Abstract
Objectives:
There is currently a lack of consensus among neuropsychologists about which cognitive assessment paradigms hold the most promise in identifying subtle cognitive deficits in preclinical AD and which are most useful for monitoring risk of cognitive deterioration. Many widely used instruments are older versions of tests originally developed for the assessment of dementia or traumatic brain injury. Current efforts to digitize these measures provides more uniform and remote assessment, which is an advancement, but does not reflect significant changes in paradigmatic underpinnings or recent advances in cognitive neuroscience.
Methods:
This work provides an overview of novel Cognitive Challenge Tests (CCTs) that employ semantic interference paradigms that uniquely measure the failure to recover from proactive semantic interference (frPSI). Other salient methods to measure meaningful cognitive change in early-stage AD are also presented, as well as how they compare with traditional neuropsychological assessments. Finally, future directions for the development of more effective assessment paradigms are discussed.
Results:
frPSI is a cognitive marker which measures the persistent inability to learn new semantically competing stimuli despite multiple opportunities to do so, and impairments in semantic inhibitory control that have repeatedly shown to be useful in the early detection of AD during its preclinical stages and related to various biomarkers of AD and neurodegeneration among culturally diverse older adults.
Conclusions:
To meet the critical needs of a rapidly evolving field, cognitive assessment instruments must show sufficient scientific rigor including robust sensitivity, specificity, and predictive utility among culturally and linguistically diverse populations and importantly, be correlated to AD biomarkers.
Keywords: preclinical Alzheimer’s disease, cognitive challenge test, Mild Cognitive Impairment, cognitive assessment, LASSI-L, memory, semantic interference, frPSI
Introduction
Memory and List-learning Paradigms
For many decades, cognitive neuroscientists and neuropsychologists have studied different aspects of episodic memory in populations at-risk for Alzheimer’s Disease (AD) and Alzheimer’s Disease Related Disorders (ADRD). List-learning paradigms in particular, have long been used as a tool with which to quantify different components of learning and memory processes and were first clinically employed in the study of head injury and stroke, and eventually AD and other neurodegenerative brain disorders (Parsons, 2011). Subsequently, these instruments were introduced in studies of persons diagnosed with mild cognitive impairment (MCI).
The foundation of the elements comprising list-learning measures was based on the procedure first published by Rey and associates in 1964 but actually used in earlier research. This led to the development and implementation of different paradigmatically similar list-learning tasks such as the Hopkins Verbal Learning Test (Brandt 1991; Brandt & Benedict, 2001), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) List Learning task (Morris et al., 1989; Welsh, et al., 1994), the Auditory Verbal Learning Test (Rey, 1941; Rey 1964; Geffen et al., 1994), California Verbal Learning Test (CVLT-II; Woods et al., 2006), Free and Cued Selective Reminding Test (FCSRT; Grober & Buschke, 1987), CogState International Shopping Test (Mielke, et al., 2015), Neuropsychological Assessment Battery List Learning Test (NAB; Stern & White, 2003), and Fuld Object Memory Evaluation (Fuld et al., 1990). Abbreviated versions of these list-learning procedures also serve as important components of the widely used Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph et al., 1998), Alzheimer’s Disease Assessment Scale, Cognition Battery (ADAS-COG; Mohs, 1996), Memory Impairment Screen (Buschke et al., 1999), as well as the Montreal Cognitive Assessment battery (MoCA; Nasreddine et al., 2005). Common features of most comprehensive list-learning tests typically include multiple learning trials (to assess initial learning, learning curves, and serial position effects). Oftentimes, these include a delayed recall trial to measure forgetting over a specified time period, typically ranging from 20 to 30 minutes, but sometimes longer. Two widely used list-learning tests, the RAVLT-II and the CVLT-II are unique in that a second to-be-recalled target word list is introduced, which can provide an indication of proactive and retroactive interference. Proactive interference (PI) is a well-studied phenomena that impacts memory performance for recently learned information (old learning interferes with new learning) while retroactive interference (RI) occurs when learning new information disrupts the memory for information recently learned (new learning interferes with old learning) (Anderson & Neely, 1996). Unlike the RAVLT-II, the CVLT-II has some semantic overlap because several words on the second list (List B) share the same semantic category as the first list (List A). While this minimal semantic overlap on the CVLT-II may not be sufficient to gauge the extent of semantic interference in memory, it is useful to determine whether semantic clustering was used as a learning strategy. Recognition memory has also been employed in typical list-learning paradigms to tease out deficits in storage versus retrieval. The efficacy of these recognition memory paradigms is largely a function of the quality and relatedness of foils of the to-be-remembered targets, as well as the nature of the forced choice responses often employed.
Despite obvious strengths, the theoretical and conceptual underpinnings underlying the aforementioned traditional list-learning paradigms are remarkably similar. However, there is increased recognition that these memory measurement paradigms are prone to several weaknesses that limit their utility and effectiveness in the assessment of early cognitive changes in preclinical and prodromal AD and ADRD (Curiel Cid and Loewenstein 2022; Loewenstein, Curiel, Duara and Buschke, 2018). For one, a of lack of controlled learning may introduce a high level of variability in performance given the many potential learning strategies that can either help or hinder performance. Further, with regards to PI and RI, the failure to maximize the semantic relatedness or other shared properties of all targets in competing lists is a major limitation of traditional list-learning tests because they fail to capitalize on the strength of these effects. Finally, although PI is common to early AD, there have been no attempts to investigate an individual’s ability to overcome the effects of PI through additional learning trials.
Novel Cognitive Challenge Tests (CCTs)
In response to these issues, our team has developed Cognitive Challenge Tests (CCTs) that have shown great promise as outcome measures to enhance screening, diagnosis, and clinical monitoring in AD clinical trials. The conceptual underpinnings of these CCTs include: 1) facilitation of learning using semantic category cues during both encoding and retrieval; 2) implementing the same cues to produce failures of inhibitory control when learning new semantically competing information ; 3) assessment of the persistence of this failure to recover from proactive semantic interference and impaired semantic inhibitory control; 4) target cognitive systems known to be vulnerable in specific disease states (e.g., prodromal AD); 5) relating CCT performance to brain biomarkers of specific etiological causes of brain dysfunction (e,g., preclinical AD). Theoretically and empirically, CCTs offer a powerful alternative to existing methods.
One such example of a CCT is the Loewenstein-Acevedo-Scales for Semantic Interference and Learning (LASSI-L). The paradigmatic elements of the LASSI-L are as follows: The participant is first instructed that they will learn common words that belong to three semantic categories (fruits, musical instruments, and articles of clothing). Examinees are then presented with 15 words at four second intervals and asked to read and repeat the word. Cued recall is elicited by presenting each semantic category and then asking participants to remember words from that category. This process for List A is repeated for an additional trial. The provision of cues at both acquisition and retrieval maximizes learning effects for List A targets (Cued A2) and sets the stage for the presentation of List B targets. The competing List B also consists of 15 words that share the same semantic categories (fruits, musical instruments, and articles of clothing). Each word is presented in the same manner as the first list (List A) and share the identical category cues. These initial category cues used in List A that served as facilitators to learning now become the very mechanisms that elicit proactive semantic interference (PSI). PSI occurs when the learning of the new list (List B) is inhibited, and semantic intrusion errors are produced (reflecting a lack of inhibitory control). A truly unique aspect of the LASSI-L that makes it distinct from other list-learning paradigms is the second learning trial of List B. Cueing List B fruits, musical instruments and articles of clothing for a second trial (Cued B2) assesses the participant’s failure to recover from proactive semantic interference (frPSI). Retroactive semantic interference is then assessed by cued recall of the original List A targets. There is a twenty-minute delay in which participants are asked to recall targets on all lists that were presented. We have recently replaced this with a delayed forced recognition test in which the participant is asked whether a word was present in the first list, the second list, or no list. This more directly probes deficits on source memory. The LASSI-L procedure is outlined in Figure 1.
