Abstract
Background
Vaccination is one of the most effective measures to prevent influenza illness and its complications; influenza vaccination remained important during the COVID-19 pandemic to prevent additional burden on health systems strained by COVID-19 demand.
Objectives
We describe policies, coverage, and progress of seasonal influenza vaccination programs in the Americas during 2019–2021 and discuss challenges in monitoring and maintaining influenza vaccination coverage among target groups during the COVID-19 pandemic.
Methods
We used data on influenza vaccination policies and vaccination coverage reported by countries/territories via the electronic Joint Reporting Form on Immunization (eJRF) for 2019–2021. We also summarized country vaccination strategies shared with PAHO.
Results
As of 2021, 39 (89 %) out of 44 reporting countries/territories in the Americas had policies for seasonal influenza vaccination. Countries/territories adapted health services and immunization delivery strategies using innovative approaches, such as new vaccination sites and expanded schedules, to ensure continuation of influenza vaccination during the COVID-19 pandemic. However, among countries/territories that reported data to eJRF in both 2019 and 2021, median coverage decreased; the percentage point decrease was 21 % (IQR = 0–38 %; n = 13) for healthcare workers, 10 % (IQR = -1.5–38 %; n = 12) for older adults, 21 % (IQR = 5–31 %; n = 13) for pregnant women, 13 % (IQR = 4.8–20.8 %; n = 8) for persons with chronic diseases, and 9 % (IQR = 3–27 %; n = 15) for children.
Conclusions
Countries/territories in the Americas successfully adapted influenza vaccination delivery to continue vaccination services during the COVID-19 pandemic; however, reported influenza vaccination coverage decreased from 2019 to 2021. Reversing declines in vaccination will necessitate strategic approaches that prioritize sustainable vaccination programs across the life course. Efforts should be made to improve the completeness and quality of administrative coverage data. Lessons learned from COVID-19 vaccination, such as the rapid development of electronic vaccination registries and digital certificates, might facilitate advances in coverage estimation.
Keywords: Influenza, Vaccination, Vaccine coverage, Americas, Caribbean
1. Introduction
The World Health Organization (WHO) estimates that each year, influenza causes 1 billion infections, including 3–5 million severe illnesses, and 290,000–650,000 influenza-related deaths globally [1], [2]. In the Americas, home to approximately 1 billion residents, an estimated 716,000–829,000 influenza-associated respiratory hospitalizations and 41,007–71,710 influenza-associated deaths occur every year, imposing a heavy burden on the health systems of the countries in the region [2], [3]. Vaccination is one of the most effective measures to prevent influenza illness and its complications; safe and effective influenza vaccines are available and are routinely used globally [4]. In 2004, the Pan American Health Organization (PAHO) Technical Advisory Group (TAG) first recommended that all countries establish an influenza vaccination policy that prioritizes groups at high risk for severe influenza illness, including children 6–23 months of age, pregnant women, individuals with underlying medical conditions, older adults, and healthcare workers [5]. Additionally, guidance from the WHO Strategic Advisory Group of Experts (SAGE), released in 2012 and updated in 2022, recommended the following target groups to be prioritized for vaccination: healthcare workers, individuals with underlying medical conditions, older adults, and pregnant women, followed by young children and persons in congregate living settings [4], [6].
In December 2019, WHO first reported the emergence of a novel coronavirus, SARS-CoV-2, that causes coronavirus disease 2019 (COVID-19). As a primarily respiratory disease, COVID-19 causes severe illness among similar groups at high risk for severe influenza infection, and therefore the highest priority groups for COVID-19 and influenza vaccination are similar [7], [8]. Influenza vaccination remained important during the COVID-19 pandemic to prevent additional burden on health systems strained by COVID-19 demand, particularly by averting influenza-associated cases, medical visits, hospitalizations, and deaths among groups at high risk for both illnesses. In March 2020, PAHO recommended the continued prioritization of influenza vaccination for healthcare workers, older adults, people with chronic diseases, and pregnant women during the COVID-19 pandemic [9]. Similarly, in September 2020, WHO SAGE defined healthcare workers and older adults as the highest priority risk groups for seasonal influenza vaccination during COVID-19 pandemic [10].
In this article, we describe policies, coverage, and the progress of seasonal influenza vaccination programs in the Americas during 2019–2021 and discuss challenges in monitoring and maintaining influenza vaccination coverage among target groups during the COVID-19 pandemic. We also describe the advances of countries in the Region in measuring influenza and COVID-19 vaccine effectiveness.
