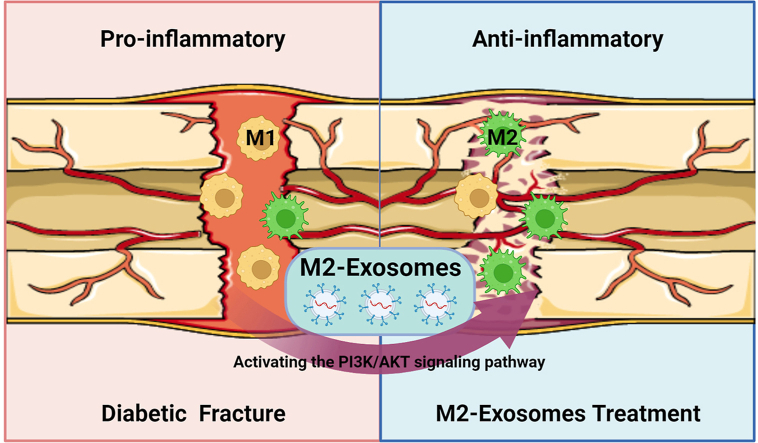
Abstract
Diabetes mellitus is a chronically inflamed disease that predisposes to delayed fracture healing. Macrophages play a key role in the process of fracture healing by undergoing polarization into either M1 or M2 subtypes, which respectively exhibit pro-inflammatory or anti-inflammatory functions. Therefore, modulation of macrophage polarization to the M2 subtype is beneficial for fracture healing. Exosomes perform an important role in improving the osteoimmune microenvironment due to their extremely low immunogenicity and high bioactivity. In this study, we extracted the M2-exosomes and used them to intervene the bone repair in diabetic fractures. The results showed that M2-exosomes significantly modulate the osteoimmune microenvironment by decreasing the proportion of M1 macrophages, thereby accelerating diabetic fracture healing. We further confirmed that M2-exosomes induced the conversion of M1 macrophages into M2 macrophages by stimulating the PI3K/AKT pathway. Our study offers a fresh perspective and a potential therapeutic approach for M2-exosomes to improve diabetic fracture healing.
Keywords: M2-exosomes, Diabetic fracture healing, Osteoimmune microenvironment, Macrophage polarization, PI3K/AKT signaling pathway
Graphical abstract
Highlights
-
•
Abnormal macrophage polarization was found in diabetic fracture model.
-
•
M2-exosomes can promote diabetic fracture healing.
-
•
M2-exosomes regulate osteoimmune microenvironment through PI3K/AKT signaling pathway.
1. Introduction
Nowadays, more than 422 million people have diabetes mellitus (DM), of which type 2 diabetes mellitus (T2DM) accounts for 90% [1]. Diabetes is prone to a variety of complications due to its persistent hyperglycemic state, which has a serious negative impact on the human skeletal system in particular [[2], [3], [4]]. Studies have shown that people with mellitus often suffer from delayed fracture healing, which brings a heavy burden to the patients and society [[5], [6], [7]].
In recent years, there has been increasing interest in the pivotal role of macrophage polarization in the process of fracture healing [8]. Macrophages are derived from monocytes and they are categorized into two primary subtypes based on their distinct functions: M1 and M2 [9]. While M1 macrophages play a pro-inflammatory part in fracture healing, M2 macrophages perform an anti-inflammatory function. These two macrophage subtypes secrete different cytokines that modulate the differentiation of osteoblasts and osteoclasts, crucial for the bone healing process [10]. In the acute inflammatory phase of fracture healing, large aggregates of inflammatory factors can recruit highly plastic macrophages to the fracture site [11]. Macrophages are initially polarized towards the M1 subtype under the influence of the inflammatory microenvironment, where they secrete large amounts of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β. This amplifies the inflammatory cascade response and induces osteoclastogenesis, ultimately leading to bone resorption and the removal of necrotic tissue from the fracture site [12,13]. Although the inflammatory response has been shown to be critical for normal fracture healing, an overly sustained inflammatory response can inhibit fracture healing [14]. Therefore, the rational regulation of macrophage subtype polarization has profound implications for fracture healing.
The microenvironment of bone trauma is constantly changing as the fracture heals, and macrophages monitor and respond to local microenvironmental signals via surface receptors to switch functional phenotypes to accommodate bone healing [15]. Under the influence of cytokines related to T helper 2 cell (Th2) activation, M1 macrophages undergo polarization and transform into M2 macrophages, resulting in the conversion of pro-inflammatory signals in the microenvironment into anti-inflammatory signals [16]. In an anti-inflammatory feedback mechanism, M2 macrophages perform a pivotal role in tissue wound healing via releasing anti-inflammatory cytokines [12]. In addition, M2 macrophages also have a positive effect on bone marrow-derived mesenchymal stem cells (BMSCs) differentiation, leading to cartilage and bone formation by regulating the expression of oncostatin M (OSM) and bone morphogenetic protein 2 (BMP-2). This further supports the formation of soft callus during fracture healing [17,18]. Even during the callus remodeling phase, M2 macrophages continue to play a vital role. They secrete growth factors that directly or indirectly facilitate increased mineralization of the matrix, thereby accelerating the formation of hard calluses and promoting fracture healing [19]. Recent studies have shown that the fracture trauma microenvironment in diabetic patients exhibits an imbalance in macrophage polarization, with a predominance of the M1 subtype [20]. If the proportion of M2 macrophages in the microenvironment is increased, it may effectively accelerate the fracture healing process in diabetic patients [21]. Therefore, appropriate interventions to promote macrophage polarization towards the M2 subtype would be beneficial in promoting diabetic fracture healing.
Exosomes are microvesicles around 50–150 nm in diameter, which are involved in the transport of biochemicals such as cytokines, mRNAs, miRNAs, and proteins [[22], [23], [24]]. Exosomes are commonly utilized due to their high biocompatibility, low immunogenicity, and stable circulation in the body [25,26]. A recent study has reported that exosomes derived from M2 macrophages (M2-exosomes) can expedite wound healing by facilitating the conversion of M1 towards M2 macrophages [27]. Nevertheless, the precise role and underlying molecular mechanisms of M2-exosomes in T2DM-associated fracture healing remain unclear.
In this research, we first explored the trend profile of bone repair capacity and macrophage polarization in a diabetic fracture model. We found that bone repair was significantly delayed due to an increased proportion of M1 inflammatory macrophages in the diabetic fracture model. Then, we extracted the M2-exosomes and used them to intervene the bone repair in diabetic fractures. The results showed that M2-exosomes remarkably regulated the osteoimmune microenvironment by reducing the proportion of M1, thereby accelerating diabetic fracture healing. Finally, we further demonstrated that M2-exosomes accelerated diabetic fracture healing by inducing the transformation of M1 macrophages into M2 macrophages by stimulating the PI3K/AKT pathway.
2. Materials and methods
2.1. Cell culture
Bone-marrow-derived macrophages (BMDMs) were obtained from the femur and tibia of two-month-old C57BL/6 mice. The bone marrow was flushed out and then cultured for a period of 7 days in 1640 media (Gibco, USA) supplemented with 30 ng/ml of macrophage colony-stimulating factor (M-CSF, Peprotech, USA). The BMDMs were cultured and induced into M1 and M2 macrophages by treating them with Interferon-γ (IFN-γ, Peprotech, USA) and IL-4 (Peprotech, USA), respectively. The cells were incubated at 37 °C in a humidified chamber with 5% CO2.
