Abstract
Guanosine has been considered a promising candidate for antidepressant responses, but if this nucleoside could modulate adenosine A1 (A1R) and A2A (A2AR) receptors to exert antidepressant-like actions remains to be elucidated. This study investigated the role of A1R and A2AR in the antidepressant-like response of guanosine in the mouse tail suspension test and molecular interactions between guanosine and A1R and A2AR by docking analysis. The acute (60 min) administration of guanosine (0.05 mg/kg, p.o.) significantly decreased the immobility time in the tail suspension test, without affecting the locomotor performance in the open-field test, suggesting an antidepressant-like effect. This behavioral response was paralleled with increased A1R and reduced A2AR immunocontent in the hippocampus, but not in the prefrontal cortex, of mice. Guanosine-mediated antidepressant-like effect was not altered by the pretreatment with caffeine (3 mg/kg, i.p., a non-selective adenosine A1R/A2AR antagonist), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX — 2 mg/kg, i.p., a selective adenosine A1R antagonist), or 4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo-{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)-phenol (ZM241385 — 1 mg/kg, i.p., a selective adenosine A2AR antagonist). However, the antidepressant-like response of guanosine was completely abolished by adenosine (0.5 mg/kg, i.p., a non-selective adenosine A1R/A2AR agonist), N-6-cyclohexyladenosine (CHA — 0.05 mg/kg, i.p., a selective adenosine A1 receptor agonist), and N-6-[2-(3,5-dimethoxyphenyl)-2-(methylphenyl)ethyl]adenosine (DPMA — 0.1 mg/kg, i.p., a selective adenosine A2A receptor agonist). Finally, docking analysis also indicated that guanosine might interact with A1R and A2AR at the adenosine binding site. Overall, this study reinforces the antidepressant-like of guanosine and unveils a previously unexplored modulation of the modulation of A1R and A2AR in its antidepressant-like effect.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11302-022-09898-8.
Keywords: A1 receptors, A2A receptors, Depression, Guanosine, Tail suspension test
Introduction
Major depressive disorder (MDD), a medical condition mainly characterized by depressed mood and anhedonia, is one of the most common and prevalent psychiatric illnesses, comprising the leading cause of disability and suicide worldwide [1]. Currently, the most prescribed antidepressant agents include monoaminergic system-targeted drugs, such as monoamine oxidase inhibitors and monoamine reuptake inhibitors [2]. Despite their widespread use, these drugs still present several challenges, particularly the treatment non-responsiveness, a delayed onset effect, and undesirable adverse effects. Therefore, these limitations underscore a significant unmet need for novel antidepressants with distinct mechanisms from conventional medications [3, 4].
Recent studies have provided evidence that purinergic signaling may represent a promising target underlying the physiopathology of MDD and antidepressant responses [5, 6]. Purinergic signaling is a complex and essential system that can shape a wide range of physiological functions, including synaptic transmission, neurotransmitter release, and neuromodulation [7]. In particular, adenosine is an adenine-based nucleoside that has been consistently reported to play a crucial role in the onset of MDD [8, 9] and antidepressant-like responses [10, 11]. More pertinently, the impact of adenosine on brain function is mainly dependent on the activity of adenosine inhibitory A1 receptors (A1R) and facilitatory A2A receptors (A2AR), which are widely distributed in the brain and are known to regulate mood [12] as well as to be implicated in antidepressant actions [6, 11, 13–15]. These findings indicate that adenosine-based purinergic signaling could represent a novel mechanism underpinning antidepressant responses.
Within this context, compelling studies have revealed surprisingly that the purine nucleoside guanosine may be a novel and promising target for antidepressant responses [16, 17]. Guanosine is an endogenous neuromodulator that has been shown to exert a plethora of activities in the central nervous system, such as neuroprotection, neuronal growth, differentiation, and survival [18–20]. Of special interest, patients with MDD have been reported to present reduced plasma levels of guanosine, suggesting that this nucleoside may be involved in the onset of MDD episodes [21, 22]. Accordingly, overwhelming evidence has demonstrated the ability of guanosine to produce antidepressant-like effects after an acute [23–27] or repeated [28–31] regimen of administration in rodents.
The key molecular targets for guanosine’s antidepressant effect include (i) modulation of NMDA receptors [24], (ii) activation of alpha-amino-3-hydroxy-methyl-5–4-isoxazole propionic acid (AMPA) receptors [25], (iii) stimulation of mitogen-activated protein kinases (MAPKs)/extracellular signal-regulated kinase (ERK) pathway [26], and (iv) activation of the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway, brain-derived neurotrophic factor (BDNF) availability, and synaptic protein translation in the hippocampus and prefrontal cortex [16, 25]. Additionally, the antidepressant-like response elicited by guanosine was also associated with increased hippocampal neuronal differentiation [29] and the suppression of oxidative stress- and neuroinflammation-related pathways [26, 28].
Interestingly, recent studies have demonstrated that guanosine is effective in affording neuroprotection by modulating A1R and A2AR [32–34]. However, the involvement of A1R and A2AR in the behavioral responses displayed by guanosine is still controversial and remains to be fully understood [35–37]. More importantly, if guanosine could modulate A1R and A2AR to exert antidepressant-like actions remains to be elucidated. Given this background, the present study aimed to investigate the role of A1R and A2AR in the antidepressant-like response displayed by guanosine in the mouse tail suspension test. Additionally, we also sought to predict the binding affinity between guanosine and A1R and A2AR using the molecular docking analysis.
Material and methods
Animals
Considering that the prevalence of depression is higher in women than in men [1], this study was performed using 192 adult female Swiss mice. The animals (30–40 g, 45–60 days of age) were maintained under controlled temperature (21 ± 1 °C) and humidity (50 ± 20%) with a 12-:12-h light/dark cycle (lights on at 7:00 a.m.). Mice were housed in groups of 8 in a cage (41 × 34 × 16 cm) with free access to food and water. The protocols were approved by the Institutional Ethics Committee (numbers 00795 and 7,485,180,518) and carried out in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Pharmacological treatments and experimental design
The first experimental approach was designed to validate the antidepressant-like effect of guanosine in the tail suspension test, as previously demonstrated [24, 25], as well as to investigate if this behavioral response could be paralleled with alterations in the immunocontent of A1R and A2AR in the hippocampus and prefrontal cortex. To address this experiment, mice (n = 8/group) received a single administration of vehicle or guanosine (0.05 mg/kg, p.o.), and 60 min after the treatments, they were subjected to the tail suspension test and open-field test (10 min apart). Guanosine, obtained from Sigma Chemical Co. (St. Louis, USA) and dissolved in distilled water, was freshly prepared and administered orally (p.o.) in a volume of 10 ml/kg body weight. All doses and time points of administration were chosen based on previous studies [24, 25]. After the behavioral tests, mice were immediately euthanized by decapitation and the hippocampus and prefrontal cortex were dissected to measure the immunocontent of adenosine A1R and A2AR by Western Blotting.
The second experimental approach was designed to evaluate if the antidepressant-like effect of guanosine is mediated by adenosine A1R and A2AR. In a first set of experiments to address this second hypothesis, mice were pretreated with vehicle, caffeine (3 mg/kg, i.p., a non-selective adenosine A1R/A2AR antagonist), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX – 2 mg/kg, i.p., a selective adenosine A1R antagonist), or 4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo-{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)-phenol (ZM241385 — 1 mg/kg, i.p., a selective adenosine A2AR antagonist). After 30 min, mice received the administration of vehicle or guanosine (0.05 mg/kg, p.o.), and then, they were subjected to the tail suspension test and open-field test (10 min apart) 60 min later. All doses and time points of administration were chosen based on previous studies [10, 13].
