PCR) and reverse transcription polymerase chain reaction (RT-PCR), which use specific primers and thermal cycling to amplify low concentrations of DNA and RNA respectively to detectable levels, are the gold standard.1 , 2 To detect protein biomarkers or host antibodies, enzyme-linked immunosorbent assays (ELISA) or ELISA-style sandwich immunoassays are the gold standard method.1 , 3 , 4 For ELISA and ELISA-style assays, antibody-antigen interactions are used to detect an analyte of interest with high sensitivity and specificity, typically by sandwiching a protein analyte between a capture antibody that is immobilized on a surface and a detection antibody—which carries a molecule that provides a detectable signal—that is introduced in a colloid mixture after sample incubation to create a high signal-to-noise ratio readout.
Although PCR and ELISA are the workhorses for clinical diagnostic laboratories, they have shortcomings that confine their use to centralized labs. Principally, operation of these assays requires robust infrastructure and highly trained users, prohibiting their applicability in low resource settings where disease outbreaks often occur. Further, these assays suffer from complex logistics associated with sample collection, transfer, testing, and readout, which delays time-to-results and forestalls clinical decision making and containment efforts.5 , 6 As a result of these limitations, much effort has been devoted to the development of POCTs to aid clinicians in the diagnosis and treatment of diseases.7 , 8 Many reviews have been written covering the importance of POCTs for infectious disease and other remote testing applications.9 , 10 However, as documented by Puig et al., technical challenges prevent the clinical translation of many POCTs.[ 11 ] This review builds on previous work to call attention to advancements in colloid and interface science that bring POCTs closer to clinical practice.
The World Health Organization (WHO) has outlined a set of criteria described by the ASSURED acronym—Affordable, Sensitive, Specific, User-friendly, Rapid, Robust, Equipment-free, and Deliverable— to serve as a reference for POCT development.[ 1 ] Other desirable attributes of POCTs include the ability to detect biomarkers of interest in low sample volumes, analyze multiple disease biomarkers in parallel (also known as multiplexing), provide a quantitative readout, and have a modular, plug-and-play design that enables reagents to be readily swapped to detect emerging targets quickly.
An example of a POCT that meets most of the ASSURED criteria is the lateral flow assay (LFA), which is the most widely used POCT. Best known for their at-home use for pregnancy testing, LFAs have proven be a powerful diagnostic and prognostic tool for other diseases as well. Detection of HIV proteins and host antibody responses by LFAs have greatly contributed to the diagnosis and monitoring of HIV infection.12 , 13 Additionally, the WHO evaluated over 200 unique LFAs for rapid diagnostic testing of malaria and identified several promising tests that have been useful to limit antimalarial drug overuse and improve disease monitoring.[ [14], [15], [16] ] Recently, their use for rapid diagnosis of COVID-19 has highlighted their utility but also made clear a major weakness —their lack of sensitivity early in infection—relative to RT-PCR tests.[ [17], [18], [19] ]
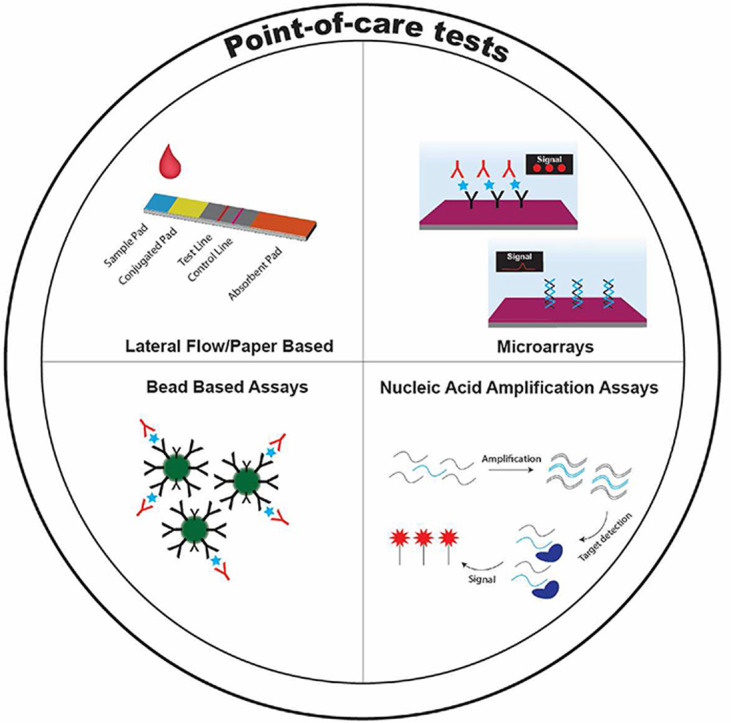
Despite their diagnostic utility for diseases like HIV, malaria, and COVID-19, POCTs still face several challenges that provide a strong rationale for the development of new assays that leverage advancements in colloid and interface science. First, disease biomarkers are commonly present in complex biological samples (i.e., blood, plasma, urine) at very low levels (picomolar concentration or lower) that can make accurate and early detection difficult by POCTs.[ [20], [21], [22], [23] ] Second, POCTs must differentiate between diseases that are caused by pathogens with similar molecular signatures and clinical symptomologies that can confound diagnosis.[ 24 ] To overcome these challenges, researchers have exploited innovations in colloid and interface science to develop various designs of POCTs for clinical applications. These assays include next-generation LFAs, paper-based assays, microarrays, and bead-based assays that match—and in some cases exceed—the analytical sensitivity and specificity of tests that are carried out in centralized lab facilities. Some of these POCTs also enable multiplexed detection—the simultaneous analysis of two or more biomarkers of interest from a single sample—to improve differentiation among diseases with similar molecular signatures.
In this review, we highlight some of the most promising bioassays where innovations in colloid and interface science enable the detection of biomarkers at the POC for infectious pathogens and other diseases. First, we discuss recent advances in LFAs and other paper based POCTs technology. Second, we discuss microfluidic DNA and protein microarrays for detection of biomarkers with quantitative readouts. Third, we describe amplification assays that rival traditional PCR-based tests but avoid the need for thermocyclers and that are hence easier to deploy as POCTs. Fourth, we discuss how bead-based, and other assay formats might be implemented as POCTs. Finally, we comment on the future direction of POCTs that includes simplified sample collection, data connectivity, and machine learning to enhance the capabilities of the next generation of POCTs.
2. Lateral flow and paper based POCTs
2.1. LFA POCTs
Lateral flow assays are one of the most widely deployed POCTs due to their simplicity, scalable manufacturing, and low cost.[ [25], [26], [27] ] LFAs typically use a hydrophilic nitrocellulose substrate to wick fluid samples along a test strip, capturing analytes of interest and reporting their presence visually by a colorimetric readout.28 , 29 LFAs fall under two categories: 1) qualitative and semi-quantitative or 2) quantitative. Qualitative and semi-quantitative LFAs produce readouts that can be observed with the naked eye to report binary (Yes/No) results or discrete (often on the magnitude of 10 ng mL-1 or larger) differences in biomarker concentration. Quantitative LFAs use a detector to report a continuous range of analyte concentrations and often have a lower limit of detection (LOD) than qualitative LFAs.[ [30], [31], [32] ] Importantly, for diagnosis of diseases like COVID-19, lower LODs are preferred because they enable earlier diagnosis.[ 33 ] In this section, we describe innovations that decrease the LODs of LFAs.
One way to improve the LOD of LFAs, is to modify samples before they are placed onto the hydrophilic paper interface. As an example of this approach, Zhang et al. developed an elaborate sample pre-enrichment protocol.[ 34 ] In this approach, they successfully reported a 10-fold improvement (2 milli-international units compared to 20 milli-international units) in the detection of human chorionic gonadotropin. However, this 10x improvement required a more user intensive workflow as follows: first, LFA test strips were assembled with the traditional conjugation pad missing––this is added later. Second, samples needed to be mixed with a silver nanoparticle-antibody conjugate colloid solution in a microcentrifuge tube, and then placed on the assembled strip. Critically, this vigorous pre-mixing of the sample containing human chorionic gonadotropin with nanoparticle-antibody conjugates increases the homogeneity of mixtures prior to LFA loading leading to improved sensitivity and more consistent results. Third, the nanoparticle-antibody-sample complex was added to the LFA strip and run through the cellulose substrate into the test and control zones. Fourth, the missing conjugation pad was placed onto the LFA in its normal position. Finally, a wash step was used to flow PBS over the newly placed conjugate pad, across the test and control zones, and into the absorption pad. Although this assay lowered the LOD by 10-fold compared to a conventional LFA, it is not clear that it can realistically be implemented clinically as a test, let alone a POCT, given the large number of user intervention steps required to carry out the assay.
A different sample preparation approach was described by Noh et al. in which magnetic rings coupled to commercially available 1 mL pipette tips were used to selectively separate influenza A antigens in PBS.[ 35 ] In an approach they call “antigen capture,” biotinylated antibodies specific for influenza A antigen, were complexed with streptavidin coated magnetic beads that could be isolated by the magnetic pipette tips during wash steps. Using this approach, the authors demonstrated a 100x improvement in LOD (102 EID50/reaction compared to 104 EIU50/reaction) for influenza A virus compared to a commercial LFA. However, this test workflow adds additional user steps and hence makes this test not deployable at the POC.
Rather than adding steps during the assay itself, Tsai et al. used a “stacking pad” LFA (sLFA) architecture, to extend the sample incubation time and improve the binding interaction of capture antibodies with antigen-silver nanoparticle-antibody complexes, which resulted in an assay with an LOD of 1 ng mL-1 for Protein A in PBS and 15.5 ng mL-1 for C-reactive protein in human serum and synovial fluid samples, surpassing conventional LFA designs.[ 36 ] Swapping out the traditional LFA test strip design of [sample pad-conjugate pad-test pad-absorbent pad] with a design that features an additional cellulose membrane, located just after the detection zone on the test pad, enabled detection of antigens at a lower concentration. Silver nanoparticle-antibody complexes implemented by the researchers, rather than the traditional gold nanoparticles, also demonstrated enhanced optical activity on the reconfigured sLFA, further demonstrating the importance of the detection colloid complex during POCT development. Overall, the addition of the stacking pad yielded a modest 2-fold improvement in the LOD for Protein A and C-reactive protein on the sLFA compared with a standard LFA that used gold nanoparticles as the reporter, but notably did so without necessitating any additional user steps.
Another way to leverage colloid sciences for POCTs involves exchanging conventional optical reporters for brighter, more efficient reporters that can improve the signal-to-noise ratios of LFAs and unlock the potential for LOD improvements without negatively impacting assay workflows or complicating test strip architectures. As an example, Li et al. developed a quantum dot LFA that beat ELISA in speed, ease-of-use, analytical sensitivity (80.9% for the quantum dot LFA and 70.2% for the ELISA), and specificity (100% for the quantum dot LFA and 98.5% for the ELISA) for detection of autoantibodies against M-type phospholipase A2 receptor in human serum samples by using quantum dots made of cadmium selenide and zinc sulfide.[ 37 ] More efficient labeling, the authors claim, is the reason the quantum dot LFA outperformed the enzymatic detection mechanism used in ELISA. Similarly, up-converting phosphor nanoparticles have been used to increase LFA capabilities as POCTs.[ [38∗], [39], [40], [41] ] Hooij et al. used up-converting phosphor nanoparticles —that convert low energy infrared excitation into higher energy emission— conjugated to the detection reagent for 4 biomarkers to develop an LFA for Mycobacterium leprae, a bacteria that is a clinical indicator of leprosy, and cytokines that are biomarkers of disease progression.[ 38 ] They reported LODs for IL-10, IP-10, CCL4, and anti-PGL-I antibodies comparable to an ELISA.
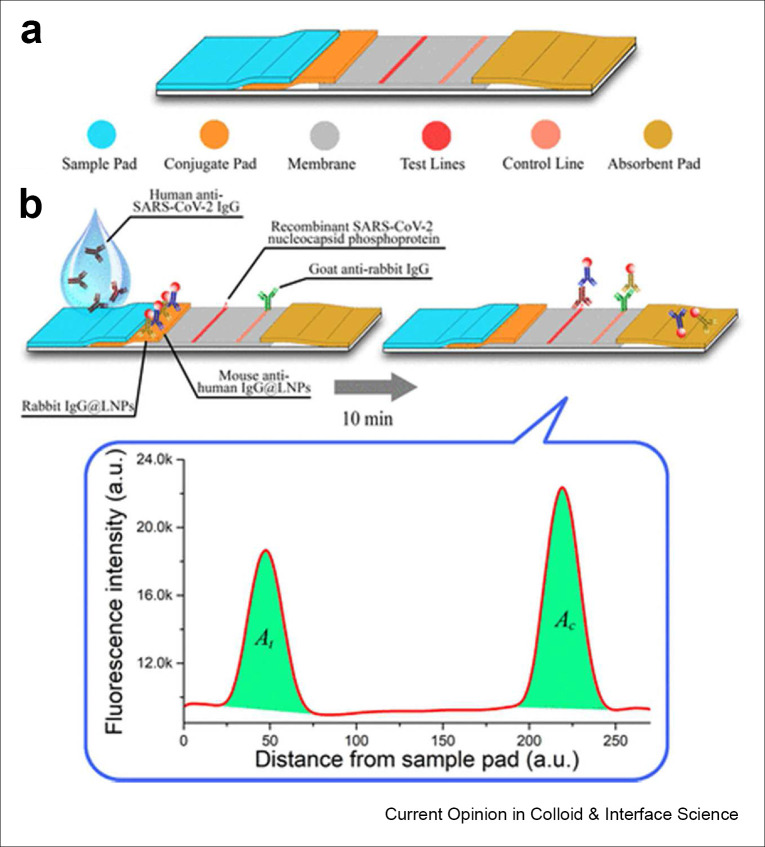
More recently, Chen et al. used lanthanide-doped polystyrene nanoparticles, a type of up-converting nanoparticle, to create a LFA to detect SARS-CoV-2 antibodies.[ 42 ] In this LFA, lanthanide-doped nanoparticles were conjugated to mouse anti-human IgG antibodies to quantify the host response to SARS-CoV-2 infection (Figure 1 ). In this design, lanthanide-doped nanoparticle conjugated antibodies bound to anti-SARS-CoV-2 IgG antibodies stuck to immobilized antigens. In a study of 7 COVID-19 patients and 51 healthy individuals, they used this LFA to classify individuals as healthy, weakly positive, or strongly positive.[ 43 ] Importantly, by swapping antiquated reporters for more efficient probes, the above detection schemes did not require significant modification to the traditional LFA strip architecture. This enabled the retention of the simplicity of the LFA workflow while significantly boosting performance that can be directly translated to the POC (see Fig. 2).
