Abstract
Background
Vasomotor symptoms, such as hot flushes and night sweats, are very common during the menopausal transition. Hormone therapy has traditionally been used as a highly effective treatment, but concerns about increased risk of some chronic diseases have markedly increased the interest of women in alternative treatments. Some of the most popular of these treatments are foods or supplements enriched with phytoestrogens—plant‐derived chemicals that have estrogenic action.
Objectives
To assess the efficacy, safety and acceptability of food products, extracts and dietary supplements containing high levels of phytoestrogens when compared with no treatment, placebo or hormone therapy for the amelioration of vasomotor menopausal symptoms (such as hot flushes and night sweats) in perimenopausal and postmenopausal women.
Search methods
Searches targeted the following electronic databases: the Cochrane Menstrual Disorders and Subfertility Group Specialised Register of randomised trials (29 July 2013), the Cochrane Register of Controlled Trials (CENTRAL; 29 July 2013), MEDLINE (inception to 29 July 2013), EMBASE (inception to 29 July 2013), AMED (1985 to 29 July 2013), PsycINFO (inception to 29 July 2013) and CINAHL (inception to 29 July 2013). Attempts were made to access grey literature by sending letters to pharmaceutical companies and performing searches of ongoing trial registers. Reference lists of included trials were also searched.
Selection criteria
Studies were included if they were randomised, included perimenopausal or postmenopausal participants with vasomotor symptoms (hot flushes or night sweats), lasted at least 12 weeks and provided interventions such as foods or supplements with high levels of phytoestrogens (not combined with other herbal treatments). Trials that included women who had breast cancer or a history of breast cancer were excluded.
Data collection and analysis
Selection of trials, extraction of data and assessment of quality were undertaken by at least two review authors. Most trials were too dissimilar for their results to be combined in a meta‐analysis, so these findings are provided in narrative 'Summary of results' tables. Studies were grouped into broad categories: dietary soy, soy extracts, red clover extracts, genistein extracts and other types of phytoestrogens. Five trials used Promensil, a red clover extract; results of these trials were combined in a meta‐analysis, and summary effect measures were calculated.
Main results
A total of 43 randomised controlled trials (4,364 participants) were included in this review. Very few trials provided data suitable for inclusion in a meta‐analysis. Among the five trials that yielded data assessing the daily frequency of hot flushes suitable for pooling, no significant difference overall was noted in the incidence of hot flushes between participants taking Promensil (a red clover extract) and those given placebo (mean difference (MD) ‐0.93, 95% confidence interval (CI) ‐1.95 to 0.10, I2 = 31%). No evidence indicated a difference in percentage reduction in hot flushes in two trials between Promensil and placebo (MD 20.15, 95% CI ‐12.08 to 52.38, I2 = 82%). Four trials that were not combined in meta‐analyses suggested that extracts with high (> 30 mg/d) levels of genistein consistently reduced the frequency of hot flushes. Individual results from the remaining trials were compared in broad subgroups such as dietary soy, soy extracts and other types of phytoestrogens that could not be combined. Some of these trials found that phytoestrogen treatments alleviated the frequency and severity of hot flushes and night sweats when compared with placebo, but many trials were small and were determined to be at high risk of bias. A strong placebo effect was noted in most trials, with a reduction in frequency ranging from 1% to 59% with placebo. No indication suggested that discrepant results were due to the amount of isoflavone in the active treatment arm, the severity of vasomotor symptoms or trial quality factors. Also, no evidence indicated that these treatments caused oestrogenic stimulation of the endometrium or the vagina or other adverse effects when used for up to two years.
Authors' conclusions
No conclusive evidence shows that phytoestrogen supplements effectively reduce the frequency or severity of hot flushes and night sweats in perimenopausal or postmenopausal women, although benefits derived from concentrates of genistein should be further investigated.
Keywords: Female, Humans, Hot Flashes, Hot Flashes/drug therapy, Isoflavones, Isoflavones/therapeutic use, Phytoestrogens, Phytoestrogens/therapeutic use, Randomized Controlled Trials as Topic, Soybeans, Sweating, Sweating/drug effects, Trifolium
Plain language summary
Phytoestrogens for vasomotor menopausal symptoms
Review question: This Cochrane review has evaluated whether phytoestrogen treatments reduce the number and severity of hot flushes and whether they are safe and acceptable.
Background: Hormone therapy is an effective treatment for controlling the most common menopausal symptoms—hot flushes and night sweats. However, it is now recommended only in low doses given for the shortest possible time because of concerns about increased risk of some chronic diseases. Many women have started to use therapies that they perceive as 'natural' and safe, but they often do not have good information about the potential benefits and risks. Some of these therapies contain phytoestrogens—a group of plant‐derived chemicals that are thought to prevent or treat disease. Phytoestrogens are found in a wide variety of plants, some of which are foods, particularly soy, alfalfa and red clover.
Study characteristics: This review found 43 RCTs conducted up to July 2013 that included 4,084 participants with hot flushes who were close to the menopause or were menopausal. Evidence obtained is current to July 2013.
Key results: Some trials reported a slight reduction in hot flushes and night sweats with phytoestrogen‐based treatment. Extracts containing high levels of genistein (a substance derived from soy) appeared to reduce the number of daily hot flushes and need to be investigated further. Overall no indication suggested that other types of phytoestrogens work any better than no treatment. No evidence was found of harmful effects on the lining of the womb, stimulation of the vagina or other adverse effects with short‐term use.
Quality of the evidence: Many of the trials in this review were small, of short duration and of poor quality, and the types of phytoestrogens used varied substantially.
Background
Description of the condition
Menopause is a significant event in the lives of most women, as it marks the end of a woman's natural reproductive life. The perimenopausal and early postmenopausal years are typically characterised by falling levels of endogenous oestrogen, which can give rise to vasomotor symptoms that are severe and disruptive, particularly in the early and late menopausal transition and in early postmenopause, as categorised by the STRAW (STages of Reproductive Aging Workshop) criteria (Harlow 2012). These vasomotor symptoms include hot flushes (also known as 'hot flashes'), sweating and sleep disturbances.
Hot flushes are described as sudden feelings of heat in the face, neck and chest (WHO 1996). Hot flushes are frequently accompanied by skin flushing and perspiration, followed by a chill as core body temperature drops (Freedman 2001; Kronenberg 1990). Flushes vary in frequency, duration and severity and may be spontaneous and unpredictable (Freedman 1995). Hot flushes that occur during the night are typically referred to as night sweats. Flushes and night sweats are events of concern in themselves because they can disrupt sleep patterns and alter daily activities, which can lead to fatigue and decreased quality of life (Ayers 2013; NAMS 2004). Hot flushes are thought to result from both the brain's response to diminished hormones and hormonal fluctuations that occur during the menopausal transition, which leads to instability of thermoregulatory mechanisms (that regulate temperature) in the hypothalamus (Deecher 2007; Freedman 2001; Kronenberg 1987).
The prevalence of vasomotor symptoms varies with ethnicity. Flushes are less common among East Asian women (median 16%) than among American and European women (median 55%) (Freeman 2007). Up to 40% of Western women are affected severely enough to seek medical help (Freeman 2007; Gold 2006). An Australian prospective study with 13‐year follow‐up reported that the mean duration of troublesome vasomotor symptoms was 5.5 years (Col 2009). A study of more than 10,000 British women 54 to 65 years of age found that more than half (54%) were currently experiencing vasomotor symptoms (averaging 34 hot flushes or night sweats per week), which were problematic in 40% of cases and were fairly stable across the age range (Hunter 2012). Although hot flushes are reported as more prevalent and intense in the perimenopausal and early postmenopausal years, they continue to be important in up to 14.6% of women in their sixties and in 8.6% of women in their seventies (Roussouw 2007).
Description of the intervention
Most therapies designed to combat menopausal vasomotor symptoms aim to supplement levels of circulating oestrogen (Sikon 2004). The treatment of choice has traditionally been hormone therapy (HT), but, despite its effectiveness for symptom reduction, a marked and global decline has occurred in the prescription and use of HT because of concerns about long‐term use, particularly worry about increased risk of chronic diseases (Bestul 2004; Haas 2004; Travers 2006). Although the combination of HT and unopposed oestrogen therapy was previously prescribed to prevent the onset of cardiovascular events as women grew older, a report of the Women's Health Initiative (WHI) trial, in 2002, indicated that the risks of this treatment outweighed the benefits (Roussouw 2002). Combined therapy was linked with increased risk of breast cancer, stroke, thromboembolism (blood clots), gallbladder disease and dementia. Unopposed oestrogen therapy increased the risk of stroke, thromboembolism and gallbladder disease, and other studies reported an increase in the incidence of breast cancer (Beral 2003). Data now available from 11 years of follow‐up provided by WHI show that risks are influenced by the age of the woman, the time since menopause and whether the HT was combined or consisted of oestrogen only (NAMS 2012). Contraindications to HT include a family history or increased risk of cardiovascular disease, blood clotting disorders, venous thromboembolism or certain hormone‐sensitive cancers (Anderson 2003; Grady 2000). Some women report adverse effects when taking HT (Bakken 2004; Bjorn 1999); potential side effects include breast tenderness, bloating and genital bleeding. Regulatory bodies around the world are now advocating that HT should be prescribed only in the smallest dose and for the shortest possible time (Europ Med Ag 2006; UK MHRA 2007).
Potential health risks associated with HT and further uncertainty surrounding actual benefits to be gained from it have caused many women to seek non‐medical alternatives (Bair 2005; Newton 2002). 'Natural' therapies appear to be very popular among women; a survey of 866 women 45 to 65 years of age reported that 61% agreed or strongly agreed with the statement that natural approaches are better than hormone pills for menopausal symptoms (Newton 2002). In a national survey on women's use of complementary alternative medicine (CAM), more than 50% of CAM users indicated that such use was consistent with their beliefs, and 55% said that they wanted a natural approach to treatment (Chao 2006).
However, sufficient research on the risks and benefits of these approaches is lacking. A survey of women seen at a university clinic reported that 70% of women taking dietary supplements did not inform their doctors about their use, and only 4% had received information about such supplements from a healthcare provider (Mahady 2003). In a national survey, when women using CAM for menopausal symptoms consulted a doctor, their disclosure rate (of CAM) was much higher, with only 36% of women reporting that they did not disclose their self treatment with CAM to their doctors (Wade 2008).
Therapies based on phytoestrogens are among the most common alternatives to HT. Phytoestrogens are nonsteroidal plant compounds of diverse structure that are found in many fruits, vegetables and grains (Knight 1996; Thompson 1991). The most common types of phytoestrogens are coumestans, lignans and isoflavones. These compounds structurally resemble oestradiol (E2) and are shown to have weak oestrogenic activity (Makela 1994; Setchell 1998). When ingested in relatively large quantities, dietary phytoestrogens have been shown to have significant biological effects in several animal species (Adlercreutz 1995) and in humans (Wilcox 1990). In humans, they appear to have both oestrogenic and anti‐oestrogenic effects, depending on the concentrations of circulating endogenous oestrogens and oestrogen receptors (Bolego 2003).
Isoflavones are among the most oestrogenically potent phytoestrogens; the major dietary isoflavones, genistein and daidzein, are found almost exclusively in legumes such as soy, chick peas, lentils and beans (Cassidy 1993). Urinary excretion of equol, a weak oestrogen, in humans eating soy‐supplemented diets can greatly exceed the concentration of urinary endogenous oestrogens; this enhances the plausibility of human physiological health effects (Setchell 1984). Other classes of phytoestrogens—lignans and prenylated flavonoids—also have potent oestrogenic activity but are not as well studied (Adlercreutz 1987; Milligan 1999). Soy, a particularly abundant source of isoflavones, is a staple ingredient in the traditional Asian diet. It is postulated that high intake of soy among Asian women may account for lower rates of some menopausal symptoms in this group. Asian populations, such as those in Japan, Taiwan and Korea, are estimated to consume 20 to 150 mg per day of isoflavones, with a mean of about 40 mg from tofu (soy bean curd) and miso (soy bean paste). Soy includes such products as tofu, miso, aburage (fried thin tofu) and fermented or boiled soy beans. Further evidence that soy might be beneficial is suggested by a cohort study of Japanese women (Nagata 2001), which found a significant inverse association between frequency of flushes and higher levels of soy consumption. However, the findings of this study are contradicted by data from a cross‐sectional study, which found that women who frequently consumed soy products were not less likely to report hot flushes or night sweats than women who never consumed soy products (Sievert 2007). Thus it is not clear whether frequent soy consumption explains the lower rate of hot flushes among different ethnic groups. Red clover (Trifolium pratense), another source of isoflavones, contains compounds that are metabolised to genistein and daidzein after consumption. The most studied red clover product is Promensil.
Potential adverse effects of phytoestrogens have included deficits in sexual behaviour in rats and impaired fertility in livestock (Bennetts 1946). No specific examples of toxicity among humans have been noted in countries in which soy is consumed regularly (Setchell 1997). It is generally considered difficult for humans to consume the quantity of isoflavones from natural soy foods needed to reach toxicological levels that induce pathological effects, as recorded in animals.
How the intervention might work
No clear explanation is known for how phytoestrogens might work in reducing hot flushes among perimenopausal and postmenopausal women.
It has been suggested that phytoestrogens act as selective oestrogen receptor modulators (SERMs), exerting anti‐oestrogenic effects in the high‐oestrogen environment of premenopause and oestrogenic effects in the low‐oestrogen environment of postmenopause, where they act as weak agonists by stimulating oestrogen receptors (Seibel 2003). Phytoestrogens appear to show greater affinity for the oestrogen receptor beta (ERβ) than for the classical oestrogen receptor alpha (ERα). As a result, they preferentially express oestrogenic effects in the central nervous system, blood vessels, bone and skin without causing stimulation of the breast or uterus (Kuiper 1997). Thus, phytoestrogens may reduce vasomotor symptoms through their action on the vascular system without causing unwanted oestrogenic effects on other body systems.
Why it is important to do this review
Current use of phytoestrogen products among perimenopausal and postmenopausal women with vasomotor symptoms is high; an American cross‐sectional analysis of more than 2,000 women (Study of Women's Health Across the Nation (SWAN)) reported that 11% of women with vasomotor symptoms used flaxseed products and 19% used soy products (Gold 2007). Several reviews have examined the efficacy of phytoestogen products in alleviating menopausal symptoms, but most have found no benefit or a very slight reduction in the frequency of daily hot flushes compared with placebo. Government agencies and healthcare organisations have also scrutinised the effects of phytoestrogens, particularly isoflavones (AFSSA 2005; Com Tox 2003). The North American Menopause Society (NAMS) position statement on the treatment of menopause‐associated vasomotor symptoms suggests that women should consider isoflavone supplementation if their menopausal flushing does not respond to other interventions (NAMS 2004; NAMS 2011). However, NAMS acknowledges that the evidence base for this recommendation is poor.
Thus, the aim of this review is to synthesise all available evidence on the efficacy, safety and acceptability of products containing phytoestrogens to assist women with vasomotor menopausal symptoms to reduce their symptoms by making good evidence‐based treatment decisions.
Objectives
To determine the efficacy, safety and acceptability of food products, extracts and dietary supplements containing high levels of phytoestrogens when compared with no treatment, placebo or hormone therapy for the amelioration of vasomotor menopausal symptoms (such as hot flushes and night sweats) in perimenopausal and postmenopausal women.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled comparisons of food products, extracts or dietary supplements containing high levels of phytoestrogens (e.g. at least 30 mg/d of isoflavones) versus placebo, HT, no treatment or products containing low levels of phytoestrogens for the alleviation of vasomotor menopausal symptoms.
Types of participants
Inclusion criteria
Perimenopausal women, defined as women in the 45‐ to 55‐year age range, who have menstruated within the past 12 months and are seeking treatment for menopausal vasomotor symptoms
Postmenopausal women, defined as women who are older than 45 years of age, who have not menstruated for longer than 12 months and are seeking treatment for menopausal symptoms
Women experiencing spontaneous or surgical menopause (bilateral oophorectomy (removal of both ovaries)) were eligible. Trials were eligible only when most women had vasomotor symptoms.
Source of recruitment Any healthcare setting or the community
Exclusion criteria
Intercurrent major disease
Previous HT (hormone therapy) within one month of commencement of the study or an oestrogen implant within the past year
Women with breast cancer or a history of breast cancer
Women with no or inconsequential vasomotor symptoms at baseline
Types of interventions
All food products or dietary supplements containing high levels of phytoestrogens (> 30 mg/d of isoflavones, > 100 µg 8‐prenylnaringenin or > 10,000 µg total lignans) versus placebo, hormone therapy, no treatment or food products with low levels of phytoestrogens given as perimenopausal or postmenopausal therapy for the alleviation of vasomotor menopausal symptoms for a period of at least 12 weeks. Studies in which phytoestrogens were combined with other therapies were excluded.
Types of outcome measures
Primary outcomes
Efficacy
Change in vasomotor menopausal symptom scores (without distinction between types of vasomotor symptoms)
Change in frequency of individual vasomotor symptoms or severity of individual vasomotor symptom scores (e.g. hot flushes and night sweats)
Incidence of vasomotor symptoms (hot flushes and night sweats) after treatment
Studies were included if they measured vasomotor symptoms on a subscale of a compendium score, for example, Greene Score, Kupperman Index, Nordin Score, MacLennan Score or any other general menopausal symptom score that derives numerical results from a combination of vasomotor menopausal symptoms.
In addition, studies were included that measured individual vasomotor symptoms, for example, severity or frequency, or both, of hot flushes and night sweats (evaluated subjectively by participants, usually in daily diaries).
Secondary outcomes
Safety
Stimulation of the endometrium (endometrial thickness, rates of atrophic endometrium)
Vaginal stimulation (pH, maturation value)
Adverse events
Acceptability
Acceptability of therapy (withdrawal due to adverse events or satisfaction rates)
Studies were included if they measured specific safety outcomes, such as measures of physiological oestrogenicity of the endometrium and vagina. Other possible safety outcomes could be measured that are related to the effects of oestrogen action on other tissue and organs, but these will be assessed in future reviews if evidence of a beneficial effect on symptoms is noted.
Search methods for identification of studies
The Trials Search Co‐ordinator designed the search strategy for use with the electronic databases. The complete search strategies are listed in the Appendices of this review.
Electronic searches
The Trials Search Co‐ordinator of the Menstrual Disorders and Subfertility Group (MDSG) searched for all published and unpublished randomised controlled trials (RCTs) of phytoestrogens for vasomotor symptoms, with no language restriction, using the following electronic databases.
MEDLINE (see Appendix 1).
EMBASE (see Appendix 2).
PsycInfo (see Appendix 3).
AMED (see Appendix 4).
Cochrane Central Register of Controlled Trials (see Appendix 5).
MDSG Specialised Register of Controlled Trials (see Appendix 6).
The principal author of the review (AL) searched the following trial registers and websites.
Trial registers for ongoing and registered trials: http://www.controlled‐trials.com
Citation indexes: http://scientific.thomson.com/products/sci
Conference abstracts in the Web of Knowledge: http://www.wokinfo.com/
LILACS database, for trials from the Portuguese‐ and Spanish‐speaking world: http://bases.bireme.br/cgibin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F
Results from clinical trials of marketed pharmaceuticals: http://www.clinicalstudyresults.org
PubMed: http://www.ncbi.nlm.nih.gov/pubmed/
OpenSIGLE database: http://opensigle.inist.fr/
Google
Searching other resources
The reference lists of retrieved potentially eligible studies and relevant reviews were also searched. Novogen, manufacturer of a standardised extract of phytoestrogens (Promensil), was contacted for details of unpublished trials.
Data collection and analysis
Selection of studies
Trials for inclusion in the review were selected at different times by two review authors (AL and FK, JM or JB) after the search strategy described previously was employed. First, titles and abstracts were scanned, and full‐text copies of those that appeared relevant were retrieved to determine whether they met the inclusion criteria for the review. If necessary, authors of potential trials for inclusion were contacted to clarify study eligibility. Disagreements over selection were resolved by consensus. The selection process for the 2013 update has been documented on a flow chart (Figure 1).
1.
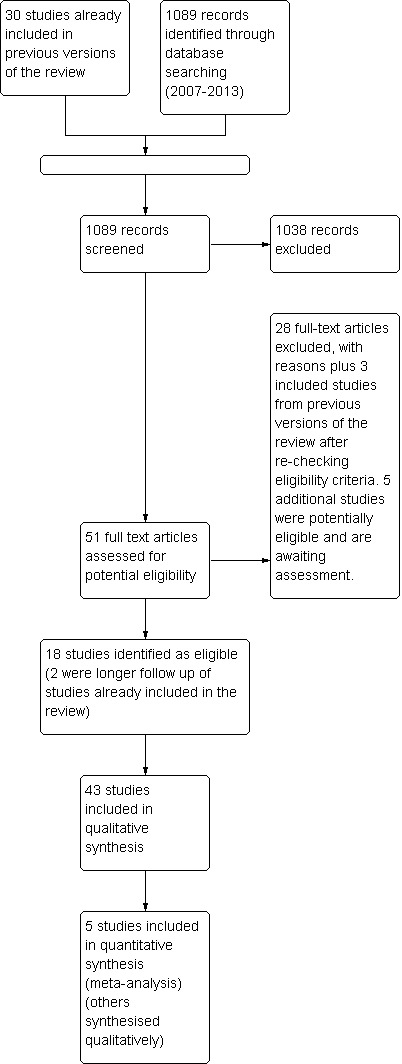
Study flow diagram.
Data extraction and management
Data were extracted independently by at least two review authors (AL and FK, JM or JB), who used a specially designed data extraction form. Any discrepancies in data extraction were resolved by consensus. When necessary, additional information on trial methodology or original trial data were sought from the principal or corresponding author of any trials that met the eligibility criteria (see Acknowledgements for details of the authors who provided additional clarification of data beyond that reported in the publications).
Data extracted included details on study characteristics (participants, interventions and comparison groups) and outcome data. When necessary, missing data were imputed from data in other, similar trials or were calculated by using formulas suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
All assessments of risk of bias were performed independently by at least two review authors (AL and FK, JM or JB), who used the Cochrane 'Risk of bias assessment tool' (Higgins 2011); results were compared. Any discrepancies were resolved by consensus. Criteria assessed included randomisation method, allocation concealment, blinding of participants and investigators, blinding of assessors, incomplete outcome data and selective outcome reporting. Summary assessments of risk of bias are presented in Figure 2 and Figure 3.
2.
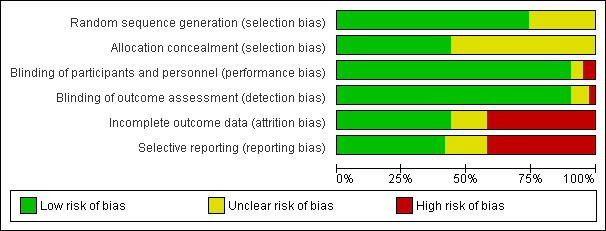
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
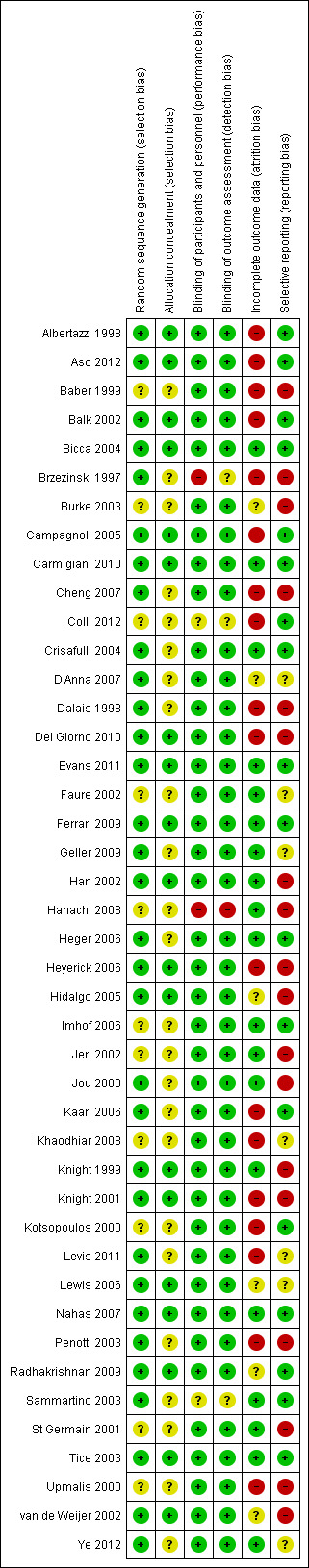
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias assessments have been incorporated into sensitivity analyses (see below).
Measures of treatment effect
When trials were combined in a meta‐analysis, summary effect measures were calculated. For dichotomous data, the numbers of events in the intervention and control groups were used to calculate risk ratios (RRs), together with their 95% confidence intervals (CIs). For continuous data, the weighted mean difference (MD) between groups, together with 95% CIs, was calculated. For all other studies, findings in the individual study publications were reported in narrative format and compared.
Unit of analysis issues
The primary unit of analysis was per woman randomised. Only first‐phase data from cross‐over trials were analysed.
Dealing with missing data
When data were missing, attempts were made to obtain these data from the authors of relevant included studies. Clarifications of data and details from the publications were received from a number of study authors (see Acknowledgements).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of included studies were sufficiently similar to warrant meta‐analysis. When meta‐analyses were performed, statistical heterogeneity was assessed by the Chi2 test (with P < 0.10 considered evidence of statistical heterogeneity) and the I2 metric. An I2 value > 50% was considered to represent substantial heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors have attempted to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert to duplication of data. No funnel plot was generated, as most of the studies were synthesised narratively because of substantial heterogeneity.
Data synthesis
A priori, it was decided that results from the included studies would be combined in meta‐analysis only if similarities were noted in the baseline experience of hot flushes among participants: the composition, type and dosage of the phytoestrogen interventions; the duration of the studies; and the outcomes measured. Significant heterogeneity was seen in the isoflavone concentration of foods and extracts used in the trials that were considered to contain high levels of phytoestrogens. Because of this variation in isoflavone concentration and the variation in the general mix of constituents of each phytoestrogen intervention, pooling of different food products, tablets and extracts was not considered appropriate, and results were reported separately for each trial in table format (see Table 1Table 2 and Table 3).
