Abstract
Esmethadone (REL-1017) is the opioid-inactive dextro-isomer of methadone and a low-affinity, low-potency uncompetitive NMDA receptor antagonist. In a Phase 2, randomized, double-blind, placebo-controlled trial, esmethadone showed rapid, robust, and sustained antidepressant effects. Two studies were conducted to evaluate the abuse potential of esmethadone. Each study utilized a randomized, double-blind, active-, and placebo-controlled crossover design to assess esmethadone compared with oxycodone (Oxycodone Study) or ketamine (Ketamine Study) in healthy recreational drug users. Esmethadone 25 mg (proposed therapeutic daily dose), 75 mg (loading dose), and 150 mg (Maximum Tolerated Dose) were evaluated in each study. Positive controls were oral oxycodone 40 mg and intravenous ketamine 0.5 mg/kg infused over 40 min. The Ketamine study included oral dextromethorphan 300 mg as an exploratory comparator. The primary endpoint was maximum effect (Emax) for Drug Liking, assessed using a bipolar 100-point visual analog scale (VAS). A total of 47 and 51 participants completed the Oxycodone Study and the Ketamine Study, respectively (Completer Population). In both studies, esmethadone doses ranging from therapeutic (25 mg) to 6 times therapeutic (150 mg) had a meaningful and statistically significantly (p < 0.001) lower Drug Liking VAS Emax compared with the positive control. Results were consistent for all secondary endpoints in both studies. In both studies, all doses of esmethadone were statistically equivalent to placebo on Drug Liking VAS Emax (p < 0.05). In the Ketamine Study, Drug Liking VAS Emax scores for esmethadone at all tested doses were significantly lower vs. dextromethorphan (p < 0.05) (exploratory endpoint). These studies indicate no meaningful abuse potential for esmethadone at all tested doses.
Subject terms: Diseases, Depression
Introduction
Major depressive disorder (MDD) is the second leading cause of disability and chronic disease burden in the United States, among all medical conditions [1]. According to data from the National Epidemiologic Survey on Alcohol and Related Conditions-III, the lifetime prevalence of MDD is 20.6% [2]. Serotonergic antidepressants take 6 to 8 weeks, on average, to produce clinical benefits and are ineffective in approximately two-thirds of patients with MDD [3]. In addition, serotonergic antidepressants have meaningful metabolic side effects, including weight gain, and cause sleep disruption and sexual dysfunction [4, 5]. Atypical antipsychotics are used as second-line adjunctive treatment; they are also marginally effective and have serious side effects [6]. There is an urgent medical need for a rapidly effective, safe, and well-tolerated treatment for MDD. Impaired neural plasticity caused by altered glutamatergic signaling has emerged as a mechanism of disease hypothesis for MDD, superseding the classic serotonergic hypothesis [7–10]. Neural plasticity is regulated by glutamatergic signaling via the N-methyl-D-aspartate receptor (NMDAR) [11, 12]. Uncompetitive NMDAR channel blockers reverse impaired neural plasticity and depressive-like behavior in animal models by restoring synaptic plasticity [13–18] and rapidly reverse MDD in patients [19–21].
Esmethadone (REL-1017) is the opioid-inactive dextro-isomer of methadone and a low affinity, low potency uncompetitive NMDAR antagonist [22, 23]. Esmethadone showed efficacy in animal models of depressive-like behavior [14, 24] acting via a brain-derived neurotrophic factor (BDNF)-dependent mechanism and interestingly esmethadone increased BDNF in humans [25]. Unlike more potent NMDAR antagonists, esmethadone does not produce Olney’s lesions or other evidence of damage to cortical neurons in rats [26]. In animal models, esmethadone has no meaningful opioid agonist effects [27–29]. In animal models predictive of human abuse potential, esmethadone did not cause physical dependence, withdrawal signs, or reinforcing effects [30, 31]. In human studies, esmethadone did not have meaningful opioid agonist effects and did not show meaningful abuse potential [32–34]. While esmethadone is not approved for any indication, it has been available to researchers since the 1940s. There have been no known cases of abuse with esmethadone. Esmethadone does not interconvert to levomethadone in vivo [35], and there is no known method for converting esmethadone to levomethadone in vitro.
Phase 1 and Phase 2 results with esmethadone provided safety, tolerability, and pharmacokinetic (PK) results across a range of doses sufficient to inform the design and conduct of the human abuse potential (HAP) studies [19, 35]. Phase 2 results with esmethadone showed rapid, robust, and sustained antidepressant effects at 25 and 50 mg oral daily doses and confirmed the favorable safety and tolerability profile seen in Phase 1 studies [19]. Phase 3 trials with esmethadone are ongoing and are expected to enroll approximately 1000 patients with MDD (ClinicalTrials.gov Identifiers: NCT04688164; NCT05081167; NCT04855747; NCT04855760). If the results of the esmethadone Phase 3 trials replicate Phase 2 results, esmethadone could offer a safe and well-tolerated rapid treatment for MDD. Although animal and human data indicate no meaningful opioid agonist effect and no ketamine-like dissociative effects with esmethadone, its potential use in a large population of patients with MDD, a patient population vulnerable to substance use disorder, warranted further evaluation. Therefore, a full HAP evaluation was conducted in two studies comparing esmethadone with oxycodone, an opioid with known abuse potential, and with ketamine, an NMDAR antagonist with known abuse potential. Racemic methadone and its isomers are currently Schedule II controlled substances in the United States and are also internationally controlled in Schedule I of the Single Convention on Narcotic Drugs, 1961. Chiral configuration is known to impart opioid activity to molecules: as a rule, for chiral molecules, only one of the two enantiomers is opioid active [7–9]. Dextromethorphan, an unscheduled, over the counter antitussive, is the opioid inactive dextro-isomer of racemetorphan, which is a schedule II narcotic, like the opioid active levo-isomer levomethorphan. While it is known that opioid receptor affinity and opioid agonist effects are stereoselective [36, 37], because of the structural similarity between esmethadone and levomethadone, an opioid agonist molecule, in the first study we compared esmethadone with oxycodone, which is a Schedule II opioid (Oxycodone Study). Because of the known NMDAR antagonist activity of esmethadone [22, 23], in the second study we compared esmethadone with ketamine, a Schedule III NMDAR antagonist (Ketamine Study). As an additional exploratory endpoint of the Ketamine Study, esmethadone was also compared to dextromethorphan (DXM), an unscheduled, over-the-counter NMDAR antagonist and antitussive medication. DXM in combination with quinidine is FDA approved for the treatment of pseudobulbar affect. DXM in combination with bupropion has shown efficacy for MDD [20, 38] and has been recently FDA approved for the treatment of MDD.
Methods
These studies were conducted in accordance with relevant federal regulations of the Declaration of Helsinki, in compliance with the International Council for Harmonisation good clinical practice guidelines, and according to the appropriate regulatory requirements in the United States. Study protocols were reviewed and approved by a qualified Institutional Review Board. All participants signed the written informed consent prior to study procedures.
Study designs
Each study utilized a single-dose, randomized, double-blind, active- and placebo-controlled crossover design to assess the abuse potential of esmethadone compared with oxycodone (Study 1—Oxycodone Study) or ketamine (Study 2—Ketamine Study) in healthy recreational drug users. All study drug administration and assessments were conducted in an inpatient setting. The overall design was consistent with FDA guidelines for assessing HAP [39]. Each study included a Screening Phase, a Qualification Phase, a Treatment Phase, and a Follow-up visit (Supplemental Figs. 1 and 2). Each Qualification Phase was conducted as a single-dose, randomized, crossover trial during which participants received single doses of oxycodone (40 mg, oral; Oxycodone Study) or ketamine (0.5 mg/kg, 40-min intravenous [IV] infusion; Ketamine Study), and placebo (oral matching placebo for the Oxycodone Study; IV and oral matching placebo for the Ketamine Study). To be eligible for the treatment phase of each study, participants had to tolerate the positive control and who could discriminate the positive control from placebo (i.e., Drug Liking bipolar visual analog scale (VAS) maximum effect (Emax) of ≥65 points for the positive control and ≥15 point difference compared with placebo). Participants had to show an appropriate response following placebo administration, which was defined as scoring within the neutral range (i.e., 40 to 60 points on Drug Liking bipolar VAS) and also acceptable neutral responses on other scales. If a participants showed a positive response on Drug Liking following placebo (>60), they were excluded. In the Treatment Phase, eligible participants were randomized to receive each of the planned study drugs in a crossover manner. Study drug administration in each treatment period was separated by a minimum washout interval of 11 days. While there is always the potential for functional unblinding, double-and triple-dummy procedures and multiple treatment sequences were put in place to mitigate this risk. The ketamine study included two comparators, further decreasing the potential for functional unblinding.
