Abstract
Inosine has robust neuroprotective effects, but it is unclear if inosine acts as direct ligand of adenosine receptors or if it triggers metabolic effects indirectly modifying the activity of adenosine receptors. We now combined radioligand binding studies with electrophysiological recordings in hippocampal slices to test how inosine controls synaptic transmission and plasticity. Inosine was without effect at 30 μM and decreased field excitatory post-synaptic potentials by 14% and 33% at 100 and 300 μM, respectively. These effects were prevented by the adenosine A1 receptor antagonist DPCPX. Inosine at 300 (but not 100) μM also decreased the magnitude of long-term potentiation (LTP), an effect prevented by DPCPX and by the adenosine A2A receptor antagonist SCH58261. Inosine showed low affinity towards human and rat adenosine receptor subtypes with Ki values of > 300 µM; only at the human and rat A1 receptor slightly higher affinities with Ki values of around 100 µM were observed. Affinity of inosine at the rat A3 receptor was higher (Ki of 1.37 µM), while it showed no interaction with the human orthologue. Notably, the effects of inosine on synaptic transmission and plasticity were abrogated by adenosine deaminase and by inhibiting equilibrative nucleoside transporters (ENT) with dipyridamole and NBTI. This shows that the impact of inosine on hippocampal synaptic transmission and plasticity is not due to a direct activation of adenosine receptors but is instead due to an indirect modification of the tonic activation of these adenosine receptors through an ENT-mediated modification of the extracellular levels of adenosine.
Keywords: Inosine, Adenosine, A1 receptor, A2A receptor, Synaptic transmission, Synaptic plasticity, Hippocampus
Introduction
Adenosine is a neuromodulator in the brain, acting mainly through inhibitory A1 receptors (A1R) and facilitatory A2AR [29]. The combined action of these two receptors is paramount to assist encoding salience in neuronal networks, whereby A1R decrease basal transmission and A2AR bolster synaptic plasticity [1]. The importance of this neuromodulation system is best heralded by the impact of its imbalance in different neuropsychiatric diseases and the neuroprotection afforded by bolstering A1R function or dampening A2AR overfunction [18]. In fact, noxious brain stimuli trigger the outflow of adenosine, which has a short half-life due to efficient re-uptake through equilibrative nucleoside transporters (ENTs) driven by an effective intracellular metabolism by astrocytic adenosine kinase and ubiquitous adenosine deaminase [64]. Noxious brain conditions also lead to inosine outflow, involving ENTs, which reach extracellular concentrations approximately 5–10 times higher than adenosine [8, 39, 55].
Inosine was reported to have very low affinity for brain A1R and A2AR [6, 47, 69] and is considered to be inactive on adenosine receptors based on the founding studies of the purinergic system [71]. Radioligand binding studies are typically performed in the presence of adenosine deaminase (ADA), an enzyme that converts adenosine present in the preparations to inosine, and the formed inosine does not disturb the studies [3]. However, several recent studies have illustrated a neuroprotective role of inosine [20, 25, 30, 38, 52, 53, 60, 62, 63], but their use of whole animals, which are not particularly amenable to dissect detailed molecular mechanisms, did not allow clarifying if inosine was directly acting through activation of adenosine receptors or rather through an indirect mechanism.
Since adenosine neuromodulation of neuronal activity in hippocampal slices is well-established to involve a combined action of A1R-mediated inhibition of synaptic transmission and an A2AR-mediated facilitation of synaptic plasticity, namely, long-term potentiation [46], with a discrete effect of A2BR [34] or A3R [24, 44], we now resorted to a hippocampal slice model in the present study to clarify if and how inosine affects neuronal activity.
Materials and methods
Animals
Male C57BL/6 J mice (8–12 weeks old; total of 30) and male Wistar rats (8–12 weeks old; total of 32) were from Charles River (Barcelona, Spain). Animals were maintained in groups of two to five per cage in a temperature-controlled room (22 ± 1 °C), with free access to food and water, and with a 12-h light/dark cycle (lights on at 7:00 am). Animals were handled according to ARRIVE guidelines, as approved by CNC Ethical Committee for Animal Research (ORBEA-128/2015).
Drugs
Inosine, DPCPX (1,3-dipropyl-8-cyclopentylxanthine; Cat# 1111), SCH58261 (2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; Cat# 2270), and dipyridamole (Cat# 0691) were from Tocris (Bristol, UK). Adenosine deaminase (ADA; Cat# 10102105001) and NBTI (S-(p-nitrobenzyl)-6-thioinosine; Cat# N2255) were from Sigma. [3H]SCH58261 (specific activity of 77 Ci/mmol; prepared by Amersham) was generously offered by Dr. Ennio Ongini (Shering-Plough, Milan, Italy) and [3H]DPCPX (specific activity of 102.1 Ci/mmol) was from DuPont NEN (Boston, MA, USA). The precursor of [3H]2-chloro-N6-cyclopentyladenosine (CCPA) [41], [3H]PSB-11 [51], and [3H]PSB-603 [10] were synthesized as previously described and tritium-labeled by Pharmaron, Cardiff, UK. [3H]CGS21680 and [3H]NECA (5′-N-ethylcarboxamido-adenosine) were purchased from PerkinElmer Life and Analytical Science (Rodgau-Jügesheim, Germany). 2-Chloroadenosine and NECA were purchased from Sigma-Aldrich (Taufkirchen, Germany) and Tocris Biosciences (Bristol, UK).
SCH58261, dipyridamole, and NBTI were made up as a 5 mM stock solution in dimethylsulfoxide (DMSO). DPCPX was made up into a 5 mM stock solution in 99% DMSO and 1% NaOH (1 M). All drug stock solutions were diluted directly into the superfusion solution to the appropriate final concentration. DMSO, in the maximal concentration applied to the preparations, was devoid of effects on synaptic plasticity experiments. Except for inosine, all drugs used in functional studied were used at supramaximal but selective concentrations based on our previous studies: 100 nM DPCPX [61], 50 nM SCH58261 [58], 2 U/mL ADA [17], 10 µM dipyridamole, and 5 µM NBTI [2].