1. Summary of findings: efficacy outcomes.
| Trial | No. | Intervention | Comparison | Duration | Efficacy outcomes | Results (between‐group comparison) | Overall risk of bias |
| SOY DIETARY SUPPLEMENTS | |||||||
| Albertazzi 1998 | 104 | 60 g soy powder (76 mg isoflavones) | Placebo (60 g casein) | 12 weeks | Number of flushes/d after treatment; percentage decrease in number of flushes | At end of study, significant difference between placebo and soy: ‐1.59 (‐1.95 to ‐1.2; P < 0.01), representing mean reduction of 1.6 flushes/d in soy group compared with placebo. 45% reduction in flushes with soy versus 30% reduction with placebo (P < 0.01) | Unclear |
| Balk 2002 | 27 | Soy and corn flour cereal (100 mg/d isoflavones) | Placebo (wheat cereal) | 24 weeks | Hot flush and night sweat symptom score after Rx (1 to 4) | NS all outcomes | High |
| Brzezinski 1997 | 145 | Phytoestrogen‐enriched diet (individualised by dietician) (isoflavone amount not given) | Control—regular diet (avoiding phytoestrogens) | 12 weeks | MSQ hot flush severity reduction subscore (0 to 3) | Greater reduction with PE‐rich diet (P = 0.004; no CI given) | High |
| Burke 2003 | 241 | (1) soy drink 1 (42 mg/d isoflavones); (2) soy drink 2 (58 mg/d isoflavones) |
Placebo (soy drink with isoflavones removed) | 2 years | Number and severity of flushes/sweats per day after Rx (symptom diary); also subgroup analysis in women with 4+ symptoms/d at baseline | NS all outcomes | High |
| Carmigiani 2010 | 60 | (1) oestradiol 1 mg + 0.5 mg norethisterone acetate; (2) dietary soy supplementation (90 mg isoflavone) in powder form + placebo tablet |
Placebo (identical powder and placebo tablets) | 16 weeks | Somatic score (hot flushes, heart discomfort, sleeping problems and muscle and joint problems) on the Menopause Rating Scale | Reduction in somatic symptoms was ‐45.6% with HT and ‐49.8% with dietary soy compared with ‐28.6% with placebo | Low |
| Cheng 2007 | 60 | (1) isoflavones 60 mg daily in fruit drink | Placebo (oatmeal drink) | 12 weeks | Hot flush and night sweat scores | Significant reduction in hot flush score with isoflavones compared with placebo | High |
| Dalais 1998 | 52 | (1) soy diet (53 mg/d isoflavones); (2) linseed diet (high in isoflavones—quantity not given) |
Placebo (wheat diet (low isoflavones)) | 12 weeks + 12 weeks | Percentage decrease in number of hot flushes | NS: 22% reduction with soy; 41% reduction with linseed; 51% reduction with wheat | High |
| Hanachi 2008 | 37 | (1) soy milk product (12.5 g soy protein with 13 mg genistein and 4.13 mg daidzein); (2) soy milk product + exercise |
Control | 12 weeks | Hot flush score on Kupperman Index | Hot flushes significantly improved with both soy interventions compared with control | High |
| Knight 2001 | 24 | Soy powder 60 g/d for beverage (134.4 mg/d isoflavones) | Placebo (casein powder for beverage) | 12 weeks | Number of flushes per week after Rx | NS: 29 flushes/wk in soy group; 46 flushes/wk in placebo group (reduction in both from baseline) | High |
| Kotsopoulos 2000 | 94 | Soy powder for beverage (118 mg/d isoflavones) | Placebo (casein powder for beverage) | 12 weeks | Hot flush symptom score (severity) (0 to 3) after Rx | NS: 0.77 score with soy; 0.83 score with placebo | High |
| Lewis 2006 | 99 | (1) soy flour muffins (42 mg/d isoflavones); (2) flaxseed muffins (50 mg/d lignans) |
Placebo (wheat flour muffins (low lignans and no isoflavones)) | 16 weeks | Menoquol vasomotor score; number of flushes per day; severity of flushes (1 to 7 scale) after Rx | NS: all outcomes | Unclear |
| Radhakrishnan 2009 | 100 | (1) soy sachets (25 mg isoflavone soy protein isolate containing 75 mg isoflavones in powder form) | Placebo powder | 6 months | Number of daily hot flushes; karyopyknotic index; maturation value; endometrial thickness; acceptability of treatment; adverse events | Significantly greater proportion of women had reduction in hot flushes with soy (84%) when compared with placebo (60%) but no significant difference in hot flush score between groups | Unclear |
| St Germain 2001 | 69 | (1) soy protein + (80.4 mg/d isoflavones); (2) soy protein – (4.4 mg/d isoflavones) in muffins and powder for cooking | Placebo (whey protein) | 24 weeks | Percentage of participants perceiving a decrease in (1) frequency, (2) duration and (3) severity of flushes; number of flushes per week after Rx; number of sweats per week after Rx | NS | High |
| SOY EXTRACT | |||||||
| Bicca 2004 | 75 | Standardised soy extract (33 mg/d isoflavones) | Placebo capsules | 25 weeks | Greene Vasomotor Subscale (intensity); percentage who experienced a decrease in frequency of flushes and sweats from baseline | NS: Greene Vasomotor Scale; 74% with soy versus 43% with placebo had decrease in number of hot flushes (P = 0.007); 68% with soy versus 46% with placebo had decrease in number of night sweats (P = 0.049). NS: severity of symptoms | Low |
| Campagnoli 2005 | 36 | Standardised soy extract (soy select) (60 mg/d isoflavones) | Placebo capsules | 12 weeks + 12 weeks | Number of flushes per week after Rx | NS | High |
| Faure 2002 | 75 | Standardised soy extract capsules (70 mg/d isoflavones) | Placebo capsules | 16 weeks | Percentage decrease in flushes per day; percentage of 'responders' (participants who had reduction of at least 50%) | 61% decrease with soy versus 21% reduction with placebo (P value not reported); 66% of soy group were responders versus 34% in placebo group (P < 0.005). Repeated measures analysis confirmed the soy‐placebo treatment effect | Unclear |
| Han 2002 | 80 | Soy capsules (100 mg/d isoflavones) | Placebo capsules | 16 weeks | Kupperman Vasomotor Symptom Score (severity) | Vasomotor score 8.2 in soy group versus 9.9 in placebo group (P < 0.01) | High |
| Jou 2008 | 96 | Soy germ extract powder (3 g) twice per day (135 mg isoflavones – 1 g = 10.9 mg daidzein and 2.85 mg genistein) in equol producers and non‐producers | Placebo (roasted wheat powder) | 6 months | Hot flushes as measured by Kupperman Index | Reduction in hot flush score of 79% in equol producers, 35% in non‐equol producers and 78% in placebo group. Results from equol producers significantly different from placebo by repeated measures analysis | High |
| Kaari 2006 | 79 | Soy extract capsules (S40/Ach‐1) (120 mg/d isoflavones) | Oestrogen + placebo capsules | 24 weeks | Percentage of participants reporting reduction (subgroup) | NS | High |
| Khaodhiar 2008 | 147 | Soy extract capsule (isoflavone quantity not known) (1) 40 mg/d; (2) 60 mg/d | Placebo capsules | 12 weeks | Percentage reduction in frequency of hot flushes (from daily diary), percentage reduction in severity of hot flushes (daily diary) | 52% and 51% reduction in hot flush frequency in 40‐mg and 60‐mg soy groups at 12 weeks compared with 39% reduction with placebo (NS). When both treatment groups were combined, an average reduction of 52% was seen in hot flush frequency per day in women taking soy compared with women taking placebo (P = 0.048) | Unclear |
| Levis 2011 | 248 | Isoflavone tablets 200 mg from soy protein (each tablet 91 mg genistein, 103 mg daidzein) | Placebo tablets | 2 years | Menopausal symptoms (hot flushes and night sweats) were secondary outcomes | Soy isoflavone group: 48.4% had hot flushes; 31.7% in placebo group had hot flushes | Unclear |
| Nahas 2007 | 80 | Soy extract (isoflavones 100 mg) | Placebo capsules | 10 months | Change in daily hot flush number; change in hot flush severity score | Significant reduction from baseline in number of daily hot flushes in both groups and significantly greater in soy versus placebo. Hot flush severity significantly reduced in soy extract group when compared with placebo | Low |
| Penotti 2003 | 62 | Soy tablets (72 mg/d isoflavones) | Placebo tablets | 24 weeks | Number of flushes per day after Rx | NS | High |
| Upmalis 2000 | 177 | Standardised soy extract tablets (50 mg/d of genistein and daidzin) | Placebo tablets | 12 weeks | Percentage change in flush severity per week; percentage change in flush frequency per week; percentage change in sweat frequency per week | 34% change in severity with soy versus 21% change in severity with placebo (P = 0.01); NS for percentage change in frequency (P = 0.078 repeated measures analysis); NS for change in reduction of night sweats | High |
| Ye 2012 | 90 | Soy germ isoflavone extract powder in capsules in two doses (84 mg and 126 mg of isoflavones) | Placebo capsules | 24 weeks | Hot flush frequency and percentage change per week | 44.3% and 48.5% change in hot flushes from baseline in the 84‐mg and 126‐mg isoflavone groups, respectively, compared with 27.8% change in placebo group (P < 0.01) | Unclear |
| RED CLOVER EXTRACTS | |||||||
| Baber 1999 | 51 | Promensil (standardised red clover extract) (40 mg/d isoflavones) | Placebo tablets | 12 weeks + 12 weeks | Number of flushes per day after Rx; percentage flush reduction | NS | High |
| Del Giorno 2010 | 120 | Trifolium pratense (red clover) 40 mg | Placebo | 12 months | Vasomotor symptoms (Kupperman Index) | NS between groups | High |
| Geller 2009 | 67 | (1) 0.625 mg CEE + 2.5 mg MPA; (2) Black cohosh (128 mg/d); (3) Red clover (398 mg/d including 120 mg isoflavones) |
Placebo | 12 months | Vasomotor symptoms (hot flushes and night sweats); frequency of hot flushes; intensity of hot flushes | NS: black cohosh and red clover versus placebo | Unclear |
| Hidalgo 2005 | 60 | Red clover supplement capsules (80 mg/d isoflavones) | Placebo capsules | 12 weeks + 12 weeks | Kupperman Index score for hot flushes and sweats (severity) (expressed as percentage) | Hot flushes: 15% with red clover versus 98% with placebo; night sweats: 30% with red clover versus 93% with placebo; P value not given | High |
| Imhof 2006 | 109 | Red clover extract capsules (80 mg/d isoflavones) | Placebo capsules | 90 days + 7 days washout + 90 days | Hot flush daily frequency, night sweats daily frequency Mean percentage decrease in hot flushes and night sweats |
Mean (SD) daily frequency (hot flushes) in red clover group (3.1 (3.5)); in placebo group (10.1 (5.5)) Mean (SD) daily frequency (night sweats) in red clover group (1.5 (2.1)); in placebo group (5.0 (2.6)) Mean percentage decrease in hot flushes in red clover group 73.5%; in placebo group 8.2% Mean percentage decrease in night sweats in red clover group 72.2%; in placebo group 0.9% All differences significant (P = 0.0001) |
Unclear |
| Jeri 2002 | 30 | Promensil (standardised red clover extract) (40 mg/d isoflavones) | Placebo tablets | 16 weeks | Percentage reduction in number of flushes per day; severity of flushes per day (scale) | Frequency: 49% reduction with red clover versus 11% reduction with placebo (P < 0.001); severity: reduction from 2.53 to 1.33 with red clover versus no reduction with placebo (P < 0.001) | High |
| Knight 1999 | 37 | Promensil (standardised red clover extract) (40 mg/d isoflavones) | Placebo tablets | 12 weeks | Number of flushes per day | NS | Low |
| Tice 2003 | 252 | (1) Promensil (standardised red clover extract) (82 mg/d isoflavones—two tablets); (2) Rimostil (standardised red clover extract) (57 mg/d isoflavones—two tablets) |
Placebo tablets | 12 weeks | Number of flushes per day; percentage reduction in flushes; rate of reduction over time | Number of flushes per day NS; percentage reductions NS; Promensil had faster reduction over time versus placebo (P = 0.03) | Low |
| van de Weijer 2002 | 30 | Promensil (standardised red clover extract) (80 mg/d isoflavones—two tablets) | Placebo tablets | 12 weeks | Number of flushes per day; median percentage change in number of flushes | 3.4 flushes/d with Promensil versus 6 flushes/d with placebo (P value not reported); 44% reduction with Promensil versus 0% reduction with placebo (P = 0.01; variation not reported) | High |
| GENISTEIN | |||||||
| Crisafulli 2004 | 90 | (1) genistein extract (54 mg/d isoflavones); (2) continuous HT (17B‐ostradiol 1 mg/d + norethisterone acetate) |
Placebo tablets | 1 year | Percentage change in number of flushes per day | 24% reduction with genistein when compared with placebo (P < 0.01); 30% reduction with HT when compared with genistein (P < 0.05) | Unclear |
| D'Anna 2007 | 247 | Genistein extract twice per day (each tablet containing 27 mg total isoflavone) | Placebo twice per day | 2 years | Number and severity of hot flushes | Significant reduction in frequency (56.4%) and severity (37.5%) of hot flushes compared with placebo | Unclear |
| Evans 2011 | 84 | Genistein (geniVida) 30 mg once daily | Placebo | 12 weeks | Percentage change in number of daily hot flushes; duration and severity of daily hot flushes | Significant reduction in frequency (51.2% vs 27.2%) and duration of hot flushes (12 minutes/d vs 23 minutes/d) at the end of treatment compared with placebo. NS severity of hot flushes | Low |
| Ferrari 2009 | 180 | Soy isoflavone extract (80 mg) with high dose of genistein (60 mg) | Placebo | 12 weeks | Change in daily frequency of hot flushes; improvement (as assessed by investigator) | Significant reduction in number of hot flushes in genistein group (41.2%) when compared with placebo (29.3%); improvement in genistein group 56.7% and in placebo group 53.1% (P value not reported) | Low |
| OTHER PHYTOESTROGENS | |||||||
| Aso 2012 | 160 | Natural S‐(‐)equol supplement twice per day (5.0 mg equol, 1.2 mg daidzein, 1.4 mg genistein) | Placebo | 12 weeks | Change in frequency and severity of hot flushes | Significant reduction in frequency of hot flushes with equol supplement (62.8%) compared with placebo (23.6%) in women with more than three hot flushes per day. Significant improvement in severity of hot flushes with equol supplement (61.2%) when compared with placebo (45%) | High |
| Colli 2012 | 90 | Flaxseed extract (1 g/d) (at least 100 mg secoisolariciresinol diglucoside (SDG)) and flaxseed meal (90 g/d) (at least 270 mg SDG) | Placebo (1 g/d collagen) | 24 weeks | Intensity of hot flush score (Kupperman Index) | In both flaxseed groups, hot flush intensity score was reduced significantly from baseline, but no significant difference was seen in the placebo group. Comparison of groups by ANOVA showed no significant difference between groups at end of study | High |
| Dalais 1998 | 52 | See above for results in the flaxseed arm | High | ||||
| Heger 2006 | 110 | Extract of ERr 731, an extract of the roots of Rheum rhaponticum | Placebo | 12 weeks | Changes in number and severity of hot flushes | Number and frequency of hot flushes significantly reduced with ERr 731 compared with placebo (P < 0.0001) (average of 5.5 fewer per day compared with 0 fewer per day) | Unclear |
| Heyerick 2006 | 67 | (1) Hop extract 1 (100 μg 8‐prenylnaringenin); (2) Hop extract 2 (250 μg 8‐prenylnaringenin) (Menohop) |
Placebo capsules | 12 weeks | Kupperman hot flush score (severity) | NS at the end of the study period | High |
| Lewis 2006 | 99 | See above for results in the flaxseed arm | Unclear |
CEE: conjugated equine oestrogen.
MPA: medroxyprogesterone acetate.
2. Summary of findings: safety outcomes.
| Trial | No. | Intervention | Comparison | Duration | Safety outcomes | Results (between‐group comparison) | Overall risk of bias |
| SOY DIETARY SUPPLEMENTS | |||||||
| Albertazzi 1998 | 104 | 60 g soy powder (76 mg isoflavones) | Placebo (60 g casein) | 12 weeks | Adverse events | NS | Unclear |
| Balk 2002 | 27 | Soy and corn flour cereal (100 mg/d isoflavones) | Placebo (wheat cereal) | 24 weeks | Endometrial stimulation; adverse events | Endometrial stimulation: NS (all participants had atrophic endometrium). Adverse events: NS | High |
| Carmigiani 2010 | 60 | (1) oestradiol 1 mg + 0.5 mg norethisterone acetate; (2) dietary soy supplementation (90 mg isoflavone) in powder form + placebo tablet |
Placebo (identical powder and placebo tablets) | 16 weeks | Endometrial thickness, vaginal maturation value; side effects | NS: side effects, endometrial thickness or vaginal maturation value with soy compared with placebo | Low |
| Cheng 2007 | 60 | (1) isoflavones 60 mg daily in fruit drink | Placebo (oatmeal drink) | 12 weeks | Endometrial thickness | No difference in endometrial thickness | High |
| Dalais 1998 | 52 | (1) soy diet (53 mg/d isoflavones); (2) linseed diet (high in isoflavones— quantity not given) |
Placebo (wheat diet—low isoflavones) | 12 weeks + 12 weeks | Vaginal maturation index (percentage increase from baseline) | Increase of 103% with soy diet (P = 0.03), 6% increase with linseed (NS), 11% increase with placebo (NS) | High |
| Knight 2001 | 24 | Soy powder 60 g/d for beverage (134.4 mg/d isoflavones) | Placebo (casein powder for beverage) | 12 weeks | Adverse events; vaginal maturation index | Total adverse events: 75% with soy versus 17% with placebo (P < 0.001); vaginal maturation index NS | High |
| Kotsopoulos 2000 | 94 | Soy powder for beverage (118 mg/d isoflavones) | Placebo (casein powder for beverage) | 12 weeks | Adverse events | Total adverse events: NS | High |
| Radhakrishnan 2009 | 100 | (1) Soy sachets (25 mg isoflavone soy protein isolate containing 75 mg isoflavones in powder form) | Placebo powder | 6 months | Karyopyknotic index; maturation value; endometrial thickness; adverse events | No difference in vaginal cytology, endometrial thickness or adverse events | Unclear |
| SOY EXTRACTS | |||||||
| Bicca 2004 | 75 | Standardised soy extract (33 mg/d isoflavones) | Placebo capsules | 25 weeks | Vaginal maturation index; vaginal pH (percentage with improvement) | Maturation: NS; pH: 21% with soy versus 11% with placebo had improvement in pH (P = 0.008) | Low |
| Campagnoli 2005 | 36 | Standardised soy extract (Soyselect) (60 mg/d isoflavones) | Placebo capsules | 12 weeks + 12 weeks | Vaginal maturation index | NS | High |
| Faure 2002 | 75 | Standardised soy extract capsules (7 mg/d isoflavones) | Placebo capsules | 16 weeks | Adverse events | NS | Unclear |
| Han 2002 | 80 | Soy capsules (100 mg/d isoflavones) | Placebo capsules | 16 weeks | Endometrial thickness | NS | High |
| Kaari 2006 | 79 | Soy extract capsules (S40/Ach‐1) (120 mg/d isoflavones) | Oestrogen + placebo capsules | 24 weeks | Vaginal pH; vaginal maturation index; endometrial thickness; endometrial stimulation; adverse events | pH: 5.5 in soy group versus 4.8 in HT group (P = 0.0012); maturation index: significant difference in intermediate and superficial cell frequency in ET group when compared with soy group (P value not given); endometrial thickness: 5.9 mm in HT group versus 3.0 mm in soy group (significant; P value not given); non‐atrophic endometrium: 54% in HT group versus 88% in soy group (P < 0.01); adverse events: 17% genital bleeding in HT group versus 0% in soy group | High |
| Khaodhiar 2008 | 207 | Soy extract capsules (isoflavone quantity not known)—two different doses: (1) 40 mg/d; (2) 60 mg/d |
Placebo | 12 weeks | Adverse events | Very few events reported—no group comparisons reported | Unclear |
| Levis 2011 | 248 | Isoflavone tablets 200 mg from soy protein (each tablet 91 mg genistein, 103 mg daidzein) | Placebo tablets | 2 years | Adverse events | 7/99 had fractures in soy group compared with 1/83 in placebo group (P = 0.03) | Unclear |
| Nahas 2007 | 80 | Soy extract (isoflavones 100 mg) | Placebo capsules | 10 months | Median endometrial thickness (mm); maturation value; vaginal pH; adverse events | 2.4 mm in intervention group and 2.8 mm in placebo group (NS) NS in vaginal outcomes and adverse events |
Low |
| Penotti 2003 | 62 | Soy tablets (72 mg/d isoflavones) | Placebo tablets | 24 weeks | Endometrial thickness | NS | High |
| Upmalis 2000 | 177 | Standardised soy extract tablets (50 mg/d of genistin and daidzin) | Placebo tablets | 12 weeks | Adverse effects; vaginal pH; vaginal maturation index; endometrial thickness | Adverse events: 34% (30/89) of soy group reported 70 events versus 45% (39/86) in placebo group reported 79 events (no P value); pH: NS; maturation index NS; endometrial thickness: NS | High |
| RED CLOVER EXTRACTS | |||||||
| Baber 1999 | 51 | Promensil | Placebo | 12 weeks + 12 weeks | Endometrial thickness | NS | High |
| Geller 2009 | 67 | (1) 0.625 mg CEE + 2.5 mg MPA; (2) black cohosh (128 mg/d); (3) red clover (398 mg/d including 120 mg isoflavones) |
Placebo | 12 months | Endometrial thickness; adverse events | NS | Unclear |
| Hidalgo 2005 | 60 | Red clover supplement capsules (80 mg/d isoflavones) | Placebo | 12 weeks + 12 weeks | Vaginal cytology (karyopyknotic index, cornification index, maturation index) | Significant changes (P < 0.05) in all indexes when compared with placebo | High |
| Imhof 2006 | 113 | Red clover extract capsules (80 mg/d isoflavones) | Placebo | 12 weeks + 12 weeks | Endometrial thickness | Significant decrease in thickness with red clover (15%) but not with placebo (placebo values not given) (P < 0.001) No side effects reported |
Unclear |
| Tice 2003 | 252 | (1) Promensil (standardised red clover extract) (82 mg/d isoflavones— two tablets); (2) Rimostil (standardised red clover extract) (57 mg/d isoflavones—two tablets) |
Placebo | 12 weeks | Adverse events | NS | Low |
| GENISTEIN EXTRACTS | |||||||
| Crisafulli 2004 | 90 | (1) genistein extract (54 mg/d isoflavones); (2) continuous HT (17B‐oestradiol 1 mg/d + norethisterone acetate) |
Placebo | 1 year | Endometrial thickness | NS | Unclear |
| D'Anna 2007 | 247 | Genistein extract twice per day (each tablet containing 27 mg total isoflavone) | Placebo twice per day | 2 years | Endometrial thickness; vaginal maturation values | NS | Unclear |
| Evans 2011 | 84 | Genistein (geniVida) 30 mg once daily | Placebo | 12 weeks | Endometrial thickness; adverse events | NS | Low |
| Ferrari 2009 | 180 | Soy isoflavone extract (80 mg) with high dose of genistein (60 mg) | Placebo | 12 weeks | Adverse events | NS | Low |
| Sammartino 2003 | 70 | Genistein extract tablets (quantity of isoflavones not given) | Placebo tablets | 1 year | Endometrial thickness | NS | Unclear |
| OTHER PHYTOESTROGENS | |||||||
| Colli 2012 | 90 | Flaxseed extract (1 g per day) (at least 100 mg secoisolariciresinol diglucoside (SDG)) and flaxseed meal (90 g per day) (at least 270 mg SDG) | Placebo (1 g per day collagen) | 24 weeks | Endometrial thickness; vaginal epithelial maturation value | NS | High |
| Dalais 1998 | 52 | See above for results in the linseed arm | High | ||||
| Heger 2006 | 110 | Extract of ERr 731, an extract of the roots of Rheum rhaponticum | Placebo | 12 weeks | Endometrial thickness; adverse events | NS | Unclear |
| Sammartino 2003 | 70 | Genistein extract tablets (quantity of isoflavones not given) | Placebo tablets | 1 year | Endometrial thickness | NS | Unclear |
HT: Hormone therapy.
3. Summary of findings: acceptability outcomes.
| Trial | No. | Intervention | Comparison | Duration | Outcomes | Results (between‐group comparison) |
| Albertazzi 1998 | 104 | 60 g soy powder (76 mg/d isoflavones) | Placebo | 12 weeks | Withdrawal due to adverse events | 20% in soy group versus 23% in placebo group (P value not reported) |
| Ferrari 2009 | 180 | Soy isoflavone extract (80 mg) with high dose of genistein (60 mg) | Placebo | 12 weeks | Satisfaction rates | 79.1% in genistein group; 69.1% in placebo group (P value not reported) |
| Heger 2006 | 110 | Extract of ERr 731, an extract of the roots of Rheum rhaponticum | Placebo | 12 weeks | Satisfaction rates | 63% in ERr 731 group satisfied at end of treatment; 32% in placebo group (P value not reported) |
| Knight 2001 | 24 | Soy powder 60 g/d for beverage (134.4 mg/d isoflavones) | Placebo | 12 weeks | Withdrawal due to adverse events | 25% in soy group versus 8% in placebo group (P value not reported) |
| Kotsopoulos 2000 | 94 | Soy powder for beverage (118 mg/d isoflavones) | Placebo | 12 weeks | Withdrawal due to adverse events or inability to tolerate treatment | 20% in soy group versus 18% in placebo group (P value not given) |
| Radhakrishnan 2009 | 100 | Soy sachets (25 mg isoflavone soy protein isolate containing 75 mg isoflavones in powder form) | Placebo powder | 6 months | Acceptability of treatment according to taste, odor or bulk of preparation | Not reported |
Data from five trials were combined in meta‐analyses because the intervention was a standardised dose of Promensil (Baber 1999; Jeri 2002; Knight 1999; Tice 2003; van de Weijer 2002). It was planned that a fixed‐effect model would be used to combine studies in the meta‐analyses, although both fixed‐effect and random‐effects estimates were calculated initially and results compared.
In the forest plots, an increase in the risk of a particular binary outcome that may be beneficial (e.g. improvement in hot flush severity) or detrimental (e.g. proportion with adverse events) is displayed graphically in the meta‐analyses to the right of the centre line, and a decrease in the risk of an outcome to the left of the centre line. Similarly, for continuous data, for some outcomes a higher value for an outcome may be considered beneficial (e.g. greater change in vasomotor symptom score) or detrimental (e.g. number of hot flushes per day), and interpretation will be guided by considering the graph labels that are reversed for benefit as opposed to detriment.
Subgroup analysis and investigation of heterogeneity
For most trials, when results were reported in tabular form, subgroup analysis was undertaken because of variation in the phytoestrogen interventions. Trials were grouped a priori according to the type of phytoestrogen given in the experimental arms. Subgroups included the following.
Trials in which the phytoestrogen given was in the form of dietary soy, such as flour, beverage or powder containing isoflavones.
Trials in which the phytoestrogen given was in the form of a soy isoflavone extract.
Trials in which the phytoestrogen given was in the form of a red clover extract.
Trials in which the phytoestrogen given was in the form of a predominantly genistein extract.
All other trials.
Statistical heterogeneity between the results of studies pooled in meta‐analyses was examined by inspecting the scatter in the data points and the overlap in their confidence intervals and, more formally, by checking results of the Chi2 test and the I2 quantity. A priori, it was planned to look at the possible contribution of differences in trial design to any heterogeneity identified in this manner. When substantial heterogeneity was indicated that could not be explained, a random‐effects model was reported as a more appropriate method for estimating an average treatment effect.
Sensitivity analysis
Sensitivity analysis was conducted to compare differences among participants, interventions, outcomes and methodological quality of included studies.
Comparison of trial results of all included studies with those studies at low risk of bias (with at least double blinding, adequate concealment, intention‐to‐treat analyses).
Comparison of trial results of all included studies with those studies in which a power calculation was performed for sample size.
Comparison of trial results of all included studies with those studies in which women were required to have at least five moderate to severe hot flushes per day before they were eligible to participate.
Comparison of trial results of all included studies with those studies using more than 50 mg/d of isoflavones in the treatment group.
Overall summaries: 'Summary of results' tables
As few of the studies could be combined in meta‐analyses, separate 'Summary of results' tables were generated to display efficacy, safety and acceptability outcomes for each trial (Table 1, Table 2 and Table 3). Study results in these tables should be considered by referring to the quality of the individual study (Figure 3) to aid in interpretation of overall results.
Results
Description of studies
Results of the search
For earlier versions of this review, 30 studies (2,730 participants) were included, 31 were excluded and 11 were awaiting classification (further details on the total number of potentially eligible trials are not available).
For the 2013 update of the review, the search retrieved 51 potentially eligible additional studies through inspection of titles and abstracts (Figure 1).
Included studies
Of 51 potentially eligible studies in the 2013 update, a further 16 new studies met the inclusion, criteria together with two additional studies, which were later publications of studies already included in the review, with longer follow‐up or additional results. A total of 43 RCTs (with 4,364 participants) is included in the review (Figure 1). Full details of the included studies are displayed in an additional table (Characteristics of included studies).
Study design and setting
A total of 38 studies used a parallel‐group design, and the remaining five used a cross‐over design. One cross‐over trial had no washout period, and in the remaining four trials, the washout period ranged from seven days to one month. One cross‐over trial was combined with parallel‐group trials in forest plots; only data from the first phase of the trial before cross‐over were used in these analyses.
Participants
Most participants were recruited solely from menopause clinics or through a mixture of advertisements and flyers placed in medical practices or in the community; the source of recruitment was not specified in 14 trials. Participants in these trials were experiencing vasomotor symptoms (hot flushes or night sweats) ranging from at least one flush per day to up to 15 flushes per day. Fifteen other trials were included in which vasomotor symptoms or scores on menopausal symptom indices were measured at baseline, although specification of the level of these symptoms was not a requirement for inclusion in the trial. Two of the trials measured the effects of treatment in subgroups (only those women with symptoms at baseline) of randomly assigned participants. One trial excluded women with severe menopausal symptoms who required medical treatment. Menopausal status was most often confirmed by follicle‐stimulating hormone (FSH), luteinising hormone (LH) and plasma oestradiol measurements and/or by amenorrhoea ranging from two months to up to 10 years. Elderly women were not included; participants usually ranged in age from 40 to 65 years, although one trial included women up to 75 years of age. Because the minimum threshold of the last menstrual period ranged from two to 12 months or longer, many trials included a mix of perimenopausal and postmenopausal women. Three trials explicitly recruited perimenopausal women; women were required to have no more than one menstrual period during the three months before recruitment (ages ranged from 45 to 55 years) in one trial; in another, women were required to have had at least one period over the past 12 months (average time since last menstrual period was 16 weeks), and in another, women were 45 to 55 years of age and showed cycle irregularity over the previous 12 months or last menstruation at least three but no longer than 12 months previously. In most of the trials, women using HT, currently or recently, were excluded. Other exclusion criteria included women on a vegetarian diet or on a soy‐rich diet, malignancy, comorbidities and taking medication that might interfere with assessment of vasomotor symptoms. It was not clear in most trials whether participants had a natural or surgical menopause, but nine trials specifically excluded women with a surgical menopause. Women in five trials were from Australia, seven trials were performed in Italy, eight in the USA, seven in Brazil and the remainder in Israel, Japan, Canada, Sweden, France, Ukraine, Belgium, Ecuador, Peru, Austria, Taiwan, the Netherlands, India, China and Iran.
Interventions
Interventions used in the trials varied substantially.
Type and method of delivery of phytoestrogen Trials were grouped into broad categories according to method of delivery and type of phytoestrogen.
Thirteen trials assessed the effects of dietary substances in the form of flour, powder or beverages derived from soy isoflavones with varying amounts of phytoestrogen enrichment.
Twelve trials assessed the effects of varying types of soy isoflavone extracts, usually in tablet form.
Nine trials assessed the effects of red clover extracts (five of the nine used a standardised extract manufactured by Novogen under the brand name Promensil).
Five trials assessed the effects of mainly genistein extracts on hot flushes.
The remaining trials (n = 6) assessed other types of phytoestrogen supplements: Three trials investigated the effects of flaxseed dietary supplements (two of which had soy dietary supplement arms and were included in the first category and the other trial also assessed the effects of a flaxseed extract in addition to the flaxseed dietary supplement); one looked at two doses of a hop extract (Humulus luputus L.), one investigated the effects of a standardised natural S‐(‐)equol containing supplement (SE5‐OH) (a metabolite of isoflavones) and another investigated the effects of an extract taken from the roots of Rheum rhaponticum (ERr 731) (which is considered a phytoestrogen supplement). The authors of this trial noted that ERr 731 has been used by perimeopausal and postmenopausal women in Germany since 1993.
Duration Duration of the interventions provided was three months in most of the trials (or three months for the first phase of cross‐over trials). Five trials had a duration of 16 weeks, nine trials had a duration of 24 weeks, one trial had a duration of 10 months, four trials had a duration of one year and three trials had a duration of two years.
Comparison groups The phytoestrogen interventions were mostly placebo controlled, although three open studies compared phytoestrogens with other types of control, either different diet with no phytoestrogens or calcium tablets. One other study included a blinded arm that compared flaxseed extract capsules with placebo capsules and another unblinded arm in which flaxseed dietary powder was used. Six placebo‐controlled studies compared different doses of the phytoestrogen intervention, and two other placebo‐controlled studies compared different types of phytoestrogens (e.g. comparison of a soy diet with a linseed diet or flaxseed muffins with soy muffins). Three studies compared phytoestrogens with HT and placebo, and another compared phytoestrogens solely with HT without a control group.