Participants
Participants were healthy individuals 18 to 55 years of age, inclusive, who were experienced with nontherapeutic (recreational) drug use. In the Oxycodone Study (Study 1), participants had prior experience with recreational opioid use (defined as ≥10 lifetime occasions of use and ≥1 use in the 12 weeks prior to Screening). In the Ketamine Study (Study 2), participants had prior experience with NMDAR antagonists (e.g., ketamine, esketamine, phencyclidine [PCP], DXM), and had specifically used ketamine ≥1 in their lifetime and had ≥1 use of drugs for nonmedical purposes by either the intranasal or IV route in the past year. Participants were recruited separately for each study and were compensated according to IRB-approved parameters. In addition, participants had a body mass index (BMI) ranging from 18 to 35 kg/m2, were healthy according to physical examination, medical history, vital signs, clinical laboratory assessments, and 12-lead electrocardiogram (ECG); and had negative urine drug screens and pregnancy tests (females) at each visit. Participants with a history/presence of drug or alcohol dependence or psychiatric disorder, according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition, text revision) or who had ever participated in a substance or alcohol rehabilitation program were excluded. This was defined as any psychiatric disease that was anticipated in the Investigator’s or Medical Monitor’s opinion to be clinically significant and to potentially compromise safety or adversely affect the evaluation of the study data. No participant was excluded in either study for failing to satisfy this inclusion/exclusion criteria. Other exclusion criteria included corrected QT interval by Fridericia (QTcF) > 450 ms, pregnancy or breastfeeding (females), and allergy to NMDAR antagonists or related drugs or allergy to opioids. Concomitant medications (except acetaminophen, hormonal contraceptives, and hormone replacement therapy) were prohibited during the study.
Study drugs and dose selection
Esmethadone doses of 25 mg, 75 mg (3x planned therapeutic dose and planned loading dose for the treatment of MDD, and 150 mg (6x therapeutic dose and the maximum tolerated dose [MTD]) [35], administered as 25 mg tablets (Patheon Pharma Services), were selected for evaluation in the Treatment Phase of both studies. The 25 mg and 75 mg doses were selected in accordance with FDA Guidance recommendations to evaluate the planned therapeutic daily dose, and a dose that is 2 to 3 times the planned therapeutic dose; the 75 mg dose is also the planned loading dose in patients. In consideration of its pharmacological class and that experienced drug users may exhibit a greater tolerance and seek higher doses, 150 mg was selected as it is 6 times the planned therapeutic dose.
Oxycodone, a Schedule II opioid, was selected as the positive control in the Oxycodone Study (Study 1). The dose of oxycodone (40 mg; administered as 20 mg over-encapsulated tablets) was consistent with those previously evaluated in HAP studies [40–49].
Ketamine, a Schedule III NMDAR antagonist, was selected as the positive control in the Ketamine Study (Study 2). The dose and infusion duration of IV ketamine (0.5 mg/kg over 40 min) was consistent with a previous HAP study (CDER review of NDA 211243 [esketamine]). DXM, an unscheduled, over-the-counter, NMDAR antagonist, and antitussive drug, was selected as an exploratory comparator in the Ketamine Study. The oral dose of DXM (300 mg capsule) was based on a prior single ascending dose study showing that this dose had detectable subjective effects but was not associated with prominent emesis that was more commonly observed at higher supratherapeutic doses of 400 to 800 mg [50].
To ensure blinding, study drugs were administered in a double- (Oxycodone Study) or triple- (Ketamine Study) dummy fashion, where participants received the same number of tablets and capsules (and a 40-min IV infusion in the Ketamine Study) administered as a combination of active or placebo product in each treatment period.
Pharmacodynamic assessments
Bipolar 100-point VAS of “at-the-moment” Drug Liking, Overall Drug Liking, and Take Drug Again were used to measure the balance of positive and negative effects. The Emax of “at-the-moment” Drug Liking VAS was defined as the primary endpoint. Unipolar “at-the-moment” 100-point VAS were used to measure positive (High, Good Effects), negative (Bad Effects), and other (Any Effects, Alertness/Drowsiness) subjective drug effects. In the Ketamine Study, perceptual/dissociative effects were assessed using “at-the-moment” 100-point Hallucinations and Bowdle VAS (13-item scale rating current feelings) [51]. At-the-moment VAS was administered predose and 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 36, and 48 h post dose. Scales that referred specifically to drug effects were not administered predose. Overall Drug Liking VAS and Take Drug Again VAS were administered 12 and 24 h post dose. Drug Similarity VAS, administered at 12 h post dose, was used to estimate the class of drugs that the participants identified as being most similar to each of the treatments. Pupillometry was performed at predose and 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h post dose in the Oxycodone Study.
Pharmacokinetic assessments
Blood samples were collected predose and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 24, 36, and 48 h post dose. In the Oxycodone Study, plasma samples were analyzed for REL-101 and oxycodone. In the Ketamine Study, plasma samples were analyzed for esmethadone, ketamine, norketamine, DXM, and dextrorphan.
Safety assessments
Safety assessments were performed throughout all phases of the studies and included adverse events (AE; spontaneous participant reports), vital signs (blood pressure, pulse rate, oxygen saturation, and respiratory rate), 12-lead ECGs, continuous cardiac telemetry, pulse oximetry (from ≥15 min predose to ≥4 h post dose), clinical laboratory testing, physical examinations, and the Columbia-Suicide Severity Rating Scale (C-SSRS) [52].
Statistical analyses
Pharmacodynamic (PD) analyses were conducted using the Completer Population, defined as participants who completed all treatment periods and had at least one response on the VAS for Drug Liking within 2 h of peak plasma concentrations (Tmax) for each treatment [39]. For both studies, PD analyses were also performed for the Modified Completer Population, which excluded participants who had similar Drug Liking Emax scores (within 5 points difference) across all study treatments (including placebo) and excluded participants with an Emax for placebo >60 and a difference between Emax for oxycodone and placebo of ≤5.
The primary PD endpoint, Drug Liking VAS Emax, was analyzed using a 1-sided hypothesis test at a significance level of α = 0.05 and reported with 95% confidence intervals (CIs) and prespecified margins for each of the hypotheses. For the validation test (positive control vs. placebo), the margin of 15 was selected based on prior studies (CDER review of NDA 211243 [esketamine]) [44, 53–55]. For the test between the positive control and esmethadone (abuse potential relative to oxycodone or ketamine), a margin of 0 was applied. A margin of 11 was selected for the statistical test between esmethadone and placebo and between DXM and placebo (abuse potential relative to placebo) for the primary endpoint, based on a meta-analysis of 8 HAP studies [56]. The tests were conducted sequentially and thus, no multiplicity adjustment was needed. No specific margins were prespecified for the inferential analysis of secondary endpoints because there is currently no supporting literature that can aid in identification of a margin. Therefore, secondary endpoint results are presented as descriptive statistics.
Pharmacodynamic endpoints (Emax), area under/over the effect curve) were initially analyzed using a linear mixed effects model containing treatment, period, sequence, and first-order carryover effect as fixed effects (SAS version 9.4 or higher, SAS Institute Inc., Cary, NC, USA). The participant nested-within-treatment sequence was included as a random effect. Baseline was also included as a covariate where applicable (i.e., for measures evaluated predose). The first-order carryover effect was the previous treatment received in the Treatment Phase. If the carryover effect was found to be nonsignificant at the 25% level, then the term was dropped from the model [57]. If the carryover effect was significant at the 25% level, but not at the 5% level, then the carryover effect term was retained in the model; if the carryover effect was significant at the 5% level, a first-period analysis was conducted. The residuals from the mixed effects model were investigated for normality using the Shapiro–Wilk W test [58]. Parameters were analyzed under the assumption of a normal distribution of errors if the p value of the test was ≥0.01. If the p value was <0.01 for the Shapiro–Wilk W test on the residuals from the mixed model, a test of skewness was conducted on each paired difference. If the distribution of the paired differences was not skewed (−0.5 < skewness value < 0.5), then the endpoint was analyzed using a paired t-test. If the distribution of the paired differences was skewed (skewness value ≤ 0.5 or skewness value > 0.5), then the endpoint was analyzed nonparametrically using the Sign Test. Based on assumptions, derived from prior studies [54] and CDER review of NDA 211243 [esketamine]). that the true mean difference between the active comparators and placebo is approximately 35 points, the estimated sample size of 43 completer subjects provided greater than 90% power.
Pharmacokinetic (PK) parameters (peak plasma concentration [Cmax], Tmax, area under the concentration-time curve from 0 to last measurable concentration [AUC0-last]) for each analyte were calculated using non-compartmental analysis (Phoenix WinNonlin, version 8.1, Certara, L.P., Princeton, NJ, USA) for the PK Population, which included all participants who received at least 1 dose of active study drug and had at least 1 measurable PK sample for the respective treatment. Derived parameters were summarized descriptively. All safety analyses were summarized descriptively using the Safety Population, which included all participants who received at least 1 dose of study drug in the Treatment Phase.