In another set of experiments, mice were treated with vehicle or guanosine (0.05 mg/kg, p.o.), and following 30 min, they received the administration of adenosine (0.5 mg/kg, i.p., a non-selective adenosine A1R/A2AR agonist), N-6-cyclohexyladenosine (CHA — 0.05 mg/kg, i.p., a selective adenosine A1 receptor agonist), or N-6-[2-(3,5-dimethoxyphenyl)-2-(methylphenyl)ethyl]adenosine (DPMA — 0.1 mg/kg, i.p., a selective adenosine A2A receptor agonist). After 30 min, mice were subjected to the tail suspension test and open-field test (10 min apart). All doses and time points of administration were chosen based on previous studies [10, 13].
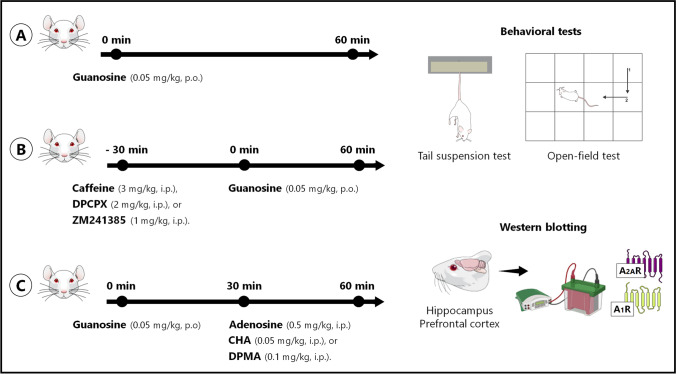
A schedule of experimental approaches, pharmacological treatments, behavioral tests, and Western blotting analysis is provided in Fig. 1.
Fig. 1.
Schedule of experimental approaches, pharmacological treatments, behavioral tests, and Western blotting analysis. In the first experimental approach, female Swiss mice were administered orally (p.o.) with vehicle or guanosine (0.05 mg/kg, p.o.), and 60 min after the treatments, they were subjected to the tail suspension test and open-field test (10 min apart). After the behavioral tests, mice were immediately euthanized by decapitation and the hippocampus and prefrontal cortex were dissected to measure the immunocontent of adenosine A1R and A2AR by Western blotting (A). In the second experimental approach, mice were pretreated with vehicle, caffeine (3 mg/kg, i.p.), DPCPX (2 mg/kg, i.p.), or ZM241385 (1 mg/kg, i.p.) and 30 min after the treatments, they received the administration of vehicle or guanosine (0.05 mg/kg, p.o.). Following 60 min of the treatments, mice were subjected to the tail suspension test and open-field test (B). In the third experimental approach, mice were treated with vehicle or guanosine (0.05 mg/kg, p.o.), and following 30 min, they received the administration of adenosine (0.5 mg/kg, i.p.), CHA (0.05 mg/kg, i.p.), or DPMA (0.1 mg/kg, i.p.). After 30 min of the treatments, mice were subjected to the tail suspension test and open-field test (C). Figure designed using images from Mind the Graph
Tail suspension test
The total immobility time of mice suspended by the tail was measured as previously proposed [38]. Briefly, visually isolated mice were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was recorded for a 6-min period by an experienced observer blind to the experimental groups. Mice were considered immobile only when they hung passively and completely motionless. The immobility time in the tail suspension test was taken as indicative of antidepressant-like responses [39].
Open-field test
The locomotor activity of mice was assessed in the open field apparatus, which consists of a wooden box (40 × 60 cm × 50 cm) with the floor divided into 12 equal squares [40]. At the start of each trial, mice were placed in the left corner of the field and allowed to freely explore the arena. The number of crossings (squares crossed with all paws) was registered for 6 min [41, 42]. The arena floor was cleaned with 10% ethanol between tests.
Western blotting
The hippocampus and prefrontal cortex were quickly dissected and snap-frozen with liquid nitrogen before storage at − 80 °C until use [43, 44]. Briefly, samples were mechanically homogenized in 50 mM TRIS pH 7.0, 1 mM ethylenediaminetetraacetic acid (EDTA), 100 mM NaF, 0.1 mM phenylmethylsulphonyl fluoride, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, and Sigma Protease Inhibitor Cocktail (P2714). Lysates were centrifuged (10,000 g for 10 min, at 4 °C) to eliminate cellular debris. The supernatants were diluted 1/1 (v/v) in 100 mM TRIS pH 6.8, 4 mM EDTA, 8% SDS, and boiled for 5 min. Thereafter, sample dilution (40% glycerol, 100 mM TRIS, bromophenol blue, pH 6.8) in the ratio 25:100 (v/v) and β-mercaptoethanol (final concentration 8%) were added to the samples. Protein content was quantified using bovine serum albumin (BSA) as a standard [45]. The samples containing 60 µg protein/track were separated by SDS-PAGE (miniVE Vertical Electrophoresis System TM, GE Healthcare Life Sciences, Piscataway, NJ, USA) using 7–10% gel, and the proteins were transferred to nitrocellulose membranes using a semi-dry blotting apparatus (1.2 mA/cm2; 1.5 h). To verify the transfer efficiency, membranes were stained with Ponceau [46], and subsequently blocked with 5% BSA in TBS (10 mM Tris, 150 mM NaCl, pH 7.5). The immunocontent of adenosine A1R (Santa Cruz Biotechnology®, CA, USA — 1:500 dilution) and A2AR (Santa Cruz Biotechnology®, CA, USA — 1:500 dilution), as well as β-actin (loading control, Cell Signaling Technology — 1:1000 dilution), were detected using antibodies diluted in TBS-T (10 mM Tris, 150 mM NaCl, 0.1% Tween-10, pH 7.5) containing 2.5% BSA and incubated overnight. Subsequently, the membranes were incubated with anti-rabbit antibody horseradish peroxidase-conjugated secondary antibody (Cell Signaling, 1:2500) for 60 min, and the immunoreactive bands were developed using a chemiluminescence kit (Amersham ECL Select, Piscataway, USA). All blocking and incubation steps were followed by three washes (5 min) of the membranes with TBS-T [47–49]. The optical density (OD) of the bands was quantified using the Image Lab Software® 4.1 (Bio-Rad Laboratories). The immunocontent of A1R and A2AR was determined as the ratio of the respective protein band over the OD of the β-actin band.
Molecular docking
The molecular docking analysis was carried out to predict the binding affinity between guanosine and A1R and A2AR. The coupling energy of the guanosine at the adenosine (A1R/A2AR non-selective agonist) binding site to A1R and A2AR (crystallographic structure) was evaluated using the DockThor 2.0 software [50]. To compare the docking properties of guanosine, we also estimated the docking energies of adenosine to A1R and to A2AR. The protein database (RSCB — Protein Data Bank) was used to obtain the structures of A1R (PDB 6D9H code) and A2AR (PDB 2YDO code). Moreover, the hydrogen-bond interaction patterns and hydrophobic contacts between guanosine and A1R or A2AR were performed using LigPlot + 2.2.5 graphical interface [51]. All binders underwent geometric optimization in the Chimera 1.14 software. The preparation of A1R and A2AR included the removal of water molecules, the addition of hydrogens, and the assignment of Gasteiger charges. Additionally, the poses of ligands were also observed using the Chimera 1.14.
Statistical analysis
The D’Agostino-Pearson test was used to assess data normality. Differences among experimental groups were determined by Student’s t-test or two-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test, when appropriate. Values of P < 0.05 were considered significant. The results are expressed as means ± standard error of the mean (S.E.M.).