Extracellular electrophysiological recordings
Electrophysiological recordings of synaptic transmission and plasticity in hippocampal slices were carried out as previously described [46]. Briefly, after deep halothane anesthesia, mice were sacrificed by decapitation and the hippocampus was dissected to prepare 400-µm-thick transverse slices from the dorsal hippocampi using a McIlwain tissue chopper (Brinkmann Instruments, USA, RRID:SCR_015798). Slices were allowed to recover functional and energetically for at least 1 h in a preincubation chamber (BSC-PC prechamber, Harvard Apparatus, Holliston, USA, No. PY2 65–0076) with gassed ACSF at 34 °C. Individual slices were then transferred to a submerged recording chamber with 1-mL capacity (Harvard Apparatus, MA, USA) and continuously superfused at a flow rate of 3 mL/min with oxygenated ACSF (control) kept at 30.5 °C (TC-202A Bipolar Temperature Controller, Harvard Apparatus, MA, USA). A bipolar concentric electrode was placed in the proximal CA1 stratum radiatum for stimulation of the Schaffer fibers every 20 s with regular pulses of 0.1 ms delivered with a Grass S44 square pulse stimulator (Grass Technologies, RI, USA). The orthodromically evoked field excitatory post-synaptic potentials (fEPSP) were recorded through an extracellular microelectrode pipette filled with NaCl 4 M yielding a 1–2-MΩ resistance and placed in the CA1 stratum radiatum targeting the distal dendrites of pyramidal neurons. Recordings were obtained with an ISO-80 amplifier (World Precision Instruments, Hertfordshire, UK), digitized using an analog-to-digital converter (BNC-2110, National Instruments, Newbury, UK), and analyzed using the WinLTP version 2.20.1 software (WinLTP Ltd., UK, RRID: SCR_008590) [5]. An input/output curve (I/O) (percentage of maximum fEPSP slope versus stimulus intensity) was obtained to select a stimulation intensity yielding ∼40% of maximal fEPSP response. After a minimum period of 30 min to fully stabilize baseline fEPSP responses in the absence or presence of tested drugs and resetting stimulation to yield ∼40% of maximal fEPSP response, LTP was induced by a high-frequency stimulation (HFS) train (100 Hz for 1 s) [46]. LTP magnitude was calculated as the percentage change between the average slope of the ten averaged potentials taken between 50 and 60 min after terminating LTP induction in relation to the average slope of the fEPSP measured during the 10 min that preceded LTP induction. The effect of drugs on LTP was assessed by comparing LTP magnitude in the absence and presence of the drug in experiments carried out in different slices from the same animal [46].
Radioligand binding assays
Radioligand binding assays at human and rat adenosine receptor subtypes were performed as previously described using membranes from CHO cells heterologously expressing the different adenosine receptors or rat brain membranes, prepared as previously described [3, 10]. All experiments were performed in the presence of 2 U/mL ADA using the suitable radioligands for each receptor subtype, as indicated below in each subsection.
After the indicated incubation time, the assay mixtures were filtered through GF/B glass fiber filters using a Brandel harvester (Brandel, Gaithersburg, MD, USA). Filters were washed three times (3–4 mL each) with ice-cold 50 mM Tris–HCl buffer, pH 7.4. Then, the filters were transferred to scintillation vials, incubated for 9 h with 2.5 mL of scintillation cocktail (Luma Safe, PerkinElmer), and counted in a liquid scintillation counter (Tri-Carb 2810 TR) with a counting efficiency of 53%. All data were analyzed with GraphPad Prism (GraphPad Inc., La Jolla, CA).
Radioligand binding assays at human and rat A1 adenosine receptors
Competition binding experiments using the agonist radioligand [3H]2-chloro-N6-cyclopentyladenosine (CCPA) were performed in a final volume of 400 µL containing 10 µL of test compound dissolved in DMSO, 190 µL buffer (50 mM Tris–HCl, pH 7.4), 100 µL of radioligand solution in the same buffer (final concentration 1 nM), and 100 µL of a suspension of a membrane preparation (human: 6 µg of protein per vial in buffer, recombinantly expressed human A1 adenosine receptor in CHO cells; lot #1604613, PerkinElmer; rat: rat brain cortical membrane preparation, 150 µg of protein per vial). Non-specific binding was determined in the presence of 2-chloroadenosine (CADO) (final concentration 10 µM) and the incubation time was 90 min at room temperature.
Radioligand binding assays at human and rat A2A adenosine receptors
Competition binding experiments using the agonist radioligand [3H]CGS21680 were performed in a final volume of 400 µL containing 10 µL of test compound dissolved in DMSO, 190 µL buffer (50 mM Tris–HCl, 10 mM MgCl2, pH 7.4), 100 µL of radioligand solution in the same buffer (final concentration 5 nM), and 100 µL of membrane preparation (human: 4 µg of protein per vial in buffer, recombinantly expressed in CHO cells; rat: rat brain striatal membrane preparation, 200 µg protein per vial). Non-specific binding was determined in the presence of 5′-N-ethylcarboxamidoadenosine (NECA; final concentration 10 µM) and the incubation period was 120 min at room temperature.
Radioligand binding assays at human and rat A2B adenosine receptors
Competition binding experiments with [3H]NECA were performed in a final volume of 1 mL containing 10 µL of test compound dissolved in 100% DMSO, 790 µL buffer (50 mM Tris–HCl, 10 mM MgCl2, pH 7.4), 100 μL of radioligand solution in the same buffer (final concentration 30 nM), and 100 μL of membrane preparation (human: 20 μg protein per vial in the same buffer of Lot #1599597 or 9.5 µg protein per vial in the same buffer of Lot # 2302323, PerkinElmer; rat: 70 µg protein per vial in buffer). Non-specific binding was determined in the presence of 250 μM (final concentration) NECA and the incubation time was 4 h at 4 °C.
Competition binding experiments with [3H]PSB-603 were performed in a final volume of 1 mL containing 10 µL of test compound dissolved in DMSO, 790 µL buffer (50 mM Tris–HCl, pH 7,4), 100 μL of radioligand solution in the same buffer (final concentration 0.3 nM), and 100 μL of membrane preparation (10 μg protein per vial in the same buffer, Lot 1599597, PerkinElmer). Non-specific binding was determined in the presence of 10 µM (final concentration) DPCPX and the incubation time was 75 min at room temperature.
Radioligand binding assays at human and rat A3 adenosine receptors
Competition binding experiments with [3H]5′-N-ethylcarboxamidoadenosine (NECA) were performed in a final volume of 400 µL containing 4 µL of test compound dissolved in DMSO, 200 µL of buffer (50 mM Tris–HCL, 10 mM MgCl2, 1 mM EDTA, pH 8.25), 100 µL of radioligand solution in the same buffer (final concentration 10 nM), and 100 µL of membrane preparation (human: 9 µg protein per vial in buffer, Lot #1619113, PerkinElmer or 30 µg of protein per vial in buffer, transiently transfected CHO-S cell membrane preparations; rat: 35 µg of protein per vial in buffer, Lot # 1648417, PerkinElmer or 30 µg of protein per vial in buffer, transiently transfected CHO-S cell membrane preparations). Non-specific binding was determined in the presence of N6-((R)-2-phenylisopropyl)adenosine (final concentration 100 µM) and the incubation time was 180 min at room temperature.
