Abstract
Purpose
This study aimed to determine whether performing bedside ultrasound impacts the occurrence of acute kidney injury (AKI) in the immediate postoperative period (POP) of high-risk surgery patients.
Methods
POP patients were randomly assigned to two groups: (i) ultrasound (US) group, in which hemodynamic management was guided with clinical parameters supplemented with the bedside US findings; (ii) control group, hemodynamic management based solely on clinical parameters. Two exams were performed in the first 24 h of admission.
Results
Fifty-one patients were randomized to the US group and 60 to the control group. There was no significant difference for incidence of AKI in both groups assessed 12 h (31.4% vs 35.0%, P = 0.84), 24 h (27.5% vs 23.3%, P = 0.66), or 7 days (17.6 vs 8.3%, P = 0.16) after surgery. No difference was found in the amounts of volume administered over the first 12 h (1000 [500–2000] vs. 1000 [500–1500], P = 0.72) and 24 h (1000 [0–1500] vs. 1000 [0–1500], P = 0.95) between the groups. Patients without AKI in the control group received higher amounts of volume during the ICU stay.
Conclusion
The use of bedside US in the immediate postoperative period of high-risk surgery did not show benefits in reducing AKI incidence.
Keywords: Ultrasound, Acute kidney injury, Postoperative period, Intensive care unit
Introduction
Over 310 million surgeries are performed annually worldwide [1]. The incidence of acute kidney injury (AKI) in the postoperative period of major noncardiac surgeries varies in the different studied series, ranging from 1 [2] to 18% [3]. One of this variability occurred mainly because of the different criteria used to define AKI and the types of surgery included in the studies. The development of AKI in the postoperative period (POP) is associated with higher mortality rates [4]. Even the initial KDIGO stage 1 after major surgeries has a higher risk of presenting a new episode of AKI within 1–2 years [5].
Hypovolemia during the perioperative period is one of the main mechanisms of AKI. To restore hemodynamics in the perioperative period, physicians often use volume resuscitation. This intervention aims to optimize oxygen delivery improve kidney perfusion, and eventually decrease AKI incidence [6]. Bedside ultrasound is a non-invasive tool that is quickly available, with no radiation emission, which has proven valuable in the monitoring volume status in different scenarios [7]. More specifically, lung ultrasound (LUS) has helped manage patients in the intensive care unit (ICU) through the assessment of the artifacts generated by the amount of fluid in the lungs, guiding the practice of fluid management [8]. As a tool to guide fluid and vasopressors management in high-risk surgical patients, the use of bedside ultrasound in POP has not been tested in clinical trials.
In this study, we sought to determine whether performing bedside ultrasound to guide hemodynamic management in the POP reduces AKI incidence in high-risk surgery patients.
Materials and methods
Study design and population
We carried out an open-labeled, single-center, randomized clinical trial in the ICU at Hospital das Clínicas da Universidade Federal de Minas Gerais, from February 2018 to March 2019 [9]. The study was registered at ClinicalTrials.gov with ID number NCT03509935 and was approved by the local Research Ethics Committee. All the patients included in the study or their next-of-kin signed the consent form. We included adult (≥ 18 years old) POP patients of high-risk surgeries, with at least one of the following criteria: use of vasoactive or inotropic drugs; mean blood pressure < 65 mmHg or systolic blood pressure < 90 mmHg; hyperlactatemia > 2 mmol/L; heart rate > 90 bpm; invasive mechanical ventilation requirement for at least 6 h on inclusion; hypoxia; length of surgery greater than 4 h; blood products transfusion requirement during the procedure; and oliguria.
The non-inclusion criteria were as follows: being in a previous renal replacement therapy program, moribund patients (i.e., death estimated to the next 24 h), and logistical impossibility to perform the first ultrasound assessment within 12 h following ICU admission.
After inclusion, the patients were randomly assigned to one of two groups: (i) US group: bedside ultrasound and clinical parameters guided the hemodynamic management; (ii) Control group: only clinical parameters guided the hemodynamic management without the bedside ultrasound exam. Randomization was performed individually at an allocation rate of 1:1, using a computer-generated random number table that was sequenced in enumerated and sealed brown envelopes. The random allocation sequence was generated and supervised by a researcher not involved with the inclusion, follow-up, or analysis of the data. Patients were screened, randomized, and assigned to the groups by the principal investigator and assistants. Due to the nature of the intervention, the investigators and the assistant physicians were aware of the group in which the patients had been included. Preparation and conduction of the study followed the recommendations of the CONSORT Statement [10]. Ultrasound evaluation performed by the hospital imaging service was allowed, and the decision about its requirement was at the discretion of the assistant physician.
