Abstract
Plumbago zeylanica L. is commonly known as chitrak, consumed since long time owing to its potent medicinal benefits. It is a major source of the yellow crystalline naphthoquinone called plumbagin, which is highly acclaimed for its anticancerous activities on different cancers i.e. prostrate, breast, ovarian, etc. The growing urges for this compound make this plant extremely demanding in the global market; hence, the plant is indiscriminately harvested from its very natural habitat. Therefore, in vitro biomass production of this plant can be a sustainable alternative for plumbagin production. In this present study, it has been found that, compared to other cytokinins, biomass production was enhanced by using aromatic cytokinin meta-topolin (mT). The highest shoot buds produced by mT (1 mg/l) was 13.60 ± 1.14 after 14 d of culture establishment. After 84 d in the same medium, 129.8 ± 2.71 shoots were produced, and the fresh weight of the total biomass was 19.72 ± 0.65 g. The highest number of roots was induced (37.80 ± 0.84) with 1.0 mg/l Indole-3-butyric acid (IBA). The well rooted plantlets were acclimatized in field condition with 87%survival. The regenerated plants' genetic fidelity was accessed through molecular markers i.e. Inter simple sequence repeat (ISSR), Start codon targeted (SCoT) and cytology studies. The monomorphic bands amplified by the primers across in vivo and in vitro plants confer the genetic homogeneity of the regenerants. The plumbagin content from different parts of the in vitro grown plants in vivo mother plant was quantified through High-Performance Liquid Chromatography (HPLC), and found that they do not differ significantly. Even all parts of the in vitro plants produce plumbagin, roots contain the maximum amount (14.67 ± 0.24 mg/g dry weight basis).
Keywords: Micropropagation, ISSR, SCoT, meta-topolin, Chromosome
Mankind recognized the immense value and effectiveness of the medicinal plant since the beginning of civilization. World Health Organization (WHO) claimed that nearly 88% of the total countries and 80% of the global population utilize traditional medicine, mainly plant-based, for their primary healthcare needs (WHO 2022). The immense usages of indigenous plant products primarily draw the attention of modern scientist to look for natural products more intensely. Plumbago zeylanica (chitrak), belonging to the family Plumbaginaceae commonly used as a traditional medicine since ancient times. It is widely distributed in tropical and subtropical regions of South East Asia, Africa and Australia. In Indian Ayurveda, the roots and other different parts of the plants are considered beneficial for enlarged liver and spleen, obesity, piles worms, dysentery, rheumatism, laryngitis, digestive disorder etc. (Shukla et al. 2021). Plumbagin is a bioactive compound mainly found in the roots of P. zeylanica also in other parts, has remarkable application as an antiproliferative agent in different cancer cell lines (Jayanthi et al. 2020). The anticancerous activities of plumbagin have been demonstrated in several different types of cancers, including breast cancer, ovarian cancer, prostate cancer, lung cancer, pancreatic cancer, and colon cancer (Roy 2021). Plumbagin suppresses various signal molecules such as NF-κB, AKT/mTOR, STAT3 and alters various signaling pathways crucial for cancer development (Tripathi et al. 2019). The remarkable application of plumbagin, owing to its pharmacological properties, increases the global market demand and requires a huge biomass of plants (Roy and Bharadvaja 2018a). In the current situation, the plant is unsystematically exploited from its wild habitat to meet the required biomass, which poses an inevitable threat to the very natural sources (Pant et al. 2012). Keeping that in mind, it is necessary to look for sustainable biomass production of the plant viz-a-viz nonconventional synthesis of plumbagin in order to deal with the current requirement of plumbagin while protecting the natural sources. In vitro biomass production through micropropagation is a reliable alternative for the sustainable production of natural products.
A certain number of research works on the mass propagation of P. zeylanica has been reported so far (Sahoo and Debata 1998; Rout et al. 1999, 2001; Selvakumar et al. 2001; Rout and Das 2002; Verma et al. 2002; Wei et al. 2006; Sivanesan and Jeong 2009; Idayat 2010; Patidar et al. 2013; Chandravanshi et al. 2014; Chatterjee and Ghosh 2015; Raja et al. 2018; Sharma and Agrawal 2018; Kakade et al. 2022). A wide range of primary cytokinin sources, like 6-Benzylaminopurine (BAP), Kinetin (KIN) etc. were reported to be efficient in the induction of multiple shoots in P. zeylanica. However, as a new alternative source of cytokinin, the efficiency of mT proved to be quite promising for an array of medicinal plants (Gantait and Mitra 2021). Yet, to date, the use of mT has not been reported in P. zeylanica in order to enhance the biomass production via multiple shoot initiation and proliferation.
Genetic homogeneity is a crucial parameter that must be considered in micropropagation, particularly when focusing on industrial applications (Chirumamilla et al. 2021). Polymerase chain reaction (PCR) based molecular markers like Inter Simple Sequence Repeats (ISSR) and Start Codon Targeted (SCoT) polymorphism are often used to determine the genetic fidelity of regenerated plants (Chirumamilla et al. 2021; Mahanta et al. 2023). The utility of ISSR in combination with SCoT marker systems for the assessment of genetic fidelity among the callus-derived regenerants along with doner plant of P. zeylanica was reported by Sharma and Agrawal (2018). Apart from this, there no report on genetic fidelity assessment on P. zeylanica, so far. Nonetheless, similar to the molecular marker, chromosome study is also severe as a potential maker in fidelity assessment of the regenerants (Subrahmanyeswari et al. 2022).