2.2. Femur fracture model
First, two-month-old C57BL/6 (control group) and db/db mice (T2DM group) were anesthetized according to the Animal Ethics Committee of Shanghai University. The hair on the surface of the skin on the right lower limb was removed, followed by disinfection of the local skin. An incision was then made along the femur. A syringe needle was inserted into the bone marrow space through the incision made along the femur. The osteotomy was performed after transecting the femur, and the incision was closed using 5-0 silk sutures. Based on previous literature [28,29], on the second day after surgery, M2-exosomes (100 μg/mL, 100 μL) were introduced into the fracture site for 5 consecutive days, while PBS was used as the control group. The samples were then gathered for follow-up studies on the 7th, 14th, and 21st days post-surgery.
2.3. Micro-CT
The right thigh femur was collected from mice at weeks 1, 2, and 3 postoperatively. The collected mouse femurs were scanned using a SkyScan CT scanner, and the application software was used to evaluate and analyze relevant parameters, such as callus total volume (TV), callus bone volume (BV), BV/TV, and BMD.
2.4. Preparation and characterization of exosomes
Once reaching 80–90% confluency, M2 macrophages were cultured in a serum-free medium for two days. The supernatant was collected and subjected to sequential centrifugation steps at 300×g for 10 min, 2000×g for 10 min, and 10,000×g for half an hour to remove dead cells and cell debris. Next, the supernatant underwent two rounds of ultracentrifugation at 110,000×g for 70 min each at 4 °C using Beckman Coulter equipment to isolate exosomes. The obtained exosomes were stored at −80 °C for further experiments. The size distribution, concentration, and morphology of the exosomes were analyzed using nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). The expression of specific labeled proteins on the exosome surface was detected by Western blotting.
2.5. Western blotting
After removal of the culture medium, M1, RM2, and M2 macrophages were washed twice with PBS. The cells were then subjected to lysis by adding RIPA Lysis Buffer (Beyotime, China) to the dish and placed on ice for 30 min. After the cell lysis, the mixture was centrifuged at 12,000×g for 15 min at 4 °C. The proteins in the supernatant were extracted and then protein quantification was performed. After overnight incubation with primary antibodies at 4 °C, the samples were treated with DyLight® 800 (Abcam, UK) the next day for blotting. Finally, the images were probed after the membrane was exposed to a far-infrared ray. Below are the antibodies used in the experiment: anti-CD206 (Abcam, UK), anti-iNOS (Abcam, UK). And the following antibodies were purchased from Cell Signaling Technology: anti-Arg-1, anti-PI3K, anti-p-PI3K, anti-AKT, anti-p-AKT.
2.6. qRT-PCR
To perform qRT-PCR, total RNA was extracted using RNAiso Plus (TaKaRa, Japan), and reverse transcription was carried out using PrimeScript™ RT Master Mix (TaKaRa, Japan). The qPCR was then performed using the MonAmp™ SYBR® Green qPCR Mix (Monad, China). The primer sequences are displayed in Supplementary Table S1.
2.7. Alkaline phosphatase and Alizarin Red-S staining
Alkaline phosphatase (ALP) and Alizarin Red-S (ARS) staining were performed on days 7 and 14 after cell culture, respectively. For ALP staining, cells were first immobilized with 4% paraformaldehyde (PFA, Solarbio, China) for 15 min. The cells were then stained using the corresponding chromogenic kit, and the staining results were then quantified using a quantitative kit. For ARS staining, the cells were first fixed and then soaked with ARS solution (Solarbio, China) for half an hour. The color development was halted by treating the samples with deionized water. The stained samples were then examined under an inverted microscope and quantified by measuring the absorbance at 562 nm using a multifunctional enzyme marker (BioTek, USA).
2.8. Immunofluorescence
First, M1, RM2, and M2 macrophages were seeded in 12-well plates. The cells were then washed with PBS, and immobilized with 4% PFA, permeabilized with 0.1% Triton X-100 for half an hour, and blocked with 10% goat serum to prevent nonspecific staining. Primary antibodies such as anti-F4/80 (Abcam, UK), anti-iNOS (Abcam, UK), and anti-CD206 (Abcam, UK) were used to localize the proteins at 4 °C. Afterward, the slices were incubated with species-matched secondary antibodies, followed by sealing and observation for further analysis.
2.9. Flow cytometry
Cultured cells were obtained and treated with anti-F4/80 antibody (Biolegend, USA) for 0.5h, followed by treatment with intracellular stain permeation washing buffer, and incubated with anti-CD206 antibody (Biolegend, USA) for 0.5h while shading. The sediment was then collected by centrifugation and washed with PBS. After repeated washing, the machine was used for measurement and analysis.
2.10. Histological analysis
The femur samples were sequentially fixed (4% PFA), decalcified (15% EDTA), and finally embedded with paraffin. Then cut the wax block into 5 μm thin slices. These sections were then subjected to deparaffinization and dehydration processes. Afterward, they were stained with hematoxylin-eosin (H&E), Safranin O-Fast Green (SOFG), and Masson for tissue analysis.
2.11. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9.3 software. All data were presented as means ± standard deviation (SD). Student's t-test was utilized for analyzing two-group data, while one-way analysis of variance (ANOVA) with Tukey's post hoc test was used to analyze the multiple-group data. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Delayed fracture healing in diabetic mice
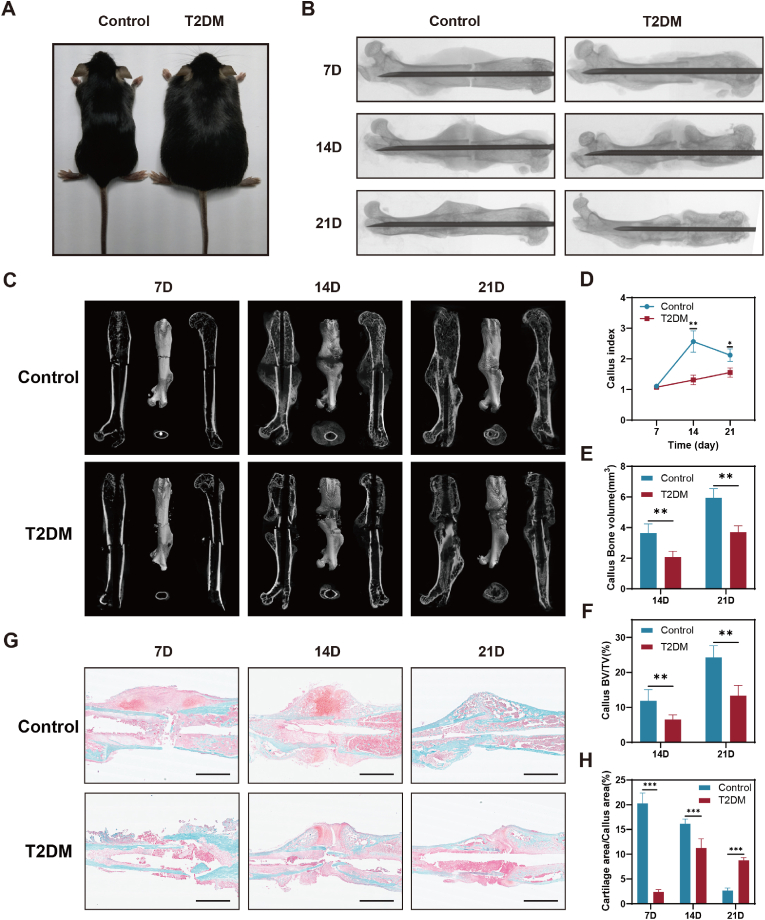
To testify whether fracture healing is delayed in diabetic mice, we selected two-month-old db/db mice and C57 BL/6 mice to establish femur fracture models (Fig. 1A). One commonly used method for monitoring the progress of fracture healing is through X-ray imaging. We observed that the T2DM group had significantly less bridging callus than the control group at the same time period after fracture surgery (Fig. 1B). By analyzing the 3D images of the femur, the woven bone formation was suppressed in the diabetes group compared to healthy controls (Fig. 1C and D). The results of quantitative statistical from micro-CT scanning revealed that the callus BV and callus BV/TV of diabetic mice were comparatively smaller than those of the control group at the same time point, as depicted in Fig. 1E and F. According to the micro-CT data, we observed that the bone mineral density (BMD) of calluses during the fracture healing process in T2DM mice was not significantly different from, or slightly higher than, that of the control (Fig. S1). Safranin O-fast green (SOFG) staining exhibited that cartilage formation and mineralization were delayed in diabetic mice (Fig. 1G and H). These findings suggest delayed fracture healing in diabetic mice.
