Abstract
Objective:
Develop and pilot test a mobile health (mHealth) cognitive behavioral coping skills training and activity coaching protocol (HCT Symptoms and Steps) for hematopoietic stem cell transplant (HCT) patients.
Design:
Two-phase, mixed methods study.
Sample:
HCT patients and healthcare providers.
Methods:
Phase I was patient (n=5) and provider (n=1) focus groups and user testing (N=5) to develop the HCT Symptoms and Steps protocol. Phase II was a pilot randomized trial (N=40) to evaluate feasibility, acceptability, and pre-to-post outcomes (e.g., physical disability, pain, fatigue, distress, physical activity, symptom self-efficacy) compared to an education control.
Findings:
Qualitative feedback on symptoms, recruitment strategies, coping skills, and mHealth components (e.g., Fitbit, mobile app) were integrated into the protocol. HCT Symptoms and Steps was feasible and acceptable. Pre-post changes suggest physical disability and activity improved while symptoms (e.g., fatigue, distress) decreased.
Conclusions:
HCT Symptoms and Steps has strong feasibility and acceptability and shows promise for benefits. Larger, fully-powered randomized trials are needed to examine intervention efficacy.
Implications:
HCT Symptoms and Steps may reduce physical disability and improve health outcomes post-transplant.
Keywords: Cancer, hematopoietic stem cell transplant, physical disability, pain, fatigue, oncology, distress, symptom management, physical activity
Introduction
Hematopoietic stem cell transplant (HCT) is an aggressive treatment for life-threatening cancers. HCT has led to improved prognosis and survival, but 70–80% of HCT patients experience significant physical disability (i.e., problems with activities of daily living and functional mobility).1–3 Physical disability is exacerbated by pain, fatigue, and psychological distress, which are among the most prevalent and debilitating symptoms following HCT.1–2 Physical disability and high symptom burden interfere with patients’ ability to engage in physical activity.
Paradoxically, physical activity is an effective way to reduce disability and alleviate symptoms.4–9 Physical activity is included in standard care guidelines following transplant; HCT patients are instructed to engage in 30 minutes of activity or 2500 steps daily.10 Evidence shows physical activity is safe and feasible for HCT patients, can alleviate symptoms, and improve quality of life and potentially survival.11–13
Despite recommendations, HCT patients report difficulty engaging in physical activity. HCT patients spend days pre- and post-transplant in the inpatient setting, followed by weeks of intensive outpatient care. Pain, fatigue, and distress during this acute recovery phase are major barriers to physical activity.2,11 HCT patients report increased symptom severity when they attempt to follow activity guidelines; thus, patients often limit their activity or stop it completely.11 Low levels of activity then exacerbate disability, and can result in impaired cardiorespiratory fitness and decreased mobility and strength.10,13 Teaching HCT patients strategies to cope with symptoms is critical to helping them increase physical activity, thereby reducing physical disability.
Cognitive behavioral (CBT)-based coping skills training interventions are an efficacious, non-pharmacological treatment for cancer-related symptoms and associated physical disability. Cognitive and behavioral factors play an important role in cancer patients’ ability to cope with symptoms and engage in physical activity. HCT patients with pain, fatigue, and distress are likely to have low confidence in their ability to control their symptoms (i.e., low self-efficacy for symptom management), and this is associated with worse symptom severity, poor adherence to activity recommendations, and greater physical disability.14–15 CBT-based coping skills training has been shown to improve symptom self-efficacy and, in turn, improve adherence to activity recommendations and reduce symptom severity and disability in cancer patients with persistent symptoms.16–18 However, the application of these protocols to HCT patients has been limited by a lack of tailoring to HCT patients’ specific needs (e.g., intervention content, length, accessible delivery format).14
Study Objectives
We sought to develop and test a mobile health (mHealth), combined coping skills training and activity coaching intervention (HCT Symptoms and Steps) that began in-person immediately following transplant and continued in the patient’s home via mHealth technology (i.e., videoconference sessions, adjunct mobile application, Fitbit activity tracker).
First, we aimed to develop and refine the HCT Symptoms and Steps protocol via patient and healthcare provider focus groups, followed by user testing (Phase I). Second, we aimed to examine the feasibility and acceptability of the developed HCT Symptoms and Steps protocol through a pilot randomized controlled trial (Phase II). We hypothesized the protocol would be feasible (i.e., accrual N=40 in 12 months; >80% adherence to protocol; <20% attrition) and acceptable (i.e., >80% intervention satisfaction). Finally, we aimed to examine outcome patterns of HCT Symptoms and Steps for improving physical disability and other outcomes compared to an attention-matched education control group (HCT Education). We hypothesized the HCT Symptoms and Steps group would demonstrate significantly improved physical disability, as well as pain, fatigue, distress, physical activity, and symptom self-efficacy compared to HCT Education.
Methods
Participants
Patients who had undergone autologous HCT at the Duke Adult Blood and Marrow Transplant (ABMT) clinic were eligible for focus groups, user testing, or the randomized trial. Additional criteria included: 1) age ≥18 years, 2) ≤3 months post-transplant, 3) at least two symptoms (pain, fatigue, or distress) >0 on 0–10 scale, 4) at least one symptom ≥3 on 0–10 scale, and 5) life expectancy ≥12 months. Exclusion criteria included: 1) cognitive impairment, 2) severe psychiatric condition, and 3) non-English speaking.