To the best of our knowledge, none of the previous studies comprehensively covered all the aspects like mT-mediated high-frequency regeneration, acclimatization, genetic fidelity assessment with cyto-genetic marker systems, and HPLC-based quantification of plumbagin from P. zeylanica, to date. Hence, based on this backdrop, the present study aims to achieve enhanced biomass production of P. zeylanica, simultaneously synthesizing its key secondary metabolites plumbagin, and ensuring genetic fidelity of the in vitro regenerants cytological and molecular marker-based approaches among the regenerants along with their mother plant.
Shoot tips from six-month-old P. zeylanica plants were collected in the college's medicinal plant garden. The shoot tips, with 2–3 auxiliary buds (3 cm long), were washed with tap water (20 min) and 2% nonionic surfactant (5 min). They were then treated with 2% Bavistin fungicide, washed, and disinfected with 0.1% HgCl2 (8 min). Finally, they were rinsed with sterile water to remove any residue.
The experiments were conducted using MS medium (Murashige and Skoog 1962) supplemented with 3% sucrose and 0.75% agar, with a pH of 5.7 and sterilized by autoclaving. The cultures were maintained in a room with a temperature of 24 ± 2 °C, 16 h photoperiod, and light intensity at 60 μM/m2/s. Shoot multiplication was tested using BAP, KIN, and mT cytokinins at four different concentrations (0.5–2.0 mg/l). Further, mT at 1.0 mg/l was combined with IAA, IBA, and NAA auxins at four different concentrations (0.05–0.50). MS basal medium without plant growth regulators was used as the control. Shoot induction percentage, number of shoots per explant, and shoot length were recorded after 4 weeks.
Shootlets (≧1.2 cm height) were transferred to rooting media using MS basal medium at full or half strength. Three different auxins (IAA, IBA, and NAA) at various concentrations (0.25–1.5 mg/l) were added to the best strength of MS medium. half strength MS medium were used as control and data on root initiation time, number of roots per explant, and root length (cm) were recorded after 3 weeks.
In vitro-rooted plantlets (of ≈ 50 d. old) were carefully washed and planted in Soilrite™ (≈200gm) in pots, covered with a plastic bag, and watered every 2 days. After 15 days, the plants were transferred to bigger earthen pots with field soil in a shade net house.
Plumbagin production was evaluated in in vivo and in vitro plants by drying, powdering, and performing ultrasound-assisted extraction to obtain plumbagin. The resulting extract was analyzed using HPLC (Waters 1525 binary HPLC pump and PDA detector) with a C-18 column and mobile phases of acetonitrile and water (65:35 ratio). Plumbagin was detected at 270 nm with a 20 µl sample injection volume and 15 min run time. Standard plumbagin was prepared separately, and the experiment was conducted with five replicates to ensure accuracy for both standards and each sample.
To assess the genetic fidelity, genomic DNA was extracted from in vivo mother plant and eight randomly selected in vitro regenerated P. zeylanica samples using the CTAB method. PCR was performed using 15 ISSR and 15 SCoT primers with a 50 μl reaction mixture containing 40 ng genomic DNA, 0.5 µM primer, 1X Taq buffer, 1 unit Taq DNA polymerase, and 10 mM dNTPs. Amplification was done using a Thermal Cycler, and the PCR cycles were standardized. Amplified products were separated by 1.2% agarose gel electrophoresis with 1X TAE buffer.
Chromosomes were prepared from root tips cells of in vivo and in vitro plants following Santra et al. (2020) to evaluate the genetic stability at chromosomal level of the regenerated plants.
All the in vitro regeneration experiments were performed thrice with 10 replicates, and the collected data were analyzed by one-way ANOVA using SPSS ver. 26. The significant difference between the sample means was estimated by Duncan's multiple range test (DMRT) at 5% level of significance (P ≤ 0.05) (Duncan 1955).
Direct shoot regeneration of P. zeylanica was achieved using shoot tip explants on MS medium supplemented with different cytokinins. Among the tested cytokinins, mT and BAP showed good responses for shoot bud initiation and proliferation over KIN (Fig. 1A). mT with a concentration of 1 mg/l showed the highest response rate and highest shoot bud induction after 14 d, with an average number of shoots and length of 9.45 ± 0.18 and 5.87 ± 0.15 cm, respectively, after 30 d (Table 1). The same concentration of BAP produced fewer shoots (7.83 ± 0.14) with an average length of 4.10 ± 0.29 cm and less total biomass. Increasing cytokinin concentration beyond optimum levels imposed a poor response of shoot multiplication. The outcomes of this study indicated that mT had greater shoot induction capacity than BAP and KIN in this plant species. The ratio of endogenous and exogenous growth hormones effectively regulates the rate of micropropagation and organ development (Nowakowska et al. 2022). Cytokinins have a significant structural variation and different bioactivities, and the optimal results of shoot multiplication rely on the type of cytokinins best suited for the particular plant species (Sakakibara 2006). Topolin is a new group of aromatic cytokinins, including mT, that becomes a suitable alternative to cytokinins such as BAP, zeatin, KIN, etc. (Turkyilmaz 2021). Cytokinins like BAP and KIN have previously been applied for direct shoot organogenesis in P. zeylanica (Verma et al. 2002; Rout and Das 2002; Patidar et al. 2013; Roy and Bharadvaja 2018b). In the present studies, the effect of mT on micropropagation was found to be superior over the others. To the best of our knowledge, the effect of mT has not been tested earlier in this species; however, similar to our result, the superior application of mT has been emphasized by several researchers with a number of different plants (Halder and Ghosh 2021; Pramanik et al. 2021). The application of mT in in vitro system is associated with better shoot proliferation, rooting, acclimatization, and effectively minimizing various physiological disorders (Aremu et al. 2012; Halder and Ghosh 2021).
Fig. 1.
