Abstract
Substituted pyridines with diverse functional groups are important structural motifs found in numerous bioactive molecules. Several methodologies for the introduction of various bio-relevant functional groups to pyridine have been reported, but there is still a need for a single robust method allowing the selective introduction of multiple functional groups. This study reports a ring cleavage methodology reaction for the synthesis of 2-alkyl/aryl 3-electron-withdrawing groups (esters, sulfones, and phosphonates) 5-aminoaryl/phenol pyridines via the remodeling of 3-formyl (aza)indoles/benzofurans. Totally ninety-three 5-aminoaryl pyridines and thirty-three 5-phenol pyridines were synthesized showing the robustness of the developed methodology. The application of this methodology further provided a privileged pyridine scaffold containing biologically relevant molecules and direct drug/natural product conjugation with ethyl 2-methyl nicotinate.
Subject terms: Synthetic chemistry methodology, Synthetic chemistry methodology, Homogeneous catalysis, Diversity-oriented synthesis
Substituted pyridines are important structural motifs present in various bioactive molecules, however their multi-functionalization remains challenging. Here, the authors report a facile approach for introducing various functional groups on the pyridine scaffold by remodeling of 3-formyl (aza)indoles/benzofurans via a ring cleavage reaction.
Introduction
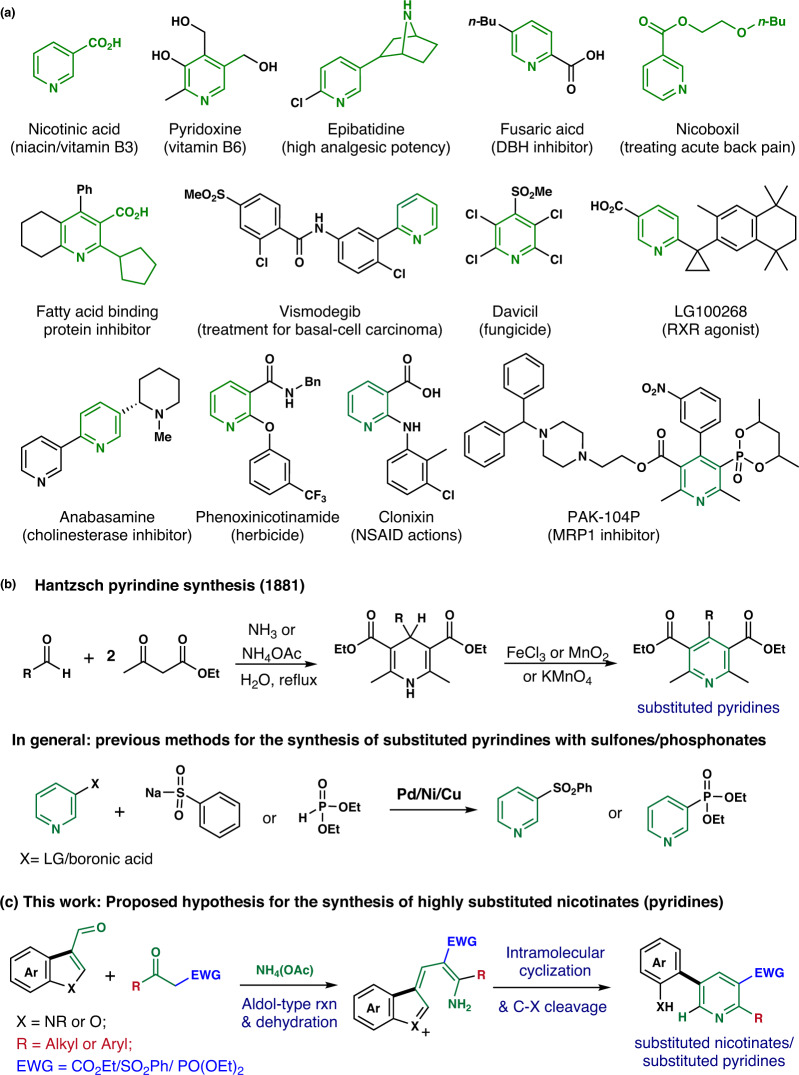
Pyridine is a simple six-membered heterocyclic scaffold found in various natural products, drug molecules, vitamins, and materials (Fig. 1a)1–12. The biological activities and physical properties of pyridine analogs can be improved by introducing various functional groups into the pyridine scaffold. For example, vitamin B3, also known as nicotinic acid and with multiple biological activities, contains the carboxylic acid moiety at the C-3 position of the pyridine13–15. Furthermore, di- and tri-substituted pyridines are frequently found in numerous drug molecules, natural products, and agrochemicals, including pyridoxine, epibatidine, fusaric acid, nicoboxil, vismodegib, phenoxynicotinamide, anabasamine, and clonixin10–12. In particular, pyridyl sulfones are widely present in diverse bioactive molecules16,17 showing anti-inflammatory and anti-viral activities18–20. Pyridyl phosphonates are also valuable in the field of medicinal chemistry. Specifically, PAK-104P is a pyridyl phosphonate that alleviates drug resistance to paclitaxel and doxorubicin21. Therefore, the development of a robust synthetic route enabling the incorporation of sulfone and phosphonate moieties on the pyridine scaffold is highly needed in medicinal and agricultural chemistry22–27.
Fig. 1. Overview of bioactive pyridines and hypothesis of this work.
a Bioactive natural products and drug molecules containing substituted pyridines. b Previous synthetic strategies for substituted pyridines. c Proposed synthetic strategy of substituted pyridines with diverse functional groups (esters/sulfones/phosphonates).
Poly-substituted pyridine moieties have been obtained by the traditional Hantzsch pyridine synthesis (Fig. 1b), Chichibabin pyridine synthesis, Bohlmann–Rahtz pyridine synthesis, etc28–35. However, the introduction of electron-withdrawing groups on the pyridine moiety is still challenging. For instance, pyridyl sulfones are generally synthesized by the metal-catalyzed coupling of sulfinate salts with halopyridines36, 37 or pyridyl boronic acids38 (Fig. 1b). Other synthetic routes include the oxidation of sulfides39,40, pyridine modification using sulfoxylate reagents41 or organometallic reagents42, and displacement reactions of the sodium salts of the corresponding sulfones with pyridyl halides43,44. However, these synthetic protocols require stench thiol compounds45 and hazardous byproducts are formed. In the case of pyridyl(heteroaryl) phosphonates, a cross-coupling reaction is still the best synthetic method, but these coupling reactions require hydrophosphorous derivatives and pyridyl(heteroaryl) halide, tosylates, and boronic acids in the presence of transition metal catalysts, including palladium46–53, nickel54, and silver55. Michaelis-Arbuzov reaction56,57 and metal-free Sandmeyer-type phosphonylation58 are alternative synthetic protocols for the formation of aryl-phosphorous bonds. However, these methods require expensive metal catalysts and ligands under harsh conditions. All of these mentioned methods have mainly focused on the synthesis of aryl sulfones and phosphonates, and a few studies have reported the heteroaryl(pyridyl) functionalization.