Results
Participant disposition and demographics
In the Oxycodone Study (Study 1), 50 participants were randomized at two clinical sites (Hassman Research Institute [n = 8] and Ohio Clinical Trials [n = 42]) to the Treatment Phase, and 47 completed all treatment periods and were included in the Completer Population. Six participants discontinued early: 5 were lost to follow-up (3 of these 5 subjects completed all 5 treatment periods but did not attend the final follow-up visit and were included in the Completer Population), and 1 was discontinued due to noncompliance or major protocol violation (repeated visit cancellation/no show). In the Ketamine Study (Study 2), 54 participants were randomized at two clinical sites (Ohio Clinical Trials [n = 32] and Woodland Research Northwest [n = 22]) to the Treatment Phase, and 51 completed all treatment periods and were included in the Completer Population. Three participants discontinued early: 1 withdrew consent, 1 discontinued for safety reasons (AEs of elevated alanine aminotransferase, aspartate aminotransferase, and blood lactate dehydrogenase at admission to treatment period 6 [last treatment: DXM 300 mg]), and 1 was discontinued for administrative reasons.
In the Oxycodone Study, most participants were male, Black or African-American and non-Hispanic, with a mean age of 36.3 years (Table 1). All participants reported prior experience with opioids, and the majority reported a history of cannabinoid use. Recreational use of depressants also was relatively common, whereas few participants reported recreational use of stimulants, hallucinogens, or dissociative anesthetics. In the Ketamine Study, most participants were male, white, and non-Hispanic, with a mean age of 34.4 years (Table 1). All participants reported prior experience with dissociative anesthetics, and the majority reported a history of cannabinoids use, hallucinogen use, and stimulant use. Recreational use of opioids also was relatively common, whereas few participants reported recreational use of depressants or nitrite inhalants.
Table 1.
Baseline characteristics—Oxycodone Study.
| Oxycodone Study | Ketamine Study | |||
|---|---|---|---|---|
| Demographic variable | Safety population N = 50 | Completer population N = 47 | Safety population N = 54 | Completer population N = 51 |
| Age, yearsa | 36.3 ± 8.9 | 36.3 ± 9.1 | 34.4 ± 9.8 | 34.4 ± 9.8 |
| Male, n (%) | 40 (80.0) | 37 (78.7) | 37 (68.5) | 35 (68.6) |
| Race, n (%) | ||||
| White | 21 (42.0) | 20 (42.6) | 34 (63.0) | 32 (62.7) |
| Black | 28 (56.0) | 27 (57.4) | 15 (27.8) | 14 (27.5) |
| American Indian or Alaskan Native | 1 (2.0) | 0 | 2 (3.7) | 2 (3.9) |
| Asian | 0 | 0 | 2 (3.7) | 2 (3.9) |
| Other | 0 | 0 | 2 (3.7) | 2 (3.9) |
| Hispanic or Latino, n (%) | 6 (12.0) | 5 (10.6) | 4 (7.4) | 4 (7.8) |
| Body mass index, kg/m2 a | 26.8 ± 4.2 | 26.8 ± 4.2 | 25.2 ± 3.8 | 25.2 ± 3.7 |
aMean ± standard deviation.
Pharmacodynamics
Oxycodone Study
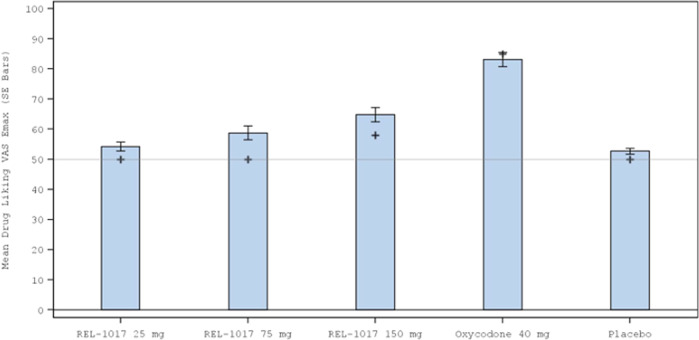
Effects on Drug Liking VAS Emax (primary study endpoint) are shown in Fig. 1. The validity of the study was determined from the comparison of Drug Liking VAS Emax between the positive control, oxycodone 40 mg, and placebo. The median (Q1, Q3) difference was 35.0 (18.0, 49.0; p < 0.001) for the Completer Population, indicating that oxycodone had a meaningful and statistically significantly higher Drug Liking VAS Emax compared with placebo, using a prespecified margin of 15 (Tables 2 and 3). The abuse potential of esmethadone relative to oxycodone was determined from the comparison of Drug Liking VAS Emax of each dose with the positive control. Drug Liking VAS Emax for each esmethadone dose was meaningfully and significantly lower than oxycodone at a prespecified margin of 0 (mean/median difference between oxycodone and each dose of esmethadone ≥19; all p < 0.001) (Tables 2 and 3). The abuse potential of esmethadone relative to placebo was determined from the comparisons of Drug Liking VAS Emax of each dose with placebo. The median difference from placebo was 0 for esmethadone 25 mg and 75 mg (p < 0.001) and 7.0 for the 150 mg dose (p = 0.036), indicating that esmethadone was not meaningfully different from placebo and was statistically equivalent to placebo at doses up to 6 times the planned therapeutic dose and MTD (Tables 2 and 3). Mean Drug Liking VAS scores were maintained close to placebo scores over 24 h (Supplemental Figure 3).
Fig. 1. Mean (standard error) Drug Liking VAS Emax by Treatment During the Treatment Phase for the Oxycodone Study (Completer Population).
+ = median value; p < 0.001 for median difference between oxycodone and placebo.
Table 2.
Drug Liking bipolar VAS Emax—Oxycodone Study.
| Oxycodone Study | |||||
|---|---|---|---|---|---|
| Statistic | Esmethadone 25 mg (N = 47) | Esmethadone 75 mg (N = 47) | Esmethadone 150 mg (N = 47) | Oxycodone 40 mg (N = 47) | Placebo (N = 47) |
| Mean (SD) | 54.2 (10.35) | 58.7 (15.82) | 64.9 (16.58) | 83.2 (16.57) | 52.7 (6.52) |
| Median | 50.0 | 50.0 | 58.0 | 85.0 | 50.0 |
| Range | 50–100 | 50–100 | 50–100 | 50–100 | 50–80 |
Drug Liking VAS is a bipolar scale where a score of 0 represents “strong disliking,” a score of 100 represents “strong liking,” and a score of 50 represents “neither like nor dislike” (neutral point). The question text is, “At this moment, my liking for this drug is?”.
Emax maximum effect, range minimum–maximum, SD standard deviation, VAS visual analog scale.
Table 3.
Inferential analysis of Drug Liking VAS Emax—esmethadone vs. oxycodone.
| Pairwise comparisons | Mean/median of intra-participant difference | 95% CI/Quartiles | P-valuea |
|---|---|---|---|
| Study validity | |||
| Oxycodone 40 mg – Placebo | 35.0 | (18.0, 49.0)b | <0.001 |
| Drug Liking VAS Emax relative to oxycodone | |||
| Oxycodone 40 mg – Esmethadone 25 mg | 34.0 | (19.0, 48.0)b | <0.001 |
| Oxycodone 40 mg – Esmethadone 75 mg | 25.0 | (12.0, 41.0)b | <0.001 |
| Oxycodone 40 mg – Esmethadone 150 mg | 19.0 | (5.0, 34.0)b | <0.001 |
| Drug Liking VAS Emax relative to placebo | |||
| Esmethadone 25 mg – Placebo | 0.0 | (0.0, 1.0)b | <0.001 |
| Esmethadone 75 mg – Placebo | 0.0 | (−1.0, 8.0)b | <0.001 |
| Esmethadone 150 mg – Placebo | 7.0 | (0.0, 23.0)b | 0.036 |
Note: Friedman’s test was used to assess overall treatment effects: p-value < 0.001 for both populations.
Study validity hypothesis (#1): Ho: µC – µP ≤ 15 vs. Ha: µC – µP > 15; 1-sided test (α = 0.05).
Abuse potential relative to oxycodone hypothesis (#2): Ho: µC – µT ≤ 0 vs. Ha: µC – µT > 0; 1-sided test (α = 0.05).
Abuse potential relative to placebo hypothesis (#3): Ho: µT – µP ≥ 11 vs. Ha: µT – µP < 11; 1-sided test (α = 0.05) where P = placebo; C = positive control; and T = test drug. In this equivalence test, a significant p-value (<0.05) indicates the response to REL-1017 was statistically equivalent to that of placebo.