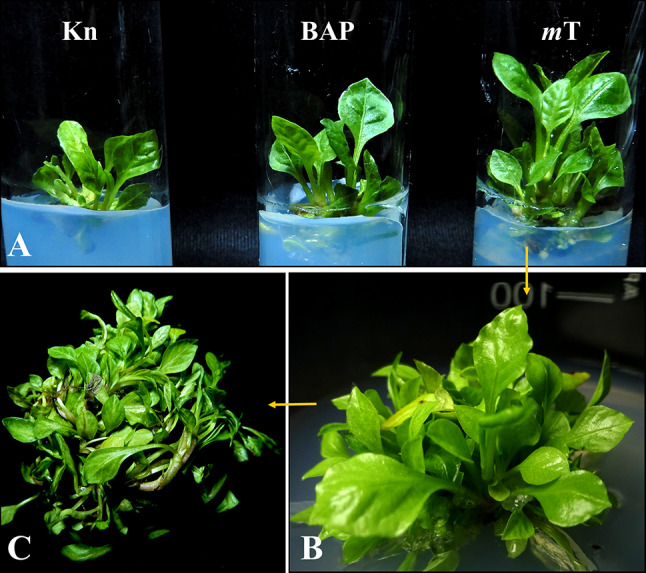
In vitro micropropagation of Plumbago zeylanica from shoot tip explants A In vitro shoot regeneration from shoot tip explants, B mT mediated high-frequency shoot regeneration, C Total number of regenerated shoots after 84 days from a single culture vessel
Table 1.
The influence of cytokinins on shoot multiplication from shoot tip explant of Plumbago zeylanica
| PGRs | Concentration of PGRs (mg/l) | Percentage of response | Mean number of shoot/explants | Mean length of shoot (cm) |
|---|---|---|---|---|
| Control | 0.0 | – | – | – |
| BAP | 0.5 | 84.12 ± 1.16e | 5.64 ± 0.18e | 3.76 ± 0.10e |
| 1.0 | 89.35 ± 0.98c | 7.83 ± 0.14c | 4.10 ± 0.29d | |
| 1.5 | 82.55 ± 1.60f | 4.11 ± 0.05h | 3.64 ± 0.24ef | |
| 2.0 | 67.32 ± 0.76k | 2.25 ± 0.05j | 2.77 ± 0.13h | |
| KIN | 0.5 | 72.66 ± 1.25j | 4.78 ± 0.07g | 3.23 ± 0.13g |
| 1.0 | 76.55 ± 1.16h | 5.81 ± 0.05e | 3.45 ± 0.15f | |
| 1.5 | 74.23 ± 0.92i | 5.16 ± 0.43f | 3.21 ± 0.15g | |
| 2.0 | 62.44 ± 0.69l | 3.30 ± 0.13i | 3.13 ± 0.11g | |
| mT | 0.5 | 93.65 ± 1.37b | 8.26 ± 0.20b | 5.21 ± 0.16b |
| 1.0 | 95.28 ± 1.39a | 9.45 ± 0.18a | 5.87 ± 0.15a | |
| 1.5 | 86.36 ± 0.93d | 7.94 ± 0.21c | 4.98 ± 0.16c | |
| 2.0 | 79.35 ± 1.06g | 6.89 ± 0.32d | 3.65 ± 0.09ef |
Each value represents the mean ± standard error of three replicates (10 samples score). Mean values followed by the same letters in each column are not significantly different (P ≤ 0.05) according to Duncan’s multiple range tests (Duncan 1955)
The combination of mT cytokinin with mild concentrations of IAA, IBA, or NAA auxins significantly increased shoot production in P. zeylanica. The best shoot production was observed when mT was combined with 0.25 mg/l IAA, resulting in 43.91% more shoots than mT alone. After 84 d of subculture, 129.8 ± 2.71 number of shoots with a fresh weight of 19.72 ± 0.65 g was obtained from a single culture bottle (Fig. 1B, C). The synergism of a high level of cytokinin and a low level of auxin potentially affects the regeneration in in vitro system (Fatima et al. 2011). The difference between the signaling pathways of auxin and cytokinin led to their interaction which may ultimately cause the superiority of the synergism (Gupta et al. 2020). Similar to the present studies, the enhancement of shoot regeneration has been observed in other plant species by combining various cytokinins and auxins (Shekhawat et al. 2021). It is also noted that the association between NAA and IAA was more influential than the IBA in effective shoot regeneration (Table 2). The effectiveness of IAA and NAA in combination with cytokinins has been demonstrated earlier on different plant species (Amoo and Van Staden 2013).
Table 2.