Outcomes
Most of the trials were pilot studies that did not use power calculations. The effect of the interventions provided in the included studies on total menopausal scores derived from general menopausal symptom questionnaires (such as that of Kupperman and Greene) was not an outcome of this review, although studies were included if they measured vasomotor symptoms on a subscale of a compendium score. Most included studies assessed the effectiveness of the intervention as the primary outcome, although effectiveness was measured in different ways (number of hot flushes per day after treatment, percentage decrease in frequency of hot flushes, severity score after treatment, proportion that reported any reduction in frequency). A few studies separately reported on the frequency and severity of night sweats. Frequency of hot flushes or night sweats was generally reported by participants themselves in a daily diary. Severity was recorded usually in the scales or subscales of general menopause symptom rating scales in different categories, but a few studies required that women record severity in prespecified categories in their daily diaries. Menopause symptom scales included Menopause Symptoms Questionnaire, Menopause Rating Scale, Kupperman Index, Greene Climacteric Scale, Menopause‐Specific Quality of Life Questionnaire, Women's Health Questionnaire and the modified Climacteric Symptom Evaluation Checklist. These instruments commonly used a 4‐point scale from 0 (no symptoms) to 3 (severe symptoms) to categorise severity, but a few scales used a larger number of categories.
Two studies specifically assessed the safety of the intervention (as measured by effects on endometrial stimulation) as the primary outcome, and 14 others assessed these measures as secondary outcomes. A few studies also assessed the effects of the intervention on the vaginal epithelium or on pH—each of which is a surrogate outcome that is a biological indicator of oestrogenic activity. Adverse events were reported in a few trials but generally were collected as spontaneous reports. Most trials provided details of withdrawals before the study was completed, and a few indicated whether these occurred because of adverse effects or because of problems with acceptability of the intervention.
Excluded studies
Of 51 potentially eligible studies for the 2013 update, 28 were excluded because women were not symptomatic at baseline, the studies were not randomised, the duration of the study was less than 12 weeks, the interventions assessed were not included in the review, women had breast cancer, the intervention was a combination treatment, the study was a dose‐finding study that did not include a control group or the interventions were not considered phytoestrogens. A further three studies, originally included in the review, were also excluded because the participants had minimal vasomotor symptoms at baseline (Dodin 2005; Duffy 2003; Woo 2003). A total of 60 studies have been excluded from the review (Characteristics of excluded studies). Five studies were considered potentially eligible in the 2013 update and are awaiting classification; a total of eight studies are now awaiting classification.
Risk of bias in included studies
Risk of bias in the included studies is summarised in chart format (Figure 2 and Figure 3). However, given that the results are mostly presented in narrative format in subgroups to reduce the variability of the intervention, the overall risk of bias of each study is also included in the 'Additional tables' summarising the results, so that the reader can judge the quality of the trial evidence for each subgroup separately.
Allocation
In all, 32 of the studies gave full descriptions of an adequate randomisation procedure and were considered at low risk of bias. The remaining 11 trials claimed that randomisation was the method of allocation, but the method was not described; these trials were considered at unclear risk of bias. Less than half of the studies (n = 19) reported methods to conceal allocation and were considered at low risk of bias; the remaining trials reported no details and were considered at unclear risk of bias.
Blinding
Nearly all of the trials reported that treatments were blind to participants, investigators and outcome assessors, but the procedures used to ensure that this occurred were not always described. In many studies, the outcome assessors were the participants, as they evaluated their own experience of hot flushes through questionnaires. In four studies, blinding was not possible because the interventions were different types of diets or because phytoestrogens were compared with calcium (although for this latter study, lack of blinding was not likely to affect measurement of the primary outcome—endometrial stimulation).
Incomplete outcome data
For 20 studies, no dropouts or withdrawals were discussed, numbers were balanced between groups or missing data were imputed; these studies were considered at low risk of bias for incomplete outcome data. Eighteen studies were considered at high risk of bias; in these studies, dropouts and withdrawals ranged from 16% to 31%. Five studies were considered at unclear risk of bias, as the percentage of dropouts ranged from 10% to 15% and/or dropouts were unbalanced between randomly assigned groups.
Selective reporting
Eighteen studies had low risk of bias, as all prespecified and potential outcomes were reported; seven had unclear risk of bias and 18 had high risk of bias because adverse events were not reported or because outcomes that had been prespecified were not reported.
Effects of interventions
Five of the included studies assessed the effects of Promensil, which is a standardised product, and their data were combined in a meta‐analysis. Because of the heterogeneity of the phytoestrogen interventions provided in the other included studies (dose, composition, type), these data could not be pooled but were synthesised in narrative format and displayed in separate tables for efficacy, safety and acceptability outcomes (seeTable 1, Table 2 and Table 3).
Dietary soy
Primary outcome: efficacy
Of the 13 included studies that used some type of substance containing dietary soy and that had efficacy analyses of any kind, seven studies indicated that no significant differences in primary efficacy outcomes were noted between the soy intervention and control groups.
Of the remaining six studies, one study assessed vasomotor symptoms specified as "somatic" symptoms on the Menopause Rating Scale. The Carmigiani study reported that both women on hormone therapy and women taking dietary soy supplementation (90 mg isoflavone) had significantly improved somatic symptoms (hot flushes and muscle/joint problems) (46% and 50%, respectively) when compared with placebo (29%) (Carmigiani 2010). This study found a significant difference in the frequency of hot flushes. The Albertazzi study of 104 women compared soy powder containing 76 mg/d of isoflavones with casein powder over 12 weeks (Albertazzi 1998). Investigators reported a mean reduction of 1.6 flushes per day (95% CI ‐1.95 to ‐1.2) for participants consuming soy powder compared with placebo. This was also expressed as a 45% reduction in the number of hot flushes with soy powder compared with a 30% reduction with placebo powder. Two studies found that severity or intensity of hot flushes was significantly reduced by the intervention. Brezinski compared a phytoestrogen‐enriched diet that was individualised for each participant by a dietician (exceeding the cutoff point of > 30 mg/d of isoflavones) versus a regular diet that avoided phytoestrogen‐containing foods consumed by a control group (Brzezinski 1997). Hot flushes (rated in a menopause symptoms questionnaire) were reduced in severity in both arms of the study but to a significantly greater extent in the phytoestrogen diet group. This study was one of the few that was not blinded, and knowledge of treatment could have affected participants' assessments. In the Radhakrishnan study, a significantly higher proportion of women (84%) reported improvement in hot flush symptoms (severity) with soy protein when compared with placebo (60%), but no evidence was found of a significant difference in the hot flush score (mean hot flushes per day) after six months (Radhakrishnan 2009). Two studies reported other significant differences, but it is unclear whether the scores represented frequency or severity or a combination of the two. The Cheng study reported that women taking 60 mg isoflavones daily had a significantly lower hot flush score (57%) than those taking placebo, but details on what the score represented are not clear (both number of daily hot flushes and intensity were recorded) (Cheng 2007). The Hanachi study reported that soy milk significantly reduced hot flushes by 72% compared with control after three months, but no details were given of the actual values for each group (Hanachi 2008).
Secondary outcomes: safety
Of the six studies that assessed adverse events, five were negative (no significant differences between randomised groups) and one was positive. The positive study (Knight 2001) found that 75% of participants in the soy group had adverse events compared with 17% of the placebo group. Side effects included bloating, nausea, weight gain and concerns about bowel function.
In all three studies that assessed the effects of phytoestrogens on the endometrium, no evidence of a significant difference between groups was found.
Of four studies that assessed the effects of a soy diet on the vaginal maturation index, three found no evidence of a significant difference between phytoestogen and control groups, but one study reported that this index increased by 103% from baseline with a soy diet compared with a 6% increase with linseed and an 11% increase with placebo (Dalais 1998).
Secondary outcomes: acceptability
Of the four studies that assessed the acceptability of the phytoestrogen intervention compared with control, one study reported a difference in the rate of withdrawal due to adverse events (Knight 2001) (P value not reported). This small study reported that 25% of participants who consumed a beverage containing soy powder withdrew from the study because of dislike of the taste compared with 8% in the placebo group.
Sensitivity analyses
Dietary food supplements varied enormously in the type of product used in the trials, the formulation and the isoflavone content (42 mg/d to 134 mg/d). Sensitivity analysis was undertaken to attempt to explain differences in efficacy outcomes between the six positive studies and the seven negative studies. In particular, the difference between positive and negative trials was not explained by the level of isoflavones in the food product. Variability in trial results could have been caused by other factors for which no controls could be applied. Intestinal florae convert soy isoflavone to equol—a more potent oestrogenic isoflavone that is absorbed along with unconverted genistein and daidzein; this conversion is variable (Adlercreutz 1990) and may have influenced the heterogeneity of the results. The severity of hot flushes at baseline could also explain the differences. In the six positive studies, severity of hot flushes among participants was variable; two trials required that women have at least five or eight moderate to severe flushes per day, but in the other trials, hot flushes were mild or were unspecified.
Quality of the trials in this subgroup was variable; only one positive trial had low risk of bias. Of the six trials with positive findings, two trials had very high dropout rates (24% and 21%) and two trials were unblinded and were thus considered at high risk of bias.
Soy extracts
Primary outcome: efficacy
Of the 12 studies that compared various types of soy extract in capsule or tablet form (11 vs placebo and one vs HT), nine studies (all vs placebo) reported significant differences in efficacy outcomes (frequency or severity). Five trials (Bicca 2004; Faure 2002; Khaodhiar 2008; Nahas 2007; Ye 2012) reported a reduction in the frequency of flushes (one also found a reduction in the frequency of night sweats); four trials found a reduction in severity of flushes as measured by the Kupperman vasomotor symptom score (Han 2002; Jou 2008; Nahas 2007) or by a subjective rating by participants on a scale of 1 to 3 (Upmalis 2000). This latter trial reported that severity of night sweats did not differ at the end of the study according to group. Not all of the positive studies described benefit from soy extracts; one trial found that women were significantly MORE likely to have hot flushes after isoflavone treatment (48.4%) than after placebo (31.7%), although this was a secondary outcome in the trial (Levis 2011). The trial that compared soy extract with oestrogen therapy (ET; Kaari 2006) reported no differences between them in the percentage of participants reporting any reduction in hot flushes (at six months, P = 0.74; Student's t test).
Secondary outcomes: safety
Of the eight studies that assessed safety outcomes, one assessed effects on endometrial stimulation, four on vaginal pH, five on endometrial thickness, six on vaginal maturation index and six on adverse events. The trial that compared soy extract with ET (unopposed oestrogen therapy) (Kaari 2006) reported significant improvement in vaginal pH and maturation index in the ET group. The soy extract group had a significantly thinner endometrium, less endometrial stimulation and fewer adverse events (all of which were genital bleeding in the ET group). One of the three other trials that compared soy extract with placebo found significantly greater improvement in vaginal pH in the soy group (Bicca 2004). One of the six studies that assessed adverse events reported that women taking soy extracts had a significant increase in rate of constipation and in fractures compared with women taking placebo (although this latter outcome was not considered to be related to treatment) (Levis 2011). For all other studies, no evidence was found of differences in endometrial thickness, vaginal maturation index or incidence of adverse events.
Secondary outcomes: acceptability
Two studies assessed the acceptability of the interventions as measured by withdrawal due to adverse events; no evidence of a difference between groups was found.
Sensitivity analyses
Sensitivity analyses exploring the effects of quality issues, levels of isoflavones in the active arm (ranging from 33 mg/d to 200 mg/d) or severity of flushes at baseline did not explain the differences in results. The five placebo‐controlled trials that found a difference in flush frequency (Bicca 2004; Faure 2002; Khaodhiar 2008; Nahas 2007; Ye 2012) out of the nine that measured this outcome reported reduction ranging from 50% to 74% with soy extract compared with reduction ranging from 21% to 43% with placebo. In contradiction to these findings, one trial actually reported a greater proportion of hot flushes after treatment with soy extracts versus placebo. Six trials had a longer duration than the more usual 12 weeks, ranging from 16 weeks to two years. The trial that compared soy extract versus ET (Kaari 2006) found no difference in the percentage of participants reporting a reduction in hot flush frequency, but participants had only mild symptoms at baseline (55% and 72% had hot flushes at baseline in the soy and ET groups, respectively). Although no placebo group was included in the study, the authors concluded that their results suggest that the soy isoflavone extract at 120 mg/d was effective in relieving the frequency of hot flushes; however, the trial was considered at high risk of bias.
Severity scores were significantly different in four of the seven trials that measured this outcome; three trials used the Kupperman vasomotor scale (rating severity from 0 to 3; Han 2002; Jou 2005; Nahas 2004), and the other used a simple severity scale (with scores of 1 to 3 representing mild, moderate and severe symptoms) scored daily by participants (Upmalis 2000). Variability in the results of included trials was not explained by sensitivity analyses of quality and other aspects of the studies.
Red clover extracts
Nine trials assessed the effects of red clover extracts on outcomes. Five of these used Promensil, and data from these trials were included in meta‐analyses. The other trials used MF11RCE (80‐mg isoflavones), a red clover supplement with 40 mg isoflavones or a red clover extract with 120 mg isoflavones. We used data from the first phase of the Baber and Imhof cross‐over trials.
Primary outcome: efficacy
Promensil: Five studies reported on the incidence of daily hot flushes after treatment with two different doses of Promensil (40 mg/d and 80 mg/d) (Baber 1999; Jeri 2002; Knight 1999; Tice 2003; van de Weijer 2002). No significant differences were reported between groups in the overall incidence of hot flushes (MD ‐0.93, 95% CI ‐1.95 to 0.10, I2 = 31%; Figure 4). Although subgroup analysis suggested benefit for a dose of 40 mg daily of Promensil, this finding should be viewed with caution, as two of the three trials had substantial risk of bias. One moderately sized trial of good quality and one small trial at high risk of bias assessed the percentage reduction in the number of hot flushes from baseline, resulting in a very imprecise pooled estimate (MD 20.15, 95% CI ‐12.08 to 52.38, I2 = 82%; Jeri 2002; Tice 2003; Analysis 1.2). A small trial (Jeri 2002) reported that a significantly greater proportion of women described hot flush severity ranging from moderate to severe to none or light in the Promensil group (RR 17.06, 95% CI 1.1 to 264.5; Analysis 1.3). One trial found no difference in the change in vasomotor score from baseline to end of study (MD 0.02, 95% CI ‐0.29 to 0.32; Tice 2003; Analysis 1.4).
4.
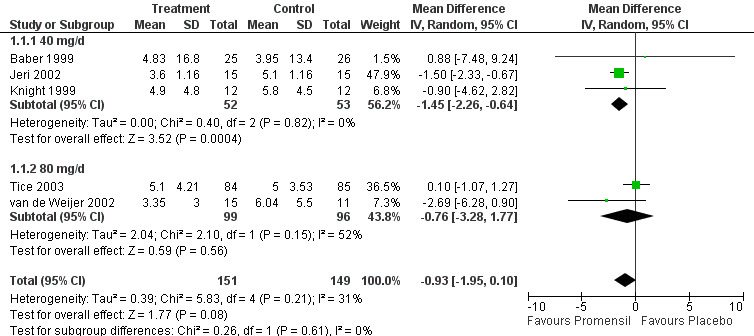
Forest plot of comparison: 1 Promensil versus placebo, outcome: 1.1 Incidence of hot flushes (number/d).
1.2. Analysis.
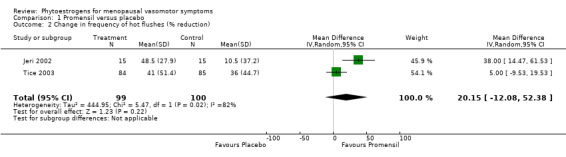
Comparison 1 Promensil versus placebo, Outcome 2 Change in frequency of hot flushes (% reduction).
1.3. Analysis.
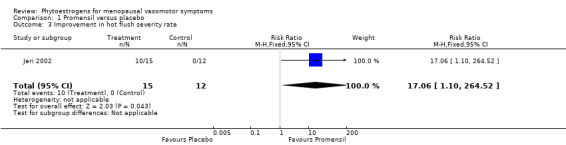
Comparison 1 Promensil versus placebo, Outcome 3 Improvement in hot flush severity rate.
1.4. Analysis.
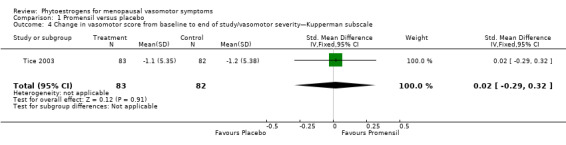
Comparison 1 Promensil versus placebo, Outcome 4 Change in vasomotor score from baseline to end of study/vasomotor severity—Kupperman subscale.
Other red clover extracts (not included in the meta‐analyses): One trial using a dose of 80 mg of red clover (Imhof 2006) found significant benefit for daily frequency of hot flushes and night sweats and for the mean percentage of decrease in these symptoms (P = 0.0001). Another study that assessed an unspecified red clover extract (same dose) reported benefits for hot flush and night sweat severity (as assessed by the Kupperman Index; Hidalgo 2005). After treatment, 15% of women taking 80 mg of red clover reported hot flushes compared with 98.1% of women taking placebo; values for night sweats were 30.2% and 92.5% for red clover and placebo, respectively (P < 0.05). The authors claimed that these values represented severity "as expressed as a percentage," but it is not clear what they meant. The other two studies assessing the efficacy of red clover (Del Giorno 2010; Geller 2009) found no difference between groups when treatment was given for 12 months.
Secondary outcomes: safety
Results are reported separately for Promensil and other red clover extracts.
One large trial of Promensil versus placebo assessed adverse events (Tice 2003). It reported no differences in the proportions of women who experienced any adverse event (RR 0.95, 95% CI 0.65 to 1.40). Also, no differences were found between groups in rates of specific adverse events, such as respiratory tract infection, headache, myalgia, nausea, arthralgia, diarrhoea and vaginal spotting. Two other studies (not included in the meta‐analysis) also did not find differences between groups with respect to adverse events.
Three trials assessed the effects of treatment on endometrial thickness (Baber 1999; Geller 2009; Imhof 2006). One trial (included in the meta‐analysis) found no difference in endometrial thickness after 12 weeks of treatment with Promensil. The other two trials reported different findings: One reported a significant decrease of 15% in endometrial thickness in women treated with red clover compared with zero change in women treated with placebo (SD of change not given, P < 0.001; Imhof 2006), and the other found no evidence of a significant difference between groups (Geller 2009).
One study that assessed an unspecified red clover extract reported significant changes in all vaginal cytology indexes (karyopyknotic index, cornification index, maturation index) when compared with placebo (P < 0.05) (Hidalgo 2005).
Secondary outcomes: acceptability
No trials assessed the acceptability of treatment.
Sensitivity analyses
Variability in study results appeared to be explained in part by trial quality. One small Peruvian study (n = 30) of poor quality (Jeri 2002) reported highly significant effects for both frequency and severity but gave no details on randomisation method, allocation concealment or baseline comparability. Inclusion of this study in the meta‐analyses, combined with findings of other, larger trials of better quality, caused highly significant heterogeneity, and a random‐effects model was chosen for presentation of results. Exclusion of this small trial of poor quality from the meta‐analyses reduced heterogeneity and the summary effect estimate, suggesting that benefit derived from a dose of 40 mg of Promensil per day was no longer significant. Another study (van de Weijer 2002) reported a significant benefit of Promensil (at a dose of two tablets per day) but did not provide an indication of the variability around the estimate so could not be included in the meta‐analysis. A large trial of good quality (n = 252) (Tice 2003) that compared two types of red clover extract—Promensil (two tablets per day) and Rimostil—versus placebo reported no significant change in the frequency of hot flushes between groups and no significant difference in the change in vasomotor score over the period of the study. One of these studies also compared a higher dose of Promensil (160 mg/d) with placebo, but substitution of these values in the meta‐analysis did not alter the results.
Four studies compared other types of red clover extract (Del Giorno 2010; Geller 2009; Hidalgo 2005; Imhof 2006), but findings were inconclusive regarding efficacy. Two of these studies found benefit for 80 mg of red clover, but two others reported no evidence of significant differences associated with a dose of 40 mg or 120 mg of red clover at the end of 12 months of treatment. Variation in the findings was explained to some extent by differing quality of the trials. Results from larger studies of better quality appeared to conflict with those from smaller studies of poorer quality.
Genistein
Five studies assessed the effects of predominantly genistein extracts on outcomes (Crisafulli 2004; D'Anna 2007; Evans 2011; Ferrari 2009; Sammartino 2003). Genistein doses ranged from 30 mg to 60 mg per day. Duration of treatment with genistein ranged from 12 weeks to two years.
Primary outcome: efficacy
All four studies assessing primary efficacy—two with unclear risk of bias and two with low risk of bias—reported that genistein significantly improved the frequency of hot flushes when compared with placebo (Crisafulli 2004; D'Anna 2007; Evans 2011; Ferrari 2009). One study also found that the duration of hot flushes was reduced compared with placebo (although this is not an outcome of this review). The Crisafulli study also compared genistein with continuous hormone therapy; it reported a 24% mean reduction in daily hot flushes with genistein compared with placebo (P < 0.05) and a 30% reduction in daily hot flushes with HT compared with genistein (P < 0.05). The other three studies reported a mean percentage reduction in daily hot flushes from baseline ranging from 41% to 61% with genistein in comparison with a mean reduction ranging from 7% to 29% with placebo. Two studies of 12 weeks' duration found no evidence that the severity or intensity of hot flushes differed between genistein and placebo groups (Evans 2011; Ferrari 2009), but a study of longer duration reported that hot flush severity declined significantly when compared with placebo over two years (D'Anna 2007).
Secondary outcomes: safety
Four studies did not find a significant difference between groups in endometrial thickness after genistein treatment, and one study found no evidence of a significant difference in the vaginal maturation value.
Two studies found no evidence of a significant difference in adverse events between randomly assigned groups. In one study, most of these adverse events were gastrointestinal. No severe adverse events were experienced by participants.
Secondary outcomes: acceptability
The proportion of participants who were satisfied with treatment was similar in both groups in one study (79% with genistein compared with 69% with placebo; Heger 2006).
Sensitivity analyses
The four studies had low or unclear risk of bias. All studies evaluating efficacy consistently reported significant reductions in the frequency of hot flushes at the end of treatment ranging from 12 weeks to two years. Individual trial characteristics were generally fairly similar. Doses of genistein ranged from 56 mg to 60 mg of genistein per day, with one trial using a 30‐mg dose. Women generally had at least four hot flushes per day, and two studies reported average numbers of eight and nine hot flushes per day. Reductions in the number of hot flushes with genistein ranged from 24% to 56% against placebo. Placebo response, when reported, was variable; the two‐year study reported a reduction from baseline of 7.2%, but two shorter studies of 12 weeks' duration reported reductions of 27% and 29%. Results of the effects of genistein on the severity of hot flushes were more mixed and could not be explained by trial characteristics or quality.
Other phytoestrogens
Three studies compared flaxseed dietary supplement or flaxseed extract versus placebo or control (Colli 2012; Dalais 1998; Lewis 2006); one compared two strengths of hop extract with placebo (Heyerick 2006), one compared a natural supplement containing S‐(‐)equol, a daidzein metabolite, with placebo in women who were equol nonproducers (Aso 2012) and one compared an extract of Rheum rhaponticum (ERr 731) with placebo in perimenopausal women (Heger 2006). Duration of treatment was 12 weeks in most of the studies, but one trial had a duration of 16 weeks and another had a duration of 24 weeks.
Primary outcome: efficacy
All six trials assessed efficacy outcomes. Two trials found no evidence of a difference between groups in frequency or intensity of hot flushes after flaxseed treatment (Dalais 1998; Lewis 2006), and both Lewis and Colli did not find evidence of a time by interaction effect of hot flush severity or intensity between groups. The study evaluating hop extracts did not find evidence of a significant difference in the Kupperman Index hot flush severity score, although a non‐significant trend favoured both hop extract doses (Heyerick 2006). The study comparing S‐Equol with placebo found that S‐Equol was associated with a significantly larger decrease in hot flush frequency from baseline (62.8%) compared with placebo (23.6%) in women with three or more hot flushes per day and a significant improvement in hot flush severity with S‐Equol (61%) compared with placebo (45%) (Aso 2012). Results of the trial were assessed in postmenopausal women with at least one hot flush per day and low rates of equol excretion, as this was considered to reduce the possibility of confounding by background isoflavone intake. Another trial investigating the effects of an extract from the roots of Rheum rhaponticum (ERr 731) in perimenopausal women in the Ukraine reported that both the frequency and the severity of moderate to severe hot flushes and sweats (Menopause Rating Scale II and Menoqol vasomotor score) were significantly reduced with ERr 731 compared with placebo (mean decrease of 2.5 points compared with mean decrease of 1.2 points) at week 12 (Heger 2006). Effects were assessed blindly by both participants and investigators.
Secondary outcomes: safety
Three trials assessed endometrial thickness after six months or one year and reported no significant difference between groups (Colli 2012; Crisafulli 2004; Heger 2006). Also no evidence of a change in vaginal maturation index was noted with flaxseed compared with placebo or control (Colli 2012; Dalais 1998). In one trial, women ingesting flaxseed meal withdrew from treatment in greater numbers than those taking flaxseed extract or placebo because of gastrointestinal complaints (Colli 2012), but these differences were not tested statistically.
Secondary outcomes: acceptability
One trial (Heger 2006) reported that 63% of women taking ERr 731 were satisfied with their treatment compared with 32% of women taking placebo (no P value reported).
Sensitivity analyses
Studies using similar interventions in this subgroup were too few for sensitivity analyses to be undertaken.
Discussion
Summary of main results
This review has assessed the effectiveness, safety and acceptability of foods, supplements or extracts containing phytoestrogens when compared with placebo, no treatment and HT in randomised studies completed by the end of July 2013. It has been able to pool only the studies that used Promensil in meta‐analyses because of the heterogeneity of the other phytoestrogen interventions.
Primary outcome: efficacy
Dietary soy
Of the 13 included studies that used some type of substance containing dietary soy and had efficacy analyses of any kind, seven studies indicated that no significant differences were seen between the soy intervention group and the control group in terms of primary efficacy outcomes. In studies that reported significant findings, interventions included phytoestrogen‐enriched diets, soy milk, fruit drinks with isoflavones and soy powders. Only one trial had low risk of bias, and participants varied in the severity of their flushes at baseline. Sensitivity analyses could not explain the variable results. Thus, the findings from these trials must be considered only tentative, as variability and significant bias influencing the findings cannot be excluded.
Overall, no evidence suggested that a diet with high levels of soy phytoestrogens had a positive effect on hot flush frequency or severity.
Soy extracts
Of the 11 trials that compared soy extracts with placebo, nine had some positive results and two were negative. Five of nine studies found significant improvement in hot flush frequency with soy extract, but one found that soy extract was associated with more hot flushes than were seen with placebo. Four of seven studies found that hot flush severity was significantly reduced with soy extract, but most of these studies were at high risk of bias. One other study at high risk of bias found no difference in the effect of soy extract or hormone therapy on hot flush symptoms (as measured by the Kupperman Index).
Given the variability in the interventions, the severity of hot flushes at baseline and the potential for risk of bias, no overall conclusive evidence showed that soy extracts had a positive effect on hot flush frequency or severity.
Red clover extracts
Five studies assessed the effects of Promensil, and four studies assessed the effects of other red clover extracts. Findings were inconclusive and could largely be explained by risk of bias. The two larger studies at low risk of bias found no evidence of benefit with red clover extracts.
Overall, no evidence suggested that red clover extracts had a positive effect on hot flush frequency or severity.
Genistein
All four studies found consistent benefit for hot flush frequency with doses of genistein ranging from 30 to 60 mg per day in women with moderate to severe hot flushes, although benefits for hot flush severity were more mixed. Although benefits were found with genistein, they were significantly less than those associated with continuous hormone therapy in one study. These positive findings should be considered tentative as, in two of the four studies, effects on hot flushes were secondary outcomes, and in one study, measurements were made in a subgroup from the total study population, which may have introduced bias (Schulz 2005).
Overall, genistein extracts appeared to significantly reduce the numbers of hot flushes experienced by symptomatic postmenopausal women but to a lesser extent than hormone therapy.
Other phytoestrogens
Among six trials that assessed the effects of other types of phytoestrogens, no evidence of an efficacy difference was noted between flaxseed or linseed diets or extracts and placebo or control in three trials (two of which also contained soy diet arms) or in one trial that assessed a phytoestrogen preparation derived from hops in two different doses in women who had two to five daily flushes. In one trial, ERr 731 was associated with a significant reduction in hot flush and sweating severity symptoms (Menopause Rating Scale II) and in Menoqol vasomotor score compared with placebo. Another trial reported that hot flush frequency and severity were significantly reduced with the S‐Equol supplement (a daidzein metabolite) when compared with placebo.
Overall, although benefits were reported from single trials investigating a phytoestrogen extract from the rhubarb plant (ERr 731) and an equol supplement (SE5‐OH), data were insufficient to permit determination of whether any other type of phytoestrogen product had significant effects on vasomotor symptoms.
Secondary outcomes
Safety
Data on oestrogenic effects on the endometrium and the vagina and rates of adverse effects were collected in only a few trials and were considered together rather than in subgroups.
Endometrial outcomes
Phytoestrogen products do not appear to have an oestrogen agonistic effect on the endometrium when given for up to one year, in contrast to hormone replacement therapy. No evidence was derived from two studies in the review to suggest that phytoestrogens promote a proliferative endometrium (Balk 2002; Kaari 2006). Most studies reported no difference in endometrial thickness between phytoestrogens and placebo. One study actually found a significant reduction in endometrial thickness from baseline (Imhof 2006). The lack of an oestrogenic effect on the endometrium was further supported by a study comparing phytoestrogens with HT; endometrial thickness significantly changed from baseline with HT, but no change was seen with phytoestrogens (Kaari 2006).