In the presence of ammonium acetate, β-keto ester/sulfone/phosphonate can be transformed in situ to substituted enamines that undergo aldol-type addition to N-substituted (aza)indole carboxaldehydes and the subsequent ring cleavage reaction to produce substituted pyridines in conjugation with o-amino(hetero)aryl moieties (Fig. 1c) and our group has previously reported the synthesis of heterobiaryls via the ring cleavage reaction of (aza)indoles59,60. This study reports a single methodological approach for introducing various bioactive functional groups on the pyridine scaffold. The synthesis of m-aryl-conjugated ο-substituted nicotinic esters and pyridine analogs with sulfone or phosphonate groups through the remodeling of (aza)indoles/benzofurans via ring cleavage reaction was investigated to address drawbacks and limitations of previous methods.
Results and discussion
Working hypothesis and plausible mechanism
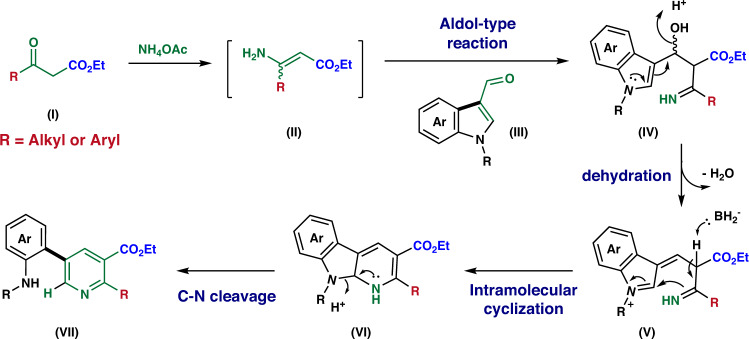
Initially, we investigated the synthesis of m-aminopyridyl-o-methyl-substituted ethyl nicotinates (3aa) via the proposed ring cleavage reaction of N-phenylsulfonyl 3-formyl 7-azaindole (1a) with ethyl acetoacetate (2a) as a model system. Ammonium acetate was the nitrogen source for the substituted enamines, which are the key intermediates of the (aza)indole ring cleavage reaction (Fig. 2). From the β-keto ester(I) and ammonium acetate is generated substituted β-amino acrylate intermediate (II). Then, aldol-type condensation between the β-amino acrylate intermediate and 3-formyl (aza)indole (III) forms intermediate (V) by dehydration of the (IV). Sequential intramolecular cyclization (VI) and C-N bond cleavage generates the desired m-aminopyridyl-o-methyl-substituted ethyl nicotinates (VII).
Fig. 2. Working hypothesis and plausible mechanism.
Plausible mechanism of the synthesis of 2-alkyl/aryl 3- esters 5-aminoaryl pyridine based on the aldol-type reaction between 3-formyl (aza)indole and β-aminoacrylate generated from β-ketoester and ammonium acetate in situ is proposed.
Reaction optimization and substrate scope
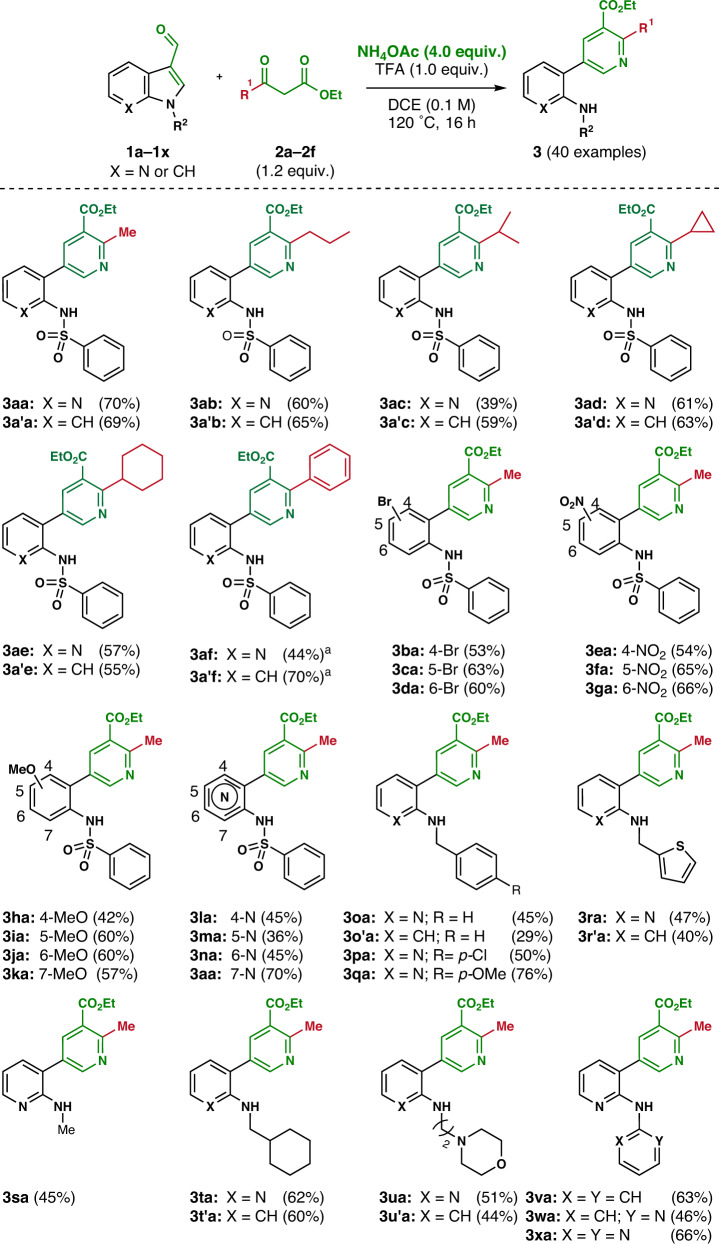
The developed methodology proceeded smoothly, even in the absence of an acid catalyst. However, the yields were significantly reduced with other β-ketoesters (2b–2f). Therefore, the reaction conditions were optimized by changing various parameters (see Supplementary Table 4): N-Phenylsulfonyl 7-azaindole 3-carboxaldehyde (1a) and ethyl acetoacetate (2a) were heated in dichloroethane (DCE) in the presence of NH4OAc and trifluoroacetic acid (TFA) at 120 °C for 16 h to deliver the desired m-aminoaryl-o-methyl nicotinate 3aa in a 70% yield. Under the optimized conditions, the substrate scope of this methodology was then investigated with various β-ketoesters (2b–2 f; see Supplementary Fig. 7), such as alkyl and aryl β-keto esters, using N-phenylsulfonyl 3-formyl 7-azaindole (1a) and indole (1a’) (Fig. 3). The ring cleavage reaction of 3-formyl (aza)indoles (1a and 1a’) with n-propyl β-ketoester (2b) afforded m-aminoaryl-ο-propyl ethyl nicotinates (3ab and 3a’b) in good yields. Isopropyl (2c) and cyclopropyl (2d) β-ketoesters were also applicable to this methodology and yielded the desired substituted nicotinates (3ac–3a’d) in moderate yields. In fact, cyclopropyl-substituted pyridine analogs have been extensively used in medicinal chemistry. As shown in Fig. 1a, LG100268 is an agonist of retinoid X receptor (RXR)61. This (aza)indole cleavage reaction was also compatible with cyclohexyl (2e) and phenyl (2f) β-ketoesters, and provided ο-cyclohexyl and ο-phenyl nicotinate analogs (3ae–3a’f) from the corresponding (aza)indoles (1a and 1a’).