Figure 1:
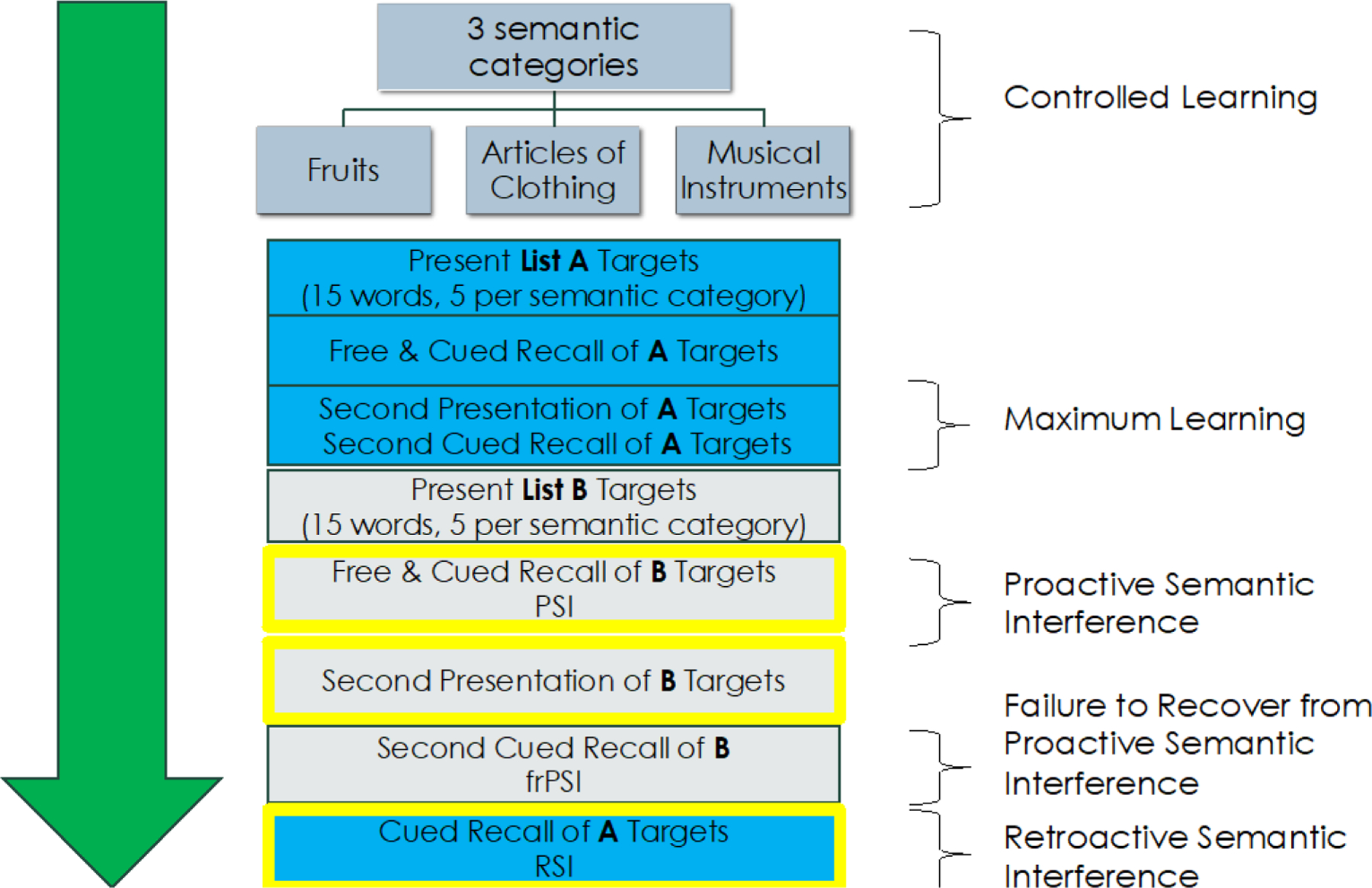
LASSI-L Semantic Interference Paradigm
Deficits on subtests of the LASSI-L, particularly PSI as well as frPSI and impairments in semantic inhibitory control, have been powerful markers of preclinical and prodromal AD that are described in detail in the section that follows. There is a growing literature that frPSI may be a distinguishing feature of prodromal AD as well as sensitive to AD biomarkers (Curiel et al., 2020; Loewenstein, et al., 2018; Matias-Guiu, et al., 2018; Loewenstein et al., 2017a; 2017b; Sanchez et al., 2017;).
In Table 1, we summarize key terms used. In Table 2 we highlight a few studies from the work of national and international groups with the LASSI-L that will be described below.
TABLE 1:
Select studies of PSI, frPSI and failures of semantic inhibitory control (e.g. semantic intrusion errors) on the LASSI-L
| Study Reference | Clinical Population Studied | Cultural Diversity of Sample | Primary LASSI-L Subtests Highlighted | Biomarker(s) Examined | General Conclusions |
|---|---|---|---|---|---|
| Loewenstein, D. A., Curiel, R. E., DeKosky, S., Bauer, R. M., Rosselli, M., Guinjoan, S. M., Adjouadi, M., Peñate, A., Barker, W. W., Goenaga, S., Golde, T., Greig-Custo, M. T., Hanson, K. S., Li, C., Lizarraga, G., Marsiske, M., & Duara, R. (2018). Utilizing semantic intrusions to identify amyloid positivity in mild cognitive impairment. Neurology, 91(10), e976–e984. | early AD (Amy+), SNAP (Amy-), or other neurologica l/psychiatric diagnosis (Amy-) | Hispanic/Latino (>30%) | Semantic intrusion errors on Cued B1 and Cued B2 | Positive association with amyloid PET and MRI | SIs on the LASSI-L related to PSI and frPSI uniquely differentiated Amy+ and Amy− participants with aMCI |
| Capp, K. E., Curiel Cid, R. E., Crocco, E. A., Stripling, A., Kitaigorodsky, M., Sierra, L. A., Melo, J. G., & Loewenstein, D. A. (2020). Semantic Intrusion Error Ratio Distinguishes Between Cognitively Impaired and Cognitively Intact African American Older Adults. Journal of Alzheimer’s disease: JAD, 73(2), 785–790. | Cognitively Unimpaired and amnestic MCI | Black/Afric an American older adults | Percentage of Intrusion Errors (PIE) on Cued B1 | None studied | PIE on LASSI-L subscales susceptible to PSI differentiated aMCI from CN and adds to emerging evidence that the LASSI-L may be culturally appropriate for use in Black/African American groups. |
| Curiel Cid, R. E., Loewenstein, D. A., Rosselli, M., Matias‐Guiu, J. A., Piña, D., Adjouadi, M., … & Duara, R. (2019). A cognitive stress test for prodromal Alzheimer’s disease: Multiethnic generalizability. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11(1), 550–559. | Cognitively Unimpaired and amnestic MCI | Hispanic/Latino (>45%) | Cued A2, Cued B1, Cued B2, Cued A3, delayed recall and semantic intrusion errors on all subtests | Associated with cortical thinning of the entorhinal cortex and precuneus on MRI in those with aMCI | Healthy controls obtained equivalent scores on all indices except retroactivesemantic interference. English-speaking and Spanish-speaking aMCI participants had equivalentscores except English speaker’s greater vulnerability to frPSI |
| Crocco, E., Curiel-Cid, R. E., Kitaigorodsky, M., González-Jiménez, C. J., Zheng, D., Duara, R., & Loewenstein, D. A. (2020). A Brief Version of the LASSI-L Detects Prodromal Alzheimer’s Disease States. Journal of Alzheimer’s disease: JAD, 78(2), 789–799. https://doi.org/10.3233/JAD-200790 | early-stage MCI, late-stage MCI to mild AD | Hispanic/Latino (>50%) | Cued A2, Cued B1 and semantic intrusion errors on Cued B1 | Positive association with amyloid PET | LASSI-L subscales taking approximately 6–8 minutes to administer, had excellent discriminative ability using established cut-offs among individuals with biomarker-confirmed prodromal AD. |
| Kitaigorodsky, M., Crocco, E., Curiel-Cid, R. E., Leal, G., Zheng, D., Eustache, M. K., Greig-Custo, M. T., Barker, W., Duara, R., & Loewenstein, D. A. (2021). The relationship of semantic intrusions to different etiological subtypes of MCI and cognitively healthy older adults. Alzheimer’s & dementia (Amsterdam, Netherlands), 13(1), e12192. https://doi.org/10.1002/dad2.12192 | Cognitively Normal: Amyloid Negative, MCI ‐ Non‐ Neurologic al: Amyloid Negative; MCI – Neurologic al: Amyloid Negative; MCI – SNAP: Amyloid Negative; MCI – AD: Amyloid Positive | Hispanic/Latino (>50%) | Cued B1, Cued B2, and semantic intrusion errors on these subtests | Positive association with amyloid PET | Semantic intrusion errors on the LASSI-L occur much less frequently in persons who have different types of non-AD-related MCI and may be used as an early cognitive marker of prodromal AD. |
| Crocco, E. A., Curiel Cid, R., Kitaigorodsky, M., Grau, G. A., Garcia, J. M., Duara, R., Barker, W., Chirinos, C. L., Rodriguez, R., & Loewenstein, D. A. (2021). Intrusion Errors and Progression of Cognitive Deficits in Older Adults with Mild Cognitive Impairment and PreMCI States. Dementia and geriatric cognitive disorders, 50(2), 135–142. https://doi.org/10.1159/000512804 | 116 older adults in the following groups: Stable Cognitively Unimpaired, amnestic MCI who progressed to dementia, PreMCI, PreMCI who Progressed to MCI or Reverted to CU | Hispanic/Latino (>50%) | Percentage of intrusion errors made in relation to total responses on Cued B1 | None studied | Percentage of Intrusion Errors (PIE) on LASSI-L subscales that measure PSI may be a useful predictor of clinical progression over time in at-risk older adults. |
| Curiel Cid, R. E., Crocco, E. A., Kitaigorodsky, M., Beaufils, L., Peña, P. A., Grau, G., Visser, U., & Loewenstein, D. A. (2021). A Novel Computerized Cognitive Stress Test to Detect Mild Cognitive Impairment. The journal of prevention of Alzheimer’s disease, 8(2), 135–141. https://doi.org/10.14283/jpad.2021.1 | Cognitively Unimpaired and amnestic MCI | Hispanic/Latino (>50%) | Cued A2, Cued B1, Cued B2 | None studied | The LASSI-BC, a brief computerized version of the LASSI-L is a valid and useful cognitive tool for the detection of aMCI among older adults. |
TABLE 2:
Descriptions of Acronyms for Commonly Used Terms
| Acronym | Name | What it is |
|---|---|---|
| LASSI-L | Loewenstein-Acevedo Scales for Semantic Interference and Learning | A Cognitive Challenge Test that employs a unique measure of the failure to recover from proactive semantic interference |
| SIT | Semantic Interference Test | A cognitive test that uses the first three trials of the Fuld Object Memory Evaluation followed by a semantically competing list in which each target had a unique semantic association with the original to-be-remembered target word. |
| PI | proactive interference | Old learning interfering with new learning |
| RI | retroactive interference | New learning interfering with recall of previously learned information |
| PSI | proactive semantic interference | Old learning interfering with learning of new semantically competing information |