Bolded p-values are statistically significant. A statistically significant p-value for the comparison of esmethadone vs. placebo indicates that esmethadone at that dose level has a response profile equivalent to placebo.
CI confidence interval, Emax maximum effect, VAS visual analog scale.
aA paired t test was used to assess the mean difference between the 2 treatments; mean and 95% CI are presented.
bThe Sign test was used to assess the median difference between the 2 treatments; median and quartiles are presented.
Consistent with the primary endpoint, oxycodone had greater effects compared with placebo on all secondary endpoints, including global effects (Overall Drug Liking, Take Drug Again VAS), positive effects (Good Effects, High VAS), and other effects (Any Effects, Alertness/Drowsiness VAS) and all doses of esmethadone had lower effects on all secondary endpoints compared with oxycodone (Table 4). Based on Drug Similarity VAS, participants rated oxycodone to be most similar to the category of Opioids (mean/median scores of 81.6 and 100, respectively). Participants did not perceive esmethadone 25 mg or 75 mg as similar to Opioids, and esmethadone 150 mg was rated as only modestly similar to Opioids, with a mean score of 38.8 and median score of 26 on unipolar 0-100 VAS (Table 4). Mean (SD) maximum pupillary constriction (MPC) values were was 0.847 (0.5372); 1.312 (0.5600); 2.114 (0.7629) mm for esmethadone 25 mg; esmethadone 75 mg; esmethadone 150 mg, respectively; 3.036 (1.0272) mm for oxycodone 40 mg; and 0.685 (0.5153) mm for placebo. Mean values were significantly lower for each esmethadone dose vs. oxycodone (p < 0.001).
Table 4.
Descriptive statistics for secondary endpoints—esmethadone vs. oxycodone.
| Oxycodone Study | |||||
|---|---|---|---|---|---|
| Statistic | Esmethadone 25 mg (N = 47) | Esmethadone 75 mg (N = 47) | Esmethadone 150 mg (N = 47) | Oxycodone 40 mg (N = 47) | Placebo (N = 47) |
| Overall Drug Liking bipolar VAS | |||||
| Mean (SD) | 53.1 (9.20) | 58.1 (19.33) | 61.4 (18.67) | 73.9 (24.02) | 52.6 (12.50) |
| Median | 50.0 | 50.0 | 51.0 | 73.0 | 50.0 |
| Range | 36–85 | 12–100 | 16–100 | 9–100 | 0–98 |
| Take Drug Again bipolar VAS | |||||
| Mean (SD) | 52.6 (17.34) | 57.8 (24.33) | 61.2 (22.94) | 76.1 (26.97) | 51.1 (16.66) |
| Median | 50.0 | 50.0 | 50.0 | 83.0 | 50.0 |
| Range | 0–100 | 0–100 | 0–100 | 7–100 | 0–100 |
| High unipolar VAS | |||||
| Emax | |||||
| Mean (SD) | 9.9 (22.65) | 21.4 (31.88) | 31.3 (34.66) | 74.8 (26.74) | 7.2 (17.33) |
| Median | 0.0 | 1.0 | 17.0 | 83.0 | 0.0 |
| Range | 0–100 | 0–100 | 0–100 | 5–100 | 0–77 |
| Good Effects unipolar VAS | |||||
| Emax | |||||
| Mean (SD) | 9.9 (23.55) | 22.3 (32.75) | 32.9 (36.07) | 73.1 (26.26) | 9.1 (21.67) |
| Median | 0.0 | 0.0 | 19.0 | 79.0 | 0.0 |
| Range | 0–100 | 0–100 | 0–100 | 7–100 | 0–96 |
| Bad Effects unipolar VAS Emax | |||||
| Emax | |||||
| Mean (SD) | 3.3 (11.81) | 7.4 (21.32) | 12.7 (24.77) | 27.4 (30.37) | 1.1 (4.79) |
| Median | 0.0 | 0.0 | 0.0 | 14.0 | 0.0 |
| Range | 0–72 | 0–100 | 0–89 | 0–100 | 0–28 |
| Alertness/Drowsiness unipolar VAS | |||||
| Emin | |||||
| Mean (SD) | 44.4 (10.44) | 41.2 (14.77) | 34.1 (16.27) | 18.4 (14.94) | 45.9 (12.16) |
| Median | 50.0 | 50.0 | 37.0 | 16.0 | 50.0 |
| Range | 0–50 | 0–50 | 0–54 | 0–50 | 0–90 |
| Any Effects unipolar VAS | |||||
| Emax | |||||
| Mean (SD) | 10.9 (24.66) | 25.9 (34.37) | 37.1 (36.45) | 78.1 (26.28) | 7.3 (17.34) |
| Median | 0.0 | 4.0 | 28.0 | 85.0 | 0.0 |
| Range | 0–100 | 0–100 | 0–100 | 5–100 | 0–72 |
| Drug Similarity unipolar VAS Scores at 12 h: | |||||
| Opioids | |||||
| n | 46 | 44 | 47 | 47 | 47 |
| Mean (SD) | 9.8 (26.11) | 17.7 (30.76) | 38.8 (40.43) | 81.6 (30.84) | 9.8 (23.19) |
| Median | 0.0 | 0.0 | 26.0 | 100.0 | 0.0 |
| Range | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 |
Overall Drug Liking VAS is a bipolar scale where a score of 0 represents “strong disliking,” a score of 100 represents “strong liking,” and a score of 50 represents “neither like nor dislike” (neutral point). The question text is, “Overall, my liking for this drug is.”
Take Drug Again VAS is a bipolar scale where a score of 0 represents “definitely not,” a score of 100 represents “definitely so,” and a score of 50 represents “neutral” (neutral point). The question text is, “I would take this drug again.”
Emax maximum effect, range minimum–maximum, SD standard deviation, VAS visual analog scale.
Ketamine Study
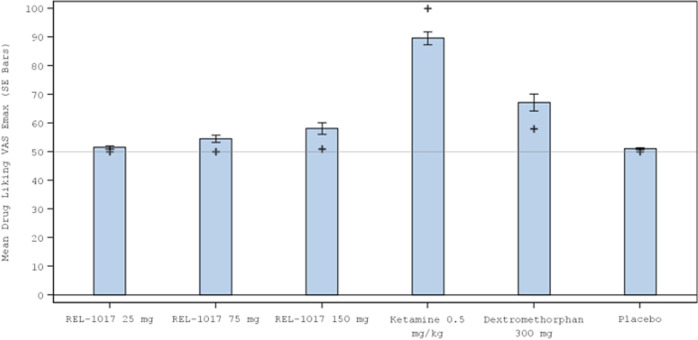
Effects on Drug Liking VAS Emax (primary endpoint) are shown in Fig. 2. The validity of the study was determined from the comparison of Drug Liking VAS Emax between the positive control, ketamine 0.5 mg/kg administered IV over 40 min, and placebo (Table 5). The median (Q1, Q3) difference was 49.0 (27.0, 50.0; p < 0.001) for the Completer Population, indicating that ketamine had a meaningful and statistically significantly higher Drug Liking VAS Emax compared with placebo, using a prespecified margin of 15. The abuse potential of esmethadone relative to ketamine was determined from the comparison of Drug Liking VAS Emax of each esmethadone dose with ketamine. Drug Liking VAS Emax for each esmethadone dose was meaningfully and statistically significantly lower than that of ketamine at a prespecified margin of 0 (mean/median difference between ketamine and each dose of esmethadone ≥34.0; all p < 0.001) (Table 6). The abuse potential of esmethadone compared to placebo was determined from the comparisons of Drug Liking VAS Emax of each dose with placebo. The median difference from placebo was 0 for all tested doses of esmethadone (p ≤ 0.003), indicating that esmethadone was equivalent to placebo at doses up to 6 times the planned therapeutic dose. In the exploratory comparisons with DXM, Drug Liking VAS Emax for DXM 300 mg was meaningfully and significantly lower than the Drug Liking VAS Emax for ketamine (mean difference: 21.6; p < 0.001). DXM was found not to be equivalent to placebo in Drug Liking VAS Emax using the margin of 11 (p = 0.39 (Table 6). Drug Liking VAS Emax for all tested doses of esmethadone were significantly lower compared to DXM (mean/median differences ≥8.0; p ≤ 0.002) (Table 6). Mean Drug Liking VAS scores were maintained close to placebo scores over 24 h (Supplemental Figure 3).
Fig. 2. Mean (standard error) Drug Liking VAS Emax by Treatment During the Treatment Phase for the Ketamine Study (Completer Population).
+ = median value; p < 0.001 for median difference between ketamine and placebo.
Table 5.