Results
The antidepressant-like effect of guanosine is paralleled with alterations on hippocampal and prefrontocortical adenosine A1R and A2AR immunocontent
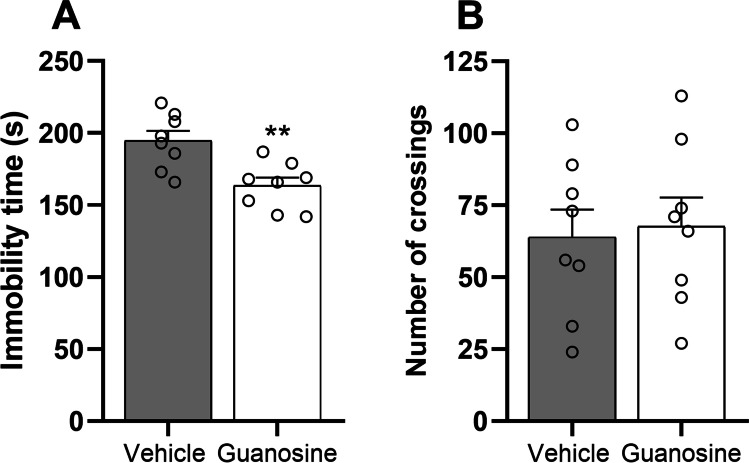
In the first experimental approach, we aimed to validate the antidepressant-like effect of guanosine in mice subjected to the tail suspension test (Fig. 2) and open-field test (Fig. 2). Statistical analysis showed that the acute administration of guanosine (0.05 mg/kg, p.o.) significantly reduced the immobility time when compared to the vehicle-treated group (t(14) = 3.52, P < 0.01), suggesting an antidepressant-like effect. No significant alterations were observed in the number of crossings (t(14) = 0.26, P = 0.79) in the open-field test. Fluoxetine, the positive control, also reduced the immobility time in the tail suspension test without altering the number of crossings in the open-field test (supplementary information).
Fig. 2.
Guanosine (0.05 mg/kg, p.o.) exerts an antidepressant-like effect in mice subjected to the tail suspension test (A) and open-field test. Values are expressed as means ± S.E.M. (n = 8). **P < 0.01 as compared with the vehicle-treated group (Student’s t-test)
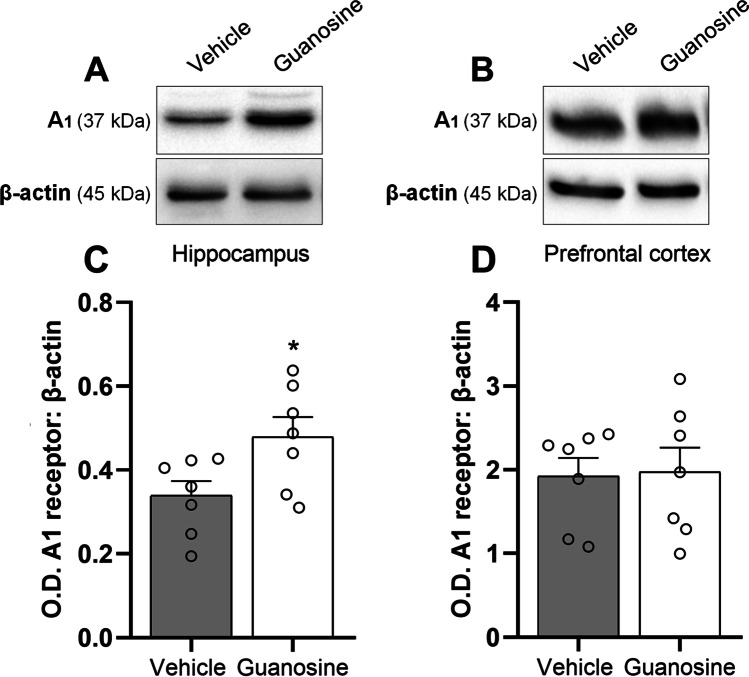
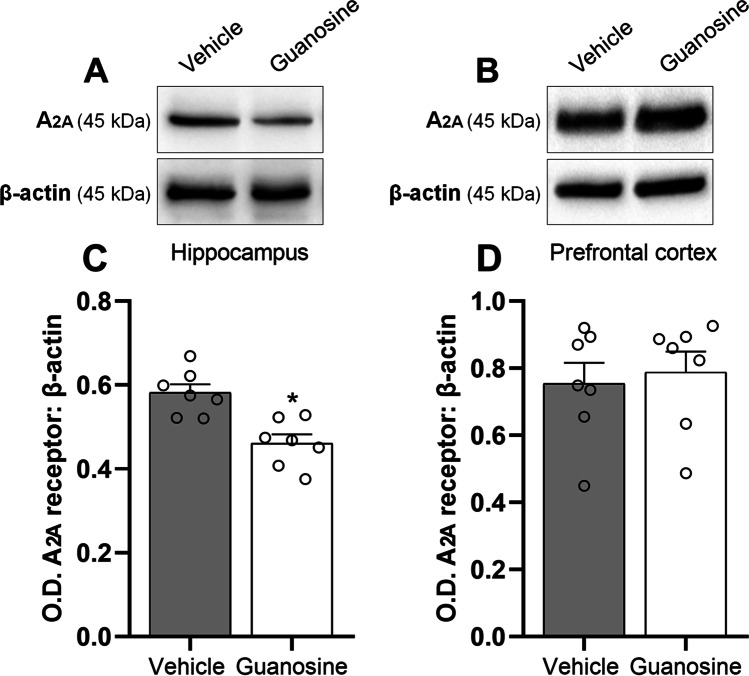
In the next step, we investigated if the antidepressant-like effect exerted by guanosine could be associated with alterations in the immunocontent of A1R (Fig. 3) and A2AR (Fig. 4) in the hippocampus and prefrontal cortex. Statistical analysis showed that the acute administration of guanosine (0.05 mg/kg, p.o.) significantly augmented the immunocontent of A1R in the hippocampus (Fig. 3 and C; t(12) = 2.41, P < 0.05), but not in the prefrontal cortex (Fig. 3 and 3; t(12) = 0.12, P = 0.89). Additionally, a single administration of guanosine (0.05 mg/kg, p.o.) significantly reduced the immunocontent of adenosine A2AR in the hippocampus (Fig. 4 and C; t(12) = 4.10, P < 0.01), but not in the prefrontal cortex (Fig. 4 and 4; t(12) = 0.38, P = 0.70). These results suggest that the antidepressant-like response of guanosine may be paralleled with alterations in the immunocontent of adenosine receptors, particularly an increment of A1R and a reduction of A2AR specifically in the hippocampus.
Fig. 3.
Guanosine selectively increases A1R immunocontent in the hippocampus of mice. Representative images of A1R/β-actin and corresponding quantification in the hippocampus (A and C) and prefrontal cortex (B and D). Values are expressed as means (percentage of control) ± S.E.M. (n = 7). *P < 0.05 as compared with the vehicle-treated group (Student’s t-test)
Fig. 4.