| frPSI | failure to recover from proactive semantic interference | The inability to recover from the effects of PSI despite multiple learning trials. |
Proactive Semantic Interference (PSI) for the Assessment of Preclinical and Prodromal AD
Experimental paradigms in the cognitive neurosciences have aptly shown that interference effects have a profound influence on short-term memory processes, and there has been an ongoing debate as to whether short and longer intervals of delayed recall is a function of decaying memory traces versus interference effects, or a combination of the two processes (Portrat et al., 2008). Howard and colleagues (2006) proposed that lexical retrieval processes shared many features including competitive selection, priming, and shared activation. A combination of these processes led to cumulative semantic interference. These conceptual underpinnings formed the basis of several subsequent investigations on the effects of semantic interference on lexical retrieval tasks (Oppenheim, 2007; Wohner, et al., 2020). Atkins (2011) has cogently argued that interference effects in and of themselves are one of the greatest contributors to short-term memory failure. PSI effects are common features of early AD, and semantic, phonological, or contextual similarity of different lists of to-be-remembered targets can enhance the PSI effects (Loewenstein, et al., 2018). Ebert and Anderson (2009) investigated memory interference on the California Verbal Learning Test and found that measuring the words that interfered from a second target list did not differ between healthy older adults and individuals with aMCI. However, these same participants were administered an experimental paradigm in which semantically related word pairs were presented in an A-B versus A-C paired associate interference paradigm (A was a stem word associated to the semantically similar target). Results indicated greater PI effects, but no RI effects were observed for aMCI participants relative to healthy older adults. It has been concluded by several studies that susceptibility to proactive and retroactive interference are not merely a retrieval phenomenon but reflect the poor storage and consolidation deficits often observed in AD (Hanseeuw et al., 2012). An experimental paradigm was developed by Loewenstein and colleagues based upon their observation that semantic intrusions occurred with much greater frequency in early AD and aMCI groups diagnosed with presumptive underlying AD compared to a relatively low incidence of such errors among individuals with MCI related to early cardiovascular disease, major depression, or who were healthy older adults (Duara et al., 1989; Loewenstein et al., 2001). This led to the development of the Semantic Interference Test (SIT; Loewenstein et al., 2003; Loewenstein et al., 2004). Correct recall of the first three trials of the Fuld Object Memory Evaluation followed by a semantically competing list (susceptible to PSI) in which each target had a unique semantic association with the original to-be-remembered targets. For example, a first list target might include lighter while the second list included matches. Similarly, a first list target might include bracelet, while the second list target might be necklace. The SIT demonstrated high levels of sensitivity in differentiating aMCI participants with presumptive AD and was a strong predictor of progression from aMCI to dementia over a 30-month period (Loewenstein et al., 2007). Further, Loewenstein et al., (2016) demonstrated that PSI rather than RSI was much more associated with total and regional amyloid load on the SIT. Rosselli et al., (2019) also established that the SIT was a culturally fair instrument for Hispanic/Latino older adults.
It is important to note that PSI and frPSI are different from the concept of “release from proactive interference.” First PSI and frPSI represents a failure to learn new information in the face of recently learned competing information, and frPSI specifically is a unique measurement of the inability to recover from the effects of PSI despite additional exposures to the semantically competing stimuli. In contrast, the concept of release from proactive interference stems from the notion that trying to memorize different dates or semantically similar targets (e.g., animals) becomes increasingly difficult until examinees are presented with a series of unrelated targets which “releases” individuals from proactive interference because of the increased processing that occurs when the examinee perceives a change in the items that demand memorization. Release from proactive interference has had equivocal support in the AD literature (Cushman et al.,1988; Binetti et al., 1996; 1995) and no current evidence exists that release from proactive interference is an adequate diagnostic tool in identifying individuals with preclinical or prodromal AD. In contrast, measures such as the SIT and LASSI-L that employ proactive semantic interference have been promising in establishing PSI in prodromal AD, were predictive of progression, and related to AD biomarkers such as amyloid PET, as previously mentioned. There are some limitations to the SIT paradigm, which have been addressed by subsequent instruments such as the LASSI-L. For example, similar to most memory tests, the SIT does not employ controlled learning by offering explicit semantic cues at both acquisition and retrieval. This type of controlled learning does not only minimize the use of individual learning strategies but uses common semantic cues during recall that enhance the semantic interference effects (Loewenstein, et al., 2018). Second, the SIT did not maximize the potential to elicit semantic intrusion errors that reflect a lack of semantic inhibitory control. The SIT fails to measure the extent to which individuals are able to overcome initial PSI effects if provided additional opportunities to learn the semantically competing targets. Indeed, a review of the literature by Loewenstein, Curiel, Buschke and Duara (2018) confirms that PSI, and more specifically, frPSI may represent one of the earliest deficits in early amnestic MCI and can be elicited when one employs an equivalent number of semantically competing targets. That is, PSI is maximized by having shared semantic categories across both the initial and competing target lists, and when recall is elicited using semantic cues. This is one of the important conceptual underpinnings of a Cognitive Challenge Test (CST) as outlined above. PSI deficits may manifest in several ways. The traditional way in which PSI is ascertained is through observing a decreased total recall on the competing list relative to the initial learning capacity exhibited on the first target list; however, as we will later discuss, taking note of the semantic intrusion errors that are made during cued recall trials that elicit PSI, and calculating the total number of semantic intrusion errors made in relation to the total number of responses on a given trial, may be equally important to gauge a subtle, yet important breakdown in cognitive function associated with prodromal AD.