2. Methods
We used data on influenza vaccination policies and vaccination coverage reported by countries and territories to PAHO-WHO and the United Nations Children’s Fund (UNICEF) via the electronic Joint Reporting Form on Immunization (eJRF) for 2019–2021 [11]. These data are published annually by PAHO in publicly accessible “Immunization in the Americas” brochures [12]. Coverage rates are expressed as a percentage of the corresponding mid-year population, as reported by the country. Influenza vaccination coverage estimates correspond to a complete annual immunization schedule (i.e., two doses among vaccine-naive children and a single dose among adults and previously vaccinated children). For calculation of the change in reported coverage from 2019 to 2021, we used data from countries/territories that reported coverage data to eJRF for a particular vaccination target group in both 2019 and 2021. Values of >100 % coverage were set as 100 % for all calculations. If data on influenza vaccination policies were not reported for 2021, the most recently reported policies were used (n = 4 countries/territories); additionally, in some instances, PAHO country immunization focal points were contacted individually by email to confirm policy details. Additionally, we summarized country vaccination strategies shared with PAHO and published in public reports.
3. Results
3.1. Current policies and vaccination target groups in the Americas
As of 2021, a total of 39 (89 %) out of 44 reporting countries/territories in the Americas have policies in place for seasonal influenza vaccination (Table 1 ). Healthcare workers are targeted for vaccination in all 39 countries/territories reporting an existing influenza vaccination policy (Table 1). Additionally, older adults are targeted for vaccination in 38 (97 %) countries/territories with a policy, pregnant women in 35 (90 %), persons with chronic diseases in 35 (90 %), and children in 30 (77 %). Among countries vaccinating older adults, eligible ages include older than 60 years (n = 16; 42 %), 65 years (n = 15; 39 %), and other cut-offs (n = 7; 18 %) (Table 2 ). Among countries vaccinating children, eligible ages include 6 months–2 years (n = 6, 19 %), 6 months–3 years (n = 6, 19 %), 6 months–5 years (n = 10, 32 %), and other cut-offs (n = 8; 26 %). The greatest policy changes since 2014 have occurred among the target group of persons with chronic diseases, with six additional countries introducing policies for vaccinating this target group between 2014 and 2021 [13].
Table 1.
Countries/territories in the Americas with policies for seasonal influenza vaccination, 2021. [Source: electronic Joint Report Form (eJRF) country reports to PAHO/WHO-UNICEF].
| Vaccination policy details | No. countries with policy | Percentage (%) |
|---|---|---|
| Any influenza vaccination policy* | 39 | 89 % |
| Vaccination target groups† | ||
| Healthcare workers | 39 | 100 % |
| Older adults | 38 | 97 % |
| Pregnant women | 35 | 90 % |
| Persons with chronic diseases | 35 | 90 % |
| Children | 30 | 77 % |
| Vaccine formulation used† | ||
| Northern Hemisphere | 25 | 64 % |
| Southern Hemisphere | 14 | 36 % |
*Percentage among 44 reporting countries/territories in the Americas. An additional seven countries/territories in the Americas region did not report to eJRF (Bonaire, French Guiana, Guadeloupe, Martinique, Puerto Rico, Saba, and St. Eustatius.).
†Among 39 countries reporting any seasonal influenza vaccination policy.
TABLE 2.
Reported influenza vaccination policies1 and coverage estimates2, by target group and country/territory in the Americas, 2019–2021. [Source: electronic Joint Report Form (eJRF) reports to PAHO/WHO-UNICEF and country reports].
| Country |
Health workers |
Older adults |
Pregnant persons |
Persons with chronic diseases |
Children |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Policy | 2019 coverage (%) | 2020 coverage (%) | 2021 coverage (%) | Policy | Eligible age group | 2019 coverage (%) | 2020 coverage (%) | 2021 coverage (%) | Policy | 2019 coverage (%) | 2020 coverage (%) | 2021 coverage (%) | Policy | 2019 coverage (%) | 2020 coverage (%) | 2021 coverage (%) | Policy | Eligible age group | 2019 coverage (%) | 2020 coverage (%) | 2021 coverage (%) | |
