Abstract
Purpose
Adhesive Capsulitis (AC) is a musculoskeletal disorder initially described by Codman in 1934. The disease is characterized by pain-limited restriction in active and passive glenohumeral range of motion (ROM) despite the lack of a structural deficit. In the last decades, arthroscopy and magnetic resonance imaging (MRI) has been the only diagnostic tools able to highlight the characteristic alterations of the glenohumeral capsular-ligament apparatus in AC; nevertheless, both arthroscopy and MRI are burdened by intrinsic limitations. The aim of this narrative review is to summarize the most significant evidence supporting the use of ultrasound (US) for the diagnosis of AC.
Methods
We extensively searched via PubMed library the terms “frozen-shoulder” and “adhesive capsulitis” each combined with “ultrasound”.
Results
We found 3723 papers on PubMed and selected those inherent to AC diagnosis, US imaging, correlation with arthroscopic and MRI findings. Forty papers which were strictly related to the topic of this narrative review were initially chosen, then 20 studies which described and exploited US for AC diagnosis were finally included.
Coracohumeral ligament (2.65 ± 0.4 mm) and axillary pouch thickening (3.34 ± 0.8 mm), as well as an increase in vascularity at rotator interval (78/214, 36.44%), represented the commonest US signs useful for AC diagnosis and for which the most significant cut-off values were reported.
Conclusions
The evidence collected in this review testify that musculoskeletal US is as reliable as MRI for AC diagnosis, therefore we believe that in this context US should be considered a first-line imaging technique.
Keywords: Ultrasound, Frozen shoulder, Adhesive capsulitis, Painful shoulder
Introduction
Adhesive capsulitis (AC) was initially described by Codman [1] in 1934 as a painful reduction of shoulder range of motion (ROM). Subsequently, Neviaser [2] defined it as a pain-limited restriction in active and passive glenohumeral ROM despite the lack of a structural deficit, introducing the term AC in 1945. AC mainly affects women between 40 and 60 years and it is characterized by both passive and active ROM limitation in the absence of an intrinsic shoulder disease: external rotation is predominantly involved but internal rotation [3], as well as arm flection/abduction, may be affected too. For many years the diagnosis has been clinical and exclusionary, indeed other diseases may cause a reduction of ROM like bone neoplasms and shoulder osteoarthritis. Nevertheless, in the last decades, the use of arthroscopy and implementation of magnetic resonance imaging (MRI) allowed highlighting characteristic alterations of the glenohumeral capsular-ligament apparatus such as thickening of the coracohumeral ligament (CHL), thickening of the axillary pouch (AP), obliteration of the fat triangle at the rotator interval (RI) and capsular contrast-enhanced inflammatory alterations [4]. However, arthroscopy and MRI are examinations burdened by intrinsic limitations: the former is an invasive technique that may expose the patients to complications, and the latter is a time-consuming investigation carrying high management costs. Technological advances in the ultrasound (US) field allow accurate visualization of structures that were considered not appreciable until a few years ago by the US. Therefore, AC study by musculoskeletal echography, allowing a dynamic and non-invasive investigation of the affected joint, represents today a valid alternative to MRI and arthroscopy. This narrative review will aim to summarize the papers that best describe US features useful for AC diagnosis.
Epidemiology of AC
The disease could be divided into primary or idiopathic and secondary [5]: the latter is mainly caused by forced immobilization due to trauma or previous surgery [6] but other pathological conditions are deemed to play a paramount role in AC pathogenesis: indeed among secondary causes of AC are listed the calcific tendinopathy of the rotator cuff tendons (during the painful resorptive phase as described by Uhthoff [7]), diabetes [8], thyroid [8], rheumatological, neurological, cardiac [8], cancer, and Dupuytren’s diseases [8]. The prevalence of AC is reported to be between 2 and 7%, reaching its highest prevalence in the diabetic population [9] and female subjects during the peri-menopausal stage [10]. Usually is a self-limiting disorder affecting the non-dominant shoulder which spontaneously subsides after 2 years from the onset, but often it can involve the contralateral joint producing a severe quality of life impairment.
Physiopathology
Early stages of AC are characterized by severe inflammation starting at RI and subsequently extending to other sites such as the anterior glenohumeral joint, AP, and the posterior portion of the glenohumeral joint. As the disease progresses, the inflammatory alterations are replaced by fibrotic changes. At the cellular level myofibroblasts appear as the main cytological characters involved in the process [8, 9]. The disease has been divided into 4 clinical phases [2–4] (Table 1) which highly correlate with arthroscopic findings and histopathological specimens. Several studies investigated the role of cytokine in favouring inflammation and progression to fibrosis, while others correlated clinical stages with imaging findings.
Table 1.
Correlation between Adhesive Capsulitis stages and symptoms (according to Neviaser [2])
| Stage | Symptoms |
|---|---|
| 1 Pre-freezing | Pain and limited ROM (0–3 months) |
| 2 Freezing | Frankly limited ROM and pain (3–9 months) |
| 3 Frozen | Severe ROM limitation, little pain (9–15 months) |
| 4 Thawing |
Gradually improvement in ROM, scarce pain (15–24 months) |
Anatomy
Rotator interval region, long head of biceps tendon (LHBT), axillary pouch (AP), and posterior joint capsule (PJC)
The RI region is superiorly and laterally delimitated by the inferior margin of the supraspinatus (SST) tendon, inferiorly and medially by the upper margin of the subscapularis (SUBT) tendon, medially by the coracoid process, laterally by the intersection of the coracohumeral (CHL) and superior glenohumeral ligament (SGHL) at LHBT, that contribute to form its pulley and prevent it from sliding medially. The coracohumeral ligament (CHL) originates from the coracoid process and goes laterally inserting into the greater humeral tubercle and to the lesser one, enveloping LHBT and helping to form its pulley (along with SST and SUBT tendon aponeurosis) (Fig. 1). Moreover, CHL goes to the anterior margin of SST tendon forming the lateral pulley complex. This part is recalled as the anterior pillar of the rotator cable which represents the most important anchorage and stress dispersion system for the SST tendon at the humeral head [11, 12]. The tension of CHL is an important determinant of shoulder mobility, particularly in external rotation and abduction. The humeral greater tuberosity and the lesser tuberosity are bone protrusions delimiting the bicipital groove. However, the groove extends cranially to the protrusions, just below the surgical neck, where the LHBT leaves an impression on the bone (Fig. 2). The proximal part of the bicipital groove is normally visible at US investigation; the CHL inserts on the upper edge of the great tuberosity, on its prominence (transversely) and on the superior crest of the lesser tuberosity, as described in the classical anatomical literature. Thus, CHL and LHBT are strictly paired when investigated by the US, displaying a specific pattern. Finally, the AP represents the inferior part of the glenohumeral capsule (Fig. 3), and it’s placed between the anterior and posterior bands of the inferior glenohumeral ligament (IGHL) [4]. Usually, the AP displays a thin wall thickness, generally not higher than 3 mm [13]. The PJC is located below the infraspinatus tendon (IST) and expands during the external rotation of the arm when the joint effusion is present; conversely, in the case of AC, it contracts.
Fig. 1.