Synergistic effect of meta-topolin (mT) (1 mg/l) with different auxins on in vitro biomass production of Plumbago zeylanica
| PGRs (auxin) | Concentration of PGRs (mg/l) | Percentage of response | Mean number of shoot/explants | Mean length of shoot (cm) |
|---|---|---|---|---|
| IAA | 0.05 | 96.11 ± 0.83d | 9.40 ± 1.34bcd | 5.88 ± 0.23b |
| 0.10 | 97.27 ± 0.49bc | 10.40 ± 1.40b | 5.94 ± 0.41b | |
| 0.25 | 97.88 ± 0.50b | 13.60 ± 1.14a | 6.85 ± 0.36a | |
| 0.50 | 95.24 ± 0.36d | 8.40 ± 1.14cde | 5.15 ± 0.18c | |
| IBA | 0.05 | 95.43 ± 0.28d | 9.20 ± 0.84bcd | 5.33 ± 0.58c |
| 0.10 | 88.55 ± 1.04f | 8.40 ± 0.55cde | 4.26 ± 0.11d | |
| 0.25 | 87.29 ± 0.53g | 7.80 ± 0.84e | 3.85 ± 0.21d | |
| 0.50 | 80.19 ± 0.94h | 6.20 ± 0.83f | 3.19 ± 0.60e | |
| NAA | 0.05 | 96.67 ± 0.50cd | 9.80 ± 1.09b | 5.92 ± 0.35b |
| 0.10 | 98.76 ± 0.34a | 12.80 ± 0.83a | 6.06 ± 0.65b | |
| 0.25 | 95.31 ± 0.29d | 9.60 ± 0.54bc | 5.88 ± 0.257b | |
| 0.50 | 91.31 ± 0.90e | 8.20 ± 0.83de | 5.17 ± 0.20c |
Each value represents the mean ± standard error of three replicates (10 samples score). Mean values followed by the same letters in each column are not significantly different (P ≤ 0.05) according to Duncan’s multiple range tests (Duncan 1955)
In vitro rhizogenesis was achieved by placing regenerated micro shoots (3–5 cm) in half-strength MS medium supplemented with different auxins (IAA, IBA, NAA) at varying concentrations. Half-strength MS medium showed higher effectiveness for rhizogenesis compared to full strength, serving as the control. The best response frequency (90.21 ± 0.68) and number of roots (37.80 ± 0.84) were obtained with 1.0 mg/l IBA after 21 d (Fig. 2A–D). The average root length was 3.67 ± 0.14 cm, and the fresh weight reached 741.2 ± 2.31 mg (Table 3). Successfully rooted plants were acclimatized ex vitro (Fig. 2E, F), with a survival rate of 87.26%. Half strength of MS medium is often associated with better response in root induction than the full strength of MS medium reported in several plant species (Halder and Ghosh 2021). In agreement with the present finding, the eminence of IBA has also been reported for root induction in P. zeylanica earlier (Sivanesan and Jeong 2009; Selvakumar et al. 2001; Sharma and Agrawal 2018). To the best of our knowledge, the highest root production per micro shoot fortified with IBA was 37.80 ± 0.84, which is greater in numbers than any of the previous reports. This might be a consequence of the cytokinin used in shoot regeneration apart from auxins' effect.
Fig. 2.

Root induction and acclimatization of the regenerated micro shoots of Plumbago zeylanica A Induction of roots from the regenerated shoot in 1/2 MS medium with IBA 1 mg/l, B–D Growth and yield of roots from a regenerated shoot, E–F acclimatization of the regenerated plants
Table 3.
In vitro rooting of regenerated shoot tips after 21 days of culture of Plumbago zeylanica
| PGRs (auxin) | Concentration of PGRs (mg/l) | Percentage of response | Mean number of roots | Mean length of root (cm) |
|---|---|---|---|---|
| Control | 0.0 | 76.27 ± 1.21e | 5.17 ± 0.39i | 1.76 ± 0.12g |
| IAA | 0.25 | 79.43 ± 1.12d | 7.32 ± 0.13g | 1.82 ± 0.18g |
| 0.5 | 80.46 ± 0.62d | 8.96 ± 0.17f | 2.31 ± 0.24ef | |
| 1.0 | 72.61 ± 0.88f | 9.66 ± 0.11f | 2.54 ± 0.13de | |
| 1.5 | 58.14 ± 0.67j | 7.54 ± 0.16g | 1.45 ± 0.07h | |
| IBA | 0.25 | 81.57 ± 0.89c | 15.60 ± 0.55d | 2.31 ± 0.17ef |
| 0.5 | 86.73 ± 0.62b | 29.20 ± 1.09b | 3.15 ± 0.14b | |
| 1.0 | 90.21 ± 0.68a | 37.80 ± 0.84a | 3.67 ± 0.14a | |
| 1.5 | 64.51 ± 0.88i | 19.40 ± 1.14c | 2.94 ± 0.19bc | |
| NAA | 0.25 | 65.76 ± 0.48h | 7.31 ± 0.52g | 2.74 ± 0.24cd |
| 0.5 | 68.21 ± 1.06g | 11.76 ± 0.35e | 3.05 ± 0.11b | |
| 1.0 | 37.55 ± 0.75k | 9.43 ± 0.27f | 2.45 ± 0.20ef | |
| 1.5 | 21.25 ± 0.64l | 6.42 ± 0.11h | 2.23 ± 0.19f |
Each value represents the mean ± standard error of three replicates (10 samples score). Mean values followed by the same letters in each column are not significantly different (P ≤ 0.05) according to Duncan’s multiple range tests (Duncan 1955)
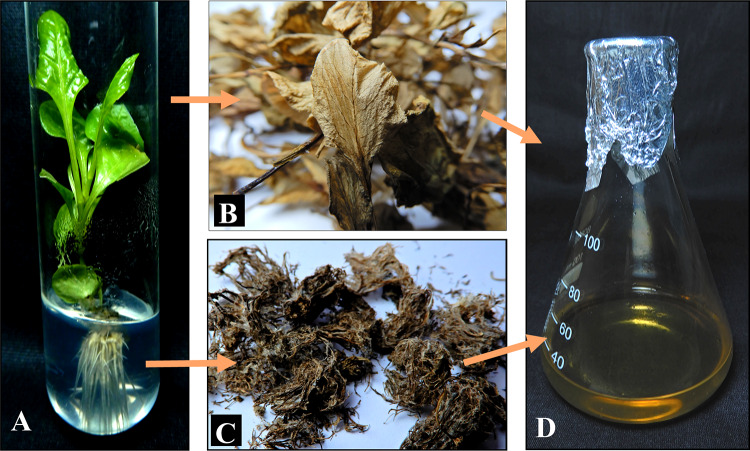
The quantitative analysis of plumbagin was carried out by HPLC from different parts of the plants viz. roots, stems and leaves (Fig. 3A–D). Though all of the regenerated biomass produces plumbagin but the root contains the maximum amount of plumbagin 14.67 ± 0.24 mg/g dw basis. The concentration of plumbagin in stem and leaves was 2.434 ± 0.19 and 2.198 ± 0.09 mg/g dw basis, respectively (Fig. 4A–D). The comparative plumbagin production for in vivo mother plants and randomly selected in vitro regenerated plants has been represented in Fig. 5. No significant variation of plumbagin content has been detected between the mother and regenerated plants; hence, the chemical fidelity of the regenerants based on the principal compound is justified. In accordance with our result plumbagin has also been obtain from this species in their natural habitat earlier (Bothiraja et al. 2011). However, the plumbagin was found mostly in the root but was also reported to be present in the leaves and stems earlier (Mallavadhani et al. 2002). Therefore, the whole regenerated biomass (root, stems and leaves) is a potential source of the plumbagin and can be considered an alternative approach for the production of plumbagin.