Vaginal outcomes
Evidence of the effects of phytoestrogens on the vaginal maturation index and on pH is mixed. Five placebo‐controlled trials found no evidence of a stimulatory effect, but two other studies described a positive oestrogenic effect of increasing cellular mitotic activity, as evidenced by improvement in maturation indices—one with a soy diet versus a wheat diet and the other with a red clover extract (Dalais 1998; Hidalgo 2005). In two studies with a hormone therapy comparator, vaginal cytology values with HT were significantly different from those obtained with soy (Carmigiani 2010; Kaari 2006). Other studies not included in this review, wherein participants were asymptomatic or had breast cancer, have also produced conflicting data. Three studies found evidence of improvement in maturation values (Baird 1995; Chiechi 2003; Uesugi 2004), but three others have not confirmed these results (Duncan 1999; Manonai 2006; Nikander 2005). Characteristics of the individual trials provided no clues that could explain these mixed results. Similarly, one unpublished study described improvement in vaginal pH with a soy extract when compared with placebo (Bicca 2004), and another, much larger study did not find evidence of a difference (Upmalis 2000). In the study that compared soy extract versus HT, vaginal pH improved significantly more with HT than with soy (Kaari 2006). It is hoped that a Cochrane systematic review will be prepared to specifically assess the effects of phytoestrogens on urogenital menopausal symptoms to provide further clarification.
Adverse events
Only three of 17 trials found a significant difference in adverse event rates (Colli 2012; Knight 2001; Levis 2011). The phytoestrogen supplement in the first small trial was give in the form of a powder, and the authors included data on the dislike of the taste of soy powder in the total incidence of adverse events. In this case, the adverse event rate was linked to the type of product used in the soy diet and was more appropriate as a measure of acceptability. Data on the total incidence of adverse effects, with dislike of taste excluded, were not available. The Levis trial, which investigated the effects of soy on bone loss and on vasomotor symptoms, reported that fracture rates were significantly higher in the group of women consuming soy, but this is likely to be a chance event, as all fractures were associated with a traumatic event rather than with osteoporosis. Women ingesting flaxseed meal as a dietary supplement in another trial were more likely to withdraw from treatment because of gastrointestinal complaints when compared with those given flaxseed extract or placebo (Colli 2012). In a trial that compared soy extract with combined HT (Kaari 2006), women in the latter arm were more likely to experience genital bleeding, which is a common symptom of HT in perimenopausal women. This symptom was not experienced by women who took phytoestrogen supplements. Adverse events were most often collected incidentally during the trials.
Acceptability
Few trials specifically assessed this outcome in spite of the high dropout rate in many of the studies. No evidence was found of a difference in acceptability of any of the phytoestrogen products used when compared with placebo, except for one trial; 63% of those taking ERr 731 were satisfied with their treatment compared with 38% taking placebo.
Summary
In summary, generally no conclusive evidence showed a benefit of phytoestrogen‐enriched or ‐derived products for menopausal vasomotor symptoms, with the exception of products containing a minimum of 30 mg per day of genistein, which have been evaluated for up to two years in four studies. Also, no evidence indicated that products derived from phytoestrogens were associated with stimulation of the endometrium and/or vagina of women with vasomotor symptoms. No evidence suggested an increase in adverse events, and limited data suggest that phytoestrogen supplements were well tolerated.
Overall completeness and applicability of evidence
The review included 43 studies, but variation in components and duration of the intervention and in types of participants and severity of their hot flushes, as well as potential variation in metabolism and absorption among individuals and a high placebo response rate, generally resulted in inconclusive findings. Although some trials reported a significant beneficial effect of phytoestrogen treatment on symptoms, strong evidence was found of a placebo effect, with improvements in frequency ranging from ‐1% to ‐59%—similar to the placebo effect found in the Cochrane review of hormone replacement for vasomotor symptoms (MacLennan 1999). When some of the included studies reported significant differences between groups, it was not possible to tease out which of the many variables that differed between included studies might have explained the results.
Studies varied according to the total amount or 'dose' of isoflavone given in the active treatment arm. The rationale for a role for isoflavones is supported by epidemiological evidence from a community‐based study, which found that the incidence of hot flushes was inversely related to the quantity of soy foods consumed and the daily intake of isoflavones (Nagata 2001). Studies of Japanese women claim a typical daily consumption of 20 to 54 mg of isoflavones (Nagata 2001; Somekawa 2001). Most of the studies in this review provided treatments with at least 50 mg per day of isoflavones; some used more than 100 mg of isoflavones per day. Examination of the pattern of results within each subgroup did not indicate that trials were more likely to be positive if they used higher doses of isoflavones. Comparison of total isoflavone levels in treatments may not be useful, as the isoflavone profiles of different supplements and extracts differ considerably. Broad groupings in this review into 'types' of phytoestrogens provided no clues as to the best way that phytoestrogens can be delivered for therapeutic effect.
In addition to the heterogeneity of interventions used in the included studies, good evidence of variability was noted in the metabolism and absorption of isoflavones by individuals, which can lead to variations in serum concentrations of parent isoflavones and their metabolites (Rowland 2003; Wiseman 2004). It has been claimed that only 20% to 30% of the general population in the United States possess gut microflorae that convert the isoflavone daidzein to the more oestrogenic dihydroxy isoflavan equol (Setchell 2002) in comparison with 50% to 60% of Asians (NAMS 2011). It has been suggested that 'equol producers' make up a distinct subpopulation that may be associated with the greatest benefit from soy isoflavones for relief of hot flushes, and they may explain anecdotal reports by many women of phytoestrogen effectiveness in relieving hot flushes. The studies included in this review generally did not control or stratify for this added potential source of variation in response to treatment with phytoestrogens, although investigators in one trial stated that isoflavone supplementation improved symptoms only in women with the ability to produce equol. Another recent trial found a significant benefit associated with use of a supplement of S‐(‐)equol in equol‐nonproducing postmenopausal Japanese women. Further research should consider the role of equol production.
In spite of variation in doses, duration and components of phytoestrogen products, extracts containing at least 30 mg of genistein, a type of isoflavone, appeared to reduce both frequency and severity of hot flushes in women, but less so than hormone therapy. A possible rationale is the suggestion that genistein is the most potent isoflavone with regard to receptor binding and transactivation (Muthyala 2004). No evidence of safety concerns in the short term was found. Further research is needed to confirm the efficacy of high doses of genistein in menopausal women.
Quality of the evidence
The quality of the trials was variable. Although most studies blinded both participants and assessors, less than half reported adequate allocation concealment to protect against selection bias, and high rates of attrition and potential selective outcome reporting in half of the trials suggest that they were at high risk of attrition and reporting bias. Substantial variation in the included studies precluded combining findings in forest plots, and this has limited any definitive conclusions.
Four trials have suggested benefit for the reduction of hot flush frequency and severity from high doses of genistein, but these findings should be considered tentative because of methodological shortcomings in some of the trials. The effect of genistein on menopausal vasomotor symptoms requires verification.
Potential biases in the review process
This review has the following limitations. Because we were unable to pool most of the studies and in many cases had no access to the original data, we accepted the statistical methods used in each study and reported study results in tabular form. Many studies did not use an appropriate statistical method to measure changes in frequency or severity over time; endpoint analysis may have obscured different patterns of response. Some trials used unvalidated menopausal symptom questionnaires to assess severity, and not all scales were similar. For example, the Greene Vasomotor Scale includes hot flushes and night sweats, whereas the vasomotor scale used by Kotospoulos includes hot flushes, lightheadedness and headache.
A second weakness of the review is the potential for publication bias. The search for relevant studies was very comprehensive, and attempts were made to access the grey literature. However, several of the studies waiting assessment are positive studies in abstract form, and their inclusion may paint a different picture. Publication bias usually operates in a differential manner, leading to a higher probability of publication of studies that indicate positive results.
Also, some potential for bias may be associated with the need for the review authors to determine which interventions would be considered as phytoestrogens, given the wide variety of sources and doses of phytoestrogenic compounds.
Agreements and disagreements with other studies or reviews
One review has suggested that phytoestrogen products might be more effective in women with more severe flushes at baseline (Huntley 2004). This hypothesis has been supported by another study, which proposed that the effectiveness of phytoestrogens for hot flush relief is seen only in those with five or more hot flushes per day (Messina 2003). Another systematic review and meta‐analysis (Howes 2006) also came to the conclusion that women more severely affected by vasomotor symptoms derived a small benefit from isoflavone supplementation and suggested a cut point of around four flushes per day. However, the authors of that review pooled highly variable studies, causing significant statistical heterogeneity, which affects the credibility of effect estimates. These suggestions have not been confirmed by the sensitivity analyses performed in this review, which compared the overall pattern of results with studies that required women to have at least five hot flushes per day. In addition, the North American Menopause Society (NAMS) position paper on isoflavones, which used strict criteria for evaluation of isoflavones, concluded that no linear dose‐response relationship was observed (NAMS 2011), and a recent systematic review (Taku 2012) concluded that the effect of baseline frequency of hot flushes is unclear. When included trials show huge variation in characteristics of participants, types of intervention and outcomes measured, it is important to consider the quality of the trials. One of the largest trials (Tice 2003) was a study of good quality with high compliance, a low dropout rate and good generalisability (including a broad cross section of the population). It required that women have at least 35 hot flushes per week to participate, and investigators found no evidence of benefit for Promensil or Rimostil.
The efficacy findings of this review are broadly in accord with those of most systematic reviews assessing the effects of phytoestrogens on menopausal symptoms published over the past decade (Bolanos 2010; Coon 2007; Geller 2005; Glazier 2001; Haimov‐Kochman 2005; Howes 2006; Huntley 2004; Jacobs 2009; Krebs 2004; Low Dog 2005; Nedrow 2006; Villaseca 2012; Williamson‐H 2006), and this review has included several more recently published studies. In general, most reviews have concluded that phytoestrogen supplementation has no effect or a very mild effect on vasomotor symptoms. However, NAMS, using strict criteria for selection of relevant trials, concluded that soy‐based isoflavones are modestly effective in relieving menopausal symptoms, and that their use in women with distressing symptoms is 'reasonable' (NAMS 2011). A recent systematic review reported that soy isoflavone supplements derived by extraction or chemical synthesis were significantly more effective than placebo in reducing the frequency and severity of hot flushes (Taku 2012). This review included trials with a shorter duration of treatment (six weeks) and women with previous breast cancer; these types of studies were excluded from this review. The Taku review authors also acknowledged substantial heterogeneity in their findings and have noted that further research is warranted to clarify the influence of additional factors, such as dose, isoflavone form, baseline hot flush frequency and duration of treatment.
The Taku review has also confirmed the findings of Williamson‐H 2006, which focused specifically on soy isoflavone extracts and stratified studies according to the amount of genistein included in the extract. Findings of this review suggest that supplements that provide at least 15 mg of genistein per day are effective, whereas those providing less genistein are not, and this hypothesis has been supported by the findings of this review. However, this hypothesis has not been supported by the longitudinal Study of Women's Health Across the Nation, which assessed the association between vasomotor symptoms and ethnicity during the menopausal transition in 3,198 women (Gold 2006). Investigators in this study reported that genistein intake in their sample was not related to vasomotor symptoms, and that it did not account for the reduced symptom reporting that they found among Asian women after adjusting for covariates. This hypothesis needs to be further investigated in randomised trials for inclusion in future updates of this review.
The safety of phytoestrogen products has been investigated in another systematic review and assessed in separate subgroups: gynecological or urinary, gastrointestinal, musculoskeletal, neurological or sensory and nonspecific (Tempfer 2009). The review authors concluded that phytoestrogen supplements had a safe side effect profile, along with moderately elevated rates of gastrointestinal side effects. Use of phytoestrogens was not associated with increased risk of endometrial or breast cancer. Most studies in the review were of limited duration. These findings support the conclusion of this review that phytoestrogen‐enriched products appear to be safe when used for up to two years. A study of women without hot flushes at baseline (not eligible for inclusion in this review) (Unfer 2004) reported that long‐term treatment (up to five years) with soy (150 mg/d isoflavones) was associated with increased occurrence of simple endometrial hyperplasia compared with placebo. This finding has not been confirmed by other studies, but the long‐term endometrial safety of high doses of phytoestrogen supplements has not been fully established.
Authors' conclusions
Implications for practice.
No conclusive evidence shows that phytoestrogen supplements effectively reduce the frequency or severity of hot flushes and night sweats in perimenopausal or postmenopausal women, although benefits reported with concentrates of genistein may be beneficial and should be further investigated. Many of the included studies were of poor quality, and results were inconsistent, providing no guidance on which type of product is likely to be more beneficial. Women need to be reassured that these symptoms usually abate over time. When therapy is desired or required, the use of unspecified phytoestrogen supplements is not based on good quality evidence of benefit, although it is possible that high doses of genistein may offer relief. No evidence shows harmful side effects in the short term resulting from the use of these supplements.
Implications for research.
More research is required to test the following hypotheses.
Supplements containing at least 15 mg of genistein are more effective than supplements containing less than 15 mg of genistein.
Phytoestrogen supplements are more effective in women with more than five moderate to severe hot flushes per day than in those with mild symptoms.
Phytoestrogen supplements are more effective in women who are equol producers.
In addition, to enhance comparability, future trials should be based on phytoestrogen products that are well characterised; they should provide strict monitoring of participants throughout the trial, should be well powered and of adequate duration and should use validated measurements of outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2013 | New citation required and conclusions have changed | Fourteen new trials added in the 2013 update of the review (two of which were longer follow‐ups of previously included trials). Three previously included trials were excluded in the 2013 update because additional information indicated that the women did not have troublesome hot flushes at baseline. Conclusions changed: Genistein extracts appeared to offer a benefit for hot flushes, but confirmation through more research is needed. |
| 31 October 2013 | New search has been performed | New studies added and review conclusions changed. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 14 May 2008 | Amended | Converted to new review format. |
| 27 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the work of the Trial Search Co‐ordinators of MDSG—Mrs Haiyan Feng and Mrs Marian Showell—in the design and implementation of comprehensive search strategies to locate trials for the review. We would also like to thank the Managing Editors—Mrs Jane Clarke and Mrs Helen Nagels—for their assistance in preparing the review for publication.
The following authors of included studies provided additional clarification of data and quality issues: Rod Baber, Marilene Bica, Amnon Brzezinski, Fabien Dalais, Peter Chedraui, Hope Ricciotti, Peter Heger, Francesco Squadrito and Mal Evans.
Appendices
Appendix 1. MEDLINE search strategy
1 exp perimenopause/ or exp postmenopause/ (17644) 2 postmenopaus$.ti,ab,sh. (41580) 3 menopaus$.ti,ab,sh. (43511) 4 exp Climacteric/ or exp Hot Flashes/ or exp Menopause/ (46568) 5 hot flash$.ti,ab. (1560) 6 hot flush$.ti,ab. (1646) 7 climacteric.ti,ab. (3594) 8 (vagina$ adj3 atroph$).ti,ab,sh. (586) 9 (vagina$ adj3 dry$).ti,ab. (645) 10 endometri$.ti,ab. (62890) 11 or/1‐10 (133776) 12 Phytoestrogens/ (2493) 13 phytoestrogen$.ti,ab. (2979) 14 Soy Foods/ (813) 15 soy$.ti,ab. (35598) 16 exp isoflavones/ or coumestrol/ or genistein/ or pterocarpans/ or rotenone/ (13154) 17 linseed.mp. or Flax/ (2037) 18 isoflavon$.ti,ab. (5734) 19 red clover.ti,ab. (715) 20 daidzein.ti,ab. (2323) 21 promensil.ti,ab. (8) 22 or/12‐21 (50378) 23 11 and 22 (2078) 24 randomized controlled trial.pt. (337111) 25 controlled clinical trial.pt. (85195) 26 randomized.ab. (252126) 27 placebo.tw. (143456) 28 clinical trials as topic.sh. (162463) 29 randomly.ab. (184559) 30 trial.ti. (108477) 31 (crossover or cross‐over or cross over).tw. (54671) 32 or/24‐31 (825664) 33 exp animals/ not humans.sh. (3782187) 34 32 not 33 (761678) 35 23 and 34 (686) 36 2012$.ed. (638938) 37 35 and 36 (43)
Appendix 2. EMBASE search strategy
1 exp "menopause and climacterium"/ or climacterium/ or early menopause/ or menopause/ or postmenopause/ (76520) 2 postmenopaus$.ti,ab. (47787) 3 menopaus$.ti,ab. (43440) 4 climacter$.ti,ab. (4494) 5 exp Hot Flush/ (9977) 6 hot flush$.ti,ab. (2262) 7 hot flash$.ti,ab. (2066) 8 (vagina$ adj3 atroph$).ti,ab,sh. (777) 9 (vagina$ adj3 dry$).ti,ab,sh. (1100) 10 endometri$.ti,ab. (75307) 11 or/1‐10 (176874) 12 exp PHYTOESTROGEN/ (4474) 13 phytoestrogen$.ti,ab. (3724) 14 plant estrogen$.ti,ab. (69) 15 Daidzein/ or Isoflavone Derivative/ or Genistein/ or Soybean Oil/ or Soybean/ or Isoflavone/ or Soybean Protein/ or soy$.mp. (57048) 16 COUMESTROL DERIVATIVE/ or COUMESTROL/ (662) 17 coumestrol.ti,ab. (404) 18 pterocarpan$.ti,ab,sh. (274) 19 rotenone$.ti,ab,sh. (5229) 20 linseed.ti,ab,sh. (1785) 21 red clover.ti,ab,sh. (947) 22 daidzein.ti,ab,sh. (4250) 23 promensil.ti,ab,sh. (14) 24 or/12‐23 (66860) 25 11 and 24 (3465) 26 Clinical Trial/ (871161) 27 Randomized Controlled Trial/ (328650) 28 exp randomization/ (59349) 29 Single Blind Procedure/ (16360) 30 Double Blind Procedure/ (110736) 31 Crossover Procedure/ (34922) 32 Placebo/ (204401) 33 Randomi?ed controlled trial$.tw. (78497) 34 Rct.tw. (9931) 35 random allocation.tw. (1183) 36 randomly allocated.tw. (17608) 37 allocated randomly.tw. (1829) 38 (allocated adj2 random).tw. (711) 39 Single blind$.tw. (12516) 40 Double blind$.tw. (130405) 41 ((treble or triple) adj blind$).tw. (280) 42 placebo$.tw. (178872) 43 prospective study/ (213060) 44 or/26‐43 (1272542) 45 case study/ (16909) 46 case report.tw. (230790) 47 abstract report/ or letter/ (843460) 48 or/45‐47 (1086430) 49 44 not 48 (1237229) 50 25 and 49 (1414) 51 limit 50 to yr="2012 ‐Current" (45)
Appendix 3. PsycINFO search strategy
1 exp menopause/ (2681) 2 postmenopaus$.tw. (1757) 3 menopaus$.tw. (3373) 4 perimenopaus$.tw. (440) 5 climacteric.tw. (392) 6 hot flash$.tw. (268) 7 hot flush$.tw. (152) 8 (vagina$ adj3 atroph$).tw. (26) 9 (vagina$ adj3 dry$).tw. (99) 10 or/1‐9 (4865) 11 Phytoestrogens.tw. (58) 12 soy.tw. (217) 13 linseed.tw. (8) 14 isoflavon$.tw. (96) 15 red clover.tw. (6) 16 daidzein.tw. (28) 17 promensil.tw. (0) 18 or/11‐17 (312) 19 10 and 18 (60) 20 limit 19 to yr="2012 ‐Current" (5)
Appendix 4. AMED search strategy
1 exp climacteric/ or exp menopause/ or exp postmenopause/ (499) 2 postmenopaus$.tw. (374) 3 menopaus$.tw. (657) 4 exp Climacteric/ (499) 5 hot flash$.tw. (47) 6 hot flush$.tw. (33) 7 climacteric.tw. (49) 8 (vagina$ adj3 atroph$).tw. (2) 9 (vagina$ adj3 dry$).tw. (10) 10 or/1‐9 (890) 11 exp Phytoestrogens/ (54) 12 phytoestrogen$.tw. (132) 13 Soy Foods/ (12) 14 soy$.tw. (226) 15 exp isoflavones/ (96) 16 linseed.tw. (7) 17 isoflavon$.tw. (278) 18 red clover.tw. (14) 19 daidzein.tw. (53) 20 promensil.tw. (0) 21 or/11‐20 (514) 22 10 and 21 (68) 23 limit 22 to yr="2012 ‐Current" (1)
Appendix 5. CENTRAL search strategy
1 exp perimenopause/ or exp postmenopause/ (3249)
2 postmenopaus$.ti,ab,sh. (7655)
3 menopaus$.ti,ab,sh. (4117)
4 exp Climacteric/ or exp Hot Flashes/ or exp Menopause/ (5212)
5 hot flash$.ti,ab. (325)
6 hot flush$.ti,ab. (570)
7 climacteric.ti,ab. (573)
8 (vagina$ adj3 atroph$).ti,ab,sh. (113)
9 (vagina$ adj3 dry$).ti,ab. (116)
10 endometri$.ti,ab. (3122)
11 or/1‐10 (13074)
12 Phytoestrogens/ (148)
13 phytoestrogen$.ti,ab. (171)
14 Soy Foods/ (67)
15 soy$.ti,ab. (1349)
16 exp isoflavones/ or coumestrol/ or genistein/ or pterocarpans/ or rotenone/ (468)
17 linseed.mp. or Flax/ (111)
18 isoflavon$.ti,ab. (514)
19 red clover.ti,ab. (35)
20 daidzein.ti,ab. (139)
21 promensil.ti,ab. (7)
22 or/12‐21 (1716)
23 11 and 22 (516)
24 limit 23 to yr="2007 ‐Current" (174)
Appendix 6. MDSG search strategy
Keywords CONTAINS "menopausal"or"*Menopause"or"perimenopause"or"perimenopausal"or "Postmenopausal"or "postmenopause"or"climacteric "or "vasomotor"or"hot flashes"or "hot flushes"or"vaginal atrophy"or "vaginal dryness"or Title CONTAINS "menopausal"or"*Menopause"or"perimenopause"or"perimenopausal"or "Postmenopausal"or "postmenopause"or"climacteric "or "vasomotor"or"hot flashes"or "hot flushes"or"vaginal atrophy"or "vaginal dryness"
AND
Keywords CONTAINS "Phyto‐Female complex" or"phytoestrogen"or "phytoestrogens" or"phytosterols"or "soy" or"soy‐protein diet" or"soy‐nut diet" or"soybean"or "soybeans"or "soyfem preparation" or"soymilk" or"isoflavones" or"isoflavonoids" or"red clover"or "daidzein"or "promensil" or Title CONTAINS "Phyto‐Female complex" or"phytoestrogen"or "phytoestrogens" or"phytosterols"or "soy" or"soy‐protein diet" or"soy‐nut diet" or"soybean"or "soybeans"or "soyfem preparation" or"soymilk" or"isoflavones" or"isoflavonoids" or"red clover"or "daidzein"or "promensil"
Data and analyses
Comparison 1. Promensil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of hot flushes (number/d) | 5 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.95, 0.10] |
| 1.1 40 mg/d | 3 | 105 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐2.26, ‐0.64] |
| 1.2 80 mg/d | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐3.28, 1.77] |
| 2 Change in frequency of hot flushes (% reduction) | 2 | 199 | Mean Difference (IV, Random, 95% CI) | 20.15 [‐12.08, 52.38] |
| 3 Improvement in hot flush severity rate | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.06 [1.10, 264.52] |
| 4 Change in vasomotor score from baseline to end of study/vasomotor severity—Kupperman subscale | 1 | 165 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.29, 0.32] |
| 5 Endometrial thickness (mm) after treatment | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐4.94, 5.06] |
| 6 Adverse event rates | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.40] |
| 7 Incidence of specific adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Cold or upper respiratory tract infection (URTI) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
| 7.2 Headache | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.27] |
| 7.3 Myalgia | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.58, 3.62] |
| 7.4 Nausea | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.91] |
| 7.5 Arthralgia | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.27, 2.66] |
| 7.6 Diarrhoea | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.94] |
| 7.7 Vaginal spotting | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.85] |
1.1. Analysis.
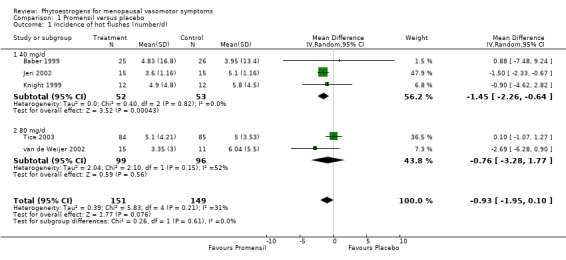
Comparison 1 Promensil versus placebo, Outcome 1 Incidence of hot flushes (number/d).
1.5. Analysis.
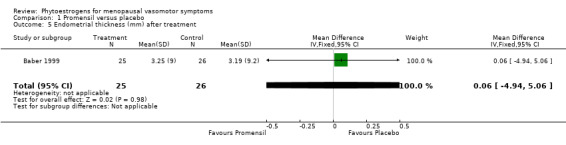
Comparison 1 Promensil versus placebo, Outcome 5 Endometrial thickness (mm) after treatment.
1.6. Analysis.

Comparison 1 Promensil versus placebo, Outcome 6 Adverse event rates.
1.7. Analysis.
Comparison 1 Promensil versus placebo, Outcome 7 Incidence of specific adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albertazzi 1998.
| Methods | Design: parallel‐group Number randomly assigned: 92 initially plus a further 12 from the reserve randomisation list = 104 Number dropped out: 21 (nine in active group: seven for gastrointestinal symptoms, one for lack of efficacy, one for non‐compliance; seven in placebo group: two for gastrointestinal symptoms, three for lack of efficacy, one for non‐compliance, one for other reasons) Number lost to follow‐up: four (two in each group) Number analysed: 79 Intention‐to‐treat analysis: no Power calculation: 90% power to detect a difference of three hot flushes per 24 hours (P = 0.05) Duration: 12 weeks Timing: not stated Location: two Italian university hospitals Funding: partial industry funding from Protein Technologies, Missouri | |
| Participants | Inclusion criteria: postmenopausal women requesting treatment for severe hot flushes, six months since last menstruation or six weeks since bilateral oophorectomy, minimum of seven moderate to severe hot flushes or night sweats per 24 hours during two out of four weeks before the study (threshold defined as warmth and sweating preventing normal daily activity), baseline FSH > 50 IU/mL, serum oestradiol < 35 pg/mL
Exclusion criteria: use of HT within six weeks of study or other drug used for climacteric symptoms during study period
Age, years: active arm 53 (48 to 61), placebo 52 (45 to 62) Recruitment: not stated |
|
| Interventions |
|
|
| Outcomes | Menopausal symptoms: change in number of daily moderate and severe hot flushes or night sweats from baseline in each month of treatment; Kupperman Index Compliance: self‐report in daily diary and sachet count Adverse effects: reported monthly at follow‐up; for each woman, only the worst symptom (in her opinion) was taken into account | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Investigator site personnel blinded to trial codes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate—12 participants added |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Aso 2012.
| Methods | Design: parallel‐group Number screened for inclusion: 1,211 Number randomly assigned: 160 Number dropped out or withdrew: 34 (placebo group: three dropped out, eight did not comply, 12 met withdrawal criteria; equol group: eight did not comply, three met withdrawal criteria) Intention‐to‐treat analysis: no Power calculation: not stated Duration: 12 weeks of intervention or placebo, assessments at week 12 and week 18 Location: four clinics/centres in Tokyo, Saitama, Chiba, Fukuoka Funding: Otsuka Pharmaceutical Company Ltd provided the intervention and financial support |
|
| Participants | Inclusion criteria: duration of amenorrhoea one to 10 years, oestradiol < 21 pg/mL, FSH > 30 mIU/d, frequency of hot flushes ≥ 1/d, equol non‐producer, SMI score ≥ 25, BMI 18.6 to 25, SDS score < 53 Exclusion criteria: surgical menopause, severe menopausal symptoms requiring medical treatment, abnormal vital signs and clinical laboratory tests outside the normal range, current or past reproductive related cancer, thyroid dysfunction or other serious medical conditions, allergy to soy, milk or egg, use of prescription medication for menopausal symptoms, OTC medical agents or health foods for relief of menopausal symptoms Age, years: 53.9 (mean) in placebo group, 53.2 (mean) in equol group Recruited using fliers, advertisements and volunteer lists and registered at a clinical research organisation |
|
| Interventions |
|
|
| Outcomes | Menopausal symptoms (as measured by the modified Climacteric Symptom Evaluation Form Checklist, Visual Analog Scale (VAS), subscales of the Greene Climacteric Scale and somatic symptom scales) Quality of life (as measured by Short Form‐36 and VAS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified permutation block method controlled for study site, years since menopause and BMI |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random permutation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff members and laboratory technicians blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large proportion of participants dropped out and not analysed |
| Selective reporting (reporting bias) | Low risk | All plausible outcomes reported |
Baber 1999.
| Methods | Randomisation method: not stated Blinding: double Allocation concealment: not stated Design: cross‐over Number screened for inclusion: not stated Number randomly assigned: 51 Number dropped out: eight (seven for personal reasons, one for medical reasons not related to study) Number lost to follow‐up: none stated Intention‐to‐treat analysis: no Power calculation: not stated Duration: three months × 2 Timing: not stated Location: tertiary menopause clinic, Australia Funding: industry (Novogen Ltd, Australia) | |
| Participants | Inclusion criteria: minimum of mean of three hot flushes per day in week preceding trial Exclusion criteria: intercurrent medical problems, HT or antibiotics in previous three months, FSH < 30 mIU/mL, menstruation in previous six months, hysterectomy, vegetarian (> 10 g legumes/d) Age, years: 54 (±4.1) Recruitment method: volunteers. Not further specified | |
| Interventions |
|
|
| Outcomes | Menopausal symptoms: daily flush frequency scored on daily diary card Quality of life: Greene Climacteric Scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Allocated randomly and blindly," but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind: analysis of data performed by separate group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Balk 2002.