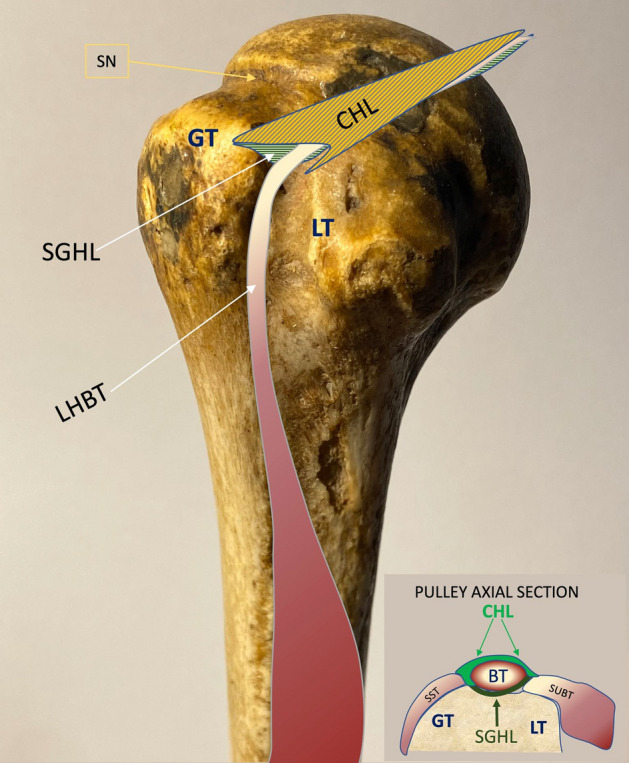
Right humerus. GT great tuberosity; LT lesser tuberosity; CHL coracohumeral ligament; SGHL superior glenohumeral ligament; LHBT long head biceps tendon; SN surgical neck. In the small box, pulley axial section of RI. SST supraspinatus tendon; SUBT subscapularis tendon; BT biceps tendon
Fig. 2.
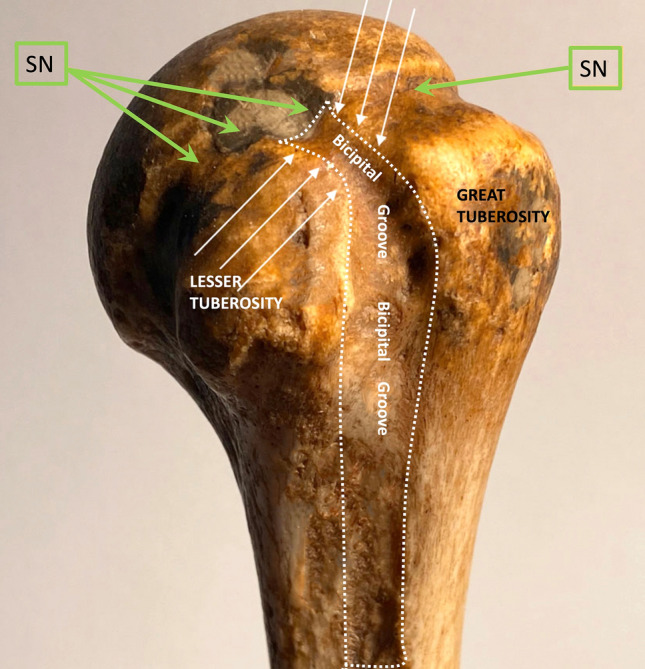
Left humeral head: the bicipital groove (white arrows and dashed white line) begins just below the surgical neck (SN, green arrows)
Fig. 3.
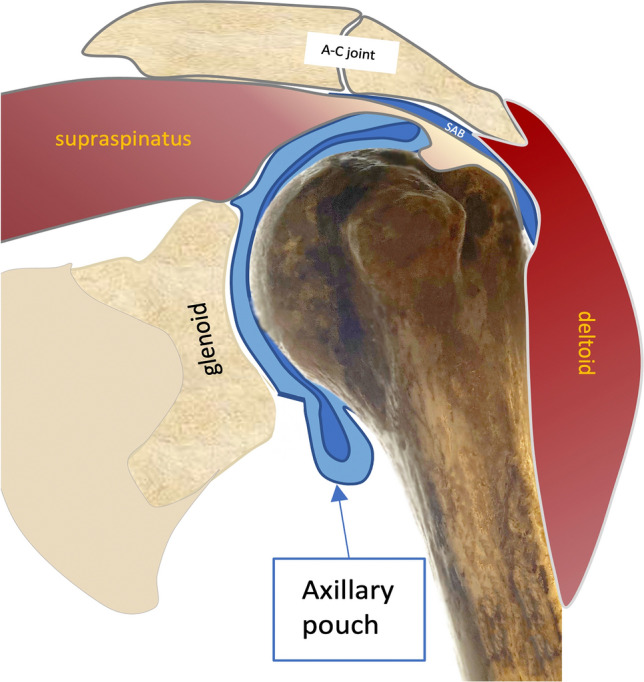
Coronal view of the axillary pouch in neutral rotation and slight abduction. SAB subacromial bursa; A-C acromioclavicular joint
Materials and methods
We extensively searched on PubMed library the terms “frozen shoulder” and “adhesive capsulitis” each combined with “ultrasound”. Were considered only papers written in English and published in impacted journals. Abstracts were excluded from being considered for this review.
Results
We found 3723 papers on PubMed, we selected those inherent to AC diagnosis, US imaging, correlation with arthroscopic and MRI findings as well clinical presentation. Of these, we selected 40 papers that were strictly related to the topic of this review and finally selected 20 studies that described and exploited the US for AC diagnosis. Tables 2 and 3 summarize the US signs and their cut-off values useful for AC diagnosis.
Table 2.