Procedures
The trial was approved by the Duke Institutional Review Board (Pro00100321) and registered on ClinicalTrials.gov (NCT03859765). Oncologists provided information on patients scheduled for transplant. Study staff conducted chart review to assess eligibility. Staff met patients at the ABMT clinic or via telephone to complete informed consent. Eligible Duke ABMT providers (e.g., oncologists, nurse practitioners) were recruited through ABMT administrators.
HCT Symptoms and Steps.
An initial HCT Symptoms and Steps intervention was developed based on study team expertise in Pain Coping Skills Training (PCST) intervention science, intervention development and mHealth applications, and experience working with HCT patients.14–15,19
The initial HCT Symptoms and Steps intervention included seven, 45–60 minute weekly sessions. The first three sessions were to be conducted at the ABMT clinic, and included one session of symptom coping skills training with a psychologist and two sessions of activity coaching with an occupational therapist (OT). The remaining four sessions were to be conducted via videoconferencing with the psychologist and integrated symptom coping skills training and activity coaching. The psychologist teaches cognitive behavioral coping skills (e.g., progressive muscle relaxation, activity pacing/planning, cognitive restructuring, pleasant imagery, goal setting) to manage pain, fatigue, and distress and increase activity. The OT worked with participants to determine prior and current level of functioning, provide activity guidelines, identify strategies for increasing activity and reengaging with activities of daily living (e.g., return to work), and set activity goals. All sessions included didactic and experiential components that were summarized in handouts. Patients were instructed to engage in at-home practice to facilitate skill acquisition and generalization.20–21
In-person and videoconference sessions were supported by a mobile app from Pattern Health (Durham, NC). The HCT Symptoms and Steps app included: 1) content on coping skills and physical activity (e.g., handouts, audio recordings, demonstration videos), 2) syncing of Fitbit data and transmission in real-time to patient and study staff, 3) coping skills use tracking, 4) 3x/week symptom assessment, and 5) push notifications from the therapist (3x/week) including reminders, encouragement, and real-time personalized feedback based on skills use and symptom assessment.
HCT Education.
HCT Education was an attention-matched, health education control intervention. It was proposed that HCT Education participants receive one in-person session prior to discharge home, followed by six telephone sessions. Sessions were 25–40 minutes and content included general cancer education, information about health and well-being, and HCT treatment guidelines. HCT Education participants downloaded an HCT Education mobile app that included: 1) handouts, 2) syncing of Fitbit data and transmission in real-time to patient and study staff, and 3) 3x/week symptom assessment. HCT Symptoms and Steps intervention content was not included in the HCT Education intervention or mobile app.
Fitbit Device.
Patients in both groups were provided with a Fitbit to track daily steps, and received a dynamic weekly step count goal that was adjusted based on performance. All participants were given an initial daily step count goal of 2,500 steps (approximately 1 mile), consistent with ABMT medical team recommendations. If a participant met the initial step count goal on at least 5 days during the week, the goal increased based on the average daily step count. In subsequent weeks, if participants met the daily goal 5/7 days, the goal increased by a maximum of 10%/week.
Treatment Delivery.
HCT Symptoms and Steps and HCT Education were delivered by doctoral-level psychologists with experience delivering cognitive behavioral interventions and working with HCT patients. Activity coaching sessions were delivered by an OT with expertise working with HCT patients. Study therapists followed a treatment manual and attended weekly supervision to discuss session delivery and content.
Phase I: Focus Groups and User Testing
Patient (n=5) and provider (n=1) focus groups were conducted to iteratively refine the study protocol. Focus groups were audio-recorded and transcribed. During focus groups, patients and providers gave verbal feedback based on a presentation and responded to structured questioning about the content, timing, and modality of the interventions. Focus group participants were compensated $20. Five user testing patients completed the 7-session HCT Symptoms and Steps intervention. Feedback on coping skills, Fitbit, and mobile app was used to further refine the protocol.
Phase II: Pilot Randomized Controlled Trial
We conducted a small pilot RCT to assess the feasibility, acceptability, and outcome patterns of HCT Symptoms and Steps compared to HCT Education. Participants were randomly assigned in equal allocation using sequentially numbered envelopes to HCT Symptoms and Steps or HCT Education. Participants who did not have a smartphone were loaned a device equipped with Internet to access mHealth features. User tester and RCT participants completed pre- (A1) and post-treatment (A2) electronic assessments and were compensated $20 per assessment.
Measures
Sociodemographic and Medical Variables.
Sociodemographic and medical variables were collected via self-report and medical record review.
Patient-Reported Outcomes.
Physical disability was assessed by the Functional Assessment of Cancer Therapy (FACT-G) 7-item Physical Well-Being subscale (Cronbach’s α=.81). Pain was assessed by the 4-item Pain Severity subscale of the Brief Pain Inventory (BPI) (α=.91).22–24 Fatigue was assessed by the 7-item PROMIS Adult Fatigue Short Form (α=.74).25–28 Psychological distress was measured with the 14-item Hospital Anxiety and Depression Scale (HADS) (α=.83).29–33 Symptom self-efficacy was assessed with the 6-item Self-Efficacy for Managing Chronic Disease Scale (α=.85).34
Physical Activity.
Physical activity (i.e., steps) was continuously monitored using Fitbit activity trackers. Patients also reported their step count for the previous day at the beginning of each session. If step data was unavailable (e.g., Fitbit malfunction), the step count for the closest available date was recorded. Studies have demonstrated the reliability, validity, and acceptability of Fitbits in research.35–37
Acceptability.