Guanosine selectively reduces A2AR immunocontent in the hippocampus of mice. Representative images of A2AR/β-actin and corresponding quantification in the hippocampus (A and C) and prefrontal cortex (B and D). Values are expressed as means (percentage of control) ± S.E.M. (n = 7). *P < 0.05 as compared with the vehicle-treated group (Student’s t-test)
The antidepressant-like effect of guanosine is associated with the modulation of A1R and A2AR
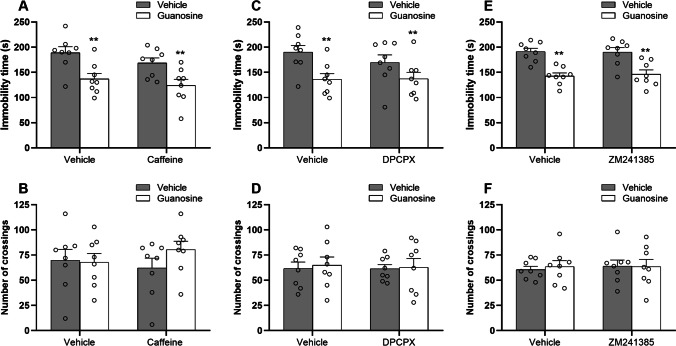
To reinforce the hypothesis that the antidepressant-like effect of guanosine may involve the modulation of A1R and A2AR, mice were pretreated with caffeine (3 mg/kg, i.p., a non-selective A1R/A2AR antagonist), DPCPX (2 mg/kg, i.p., a selective A1R antagonist), or ZM241385 (1 mg/kg, i.p., a selective A2AR antagonist). The results illustrated in Fig. 5 show the effect of the pretreatment with caffeine (Fig. 5 and 5), DPCPX (Fig. 5 and 5), or ZM241385 (Fig. 5 and 5) in the antidepressant-like effect of guanosine in the tail suspension test and open-field test. Two-way ANOVA revealed a significant main effect of guanosine [F(1, 28) = 17.95, P < 0.01], with guanosine treated-mice presenting a significant reduction in the immobility time (P < 0.01) regardless of the administration with vehicle or caffeine. However, two-way ANOVA revealed no significant differences for caffeine [F(1, 28) = 2.23, P = 0.14], and guanosine × caffeine interaction [F(1, 28) = 0.13, P = 0.72] in the immobility time (Fig. 5). No alterations were detected in the number of crossings (Fig. 5) in the open-field test (guanosine [F(1, 28) = 0.06, P = 0.76], caffeine [F(1, 28) = 0.17, P = 0.67], and guanosine × caffeine interaction [F(1, 28) = 0.22, P = 0.63]. Furthermore, two-way ANOVA revealed a significant main effect of guanosine [F(1, 28) = 13.44, P < 0.01], with guanosine treated-mice presenting a significant reduction in the immobility time (P < 0.01) regardless of the administration with vehicle or DPCPX. However, two-way ANOVA revealed no significant differences for DPCPX [F(1, 28) = 0.18, P = 0.67], and guanosine × DPCPX interaction [F(1, 28) = 0.25, P = 0.61] in the immobility time (Fig. 5). No alterations were detected in the number of crossings (Fig. 5) in the open-field test (guanosine [F(1, 28) = 0.10, P = 0.74], DPCPX [F(1, 28) = 0.03, P = 0.85], and guanosine × DPCPX interaction [F(1, 28) = 0.01, P = 0.89]. Likewise, two-way ANOVA revealed a significant main effect of guanosine [F(1, 28) = 36.60, P < 0.01], with guanosine treated-mice presenting a significant reduction in the immobility time (P < 0.01) regardless of the administration with vehicle or ZM241385. However, two-way ANOVA revealed no significant differences for ZM241385 [F(1, 28) = 0.03, P = 0.85], and guanosine × ZM241385 interaction [F(1, 28) = 0.08, P = 0.77] in the immobility time (Fig. 5). No alterations were detected in the number of crossings (Fig. 5) in the open-field test (guanosine [F(1, 28) = 0.04, P = 0.83], ZM241385 [F(1, 28) = 0.09, P = 0.75], and guanosine × ZM241385 interaction [F(1, 28) = 0.08, P = 0.77]. These results suggest that the acute antidepressant-like effect displayed by guanosine in the tail suspension test may not involve, at least partially, the activation of adenosine A1R and A2AR.
Fig. 5.
The effects of caffeine (3 mg/kg, i.p., A and B), DPCPX (2 mg/kg, i.p., C and D), or ZM241385 (1 mg/kg, i.p., E and F) in the antidepressant-like effect of guanosine in mice subjected to the tail suspension test and open-field test. Values are expressed as means ± S.E.M. (n = 8) followed by two-way ANOVA. *P < 0.05 and **P < 0.01 as compared with the vehicle-treated group (i.e., significant main effect of guanosine)
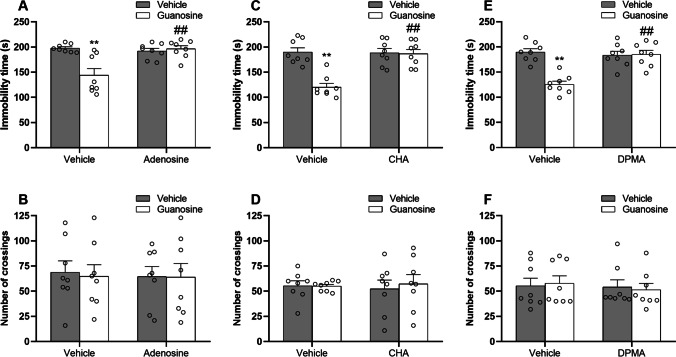
Subsequently, we assessed the influence of the administration of adenosine (0.5 mg/kg, i.p., a non-selective A1R/A2AR agonist), CHA (0.05 mg/kg, i.p., a selective adenosine A1 receptor agonist), or DPMA (0.1 mg/kg, i.p., a selective adenosine A2A receptor agonist) in the antidepressant-like responses elicited by guanosine. The results illustrated in Fig. 6 show the effect of the pretreatment with adenosine (Fig. 6 and 6), CHA (Fig. 6 and 6), or DPMA (Fig. 6 and 6) in the antidepressant-like effect of guanosine in the tail suspension test and open-field test. Two-way ANOVA revealed significant differences for guanosine [F(1, 28) = 9.85, P < 0.01], adenosine [F(1, 28) = 8.88, P < 0.01], and guanosine × adenosine interaction [F(1, 28) = 13.67, P < 0.01] in the immobility time (Fig. 6). Post hoc analysis indicated that guanosine administration significantly reduced the immobility time (P < 0.01), but this anti-immobility effect was significantly abolished by adenosine (P < 0.01). No alterations were detected in the number of crossings (Fig. 6) in the open-field test (guanosine [F(1, 28) = 0.02, P = 0.93], adenosine [F(1, 28) = 0.10, P = 0.75], and guanosine × adenosine interaction [F(1, 28) = 0.01, P = 0.97]. Furthermore, two-way ANOVA revealed significant differences for guanosine [F(1, 28) = 18.27, P < 0.01], CHA [F(1, 28) = 14.98, P < 0.01], and guanosine × CHA interaction [F(1, 28) = 16.28, P < 0.01] in the immobility time (Fig. 6). Post hoc analysis indicated that the anti-immobility effect of guanosine in the immobility time (P < 0.01) was significantly occluded by CHA administration (P < 0.01). No alterations were observed in the number of crossings (Fig. 6) in the open-field test (guanosine [F(1, 28) = 0.10, P = 0.74], CHA [F(1, 28) = 0.01, P = 0.95], and guanosine × CHA interaction [F(1, 28) = 0.14, P = 0.70]. Likewise, two-way ANOVA revealed significant main effects for guanosine [F(1, 28) = 17.83, P < 0.01], DPMA [F(1, 28) = 12.94, P < 0.01], and guanosine × DPMA interaction [F(1, 28) = 19.73, P < 0.01] in the immobility time (Fig. 6). Post hoc analysis indicated that the ability of guanosine in reducing the immobility time (P < 0.01) was significantly counteracted by DPMA administration (P < 0.01). No alterations were detected in the open-field test (Fig. 6) in the open-field test (guanosine [F(1, 28) = 0.01, P = 0.98], DPMA [F(1, 28) = 0.28, P = 0.59], and guanosine × DPMA interaction [F(1, 28) = 0.14, P = 0.71]. These results suggest that the antidepressant-like effect exerted by guanosine in the tail suspension test may involve the modulation of adenosine A1R/A2AR.