Failure to Recover from Semantic Interference (frPSI) is a Unique and AD-Relevant Assessment Paradigm
The ability to elicit PSI effects is important; however, until recently, there had been a paucity of data regarding the extent to which PSI can be reduced or ameliorated with a second and additional learning trials of the competing target list.
A number of studies employing the LASSI-L have confirmed the unique importance of frPSI in differentiating between aMCI and normal control groups, even after adjusting for initial learning (Crocco et al, 2014; Curiel et al., 2018; Loewenstein et al., 2021). There are intriguing results across a wide variety of studies conducted both nationally and internationally on frPSI that is uniquely assessed by the LASSI-L. In Madrid, Matias-Guiu and colleagues (2018) compared the LASSI-L Cued B2 recall and its original delayed recall trial against the FCSRT, a widely used traditional list-learning measure in AD, in differentiating MCI patients with and without AD patterns on FDG PET. Results indicated that the LASSI-L accounted for a significantly greater area under the Receiver Operating Characteristic Curve (auROC) of .894 compared to .708 for the FCSRT. Given that LASSI-L Cued B2 was such an important component of the predictive model and frPSI is not assessed by the FCSRT, the potential importance of frPSI was further highlighted. frPSI has also been uniquely related to volumetric reductions in AD prone regions on MRI in aMCI participants. In a study with 29 aMCI participants, correct Cued B2 recall (measuring frPSI) was the most highly related neuropsychological measure to volumetric reductions in AD prone regions on structural MRI such as the hippocampus, precuneus, inferior temporal, temporal pole, superior parietal, and rostral middle frontal lobes (Spearman’s rho ranged from rs=.44 to rs=.54). Measures such as the HVLT-R total recall and delayed recall, LASSI-L Cued A2 (maximum learning) and LASSI-L Cued B1 (proactive semantic interference) did not relate to any volumetric measurements. NACC delayed recall was only related to volumetric reductions in the inferior temporal lobe. In a larger replication and extension study of 45 aMCI patients from an entirely different cohort, only performance on Cued B2 recall was again strongly associated with volumetric reductions in the hippocampus, precuneus, inferior temporal lobe and superior parietal lobe (Loewenstein et al., 2017b). Cued B2 was also strongly related to volumetric reductions and cortical thickness in the entorhinal cortex in the second cohort where a greater percentage of participants were recruited from a specialty memory disorders clinic and had underlying AD. In this second larger study, again no other LASSI-L measures, HVLT-R or NACC delayed passages were related to any of the volumetric MRI measures. In both studies, the correct number of targets recalled on Cued B2 recall (frPSI) was also related to dilatation of the inferior lateral ventricle. Taken together, these structural MRI studies among participants were suggestive of the notion that among persons with aMCI, LASSI-L Cued B2 Recall (susceptible to frPSI), had a unique and strong relationship with brain volume on MRI. These associations were not obtained for traditional memory measures very commonly used in the assessment of MCI such as the HVLT-R or delayed NACC story passages. Similar results were obtained by Crocco and colleagues (2018) examining volumetric reductions in AD prone regions in a combined sample of PreMCI and cognitively unimpaired community-dwelling older adults.
PSI, frPSI, and Semantic Intrusion Errors
In 2018, our group studied 34 participants diagnosed with aMCI due to AD with positive amyloid PET imaging (that was visually read as positive by an expert rater) versus 29 individuals with aMCI who experienced a clinical course suggestive of AD but were amyloid negative (n=29) (Loewenstein et al., 2018). The latter cases were referred to as having suspected non-Alzheimer’s pathology (SNAP). Finally, there was a comparison group of 25 amyloid negative aMCI patients who had a variety of clinical conditions including cerebrovascular disease, diffuse Lewy body disease, frontal-temporal lobar degeneration, or neuropsychiatric conditions. We found that the amyloid positive aMCI group made considerably greater numbers of semantic intrusion errors on LASSI- L Cued B1 and Cued B2 subtests sensitive to PSI and frPSI relative to the aMCI amyloid negative group, even when adjusting for hippocampal atrophy, global cognition measured by the MMSE, and other demographic factors. However, no such differences were noted as it relates to the number of correct responses produced on Cued B1 and Cued B2 trials. These results suggested that Cued B1 and Cued B2 intrusion errors might be more specific to AD pathology in the MCI stage. We recognized at the time that this performance deficit was unlikely to be related to amyloidosis itself, but instead, another downstream process such as synaptic dysfunction, tau accumulation and/or another aspect of the cascade of pathology that occurs in early AD that might precede actual cell death that is reflected in neurodegeneration. The dissociation between correct responses on LASSI-L and semantic intrusion errors caused us to reflect on how PSI and frPSI might represent different cognitive processes in AD. Using confirmatory path analysis (Zheng et al., 2021) found that among 212 participants, semantic intrusion errors on both Cued B1 and Cued B2 of the LASSI-L were related to both amyloid positivity and decreased brain volumes on MRI using a composite of AD-prone regions (Dickerson et al., 2011). APOE ε4 status exerted indirect effects through its relationship with amyloid positivity while increased age also exerted its effects indirectly through both amyloid positivity and volumetric reductions. Adjustment for MMSE in the model did not alter the pattern of obtained results. A subsequent study examined older adults who were cognitively unimpaired and amyloid PET negative, and three groups with MCI due to varying etiologies: aMCI due to psychiatric conditions and were amyloid PET negative, aMCI who were amyloid PET negative and had neurological conditions, and aMCI who were amyloid PET positive. The amyloid negative groups made the fewest number of LASSI-L semantic intrusion errors on Cued B2 (6.5% and 5.9%) followed by 23.7% of amyloid negative SNAP participants, and 40% of amyloid negative patients with other neurological conditions. In contrast, 75% of aMCI amyloid positive individuals (prodromal AD) made semantic intrusion errors on Cued B2 (Kitaigorodsky et al., 2021). Semantic intrusion errors did not differ in those who were predominant English-speakers as compared to Spanish-speakers.
In an intriguing study, regarding frPSI, conducted at the Fleni Institute in Buenos Aires, Argentina, Sanchez and colleagues (2017) studied the adult children of parents with late onset AD and found that 50% of this largely asymptomatic group exhibited Cued B2 errors compared to 0% of controls. Interestingly, the number of Cued B2 but not Cued B1 errors were highly related to cortico-limbic dysconnectivity on fMRI.