Fig. 3.
Extraction of plumbagin from in vitro regenerated plants of Plumbago zeylanica A Whole in vitro regenerated plant, B Dried aerial parts of regenerated plants, C Dried roots of the regenerated plants, D Extracted plumbagin from different parts of the regenerated plants
Fig. 4.
HPLC chromatogram of plumbagin from in vitro regenerated plants A standard plumbagin B roots, C stems, D leaves
Fig. 5.
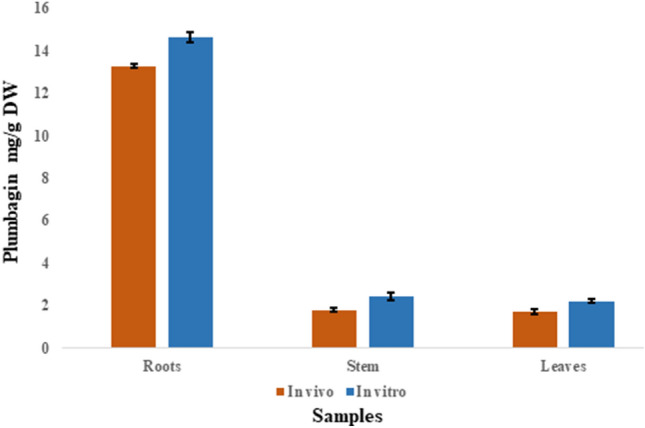
Plumbagin content from different parts of in vivo mother plant and in vitro regenerated plants of Plumbago zeylanica
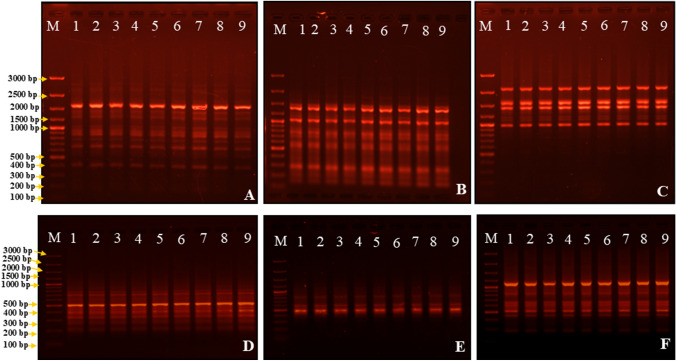
In the present study, the clonal fidelity of regenerated P. zeylanica plants was assessed using 15 ISSR and 15 ScoT markers (Table 4). These markers provide valuable insights into the genetic uniformity of the regenerants and their similarity to the mother plants. A total of 785 bands were scored from one mother plant and eight randomly selected regenerated plants using the 15 SCoT primers (Fig. 6A–C). The amplification by SCoT primers resulted in 100 loci ranging between 200 and 2500 bp. Similarly, the 15 ISSR primers produced 111 loci ranging between 200 and 2000 bp, and a total of 1107 bands were scored (Fig. 6D–F). Notably, all the bands amplified by both sets of primers were found to be monomorphic, indicating that no variation was detected among the regenerated plants. This result demonstrates the clonal uniformity of the regenerated P. zeylanica plants studied. The use of both ISSR and SCoT markers in this analysis offers several advantages. These markers have been extensively employed by other researchers to study clonal fidelity in different plant species, highlighting their reliability and accuracy. Additionally, ISSR markers target the region between microsatellites, while SCoT markers amplify highly conserved regions of functional genes. Combining the use of both markers provides a more comprehensive and precise assessment of genetic homogeneity in the regenerated plants.
Table 4.