Studies which evaluated US for AC diagnosis
| Authors | Duration | Age | Study type | Probe | n° | CHL | SGHL | Axillary pouch | LHBT | SST | ISP | PD | CEUS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ryu KN et al. 1993 [27] |
n.a |
56 yo |
Prosp |
5 MHz linear |
23 | n.a | n.a | n.a |
Effusion 23/26 |
SGLS SE 91% SP 100% |
n.a | n.a | n.a |
|
Lee J. C. et al. 2005 [14] |
< 12 months (26) > 12 months 4 |
50 yo |
Case–control | 15 L8 W | 30 | n.a | n.a | n.a | Synovitis | n.a | n.a | 26/30 ↑ | n.a |
|
Homsi C. et al. 2006 [18] |
n.a | n.a | Case–control, prosp | 5–13 MHz linear | 17 | 3 mm (15) vs 1.34 and 1.39 mm (asymptomatic and painful) | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Kim I, et al. 2012 [29] |
n.a |
43.2 yo |
Prosp |
7–12 MHz Linear |
47 | n.a | n.a | n.a | n.a | SGLS | n.a | n.a | n.a |
|
Michelin P. et al. 2012 [22] |
n.a |
54 yo |
Retrosp |
6–18 MHz linear 4–9 MHz linear 5–12 MHz linear |
20 | n.a | n.a | 4.0 mm vs 1.3 mm | n.a | n.a | n.a | n.a | n.a |
|
Walmsley S. et al.2013 [30] |
5.4 ± 1.8 months |
56.0 ± 7.2 yo |
Prosp |
12 MHz Linear |
41 | n.a | n.a | n.a | n.a | n.a | n.a | ↑ | n.a |
|
C.H. Wu et. al.,2016 [31] |
n.a | 30.5 yo | Prosp |
4–15 MHz Linear |
20 | 3.1 mm (2.4–3.4) vs. 2.3 (2–2.6) | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Cheng X. et al. 2017 [15] |
10 months (1.5 ± 24) |
54 yo | Case–control |
5–12; 3–9 MHz Linear |
45 |
3.1 ± 0.67a mm SE 64.4% SP 88.9% |
n.a |
3.5 ± 1.06a mm SE 66.7% SP 93.3% |
55.6% vs 48.9%, (p = 0.53) |
n.a | n.a | ↑ | Higher sensitivity vs B mode |
|
Park G. et al. 2017 [19] |
5.4 ± 2.8 months | 55.3 ± 9.8 | Prosp |
5–18 MHz Linear |
75 | 1.3 ± 0.3 vs 0.9 ± 0.2 mm (p < 0.01) | n.a | 5.4 ± 1.0 vs 3.0 ± 0.7 mm (p < 0.01) | n.a | n.a | n.a | n.a | n.a |
|
Tandon A. et al.2017 [16] |
n.a | n.a | Case–control, prosp |
5–12 MHz Linear |
30 |
0.7 mma (cut-off) SE 93.1% SP 94.4% |
n.a | n.a | n.a | n.a | n.a | ↑ | n.a |
|
Kim D. H. et al. 2018 [23] |
7.3 months (4–14) | 52.4 ± 8.3 yo | Retrosp |
5–13 MHz Linear |
44 | n.a | n.a | 4.4 ± 1.1 mm vs 2.2 ± 0.5 mm | n.a | n.a | n.a | n.a | n.a |
|
Kim D. H. et al. 2019 [17] |
3 months |
56 ± 9.25 yo |
Case–control prosp |
14 MHz Linear |
39 |
3 mma (cut-off) SE 51.28% SP 91.43% |
n.a | n.a | ↑ | n.a | n.a |
↑ 0.45a SE 61.54% SP 94.2 |
n.a |
|
Sernik R. A. et al. 2019 [24] |
n.a | 56 yo | Prosp |
6–15; 5–12 MHz Linear |
23 | n.a | n.a |
2 mma SE 100% SP 96.4% |
n.a | n.a | n.a | n.a | n.a |
|
Wada T. et al. 2019 [32] |
n.a | 59.4 yo | Prosp | SL10-2 linear | 32 | 3.7 ± 1.0 mm (freezing) and 4.4 ± 1.4 (frozen) vs. 3.3 ± 1.1 (unaffected side) | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Yun S.J. et al.2019 [33] |
> 6 months | 53.5 ± 7.9 yo | Case–control, prosp | n.a | 25 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Wu P.Y. et al.2021 [20] |
7.43 ± 6.2 | 54.38 ± 7.2 yo | Prosp | 7–14-MHz linear | 65 | 3.12 ± 0.7 mm | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Do J.G. et al.2021 [21] |
19.6 ± 14.5 | 56.2 ± 8.9 yo | Retrosp |
3–12 MHz linear |
61 |
2.2 mma SE 77%, SP 91.8% |
n.a |
4 mma SE 68.9%, SP 90.2% |
Effusion (34) | n.a | n.a | ↑ | n.a |
|
Lee G-J. et al. 2021 [25] |
6.7 ± 3.2 | 58.2 ± 9.9 yo | Prosp |
5–12 Mhz Linear |
71 | n.a | n.a |
3,2 mma SE 73.2% SP 77.5% |
n.a | n.a | n.a | n.a | n.a |
|
Stella S.M. et al. 2021 [13] |
n.a | 55.6 ± 15.3 yo | Prosp |
12; 6–15 MHz Linear |
106 |
2.3 ± 0.25a mm SE 88% SP 52% |
2.3 ± 0.25a SE 88% SP 52% |
4 mma SE 93.4%, SP 98% |
Effusion (71) |
« U» fold 77/106 SE 72,6% SP 100% « Bouncing” 44/106 SE 41,5% SP 100% |
|||
|
Zhang J. et al. 2021 [34] |
n.a | n.a | Prosp |
L-9 Linear |
64 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
n.a. not assessed; CHL coracohumeral ligament; LHBT long head biceps tendon; SST supraspinatus; ISP infraspinatus; PD power doppler; CEUS contrast enhanced ultrasonography; SGHL superior glenohumeral ligament; SGLS subacromial gliding limitation of supraspinatus tendon; SE sensitivity; SP specificity
aCut-off value; Retrosp: retrospective; Prosp.:prospective
Table 3.
Studies which evaluated US for AC diagnosis
| Authors | M/F | GS changes | RI thickness | PCT | IGHL | SWE CHL |
SWE/strain SST |
SWE/strain ISP |
SWE/strain LBH |
SMI® | CORRELATION |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ryu KN et al. 1993 [27] |
15/11 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | Arthrography |
|
Lee J. C. et al. 2005 [14] |
10/20 |
↑ Hypoechogenicity of RI in 26/30 SE 97% SP 100% |
n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Homsi C et al. 2006 [18] |
n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | Arthrography |
|
Kim I, et al. 2012 [29] |
17/30 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | MRA |
|
Michelin P et al. 2012 [22] |
6/14 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
MRI 8/20 |
|
Walmsley S. et al.2013 [30] |
22/19 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.p |
|
C.H. Wu et al.2016 [31] |
11/19 | n.a | n.a | n.a | n.a |
234.8 (174.4 -256.7) |
n.a | n.a | n.a | n.a | |
|
Cheng X. et al. 2017 [15] |
15/30 | ↑ Hypoechogenicity | n.a | n.a | n.a | ↑ | ↑ | ↑ | ↑ | n.a | MRI |
|
Park G. et al. 2017 [19] |
27/48 | n.a | n.a | n.a | 5.4 ± 1.0 mm | n.a | n.a | n.a | n.a | n.a | MRI, arthrography |
|
Tandon A. et al.2017 [16] |
n.a |
↑ Hypoechogenicity SE 86,2% SP 92,8% |
n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | MRI |
|
Kim D. H. et al. 2018 [23] |
19/25 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | MRI |
|
Kim D. H. et al. 2019 [17] |
21/18 |
↑ Hypoechogenicity SE 66.67% SP 94.29% |
n.a | n.a | n.a | n.a | n.a | n.a | n.a |
1.31 aSE 76.92% SP 91.43% |
|
|
Sernik R. A. et al. 2019 [24] |
12/12 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | MRI |
|
Wada T. et al. 2019 [32] |
13/19 | n.a | n.a | 1,3 ± 0,2 (freezing) and 1,2 ± 0,2 (frozen) | n.a | 270.3 ± 142.5 kPa (freezing) vs 239.9 ± 113.8 kPa and 287.2 ± 135.3 kPa vs 214.1 ± 91.1 kPa (frozen) |
27.9 ± 9 kPa vs 25.4 ± 7.6 kPa (freezing) and 21.8 ± 6.5 kPa vs 22.5 ± 8.2 kPa (frozen) |
20.5 ± 9.7 kPa vs 19.8 ± 4.8 kPa (freezing) vs 18.5 ± 4.9 kPa vs 21.5 ± 9.1 kPa (frozen) |
210 ± 103.2 kPa vs 194.3 ± 98.8 kPa (freezing) and 241.2 ± 113.6 kPa vs 233.3 ± 119.4 kPa (frozen) |
n.a | n.a |
|
Yun S.J. et al. 2019 [33] |
0/14 | n.a | n.a | n.a | n.a | n.a |
8.53 kPaa SE 84.0% SP 100% and 1.69 m/s SE 88% SP 100% |
9.76 kPaa SE 92% SP 100% and 1.80 m/s SE 92% SP 100% |
n.a | n.a | n.a |
|
Wu P.Y. et al.2021 [20] |
27/38 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Do J.G. et al.2021 [21] |
31/30 | n.a | ↑ | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Lee G-J. et al. 2021 [25] |
26/45 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Stella S.M. et al.2021 [13] |
n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
|
Zhang J. et al. 2021 [34] |
n.a | n.a | n.a | n.a | n.a |
Stage I 110 (90.3–130.5)b kPa vs 89.7 (56.3–92.4)b Stage II 158.8 (120–220)b vs 96 (72.5–120)b kPa Stage III 151.6 (110.6–210.7)b kPa vs 95.4 (68.3–121.5)b Kpa |
n.a | n.a | n.a | n.a |
n.a. not assessed; GS gray-scale; RI rotator interval; SWE shear-wave elastosonography (kPa); Strain strain ultrasound elastography (Kpa); SMI superb microvascular imaging; SE sensitivity; SP specificity; PCT posterior capsule
aCut-off value; MRA magnetic resonance arthrography
bInterquartile range; kPa kilopascal
Grey scale US changes
Lee et al. 2005 [14] were the first to document hypoechogenic changes as well as an increase of Power Doppler ultrasound (PD-US) at RI in 26 AC patients. In 2017, Cheng et al. [15] highlighted in their case–control study RI hypoechogenicity in 32 out of 45 patients and LHBT effusion in 25 individuals diagnosed with AC. The same year Tandon et al. [16] documented hypoechoic changes suggestive of synovitis at RI in patients with AC reporting a sensitivity of 86.2% and a specificity of 92.8%. Subsequently, Kim et al. [17] in 2019 found RI hypoechogenicity in 26 patients with AC reporting a sensitivity of 66.67% (49.8–80.9) and a specificity of 94.29% (80.8–99.3).