| Methods | Design: parallel‐group Number of women screened: "hundreds" (most not amenorrhoeic for one year) Number randomly assigned: 27 Number dropped out: seven (five in active arm: two for family reasons, three for adverse effects; two in placebo arm: one for lack of efficacy, one disliked taste) Number lost to follow‐up: one (active arm) Number analysed: 19 Intention‐to‐treat analysis: no Power calculation: powered to detect endometrial changes, but baseline proliferation rate underestimated and study thus underpowered for primary outcome Duration: six months Timing: January 1998 to June 2000 Location: university hospital clinic Funding: academic research grants | |
| Participants | Inclusion criteria: postmenopausal women 40 years of age with no vaginal bleeding for one year or over 30 years of age with oophorectomy or premature ovarian failure, omnivorous, intact uterus, normal endometrium on Pipelle biopsy, normal mammogram within previous year Exclusion criteria: tamoxifen usage, endometrial cancer, allergy to soy, hormone therapy on past year, using phytoestrogen supplements (diet logged for two weeks before study) Age, years: active arm 56.8 ± 5.9; placebo arm 57.9 ± 8.2 Recruitment method: primary and tertiary clinics, newspaper and radio advertisements, research institute website | |
| Interventions |
Given list of soy‐ and phytoestrogen‐containing foods to avoid |
|
| Outcomes | Menopausal symptoms: weekly log monitoring nine specific symptoms on 4‐point scale Compliance: daily dietary logs, check of unused cereal Adverse effects: weekly log monitored specific symptoms such as nausea, breast tenderness and gastrointestinal effects | |
| Notes | Authors reported that recruitment was difficult. Participants were not required to have menopausal symptoms to be eligible for the study, although these were measured at baseline. Mean symptom score at baseline indicated that, on average, participants had mild to moderate hot flushes and/or night sweats | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in blocks of six |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very high dropout (8/27) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Bicca 2004.
| Methods | Design: parallel‐group Number of women screened: 90 Number analysed: 75 Number dropped out: one lost to follow‐up in soy group, three lost in placebo group (two adverse events, one other) Intention‐to‐treat analysis: no Power calculation: yes Duration: 25 weeks Timing: not specified Location: university in Brazil Funding: not specified | |
| Participants | Inclusion criteria: women 42 to 61 years of age, symptomatic and no menses for 12 months Exclusion criteria; oral HT in previous three months, topical HT in previous 30 days, use of medication that could influence the results, concomitant severe disease Mean age, years: 54 in soy group, 52 in placebo group Recruitment method: advertisements | |
| Interventions |
|
|
| Outcomes | Decrease in frequency of hot flushes and night sweats; vasomotor symptom intensity; change in vaginal pH | |
| Notes | Study not published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers kept by separate organisation |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as triple‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as triple‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Brzezinski 1997.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 145 Number dropped out: 31 (17 in active group: two for unbearable symptoms, 15 for personal reasons; 14 in control group: seven for unbearable symptoms, seven for personal reasons) Number lost to follow‐up: none stated Number analysed: 114 Intention‐to‐treat analysis: no Power calculation: power calculation performed, but anticipated effect size not specified Duration: 12 weeks Timing: not stated Location: menopause clinic in Jerusalem, Israel Funding: academic grants | |
| Participants | Inclusion criteria: perimenopausal and postmenopausal women 43 to 65 years of age, natural or surgical menopause with at least three months of amenorrhoea, FSH > 30 IU, LH > 20 IU, plasma oestradiol < 200 pmol/mL, experiencing hot flushes, night sweats, insomnia, vaginal dryness or dyspareunia Exclusion criteria: acute medical illness, use of gonadal hormones or any medicine known to influence menopausal symptoms or endocrine variables, known or suspected food allergies Age, years: active arm 53.66 (SE 0.74), control arm 51.32 (SE 0.71) Recruitment method: women requesting help for climacteric complaints at outpatient menopause clinic | |
| Interventions |
|
|
| Outcomes | Menopausal symptoms: menopause symptoms questionnaire | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence, non‐computerised |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported and unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout |
| Selective reporting (reporting bias) | High risk | Authors did not report on adverse events |
Burke 2003.
| Methods | Design: parallel‐group Number screened for inclusion: 1,571 (1,230 ineligible: main reasons: lack of menopausal symptoms (293), refusal to stop HT (241), cycle not perimenopausal (206)) Number randomly assigned: 241 Number dropped out: none stated Number lost to follow‐up: none stated Number analysed: 211 (30 had data missing from symptom diaries) Intention‐to‐treat analysis: no Power calculation: Duration: two years Timing: August 1996 to August 1997 Location: Wake University Clinic,Carolina, USA Funding: soy supplements supplied by industry (Soy Technologies, St Louis, Missouri, USA) | |
| Participants | Inclusion criteria: perimenopausal women (no more than one menstrual period in three months before randomisation), at least one vasomotor symptom per day, not using HT for three months before recruitment, willingness to participate in one‐week run‐in with isoflavone‐free supplement Exclusion criteria: acute MI or stroke within previous six months, history of breast or endometrial cancer, invasive cancer within previous five years, active thromboembolic disease, previous osteoporosis‐related fractures treated with hormones, low baseline bone density, previous exposure to diethylboestrol, dyslipidaemia, endometrial biopsy showing hyperplasia, consumption of soy products on a daily basis and unwillingness to reduce consumption to once a week Age, years: mean 50.8 (SE 0.2) Recruitment method: "recruited from the community" | |
| Interventions |
|
|
| Outcomes | Menopausal symptoms: hot flushes, night sweats recorded in monthly calendar with daily entry field: participants asked to record number and severity of symptoms for one full week per month Compliance: compliance calendars | |
| Notes | 37 women (18%) took HT during trial: 16 in high‐dose group (25%), 11 in middle group (16%), 10 in control group (13%). Data analysed with and without these women, and pattern of results not affected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderate dropout: missing data from questionnaires because not filled in |
| Selective reporting (reporting bias) | High risk | Authors did not report on all prespecified outcomes |
Campagnoli 2005.
| Methods | Design: cross‐over Number of women screened: not stated Number randomly assigned: 36 Number analysed: 29 Number dropped out: seven (not clear which group: three medical reasons, four family reasons) Intention‐to‐treat: no Power calculation: yes: 95% power to detect at least a 20% greater reduction in hot flushes in active arm compared with placebo Duration: 12 + 12 weeks Timing: November 1999 to December 2000 Location: hospital in Torino, Italy Funding: Medestea International (manufacturer of active treatment) | |
| Participants | Inclusion criteria: minimum of five moderate to severe hot flushes/d, good general health, 45 to 58 years, BMI 18 to 28, surgical menopause (bilateral oophorectomy for at least three months or in spontaneous menopause with no menses for over six months), menopausal hormone profile (oestradiol < 30 pg/mL, FSH > 40 UI/L) Exclusion criteria: use of drugs that influence vasomotor symptoms, hormone therapy or tibolone in previous six months, consumption of soy‐based food more than once per week, use of drugs that might reduce absorption of isoflavones Mean age of completers, years: 51 Recruitment method: menopause clinic | |
| Interventions |
|
|
| Outcomes | Number of hot flushes per week after treatment (at end of first phase of study) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Schedule prepared by the manufacturer of the product using computer‐generated randomisation list and distributed sequentially |
| Allocation concealment (selection bias) | Low risk | Adequate: investigators blind to treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout |
| Selective reporting (reporting bias) | Low risk | All potential outcomes reported |
Carmigiani 2010.
| Methods | Design: parallel‐group Number of women screened: 1,520 Number of women randomly assigned: 60 Number of women analysed: 60 Number of dropouts or lost to follow‐up: Intention‐to‐treat: yes Power calculation: yes, 16 subjects required in each group to reach power of 90%, assuming a difference of three hot flushes in a 24‐hour period and SD of 3.8 hot flushes/d Duration: 16 weeks Timing: January to October 2007 Location: two menopause outpatient clinics at the Centre for Women's Integrated Healthcare of the University of Campinas, Campinas, Sao Paolo, Brazil Funding: Sao Paolo Foundation for the Support of Research |
|
| Participants | Inclusion criteria: postmenopausal women between 40 and 60 years of age who had LMP > 12 months previously; FSH > 30 mUI/mL and oestradiol levels < 20 pg/mL; more than eight hot flushes in 24 hours; not using any form of hormonal treatment during previous six months; not currently using any lipid‐lowering drugs, antidiabetic drugs, soybean‐derived products or herbal supplements Exclusion criteria: previous hysterectomy; chronic gastrointestinal disorder; contraindication to hormone therapy; patients participating in a conflicting clinical trial; known allergy or hypersensitivity to soy or cow's milk; not willing to cease consumption of soy products for the term of the trial Mean age, years: 53 in HT and soy groups; 51 in placebo group Recruitment method: menopause outpatient clinic |
|
| Interventions |
|
|
| Outcomes | Primary: menopause rating scale. Also side effects, endometrial thickness, maturation index and compliance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation list with numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded for the duration of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was gynecologist, who did not participate in the screening process or dispense drugs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout or lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Cheng 2007.
| Methods | Design: parallel‐group Number of women screened: not reported Number of women randomly assigned: 60 Number of women analysed: 51 (two dropped out during the first month, seven continued treatment but missed some examinations) Intention‐to‐treat: no Power calculation for sample size: no Duration: 12 weeks Timing: not stated Location: Sweden; other details not reported Funding: Stockholm County Council, Swedish Cancer Society, European Union Specific Targeted Research Project and Susan G. Komen Breast Cancer Foundation |
|
| Participants | Inclusion criteria: postmenopausal women, at least one year since last menstruation, FSH levels > 30 IU/mL, at least six months without taking hormone therapy. All women experienced hot flashes and night sweats Exclusion criteria: not stated Age, years: between 49 and 69; mean age placebo group 56.9 ± 4.2; isoflavone group 58.4 ± 5.0 Recruitment method: not stated |
|
| Interventions |
Women were encouraged not to alter dietary, alcohol or physical activity habits |
|
| Outcomes | Compliance, endometrial thickness, lipoprotein levels, hormone levels, immunohistochemistry, vasomotor symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned by computer to two groups' |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Treatment was blinded both to the women and doctor' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assume that the doctors evaluated outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two dropped out during the first month and seven women continued treatment but missed some of the examinations. ITT analysis was not conducted for all outcomes |
| Selective reporting (reporting bias) | High risk | Some prespecified outcomes not reported in the results section |
Colli 2012.
| Methods | Design: parallel‐group randomised placebo‐controlled trial Number of women randomly assigned: 90 Number of women analysed: 75 Intention‐to‐treat analysis: no Power calculation for sample size: no Duration: six months Timing: October 2009 to March 2010 Location: gynaecology service in Parana, Brazil Funding: Conselho Nacional de Desenvolvimiento Cientifico e Tecnologico |
|
| Participants | Inclusion criteria: women 46 to 68 years of age; FSH > 40 mIU/L, oestradiol < 30 pg/mL, amenorrhoea > 12 months; climacteric symptoms Exclusion criteria: one or more contraindications to the use of synthetic HT, use of any synthetic HT in the past six months; use of antibiotics in past six months; signs of gastrointestinal malabsorption syndrome Mean age of participants, years: 54 to 57 Recruitment: from gynaecology clinic |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Group 2 was not blinded, as the women took a different product from placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly distributed": method not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Group 1 blinded but group 2 not blinded (non‐identical placebo) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial dropouts with no reasons stated and uneven between groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Crisafulli 2004.
| Methods | Design: parallel‐group Number randomly assigned: 90 Number dropped out: seven (no reasons given) Number lost to follow‐up: zero Number analysed: 90 Intention‐to‐treat analysis: yes Power calculation: 90% power to detect a 2.5‐mm difference in endometrial thickness among the three treatment groups (P = 0.05) Duration: 12 months Timing: not stated Location: university clinic in Italy Funding: not stated | |
| Participants | Inclusion criteria: healthy and ambulatory; 47 to 57 years of age; not undergone surgically induced menopause; no menstrual period in the preceding year; FSH > 50 IU/L; serum 17B‐oestradiol level of 100 pmol/L or less
Exclusion criteria: clinical/laboratory abnormalities that suggested cardiovascular, hepatic or renal disorders; coagulopathy; use of oral or transdermal oestrogen, progestin, androgen or other steroids in the preceding year; smoking more than 10 cigarettes/d
Age, years: mean in placebo group 51; mean in other two groups 52 Recruitment: referred by university department |
|
| Interventions |
All participants had a four‐week stabilisation diet (isocaloric, fat restriction) before treatment |
|
| Outcomes | Menopausal symptoms: daily number of hot flushes at three, six and 12 months Adverse effects: endometrial thickness at six and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind (participants and investigators) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout but no reasons given |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
D'Anna 2007.
| Methods | Design: parallel‐group Number randomly assigned: 265 (substudy of larger trial) Number dropped out: by year one, 10/125 in genistein group (after six months of follow‐up) and 8/122 in placebo group (four after three months and four after six months). Reasons given for dropping out were mainly gastrointestinal side effects but also presence of other diseases, loss to follow‐up and inadequate interaction with the woman's family doctor. None withdrew because of treatment inefficacy. By year two, 16/125 in genistein group and 13/122 in placebo group Number analysed: year one: 247; year two: 236 Intention‐to‐treat analysis: no Power calculation for sample size: yes: difference of at least 20% between groups required 97 participants in each group Duration: one and two years (two publications) Timing: not stated Location: two separate university centres in Italy Funding: Italian Ministry of Scientific Research and Technology and University of Messina, Italy |
|
| Participants | Inclusion criteria: 50 to 70 years old; postmenopausal; FSH > 50 IU/L; serum oestradiol < 100 pmol/L. In the substudy of 247 participants, only women with vasomotor symptoms were evaluated Exclusion criteria: clinical or laboratory abnormalities that suggested various disorders; coagulopathy; use of oral or transdermal oestrogen, progestin, androgen or other steroids; use of bisphosphonates, cholesterol‐lowering therapy or cardiovascular medications in previous six months; smoking > 10 cigarettes/d; BMD of the femoral neck > 0.795 g/cm3 Age, years: mean 53 in both groups Recruitment: women referred from university departments |
|
| Interventions |
|
|
| Outcomes | Number and severity of hot flushes Endometrial thickness |
|
| Notes | The study is a substudy of a larger study evaluating bone loss and CVD prevention. The substudy consisted of women with vasomotor symptoms, and characteristics of participants did not differ from those in the parent study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerised database" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although dropouts were minimal at both one and two years, outcomes were measured in a subgroup of the total study population |
| Selective reporting (reporting bias) | Unclear risk | Side effects were presumably collected, as the authors stated that treatments were "well tolerated" but the results were not reported |
Dalais 1998.
| Methods | Design: cross‐over Number randomly assigned: 52 Number dropped out: eight (seven for personal reasons, one for lack of compliance) Number lost to follow‐up: none stated Number analysed: 44 Intention‐to‐treat analysis: no Power calculation: yes, 80% chance of detecting a 40% decrease in hot flush rate Duration: 2 × 12 weeks with a four‐week washout period Timing: not stated Location: Australia Funding: industry support (George Weston foods) |
|
| Participants | Inclusion criteria: postmenopausal women 45 to 65 years of age, FSH > 40 IU/mL, > 14 hot flushes/wk, 12 months of amenorrhoea, non‐smoking, non‐vegetarian Exclusion criteria: antibiotics or hormone therapy during preceding three months Age, years: mean 53.6 to 54.6 Recruitment method: not stated |
|
| Interventions |
Participants completed a food diary for two weeks before randomisation and were asked to repeat the same two‐weekly diet and to note in their hot flush diary if they diverged from it |
|
| Outcomes | Measures of frequency and severity of menopausal symptoms Measures of change in quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate |
| Selective reporting (reporting bias) | High risk | Adverse events not measured |
Del Giorno 2010.
| Methods | Design: parallel‐group Number randomly assigned: 120 Number dropped out: eight because they did not take more than 80% of drug and 12 for personal reasons (not given per group) Number analysed: 100 Intention‐to‐treat analysis: no Power calculation: yes, 80% power, beta 10%, 38 participants per group Duration: follow‐up at four, eight and 12 months Timing: December 2005 to December 2008 Location: University of Sao Paolo, Brazil Funding: not stated |
|
| Participants | Inclusion criteria: 45 to 65 years of age with menopausal symptoms; > 12 months amenorrhoea; FSH > 30 mIU/mL; oestradiol < 30 pg/mL Exclusion criteria: diabetes mellitus; CVD; hypersensitivity to drugs used in the study; oestrogen‐dependent cancer; liver failure; nephropathy; systemic lupus erythematosus; porphyia; altered cervicovaginal cytology; osteoporosis; endometrial thickness > 6 mm; uterine volume > 200 cm3; BI‐RADS category 3, 4 or 5 mammograms; hormone treatment with sex steroids or phytoestrogens in past six months Mean age, years: 56 in phytoestrogen group; 55 in placebo group Recruitment: from outpatient clinic |
|
| Interventions |
|
|
| Outcomes | Vasomotor symptom score (Kupperman): sexual satisfaction (as measured by GRISS questionnaire) | |
| Notes | Vasomotor symptoms not further defined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer program" |
| Allocation concealment (selection bias) | Low risk | "Sample blinding codes" not disclosed during study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout; appears that women were allocated to groups after dropouts were excluded |
| Selective reporting (reporting bias) | High risk | Insufficient information on outcomes assessed |
Evans 2011.
| Methods | Design: parallel‐group Number randomly assigned: 84 Number dropped out: by 4 weeks, n = 2 (in genistein group); by 12 weeks, n = 14 more (eight in genistein group, six in placebo group). Reasons were given Number analysed: 82 Intention‐to‐treat analysis: yes, modified; all those who had at least four weeks of treatment for efficacy Power calculation: yes, 42 participants per group required to detect a clinically important difference of 35% at 5% level of significance and 80% power Duration: four, eight and 12 weeks of follow‐up Timing: not stated Location: five study sites in southwestern Ontario, Canada Funding: DSM Nutritional Products (manufacturer of the genistein tested) |
|
| Participants | Inclusion criteria: minimum of 40 hot flushes/wk; between ages of 40 and 65 years; physiological state of natural or surgical menopause (amenorrhoeic for ≥ three months + serum FSH > 35 IU/mL or > 42 days post surgery) Exclusion criteria: clinical or laboratory abnormalities; use of conventional hormone therapy or selective oestrogen receptor modulators within four weeks of study start; known allergy or hypersensitivity to soy, peanuts, purified isoflavones, genistein, lactose and/or cow's milk; consumed soy products within four weeks before screening visit; unpredictable vaginal bleeding; uterine fibroids or endometriosis that required treatment; untreated polycystic ovarian syndrome; history of abnormal PAP smear; use of gonadotropin agonists within 24 weeks; glucocorticoids or chronic high‐dose prednisone or equivalent for the past 12 weeks Mean age, years: 53 Recruitment: not stated |
|
| Interventions |
|
|
| Outcomes | Primary: percentage change in number of daily hot flushes from pretreatment to week 12 Secondary: duration and severity of daily hot flushes, Greene Climacteric Scale score; FSH, 17B‐oestradiol, endometrial thickness |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes for each randomisation number" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patient, Medical Directors and research staff blinded to the treatment assignment for the duration of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patient, Medical Directors and research staff blinded to the treatment assignment for the duration of the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified intention‐to‐treat analysis: very low dropout by week four |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Faure 2002.
| Methods | Design: parallel‐group Number randomly assigned: 75 Number dropped out: 17 for inefficacy (six from active group, 11 from placebo group) Number lost to follow‐up: three (placebo group) Number analysed: 75 Intention‐to‐treat analysis: yes (missing data imputed by "last observation carried forward" principle), also per‐protocol analysis Power calculation: 630 in each study arm required to give 90% power to detect a difference of three hot flushes per day, assuming a standard deviation of 3.8 hot flushes/d (P = 0.05) Duration: 16 weeks Timing: not stated Location: outpatient clinic, Nimes, France Funding: industry funded (Arkopharma Laboratories) | |
| Participants | Inclusion criteria: postmenopausal women requesting treatment for hot flushes, at least six months since last menstrual period, minimum of seven moderate to severe hot flushes or night sweats during two weeks before study. Baseline FSH > 40 IU/L and serum oestradiol < 35 pg/mL
Exclusion criteria: use of any other drug for treatment of climacteric symptoms during study period, HT within six weeks before the study
Age, years: active group 53 (SD 5.6), placebo group 53.9 (SD 4.1) Recruitment: not reported |
|
| Interventions |
|
|
| Outcomes | Menopausal symptoms: daily diary card recording number of moderate/severe hot flushes and night sweats Adverse events recorded at each follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High dropout rates but missing data imputed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information on one of the prespecified outcomes: adverse events |
Ferrari 2009.
| Methods | Design: parallel‐group Number randomly assigned: 180 Number dropped out: 30/85 in genistein group (three before treatment intake); 29/95 in placebo group (one before treatment intake). Detailed reasons given Number analysed: 170 (exclusions were those with violation of selection criteria at entry) Intention‐to‐treat analysis: yes, modified; women excluded with violation of selection criteria at entry Power calculation: yes, 20% reduction in frequency of hot flushes for isoflavone group compared with placebo group—required 176 participants Duration: 12 weeks Timing: September 2004 to April 2006 Location: 16 different hospitals in Italy Funding: not stated |
|
| Participants | Inclusion criteria: women 40 to 65 years of age; reporting a minimum of five moderate to severe hot flushes per day over the last seven days of the run‐in period; absence of menstruation for at least six months or six weeks after bilateral oophorectomy; FSH ≥ 30 IU/mL; 17B‐oestradiol ≤ 40 pg/mL Exclusion criteria: HT or any other hormone therapy in last three months before inclusion or one month if participant had been treated with megestrol acetate, clonidine, vitamin E, phenobarbital, ergotamine or antidepressant drugs; presence of suspected or confirmed breast nodule; severe liver or renal dysfunction; type 1 or 2 diabetes mellitus; heart failure NYHA class II to IV, severe neurological disease; history of drug abuse or alcoholism; known hypersensitivity to soy or soy derivatives Mean age, years: 53 in isoflavone group; 55 in placebo group Recruitment: not reported |
|
| Interventions |
Initial two‐week run‐in period, then a baseline visit |
|
| Outcomes | Average number of hot flushes in last seven days compared with baseline; improvement in hot flushes; change in severity of hot flushes; satisfaction; Kupperman Index; compliance; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list. . .generated by blocks of four patients" |
| Allocation concealment (selection bias) | Low risk | Randomisation done off‐site, with consecutive random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High dropout but modified intention‐to‐treat analysis, with the only exclusions those who violated inclusion criteria |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Geller 2009.
| Methods | Design: parallel‐group Number randomly assigned: 67 (black cohosh treatment arm not included) Number dropped out: seven (reasons given) Number analysed: 67 Intention‐to‐treat analysis: yes, all women who had been in study for at least three months Power calculation: yes, for primary outcomes only, comparing each treatment with placebo: 22 women per arm, treatments would reduce vasomotor symptoms by approx 60% with anticipated placebo effect of 35% Duration: 12 months Timing: February 2003 to December 2007 Location: two university medical centres in the USA Funding: Office of Dietary Supplements, National Center for Complementary and Alternative Medicine, National Institute for General Medical Sciences and Office for Research on Women's Health |
|
| Participants | Inclusion criteria: experiencing at least 35 vasomotor symptoms per week, perimenopausal or postmenopausal with intact uterus, amenorrhoea for longer than six months to < 10 years, FSH > 40 mlU/mL, hormone therapy not contraindicated, able to give informed consent Exclusion criteria: previous hysterectomy, < 35 vasomotor symptoms/wk, last menstrual period > 10 years, positive pregnancy test or breastfeeding, BMI > 38 kg/m2, previous history of endometrial hyperplasia or neoplasia, previous history of cancers of the reproductive tract or breast cancer, history or presence of myocardial infarction or stroke, history of severe recurrent depression or severe psychiatric disturbances, history or presence of cerebrovascular accident, severe varicose veins, sickle cell anaemia, history of alcohol or drug abuse, abnormal vaginal bleeding of undetermined cause, untreated or uncontrolled hypertension defined as systolic blood pressure > 165 mmHg or diastolic > 95 mmHg, concurrent administration of medication‐containing oestrogen/progestin, SERM, St John's Wort, bisphosphonates or dietary phytoestrogens, history of migraine associated with hormone use, history or presence of deep vein thrombosis, thrombophlebitis or thromboembolic disorder, current participation in any other clinical trial within 30 d of enrolment, >5 alcoholic drinks per week, smoker, diabetes, abnormal transvaginal ultrasound defined as > 7 mm thickness, abnormal endometrial biopsy or mammogram, vegans Mean age, years: 52 in placebo and red clover groups; 53 in hormone therapy group Recruitment: not reported |
|
| Interventions |
Women taking oral hormone therapy had a two‐month washout period, and women using transdermal patches had a one‐month washout period |
|
| Outcomes | Primary outcome: relief of vasomotor symptoms Secondary outcomes: relief of somatic symptoms, mood changes, sexual dysfunction, health‐related quality of life, use of validated questionnaires, adverse events Participants completed diaries |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random, computer‐generated code' |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by intention‐to‐treat Placebo = one dropout due to life changes; CEE/MPA = four dropouts due to two adverse events, one relocation and one life change; black cohosh = two dropouts, one loss to follow‐up and one lack of efficacy; red clover = two dropouts due to lack of efficacy |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported, but insufficient information on adverse effects |
Han 2002.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 82 Number of dropouts: two (one from each arm, one due to poor response, one due to nausea—not stated which arms they were on) Number analysed: 80 Intention‐to‐treat analysis: no Power calculation: none stated Duration: four months Timing: August 1999 to February 2000 Location: university clinic, Brazil Funding: unclear. Investigators acknowledge the co‐operation of a doctor employed by food supplement manufacturer Eugenbio | |
| Participants | Inclusion criteria: women 45 to 55 years of age; "in menopause" at least 12 months, no hormonal treatment for at least 12 months, intact uterus, FSH > 25 U/L, oestradiol < 20 pg/mL, having hot flushes Exclusion criteria: taking lipid‐lowering drugs, antidiabetic medications, soybean‐derived products or herbal supplements; uncontrolled hypertension, stroke or transient ischaemic attack, cancer diagnosed within past five years, previous myocardial infarction Age, years: mean active arm 48 (SE 1.1), placebo arm 49 (SE 1.3) Recruitment method: not stated | |
| Interventions |
|
|
| Outcomes | Menopausal symptoms: hot flashes (Kupperman Index)
Compliance: examination of prescriptions/pills Endometrial thickness |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Numbered coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded researchers and participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes recorded by an independent gynaecologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Hanachi 2008.
| Methods | Design: parallel‐group Number randomly assigned: 37 Number dropped out: not reported Number analysed: assume this is 37 Intention‐to‐treat analysis: not reported Power calculation for sample size: not reported Duration: three months Timing: not stated Location: not stated Funding: not stated |
|
| Participants | Inclusion criteria: non‐smokers; free from diseases; not on any type of hormone treatment during previous 12 months; not currently using lipid‐lowering drugs, antidiabetic medications, soybean‐derived products or herbal supplements; intact uterus; FSH > 25 U/L; oestradiol < 100 pg/mL; presence of hot flushes Exclusion criteria: history of uncontrolled hypertension, stroke or transient ischemic attack, cancer diagnosed less than five years ago, previous myocardial infarction Mean age, years: 52 Recruitment method: not stated |
|
| Interventions |
|
|
| Outcomes | Kupperman Index (hot flushes); lipids | |
| Notes | Baseline values not given, only percentage decrease from baseline. Control values also not given. Nature of control not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly assigned', but no other description provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear there were any dropouts |
| Selective reporting (reporting bias) | High risk | Outcomes not clearly described |
Heger 2006.
| Methods | Design: parallel‐group, multi‐centre Number of women randomly assigned: 110 Number of dropouts: n = 16 in ERr 731 group (one lack of efficacy, three violations of smoking ban, three adverse events, two organisational reasons, seven other reasons); n = 48 in placebo group (31 lack of efficacy, one violation of smoking ban, one adverse events, 16 other reasons) Number analysed: n = 109 (one woman refused to take the intervention) Intention‐to‐treat: yes, modified—only the women who took the interventions Power calculation: yes, using a group sequential design according to O'Brien and Fleming Duration: 12 weeks Timing: February 2003 to May 2004 Location: nine gynaecological outpatient departments in the Ukraine Funding: Health Research Services Ltd, Germany, and Chemisch‐Pharmazeutische Fabrik Goeppingen, Carl Muller, Apotheker, GmbH u Co KG, Goeppingen, Germany (manufacturer of the supplement) |
|
| Participants | Inclusion criteria: climacteric complaints with MRS II total score > 22 points; perimenopause, defined as 45 to 55 years of age with cycle irregularity during the past 12 months or LMP at least three but no longer than 12 months ago Exclusion criteria: regular cycles during the past three months; mandatory indication for HT; treatment with drugs containing oestrogen/progestogen during past six months or any other Rx in past three months; PAP smear class III/IV and/or endometrial hyperplasia; known or suspected hypersensitivity to experimental intervention; concomitant medications that might influence trial results; BMI < 18 kg/m2 or > 30 kg/m2 and/or abnormal eating habits; wish to become pregnant or to be breast‐feeding; previous or existing major diseases; previous or existing psychiatric disorders including depression; smoking, moderate alcohol intake, coffee/chocolate intake of 500 mg or more of caffeine per day and/or suspected drug abuse; participation in another clinical trial during past six months; incompetence or incapability of understanding the trial Mean age, years: 49 Recruitment method: from outpatient departments (women seeking treatment for climacteric complaints) |
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation list with a balanced 1:1 randomisation using a block size of 4" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators and data monitoring committee—all blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators and data monitoring committee—all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One woman did not take the intervention and was not included. When discontinuations were reported, the LOCF method was used for missing data |
| Selective reporting (reporting bias) | Low risk | Most prespecified outcomes reported; authors stated that an additional paper will report on the remaining outcomes |
Heyerick 2006.
| Methods | Design: parallel‐group Number of women screened: 84 Number randomly assigned: 67 Number of dropouts: 18% (12/67): four in high‐phytoE group (three no efficacy, one other), one in low‐phytoE group (no efficacy), seven in placebo group (all no efficacy) Number analysed: 55 Intention‐to‐treat analysis: no Power calculation: not reported Duration: 12 weeks Timing: December 2003 to April 2004 Location: Ghent, Belgium Funding: IWT‐Vlaanderen in Belgium; Biodynamics, Belgium | |
| Participants | Inclusion criteria: healthy, 45 to 60 years of age, intact uterus, no menses for past 12 months, at least two to five hot flushes per day, abstention from HT for past three months Exclusion criteria: score < 2 on Kupperman Hot Flush Index Mean age, years: 52 in placebo and low‐dose groups; 53 in high‐dose group Recruitment method: not stated | |
| Interventions |
Dose, duration and timing of administration: one capsule a day for 12 weeks |
|
| Outcomes | Hot flush score on Kupperman Index (severity) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On‐line randomiser |
| Allocation concealment (selection bias) | Low risk | Random codes kept separate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not measured |
Hidalgo 2005.