Fig. 1.
Delayed fracture healing in diabetic mice. (A) Images of two-month-old control and diabetic mice. (B) Radiographs of the samples from control and diabetic mice at different days after surgery. (C) Micro-CT 3D images of the whole femur after fracture. (D) Quantitative statistics of callus formation at different time points post-fracture was conducted. n = 5. (E–F) A quantitative statistical analysis of the micro-CT results of the healing tissues was performed. n = 5. (G–H) SOFG staining and quantitative statistics of callus sections. Scale bar in all images: 2.5 mm. n = 5. *P < 0.05; **P < 0.01; ***P < 0.001.
3.2. The imbalance of macrophage polarization in diabetic fracture healing
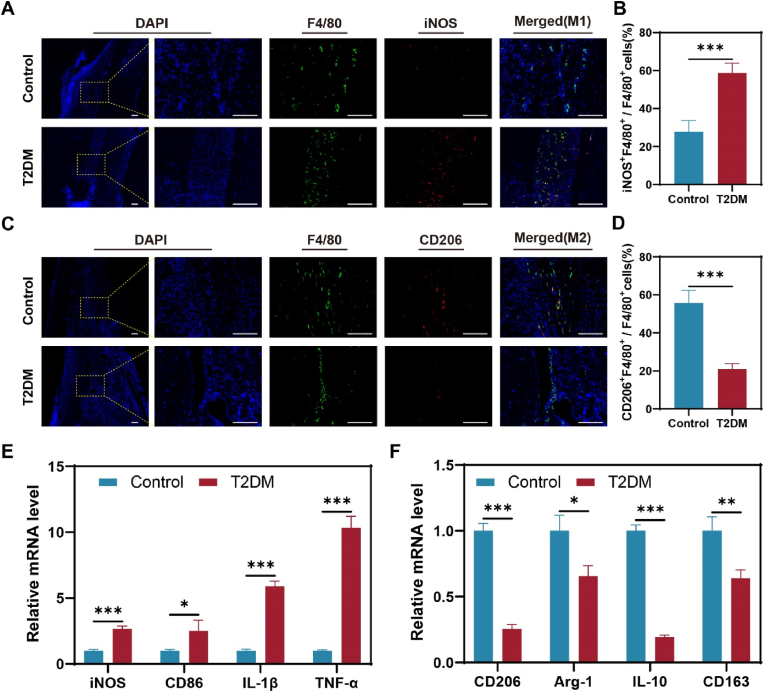
To investigate the cause of delayed fracture healing in diabetic mice, immunofluorescence staining was used to identify the macrophage phenotype at 7 days post-fracture (dpf). We conducted immunolocalization studies of F4/80, iNOS, and CD206 (Fig. 2A, C) and found that there were more F4/80+/iNOS+ (M1) macrophages in the diabetic mice at 7dpf than controls (Fig. 2A and B). However, M2 macrophages (F4/80+/CD206+) were significantly reduced at 7 dpf in diabetic mice compared to the control group (Fig. 2C and D). In addition, mRNA expression levels of M1 and M2 macrophage-related marker genes in callus were analyzed by PCR. The mRNA levels of iNOS, CD86, IL-1β, and TNF-α were significantly higher in diabetic mice, as shown in Fig. 2E. Conversely, the mRNA levels of CD206, Arg-1, IL-10, and CD163 were significantly lower in diabetic mice, as demonstrated in Fig. 2F. These results revealed the imbalance of macrophage polarization in diabetic fracture healing.
Fig. 2.
The imbalance of macrophage polarization in T2DM fracture healing. (A) Localization of M1 (F4/80+ and iNOS+) macrophages. (B) M1 (F4/80+/iNOS+) macrophage quantization. n = 5. (C) Localization of M2 (F4/80+ and CD206+) macrophages. (D) M2 (F4/80+ and CD206+) macrophage quantization. n = 5. (E) iNOS, CD86, IL-1β, and TNF-α mRNA level. n = 3. (F) CD206, Arg-1, IL-10, and CD163 mRNA level. n = 3. Scale bar in all images: 100 μm. *P < 0.05; **P < 0.01; ***P < 0.001.
3.3. Preparation and characterization of M2-exosomes
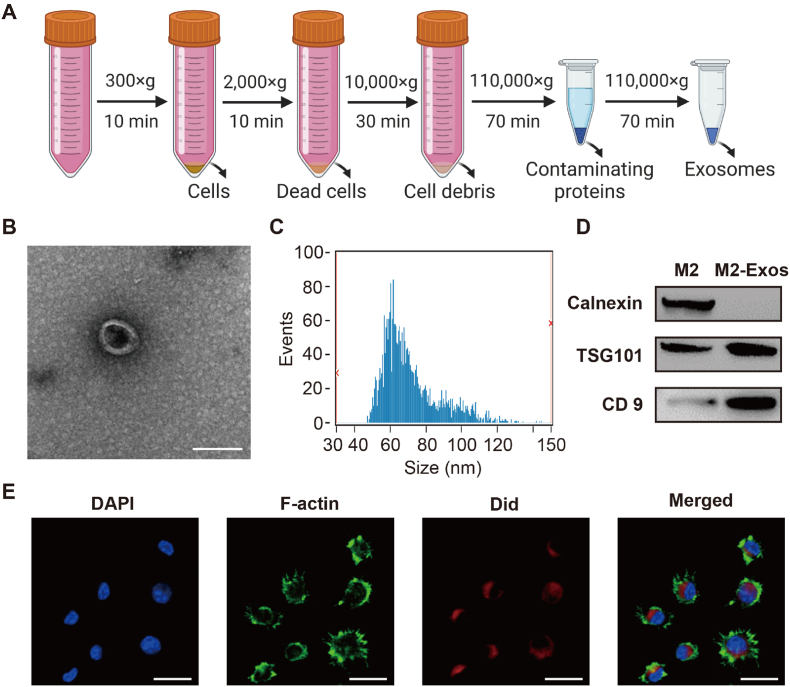
Next, we isolated hematopoietic precursor cells from the femur and tibia of mice. Then, they were treated with monocyte colony-stimulating factor (M-CSF) for a week to obtain uncommitted M0. We stimulated M0 with Th2 cytokines such as IL-4 to acquire the M2 macrophages we needed. To characterize M2 macrophage-derived exosomes, exosomes were obtained from the supernatant of M2 macrophages using ultracentrifugation (Fig. 3A). As shown in Fig. 3B, M2-exosomes showed spherical vesicles and bilayer lipid membranes under transmission electron microscopy (TEM). We measured the size distribution with nanoparticle tracking analysis (NTA) and found that the M2-exosomes were mainly between 50 and 150 nm (Fig. 3C). Subsequently, Western blotting showed the M2-exosomes expressed the TSG101and CD9 without the endoplasmic reticulum specific marker calnexin (Fig. 3D). To investigate the uptake of M2-exosomes by M1 macrophages, we labeled the M2-exosomes with Did and co-cultured them with M1 macrophages. Our results indicated that M1 macrophages were able to endocytose the M2-exosomes (Fig. 3E). Moreover, we also explored the uptake of M2-exosomes by BMSCs, and we found that BMSCs had a good phagocytosis of M2-exosomes (Fig. S2). Collectively, we successfully extracted M2-exosomes and found that M2-exosomes can be effectively taken up by M1 macrophages.