CHL and other ligaments thickening
A characteristic thickening of the CHL (Fig. 4), that lay among the first US signs described in AC, is reported by several studies. Homsi et al. in 2006 [18] in a case–control study compared the average thickness of CHL in 15 AC patients with asymptomatic (92) and painful (227) shoulders reporting a higher thickness of the ligament in patients affected by the disease (3 mm vs 1.34 mm and 1.39 mm respectively). Nevertheless, they did not provide any cut-off value. Cheng X. et al. [15] in 2017 evaluated 45 patients with AC and confirmed the usefulness of CHL thickening evaluation, as well as AP, in AC patients compared to controls (CHL: 3.1 mm ± 0,67 vs 1.4 ± 0.55). The same year Tandon A. et al. [16] in a case–control study, evaluated CHL thickness in 30 AC patients proposing their cut-off value measured at the coracoid insertion of the ligament (0,7 mm; sensitivity 93.1% specificity 94.4%); furthermore, they reported hypoechoic changes suggestive for synovitis at RI in 86% and hypervascular PD-US signal in 10% of the patients. Combining all these parameters the authors reported an overall sensitivity of 87% and specificity of 100% of US in diagnosing AC. Park et al. [19] studied with the US the CHL, IGHL, and RI thickness in a group of patients diagnosed with primary AC using MRI and arthrography as reference. CHL and IGHL thickness was reported as significantly higher in affected shoulders, moreover, they documented a negative correlation between shoulder external rotation and ligamentous apparatus thickening. Subsequently, Kim et al. [17] documented a higher CHL thickness in AC patients compared to healthy controls (p < 0.01) reporting a sensitivity of 51% and a specificity of 91% with an area under the curve (AUC) of 0.82. Wu et al. [20] evaluating a cohort of 65 patients demonstrated that CHL thickness was strictly correlated with disease duration as well as negatively related to external and internal rotation. Another group of study [21] retrospectively evaluated AC with the US correlating CHL, AP, and RI thickness as well as PD signal with disease duration and ROM limitation reporting a wider capsule-ligamentous apparatus in affected joints; furthermore, they described a thicker CHL in stage II vs stage I AC and a wider RI in stage II compared to stage III proposing their optimal cut-off values (2.2 mm; sensitivity 77%; specificity 91.8%). Stella et al. [13] in a cross-sectional study of 106 patients with clinically suspected AC confirmed CHL and SGHL thickening as useful parameters in AC diagnosis. Moreover, pulley thickening (CHL and/or SGHL) in comparison with the contralateral shoulder was detected in 93 out of 106 shoulders in AC diagnosed patients (88%). The mean of CHL thickening derived from the studies presented in this review is 2.65 ± 0.4 mm [13, 15, 17, 21].
Fig. 4.

Left shoulder: axial section of the RI showing conspicuous coracohumeral ligament biceps tendon thickening (with double arrow). LHBT long head of the biceps tendon
Axillary pouch thickening
Since Michelin et al. in 2012 [22] firstly investigated the inferior glenohumeral capsule thickening by studying AP/IGHL width in 20 patients with AC, and describing a significant widening of the AP and/or IGHL in contracted joints (4 mm), many papers documented the importance of evaluating AP in suspected AC [25, 26, 29] (Figs. 5, 6). Similar results are reported by Kim [23] who confirmed in a larger study the thickening of the AP in a larger cohort. Subsequently, Sernik et al. [24] documented a good correlation between AP thickening via the US (when higher than 2 mm) with MRI findings in 23 patients diagnosed with AC. Do et al. in 2021 [21] too, studying AP thickening, provided highly sensitive cut-off values useful for diagnosis. Meanwhile, Lee et al. [25], investigating the inferior joint capsule thickness in 71 patients with AC, documented that an AP of 3.2 mm thickness (with the arm held in a neutral position) had the highest diagnostic accuracy with a sensitivity of 73.2% and specificity of 77.5%. In this regard, Stella et al. [13] confirmed the importance of evaluating the AP thickening in patients with AC: an AP larger than 4 mm (mean 5.3 ± 1.15 mm) was detected in 99 patients (sensitivity 93.4% and specificity 98%) in comparison with the controls and with the contralateral shoulder (p < 0.0001); moreover, a difference in thickness higher than 60% was diagnostic for AC. Indeed, in this paper it is mentioned that in a previous preliminary study, using early-generation US equipment and poor-definition probes (7.5 MHz), there was the first identification of the AP abnormality, initially interpreted as a liquid (hypoechoic) distension of the axillary recess; the subsequent use of modern musculoskeletal US equipment revealed (2012) the hypoechoic “distension” as being the thickening of the AP. The mean AP thickening obtained from the studies presented in this review is 3.34 ± 0.8 mm [13, 15, 17, 21].
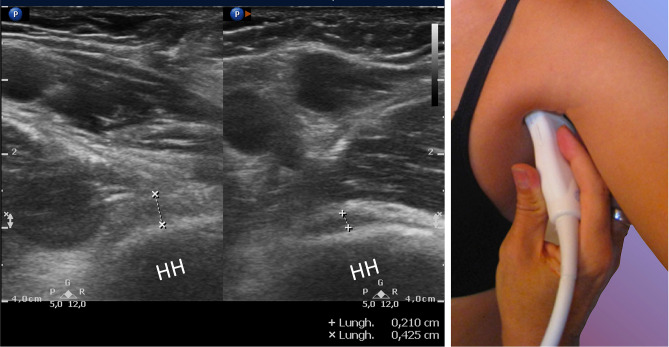
Fig. 5.
