Abstract
Purpose
Respiratory distress syndrome (RDS), also known as hyaline membrane disease, is the most common clinical syndrome encountered among preterm infants, and the complications of the disease account for substantial mortality. Diagnosis of RDS is based on the clinical status of patients in correlation with laboratory parameters and chest X-ray. Lung ultrasound despite its wide use still is not incorporated into diagnostic algorithms. The aim of the study was to evaluate the diagnostic ability of lung ultrasound in diagnosing respiratory distress syndrome as well as in the monitoring of the response to treatment. A secondary aim was to propose a modified ultrasound grading scale.
Methods
The prospective study included 150 neonates with clinical and radiographic signs of neonatal respiratory distress syndrome within the first 24 h of life, with different gestational age (≤ 35 weeks). Lung ultrasound was performed by two radiologists and correlated with a chest X-ray. Two gradation scales (ultrasound and X-ray) were compared and each scale was correlated with the patient’s clinical data.
Results
In comparison between ultrasound findings and X-ray results showed a statistically significant difference in a favor of ultrasound. Based on the presence of subpleural consolidations, further differentiation of ultrasound profiles were made into subgroups and new ultrasound classification have been proposed.
Conclusion
Our study showed that lung ultrasound enables the diagnosing of respiratory distress syndrome in premature neonates and also shows a significant correlation with chest X-ray, which is considered as a radiological method of choice for the diagnosis of RDS.
Keywords: Lung, Ultrasound, Neonates, Respiratory distress, X-ray
Introduction
Respiratory distress syndrome (RDS), also known as hyaline membrane disease, is the most common clinical syndrome encountered among preterm infants born at less than 32 weeks of gestation, and the complications of the disease account for substantial mortality [1]. According to Euro-peristat report, severity and incidence of RDS are in inverse correlation with gestational age with 92% of neonates born at 24–25 weeks affected, 88% at 26–27 weeks, 76% at 28–29 weeks, and 57% at 30–31 weeks [2]. Also, neonates with low gestational weight have more severe types of RDS.
For the Vermont Oxford Network, an infant defines as having RDS when it has PaO2 less than 50 mmHg with room air, central cyanosis in room air, a necessity for additional oxygen to maintain a PaO2 higher than 50 mmHg, or a blood oxygenation level over 85% within the first 24 h of life [4].
Diagnosis of RDS is based on the clinical status of patients in correlation with laboratory parameters and chest X-ray. Positive clinical signs of RDS include grunting, cyanosis, nasal flaring tachypnea, intercostal retractions, hypoventilation, hypoxemia, and respiratory acidosis [3].
Clinical staging correlates well with X-ray findings [5].
Despite lung ultrasound wide use, it is not still incorporated into diagnostic algorithm [6, 7].
The main aim of the study was to evaluate the diagnostic ability of lung ultrasound compared to X-ray in the detection of pulmonary manifestations of respiratory distress syndrome. The secondary aim was to propose a modified ultrasound grading score.
Material and methods
The prospective study included 150 neonates with clinical and radiographic signs of neonatal respiratory distress syndrome within the first 24 h of life, with different gestational age (≤ 35 weeks), examined in the NICU department of the tertiary level University Hospital.
Ethics Committee Approval and Inform consent of the parents were obtained for the study.
Examinations were performed by two pediatric radiologists with 6 and 10 years of experience. Lung ultrasound was correlated with chest X-ray and clinical signs (positive for impaired respiratory function in RDS) which are considered as the gold standard for RDS.
Examinations were conducted by using GE Ultrasound machine, Voluson 5, with 7,5 and 10 MHz linear probes in supine and both lateral decubitus positions of the anterior lung area (between the sternum and anterior axillary line), lateral lung area (between anterior and posterior axillary lines) and posterior lung area (between the posterior axillary line and the spine) in caudo-cranial direction. A complete evaluation of both lungs was mandatory [8].
During the examination, the position of the patient is changed, first we examined both anterior and lateral segments of the lungs with transverse and sagittal probe orientation, then in right or left lateral decubitus we examined both posterior parts of the lung, also with transverse and sagittal probe orientation. If the patient is intubated, the examiner needed help during the examination (from a nurse or other colleague).
We analyzed the presence of the following signs (Pictures 1, 2, 3, 4):
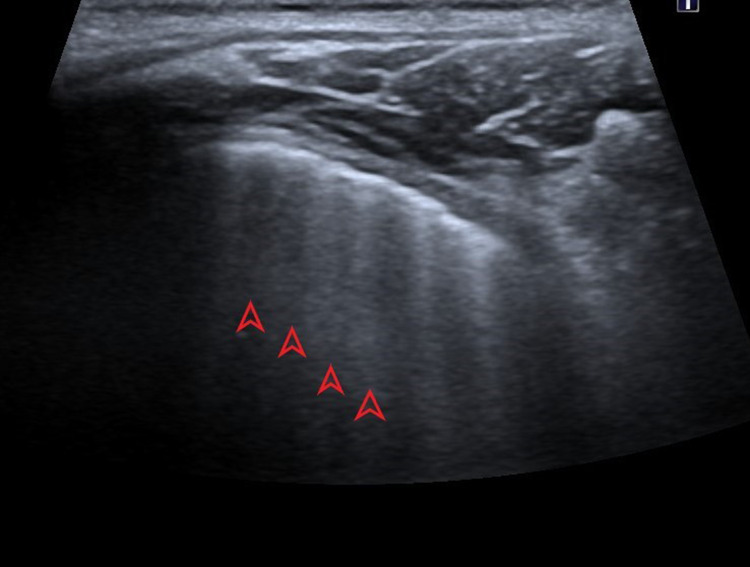
Picture 1.