Acceptability was assessed using the 8-item Client Satisfaction Questionnaire (CSQ).38 Items were rated on a 4-point scale from 1=low acceptability to 4=high acceptability and summed to obtain an overall satisfaction score (α =.92).
Analytic Strategy
Phase I
Aim 1 Analyses.
Focus group audio-recordings and transcriptions were reviewed (SK, TS) and informed the refinement of the discussion guide for subsequent focus groups. Qualitative analysis was conducted using rapid analysis, which can inform intervention development and refinement more quickly than traditional, often lengthy qualitative procedures.39–40 Interview questions were used to create topic domains. Transcriptions were reviewed by two independent coders (KH, HF) to summarize participant responses relevant to each domain. Within domains, responses were analyzed to identify key themes. Findings informed intervention procedures and material refinement. A similar approach has been previously used by our team to develop psychosocial interventions for cancer patients.15
Phase II
Aim 2 Analyses.
Feasibility was indexed by accrual, adherence, and attrition. Accrual was indicated by meeting the recruitment goal of 40 participants in 12 months. Adherence was indicated by 80% completion rates for sessions and assessments across groups. Attrition was indicated by <20% of participants withdrawing from the study. Acceptability was indicated by patients reporting 80% satisfaction with HCT Symptoms and Steps (M=25.6/32) on the CSQ.
Aim 3 Analyses.
Independent two-sample t-tests and chi-square tests of independence were used to compare baseline demographic, medical, and psychosocial characteristics between groups. Intent-to-treat linear mixed model analyses were used to evaluate change in continuous outcome variables from baseline to post-assessment (i.e., time) as a function of group assignment. Mixed model analyses included all participants who were randomized and completed the baseline assessment. Findings for the main effects (group, time) and interaction effect (group*time) are presented for physical disability, as well as pain, fatigue, psychological distress, physical activity, and symptom self-efficacy. These analyses should be considered exploratory given the pilot nature of this work.41
Sample Size
The number of focus groups was informed by empirical work suggesting 90% of discoverable themes are identified in 3 to 6 groups. Consistent with these findings, our team determined that we had likely reached thematic saturation (i.e., the point at which no new themes are likely to be identified) after 5 patient groups.42 The number of user testers was determined based on methods used in prior studies.15 Consistent with pilot trial guidelines,43 the study team determined that 40 participants would be sufficient for identifying feasibility concerns (e.g., recruitment difficulties) that would impact a future efficacy trial.
Results
Phase I Findings: Focus Groups and User Testing
Seventeen patients (n=5 groups) and six providers (n=1 group) participated in focus groups. Five patients completed user testing. Findings from focus groups are summarized in Supplementary Table 1.
Learnings and Protocol Updates
Both patients and providers reported considerable physical and psychological symptom burden associated with HCT. Fatigue was highlighted as particularly bothersome. There were several key suggestions that were incorporated into the protocol. First, patients shared it would be helpful to learn about the study prior to transplant. This timeline was corroborated by providers and implemented to ensure recruitment procedures were appropriate during rigorous medical treatment. Second, staff offered assistance with study technologies (i.e., mobile app, Fitbit) at enrollment, and were available for ongoing support thereafter. Likewise, contact information for the study team were incorporated directly into the intervention materials and mobile app. User testing participants (n=5) described an enjoyable experience with the protocol and offered positive feedback regarding study content. Pleasant activity scheduling, cognitive restructuring, and mini-relaxation practices were described as particularly helpful. User testers noted some difficulties securing the Fitbit onto the wrist and syncing with the app. They also made suggestions to make the app more patient-friendly and personalized (e.g., free text entry). In response to feedback, the study team offered different size Fitbit wristbands and simplified the patient-facing dashboard. Assessment tracking programming was improved and a free text entry option was added. Finally, the study team created a thorough technology support guide for using the app and Fitbit.
Phase II Findings: Pilot Randomized Controlled Trial
Feasibility
Accrual.
Forty patients were enrolled from 06/30/2020 to 10/25/2021 (16 months; see Figure 1). The recruitment timeline was longer than the proposed feasibility benchmark (40 patients in 12 months) due to the COVID-19 pandemic. Safety restrictions at the Duke University Health System necessitated a temporary pause on recruitment and transition to an entirely remote recruitment and intervention format. Additionally, the frequency of HCTs was reduced during the initial months of recruitment while the ABMT clinic navigated new COVID-19 protocols. All consented participants were randomized to HCT Symptoms and Steps (n=20) or HCT Education (n=20).
Figure 1.