Oblique axial section: calipers shows the thickness of the axillary pouch in a patient with AC; the lest image is the contralateral side. HH humeral head
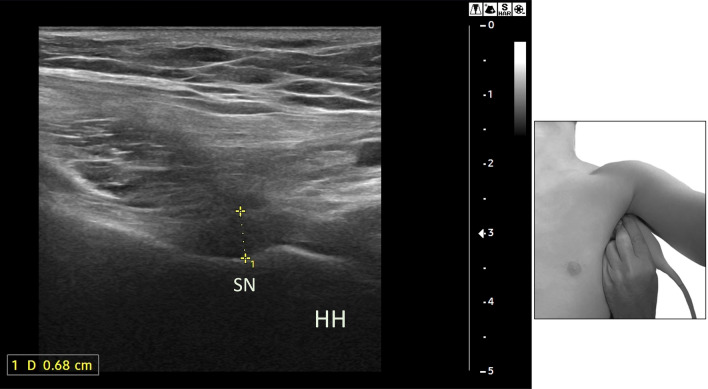
Fig. 6.
Coronal section: the calipers show the thickness of the AP in patient with AC. HH, humeral head, SN, surgical neck
LHBT sheath alterations
Lee et al. in 2005 [14] studying the RI of 30 patients with frozen shoulder documented increased hypoechogenicity and soft tissue invasion of RI as well as of LHBT sheath in presence of augmented vascularity at PD-US, suggestive of inflammatory alterations (synovitis). Albeit other papers documented the increased hypoechogenicity of LHBT, sensitivities, and specificities are not reported [13, 14, 20, 21, 26, 27]. Stella et al.[13] described the effusion of LBHT in 75 out of 106 patients with AC, moreover, they reported in 43/106 patients a thickening of the CHL that may produce, in a transverse US section of the pulley, a ‘‘pseudo-double tendon’’ appearance with the adjacent LHBT [13, 28].
Supraspinatus and infraspinatus alterations
Ryu et al. [27] in 1993 suggested the usefulness of dynamic ultrasonography to detect AC by studying subacromial gliding limitation of the supraspinatus tendon (SGLS) in patients subsequently diagnosed with AC, reporting a sensitivity of 91% and a specificity of 100%. Moreover, as an ancillary finding, they reported the presence of LHBT effusion in 23/26 patients (88%). In a later study, SGLS has been studied [29] in 47 patients documenting a strong correlation with MRI, confirming the utility of evaluating this US sign. Stella et al. [13] in their study described two novel signs useful for AC diagnosis: the former describes the folding of the infraspinatus (ISP) tendon sliding backwards showing a characteristic change in shape from flat to concave toward the joint capsule during dynamic examination, individuated in 41,5% of patients; the latter documents the “bounce sign”, described as a plucked guitar string, reported in nearly 57% of those with tendon folding during passive external rotation: the sign is a slight, rapid and fleeting jolt of the ISP tendon folding toward the articular capsule during external rotation, that quickly returns to baseline resting position.
Power Doppler ultrasound
Walmsley et al. in 2013 [30] in a prospective study evaluating the RI of 41 early-stage AC patients documented in 12 (29%) and increased vascularity at PD-US (Fig. 7), confirming the usefulness of detecting an inflammatory pattern in early AC. Subsequently, [17] a case–control study compared Superb Microvascular Imaging® (SMI; Canon Medical Systems, Otawara, Japan) with conventional PD-US in 39 AC patients and 35 healthy subjects displaying a higher sensitivity and specificity in detecting inflammatory flows at the RI for SMI against conventional PD-US (AUC 0,9 vs 0,78 p < 0.01). Furthermore, SMI signal strength was inversely related to shoulder external rotation, ROM, and forward flexion in the AC group (p < 0.05). Five studies documented an increase of vascularization at the RI of patients with frozen shoulder [14, 16, 17, 21, 30]: for a total of 214 patients evaluated, 78 of these (38.44%) displayed an increase in PD-US signal.
Fig. 7.
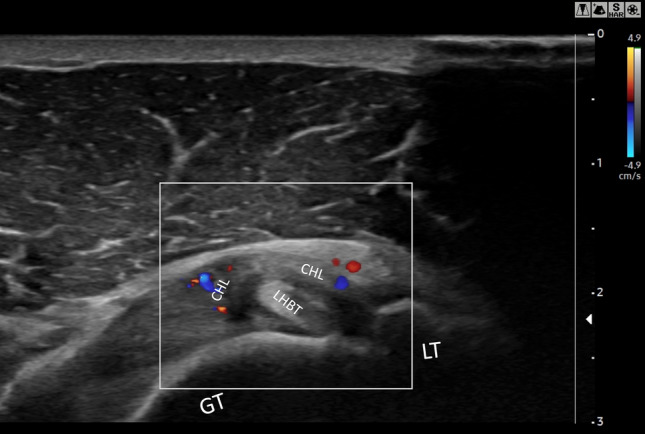
Directional PD US shows hypervascularity in the RI: axial section of the rotator interval of a right shoulder in a patient with AC. CHL, Coracohumeral ligament; LHBT, Long Head Beceps Tendon; GT, greater tuberosity; LT, lesser tubercle
Contrast-enhanced ultrasonography (CEUS)
Cheng et al. in 2017 [15] compared US-arthrography, by injecting the contrast agent SonoVue® (Bracco, Milan, Italy) into the glenohumeral joint, with the conventional US, detecting a specific decrease of axillary recess volume in AC patients. CEUS US-arthrography showed also filling defects (91.1%), synovitis-like abnormalities (75.6%) in the glenohumeral joint, and RI defects (71.1%).
Shear wave elastosonography (SWE) and strain elastography ultrasound (SE)
Wu et al. [31], in a case–control analysis, prospectively studied the thickness and elasticity of CHL with SWE in 20 subjects with a clinical suspicion of AC. Both thickness (3.1 vs 2.3 p < 0.001) and elastosonography findings were higher in the affected shoulders against the contralateral unaffected joint (234.8 kPa vs 203.3 kPa p < 0.04). Two years later, another study [32] investigated the efficacy of SWE in 32 patients and analyzed the mechanical stiffness of the CHL, SST, and ISP tendons as well as the LHBT in the pre-freezing and frozen stages of AC. Yun et al. [33] also examined the stiffness of the SST and ISP tendons using SWE and SE in a prospective case–control study of 20 patients with primary AC and described stiffer tendons in these shoulders. In addition, they proposed their cut-off values for AC using SWE and SE: mean SST velocity (cut-off, 1.69 m/s) and mean ISP velocity (cut-off, 1.80 m/s) showed high AUC (> 0.970); SST (cut-off, 8.53 kPa; sensitivity 84.0% specificity 100%) and ISP stiffness (cut-off, 9.76 kPa; sensitivity 92% specificity 100%) also displayed high diagnostic accuracy. Subsequently, Zhang et al. [34] documented a strict correlation between CHL stiffness, disease duration, the shoulder ROM, and their correlation with the visual analogue scale score (VAS), offering insights into the possibility of diagnosing disease stage by US examination.
Discussion
AC is a 'not easy' disease to diagnose, especially in the early stages. Several papers document the usefulness of musculoskeletal US examination to exclude other pathologies that may mimic AC, such as tendon rupture, calcified tendinopathy, arthritis, and chronic adhesive bursitis [35]. Musculoskeletal US examination is successfully used in the outpatient setting as a reliable tool for AC diagnosis and provides pinpoint anatomical and functional information expressed either as grey-scale changes or PD-US signals. In addition, a recently published meta-analysis examined the diagnostic accuracy of CHL thickness, RI abnormalities, and AP widening and concluded that musculoskeletal US has comparable diagnostic value to MRI [36].