Competition binding experiments with [3H]PSB-11 were performed in a final volume of 400 µL containing 4 µL of test compound dissolved in DMSO, 196 µL buffer (50 mM Tris–HCL, pH 7.4), 100 µL of radioligand solution in the same buffer (final concentration 1 nM), and 100 µL of membrane preparation (150 µg protein per vial in buffer). Non-specific binding was determined in the presence of N6-((R)-2-phenylisopropyl)adenosine (final concentration 100 µM) and the incubation time was 45 min at room temperature.
Radioligand binding assays at synaptic A1R and A2AR
The competition by inosine (0.1–1000 μM) of the binding of the selective A1R antagonist [3H]DPCPX (2 nM) or of the selective A2AR antagonist [3H]SCH58261 (2 nM) was performed as previously described [45] using synaptosomal membranes from rat cerebrocortical synaptosomes, prepared as previously described [57]. Briefly, the binding of [3H]DPCPX (2 nM) or of [3H]SCH58261 (2 nM) was carried out for 2 h at 37 °C and for 4 h at room temperature (23–25 °C), with 62–110 μg of membrane protein in a final volume of 200 μL in an incubation solution containing 50 mM Tris–HCl and MgCl2 (2 mM for A1R, 10 mM for A2AR) at pH 7.4, with 4 U/mL adenosine deaminase. Specific binding was determined by subtraction of the non-specific binding which was measured in the presence of 2 μM XAC. The binding reactions were stopped by vacuum filtration through Whatman GF/C glass fiber filters, followed by washing of the filters and reaction tubes with 5 mL of the incubation solution, kept at 4 °C. The filters were then placed in scintillation vials, and 2 mL of scintillation liquid (Scintran Cocktail T; Wallac, Turku, Finland) was added. Radioactivity bound to the filters was determined after 12 h with an efficiency of 55–60% for 2 min. Binding data was expressed as the percentage of binding of [3H]DPCPX (2 nM) or [3H]SCH58261 (2 nM) in the absence of competitor and adjusted by non-linear regression assuming one single binding site and complete displacement to first calculate the IC50 values and then the corresponding Ki values according to the Cheng and Prusoff equation using the previously determined KD values of [3H]DPCPX (1.2 nM) and of [3H]SCH58261 (0.6 nM) binding to synaptosomal membranes [45].
Statistics
In all experimental procedures, four or more animals were used for each parameter analyzed and the individual sample size (n = number of animals) is specified for each experiment. All data are presented as mean ± SEM and significance was considered at p < 0.05 using a Student’s t test to determine the effects of a drug versus baseline, whereas comparison between two groups was carried out using a paired Student’s t test.
Results
To investigate the impact of inosine on synaptic transmission and plasticity in representative neuronal circuits such as in the hippocampus, it is first important to define the concentration levels of inosine expected to occur in the brain parenchyma. Typically, the measured concentration of extracellular inosine in brain preparations is approximately 10 times greater than that of adenosine [8, 39, 55]. Since extracellular adenosine levels under non-noxious conditions in hippocampal excitatory synapses are in the low micromolar range [17, 21, 23], we tested inosine in concentrations ranging from 30 to 300 μM.
Involvement of adenosine receptors in the ability of inosine to control synaptic transmission and plasticity
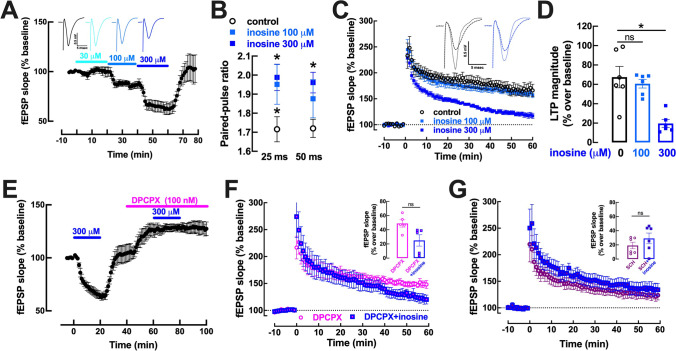
In Schaffer fibers-CA1 pyramid synapses of hippocampal slices from adult mice, inosine did not significantly affect the evoked field excitatory post-synaptic potential (fEPSPs) at a concentration of 30 μM (Fig. 1A), but depressed fEPSPs in an apparent concentration-dependent manner at higher concentrations with an inhibition of 13.8 ± 3.5% (n = 6, p = 0.011) at 100 μM and of 32.6 ± 2.9% (n = 6, p < 0.001) at 300 μM (Fig. 1A), essentially as previously reported by others [4]. This likely resulted from a presynaptic effect, since 100–300 μM inosine increased paired-pulse facilitation using inter-pulse intervals of either 25 ms or 50 ms (Fig. 1B). When studying long-term potentiation (LTP) as a representative plastic process in hippocampal synapses, we observed that 100 μM inosine did not significantly alter LTP magnitude (9.65 ± 1.73% inhibition; n = 6, p = 0.736), but 300 μM inosine decreased LTP magnitude by 71.0 ± 18.8% (n = 6, p < 0.001) (Fig. 1C, D).
Fig. 1.
The inhibitory effect of inosine on synaptic transmission and plasticity in mouse hippocampal slices is mediated by adenosine A1 and A2A receptors. A Although inactive at a concentration of 30 μM, inosine concentration dependently inhibited synaptic transmission at concentrations of 100–300 μM. The inserts correspond to representative fEPSPs under each condition. B Inosine (100–300 μM) increase paired pulse facilitation with inter-pulse intervals of 25 and 50 ms, suggesting that inosine-induced inhibition of synaptic transmission is exerted presynaptically. C Although devoid of significant effects at 100 μM, at 300 μM inosine depressed the magnitude of long-term potentiation induced by a high-frequency (HF) train (100 Hz for 1 s) applied at time 0. The inserts correspond to representative fEPSPs under each condition, dashed lines before delivering the HF, and filled lines 60 min after the HF train. D Average values of LTP magnitude calculated as the variation of fEPSP slope before and 60 min after HF stimulation. E The selective A1 receptor antagonist DPCPX (100 nM) prevented the ability of 300 μM inosine to decrease synaptic transmission. F DPCPX also curtailed the ability of inosine to inhibit LTP (insert with average values). G The selective A2A receptor antagonist SCH58261 (50 nM) also fully eliminated the ability of inosine to inhibit LTP (insert with average values). Data are mean ± SEM of 5–6 determination (independent mice). *p < 0.05 with paired Student’s t test, ns: non-significant
Since it is well established that hippocampal synaptic transmission in adult rodents is mostly under the control of adenosine A1 receptors (A1R), without a relevant participation of A2AR [16, 19] with only discrete modulatory roles of A3R and A2BR that seem strictly dependent on A1R function [24, 34, 44], we tested the impact of a supramaximal concentration of the selective A1R antagonist DPCPX (100 nM; see [61] on the inhibition by inosine of synaptic transmission and plasticity. DPCPX (100 nM) caused the expected increase of fEPSP slopes (25.5 ± 7.8%; n = 6, p = 0.022; Fig. 1E), due to the disinhibition of synaptic transmission by eliminating the tonic activation of inhibitory A1R by endogenous adenosine (e.g., [61]. As shown in Fig. 1E, F, DPCPX abrogated the ability of 300 μM inosine to depress synaptic transmission (Fig. 1E,n = 6, p = 0.458 vs. DPCPX alone) and LTP magnitude (Fig. 1F; n = 5, p = 0.104 vs. DPCPX alone). As an important control, we confirmed that two successive application of 300 μM inosine separated by 30 min caused a similar depression of synaptic transmission (29.9 ± 2.8% inhibition in the first application and 39.1 ± 3.4% inhibition in the second application; n = 4, p = 0.066).