| Methods | Design: cross‐over Number of women screened: not stated Number randomly assigned: 60 Number of dropouts: 12% (7/60): not clear which group: five no reason, two adverse events Intention‐to‐treat analysis: no Power calculation: yes, but insufficient Duration: 12 weeks, seven‐day washout, then another 12 weeks on alternate treatment Timing: July 2003 to August 2004 Location: Guayaquil, Ecuador Funding: Melbrosin International (provision of intervention and control) | |
| Participants | Inclusion criteria: over 40 years of age, no menses for past 12 months, non‐users of HT, moderate to severe menopausal symptoms (Kupperman Index score ≥ 15, basal determination Exclusion criteria: no consent, indication of non‐compliance, conventional HT, isoflavone supplements, thyroid medication or history of thyroid disease, medication that could interfere with vasomotor symptoms and/or lipid serum levels Mean age, years: 51 Recruitment method: clinical database, private practice or from the general population through advertising and flyers | |
| Interventions |
Dose, duration and timing of administration: Participants acted as their own control—two capsules a day of the randomly assigned treatment for 90 days, a seven‐day washout period, then alternate treatment for a further 90 days |
|
| Outcomes | Hot flush and night sweat Kupperman scores (severity) expressed as percentages Vaginal cytology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generation |
| Allocation concealment (selection bias) | Low risk | Opaque containers with investigators blinded to codes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear whether dropouts likely to influence results |
| Selective reporting (reporting bias) | High risk | Adverse effects not measured |
Imhof 2006.
| Methods | Design: cross‐over Number of women screened: not stated Number randomly assigned: 113 Number dropped out: four (three in active arm and one in placebo arm—all started hormone therapy) Number analysed: 109 Intention‐to‐treat analysis: yes—modified, based only on the women who started the trial and excluded the women who started hormone treatment Power calculation: not reported Timing: not stated Location: general hospital and study centre in Vienna, Austria Funding: Melbrosin International (manufacturer of interventions) | |
| Participants | Inclusion criteria: postmenopausal (no menses for > 12 months), 40+ years, negative pregnancy test, willingness for adherence to control dates and to take prescribed medications, moderate to severe menopausal symptoms (Kupperman Index ≥ 15) Exclusion criteria: constant ET, known isoflavone hypersensitivity Mean age, years: 55 in active arm and 54 in placebo arm Recruitment method: menopause clinic | |
| Interventions |
|
|
| Outcomes | Endometrial thickness, daily hot flush and night sweat frequency and side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Jeri 2002.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 30 Number dropped out: none stated Number lost to follow‐up: none stated Number analysed: 30 Intention‐to‐treat analysis: not mentioned Power calculation: not stated Duration: 16 weeks Timing: not stated Location: Peru Funding: not stated |
|
| Participants | Inclusion criteria: healthy, non‐vegetarian women; postmenopausal for longer than one year; younger than 60 years of age; FSH level > 30 mIU/mL; having at least five hot flushes daily, averaged over one week; not using HT, antidepressants or other medications, or soy or other oestrogen‐active plant products for the past 16 weeks
Exclusion criteria:
Age, years: 51
Other characteristics of participants: All were Hispanic "with a middle class income and good education" Recruitment method: not reported |
|
| Interventions |
|
|
| Outcomes | Menopausal symptoms: hot flush frequency and severity recorded at beginning and end of study | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently no dropouts |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Jou 2008.
| Methods | Design: parallel‐group Number randomly assigned: 96 Number dropped out: 4/30 in placebo group (one unwell and three for personal reasons), 2/34 in the equol‐producing group (personal reasons), one in the non–equol‐producing group (personal reasons) Number analysed: 89 Intention‐to‐treat analysis: no Power calculation for sample size: 81 participants (27 per group) would have 85% power to detect a difference of 10 in total Kupperman score between groups Duration: six months Timing: April to December 2005 Location: clinics at hospital and university in Taiwan Funding: not stated |
|
| Participants | Inclusion criteria: healthy menopausal women (confirmed by FSH ≥ 40 mIU/mL); did not receive hormone therapy during previous year; Kupperman Index score > 0 Exclusion criteria: taking medications containing hormones; major systemic disease such as diabetes mellitus, hypertension, hypothyroidism, chronic renal disease, breast disease, CVD, cancer Mean age, years: 54 Recruitment method: from gynaecology clinics |
|
| Interventions |
|
|
| Outcomes | Hot flushes as measured by Kupperman Index together with other symptoms | |
| Notes | Hot flush status at baseline not reported. Menopausal symptom score at baseline not comparable between groups (higher in the equol‐producing group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random number generator' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Kaari 2006.
| Methods | Design: parallel‐group Number of women screened: 150 Number randomly assigned: 79 Number of dropouts: 14% (11/79): seven from soy group (three medical, four personal), four from ET group (one medical, one no reason, one scared of biopsy, one personal) Number analysed: 68 Intention‐to‐treat analysis: no Power calculation: not reported Duration: 24 weeks Timing: July 2001 to November 2002 Location: University of Sao Paolo, Brazil Funding: ACHE Laborotorio Ldta (made the intervention) | |
| Participants | Inclusion criteria: ≥ 45 years, good overall health, no menses for past 12 months, FSH ≥ 30 mU/mL, intact uterus, endometrial thickness < 5 mm, atrophic endometrium (biopsy) Exclusion criteria: strict vegetarian, high‐fiber/high‐soy diet, regular consumption of vitamin and mineral supplementation > Recommended Dietary Allowances, antibiotic or hormone use in past six months, history of chronic disorders (incl benign breast disease), regular use of medication known to interfere with study endpoints, BMI > 30 Mean age, years: 54 Recruitment method: menopause clinic | |
| Interventions |
|
|
| Outcomes | In those who were symptomatic at baseline: percentage of participants who reported any reduction in hot flush severity (Kupperman score) Endometrial thickness Endometrial proliferation Adverse events | |
| Notes | Participants were recruited from a menopause clinic, but 18% of soy group and 26% of ET group were asymptomatic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Moderate dropout |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Khaodhiar 2008.
| Methods | Design: parallel‐group
Number of women screened: 235 recruited and 191 randomly assigned
Number of dropouts: 49/191 (26%) dropped out, were lost to follow‐up or were excluded (not clear which group they came from: 30 unable to comply with protocol or lost to follow‐up, eight withdrew because they started taking other meds, two were withdrawn because they resumed menstruation, two were withdrawn because of abnormal liver function tests, two discontinued because of side effects, five did not complete their diaries) Number analysed: 142 Intention‐to‐treat analysis: no Power calculation: yes, 50 women per group for 80% power to detect differences in hot flush activity of 0.58 SD, average shift of 1.2 hot flushes/d or a hot flush score of 3 units/d Duration: 12 weeks Timing: not stated Location: medical centre in Harvard Medical School, Boston, USA Funding: Nichimo Co Ltd, Japan—manufacturer of daidzein‐rich isoflavone aglycone extract |
|
| Participants | Inclusion criteria: postmenopausal, no menses for past six months, 38 to 60 years of age, between five and 15 hot flushes per day Exclusion criteria: active smoker; use of dietary supplements containing soy isoflavones, vitamin E, flaxseed or red clover; use of HT or any medication for hot flushes within past six weeks; BMI ≥ 40; history of breast, endometrial or cervical cancer; positive pregnancy test; history of undiagnosed vaginal bleeding, thromboembolic disease, cardiovascular disease, liver or kidney disease, diabetes mellitus or major illness Mean age, years: between 52 and 54 in the three groups Recruitment method: referring physicians at the medical centre and newspaper advertisements | |
| Interventions |
|
|
| Outcomes | Mean change from baseline to week 12 in frequency of hot flushes (daily diary) Severity of hot flushes (measured morning and evening on a scale of 1 to 4) (daily diary) Outcomes were measured as percentage change for both | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis—30 women withdrew from study as unable to comply with protocol or lost to follow‐up (not clear from which group); eight withdrew because they started to take other medications or supplements, two women resumed menses after randomisation and were excluded, two women were withdrawn after randomisation because of abnormal liver function tests, two women in the 60‐mg isoflavone group withdrew because of side effects and five women did not complete hot flush diaries and were excluded Number analysed: 142 had analysable data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data on adverse events and quality of life |
Knight 1999.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 37 initially. When one woman dropped out after randomisation and before commencing treatment, another was recruited to the same treatment Number dropped out: two in high‐dose group withdrawn on GP advice Number lost to follow‐up: Number analysed: 35 Intention‐to‐treat analysis: no Power calculation: not reported Duration: 12 weeks Location: hospital outpatient clinic, Sydney, Australia Funding: industry funded (Novogen, Australia) | |
| Participants | Inclusion criteria: postmenopausal women (bilateral oophorectomy or amenorrhoea for least six months with typical symptoms of menopause, FSH > 40 IU/L), having at least three hot flushes daily, 40 to 65 years of age Exclusion criteria: HT use within previous six weeks, allergy to isoflavones, current bowel, liver or gallbladder disease, diabetes requiring medication, malignancy other than skin cancers, contraindications to HT use, vegetarians, regular soy product users, taking liver enzyme–inducing medications Age, years: high dose 51.1 (± 8.8), medium dose 54.5 (± 4.4), placebo 53.1 (± 2.5) Recruitment method: through university department of obstetrics and gynaecology | |
| Interventions |
Participants advised not to alter their usual diet during the study |
|
| Outcomes | Menopausal symptoms: daily flush diary Quality of life: Greene Menopause Scale Compliance: pill counts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Randomisation performed remotely by external statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Knight 2001.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 24 Number dropped out: three (from active arm—all disliked taste) Number lost to follow‐up: one (from placebo arm) Number analysed: 20 Intention‐to‐treat analysis: no Power calculation: not stated Duration: 12 weeks Timing: not stated Location: Australia Funding: industry funded (Protein Technology Industries) | |
| Participants | Inclusion criteria: postmenopausal women (bilateral oophorectomy or amenorrhoea for least six months with typical symptoms of menopause, FSH > 40 IU/L), having at least three hot flushes daily, 45 to 60 years of age Exclusion criteria: HT use within previous six weeks; allergy to isoflavones; current bowel, liver or gallbladder disease; diabetes requiring medication; malignancy other than skin cancers; contraindications to HT use; vegetarians; regular soy product users (> once a week), taking liver enzyme–inducing medications Age, years: Recruitment method: two university hospital obstetrics and gynaecology clinics | |
| Interventions |
Participants advised not to alter their usual diet during the study |
|
| Outcomes | Menopausal symptoms: flush count Quality of life: Greene Menopause Scale Compliance: sachet counts Adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly permuted blocks of six, using computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Randomisation performed remotely—external statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Moderate dropout, which could influence findings—25% in active arm |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Kotsopoulos 2000.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 94 Number dropped out: 19 (10 on active treatment, nine on placebo, because of adverse effects) Number lost to follow‐up: none Number analysed: 73 (two excluded from analysis as FSH in normal range) Intention‐to‐treat analysis: no Power calculation: not stated Duration: three months Timing: not stated Location: Australia Funding: academic research grant | |
| Participants | Inclusion criteria: 50 to 75 years of age, postmenopausal (12 months of amenorrhoea and FSH > 20 U/L) Exclusion criteria: taking antibiotics within three months before study, receiving HT during 12 months before study, smoker, vegetarian, ingesting phytoestrogens or soy‐based products Mean age, years: 59 Recruitment method: subgroup of a larger trial | |
| Interventions |
|
|
| Outcomes | Menopausal symptoms: validated questionnaire to record psychological, vasomotor, musculoskeletal, genitourinary and other symptoms on 4‐point scale. Completed at baseline and after treatment Compliance: returned sachet count Adverse effects: recorded adverse effects causing women to drop out | |
| Notes | No requirement for vasomotor symptoms was given for eligibility for the study. 80% of participants had mild symptoms at baseline, and analyses were undertaken in this subgroup of women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Levis 2011.
| Methods | Design: parallel‐group Number randomly assigned: 248 Number dropped out: 11 in soy group (but they completed all study visits) and 12 in placebo group (but they completed all study visits) Number lost to follow‐up: 23 in soy group (18 before 12 months, five between 12 and 24 months), 43 in placebo group (40 before 12 months and three between 12 and 24 months) Number analysed: 182 (99 in soy group and 83 in placebo group) Intention‐to‐treat analysis: no Power calculation: yes, 80% power to detect a 4% or greater difference in BMD of the lumbar spine, with the assumption that the control group will lose 4% to 5% of bone mass. Target total sample size was 306 with 15% attrition rate expected Duration: two years Timing: July 2004 to March 2009 Location: University of Miami Miller School of Medicine, South Florida, USA Funding: National Institute of Arthritis, Musculoskeletal and Skin Diseases |
|
| Participants | Inclusion: women 45 to 60 years of age, menopausal for longer than 12 months but less than five years; or absence of menses for six to 12 months and FSH > 40 IU/L Exclusion: osteoporotic fractures, a bone mineral density T score in the lumbar spine or total hip of < ‐2, BMI of 32 or higher, abnormal mammogram findings, cancer in previous 10 years (except skin cancer), taking bone active drugs, corticosteroids, or herbal products. Taking menopausal hormone therapy within six months before trial Mean age, years: 53 ± 3.3 in soy group; 52 ± 3.3 in placebo group Recruitment method: from South Florida area by direct mailings, posters and presentations to community organisations |
|
| Interventions |
Treatment for two years taken in the morning before breakfast. Additional calcium supplements were provided to participants as required. Followed up at baseline and at 12 and 24 months |
|
| Outcomes | Bone mineral density Bone collagen Menopausal symptoms Vaginal oestrogenisation Serum lipids Thyroid function |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence in blocks of ten |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel masked to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and study personnel masked to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, 66 participants were lost to follow‐up (26.6%)—18.8% from the soy group and 34.1% in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Quality of life was indicated as a secondary outcome in the protocol but was not reported in the 2011 paper and was not a primary outcome of this review. Not all of the exclusion criteria were listed in the paper |
Lewis 2006.
| Methods | Randomisation method: separate site prepared randomisation codes Blinding: double Allocation concealment: adequate Design: parallel‐group Number of women screened: 792 Number randomly assigned: 99 Number of dropouts: 12% (12/99): two in soy group (one could not accept Rx, one adverse event), five in flaxseed group (two could not accept Rx, one adverse event, one medical, one personal), five in placebo group (one could not accept Rx, one adverse event, two medical, one protocol violation) Number analysed: 87 Intention‐to‐treat analysis: no Power calculation: not reported Duration: 16 weeks Timing: not stated Location: Toronto and Calgary, Canada Funding: Canadian Institutes of Health Research | |
| Participants | Inclusion criteria: 45 to 60 years old, natural menopause with last menses in previous one to eight years, Menoquol vasomotor score > 3.0 Exclusion criteria: medical or surgical menopause; inflammatory bowel disease; malabsorption syndrome; uncontrolled thyroid disorder; known allergy or intolerance to muffin ingredients or any serious and active medical or social condition likely to affect quality of life during the study; no ET in past three months; no phytoestrogens, steroids or antibiotics in past month Mean age, years: 53 Recruitment method: mailings to family practice, to previous participants of menopause workshops and to gynaecologists and family physicians | |
| Interventions |
Dose, duration and timing of administration: one muffin per day |
|
| Outcomes | Primary: Menoquol vasomotor score (0 to 6)
Secondary: number of flushes per day
Severity of flushes (0 to 6) Gastrointestinal side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Separate site prepared the randomisation codes |
| Allocation concealment (selection bias) | Low risk | Separate site ensured concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderate dropout—not balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Inadequate data on side effects |
Nahas 2007.
| Methods | Design: parallel‐group Number randomly assigned: 80 Number dropped out: four (two in each group—reasons given) Number analysed: 76 Intention‐to‐treat analysis: no Power calculation: not reported Duration: 10 months Timing: not stated Location: Climacterium Outpatient Service of the Department of Gynecology in Sao Paolo University Funding: Ativus Farmaceutica Brazil and Fundacao Lucentis de Apoio a Cultura, Ensino, Pesquisa e Extensao |
|
| Participants | Inclusion: postmenopausal, 45 years of age or older with good overall health, spontaneous amenorrhoea for at least 12 months, FSH level > 40 mIU/mL, average of five or more vasomotor symptoms per day Exclusion: strict vegetarian; high‐fiber or high‐soy diet; history of breast cancer, endometrial carcinoma, cardiovascular disease, thromboembolic disorders, undiagnosed vaginal bleeding, chronic alcoholism or chronic gastrointestinal diseases. Not on hormonal therapy or phytoestrogens for previous six months Mean age, years: 55.7 Recruitment method: from outpatient menopause clinic at university |
|
| Interventions |
Followed up for 10 months with evaluations at baseline and at four, seven and 10 months |
|
| Outcomes | Kupperman Menopausal Index Daily diary of hot flushes Symptoms Body mass index Vaginal cytology (maturational value, pH) Transvaginal ultrasonography (endometrial thickness) Adverse events Mammography Lipids, plasma levels of isoflavones |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned to one of two groups'. Computer randomised |
| Allocation concealment (selection bias) | Low risk | Centralised computerised randomisation using software by a statistician who was unaware of the study protocol |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Isoflavone group: Two withdrew because of flatulance and epigastralgia; control group: two withdrew because of depression and family problems and not wishing to continue |
| Selective reporting (reporting bias) | Low risk | Original protocol was not viewed, but outcomes listed in the methods section of the paper were reported in the results |
Penotti 2003.
| Methods | Design: parallel‐group Number randomly assigned: 62 Number dropped out: 13 did not complete six months of treatment (six in active group: one because of diarrhoea and five because of persistent hot flushes; seven in placebo group because of persistent hot flushes) Number analysed: 49 at six months Number lost to follow‐up: nil Intention‐to‐treat analysis: no Power calculation: not stated Duration: six months Timing: not stated Location: outpatient menopause clinics, Italy Funding: not stated | |
| Participants | Inclusion criteria: postmenopausal for at least six months; 45 to 60 years of age; FSH and 17‐B E2 levels within postmenopausal range; experiencing at least seven hot flushes daily (evaluated by participant diary completed over 15 days prerandomisation); computerised bone mineralometry score greater than ‐2.5 at level of lumbar spine Exclusion criteria: serious disease such as hypertension, heart disease, diabetes, renal disease, peripheral vascular disease Age, years: 52.5 (49 to 58) Recruitment method: from outpatient menopause clinic at gynaecology department |
|
| Interventions |
|
|
| Outcomes | Menopausal symptoms: mean (=/‐SD) daily number of hot flushes per month | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers list, balanced in blocks of 10 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout |
| Selective reporting (reporting bias) | High risk | Adverse events not measured |
Radhakrishnan 2009.
| Methods | Design: parallel‐group Number randomly assigned: 100 Number dropped out: 15 (six in soy group and nine in control group) Number analysed: 85 Intention‐to‐treat analysis: no Power calculation: yes, 7% decrease in cholesterol with 80% power and 20% withdrawal rate Duration: six months Timing: not stated Location: Department of Obstetrics and Gynecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India Funding: Dupont Protein Technology International–manufactured soy and protein supplements |
|
| Participants | Inclusion: last menstruation at least 12 months previous or six weeks since bilateral oophorectomy; FSH level 40 mlU/mL; unwillingness to take or intolerance to HT; not currently taking lipid‐lowering drugs, antidiabetic medications or herbal supplements; discontinued HT more than three months previously Exclusion: unexplained vaginal bleeding, hypertension, diabetes, liver dysfunction, renal or cardiac disease, active thromboembolic disease, deep vein thrombosis, coronary artery disease, cerebrovascular accident or past history of thromboembolic disease associated with oestrogen use; present or past oestrogen‐dependent malignancy such as breast or endometrial carcinomas, known peanut/legume allergy Mean age, years: 48.07 ± 5.44 in the soy group; 49.71 ± 7.27 in the placebo group Recruitment method: from outpatient clinic |
|
| Interventions |
|
|
| Outcomes | Kupperman Menopausal Index Karyopyknotic Index Maturation value Endometrial thickness Laboratory investigations Bone mineral density Acceptability of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using computer‐generated randomised numbers in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Precoded sachets were provided by DUPONT Protein Technology International |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 stopped the trial prematurely during the initial two months: six in the study group and nine in the control group. Main reasons were gastrointestinal side effects and food intolerance. Three cases in each group were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Original protocol not viewed. Outcomes listed in the methods sections were reported in the results |
Sammartino 2003.
| Methods | Design: cross‐over (no washout) Number of women screened: not stated Number randomly assigned: 70 Number of dropouts: seven (three in genistein group: two no compliance, one personal; four in calcium group: three no compliance, one personal) Number analysed: 63 Intention‐to‐treat analysis: no Power calculation: not reported Duration: one year Timing: not stated Location: menopause clinic at university department in Naples, Italy Funding: not stated | |
| Participants | Inclusion criteria: minimum number of seven moderate to severe hot flushes (including night sweats)/d (defined), postmenopausal (hormones in the menopausal range: FSH > 40 IU/L; oestradiol < 20 pg/mL); no menses for 12 months Exclusion criteria: neoplastic, metabolic and infectious diseases; concomitant use of any drug; BMI > 30; past or concomitant use of HRT or any other drug used for menopausal symptoms; endometrial thickness > 5 mm or endometrial abnormalities Mean age, years: 52 in both groups Recruitment method: menopause clinic | |
| Interventions |
|
|
| Outcomes | Endometrial thickness and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study not blinded, but lack of blinding unlikely to affect measurement of the main outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study not blinded, but lack of blinding unlikely to affect measurement of the main outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts balanced between groups and unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
St Germain 2001.
| Methods | Design: parallel‐group Number screened for inclusion: 1,000 (of whom 102 passed first screening. Subsequently, 22 found to be ineligible, six could not tolerate treatment, one died, one had a family member die, two had medical conditions preventing continuance, one was non‐compliant) Number randomly assigned: 69 Number dropped out: nil Number lost to follow‐up: nil Number analysed: 69 Intention‐to‐treat analysis: no Power calculation: not stated Duration: 24 weeks Timing: not stated Location: Human Metabolic Unit, Iowa State University, USA Funding: academic grants and industry donations of muffin ingredients and laboratory tests | |
| Participants | Inclusion criteria: perimenopausal women, one or both ovaries remaining, FSH at least 30 IU/L, BMI 20 to 31,experiencing at least 10 hot flushes or night sweats per week, within 12 months of last menstrual cycle
Exclusion criteria: smokers, any chronic disease such as known cardiovascular disease or osteoporosis, long‐term medication use, taking HT at time of study or during the previous 12 months
Age, years: median 50 (42 to 62) Recruitment method: not reported |
|
| Interventions |
Participants advised to avoid food items containing soy isoflavones and not to take their own supplements |
|
| Outcomes | Menopausal symptoms: Menopausal Index of hot flushes, night sweats and other symptoms Measures of change in quality of life: Compliance: self‐reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all randomly assigned participants were analysed |
| Selective reporting (reporting bias) | High risk | Adverse effects not reported |
Tice 2003.
| Methods | Design: parallel‐group, active arms versus placebo Number screened: 1,191 (principal reasons for ineligibility: too few hot flushes (251), not interested (216) and medical conditions/medications (192)) Number randomly assigned: 252 Number dropped out: six (two taking Promensil (one too busy, one no improvement), two taking Rimostil (one nauseated, one on physician's advice), two taking placebo (one feared possible placebo, one too busy)) Number lost to follow‐up: Number analysed: 252 Intention‐to‐treat analysis: yes (also per‐protocol analysis) Power calculation: 90% power to detect at least a 15% greater reduction in hot flush frequency in the active arm compared with placebo; 25% placebo effect anticipated Duration: 12 weeks Timing: November 1999 to March 2001 Location: three US academic clinical research sites Funding: industry (Novogen Inc) | |
| Participants | Inclusion criteria: women 45 to 60 years of age, experiencing at least 35 hot flushes/wk, FSH level 30 mIU/mL, documented bilateral oophorectomy or at least two consecutive months of amenorrhoea before enrolment, with at least six months of amenorrhoea during the year before entry Exclusion criteria: vegetarian, ate soy products > once a week, taking medications affecting isoflavone absorption or hormonal preparations during previous three months, significant gastrointestinal disease, > two alcoholic beverages per day, allergic to red clover, consumed < 80% of expected study tablets during the two‐week placebo run‐in Age, years: 52.3 (SD 2.8 to 3.4 in different arms) Recruitment method: newspaper and radio advertising, flyers, directed mailings | |
| Interventions |
|
|
| Outcomes | Menopausal symptoms: daily diary cards for recording hot flushes and night sweats Quality of life: Greene Climacteric Scale Compliance: pill count Adverse effects: assessed at follow‐up, specific method unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in random blocks of six, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Allocation schedule maintained remotely, at pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind: participants and researchers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind: participants and researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Upmalis 2000.
| Methods | Design: parallel‐group Number screened for inclusion: not stated Number randomly assigned: 177 Number dropped out: 40 (21 in active arm: one did not take supplement, nine ineligible, 11 violated protocol, one had urinary tract infection; 18 in placebo arm: one did not take supplement, four ineligible, 13 violated protocol) Number lost to follow‐up: 15 (nine in active group, six in placebo group) discontinued before week 12 Number analysed: 122 (for efficacy) Intention‐to‐treat analysis: no Power calculation: not stated Duration: 12 weeks Timing: not stated Location: 15 outpatient sites in USA Funding: industry funded (Advanced Care Products) | |
| Participants | Inclusion criteria: postmenopausal women experiencing average of at least five hot flushes per day, over 50 years of age, in good health, weight within ± 35% range for BMI, FSH at least 40 mIU/mL, oestradiol level 25 pg/mL or less, no menses for at least six months, discontinued HT at least 60 days before study entry
Exclusion criteria: history of breast cancer, hyperplasia, endometrial carcinoma or cervical neoplasia, positive pregnancy test, undiagnosed abnormal vaginal bleeding, bilateral oophorectomy or hysterectomy, thromboembolic disorders, cardiovascular disease, liver disease, chronic alcoholism, medication hypersensitivity, allergy to dietary supplement ingredients, uncontrolled addiction, severe depression, acute systemic infection or abnormal laboratory values
Age, years: mean 55 Recruitment method: not stated |
|
| Interventions |
Intake of other soy products and dietary supplements restricted during the study |
|
| Outcomes | Menopausal symptoms: daily diary card for number and severity of hot flushes and night sweats; 3‐point scale for severity
Compliance: check of unused medication at week six and week 12 Endometrial thickness Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very high rate of dropout |
| Selective reporting (reporting bias) | High risk | Quality of life prespecified as an outcome in the methods section but not reported on in the results section |
van de Weijer 2002.
| Methods | Design: parallel‐group Number screened for inclusion: 42 (six ineligible, 24 did not return to clinic, tqo recorded inadequate data in diary during screening phase) Number randomly assigned: 30 Number dropped out: six (three in each group, mainly because of lack of efficacy) Number lost to follow‐up: nil Number analysed: 26 Intention‐to‐treat analysis: no Power calculation: not stated Duration: 12 weeks Timing: not stated Location: university clinic, The Netherlands Funding: industry funded (Novogen Ltd, Australia) | |
| Participants | Inclusion criteria: postmenopausal women 49 to 65 years of age with at least 12 months' amenorrhoea, average of > five hot flushes daily
Exclusion criteria: HT or antibiotics within 12 weeks of study entry, undiagnosed vaginal bleeding, active liver or renal disease, history of allergy to foodstuffs, cardiovascular disease or thromboembolism
Age, years: active arm 52.5 (SD 5.2), placebo 54.2 (SD 7.4) Recruitment method: not stated |
|
| Interventions |
Participants given list of foods to avoid, including legumes and isoflavone supplements |
|
| Outcomes | Menopausal symptoms: daily diary for number of hot flushes, list of 21 symptoms to score on 4‐point scale Measures of change in quality of life | |
| Notes | Baseline hot flush count = average count of last seven days from four‐week screening phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blank envelopes containing allocation shuffled, numbered consecutively, then given consecutively to participants |
| Allocation concealment (selection bias) | Low risk | Participants took envelope to pharmacy, where number in envelope was matched with batch number of medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and researchers blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both participants and researchers blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition > 10% and unbalanced between groups |
| Selective reporting (reporting bias) | High risk | Adverse events not reported |
Ye 2012.
| Methods | Design: parallel‐group Number of women randomly assigned: 90 Number of women analysed: 84 (two withdrew in low‐dose group; four withdrew in high‐dose group because of adverse events, traffic accident and emigration) Intention‐to‐treat analysis: yes—last observation carried forward used for missing data Power calculation: not stated Duration: six months Timing: not stated Location: Sun Yat‐sen University, Guangzhou, China Funding: Guangzhou Sciences and Technology Bureau, Department of Health of Guangdong Province and extract provided by pharmaceutical company |
|
| Participants | Inclusion criteria: Chinese, between 45 and 60 years of age, within the five‐year period after natural menopause (12 months since last menstrual cycle), BMI < 30 kg/m2, FSH > 30 IU/L, Kupperman Climacteric Scale score > 15 Exclusion criteria: detectable diseases such as chronic diseases of the kidney, liver, heart or endocrine system; cancer; diabetes; taking medications known to affect bone health or lipid metabolism Mean age of participants, years: 52 to 53 Recruitment method: advertisements in local media |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation (SPSS) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not applicable as hot flushes assessed by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for dropouts and missing data imputed by last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not measured |
Aglycone: unconjugated parent form of isoflavone. Dropouts: did not complete treatment as per protocol; reason for dropping out and/or outcome reported. Intention‐to‐treat analysis: all randomly assigned women included in analysis, in the groups to which they were assigned. Greene Climacteric Scale: measures quality of life in women experiencing symptoms attributed to menopause (11 psychological symptoms, seven somatic, two vasomotor, one sexual, on a 4‐point scale ranging from none to severe). Kupperman Index: numerical conversion index of 11 menopausal symptoms on a 4‐point scale from no complaints to severe. Per‐protocol analysis: analysis according to treatment actually received. Lost to follow‐up: women whose reasons for withdrawing and/or outcomes are unknown or are not reported.