Fig. 3. Substrate scope investigation.
Reaction conditions: 1 (0.2 mmol), 2 (1.2 equiv.), NH4OAc (4.0 equiv.), TFA (1.0 equiv.) in DCE (2 mL) at 120 °C for 16 h. Yields of isolated products (3) are given in parenthesis. a: Reaction performed in ethanol and without trifluoroacetic acid.
Next, we investigated the substitution effects of indole substrates (1b–1k; see Supplementary Fig. 6) in the ring cleavage reaction with ethyl acetoacetate (2a) and confirmed the formation of desired substituted pyridine analogs in good yields, regardless of the electronic effects of the substituents (Fig. 3). N-Phenylsulfonyl 3-formyl indoles containing electron-withdrawing bromo (1b–1d) and nitro (1e–1g) group at the C-4, C-5, and C-6 positions, respectively, provided the desired substituted pyridines (3ba–3ga) in moderate to good yields. In the case of electron-donating methoxy group (1h–1k), the desired substituted pyridines (3ha–3ka) were obtained in comparable yields. Furthermore, the substrate scope of this methodology was also examined using regioisomeric azaindoles (1l–1n) to generate diverse o-aminopyridyl-conjugated pyridine analogs (3la–3na).
The compatibility and substrate scopes of this methodology with diverse N-substituted (aza)indoles (1o–1x; see Supplementary Fig. 6) using ethyl acetoacetate (2a) were then investigated (Fig. 3). 3-Formyl (aza)indoles containing N-benzyl substituents (1o–1q), regardless of the functional groups on the benzene ring, successfully proceeded the desired reaction in good to excellent yields. Furthermore, when the benzene ring was substituted with thiophene (1r and 1r’), the desired transformation was well achieved. 3-Formyl (aza)indoles containing alkyl groups, such as methyl (1s), cyclohexylmethyl (1t and 1t’), 2-morpholinoethyl (1u and 1u’), were also converted to the desired pyridine analogs (3sa–3u’a). It is worth mentioning that N-aryl-substituted 3-formyl azaindoles, such as phenyl (1v), pyridyl (1w), and pyrimidyl (1x) moieties, suited well with this ring cleavage methodology, and N-aryl-substituted aminopyridyl nicotinates (3va–3xa) were obtained in moderate to good yields.
Use of β-keto sulfones and β-keto phosphonates
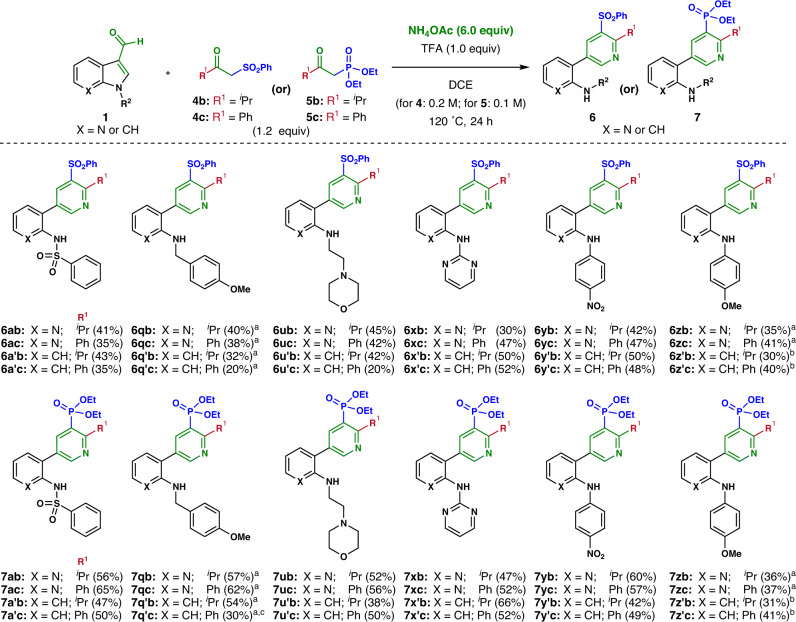
We next examined the robust synthesis of pyridine analogs containing various sulfones and phosphonates using our methodology (Fig. 4). The reaction compatibility of N-substituted 3-formyl (aza)indole (1a and 1a’) with phenyl sulfonyl acetone (4a; see Supplementary Fig. 2) was initially explored, but the undesired o-methylsulfonyl pyridine (6aa’) was formed along with the desired o-methyl-m-sulfonyl pyridine analog (6aa) due to the regioselectivity of enamine formation (see Supplementary Fig. 2). A similar reactivity pattern was observed in the case of diethyl (2-oxopropyl)phosphonate (5a; see Supplementary Fig. 3). To address this issue, we used 3,3-dimethyl phenylsulfonyl acetone (4b) as the source of sulfonyl enamine, and successfully obtained the desired o-isopropyl-m-sulfonyl pyridine (6ab) without forming its regioisomers (see Supplementary Fig. 4). Unlike β-ketoesters, the corresponding sulfones and phosphonates were not sufficiently reactive. Therefore, we further optimized the reaction conditions, and confirmed that isopropyl (4b) and phenyl β-ketosulfones (4c) were less reactive than their phosphonate analogs (5b and 5c). A higher reaction concentration was thus needed. Under the re-optimized conditions (as shown in Fig. 4), the reactivities of N-phenylsulfonyl 3-formyl 7-azaindole (1a) and indole (1a’) with β-keto sulfones (4b–4c) or