Genetic fidelity assessment of in vitro regenerants and the mother plant of Plumbago zeylanica using ISSR and SCoT primers, their sequences with number and size range of amplicons
| Primer | Sequence (5'-3') | Tm (°C) | Number of amplified bands per primer | Total number of bands | Size of amplicon |
|---|---|---|---|---|---|
| ISSR primers | |||||
| UBC 808 | AGAGAGAGAGAGAGAGC | 48.8 | 4 | 36 | 300–1200 |
| UBC 809 | AGAGAGAGAGAGAGG | 57.5 | 7 | 63 | 500–2000 |
| UBC 825 | ACACACACACACACACT | 51.4 | 9 | 81 | 800–800 |
| UBC 840 | GAGAGAGAGAGAGAGAGACTT | 48.8 | 5 | 45 | 600–900 |
| UBC841 | GAGAGAGAGAGAGAGACTC | 51.9 | 9 | 81 | 500–2000 |
| UBC 848 | CACACACACACACACAAGG | 54.2 | 7 | 63 | 500–1000 |
| UBC 850 | GTGTGTGTGTGTGTGTCTC | 53.6 | 8 | 72 | 300–1000 |
| UBC 854 | TCTCTCTCTCTCTCTCAGG | 51.5 | 4 | 36 | 800–1700 |
| UBC 855 | ACACACACACACACACCTT | 54.6 | 10 | 90 | 200–1200 |
| UBC 856 | ACACACACACACACACTA | 51.0 | 8 | 72 | 800–1700 |
| UBC 861 | ACCACCACCACCACCACC | 60.0 | 7 | 63 | 300–1500 |
| UBC 880 | GGAGAGGAGAGGAGA | 47.9 | 6 | 54 | 500–2000 |
| UBC 888 | CGTAGTCGTCACACACACACACA | 59.1 | 8 | 72 | 400–1500 |
| UBC 889 | AGTCGTAGTACACACACACACAC | 56.6 | 10 | 90 | 200–1000 |
| UBC 890 | ACGACTACGGTGTGTGTTTGTGT | 58.8 | 9 | 81 | 500–1000 |
| SCoT primers | |||||
| SCoT-1 | CAACAATGGCTACCACCA | 52.6 | 7 | 63 | 500–2000 |
| SCoT-2 | CAACAATGGCTACCACCC | 53.6 | 8 | 72 | 200–1700 |
| SCoT-3 | CAACAATGGCTACCACCG | 53.9 | 5 | 45 | 500–2000 |
| SCoT-4 | CAACAATGGCTACCACCT | 52.3 | 5 | 45 | 500–2000 |
| SCoT-5 | CAACAATGGCTACCACGA | 52.6 | 3 | 27 | 1000–1500 |
| SCoT-6 | CAACAATGGCTACCACGC | 54.4 | 6 | 54 | 1000–2600 |
| SCoT-7 | CAACAATGGCTACCACGG | 53.9 | 6 | 54 | 300–2500 |
| SCoT-8 | CAACAATGGCTACCACGT | 52.9 | 2 | 18 | 200–500 |
| SCoT-9 | CAACAATGGCTACCAGCA | 52.9 | 3 | 27 | 300–1000 |
| SCoT-10 | CAACAATGGCTACCAGCC | 53.9 | 2 | 18 | 400–1000 |
| SCoT-11 | AAGCAATGGCTACCACCA | 54.4 | 8 | 72 | 200–2000 |
| SCoT-12 | ACGACATGGCGACCAACG | 58.4 | 7 | 56 | 400–2200 |
| SCoT-13 | ACGACATGGCGACCATCG | 58.0 | 5 | 45 | 400–2500 |
| SCoT-14 | ACGACATGGCGACCACGC | 61.3 | 12 | 108 | 200–1000 |
| SCoT-15 | ACGACATGGCGACCGCGA | 62.6 | 9 | 81 | 300–1500 |
Fig. 6.
Genetic fidelity of regenerated Plumbago zeylanica and their mother plant based on SCoT and ISSR primers A SCoT 12, B SCoT 2, C SCoT 6, D ISSR-UBC 825, E ISSR-UBC 840, F ISSR-UBC 850
The cytological analysis is one of the crucial parts of confirming genetic fidelity at the chromosomal level. The limitation of the molecular markers is that they cannot detect numerical chromosomal aberrations. Eventually, variation may occur in chromosome number within the regenerated plants (Lee and Phillips 1988). In the present study, the mother plant and five randomly selected regenerated plants have the chromosome number 2n = 28 in their somatic cells (Fig. 7A, B). The result described above confirms the chromosomes of the regenerants are numerically stable, and no ploidy change has been detected during this study. The chromosome counts have been taken as a parameter for assessing genetic fidelity in different plants (Halder and Ghosh 2021; Subrahmanyeswari et al. 2022). Similar to our results, chromosome count has been recorded in P. zeylanica earlier but not applied as a part of genetic fidelity assessment before (Khatun et al. 2019). The EMA-based chromosome preparation allows cytoplasm-free chromosomal spread, making it easier to observe the chromosomes more prominently; hence, it is beneficial for those with dense cytoplasm content.
Fig. 7.
Numerical stability of chromosome showing 2n = 28 of regenerated plants along with mother plant of Plumbago zeylanica A in vivo mother plant, B in vitro regenerated plant
Meta-topolin is a unique cytokinin applied for the first time in P. zeylanica to improve in vitro regeneration. The increased micropropagation with enhanced qualities of the regenerants manifests the excellence of mT over other BAP and KIN in the present study. Production of genetically homogeneous plants is one of the primary objectives for in vitro clonal propagation. Molecular primer-based genetic fidelity assessment shows the regenerated plants were genetically similar to the mother plants. Two different primers were employed to amplify different selected regions of DNA to ensure more promising data. In addition, the regenerated plants have the same chromosome number as the mother plants conferring their stable ploidy. The plumbagin content of the mother plant and regenerated plants remain the same; hence, the regenerants retain their medicinal efficacy. All of the plant parts can produce plumbagin though the amount is much greater in the roots. The optimized protocols for mass propagation with mT in this study can be a sustainable option for the production of plumbagin.
Acknowledgements
IS acknowledge the Government of West Bengal for providing Swami Vivekananda Merit-cum-Means Scholarship. Authors are thankful to Swami Kamalasthananda, Principal, Ramakrishna Mission Vivekananda Centenary College, Rahara, Kolkata (India), for the facilities provided for the present study. Also acknowledge DST-FIST programme for instrumental facilities.
Author contributions
IS did all the experimental work and prepared the manuscript. BG was involved in result interpretation and made necessary correction in the write up. Final approval of the article was done by BG. All authors read and approved the final manuscript.
Data availability
The dataset generated during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Indranil Santra, Email: nil.indra@yahoo.in.
Biswajit Ghosh, Email: ghosh_b2000@yahoo.co.in.