Abbreviations
SDS: Self Depression Scale.
BMI: Body mass index.
SMI: Simplified Menopausal Index.
HT: Hormone therapy.
ET: Oestrogen therapy.
FSH: Follicle‐stimulating hormone.
LMP: Last menstrual period.
MRS II: Menopause Rating Scale II.
LOCF: Last observation carried forward.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albert 2001 | No control group |
| Albertazzi 2005 | Duration of trial only 6 weeks. |
| Aly 2009 | Used Black Cohosh |
| Amato 2005 | Low prevalence of menopausal symptoms |
| Atkinson 2003 | About half of the women in each group had hot flushes but this was not a requirement for participation in the study. |
| Bai 2007 | Used Black Cohosh |
| Baird 1995 | The study assessed oestrogenicity of dietary soy but did not assess any of the primary outcomes in this review, frequency or severity of vasomotor symptoms. |
| Cancellieri 2007 | The soy intervention included other plant extracts. It was not possible to separate out the effects of phytoestrogens alone. |
| Carranza‐Lira 2001 | Only one month of treatment |
| Caserta 2005 | Not clear if women symptomatic at baseline and did not state that the two groups were randomised |
| Chandeying 2007 | No details on the dosage of phytoestrogens in the product and both groups received some form of oestrogen therapy |
| Chandeying 2007a | Dose comparison study |
| Chiechi 2003 | Analyses effects of soy‐rich diet on vaginal epithelium of asymptomatic postmenopausal women. Symptomatic women excluded. |
| Cianci 2012 | Combination treatment: isoflavones and berberine combined |
| Cohn 2009 | Not clear if intervention is a phytoestrogen |
| Colacurci 2004 | There is no evidence that the control group was randomised. |
| Dodin 2005 | Women had very mild symptoms of hot flushes at baseline ‐ women requesting treatment for hot flushes unlikely to agree to be randomised to the trial ‐ only a subset of women analysed. |
| Duffy 2003 | Women had low incidence of symptoms ‐ aim of study to assess effects on cognitive function |
| Duncan 1999 | No outcomes of interest ‐ outcomes were physiological rather than clinical |
| Ehsanpour 2012 | Trial duration only 8 weeks |
| Erkkola 2010 | Only treated for 8 weeks in first arm of crossover |
| Hale 2001 | Women did not have vasomotor symptoms at baseline |
| Harding 1996 | Treatment only given for 2 months |
| Hochanadel 1999 | Only cognitive outcomes assessed; no flush outcomes or endometrial safety outcomes. |
| Ishiwata 2009 | A proportion of women were premenopausal at baseline (39/134) ‐ not clear if they were symptomatic at baseline |
| Jenks 2012 | Treatment for only 8 weeks |
| Jou 2005 | Treatment for only 6 weeks. |
| Katz 2007 | Treatment was only for 6 weeks |
| Kok 2005 | Participants were asymptomatic and effects measured on quality of life, not vasomotor symptoms |
| Kolarov 2001 | Does not appear to be randomised. |
| Krzysztof 2007 | Study not randomised |
| Lamlertkittikul 2004 | This was a dose finding study with no control group |
| Manonai 2006 | No evidence that women had vasomotor symptoms at baseline. |
| Manonai 2008 | Dosage study |
| Mittal 2011 | Not clear if symptomatic at baseline ‐ cannot separate out the effects of isoflavone on vasomotor symptoms |
| Murkies 1995 | It was not possible to determine the level of phytoestrogens in the experimental intervention. |
| Nahas 2004 | 38% of the participants had breast cancer. |
| Nasrin 2011 | Women were treated for only 6 weeks |
| Newton 2006 | The soy intervention was not standardised and was included with another preparation, a multibotannical tablet, so it was difficult to separate out the individual effects of soy alone. Also, women were given only dietary soy counseling and there was no measurement of their actual soy consumption. |
| Nikander 2005 | Women had a history of breast cancer |
| Palacios 2010 | Women were asymptomatic at the start of the trial and no control group ‐ designed to assess endometrial safety |
| Pedro 2012 | No evidence that the participants had hot flushes at baseline |
| Pop 2008 | This was not a randomised trial. The women were randomly stratified based on equol production to placebo or soy group |
| Pruthi 2012 | Treated for only 6 weeks |
| Quaas 2012 | No evidence of hot flushes at baseline in the participants |
| Quella 2000 | Participants had breast cancer. |
| Rotem 2007 | Combination treatment with black cohosh, dong quai, milk thistle, red clover, American ginseng and chaste‐tree berry |
| Russo 2003 | The intervention was a composite of phytoestrogens and black cohosh. It was not possible to determine whether effects solely due to phytoestrogens. |
| Sammartino 2006 | The intervention was a combination of isoflavones, lignans and cimicifugua racemosa. The effects of phytoestrogens alone could not be separated out. |
| Scambia 2000 | Co‐intervention of HT. First part of the trial, soy vs placebo, only for 6 weeks. |
| Schwen 2010 | Animal model |
| Secreto 2003 | About 10% of the participants had breast cancer |
| Steinberg 2011 | Women were not symptomatic |
| Uesugi 2004 | The participants in the study were a mixture of asymptomatic and symptomatic women. Data were not available separately just for symptomatic women. Treatment also only for 4 weeks. |
| Unfer 2004 | Participants were asymptomatic. |
| van Patten 2002 | Women had breast cancer |
| Verhoeven 2005 | Intervention was a combination of phytoestrogens and black cohosh. It was not possible to separate out the effects of phytoestrogens alone. |
| Virojchaiwong 2011 | Head to head study |
| Washburn 1999 | Treatment given for only 6 weeks. |
| Woo 2003 | Low prevalence of menopausal vasomotor symptoms |
| Xue 2004 | Outcome was a composite of menopausal symptoms. Effects on vasomotor symptoms could not be separated. |
| Yang 2012 | No control group ‐ comparison of 2 different strengths of soy extract |
Characteristics of studies awaiting assessment [ordered by study ID]
Agrawal 2005.
| Methods | Randomised placebo‐controlled trial in menopause clinic of a hospital in India |
| Participants | Not reported |
| Interventions |
|
| Outcomes | Quality of life, hot flushes, night sweats, urinary outcomes, psychological symptoms, sexual desire, dyspareunia |
| Notes | Author contacted and awaiting reply |
Baker 2011.
| Methods | Double‐blind placebo‐controlled randomised study (abstract) |
| Participants | Symptomatic perimenopausal and postmenopausal women (n = 51) |
| Interventions |
|
| Outcomes | Number and severity of hot flushes, nighttime wakenings, vaginal dryness, endometrial thickness |
| Notes | Abstract published in 2011; author contacted |
Garcia‐Martin 2012.
| Methods | Parallel‐group double‐blind randomised trial |
| Participants | Postmenopausal Spanish women (n = 99) |
| Interventions |
|
| Outcomes |
|
| Notes | Author contacted for full text of the publication |
Mucowski 2013.
| Methods | Randomised placebo‐controlled trial |
| Participants | Menopausal women (n = 110), secondary analysis of WISH trial in women who reported at least seven weekly hot flushes of any level at baseline |
| Interventions |
|
| Outcomes | Composite flushing score (frequency times intensity) |
| Notes | Secondary analysis of larger trial. Author contacted for data |
Nahidi 2009.
| Methods | Randomised double‐blind clinical trial |
| Participants | Menopausal women referred to healthcare centres (n = 68) |
| Interventions |
|
| Outcomes | Number of nocturnal hot flushes |
| Notes | Author contacted |
Paixao 2005.
| Methods | Double‐blind randomised study, duration 12 months |
| Participants | Menopausal women (n = 34) with age ranging from 38 to 54 years |
| Interventions |
|
| Outcomes | Climacteric symptoms (as measured by Kupperman's Menopausal Index), endometrial thickness (as measured by vaginal echography) |
| Notes |
Sekhavat 2012.
| Methods | Not reported |
| Participants | Not reported |
| Interventions | Not reported |
| Outcomes | Not reported |
| Notes | Awaiting translation to determine whether study meets eligibility criteria |
Stanosz 2006.
| Methods | Randomised study, duration 12 months |
| Participants | Women in the early postmenopausal period (n = 71) |
| Interventions |
|
| Outcomes | Kupperman Index |
| Notes | Not clear whether vasomotor symptoms alone measured |
Contributions of authors
Anne Lethaby registered the title; undertook searches, selection of studies, data extraction, quality assessment and data entry; and wrote the review. Julie Brown undertook searches, selection of studies, data extraction, contact with authors and quality assessment and commented on the final version of the review. Jane Marjoribanks undertook selection of studies, data extraction, quality assessment and preparation of tables and commented on the final version of the review. Fredi Kronenberg undertook selection of studies, data extraction and quality assessment and commented on both the protocol and the final review. Helen Roberts provided clinical input and commented on the final version of the review. John Eden commented on the final version of the review.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, University of Auckland, New Zealand.
External sources
No sources of support supplied
Declarations of interest
Anne Lethaby provided advice and suggestions to the author of the unpublished Brazilian study (Bicca 2004) that has been included in this review. She is included as an author of that unpublished paper.
John Eden is an author of two of the included studies (Knight 1999; Knight 2001).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Albertazzi 1998 {published data only}
- Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstetrics and Gynecology 1998;91:6‐11. [DOI] [PubMed] [Google Scholar]
- Albertazzi P, Pansini F, Bottazzi M, Bonaccorsi G, Aloysio D, Morton S. Dietary soy suplementation and phytooestrogen levels. Obstetrics and Gynecology 1999;94(2):229‐31. [DOI] [PubMed] [Google Scholar]
- Pansini F, Albertazzi P, Bonaccorsi G, Zanotti C, Campobasso C, Negri C, et al. Soy and Hot Flushes. 8th International Congress on the Menopause, Sydney, Australia. 3‐7 November 1996.
Aso 2012 {published data only}
- Aso T. Equol improves menopausal symptoms in Japanese women. Journal of Nutrition 2010;140:1386S‐9S. [DOI] [PubMed] [Google Scholar]
- Aso T, Uchiyama S, Matsumura Y, Taguchi M, Nozaki M, Takamatsu K, et al. A natural S‐Equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. Journal of Women's Health 2012;21(1):92‐100. [DOI] [PubMed] [Google Scholar]
Baber 1999 {published data only}
- Baber RJ, Templeman C, Morton T, Kelly GE, West L. Randomized placebo‐controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999;2:85‐92. [DOI] [PubMed] [Google Scholar]
Balk 2002 {published data only}
- Balk JL, Whiteside DA, Naus G, DeFerrari E, Roberts JM. A pilot study of the effects of phytoestrogen supplementation on the postmenopausal endometrium. Journal of the Society for Gynecologic Investigation 2002;9:238‐42. [PubMed] [Google Scholar]
Bicca 2004 {unpublished data only}
- Bicca ML de O, Horta BL, Lethaby AE. Double‐blind randomized clinical trial to assess the effectiveness of soy isoflavones in the relief of climacteric symptoms.
Brzezinski 1997 {published data only}
- Brzezinski A, Adlercreutz H, Shaoul R, Rosler A, Shmueli A, Tanos V, Schenker JG. Short‐term effects of phytoestrogen‐rich diet on postmenopausal women. Menopause 1997;4(2):89‐94. [Google Scholar]
Burke 2003 {published data only}
- Burke GL, Legault C, Anthony M, Bland DR, Morgan TM, Naughton MJ, et al. Soy protein and isoflavone effects on vasomotor symptoms in peri‐ and postmenopausal women: the Soy Estrogen Alternative Study. Menopause 2003;10(2):147‐53. [DOI] [PubMed] [Google Scholar]
Campagnoli 2005 {published data only}
- Campagnoli C, Abba C, Ambroggio S, Peris C, Perona M, Sanseverino P. Polyunsaturated fatty acids (PUFAs) might reduce hot flushes: an indication from two controlled trials on soy isoflavones alone and with a PUFA supplement. Maturitas 2005;51:127‐34. [DOI] [PubMed] [Google Scholar]
Carmigiani 2010 {published data only}
- Carmigiani LO, Pedro AO, Cost‐Paiva LH, Pinto‐Neto AM. The effect of dietary soy supplementation compared to estrogen and placebo on menopausal symptoms: a randomised controlled trial. Maturitas 2010;67:262‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2007 {published data only}
- Cheng G, Wilczek B, Warner M, Gustafsson J‐A, Landgren B‐L. Isoflavone treatment for acute menopausal symptoms. Menopause 2007;14(3, Part 1):468‐73. [DOI] [PubMed] [Google Scholar]
Colli 2012 {published data only}
- Colli MC, Bracht A, Soares AA, Oliveira AL, Boer CG, Souza CGM, et al. Evaluation of the efficacy of flaxseed meal and flaxseed extract in reducing menopausal symptoms. Journal of Medicinal Food 2012;15(9):840‐5. [DOI] [PubMed] [Google Scholar]
Crisafulli 2004 {published data only}
- Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, et al. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double‐blind EPT‐ and placebo‐ controlled study. Menopause 2004;11:400‐4. [DOI] [PubMed] [Google Scholar]
D'Anna 2007 {published data only}
- D'Anna R, Cannata M, Atteritano M, Cancellieri F, Corrado F, Baviera G, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 1 year randomized, double‐blind, placebo controlled study. Menopause 2007;14(4):648‐55. [DOI] [PubMed] [Google Scholar]
- D'Anna R, Cannata ML, Marini H, Atteritano M, Cancellieri F, Corrado F, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium and vaginal epithelium in postmenopausal women: a 2‐year randomised, double‐blind, placebo‐controlled study. Menopause 2009;16(2):301‐6. [DOI] [PubMed] [Google Scholar]
Dalais 1998 {published data only}
- Dalais FS, Rice GE, Wahlqvist ML, Grehan M, Murkies AL, Medley G, et al. Effects of dietary phytoestrogens in postmenopausal women. Climacteric 1998;1:124‐9. [DOI] [PubMed] [Google Scholar]
Del Giorno 2010 {published data only}
- Giorno C, Maggio da Fonseca A, Bagnoli VR, Serrano de Assis J, Soares JM, Baracat EC. Effects of trifolium pratense on climacteric and sexual symptoms in postmenopausal women. Revista da Associacao Medica Brasileira 2010;56(5):558‐62. [DOI] [PubMed] [Google Scholar]
Evans 2011 {published data only}
- Evans M, Ellito JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi‐center, randomised, placebo‐controlled study. Maturitas 2011;68:189‐96. [DOI] [PubMed] [Google Scholar]
Faure 2002 {published data only}
- Faure ED, Chantre P, Mares P. Effects of a standardised soy extract on hot flushes: a multicentre, double‐blind, randomised placebo‐controlled study. Menopause 2002;9(5):329‐34. [DOI] [PubMed] [Google Scholar]
Ferrari 2009 {published data only}
- Ferrari A. Soy extract phytoestrogens with high dose of isoflavones for menopausal symptoms. Journal of Obstetric and Gynaecological Research 2009;35(6):1083‐90. [DOI] [PubMed] [Google Scholar]
Geller 2009 {published data only}
- Geller SE, Shulman LP, Breemen RB, Banuvar S, Zhou Y, Epstein G, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomised controlled trial. Menopause 2009;16(6):1156‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2002 {published data only}
- Han KK, Soares JM, Haidar MA, Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstetrics and Gynecology 2002;99:389‐94. [DOI] [PubMed] [Google Scholar]
Hanachi 2008 {published data only}
- Hanachi P, Golkho S. Assessment of soy phytoestrogens and exercise on lipid profiles and menopause symptoms in menopausal women. Journal of Biological Sciences 2008;8(4):789‐93. [Google Scholar]
Heger 2006 {published data only}
- Heger M, Ventskovskiy BM, Borzenko I, Kneis KC, Rettenberger R, Kaszkin‐Bettag M, et al. Efficacy and safety of a special extract of Rheum rhaponticum (ERr 731) in perimenopausal women with climacteric complaints: a 12‐week randomised double‐blind placebo‐controlled trial. Menopause 2006;13(5):744‐59. [DOI] [PubMed] [Google Scholar]
Heyerick 2006 {published data only}
- Heyerick A, Vervarcke S, Depypere H, Bracke M, Keukeleire D. A first prospective, randomized, double‐blind, placebo‐controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas 2006;54:164‐75. [DOI] [PubMed] [Google Scholar]
Hidalgo 2005 {published data only}
- Hidalgo LA, Chedraui PA, Morocho N, Ross S, San Miguel G. The effect of red clover isoflavones on menopausal symptoms, lipids and vaginal cytology in menopausal women: a randomized, double‐blind, placebo‐controlled study. Gynecological Endocrinology 2005;21(5):257‐64. [DOI] [PubMed] [Google Scholar]
Imhof 2006 {published data only}
- Imhof M, Gocan A, Reithmayer F, Lipovac M, Schimitzek C, Chedraui P, et al. Effects of a red clover extract (MF11RCE) on endometrium and sex hormones in postmenopausal women. Maturitas 2006;55:76‐81. [DOI] [PubMed] [Google Scholar]
- Lipovac M, Chedraui P, Gruenhut C, Gocan A, Kurz C, Neuber B, et al. The effect of red clover isoflavone supplementation over vasomotor and menopausal symptoms in postmenopausal women. Gynecological Endocrinology 2012;28(3):203‐7. [DOI] [PubMed] [Google Scholar]
Jeri 2002 {published data only}
- Jeri A. The use of an isoflavone supplement to relieve hot flushes. The Female Patient 2002;27:47‐9. [Google Scholar]
Jou 2008 {published data only}
- Jou H‐J, Wu S‐C, Chang F‐W, Ling P‐Y, Chu KS, Wu W‐H. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. International Journal of Gynecology and Obstetrics 2008;102:44‐9. [DOI] [PubMed] [Google Scholar]
Kaari 2006 {published data only}
- Kaari C, Haidar MA, Soares Junior JM, Nunes MG, Azevedo Quadros LG, Kemp C, et al. Randomized clinical trial comparing conjugated equine estrogens and isoflavones in postmenopausal women: a pilot study. Maturitas 2006;53:49‐58. [DOI] [PubMed] [Google Scholar]
Khaodhiar 2008 {published data only}
- Khaodhiar L, Ricciotti HA, Li L, Pan W, Schickel M, Zhou J, et al. Daidzein‐rich isoflavone‐aglycones are effective in reducing hot flashes in menopausal women. Menopause 2008;15(1):125‐32. [PMC free article] [PubMed] [Google Scholar]
Knight 1999 {published data only}
- Knight DC, Howes JB, Eden JA. The effect of Promensil, an isoflavone extract, on menopausal symptoms. Climacteric 1999;2:79‐84. [DOI] [PubMed] [Google Scholar]
Knight 2001 {published data only}
- Knight DC, Howes JB, Eden JA, Howes LG. Effects on menopausal symptoms and acceptability of isoflavone‐containing soy powder dietary supplementation. Climacteric 2001;4:13‐8. [PubMed] [Google Scholar]
Kotsopoulos 2000 {published data only}
- Kotsopoulos D, Dalais FS, Liang Y‐L, McGrath BP, Teede HJ. The effects of soy protein containing phytoestrogens on menopausal symptoms in postmenopausal women. Climacteric 2000;3:161‐7. [DOI] [PubMed] [Google Scholar]
Levis 2011 {published data only}
- Levis S, Strickman‐Stein N, Ganjei‐Azar P, Xu P, Doerge D, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms. Archives of Internal Medicine 2011;171(15):1363‐8. [DOI] [PubMed] [Google Scholar]
Lewis 2006 {published data only}
- Lewis JE, Nickell LA, Thompson LU, Szalai JP, Kiss A, Hilditch JR. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause 2006;13(4):631‐42. [DOI] [PubMed] [Google Scholar]
Nahas 2007 {published data only}
- Nahas E, Nahas‐Neto J, Orsatti F, Carvalho E, Oliveira M, Dias R. Efficacy and safety of a soy isoflavone extract in postmenopausal women: a randomized double‐blind, and placebo controlled study. Maturitas 2007;58:249‐58. [DOI] [PubMed] [Google Scholar]
Penotti 2003 {published data only}
- Penotti M, Fabio E, Modena AB, Rinaldi M, Omodei U, Vigano P. Effect of soy‐derived isoflavones on hot flushes, endometrial thickness and the pulsatility index of the uterine and cerebral arteries. Fertility and Sterility 2003;79(5):1112‐7. [DOI] [PubMed] [Google Scholar]
- Penotti M, Fabio E, Rinaldi M, Omodei U, Bacchi M. Effects of soy‐derived isoflavones on hot flushes and vascular reactivity. The 10th World Congress on the Menopause, Berlin, Germany. 10‐14 June 2002:Abstract F‐27‐01.
Radhakrishnan 2009 {published data only}
- Radhakrishnan G, Agarwal N, Vaid N. Evaluation of isoflavone rich soy protein supplementation for postmenopausal therapy. Pakistan Journal of Nutrition 2009;8(7):1009‐17. [Google Scholar]
Sammartino 2003 {published data only}
- Sammartino A, Carlo C, Mandato VD, Bifulco G, Stefano M, Nappi C. Effects of genistein on the endometrium: ultrasonographic evaluation. Gynecological Endocrinology 2003;17(1):45‐9. [PubMed] [Google Scholar]
St Germain 2001 {published data only}
- Germain A, Peterson CT, Robinson JG, Alekel DL. Isoflavone‐rich or isoflavone‐poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2002;8(1):17‐26. [DOI] [PubMed] [Google Scholar]
Tice 2003 {published data only}
- Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings SR. Phytoestrogen supplements for the treatment of hot flashes: the isoflavone clover extract (ICE) study. JAMA 2003;290(2):207‐14. [DOI] [PubMed] [Google Scholar]
Upmalis 2000 {published data only}
- Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter double‐blind randomized placebo‐controlled study. Menopause 2000;7(4):236‐42. [DOI] [PubMed] [Google Scholar]
van de Weijer 2002 {published data only}
- Weijer PHM, Barentsen R. Isoflavones from red clover (Promensil) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas 2002;42:187‐93. [DOI] [PubMed] [Google Scholar]
Ye 2012 {published data only}
- Ye Y‐B, Wang Z‐L, Zhuo S‐Y, Lu W, Liao H‐F, Verbruggen MA, et al. Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women: a randomised placebo‐controlled trial. Menopause 2012;19(7):791‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albert 2001 {published data only}
- Albert A, Altabre C, Baro F, Cabero A, Cancelo MJ, Castelo‐Branco C, et al. Assessment of the efficiency and safety of soya‐extracted phytoestrogens (Glycine Max L), in the treatment of climacteric symptoms. Results of a mulicenter, open, prospective pilot study [Valoracion de la eficacia y seguridad de una preparacion de fitoestrogenos extraidos de la soja (Glycine Max L.) en al tratamiento de la sintomatologia climaterica. Resultados de un ensayo piloto multicentrico, abierto y prospectivo]. Toko‐ginecologia Practica 2001;60(5):257‐64. [Google Scholar]
Albertazzi 2005 {published data only}
- Albertazzi P, Steel SA, Bottazzi M. Effect of pure genistein on bone markers and hot flushes. Climacteric 2005;8(4):371‐9. [DOI] [PubMed] [Google Scholar]
Aly 2009 {published data only}
- Aly M. Use of black cohosh (Cimicifuga racemosa) in postmenopausal women: a randomized controlled study. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S413‐729. [Google Scholar]
Amato 2005 {published data only}
- Amato P, Young R, Cramer M, Lewis R, Murray M, Steinberg F, et al. The effect of soy isoflavone supplementation on menopausal quality of life: a multicenter, randomised controlled trial. 16th Annual Meeting of NAMS, San Diego, CA, USA. September‐October 2005.
- Amato P, Young RL, Steinberg FM, Murray MJ, Lewis RD, Cramer MA, et al. Effect of soy isoflavone supplementation on menopausal quality of life. Menopause 2013;20(4):443‐7. [DOI] [PubMed] [Google Scholar]
Atkinson 2003 {published data only}
- Atkinson C, Warren RML, Sala E, Dowsett M, Dunning AM, Healey CS, et al. Red clover‐derived isoflavones and mammographic breast density: a double‐blind, randomized, placebo‐controlled trial. Breast Cancer Research 2004;6(3):R170‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bai 2007 {published data only}
- Bai W, Henneicke‐von Zepelin H‐H, Wang S, Zheng S, Liu J, Zhang Z, et al. Efficacy and tolerability of a medicinal product containing isopropanolic black cohosh extract in Chinese women with menopausal symptoms: a randomized, double blind, parallel‐controlled study versus tibolone. Maturitas 2007;58:31‐41. [DOI] [PubMed] [Google Scholar]
Baird 1995 {published data only}
- Baird DB, Umbach DM, Lansdell L, Hughes CL, Setchell KDR, Weinberg CR, et al. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. Journal of Clinical Endocrinology and Metabolism 1995;80(5):1685‐90. [DOI] [PubMed] [Google Scholar]
Cancellieri 2007 {published data only}
- Cancellieri F, Leo V, Genazzani AD, Nappi C, Parenti GL, Polatti F, et al. Efficacy on menopausal neurovegetative symptoms and some plasma lipids blood levels of an herbal product containing isoflavones and other plant extracts. Maturitas 2007;56:249‐56. [DOI] [PubMed] [Google Scholar]
Carranza‐Lira 2001 {published data only}
- Carranza‐Lira SC, Barahona OF, Ramos DP, Herrera J, Olivares‐Segura A, Cardoso G, et al. Changes in symptoms, lipid and hormone levels after the administration of a cream with phytoestrogens in the climacteric: preliminary report. International Journal of Fertility 2001;46(6):296‐9. [PubMed] [Google Scholar]
Caserta 2005 {published data only}
- Caserta L, Caserta R, Torella M, Nappo C, Lucia D, Panariello S. The effects of phytoestrogen therapy on the endometrium in postmenopausal women. Minerva Ginecologica 2005;57(5):551‐5. [PubMed] [Google Scholar]
Chandeying 2007 {published data only}
- Chandeying V, Sangthawan M. Efficacy comparison of Pueraria mirifica (PM) against conjugated equine estrogen (CEE) with/without medroxyprogesterone acetate (MPA) in the treatment of climacteric symptoms in perimenopausal women: Phase III study. Journal of the Medical Association of Thailand 2007;90(9):1720‐6. [PubMed] [Google Scholar]
Chandeying 2007a {published data only}
- Chandeying V, Lamlertkittikul S. Challenges in the conduct of Thai herbal scientific study: efficacy and safety of phytoestrogen, Pueraria mirifica (Kwao Keur Kao), phase I, in the alleviation of climacteric symptoms in perimenopausal women. Journal of the Medical Association of Thailand 2007;90(7):1274‐80. [PubMed] [Google Scholar]
Chiechi 2003 {published data only}
- Chiechi LM, Putignano G, Guerra V, Schiavelli MP, Cisternino AM, Carriero C. The effect of a soy rich diet on the vaginal epithelium in postmenopause: a randomized double blind trial. Maturitas 2003;45:241‐6. [DOI] [PubMed] [Google Scholar]
Cianci 2012 {published data only}
- Cianci A, Cicero AFG, Colacurci N, Matarazzo MG, Leo V. Activity of isoflavones and berberine on vasomotor symptoms and lipid profile in menopausal women. Gynecological Endocrinology 2012;28(9):699‐702. [DOI] [PubMed] [Google Scholar]
Cohn 2009 {published data only}
- Cohn RD, Bornhauser C, MacManus D, Sadakane M, Read W. A study of lavender and tea tree oils on postmenopausal FSH levels and hot flash severity. Journal of Clinical Oncology, 2009 ASCO Annual Meeting Proceedings 2009;27(15S):1516. [Google Scholar]
Colacurci 2004 {published data only}
- Colacurci N, Zarcone R, Borrelli A, Franciscis P, Fortunato N, Cirillo M, et al. Effects of soy isoflavones on menopausal neurovegetative symptoms. Minerva Ginecologica 2004;56(5):407‐12. [PubMed] [Google Scholar]
Dodin 2005 {published data only}
- Dodin S, Lemay A, Jacques H, Legare F, Forest J‐C, Masse B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density and symptoms in menopausal women: a randomized, double‐blind, wheat germ placebo‐controlled clinical trial. Journal of Clinical Endocrinology and Metabolism 2004;90(3):1390‐7. [DOI] [PubMed] [Google Scholar]
Duffy 2003 {published data only}
- Duffy R, Wiseman H, File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacology, Biochemistry and Behaviour 2003;75:721‐9. [DOI] [PubMed] [Google Scholar]
Duncan 1999 {published data only}
- Duncan AM, Underhill KEW, Xu X, LaValleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. Journal of Clinical Endocrinology and Metabolism 1999;84(10):3479‐84. [DOI] [PubMed] [Google Scholar]
Ehsanpour 2012 {published data only}
- Ehsanpour S, Salehi K, Zolfaghari B, Bakhtiari S. The effects of red clover on quality of life in postmenopausal women. Iranian Journal of Nursing and Midwifery 2012;17(1):34‐40. [PMC free article] [PubMed] [Google Scholar]
Erkkola 2010 {published data only}
- Erkkola R, Vervarcke S, Vansteelandt S, Rompotti P, Keeukeleire D, Heyerick A. A randomized, double‐blind, placebo‐controlled, cross‐over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine 2010;17(6):389‐96. [DOI] [PubMed] [Google Scholar]
Hale 2001 {published data only}
- Hale GE, Hughes CL, Robboy SJ, Agarwal SK, Bievre M. A double‐blind randomised study on the effects of red clover isoflavones on the endometrium. Menopause 2001;8(5):338‐46. [DOI] [PubMed] [Google Scholar]
Harding 1996 {published data only}
- Harding C, Morton M, Gould V, McMichael Phillips D, Howell A, Bundred NJ. Dietary soy supplementation is oestrogenic in menopausal women (poster abstract). Second International Symposium on the role of soy in preventing and treating chronic disease, September 15‐18 1996, Brussels, Belgium. 1996.