Curiel Cid and associates (2020), recognized that a potential issue in solely accounting for the absolute number of semantic intrusions might fail to capture individuals with a low number of total responses. Persons who generated a low number of total correct responses might not necessarily make a large number of intrusions to meet the threshold of impairment, but still exhibit impaired inhibitory processes due to subtle underlying brain pathology. To address this issue, a ratio was developed to examine the percentage of intrusion errors in relation to the total number of responses (PIE). For example, to examine the PIE for measures such as Cued B2 recall the formula would be: Total Intrusion Errors/(Total Intrusion Errors + Total Correct Responses). In this initial study examining the utility of PIE on Cued B2, older adults who were amyloid PET negative and cognitively unimpaired, and amyloid PET negative and aMCI were clearly separated from amyloid positive aMCI patients (prodromal AD). Of particular interest is that among those with cognitive impairments, PIE on both Cued B1 and Cued B2 were significantly related to amyloid load even after adjusting for the degree of hippocampal atrophy. Crocco and colleagues (2021) used the percentage of intrusion errors (PIE) as a function of total responses on Cued B1 of the LASSI-L and found that a cutoff of 44% intrusion errors identified 83.3% of aMCI participants that progressed to dementia over an average 26-month period. Importantly, PIE also predicted those with PreMCI who reverted to cognitively normal status over time versus those PreMCI individuals who progressed to meet criteria for aMCI.
Understanding the Nature of the Deficits Underlying PSI and frPSI
There is a growing literature based on fMRI studies that reduced inhibition may be related to deficits in the right inferior frontal lobe and that self-monitoring and ability to manage distracting and competing material is related to bilateral prefrontal lobe deficits (Johnson & Anderson, 2004; Marko & Riečanský, 2021). Because of rich interconnections between these regions and posterior areas of the brain, including the hippocampus and other medial temporal lobe circuits, disruptions in any aspects of these integrated brain circuits may produce semantic interference effects and frPSI (Curiel and Loewenstein, 2022; Loewenstein, Curiel, Buschke, et al., 2018; Sanchez et al., 2017). Earlier notions that purely monolithic processes involving the hippocampus, entorhinal cortex, and other medial temporal regions accounted for semantic interference because of deficits in source memory, deficits in learning and working memory has been increasingly viewed as overly “locationist” and ignores the importance of interactive networks of other brain-regions such as the frontal regions in inhibition, self-monitoring, allocation of attentional resources, and other factors that may influence, PSI, frPSI and semantic intrusion errors.
There is increased evidence that inhibitory processes on PSI and frPSI involving correct recall are distinct from failures in semantic inhibitory control that result in cognitive breakdowns such as semantic intrusion errors. Lack of inhibitory control and deficits in self-monitoring that give rise to semantic intrusions on tasks sensitive to PSI and frPSI may reflect an intricate interaction between memory and executive function deficits. Interestingly, the default node network (which includes the posterior cingulate, precuneus as well as frontal temporal regions) has been associated with initial amyloid deposition in preclinical AD which typically start in frontal and lateral temporal lobe regions but extend to other regions as well (Bullich et al., 2020). Abnormal tau accumulation typically starts in the medial temporal regions as progressive patterns of tau accumulate in other areas of the neocortex (Braack & Braack, 1991). Thus, patterns of previously observed cortico-limbic dysconnectivity as well as structural regions prone to early neurodegeneration in AD point to possible disruption in a number of functional subsystems prone to early AD pathology. The persistent failure of aMCI patients to recover from the effects of PSI, even when accounting for initial learning and repeated presentation of the competing List B target lists, is a notable cognitive feature in aMCI and PreMCI states. Continued examination of these theoretical underpinnings is critical for future research efforts. frPSI and failures in semantic inhibitory control continue to be studied by different research groups and may yield invaluable insights into AD and related disorders.
Future Directions
There have been increasing attempts to digitize and create computerized versions of neuropsychological measures (Zygouris et al., 2015). While these may facilitate standardized and remote administration, these computerized tests are generally limited by the same paradigmatic underpinnings of the paper-and-pencil tests that they are meant to replace. Curiel et al., (2021) developed a computerized version of the LASSI-L, a novel cognitive paradigm based on semantic interference that evidences the same excellent discriminative properties as the paper-and-pencil version but can be administered on a tablet or computer and is web-based so that it can be administered remotely through a secure interface. Importantly, the LASSI-BC was built using advanced speech detection software and can audio record the entire testing session so that through machine learning, the program becomes increasingly accurate in recognizing both correct responses and intrusion errors. Many hundreds of individuals have been administered the LASSI-BC and ongoing work in our laboratory aims to relate these novel and AD-relevant cognitive assessment paradigms to multiple biomarkers of AD and neurodegeneration in different cultural and language groups (Capp et al., 2019; Curiel et al., 2020; Matias-Guiu et al., 2018; Sanchez et al., 2017). The LASSI-BC can be administered in both English and Spanish—and other languages—and compares quite favorably to the paper-and-pencil LASSI-L in discriminating aMCI from normal control participants (Curiel et al., In Preparation). Three equivalent alternate forms were developed to minimize practice effects and are currently under study.
We have recently innovated upon the validated LASSI-L to make the test more brief and able to capture additional discrete cognitive processes. Crocco and colleagues (2021) found that the first four subtests of the LASSI-L, which take 6 minutes to administer (see Figure 1), completely capture the optimal frPSI and semantic inhibitory effects as administering the full LASSI-L. In this study, the Brief LASSI-L showed excellent sensitivity and specificity in distinguishing persons with aMCI from cognitively unimpaired controls. Importantly, the cognitive subtests included in the Brief LASSI-L have shown significant associations with amyloid deposition on PET and are related to volumetric loss and cortical thinning in AD prone brain regions, which make the Brief-LASSI-L a potentially promising screening tool for clinical trials and clinical practice.
A gap in the previous LASSI-L literature is the lack of the assessment of delayed source memory deficits which have been related to amyloid load, as well as dysconnectivity on fMRI, and appear to involve different functional networks within the brain (Loewenstein et al., 2018; Curiel and Loewenstein, 2022)
For example, there is emerging evidence that disconnections between the left posterior parietal cortex and medial temporal lobe (MTL) regions interfere with source memory on semantically based delayed recognition paradigms. Moreover, deficits in source memory have also been related to amyloid load (Choi, et al., 2021). We have addressed this by developing a twenty-minute delayed recognition subtest for the LASSI-L and computerized LASSI-BC test. After a delay period, the examinee is presented with a target word or foil and must identify whether the word was on List A, List B or whether the word was on neither list. This provides an unprecedented opportunity to identify deficits in source memory for two semantically competing lists. This novel delayed semantic source memory paradigm will also allow us to gain knowledge about whether delayed semantic source memory deficits are distinct from failures in semantic inhibitory control (intrusion errors).
One question that arises from our previous work, and that of our national and international colleagues, was whether semantic interference effects persist given repeated learning trials to attempt to ameliorate these deficits in those at risk for AD. To address this important question, we developed a computerized Cognitive Stress Test (CST). The CST employs a similar computer interface as the LASSI-BC computerized test (Curiel et al, 2021) to administer semantically competing word lists over three (rather than two) trials. This provides a unique opportunity to examine the extent to which frPSI can be attenuated over time among persons with aMCI. To explore the utility of this measurement in preclinical AD, we are also studying the occurrence of this cognitive impairment in otherwise cognitively unimpaired older adults who are at risk of progressing to MCI. Loewenstein and associates (2021) have demonstrated that persons with aMCI improve in learning List B targets over time but fail to completely recover from proactive semantic interference effects. Cued B3 recall, as well as Cued B2 intrusions, are two CST measures that were particularly effective in distinguishing between aMCI and older adults. The extended word list (18) for the CST and three trials of List A and List B targets makes it suitable for even younger populations.