Ultrasound profile 3, presence of A-lines (red arrows), parallel to horizontal hyperechoic pleural line (pointed red arrow)
Picture 2.
Ultrasound profile 2, presence of B-lines, hyperechoic vertical lines (pointed red arrows)
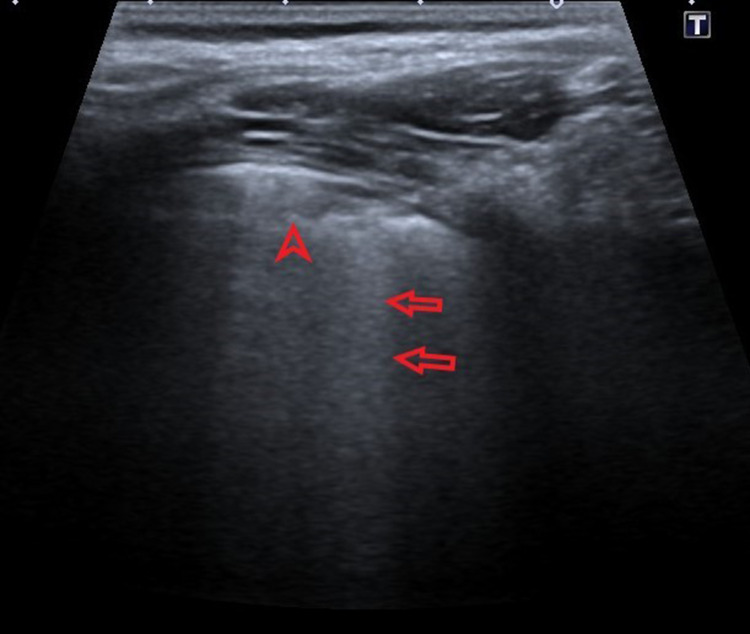
Picture 3.
Ultrasound profile 2, confluent B lines (red arrows) with subpleural consolidation (pointed red arrow)
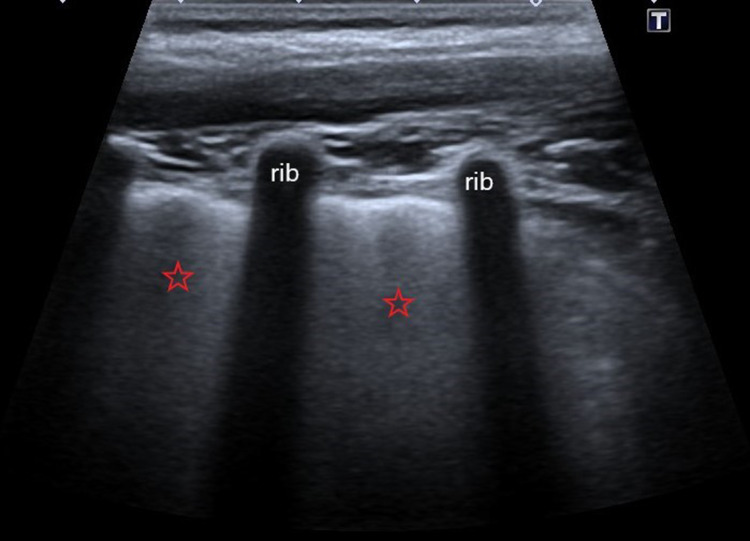
Picture 4.
Ultrasound profile 1, confluent B-lines or “white lungs” (red stars)
(a) B-lines which represent an accumulation of fluid into interstitial and alveolar space. B lines are hyperechoic vertical lines, positioned perpendicular to A-lines, erase them, and distributed from the pleural line to the bottom of the screen.
Distance between B lines points to alveolar or interstitial presentation, so we can differentiate between “white lung”—alveolar presentation or confluent B lines-interstitial presentation [9].
(b) Subpleural consolidation which can be seen as isoechoic to hypoechoic areas of the lung parenchyma, with irregular shape (round or triangular), predominantly found in subpleural space and posterior parts of the lungs.
(c) A lines—horizontal hyperechoic lines, parallel with a pleural line which represents normal findings.
(d) Thickness of pleura- hyperechoic horizontal line found just below subcutaneous and muscular layers.
Ultrasound scans were classified into three profiles, grading based on the severity of respiratory distress syndrome, exponentially from most severe to mild forms or resolution of disease [10]. Ultrasound (US) gradations are shown in Table 1 and Pictures 1, 2, 3, 4. A lung ultrasound was performed after a chest X-ray. Repeated lung ultrasound was performed after 24/36 or 48 h, respectively, according to clinical signs and clinical conditions of the patient and in some cases, simultaneously with a repeated chest X-ray.
Table 1.
Ultrasound profiles
| Profiles | Ultrasound findings |
|---|---|
| 1 | A full hyperechoic image of the lung fields or “white lung” |
| 2 | Prevalence of B-lines, lung sliding sign present |
| 3 | A-lines predominance, lung sliding sign present |
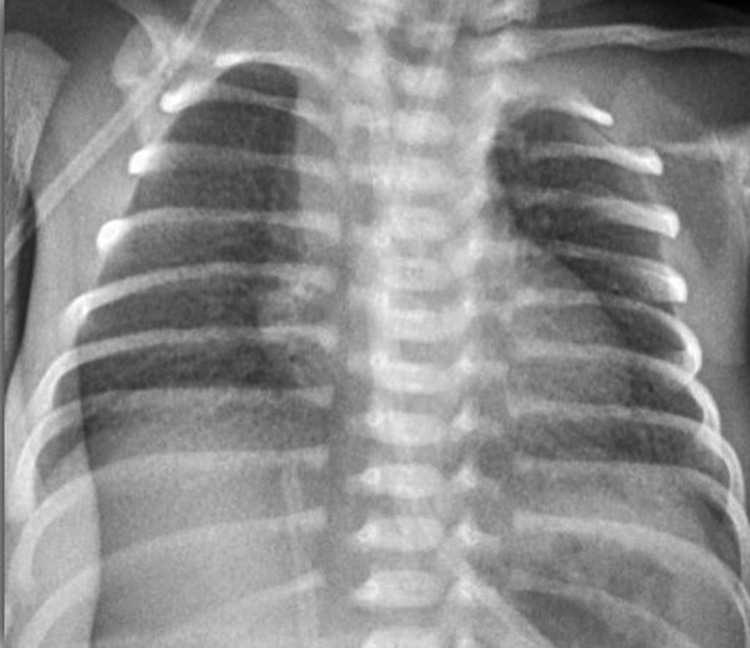
Radiographic findings were classified into four stages, from mild to severe disease [10] Table 2 and Pictures 5, 6, 7, 8.