Given that the A2AR is the predominant adenosine receptor controlling hippocampal LTP (reviewed in [18], with inconsistent effects of A3R [16, 48] and A2AR control A1R function in the hippocampus [42, 43], we further tested the impact of a supramaximal concentration of the selective A2AR receptor SCH58261 (50 nM; see [58] on the inhibitory effects of inosine on synaptic plasticity. As shown in Fig. 1G, SCH58261 (50 nM) abrogated the ability of 300 μM inosine to significantly reduce LTP magnitude (n = 6, p = 0.311 vs. SCH58261 alone).
Overall, these findings indicate that the ability of inosine to affect synaptic transmission and plasticity is strictly dependent on the functioning of the two adenosine receptors — A1R and A2AR — with more evident effects on the control of synaptic transmission and plasticity (reviewed in [29].
Impact of inosine on the binding of radioligands to adenosine receptors
To test the ability of inosine to directly bind to adenosine receptors, we first carried out radioligand binding studies using cell membrane preparations of cells heterologously expressing the different human adenosine receptor subtypes or membrane preparations obtained from the rat cerebral cortex, where A1R are predominant, and from rat striatum, where A2AR is most abundant (e.g., [57]. Agonist rather than antagonist radioligands were employed in order to label the high-affinity conformation for agonists. As can be seen in Table 1, inosine was only able to displace binding to either human or rat A1R, albeit with very low potency (Ki approximately 100 µM) and was essentially ineffective in displacing binding to human or rat A2AR and A2BR within the estimated concentrations of inosine occurring in the brain (n = 3–4). Likewise, it showed no affinity for the human A3R, but significant affinity for the rat A3R (Ki value of 1.37 ± 1.08 µM; n = 3) (Fig. 2A).
Table 1.
Affinity of inosine at human and rat adenosine receptor subtypes determined in radioligand binding assays
| Ki (µM)a (or percent inhibition at indicated concentration) | |||
|---|---|---|---|
| A1 vs. [3H]CCPA | A2A vs. [3H]CGS21680 | A2B vs. [3H]NECA | A3 vs. [3H]NECA |
|
Human 94 ± 18 µM Rat 101 ± 22 µM |
Human > 300 µM (6%) Rat > 300 µM (15%) |
Human > 300 µM (40%)b Rat > 300 µM (0%) |
Human > 300 µM (20%)c Rat 1.37 ± 1.08 µM |
aDetermined in 3 to 4 separate experiments. Ki values are shown in bold
bVersus the antagonist radioligand [3H]PSB-603, the following data were determined: 0–4% inhibition at 300 µM (in the presence of ADA)
cVersus [3H]PSB-11
Bold indicates values of potentially physiological significance
Fig. 2.
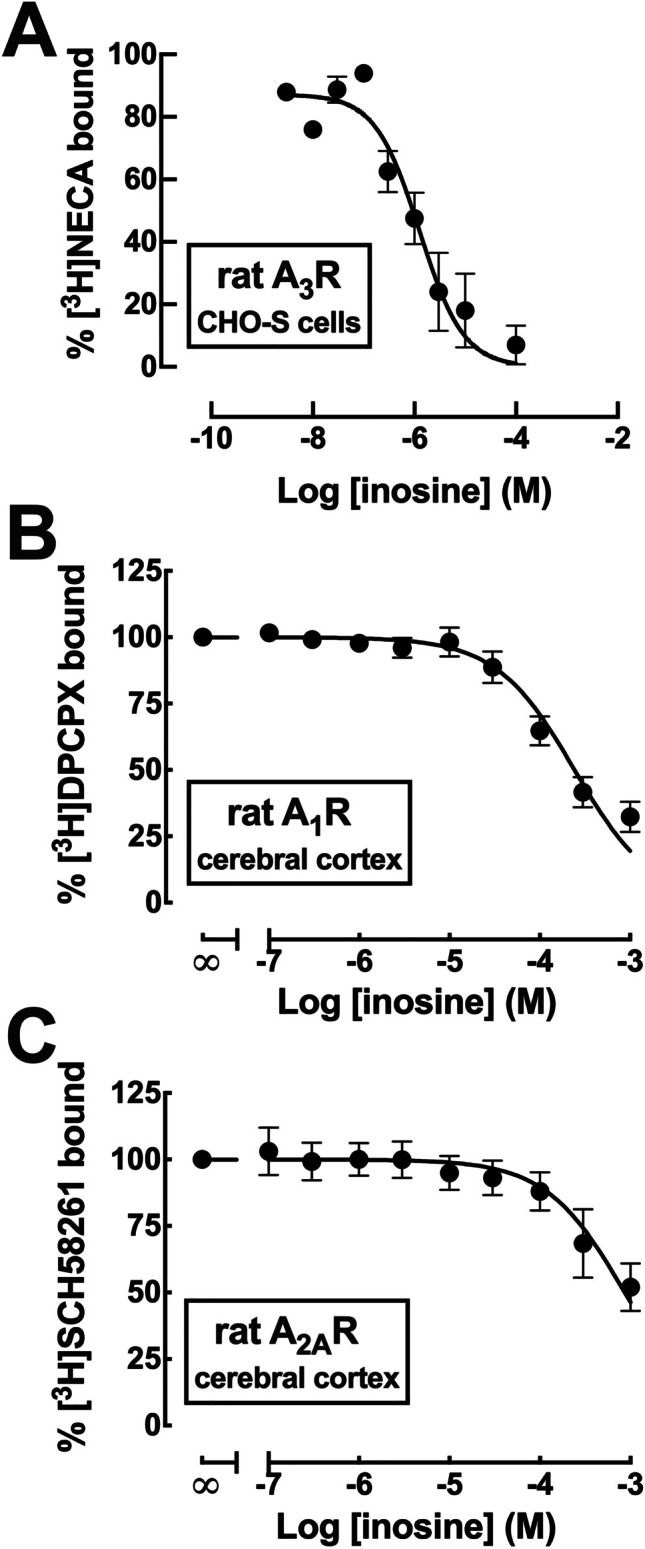
Ability of inosine to displace radioligand binding to rat A3 adenosine receptors, but not to rat A1 or A2A adenosine receptors. A Concentration-dependent inhibition by inosine of the binding of the non-selective adenosine receptor ligand [3H]NECA to membranes of Chinese hamster ovary suspension (CHO-S) cells recombinantly expressing rat adenosine A3 receptors. The data are mean ± SEM of 3 experiments, yielding a Ki value 1.37 µM. B Concentration-dependent inhibition by inosine of the binding of the adenosine A1 receptor selective antagonist [3H]DPCPX to membranes of synaptosomes from the rat cerebral cortex. The data are mean ± SEM of 4 experiments, yielding a Ki value 108 μM. C Concentration-dependent inhibition by inosine of the binding of the adenosine A2A receptor selective antagonist [3H]SCH58261 to membranes of synaptosomes from the rat cerebral cortex. The data are mean ± SEM of 4 experiments, yielding a Ki value 200 μM. Note that the displayed data only allow a direct estimation of the IC50 values of inosine but not of the Ki values, which require computing the concentration of the radioligand and is KD for the receptor
Given that synaptic transmission and plasticity are controlled by synaptic A1R and A2AR, we next assessed the ability of inosine to compete for A1R and A2AR binding in synaptic membranes. In the absence of competitors, the specific binding of the selective A1R antagonist, [3H]DPCPX (2 nM), to cerebrocortical synaptosomal membranes was 1230 ± 30 fmol/mg protein (n = 4) and that of the selective A2AR antagonist, [3H]SCH58261 (2 nM), was 42.2 ± 4.2 fmol/mg protein (n = 4). Inosine displayed low affinity to displace binding to synaptic A1R and A2AR, with estimated Ki values of 108 μM (95% confidence interval of 84–138 μM, n = 4) and of 200 μM (95% confidence interval of 130–307 μM, n = 4), respectively (Fig. 2B, C).
Involvement of endogenous extracellular adenosine in the ability of inosine to control synaptic transmission and plasticity
In order to unravel if the effects of inosine were due to a control of the levels of extracellular adenosine tonically activating adenosine receptors, we tested the effects of inosine in the presence of a supramaximal concentration of adenosine deaminase (ADA; 2 U/mL) to eliminate extracellular adenosine levels [17]. As shown in Fig. 3A–C, ADA (2 U/mL) caused the expected increase of fEPSP slopes (31.8 ± 5.0%; n = 6, p = 0.001; Fig. 3A) and abrogated the ability of 300 μM inosine to depress synaptic transmission (Fig. 3A; n = 6, p = 0.753 vs. ADA alone) and LTP magnitude (Fig. 3B, C; n = 4–5, p = 0.156 vs. ADA alone).