Bedside ultrasound protocol
Patients allocated to the US group underwent two sequential ultrasound assessments: the first exam was performed in the first 12 h of ICU admission. The second exam was performed between 12 and 24 h after the first one. Four trained physicians (CGR, TBLSA, RDB, CCM) with vast experience in ultrasound exams carried out all ultrasound assessments. All of them are certified in ultrasound point of care at the ICU. The agreement between examiners was assessed in a previous study conducted by the same group, with the Kappa correlation coefficient of 0.799 (P < 0.001) [11].
For the ultrasound assessments, we used the following protocol:
Lung ultrasound—4 areas in each hemithorax: anterior (2° intercostal space—ICS—at a midclavicular line), lateral (anterior axillary line at 5° ICS), posterior region (posterolateral alveolar and/or pleural syndrome—PLAPS), and costophrenic region (supplementary material 1) [12].
Inferior vena cava (IVC) ultrasound: collapsibility or distensibility index according to the patient’s condition, in spontaneous or controlled ventilation, respectively. Patients with IVC collapsibility index > 40% and distensibility index > 12% were considered as fluid responders.
Cardiac ultrasound: subjective assessment of the left ventricular contractility in normal, moderately reduced or severely reduced.
Patients were placed in the semi-recumbent position (between 30° and 45°). The background light was dimmed to enhance the quality of the obtained images.
Terason 3000 ultrasound (Burlington, MA, USA) was used with the sectorial transducer, 10–15 cm deep. For better visualization of the pleura, a linear probe was used whenever required.
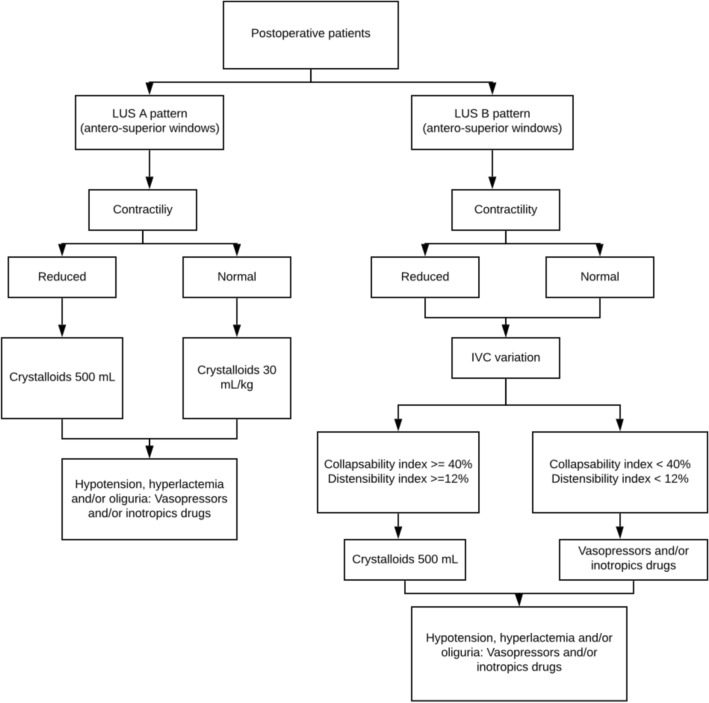
After each exam, the ultrasound findings were informed to the assistance physicians, with the recommendations for the fluids administration or fluids withdrawal, administration of vasoactive or inotropic drugs. These recommendations were pre-specified according to a decision flow chart defined in the study protocol (Fig. 1).
Fig. 1.
Decision making flowchart in the ultrasound group
In both study groups, patient management was based on conventional parameters used in the ICU, aiming to preserve adequate organ functioning, with a goal of mean blood pressure ≥ 65 mmHg or systolic pressure ≥ 90 mmHg and diuresis ≥ 0.5 mL/kg/h. Volume expansion was predominantly performed with crystalloid fluids (Normal saline or Ringer’s lactate). Patients remaining hypotensive despite volume replacement received vasopressors (noradrenaline) and/or inotropic drugs (dobutamine) at the discretion of the treating physician. In all the patients included in the study, organ perfusion was monitored by laboratory variables (e.g., arterial lactate and central venous saturation) as well as by dynamic clinical variables, such as pulse pressure variation when indicated. The hemoglobin goal was ≥ 7 g/dL. Ventilatory support, either invasive or not, was offered as needed. In patients under invasive mechanical ventilation, protective ventilation parameters were used, namely: plateau pressure < 30 cmH20, tidal volume ranging from 4 to 8 mL/kg, and driving pressure < 15 cmH20.