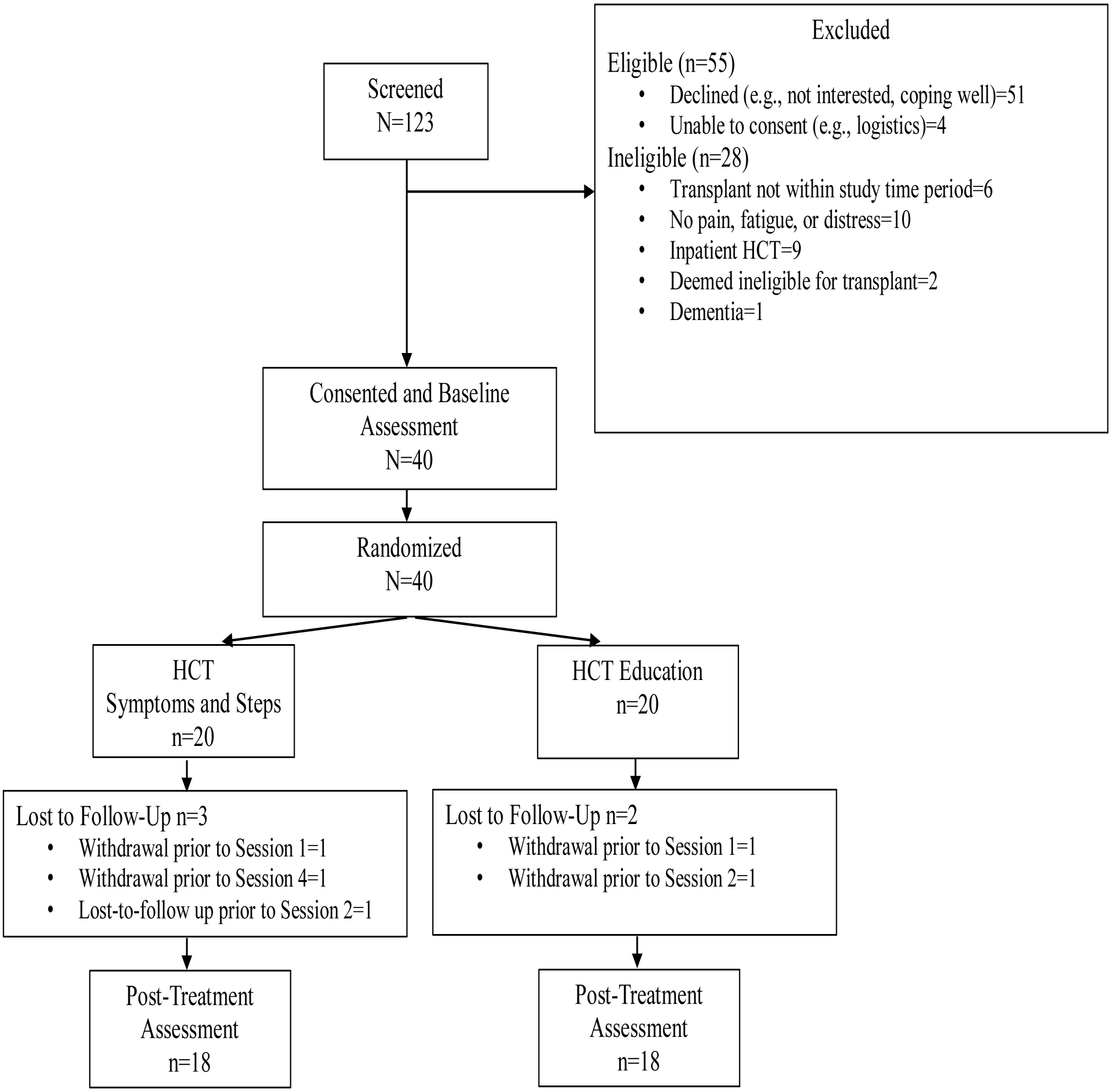
CONSORT
Note. Participant that was lost-to-follow up prior to Session 2 in HCT Symptoms and Steps completed the post-treatment assessment.
Adherence.
Eighty-five percent of HCT Symptoms and Steps participants completed all intervention sessions (n=17). Two participants declined sessions prior to session one and four, respectively, due to time constraints. One participant was lost to follow-up after session one. Ninety percent of HCT Education participants completed all intervention sessions (n=18). All participants (100%) assigned to HCT Symptoms and Steps completed the A1 assessment and 85% completed A2. All HCT Education participants (100%) completed the A1 assessment and 90% completed A2. Across groups, intervention session and assessment completion exceeded the 80% feasibility benchmark.
Attrition.
Of the 40 participants randomized, 87% completed the study (n=35). Five participants (13%) did not complete the study. This met the feasibility benchmark of <20% attrition.
Acceptability
Participants found the HCT Symptoms and Steps intervention to be highly acceptable with a mean satisfaction rating of 27.89/32 (SD=3.46). This exceeded the 80% threshold (M=25.6/32).
Descriptive Results
Participants were on average 59.32 years old, and the majority were male (58%) and non-Hispanic White (90%). Mean time since cancer diagnosis was 26 months, and most patients were diagnosed with multiple myeloma (78%). Mean time since transplant was 19.65 days. There were no statistically significant group differences on demographic or medical variables at baseline (Table 1).
Table 1.