Figure 1.
A) Design of a LFA to detect anti-SARS-CoV-2 antibodies. B) Simulated assay using lanthanide doped up-converting phosphor nanoparticles as the fluorescent reporter. Reproduced with permission from ACS:.https://pubs.acs.org/doi/10.1021/acs.analchem.0c00784. Please note that further permissions related to the use of this material should be directed to the ACS. Copyright (2020), (ACS Analytical Chemistry).
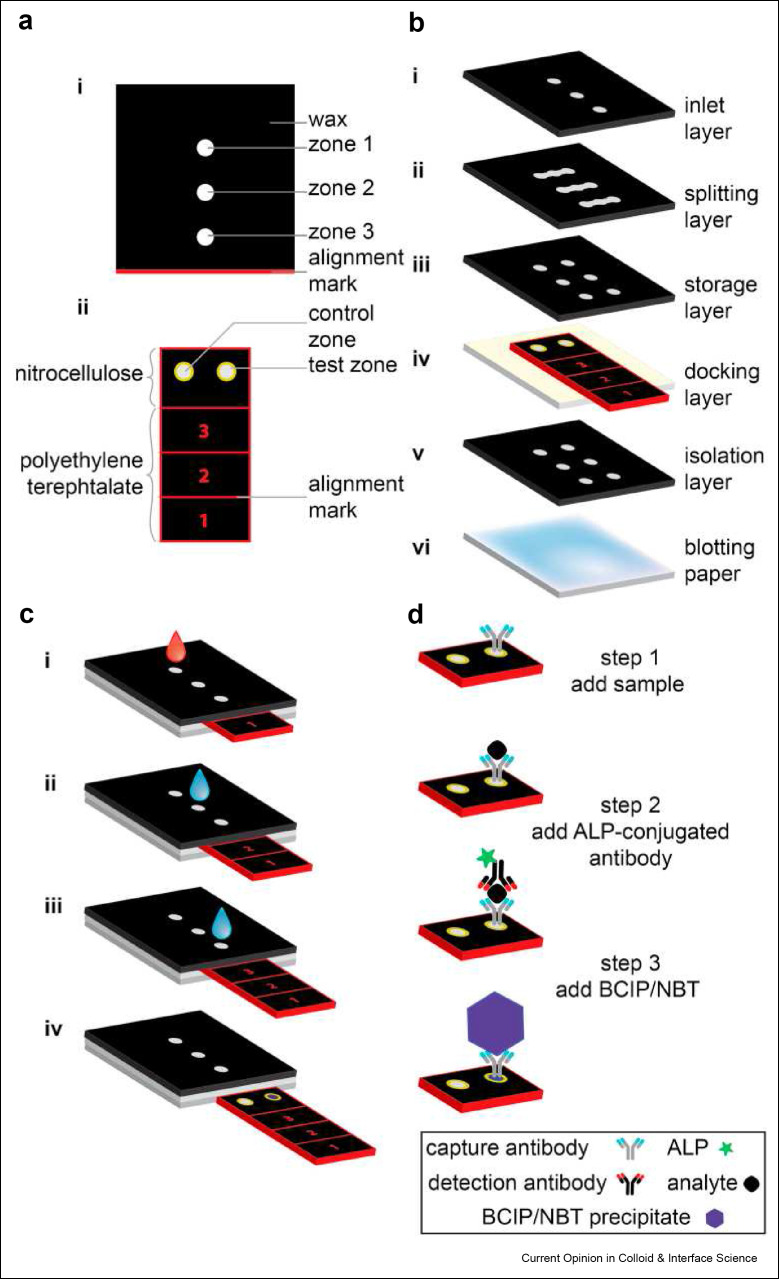
Figure 2.
Design schematic of the sliding strip microfluidic device by Verma et al. A) Top view of the device. B) Exploded view of each component of the sliding strip microfluidic. C) Stepwise operation of the sliding strip device. Step 1, addition of sample and water wash. Step 2, water addition to dissolve stored detection antibodies and buffer. Step 3, addition of more water to dissolve substrates and a buffer needed to complete the reaction and generate a colorimetric signal. Step 4, removal of sliding strip for results readout. Results can be captured visually or by using a desktop scanner. D) Secondary outline of steps with an emphasis on the molecular interactions happening at each stage of the sliding strips checkpoints. Reused with permission under creative copyright license CC-BY 4.0 2018 (Elsevier).
LFA provides advantages in ease-of-use and the ability to be used with or without a companion detector in many cases that makes it overwhelmingly likely that LFA will continue to be a part of point-of-care testing moving forward. This is especially true whenever the results clinicians and people care about is a binary result indicating the presence or absence of a biomarker of interest with less emphasis on the absolute quantity of that marker. More commonly now however, likely due to the increased use of LFAs for COVID-19 antigen and serologic testing, limitations of LFA technologies have been brought into the forefront that support investigation towards other POCT strategies. One notable limitation of the LFA include the subjectiveness of the tests in which error can increase based on the subjectivity of results depending on who is reading the test. This limitation is often overcome using a companion detector that also increases the ability of partnered POCTs to discriminate smaller changes in biomarker amount leading to more robust quantitative performance, a topic that will become a focal point of our discussion of POCTs moving forward in this review.
2.2. Non-LFA paper based POCTs
The hydrophilic nature of paper easily wicks biofluid samples like blood, serum, plasma, and urine along flow paths that enable disease detection and monitoring. As a result, paper remains an excellent choice as a substrate material for POCTs. In addition to the LFA, paper has been extensively explored for the fabrication of other types of POCTs.[ [44∗], [45], [46∗∗], [47] ] Paper based microfluidics have successfully been used to detect conventional biomarkers like glucose[ 48 ] and proteins using novel colorimetric and fluorescent readouts.[ 44 ] Whitesides and coworkers invented a broad range of paper based microfluidic devices, including a sliding-strip microfluidic device that enables ELISA-style assays to be run at the point-of-care.[ 49 ] This device is sectioned into two discrete regions; the first contains the sensing area and the second region stores the reagents for the assay. By moving the sliding strip at scheduled times, this paper microfluidic assay greatly reduces user intervention compared to a conventional ELISA. The sliding-strip microfluidic assay was used to detect C-reactive protein with a LOD of 1 ng mL-1 in diluted blood. Beyond the sliding-strip device, the Whitesides group also designed a “paper machine” POCT that allowed detection of the Escherichia coli malB gene by a loop mediated isothermal amplification (LAMP) reaction––a technique described in detail later in the Nucleic Acid Amplification section of this review––using a portable smartphone detector and a hand-held UV source.[ 50 ] Using the “paper machine”, they could detect E. coli spiked into human plasma samples with a LOD of 5 cells per sample in 1 h.
Patterned paper was also used by Mu et al. to combine LFA- and ELISA-like technologies for the serological monitoring of IgG antibody responses to hepatitis C infections.[ 51 ] This “multiplexed” system responds directly to CDC recommendations for hepatitis C diagnosis by coupling anti-hepatitis C IgG antibody detection using immobilized hepatitis C antigens with a recombinant immunoblot to confirm anti-IgG antibodies and greatly reduce false positives. Using only a 6 μL volume (0.3 μL of human serum + 5.7 μL of buffer), the patterned paper POCT has a LOD of 267 aM for chemiluminescence readout and 26.7 fM for colorimetric readout. Additionally, this test takes only ∼30 min in total whereas a comparable ELISA take ∼1 hour and the recombinant immunoblot assay takes at least 6 h to complete.
3. Hard substrate-based microarrays
3.1. Hard substrate-based microarray POCTs
In hard substrate-based microarrays, similar to LFAs, biomarkers of interest are generally captured by “receptors” that are immobilized on a substrate in discrete spatial addresses —typically spots— and a signal is generated by binding of secondary “detection” probes in the spots. Unlike LFAs and paper based POCTs, microarrays typically use hard substrates such as glass or semiconductors.52 , 53 Additionally, because of the diversity of substrate material for hard substrate-based microarrays, “label-free” sensing is also possible, where the capture of a target analyte directly generates a readout.54 , 55 Microarrays to detect protein and DNA targets have been around for several decades; however, these assays were limited to centralized testing facilities due to the many user steps that required expert technicians. Advances in microfluidic technologies, specifically passive microfluidic technologies, have enabled the integration of microarray techniques into POCTs. This section describes microarray technologies that have been successfully adopted into sample-in-results-out platforms that provide important disease information at the POC.
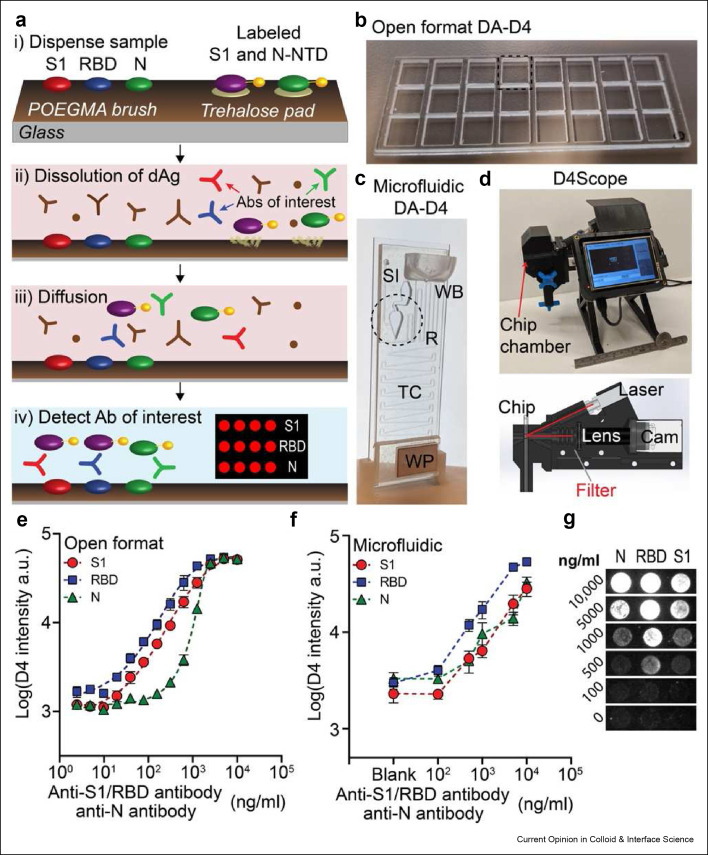
For more than a decade, Chilkoti and coworkers have developed an ELISA-like assay that achieves results that match that of clinical lab tests in a “self-contained” platform called the D4.56 , 57 These results are enabled by using a PEG-like bottlebrush of poly(oligo(ethylene glycol) methyl ether methacrylate (POEGMA) “grafted from” glass substrates via surface-initiated atom transfer radical polymerization (SI-ATRP).[ [58], [59], [60∗∗], [61∗∗] ] Importantly, POEGMA is a non-fouling surface that eliminates virtually all non-specific protein adsorption to avoid the blocking and manual wash steps that traditional lab tests rely on.[56∗], [57], [58] , 62 These steps are traditionally necessary because the samples containing the analyte of interest, commonly blood —potentially the richest source of analytes for many diseases— also contain many proteins that can lead to non-specific binding that reduces the signal to noise of the assay and compromise its performance.62 , 63 Additionally, POEGMA stabilizes reagents “on-chip” eliminating the need for cold chains and further expanding the D4’s potential at the point-of-care. In this platform, reagents like proteins and antibodies, can be functionalized using inkjet printing.56 , 57 Most commonly, two different types of antibodies —stable capture antibodies and soluble detection antibodies— are printed onto the POEGMA surface to create a fully self-contained POCT that runs to completion in 4 steps: 1) a sample is Dispensed on the assay, 2) detection antibodies Dissolve from the POEGMA brush, 3) detection antibodies and the analyte of interest Diffuse to the capture antibodies, and 4) the analyte of interested is Detected by a fluorescence signal that scales with the concentration of analyte in the sample (Figure 3 ).[ 57 ] The D4 POCT has a LOD in the femtomolar range for many analytes, including IL-6 and tumor necrosis factor alpha.57 , 64
Figure 3.