| Anguilla | ✓ | – | – | 2 | ✓ | >65y | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Antigua and Barbuda | ✓ | – | – | – | ✓ | >60y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | 6 m-<3y | – | – | – |
| Argentina | ✓ | 100+ | 100+ | 62 | ✓ | ≥65y | – | 71 | 54 | ✓ | 75 | 77 | 54 | ✓ | 52 | 68 | 41 | ✓ | 6 m-<2y | 75 | 69 | 42 |
| Aruba | ✓ | – | – | – | ✓ | >60y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | 6 m-<2y | – | – | – |
| Bahamas | ✓ | – | – | – | ✓ | ≥65y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | ≥6m | – | – | – |
| Barbados | ✓ | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||||
| Belize | ✓ | 46 | 12 | 3 | ✓ | ≥65y | 36 | 6 | 2 | ✓ | 72 | 21 | 6 | – | – | – | ✓ | 6 m-<3y | 83 | 54 | 85 | |
| Bermuda | ✓ | 1 | – | – | ✓ | >65y | 12 | 12 | – | ✓ | 10 | 11 | – | ✓ | 2 | – | – | ✓ | 6 m-18y | 17 | 13 | – |
| Bolivia | ✓ | 100+ | 64 | 21 | ✓ | ≥60y | 75 | 59 | 25 | ✓ | 96 | 83 | 36 | ✓ | 100+ | 55 | 65 | ✓ | 6 m-<2y | 80 | 61 | 28 |
| Brazil | ✓ | 91 | 100+ | – | ✓ | ≥60y | 99 | 96 | – | ✓ | 85 | 77 | – | ✓ | 88 | – | – | ✓ | 6 m-<5y | 85 | 67 | – |
| British Virgin Islands | ✓ | 2 | 5 | – | ✓ | ≥75y | – | – | – | ✓ | – | – | – | ✓ | 78 | 44 | – | – | – | – | – | |
| Canada | ✓ | – | – | – | ✓ | ≥65y | 70 | 70 | 70 | ✓ | – | 45 | 45 | ✓ | – | – | – | ✓ | 6 m-<5y | – | – | – |
| Cayman Islands | ✓ | – | – | – | ✓ | ≥60y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | ≥6m | – | – | – |
| Chile | ✓ | 97 | 100+ | 100+ | ✓ | ≥65y | 68 | 85 | 72 | ✓ | 94 | 75 | 58 | ✓ | 94 | 100+ | 78 | ✓ | 6 m-<5y | 74 | 78 | 72 |
| Colombia | ✓ | – | – | – | ✓ | ≥60y | 72 | 89 | – | ✓ | 70 | 86 | – | ✓ | – | – | – | ✓ | 6 m-<2y | 68 | 74 | 63 |
| Costa Rica | ✓ | – | – | 88 | ✓ | >58y | – | – | – | ✓ | – | – | 69 | ✓ | – | – | – | ✓ | 6 m-<7y | – | – | 66 |
| Cuba | ✓ | 99 | 93 | 100 | ✓ | ≥75y | 86 | 100 | – | ✓ | – | 75 | 82 | ✓ | 85 | 87 | 70 | ✓ | 6 m-<2y | 82 | 56 | 92 |
| Curaçao | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Dominica | ✓ | 92 | – | – | ✓ | ≥65y | 5 | – | – | ✓ | 20 | – | – | ✓ | – | – | – | ✓ | 6 m-<3y | 1 | – | – |
| Dominican Republic | ✓ | – | 31 | 51 | ✓ | ≥65y | – | 8 | 79 | ✓ | – | 9 | 70 | ✓ | – | 58 | – | ✓ | 6 m-<2y | – | 7 | 13 |
| Ecuador | ✓ | 94 | 94 | 83 | ✓ | >65y | 76 | 76 | 6 | ✓ | 56 | 75 | 25 | ✓ | 100+ | 100+ | 12 | ✓ | 6 m-<5y | 99 | 88 | 62 |
| El Salvador | ✓ | 100+ | 100+ | 100 | ✓ | ≥60y | 42 | 37 | 45 | ✓ | 48 | 49 | 60 | ✓ | – | – | – | ✓ | 6 m-<5y | 57 | 39 | 54 |
| Grenada | ✓ | – | – | – | ✓ | >65y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | 6 m-<5y | – | – | – |
| Guatemala | ✓ | – | 47 | 32 | ✓ | ≥60y | – | – | 3 | ✓ | – | 16 | 10 | ✓ | – | – | 25 | ✓ | 6 m-<3y | 88 | 10 | 4 |
| Guyana | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Haiti | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Honduras | ✓ | 85 | 82 | 60 | ✓ | ≥60y | 68 | 56 | 45 | ✓ | 85 | 84 | 63 | ✓ | 100+ | 100+ | 100 | ✓ | 6 m-<1y | 57 | 53 | 47 |
| Jamaica | ✓ | 23 | 18 | 2 | ✓ | ≥65y | 25 | 20 | – | ✓ | – | 7 | – | ✓ | – | – | – | ✓ | 6 m-<18y | 6 | 1 | 0.1 |
| Mexico | ✓ | 100+ | 100+ | 100 | ✓ | ≥60y | 94 | 94 | 95 | ✓ | 81 | 78 | 88 | ✓ | 100 | 100+ | 94 | ✓ | 6 m-<3y | 91 | 87 | 82 |
| Montserrat | ✓ | – | – | – | ✓ | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Nicaragua | ✓ | 96 | 100 | 100 | ✓ | – | – | – | 96 | ✓ | 98 | 99 | 89 | ✓ | 100+ | 100 | 99 | – | – | – | – | |
| Panama | ✓ | 95 | 89 | 42 | ✓ | ≥60y | 83 | 99 | 18 | ✓ | 63 | 73 | 58 | ✓ | – | – | 100 | ✓ | 6 m-<5y | 71 | 61 | 57 |
| Paraguay | ✓ | – | – | – | ✓ | ≥60y | 30 | 47 | 21 | ✓ | 31 | 16 | 3 | ✓ | – | – | – | ✓ | 6 m-<3y | 32 | 23 | 29 |
| Peru | ✓ | – | – | – | ✓ | >60y | 47 | 69 | 36 | ✓ | 37 | 44 | 18 | ✓ | – | – | – | ✓ | 6 m-<5y | 58 | 81 | 72 |
| Saint Kitts and Nevis | ✓ | – | – | – | ✓ | ≥18y | – | – | – | ✓ | 7 | – | – | ✓ | – | – | – | – | – | – | – | |
| Saint Lucia | ✓ | – | – | – | ✓ | ≥60y | – | – | – | ✓ | – | – | 1 | ✓ | – | – | – | – | – | – | – | |
| Saint Vincent and the Grenadines | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Sint Maarten | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Suriname | ✓ | – | – | – | ✓ | >60y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | – | – | – | – | |
| Trinidad and Tobago | ✓ | – | – | – | ✓ | >65y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | 6 m-5y | – | – | – |