References
- Amoo SO, Van Staden J. Influence of plant growth regulators on shoot proliferation and secondary metabolite production in micropropagated Huernia hystrix. Plant Cell Tissue Organ Cult. 2013;112:249–256. doi: 10.1007/s11240-012-0230-x. [DOI] [Google Scholar]
- Aremu AO, Bairu MW, Doležal K, Finnie JF, Van Staden J. Topolins: a panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012;108:1–16. doi: 10.1007/s11240-011-0007-7. [DOI] [Google Scholar]
- Bothiraja C, Joshi PP, Dama GY, Pawar AP. Rapid method for isolation of plumbagin, an alternative medicine from roots of Plumbago zeylanica. Eur J Integr Med. 2011;3:39–42. doi: 10.1016/j.eujim.2011.02.008. [DOI] [Google Scholar]
- Chandravanshi M, Sahu Y, Agrawal A, Raja W. In vitro micropropagation of important commorcial medicinal plant: Plumbago zeylanica. Adv Biol Res. 2014;8:139–142. [Google Scholar]
- Chatterjee T, Ghosh B. Simple protocol for micropropagation and in vitro conservation of Plumbago zeylanica L: an important indigenous medicinal plant. Int J Bio-Resour Stress Manag. 2015;6:068–075. doi: 10.5958/0976-4038.2015.00012.3. [DOI] [Google Scholar]
- Chirumamilla P, Gopu C, Jogam P, Taduri S. Highly efficient rapid micropropagation and assessment of genetic fidelity of regenerants by ISSR and SCoT markers of Solanum khasianum clarke. Plant Cell Tissue Organ Cult. 2021;144:397–407. doi: 10.1007/s11240-020-01964-6. [DOI] [Google Scholar]
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Fatima N, Ahmad N, Anis M. Enhanced in vitro regeneration and change in photosynthetic pigments, biomass and proline content in Withania somnifera L. (Dunal) induced by copper and zinc ions. Plant Physiol Biochem. 2011;49:1465–2147. doi: 10.1016/j.plaphy.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Gantait S, Mitra M. Role of meta-topolin on in vitro shoot regeneration: an insight. In: Ahmad N, Strnad M, editors. Meta-topolin: a growth regulator for plant biotechnology and agriculture. Singapore: Springer; 2021. pp. 143–168. [Google Scholar]
- Gupta S, Kachhwaha S, Kothari SL, Jain R. Synergistic effect of cytokinins and auxins enables mass clonal multiplication of drumstick tree (Moringa oleifera Lam.): a wonder. In Vitro Cell Dev Biol Plant. 2020;56:458–469. doi: 10.1007/s11627-020-10065-0. [DOI] [Google Scholar]
- Halder T, Ghosh B. Cytological, genetical and phytochemically stable meta-Topolin (mT)-induced mass propagation of underutilized Physalis minima L. for production of withaferin A. Biocatal Agric Biotechnol. 2021;33:1012. doi: 10.1016/j.bcab.2021.102012. [DOI] [Google Scholar]
- Idayat TG. Micropropagation of Plumbago zeylanica L. (Plumbaginaceae) in Ibadan, Southwestern. Nigeria J Med Plant Res. 2010;4:293–297. [Google Scholar]
- Jayanthi M, Gokulanathan A, Haribalan P, Ashakiran K, Kumar CD, Kamla D, Renukadevi BS. Plumbagin from two Plumbago species inhibits the growth of stomach and breast cancer cell lines. Ind Crops Prod. 2020;146:112147. doi: 10.1016/j.indcrop.2020.112147. [DOI] [Google Scholar]
- Kakade PS, Zimare SB, Malpathak NP. Effects of Sargassum ilicifolium seaweed extract on enhanced in vitro seed germination, mass propagation, and accumulation of plumbagin in Plumbago zeylanica L. Plant Cell Tissue Organ Cult. 2022;149:399–410. doi: 10.1007/s11240-022-02242-3. [DOI] [Google Scholar]
- Khatun R, Dash CK, Alam SS, Sultana SS. Chromosomal Characterization in Plumbago zeylanica and P. indica from Bangladesh. Cytologia. 2019;84:153–155. doi: 10.1508/cytologia.84.153. [DOI] [Google Scholar]
- Lee M, Phillips RL. The chromosomal basis of somaclonal variation. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:413–437. doi: 10.1146/annurev.pp.39.060188.002213. [DOI] [Google Scholar]
- Mahanta M, Gantait S, Mukherjee E, Bhattacharyya S. meta-Topolin-induced mass propagation, acclimatization and cyto-genetic fidelity assessment of gerbera (Gerbera jamesonii Bolus ex Hooker f.) S Afr J Bot. 2023;153:236–245. doi: 10.1016/j.sajb.2022.11.032. [DOI] [Google Scholar]
- Mallavadhani UV, Sahu G, Muralidhar J. Screening of Plumbago species for the bio-active marker plumbagin. Pharm Biol. 2002;40(7):508–511. doi: 10.1076/phbi.40.7.508.14685. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nowakowska K, Pińkowska A, Siedlecka E, Pacholczak A. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron' Kazimierz Odnowiciel'in the in vitro cultures. Plant Cell Tissue Organ Cult. 2022;149:675–684. doi: 10.1007/s11240-021-02206-z. [DOI] [Google Scholar]
- Pant M, Lal A, Rana S, Rani A. Plumbago zeylanica L.: a mini review. Int J Pharm Appl. 2012;3:399–405. [Google Scholar]
- Patidar SL, Tripathi MK, Tiwari G, Chundawat RS, Pandey A, Patidar H, Pandey G. In vitro micropropagation of Plumbago zeylanica Linn. through nodal segment and leaf explants. Plant Cell Biotech Mol Biol. 2013;14:72–83. [Google Scholar]