A) Design of the D4 assay for anti-SARS-CoV-2 antibodies. B) POCT format at the open format D4 assay. C) Image of the POCT microfluidic D4 cassette. D) Image of the D4Scope. E) Quantitative performance of the open format D4 assay for anti-SARS-CoV-2 antibodies. F) Quantitative performance of the microfluidic D4 assay for anti-SARS-CoV-2 antibodies. G) Spot image representation for assay intensity at each dose point. Reused with permission under creative copyright license CC-BY-NC 4.0 2021 (AAAS).
Recently, the D4 has been integrated into a microfluidic cassette enabling its use as a true POCT.[ 65 ] The microfluidic version of the D4-POCT is a gravity-assisted microfluidic cassette that delivers printed reagents into a “reaction chamber” where a fluorescence signal can be recorded using a handheld detector. Heggestad et al reported the detection of anti-SARS-CoV-2 antibodies in human serum against three SARS-CoV-2 viral antigens– spike protein (S1), receptor-binding domain (RBD), and nucleocapsid (N) protein– in the “open-format” D4 assay and the microfluidic D4 POCT.[65] In a clinical test of the assay with 46 COVID-19–positive plasma samples and 41 negative samples, there was a statistically significant difference between the mean intensity for COVID-19-positive and COVID-19–-negative samples (P < 0.0001) for all three markers. Importantly, this study demonstrates the utility of microfluidics as an enabling technology that helps minimize user intervention and automate the assay. In the case of the D4 POCT, this is true even for undiluted whole blood samples which unlocks the potential to automate and streamline many of the most common diagnostic assays run in clinical laboratories.
Laksanasopin et al. also created an ELISA-like POCT using a microfluidic dongle for detection HIV and syphilis as an accessory to an iPod.[ 66 ] Impressively, the dongle was able to perform all three assays in parallel on a single chip in 15 minute. The iPod delivers power to the dongle that houses detection zones with capture proteins for each antibody target. While designed for the iPod, a technology that is now mostly out-of-date, Laksanasopin et al.’s design can be adopted broadly to other smartphones and portable consumer electronic devices. To run the assay, 1 μL of whole blood is mixed with 9 μL of diluent, and a 2 μL volume of the diluted blood added to the chip. The assay begins with the push of a button on the smartphone-paired app that pulls a vacuum. Gold labeled antibodies and wash buffer diffuse through microchannels in the cassette. After completion of these steps, which takes ∼5 min, the user then slides a toggle that activates a silver autocatalysis reaction that enhances the signal of the gold particle conjugated antibodies. The optical density is then read on screen via the app. HIV antibodies were detected with a sensitivity of 100% and a specificity of 87%, treponemal antibodies were detected with a sensitivity of 92% and a specificity of 92%, and anti-cardiolipin antibodies were detected with a sensitivity of 100% and a specificity of 79% from fingerpick blood. Venipuncture blood yielded 100% specificity and 91% sensitivity for HIV, 77% specificity and 89% sensitivity for treponemal syphilis, and 80% specificity and 82% sensitivity for nontreponemal syphilis.[ 66 ] Notably, the fully passive microfluidic D4 and the active microfluidics of the ELISA-like dongle exemplify two major categories of microfluidics; each with distinct advantages. Passive microfluidics enable the running of many tests in parallel which may be critical for global health diagnostics, especially in low resource settings. Active microfluidics, on the other hand, are limited because they need a connected power source to run; but they may enable the detection of biomarkers using complex assays formats as we will discuss later in the nucleic acid amplification section of this review.
3.2. Promising hard substrate-based microarray technologies for future POCT development
Other promising microarray technologies exist that have not yet been integrated into POCTs but are worth mentioning because they may enable the development of new POCT platforms. Microarrays with electrochemical reporters, for example, are of interest as they can provide results in real-time with detectors that can be easily miniaturized.67 , 68 Lee et al. created a multiplexed giant magnetoresistive electrochemical sensor for the monitoring of systemic lupus erythematosus, an autoimmune disease characterized by a high titer of autoantibodies.[ 69 ] To fabricate the giant magnetoresistive sensor, capture antigens were printed onto a semiconductor surface using inkjet printing. When the sample is applied, autoantibodies in solution bind to the printed antigen. Detection antibodies conjugated to magnetic nanoparticles (MNP) then bind the target antibodies creating a change in the electromagnetic field surrounding the chip. While the authors do not provide a direct report of this sensor’s LOD, the change in proximity of the bound MNP to the sensor chips yielded a sensitivity comparable to ELISA (R2 >0.99), with results available in real-time. As reported, the giant magnetoresistive sensor is sensitive but requires a blocking step and several wash steps that limit its use as a POCT. One potential solution to translate this technology into a POCT would be to explore incorporating a non-fouling layer on the semiconductor surface to potentially eliminate the blocking and wash steps.
Graphene as a substrate material may be ideal for electrochemical biosensor applications due to its electrical conductivity.[ 70 ] Hwang et al. modified graphene by bending it into a “bent and crumpled state” to create a nucleic acid field effect transistor for DNA detection (Figure 3).[ 71 ] The authors state that orienting the graphene into this crumpled state generates exponential signal changes, even at low DNA and RNA concentrations, that leads to improved sensitives (down to 600zM) compared to other sensors fabricated with planar graphene. Specifically, this graphene field effect transistor (FET) was fabricated by conjugated DNA probe strands to a pyrenebutanoic acid succinimidyl ester intermediate that was bound to the graphene surface through π-π stacking. Impressively, this sensor demonstrated a zeptomolar LOD (10-21 M) in buffer and attomolar (10-18 M) LOD in human serum for the cancer-related biomarker miRNA let-7b.
Likewise, Hajian et al. used graphene combined with clustered regulatory interspaced short palindromic repeats (CRISPR) and Cas9 protein to detect DNA in the femtomolar range using a FET without amplification in a platform they call the CRISPR-Chip.[ 72 ] Sensing DNA without the need for amplification greatly reduces the complexity of the overall system extending this platform to point-of-care applications. In the CRISPR-Chip, a CRISPR-Cas9 complex is covalently conjugated to a graphene substrate. This CRISPR-Cas9 complex contains guide RNA that creates gene-targeting specificity so that when samples are added on the surface of the chip, the CRISPR-Cas9 complex searches double stranded DNA for DNA sequences of interest. If DNA sequences of interest are found, they bind and generate an electrical readout. The authors used the CRISPR-Chip to detect two Duchenne muscular dystrophy-associated DNA mutations in clinical samples. In these tests, the LOD of the CRISP-Chip was determined to be 1.7 fM for unamplified sample volumes that can be obtained with a buccal swap (the current collection standard for Duchenne muscular dystrophy diagnosis). The authors highlight that these results are directly relevant to other important genetic mutation diseases such as Huntington’s and hemophilia B.
4. Nucleic acid amplification assays
Nucleic acids are of great importance for the diagnosis of infectious disease because pathogens can be readily identified by their unique DNA or RNA sequences. Though microarrays have been used to detect nucleic acids, their LOD is typically worse than PCR-based detection methods because they lack the amplification that is built into all PCR-based methods.2 , [73], [74], [75∗∗] PCR-based detection methods, are however, difficult to implement in a POCT because of the need for thermocycling instrumentation. Due to this limitation, an enormous amount of effort has been poured into miniaturizing thermocycler technology and to incorporate isothermal amplification methods so thermocycling is no longer needed. Loop mediated isothermal amplification (LAMP), and recombinase polymerase amplification (RPA) are methods for detection of nucleic acids that have shown success as point-of-care amplification methods.[ [76], [77], [78], [79], [80], [81], [82] ] LAMP uses a set of 4-8 sequence specific primers to isothermally drive cycling reactions with high specificity, which eliminates much of the bulky equipment used for thermocycling in PCR.79 , 83 RPA uses protein mediated reactions, rather than heat, to drive isothermal DNA or RNA amplification, with minimal sample preparation.[ 84 ] This section describes ways researchers are amplifying nucleic acid sequences at the POC.
4.1. Loop mediated isothermal amplification assays
LAMP has been shown, in some cases, to outperform conventional PCR as demonstrated by Soto et al. in a LAMP assay for Schistosoma mansoni.[ 85 ] They used this LAMP assay to evaluate stool samples in a completely noninvasive protocol. They also extended their LAMP assay closer to the point-of-care by desiccating reagents in a single tube.[ 86 ] Significantly, the results could be read by portable detectors that are less bulky than the thermocyclers used for PCR, but they still require electrical power, which can limit their applications in low resource settings. A solution to the need for uninterrupted AC power was reported by Snodgrass et al. who created a portable nucleic acid quantification device that can be powered by electricity when available, or by sunlight or fire in low resource settings if AC power is unavailable.[ 87 ] Their device, the Tiny Isothermal Nucleic acid quantification sYstem (TINY), is fabricated using PureTemp 68 phase-change material that stores heat and transforms the stored energy into power when needed. TINY was used to detect Kaposi’s sarcoma-associated herpesvirus, using the three different heating methods.[87] This study showed that TINY provides reliable results using all three power sources and promises to turn LAMP into a POCT that can be deployed in low resource settings. Alternatively, in 2016, Ball et al. reported the use of a technique called quenching of unincorporated amplification signal reporters (QUASR),[88] that was later implemented with a smartphone detection system by Priye et al. in a reverse transcriptase-LAMP (RT-LAMP) assay with a LOD of <50 RNA molecules per reaction for the detection RNA biomarkers of West Nile Virus and Chikungunya virus in whole blood.[ 89 ]
For COVID-19, Panpradist et al. used reverse transcription RT-LAMP to convert single stranded RNA into DNA that could be detected in a tube-based POCT called Harmony COVID-19.[ 90 ] This platform uses lyophilized reagents in a sequence of microtubes to enable the detection of 15-20 RNA copies per reaction in purified, synthetic SARS-CoV-2 samples. Further, the authors incorporated an “internal amplification control” to reduce the number of false negatives reported. Importantly, the authors note that when analyzing simulated nasal matrix samples, the interference in their assay increases but clinical tests using the Harmony COVID-19 system demonstrated 96% accuracy, 87% sensitivity, and 100% specificity compared to RT-PCR when using RNA samples extracted from viral transport media. The authors then went further analyze the performance of their device in viral transport media samples without RNA extraction in which the Harmony COVID-19 system detected 95% of SARS-CoV-2 samples with at least one RNA copy per microliter.
Also developed for COVID-19, the LuciraTM Check It COVID-19 Test (Lucira) is a commercially available LAMP-based nucleic acid amplification POCT.[ 105 ] The Lucira uses LAMP To detect the presence of COVID-19 RNA sequences in nasopharyngeal samples in less than 30 mins using a battery powered handheld device.[ 105 ] The Lucira has been designed for at-home use, and thus includes everything user needs to complete the test, including two AA batteries. Critically, the Lucira has demonstrated good performance, with one study reporting that it was able to accurately identify all COVID-19 negative patients (n=100) while capturing 82 out of 90 possible (91.1%) positive cases. The combination of accuracy, portability, and ease-of-use make the Lucira a very attractive option for remote COVID-19 detection. Despite its advantages, one notable limitation of the Lucira is its’ reliance on the companion device to run the tests. This limits the ability of the Lucira to test multiple samples in parallel (without adding multiple detectors) and thus may decrease its applicability for community-based testing.
4.2. Recombinase polymerase amplification assays
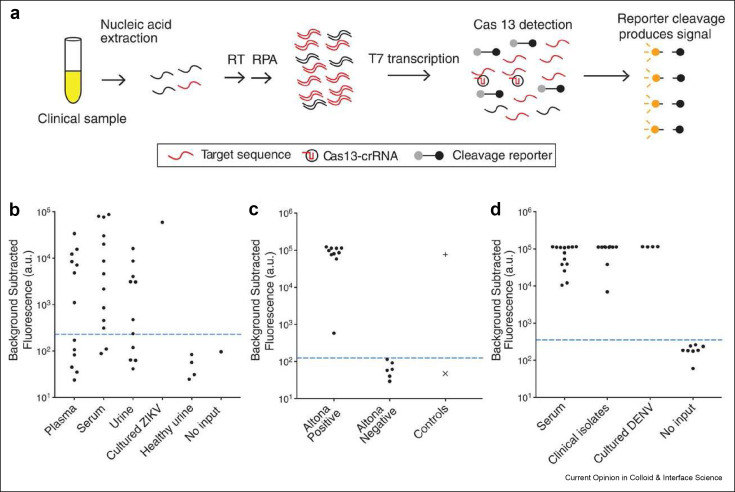
Using RPA, one system that has become increasingly noteworthy is the Specific High Sensitivity Enzymatic Reporter UnLOCKing (SHERLOCK) system introduced by Gootenberg et al.91 , 92 Members of the same group later combined SHERLOCK with a Heating Unextracted Diagnostic Samples to Obliterate Nucleases (HUDSON) protocol to quickly isolate nucleic acid targets from body fluid.92 , 93 Figure 5 A shows the workflow HUDSON-SHERLOCK in 3 general steps. Step 1: either 1) target DNA sample sequences are amplified by standard RPA reactions or 2) RNA is amplified and reverse transcribed to DNA by reverse transcriptase-RPA reactions. Step 2: T7 polymerase converts the amplified DNA products of Step 1 into RNA that can be identified by CRISPR/Cas13 Step 3: Collateral cleavage, induced by CRISPR RNA guided Cas13 protein binding, liberates fluorescent reporters from quenchers to generate a fluorescence signal that can be detected with either conventional qPCR detectors or on LFA-style strip. Using the commercially available Altona Zika Virus RT-PCR kit as a benchmark, the SHERLOCK assay demonstrated 100% sensitivity (no false negatives) and 100% specificity (no false positives) in identifying Zika Virus in human serum and saliva.[ 93 ] Specifically, LODs of 90 aM (45 cp.μl-1) in whole blood or serum, 0.9 aM (∼1 cp.μl-1) in saliva, and 20 aM (10 cp μl-1) in urine were reported The same group also used HUDSON-SHERLOCK to analyze urine and saliva to discriminate Zika virus infection from Dengue virus, West Nile virus and yellow fever virus with 100% specificity.