Studied variables
Patients’ information on demographics, clinical, and laboratory data was collected. The prevalence of comorbidities such as chronic obstructive pulmonary disease, asthma, systemic arterial hypertension, diabetes mellitus, coronary artery disease, obesity, solid organ neoplasm, hematological neoplasm, and cirrhosis was registered. Patients' surgical risk was gauged using the American Society of Anesthesiology (ASA) score [13]. Surgical procedures were further stratified into elective or emergency. Finally, we calculated the APACHE II [14], SOFA [15], and SAPS 3 [16] scores for all patients included.
Outcomes
The primary outcome was the prevalence of AKI, as defined by the KDIGO classification, measured during the first seventh day of ICU admission. The reference basal creatinine was considered the most recent result within the seven days previous to the surgery [17]. The sample size was calculated considering a 20% prevalence of postoperative AKI among patients undergoing major surgeries [3, 18]. It would be necessary 89 patients per group in a 1:1 allocation scheme to obtain an absolute reduction in 10% of the incidence of AKI. The significance level was set at p < 0.05 with a predicted power of 80% (GPower 3.1®).
The secondary outcomes were defined as the length of ICU (in hours), length of hospital stay (in days), ICU and 28-day mortality rates, length of invasive mechanical ventilation, total volume replacement within the first 36 h of ICU admission, use of vasoactive drugs during ICU stay.
Statistical analysis
Qualitative variables were compared with Chi-square tests or Fisher’s exact test as recommended. Quantitative variables were compared by the Mann–Whitney’s U test, according to its non-normal distribution. The p value with statistical significance was 0.05, bicaudal. SPSS 22.0 (SPSS, Inc, Chicago, IL) program was used for statistical analysis.
Primary and secondary outcomes were analyzed according to the intention to treat (ITT) approach.
Results
During the studied period, 282 patients were evaluated for potential eligibility. From these, 168 were not included for the reasons shown in Fig. 2. The main reason for not inclusion was a screening process exceeding 12 h (i.e., the first ultrasound assessment would be performed more than 12 h after ICU admission), which occurred in 147 cases. Thus, 111 were randomized, followed the study protocol and were included in the intention to treat (ITT) analysis.
Fig. 2.
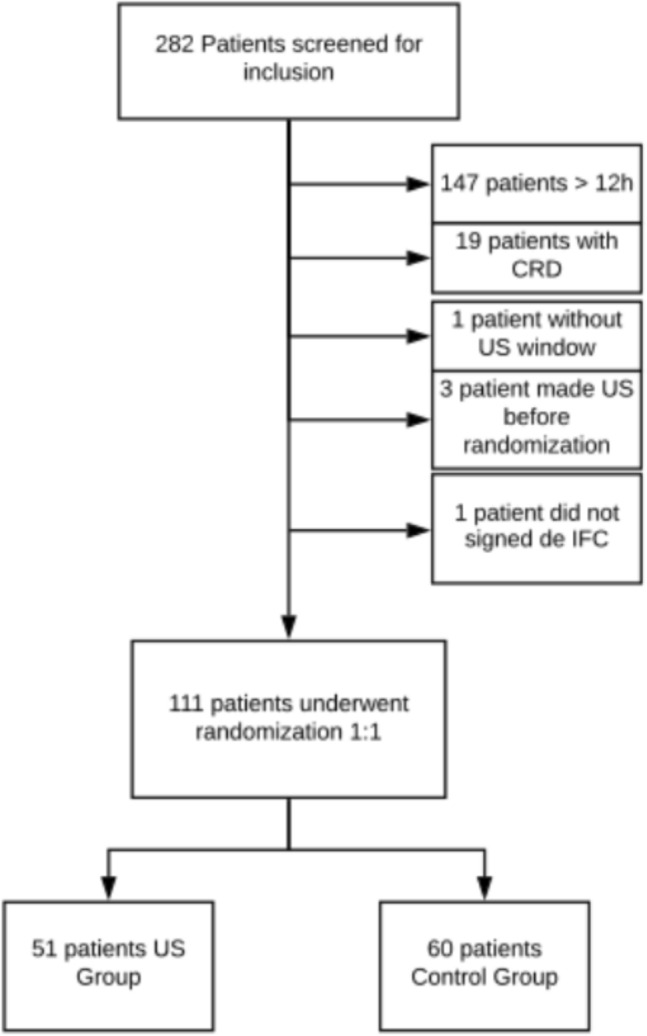
Flowchart of eligible patients. CRD chronic renal disease, ICF informed consent form
The main causes of ICU admission were digestive tract surgeries (31.5%) followed by neurosurgeries (28.8%) and liver and biliary tract surgeries (15.3%), with no difference between control and US groups. A higher frequency of noradrenaline use during surgery was observed in the control group compared to the US group (48.3% vs. 29.4%, P = 0.03). The median (Q1–Q3) age of all the patients included was 56 years (41–68). There was no difference between the groups regarding the severity scores, including SOFA on admission. The mains patients’ characteristics are shown in Table 1.