Fig. 3.
Preparation and characterization of M2-exosomes. (A) Schematic diagram of M2-exosomes isolation. (B) The morphology of the M2-exosomes was visualized with TEM. Scale bar: 100 nm. (C) The size distribution of M2-exosomes was examined with NTA. (D) Western blot experiments showing the protein levels of TSG101, CD9, and calnexin (E) Did-labeled M2-exosomes uptake by M1 macrophages was investigated with confocal microscopy. Scale bar: 20 μm.
3.4. M2-exosomes promote macrophage reprogramming and osteogenesis in vitro
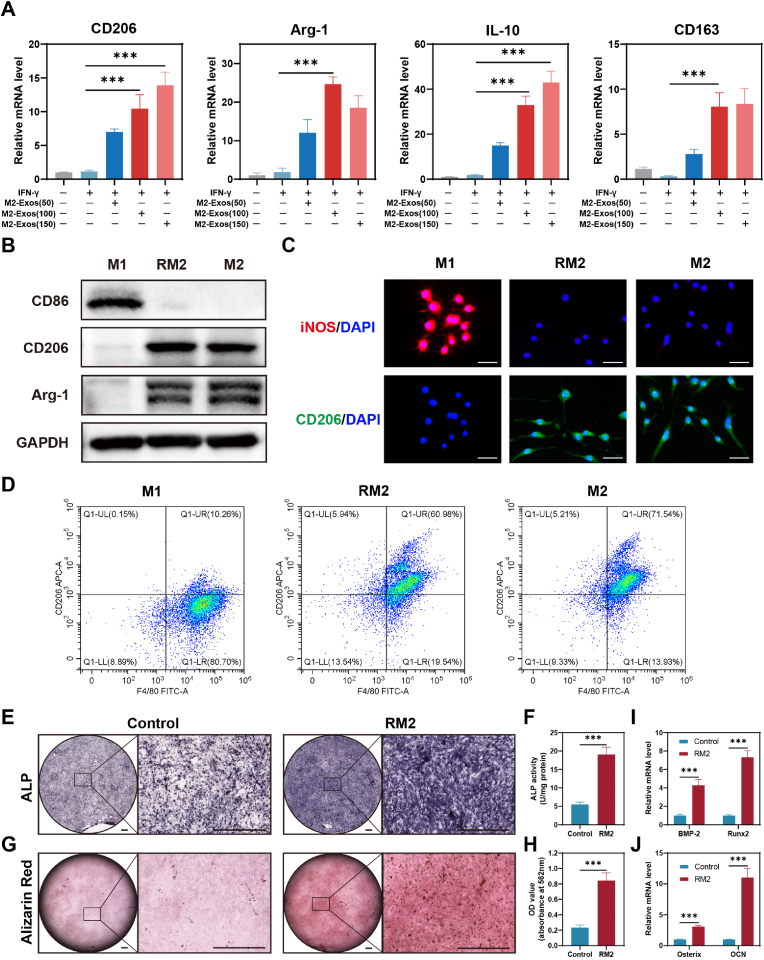
To investigate the optimal concentration of M2-exosomes to facilitate macrophage polarization (M1 to M2), we used three concentrations of M2-exosomes (50 μg/mL, 100 μg/mL, 150 μg/mL) to treat M1 macrophages. And the mRNA level of CD206, Arg-1, IL-10, and CD163 was increased after treatment with M2-exosomes (Fig. 4A), i.e., M1 repolarized into M2 macrophages, which we termed RM2 macrophages. Considering the economy, we finally chose 100 μg/mL concentration of M2-exosomes for the subsequent cell experiments. Subsequently, Western blotting and immunofluorescence analysis showed that RM2 could express M2 macrophage-specific marker proteins, such as CD206 and Arg-1 (Fig. 4B and C). We can also observe that RM2 exhibits spindle-shaped cells similar to those of M2 macrophages. In addition, to accurately quantify the extent to which M2-exosomes induce reprogramming from M1 to M2, we compared CD206 levels in M1, M2, and RM2 by using flow cytometry. Flow cytometry analysis showed similar levels of CD206 expression in RM2 and M2 macrophages (Fig. 4D).
Fig. 4.
M2-exosomes promote macrophage reprogramming and osteogenesis. (A) qRT-PCR analysis of the mRNA levels of CD206, Arg-1, IL-10, and CD163 in macrophages from M2-exosomes-treated groups. n = 3. (B) Western blot experiments show the protein level of the M1 and M2 macrophage markers, including CD86, CD206, and Arg-1. (C) Immunofluorescence analysis of the M1 and M2 macrophages markers, including iNOS and CD206. Scale bar: 50 μm. (D) M2 macrophages were co-located using F4/80 and CD206 and analyzed by FCM. (E) Representative images of ALP staining of BMSCs after 7 days of continuous induction of RM2 macrophages supernatant. Scale bars: 1000 μm. (F) ALP staining of BMSCs corresponding quantitative analysis, n = 3. (G) Representative picture of Alizarin Red S analysis of BMSCs cultured with RM2 macrophage supernatant for two weeks. Scale bars: 1000 μm. (H) ARS staining of BMSCs corresponding quantitative analysis, n = 3. (I–J) qRT-PCR analysis of mRNA levels of BMP-2, Runx2, Osterix and OCN in BMSCs of RM2 macrophages supernatant treatment group. ***P < 0.001.
Generally, the key link of fracture healing is osteogenic differentiation of BMSCs, and the differentiated osteoblasts play their role in bone regeneration and remodeling. Subsequently, we investigated the influence of reprogramming RM2 on bone formation in BMSCs. We co-incubated BMSCs with RM2 macrophage supernatant for 7 or 14 days and subsequently analyzed them by using ALP and ARS staining. The staining depth of ALP in RM2 group was higher than that in control, as evidenced by quantitative analysis showing that BMSCs in the RM2 group had higher ALP activity. (Fig. 4E and F). In addition, the qualitative and quantitative findings of the ARS assay were consistent with those of the ALP assay. Specifically, the RM2 group exhibited a greater number of bone-mineralized nodules compared to the control group, indicating superior osteogenic potential. (Fig. 4G and H). In addition, we detected and analyzed the mRNA levels of osteogenesis-related genes, including BMP-2, Runx2, Osterix, and OCN, using RT-qPCR. Our results, as depicted in Fig. 4I and J, showed elevated mRNA levels in the RM2 group compared to the control. These results revealed that the M2-exosomes possess the ability to induce macrophage reprogramming from M1 to M2 and that reprogramming RM2 macrophages facilitates osteogenic differentiation of BMSCs.