Figure 5.
Schematic of SHERLOCK. A) RNA is extracted from clinical samples and amplified by either reverse transcriptase RPA or standard RPA before being placed into a reaction mixture that contains T7 RNA polymerase, Cas13, target specific crRNA, and a fluorescent RNA reporter, which produces a fluorescence signal. B) Fluorescence read-out from SHERLOCK of patient samples acquired during the 2015-2016 Zika pandemic. C) Comparison of SHERLOCK (left and middle columns) with Altona RT-PCR results (right column). D) Fluorescence output from SHERLOCK for DENV positive clinical samples and controls. Reused with permission under creative copyright license CC-BY 4.0 2018 (AAAS).
Another impactful implementation of RPA was demonstrated by Chen et al. called the DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR).[ 94 ] Rather than using Cas13, as in SHERLOCK, DETECTR uses Cas12a to collaterally cleave reporter molecules when crRNA mediated binding occurs with the target DNA. Similar to SHERLOCK, when a Cas12a-crRNA complex binds a target DNA, a fluorescence signal is generated that can be quantified in a point-of-care setting. Rapid and accurate detection of human papilloma virus in human anal swab samples were reported with attomolar sensitivities and 100% specificity and 92% specificity for HPV16 and HPV18d respectively.[ 94 ]
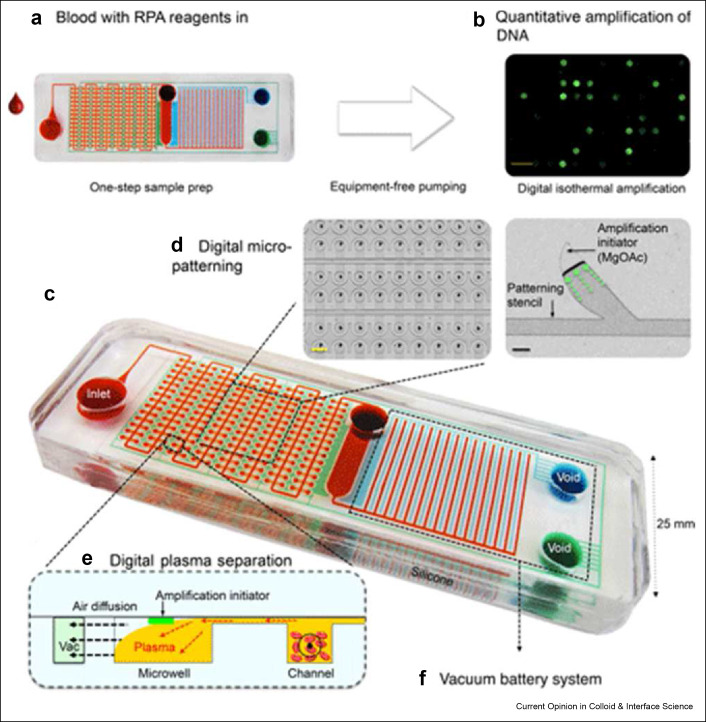
Yeh et al. created a self-powered and integrated microfluidic point-of-care low-cost enabling (SIMPLE) microfluidic based on RPA for detection of methicillin-resistant Staphylococcus aureus.[ 95 ] This device uses a vacuum battery to power a sample separation system that delivers plasma to a magnesium acetate RPA initiator where amplification occurs (Figure 7 ). The flow of blood samples over a microcliff removes red blood cells without hemolysis to improved sensitivity levels. SIMPLE can quantify nucleic acid sequences from human whole blood in ∼30 min with significant improvement over a traditional PCR test in terms of speed, cost, and user-friendliness. More broadly, the modular nature of the detection platform suggests it can be used to easily diagnose infectious diseases pathogens.
Figure 7.
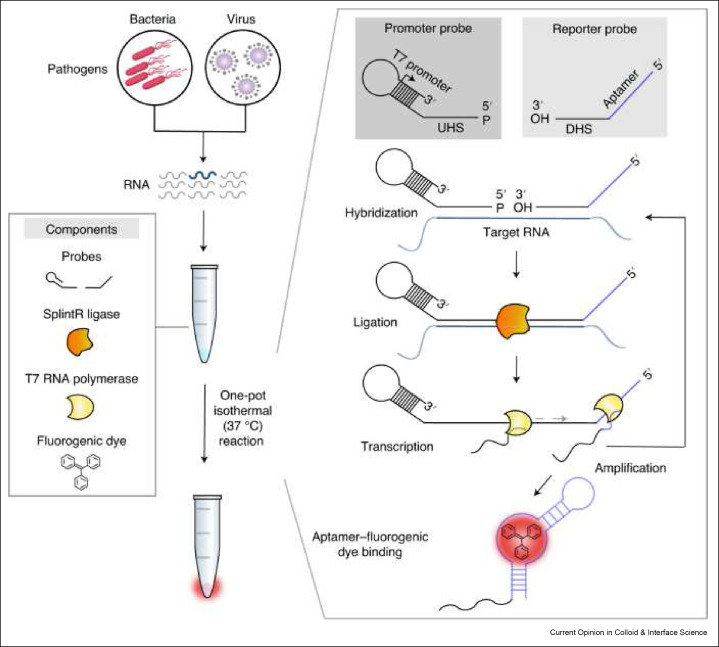
Schematic of SENSR, a one-pot isothermal reaction cascade for the rapid detection of RNA. The reaction is composed of four main components: a set of probes, SplintR ligase, T7 RNA polymerase and a fluorogenic dye. In the presence of target RNA, hybridization, ligation, transcription and aptamer–dye binding reactions occur sequentially in a single reaction tube at a constant temperature and yields a fluorescence signal. Reused with permission under creative copyright license CC-BY 4.0 2020 (Springer Nature).
4.3. Other nucleic acid assay types
In addition to LAMP and RPA based nucleic acid amplification, ligation dependent isothermal amplification has also been used for disease diagnosis.[ 96 ] In perhaps the most notable example of this method, Woo et al. demonstrated the detection of SARS-CoV-2, influenza A, and MRSA by a method they have named the splint-based one-pot isothermal RNA detection (SENSR).[ 97 ] SENSR uses two cleverly designed DNA probes to improve sensitivity for the RNA target and generate a fluorescence read-out (Figure 6 ). The promoter probe consists of an upstream hybridization sequence that is complementary to the target RNA and a stem-loop region that is a T7 promoter. The reporter probe consists of a downstream hybridization sequence that is complementary to a different region of the target RNA and a template for a dye-binding aptamer. The two promoters, SplintR ligase, and T7 RNA polymerase are added to sample RNA. In the presence of the target RNA, the SplintR ligase ligates the two DNA probes together to act as the DNA template for the T7 RNA polymerase, which synthesizes the RNA aptamer capable of binding a fluorogenic dye whose fluorescence is greatly enhanced upon binding by the aptamer. SENSR is a user-friendly POCT because all reagents required for this assay are combined into one sample tube.
Figure 6.
SIMPLE microfluidic device. A) User places whole blood samples onto the chip and sample processing proceeds automatically. B) DNA is amplified via a reusable heat pack at a constant temperature. C) Dye loaded chip for visual presentation. Red dye illustrates the microfluidic channels, blue dye illustrates the primary vacuum and battery system, green dye shows the auxiliary vacuum. D) Digital micropatterning for red blood cell separation (left) and an amplification initiator stencil for RPA (right) scale bars are 1 mm (yellow) and 100 μm (black). E) Profile view of whole blood separation within the microfluidic region. F) On-board vacuum to provide a portable power supply. Reused with permission under creative copyright license CC-BY 4.0 2017 (AAAS).
Another way nucleic acid amplification assays have been successfully commercialized is by using a technology called digital microfluidics (DMF). DMF uses electrical current to control fluid droplets in a process termed electrowetting-based actuation first introduced by Washizu et al. in 1998[ 98 ] and Pollack and Fair in 2002[ 99 ] to drive sequential, PCR-like reactions[ 100 ] and has gone on to commercial success.[ 101 ] The most prominent example of DMF is the commercialization of the technology by Baebies© to diagnose and monitor newborn illnesses and recently to test for COVID-19 infection. Baebies© SEEKER and FINDER devices automatically analyze samples rapidly at the clinical bedside using low sample volumes.102 , 103 Here, fluid samples are sandwiched between two plates where the top plate contains the fluid and the bottom plate contains addressable electrodes that drive PCR reactions that can be used for a diverse set of disease biomarkers.[ 104 ] The overall advantages of DMF have been well covered in other reviews but one important opportunity moving forward is the promise of tailored, high-throughput whole blood testing.[ 103 ] In this way, DMF can be used to tackle the specific needs of newborn genetic screening (specifically low samples volume requirements) while providing a platform that may enable broad blood-based genetic testing that can be run rapidly and in a more cost effective manner than traditional PCR. It is important to note that while DMF is extremely valuable, its reliance on electrical power may limit its utility in global health diagnostic applications when power is not available.
5. Bead-based assays
Bead-based, or suspension, arrays can improve the LOD compared to conventional planar ELISA-type assays due to the increased surface area-to-volume ratios offered by beads compared to planar surfaces.[ 106 ] This is because the higher surface area to volume of beads provide a greater number of capture molecules per unit volume and consequently a higher signal.[ 106 ] Because no reagent is tethered to a surface however, bead based assays are difficult to implement as POCTs. This section describes notable bead-based technologies in the literature that are approaching point-of-care use and other technologies that may eventually be incorporated into POCTs.
The most well-known bead-based assay is Luminex’s xMAP system that allows expansive multiplexed detection of analytes. In the xMAP system, multiplex detection of n analytes is accomplished with n sets of capture microbeads, where each type of capture microbead has a unique fluorescence signature. A unique capture reagent specific for an analyte is covalently conjugated to each type of bead. The functionalized capture beads are then mixed and incubated with a sample that can contain one or more of the analytes of interest. After washing the beads to remove loosely adsorbed analytes, the suspension is then incubated with a mixture of n beads, where each type of bead is conjugated with a detection reagent against one of the analytes, and all detection antibodies are labeled with the same fluorophore. To read the assay, the fluorescence signals are measured from each bead—one from the capture microbead to identify the biomarker it targets, and another for the detection antibody to quantify the amount of the biomarker bound to the bead. xMAP has been used for nucleic acid assays and immunoassays to diagnose diseases like human papillomavirus,107 , 108 colorectal cancer,[ 109 ] Alzheimer’s disease,[ 110 ] and Salmonella, Cryptosporidium infection.[ 111 ] The xMAP system has also been used to quantify the host antibody response to infection by Sendai virus, rabbit hemorrhagic disease virus, rabbit rotavirus,[ 112 ] Newcastle disease virus, avian influenza virus,[ 113 ] bronchitis virus,[ 114 ] and adenovirus.[ 115 ]
Another bead-based platform—the Single Molecule Array (SiMoA) assay—has been commercialized by Quanterix.[ 116 ] SiMoA is an enzyme-linked immunoassay capable of detecting analytes with single molecule resolution that might ultimately prove useful at the point-of-care. In SiMoA, analytes are first captured by antibodies immobilized on magnetic beads before being bound by a secondary antibody conjugated to an enzyme. The beads diffuse into femtoliter volume wells based on Poisson statistics, such that each well accommodates only one bead (Figure 4 ).[ 117 ] The fluorescence signal from each well is then measured. LODs as low as fg.mL-1 have been reported for SiMoA.[ 116 ] Critically, the SiMoA technology enables the quantification of single molecules because when protein concentrations are low, each microbead complex binds to only one molecule of the analyte and isolates it within a single well.117 , 118 SiMOA assays have been developed to detect TNF-α and prostate specific antigen (PSA) in human serum with an LOD of 14 fg mL-1 [ 116 ] and detection of cytokines IL-6, IL-8, IL-18, and VEGF as a rapid triage test for pulmonary tuberculosis.[ 119 ]
Figure 4.
A) Comparison of graphene FET in planar (left) and bent and crumpled (right) orientations. B) Fabrication process for graphene shrinking and probe DNA immobilization (black) and target DNA (red) hybridization. C) SEM images of graphene in the bent and crumpled state, left scale bar is 5 μM, right scale bar is 500 nm. D) Raman spectrum of graphene on the polystyrene substrate. E) Charge transfer of the crumpled graphene highlighting the shift in the electron transfer at the Dirac point. F) Dirac point shift as a function of pH. Reused with permission under creative copyright license CC-BY 4.0 2020 (Springer Nature).