Fig. 6.
The effects of adenosine (0.5 mg/kg, i.p., A and B), CHA (0.05 mg/kg, i.p., C and D), or DPMA (0.1 mg/kg, i.p., E and F) in the antidepressant-like effect of guanosine in mice subjected to the tail suspension test and open-field test. Values are expressed as means ± S.E.M. (n = 8). **P < 0.01 compared with the vehicle-treated group; ##P < 0.01 compared with the guanosine-treated group (two-way ANOVA followed by Newman-Keuls post hoc test)
Guanosine might interact with A1R and A2AR at the adenosine binding site
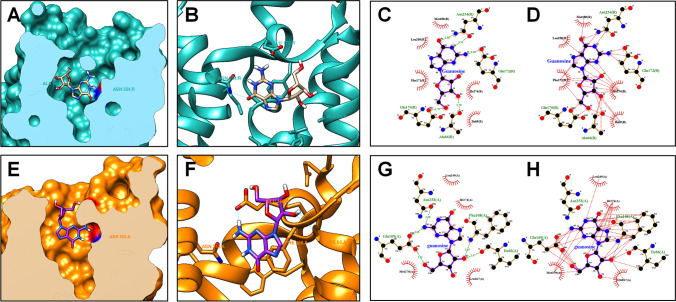
In the first in silico approach, we aimed to predict the binding affinity between guanosine and A1R as well as the guanosine–A1R interactions (Fig. 7 and 7). The prediction of the guanosine binding affinity to A1R was − 8140 kcal/mol, according to the DockThor 2.0 software. Moreover, LigPlot + analysis revealed 5 hydrogen-bond interactions (amino acid residues Asn254, Glu172, Glu170, and Ala66) (Fig. 7) and 28 hydrophobic contacts (amino acid residues Asn254, Glu172, Glu170, Ala66, Met180, Leu250, Phe171, Ile274, and Ile69) (Fig. 7) between guanosine and A1R. In the second in silico approach, we aimed to predict the binding affinity between guanosine and A2AR as well as the guanosine–A2AR interactions (Fig. 7 and 7). The coupling energy obtained between guanosine and A2AR was − 8,069 kcal/mol. Additionally, guanosine–A2AR interactions using the LigPlot + software revealed 5 hydrogen-bond interactions (amino acid residues Asn253, Glu169, Phe168, and Ile66) (Fig. 7) and 39 hydrophobic contacts (amino acid residues Phe168, Ile66, Glu169, Leu249, Ile274, Leu167, and Met270) (Fig. 7). This prediction of A1R/A2AR –guanosine binding data suggests that guanosine might interact with A1R and A2AR at the adenosine binding site. Additionally, the prediction of the adenosine binding affinity to A1R was − 8291 kcal/mol while to A2AR was − 7998 kcal/mol. Interestingly, most of the hydrogen-bond interactions and hydrophobic contacts observed herein with A1R/A2AR–guanosine binding prediction were shown to be shared between adenosine and A1R (hydrogen-bond interactions at amino acid residues Asn254 and Glu172; hydrophobic contacts at amino acid residues Asn254, Glu172, Met180, Leu250, Phe171, and Ile274) or A2AR (hydrogen-bond interactions at amino acid residues Asn253 and Glu169; hydrophobic contacts at amino acid residues Phe168, Ile66, Glu169, Leu249, Ile274, Leu167, and Met270).
Fig. 7.
Molecular interactions between guanosine and A1R or A2AR. The putative binding site of guanosine to A1R (A). Structure of the A1R complexed with guanosine (B). Representative pose of guanosine complexed with A1R, in which guanosine–A2AR hydrogen bond are represented as green dashed lines (C). Representative pose of guanosine complexed with A1R, in which guanosine–A1R hydrophobic contacts are represented as red dashed lines (D). The putative binding site of guanosine to A2AR (E). Structure of the A2AR complexed with guanosine (F). Representative pose of guanosine complexed with A2AR, in which guanosine–A2AR hydrogen bonds are represented as green dashed lines (G). Representative pose of guanosine complexed with A2AR, in which guanosine–A1R hydrophobic contacts are represented as red dashed lines (H)
Discussion
Several lines of evidence have demonstrated that disturbances in plasma levels of guanosine, an endogenous guanine-based purine, are associated with depressive symptoms in clinical studies [21, 22] as well as the ability of this nucleoside to produce antidepressant-like actions in preclinical reports [24–27]. In the present research, we corroborated these findings by showing that acute administration of guanosine (0.05 mg/kg, p.o.) significantly decreased the immobility time in the tail suspension test compared to vehicle-treated mice without altering the number of crossings in the open-field test. These results indicate that the anti-immobility effect displayed by this nucleoside in the tail suspension test was not affected by locomotor activity, as previously demonstrated [24–27]. Moreover, a study by Lara et al. [52] that administered guanosine at higher doses (2 and 7.5 mg/kg) to male Swiss mice reported no alteration in the rotarod performance in guanosine-treated mice. Also, in agreement, a study by Schmidt et al. [53] showed that guanosine administered by i.t. route did not alter sensorimotor coordination of male Swiss mice in the rotarod and caused no significant alteration in episodes of rearing, crossing, grooming, and fecal boluses. Although the reduction of immobility time caused by drugs in the tail suspension test is not a definitive conclusion that this drug has an antidepressant effect, it is interesting to mention that guanosine has been consistently shown to elicit antidepressant-like responses in naïve mice and rodents subjected to models that induce depressive-like behavior [16, 17, 23–26, 54].
To further unravel the molecular basis associated with the antidepressant-like effect of guanosine, we next assessed the role of the modulation of A1R and A2AR levels in the hippocampus and prefrontal cortex, two brain regions afflicted in MDD [55], in mice that exhibited a behavioral response to guanosine in the tail suspension test. Compelling studies have demonstrated that adenosine inhibitory A1R and facilitatory A2AR may underlie the onset of depressive symptoms and antidepressant responses [12, 56]. Moreover, the overexpression of A2AR in forebrain neurons is paralleled with depression-like behavior [57] and chronic unpredictable stress increases A2AR immunocontent in the hippocampus [11]. Conversely, A1R overexpression in forebrain neurons is associated with antidepressant-like effects and A1R knockout mice displayed depressive-like phenotypes [58]. Furthermore, A2AR antagonists have been shown to evoke antidepressant-like effects in rodents subjected to the forced swim test and tail suspension test [59, 60], while increased A1R function elicits antidepressant-like responses in these same behavioral paradigms [58, 61]. Here, to the best of our knowledge, we showed for the first time that acute administration of guanosine significantly increased A1R and reduced A2AR immunocontent in the hippocampus, but not in the prefrontal cortex, of mice. Therefore, one may speculate that the ability of guanosine in inducing a hippocampal region-specific modulation of A1R and A2AR immunocontent could contribute to the antidepressant-like effect of this nucleoside in the tail suspension test, although further studies are necessary to ascertain this hypothesis.