| Turks and Caicos Islands | ✓ | – | – | – | ✓ | ≥50y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | ≥6m | – | – | – |
| United States of America3 | ✓ | – | – | – | ✓ | ≥65y | – | – | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | 6 m-17y | – | – | – |
| Uruguay | ✓ | 50 | 100+ | 28 | ✓ | ≥65y | 31 | 52 | 38 | ✓ | 30 | 55 | 28 | ✓ | – | – | – | ✓ | 6 m-<5y | 27 | 7 | 6 |
| Venezuela | ✓ | – | 51 | 77 | ✓ | ≥60y | – | 11 | 30 | – | – | – | ✓ | – | 73 | 64 | – | – | – | – | ||
| Number reporting | 39 | 17 | 19 | 18 | 38 | – | 19 | 21 | 17 | 33 | 11 | 11 | 11 | 35 | 18 | 22 | 19 | 30 | – | 20 | 20 | 17 |
| Median coverage | 94 | 89 | 61 | 68 | 69 | 38 | 66.5 | 69.5 | 54 | 94 | 87 | 70 | 69.5 | 58.5 | 54 | |||||||
| Interquartile range | 50–99 | 49–100 | 29–97 | 33.5–75.5 | 37–85 | 21–70 | 32.5–84 | 26.8–77 | 21.5–66 | 81.5–100 | 63–100 | 52.5–96.5 | 50.8–82.3 | 20.5–70.3 | 28–66 | |||||||
Reflects influenza vaccination policies reported to eJRF in 2021 (or most recent year of data reported).
Coverage estimates of >100 % are marked as “100+” but were set to 100 % for median and interquartile range calculations.
Administrative estimates of influenza coverage are not available in the United States; however, survey-based estimates are available online at https://www.cdc.gov/flu/fluvaxview/index.htm.
3.2. Influenza vaccine formulations and procurement in the Americas
In 2021, 25 (64 %) countries/territories with a seasonal influenza vaccination policy used the Northern Hemisphere vaccine formulation and 14 (36 %) used the Southern Hemisphere vaccine formulation (Table 1). To date, no country/territory in Latin America and the Caribbean has reported the public-sector use of live attenuated influenza vaccines (LAIV) to PAHO/WHO; countries use only trivalent or quadrivalent inactivated influenza vaccines. However, LAIV is used in North American countries; a quadrivalent LAIV is authorized in Canada for individuals aged 2–59 years [14] and in the United States for healthy non-pregnant people aged 2–49 years [15].
Thirty-two of 39 (82 %) countries/territories with seasonal influenza vaccination policies purchase influenza vaccines through the PAHO Revolving Fund for Access to Vaccines [16]. During 2019–2021, >25 million influenza vaccine doses were purchased and procured for countries/territories each year through the Revolving Fund mechanism, including 27.1 million doses in 2019, 27.2 million doses in 2020, and 34.9 million doses in 2021. The Revolving Fund’s average price per dose for trivalent vaccine ranged from $1.10–3.29 US Dollars in 2019, $1.20–4.50 in 2020, and $1.31–4.50 in 2021, depending on the vaccine presentation (pre-filled syringes, single-dose vials, and multi-dose vials); for quadrivalent vaccine, the price per dose ranged from $4.40–5.40 in 2019, $4.40–6.00 in 2020, and $5.10–6.00 in 2021 [16]. Notably, the Revolving Fund also facilitates distribution of vaccines for emergencies, including COVID-19 vaccines [17]. In addition to vaccine procurement through the Revolving Fund, countries in the region also procure influenza vaccine through private–public partnerships for vaccine production and vaccine technology transfer agreements for regional vaccine production [18], [19], [20].
3.3. Influenza vaccination strategies during the COVID-19 pandemic
Countries/territories of the Americas use different vaccination strategies to prevent influenza, including provision of vaccine through routine healthcare services as well as annual vaccination campaigns, including fixed-post, door-to-door, and mobile vaccination strategies. At least 14 countries in the Americas schedule intensive vaccination campaigns spanning 1–3 months. Other countries such as Argentina, Chile, the United States, and Canada, organize longer “winter campaigns” of >3 months duration. Many countries in the Southern Hemisphere time their vaccination activities with the “Vaccination Week of the Americas (VWA)” initiative in late April; from April 24–30, 2021, the Region of the Americas celebrated the 19th VWA campaign and the 10th World Immunization Week. During VWA in 2021, at least seven countries used VWA as a platform to vaccinate their population against influenza, vaccinating >65 million people combined [21].