phosphonates (5b–5c) were investigated. The desired m-(hetero)aryl pyridyl sulfones (6ab–6a’c) and phosphonates (7ab–7a’c) were obtained in moderate to good yields. In the case of the N-alkyl substituents, N-p-methoxybenzyl (1q and 1q’) and N-(2-morpholinoethyl)-3-formyl (aza)indoles (1u and 1u’) afforded the desired ring cleavage products (6qb–6u’c and 7qb–7u’c) in moderate yields. This reactivity pattern was further confirmed with N-pyrimidyl (1x and 1x’), N-p-nitrophenyl (1y and 1y’), N-p-methoxyphenyl (1z and 1z’) 3-formyl (aza)indoles. Compared to the electron-donating N-p-methoxyphenyl analogs (6zb–6z’c and 7zb–7z’c), the electron-withdrawing N-pyrimidyl and N-p-nitrophenyl 3-formyl (aza)indoles provided the desired pyridyl sulfones and phosphonates in better yields (6xb–6x’c and 7xb–7x’c; 6yb–6y’c and 7yb–7y’c).
Fig. 4. Use of various enamine sources for the synthesis of pyridyl sulfones/phosphonates.
Reaction conditions: 1 (0.2 mmol), β-keto sulfones/phosphonates (4b–c/5b–c, 1.2 equiv.), NH4OAc (6.0 equiv.), TFA (1.0 equiv.) in DCE (2 mL/1 mL) at 120 °C for 16 h to 48 h. Yields of isolated products (6 and 7) are given in parenthesis. a: 30 h reaction time. b: 48 h reaction time. c: 55% Crude NMR yield.
Use of benzofurans as substrates
Once the general reactivity of N-substituted 3-formyl (aza)indoles with a series of enamines (in situ generated from β-keto esters, sulfones, and phosphonates) was confirmed for the synthesis of highly functionalized pyridines, the scope of the ring cleavage methodology was extended to benzofuran derivatives. Benzofuran is an oxygen-containing heterocycle found in diverse natural products and bioactive molecules62, but up to our knowledge the remodeling of benzofuran skeletons to N-heterocycles has not yet been reported. The reactivity of 3-formyl benzofurans with representative β-ketoesters using this methodology to harness o-substituted-m-phenol-conjugated nicotinates was investigated (Fig. 5). In fact, the phenol and heterobiaryl moieties are one of the most abundant structural units found in numerous bioactive natural products and therapeutic agents63,64. Unlike indoles and azaindoles, most of 3-formyl benzofurans are not commercially available. Therefore, the substituted 3-formyl benzofuran analogs (8b–8j) were prepared from their corresponding salicylaldehydes (see Supplementary Fig. 14). As a model system, methyl (2a), cyclopropyl (2d), and phenyl (2f) β-ketoesters were chosen as the enamine sources. Under the optimized conditions, 3-formyl benzofuran (8a) was reacted with three representative β-ketoesters to deliver the desired ring cleavage product containing methyl (9aa), cyclopropyl (9ad), and phenyl (9af) moieties at the C-2 position. We then examine the substrate scope of this ring cleavage reaction using various 5- and 6-substituted 3-formyl benzofurans (8b–8i) and obtained highly functionalized nicotinate derivatives (9ba–9ia, 9bd–9id, and 9bf–9if) in moderate to good yields. In particular, the reaction of 3-formyl benzofurans with both electron-withdrawing groups (chloro, bromo, and nitro; 8b–8e) and electron-donating groups (methyl and methoxy; 8f–8i) at the C-5 and C-6 positions afforded the desired (hetero)biaryl products. 3-Formyl naphthofuran (8j) also provided the o-substituted nicotinate analogs containing a naphthol moiety (9ja, 9jd, and 9jf).
Fig. 5. Reaction with benzofuran analogs.
Reaction conditions: 8 (0.2 mmol), β-ketoesters (2a, 2d, or 2f, 1.2 equiv.), NH4OAc (4.0 equiv.), TFA (1.0 equiv.) in DCE (2 mL) at 120 °C for 16 h. Yields of isolated products (9) are reported.