- Pramanik B, Sarkar S, Bhattacharyya S, Gantait S. meta-Topolin-induced enhanced biomass production via direct and indirect regeneration, synthetic seed production, and genetic fidelity assessment of Bacopa monnieri (L.) Pennell, a memory-booster plant. Acta Physiol Plant. 2021;43:1–14. doi: 10.1007/s11738-021-03279-1. [DOI] [Google Scholar]
- Raja HD, Jenifer AM, Steffi PF, Thamilmaraiselvi B, Srinivasan P, Tamilvanan R. Micropropagation of Plumbago zeylanica–an important medicinal plant. World J Pharm Pharm Sci. 2018;7:1823–1829. [Google Scholar]
- Rout GR, Das G. An assessment of genetic integrity of micropropagated plants of Plumbago zeylanica by RAPD markers. Biol Plant. 2002;45:27–32. doi: 10.1023/A:1015186709691. [DOI] [Google Scholar]
- Rout GR, Saxena C, Das P, Samantaray S. Rapid clonal propagation of Plumbago zeylanica Linn. Plant Growth Regul. 1999;28:1–4. doi: 10.1023/A:1006146713143. [DOI] [Google Scholar]
- Rout GR, Das G, Samantaray S, Das P. Micropropagation of Plumbago zeylanica L. by encapsulated nodal explants. J Hortic Sci Biotechnol. 2001;76:24–29. doi: 10.1080/14620316.2001.11511321. [DOI] [Google Scholar]
- Roy A. Plumbagin: a potential anti-cancer compound. Mini Rev Med Chem. 2021;21:731–737. doi: 10.2174/1389557520666201116144421. [DOI] [PubMed] [Google Scholar]
- Roy A, Bharadvaja N. Biotechnological approaches for the production of pharmaceutically important compound: plumbagin. Curr Pharm Biotechnol. 2018;19:372–381. doi: 10.2174/1389201019666180629143842. [DOI] [PubMed] [Google Scholar]
- Roy A, Bharadvaja N. Effect of various culture conditions on shoot multiplication and GC–MS analysis of Plumbago zeylanica accessions for plumbagin production. Acta Physiol Plant. 2018;40:1–11. doi: 10.1007/s11738-018-2766-9. [DOI] [Google Scholar]
- Sahoo S, Debata BK. Micropropagation of Plumbago zeylanica Linn. J Herbs Spices Med Plants. 1998;5:87–93. doi: 10.1300/J044v05n04_10. [DOI] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Santra I, Haque SM, Ghosh B. Giemsa C-banding karyotype and detection of polymorphic constitutive heterochromatin in Nigella sativa L. Cytologia. 2020;85:85–90. doi: 10.1508/cytologia.85.85. [DOI] [Google Scholar]
- Selvakumar V, Anbudurai PR, Balakumar T. In vitro propagation of the medicinal plant Plumbago zeylanica L. through nodal explants. In Vitro Cell Dev Biol Plant. 2001;37:280–284. doi: 10.1007/s11627-001-0050-x. [DOI] [Google Scholar]
- Sharma U, Agrawal V. In vitro shoot regeneration and enhanced synthesis of plumbagin in root callus of Plumbago zeylanica L.: an important medicinal herb. In Vitro Cell Dev Biol Plant. 2018;54:423–435. doi: 10.1007/s11627-018-9889-y. [DOI] [Google Scholar]
- Shekhawat JK, Rai MK, Shekhawat NS, Kataria V. Synergism of m-topolin with auxin and cytokinin enhanced micropropagation of Maytenus emarginata. In Vitro Cell Dev Biol Plant. 2021;57:418–426. doi: 10.1007/s11627-020-10132-6. [DOI] [Google Scholar]
- Shukla B, Saxena S, Usmani S, Kushwaha P. Phytochemistry and pharmacological studies of Plumbago zeylanica L.: a medicinal plant review. Clin Phytoscience. 2021;7:1–11. doi: 10.1186/s40816-021-00271-7. [DOI] [Google Scholar]
- Sivanesan I, Jeong B. Micropropagation of Plumbago zeylanica L. Afr J Biotechnol. 2009;8:3761–3768. [Google Scholar]
- Subrahmanyeswari T, Gantait S, Sarkar S, Bhattacharyya S. Accelerated mono-phasic in vitro mass production of banana propagules and their morpho-cyto-genetic stability assessment. S Afr J Bot. 2022;146:794–806. doi: 10.1016/j.sajb.2022.02.011. [DOI] [Google Scholar]
- Tripathi SK, Panda M, Biswal BK. Emerging role of plumbagin: cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem Toxicol. 2019;125:566–582. doi: 10.1016/j.fct.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz BU. The use of meta-topolin in cell and tissue cultures for increasing production of secondary metabolites. In: Ahmad N, Strnad M, editors. Meta-topolin: a growth regulator for plant biotechnology and agriculture. Singapore: Springer; 2021. pp. 253–263. [Google Scholar]
- Verma PC, Singh D, ur Rahman L, Gupta MM, Banerjee S. In vitro-studies in Plumbago zeylanica: rapid micropropagation and establishment of higher plumbagin yielding hairy root cultures. J Plant Physiol. 2002;159:547–552. doi: 10.1078/0176-1617-00518. [DOI] [Google Scholar]
- Wei X, Gou X, Yuan T, Russell SD. A highly efficient in vitro plant regeneration system and Agrobacterium-mediated transformation in Plumbago zeylanica. Plant Cell Rep. 2006;25:513–521. doi: 10.1007/s00299-006-0114-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2022) WHO establishes the Global Centre for Traditional Medicine in India. https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india. Accessed 13 Dec 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during the current study are available from the corresponding author on reasonable request.