In March 2020, many countries/territories in the Americas implemented movement restrictions and lockdowns to slow transmission of the SARS-CoV-2 virus; however, vaccination against influenza continued. Countries/territories adapted health services and immunization delivery strategies to ensure continuation of routine and influenza vaccination service, while protecting community members and vaccinators from SARS-CoV-2 transmission. Innovative approaches to influenza vaccination activities implemented during the COVID-19 pandemic included staggered appointments or appointments with specific schedules (based on demographics like gender, identification number, or last name); “drive-through” vaccination posts; vaccination in outdoor spaces; vaccination posts in strategic locations such as pharmacies, grocery stores, banks, schools, and cultural and religious buildings; and on-site vaccination in homes, long-term care facilities, and correctional facilities [22]. Other strategies used to promote vaccination uptake during the pandemic included use of social media, extended vaccination hours, and active engagement of health workers and community leaders. Additionally, most countries extended the length of their annual influenza vaccination campaigns during the COVID-19 pandemic. By September 2020, countries in the Americas administered >100 million doses of influenza vaccine using these strategies [23].
3.4. Influenza vaccination coverage in the Americas (2019–2021)
Although 39 countries/territories in the Americas have policies for seasonal influenza vaccination, not all reported vaccination coverage estimates to PAHO/WHO systematically during 2019–2021. Of 39 countries/territories that target healthcare workers for vaccination, 18 (46 %) reported vaccination coverage estimates to PAHO/WHO for 2021 (Table 2); median vaccination coverage for this target group was 61 % interquartile range (IQR) = 29–97 %). Of 38 countries/territories that target older adults, 17 (45 %) reported coverage estimates for 2021; median coverage was 38 % (IQR = 21–70 %). Of 35 countries/territories that target pregnant women, 19 (54 %) reported coverage estimates for 2021; median coverage was 54 % (IQR = 21.5–66 %). Of 33 countries/territories that target persons with chronic diseases, 11 (33 %) reported coverage estimates for 2021; median coverage was 70 % (IQR = 52.5–96.5 %). Finally, of 30 countries/territories that target children ≥ 6 months for vaccination, 17 (57 %) reported vaccination coverage estimates for 2021; median coverage was 54 % (IQR = 28–66 %).
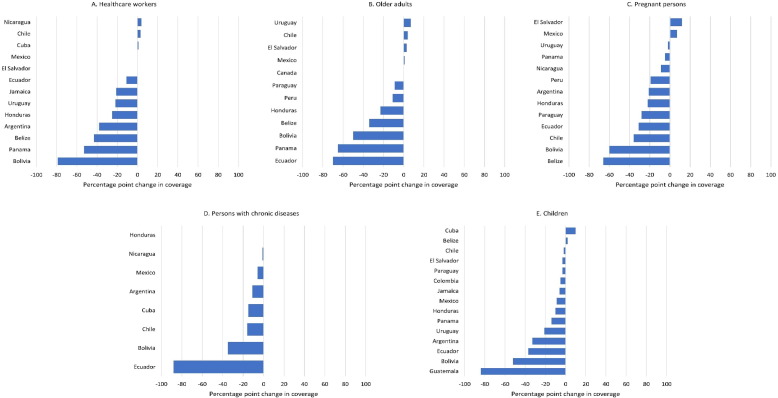
For all target groups, the median reported coverage decreased from 2019 (pre-pandemic) to 2021 (Table 2). Similarly, among countries/territories that reported estimates in both 2019 and 2021, the median percentage point decrease in coverage was 21 % (IQR = 0–38 %; n = 13) for healthcare workers, 10 % (IQR = -1.5–38 %; n = 12) for older adults, 21 % (IQR = 5–31 %; n = 13) for pregnant women, 13 % (IQR = 4.8–20.8 %; n = 8) for persons with chronic diseases, and 9 % (IQR = 3–27 %; n = 15) for children (Fig. 1 ).
Fig. 1.
Change in reported influenza vaccination coverage in 2021 compared to 2019, by target group, among countries/territories in the Americas [Source: electronic Joint Report Form (eJRF) reports to PAHO/WHO-UNICEF] Only among countries/territories that reported coverage data to eJRF in both 2019 and 2021. Values of >100 % were set as 100 % for calculations.
3.5. Monitoring and evaluation of influenza and COVID-19 vaccination
Since January 2020, COVID-19 surveillance has been gradually integrated into ongoing severe acute respiratory infection (SARI) and influenza-like illness (ILI) surveillance. The Network for the Evaluation of the Effectiveness of the Vaccine in Latin America and the Caribbean-influenza (REVELAC-i) [24], was formed in 2012 and leverages the SARI sentinel surveillance systems existing in the countries to produce regular regional and national estimates of influenza vaccine effectiveness and to evaluate the impact of the vaccine on influenza morbidity and mortality [25]. As of 2022, six countries in the Region (Chile, Costa Rica, Ecuador, Guatemala, Paraguay, and Uruguay) are collecting and analysing SARI surveillance and immunization data to generate influenza and COVID-19 vaccines effectiveness against hospitalizations. Estimates are obtained by vaccine type, age group and by circulating subtype of influenza virus and variant of SARS-CoV-2. Since there was limited circulation of influenza in the Americas during the COVID-19 pandemic (seasons 2020 and 2021) [26], estimates of effectiveness for influenza vaccines were not obtained during these years.