Fig. 3.
The inhibitory effect of inosine on synaptic transmission and plasticity in mouse hippocampal slices is strictly dependent on extracellular adenosine, likely released through equilibrative nucleoside transporters. A The removal of extracellular adenosine by superfusing adenosine deaminase (ADA; 2 U/mL) prevented the ability of 300 μM inosine to decrease field excitatory post-synaptic potentials (fEPSPs) in Schaffer fiber-CA1 pyramid synapses of mouse hippocampal slices. B Likewise, inosine did not significantly modify the magnitude of long-term potentiation (LTP) induced by a high-frequency train (100 Hz for 1 s) applied at time 0, as quantified in (C). D The blockade of equilibrative nucleoside transporters with the simultaneous presence of NBTI (5 µM) and dipyridamole (10 µM) eliminated the ability of 300 μM inosine to inhibit synaptic transmission. E Likewise, inosine did not significantly modify LTP magnitude, as quantified in (C). Data are mean ± SEM of 4–6 determination (independent mice). ns: non-significant with paired Student’s t test
It should be kept in mind that the basal extracellular levels of adenosine within hippocampal excitatory synapses have been estimated to be in the range of 0.14–4 μM [17, 21, 23], making it unlikely that the full conversion of extracellular adenosine by exogenously added ADA would generate an amount of extracellular inosine able to occlude any eventual effect of exogenously added inosine, especially since we observed that a concentration of inosine (30 μM, which is over 10 times greater than that estimated for extracellular adenosine) was devoid of effects on synaptic transmission (see Fig. 3B). Therefore, the ability of ADA to eliminate the effects of inosine indicates that the ability of inosine to affect synaptic transmission and plasticity is mostly dependent on its ability to control of the extracellular levels of adenosine, thereby indirectly affecting adenosine receptor activation.
Role of nucleoside transporter-derived extracellular adenosine in the ability of inosine to control synaptic transmission and plasticity
Since equilibrative nucleoside transporters (ENTs) use both inosine and adenosine as substrates [54], the most parsimonious explanation to understand the strict dependency on extracellular adenosine for inosine to control synaptic transmission and plasticity would be that the exposure to increase extracellular levels of inosine would drive an exchange with adenosine through ENTs. To test this hypothesis, we studied the effects of inosine in the simultaneous presence of effective concentrations (see [2] of an ENT-1 inhibitor, NBTI (5 μM), and a mixed ENT1/2 inhibitor dipyridamole (10 μM). As shown in Fig. 3D, E, the simultaneous presence of NBTI and dipyridamole abrogated the ability of 300 μM inosine to depress synaptic transmission (Fig. 3D,n = 4, p = 0.121 vs. NBTI + dipyridamole alone) and LTP magnitude (Fig. 3E, F,n = 5, p = 0.229 vs. NBTI + dipyridamole alone).
Discussion
The present study shows that inosine does not appear to directly engage neuronal A1R or A2AR, but instead it indirectly modifies the tonic activation of these adenosine receptors through an ENT-mediated alteration of the extracellular levels of adenosine. In fact, we observed that the selective blockade of A1R and A2AR blunted the ability of inosine to affect synaptic transmission and plasticity, indicating that these receptors are critically involved in the ability of inosine to affect neuronal function in hippocampal slices. Moreover, the removal of extracellular adenosine with ADA abrogated the effects of inosine on synaptic transmission and plasticity, showing that inosine does not directly engage A1R and/or A2AR, but strictly requires extracellular adenosine to modulate synaptic transmission and plasticity. Finally, the observation that the effects of inosine are blunted by the blockade of ENTs through the combined use of NBTI and dipyridamole shows that inosine affects synaptic transmission and plasticity via an ENT-mediated modification of the extracellular levels of adenosine controlling adenosine receptor-mediated neuromodulation. This adenosine-mediated control of synaptic transmission in hippocampal synapses is mostly operated by presynaptic adenosine receptors [43], although the engagement of post-synaptic adenosine receptors may also play a role in fine-tuning adaptive changes of the adenosine modulation system upon repeated recruitment [13]. Notably, we observed an increased variability in synaptic transmission upon washout of inosine, which is also abrogated by ADA and by inhibitors of ENTs, hinting at an eventual alteration of the bioavailability of adenosine receptors that remains to be investigated. This further indicates that different effects of inosine on synaptic function seem all dependent on extracellular adenosine released through ENTs.