Coraco-humeral ligament alterations in AC
The main US sign of AC is the thickening of the CHL. It is a ligament that can be easily visualised even by inexperienced sonographers (although its curvature makes it subject to anisotropy and lateral acoustic shadowing caused by refractive phenomena due to its curvilinearity) and provides useful information about shoulder health. Since the study of Homsi [18] many papers mainly focusing on CHL thickness confirmed the utility of this ligament evaluation. Park et al. [19] studied the thickness of CHL and IGHL (the latter, a component of the AP) in patients in the pre-freezing and freezing stage documenting a thicker ligamentous apparatus in AC patients. Their results displayed that the thickening of CHL is an event that can be documented also in the early stages of the disease. Furthermore, Wu et al. [20] confirmed these observations by evaluating CHL thickness in correlation with disease stages and ROM reduction: an inverse correlation between CHL thickening, shoulder ROM, and disease duration was documented in patients with disease duration higher than 6 months. Wada et al. [32] too correlated CHL thickness and disease stage by discriminating between pre-freezing and frozen shoulders: both stages displayed higher thickness of the ligament in AC patients against controls, but pre-freezing shoulders displayed a thinner CHL than frozen ones. Therefore, CHL thickening can be considered an early epiphenomenon of AC which strictly correlates with ROM reduction of the affected joint that may persist during the natural evolution of the disease. The cut-off evaluating CHL thickening proposed by Tandon A. et al. is the lesser one reported in the literature [16] (0.7 mm; sensitivity 93.1%; specificity 94.4%); however, in this study, the measurement is taken at the level of the origin of the CHL and in our opinion, this threshold is too low as it may overlap with the thickness of the ligament under normal conditions.
In fact, thickening of the CHL is not specific of AC, as it can also occur in other conditions such as ligament injuries, overuse syndromes, and in patients with anterior glenohumeral instability: in these cases, the ligament is not shortened [28] as in frozen shoulder, but only thickened, so that the distinction between these conditions is not always easy [28].
Axillar pouch and inferior glenohumeral joint in AC
Michelin et al. [22], were the first to document a significant thickening of the inferior glenohumeral joint capsule in patients with clinically and/or MRI-proven capsular contraction. Nevertheless, Stella et al. [13], affirmed that in 2004 they had begun a preliminary study of the features of the AP in patients with AC, noting an apparent expansion of the recess which was initially misinterpreted as hypoechoic joint effusion; the subsequent use of modern US equipment demonstrated (2012) that the hypoechoic image was a sign of the thickening of the joint capsule. However, a little amount of effusion may be present (in 13% of patients with AC) [13] between the meshes of the fibrous thickening of the AP due to the entrapment of synovial fluid as a result of capsular contraction. Subsequently, another group of study [15] documented AP and CHL thickening using MRI as reference: not only did they evaluate shoulders with B-mode, but by using CEUS they obtained higher sensitivities and specificities than sole grey-scale analysis. In recent years, several studies have evaluated AP thickening and provided useful cut-off values for AC diagnosis [13, 14, 16, 18, 21, 24], nevertheless, the group of study of Sernik et al. furnished those with the highest sensitivity and specificity [24]. Lately, Stella et al. [13], who examined the largest group (106 patients) ever studied with the US for AC, suggested their cut-off values for AP thickening. Furthermore, they also considered diagnostic for AC a difference in thickness of more than 60% between the contralateral pouch.
LHBT alterations in AC
Few papers have systematically studied the LHBT sheath, although its effusion is frequently reported [14, 20, 21, 26, 27]; Stella et al. [13] documented an LHBT sheath effusion in 75 out of 106 patients diagnosed with AC. Again, the LHBT sheath represents a capsular recess, and assuming that the joint contracture is appreciable in the overt stage of the disease, we can speculate that the LHBT effusion may be an early finding. In addition, the effusion may coexist with many other pathologies: tendon tears, rheumatic polymyalgia, arthritis, etc. To date, no study has investigated the diagnostic accuracy of LHBT sheath effusion for AC but given that many pathological conditions may share this US sign, in our opinion a high sensitivity and specificity seems unlikely. Nevertheless, in subjects with few US signs suggestive of disease and in the presence of a painful ROM reduction, LHBT sheath effusion should raise suspicion of AC and prompt a search for the US changes suggestive of frozen shoulder.
Supraspinatus and infraspinatus tendons alterations in AC
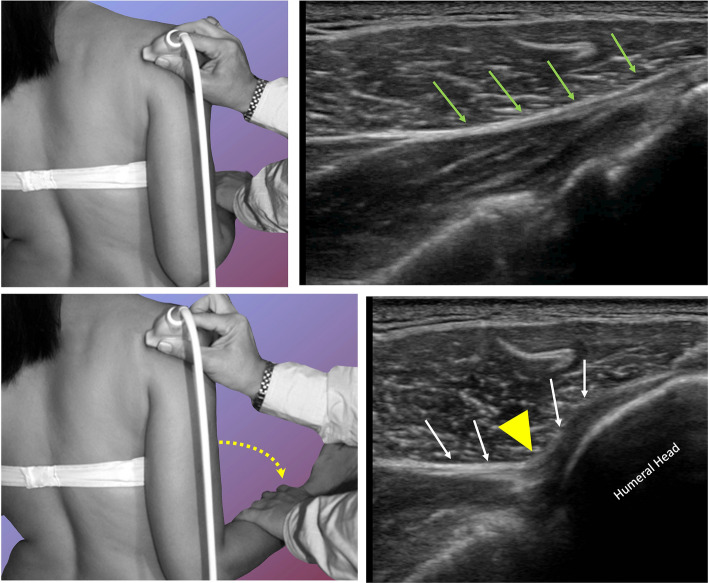
Few papers evaluated the alteration of rotator cuff tendons sliding: Ryu et al. in 1993 [27] demonstrated that dynamic ultrasonography could make a difference in assessing AC reporting high diagnostic accuracy of SST tendon sliding alterations. Lately, Kim et al. [29] too, studying SST sliding alterations, documented a high correlation between musculoskeletal US and MRA. Moreover, they highlighted a reduction in total injectable volume in AC shoulders compared to controls, confirming thus the joint capsule contraction importance in limiting patients’ ROM. Stella et al. [13] documented the ISP sliding alterations in patients with AC: a dynamic study of this tendon during a passive external rotation demonstrated in 73% of cases a reduced sliding with the tendon folding towards the joint capsule and changing its profile from flat to concave (Fig. 8). The reduced tendon sliding was often associated with a ‘bouncing’ movement (“bounce sign”): the tendon, which resembled a plucked guitar string, returned to its baseline resting position after the jolt. These signs were never previously reported. The folding of the tendon towards the joint capsule, ‘U’ shaped, was seen in 77/106 patients and displayed good sensitivity and specificity (sensitivity 72.6% specificity 100%) while “bouncing sign” was observed in 44/106 with good diagnostic accuracy (sensitivity 41.5% specificity 100%). Since no other authors evaluated these features, we cannot compare these findings with previous works. Nevertheless, it is worth noting that these features may be observable also in the early stages of the disease, and often lasting to the end-stage and even after healing.
Fig. 8.