4. Discussion
As in other regions of the world [27], countries/territories in the Americas successfully adapted vaccine delivery using novel strategies to maintain influenza vaccination services during the COVID-19 pandemic. Influenza vaccination remained a key intervention to reduce annual influenza morbidity and mortality and prevent additional burden to the health system, especially prioritizing high-risk groups because of their vulnerability to complications from influenza infection and risk of severe COVID-19, as well as healthcare workers [28]. Innovative vaccine delivery strategies, such as adapted vaccination sites and schedules, were key to maintaining vaccine access and uptake while taking precautions to prevent SARS-CoV-2 transmission, particularly for healthcare workers and persons at high risk for severe disease.
Unfortunately, despite rapid adaptation and use of these innovative vaccination strategies, challenges such as movement restrictions during the COVID-19 pandemic, increased burden on healthcare systems, redirection of resources to COVID-19 response negatively impacted many health services globally, including immunization programs. A 2021 survey of PAHO member states indicated that nearly all countries/territories reported disruptions to essential health services, with 29 % reporting severe disruptions to routine immunization services (over 50 % of users not being served as usual) [29]. Our results indicate that influenza vaccination coverage decreased on average from 2019 to 2021 for all vaccination target groups among reporting countries/territories in the Americas. This was consistent with findings for other vaccines in the region, such as those for diphtheria-tetanus-pertussis and measles among children [30]. Reversing these declines in vaccination will necessitate strategic approaches that prioritize sustainable routine and seasonal vaccination programs and improve access across the life course, including strategies to integrate vaccination for multiple antigens. Additionally, increased vaccine misinformation/disinformation and hesitancy during the COVID-19 pandemic likely also contributed to decreased vaccination coverage and should be further evaluated and addressed to reverse declines in coverage [31], [32]. Strategies specific to country contexts and target groups should be developed bolster vaccine confidence and reduce vaccine complacency [33].
Long-term solutions for vaccination program sustainability, as well as preparedness for future pandemic vaccination rollout, can leverage lessons learned from influenza vaccination. Having a strong influenza program in place has been beneficial for influenza pandemic response. For example, an analysis of the 2009 H1N1 pandemic influenza experience revealed that countries that had a seasonal influenza vaccination program were faster in introducing the H1N1 pandemic influenza vaccine [34]. Similarly, having an existing influenza vaccination program likely contributed to successful COVID-19 vaccine rollout, particularly as vaccination strategies for COVID-19 prioritized similar target groups across the life course; global efforts are underway to characterize the specific benefits of influenza vaccination programs to COVID-19 vaccine implementation [35]. Additionally, lessons learned from coadministration of both influenza and COVID-19 vaccines during the same vaccination encounter, as recommended by WHO SAGE in October 2021 [36], could facilitate implementation of integrated vaccine programs, improve vaccination uptake, and decrease the overall burden on health services. Integrating vaccination programs for respiratory viruses into routine health services, such as scheduled immunization visits or antenatal care visits, can contribute to the lasting sustainability of vaccination programs at large.
Countries should maintain pre-pandemic gains in influenza vaccination introduction and coverage and move to introduce or expand the influenza vaccination in additional target groups. Strategies for pooled procurement, as offered through the Revolving Fund, can serve to increase vaccine access across lower income group countries, facilitating the development and sustainability of vaccination programs [17]. In addition to vaccine access and cost, other implementation barriers to establishing and sustaining influenza vaccination programs should be re-evaluated following the COVID-19 pandemic, including logistics for integrated annual vaccination programs and definition of local public health priorities [37], [38]. Systematic monitoring of influenza and COVID-19 vaccine effectiveness, as is done through the REVELAC-i network, can be helpful in addressing public, political and media concerns, contributing to inform future vaccine strain selection, guiding vaccination policy, evaluating health and economic impact, and sustaining investments in influenza vaccination programs [17]. Lessons learned from COVID-19 vaccination might improve monitoring and evaluation of influenza vaccination, such as the rapid development of electronic vaccination registries and digital certificates, estimation of updated population denominators for evaluating coverage, and use of programs and networks developed to assess the impact of vaccination.