3.5. RNA sequencing revealed M2-exosomes promote macrophage polarization through PI3K/AKT signaling pathway
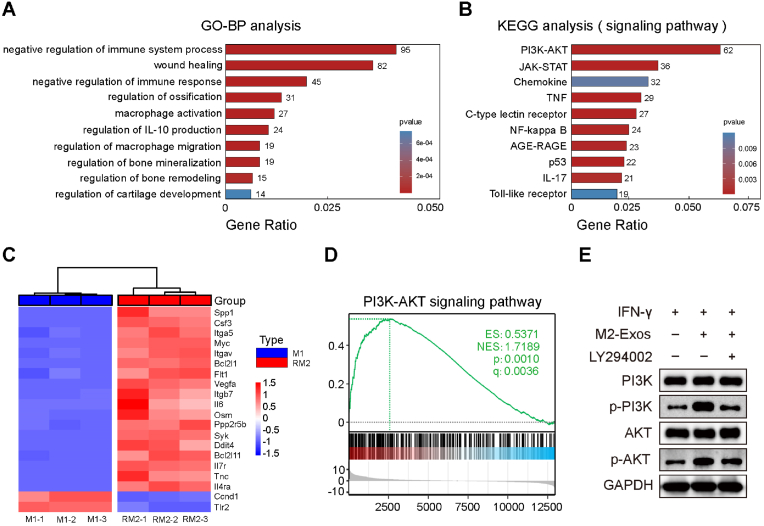
To investigate the molecular mechanism of M2-exosomes promote macrophage polarization from M1 to M2, we conducted RNA sequencing. We performed statistical analysis on genes that were differentially expressed by more than four-fold and were statistically significant, and presented them in the form of volcano plots (Fig. S3A). Our analysis revealed that a substantial number of genes exhibited significant differential expression in RM2 macrophages compared to M1 macrophages, with 747 genes being significantly down-regulated and 1531 genes being significantly up-regulated (Fig. S3B). The statistical analysis of gene ontology (GO) biological processes indicated that upregulated genes in RM2 macrophages were enriched in responses related to the immune system and wound healing. Differently expressed genes were enriched in regulation of bone mineralization and remodeling, etc. (Fig. 5A). Moreover, Kyoto encyclopedia of genes and genomes (KEGG) statistics showed that the addition of M2-exosomes affected PI3K/AKT, JAK-STAT, Chemokine, TNF signaling pathways (Fig. 5B). Among them, PI3K/AKT is associated with macrophage polarization toward M2. The relative levels of key genes in the PI3K/AKT signaling pathway are presented as a heatmap, such as Spp1, Csf3, Itga5 and Myc, were significantly increased in RM2 compared to M1 macrophages (Fig. 5C). Furthermore, signature genes for PI3K/AKT were upregulated in RM2 indicated by gene set enrichment analysis (GSEA) (Fig. 5D). LY294002 can permeabilize cells and specifically inhibit the PI3K/AKT. We found that M2-exosomes promote the phosphorylation of PI3K and AKT at the protein level. However, this stimulative effect can be partially suppressed with LY294002 (Fig. 5E). These results revealed that M2-exosomes promote the reprogramming of M1 to M2 by activating the PI3K/AKT pathway.
Fig. 5.
RNA sequencing revealed M2-exosomes promote macrophages polarization through PI3K/AKT signaling pathway. (A) GO biological process analysis of upregulated genes in RM2. (B) KEGG analysis of upregulated genes in RM2. (C) The heatmap presents the gene levels associated with the PI3K/AKT pathways of M1 and RM2. (D) GSEA showed a significant increase of PI3K/AKT pathway gene enrichment score in RM2. (E) Western blot experiments showing the protein levels of PI3K, p-PI3K, AKT, and p-AKT.
3.6. M2-exosomes promote diabetic fracture healing in vivo
To investigate whether M2-exosomes promote M1 reprogramming through activation of PI3K/AKT to accelerate diabetic fracture healing, we plan to conduct in vivo experiments. Firstly, the distribution of M2-exosomes in mice was assessed. Some studies have shown that the biological distribution of exosomes can be evaluated using fluorescence microscopic images [30]. However, callus tissue sections require 3 weeks of decalcification prior to sectioning, which can result in high susceptibility to fluorescence bursts. Therefore, we labeled the exosomes with Cy5 dye and used small animal imaging to indirectly evaluate the in vivo distribution of M2-exosomes. We injected Cy5-labeled M2-exosomes locally into the fracture gap of model mice. After 4 h, the mice were euthanized, and the distribution of exosomes in each organ was examined. The results indicated a significant enrichment of exosomes in the femur (Fig. S4).
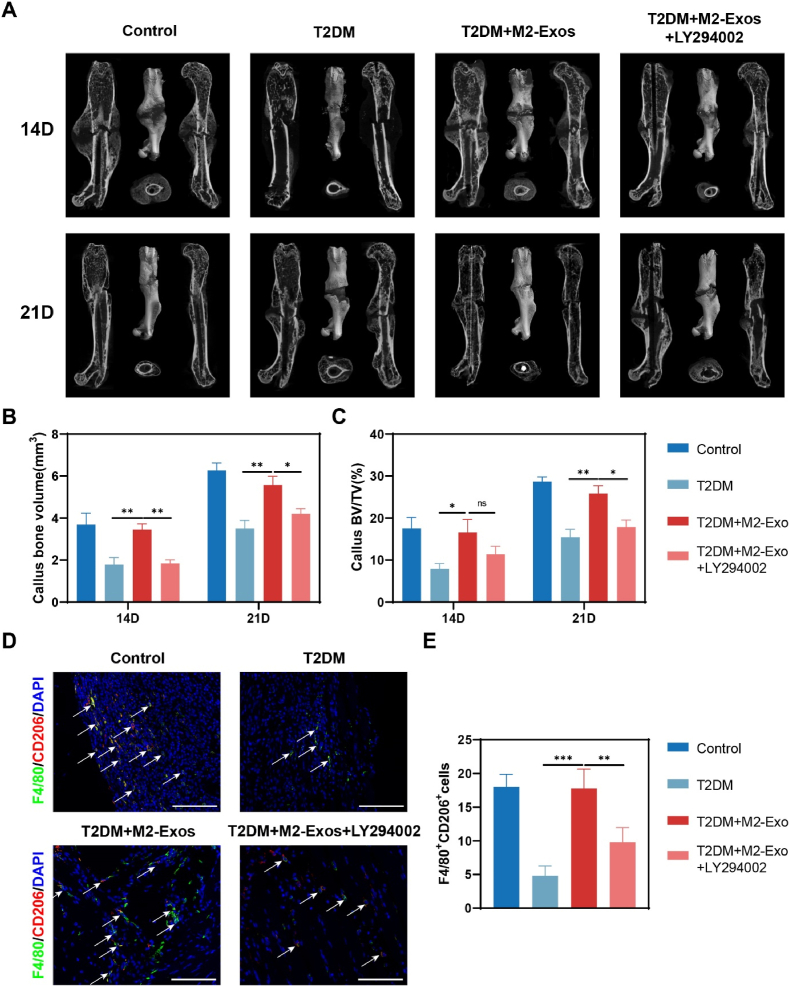
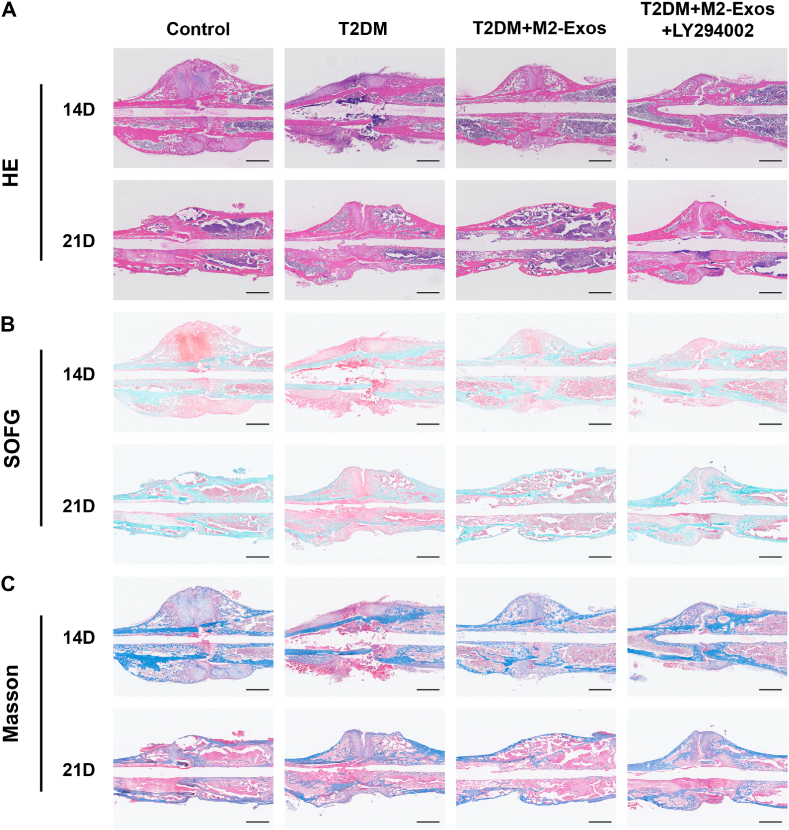
Following surgery, samples from different time points were analyzed using CT scans. The results of the scans indicated that diabetic mice treated with M2-exosomes had more calluses and a significantly reduced fracture gap compared to the diabetic groups. Furthermore, on day 21 after the fracture, the callus no longer had significant gaps in the M2-exosomes-treated diabetic mice, indicating that bone remodeling had occurred. However, the stimulative effects of M2-exosomes on T2DM mice were partially inhibited when mixed with LY294002 (Fig. 6A). The results of quantitative statistical analysis from micro-CT scanning revealed that the callus BV and callus BV/TV in M2-exosomes-treated diabetic mice were higher than those in diabetic mice on the same day after fracture surgery (Fig. 6B and C). However, there was no significant difference observed in bone mineral density (BMD) compared to controls after exosome intervention (Fig. S5). Furthermore, we evaluated the polarization of M2 macrophages in the femur through immunofluorescence on day 7 after surgery. The results showed a considerable rise in M2 macrophages in diabetic mice treated with M2-exosomes compared to diabetic controls (Fig. 6D and E). Histological staining further showed that cartilage area and bone formation were increased at the gap in diabetic mice treated with M2-exosomes, whereas the addition of PI3K/AKT signaling pathway inhibitors reduced this effect (Fig. 7A–C). In summary, our study indicated that M2-exosomes primarily facilitate macrophage reprogramming by stimulating the PI3K/AKT pathway, and LY294002 inhibits this process.
Fig. 6.
M2-exosomes promote diabetic fracture healing in vivo. (A) Micro-CT 3D images of the whole femur after fracture. (B–C) A quantitative statistical analysis of the micro-CT results of the healing tissues was performed. n = 5. (D) Immunofluorescence localization of M2 (F4/80+ and CD206+) macrophages. Scale bar in all images: 100 μm. (E) M2 (F4/80+ and CD206+) macrophage quantization. n = 5. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 7.
Histological staining analysis in vivo. HE, SOFG and Masson staining, of fracture areas in Control, T2DM, T2DM+M2-exosomes and T2DM+M2-exosomes+LY294002. Scale bar in all images: 2.5 mm.
4. Discussion
In the present study, we extracted exosomes from M2 macrophages, and in vitro experiments demonstrated that M2-exosomes modulated macrophages from M1 to M2 by targeting the PI3K/AKT pathway. In vivo experiments showed that M2-exosomes accelerated fracture healing in diabetic mice by inducing macrophage polarization. These findings suggested that M2-exosomes improve the osteoimmune microenvironment, thereby speeding up diabetic fracture healing.
Much evidence suggests that diabetes adversely affects the skeletal system [31]. The long-term accumulation of pro-inflammatory cytokines and advanced glycation end products (AGEs) severely disrupts immune microenvironmental homeostasis and impairs bone regeneration [32]. The immune microenvironment is a complex milieu comprising diverse immune cells, growth factors, extracellular matrix, and relevant signaling molecules [33]. These components play an important role in tissue regeneration and repair [34]. Immune cells are among the first to enter the damaged site during fracture healing. After the formation of a hematoma, a series of immune cells, including neutrophils, monocytes, and macrophages, infiltrate the area and secrete cytokines that modulate the proliferation and differentiation of BMSCs [35,36]. Different types of immune cells play distinct roles in the bone regeneration process. During the initial phase of inflammation, M1 macrophages activate the inflammatory response by releasing pro-inflammatory cytokines to clear debris from the damaged area [37]. Conversely, M2 macrophages accelerate bone regeneration by releasing growth factors that promote the proliferation and differentiation of osteoblasts [38,39]. Besides macrophages, T cells and B cells, which are other types of immune cells, also play a pivotal role in the process of bone regeneration [40]. Multiple studies have indicated that they can release specific cytokines and antibodies that promote osteoblast proliferation and differentiation [41]. In short, the immune microenvironment unleashes a crucial regulatory function in bone regeneration. Moreover, diabetes elevates the viscosity of the blood, increasing the likelihood of tissue hypoxia in patients and subsequently delaying the inflammatory response [42]. Persistent inflammation predisposes to impairment of fracture healing and bone regeneration, and the underlying mechanisms are not known. Shen et al. [20] found that tooth extraction sockets (TES) were accompanied by severe inflammation and delayed healing in diabetic mice. They demonstrated that imbalanced macrophage polarization impaired bone healing through disrupting paracrine signaling, leading to the interference of osteogenesis and angiogenesis in the bone microenvironment. Hence, macrophage polarization appears to be a crucial factor affecting bone regeneration.