Table 2.
X-ray grades
| Grades | X-ray findings |
|---|---|
| 1 | Fine homogenous ground glass shadowing |
| 2 | Bilateral widespread air bronchogram |
| 3 | Confluent alveolar shadowing; |
| 4 | Alveolar shadowing obscuring cardiac border |
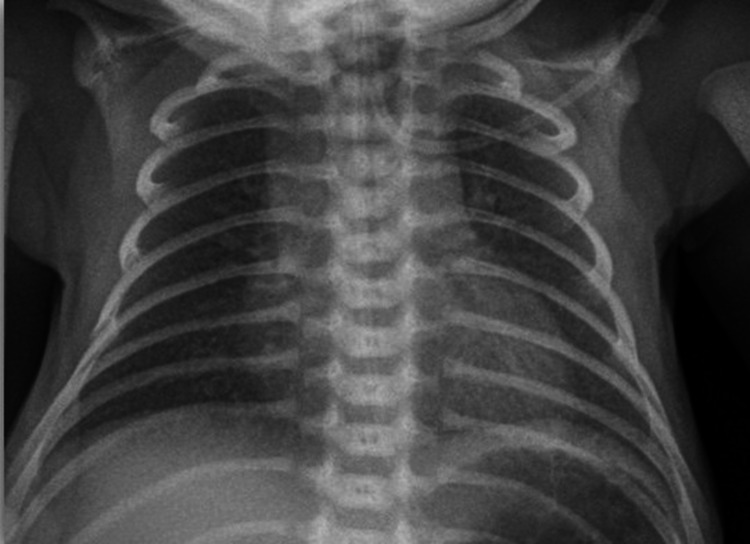
Picture 5.
Grade 1—X ray
Picture 6.
Grade 2—X ray
Picture 7.
Grade 3—X ray
Picture 8.
Grade 4—X ray
On plain X-ray radiography, there is reticulogranular or ground-glass opacification, progressive hypoaeration, and air bronchograms [11].
Chest radiography at NICU was performed with mobile X-ray machines, GE TMX+ (General Electric, Boston, MA, USA), and Agfa CR30-X computed radiography (CR) imaging system (Agfa-Gevaert, Mortsel, Belgium).
Gradation scales have inverse relation, meaning that Ultrasound profile 1 corresponds to X-ray grades 3 and 4, which are the most severe forms of RDS.
It is important to describe ultrasound findings and the terminology that is used for lung ultrasound.
Terms that are used in lung ultrasonography, besides A and B lines are lung sliding sign, parenchymal consolidation, alveolar—interstitial syndrome, lung point, double lung point, white lungs, and for M mode—“stratosphere” sign and “sandy beach” pattern in pneumothorax diagnosing.
Normal transthoracic lung ultrasound (LUS): On the interface where the parietal and visceral pleura have contact there is a reflexing surface, and the pleura is seen as a smooth, hyperechoic, horizontal line, which is moving during the respiratory cycle (lung sliding sign). Pleura thickness considers normal ≤ 5 mm [12].
Lungs are filled with air, and due to high acoustic impedance between the visceral pleura and the lung, visualization of lungs parenchyma in normal conditions is difficult, so the interpretation of ultrasound findings is based on the analysis of two types of artifacts that appear below pleural line called A and B lines, which are perpendicular to each other [13].
A-lines are horizontal lines with equal mutual distance, parallel with the pleural line, and they represent normal aeration of lung parenchyma.
B lines are vertically oriented lines, propagating from the pleural line distally. In pathological conditions, B lines erase A-lines, but usually few B-lines that do not reach the end of the screen can be found. B lines can be with more or less dense distribution, depending on the underlying pathological condition. The abundance of B-lines reflects the extent of pulmonary edema [14–16]. The presence of a B-profile with present lung sliding is a sign of interstitial edema. Contrary to this, finding the abolition of lung sliding with B profile and consolidation of lung tissue is characteristic of pneumonia due to inflammatory adherence and the presence of exudate effusion [16].
Lung ultrasound is based on the interpretation of the artifacts. In the healthy neonate with normally aerated lungs, there is a prevalence of A-lines.
Results
Descriptive statistics analyzed overall statistical data regarding gender, birth weight, gestational age, Apgar scores, type of delivery, antenatal dexamethasone therapy, and premature rupture of membranes, regardless of RDS grade. Also, we analyzed results for applied therapy CPAP, surfactant, mechanical ventilation, or Oxygen therapy as an overall estimation. Out of all conducted analyses, only results with statistical significance will be presented, besides the data of descriptive statistics.
In order to estimate the interobserver agreement between X-ray and ultrasound, a weighted Kappa test was applied.