Table 1.
Patient’s characteristics in ultrasound and control groups
| All (111) | US (51) | No US (60) | P value | |
|---|---|---|---|---|
| Male sex | 53 (47.7) | 25 (49) | 28 (46.7) | 0.85 |
| Age | 56 (41–68) | 56 (41–68) | 56 (41–68) | 0.67 |
| APACHE II score | 10 (7–12) | 10 (7–11) | 11 (7–13) | 0.3 |
| SOFA D0 | 4 (1–6) | 2 (1–5) | 4 (1–6) | 0.1 |
| SOFA 12 h | 3 (1–5) | 2 (0–6) | 3 (1–5) | 0.28 |
| Type of surgery | 0.71 | |||
| Digestive system | 35 (31.5) | 16 (31.4) | 19 (31.7) | |
| NC | 32 (28.8) | 17 (33.3) | 15 (25) | |
| Hepatic and biliary tract | 17 (15.3) | 9 (17.7) | 8 (13.4) | |
| Others | 27 (24.3) | 9 (17.7) | 18 (30) | |
| Electives | 94 (84.7) | 42 (82.4) | 52 (86.7) | 0.6 |
| ASA score | 0.95 | |||
| ASA 1 | 17 (15.3) | 8 (16.7) | 9 (15.3) | |
| ASA 2 | 55 (49.5) | 25 (52.1) | 30 (50.8) | |
| ASA 3 | 18 (16.2) | 7 (14.6) | 11 (18.6) | |
| ASA 4 | 17 (15,3) | 8 (16,7) | 9 (15,3) | |
| Hypertension | 47 (42.3) | 23 (45.1) | 24 (40) | 0.7 |
| Diabetes | 17 (15.3) | 7 (13.7) | 10 (16.7) | 0.79 |
| Solid neoplasm | 65 (58.6) | 29 (56.9) | 36 (60) | 0.84 |
| Preop Creatinine | 0.81 (0.63–1.0) | 0.78 (0.58–0.95) | 0.83 (0.66–1) | 0.19 |
| Surgery duration, h | 6:40 (5–8) | 6:55 (5:00–8:11) | 6:15 (5 -8) | 0.70 |
| NaCl_surgery, mL | 1500 (500–2,500) | 1500 (500–2500) | 1500 (500–2500) | 0.89 |
| RL_ surgery, mL | 1500 (0–2500) | 1500 (0–2000) | 1500 (125–2500) | 0.51 |
| Crystalloid_surgery, mL | 3000 (2000–4000) | 3000 (2000–4500) | 3500 (2100–4000) | 0.49 |
| Noradrenaline_surgery | 44 (39.6) | 15 (29.4) | 29 (48.3) | 0.03 |
| Hemotransfusion _surgery | 23 (20.7) | 9 (17.6) | 14 (23.3) | 0.46 |
| IMV_adm | 52 (46.8) | 21 (41.2) | 31 (51.7) | 0.34 |
Data are presented as n (%) or median (Q1–Q3)
APACHE II Acute Physiology and Chronic Health Evaluation II, SOFA Sequential organ failure assessment, ASA American society of anesthesiologists, RL Ringer’s Lactate, ICU intensive care unit, IMV invasive mechanical ventilation, LOS length of stay
Outcomes
The frequency of AKI after surgery—KDIGO 1, 2, or 3—assessed at 12 h (31.4% vs. 35%, P = 0.84), 24 h (27.5% vs. 23.3%, P = 0.66), and 7 days (17.6 vs. 8.3%, P = 0.16) did not show a statistical difference between patients allocated to the US and the control group, respectively.