Demographic and Clinical Data (N=40)
| Overall (N=40) |
HCT Symptoms and Steps (n=20) | HCT Education (n=20) | p value | |
|---|---|---|---|---|
| N (%) or M (SD) | N (%) or M (SD) | N (%) or M (SD) | ||
| Age (years) | 59.32 (10.94) | 60.59 (11.04) | 58.5 (10.96) | .469 |
| Gender | .337 | |||
| Male | 23 (57.5%) | 10 (50%) | 13 (65%) | |
| Female | 17 (42.5%) | 10 (50%) | 7 (35%) | |
| Race | .135 | |||
| White | 28 (70%) | 14 (70%) | 14 (70%) | |
| Black or African American | 9 (22.5%) | 6 (30%) | 3 (15%) | |
| Declined | 3 (7.5%) | 0 | 3 (15%) | |
| Ethnicity | .540 | |||
| Non-Hispanic or Latino | 36 (90%) | 19 (95%) | 17 (85%) | |
| Hispanic-Cuban | 1 (7.5%) | 0 | 1 (5%) | |
| Declined | 3 (2.5%) | 1 (5%) | 2 (10%) | |
| Cancer Type | .193 | |||
| Lymphoma | 6 (15%) | 1 (5%) | 5 (25%) | |
| Multiple myeloma | 31 (77.5%) | 17 (85%) | 14 (70%) | |
| Other | 3 (7.5%) | 2 (10%) | 1 (5%) | |
| First Cancer or Recurrence | .144 | |||
| First Cancer | 30 (75%) | 17 (85%) | 13 (65%) | |
| Recurrence | 10 (25%) | 3 (15%) | 7 (35%) | |
| Time since Diagnosis (months) | 26.16 (50.73) | 33.46 (65.08) | 18.86 (30.60) | .369 |
| Time since Transplant (days) | 19.65 (32.33) | 25.10 (44.30) | 14.20 (10.98) | .292 |
| Chemotherapy | 15 (39.5%) | 7 (36.8%) | 8 (42.1%) | .740 |
| Anti-Cancer Drug | 5 (13.2%) | 3 (15.8%) | 2 (10.5%) | .631 |
M=mean; SD=standard deviation. Other cancer type=Mantle Cell, Germ Cell; Received chemotherapy/anti-cancer drug=in last 7 days; Significance is 2-tailed.
Descriptive statistics for study variables by group and assessment are shown in Table 2. Changes from pre- to post-intervention in physical disability, symptoms (i.e., pain, fatigue, distress), and physical activity were in the anticipated direction, with physical disability and activity improving while symptoms decreased. Physical disability improved in all participants, with a significant effect for time (p<.001), but not group or group*time. Fatigue improved in all participants, with a significant effect for time (p=.008), but not group or group*time. Distress demonstrated a significant effect for time (p=.002), but the main effect for group and interaction effect were non-significant. There were no significant effects for pain or physical activity. For symptom self-efficacy, there were no significant main effects, but the interaction effect was significant due to a cross-over interaction (p=.014), with symptom self-efficacy decreasing slightly in the HCT Symptoms and Steps group and increasing slightly in HCT Education.
Table 2.
Change in Outcome Measures by Group
| HCT Symptoms and Steps (n=20) | HCT Education (n=20) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre (n=20) | Post (n=18) | Pre (n=20) | Post (n=18) | Linear Mixed Models | ||||||
| M(SD) | M(SD) | M(SD) Difference Pre-Post |
M(SD) | M(SD) | M(SD) Difference Pre-Post |
Group Difference at Follow-up | p-value Group Effect | p-value Time Effect | p-value Group*Time Interaction | |
| FACT-G | 16.45 (3.83) | 21.44 (4.22) | 4.78 (4.91) | 13.25 (6.41) | 20.27 (5.01) | 6.94 (6.66) | 1.17 | .495 | <.001* | .280 |
| BPI | 3.15 (2.12) | 2.38 (2.13) | −.79 (2.19) | 2.43 (2.13) | 2.26 (1.98) | 0.02 (1.54) | .12 | .988 | .902 | .240 |
| PROMIS Fatigue | 53.46 (5.83) | 50.67 (6.17) | −2.62 (6.98) | 58.30 (7.39) | 53.34 (8.22) | −4.72 (8.73) | −2.67 | .232 | .008* | .392 |
| HADS | 9.80 (6.01) | 7.50 (4.11) | −2.56 (3.90) | 12.15 (5.52) | 8.22 (5.47) | −3.94 (6.21) | −0.72 | .622 | .002* | .372 |
| Anxiety | 5.00 (2.92) | 3.83 (2.36) | −1.28 (1.96) | 4.85 (3.52) | 3.44 (2.83) | −1.22 (2.37) | 0.39 | .854 | .015* | .974 |
| Depression ^ | 4.81 (3.52) | 3.67 (2.28) | −1.28 (2.49) | 7.3 (4.04) | 4.78 (3.34) | −2.72 (5.26) | −1.11 | .319 | .008* | .286 |
| Steps | 3317.65 (2586.49) | 5004.00 (3211.63) | 1686.35 (3894.57) | 3481.06 (2620.09) | 4482.56 (2494.84) | 1001.50 (2471.15) | 521.44 | .564 | .186 | .524 |
| Symptom SE ^ | 7.71 (1.21) | 6.75 (2.16) | −.98 (2.47) | 6.60 (1.60) | 7.29 (1.92) | .85 (1.52) | −0.54 | .296 | .121 | .014* |
Note: M=mean; SD=standard deviation; FACT-G=Functional Assessment of Cancer Therapy Physical Well-Being; BPI=Brief Pain Inventory; HADS=Hospital Anxiety Depression Scale; SE=Self-Efficacy; n=17 for steps for HCT Symptoms and Steps;
significant difference between groups at baseline (p<.05); Unadjusted values shown; Group difference at follow-up calculated by: M HCT Symptoms and Steps Post–M HCT Education Post;
p<.05.
Discussion
We developed and tested HCT Symptoms and Steps, a mHealth coping skills training and activity coaching intervention. This intervention is the first to concurrently target physical activity and the symptoms that most interfere with physical activity (i.e., pain, fatigue, distress) in patients who have undergone HCT.