The likeliness of indirect effects of inosine mediated by ENT-derived control of the extracellular levels of adenosine to control synaptic transmission and plasticity is further supported by our binding studies evaluating the influence of inosine on the binding of radioligands to adenosine receptor subtypes. In fact, we observed that inosine could only consistently affect the binding to A1R, but with a low potency of around 100 μM for either heterologously expressed human A1R or rat brain A1R, in agreement with previous reports by others obtained in different preparations [28, 53, 56, 69]. In contrast, inosine poorly displaced the binding of selective A2AR ligands to human A2AR or to rat brain A2AR, although it could displace binding to synaptic A2AR with a low potency. This is in agreement with some reports [6], but in contrast to theoretical predictions [68], and these differences may be due to an eventual impact on the binding properties of A2AR upon its homomerization and heteromerization (reviewed in [27] and interaction with different intracellular partners (reviewed in [18]. Finally, whereas it seems that inosine does not significantly displace A2BR binding in spite of previous suggestions [22], the present observation indicates that inosine fails to affect binding to human A3R but displays affinity for rodent A3R. This is consonant with findings that inosine may displace A3R binding and directly activate A3R in different peripheral preparations [15, 28, 31, 33, 35, 37, 65]. However, it should be considered that A3R displays the largest inter-species pharmacological differences among adenosine receptors [50] and its distribution, subcellular localization, and function in the brain are still poorly studied, with discrete effects on synaptic transmission and plasticity in healthy rodents [12, 24, 44].
Although the present findings exclude the hypothesis that inosine may directly activate adenosine receptors to control hippocampal synaptic transmission and plasticity, they do not discard the possibility that inosine may eventually affect non-synaptic adenosine receptors to afford brain neuroprotection. Brain dysfunction mostly involves a deregulation of synaptic function at its onset [67], but there is abundant evidence that non-synaptic mechanisms can precipitate synaptic dysfunction to trigger or amplify brain dysfunction [49]. Accordingly, adenosine-mediated control of neurodegeneration can be mostly accounted by the alteration of A1R and A2AR controlling synaptic function [18], but there is evidence that non-neuronal adenosine receptors might also affect neurodegeneration [1]. In particular, although the role of A3R in the control of neurodegeneration is still unclear [11, 14, 26, 40, 59, 66], it is possible that inosine may directly engage non-neuronal A3R, namely, glial A3R (see [9, 36], A3R in myeloid/lymphoid cells (e.g., [7, 32], or A3R in mast cells [70], to affect neurodegeneration in rodents. However, the assumption of this A3R-mediated mechanism of inosine neuroprotection requires excluding the presently reported ability of inosine to increase the extracellular levels of adenosine likely through competitive inhibition of ENTs’ activity, which emerges as the most likely mechanism of inosine-mediated neuroprotection.
In conclusion, the present study shows that inosine does not directly affect neuronal A1R and A2AR but rather indirectly controls adenosine-mediated neuromodulation by altering ENT-mediated control of the extracellular levels of adenosine. Although the present study does not rule out the possibility that inosine may directly affect non-synaptic adenosine receptors to afford neuroprotection or other non-synaptic responses, it clearly indicates that the proposal for the involvement of a direct modulation by inosine of adenosine receptor function requires excluding the involvement of an indirect effect on adenosine receptors resulting from inosine-induced adenosine outflow through ENTs. It is hoped that future studies with ENT1 and/or ENT2 knockout mice may be helpful to further confirm this hypothesis.
Author contribution
P. V., J. P. L., and C. R. L. carried out the electrophysiological experiments and analyzed the data; S. H. and C. V. carried out the receptor binding studies and analyzed the data; and J. P. L., R. A. C., and C. E. M. supervised the project and wrote the manuscript.
Funding
This study was supported by La Caixa Foundation (HP17/00523), Centro 2020 (CENTRO-01–0145-FEDER-000008: BrainHealth2020 and CENTRO-01–0246-FEDER-000010), FCT (POCI-01–0145-FEDER-03127, UIDB/04539/2020, and IF/01492/2015), and Deutsche Forschungsgemeinschaft (FOR2685, SFB1328).
Data availability
Data can be made available upon reasonable request.
Declarations
Ethics approval
This study was approved by the ORBEA_128_2015/04122015 and certified by Direção Geral de Alimentação e Veterinária (DGAV; 0421/000/000/2016 Ref. 014420).