Similarly, the median of fluid balance measured at 12 h (1240 [473–2400] vs. 1635 [570–2387], P = 0.6), at 24 h (1478 [− 30–2951] vs. 1500 [674–3186]), and accumulated over the first 36 h of ICU stay (3320 [196–5330] vs. 3217 [2077–4137], P = 0.91) was not different among the US and control group, respectively. In addition, there were no statistical differences for the amount of volume administered over the first 12 h (1000 [500–2000] vs. 1000 [500–1500], P = 0.72) and 24 h (1000 [0–1500] vs. 1000 [0–1500], P = 0.95) between these groups (Table 2).
Table 2.
Interventions during ICU stay
| All (111) | US(51) | No US (60) | P-value | |
|---|---|---|---|---|
| Fluid balance-12 h, mL | 1495 (560–2400) | 1240 (473–2400) | 1635 (570–2387) | 0.6 |
| Crystalloids_12h, mL | 1000 (500–2000) | 1000(500–2000) | 1000 (500 -1500) | 0.72 |
| Positive Fluid balance_12h | 99 (89.2) | 45 (88.2) | 54 (90) | 0.77 |
| Blood products transfusion_12h | 8 (7.2) | 5 (9.8) | 3 (5) | 0.46 |
| Fluid balance_24h, mLa | 1489 (363–3031) | 1478 (-30–2951) | 1500 (674–3186) | 0.56 |
| Crystalloids_24ha | 1000 (0–1500) | 1000 (0–1500) | 1000 (0–1500) | 0.95 |
| Crystalloids_36ha | 2000 (1000–2625) | 2000 (1500–3500) | 2000 (1500–3500) | 0.51 |
| ICU Fluid balance, mLa | 3234 (1826–4783) | 3320 (196–5330) | 3217 (2077–4137) | 0.91 |
| Noradrenaline_ICU | 60 (54.1) | 25 (49) | 35 (58.3) | 0.34 |
| Hemodialysis | 8 (7.2) | 5 (9.8) | 3 (5) | 0.46 |
| ICU LOS, hs | 40 (20–88) | 40 (20–88) | 42 (24–96) | 0.67 |
| Hospital LOS, days | 19 (9–36) | 18 (10–39) | 20 (9–36) | 0.76 |
| ICU mortality | 8 (7.2) | 3 (5.9) | 5 (8.3) | 0.72 |
| 28 days mortality | 11 (12.6) | 6 (14.3) | 5 (11.1) | 0.75 |
| KDIGO 1_12h | 23 (20,7) | 7 (13.7) | 16 (26.7) | 0.1 |
| KDIGO 2_12h | 11 (9.9) | 6 (11.8) | 5 (8.3) | 0.75 |
| KDIGO 3_12h | 4 (3.6) | 3 (5.9) | 1 (1.7) | 0.33 |
| KDIGO_Pos_12h | 37 (33.3) | 16 (31.4) | 21(35) | 0.84 |
| KDIGO 1_24h | 8 (7.2) | 3 (5.9) | 5 (8.3) | 0.72 |
| KDIGO 2_24h | 11 (9.9) | 6 (11.8) | 5 (8.3) | 0.75 |
| KDIGO 3_24h | 9 (8.1) | 5 (9.8) | 4 (6.7) | 0.73 |
| KDIGO_Pos_24h | 28 (25.2) | 14 (27.5) | 12 (23.3) | 0.66 |
| KDIGO 1_7d | 3 (2.7) | 3 (5.9) | 0 | 0.09 |
| KDIGO 2_7d | 3 (2.7) | 1 (2) | 2(3.3) | 1.0 |
| KDIGO 3_7d | 7 (6.3) | 5 (9.8) | 2 (3.3) | 0.24 |
| KDIGO_Pos_7d | 14 (12.6) | 9(17.6) | 5 (8.3) | 0.16 |
| Positive KDIGO during Hosp stay | 47 (42.3) | 21 (41.2) | 26 (43.3) | 0.84 |
Data are presented as n (%) or median (Q1–Q3)
KDIGO Kidney disease improving global outcomes, h hours, d days, LOS length of stay, Pos positive
aRelated to 75 patients
No statistical difference was found in the ICU and hospital mortality rates between the US and control groups; similarly, no difference was found in the median length of ICU and hospital stay (Table 2).