Drug Liking bipolar VAS Emax—Ketamine Study.
| Ketamine Study | ||||||
|---|---|---|---|---|---|---|
| Statistic | Esmethadone 25 mg (N = 51) | Esmethadone 75 mg (N = 51) | Esmethadone 150 mg (N = 51) | Ketamine 0.5 mg/kg (N = 51) | DXM 300 mg (N = 51) | Placebo (N = 51) |
| Mean (SD) | 51.4 (3.28) | 54.9 (9.58) | 59.2 (14.38) | 90.0 (14.52) | 68.4 (18.39) | 50.9 (2.23) |
| Median | 50.0 | 50.0 | 51.0 | 100.0 | 60.0 | 50.0 |
| Range | 50–66 | 50–100 | 50–100 | 50–100 | 50–100 | 50–63 |
Drug Liking VAS is a bipolar scale where a score of 0 represents “strong disliking,” a score of 100 represents “strong liking,” and a score of 50 represents “neither like nor dislike” (neutral point). The question text is, “At this moment, my liking for this drug is?”.
Emax maximum effect, range minimum–maximum, SD standard deviation, VAS visual analog scale. Esmethadone and DXM were oral administration; ketamine IV administration.
Table 6.
Inferential analysis of Drug Liking VAS Emax—esmethadone vs. ketamine and DMX.
| Study validity | |||
| Ketamine 0.5 mg/kg – Placebo | 49.0 | (27.0, 50.0)b | <0.001 |
| Drug Liking VAS Emax relative to ketamine | |||
| Ketamine 0.5 mg/kg – Esmethadone 25 mg | 48.0 | (27.0, 50.0)b | <0.001 |
| Ketamine 0.5 mg/kg – Esmethadone 75 mg | 40.0 | (25.0, 50.0)b | <0.001 |
| Ketamine 0.5 mg/kg – Esmethadone 150 mg | 34.0 | (18.0, 49.0)b | <0.001 |
| Drug Liking VAS Emax relative to placebo | |||
| Esmethadone 25 mg – Placebo | 0.0 | (0.0, 0.0)b | <0.001 |
| Esmethadone 75 mg – Placebo | 0.0 | (0.0, 3.0)b | <0.001 |
| Esmethadone 150 mg – Placebo | 0.0 | (0.0, 14.0)b | 0.003 |
| Exploratory comparisons | |||
| DXM 300 mg – Placebo | 8.0 | (0.0, 35.0)b | 0.39 |
| DXM 300 mg – Esmethadone 25 mg | 10.0 | (0.0, 34.0)b | <0.001 |
| DXM 300 mg – Esmethadone 75 mg | 13.5 | (8.6, ∞)a | <0.001 |
| DXM 300 mg – Esmethadone 150 mg | 9.2 | (4.1, ∞)a | 0.002 |
| Ketamine 0.5 mg/kg – DXM 300 mg | 21.6 | (17.1, ∞)a | <0.001 |
Note: Friedman’s test was used to assess overall treatment effects: p value < 0.001 for both populations.
Study validity hypothesis (#1): Ho: µC – µP ≤ 15 vs. Ha: µC – µP > 15; 1-sided test (α = 0.05).
Abuse potential relative to ketamine hypothesis (#2): Ho: µC – µT ≤ 0 vs. Ha: µC – µT > 0; 1-sided test (α = 0.05).
Abuse potential relative to placebo hypothesis (#3): Ho: µT – µP ≥ 11 vs. Ha: µT – µP < 11; 1-sided test (α = 0.05) where P = placebo; C = positive control; and T = test drug.
Exploratory hypotheses:
Ho: μC2 – μP ≤ 0 vs. Ha: μC2 – μP > 0; 1-sided test (α = 0.05).
Ho: μC2 – μT ≤ 0 vs. Ha: μC2 – μT > 0; 1-sided test (α = 0.05).
Ho: μC1 – μC2 ≤ 0 vs. Ha: μC1 – μC2 > 0; 1-sided test (α = 0.05), where P = placebo; C1 = positive control (ketamine); C2 = exploratory comparator (DXM) and T = test drug.
CI confidence interval, Emax maximum effect, VAS visual analog scale.
aA paired t test was used to assess the mean difference between the 2 treatments; mean and 95% CI are presented.
bThe Sign test was used to assess the median difference between the 2 treatments; median and quartiles are presented.
Bolded p-values are statistically significant. A statistically significant p-value for the comparison of esmethadone vs. placebo indicates that esmethadone at that dose level has a response profile equivalent to placebo. The nonsignificant p-value reported for DXM vs. placebo indicates that DXM at that dose level lacks placebo equivalency.
Ketamine had greater effects compared with placebo on all secondary endpoints (Table 7). All doses of esmethadone showed lower effects on all endpoints compared with ketamine, including Overall Drug Liking VAS, Take Drug Again VAS, Good Effects VAS, High VAS, and other effects including perceptual effects. In contrast with ketamine and DXM, esmethadone did not cause hallucinations or perceptual effects. Mean (SD) unipolar Emax scores for Hallucinations VAS were 0.2 (0.55); 0.3 (0.77); 0.6 (0.24) points for esmethadone 25 mg; esmethadone 75 mg; esmethadone 150 mg, respectively; 23.1 (38.5) points for ketamine; 7.5 (19.21) points for DXM; and 0.2 (0.45) points for placebo. Based on Drug Similarity VAS, ketamine was rated to be most similar to the category of Ketamine (mean/median scores of 93.1 and 100, respectively) and less so with Opioids (22.8 and 4.0, respectively). Participants did not perceive esmethadone as similar to Ketamine or Opioids, with mean scores <20 and median scores of 0.
Table 7.
Descriptive statistics for secondary endpoints—esmethadone vs. ketamine and DMX.
| Ketamine Study | ||||||
|---|---|---|---|---|---|---|
| Statistic | Esmethadone 25 mg (N = 51) | Esmethadone 75 mg (N = 51) | Esmethadone 150 mg (N = 51) | Ketamine 0.5 mg/kg (N = 51) | DXM 300 mg (N = 51) | Placebo (N = 51) |
| Overall Drug Liking bipolar VAS | ||||||
| Mean (SD) | 51.3 (7.98) | 50.8 (13.72) | 52.9 (20.13) | 87.4 (19.35) | 57.7 (30.82) | 47.7 (9.68) |
| Median | 50.0 | 50.0 | 50.0 | 100.0 | 59.0 | 50.0 |
| Range | 43–100 | 0–00 | 0–100 | 41–100 | 0–100 | 0–54 |
| Take Drug Again bipolar VAS | ||||||
| Mean (SD) | 50.5 (10.87) | 50.0 (18.25) | 53.5 (24.40) | 88.2 (21.95) | 55.2 (32.44) | 48.8 (13.29) |
| Median | 50.0 | 50.0 | 50.0 | 100.0 | 51.0 | 50.0 |
| Range | 0–100 | 0–100 | 0–100 | 1–100 | 0–100 | 0–100 |
| High unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 2.9 (6.44) | 10.2 (18.94) | 17.3 (25.92) | 87.7 (21.70) | 60.8 (36.68) | 2.1 (4.26) |
| Median | 0.0 | 0.0 | 4.0 | 100.0 | 73.0 | 0.0 |
| Range | 0–27 | 0–79 | 0–100 | 19–100 | 1–100 | 0–19 |
| Good Effects unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 2.9 (8.73) | 10.2 (20.36) | 19.5 (28.59) | 86.3 (22.26) | 47.2 (35.56) | 2.7 (8.63) |
| Median | 0.0 | 0.0 | 5.0 | 100.0 | 49.0 | 0.0 |
| Range | 0–50 | 0–100 | 0–100 | 14–100 | 0–100 | 0–57 |
| Bad Effects unipolar VAS Emax | ||||||
| Emax | ||||||
| Mean (SD) | 2.6 (11.62) | 5.2 (14.55) | 7.1 (18.20) | 14.0 (28.36) | 34.7 (37.47) | 3.3 (9.19) |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | 22.0 | 0.0 |
| Range | 0–80 | 0–71 | 0–90 | 0–100 | 0–100 | 0–50 |
| Alertness/Drowsiness unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 51.4 (7.03) | 52.6 (9.51) | 53.6 (10.00) | 69.0 (21.29) | 58.9 (15.82) | 51.2 (6.99) |
| Median | 50.0 | 50.0 | 50.0 | 54.0 | 50.0 | 50.0 |
| Range | 50–100 | 50–100 | 50–100 | 50–100 | 50–100 | 50–100 |
| Any Effects unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 4.3 (11.56) | 13.5 (21.48) | 21.4 (28.47) | 90.1 (20.11) | 66.8 (35.71) | 4.3 (8.03) |
| Median | 0.0 | 2.0 | 8.0 | 100.0 | 79.0 | 1.0 |
| Range | 0–73 | 0–89 | 0–100 | 11–100 | 1–100 | 0–36 |
| Hallucinations unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 0.2 (0.55) | 0.3 (0.77) | 0.6 (2.24) | 23.1 (38.05) | 7.5 (19.21) | 0.2 (0.45) |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Range | 0–3 | 0–5 | 0–14 | 0–100 | 0–100 | 0–2 |
| Bowdle—External Perception unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 0.2 (0.58) | 1.1 (3.21) | 1.9 (4.56) | 33.9 (31.61) | 11.1 (17.83) | 0.2 (0.63) |
| Median | 0.0 | 0.0 | 0.2 | 21.3 | 2.3 | 0.0 |
| Range | 0–4 | 0–18 | 0–21 | 0–100 | 0–100 | 0–4 |
| Bowdle—Internal Perception unipolar VAS | ||||||
| Emax | ||||||
| Mean (SD) | 0.1 (0.17) | 0.4 (1.04) | 0.3 (0.59) | 17.5 (24.26) | 6.9 (10.64) | 0.3 (1.35) |
| Median | 0.0 | 0.0 | 0.0 | 9.6 | 1.0 | 0.0 |
| Range | 0–1 | 0–5 | 0–3 | 0–100 | 0–40 | 0–10 |
| Drug Similarity at 12 h unipolar VAS | ||||||
| Ketamine | ||||||
| n | 51 | 51 | 51 | 50 | 51 | 51 |
| Mean (SD) | 0.5 (2.34) | 5.5 (15.26) | 10.1 (24.20) | 93.1 (21.64) | 37.9 (36.35) | 1.2 (6.79) |
| Median | 0.0 | 0.0 | 0.0 | 100.0 | 34.0 | 0.0 |
| Range | 0–14 | 0–85 | 0–100 | 0–100 | 0–100 | 0–48 |
| Opioids | ||||||
| n | 30 | 30 | 30 | 29 | 30 | 30 |
| Mean (SD) | 5.8 (19.06) | 11.7 (28.54) | 16.7 (29.03) | 22.8 (34.35) | 23.8 (33.72) | 5.5 (19.59) |
| Median | 0.0 | 0.0 | 0.0 | 4.0 | 0.0 | 0.0 |
| Range | 0–100 | 0–98 | 0–100 | 0–100 | 0–100 | 0–100 |
Overall Drug Liking VAS is a bipolar scale where a score of 0 represents “strong disliking,” a score of 100 represents “strong liking,” and a score of 50 represents “neither like nor dislike” (neutral point). The question text is, “Overall, my liking for this drug is.”