Finally, we realize that PSI, frPSI, and semantic intrusion errors represent only a few of many potential cognitive markers present during preclinical and prodromal AD and have worked to develop additional CCTs. We are actively investigating other processes such as the binding and unbinding of semantic paired associates. Our computerized Semantic Paired Associates Test is a unique paradigm that is theoretically and empirically based on our previous work and the literature (Buschke et al., 2013; Curiel et al., 2016; della Sala et al., 2012; Mowrey et al., 2018; Parra et al., 2010) regarding the vulnerabilities of memory binding among persons at risk for AD, but was specifically designed as a more challenging task to detect the subtle deficits inherent in preclinical and prodromal AD. This CCT requires that the examinee bind and later decouple or unbind semantically competing stimuli, which we believe is a challenge that extends beyond the typical memory binding paradigm. The semantic category selected was based on cross-cultural relevance where common birds represent the superordinate semantic category. This fully digitized test consists of a 6-item semantic paired associate learning task. Each pair consists of a stem word and its association. For example, the first stem word Pigeon is coupled with Duck, the second stem word Chicken is coupled with Swan, the third stem word Eagle is coupled with Sparrow. The examinee learns a total of six stem words and their associations over two consecutive trials (A Pairs). Then, the examinee is presented with the stem word on a computer screen and is asked to recall the bird that is associated with the stem. To challenge the cognitive system, the stem word becomes associated with another bird (B Pairs). For example, Pigeon is now coupled with Goose; Chicken is now coupled with Crow and Eagle is now coupled with Canary. All six stem words remain the same, but each new paired associate is unique. Just as with the first pairs, the examinee learns the stem words and their new associations over two consecutive trials. Failure to unbind previously learned semantic associations (measured by total correct B Pairs during the second trial) is the primary measure. Although paired associate learning detected aMCI, in our original published version, initial learning was adversely affected by the number of paired associates and their presentation (in a recognition memory format), making it difficult to ascertain semantic unbinding effects (Curiel et al., 2016).
Finally, our group is in the process of developing a fully digitized time and event-related prospective memory test as well as more fine-grained tests of fluid executive function and allocation of attentional resources. The LASSI-L, LASSI-BC, CST, and our modified Semantic Paired Association Test are all examples of cognitive stress tests. The underlying premise of the CCT paradigms are analogous to exercise EKGs in cardiology that stress the heart to uncover deficits not observed on resting EKG. Traditional neuropsychological measures whether administered singularly or in combination are typically administered in quiet places that are free of distraction, encourages optimal performance and provides an opportunity for individuals to use individual compensatory techniques. Alternatively, cognitive stress tests are designed to elicit and measure subtle cognitive deficits that may have otherwise gone undetected.
Our laboratory and international collaborators are also examining CCTs as they relate to examining amyloid and tau PET scans and are attempting to develop activation fMRI tasks to better elucidate the functional circuitry of underlying neural networks. Importantly, we have extended our cross-cultural work from Hispanic/Latino populations to Black/African American (Capp et al., 2019) and other groups under-represented in AD and ADRD research. Doing so will improve the field’s understanding of the extent to which emerging cognitive assessment methods, neuroimaging, fluid biomarkers, epigenetics and proteomics generalize to older adults from varied ethnic/cultural groups. Since persons rarely come to autopsy with AD alone, it is also important to attempt to determine the extent to which common co-morbidities such as cerebrovascular and metabolic risk, and inflammation may contribute to the Alzheimer’s cascade in different ethnic and cultural groups, particularly among persons who are still asymptomatic for Alzheimer’s disease.
As we continue to expand and refine our genetic, neuroimaging, and fluid biomarker assays in AD and ADRD, it is important for us to continue drawing from new discoveries in cognitive neurosciences to innovate upon our approaches to assess more distinct aspects of memory and other cognitive processes, recognizing that that cognitive processes and the functional systems in the brain that underlie these processes are quite sophisticated and that many available tests do not capture an orthogonal construct but rather involve a complex interplay between different aspects of memory, attention, and other processes. Neural networks that underlie different aspects of cognitive performance are complex and we believe that ongoing advances in cognitive neurosciences will continue to provide us with better tools to detect, monitor, and better understand the cognitive manifestations of specific disease states. The field and the people that we serve deserve no less.
Figure 2:
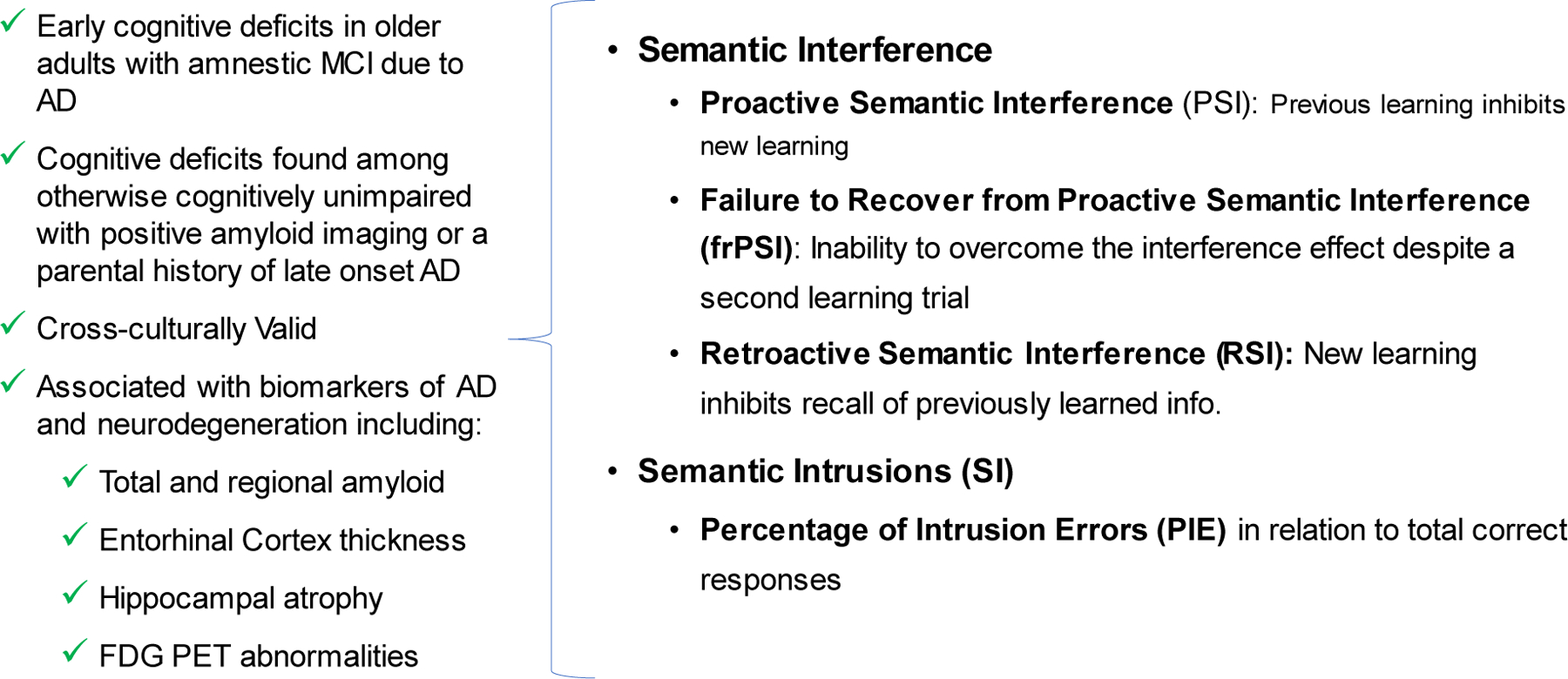
Salient Cognitive Deficits in Preclinical and Prodromal AD
Key Points.
Question:
What are novel Cognitive Challenge Tests (CCTs) and how are they useful for the assessment of preclinical Alzheimer’s Disease (AD)?
Findings:
Proactive semantic interference, the failure to recover from proactive semantic interference and semantic intrusion errors are salient cognitive markers of decline in MCI due to AD and associated with AD biomarkers, even during preclinical stages of the disease.
Importance:
The field of early detection of AD and AD related disorders is admonished to develop sensitive, specific, and cross-culturally relevant tests to improve the detection and monitoring of AD during preclinical and prodromal phases.
Next Steps:
As the field continues to develop and refine our genetic, neuroimaging and fluid biomarker assays in AD and ADRD, it is important for neuropsychologists to draw new discoveries in the cognitive neurosciences such as CCTs and refinements to these paradigms to sharpen our approaches to the assessment of memory and other cognitive processes implicated early in the disease process.