Synthetic application
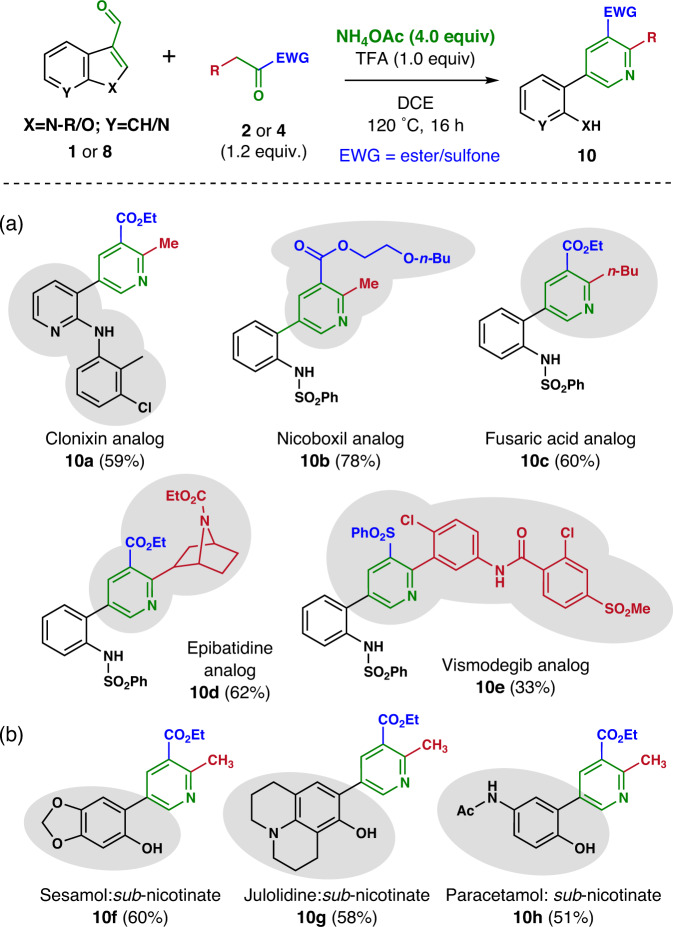
This (aza)indole ring cleavage reaction was then applied to the synthesis of analogs of various bioactive pyridines. Initially, privileged structural units were extracted from bioactive natural products (fusaric acid and epibatidine) and drug molecules (clonixin, nicoboxil, and vismodegib). These privileged pyridine scaffolds were then synthesized using (aza)indole ring cleavage methodology. Highly functionalized pyridine analogs (10a–10e) were synthesized from N-substituted 3-formyl (aza)indoles (Fig. 6a) with diverse β-ketoesters/sulfones (2a, 2g, 2h, 2i, and 4d; see Supplementary Fig. 7). For example, N-aryl 3-formyl azaindole (1aa) was reacted with ethyl acetoacetate (2a) to furnish the non-steroidal anti-inflammatory drug (NSAID) clonixin analog (10a) in a 59% yield. Other bio-relevant molecules were synthesized in a single step from N-phenylsulfonyl 3-formyl indole (1a’) via the ring cleavage reaction with the corresponding β-ketoesters (2g–2i) to afford the nicoboxil analog (10b), the fusaric acid analog (10c), and the epibatidine analog (10d) in 78%, 60%, and 62% yields, respectively. Nicoboxil is an FDA-approved drug used for the treatment of acute back pain. Fusaric acid and epibatidine are pyridine scaffold-containing natural products whereby fusaric acid is an antibiotic isolated from the fungus Fusarium heterosporium and used for the synthesis of vasodilator bupicomide65, while epibatidine is known as a modulator of the nicotinic acetylcholine receptor66, 67. The ring cleavage reaction of N-phenylsulfonyl 3-formyl indole (1a’) with aryl β-ketosulfone (4d) also furnished the desired vismodegib analog (10e). Vismodegib is an FDA-approved drug used for the treatment of basal cell carcinoma.
Fig. 6. Synthetic application.
a Synthesis of privileged pyridine scaffold-containing bio-relevant molecules. b Drug/natural product conjugation with ethyl 2-methyl nicotinate. See Supplementary information for reaction condition and further details.
Consequently, we applied this late-stage transformation method to the field of drug-drug or drug-natural product conjugation (Fig. 6b). Substituted 3-formyl benzofurans (8k and 8l) were reacted with ethyl acetoacetate (2a), which allowed the formation of sesamol-conjugated 2-methyl nicotinate (10g, natural product-drug conjugate) and julolidine-nicotinate conjugate (10h) in 60% and 58% yields, respectively. Naturally occurring sesamol exhibits anti-fungal activity and can be used to synthesize paroxetine (sold under the brand names Paxil® and Seroxat®), a type of antidepressant drug under the class of selective serotonin reuptake inhibitors (SSRI). Julolidine is a heterocyclic aromatic moiety extensively used in therapeutic agents, photoconductive materials, and chemiluminescence substances. Paracetamol-conjugated 2-methyl nicotinate (10f, drug-drug conjugate) was also obtained from the corresponding 3-formyl benzofuran (8m) in a 51% yield. Paracetamol, or acetaminophen, is used as an analgesic and antipyretic. These examples demonstrated that the proposed ring cleavage reaction could be beneficial for synthesizing highly functionalized privileged pyridines via the late-stage remodeling of (aza)indoles and benzofurans.
Conclusions
In conclusion, this study reported the successful application of the proposed ring cleavage strategy for the synthesis of o-substituted m-aminoaryl-conjugated pyridines from N-substituted 3-formyl (aza)indoles. In fact, this reaction afforded diversely substituted pyridine analogs containing multiple functional groups, such as esters, sulfones, and phosphonates, at the C-3 position of pyridine with a wide range of substrate scope, which is not easily accessible by conventional methods. Furthermore, this ring cleavage reaction was extended to benzofuran derivatives for synthesizing m-phenol-conjugated (hetero)biaryl nicotinates. Though o-aniline/phenol were inevitably incorporated on the m-position of pyridine, these moieties can enhance the bio-relevancy of the final biaryl structures due to their abundancy in drugs and bioactive molecules. synthetic methodology allowed access to various analogs of drugs and biologically relevant molecules containing privileged pyridine scaffolds. Finally, this methodology allowed the late-stage conjugation of substituted nicotinates with paracetamol, sesamol, and julolidine as drug-drug and natural product-drug conjugates from 3-formyl benzofurans. Biological studies on all the synthesized compounds are currently in progress, and the outcomes will be reported in due course.
Methods
General methods
For instrumentation and materials, see Supplementary Method - General Information. For Additional experiments concerning optimization of the reaction conditions, see Supplementary Figures – (2) Reaction Optimization.
General procedure for the reaction of N-substituted 3-formyl (aza)indoles with diverse β-ketoesters (2a–2f)
A 4-mL vial equipped with a magnetic bar and a Teflon-lined screwed cap was charged with 1 (0.2 mmol), β-ketoesters (2a–2 f, 1.2 equiv.), trifluoroacetic acid (TFA, 22.80 mg, 14.86 μL, 1.0 equiv.), and NH4OAc (61.66 mg, 4.0 equiv.) in dichloroethane (DCE, 2.0 mL). The vial was then sealed and heated at 120 °C for 16 h. Upon reaction completion checked by LC-MS and TLC analysis, the reaction mixture was diluted with dichloromethane (DCM), quenched with saturated aqueous NaHCO3 solution, and extracted with DCM (3 × 10 mL). The combined organic layer was washed with brine (10 mL), dried over anhydrous Na2SO4(s), filtered, and concentrated under reduced pressure. The crude mixture was purified by silica-gel flash column chromatography to obtain the desired product.
Note: The general procedure for the above methodology was slightly modified in the case of ethyl benzoylacetate (2f); the reaction was performed in ethanol without TFA.