US dynamic study of the infraspinatus tendon (green above and white arrows below) sliding backwards during passive external rotation from neural rotation (above) to external passive rotation (below). Note the change from a flat to a concave profile (yellow arrowhead) of the tendon. The sliding of the tendon folds towards the joint capsule because of the close contiguity
Power Doppler ultrasound in AC
Despite the nature of AC, which is recognized to start as synovitis and end as fibrosis, few papers evaluated the accuracy of PD-US. Walmsley et al. [30] documented an inflammatory pattern at RI in a subset of patients with early-stage AC. Moreover, those who displayed a positive PD-US signal at RI had a lower duration of symptoms compared to the patients without hypervascular changes. In their cohort of study, they included patients in pre-freezing (0–3 months) and freezing stage (3–9), thus with a heterogenous disease stage; assuming that hypervascular changes at RI are observable only in the early phases of the disease, the group’s heterogeneity could explain the lack of observation of hypervascular signal in most of the examined shoulders. Surprisingly, even if inflammation is held responsible for the pain experienced by patients in early stage AC, Walmsley et al. [30] reported lower pain values for the subjects who displayed a positive PD-US. Subsequently, in a case–control analysis, Kim et al. [17] compared the conventional PD-US with Superb microvascular imaging (SMI®). SMI® is a software that allows the detection of weaker signals compared to conventional PD-US [37]. They reported higher sensitivity and specificity of SMI® than conventional PD-US in detecting slow vascular flows. Moreover, they confirmed CHL thickness and RI hypoechogenicity as useful for AC diagnosis. Although SMI® performance is reported to be higher when compared to PD-US, they reported a good diagnostic accuracy for the latter too. Furthermore, PD-US is an examination that is more accessible in everyday clinical practice in most settings, which makes it easier to be adopted.
Contrast-enhanced ultrasonography (CEUS)
Cheng et al. in 2017 [15] compared US-arthrography, by injecting the contrast agent into the glenohumeral joint, with MRI as reference. Although the use of CEUS US arthrography may be tempting for AC diagnosis, it is an invasive technique, and its use is limited to the hospital setting, so its application in daily clinical practice may not be straightforward.
Share-wave and strain elastosonography in AC
To the best of our knowledge Wu et al. [31] was the first to apply SWE for AC diagnosis: their case–control study documented both higher CHL thickness and stiffness of affected shoulders against normal joints. Wada et al. [32] documented a lower stiffness for CHL and LHBT in the pre-freezing stage compared to the frozen one and a higher stiffness for SST and ISP in the pre-freezing stage compared to the frozen, allowing to suggest that SWE and SE could help define the stage of the disease. Zhang et al. [34], per what was already reported [22], documented a strict correlation between CHL stiffness, disease duration, and VAS pain: in the early phases of AC, CHL stiffness was lower in pathological shoulders than in healthy controls, conversely in the later stages of the disease CHL appeared harder at SWE. Moreover, the stiffness of CHL between the frozen and defrosting stages was not significantly different, testifying that the CHL stiffness reached its maximum in the middle phases. VAS pain was reported higher in the early stages of the disease while it appeared to subside in the later ones. Furthermore, CHL may not be visualized in 10% of individuals [38], therefore SWE and SE in this subset of patients could be of utmost importance. Indeed, elastosonography can add functional data (unavailable to MRI) to US morphological information. Nevertheless, SE and SWE are operator-dependent techniques and provide only a qualitative measure of tissue elasticity (also with Strain-Ratio evaluation), with limitations in their reproducibility. Moreover, although SWE has the potential to revolutionize bone, joint, and muscle imaging, its clinical application has been hindered by technical and artefactual challenges. Many of the stumbling blocks encountered during musculoskeletal SWE imaging are readily recognizable and can be overcome, but progressive advances in technology and a better understanding of image acquisition are required before SWE can reliably be used in MSK imaging. An important limitation in the application of SWE to tendons and ligaments in AC diagnosis is the proximity of these to bone structures (rotator cuff, pulley): the impact of bone proximity may provoke hardening artifacts which may affect the quantitative data and the inter-observer agreement [39]. Albeit studies investigating the role of SWE and SE are limited and with a validation yet to be clarified, they undoubtedly prove the interesting field of application of elastosonography: with new technological implementations, they will be able to provide further important information on the anatomical state of the ligaments and tendons structures involved in AC.
Conclusions
To date, the European Society of Musculoskeletal Radiology does not recommend musculoskeletal US as a reliable diagnostic tool for AC [40]. The evidence collected in this review testifies that musculoskeletal US is as reliable as MRI for AC diagnosis. Although CHL and AP thickening evaluation remain the cornerstone of musculoskeletal US diagnosis of AC, the combination of all the above-mentioned US parameters allows a correct diagnosis, thus assisting the physician in everyday clinical practice. For these reasons, we believe that the US should be inserted as a first-line imaging in suspected AC.
Acknowledgements
This study is dedicated to the memory of Giovanni Serafini, MD.
Funding
The authors deny any funding.
Declarations
Conflict of interest
The authors deny any conflict of interest.
Ethical committee approval
Not applicable
Human and animal rights
Not applicable
Informed consent
Not applicable
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Codman EA (1934) The shoulder: rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa. Thomas Todd Co., Boston
- 2.Neviaser JS. Adhesive capsulitis of the shoulder. J Bone Jt Surg Am. 1945;27(2):211. [Google Scholar]
- 3.Neviaser AS, Hannafin JA. Adhesive capsulitis: a review of current treatment. Am J Sports Med. 2010;38:2346–2356. doi: 10.1177/0363546509348048. [DOI] [PubMed] [Google Scholar]
- 4.Fields BKK, Skalski MR, Patel DB, et al. Adhesive capsulitis: review of imaging findings, pathophysiology, clinical presentation, and treatment options. Skeletal Radiol. 2019;48:1171–1184. doi: 10.1007/s00256-018-3139-6. [DOI] [PubMed] [Google Scholar]
- 5.Binder AI, Bulgen DY, Hazleman BL, Roberts S. Frozen shoulder: a long-term prospective study. Ann Rheum Dis. 1984;43:361–364. doi: 10.1136/ard.43.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BJ. L, The frozen shoulder. Clinical and radiographical observations. The effect of manipulation under general anesthesia. Structure and glycosaminoglycan content of the joint capsule. Local bone metabolism. Acta Orthop Scand Suppl. 1969;119:1–59. [PubMed] [Google Scholar]