Take Drug Again VAS is a bipolar scale where a score of 0 represents “definitely not,” a score of 100 represents “definitely so,” and a score of 50 represents “neutral” (neutral point). The question text is, “I would take this drug again.”
Emax maximum effect; range minimum–maximum; SD standard deviation; VAS visual analog scale.
Pharmacodynamic analyses were also performed for the Modified Completer Population. The results for the Modified Completer Population for the Oxycodone Study and for the Ketamine Study are presented in Supplemental Tables 1 to 6. It should be noted that the Modified Completer Population excludes placebo responders; therefore, descriptive and inferential statistical comparisons of the test drug against placebo in the Modified Completer Population are biased towards showing lack of equivalency with placebo (placebo responders are eliminated but test drug responders are not). Inferential statistical comparisons of the test drug against the positive control in the Modified Completer Population may also be biased towards showing a greater difference between positive control and test drug by eliminating participants with low scores for the comparator drug. Whereas the performance of analyses on the Completer Population is established and accepted [39], the performance of additional analyses on the Modified Completer Population is evolving. The intent of making this analysis available is to stimulate interest in population enrichment strategies that may enhance the interpretation of HAP studies.
Pharmacokinetics
In both studies, esmethadone geometric mean plasma exposures (Cmax and AUC0-last) increased with increasing dose of esmethadone. Median Tmax ranged from approximately 2 to 3 h post dose for all esmethadone doses in both studies, whereas Tmax for oxycodone occurred at approximately 1 h post dose (Supplemental Figure 4 and Supplemental Table 7). The Tmax for ketamine occurred at approximately 1 h post dose, the first post infusion timepoint, and Tmax for norketamine was observed at 1.5 h post dose. Exposure to DXM and dextrorphan was highly variable, with a median Tmax of 3 and 2 h, respectively (Supplemental Figure 5).
Safety
Oxycodone Study
Overall, the highest incidence (≥5% participants at any dose) of treatment-emergent AEs (TEAEs) was observed with oxycodone 40 mg (52.1%), followed by esmethadone 150 mg (28.6%) (Supplemental Table 8). The incidence of TEAEs for placebo, esmethadone 25 mg, and esmethadone 75 mg was 12.2%, 12.8%, and 12.2%, respectively. The most common TEAEs with oxycodone were nausea, somnolence, pruritus, vomiting, dizziness, and hot flush. The most common AEs with esmethadone were nausea, headache, somnolence, and vomiting. The incidence of nausea, vomiting, and somnolence appeared to increase with the esmethadone dose; however, the incidence was lower compared with oxycodone. There were no reports of dizziness or hot flush with esmethadone. There were no deaths or serious AEs. No notable treatment-related changes or trends were observed for clinical laboratory, vital signs, ECG or C-SSRS results following esmethadone administration. Notably, there were no TEAEs related to QTc prolongation. Drug-induced QTcF prolongation was modest and was slightly higher for oxycodone compared to each dose of esmethadone, including the 150 mg dose (Supplemental Table 7). Overall, esmethadone was well-tolerated at doses up to 150 mg.
Ketamine Study
The highest incidence of TEAEs was observed with DXM (74.1%), while a similar incidence of TEAEs occurred with esmethadone 75 mg (41.5%) and 150 mg (39.5%) (Supplemental Table 8). The incidence of TEAEs with esmethadone 25 mg and ketamine (24.5% and 25.0%, respectively) was slightly lower compared to placebo (30.8%). The most common TEAEs with ketamine were headache and somnolence. The most common TEAEs with esmethadone were nausea, headache, and somnolence. The incidence of nausea appeared to increase with the esmethadone dose. DXM was associated with the highest incidence of nausea and vomiting; headache, somnolence, and pruritus also were common. There were no deaths or serious AEs. No TEAEs related to QTc prolongation occurred (Supplemental Table 8). No notable treatment-related changes or trends in clinical laboratory findings, vital signs or ECG results were observed following esmethadone administration. Overall, esmethadone was well-tolerated at doses up to 150 mg.
Discussion
Esmethadone is a low potency uncompetitive NMDAR antagonist [22, 23] and promising rapid antidepressant candidate. There is experimental evidence that esmethadone may have opioid antagonistic activity to the agonist effects of levomethadone [59] and there is clinical evidence that esmethadone may have weak antagonistic activity on the respiratory depressant [60] and on subjective opioid effects [61] induced by levomethadone. Esmethadone is not yet approved for any indication and is currently a Schedule II controlled substances in the United States. If esmethadone is approved for the treatment of MDD, a rescheduling decision will be sought.
HAP studies provide important data for predicting recreational use in the community and provide information for labeling and for drug scheduling under the Controlled Substances Act (CSA). In both HAP studies, all three esmethadone doses (25 mg [therapeutic], 75 mg [3x therapeutic], and 150 mg [6x therapeutic and MTD]) had meaningful and statistically significant lower scores compared with positive control (oxycodone or ketamine) on the primary endpoint of Drug Liking VAS Emax, and a similar pattern was observed for all secondary endpoints. Furthermore, Drug Liking VAS Emax for all doses of esmethadone were statistically equivalent to placebo, including the MTD, 150 mg, in both studies, using the Completer Population. The modest dose-effect relationship observed in both studies, which was confined to the range of placebo effects [56], indicates that there would be no meaningful incentive for drug abusers to increase the dose of esmethadone to achieve a greater effect. Pupillary constriction was less with esmethadone relative to oxycodone. Pupillary constriction with esmethadone in this study was similar to pupillary constriction induced by esmethadone in prior studies [35]. While pupillary constriction is a well-known opioid agonist effect, it is not always associated with other clinically meaningful opioid agonist effects. In fact, naloxone, a well-known opioid antagonist with no described opioid agonist effects, may cause pupillary constriction [62].
We presented results for the Completer Population as indicated in the FDA 2017 Guidance [39] (Tables 3 to 6) and for the Modified Completer Population (Supplemental Tables 1–6), as suggested by recent research trends in methodology for HAP studies. For the two studies presented here, the application of Modified Completer Population parameters did not meaningfully change the results and conclusions obtained with the Completer Population.