A requirement of the SiMoA assay and other bead-based assays that limit their use as POCTs is that the detectors required to image and quantify the fluorescence signal in these assays are bulky and rely on a stationary power supply. To address this limitation, Yelleswarapu et al. invented the microdroplet Megascale Detector (μMD).[ 120 ] In μMD, microbeads conjugated to specific capture antibodies for each biomarker are directed along an optofluidic path at a controlled rate. Like the xMAP system, different colored beads are assigned to different biomarkers to enable multiplexed detection. Unlike the xMAP however, the μMD system controls the flow of sample droplets and registers the detection of the fluorescence signals in parallel rather than sequentially, thus enabling the platform to be miniaturized. As a proof-of-concept demonstration of μMD, Yelleswarapu et al. measured the levels of IL-6 and granulocyte-macrophage colony-stimulating factor in human serum with a LOD that matches the benchtop device used for SiMoA. Still, even with the possible addition of a portable detector for the SiMOA platform, further work is needed to simplify its workflow before it can be realized as a fully POCT.
Although less common, bead-based assays have also been used for electrochemical detection. Cortina et al. monitored the humoral immune response to protozoa, bacteria, and virus pathogens by capturing antibodies specific to these pathogens in human serum using an antigen functionalized magnetic bead based system.[ 121 ] This electrochemical, magnetic microbead-based biosensor uses magnetic beads conjugated with antigens for each disease that can selectively capture circulating antibodies in human serum. Samples are incubated with antigen-functionalized magnetic beads that capture antibodies specific for each protein. Then, antigen-coated microbeads are isolated from the bulk suspension by magnetic capture and washed. Next, a solution with antibodies conjugated to horseradish peroxidase is added to the isolated antigen-functionalized magnetic beads. Horseradish peroxidase labelled detection antibodies bind to the antibody-antigen-magnetic bead complex and, following a wash step, conversion of applied hydrogen peroxide to hydroquinone is recorded by portable potentiostat to generate a readout.
6. Future directions
We have discussed in this review some of the most impactful technologies enabling point-of-care diagnostics with an emphasis on platforms that may be used for the detection of infectious diseases. As this review showcases, new discoveries in the basic sciences lead to new technologies that —increasingly rapidly— find their way into new assays; the relatively quick adoption of CRISPR/Cas DNA and RNA editing tools for clinical diagnostics are one such example. Similarly, advances in physics and instrumentation for single molecule detection have led to single molecule stochastic sensing, as exemplified by the SiMoA platform.
6.1. The REASSURED acronym for POCTs
Land et al. have suggested an amended acronym for the future development of POCT, REASSURED, which includes the original WHO ASSURED criteria but extends the functionality of POCT to include Real-time connectivity and the Ease of specimen collection.[ 122 ] Real-time connectivity involves the detection of quantified signals, integrating new computational tools, and transmitting data remotely to help facilitate health care advances in all resource settings.[ 123 ] One notable example of a POCT, featuring real-time connectivity, is a 5G-enabled fluorescent sensor for COVID-19 prognosis by Guo et al.[ 124 ] This assay builds upon developments in internet of things research to approach a type of internet of medical things, or IoMT. This sensor passes data to 5G cloud servers for analysis and results reporting. Critically, the integration of 5G servers acts to reduce data transmission latency to near real-time and provides a platform for which big data analysis can take place. Another example of real-time results reporting from POCTs, also by Guo et al., leverages the broadcast television network infrastructure already in place to transmit cardiovascular health data from a cholesterol and triglyceride test strip POCT.[ 125 ]
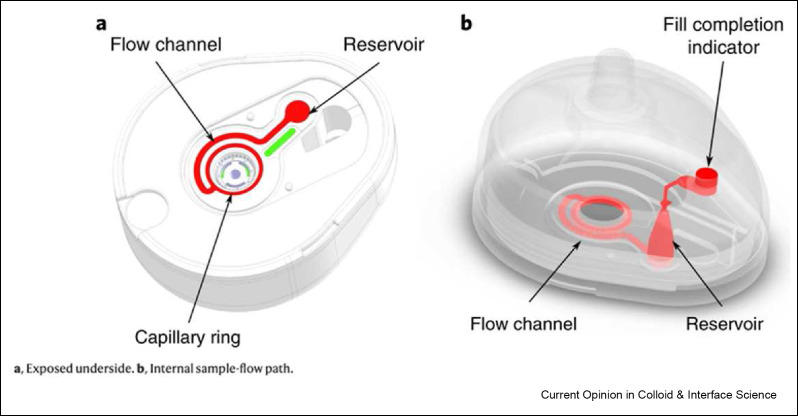
Ease of specimen collection refers to any actions prior to completing biological assays on POCTs, which include the collection, processing, and transfer of samples from patients to POCTs. To create sensitive assays that are also safe and streamlined, simple and safe sample collection methods are essential. The safety of specimen collection is especially important for infectious diseases pathogens due to their highly contagious nature. For the handling of specimens during collection, microneedles have been used to collect patient blood samples in a safe and pain-free manner [126]. The TAP® device from Blicharz et al. is an example of automatic blood collection and storage that can be deployed with POCT platforms.[ 127 ] This device punctures the skin with microneedles that are returned inside the device once the skin has been pieced. Capillary blood is then vacuum aspirated through the pores into the TAP® device with heparin to prevent clotting (Figure 8 ). The collected blood can then be transported, stored, and tested as needed. Overall, this collection method reduces the risk of infection to health care providers and eliminates the demand for trained phlebotomists on site.
Figure 8.
SiMoA assay overview. A) Bead labeled with capture antibodies capture a target biomarker —the analyte— which is then bound by a second antibody that is conjugated to an enzymatic reporter. B) Beads are then loaded into femtoliter wells for fluorescence detection. C) SEM of femtoliter wells after bead loading. D) Fluorescence image of the beads in wells. Reused with permission under creative copyright license CC-BY 4.0 2010 (Springer Nature).
Sample collection devices like the TAP® help push POCTs closer to the goal of delivering diagnostic solutions to people in all parts of the world. The next step is to integrate such sample collection tools directly with POCTs to achieve a truly all-in-one platform that includes sample collection, assay, and detection into small and cheap devices. These next generation devices will unlock the potential for POCTs to provide state-of-the art diagnostics across the globe at a fraction of the current cost of centralized laboratories.
The integration of data science with point-of-care diagnostics is another emerging area that will impact the next generation of bioassays: see a review by Qin et al. on this topic[ 128 ] In a study relevant for POCTs, Ballard et al. integrated deep learning algorithms into a multiplexed, paper-based vertical flow assay to enhance the detection of C-reactive protein compared to a standard LFA.[ 129 ] This LFA analyzes biomarkers using a multiplex framework in spatially separated immunoreaction spots. The deep learning algorithm instantly compares each of these spatially resolved areas and eliminates the worst performing spots to drive the LOD into a clinically relevant range. Tangentially, this group also used machine learning to optimize the design of a portable detector to improve the sensitivity and cost effectiveness in a localized surface plasmon resonance sensor.[ 130 ]
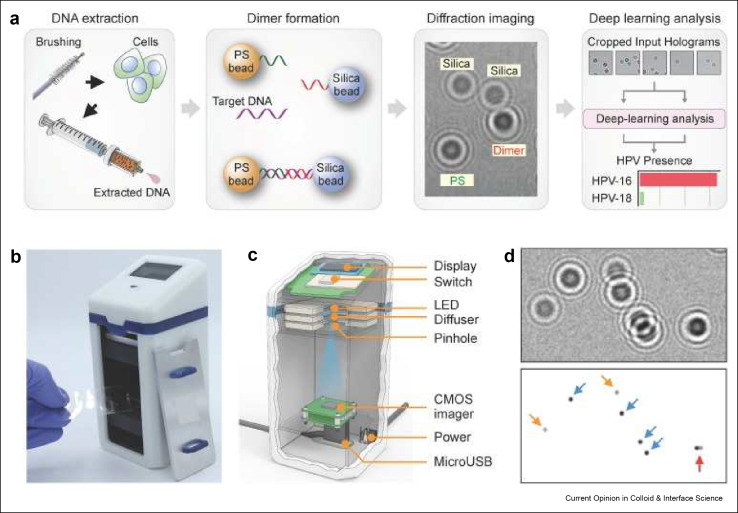
Machine learning may also lead to alternatives to pathology at the point-of-care to solve the critical shortage of trained pathologists in low resource settings.[ 131 ] Pathania et al. developed a holographic assay that could separately identify microbead monomers or dimers that formed from the DNA hybridization of capture probes bound to polystyrene beads and silica microbeads.[ 132 ] In the presence of high-risk HPV16 and HPV18 target DNA sequences, probes tethered to polystyrene beads and silica microbeads create dimers that lead to holographic images that can be read by their portable detector. Signals were analyzed by a deep learning algorithm to separate patient populations with sensitivities down to a single cell level in a process flow that is illustrated in Figure 9 .
Figure 9.
TAP microneedle blood collection device. Left: underside of the device showing the flow channel and heparinized reservoir. Right: 3D transparent device view of the blood flow channel, heparinized reservoir, and fill completion indicator. Reused with permission under creative copyright license CC-BY 4.0 2018 (Springer Nature).
Artificial intelligence (AI) is becoming increasingly important in a wide range of scientific applications (see Fig. 10). In POCT development, AI algorithms for computer vision are being used to amplify the abilities of companion detectors for POCTs. These innovations are commonly applied to the use of smartphones as a detector, due to their widespread use, inherent computational power, and access to cloud-connected networks. While Wang et al. has created an extensive review to cover image-based AI technologies, with a specific focus on the quantitative output of microfluidic POCTs[ 133 ], we will highlight a few recent works in this field. A paper by Tong et al. demonstrates how AI can be used to create a versatile POCT by using a convolutional neural network[ 134 ]. Doing so, they were able to automatically quantify COVID-19 neutralizing antibody titers using a colorimetric polydopamine nanoparticle (PDA)-based LFIA with high sensitivity and specificity while using a variety of smartphones (e.g., Apple, Samsung, and Huawei). This would allow users of these POCT strips to capture results with whatever smartphone is available. Moving beyond spatial identification, AI can also add features to POCTs like multiplexibility without the use of multiple fluorophores, as demonstrated by Coa et al.[ 135 ] Here, rather than using two different colored probes (e.g., red and green) to accomplish multiplexed detection of the genes bla NDM and bla VIM, Coa et al. used two similar colored probes (both green). To do this deep learning-based algorithm was trained on a set of 262,000 microwells with the similar colored fluorescence output. Afterward the system was able to achieve highly accurate classification of the two probes (>98%). This is important, as it can simplify multiplex testing by only requiring the detector to have a single excitation source, instead of multiple. Moving beyond biomarker quantification, D’Ambrosio et al. developed a POCT using a mobile phone as a microscope in which they analyzed the motion of Loa loa parasitic worms in human blood samples as a diagnostic tool rather than detecting a specific biomarker for the pathogen which is more conventional.[ 136 ] Using the mobile phone as a video recording device, the “wriggling” of the worms could be parsed by quantifying the displacement of red blood cells within the samples. Ultimately, this platform led to the accurate detection of Loa loa infections in 33 subjects with 100% sensitivity and 94% specificity. Finally, not all AI innovations in diagnostic medicine specifically improve the platforms facilitating the tests. This is exemplified by Fox et al. in their description of a AI system designed to disseminate curated and individualized clinical guidelines to patients based on their specific medical conditions in an effort to improve patient outcome with more personalized treatment at the point-of-care.[ 137 ] As the ubiquity of POCT continue to increase, the need for AI systems to digest and summarize data for human decision makers will become increasingly important.
Figure 10.
Artificial intelligence enhanced POCT For HPV detection. A) Assay procedure including obtaining cells from cervical brushing (far left), hybridization to PS and silica beads (middle left), diffraction imaging of the dimer hybrids (middle right), and deep learning analysis algorithm (far right). B) Photograph of AIM-HPV device. C) 3-D rendering of AIM-HPV device showing the display, switch, LED, diffuser, pinhole, CMOS imager, power port, and microUSB port. D) Diffraction images showing PS bead monomers (blue arrows), silica bead monomers (orange arrows), and PS-silica dimer (red arrow). Reused with permission under creative copyright license CC-BY 4.0 2019 (Ivyspring International).
7. Conclusion
We have described herein how POCTs can be used to diagnose, contact trace, and contain diseases, and to track the effectiveness of vaccination and treatment efforts, especially in low resource settings. As demonstrated during the recent COVID-19 pandemic, where POCTs were created and deployed in record time, POCTs can be quickly adapted to help health care professionals manage emerging global health crises.138 , 139 The POCTs described in this review achieve analytical sensitivities and specificities that meet and exceed clinical gold standard test with user-friendly workflows. The integration of these assays into automated, microfluidic chips and with cloud-connected, portable detectors further enhance their disease detection and monitoring capabilities. Next generation POCTs may integrate with sample collection technologies to create all-in-one molecular diagnostic solutions. New developments in machine learning promise to take POCTs beyond molecular diagnostics into the broader realm of pathology, which will provide an even larger and more powerful set of diagnostic tools to manage global health crises like infectious diseases. Continued development of POCT technologies will be essential to enable new early disease diagnostic pathways, optimize acute and long-term treatment plans, and contain future potential pandemics.