Our findings are subject to limitations. Firstly, countries/territories report administrative coverage estimates to the eJRF system, calculated using the number of doses administered in target groups. Unfortunately, administrative coverage estimates are frequently inaccurate or exceed 100 % because of errors both in the total target population (coverage denominator) from infrequent census estimations and challenges of enumerating special populations, as well as errors in recording vaccinations at health facilities (coverage numerator) or compiling the data to report to higher levels [39], [40]. Accurate denominators may be especially hard to obtain for target groups not specifically captured in national census estimates, such as persons with chronic diseases, pregnant women, and healthcare workers. Efforts should be focused to improve administrative coverage estimates, including regular data quality self-assessments, development and rollout of registry-based data systems, and increased use of digital technology for vaccination reporting (e.g., electronic immunization registries, digital certificates). Additionally, use of mobile-based or household-level surveys or special studies to validate administrative coverage estimates, while time- and resource-intensive, can complement efforts to improve administrative coverage data [41].
Additional limitations of these findings include that not all countries/territories report consistently to eJRF; when policy data were not reported for 2021, the most recently reported year of data was used, which might have missed any changes that occurred in recent years. Moreover, the definition of what constitutes a “policy” might vary across countries/territories; future analyses could explore the characteristics of policies in the Americas and their relationship with other indicators, such as vaccination coverage. Additionally, reporting of coverage estimates decreased during the pandemic, with fewer countries reporting in 2021 than 2019; the finding of decreased coverage following the COVID-19 pandemic was obtained from only a small proportion of countries that reported data in both years. Coverage estimates do not include statistical uncertainty, and thus estimated declines in coverage could be underestimated or overestimated. Finally, the observed changes in influenza vaccine coverage cannot be directly attributed to any specific factor associated with the COVID-19 pandemic; data suggest that vaccination coverage for other antigens (polio, measles, rubella, diphtheria, and other vaccine preventable diseases) have been decreasing for the past decade, though decline in coverage was greatest during the COVID-19 pandemic [42].
5. Conclusions
Globally, the Americas remains one of the regions with the greatest proportion of member countries/territories with seasonal influenza vaccination policies [37] and is the region with the greatest percentage vaccine distribution per population [43], representing a great regional accomplishment. Sustaining influenza vaccination programs is critical for the control of future influenza seasons, particularly after COVID-19-related prevention measures (e.g., masking, social distancing) are relaxed and influenza virus circulation increases. Influenza vaccination averts illnesses, hospitalizations, and deaths and provides a framework for future pandemic vaccine rollout, vaccinating target groups across the life course.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank country/territory focal points for their contribution of the data included in this report and thank Alba María Ropero, Carmen Sofia Arriola, Annette Regan, Kathryn Lafond, and Shoshanna Goldin for their review of the manuscript.
Data availability
The manuscript contains published, publicly-accessible data (as described in the Methods).
References
- 1.World Health Organization Global Influenza Programme. Burden of disease. 2022 [cited 2022 November 02]; Available from: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/burden-of-disease.
- 2.Iuliano A.D., et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palekar R.S., et al. Burden of influenza-associated respiratory hospitalizations in the Americas, 2010–2015. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0221479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Vaccines against influenza: WHO position paper—May 2022. Wkly Epidemiol Rec. 2022;19:185–208. [Google Scholar]
- 5.Pan American Health Organization, Final report of the XVI TAG Meeting, held in Mexico City, Mexico, November 3-5, 2004. 2004.
- 6.World Health Organization Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87(47):461–476. [PubMed] [Google Scholar]
- 7.World Health Organization . WHO SAGE Roadmap for prioritizing uses of COVID-19 vaccines. 2022. [Google Scholar]
- 8.World Health Organization, WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. 2020.
- 9.Pan American Health Organization, The Immunization Program in the Context of the COVID-19 Pandemic - March 2020. 2020.
- 10.World Health Organization, WHO SAGE Seasonal Influenza Vaccination Recommendations during the COVID-19 Pandemic. 2020.
- 11.World Health Organization. WHO/UNICEF Joint Reporting Process. 2021 [cited 2022 November 04]; Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/who-unicef-joint-reporting-process.
- 12.Pan American Health Organization. Immunization Brochure. 2022 [cited 2022 November 04]; Available from: https://www.paho.org/en/tag/immunization-brochure.
- 13.Ropero-Álvarez A.M., et al. Influenza vaccination in the Americas: Progress and challenges after the 2009 A(H1N1) influenza pandemic. Hum Vaccin Immunother. 2016;12(8):2206–2214. doi: 10.1080/21645515.2016.1157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Public Health Agency of Canada Recommendations on the use of live, attenuated influenza vaccine (FluMist®), supplemental statement on seasonal influenza vaccine for 2011–2012. Can Commun Dis Rep. 2011;37:21–30. doi: 10.14745/ccdr.v37i00a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). 2022 [cited 2022 November 30]; Available from: https://www.cdc.gov/flu/prevent/nasalspray.htm.