Phase I: Focus Groups and User Testing
A key suggestion was to emphasize strategies for coping with fatigue. Patients described fatigue as their most prevalent and troublesome symptom after HCT. It was noted that fatigue and other symptoms (i.e., pain, distress) interfere with physical activity; however, patients acknowledged physical activity is one of the best ways to alleviate symptoms. The activity-rest cycle was presented as a tool to help patients engage in more activity without exacerbating fatigue. Participants shared that symptom management protocols should highlight what patients can do versus restrictions. Thus, coping skills and daily step count tracking were introduced as strategies to increase symptom self-efficacy and participation in important activities (e.g., physical activity, work, social). Patients and providers emphasized that learning about the study prior to transplant, then initiating the intervention when patients transition home post-transplant could offer the most benefit. This timeline proved effective for recruitment, and the appropriateness of intervention timing is reflected in improved pre- to post-intervention outcomes. Improvements in distress are particularly notable, as providers highlighted the transition home can be distressing for patients.
User testers described a positive experience with the intervention and shared that coping strategies were helpful in managing symptoms. Participants offered recommendations to improve the mobile app and fit of the Fitbit, which were addressed to improve user experience. Technology support continued to be a primary concern for participants, highlighting the importance of comprehensive instruction and mHealth technology demonstration throughout the study.
Phase II: Pilot RCT
Findings suggest the protocol is feasible, as demonstrated by meeting the target recruitment goal (N=40) in a 16-month timeframe. Of the 40 participants randomized, only five did not complete intervention sessions. Assessment completion ranged from 85–100%. Results also demonstrated excellent acceptability of HCT Symptoms and Steps. High rates of accrual, protocol adherence, and acceptability indicate the recruitment and assessment procedures are feasible, and program material is applicable and helpful.
Participants in both HCT Symptoms and Steps and HCT Education demonstrated improvements in physical disability, fatigue, and distress from baseline to post-intervention. Given the small sample and pilot nature of the study, all findings should be considered preliminary and warrant exploration in larger samples. There were baseline differences between groups on several variables of interest, with HCT Education participants endorsing significantly worse depression and symptom self-efficacy. Given the lack of a standard care condition, it is not possible to determine how much improvement may be attributable to the interventions versus expected improvement with time post-transplant. Additionally, due to the limited follow-up period in the current pilot study, we were not able to evaluate the longer-term impact of HCT Symptoms and Steps beyond the immediate post-transplant window. Literature suggests CBT-based interventions may produce greater benefit over a longer period.44
Participants in both groups demonstrated clinically significant improvements (>4 points) on the FACT Physical Functioning subscale over approximately two months. This is promising, as post-transplant recovery can be a challenging and lengthy process. HCT Symptoms and Steps participants reported the cognitive behavioral tools were helpful for coping with symptoms, which may explain reductions in physical disability. While HCT Education participants did not receive the same active tools, it is possible they benefitted from education on topics relevant to post-transplant recovery (e.g., sleep). Simply acknowledging symptoms may be validating and normalize patients’ experience with physical disability. Participants in both groups were given a Fitbit and app designed for their respective intervention. These mHealth tools might improve self-monitoring of symptoms and activity, which may have helped patients to better manage symptoms and reduce physical disability. Improved physical disability may partly reflect expected improvements in functioning immediately post-transplant. However, post-transplant recovery spans several months to years. A larger efficacy trial with longer-term follow-up will better elucidate the positive impact of HCT Symptoms and Steps over time.
Though not statistically significant, participants in both groups increased their average daily count by 1000+ steps (approximately 0.5 miles). Notably, HCT Symptoms and Steps participants walked on average 600 more steps than HCT Education participants at post-intervention. All participants reported liking the Fitbit to track activity and many planned to continue using it after study completion. There were no significant effects for pain by group, time, or group*time. This may be a product of low levels of pain reported across time and groups. Qualitative interviews suggested that pain was a significant issue for some but not endorsed by all.
There was a significant interaction effect for symptom self-efficacy, such that it improved by <1 point in HCT Education and decreased by <1 point in HCT Symptoms and Steps. Findings may reflect a ceiling effect, given that participants endorsed high levels of self-efficacy at baseline. It is possible both interventions helped patients to maintain already high self-efficacy. Future trials should evaluate whether symptom self-efficacy is a mediator of intervention effects.
This pilot study is a successful first step towards a larger randomized efficacy trial. In addition to initial feasibility and acceptability results, we present promising preliminary data on clinically relevant variables for patients recovering from HCT. The protocol was shifted to a fully remote format; this is a strength and highlights the utility of mHealth technology to disseminate such protocols. Another strength was the use of the mobile app and Fitbit to support the therapist-led intervention sessions. The Fitbit provided an objective measure of physical activity and was integrated with the app to provide real-time step data and a dynamic weekly step goal that was adjusted based on performance.