General procedure for the reaction of N-substituted 3-formyl (aza)indole with β-ketosulfones (4b–c)/β-ketophosphonates (5b–c)
A 4-mL vial equipped with a magnetic bar and a Teflon-lined screwed cap was charged with 1 (0.2 mmol), β-keto sulfones/phosphonates (4b–c/5b–c, 1.2 equiv.), TFA (22.80 mg, 14.86 μL, 1.0 equiv.), and NH4OAc (92.50 mg, 6.0 equiv.) in DCE (1.0 mL (4b–c)/2.0 mL (5b–c)). The vial was then sealed and heated at 120 °C for 16 h to 48 h. Upon reaction completion checked by LC-MS and TLC analysis, the reaction mixture was diluted with DCM, quenched with saturated aqueous NaHCO3 solution, and extracted with DCM (3 × 10 mL). The combined organic fraction was washed with brine (10 mL), dried over anhydrous Na2SO4(s), filtered, and concentrated under reduced pressure. The crude compound was purified by silica-gel flash column chromatography to obtain the desired product bearing 3-pyridylsulfones (6)/3-pyridyl phosphonates (7).
General procedure for the reaction of benzofuran-3-carboxaldehydes with β-ketoesters
A 4-mL vial equipped with a magnetic bar and a Teflon-lined screwed cap was charged with 8 (0.2 mmol), β-ketoesters (2a, 2d, or 2f, 1.2 equiv.), TFA (22.80 mg, 14.86 μL, 1.0 equiv.), and NH4OAc (61.66 mg, 4.0 equiv.) in DCE (2.0 mL). The vial was then sealed and heated at 120 °C for 16 h. Upon reaction completion checked by LC-MS and TLC analysis, the reaction mixture was diluted with DCM, quenched with saturated aqueous NaHCO3 solution, and extracted with DCM (3 × 10 mL). The combined organic fraction was washed with brine (10 mL), dried over anhydrous Na2SO4(s), filtered, and concentrated under reduced pressure. The crude compound was purified by silica-gel flash column chromatography to obtain the desired phenol-conjugated product (9).
Preparation of substrates
Spectroscopic data of products
See Supplementary Data 1.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This research was funded by National Creative Research Initiative Grant (2014R1A3A2030423) through the National Research Foundation of Korea (NRF) funded by the Korean Government (Ministry of Science & ICT). Yi, S. and Lee, J.H. are grateful for the fellowship by BK21 Plus Program.
Author contributions
K.V. and S.Y. designed and optimized the methodology, performed synthetic experiments, characterized compounds, and prepared the manuscript. J.H.L. and B.V.V. performed the synthesis and characterization. S.B.P. directed the whole study and involved in all aspects of the experimental design, data analysis, and manuscript preparation. All authors critically reviewed the text and figures.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated and analyzed during this study are included in this article, its Supplementary Information, and Supplementary Data, and also available from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kannan Vaithegi, Sihyeong Yi.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-023-00914-5.
References
- 1.Daly, J. W., Garraffo, M. & Spande, T. F. Alkaloids from amphibian skins. in Alkaloids: chemical and biological perspectives (ed. Pelletier, S. W.) 13, 1–161 (Pergamon), (1999).
- 2.De S, et al. Pyridine: the scaffolds with significant clinical diversity. RSC Adv. 2022;12:15385–15406. doi: 10.1039/D2RA01571D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada, Y. Role of pyridines in medicinal chemistry and design of BACE1 inhibitors possessing a pyridine scaffold. in Pyridine (ed. Pandey, P. P.) (Intech), (2018).
- 4.Ling Y, et al. The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des. Devel. Ther. 2021;15:4289–4338. doi: 10.2147/DDDT.S329547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altaf AA, et al. A review on the medicinal importance of pyridine derivatives. J. Drug Des. Med. Chem. 2015;1:1–11. [Google Scholar]
- 6.Lou X-Y, Yang Y-W. Pyridine-conjugated pillar[5]arene: from molecular crystals of blue luminescence to red-emissive coordination nanocrystals. J. Am. Chem. Soc. 2021;143:11976–11981. doi: 10.1021/jacs.1c07006. [DOI] [PubMed] [Google Scholar]
- 7.Blatchford J, et al. Photoluminescence in pyridine-based polymers: Role of aggregates. Phys. Rev. B. 1996;54:9180–9189. doi: 10.1103/PhysRevB.54.9180. [DOI] [PubMed] [Google Scholar]
- 8.Tahir T, et al. Pyridine scaffolds, phenols and derivatives of azo moiety: Current therapeutic perspectives. Molecules. 2021;26:4872. doi: 10.3390/molecules26164872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SX, Curtis MA, Sperry J. Pyridine alkaloids with activity in the central nervous system. Bioorg. Med. Chem. 2020;28:115820. doi: 10.1016/j.bmc.2020.115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Raheem SAA, et al. Synthesis and biological activity of 2-((3-Cyano-4,6-distyrylpyridin-2-yl) thio) acetamide and its cyclized form. Alger. J. Biosci. 2020;1:46–50. doi: 10.57056/ajb.v1i2.26. [DOI] [Google Scholar]
- 11.Guan AY, Liu C-L, Sun X-F, Xie Y, Wang M-A. Discovery of pyridine-based agrochemicals by using intermediate derivatization methods. Bioorg. Med. Chem. 2016;24:342–353. doi: 10.1016/j.bmc.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Matolcsy, G., Nadasy, M. & Andriska, V. Pesticide Chemistry32. (Elsevier Science), (1989).
- 13.Dipalma JR, Thayer WS. Use of niacin as a drug. Annu. Rev. Nutr. 1991;11:169–187. doi: 10.1146/annurev.nu.11.070191.001125. [DOI] [PubMed] [Google Scholar]
- 14.Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu. Rev. Pharmcol. Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- 15.Henderson LM. Niacin. Annu. Rev. Nutr. 1983;3:289–307. doi: 10.1146/annurev.nu.03.070183.001445. [DOI] [PubMed] [Google Scholar]
- 16.Booker, S. et al. Amgen Inc. Pi3 kinase modulators and methods of use. US20090054405A1 (26.02.2009)
- 17.Lahm, G. P. & Smith, B. K. E. I. Du Pont De Nemours And Company. Heterocyclic compounds for treating helminth infections. WO2012177668A1 (27.12.2012).