- 16.PAHO Revolving Fund. 2022 https://www.paho.org/en/revolving-fund [cited 2022 November 02]; Available from: [Google Scholar]
- 17.Vicari A.S., et al. Seasonal influenza prevention and control progress in Latin America and the Caribbean in the context of the global influenza strategy and the COVID-19 Pandemic. Am J Trop Med Hyg. 2021;105(1):93–101. doi: 10.4269/ajtmh.21-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz-Prado E., et al. Vaccine market and production capabilities in the Americas. Trop Dis Travel Med Vaccines. 2021;7(1) doi: 10.1186/s40794-021-00135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friede M., et al. WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: supporting developing country production capacity through technology transfer. Vaccine. 2011;29(Suppl 1):A2–A7. doi: 10.1016/j.vaccine.2011.02.079. [DOI] [PubMed] [Google Scholar]
- 20.Pan American Health Organization, Increasing Production Capacity for Essential Medicines and Health Technologies. 2021.
- 21.Pan American Health Organization, Vaccination Week in the Americas 2021. Final Report. 2022.
- 22.Pan American Health Organization, Summary of the Status of National Immunization Programs during the COVID-19 Pandemic, July 2020. 2020.
- 23.Pan American Health Organization, Vaccination Week in the Americas 2020: Final Report. 2021.
- 24.Pan American Health Organization. Network for the Evaluation of Vaccine Effectiveness in Latin America and the Caribbean - influenza, (REVELAC-i). 2022 [cited 2022 November 04]; Available from: https://www.paho.org/en/network-evaluation-vaccine-effectiveness-latin-america-and-caribbean-influenza-revelac-i.
- 25.Arriola C.S., et al. Influenza vaccine effectiveness against hospitalizations in children and older adults-data from South America, 2013–2017. A test negative design. Vaccine X. 2019;3 doi: 10.1016/j.jvacx.2019.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan American Health Organization. Influenza Situation Report. 2022 [cited 2022 November 04]; Available from: https://www.paho.org/en/influenza-situation-report.
- 27.Wang X., et al. Influenza vaccination strategies for 2020–21 in the context of COVID-19. J Glob Health. 2020;10(2) doi: 10.7189/jogh.10.021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maltezou H.C., Theodoridou K., Poland G. Influenza immunization and COVID-19. Vaccine. 2020;38(39):6078–6079. doi: 10.1016/j.vaccine.2020.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan American Health Organization, Third round of the National Survey on the Continuity of Essential Health Services during the COVID-19 Pandemic: November-December 2021. Interim report for the Region of the Americas, January 2022. 2022.
- 30.Rachlin A., et al. Routine vaccination coverage - worldwide, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(44):1396–1400. doi: 10.15585/mmwr.mm7144a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puertas E.B., et al. Concerns, attitudes, and intended practices of Caribbean healthcare workers concerning COVID-19 vaccination: A cross-sectional study. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shet A., et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. 2022;10(2):e186–e194. doi: 10.1016/S2214-109X(21)00512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Block M., et al. Influenza vaccination hesitancy in five countries of South America. Confidence, complacency and convenience as determinants of immunization rates. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter R.M., et al. Does having a seasonal influenza program facilitate pandemic preparedness? An analysis of vaccine deployment during the 2009 pandemic. Vaccine. 2020;38(5):1152–1159. doi: 10.1016/j.vaccine.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Insights to inform influenza health worker vaccination programmes. 2022 [cited 2022 November 04]; Available from: https://www.who.int/news/item/21-03-2022-insights-to-inform-influenza-health-worker-vaccination-programmes.
- 36.World Health Organization, Coadministration of seasonal inactivated influenza and COVID-19 vaccines. 2021.
- 37.Morales K.F., et al. Seasonal influenza vaccination policies in the 194 WHO Member States: The evolution of global influenza pandemic preparedness and the challenge of sustaining equitable vaccine access. Vaccine X. 2021;8 doi: 10.1016/j.jvacx.2021.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraigsley A.M., et al. Barriers and activities to implementing or expanding influenza vaccination programs in low- and middle-income countries: A global survey. Vaccine. 2021;39(25):3419–3427. doi: 10.1016/j.vaccine.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 39.Cutts F.T., et al. Monitoring vaccination coverage: Defining the role of surveys. Vaccine. 2016;34(35):4103–4109. doi: 10.1016/j.vaccine.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cutts F.T., Izurieta H.S., Rhoda D.A. Measuring coverage in MNCH: design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PLoS Med. 2013;10(5) doi: 10.1371/journal.pmed.1001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edelstein M. Measuring vaccination coverage better will help achieve disease control. Int Health. 2017;9(3):142–144. doi: 10.1093/inthealth/ihx013. [DOI] [PubMed] [Google Scholar]
- 42.Pan American Health Organization. COVID-19 pandemic fuels largest continued backslide in vaccinations in three decades. 2022 [cited 2023 February 15]; Available from: https://www.paho.org/en/news/15-7-2022-covid-19-pandemic-fuels-largest-continued-backslide-vaccinations-three-decades.
- 43.Palache A., et al. Vaccine complacency and dose distribution inequities limit the benefits of seasonal influenza vaccination, despite a positive trend in use. Vaccine. 2021;39(41):6081–6087. doi: 10.1016/j.vaccine.2021.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript contains published, publicly-accessible data (as described in the Methods).