In our study, we had an almost equal number of male and female patients (75/74). The average gestational age was 31 weeks (range between 24–35 weeks), and the average birth weight was 1660 g. Delivery with cesarean section occurred in 68% of patients while spontaneous delivery happened in 32% of patients.
In the total cohort, 89.9% of patients survived, and 10.1% died.
In comparison between ultrasound findings on the first day and X-ray (4 grades scale), Wilcoxon paired samples test showed a statistically significant difference in a favor of ultrasound (Fig. 1).
Fig. 1.
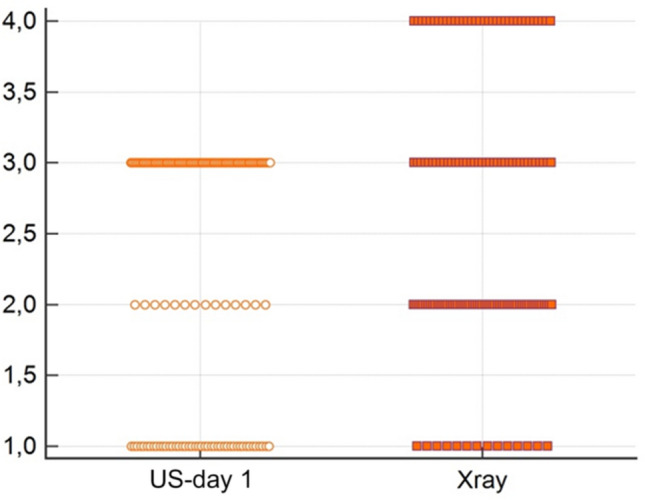
Comparison between US and X-ray on day 1
Figure 2 shows a comparison and the distribution between ultrasound profiles for days 1, 2, and 3.
Fig. 2.

Comparison of grades between ultrasound day 1, 2 and 3
Based on the presence of subpleural consolidations, further differentiation of ultrasound profiles was made into subgroup 1 and subgroup 2, where subgroup 1 represents findings with existing consolidation. In the analysis of these patients, we found a statistically significant correlation for this parameter, and these patients that have an initial ultrasound exam with the presence of subpleural consolidations had severe forms of RDS and worse outcomes (Figs. 3, 4). We consider these results as one of the most significant findings in this study.
Fig. 3.

Absolute frequency within ultrasound subgroup 1 outcome
Fig. 4.
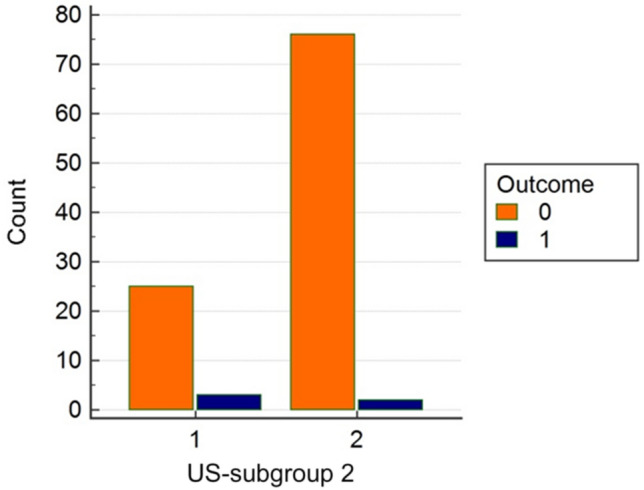
Absolute frequency within ultrasound subgroup 2 outcome
There is a statistically significant difference in gestational age and weight between US day 1-subgroup 1 and subgroup 2, where US-subgroup 1 patients (patients with consolidations), have a lower gestational age of 27.7 weeks in comparison to US-subgroup 2 with a mean gestational age of 30.5 weeks (Fig. 5).
Fig. 5.

Comparison of gestational age within ultrasound subgroup 1 and 2
Also, a significant difference was founded in the US subgroup 1 for outcome and median weight values, where in subgroup 1, the mean weight was 1101 g, and for subgroup 2 it was 1516 g (Fig. 6).
Fig. 6.
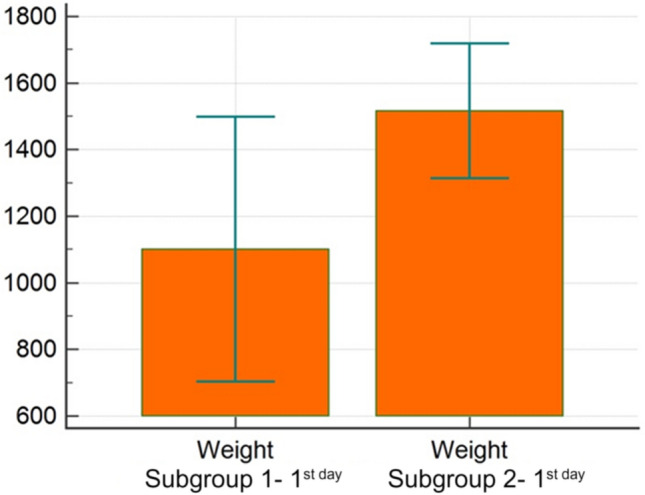
Comparison of gestational weight within ultrasound subgroup 1 and 2
In summary most patients, on US examination, had RDS grade 2, and on X-ray, it was RDS grade 1 in 70 patients (Figs. 7, 8). On ultrasound, when we analyzed each day separately, profile 2 dominated.
Fig. 7.

RDS grades on X ray
Fig. 8.

RDS profiles on US
On the second day, we found a statistically significant difference in subgroup 1 for applied therapy with O2 that shows higher values (Fig. 9) which can be explained by a higher need for oxygen in a severe grade of RDS.
Fig. 9.