Conflict of interest
RAC is a scientific consultant for the Institute for Scientific Information on Coffee (ISIC). All other authors declare no conflict of interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agostinho P, Madeira D, Dias L, Simões AP, Cunha RA, Canas PM. Purinergic signaling orchestrating neuron-glia communication. Pharmacol Res. 2020;162:105253. doi: 10.1016/j.phrs.2020.105253. [DOI] [PubMed] [Google Scholar]
- 2.Almeida T, Rodrigues RJ, de Mendonça A, Ribeiro JA, Cunha RA. Purinergic P2 receptors trigger adenosine release leading to adenosine A2A receptor activation and facilitation of long-term potentiation in rat hippocampal slices. Neuroscience. 2003;122:111–121. doi: 10.1016/s0306-4522(03)00523-2. [DOI] [PubMed] [Google Scholar]
- 3.Alnouri MW, Jepards S, Casari A, Schiedel AC, Hinz S, Müller CE. Selectivity is species-dependent: characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015;11:389–407. doi: 10.1007/s11302-015-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer C, Kargl L, ten Bruggencate G. Adenosinergic inhibition in hippocampus is mediated by adenosine A1 receptors very similar to those of peripheral tissues. Eur J Pharmacol. 1991;196:313–317. doi: 10.1016/0014-2999(91)90445-v. [DOI] [PubMed] [Google Scholar]
- 5.Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods. 2007;162:346–356. doi: 10.1016/j.jneumeth.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Askalan R, Richardson PJ. Role of histidine residues in the adenosine A2a receptor ligand binding site. J Neurochem. 1994;63:1477–1484. doi: 10.1046/j.1471-4159.1994.63041477.x. [DOI] [PubMed] [Google Scholar]
- 7.Barczyk K, Ehrchen J, Tenbrock K, Ahlmann M, Kneidl J, Viemann D, Roth J. Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood. 2010;116:446–455. doi: 10.1182/blood-2009-10-247106. [DOI] [PubMed] [Google Scholar]
- 8.Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, Clark RS, Dixon CE, Marion DW, Jackson E. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma. 1998;15:163–170. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- 9.Björklund O, Shang M, Tonazzini I, Daré E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Borrmann T, Hinz S, Bertarelli DC, Li W, Florin NC, Scheiff AB, Müller CE. 1-Alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem. 2009;52:3994–4006. doi: 10.1021/jm900413e. [DOI] [PubMed] [Google Scholar]
- 11.Bozdemir E, Vigil FA, Chun SH, Espinoza L, Bugay V, Khoury SM, Holstein DM, Stoja A, Lozano D, Tunca C, Sprague SM, Cavazos JE, Brenner R, Liston TE, Shapiro MS, Lechleiter JD. Neuroprotective roles of the adenosine A3 receptor agonist AST-004 in mouse model of traumatic brain injury. Neurotherapeutics. 2021;18:2707–2721. doi: 10.1007/s13311-021-01113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A1 and A3 receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Xiong C, Pancyr C, Stockwell J, Walz W, Cayabyab FS. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J Neurosci. 2014;34:9621–9643. doi: 10.1523/JNEUROSCI.3991-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi IY, Lee JC, Ju C, Hwang S, Cho GS, Lee HW, Choi WJ, Jeong LS, Kim WK. A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am J Pathol. 2011;179:2042–2052. doi: 10.1016/j.ajpath.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinalli AR, Guarracino JF, Fernandez V, Roquel LI, Losavio AS. Inosine induces presynaptic inhibition of acetylcholine release by activation of A3 adenosine receptors at the mouse neuromuscular junction. Br J Pharmacol. 2013;169:1810–1823. doi: 10.1111/bph.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costenla AR, Lopes LV, de Mendonça A, Ribeiro JA. A functional role for adenosine A3 receptors: modulation of synaptic plasticity in the rat hippocampus. Neurosci Lett. 2011;302:53–57. doi: 10.1016/s0304-3940(01)01633-0. [DOI] [PubMed] [Google Scholar]
- 17.Cunha RA. Release of ATP and adenosine and formation of extracellular adenosine in the hippocampus. In: Okada Y, editor. The role of Adenosine in the Nervous System. Amsterdam: Elsevier; 1997. pp. 135–142. [Google Scholar]
- 18.Cunha RA. How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem. 2016;139:1019–1055. doi: 10.1111/jnc.13724. [DOI] [PubMed] [Google Scholar]
- 19.Cunha RA, Johansson B, van der Ploeg I, Sebastião AM, Ribeiro JA, Fredholm BB. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res. 1994;649:208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- 20.Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E. Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 2014;1555:78–88. doi: 10.1016/j.brainres.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526:143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle C, Cristofaro V, Sack BS, Lukianov SN, Schäfer M, Chung YG, Sullivan MP, Adam RM. Inosine attenuates spontaneous activity in the rat neurogenic bladder through an A2B pathway. Sci Rep. 2017;7:44416. doi: 10.1038/srep44416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunwiddie TV, Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- 24.Dunwiddie TV, Diao L, Kim HO, Jiang JL, Jacobson KA. Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus. J Neurosci. 1997;17:607–614. doi: 10.1523/JNEUROSCI.17-02-00607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Shamarka MEA, Kozman MR, Messiha BAS. The protective effect of inosine against rotenone-induced Parkinson’s disease in mice; role of oxido-nitrosative stress, ERK phosphorylation, and A2AR expression. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1041–1053. doi: 10.1007/s00210-019-01804-1. [DOI] [PubMed] [Google Scholar]
- 26.Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol. 2003;23:431–447. doi: 10.1023/a:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferré S, Ciruela F. Functional and neuroprotective role of striatal adenosine A2A receptor heterotetramers. J Caffeine Adenosine Res. 2019;9:89–97. doi: 10.1089/caff.2019.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 29.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 30.Ganzella M, Faraco RB, Almeida RF, Fernandes VF, Souza DO. Intracerebroventricular administration of inosine is anticonvulsant against quinolinic acid-induced seizures in mice: an effect independent of benzodiazepine and adenosine receptors. Pharmacol Biochem Behav. 2011;100:271–274. doi: 10.1016/j.pbb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Gao Z, Li BS, Day YJ, Linden J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol Pharmacol. 2001;59:76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- 32.Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, Fortini C, Leung E, Mac Lennan S, Borea PA. Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation. Mol Pharmacol. 2004;65:711–719. doi: 10.1124/mol.65.3.711. [DOI] [PubMed] [Google Scholar]
- 33.Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- 34.Gonçalves FQ, Pires J, Pliassova A, Beleza R, Lemos C, Marques JM, Rodrigues RJ, Canas PM, Köfalvi A, Cunha RA, Rial D. Adenosine A2b receptors control A1 receptor-mediated inhibition of synaptic transmission in the mouse hippocampus. Eur J Neurosci. 2015;41:878–888. doi: 10.1111/ejn.12851. [DOI] [PubMed] [Google Scholar]
- 35.Guinzberg R, Cortés D, Díaz-Cruz A, Riveros-Rosas H, Villalobos-Molina R, Piña E. Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am J Physiol Endocrinol Metab. 2006;290:E940–E951. doi: 10.1152/ajpendo.00173.2005. [DOI] [PubMed] [Google Scholar]
- 36.Hammarberg C, Schulte G, Fredholm BB. Evidence for functional adenosine A3 receptors in microglia cells. J Neurochem. 2003;86:1051–1054. doi: 10.1046/j.1471-4159.2003.01919.x. [DOI] [PubMed] [Google Scholar]