Limitations should be noted. Due to the pilot nature of this work, the sample size was small, and only 33% of screened patients consented to the study. HCT is stressful and time-consuming and this study was conducted during the height of COVID-19; it is possible that consented patients were those already coping well with these stressors. Larger samples will provide adequate power to test for group differences in outcomes at post-treatment and longer-term follow-up assessments, and offer more conclusive evidence regarding intervention efficacy. Participants in this study reported low levels of pain across time and groups reflecting a possible selection bias (i.e., patients in less pain were more likely to participate). This trial lacked an objective measure of physical disability. The six-minute walk test (6MWT) is used to index physical disability and was intended for use in this study but removed due to the pandemic-related switch to remote procedures; it should be considered for future trials. Due to COVID-19, we were not able to conduct the first three sessions of HCT Symptoms and Steps in-person. Future efficacy trials should implement the hybrid delivery of sessions, as designed; we expect this will lead to more robust between-group differences. The HCT Education intervention represents a strong control condition that is much more than what patients receive as usual care. A possible next step is to use a Multiphase Optimization Strategy (MOST) to identify the strongest components from both interventions to optimize HCT Symptoms and Steps for a larger efficacy trial. It is also important that future work consider including other comparators (e.g., educational pamphlet only, Fitbit only, usual care).
Implications for Psychosocial Providers
Feedback from HCT patients and providers highlighted fatigue as a particularly prevalent and challenging symptom after HCT. Psychosocial interventions for HCT patients should include strategies (e.g., activity-rest cycle) geared towards managing fatigue. HCT Symptoms and Steps was delivered post-transplant as patients were transitioning home from intensive outpatient care. This timing was endorsed by HCT patients and providers, and may be a useful model for other psychosocial interventions in this patient population. Overall, the protocol was feasible and acceptable, and showed promise for improving physical disability and activity, and reducing symptoms. Psychosocial providers and researchers should consider incorporating mHealth technologies (e.g., Fitbit, mobile app) into interventions, as these features are well-received and may enhance intervention effects.
Conclusion
HCT Symptoms and Steps is designed to concurrently and synergistically increase physical activity while providing strategies to decrease the symptoms that most interfere with physical activity. This comprehensive approach is further enhanced with mHealth technologies to improve continuity of care, increase access to treatment, and alleviate physical disability and psychosocial burden. Findings provide a foundation for larger randomized efficacy trials where the benefits of the HCT Symptoms and Steps protocol can be more robustly tested.
Supplementary Material
Funding:
This study was funded through 1R21CA235083-01 awarded to first author, Sarah A. Kelleher, PhD. The work of Joseph G. Winger, Ph.D. was supported, in part, by a Kornfeld Scholars Program Award from the National Palliative Care Research Center.
Footnotes
Disclosure Statement: The authors have no relevant financial or non-financial interests to disclose.
Clinical Trial Registration Number: NCT03859765
Ethics Approval: Procedures complied with ethical guidelines and received Duke University Institutional Review Board approval (Pro00100321).
Consent to Participate: Informed consent was obtained from all individual participants included in this study.
Consent for Publication: The authors affirm that human research participants provided informed consent for publication of the data included in this publication.
References
- 1.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology. 2009;18(2):113–127. doi: 10.1002/pon.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaher EH, Ferrans C, Verlen E, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nursing Forum. 2006;33(3):614–624. doi: 10.1188/06.ONF.614-624 [DOI] [PubMed] [Google Scholar]
- 3.Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS). Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiskemann J, Huber G. Physical exercise as adjuvant therapy for patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(4):321–329. doi. 10.1038/sj.bmt.1705917 [DOI] [PubMed] [Google Scholar]
- 5.Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117(9):2604–2613. doi: 10.1182/blood-2010-09-306308 [DOI] [PubMed] [Google Scholar]
- 6.Bergenthal N, Will A, Streckmann F, et al. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014(11):CD009075. doi: 10.1002/14651858.CD009075.pub2 [DOI] [PubMed] [Google Scholar]
- 7.Carlson LE, Smith D, Russell J, Fibich C, Whittaker T. Individualized exercise program for the treatment of severe fatigue in patients after allogeneic hematopoietic stem-cell transplant: a pilot study. Bone marrow transplantation. 2006;37(10):945–954. doi: 10.1038/sj.bmt.1705343 [DOI] [PubMed] [Google Scholar]
- 8.Wilson RW, Jacobsen PB, Fields KK. Pilot study of a home-based aerobic exercise program for sedentary cancer survivors treated with hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(7):721–727. doi: 10.1038/sj.bmt.1704815 [DOI] [PubMed] [Google Scholar]
- 9.Steinberg A, Asher A, Bailey C, Fu JB. The role of physical rehabilitation in stem cell transplantation patients. Support Care Cancer. 2015;23(8):2447–2460. doi: 10.1007/s00520-015-2744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillis TA, Donovan ES. Rehabilitation following bone marrow transplantation. Cancer. 2001;92(4 Suppl):998–1007. doi: [DOI] [PubMed] [Google Scholar]
- 11.Craike MJ, Hose K, Courneya KS, Harrison SJ, Livingston PM. Perceived benefits and barriers to exercise for recently treated patients with multiple myeloma: a qualitative study. BMC Cancer. 2013;13:319. doi: 10.1186/1471-2407-13-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones LW, Courneya KS, Vallance JK, et al. Association between exercise and quality of life in multiple myeloma cancer survivors. Support Care Cancer. 2004;12(11):780–788. doi: 10.1007/s00520-004-0668-4 [DOI] [PubMed] [Google Scholar]