To obtain deeper insights into the role of A1R and A2AR in the antidepressant-like effect displayed by guanosine, we next investigated the impact of antagonists and agonists of A1R and A2AR in the guanosine-mediated anti-immobility effect in the tail suspension test. Our results unveiled that the ability of guanosine in reducing the immobility time in this test was not altered by the pretreatment with a non-selective A1R/A2AR antagonist, caffeine, or A1R-A2AR-related selective antagonists, DPCPX and ZM241385, respectively. Interestingly, in another set of experiments, we observed that the anti-immobility response exerted by guanosine in mice that underwent the tail suspension test was completely occluded by adenosine (a non-selective A1R/A2AR agonist) as well as CHA (a selective adenosine A1R agonist) and DPMA (a selective adenosine A2AR agonist). Importantly, these agonists were effective in blocking the guanosine-mediated antidepressant-like responses at doses that did not alter the immobility time in the tail suspension test per se, as previously demonstrated [10, 13]. At the same time, neither guanosine nor A1R/A2AR agonists and antagonists, at given doses, modified basal locomotor activity. Considering that the behavioral response elicited by guanosine in the tail suspension test was blocked by A1R and A2AR agonists, but not by A1R and A2AR antagonists, these results suggest that a modulation of adenosine receptors may be related to the ability of guanosine to reduce the immobility time in the tail suspension test [37]. In line with our results, a recent study demonstrated that the ability of guanosine in producing anxiolytic-related responses involves the modulation of A1R and A2AR [37]. Importantly, the authors have shown that the anxiolytic-like effect induced by guanosine in rats subjected to the elevated plus-maze test was blocked by A1R/A2AR non-selective agonist (adenosine) and A1R and A2AR selective agonists (CPA, CCPA, and CGS21680, respectively), but not by A1R and A2AR antagonists (caffeine, DPCPX, and ZM241385), which resembles our findings. One possibility that has been raised to explain these results is that a functional competition between guanosine and adenosine or A1R-A2AR selective agonists [37] may occur, or even an A1-A2A receptor-receptor interaction, which have been shown to contribute to the guanosine-mediated responses [62]. Interestingly, a study by Kaster et al. [10] showed that both the intraperitoneal administration of adenosine and the intracerebroventricular (i.c.v.) administration of this nucleoside reduced the immobility time in the forced swimming test in mice, suggesting a centrally mediated modulation of adenosine receptors in the antidepressant-like responses in mice subjected to the forced swimming test following i.c.v. adenosine administration.
Providing additional support for our results, mounting evidence has indicated a functional crosstalk between guanosine and A1R and A2AR agonists or antagonists [32–37]. Guanosine’s ability to prevent oxygen/glucose deprivation-induced reduced hippocampal slices viability and glutamate uptake was abolished by CGS21680 (A2AR selective agonist), but not by ZM241385 (A2AR selective antagonist). The pretreatment of hippocampal slices with A1R antagonist, DPCPX, occluded the effects of guanosine against oxygen/glucose deprivation-induced reduced slices viability but did not affect glutamate uptake [32]. Moreover, guanosine has also been shown to prevent reactive oxygen species production in hippocampal slices subjected to oxygen/glucose deprivation by modulating A1R and A2AR, as these responses were effectively abrogated by A1R antagonist, DPCPX, and A2AR agonist, CGS21680 [33]. Guanosine-mediated anti-tremor and antioxidant effects in reserpinized mice were shown to be A1R-dependent and A2AR-independent, as these effects were abolished by A1R antagonist DPCPX, but still present in A2AR-knockout mice [35]. Furthermore, guanosine-induced protective effects against oxidative burst and mitochondrial dysfunction induced by 6-OHDA in striatal slices were precluded by A1R antagonist DPCPX and A2AR agonist CGS21680, but not by A1R agonist CCPA [34]. Additionally, the neuroprotective effect displayed by guanosine against traumatic brain injury in rats was abrogated by A1R antagonist DPCPX, but unaltered by the A2AR antagonist SCH58261 [63].
Our in silico results also uncovered that guanosine might interact with A1R and A2AR at the adenosine binding site, reinforcing that A1R and A2AR as likely targets for guanosine-mediated antidepressant-like responses. Of note, docking computational prediction of adenosine receptors–guanosine coupling affinities revealed a strong binding affinity between guanosine and A1R or A2AR, a response comparable to the binding affinity of adenosine to A1R or A2AR. Further analysis revealed critical hydrogen-bond interactions and hydrophobic contacts between guanosine and A1R or A2AR. Interestingly, most of the hydrogen-bond interactions and hydrophobic contacts observed herein with A1R/A2AR–guanosine binding prediction were shown to be shared between adenosine and A1R or A2AR (A1R — PDB 6D9H code; A2AR — PDB 2YDO code), further reinforcing our experiments. These in silico results are in line with our in vivo data by showing that guanosine could act as a modulator or even an agonist of A1R or A2AR. However, we cannot rule out the possibility that guanosine interacts with putative guanosine receptors that may share some features with adenosine receptors, as has been suggested in previous studies. Although these receptors have not been fully characterized, specific receptor binding sites for [3H]guanosine in rat brain membranes have been reported in the last few years [64, 65]. Of special interest, [3H]guanosine binding sites in hippocampal membranes may be displaced by A1R/A2AR non-selective agonist (adenosine) and A1R or A2AR selective agonists (CPA and CGS21680, respectively) [37].
Taken together, our study reinforces the notion that guanosine is effective in exerting antidepressant-like responses and unveils a previously unexplored role of the modulation of A1R and A2AR in the antidepressant-like effect of this nucleoside. Although our experiments did not fully elucidate if guanosine binds and acts through A1R/A2AR, we provided novel evidence that the anti-immobility effect of guanosine in the tail suspension test is paralleled with the hippocampal region-specific modulation of A1R and A2AR levels. In addition, we suggest that guanosine may evoke the modulation of adenosine A1R and A2AR to produce antidepressant-like responses. Our bioinformatics data also indicated that guanosine might interact with A1R and A2AR at the adenosine binding site. Considering that modulation of A1R and A2AR has been shown to evoke antidepressant-like effects in rodents [58–61] and caffeine (A1R/A2AR non-selective antagonist) is protective against MDD in humans [66, 67] and depressive-like behavior in rodents [11], here, we added new pieces of evidence indicating that the modulation of these receptors may comprise an important mechanism by which guanosine exerts its behavioral effects in the tail suspension test. However, it is essential to bear in mind that the tail suspension test is not a model of depression and that the results obtained in this test should be taken with care from the translational point of view. However, this test has been well recognized as a useful tool to elucidate some critical molecular targets implicated in antidepressant-like responses [38, 39]. Specifically, in the present study, our behavioral data associated with docking analysis help to characterize the possible existence of guanosine binding sites in adenosine receptors. Importantly, the docking properties of guanosine and adenosine are similar regarding the coupling energy. The results reported herein extend the assumption that guanosine may be further investigated as a useful approach for the management of MDD [16]. Importantly, futures studies are welcome to characterize the role of A1R and A2AR in the antidepressant-like effect of guanosine using further molecular techniques, and animal models that induce depressive-like behavior.
Supplementary information
Below is the link to the electronic supplementary material.
Anderson Camargo
obtained his Bachelor's degree in Biological Sciences at the Regional University of Blumenau (FURB), Master’s degree in Neuroscience at the Federal University of Santa Catarina (UFSC), and Ph.D. in Neuroscience at the Federal University of Santa Catarina (UFSC). Currently, He is working as a postdoctoral researcher at the Karolinska Institutet. He researches the mechanisms underpinning the pathophysiology of stress-related disorders and fast-acting antidepressant agents, as well as the molecular targets underlying the mechanisms of resilience and vulnerability to stress and pro-resilience strategies.
Author contribution
Ana Lúcia S. Rodrigues designed the study and wrote the protocol. Anderson Camargo, Luis E. B. Bettio, Priscila B. Rosa, and Julia M. Rosa administered the drugs and performed the behavioral tests. Anderson Camargo performed Western blotting analysis. Glorister A. Altê carried out the bioinformatics studies. Anderson Camargo and Ana Lúcia S. Rodrigues contributed to undertake the statistical analysis and wrote the first draft of the manuscript, as well as approved the final manuscript.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, #421143/2018–5 and #312215/2021–5) and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). ALSR is CNPq Research Fellow.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Institutional Ethics Committee.