Many studies have revealed the relationship between macrophage polarization and fracture healing. For example, Zhang et al. [43] showed that the addition of IL-4 to hydrogel beads can induce M2 polarization and improve the osteogenic differentiation ability of BMSCs. Liu et al. [44] developed an engineered exosomes containing miRNA-181b to investigate its effect on bone regeneration. Their study showed that miRNA-181b facilitated M2 macrophage polarization, suppressed inflammation, and promoted osteointegration in vivo. Therefore, there is consensus to regulate macrophage polarization towards M2 in bone healing. However, M1 macrophages perform an indispensable role in the hematoma-inflammatory stage of fracture healing, and it has been suggested that depletion of M1 macrophages during the early stages of bone healing may lead to delayed fracture healing [45,46]. Compared with previous studies, we paid more attention to the ratio of M1 and M2 macrophages in the bone microenvironment. Our study shows that M2-exosomes can effectively reprogram M1 macrophages, and we have uncovered the molecular mechanism underlying this process through many experiments. These findings highlight the potential of M2-exosomes as a therapeutic strategy to regulate macrophage polarization in bone healing. Furthermore, we also demonstrated that reprogrammed M2 can facilitate osteogenic differentiation of BMSCs and accelerate bone regeneration.
Exosomes have gained significant attention in fracture healing research in recent years due to their distinct advantages [47]. For instance, it has been found that BMDM-derived exosomes transferred into BMSCs and delayed fracture healing [48]. Xiong et al. [28] demonstrated that macrophage-derived exosomes targeted SIK2 and SIK3 genes to enhance osteogenesis. These studies indicated that exosomes have great potential to accelerate bone regeneration by regulating osteogenic differentiation of BMSCs in the bone microenvironment. Moreover, it has also been reported that exosomes acting as immunosuppressants could effectively improve fracture repair [49]. M2-exosomes activate the IL-10/IL-10R pathway by delivering IL-10 mRNA, thereby regulating the differentiation of osteoblast progenitors and osteoclast progenitors [50]. To investigate the regulatory role and function of M2 macrophage-derived exosomes in diabetic fracture healing, we extracted them and found that they can target the PI3K/AKT pathway in M1 macrophages, thereby upregulating the anti-inflammatory response and stimulating the reprogramming of M1 macrophages into M2 macrophages in vitro. Additionally, our in vivo studies have demonstrated that M2 exosomes can reshape the disrupted osteoimmune microenvironment and improve the delayed healing of diabetic fractures.
While we have elucidated the mechanism by which M2 exosomes improve the diabetic osteoimmune microenvironment, our research has certain limitations. Specifically, it remains unclear which constituents of M2 exosomes are responsible for stimulating the PI3K/AKT pathway in M1 macrophages. Additional research is necessary to determine the role of specific miRNAs or proteins in the process of macrophage reprogramming. In addition, diabetic mice are overweight, which tends to compress the fracture ends and cause fracture instability, thus preventing fracture healing. However, effective methods to avoid the deviation associated with this instability have not been established in diabetic animal fracture models. Finally, the current capacity of M2 macrophages to generate exosomes is insufficient for clinical use, and further research is needed to improve their exosome production and regenerative capabilities.
5. Conclusions
To sum up, we investigated the trend profile of bone repair capacity and macrophage polarization in diabetic fracture healing. Our findings suggested that bone repair was significantly delayed in the diabetic fracture model due to an imbalance in the osteoimmune microenvironment, characterized by an abnormal increase in M1 and a decrease in M2 macrophages. The results showed that M2-exosomes, as an immunomodulator, could promote macrophage transformation from M1 to M2 by targeting the PI3K/AKT pathway. Our study provides a fresh perspective and a potential therapeutic approach for M2-exosomes to improve the diabetic osteoimmune microenvironment.
Ethics approval and consent to participate
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shanghai University and approved by the Animal Ethics Committee of Shanghai University.
CRediT authorship contribution statement
Yili Wang: Conceptualization, Data curation, Writing – original draft. Qiushui Lin: Investigation, Data curation, Writing – original draft. Hao Zhang: Investigation, and, Formal analysis. Sicheng Wang: Visualization. Jin Cui: Technical assistance. Yan Hu: Data collection. Jinlong Liu: Formal analysis. Mengmeng Li: Visualization. Kun Zhang: Investigation, and, Resources. Fengjin Zhou: Supervision, and, Validation. Yingying Jing: Supervision, and, Validation. Zhen Geng: Conceptualization, revised the manuscript. Jiacan Su: Conceptualization, Supervision, and revised the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303), Key Project of the National Natural Science Foundation of China (82230071) and National Natural Science Foundation of China (32101084, 82202344). We thank Shanghai OE Biotech to perform the RNA sequencing.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.05.018.
Contributor Information
Fengjin Zhou, Email: dr.zhoufj@163.com.
Yingying Jing, Email: jingy4172@shu.edu.cn.
Zhen Geng, Email: nanboshan1987@163.com.
Jiacan Su, Email: drsujiacan@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Inaishi J., Saisho Y. Beta-cell mass in obesity and type 2 diabetes, and its relation to pancreas fat: a mini-review. Nutrients. 2020;12(12) doi: 10.3390/nu12123846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shu L., Beier E., Sheu T., Zhang H., Zuscik M.J., Puzas E.J., Boyce B.F., Mooney R.A., Xing L. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif. Tissue Int. 2015;96(4):313–323. doi: 10.1007/s00223-015-9954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecka-Czernik B., Stechschulte L.A., Czernik P.J., Dowling A.R. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol. Cell. Endocrinol. 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Hunt H.B., Miller N.A., Hemmerling K.J., Koga M., Lopez K.A., Taylor E.A., Sellmeyer D.E., Moseley K.F., Donnelly E. Bone tissue composition in postmenopausal women varies with glycemic control from normal glucose tolerance to type 2 diabetes mellitus. J. Bone Miner. Res. 2021;36(2):334–346. doi: 10.1002/jbmr.4186. [DOI] [PubMed] [Google Scholar]
- 5.Russo V., Chen R., Armamento-Villareal R. Hypogonadism, type-2 diabetes mellitus, and bone health: a narrative review. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.607240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koromani F., Ghatan S., van Hoek M., Zillikens M.C., Oei E.H.G., Rivadeneira F., Oei L. Type 2 diabetes mellitus and vertebral fracture risk. Curr. Osteoporos. Rep. 2021;19(1):50–57. doi: 10.1007/s11914-020-00646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown M.L., Yukata K., Farnsworth C.W., Chen D.G., Awad H., Hilton M.J., O'Keefe R.J., Xing L., Mooney R.A., Zuscik M.J. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Zhang H., Hu Y., Jing Y., Geng Z., Su J. Bone repair biomaterials: a perspective from immunomodulation. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 9.Nakagawa M., Karim M.R., Izawa T., Kuwamura M., Yamate J. Immunophenotypical characterization of M1/M2 macrophages and lymphocytes in cisplatin-induced rat progressive renal fibrosis. Cells. 2021;10(2) doi: 10.3390/cells10020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahney C.S., Zondervan R.L., Allison P., Theologis A., Ashley J.W., Ahn J., Miclau T., Marcucio R.S., Hankenson K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019;37(1):35–50. doi: 10.1002/jor.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 12.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batoon L., Millard S.M., Raggatt L.J., Pettit A.R. Osteomacs and bone regeneration. Curr. Osteoporos. Rep. 2017;15(4):385–395. doi: 10.1007/s11914-017-0384-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B., Han F., Wang Y., Sun Y., Zhang M., Yu X., Qin C., Zhang H., Wu C. Cells-micropatterning biomaterials for immune activation and bone regeneration. Adv. Sci. 2022;9(18) doi: 10.1002/advs.202200670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren X., Chen X., Geng Z., Su J. Bone-targeted biomaterials: strategies and applications. Chem. Eng. J. 2022;446 [Google Scholar]