Acknowledgments
This work was funded by NIH/NIA grants R01AG055638 (Curiel Cid, Principal Investigator) and R01AG061106 (Loewenstein, Principal Investigator).
Footnotes
Drs. Loewenstein and Curiel Cid are co-inventors of intellectual property presented in Dr. Matias-Guiu has no potential conflicts to disclose.
References
- Anderson MC, & Neely JH (1996). Interference and inhibition in memory retrieval. In Memory (pp. 237–313). Academic Press. [Google Scholar]
- Atkins AS, Berman MG, Reuter-Lorenz PA, Lewis RL, & Jonides J (2011). Resolving semantic and proactive interference in memory over the short-term. Memory & cognition, 39(5), 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, & Trabucchi M (1996). Executive dysfunction in early Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 60(1), 91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binetti G, Magni E, Cappa SF, Padovani A, Bianchetti A, & Trabucchi M (1995). Semantic memory in Alzheimer’s disease: An analysis of category fluency. Journal of Clinical and Experimental Neuropsychology, 17(1), 82–89. [DOI] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica, 82(4), 239–259. [DOI] [PubMed] [Google Scholar]
- Brandt J, & Benedict RH (2001). Hopkins verbal learning test--revised: professional manual. Psychological Assessment Resources
- Brandt J (1991). The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The clinical neuropsychologist, 5(2), 125–142. [Google Scholar]
- Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eckholdt HM, & Lipton RB (1999). Screening for dementia with the memory impairment screen. Neurology, 52(2), 231–231. [DOI] [PubMed] [Google Scholar]
- Capp KE, Curiel Cid RE, Crocco EA, Stripling A, Kitaigorodsky M, Sierra LA, … & Loewenstein DA. (2020). Semantic intrusion error ratio distinguishes between cognitively impaired and cognitively intact African American older adults. Journal of Alzheimer’s Disease, 73(2), 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E-J, Kim BJ, Kim H-J, Kwon M, Han NE, Lee S-M, Jo S, Lee S, & Lee J-H (2021). False Memory and Alzheimer’s Disease Pathology in Patients with Amnestic Mild Cognitive Impairment: A Study with Amyloid PET. Dementia and Geriatric Cognitive Disorders Extra, 11(2), 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocco EA, Curiel Cid R, Kitaigorodsky M, Grau GA, Garcia JM, Duara R, Barker W, Chirinos CL, Rodriguez R, & Loewenstein DA (2021). Intrusion Errors and Progression of Cognitive Deficits in Older Adults with Mild Cognitive Impairment and PreMCI States. Dementia and geriatric cognitive disorders, 1–8. Advance online publication. 10.1159/000512804 [DOI] [PMC free article] [PubMed]
- Crocco EA, Loewenstein DA, Curiel RE, Alperin N, Czaja SJ, Harvey PD, … & Cardenas K. (2018). A novel cognitive assessment paradigm to detect Pre-mild cognitive impairment (PreMCI) and the relationship to biological markers of Alzheimer’s disease. Journal of psychiatric research, 96, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocco E, Curiel RE, Acevedo A, Czaja SJ, & Loewenstein DA (2014). An evaluation of deficits in semantic cueing and proactive and retroactive interference as early features of Alzheimer’s disease. The American Journal of Geriatric Psychiatry, 22(9), 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel Cid RE, & Loewenstein DA (2022). Salient Cognitive Paradigms to Assess Preclinical Alzheimer’s Disease. Neurotherapeutics, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel Cid RE, Crocco EA, Duara R, Garcia JM, Rosselli M, DeKosky ST, Smith G, Bauer R, Chirinos CL, Adjouadi M, Barker W, & Loewenstein DA (2020). A novel method of evaluating semantic intrusion errors to distinguish between amyloid positive and negative groups on the Alzheimer’s disease continuum. Journal of psychiatric research, 124, 131–136. 10.1016/j.jpsychires.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel Cid RE, Loewenstein DA, Rosselli M, Matias‐Guiu JA, Piña D, Adjouadi M, … & Duara R, (2019). A cognitive stress test for prodromal Alzheimer’s disease: Multiethnic generalizability. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11(1), 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel RE, Crocco EA, Raffo A, Guinjoan SM, Nemeroff CB, Piña D, Peñate A, Loewenstein DA (2018). Failure to Recover from Proactive Semantic Interference Differentiates Amnestic Mild Cognitive Impairment and PreMCI from Normal Aging after Adjusting for Initial Learning Ability. Advances in Alzheimer’s Disease Vol. 7, No.2 50–61. 10.4236/aad.2018.72004. [DOI] [Google Scholar]
- Curiel RE, Crocco E, Rosado M, Duara R, Greig MT, Raffo A, & Loewenstein DA (2016). A brief computerized paired associate test for the detection of mild cognitive impairment in community-dwelling older adults. Journal of Alzheimer’s disease, 54(2), 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel RE, Crocco E, Acevedo A, Duara R, Agron J, & Loewenstein DA (2013). A new scale for the evaluation of proactive and retroactive interference in mild cognitive impairment and early Alzheimer’s disease. Aging, 1(1), 1000102. [Google Scholar]
- Cushman LA, Como PG, Booth H, & Caine ED (1988). Cued recall and release from proactive interference in Alzheimer’s disease. Journal of clinical and experimental neuropsychology, 10(6), 685–692. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California verbal learning test. Assessment [DOI] [PubMed]
- Della Sala S, Parra MA, Fabi K, Luzzi S, & Abrahams S (2012). Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia, 50(5), 833–840. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, … & Detoledo-Morrell L. (2011). Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology, 76(16), 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duara R, Barker W, Loewenstein D, Pascal S, & Bowen B (1989). Sensitivity and specificity of positron emission tomography and magnetic resonance imaging studies in Alzheimer’s disease and multi-infarct dementia. European neurology, 29(Suppl. 3), 9–15. [DOI] [PubMed] [Google Scholar]
- Ebert PL, & Anderson ND (2009). Proactive and retroactive interference in young adults, healthy older adults, and older adults with amnestic mild cognitive impairment. Journal of the International Neuropsychological Society, 15(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Fuld PA, Masur DM, Blau AD, Crystal H, & Aronson MK (1990). Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. Journal of Clinical and Experimental Neuropsychology, 12(4), 520–528. [DOI] [PubMed] [Google Scholar]
- Geffen GM, Butterworth P, & Geffen LB (1994). Test-retest reliability of a new form of the auditory verbal learning test (AVLT). Archives of Clinical Neuropsychology, 9(4), 303–316. [PubMed] [Google Scholar]
- Grober E, & Buschke H (1987). Genuine memory deficits in dementia. Developmental neuropsychology, 3(1), 13–36. [Google Scholar]
- Hanseeuw BJ, Seron X, & Ivanoiu A (2012). Increased sensitivity to proactive and retroactive interference in amnestic mild cognitive impairment: new insights. Brain and cognition, 80(1), 104–110. [DOI] [PubMed] [Google Scholar]
- Howard D, Nickels L, Coltheart M, & Cole-Virtue J (2006). Cumulative semantic inhibition in picture naming: Experimental and computational studies. Cognition, 100(3), 464–482. [DOI] [PubMed] [Google Scholar]
- Johnson SK, & Anderson MC (2004). The Role of Inhibitory Control in Forgetting Semantic Knowledge. Psychological Science, 15(7), 448–453. 10.1111/j.0956-7976.2004.00700.x [DOI] [PubMed] [Google Scholar]