Informed consent.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2017) Depression and other common mental disorders: global health estimates. World Health Organization 1–24
- 2.Otte C, Gold S, Penninx B, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:1–20. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 3.Kaster MP, Moretti M, Cunha MP, Rodrigues ALS. Novel approaches for the management of depressive disorders. Eur J Pharmacol. 2016;771:236–240. doi: 10.1016/j.ejphar.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Papakostas GI, Ionescu DF. Towards new mechanisms: an update on therapeutics for treatment-resistant major depressive disorder. Mol Psychiatry. 2015;20:1142–1150. doi: 10.1038/mp.2015.92. [DOI] [PubMed] [Google Scholar]
- 5.Bartoli F, Burnstock G, Crocamo C, Carrà G. Purinergic signaling and related biomarkers in depression. Brain Sci. 2020;10:1–12. doi: 10.3390/BRAINSCI10030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szopa A, Socała K, Serefko A, et al. Purinergic transmission in depressive disorders. Pharmacol Ther. 2021;224:107821. doi: 10.1016/J.PHARMTHERA.2021.107821. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Introduction to purinergic signaling. Methods Mol Biol. 2020;2041:1–15. doi: 10.1007/978-1-4939-9717-6_1. [DOI] [PubMed] [Google Scholar]
- 8.Fredholm BB, Chen J, Cunha RA, Svenningsson P. Adenosine and brain function. Int Rev Neurobiol. 2005;63:7742. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 9.Gomes JI, Farinha-Ferreira M, Rei N, et al. Of adenosine and the blues: the adenosinergic system in the pathophysiology and treatment of major depressive disorder. Pharmacol Res. 2021;163:105363. doi: 10.1016/J.PHRS.2020.105363. [DOI] [PubMed] [Google Scholar]
- 10.Kaster MP, Rosa AO, Rosso MM, et al. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett. 2004;355:21–24. doi: 10.1016/J.NEULET.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proceed National Acad Sci USA. 2015;112:7833–7838. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Calker D, Biber K, Domschke K, Serchov T. The role of adenosine receptors in mood and anxiety disorders. J Neurochem. 2019;151:11–27. doi: 10.1111/JNC.14841. [DOI] [PubMed] [Google Scholar]
- 13.Cunha MP, Pazini FL, Rosa JM, et al. Creatine, similarly to ketamine, affords antidepressant-like effects in the tail suspension test via adenosine A1and A2Areceptor activation. Purinergic Signalling. 2015;11:215–227. doi: 10.1007/s11302-015-9446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobato KR, Binfaré RW, Budni J, et al. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:994–999. doi: 10.1016/J.PNPBP.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Lazarevic V, Yang Y, Flais I, Svenningsson P. Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors. Mole Psychiatry. 2021;26(12):7425–7435. doi: 10.1038/s41380-021-01246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo A, Rodrigues ALS. Guanosine as a promising target for fast-acting antidepressant responses. Pharmacol Biochem Behav. 2022;218:173422. doi: 10.1016/J.PBB.2022.173422. [DOI] [PubMed] [Google Scholar]
- 17.Almeida RF, Ferreira TP, David CVC, et al. Guanine-based purines as an innovative target to treat major depressive disorder. Front Pharmacol. 2021;547:1–6. doi: 10.3389/FPHAR.2021.652130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.di Liberto V, Mudò G, Garozzo R, et al. The guanine-based purinergic system: The tale of an orphan neuromodulation. Front Pharmacol. 2016;7:1–15. doi: 10.3389/fphar.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettio LEB, Gil-Mohapel J, Rodrigues ALS. Guanosine and its role in neuropathologies. Purinergic Signalling. 2016;12:411–426. doi: 10.1007/s11302-016-9509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanznaster D, Dal-Cim T, Piermartiri TCB, Tasca CI. Guanosine: a neuromodulator with therapeutic potential in brain disorders. Aging Dis. 2016;7:657–679. doi: 10.14336/AD.2016.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali-Sisto T, Tolmunen T, Toffol E, et al. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology. 2016;70:25–32. doi: 10.1016/j.psyneuen.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Mocking RJT, Naviaux JC, Li K, et al. Metabolic features of recurrent major depressive disorder in remission, and the risk of future recurrence. Translational Psychiatry. 2021;11(1):1–13. doi: 10.1038/s41398-020-01182-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida RF, Pocharski CB, Rodrigues ALS, et al. Guanosine fast onset antidepressant-like effects in the olfactory bulbectomy mice model. Sci Rep. 2020;10:8429. doi: 10.1038/s41598-020-65300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettio LEB, Cunha MP, Budni J, et al. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res. 2012;234:137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Rosa PB, Bettio LEB, Neis VB et al (2021) Antidepressant-like effect of guanosine involves activation of AMPA receptor and BDNF/TrkB signaling. Purinergic Signal 17:285–301. 10.1007/s11302-021-09779-6 [DOI] [PMC free article] [PubMed]
- 26.Rosa PB, Bettio LEB, Neis VB, et al. The antidepressant-like effect of guanosine is dependent on GSK-3β inhibition and activation of MAPK/ERK and Nrf2/heme oxygenase-1 signaling pathways. Purinergic Signalling. 2019;15:491–504. doi: 10.1007/s11302-019-09681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camargo A, Dalmagro AP, Zeni ALB, Rodrigues ALS. Guanosine potentiates the antidepressant-like effect of subthreshold doses of ketamine: possible role of pro-synaptogenic signaling pathway. J Affect Disord. 2020;271:100–108. doi: 10.1016/j.jad.2020.03.186. [DOI] [PubMed] [Google Scholar]
- 28.Almeida RF, Nonose Y, Ganzella M, et al. Antidepressant-like effects of chronic guanosine in the olfactory bulbectomy mouse model. Front Psych. 2021;1268:1–14. doi: 10.3389/FPSYT.2021.701408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettio L, Neis V, Pazini F, et al. The antidepressant-like effect of chronic guanosine treatment is associated with increased hippocampal neuronal differentiation. Eur J Neurosci. 2016;43:1006–1015. doi: 10.1111/ejn.13172. [DOI] [PubMed] [Google Scholar]
- 30.Marques NF, Binder LB, Roversi K, et al. Guanosine prevents depressive-like behaviors in rats following bilateral dorsolateral striatum lesion induced by 6-hydroxydopamine. Behav Brain Res. 2019;372:112014. doi: 10.1016/j.bbr.2019.112014. [DOI] [PubMed] [Google Scholar]
- 31.Piermartiri T, dos Santos B, Barros-Aragão F, et al. Guanosine promotes proliferation in neural stem cells from hippocampus and neurogenesis in adult mice. Mol Neurobiol. 2020;57:3814–3826. doi: 10.1007/S12035-020-01977-4. [DOI] [PubMed] [Google Scholar]
- 32.Dal-Cim T, Ludka FK, Martins WC, et al. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem. 2013;126:437–450. doi: 10.1111/jnc.12324. [DOI] [PubMed] [Google Scholar]
- 33.Dal-Cim T, Poluceno GG, Lanznaster D, et al. Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: involvement of A1 and A2A adenosine receptors and PI3K, MEK, and PKC pathways. Purinergic Signalling. 2019;15:465. doi: 10.1007/S11302-019-09679-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massari CM, Constantino LC, Tasca CI. Adenosine A1 and A2A receptors are involved on guanosine protective effects against oxidative burst and mitochondrial dysfunction induced by 6-OHDA in striatal slices. Purinergic Signalling. 2021;17:247–254. doi: 10.1007/S11302-021-09765-Y/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massari C, Constantino L, Marques N, et al. Involvement of adenosine A 1 and A 2A receptors on guanosine-mediated anti-tremor effects in reserpinized mice. Purinergic Signalling. 2020;16:379–387. doi: 10.1007/S11302-020-09716-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almeida RF, Comasseto DD, Ramos DB, et al. Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol Neurobiol. 2017;54:423–436. doi: 10.1007/s12035-015-9660-x. [DOI] [PubMed] [Google Scholar]