Declaration of Competing Interest
☒ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ashutosh Chilkoti reports financial support was provided by National Institutes of Health. Ashutosh Chilkoti has patent #WO/2020/223713 issued to Duke University. Ashutosh Chilkoti has patent #PCT/US2021/046833 issued to Duke University. Ashutosh Chilkoti has patent #63/429,316 pending to Duke University. David Kinnamon has patent #PCT/US2021/046833 issued to Duke University. Jacob Heggestad has patent #PCT/US2021/046833 issued to Duke University. David Kinnamon has patent #63/429,316 pending to Duke University. Jacob Heggestad has patent #63/429,316 pending to Duke University. Immucor Inc. has acquired the rights to the D4 assay on POEGMA brushes for in vitro diagnostics from Sentilus Inc. (cofounded by A.C. and others). D.T.B declares no competing interests.
Acknowledgement
This work was supported by grants (R01AI159992, R01AI150888, UH3CA211232 from the National Institutes of Health to A.C.
Data availability
No data was used for the research described in the article.
References
- 1.Kozel T.R., Burnham-Marusich A.R. Point-of-Care Testing for Infectious Diseases: Past, Present, and Future. Journal of Clinical Microbiology. 2017;55(8):2313. doi: 10.1128/JCM.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S., Rothman R.E. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. The Lancet Infectious Diseases. 2004;4(6):337–348. doi: 10.1016/S1473-3099(04)01044-8. 2004/06/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S., Lifson M.A., Inci F., Liang L.G., Sheng Y.F., Demirci U. Advances in addressing technical challenges of point-of-care diagnostics in resource-limited settings. Expert Rev Mol Diagn. 2016;16(4):449–459. doi: 10.1586/14737159.2016.1142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosnik A., Amiji M. Nanotechnology solutions for infectious diseases in developing nations. Advanced Drug Delivery Reviews. 2010;62(4):375–377. doi: 10.1016/j.addr.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W., et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. Jama. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St John A., Price C.P. Existing and Emerging Technologies for Point-of-Care Testing. The Clinical biochemist. Reviews. 2014;35(3):155–167. [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S. Powering point-of-care diagnostic devices. Biotechnol Adv. 2016;34(3):321–330. doi: 10.1016/j.biotechadv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen H., Liu K., Li Z., Wang P. Point of care testing for infectious diseases. Clinica Chimica Acta. 2019;493:138–147. doi: 10.1016/j.cca.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y., Dai L., Yang Y. Microfluidic technology and its application in the point-of-care testing field. Biosens Bioelectron X. 2022;10:100109. doi: 10.1016/j.biosx.2022.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Puig H., Bosch I., Gehrke L., Hamad-Schifferli K. Challenges of the Nano-Bio Interface in Lateral Flow and Dipstick Immunoassays. Trends Biotechnol. 2017;35(12):1169–1180. doi: 10.1016/j.tibtech.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora D.R., Maheshwari M., Arora B. Rapid Point-of-Care Testing for Detection of HIV and Clinical Monitoring. ISRN AIDS. 2013;2013:287269. doi: 10.1155/2013/287269. 287269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston B., Conly J. Point-of-care testing for HIV: HIV counselling and testing. The Canadian journal of infectious diseases = Journal canadien des maladies infectieuses. 2002;13(2):85–88. doi: 10.1155/2002/480403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray C.K., Gasser R.A., Jr., Magill A.J., Miller R.S. Update on rapid diagnostic testing for malaria. Clinical microbiology reviews. 2008;21(1):97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbanefo A., Kumar N. Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Tropical medicine and infectious disease. 2020;5(2):102. doi: 10.3390/tropicalmed5020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham J., et al. A review of the WHO malaria rapid diagnostic test product testing programme (2008–2018): performance, procurement and policy. Malaria Journal. 2019;18(1):387. doi: 10.1186/s12936-019-3028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkel B., et al. Screening for SARS-CoV-2 infection in asymptomatic individuals using the Panbio COVID-19 antigen rapid test (Abbott) compared with RT-PCR: a prospective cohort study. BMJ Open. 2021;11(10):e048206. doi: 10.1136/bmjopen-2020-048206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frediani J.K., et al. Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration. Scientific Reports. 2021;11(1):14604. doi: 10.1038/s41598-021-94055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houston H., Gupta-Wright A., Toke-Bjolgerud E., Biggin-Lamming J., John L. Diagnostic accuracy and utility of SARS-CoV-2 antigen lateral flow assays in medical admissions with possible COVID-19," (in eng) J Hosp Infect. 2021;110:203–205. doi: 10.1016/j.jhin.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontes C.M., et al. Engineering the Surface Properties of a Zwitterionic Polymer Brush to Enable the Simple Fabrication of Inkjet-Printed Point-of-Care Immunoassays. Langmuir. 2019;35(5):1379–1390. doi: 10.1021/acs.langmuir.8b01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabe M., Verdes D., Seeger S. Understanding protein adsorption phenomena at solid surfaces. Adv Colloid Interface Sci. 2011;162(1-2):87–106. doi: 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Song Y., Huang Y.Y., Liu X., Zhang X., Ferrari M., Qin L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014;32(3):132–139. doi: 10.1016/j.tibtech.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarei M. Infectious pathogens meet point-of-care diagnostics. Biosensors and Bioelectronics. 2018;106:193–203. doi: 10.1016/j.bios.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Parekh B.S., et al. Diagnosis of Human Immunodeficiency Virus Infection. Clinical microbiology reviews. 2018;32(1):e00064. doi: 10.1128/CMR.00064-18. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu J., et al. Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays. Lab on a Chip. 2013;13(22):4352–4357. doi: 10.1039/C3LC50672J. 10.1039/C3LC50672J. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T., Saito S., Ikeda S. A multilayer membrane amperometric glucose sensor fabricated using planar techniques for large-scale production. Journal of Biotechnology. 2006;122(2):267–273. doi: 10.1016/j.jbiotec.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Li F., et al. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnology Advances. 2020;39:107442. doi: 10.1016/j.biotechadv.2019.107442. [DOI] [PubMed] [Google Scholar]
- 28.Consden R., Gordon A.H., Martin A.J. Qualitative analysis of proteins: a partition chromatographic method using paper. The Biochemical journal. 1944;38(3):224–232. doi: 10.1042/bj0380224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yalow R.S., Berson S.A. IMMUNOASSAY OF ENDOGENOUS PLASMA INSULIN IN MAN. The Journal of Clinical Investigation. 1960;39(7):1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mak W.C., Beni V., Turner A.P.F. Lateral-flow technology: From visual to instrumental. TrAC Trends in Analytical Chemistry. 2016;79:297–305. doi: 10.1016/j.trac.2015.10.017. [DOI] [Google Scholar]
- 31.Jiang N., Ahmed R., Damayantharan M., Ünal B., Butt H., Yetisen A.K. Lateral and Vertical Flow Assays for Point-of-Care Diagnostics. Advanced Healthcare Materials. 2019;8(14):1900244. doi: 10.1002/adhm.201900244. [DOI] [PubMed] [Google Scholar]
- 32.Urusov A.E., Zherdev A.V., Dzantiev B.B. Towards Lateral Flow Quantitative Assays: Detection Approaches. Biosensors. 2019;9(3) doi: 10.3390/bios9030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R. Arnaout et al., "SARS-CoV2 Testing: The Limit of Detection Matters," (in eng), bioRxiv : the preprint server for biology, p. 2020.06.02.131144, 2020, doi: 10.1101/2020.06.02.131144.
- 34.Zhang Y., et al. Improvement in Detection Limit for Lateral Flow Assay of Biomacromolecules by Test-Zone Pre-enrichment. Scientific Reports. 2020;10(1):9604. doi: 10.1038/s41598-020-66456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noh J.Y., et al. Pipetting-based immunoassay for point-of-care testing: Application for detection of the influenza A virus. Scientific Reports. 2019;9(1):16661. doi: 10.1038/s41598-019-53083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai T.-T., Huang T.-H., Chen C.-A., Ho N.Y.-J., Chou Y.-J., Chen C.-F. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay. Scientific Reports. 2018;8(1):17319. doi: 10.1038/s41598-018-35694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C., et al. Rapid, quantitative, and high-sensitivity detection of anti-phospholipase A2 receptor antibodies using a novel CdSe/ZnS-based fluorescence immunosorbent assay. Scientific Reports. 2021;11(1):8778. doi: 10.1038/s41598-021-88343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38∗.van Hooij A., et al. Quantitative lateral flow strip assays as User-Friendly Tools To Detect Biomarker Profiles For Leprosy. Scientific reports. 2016;6:34260. doi: 10.1038/srep34260. 34260. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Chen et al. reports a lateral flow assay to detect anti-SAV-CoV-2 IgG antibodies in human serum. This paper demonstrates the effectiveness of the lateral flow technology to be quickly adopted to new challenges, like the COVID-19 pandemic, and demonstrates the use of novel signal ers like lanthanide-doped polysterene nanoparticles in a portable and rapid test.
- 39.Corstjens P.L., et al. Lateral flow assay for simultaneous detection of cellular- and humoral immune responses. Clin Biochem. 2011;44(14-15):1241–1246. doi: 10.1016/j.clinbiochem.2011.06.983. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corstjens P.L., Zuiderwijk M., Tanke H.J., van der Ploeg-van Schip J.J., Ottenhoff T.H., Geluk A. A user-friendly, highly sensitive assay to detect the IFN-gamma secretion by T cells," (in eng) Clin Biochem. 2008;41(6):440–444. doi: 10.1016/j.clinbiochem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., et al. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci Rep. 2016;6:21342. doi: 10.1038/srep21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z., et al. Rapid and Sensitive Detection of anti-SARS-CoV-2 IgG, Using Lanthanide-Doped Nanoparticles-Based Lateral Flow Immunoassay. Analytical Chemistry. 2020;92(10):7226–7231. doi: 10.1021/acs.analchem.0c00784. [DOI] [PubMed] [Google Scholar]
- 43.S. R. Stowell and J. Guarner, "Role of Serology in the Coronavirus Disease 2019 Pandemic," Clinical Infectious Diseases, 2020, doi: 10.1093/cid/ciaa510. [DOI] [PMC free article] [PubMed]
- 44∗.Martinez A.W., Phillips S.T., Carrilho E., Thomas S.W., Sindi H., Whitesides G.M. Simple Telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis. Analytical Chemistry. 2008;80(10):3699–3707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Martinez et al. demonstrates the use of hydrophillic and hydropohboic patterning on paper as a low cost and highly modular strategy to develop point-of-care bioassays. This fully passive system successfully analyzed glucose and proteins in urine in a sample volume of only 5 microliters.
- 45.Martinez A.W., Phillips S.T., Whitesides G.M., Carrilho E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Analytical Chemistry. 2010;82(1):3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 46∗∗.Carrilho E., Martinez A.W., Whitesides G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Analytical Chemistry. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]; This paper by Connelly et al. described the development of “paper machines” that perform loop-mediated isothermal amplification of Escherichia coli malB gene targets with an analytical sensitivity of 1 double-stranded copy. This paper machine uses a hand-held UV light source and a camera phone to analyze assay results.
- 47.Liana D.D., Raguse B., Gooding J.J., Chow E. Recent Advances in Paper-Based Sensors. Sensors. 2012;12(9):11505–11526. doi: 10.3390/s120911505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez A.W., Phillips S.T., Butte M.J., Whitesides G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed Engl. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verma M.S., et al. Sliding-strip microfluidic device enables ELISA on paper. Biosensors & bioelectronics. 2018;99:77–84. doi: 10.1016/j.bios.2017.07.034. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connelly J.T., Rolland J.P., Whitesides G.M. Paper Machine” for Molecular Diagnostics. Analytical Chemistry. 2015;87(15):7595–7601. doi: 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- 51.Mu X., Zhang L., Chang S., Cui W., Zheng Z. Multiplex Microfluidic Paper-based Immunoassay for the Diagnosis of Hepatitis C Virus Infection. Analytical Chemistry. 2014;86(11):5338–5344. doi: 10.1021/ac500247f. [DOI] [PubMed] [Google Scholar]
- 52∗∗.Delehanty J.B., Ligler F.S. A microarray immunoassay for simultaneous detection of proteins and bacteria. Anal Chem. 2002;74(21):5681–5687. doi: 10.1021/ac025631l. [DOI] [PubMed] [Google Scholar]; This paper by Hucknall et al. presents a simple and efficient method to fabricatie antibody microarrays on a nonfouling polymer brush with exceptional sensitivity to protein analytes in serum and blood, down to the femtomolar level. This platform provides a promising tool for the multiplexed quantification of various disease biomarkers forms amicroliter volumes.
- 53∗.Dincer C., Bruch R., Kling A., Dittrich P.S., Urban G.A. Multiplexed Point-of-Care Testing – xPOCT. Trends in Biotechnology. 2017;35(8):728–742. doi: 10.1016/j.tibtech.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Joh et al. reports a self-contained immunoassay platform––the "D4"––that matches the analytical figures of merit of standard laboratory based tests, namely the ELISA, in a point-of-care-test. In this paper, the D4 assay is used to detect multiple biomarkers in serum and blood using with a portable smartphone detector. The D4 point-of-care test enables testing in resource limited settings without sacrificing analytical performance.
- 54∗.Yu X., Xu D., Cheng Q. Label-free detection methods for protein microarrays. PROTEOMICS. 2006;6(20):5493–5503. doi: 10.1002/pmic.200600216. https://doi.org/10.1002/pmic.200600216. [DOI] [PubMed] [Google Scholar]; This paper by Ma et al. is the first demonstration of growing a nonfouling poly(oligoethylene glcol methacrylate) brush of tunable thickness on gold using surface-initiated polymerization. Synthesizing brushes in this way greatly simplifies fabrication of nrobust non-fouling coatings for diverse applications, including point-of-care bioassays.