- Kitaigorodsky M, Crocco E, Curiel-Cid RE, Leal G, Zheng D, Eustache MK, Greig-Custo MT, Barker W, Duara R, & Loewenstein DA (2021). The relationship of semantic intrusions to different etiological subtypes of MCI and cognitively healthy older adults. Alzheimer’s & dementia (Amsterdam, Netherlands: ), 13(1), e12192. 10.1002/dad2.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel Cid RE, Kitaigorodsky M, Crocco EA, Zheng DD, & Gorman KL (2021). Amnestic Mild Cognitive Impairment is Characterized by the Inability to Recover from Proactive Semantic Interference across Multiple Learning Trials. The journal of prevention of Alzheimer’s disease, 8(2), 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, DeKosky S, Bauer RM, Rosselli M, Guinjoan SM, … & Duara R. (2018). Utilizing semantic intrusions to identify amyloid positivity in mild cognitive impairment. Neurology, 91(10), e976–e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, Duara R, & Buschke H (2018). Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer’s disease. Assessment, 25(3), 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, Wright C, Sun X, Alperin N, Crocco E, Czaja SJ, Raffo A, Penate A, Melo J, Capp K, Gamez M, Duara R (2017) Recovery from Proactive Semantic Interference in MCI and Normal Aging: Relationship to atrophy in brain regions vulnerable to Alzheimer’s Disease, Journal of Alzheimer’s Disease, 56:3 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, DeKosky S, Rosselli M, Bauer R, Grieg-Custo M, … & Duara R. (2017). Recovery from proactive semantic interference and MRI volume: A replication and extension study. Journal of Alzheimer’s Disease, 59(1), 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, Greig MT, Bauer RM, Rosado M, Bowers D, … & Duara R. (2016). A novel cognitive stress test for the detection of preclinical Alzheimer disease: discriminative properties and relation to amyloid load. The American Journal of Geriatric Psychiatry, 24(10), 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Greig MT, Curiel R, Rodriguez R, Wicklund M, Barker WW, … & Duara R. (2015). Proactive semantic interference is associated with total and regional abnormal amyloid load in non-demented community-dwelling elders: A preliminary study. The American Journal of Geriatric Psychiatry, 23(12), 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Agron J, & Duara R (2007). Vulnerability to proactive semantic interference and progression to dementia among older adults with mild cognitive impairment. Dementia and geriatric cognitive disorders, 24(5), 363–368. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, & Duara R (2004). Semantic interference deficits and the detection of mild Alzheimer’s disease and mild cognitive impairment without dementia. Journal of the International Neuropsychological Society, 10(1), 91–100. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Schram L, Ownby R, White G, Mogosky B, … & Duara R. (2003). Semantic interference in mild Alzheimer disease: preliminary findings. The American journal of geriatric psychiatry, 11(2), 252–255. [PubMed] [Google Scholar]
- Loewenstein DA, Argüelles T, Acevedo A, Freeman RQ, Mendelssohn E, Ownby RL, … & Duara R. (2001). The utility of a modified object memory test in distinguishing between different age groups of Alzheimer’s disease patients and normal controls. Journal of Mental Health and Aging, 7(3), 317–324. [Google Scholar]
- Marko M, & Riečanský I (2021). The left prefrontal cortex supports inhibitory processing during semantic memory retrieval. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 134, 296–306. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu JA, Cabrera-Martin MN, Curiel RE, Valles-Salgado M, Rognoni T, Moreno-Ramos T, Carreras JL, Loewenstein DA, Matias-Guiu J (2018) Comparison between FCSRT and LASSI-L to detect early-stage Alzheimer’s Disease, Journal of Alzheimer’s Disease 61 (1) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matías-Guiu JA, Curiel RE, Rognoni T, Valles-Salgado M, Fernández-Matarrubia M, Hariramani R, … & Matías-Guiu J. (2017). Validation of the Spanish version of the LASSI-L for diagnosing mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s Disease, 56(2), 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Machulda MM, Hagen CE, Edwards KK, Roberts RO, Pankratz VS, … & Petersen RC. (2015). Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimer’s & Dementia, 11(11), 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs RC (1996). The Alzheimer’s disease assessment scale. International psychogeriatrics, 8(2), 195–203. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum GDME, … & Clark C. (1989). The consortium to establish a registry for Alzheimer’s disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology [DOI] [PubMed]
- Mowrey WB, Lipton RB, Katz MJ, Ramratan WS, Loewenstein DA, Zimmerman ME, & Buschke H (2018). Memory binding test predicts incident dementia: results from the einstein aging study. Journal of Alzheimer’s Disease, 62(1), 293–304. [DOI] [PubMed] [Google Scholar]
- Multhaup KS, Balota DA, & Faust ME (2003). Exploring semantic memory by investigating buildup and release of proactive interference in healthy older adults and individuals with dementia of the Alzheimer type. Journal of the International Neuropsychological Society, 9(6), 830–838. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, … & Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Oppenheim GM, Tainturier MJ, & Barr P (2015). Preserved cumulative semantic interference despite amnesia. In Front. Psychol. Conference Abstract: Academy of Aphasia 53rd Annual Meeting
- Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, & Della Sala S (2010). Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain, 133(9), 2702–2713. [DOI] [PubMed] [Google Scholar]
- Parsons TD (2011). Neuropsychological assessment using virtual environments: Enhanced assessment technology for improved ecological validity. In Advanced computational intelligence paradigms in healthcare 6. Virtual reality in psychotherapy, rehabilitation, and assessment (pp. 271–289). Springer Berlin Heidelberg. [Google Scholar]
- Portrat S, Barrouillet P, & Camos V (2008). Time-related decay or interference-based forgetting in working memory? Journal of Experimental Psychology: Learning, Memory, and Cognition, 34(6), 1561–1564. 10.1037/a0013356 [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, & Chase TN (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of clinical and experimental neuropsychology, 20(3), 310–319. [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie Presses Universitaires de France. City. [Google Scholar]
- Rey A. (1941). L’examen psychologique dans les cas d’encéphalopathie traumatique.(Les problems.). Archives de psychologie
- Rosselli M, Tappen RM, & Newman D (2019). Semantic Interference Test: Evidence for Culture and Education Fairness from an Ethnically Diverse Sample in the USA. Archives of Clinical Neuropsychology, 34(3), 337–349. [DOI] [PubMed] [Google Scholar]
- Sanchez SM, Abulafia C, Duarte-Abritta B, Ladron de Guevara S, Castro M, Drucaroff L, Sevlever G, Nemeroff CB, Vigo DE, Loewenstein DA, Villarreal M, Guinjoan S (2017) Failure to recover from proactive semantic interference and abnormal limbic connectivity in asymptomatic, middle-aged offspring of patients with late-onset Alzheimer’s Disease. Journal of Alzheimer’s Disease, 60 (3): 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram LL, Rubert M, & Loewenstein DA (1995). A qualitative analysis of semantic intrusive errors in Alzheimer’s disease. Archives of Clinical Neuropsychology, 10(3), 255–263. [PubMed] [Google Scholar]
- Stern RA, & White T (2003). NAB, Neuropsychological Assessment Battery: Administration, scoring, and interpretation manual. Lutz: Psychological Assessment Resources
- Torres VL, Rosselli M, Loewenstein DA, Curiel RE, Vélez Uribe I, Lang M, … & Duara R. (2019). Types of errors on a semantic interference task in mild cognitive impairment and dementia. Neuropsychology, 33(5), 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, & Heyman A (1994). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology, 44(4), 609–609. [DOI] [PubMed] [Google Scholar]
- Wöhner S, Jescheniak JD, & Mädebach A (2020). Semantic interference is not modality specific: Evidence from sound naming with distractor pictures. Quarterly Journal of Experimental Psychology, 73(12), 2290–2308. [DOI] [PubMed] [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, & Holdnack JA (2006). The California Verbal Learning Test–second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of clinical neuropsychology, 21(5), 413–420. [DOI] [PubMed] [Google Scholar]
- Zheng DD, Curiel Cid RE Duara R., Kitaigorodsky M., Crocco E., & Loewenstein DA. (2021). Semantic intrusion errors as a function of age, amyloid, and volumetric loss: a confirmatory path analysis. International psychogeriatrics, 1–11. [DOI] [PMC free article] [PubMed]