Comparison of O2 therapy within ultrasound day 2 subgroups
In Table 3 data for the percentage of different complications are shown during the treatment of RDS. Most often, in 4.6% of patients, the complication was pneumothorax, followed by 3.3% of patients who had a pulmonary hemorrhage and 3.3% with BPD, whilst only 1.3% had sepsis.
Table 3.
Complications of RDS
| Type of complication | Number of patients | % |
|---|---|---|
| Pneumothorax | 7 | 4.6 |
| Pulmonary hemorrhage | 5 | 3.3 |
| BPD | 5 | 3.3 |
| Sepsis | 2 | 1.3 |
| Total | 19 | 12.6 |
We found statistically significant results with ROC analysis for gestational age and birth weight with cut-off values, meaning that a baby with a gestational age of ≤ 28 weeks and birth weight of ≤ 1210 g will have a higher probability of developing the more severe grade of respiratory distress syndrome and worse outcome (Figs. 10, 11). These results show high sensitivity and specificity, especially for birth weight (sensitivity 100%; specificity 90.3%) and respectively for gestational age (sensitivity 80%; specificity 89.5%).
Fig. 10.

Receiver operating characteristic (ROC) curve for gestational age
Fig. 11.
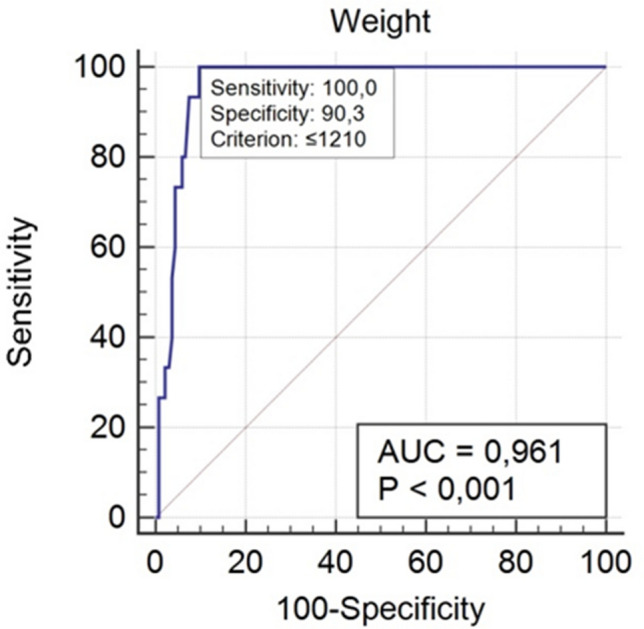
Receiver operating characteristic (ROC) curve for weight
Statistically significant results were found for Apgar scores in the 1st and 5th minute, and these scores contribute significantly to the outcome with the results even stronger for Apgar scores in the 5th minute (Tables 4, 5).
Table 4.
Descriptive statistical parameters for—Apgar score in first minute
| Sample size | 144 |
| Lowest value | 1.0000 |
| Highest value | 10.0000 |
| Arithmetic mean | 6.8472 |
| 95% CI for the arithmetic mean | 6.5347 to 7.1597 |
| Median | 7.0000 |
| 95% CI for the median | 7.0000 to 7.2404 |
| Variance | 3.5989 |
| Standard deviation | 1.8971 |
| Standard error of the mean | 0.1581 |
Table 5.
Descriptive statistical parameters for Apgar score in fifth minute
| Sample size | 134 |
| Lowest value | 2.0000 |
| Highest value | 10.0000 |
| Arithmetic mean | 7.8657 |
| 95% CI for the arithmetic mean | 7.6383 to 8.0931 |
| Median | 8.0000 |
| 95% CI for the median | 8.0000 to 8.0000 |
| Variance | 1.7713 |
| Standard deviation | 1.3309 |
| Standard error of the mean | 0.1150 |
Inter-rater agreement was calculated with the Kappa test and show moderate results (Fig. 12).
Fig. 12.
Inter-rater agreement test between X-ray and ultrasound day 1
Discussion
Lung ultrasound, despite its extensive use in clinical settings, is not still a part of official recommendations or algorithms in the patient management of various respiratory and thoracic pathological conditions. Clinical diagnosis is the preferred method for diagnosing RDS in comparison to radiological findings, although it could be used in the differentiation of extrapulmonary and intrapulmonary causes [16–18, 20].
Children are more vulnerable to potentially harmful effects of ionizing radiation, especially premature ones with immature tissues and cells which have high growth potential.
Because ultrasound is not harmful to the patient, it can be repeated as many times as is necessary, contrary to the chest X-ray examination which implies ionizing radiation. It is indisputable that the X-ray exam has its advantages in the diagnosing of respiratory distress syndrome and it can be done as an initial exam together with tube insertion checking examination (umbilical catheters or nasogastric tube positioning checking).
Analysis of RDS grades has been based on the evaluation of different scores on X-ray and ultrasound examinations, as explained in the Material and methods section.
A comparison was made between two radiological methods, X-ray as a standard way of diagnosing respiratory distress syndrome, and lung ultrasound as a relatively new tool, but still not incorporated into a diagnostic algorithm and therefore not routinely used.
Two gradation scales (ultrasound and X-ray) were compared, and each scale was correlated with patients’ clinical data.
Comparison between a chest X-ray and lung ultrasound in the diagnosis of neonatal respiratory disease was previously investigated by different authors, who published significant results in favor of lung ultrasound as a highly sensitive method in the evaluation of neonatal lungs [17–19].