In summary, the results of these two, randomized, double-blind, active- and placebo-controlled, crossover studies testing the abuse potential of esmethadone in recreational drug users are consistent with the profile of a drug without meaningful abuse potential and are similar to results observed for unscheduled drugs without abuse potential such as eslicarbazepine and difelikefalin [42, 63] and Schedule V drugs such as lacosamide [64]. Furthermore, the Ketamine Study, in addition to showing meaningful and statistically significant lower abuse potential compared to ketamine, also showed statistically significant lower effects compared to DXM (Table 6), an unscheduled and over-the-counter NMDAR antagonist and antitussive medication. Mean values for Drug Liking tended to be slightly higher with the 150 mg dose of esmethadone, but no significant differences from placebo were observed.
Considering that MDD is a highly prevalent and life-threatening condition and available treatments have limited and delayed efficacy and metabolic and neurological side effects, an unmet need exists for safe, well-tolerated, and rapidly effective antidepressants. Patients in need should not have restricted or delayed access to potentially safe, well-tolerated, and life-saving drugs [36].
In conclusion, the results of these two HAP studies, in accordance with results in preclinical models [30, 59], indicate no meaningful abuse potential for esmethadone at all tested doses. These results confirm a recent Drug Enforcement Administration (DEA) publication stating: “The d-isomer lacks significant respiratory depressant action and addiction liability…” [65]. If Phase 3 results confirm the rapid, robust, and sustained antidepressant effects and the favorable tolerability and safety profiles of the Phase 2 study, esmethadone has the potential to become an important first-line adjunctive treatment for MDD.
Supplementary information
Acknowledgements
Editorial support for the current manuscript was performed by Richard Perry, PharmD, supported by Relmada Therapeutics, Inc., according to Good Publication Practices (GPP3). All opinions, conclusions, and data interpretation lie with the authors.
Author contributions
Conceptualization: MS, JH, GA, PLM, and MP; methodology: MS, JH, GA, SDM, PLM, and MP; software: SDM and FF; validation: FF; formal analysis: SDM; investigation: MS, JH, GA, PLM, and MP; resources: ST; data curation: MS, SDM, FF, CG, and PLM; writing—original draft preparation: MS, JH, GA, PLM, and MP; writing—review and editing: MS, JH, GA, CG, FV, FLS, TK, FF, CG, JH, AB, JA, RL, CEI, ST, PLM, and MP; visualization: FF and PLM; supervision: FF and PLM; project administration: GA, ST, and MP; funding acquisition: ST. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Relmada Therapeutics, Inc., Coral Gables, Florida. Relmada Therapeutics participated in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
Competing interests
Drs. Inturrisi and Manfredi are inventors on patents related to esmethadone. Drs. Traversa and O’Gorman are employees of Relmada Therapeutics. Drs. Manfredi, Pappagallo, Inturrisi, Folli, and Kosten have received compensation from Relmada Therapeutics as consultants. Drs. Shram, Henningfield, Apseloff, Gorodetzky, De Martin, Vocci, Sapienza, Huston, Buchhalter, Ashworth, Lanier, Mattarei, and Guidetti are employed or have received compensation from companies or institutions that received funding from Relmada Therapeutics.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Paolo L. Manfredi, Marco Pappagallo.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-023-02473-8.
References
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336–46. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/S0193-953X(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson JM. SSRI Antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3:22–27. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakobsen JC, Gluud C, Kirsch I. Should antidepressants be used for major depressive disorder? BMJ Evid Based Med. 2020;25:130. doi: 10.1136/bmjebm-2019-111238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Keitner GI, Qin B, Raindran AV, Bauer M, Del Giovane C, et al. Atypical antipsychotic augmentation for treatment-resistant depression: A systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060. doi: 10.1093/ijnp/pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boku S, Nakagawa S, Toda H, Hishimoto A. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72:3–12. doi: 10.1111/pcn.12604. [DOI] [PubMed] [Google Scholar]
- 8.Henter ID, de Sousa RT, Zarate CA., Jr Glutamatergic modulators in depression. Harv Rev Psychiatry. 2018;26:307–19. doi: 10.1097/HRP.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder; rationale and progress to date. Drugs. 2012;72:1313–33. doi: 10.2165/11633130-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol. Psychiatry. 2022. 10.1038/s41380-022-01661-0. [DOI] [PMC free article] [PubMed]
- 11.Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ, et al. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150:1081–105. 10.1085/jgp.201812032. [DOI] [PMC free article] [PubMed]
- 12.Nicoll RA. A brief history of long-term potentiation. Neuron. 2017;93:281–90. doi: 10.1016/j.neuron.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Autry AE, Adachi M, Nosyreva E, Na ES, Lo MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogaça MV, Fukumoto K, Franklin T, Liu RJ, Duman CH, Vitolo OV, et al. N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44:2230–8. doi: 10.1038/s41386-019-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteggia LM, Gideons E, Kavalali ET. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry. 2013;73:1199–203. doi: 10.1016/j.biopsych.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl SM, De Martin S, Mattarei A, Bettini E, Pani L, Guidetti C, et al. Esmethadone (REL-1017) and other uncompetitive NMDAR channel blockers may improve mood disorders via modulation of synaptic kinase-mediated signaling. Int J Mol Sci. 2022;23:12196.. doi: 10.3390/ijms232012196.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fava M, Stahl S, Pani L, De Martin S, Pappagallo M, Guidetti C, et al. REL-1017 (Esmethadone) as adjunctive treatment in patients with major depressive disorder: a Phase 2a randomized double-blind trial. Am J Psychiatry. 2022;179:122–31. doi: 10.1176/appi.ajp.2021.21020197.. [DOI] [PubMed] [Google Scholar]
- 20.Tabuteau H, Jones A, Anderson A, Jacobson M, Iosifescu DV. Effect of AXS-05 (dextromethorphan-bupropion) in major depressive disorder: A randomized double-blind controlled trial. Am J Psychiatry. 2022;179:490–9. doi: 10.1176/appi.ajp.21080800. [DOI] [PubMed] [Google Scholar]
- 21.Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh L, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–64. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettini E, De Martin SA, Mattarei A, Pappagallo M, Stahl SM, Bifari F, et al. The N-Methyl-D-Aspartate receptor blocker REL-1017 (esmethadone) reduces calcium influx induced by glutamate, quinolinic acid, and gentamicin. Pharmaceuticals. 2022;15:882. doi: 10.3390/ph15070882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223:5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- 24.Hanania T, Manfredi P, Inturrisi C, Vitolo OV. The N-methyl-D-aspartate receptor antagonist d-methadone acutely improves depressive-like behavior in the forced swim test performance of rats. Exp Clin Psychopharmacol. 2020;28:196–201. doi: 10.1037/pha0000310. [DOI] [PubMed] [Google Scholar]
- 25.De Martin S, Gabbia D, Folli F, Bifari F, Fiorina P, Ferri N, et al. REL-1017 (Esmethadone) increases circulating BDNF levels in healthy subjects of a Phase 1 clinical study. Front Pharmacol. 2021;12:671859. doi: 10.3389/fphar.2021.671859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bifari F, Pappagallo M, Bleavins M, Traversa S, Folli F, Manfredi P. REL-1017 (Esmethadone), A novel NMDAR Blocker for the treatment of MDD is not neurotoxic in Sprague-Dawley rats. Front Pharm. 2022;13:863959. doi: 10.3389/fphar.2022.863959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolan EA, Tallarida RJ, Pasternak GW. Synergy between mu opioid ligands: evidence for functional interactions among mu opioid receptor subtypes. J Pharm Exp Ther. 2002;303:557–62. doi: 10.1124/jpet.102.035881. [DOI] [PubMed] [Google Scholar]
- 28.Lemberg K, Kontinen VK, Viljakka K, Kylanlahti I, Yli-Kauhaluoma J, Kalso E. Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg. 2006;102:1768–74. doi: 10.1213/01.ane.0000205751.88422.41. [DOI] [PubMed] [Google Scholar]