- 37.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaster MP, Budni J, Gazal M, Cunha MP, Santos AR, Rodrigues AL. The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A2A receptors. Purinergic Signal. 2013;9:481–486. doi: 10.1007/s11302-013-9361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kékesi KA, Kovács Z, Szilágyi N, Bobest M, Szikra T, Dobolyi A, Juhász G, Palkovits M. Concentration of nucleosides and related compounds in cerebral and cerebellar cortical areas and white matter of the human brain. Cell Mol Neurobiol. 2006;26:833–844. doi: 10.1007/s10571-006-9103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Li X, Deng P, Wang D, Bai X, Li Y, Luo C, Belguise K, Wang X, Wei X, Xia Z, Yi B. Activation of adenosine A3 receptor reduces early brain injury by alleviating neuroinflammation after subarachnoid hemorrhage in elderly rats. Aging. 2020;13:694–713. doi: 10.18632/aging.202178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohse MJ, Klotz KN, Schwabe U, Cristalli G, Vittori S, Grifantini M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- 42.Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 43.Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience. 2002;112:319–329. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 44.Lopes LV, Rebola N, Costenla AR, Halldner L, Jacobson MA, Oliveira CR, Richardson PJ, Fredholm BB, Ribeiro JA, Cunha RA. Adenosine A3 receptors in the rat hippocampus: lack of interaction with A1 receptors. Drug Dev Res. 2003;58:428–438. doi: 10.1002/ddr.10188. [DOI] [Google Scholar]
- 45.Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br J Pharmacol. 2004;141:1006–1014. doi: 10.1038/sj.bjp.0705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopes JP, Pliássova A, Cunha RA. The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem Pharmacol. 2019;166:313–321. doi: 10.1016/j.bcp.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Lorenzen A, Nitsch-Kirsch M, Vogt H, Schwabe U. Characterization of membrane-bound and solubilized high-affinity binding sites for 5′-N-ethylcarboxamido[3H]adenosine from bovine cerebral cortex. J Neurochem. 1993;60:745–751. doi: 10.1111/j.1471-4159.1993.tb03210.x. [DOI] [PubMed] [Google Scholar]
- 48.Maggi L, Trettel F, Scianni M, Bertollini C, Eusebi F, Fredholm BB, Limatola C. LTP impairment by fractalkine/CX3CL1 in mouse hippocampus is mediated through the activity of adenosine receptor type 3 (A3R) J Neuroimmunol. 2009;215:36–42. doi: 10.1016/j.jneuroim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Matejuk A, Ransohoff RM. Crosstalk between astrocytes and microglia: an overview. Front Immunol. 2020;11:1416. doi: 10.3389/fimmu.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller CE. Medicinal chemistry of adenosine A3 receptor ligands. Curr Top Med Chem. 2003;3:445–462. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- 51.Müller CE, Diekmann M, Thorand M, Ozola V. [3H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1–i]-purin-5-one ([3H]PSB-11), a novel high-affinity antagonist radioligand for human A3 adenosine receptors. Bioorg Med Chem Lett. 2002;12:501–503. doi: 10.1016/s0960-894x(01)00785-5. [DOI] [PubMed] [Google Scholar]
- 52.Muto J, Lee H, Lee H, Uwaya A, Park J, Nakajima S, Nagata K, Ohno M, Ohsawa I, Mikami T. Oral administration of inosine produces antidepressant-like effects in mice. Sci Rep. 2014;4:4199. doi: 10.1038/srep04199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nascimento FP, Macedo-Júnior SJ, Pamplona FA, Luiz-Cerutti M, Córdova MM, Constantino L, Tasca CI, Dutra RC, Calixto JB, Reid A, Sawynok J, Santos AR. Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological, genetic and biochemical aspects. Mol Neurobiol. 2015;51:1368–1378. doi: 10.1007/s12035-014-8815-5. [DOI] [PubMed] [Google Scholar]
- 54.Parkinson FE, Damaraju VL, Graham K, Yao SY, Baldwin SA, Cass CE, Young JD. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr Top Med Chem. 2011;11:948–972. doi: 10.2174/156802611795347582. [DOI] [PubMed] [Google Scholar]
- 55.Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab. 1987;7:679–686. doi: 10.1038/jcbfm.1987.122. [DOI] [PubMed] [Google Scholar]
- 56.Ragazzi E, Wu SN, Shryock J, Belardinelli L. Electrophysiological and receptor binding studies to assess activation of the cardiac adenosine receptor by adenine nucleotides. Circ Res. 1991;68:1035–1044. doi: 10.1161/01.res.68.4.1035. [DOI] [PubMed] [Google Scholar]
- 57.Rebola N, Porciúncula LO, Lopes LV, Oliveira CR, Soares-da-Silva P, Cunha RA. Long-term effect of convulsive behavior on the density of adenosine A1 and A2A receptors in the rat cerebral cortex. Epilepsia. 2005;46(Suppl 5):159–165. doi: 10.1111/j.1528-1167.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- 58.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Rosito M, Deflorio C, Limatola C, Trettel F. CXCL16 orchestrates adenosine A3 receptor and MCP-1/CCL2 activity to protect neurons from excitotoxic cell death in the CNS. J Neurosci. 2012;32:3154–3163. doi: 10.1523/JNEUROSCI.4046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruhal P, Dhingra D. Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacology. 2018;26:1317–1329. doi: 10.1007/s10787-018-0476-y. [DOI] [PubMed] [Google Scholar]
- 61.Sebastião AM, Cunha RA, de Mendonça A, Ribeiro JA. Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br J Pharmacol. 2000;131:1629–1634. doi: 10.1038/sj.bjp.0703736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–659. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira FC, de Mattos BDS, Mello JE, Cardoso J, Spohr L, Luduvico KP, Soares MSP, Carvalho FB, Gutierres JM, Oliveira Campello Felix A, Stefanello FM, Spanevello RM. Protective effects of inosine on memory consolidation in a rat model of scopolamine-induced cognitive impairment: involvement of cholinergic signaling, redox status, and ion pump activities. Neurochem Res. 2022;47:446–460. doi: 10.1007/s11064-021-03460-5. [DOI] [PubMed] [Google Scholar]
- 64.Tescarollo FC, Rombo DM, DeLiberto LK, Fedele DE, Alharfoush E, Tomé ÂR, Cunha RA, Sebastião AM, Boison D. Role of adenosine in epilepsy and seizures. J Caffeine Adenosine Res. 2020;10:45–60. doi: 10.1089/caff.2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J Clin Invest. 2000;105:361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Von Lubitz DK, Lin RC, Boyd M, Bischofberger N, Jacobson KA. Chronic administration of adenosine A3 receptor agonist and cerebral ischemia: neuronal and glial effects. Eur J Pharmacol. 1999;367:157–163. doi: 10.1016/s0014-2999(98)00977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waites CL, Garner CC. Presynaptic function in health and disease. Trends Neurosci. 2011;34:326–337. doi: 10.1016/j.tins.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Welihinda AA, Kaur M, Greene K, Zhai Y, Amento EP. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal. 2016;28:552–560. doi: 10.1016/j.cellsig.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu PH, Phillis JW, Balls K, Rinaldi B. Specific binding of 2-[3H]chloroadenosine to rat brain cortical membranes. Can J Physiol Pharmacol. 1980;58:576–579. doi: 10.1139/y80-096. [DOI] [PubMed] [Google Scholar]
- 70.Xiao C, Liu N, Jacobson KA, Gavrilova O, Reitman ML. Physiology and effects of nucleosides in mice lacking all four adenosine receptors. PLoS Biol. 2019;17:e3000161. doi: 10.1371/journal.pbio.3000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribeiro JA, Sebastião AM (1987) On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol 384:571–585. 10.1113/jphysiol.1987.sp016470 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon reasonable request.