Many articles evaluated lung pathology in neonates by using an ultrasound scoring system, including examination of 6 lung areas (anterior and lateral), where each area can be scored from 0–3, with a total maximum of 18 points [6, 20, 21]. In contrary to this scoring system, in our study different grading system was used, with a 3-profile ultrasound scale, which included evaluation of the posterior parts of the lungs where we found the most severe findings. These findings can be explained with a supine position of a patient where gravitation dependents parts of the lungs accumulate fluid with the presence of consolidated parenchyma. It would be interesting to compare these two different ultrasound gradation scales and to evaluate the significance and correspondence to the clinical status of a patient and the outcome.
Initially, when we compare subgroups of X-ray, grades 3 and 4 we did not find any statistical difference between them, which enables the modification of the radiographic (X-ray) scale to inverse a 3-grade scale. In this way, the comparison between ultrasound and X-ray was balanced with scales that have even gradation scoring with mutual correspondence in numbers.
The initial X-ray exam was compared to the US–day 1. The reason for not including other US examinations on day-2 and 3 in comparison to X-ray, is because repeated X-ray exams had not been performed at regular intervals whilst they were dependent on the clinical status of patients, based on the judgment of the neonatologist.
Subsequently, we made a subgroup analysis of ultrasound findings. Evaluation of ultrasound profiles was made according to the three-profile scale, previously explained in the material and methods and introduction sections. Apart from the classification in one of three profiles, different ultrasound findings were observed. One of the main pathological/ ultrasound signs is the presence of B-lines, which represent the accumulation of fluid in interstitial space and alveoli. All our patients had this positive sign, meaning that one of the conditions for the diagnosis of respiratory distress syndrome is the presence of B lines. Thick pleura was also very common in our cohort, although it is not a especially characteristic sign for the diagnosis of RDS. Partial atelectasis also was described as one of the pathological findings in these patients.
Based on the findings of subpleural consolidations on day 1, we made an additional classification of ultrasound findings into subgroup 1 and subgroup 2, depending on the presence or absence of subpleural consolidations, regardless of the ultrasound profile. Subgroup 1 included patients with consolidations.
These findings are also connected to the grade of RDS, so the combination of subpleural consolidation with completely "white lungs" or grade 1 RDS will have a worse prognosis. In different papers, subpleural consolidations were mentioned as one of the pathological ultrasound findings, but without correlation to prognosis, treatment, and outcome [22, 23].
Based on our results, we concluded that the current classification of ultrasound findings into three profiles is insufficient and too “wide” for the classification of patients with RDS, especially for the patients classified into Profile 2. Considering that subpleural consolidations represent significant findings, we proposed a modified ultrasound classification, which, in our opinion, will be more useful for the evaluation of the pathological conditions in respiratory distress syndrome (Table 6).
Table 6.
Modified ultrasound classification
| US profile | Findings | ||
|---|---|---|---|
| Profile 1 | “White” lungs—confluent B lines without spared area of lungs | 1a | With subpleural consolidations |
| Profile 2 | Prevalence of confluent B lines | 2a | With subpleural consolidations |
| Profile 3 | Prevalence of A-lines | ||
In our study birth weight was shown to be a better predictor of patient morbidity and outcome. This result is contrary to studies that concluded that gestational age as a single parameter is more appropriate than birth weight for the evaluation of premature outcomes and assessment of potentially severe postnatal morbidities [24, 25]. Although, a recently published paper described that there was not a significant difference between gestational age and birth weight to morbidity and outcome in premature patients [26].
In our study results of the Non-parametric MANOVA test showed specific cut-off values for the evaluation of patient’s prognosis and outcome as follows: patients with gestational age ≤ 28 weeks, less than 1210 g and Apgar score in 5th minute values ≤ 7 with RDS grade 3 or 4 (on X-ray) or grade 1 (on ultrasound) and US subgroup 1 had a worse outcome.
According to a study published as a part of Euro-Peristat report, there is considerable variation between Apgar score values in neonates in different countries, so this variety is not a reliable marker for the prediction of patient morbidity and mortality. The conclusion of this study refers to the evaluation of the distribution of the Apgar score during the time, rather than to the value of the Apgar score itself [27].
Radiation protection is one of the most important issues in pediatric radiology. Children are more vulnerable to potentially harmful effects of ionizing radiation, especially premature with immature tissues and cells which have high growth potential. In a paper published by Zawada et al., the advantages of lung ultrasound were described with the purpose to reduce the use of ionizing radiation at an early age [28].
In medical exposures, biological effects depend on the radiosensitivity of target tissue and organ that was exposed to ionizing radiation [29]. Justification of radiological procedure and X-ray exposures in the pediatric population have to respect the ALARA principle (“as low as reasonably achievable”), and in papers published by the American College of Radiologists and IAEA (International Agency for Atomic Energy), there are defined recommendations for the use of ionizing radiation in medical purposes [30–32].
Lung ultrasound has been used in clinical practice for more than three decades [14]. This method has been used first in adult patients, mostly in emergency settings. As a result of that approach, specific protocols were created for different pathological intrathoracic conditions [16, 33, 34].
In international evidence-based recommendations for point-of-care ultrasound, there is a consensus statement regarding the use of lung ultrasound for different pathological conditions, including pneumothorax as one of the complications of RDS with strong evidence for its use in this particular condition [35].
The use of lung ultrasound in children is even more comfortable to perform, since the body size and weight of children allow a detailed evaluation of the thorax, especially in the neonatal period. Lung ultrasound has been used in the assessment and monitoring of a variety of pathological conditions, including respiratory distress syndrome as one of the most frequent pathologies in neonates [22, 36–38].
We did not find any major limitations in our study.
Due to the high sensitivity of ultrasound, subtle changes can be noticed in a more prolonged period, which could potentially influence more extended follow-up and the duration of a patient hospital stay.