- 16.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012;8(3):133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Bose T., Unger R.E., Jansen J.A., Kirkpatrick C.J., van den Beucken J. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017;369(2):273–286. doi: 10.1007/s00441-017-2598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guihard P., Danger Y., Brounais B., David E., Brion R., Delecrin J., Richards C.D., Chevalier S., Redini F., Heymann D., Gascan H., Blanchard F. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cell. 2012;30(4):762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 19.Pajarinen J., Lin T., Gibon E., Kohno Y., Maruyama M., Nathan K., Lu L., Yao Z., Goodman S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen X., Shen X., Li B., Zhu W., Fu Y., Xu R., Du Y., Cheng J., Jiang H. Abnormal macrophage polarization impedes the healing of diabetes-associated tooth sockets. Bone. 2021;143 doi: 10.1016/j.bone.2020.115618. [DOI] [PubMed] [Google Scholar]
- 21.Sun X., Ma Z., Zhao X., Jin W., Zhang C., Ma J., Qiang L., Wang W., Deng Q., Yang H., Zhao J., Liang Q., Zhou X., Li T., Wang J. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2021;6(3):757–769. doi: 10.1016/j.bioactmat.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J., Wang F., Hu Y., Luo Y., Wei Y., Xu K., Zhang H., Liu H., Bo L., Lv S., Sheng S., Zhuang X., Zhang T., Xu C., Chen X., Su J. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023;4(1) doi: 10.1016/j.xcrm.2022.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H., Zhang H., Wang S., Cui J., Weng W., Liu X., Tang H., Hu Y., Li X., Zhang K., Zhou F., Jing Y., Su J. Bone-targeted bioengineered bacterial extracellular vesicles delivering siRNA to ameliorate osteoporosis. Compos. B Eng. 2023;255 [Google Scholar]
- 24.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi B., Chen L., Xiong Y., Yang Y., Panayi A.C., Xue H., Hu Y., Yan C., Hu L., Xie X., Lin Z., Zhou W., Cao F., Xiao X., Feng Q., Liu G. Osteoblast/osteoclast and immune cocktail therapy of an exosome/drug delivery multifunctional hydrogel accelerates fracture repair. ACS Nano. 2022;16(1):771–782. doi: 10.1021/acsnano.1c08284. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y., Li X., Zhang Q., Gu Z., Luo Y., Guo J., Wang X., Jing Y., Chen X., Su J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact. Mater. 2021;6(9):2905–2913. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H., Wang S.Y., Kwak G., Yang Y., Kwon I.C., Kim S.H. Exosome-Guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv. Sci. 2019;6(20) doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y., Chen L., Yan C., Zhou W., Yu T., Sun Y., Cao F., Xue H., Hu Y., Chen D., Mi B., Liu G. M2 Macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J. Nanobiotechnol. 2020;18(1):66. doi: 10.1186/s12951-020-00622-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Ma L., Li G., Lei J., Song Y., Feng X., Tan L., Luo R., Liao Z., Shi Y., Zhang W., Liu X., Sheng W., Wu S., Yang C. Nanotopography sequentially mediates human mesenchymal stem cell-derived small extracellular vesicles for enhancing osteogenesis. ACS Nano. 2022;16(1):415–430. doi: 10.1021/acsnano.1c07150. [DOI] [PubMed] [Google Scholar]
- 30.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20(6):4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 31.Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L., Bone I.O.F., Diabetes Working G. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13(4):208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 32.Rubin M.R., Patsch J.M. Assessment of bone turnover and bone quality in type 2 diabetic bone disease: current concepts and future directions. Bone Res. 2016;4 doi: 10.1038/boneres.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong Y., Mi B.B., Lin Z., Hu Y.Q., Yu L., Zha K.K., Panayi A.C., Yu T., Chen L., Liu Z.P., Patel A., Feng Q., Zhou S.H., Liu G.H. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9(1):65. doi: 10.1186/s40779-022-00426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Y., Lin Z., Bu P., Yu T., Endo Y., Zhou W., Sun Y., Cao F., Dai G., Hu Y., Lu L., Chen L., Cheng P., Zha K., Shahbazi M.A., Feng Q., Mi B., Liu G. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv. Mater. 2023 doi: 10.1002/adma.202212300. [DOI] [PubMed] [Google Scholar]
- 35.Chang M.K., Raggatt L.J., Alexander K.A., Kuliwaba J.S., Fazzalari N.L., Schroder K., Maylin E.R., Ripoll V.M., Hume D.A., Pettit A.R. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 36.Wu A.C., Raggatt L.J., Alexander K.A., Pettit A.R. Unraveling macrophage contributions to bone repair. BoneKEy Rep. 2013;2:373. doi: 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiller K.L., Anfang R.R., Spiller K.J., Ng J., Nakazawa K.R., Daulton J.W., Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M., Hou Q., Zhong L., Zhao Y., Fu X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.681710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arabpour M., Saghazadeh A., Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharm. 2021;97 doi: 10.1016/j.intimp.2021.107823. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Wang R., Wang G., Zhang B., Wang C., Li D., Ding C., Wei Q., Fan Z., Tang H., Ji F. Single-cell RNA sequencing reveals B cells are important regulators in fracture healing. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.666140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer V., Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022;123:14–21. doi: 10.1016/j.semcdb.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Gerber P.A., Rutter G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxidants Redox Signal. 2017;26(10):501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J., Shi H., Zhang N., Hu L., Jing W., Pan J. Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-beta1/Smad pathway for repair of bone defect. Cell Prolif. 2020;53(10) doi: 10.1111/cpr.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W., Yu M., Chen F., Wang L., Ye C., Chen Q., Zhu Q., Xie D., Shao M., Yang L. A novel delivery nanobiotechnology: engineered miR-181b exosomes improved osteointegration by regulating macrophage polarization. J. Nanobiotechnol. 2021;19(1):269. doi: 10.1186/s12951-021-01015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hozain S., Cottrell J. CDllb+ targeted depletion of macrophages negatively affects bone fracture healing. Bone. 2020;138 doi: 10.1016/j.bone.2020.115479. [DOI] [PubMed] [Google Scholar]
- 46.Gu Q., Yang H., Shi Q. Macrophages and bone inflammation. J. Orthop. Translat. 2017;10:86–93. doi: 10.1016/j.jot.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Li M., Zhang T., Liu X., Zhang H., Geng Z., Su J. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem. Eng. J. 2022;450 [Google Scholar]
- 48.Zhang D., Wu Y., Li Z., Chen H., Huang S., Jian C., Yu A. MiR-144-5p, an exosomal miRNA from bone marrow-derived macrophage in type 2 diabetes, impairs bone fracture healing via targeting Smad1. J. Nanobiotechnol. 2021;19(1):226. doi: 10.1186/s12951-021-00964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z., Xiong Y., Meng W., Hu Y., Chen L., Chen L., Xue H., Panayi A.C., Zhou W., Sun Y., Cao F., Liu G., Hu L., Yan C., Xie X., Lin C., Cai K., Feng Q., Mi B., Liu G. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact. Mater. 2022;13:300–311. doi: 10.1016/j.bioactmat.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., Wan Z., Yang L., Song S., Fu Z., Tang K., Chen L., Song Y. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J. Nanobiotechnol. 2022;20(1):110. doi: 10.1186/s12951-022-01314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.