- 7.Uhthoff HKLJ. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5(4):183–191. doi: 10.5435/00124635-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ryan V, Brown H, Lowe CJM, Lewis JS. The pathophysiology associated with primary (idiopathic) frozen shoulder: a systematic review. BMC Musculoskelet Disord. 2016 doi: 10.1186/s12891-016-1190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le HV, Lee SJ, Nazarian A, Rodriguez EK. Adhesive capsulitis of the shoulder: review of pathophysiology and current clinical treatments. Shoulder Elb. 2017;9:75–84. doi: 10.1177/1758573216676786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunker TD. Frozen shoulder: unravelling the enigma. Ann R Coll Surg Engl. 1997;79:210–213. [PMC free article] [PubMed] [Google Scholar]
- 11.Tamborrini G, Möller I, Bong D, et al. the rotator interval-a link between anatomy and ultrasound. Ultrasound Int Open. 2017;3:E107–E116. doi: 10.1055/s-0043-110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge”. Arthroscopy. 1993;9:611–616. doi: 10.1016/S0749-8063(05)80496-7. [DOI] [PubMed] [Google Scholar]
- 13.Stella SM, Gualtierotti R, Ciampi B, Trentanni C, Sconfienza LM, Del Chiaro A, Pacini P, Miccoli M, Galletti S. Ultrasound features of adhesive capsulitis. Rheumatol Ther. 2021 doi: 10.1007/s40744-021-00413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JC, Sykes C, Saifuddin A, Connell D. Adhesive capsulitis: sonographic changes in the rotator cuff interval with arthroscopic correlation. Skelet Radiol. 2005;34:522–527. doi: 10.1007/s00256-005-0957-0. [DOI] [PubMed] [Google Scholar]
- 15.Cheng X, Zhang Z, Xuanyan G, et al. Adhesive capsulitis of the shoulder: evaluation with US-arthrography using a sonographic contrast agent. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-05491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon A, Dewan S, Bhatt S, et al. Sonography in diagnosis of adhesive capsulitis of the shoulder: a case-control study. J Ultrasound. 2017 doi: 10.1007/s40477-017-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Choi YH, Oh S, et al. Ultrasound microflow imaging technology for diagnosis of adhesive capsulitis of the shoulder. J Ultrasound Med. 2020;39:967–976. doi: 10.1002/jum.15181. [DOI] [PubMed] [Google Scholar]
- 18.Homsi C, Bordalo-Rodrigues M, da Silva JJ, Stump XMGRG. Ultrasound in adhesive capsulitis of the shoulder: is assessment of the coracohumeral ligament a valuable diagnostic tool? Skelet Radiol. 2006;35:673–678. doi: 10.1007/s00256-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 19.Park GY, Park JH, Kwon DR, et al. Do the findings of magnetic resonance imaging, arthrography, and ultrasonography reflect clinical impairment in patients with idiopathic adhesive capsulitis of the shoulder? Arch Phys Med Rehabil. 2017;98:1995–2001. doi: 10.1016/j.apmr.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Wu PY, Hsu PC, Chen TN, et al. Evaluating correlations of coracohumeral ligament thickness with restricted shoulder range of motion and clinical duration of adhesive capsulitis with ultrasound measurements. PMR. 2021;13:461–469. doi: 10.1002/pmrj.12432. [DOI] [PubMed] [Google Scholar]
- 21.Do JG, Hwang JT, Yoon KJ, Lee Y-T. Correlation of ultrasound findings with clinical stages and impairment in adhesive capsulitis of the shoulder. Orthop J Sports Med. 2021 doi: 10.1177/23259671211003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelin P, Delarue Y, Duparc F, Dacher JN. Thickening of the inferior glenohumeral capsule: an ultrasound sign for shoulder capsular contracture. Eur Radiol. 2013;23:2802–2806. doi: 10.1007/s00330-013-2874-2. [DOI] [PubMed] [Google Scholar]
- 23.Hwan Kim D, Cho C-H, Hyun SD. Ultrasound measurements of axillary recess capsule thickness in unilateral frozen shoulder: study of correlation with MRI measurements. Skelet Radiol. 2018 doi: 10.1007/s00256-018-2959-8. [DOI] [PubMed] [Google Scholar]
- 24.Sernik RA, Vidal Leão R, Luis Bizetto E, et al. Thickening of the axillary recess capsule on ultrasound correlates with magnetic resonance imaging signs of adhesive capsulitis. Ultrasound. 2019;27:183–190. doi: 10.1177/1742271X19840063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J-G, Peo H, Cho J-H, et al. Dynamic ultrasonographic measurement of inferior joint capsule thickness in patients with unilateral frozen shoulder. Diagnostics. 2021 doi: 10.3390/diagnostics11050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Cho CH, Sung DH. Ultrasound measurements of axillary recess capsule thickness in unilateral frozen shoulder: study of correlation with MRI measurements. Skelet Radiol. 2018;47:1491–1497. doi: 10.1007/s00256-018-2959-8. [DOI] [PubMed] [Google Scholar]
- 27.Ryu KN, Lee SW, Rhee YG, Lim JH. Adhesive capsulitis of the shoulder joint: usefulness of dynamic sonography. J Ultrasound Med. 1993;12(8):445–449. doi: 10.7863/jum.1993.12.8.445. [DOI] [PubMed] [Google Scholar]
- 28.Stella SM, Gualtierotti R, Trentanni C, Ciampi B, Del Chiaro A, Galletti S. A response to: letter to the editor regarding ultrasound features of adhesive capsulitis. Rheumatol Ther. 2022;9(4):1225–1228. doi: 10.1007/s40744-022-00451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim I, Yi JH, Lee J, et al. Limited subacromial gliding of the supraspinatus tendon during dynamic ultrasonography can predict a decrease in capacity and MR arthrographic features of the shoulder joint. Eur Radiol. 2012 doi: 10.1007/s00330-012-2513-3. [DOI] [PubMed] [Google Scholar]
- 30.Walmsley S, Osmotherly PG, Walker CJ, Rivett DA. Power Doppler ultrasonography in the early diagnosis of primary/idiopathic adhesive capsulitis: an exploratory study. J Manip Physiol Ther. 2013;36:428–435. doi: 10.1016/j.jmpt.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Wu CH, Chen WS, Wang TG. Elasticity of the coracohumeral ligament in patients with adhesive capsulitis of the shoulder. Radiology. 2016;278:458–464. doi: 10.1148/radiol.2015150888. [DOI] [PubMed] [Google Scholar]
- 32.Wada T, Itoigawa Y, Yoshida K, et al. Increased stiffness of rotator cuff tendons in frozen shoulder on shear wave elastography. J Ultrasound Med. 2020;39:89–97. doi: 10.1002/jum.15078. [DOI] [PubMed] [Google Scholar]
- 33.Yun SJ, Jin W, Cho NS, et al. Shear-wave and strain ultrasound elastography of the supraspinatus and infraspinatus tendons in patients with idiopathic adhesive capsulitis of the shoulder: a prospective case-control study. Korean J Radiol. 2019;20:1176–1185. doi: 10.3348/kjr.2018.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Zhang L, Guo FZT. Shear wave elastography of the coracohumeral ligament with frozen shoulder in different stages. J Ultrasound Med. 2022 doi: 10.1002/jum.15942. [DOI] [PubMed] [Google Scholar]
- 35.Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2014 doi: 10.1016/j.ejrad.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Tian H, Dong F, et al. The role of grey-scale ultrasound in the diagnosis of adhesive capsulitis of the shoulder: a systematic review and meta-analysis. Med Ultrason. 2020;22:305–312. doi: 10.11152/mu-2430. [DOI] [PubMed] [Google Scholar]
- 37.Lim AKP, Satchithananda K, Dick EA, et al. Microflow imaging : new Doppler technology to detect low-grade inflammation in patients with arthritis. Eur Radiol. 2018;28:1046–1053. doi: 10.1007/s00330-017-5016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Tang K, Wang J, et al. MRI findings for frozen shoulder evaluation: is the thickness of the coracohumeral ligament a valuable diagnostic tool? PLoS ONE. 2011 doi: 10.1371/journal.pone.0028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bortolotto C, Turpini E, Felisaz P, et al. Median nerve evaluation by shear wave elastosonography : impact of “ bone - proximity ” hardening artifacts and inter-observer agreement. J Ultrasound. 2017;20:293–299. doi: 10.1007/s40477-017-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sconfienza LM, Albano D, Allen G, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018 doi: 10.1007/s00330-018-5474-3. [DOI] [PubMed] [Google Scholar]