- 29.Shimoyama N, Shimoyama M, Elliott KJ, Inturrisi CE. d-methadone is antinociceptive in the rat formalin test. J Pharm Exp Ther. 1997;283:648–52. [PubMed] [Google Scholar]
- 30.Henningfield L, Gauvin D, Bifari F, Fant R, Shram M, Buchhalter A, et al. REL-1017 (esmethadone; d-methadone) does not cause reinforcing effect, physical dependence and withdrawal signs in Sprague Dawley rats. Sci Rep. 2022;12:11389. doi: 10.1038/s41598-022-15055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramabadran K. An analysis of precipitated withdrawal in rats acutely dependent on morphine. Jpn J Pharm. 1985;37:307–16. doi: 10.1254/jjp.37.307. [DOI] [PubMed] [Google Scholar]
- 32.Eddy NB, Halbach H, Braenden OJ. Synthetic substances with morphin-like effect. Relationship between analgesic action and addiction liability, with a discussion of the chemical structure of addiction-producing substances. Bull WHO. 1957;14:353. [PMC free article] [PubMed] [Google Scholar]
- 33.Fraser HF, Isbell H. Human pharmacology and addictiveness of certain dextroisomers of synthetic analgesics: I. d-3-Hydroxy-N-phenethylmorphinan, II. d-3-Methoxy-N-phenethylmorphinan, III. d-Methadone. Bull Narcotics. 1962;14:25–35. [Google Scholar]
- 34.Isbell H, Eisenman AJ. ‘The addiction liability of some drugs of the methadon series’. J Pharm Exp Ther. 1947;93:305–13. [PubMed] [Google Scholar]
- 35.Bernstein G, Davis K, Mills C, Wang L, McDonnell M, Oldenhof J, et al. Characterization of the safety and pharmacokinetic profile of d-methadone, a novel n-methyl-d-aspartate receptor antagonist in healthy, opioid-naïve subjects: results of two phase 1 studies. J Clin Psychopharmacol. 2019;39:226–37. doi: 10.1097/JCP.0000000000001035. [DOI] [PubMed] [Google Scholar]
- 36.Pappagallo M, Inturrisi CE, Manfredi PL. Comment on “Novel glutamatergic modulators for the treatment of mood disorders: Current status”. CNS Drugs 2022;36:203-4. 10.1007/s40263-021-00891-0. [DOI] [PMC free article] [PubMed]
- 37.Codd EE, Shank RP, Schupsky JJ, Raffa RB. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharm Exp Ther. 1995;274:1263–70. [PubMed] [Google Scholar]
- 38.Iosifescu DV, Jones A, O’ Gorman C, Streicher C, Feliz S, Fava M, et al. Efficacy and safety of AXS-05 (Dextromethorphan-Bupropion) in patients with major depressive disorder: a Phase 3 randomized clinical trial (GEMINI) J Clin Psychiatry. 2022;83:21m14345. doi: 10.4088/JCP.21m14345. [DOI] [PubMed] [Google Scholar]
- 39.FDA. Food and Drug Administration. Assessment of abuse potential of drugs. Guidance for Industry. January 2017. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
- 40.Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013;129:116–24. doi: 10.1016/j.drugalcdep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge X, Henningfield JE, Siddhanti S, Jobes J, Lu L, Xie S, et al. Human abuse potential of oral NKTR-181 in recreational opioid users: A randomized, double-blind, crossover study. Pain Med. 2020;21:e114–e126. doi: 10.1093/pm/pnz232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy-Cooperman N, McIntyre G, Bonifacio L, McDonnell M, Davenport JM, Covington PS, et al. Abuse potential and pharmacodynamic characteristics of oral and intranasal eluxadoline, a mixed μ- and κ-opioid receptor agonist and δ-opioid receptor antagonist. J Pharm Exp Ther. 2016;359:471–81. doi: 10.1124/jpet.116.236547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meske D, Kopecky EA, Passik S, Shram MJ. Evaluation of the oral human abuse potential of Oxycodone DETERx® formulation (Xtampza® ER) J Opioid Manag. 2018;14:359–72. doi: 10.5055/jom.2018.0468. [DOI] [PubMed] [Google Scholar]
- 44.Pathak S, Vince B, Kelsh D, Setnik B, Nangia N, DiPetrillo L, et al. A Phase I, oxycodone-, pentazocine-, naltrexone, and placebo-controlled study. J Clin Pharm. 2019;59:218–28. doi: 10.1002/jcph.1343. [DOI] [PubMed] [Google Scholar]
- 45.Setnik B, Roland CL, Cleveland JM, Webster L. The abuse potential of Remoxy(®), an extended-release formulation of oxycodone, compared with immediate- and extended-release oxycodone. Pain Med. 2011;12:618–31. doi: 10.1111/j.1526-4637.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 46.Setnik B, Roland CL, Pixton G, Webster L. Measurement of drug liking in abuse potential studies: a comparison of unipolar and bipolar visual analog scales. J Clin Pharm. 2017;57:266–74. doi: 10.1002/jcph.801. [DOI] [PubMed] [Google Scholar]
- 47.Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webster LR, Bath B, Medve RA, Marmaon T, Stoddard GJ. Randomized, double-blind, placebo-controlled study of the abuse potential of different formulations of oral oxycodone. Pain Med. 2012;13:790–801. doi: 10.1111/j.1526-4637.2012.01380.x. [DOI] [PubMed] [Google Scholar]
- 49.Webster L, Henningfield J, Buchhalter AR, Siddhanti S, Lu L, Odinecs A, et al. Human abuse potential of the new opioid analgesic molecule NKTR-181 compared with oxycodone. Pain Med. 2018;19:307–18. doi: 10.1093/pm/pnw344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reissig CJ, Carter LP, Johnson MW, Mintzer MJ, Klinedinst MA, Griffiths RR. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology. 2012;223:1–15. doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowdle T, Radant A, Cowley D, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers. Anesthesiology. 1998;8:82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shram MJ, Sathyan G, Khanna S, Tudor IC, Nath R, Thipphawong J, et al. Evaluation of the abuse potential of extended release hydromorphone versus immediate release hydromorphone. J Clin Psychopharmacol. 2010;30:25–33. doi: 10.1097/JCP.0b013e3181c8f088. [DOI] [PubMed] [Google Scholar]
- 54.Shram MJ, Sellers EM, Romach MK. Oral ketamine as a positive control in human abuse potential studies. Drug Alcohol Depend. 2011;114:185–93. doi: 10.1016/j.drugalcdep.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Wilbraham D, Berg PH, Tsai M, Liffick E, Loo LS, Doty EG, et al. Abuse potential of lasmiditan: a phase 1 randomized, placebo- and alprazolam-controlled crossover study. J Clin Pharm. 2020;60:495–504. doi: 10.1002/jcph.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, Bonson KR. An equivalence test for the comparison between a test drug and placebo in human abuse potential studies. J Biopharm Stat. 2013;23:294–306. doi: 10.1080/10543406.2011.616972. [DOI] [PubMed] [Google Scholar]
- 57.Tsong Y, Chen WJ. Noninferiority testing beyond simple two-sample comparison. J Biopharm Stat. 2007;17:289–308. doi: 10.1080/10543400601177368. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro SS, Wilk MB. “An analysis of variance test for normality (complete samples)”. Biometrika. 1965;52:591–611. doi: 10.1093/biomet/52.3-4.591. [DOI] [Google Scholar]
- 59.Michaelides M, Levinstein M, De Oliveira P, Casajuana-Martin N, Quiroz C, Budinich R, et al. Unique pharmacodynamic properties and low abuse liability of the µ-opioid receptor ligand (S)-methadone. Res Sq. 2023;Mar:rs.3.rs-2644719. 10.21203/rs.3.rs-2644719/v1. [DOI] [PMC free article] [PubMed]
- 60.Olsen GD, Wendel HA, Livermore JD, Leger RM, Lynn RK, Gerber N. Clinical effects and pharmacokinetics of racemic methadone and its optical isomers. Clin Pharm Ther. 1977;21:147–57. doi: 10.1002/cpt1977212147. [DOI] [PubMed] [Google Scholar]
- 61.Soyka M, Zingg C. Feasability and safety of transfer from racemic methadone to (R)-methadone in primary care: clinical results from an open study. World J Biol Psychiatry. 2009;10:217–24. doi: 10.1080/15622970802416057. [DOI] [PubMed] [Google Scholar]
- 62.Loimer N, Schmid R, Grünberger J, Linzmayer L. Naloxone induces miosis in normal subjects. Psychopharmacology. 1990;101:282–3. doi: 10.1007/BF02244141. [DOI] [PubMed] [Google Scholar]
- 63.Shram MJ, Spencer RH, Qian J, Munera CL, Lewis ME, Henningfield JE, et al. Evaluation of the abuse potential of difelikefalin, a selective kappa-opioid receptor agonist, in recreational polydrug users. Clin Transl Sci. 2022;15:535–47. doi: 10.1111/cts.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoedel KA, Andreas JO, Doty P, Eckhardt K, Sellers EM. Randomized, double-blind, placebo- and active comparator-controlled crossover study evaluating the abuse potential of the antiepileptic drug lacosamide in healthy recreational drug users. J Clin Psychopharmacol. 2017;37:675–83. doi: 10.1097/JCP.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 65.Drug Enforcement Administration (DEA). Diversion Control Division. Drug & Chemical Evaluation Section. Drug and Chemical Information: Methadone. December 2019. Drug and Chemical Information - DEA Diversion Control Division https://www.deadiversion.usdoj.gov › drug_chem_info.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.