The relative limitation of this method is related to the fact that ultrasound examination is highly operator dependent and prone to subjectivity. Although there are exiting profiles and ultrasound artifacts for the classification of different respiratory pathology, there is always a question regarding variability in the technique of the examination. In our opinion, this obstacle could be avoided with continuous training and practice work, with the use of standardized protocol of the examination.
Lacking standardization of US is also an obstacle, but there are existing, widely used and explained terms (A lines, B lines, lung consolidation, dynamic and static bronchogram, lung sliding sign, M-mode and shore sign, etc.) and the necessity of the examination of complete area (both lung, anterior, posterior and lateral, from apex to bottom) is essential for making a conclusion and report, together with all other relevant patient data, laboratory tests, and clinical history. Introducing the unique, validated scoring system would decrease the subjectivity of the operator and provide an operator-independent assessment [39].
Conclusion
Our study showed that lung US enables the diagnosis and follow-up of respiratory distress syndrome in preterm neonates, with a good correlation with chest X-ray, which is considered the radiological method of choice for the diagnosis of RDS.
Subpleural consolidations on US examinations are a significant finding in patients with RDS in terms of prognosis and outcome and the proposed modification of the exiting three profile ultrasound scale can serve for a more detailed classification of patients with RDS.
Lung US should be included as a complementary method to chest X-ray in the diagnostic algorithms for diagnosing RDS. With the introduction of ultrasound as a non-harmful imaging method, it would be possible to reduce the number of X-ray examinations, replacing some of them with ultrasound and thereby reducing the amount of ionizing radiation delivered to neonates.
Funding
The authors did not receive support from any organization for the submission of the manuscript. No funding was received to assist with the preparation of this manuscript. No funding was received for conducting this study. No funds, grants, or other support was received.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interest to declare that are relevant to the content of this article. All authors certify that they no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interest in any material discussed in this article.
Ethical approval
Study approved by Ethical committee of Clinical Center University of Sarajevo (no. 0302-19064).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Irmina Sefic Pasic, Email: irmina.sefic@gmail.com.
L. Riera Soler, Email: lriera@vhebron.net
E. Vazquez Mendez, Email: evazquez@vhebron.net
F. Castillo Salinas, Email: fecastillo@vhebron.net
References
- 1.Jeenakeri R, Drayton M. Management of respiratory distress syndrome. Paediatr Child Health (Oxf) 2009;19(4):158–164. doi: 10.1016/j.paed.2008.12.004. [DOI] [Google Scholar]
- 2.Euro-Peristat Project Core indicators of the health and care of pregnant women and babies in Europe in 2015. Eur Perinat Heal Rep. 2018 doi: 10.1080/03639045.2017.1415927. [DOI] [Google Scholar]
- 3.Fogg MF, Drorbaugh JE. Respiratory distress in the newborn infant. Am J Nurs. 2015;56(10):1559–1562. [PubMed] [Google Scholar]
- 4.Horbar JD, Soll RF, Edwards WH. The Vermont Oxford Network: a community of practice. Clin Perinatol. 2010;37(1):29–47. doi: 10.1016/J.CLP.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Ezz- Eldin ZM, Abdel Hamid TA, Labib Youssef MR, Nabil HED. Clinical risk index for babies (CRIB II) scoring system in prediction of mortality in premature babies. J Clin Diagn Res. 2015;9(6):SC08–SC11. doi: 10.7860/JCDR/2015/12248.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) Crit Care. 2020;24(1):1–16. doi: 10.1186/s13054-020-2787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovrenski J. Pediatric lung ultrasound—pros and potentials. Pediatr Radiol. 2020;50(3):306–313. doi: 10.1007/s00247-019-04525-y. [DOI] [PubMed] [Google Scholar]
- 8.Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: bedside lung ultrasound in critical care practice. Crit Care. 2007;11(1):1–9. doi: 10.1186/cc5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man MA, Dantes E, Hancu BD, et al. Correlation between transthoracic lung ultrasound score and HRCT features in patients with interstitial lung diseases. J Clin Med. 2019 doi: 10.3390/jcm8081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Malah HEDGM, Hany S, Mahmoud MK, Ali AM. Lung ultrasonography in evaluation of neonatal respiratory distress syndrome. Egypt J Radiol Nucl Med. 2015;46(2):469–474. doi: 10.1016/j.ejrnm.2015.01.005. [DOI] [Google Scholar]
- 11.M.C. L, E.Y. L. Neonatal lung disorders: Pattern recognition approach to diagnosis. Am J Roentgenol. 2018;210(5):964–975. doi:10.2214/AJR.17.19231 LK. http://sfx.metabib.ch/sfx_uzh?sid=EMBASE&sid=EMBASE&issn=15463141&id=doi:10.2214%2FAJR.17.19231&atitle=Neonatal+lung+disorders%3A+Pattern+recognition+approach+to+diagnosis&stitle=Am.+J.+Roentgenol.&title=American+Journal+of+Roentgenology&volume=210&issue=5&spage=964&epage=975&aulast=Liszewski&aufirst=Mark+C.&auinit=M.C.&aufull=Liszewski+M.C.&coden=AJROA&isbn=&pages=964-975&date=2018&auinit1=M&auinitm=C. [DOI] [PubMed]
- 12.Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811–816. doi: 10.1002/jhm.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills GH. General ultrasound in the critically ill. Br J Anaesth. 2005;95(2):279. doi: 10.1093/bja/aei577. [DOI] [Google Scholar]