- 18.Kozlowki, J. A., Shankar, B. B., Shih, N.-Y. & Tong, L. Schering Corporation. Cannabinoid receptor agonists. WO2004000807A1 (31.12.2003).
- 19.Wassmundt, F. W. University of Connecticut. Aryl and heteroaryl compounds having anti-retrovirus activity. CA2094139A1 (23.04.1992).
- 20.Reitz, D. B., Manning, R. E., Huang, H.-C. & Li, J. GD Searle LLC. Substituted spiro compounds for the treatment of inflammation. US5393790A (28.02.1995).
- 21.Vanhoefer U, et al. PAK-104P, a pyridine analogue, reverses paclitaxel and doxorubicin resistance in cell lines and nude mice bearing xenografts that overexpress the multidrug resistance protein. Clin. Cancer Res. 1996;2:369–377. [PubMed] [Google Scholar]
- 22.Galambos, J. et al. Richter Gedeon Nyrt. Sulfonyl-quinoline derivatives. US8063220B2 (12.02.2009).
- 23.Adams, J. B. EIDP Inc. Pyridyl sulfone herbicides. US4605432A (12.08.1984).
- 24.Hartz RA, et al. Synthesis and evaluation of 2-anilino-3-phenylsulfonyl-6-methylpyridines as corticotropin-releasing factor1 receptor ligands. Bioorg. Med. Chem. Lett. 2006;16:934–937. doi: 10.1016/j.bmcl.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 25.Deng L, et al. Inhibition of 1-deoxy-d-xylulose-5-phosphate reductoisomerase by lipophilic phosphonates: SAR, QSAR, and crystallographic studies. J. Med. Chem. 2011;54:4721–4734. doi: 10.1021/jm200363d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshino K, Kohno T, Morita T, Tsukamoto G. Organic phosphorus compounds. 2. Synthesis and coronary vasodilator activity of (benzothiazolylbenzyl)phosphonate derivatives. J. Med. Chem. 1989;32:1528–1532. doi: 10.1021/jm00127a021. [DOI] [PubMed] [Google Scholar]
- 27.Rendošov MR, et al. Silver(I) pyridylphosphonates – synthesis, structure, stability and light-insensitivity investigation. RSC Adv. 2019;9:1570–1575. doi: 10.1039/C8RA10136A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Raheem SAA, et al. A concise review on some synthetic routes and applications of pyridine scaffold compounds. Curr. Chem. Lett. 2021;10:337–362. doi: 10.5267/j.ccl.2021.7.001. [DOI] [Google Scholar]
- 29.Vessally E, Hosseinian A, Edjlali L, Bekhradnia A, Esrafili MD. New page to access pyridine derivatives: synthesis from N-propargylamines. RSC Adv. 2016;6:71662–71675. doi: 10.1039/C6RA08720E. [DOI] [Google Scholar]
- 30.Bull JA, Mousseau JJ, Pelletier G, Charette AB. Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N-activated pyridines. Chem. Rev. 2012;112:2642–2713. doi: 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]
- 31.Henry GD. De novo synthesis of substituted pyridines. Tetrahedron. 2004;60:6043–6061. doi: 10.1016/j.tet.2004.04.043. [DOI] [Google Scholar]
- 32.Liéby-Muller, F., Allais, C., Constantieux, T. & Rodriguez, J. Metal-free Michael addition initiated multicomponent oxidative cyclodehydration route to polysubstituted pyridines from 1,3-dicarbonyls. Chem. Commun. 4207–4209 (2008). [DOI] [PubMed]
- 33.Jiang Y, Park C-M, Loh T-P. Transition-metal-free synthesis of substituted pyridines via ring expansion of 2-allyl-2H-azirines. Org. Lett. 2014;16:3432–3435. doi: 10.1021/ol501010k. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Tang Z, Song Q. Lewis acid-mediated [3+3] annulation for the construction of substituted pyrimidine and pyridine derivatives. Adv. Synth. Catal. 2017;359:952–958. doi: 10.1002/adsc.201601386. [DOI] [Google Scholar]
- 35.Allais C, Grassot J-M, Rodriguez J, Constantieux T. Metal-free multicomponent syntheses of pyridines. Chem. Rev. 2014;114:10829–10868. doi: 10.1021/cr500099b. [DOI] [PubMed] [Google Scholar]
- 36.Liu NW, et al. Nickel-catalyzed synthesis of diaryl sulfones from aryl halides and sodium sulfinates. Eur. J. Org. Chem. 2018;2018:1208–1210. doi: 10.1002/ejoc.201701478. [DOI] [Google Scholar]
- 37.Liu NW, Hofman K, Herbert A, Manolikakes G. Visible-Light photoredox/nickel dual catalysis for the cross-coupling of sulfinic acid salts with aryl iodides. Org. Lett. 2018;20:760–763. doi: 10.1021/acs.orglett.7b03896. [DOI] [PubMed] [Google Scholar]
- 38.Bandgar BP, Bettigeri SV, Phopase J. Unsymmetrical diaryl sulfones through palladium-catalyzed coupling of aryl boronic acids and arylsulfonyl chlorides. Org. Lett. 2004;6:2105–2108. doi: 10.1021/ol049692c. [DOI] [PubMed] [Google Scholar]
- 39.Trankle WG, Kopach ME. Green chemical synthesis of 2-benzenesulfonyl-pyridine and related derivatives. Org. Process Res. Dev. 2007;11:913–917. doi: 10.1021/op700060e. [DOI] [Google Scholar]
- 40.Guilbaud J, et al. Palladium-catalyzed heteroaryl thioethers synthesis overcoming palladium dithiolate resting states inertness: practical road to sulfones and NH-sulfoximines. Catal. Commun. 2018;111:52–58. doi: 10.1016/j.catcom.2018.03.025. [DOI] [Google Scholar]
- 41.Kim DK, et al. Silyloxymethanesulfinate as a sulfoxylate equivalent for the modular synthesis of sulfones and sulfonyl derivatives. Chem. Sci. 2020;11:13071–13078. doi: 10.1039/D0SC02947E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margraf N, Manolikakes G. One-pot synthesis of aryl sulfones from organometallic reagents and iodonium salts. J. Org. Chem. 2015;80:2582–2600. doi: 10.1021/jo5027518. [DOI] [PubMed] [Google Scholar]