- 55.Syahir A., Usui K., Tomizaki K.-y., Kajikawa K., Mihara H. Label and Label-Free Detection Techniques for Protein Microarrays. Microarrays. 2015;4(2):228–244. doi: 10.3390/microarrays4020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56∗.Hucknall A., Kim D.H., Rangarajan S., Hill R.T., Reichert W.M., Chilkoti A. Simple Fabrication of Antibody Microarrays on Nonfouling Polymer Brushes with Femtomolar Sensitivity for Protein Analytes in Serum and Blood. Adv Mater. 2009;21(19):1968–1971. doi: 10.1002/adma.200803125. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Ma et al. extends the technolgoy reported in reference 54 to glass, which is theerpefrr ssubstare for clicnical assays.
- 57.Joh D.Y., et al. Inkjet-printed point-of-care immunoassay on a nanoscale polymer brush enables subpicomolar detection of analytes in blood. Proc Natl Acad Sci U S A. 2017;114(34):E7054–E7062. doi: 10.1073/pnas.1703200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma H., Hyun J., Stiller P., Chilkoti A. Non-Fouling” Oligo(ethylene glycol)- Functionalized Polymer Brushes Synthesized by Surface-Initiated Atom Transfer Radical Polymerization. Advanced Materials. 2004;16(4):338–341. doi: 10.1002/adma.200305830. https://doi.org/10.1002/adma.200305830. [DOI] [Google Scholar]
- 59.Ma H., Hyun J., Zhang Z., Beebe T.P., Jr., Chilkoti A. Fabrication of Biofunctionalized Quasi-Three-Dimensional Microstructures of a Nonfouling Comb Polymer Using Soft Lithography. Advanced Functional Materials. 2005;15(4):529–540. doi: 10.1002/adfm.200400088. https://doi.org/10.1002/adfm.200400088. [DOI] [Google Scholar]
- 60∗∗.Ma H., Li D., Sheng X., Zhao B., Chilkoti A. Protein-Resistant Polymer Coatings on Silicon Oxide by Surface-Initiated Atom Transfer Radical Polymerization. Langmuir. 2006;22(8):3751–3756. doi: 10.1021/la052796r. [DOI] [PubMed] [Google Scholar]; This paper by Fontes et al. reports the detection of the Ebola virus's secreted glycoprotein by the D4 point of care test a day earlier than PCR. This also paper introduces a customized portable fluorescence detector—the D4Scope—that is optimized for the D4 assay.
- 61∗∗.Ma H., Wells M., Beebe T.P., Jr., Chilkoti A. Surface-Initiated Atom Transfer Radical Polymerization of Oligo(ethylene glycol) Methyl Methacrylate from a Mixed Self-Assembled Monolayer on Gold. Advanced Functional Materials. 2006;16(5):640–648. doi: 10.1002/adfm.200500426. [DOI] [Google Scholar]; This paper by Fontes et al. reports the detection of the Ebola virus's secreted glycoprotein by the D4 point of care test a day earlier than PCR. This also paper introduces a customized portable fluorescence detector—the D4Scope—that is optimized for the D4 assay.
- 62.Hucknall A., Rangarajan S., Chilkoti A. Pursuit of Zero: Polymer Brushes that Resist the Adsorption of Proteins. Advanced Materials. 2009;21(23):2441–2446. doi: 10.1002/adma.200900383. [DOI] [Google Scholar]
- 63.Heggestad J.T., Fontes C.M., Joh D.Y., Hucknall A.M., Chilkoti A. Pursuit of Zero 2.0: Recent Developments in Nonfouling Polymer Brushes for Immunoassays. Advanced Materials. 2020;32(2):1903285. doi: 10.1002/adma.201903285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fontes C.M., et al. Ultrasensitive point-of-care immunoassay for secreted glycoprotein detects Ebola infection earlier than PCR. Science Translational Medicine. 2021;13(588):eabd9696. doi: 10.1126/scitranslmed.abd9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heggestad J.T., et al. Multiplexed, quantitative serological profiling of COVID-19 from blood by a point-of-care test. Sci Adv. 2021;7(26) doi: 10.1126/sciadv.abg4901. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laksanasopin T., et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Science Translational Medicine. 2015;7(273):273re1. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 67∗.Pashchenko O., Shelby T., Banerjee T., Santra S. A Comparison of Optical, Electrochemical, Magnetic, and Colorimetric Point-of-Care Biosensors for Infectious Disease Diagnosis. ACS infectious diseases. 2018;4(8):1162–1178. doi: 10.1021/acsinfecdis.8b00023. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Hwang et al. reports the integration of deformed graphene––graphene that has been "bent" or "crumpled"––in a field-effect transistor-based biosensor to enable the detection of DNA and RNA molecules in the zemptomolar regeime. Importantly, by using graphene in this deformed state, this FET-biosesnsor circumvents previously documented limitations for electrochemical biosensing by modulating the Debye screening length.
- 68∗.Zarei M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends in Analytical Chemistry. 2017;91:26–41. doi: 10.1016/j.trac.2017.04.001. [DOI] [Google Scholar]; This paper by Hajian et al. details the CRISPR-Chip, a graphene-based field-effect transistor that uses CRISPR-Cas9 to detect target DNA sequences using a fluorescence response. Importantly, by using the CRISPR-Cas9 system, the CRISPR-Chip facilitates the detection of unamplified DNA with femtomolar sensitivity in 15 minutes.
- 69.Lee J.-R., et al. Multiplex giant magnetoresistive biosensor microarrays identify interferon-associated autoantibodies in systemic lupus erythematosus. Scientific Reports. 2016;6(1):27623. doi: 10.1038/srep27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peña-Bahamonde J., Nguyen H.N., Fanourakis S.K., Rodrigues D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. Journal of Nanobiotechnology. 2018;16(1):75. doi: 10.1186/s12951-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang M.T., et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nature Communications. 2020;11(1):1543. doi: 10.1038/s41467-020-15330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajian R., et al. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nature Biomedical Engineering. 2019;3(6):427–437. doi: 10.1038/s41551-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patterson A.S., Hsieh K., Soh H.T., Plaxco K.W. Electrochemical real-time nucleic acid amplification: towards point-of-care quantification of pathogens. Trends in Biotechnology. 2013;31(12):704–712. doi: 10.1016/j.tibtech.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Ballard Z., Ozcan A. Nucleic acid quantification in the field. Nature Biomedical Engineering. 2018;2(9):629–630. doi: 10.1038/s41551-018-0292-0. [DOI] [PubMed] [Google Scholar]
- 75∗∗.Dave V.P., et al. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Laboratory Investigation. 2019;99(4):452–469. doi: 10.1038/s41374-018-0143-3. [DOI] [PubMed] [Google Scholar]; This seminal paper by Notomi et al. introduces the loop-mediated isothermal amplification (LAMP) technology as a way to amplify specified nucleic acid sequences without the use of PCR. This greatly simplifies the hardware required for nucleic acid amplification to enable new methods for point-of-care DNA/RNA-based disease diagnosis.
- 76.Mori Y., Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother. 2009;15(2):62–69. doi: 10.1007/s10156-009-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nzelu C.O., Kato H., Peters N.C. Loop-mediated isothermal amplification (LAMP): An advanced molecular point-of-care technique for the detection of Leishmania infection. PLoS neglected tropical diseases. 2019;13(11):e0007698. doi: 10.1371/journal.pntd.0007698. e0007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.James A., Macdonald J. Recombinase polymerase amplification: Emergence as a critical molecular technology for rapid, low-resource diagnostics. Expert Review of Molecular Diagnostics. 2015;15(11):1475–1489. doi: 10.1586/14737159.2015.1090877. [DOI] [PubMed] [Google Scholar]
- 79.Notomi T., et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research. 2000;28(12):e63. doi: 10.1093/nar/28.12.e63. e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS biology. 2006;4(7):e204. doi: 10.1371/journal.pbio.0040204. e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park S., Zhang Y., Lin S., Wang T.H., Yang S. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol Adv. 2011;29(6):830–839. doi: 10.1016/j.biotechadv.2011.06.017. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y., Liu Y. Advances in integrated digital microfluidic platforms for point-of-care diagnosis: a review. Sensors & Diagnostics. 2022;1(4):648–672. doi: 10.1039/D2SD00031H. 10.1039/D2SD00031H. [DOI] [Google Scholar]
- 83∗.Roy S., Wei S.X., Ying J.L.Z., Safavieh M., Ahmed M.U. A novel, sensitive and label-free loop-mediated isothermal amplification detection method for nucleic acids using luminophore dyes. Biosensors and Bioelectronics. 2016;86:346–352. doi: 10.1016/j.bios.2016.06.065. [DOI] [PubMed] [Google Scholar]; This paper by Snodgrass et al. describes the development of a portable device that can perform isothermal amplification of nucleic acid sequences and can operate in low resource settings, that is powered by electricity, sunlight, or a flame. As a proof-of-concept, this device was used in two Ugandan health clinicals, where it opererated through power outages with comparable performance to commcical PCR tests.
- 84.Daher R.K., Stewart G., Boissinot M., Bergeron M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clinical chemistry. 2016;62(7):947–958. doi: 10.1373/clinchem.2015.245829. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernández-Soto P., Gandasegui Arahuetes J., Sánchez Hernández A., López Abán J., Vicente Santiago B., Muro A. A Loop-Mediated Isothermal Amplification (LAMP) Assay for Early Detection of Schistosoma mansoni in Stool Samples: A Diagnostic Approach in a Murine Model. PLOS Neglected Tropical Diseases. 2014;8(9):e3126. doi: 10.1371/journal.pntd.0003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.García-Bernalt Diego J., et al. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: towards a ready-to-use test. Scientific Reports. 2019;9(1):14744. doi: 10.1038/s41598-019-51342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87∗∗.Snodgrass R., et al. A portable device for nucleic acid quantification powered by sunlight, a flame or electricity. Nature Biomedical Engineering. 2018;2(9):657–665. doi: 10.1038/s41551-018-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Gootenberg et al. introduces the Specific High Sensitivty Enzymatic Reporter UnLOCKing (SHERLOCK) platform, a groundbreaking technology for isother,ally detecting nucleic acid sequences using CRISPR-Cas13 collateral cleavage as a point-of-care test. SHERLOCK can detect DNA and RNA in real-time. Its performance was demonstrated for Zika and dengue virus, bacterial pathogens, and human DNA and DNA mutations.
- 88∗∗.Ball C.S., Light Y.K., Koh C.-Y., Wheeler S.S., Coffey L.L., Meagher R.J. Quenching of Unincorporated Amplification Signal Reporters in Reverse-Transcription Loop-Mediated Isothermal Amplification Enabling Bright, Single-Step, Closed-Tube, and Multiplexed Detection of RNA Viruses. Analytical Chemistry. 2016;88(7):3562–3568. doi: 10.1021/acs.analchem.5b04054. [DOI] [PubMed] [Google Scholar]; This paper by Gootenberg et al. reports the evolution of the SHERLOCK platform as a multiplexable, portable, and low cost point-of-care test. Here, SHERLOCK is integrated into a lateral flow test that combines the advantages of SHERLOCK nucleic acid detection with the user-friendliness of the lateral flow assay for enhanced deployability to detect Zika and dengue viral ssRNA in patent liquid biopsy samples.
- 89∗∗.Priye A., Bird S.W., Light Y.K., Ball C.S., Negrete O.A., Meagher R.J. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Scientific Reports. 2017;7(1):44778. doi: 10.1038/srep44778. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports a protocol—Heating Unextracted Diagnostic Samples to Obliterate Nucleases (HUDSON)—as companion to SHERLOCK. HUDSON lyses viral particles and inactivates RNases in bodily fluids and is used to detect Zika and dengue virus directly from bodily fluids by SHERLOCK. The combination of HUDSON and SHERLOCK enables instrument-free dengue virus detection in less than two hours in a variety of sample types including urine, whole blood, plasma, serum, and saliva.
- 90.N. Panpradist et al., "Harmony COVID-19: A ready-to-use kit, low-cost detector, and smartphone app for point-of-care SARS-CoV-2 RNA detection," Science Advances, vol. 7, no. 51, p. eabj1281, doi: 10.1126/sciadv.abj1281. [DOI] [PMC free article] [PubMed]
- 91∗.Gootenberg J.S., et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356(6336):438. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Yeh et al. describes a self-powered integrated ow-cost microfluidic point-of-care test called the SIMPLE chip. The SIMPLE chip works by using an on-board vacuum to drive whole blood samples through a series of steps, leading to the detection of methicllin-resistant Staphylococcus aureus DNA by an isothermal amplification technique smd operates without the use of an external power supply or accessory instruments. Its utilty was demonstrated by the detection of methicllin-resistant Staphylococcus aureus.
- 92.Gootenberg J.S., Abudayyeh O.O., Kellner M.J., Joung J., Collins J.J., Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360(6387):439–444. doi: 10.1126/science.aaq0179. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93∗.Myhrvold C., et al. Field-deployable viral diagnostics using CRISPR-Cas13. eng, editor. Science (New York, N.Y.) 2018;360(6387):444–448. doi: 10.1126/science.aas8836. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Woo et al. describes the detection of SARS-CoV-2 RNA in nasopharyngeal swap samples in 40 patients by a sensitive splint-based one-pot isothermal RNA detection, or SENSR. SENSR combines two enzymatic reactions, a ligation by SplintR ligase and transcription by T7 RNA polymerase to form an RNA aptamer that binds to fluorescent reporters only when the target RNA is detected. In addition to SARS-CoV-2, SENSR was also used to successfully detect Vibrio vulnificus, Escherichia coli O157:H7, MERS-CoV, and influenza A.