- 37.Frinchi M, Verdi V, Plescia F, et al. Guanosine-mediated anxiolytic-like effect: interplay with adenosine A1 and A2A receptors. Int J Mol Sci. 2020;21:1–15. doi: 10.3390/IJMS21239281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 39.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/J.NEUBIOREV.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Dalmagro AP, Camargo A, Rodrigues ALS, Zeni ALB. Involvement of PI3K/Akt/GSK-3β signaling pathway in the antidepressant-like and neuroprotective effects of Morus nigra and its major phenolic, syringic acid. Chem Biol Interact. 2019;314:108843. doi: 10.1016/j.cbi.2019.108843. [DOI] [PubMed] [Google Scholar]
- 41.Camargo A, Dalmagro AP, Wolin IAV, et al. The resilient phenotype elicited by ketamine against inflammatory stressors-induced depressive-like behavior is associated with NLRP3-driven signaling pathway. J Psychiatr Res. 2021;144:118–128. doi: 10.1016/J.JPSYCHIRES.2021.09.057. [DOI] [PubMed] [Google Scholar]
- 42.Camargo A, Torrá ACNC, Dalmagro AP, et al. Prophylactic efficacy of ketamine, but not the low-trapping NMDA receptor antagonist AZD6765, against stress-induced maladaptive behavior and 4E-BP1-related synaptic protein synthesis impairment. Prog Neuropsychopharmacol Biol Psychiatry. 2022;115:110509. doi: 10.1016/J.PNPBP.2022.110509. [DOI] [PubMed] [Google Scholar]
- 43.Camargo A, Dalmagro AP, de Souza MM, et al. Ketamine, but not guanosine, as a prophylactic agent against corticosterone-induced depressive-like behavior: possible role of long-lasting pro-synaptogenic signaling pathway. Exp Neurol. 2020;334:113459. doi: 10.1016/j.expneurol.2020.113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camargo A, Dalmagro AP, Fraga DB, et al. Low doses of ketamine and guanosine abrogate corticosterone-induced anxiety-related behavior, but not disturbances in the hippocampal NLRP3 inflammasome pathway. Psychopharmacology. 2021;238(9):2555–2568. doi: 10.1007/S00213-021-05879-8. [DOI] [PubMed] [Google Scholar]
- 45.Peterson GLA. A simplification of the protein assay method of Lowry et al which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 46.Camargo A, Dalmagro AP, Wolin IAV, et al. A low-dose combination of ketamine and guanosine counteracts corticosterone-induced depressive-like behavior and hippocampal synaptic impairments via mTORC1 signaling. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110371. doi: 10.1016/j.pnpbp.2021.110371. [DOI] [PubMed] [Google Scholar]
- 47.Fraga DB, Camargo A, Olescowicz G, et al. A single administration of ascorbic acid rapidly reverses depressive-like behavior and hippocampal synaptic dysfunction induced by corticosterone in mice. Chem Biol Interact. 2021;342:109476. doi: 10.1016/j.cbi.2021.109476. [DOI] [PubMed] [Google Scholar]
- 48.Camargo A, Pazini FL, Rosa JM, et al. Augmentation effect of ketamine by guanosine in the novelty-suppressed feeding test is dependent on mTOR signaling pathway. J Psychiatr Res. 2019;115:103–112. doi: 10.1016/j.jpsychires.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Camargo A, Dalmagro AP, Rosa MJ, et al. Subthreshold doses of guanosine plus ketamine elicit antidepressant-like effect in a mouse model of depression induced by corticosterone: Role of GR/NF-κB/IDO-1 signaling. Neurochem Int. 2020;139:104797. doi: 10.1016/j.neuint.2020.104797. [DOI] [PubMed] [Google Scholar]
- 50.Guedes IA, Costa LSC, dos Santos KB, et al. Drug design and repurposing with DockThor-VS web server focusing on SARS-CoV-2 therapeutic targets and their non-synonym variants. Sci Rep. 2021;11:5543. doi: 10.1038/S41598-021-84700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laskowski RA, Swindells MB. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/CI200227U/ASSET/IMAGES/LARGE/CI-2011-00227U_0005.JPEG. [DOI] [PubMed] [Google Scholar]
- 52.Lara DR, Schmidt AP, Frizzo MES, et al. Effect of orally administered guanosine on seizures and death induced by glutamatergic agents. Brain Res. 2001;912:176–180. doi: 10.1016/S0006-8993(01)02734-2. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt AP, Böhmer AE, Schallenberger C, et al. Spinal mechanisms of antinociceptive action caused by guanosine in mice. Eur J Pharmacol. 2009;613:46–53. doi: 10.1016/j.ejphar.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Bettio LEB, Freitas AE, Neis VB, et al. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol Biochem Behav. 2014;127:7–14. doi: 10.1016/j.pbb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Liu W, Ge T, Leng Y, et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast. 2017;2017:6871089. doi: 10.1155/2017/6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada K, Kobayashi M, Kanda T. Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol. 2014;119:373–393. doi: 10.1016/B978-0-12-801022-8.00015-5. [DOI] [PubMed] [Google Scholar]
- 57.Coelho JE, Alves P, Canas PM, et al. Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front Psych. 2014;5:1–8. doi: 10.3389/FPSYT.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serchov T, Clement HW, Schwarz MK, et al. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron. 2015;87:549–562. doi: 10.1016/J.NEURON.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodgson RA, Bertorelli R, Varty GB, et al. Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression. J Pharmacol Exp Ther. 2009;330:294–303. doi: 10.1124/JPET.108.149617. [DOI] [PubMed] [Google Scholar]
- 60.Yamada K, Kobayashi M, Mori A, et al. Antidepressant-like activity of the adenosine A(2A) receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents. Pharmacol Biochem Behav. 2013;114–115:23–30. doi: 10.1016/J.PBB.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Hines DJ, Schmitt LI, Hines RM, et al. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Translational Psychiatry. 2013;3(1):1–9. doi: 10.1038/tp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lanznaster D, Massari CM, Marková V, et al. Adenosine A1–A2A receptor-receptor interaction: contribution to guanosine-mediated effects. Cells. 2019;8:1–16. doi: 10.3390/CELLS8121630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobrachinski F, Gerbatin RR, Sartori G, et al. Guanosine attenuates behavioral deficits after traumatic brain injury by modulation of adenosinergic Receptors. Mol Neurobiol. 2019;56:3145–3158. doi: 10.1007/S12035-018-1296-1. [DOI] [PubMed] [Google Scholar]
- 64.Traversa U, Bombi G, di Iorio P, et al. Specific [(3)H]-guanosine binding sites in rat brain membranes. Br J Pharmacol. 2002;135:969–976. doi: 10.1038/sj.bjp.0704542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traversa U, Bombi G, Camaioni E, et al. Rat brain guanosine binding site: Biological studies and pseudo-receptor construction. Bioorg Med Chem. 2003;11:5417–5425. doi: 10.1016/j.bmc.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 66.Jee HJ, Lee SG, Bormate KJ, Jung YS. Effect of caffeine consumption on the risk for neurological and psychiatric disorders: sex differences in Human. Nutrients. 2020;12:1–20. doi: 10.3390/NU12103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223–234. doi: 10.1002/MNFR.201500620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.