- 13.De Lisio M, Baker JM, Parise G. Exercise promotes bone marrow cell survival and recipient reconstitution post-bone marrow transplantation, which is associated with increased survival. Exp Hematol. 2013;41(2):143–154. doi: 10.1016/j.exphem.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan ML, Shelby RA, Dorfman CS, et al. The effect of pre-transplant pain and chronic disease self-efficacy on quality of life domains in the year following hematopoietic stem cell transplantation. Support Care Cancer. 2017;26(4):1243–1252. doi: 10.1007/s00520-017-3947-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somers TJ, Kelleher SA, Dorfman CS, et al. An mHealth Pain Coping Skills Training Intervention for Hematopoietic Stem Cell Transplantation Patients: Development and Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth. 2018;6(3):e66. doi. 10.2196/mhealth.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients. J Consult Clin Psychol. 2002;70(3):590–610. doi: 10.1037//0022-006x.70.3.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrykowski MA, Manne SL. Are psychological interventions effective and accepted by cancer patients? I. Standards and levels of evidence. Ann Behav Med. 2006;32(2):93–97. doi: 10.1207/s15324796abm3202_3 [DOI] [PubMed] [Google Scholar]
- 18.Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med. 2006;36(1):13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L [DOI] [PubMed] [Google Scholar]
- 19.Keefe FJ, Shelby RA, Somers TJ, et al. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain. 2011;152(4):730–741 doi: 10.1016/j.pain.2010.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant MJ, Simons AD, Thase ME. Therapist skill and patient variables in homework compliance: Controlling an uncontrolled variable in cognitive therapy outcome research. Cognitive Ther Res. 1999;23(4):381–399. doi: 10.1023/A:1018703901116 [DOI] [Google Scholar]
- 21.Kazantzis N, Deane FP, Ronan KR. Homework assignments in cognitive and behavioral therapy: A meta-analysis. Clin Psychol (New York). 2000;7(2):189–202. doi: 10.1093/clipsy.7.2.189 [DOI] [Google Scholar]
- 22.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 23.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Turk DC, Dworkin HD, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi: 10.1016/j.pain.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 25.Riley WT, Rothrock N, Bruce B, et al. Patient-reported outcomes measurement information system (PROMIS) domain names and definitions revisions: further evaluation of content validity in IRT-derived item banks. Qual Life Res. 2010;19(9):1311–1321. doi: 10.1007/s11136-010-9694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92(10 Suppl):S20–7. doi: 10.1016/j.apmr.2010.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristian A Assessing Physical Function and Fatigue in Patients Selected to Undergo Bone Marrow Transplant. Biol Blood Marrow Transplant. 2020;26(3):S364. Doi: 10.1016/j.bbmt.2019.12.197 [DOI] [Google Scholar]
- 28.Reeve BB, Pinheiro LC, Jensen RE, et al. Psychometric evaluation of the PROMIS Fatigue measure in an ethnically and racially diverse population-based sample of cancer patients. Psychol Test Assess Model. 2016;58(1):119–139. [Google Scholar]
- 29.Pochard F, Azoulay E, Chevret S, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: ethical hypothesis regarding decision making capacity. Crit Care Med. 2001;29(10):1893–1897. doi: 10.1097/00003246-200110000-00007 [DOI] [PubMed] [Google Scholar]
- 30.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–478. doi: 10.1056/NEJMoa063446 [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 32.Herrmann C International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi: 10.1016/s0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 33.Annunziata MA, Muzzatti B, Bidoli E, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer. 2020;28(8):3921–3926. doi: 10.1016/s0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 34.Gruber-Baldini AL, Velozo C, Romero S, Shulman LM. Validation of the PROMIS((R)) measures of self-efficacy for managing chronic conditions. Qual Life Res. 2017;26(7):1915–1924. doi: 10.1007/s11136-017-1527-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrante JM, Lulla A, Williamsonv JD, Devinev KA, Ohman-Strickland P, Bandera EV. Patterns of Fitbit use and activity levels among African American breast cancer survivors during an eHealth weight loss randomized controlled trial. Am J Health Promot. 2022;36(1):94–105. doi: 10.1177/08901171211036700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gell NM, Grover KW, Humble M, et al. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support Care Cancer. 2017;25:1291–1300. doi: 10.1007/s00520-016-3523-5 [DOI] [PubMed] [Google Scholar]
- 37.Rossi A, Frechette L, Miller D, et al. Acceptability and feasibility of a Fitbit physical activity monitor for endometrial cancer survivors. Gynecol Oncol. 2018;149(3):470–475. doi: 10.1016/j.ygyno.2018.04.560 [DOI] [PubMed] [Google Scholar]
- 38.Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5(3):233–237. doi: 10.1016/0149-7189(82)90074-x [DOI] [PubMed] [Google Scholar]
- 39.Lewinski AA, Crowley MJ, Miller C, et al. Applied rapid qualitative analysis to develop a contextually appropriate intervention and increase the likelihood of uptake. Medical Care. 2021;59:S242–S251. doi: 10.1097/MLR.0000000000001553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis. BMJ Open. 2018;8(10):e019993. doi: 10.1136/bmjopen-2017-019993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol. 2014;14(1):1–8. doi: 10.1186/1471-2288-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guest G, Namey E, McKenna K. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods. 2017;29(1):3–22. doi: 10.1177/1525822X16639015 [DOI] [Google Scholar]
- 43.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelleher SA, Winger JG, Fisher HM, et al. Behavioral cancer pain intervention using videoconferencing and a mobile application for medically underserved patients: Rationale, design, and methods of a prospective multisite randomized controlled trial. Contemp Clin Trials. 2021;102:106287. doi: 10.1016/j.cct.2021.106287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