- 94.Chen J.S., et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science (New York, N.Y.) 2018;360(6387):436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh E.-C., Fu C.-C., Hu L., Thakur R., Feng J., Lee L.P. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Science Advances. 2017;3(3):e1501645. doi: 10.1126/sciadv.1501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96∗.Deng S., et al. Three isothermal amplification techniques for rapid identification of Cladophialophora carrionii, an agent of human chromoblastomycosis. Journal of clinical microbiology. 2014;52(10):3531–3535. doi: 10.1128/JCM.01033-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Zhong et al. reviews the use of digital microfluidic biochips for DNA sequencing and point-of-care diagnostics. Critically, this paper provides a description of autonomous nanoliter fluid control that enables the development of novel biological assays.
- 97.Woo C.H., Jang S., Shin G., Jung G.Y., Lee J.W. Sensitive fluorescence detection of SARS-CoV-2 RNA in clinical samples via one-pot isothermal ligation and transcription. Nature Biomedical Engineering. 2020 doi: 10.1038/s41551-020-00617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Washizu M. Electrostatic actuation of liquid droplets for micro-reactor applications. IEEE Transactions on Industry Applications. 1998;34(4):732–737. doi: 10.1109/28.703965. [DOI] [Google Scholar]
- 99.Pollack M.G., Fair R.B., Shenderov A.D. Electrowetting-based actuation of liquid droplets for microfluidic applications. Applied Physics Letters. 2000;77(11):1725–1726. doi: 10.1063/1.1308534. [DOI] [Google Scholar]
- 100.Zhong Z., Li Z., Chakrabarty K., Ho T.Y., Lee C.Y. Micro-Electrode-Dot-Array Digital Microfluidic Biochips: Technology, Design Automation, and Test Techniques. IEEE Transactions on Biomedical Circuits and Systems. 2019;13(2):292–313. doi: 10.1109/TBCAS.2018.2886952. [DOI] [PubMed] [Google Scholar]
- 101.Li J., Kim C.C. Current commercialization status of electrowetting-on-dielectric (EWOD) digital microfluidics. Lab Chip. 2020;20(10):1705–1712. doi: 10.1039/d0lc00144a. (in eng) [DOI] [PubMed] [Google Scholar]
- 102.Millington D., Norton S., Singh R., Sista R., Srinivasan V., Pamula V. Digital microfluidics comes of age: high-throughput screening to bedside diagnostic testing for genetic disorders in newborns. Expert Rev Mol Diagn. 2018;18(8):701–712. doi: 10.1080/14737159.2018.1495076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.R. S. A. U. N. R. N. M. B. M. C. J. E. J. H. D. K. K. K. M. R. C. W. D. K. A. D. A. Sista and P. Pediatric, Diagnostics, vol. 10, no. 1, doi: 10.3390/diagnostics10010021.
- 104.Millington D.S., et al. Digital microfluidics: a future technology in the newborn screening laboratory? eng, editor. Semin Perinatol. 2010;34(2):163–169. doi: 10.1053/j.semperi.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.M. Zahavi, H. Rohana, M. Azrad, B. Shinberg, and A. Peretz, "Rapid SARS-CoV-2 Detection Using the Lucira™ Check It COVID-19 Test Kit," Diagnostics, vol. 12, no. 8, doi: 10.3390/diagnostics12081877. [DOI] [PMC free article] [PubMed]
- 106.Chou J., Wong J., Christodoulides N., Floriano P.N., Sanchez X., McDevitt J. Porous bead-based diagnostic platforms: bridging the gaps in healthcare. Sensors (Basel, Switzerland) 2012;12(11):15467–15499. doi: 10.3390/s121115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perdomo-Lara S.J., et al. Human papilloma virus genotypes in dysplasia and epithelial hyperplasia of oral cavity using the luminex xmap technology. A multicenter study. Med Oral Patol Oral Cir Bucal. 2020;25(1):e61–e70. doi: 10.4317/medoral.23188. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao P.Y., et al. Comparison of the cervista HPV HR test and luminex XMAP technology for the diagnosis of cervical intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2017;214:150–155. doi: 10.1016/j.ejogrb.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 109.Taniguchi H., et al. Clinical Validation of Newly Developed Multiplex Kit Using Luminex xMAP Technology for Detecting Simultaneous RAS and BRAF Mutations in Colorectal Cancer: Results of the RASKET-B Study. Neoplasia. 2018;20(12):1219–1226. doi: 10.1016/j.neo.2018.10.004. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang J.H., Vanderstichele H., Trojanowski J.Q., Shaw L.M. Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease. Methods. 2012;56(4):484–493. doi: 10.1016/j.ymeth.2012.03.023. (in eng) [DOI] [PubMed] [Google Scholar]
- 111.Reslova N., Michna V., Kasny M., Mikel P., Kralik P. xMAP Technology: Applications in Detection of Pathogens. Frontiers in microbiology. 2017;8:55. doi: 10.3389/fmicb.2017.00055. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112∗∗.Wu M., et al. High-throughput Luminex xMAP assay for simultaneous detection of antibodies against rabbit hemorrhagic disease virus, Sendai virus and rabbit rotavirus. Arch Virol. 2019;164(6):1639–1646. doi: 10.1007/s00705-019-04226-9. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper by Rissin et al. outlines the Single Molecule Array (SiMOA) technology capable of fluorescently detecting individual protein molecules. Single molecule detection in SiMOA is enabled by the isolation of microscopic beads that are either carrying enzyme-labled proteins or not into femotoliter-sized reaction chambers based on Poisson distributions. The authors demonstrate that SiMOA outperforms conventional ELISA for detection of prostate specific antigen in patient serum samples.
- 113∗.Wang H., et al. Establishment of xMAP for the simultaneous detection of antibodies to Newcastle disease virus and avian influenza virus. Poult Sci. 2019;98(3):1494–1499. doi: 10.3382/ps/pey510. [DOI] [PubMed] [Google Scholar]; This paper by Rissin and Walt describes the use of femtoliter arrays and Poisson statistics for single molecule detection. The authors speculate that the use of this technology could enable ultra-sensitive protein detection which was later demonstrated using the SiMOA technology.
- 114.Wang H., et al. Development of a sensitive and specific xMAP assay for detection of antibodies against infectious laryngotracheitis and bronchitis viruses. Virol J. 2018;15(1):146. doi: 10.1186/s12985-018-1048-x. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Washington C., et al. Multiplexed Luminex xMAP assay for detection and identification of five adenovirus serotypes associated with epidemics of respiratory disease in adults. J Clin Microbiol. 2010;48(6):2217–2222. doi: 10.1128/jcm.00029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rissin D.M., et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nature biotechnology. 2010;28(6):595–599. doi: 10.1038/nbt.1641. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rissin D.M., Walt D.R. Digital Concentration Readout of Single Enzyme Molecules Using Femtoliter Arrays and Poisson Statistics. Nano Letters. 2006;6(3):520–523. doi: 10.1021/nl060227d. [DOI] [PubMed] [Google Scholar]
- 118∗.Rissin D.M., Walt D.R. Digital readout of target binding with attomole detection limits via enzyme amplification in femtoliter arrays. J Am Chem Soc. 2006;128(19):6286–6287. doi: 10.1021/ja058425e. [DOI] [PubMed] [Google Scholar]; This paper by Land et al. reimagines the ASSURED acronym develoeped by the World Health Organization to outline desirable qualities for point-of-care tests. A new acronym, REASSURED, is introduced that adds the features of 1) Real-time connectivity and 2) Ease of speciment collection to the list of desired attributes of future point-of-care tests.
- 119.Ahmad R., et al. A rapid triage test for active pulmonary tuberculosis in adult patients with persistent cough. Science Translational Medicine. 2019;11(515):eaaw8287. doi: 10.1126/scitranslmed.aaw8287. [DOI] [PubMed] [Google Scholar]
- 120.Yelleswarapu V., Buser J.R., Haber M., Baron J., Inapuri E., Issadore D. Mobile platform for rapid sub–picogram-per-milliliter, multiplexed, digital droplet detection of proteins. Proceedings of the National Academy of Sciences. 2019;116(10):4489. doi: 10.1073/pnas.1814110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121∗.Cortina M.E., et al. Electrochemical magnetic microbeads-based biosensor for point-of-care serodiagnosis of infectious diseases. Biosensors and Bioelectronics. 2016;80:24–33. doi: 10.1016/j.bios.2016.01.021. [DOI] [PubMed] [Google Scholar]; This paer by Blicharz et al. describes a new microneedle device for the automated, painless collection of capillary blood from humans. This device collects 100 microliters of blood and mixes it with an anti-coagulant for blood storage and transport t. The blood collection device can be used as a companion technology for central lab based tests or point-of-care tests. The authors validated the microneedle blood collection device by comparing results of haemoglobin A1c tests using blood collected from the new device and blood collected by a venous blood draw.
- 122.Land K.J., Boeras D.I., Chen X.-S., Ramsay A.R., Peeling R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nature Microbiology. 2019;4(1):46–54. doi: 10.1038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Christodouleas D.C., Kaur B., Chorti P. From Point-of-Care Testing to eHealth Diagnostic Devices (eDiagnostics) ACS Central Science. 2018;4(12):1600–1616. doi: 10.1021/acscentsci.8b00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo J., et al. 5G-enabled ultra-sensitive fluorescence sensor for proactive prognosis of COVID-19. Biosensors and Bioelectronics. 2021;181:113160. doi: 10.1016/j.bios.2021.113160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo J., et al. Cyber–Physical Healthcare System With Blood Test Module on Broadcast Television Network for Remote Cardiovascular Disease (CVD) Management. IEEE Transactions on Industrial Informatics. 2021;17(5):3663–3670. doi: 10.1109/TII.2020.3010280. [DOI] [Google Scholar]
- 126.Xue P., Zhang L., Xu Z., Yan J., Gu Z., Kang Y. Blood sampling using microneedles as a minimally invasive platform for biomedical diagnostics. Applied Materials Today. 2018;13:144–157. doi: 10.1016/j.apmt.2018.08.013. [DOI] [Google Scholar]
- 127∗.Blicharz T.M., et al. Microneedle-based device for the one-step painless collection of capillary blood samples. Nature Biomedical Engineering. 2018;2(3):151–157. doi: 10.1038/s41551-018-0194-1. [DOI] [PubMed] [Google Scholar]; This paper by D'Ambrosio et al. describes a mobile phone-based point-of-care video microscope device that can quantify L. loa microfilariae in whole blood samples without the need for sample preparation. This device was successfully validated by comparing results with manual blood smears for Loa-infected patient samples.
- 128.Qin Q., et al. Algorithms for immunochromatographic assay: review and impact on future application. Analyst. 2019;144(19):5659–5676. doi: 10.1039/c9an00964g. (in eng) [DOI] [PubMed] [Google Scholar]
- 129.Ballard Z.S., et al. Deep learning-enabled point-of-care sensing using multiplexed paper-based sensors. npj Digital Medicine. 2020;3(1):66. doi: 10.1038/s41746-020-0274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballard Z.S., Shir D., Bhardwaj A., Bazargan S., Sathianathan S., Ozcan A. Computational Sensing Using Low-Cost and Mobile Plasmonic Readers Designed by Machine Learning. ACS Nano. 2017;11(2):2266–2274. doi: 10.1021/acsnano.7b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Horton S., et al. Delivering modern, high-quality, affordable pathology and laboratory medicine to low-income and middle-income countries: a call to action. The Lancet. 2018;391(10133):1953–1964. doi: 10.1016/S0140-6736(18)30460-4. [DOI] [PubMed] [Google Scholar]
- 132.Pathania D., et al. Point-of-care cervical cancer screening using deep learning-based microholography. Theranostics. 2019;9(26):8438–8447. doi: 10.7150/thno.37187. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang B., et al. Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence. Nature Communications. 2023;14(1):1341. doi: 10.1038/s41467-023-36017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong H., et al. Artificial intelligence-assisted colorimetric lateral flow immunoassay for sensitive and quantitative detection of COVID-19 neutralizing antibody. Biosens Bioelectron. 2022;213:114449. doi: 10.1016/j.bios.2022.114449. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cao C., et al. Similar color analysis based on deep learning (SCAD) for multiplex digital PCR via a single fluorescent channel. Lab on a Chip. 2022;22(20):3837–3847. doi: 10.1039/D2LC00637E. 10.1039/D2LC00637E. [DOI] [PubMed] [Google Scholar]
- 136.D'Ambrosio M.V., et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med. 2015;7(286):286re4. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.John F., Matthew S., Omar K., Catriona K., Peter A., John B. OpenClinical.net: Artificial intelligence and knowledge engineering at the point of care. BMJ Health & Care Informatics. 2020;27(2):e100141. doi: 10.1136/bmjhci-2020-100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kevadiya B.D., et al. Diagnostics for SARS-CoV-2 infections. Nature Materials. 2021/05/01 2021;20(5):593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Verma N., Patel D., Pandya A. Emerging diagnostic tools for detection of COVID-19 and perspective. Biomedical Microdevices. 2020;22(4):83. doi: 10.1007/s10544-020-00534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.