- 43.Maloney KM, Kuethe JT, Linn K. A practical, one-pot synthesis of sulfonylated pyridines. Org. Lett. 2011;13:102–105. doi: 10.1021/ol102629c. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Wang F, Song G, Li X. Diverse reactivity in a rhodium(III)-catalyzed oxidative coupling of N-allyl arenesulfonamides with alkynes. Angew. Chem. Int. Ed. 2012;51:12348–12352. doi: 10.1002/anie.201206918. [DOI] [PubMed] [Google Scholar]
- 45.Scalone M, Waldmeier P. Efficient enantioselective synthesis of the NMDA 2B receptor antagonist Ro 67-8867. Org. Process Res. Dev. 2003;7:418–425. doi: 10.1021/op034006v. [DOI] [Google Scholar]
- 46.Fu WC, So CM, Kwong FY. Palladium-catalyzed phosphorylation of aryl mesylates and tosylates. Org. Lett. 2015;17:5906–5909. doi: 10.1021/acs.orglett.5b03104. [DOI] [PubMed] [Google Scholar]
- 47.Belabassi Y, Alzghari S, Montchamp J-L. Revisiting the Hirao cross-coupling: improved synthesis of aryl and heteroaryl phosphonates. J. Organomet. Chem. 2008;693:3171–3178. doi: 10.1016/j.jorganchem.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalek M, Jezowska M, Stawinski J. Preparation of arylphosphonates by palladium(0)-catalyzed cross-coupling in the presence of acetate additives: synthetic and mechanistic studies. Adv. Synth. Catal. 2009;351:3207–3216. doi: 10.1002/adsc.200900590. [DOI] [Google Scholar]
- 49.Zhang Y, et al. Palladium-catalyzed one-pot phosphorylation of phenols mediated by sulfuryl fluoride. Chem. Commun. 2021;57:4588–4591. doi: 10.1039/D1CC00769F. [DOI] [PubMed] [Google Scholar]
- 50.Kalek M, Ziadi A, Stawinski J. Microwave-assisted palladium-catalyzed cross-coupling of aryl and vinyl halides with H-phosphonate diesters. Org. Lett. 2008;10:4637–4640. doi: 10.1021/ol801935r. [DOI] [PubMed] [Google Scholar]
- 51.Petrakis KS, Nagabhushan TL. Palladium-catalyzed substitutions of triflates derived from tyrosine-containing peptides and simpler hydroxyarenes forming 4-(diethoxyphosphinyl)phenylalanines and diethyl arylphosphonates. J. Am. Chem. Soc. 1987;109:2831–2833. doi: 10.1021/ja00243a050. [DOI] [Google Scholar]
- 52.Hirao T, Masunaga T, Yamada N, Ohshiro Y, Agawa T. Palladium-catalyzed new carbon-phosphorus bond formation. Bull. Chem. Soc. Jpn. 1982;55:909–913. doi: 10.1246/bcsj.55.909. [DOI] [Google Scholar]
- 53.Hirao T, Masunaga T, Ohshiro Y, Agawa T. A novel synthesis of dialkyl arenephosphonates. Synth. 1981;1981:56–57. doi: 10.1055/s-1981-29335. [DOI] [Google Scholar]
- 54.Li C-j. Nickel-catalyzed phosphorylation of tosylates. Russ. J. Gen. Chem. 2020;90:725–730. doi: 10.1134/S1070363220040258. [DOI] [Google Scholar]
- 55.Geng Z, et al. Pd-catalyzed C–P coupling of heteroaryl boronic acid with H-phosphonate diester. Tetrahedron Lett. 2016;57:3063–3066. doi: 10.1016/j.tetlet.2016.05.038. [DOI] [Google Scholar]
- 56.Qiu D, et al. Visible light-driven, photocatalyst-free Arbuzov-like reaction via arylazo sulfones. Adv. Synth. Catal. 2019;361:5239–5244. doi: 10.1002/adsc.201900953. [DOI] [Google Scholar]
- 57.Shaikh RS, Düsel SJS, König B. Visible-light photo-Arbuzov reaction of aryl bromides and trialkyl phosphites yielding aryl phosphonates. ACS Catal. 2016;6:8410–8414. doi: 10.1021/acscatal.6b02591. [DOI] [Google Scholar]
- 58.Wang S, Qiu D, Mo F, Zhang Y, Wang J. Metal-free aromatic carbon-phosphorus bond formation via a Sandmeyer-type reaction. J. Org. Chem. 2016;81:11603–11611. doi: 10.1021/acs.joc.6b01820. [DOI] [PubMed] [Google Scholar]
- 59.Lee S, Park SB. An efficient one-step synthesis of heterobiaryl pyrazolo[3,4-b] pyridines via indole ring opening. Org. Lett. 2009;11:5214–5217. doi: 10.1021/ol902147u. [DOI] [PubMed] [Google Scholar]
- 60.Varun BV, Vaithegi K, Yi S, Park SB. Nature-inspired remodeling of (aza)indoles to meta-aminoaryl nicotinates for late-stage conjugation of vitamin B3 to (hetero)arylamines. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-19610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leal AS, et al. Retinoid X receptor agonist LG100268 modulates the immune microenvironment in preclinical breast cancer models. npj Breast Cancer. 2019;5:1–15. doi: 10.1038/s41523-019-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao YH, et al. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019;9:27510–27540. doi: 10.1039/C9RA04917G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahim MA, Kristufek SL, Pan S, Richardson JJ, Caruso F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019;58:1904–1927. doi: 10.1002/anie.201807804. [DOI] [PubMed] [Google Scholar]
- 64.Scott KA, Cox PB, Njardarson JT. Phenols in pharmaceuticals: analysis of a recurring motif. J. Med. Chem. 2022;65:7044–7072. doi: 10.1021/acs.jmedchem.2c00223. [DOI] [PubMed] [Google Scholar]
- 65.Tung TT, et al. Fusaric acid and analogues as gram-negative bacterial quorum sensing inhibitors. Eur. J. Med. Chem. 2017;126:1011–1020. doi: 10.1016/j.ejmech.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 66.Salehi B, et al. Epibatidine: a promising natural alkaloid in health. Biomol. 2019;9:6. doi: 10.3390/biom9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W, et al. Antinociceptive effects of novel epibatidine analogs through activation of α4β2 nicotinic receptors. Sci. China Life Sci. 2018;61:688–695. doi: 10.1007/s11427-017-9062-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated and analyzed during this study are included in this article, its Supplementary Information, and Supplementary Data, and also available from the authors upon reasonable request.