Hochanadel 1999 {published data only}
- Hochanadel G, Shifren I, Zhdanova I, Maher T, Spiers P. Soy isoflavones (phytoestrogens) in the treatment of the cognitive and somatic symptoms of menopause. Fertility and Sterility 1999;71(4):22. [Google Scholar]
Ishiwata 2009 {published data only}
- Ishiwata N, Melby MK, Mizuno S, Watanabe S. New equol supplement for relieving menopausal symptoms: randomised, placebo‐controlled trial of Japanese women. Menopause 2009;16(1):141‐8. [DOI] [PubMed] [Google Scholar]
Jenks 2012 {published data only}
- Jenks BH, Iwashita S, Nakagawa Y, Ragland K, Lee J, Carson WH, et al. A pilot study on the effects of S‐Equol compared to soy isoflavones on menopausal hot flash frequency. Journal of Women's Health 2012;21(6):674‐82. [DOI] [PubMed] [Google Scholar]
Jou 2005 {published data only}
- Jou HJ, Ling PY, Wu SC. Comparison of 70mg and 35mg isoflavone soya supplement for menopause symptoms. International Journal of Gynaecology and Obstetrics 2005;90(2):156‐60. [DOI] [PubMed] [Google Scholar]
Katz 2007 {published data only}
- Katz D, Evans M, Njike V, Hoxley M, Nawax H, Comerford B, et al. Raloxifene, soy phytoestrogens and endothelial function in postmenopausal women. Climacteric 2007;10(6):500‐7. [DOI] [PubMed] [Google Scholar]
Kok 2005 {published data only}
- Kok L, Kreijkamp‐Kaspers S, Grobbee DE, Lampe JW, Schouw YT. A randomized placebo‐controlled trial on the effects of soy protein containing isoflavones on quality of life in postmenopausal women. Menopause 2005;12(1):56‐62. [DOI] [PubMed] [Google Scholar]
- Kok L, Kreijkamp‐Kaspers S, Grobbee DE, Schouw YT. Design and baseline characteristics of a trial on health effects of soy protein with isoflavones in postmenopausal women. Maturitas 2004;47:21‐9. [DOI] [PubMed] [Google Scholar]
Kolarov 2001 {published data only}
- Kolarov G, Nalbanski B, Kamenov Z. [Possibilities for an individualised approach to the treatment of climacteric symptoms with phytoestrogens] Bulgarian. Source not reported 2001;40:18‐21. [PubMed] [Google Scholar]
Krzysztof 2007 {published data only}
- Krzysztof D, Agnieszka S‐M, Ewa P, Agnieszka K‐S, Magdalena M, Agnieszka K. Efficacy of standardised isoflavones extract (Soyfem) (52‐104 mg/24h) in moderate and medium‐severe climacteric syndrome. Ginekologia Polska 2007;78:307‐11. [PubMed] [Google Scholar]
Lamlertkittikul 2004 {published data only}
- Lamlertkittikul S, Chandeying V. Efficacy and safety of Pueraria mirifica (Kwao Kruea Khao) for the treatment of vasomotor symptoms in perimenopausal women: phase II study. Journal of the Medical Association of Thailand 2004;87:33‐40. [PubMed] [Google Scholar]
Manonai 2006 {published data only}
- Manonai J, Songchitsomboon S, Chanda K, Hong JH, Komindr S. The effect of a soy‐rich diet on urogenital atrophy: a randomized crossover trial. Maturitas 2006;54:135‐40. [DOI] [PubMed] [Google Scholar]
Manonai 2008 {published data only}
- Manonai J, Chittacharoen A, Udomsubpayakul U, Theppisai HY, Theppisai U. Effects and safety of Puerarua mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy post‐menopausal women. Menopause 2008;15(3):530‐5. [DOI] [PubMed] [Google Scholar]
Mittal 2011 {published data only}
- Mittal N, Hota D, Dutta P, Bhansali A, Suri V, Aggarwal N, et al. Evaluation of effect of isoflavone on thyroid economy and autoimmunity in oophorectomised women: a randomised, double‐blind, placebo controlled trial. Indian Journal of Medical Research 2011;133:633‐40. [PMC free article] [PubMed] [Google Scholar]
Murkies 1995 {published data only}
- Murkies AL, Lombard C, Strauss BJG, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases postmenopausal hot flushes: effect of soy and wheat. Maturitas 1995;21:189‐95. [DOI] [PubMed] [Google Scholar]
Nahas 2004 {published data only}
- Nahas EP, Neto JN, Luca L, Traiman P, Pontes A, Dalben I. Benefits of soy germ isoflavones in postmenopausal women with contraindication for conventional hormone replacement therapy. Maturitas 2004;48(4):372‐80. [DOI] [PubMed] [Google Scholar]
Nasrin 2011 {published data only}
- Nasrin B, Azam A, Sedigheh A, Ahmad E, Habibollah E. Effect of powder of flaxseed on hot flashes in women at the climacteric. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;14(1):37‐47. [Google Scholar]
Newton 2006 {published data only}
- Newton KM, Reed SD, Croix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Principal results of the herbal alternatives for menopause (HALT) study. 16th Annual Meeting of the North American Menopause Society, San Diego, CA. 2005 September 28‐October 5:S‐13.
- Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy or placebo. Annals of Internal Medicine 2006;145:869‐79. [DOI] [PubMed] [Google Scholar]
- Reed SD, Newton KM, Grothaus L, Ehrlich K, Grieco V, Croix AZ. Vaginal findings from a randomized placebo‐controlled trial of black cohosh, multibotannical herbs and dietary soy for hot flushes: the herbal alternatives (HALT) study. 7th European Congress on Menopause, Istanbul, Turkey. 2006 June 3‐7:s102‐3.
- Reed SD, Newton KM, Grothaus L, Ehrlich K, Schenk J, Croix AZ. The herbal alternative for menopause study (HALT: dietary soy intervention). 7th European Congress on Menopause, Istanbul, Turkey. 2006 June 3‐7:s63.
- Reed SD, Newton KM, Croix AZ, Grothaus LA, Ehrlich K, Guiltinan K. The herbal alternatives for menopause (HALT) study: endometrial safety. 16th Annual Meeting of the North American Menopause Society, San Diego, CA. 2005 September 28‐October 5:P‐70.
- Reed SD, Newton KM, LaCroix AZ, Grothaus LC, Grieco VS, Ehrlich K. Vaginal, endometrial and reproductive hormone findings: randomised placebo‐controlled trial of black cohosh, multibotannical herbs, and dietary soy for vasomotor symptoms: the Herbal Alternatives for Menopause (HALT) study. Menopause 2008;15(1):51‐8. [PubMed] [Google Scholar]
Nikander 2005 {published data only}
- Nikander E, Rutanen E‐M, Nieminen P, Wahlstrom T, Ylikorkala O, Tiitinen A. Lack of effect of isoflavonoids on the vagina and endometrium in postmenopausal women. Fertility and Sterility 2005;83(1):137‐42. [DOI] [PubMed] [Google Scholar]
Palacios 2010 {published data only}
- Palacios S, Pornel B, Vazquez F, Aubert L, Chantre P, Mares P. Long‐term endometrial and breast safety of a specific, standardized soy extract. Climacteric 2010;43(4):368‐75. [DOI] [PubMed] [Google Scholar]
Pedro 2012 {published data only}
- Pedro AO, Carmigiani LO, Pinto‐Nero AM, Costa‐Paiva LS. The effect of a soy‐based dietary supplement on the vaginal epithelium and endometrium in postmenopause: a randomised double blind controlled clinical trial. Menopause 2012;19(12):1402‐3. [DOI] [PubMed] [Google Scholar]
Pop 2008 {published data only}
- Pop E, Fischer L, Coan A, Gitzinger M, Nakamura J, Zeisel S. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: a phase 1 clinical trial. Menopause 2008;15(4):684‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruthi 2012 {published data only}
- Pruthi S, Qin R, Terstreip S, Liu H, Loprinzi C, Shah T, et al. A phase III, randomized, placebo‐controlled, double‐blind trial of flaxseed for the treatment of hot flashes: North Central Cancer Treatment Group N08C7. Menopause 2012;19(1):48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quaas 2012 {published data only}
- Quaas AM, Kono N, Mack WJ, Hodis H, Paulson RJ, Shoupe D. The effect of isoflavone soy protein (ISP) supplementation on endometrial thickness, hyperplasia and endometrial cancer risk in postmenopausal women: a randomised controlled trial. Fertility and Sterility 2012;97(3 Suppl):S6 (abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
Quella 2000 {published data only}
- Quella SK, Loprinzi CL, Barton DL, Knost JA, Sloan JA, LaVasseur BI, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment trial. Journal of Clinical Oncology 2000;18(5):1068‐74. [DOI] [PubMed] [Google Scholar]
Rotem 2007 {published data only}
- Rotem CA, Kaplan B. Phyto‐female complex for the relief of hot flushes, night sweats and quality of sleep: randomised controlled double‐blind pilot study. Gynecological Endocrinology 2007;23(2):117‐22. [DOI] [PubMed] [Google Scholar]
Russo 2003 {published data only}
- Russo R, Corosu R. The clinical use of a preparation based on phytoestrogens in the treatment of menopausal disorders. Acta Bio‐Medica de l Ateneo Parmense 2003;74(3):137‐43. [PubMed] [Google Scholar]
Sammartino 2006 {published data only}
- Sammartino A, Tommaselli GA, Gargano V, Carlo C, Attianese W, Nappi C. Short‐term effects of a combination of isoflavones, lignans and Cimicifuga racemosa on climacteric‐related symptoms in postmenopausal women: a double‐blind randomized placebo‐controlled trial. Gynecological Endocrinology 2006;22(11):646‐50. [DOI] [PubMed] [Google Scholar]
Scambia 2000 {published data only}
- Scambia G, Mango D, Signorile PG, Angeli RA, Palena C, Gallo D, et al. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause 2000;7(2):105‐11. [DOI] [PubMed] [Google Scholar]
Schwen 2010 {published data only}
- Scwen R, Griewe J, Desai P, Jackson R, Ausio Pharmaceuticals and Cincinnati College of Pharmacy. Development of S‐equol, a natural selective estrogen receptor beta agonist for the treatment of menopausal symptoms. Menopause 2010;17(6):12‐7. [Google Scholar]
Secreto 2003 {published data only}
- Secreto G, Chiechi LM, Amadori A, Miceli R, Venturelli E, Valerio T, et al. Soy isoflavones and melatonin for the relief of climcteric symptoms: a multicenter, double blind, randomized study. Maturitas 2004;47:11‐20. [DOI] [PubMed] [Google Scholar]
Steinberg 2011 {published data only}
- Steinberg F, Murray M, Lewis R, Cramer M, Amato P, Young R, et al. Clinical outcomes of a 2‐y soy isoflavone supplementation in menopausal women. American Journal of Clinical Nutrition 2011;93(2):356‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uesugi 2004 {published data only}
- Uesugi S, Watanabe S, Ishiwata N, Uehara M, Ouchi K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. BioFactors 2004;22:221‐8. [DOI] [PubMed] [Google Scholar]
Unfer 2004 {published data only}
- Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Renzo GC. Endometrial effects of long‐term treatment with phytoestrogens: a randomized double‐blind placebo‐controlled study. Fertility and Sterility 2004;82(1):145‐8. [DOI] [PubMed] [Google Scholar]
van Patten 2002 {published data only}
- Patten CL, Olivotto IA, Chambers GK, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomised controlled clinical trial. Journal of Clinical Oncology 2002;20(6):1449‐55. [DOI] [PubMed] [Google Scholar]
Verhoeven 2005 {published data only}
- Verhoeven MO, Mooren MJ, Weijer PHM, Verhegem PJE, Burgt LMJ, Kenemans P. Effect of a combination isoflavones and Actaea racemosa Linnaeus on climacteric symptoms in healthy symptomatic perimenopausal women: a 12‐week randomized, placebo‐controlled, double‐blind study. Menopause 2005;12(4):412‐20. [DOI] [PubMed] [Google Scholar]
Virojchaiwong 2011 {published data only}
- Virojchaiwong P, Suvithayasiri V, Itharat A. Comparison of Pueraria mirifica 25 and 50 mg for menopausal symptoms. Archives of Gynecology & Obstetrics 2011;284(2):411‐9. [DOI] [PubMed] [Google Scholar]
Washburn 1999 {published data only}
- Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6(1):7‐13. [PubMed] [Google Scholar]
Woo 2003 {published data only}
- Woo J, Lau E, Ho SC, Cheng F, Chan C, Chan AS, et al. Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause 2003;10(4):352‐61. [DOI] [PubMed] [Google Scholar]
Xue 2004 {published data only}
- Xue X, Niu J, Wang J, Ai H. A prospective controlled study on the clinical therapeutic effect of treatment of menstrual syndrome by isoflavone of soybean. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004;24(9):835‐6. [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang T‐S, Wang S‐Y, Yang Y‐C, Su C‐H, Lee F‐K, Chen S‐C, et al. Effects of standardised phytoestrogen on Taiwanese menopausal women. Taiwanese Journal of Obstetrics and Gynecology 2012;51(2):229‐35. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Agrawal 2005 {unpublished data only}
- Agrawal S, Shah D. Phytoestrogens and quality of life. Royal College of Obstetricians and Gynaecologists 6th International Meeting, Cairo, Egypt. 2005 September 27‐30.
Baker 2011 {unpublished data only}
- Baker MB, Ciccone M, Wilson M, Stanczyk F, Shoupe D. A double‐blind placebo controlled trial on the effects of a licorice extract on menopausal symptoms. Fertility and Sterility 2011;96(3 Suppl):S5. [Google Scholar]
Garcia‐Martin 2012 {published data only}
- Garcia‐Martin A, Quesada Charneco M, Alvarez Guisado A, Jimenez Moleon JJ, Fonolla Joya J, Munoz‐Torres M. Effect of milk product with soy isoflavones on quality of life and bone metabolism. Medicina Clinica 2012;138(2):47‐51. [DOI] [PubMed] [Google Scholar]
Mucowski 2013 {published data only}
- Mucowski SJ, Shoupe D, Dang H, Henderson V, Kono N, Hodis N, et al. The effect of soy isoflavones on menopausal vasomotor flushing. Fertility and Sterility 2012;99(Suppl 1):S35 (abstract). [Google Scholar]
Nahidi 2009 {published data only}
- Nahidi F, Zare E, Mojab F, Alavi Majd H. Effects of licorice root extract on the number of nocturnal hot flashes in menopausal women. Journal of Nursing and Midwifery 2009;19(67). [Google Scholar]
Paixao 2005 {published data only}
- Paixao JS, Fonseca AM, Bagnoli VR, Arie WMY, Penteado SRL, Junqueira PA, et al. Effects of genistein on endometrial thickness and climacteric symptoms: a double blind randomized controlled study. 16th Annual Meeting of North American Menopause Society, San Diego, CA, USA. 2005 September 28‐October 5.
Sekhavat 2012 {published data only}
- Sekhavat L, Firouzabadi RD. Effect of soya protein on symptoms of hot flash in menopausal women in Yazd, Iran. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15(6):10‐5. [Google Scholar]
Stanosz 2006 {published data only}
- Stanosz S, Puk E, Grobelny W, Stanosz M, Kazikowska A. Evaluation of the efficacy and tolerance of Soyfem in women in the early menopausal period [Polish]. Przeglad Menopauzalny 2006;5(3):182‐90. [Google Scholar]
Additional references
Adlercreutz 1987
- Adlercreutz H, Hockerstedt K, Bannwart C, Bloigu S, Hamalainen E, Fotsis T, et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone blinding globulin (SHBG). Journal of Steroids and Biochemistry 1987;27:1135‐44. [DOI] [PubMed] [Google Scholar]
Adlercreutz 1990
- Adlercreutz H. Western diet and western diseases: some hormonal and biochemical mechanisms and associations. Scandinavian Journal of Clinical Laboratory Investigations 1990;50(Suppl):3‐23. [PubMed] [Google Scholar]
Adlercreutz 1995
- Adlercreutz H. Phytoestrogens: epidemiology and a possible role in cancer protection. Environmental Health Perspectectives 1995;103(Suppl 7):103‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
AFSSA 2005
- AFSSA Working Group. Safety and benefit of phytoestrogens—recommendations. INSERM‐CRLC M France 2005:1‐8.
Anderson 2003
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA 2003;290(13):1739‐48. [DOI] [PubMed] [Google Scholar]
Ayers 2013
- Ayers B, Hunter MS. Health‐related quality of life of women with menopausal hot flushes and night sweats. Climacteric 2013;16:235‐9. [DOI] [PubMed] [Google Scholar]
Bair 2005
- Bair YA, Gold EB, Azari RA, et al. Use of conventional and complementary health care during the transition to menopause: longitudinal results from the Study of Women's Health Across the Nation (SWAN). Menopause 2005;12:31‐9. [DOI] [PubMed] [Google Scholar]
Bakken 2004
- Bakken K, Eggen AE, Lund E. Side‐effects of hormone replacement therapy and influence in pattern of use among women aged 45‐64 years. The Norwegian Women and Cancer (NOWAC) study 1997. Acta Obstetrica Gynecologica Scandinavica 2004;83(9):850‐6. [DOI] [PubMed] [Google Scholar]
Bennetts 1946
- Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Australian Vetinary Journal 1946;22:2‐12. [DOI] [PubMed] [Google Scholar]
Beral 2003
- Beral V. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet 2003;362(9382):419‐27. [DOI] [PubMed] [Google Scholar]
Bestul 2004
- Bestul MB, McCollum M, Hansen LB, Saseen JJ. Impact of the women's health initiative trial results on hormone replacement therapy. Pharmacotherapy 2004;24(4):495‐9. [DOI] [PubMed] [Google Scholar]
Bjorn 1999
- Bjorn I, Backsrom T. Drug related negative side‐effects is a common reason for poor compliance in hormone replacement therapy. Maturitas 1999;32(2):77‐86. [DOI] [PubMed] [Google Scholar]
Bolanos 2010
- Bolanos R, Castillo A, Francia J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: systematic review and meta‐analysis. Menopause 2010;17(3):660‐6. [PubMed] [Google Scholar]
Bolego 2003
- Bolego C, Poli A, Cignarella A, Paoletti R. Phytoestrogens: pharmacological and therapeutic perspectives. Current Drug Targets 2003;4:77‐87. [DOI] [PubMed] [Google Scholar]
Cassidy 1993
- Cassidy A, Bingham S, Carlson J, Setchell KDR. Biological effects of plant estrogens in premenopausal women. FASEB J 1993;A:866. [Google Scholar]
Chao 2006
- Chao MT, Wade C, Kronenberg F, Kalmuss D, Cushman LF. Women's reasons for complementary and alternative medicine use: racial/ethnic differences. Journal of Alternative and Complementary Medicine 2006;12(8):719‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Col 2009
- Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle aged women: a longitudinal study. Menopause 2009;16:453‐7. [DOI] [PubMed] [Google Scholar]
Com Tox 2003
- Committee on Toxicity. Phytoestrogens and health. London, 2003:1‐444. [Google Scholar]
Coon 2007
- Coon JT, Pittler MH, Ernst E. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta‐analysis. Phytomedicine 2007;14:153‐9. [DOI] [PubMed] [Google Scholar]
Deecher 2007
- Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Archives of Women's Mental Health 2007;10(6):247‐57. [DOI] [PubMed] [Google Scholar]
Europ Med Ag 2006
- European Medicines Agency. Evaluation of medicines for human use: guidelines for hormone replacement therapy. www.emea.eu.int (accessed on 20 January 2006).
Freedman 1995
- Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. Clinical Endocrinology and Metabolism 1995;80(8):2354‐8. [DOI] [PubMed] [Google Scholar]
Freedman 2001
- Freedman RR. Physiology of hot flashes. American Journal of Human Biology 2001;13(4):453‐64. [DOI] [PubMed] [Google Scholar]
Freeman 2007
- Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric 2007;10:197‐214. [DOI] [PubMed] [Google Scholar]
Geller 2005
- Geller SE, Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. Journal of Women's Health 2005;14(7):634‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glazier 2001
- Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Archives of Internal Medicine 2001;161:1161‐72. [DOI] [PubMed] [Google Scholar]
Gold 2006
- Gold EB, Colvin A, Avis N, Bromberger J, Greendate GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. American Journal of Public Health 2006;96(7):1226‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gold 2007
- Gold EB, Bair Y, Zhang G, Utts J, Greendate GA, Upchurch D, et al. Cross‐sectional analysis of specific complementary and alternative medicine (CAM) use by racial/ethnic group and menopausal status: the Study of Women's Health Across the Nation (SWAN). Menopause 2007;14(4):612‐23. [DOI] [PubMed] [Google Scholar]
Grady 2000
- Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/Progestin Replacement Study. Annals of Internal Medicine 2000;132(9):689‐96. [DOI] [PubMed] [Google Scholar]
Haas 2004
- Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Annals of Internal Medicine 2004;140(3):184‐8. [DOI] [PubMed] [Google Scholar]
Haimov‐Kochman 2005
- Haimov‐Kochman R, Hchner‐Celnikier D. Hot flashes revisited: pharmacological and herbal options for hot flush management. What does the evidence tell us?. Acta Obstetrica Gynecologica Scandinavica 2005;84:972‐9. [DOI] [PubMed] [Google Scholar]
Harlow 2012
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertility and Sterility 2012;97(4):843‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Howes 2006
- Howes LG, Howes JB, Knight DC. Isoflavone therapy for menopausal flushes: a systematic review and meta‐analysis. Maturitas 2006;55:203‐11. [DOI] [PubMed] [Google Scholar]
Hunter 2012
- Hunter MS, Gentry‐Maharaj A, Ryan A, Burnell M, Lanceley A, Fraser L, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross‐sectional cohort study of 10 418 British women aged 54–65. BJOG 2012;119:40‐50. [DOI] [PubMed] [Google Scholar]
Huntley 2004
- Huntley AL, Ernst E. Soy for the treatment of perimenopausal symptoms—a systematic review. Maturitas 2004;47:1‐9. [DOI] [PubMed] [Google Scholar]
Jacobs 2009
- Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A. Efficacy of isoflavones in relieving vasomotor menopausal symptoms—a systematic review. Molecular Nutrition and Food Research 2009;53:1084‐97. [DOI] [PubMed] [Google Scholar]
Knight 1996
- Knight D, Eden JA. A review of the clinical effects of phytoestrogens. Obstetrics and Gynecology 1996;87:897‐904. [PubMed] [Google Scholar]
Krebs 2004
- Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstetrics and Gynecology 2004;104(4):824‐36. [DOI] [PubMed] [Google Scholar]
Kronenberg 1987
- Kronenberg F, Downey JA. Thermoregulatory physiology of menopausal hot flashes: a review. Canadian Journal of Physiology and Pharmacology 1987;65(6):1312‐24. [DOI] [PubMed] [Google Scholar]
Kronenberg 1990
- Kronenberg F. Hot flashes: epidemiology and physiology. Annals of the New York Academy of Sciences 1990;592:52‐86. [DOI] [PubMed] [Google Scholar]
Kuiper 1997
- Kuiper G, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997;138:863‐70. [DOI] [PubMed] [Google Scholar]
Low Dog 2005
- Low Dog T. Menopause: a review of botanical dietary supplements. American Journal of Medicine 2005;118(12B):98‐108. [DOI] [PubMed] [Google Scholar]
MacLennan 1999
- MacLennan A. The four harms of harmless therapies (editorial). Climacteric 1999;2:73‐4. [DOI] [PubMed] [Google Scholar]
Mahady 2003
- Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri‐ and postmenopausal women. Menopause 2003;10:65‐72. [DOI] [PubMed] [Google Scholar]
Makela 1994
- Makela S, Davis VL, Tally WC. Dietary estrogens act through estrogen‐receptor mediated processes and show no antiestrogenicity in cultured breast cancer cells. Environmental Health Perspectives 1994;102:572‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Messina 2003
- Messina M, Hughes C. Efficacy of soy foods and soybean isoflavone supplements for alleviating menopausal symptoms is positively related to initial hot flush frequency. Journal of Medicinal Food 2003;6:1‐11. [DOI] [PubMed] [Google Scholar]
Milligan 1999
- Milligan SR, Kalita JC, Heyerick A, Rong H, Cooman L, Keukeleire D. Identification of a potent phytoestrogen in hops (Humulus luputus L.) and beer. Journal of Clinical Endocrinology and Metabolism 1999;84:2249‐52. [DOI] [PubMed] [Google Scholar]
Muthyala 2004
- Muthyala RS, Ju YH, Sheng S. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R‐ and S‐equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorganic and Medicinal Chemistry Letters 2004;12:1559‐67. [DOI] [PubMed] [Google Scholar]
Nagata 2001
- Nagata C, Takatsuka N, Kawakami N, Shimizu H. Soy product intake and hot flashes in Japanese women: results from a community‐based prospective study. American Journal of Epidemiology 2001;153:790‐3. [DOI] [PubMed] [Google Scholar]
NAMS 2004
- North American Menopause Society. Treatment of menopause‐associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause 2004;11(1):11‐33. [DOI] [PubMed] [Google Scholar]
NAMS 2011
- North American Menopause Society. The role of soy isoflavones in menopausal health: report of the North American Menopause Society. Menopause 2011;18(7):732‐53. [DOI] [PubMed] [Google Scholar]
NAMS 2012
- North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause 2012;19(3):257‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nedrow 2006
- Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause‐related symptoms. Archives of Internal Medicine 2006;166:1453‐65. [DOI] [PubMed] [Google Scholar]
Newton 2002
- Newton KM, Buist DSM, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population‐based survey. Obstetrics and Gynecology 2002;100:18‐25. [DOI] [PubMed] [Google Scholar]
Roussouw 2002
- Roussouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized trial. JAMA 288;3:321‐33. [DOI] [PubMed] [Google Scholar]
Roussouw 2007
- Roussouw JE, Prentice RL, Manson JE. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297:1465‐77. [DOI] [PubMed] [Google Scholar]
Rowland 2003
- Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto‐oestrogens. British Journal of Nutrition 2003;(Suppl):45‐8. [DOI] [PubMed] [Google Scholar]
Schulz 2005
- Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet 2005;365:1657‐61. [DOI] [PubMed] [Google Scholar]
Seibel 2003
- Seibel MM. Treating hot flushes without hormone replacement therapy. Journal of Family Practice 2003;52(4):291‐6. [PubMed] [Google Scholar]
Setchell 1984
- Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal oestrogens of dietary origin: possible role in hormone dependent disease. American Journal of Clinical Nutrition 1984;2:49‐56. [DOI] [PubMed] [Google Scholar]
Setchell 1997
- Setchell KDR, Zimmer‐Nechemias L, Cai J, Heubi JE. Exposure of infants to phytoestrogens from soy‐based infant formula. Lancet 1997;350:23‐7. [DOI] [PubMed] [Google Scholar]
Setchell 1998
- Setchell KDR. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. American Journal of Clinical Nutrition 1998;68(Suppl):1333‐46. [DOI] [PubMed] [Google Scholar]
Setchell 2002
- Setchell KD, Brown NM, Lydeking‐Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. Journal of Nutrition 2002;132:3577‐84. [DOI] [PubMed] [Google Scholar]
Sievert 2007
- Sievert LL, Morrison L, Brown DE, Reza AM. Vasomotor symptoms among Japanese‐American and European‐American women living in Hilo, Hawaii. Menopause 2007;14(2):261‐9. [DOI] [PubMed] [Google Scholar]
Sikon 2004
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Seminars in Reproductive Medicine 2004;71:578‐82. [DOI] [PubMed] [Google Scholar]
Somekawa 2001
- Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids and bone mineral density in postmenopausal Japanese women. Obstetrics and Gynecology 2001;97:109‐15. [DOI] [PubMed] [Google Scholar]
Taku 2012
- Taku K, Melby MK, Kronenberg F, Kurzer MS, Messina M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta‐analysis of randomised controlled trials. Menopause 2012;19(7):776‐90. [DOI] [PubMed] [Google Scholar]
Tempfer 2009
- Tempfer CB, Froese G, Heinze G, Bentz EK, Hefler LA, Huber JC. Side effects of phytoestrogens: a meta‐analysis of randomised trials. American Journal of Medicine 2009;122:939‐46. [DOI] [PubMed] [Google Scholar]
Thompson 1991
- Thompson LU, Robb P, Serraino M. Mammalian lignan production from various foods. Nutrition and Cancer 1991;16:43‐52. [DOI] [PubMed] [Google Scholar]
Travers 2006
- Travers C, O'Neill SM, Khoo S‐K, King R. Hormones down under. Hormone therapy use after the Women's Health Initiative. Australian and New Zealand Journal of Obstetrics and Gynaecology 2006;46:330‐5. [DOI] [PubMed] [Google Scholar]
UK MHRA 2007
- Medicines and Healthcare Products Regulatory Agency. Hormone‐replacement therapy: safety update—UK Public Assessment Report. http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product‐specificinformationandadvice/Product‐specificinformationandadvice‐G‐L/Hormonereplacementtherapy%28HRT%29/ (accessed 26 June 2013).
Villaseca 2012
- Villaseca P. Non‐estrogen conventional and phytochemical treatments for vasomotor symptoms: what needs to be known for practice. Climacteric 2012;15:115‐24. [DOI] [PubMed] [Google Scholar]
Wade 2008
- Wade C, Chao M, Kronenberg F, Cushman L, Kalmuss D. Medical pluralism among American women: results of a national survey. Journal of Women's Health 2008;17(5):829‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1996
- World Health Organisation Scientific Group. Menopause. WHO Technical Report Series 866, 1996; Vol. 7, issue 4:236‐42. [PubMed]
Wilcox 1990
- Wilcox G, Wahlqvist ML, Burger HG, Medley G. Oestrogenic effects of plant foods in postmenopausal women. BMJ 1990;301:905‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson‐H 2006
- Williamson‐Hughes PS, Flickinger BD, Messina MJ, Empie MW. Isoflavone supplements containing predominantly genistein reduce hot flash symptoms: a critical review of published studies. Menopause 2006;13(5):831‐9. [DOI] [PubMed] [Google Scholar]
Wiseman 2004
- Wiseman H, Casey K, Bowey EA, et al. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. American Journal of Clinical Nutrition 2004;80(3):692‐9. [DOI] [PubMed] [Google Scholar]