- 14.Lichtenstein DA. Lung ultrasound in the critically ill. J Med Ultrasound. 2009;17(3):125–142. doi: 10.1016/S0929-6441(09)60120-X. [DOI] [Google Scholar]
- 15.Chiumello D, Froio S, Colombo A, Coppola S. Lung ultrasound in the critically ill patient. Top Issues Anesth Intensive Care. 2016;17(3):55–67. doi: 10.1007/978-3-319-31398-6_3. [DOI] [Google Scholar]
- 16.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure*: the BLUE protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Sorantin E, Cao H. Neonatal lung ultrasonography. 2019. 10.1007/978-94-024-1549-0
- 18.Raimondi F, Migliaro F, Sodano A, Vallone G, Ferrara T, Maddaluno S, Coppola C, Capasso L. Point-of-care chest ultrasound in the neonatal intensive care unit. J Pediatr Neonatal Individ Med. 2013;2(2):e020214. doi: 10.1177/1742271X16689374. [DOI] [Google Scholar]
- 19.Hiles M, Culpan AM, Watts C, Munyombwe T, Wolstenhulme S. Neonatal respiratory distress syndrome: chest X-ray or lung ultrasound? A systematic review. Ultrasound. 2017;25(2):80–91. doi: 10.1177/1742271X16689374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos TM, Franci D, Coutinho CMG, et al. A simplified ultrasound-based edema score to assess lung injury and clinical severity in septic patients. Am J Emerg Med. 2013;31(12):1656–1660. doi: 10.1016/J.AJEM.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 21.Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. 2015;169(8):e151797–e151797. doi: 10.1001/jamapediatrics.2015.1797. [DOI] [PubMed] [Google Scholar]
- 22.Lovrenski J. Lung ultrasonography of pulmonary complications in preterm infants with respiratory distress syndrome. Ups J Med Sci. 2012;117(1):10–17. doi: 10.3109/03009734.2011.643510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Wang Y, Fu W, Yang CS, Huang JJ. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine (United States) 2014;93(27):23–28. doi: 10.1097/MD.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazemi K, Rakhsha M, Pourali L, Ayati S, Boskabadi H, Shakeri MT. Effective maternal and neonatal factors associated with the prognosis of preterm infants A R T I C L E I N F O. 2014;1(Md). http://psj.mums.ac.ir/article_6304_1d0ca0811b289a3d70039f800a98aae0.pdf. .
- 25.Navaei F, Aliabady B, Moghtaderi J, Moghtaderi M, Kelishadi R. Early outcome of preterm infants with birth weight of 1500 g or less and gestational age of 30 weeks or less in Isfahan city, Iran. World J Pediatr. 2010;6(3):228–232. doi: 10.1007/s12519-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 26.Koller-Smith LI, Shah PS, Ye XY, et al. Comparing very low birth weight versus very low gestation cohort methods for outcome analysis of high risk preterm infants. BMC Pediatr. 2017;17(1):166. doi: 10.1186/s12887-017-0921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqui A, Cuttini M, Wood R, et al. Can the Apgar score be used for international comparisons of newborn health? Paediatr Perinat Epidemiol. 2017;31(4):338–345. doi: 10.1111/ppe.12368. [DOI] [PubMed] [Google Scholar]
- 28.Zawada T, Wieczorek A, Garba P. Point of care ultrasound—a way to reduce radiation exposure of patients and medical staff. Intensive Care Med Exp. 2015;3(Suppl 1):2197. doi: 10.1186/2197-425X-3-S1-A273. [DOI] [Google Scholar]
- 29.Yu CC. Radiation safety in the neonatal intensive care unit: too little or too much concern? Pediatr Neonatol. 2010;51(6):311–319. doi: 10.1016/S1875-9572(10)60061-7. [DOI] [PubMed] [Google Scholar]
- 30.Malone J, Guleria R, Craven C, et al. Justification of diagnostic medical exposures: some practical issues. Report of an International Atomic Energy Agency Consultation. Br J Radiol. 2012;85(1013):523–538. doi: 10.1259/bjr/42893576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amis ES, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272–284. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Edison P, Chang PS, Toh GH, Lee LN, Sanamandra SK, Shah VA. Reducing radiation hazard opportunities in neonatal unit: quality improvement in radiation safety practices. BMJ Open Qual. 2017;6(2):e000128. doi: 10.1136/bmjoq-2017-000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtenstein D, Axler O. Intensive use of general ultrasound in the intensive care unit. Intensive Care Med. 1993;19(6):353–355. doi: 10.1007/BF01694712. [DOI] [PubMed] [Google Scholar]
- 34.Neethling E, Roodt F, Beck C, Swanevelder JLC. Point-of-care and lung ultrasound incorporated in daily practice. S Afr Med J. 2018;108(5):376–381. doi: 10.7196/SAMJ.2018.v108i5.13313. [DOI] [Google Scholar]
- 35.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 36.Raimondi F, Migliaro F, Sodano A, et al. Can neonatal lung ultrasound monitor fluid clearance and predict the need of respiratory support? Crit Care. 2012;16(6):R220. doi: 10.1186/cc11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cattarossi L, Copetti R, Poskurica B, Miserocchi G. Surfactant administration for neonatal respiratory distress does not improve lung interstitial fluid clearance: echographic and experimental evidence. Perinat Med. 2010;38:557. doi: 10.1515/jpm.2010.096. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Fanjul J, Moreno Hernando J, Iriondo Sanz M. PS-375 can lung ultrasound change respiratory distress management in newborns? Abstract PS-375 Table 1. Arch Dis Child. 2014;99(Suppl 2):A247.2–A248. 10.1136/archdischild-2014-307384.674
- 39.Corradi F, Via G, Forfori F, Brusasco C, Tavazzi G. Lung ultrasound and B-lines quantification inaccuracy: B sure to have the right solution. Intensive Care Med. 2020;46(5):1081–1083. doi: 10.1007/s00134-020-06005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]