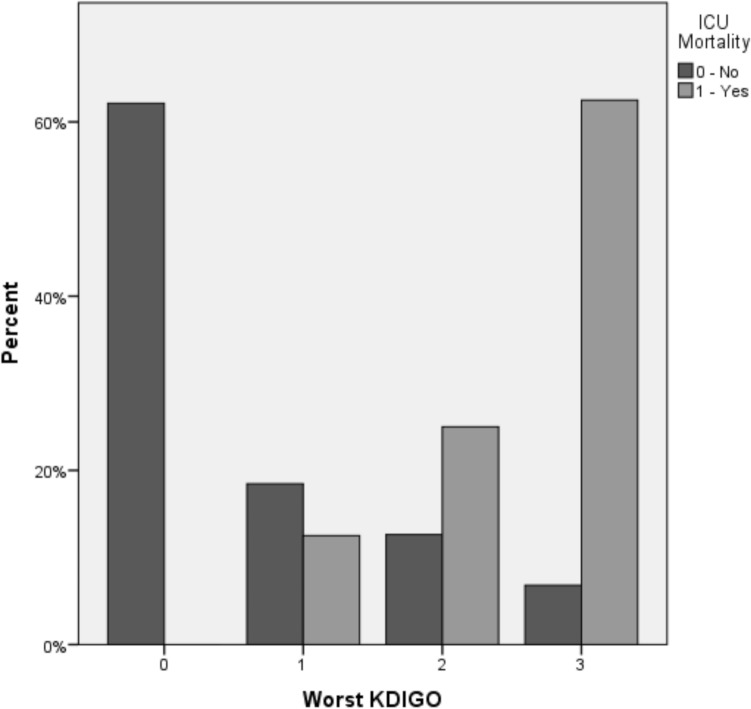
The ICU mortality was significantly higher among patients who developed any AKI level (17% vs. 0%, P < 0.001) as compared to those without this complication, being proportionally higher according to the KDIGO level (5% in KDIGO 1, 13.3% in KDIGO 2 and 41.7% in KDIGO 3, P < 0.001), as shown in Fig. 3.
Fig. 3.
KDIGO score and mortality in ICU
Patients without AKI have shown less fluid balance during the ICU stay in the US group than the control group (747.5 [− 20–1928] vs. 2365 [7532–3507], P < 0.001).
Among the 51 patients randomized to the US group, those who develop AKI during the follow-up had a higher collapsibility index of the inferior vena cava (0.51 [0.42–0.74] vs. 0.35 [0.17–0.65], P = 0.04); and were therefore considered fluid-responsive more frequently (14 [77.7%] vs. 12 [42.9%], P = 0.03) as compared with those without AKI. Consequently, AKI patients received higher amounts of crystalloids boluses during ICU stay (2500 [1500–3750] vs. 1000 [500–2000], P < 0.001). Conversely, no difference in the amount of crystalloids was found in patients with or without AKI in the control group (2000 [1000–2625] vs. 1550 [1000–2500] P = 0.29).
Finally, the subgroup of AKI patients presented higher lung score values at inclusion and at 12–24 h of follow up (Table 3), even though this difference did not reach statistical significance.
Table 3.
Characteristics of patients included in the US group
| All (51) | KDIGO positive | KDIGO negative | P value | |
|---|---|---|---|---|
| LUS D1 | 5 (2–10) | 7 (3.5–11) | 4 (1.7–8) | 0.07 |
| Major IVC diameter D1 | 1.25 (0.97–1.63) | 1.15 (0.88–1.51) | 1.28 (0.97–1.65) | 0.59 |
| Collapsibility index IVC D1 | 0.45 (0.27–0.65) | 0.51 (0.42–0.74) | 0.35 (0.17–0.65) | 0.04 |
| Fluid responsiveness D1 | 26 (56.5) | 14 (77.7) | 12 (42.9) | 0.03 |
| LUS D2 | 9 (6–13) | 12.5 (5–15) | 9 (7–11) | 0.09 |
| Collapsibility index IVC | 0.37 (0.19–0.61) | 0.45 (0.3–0.7) | 0.33 (0.17–0.48) | 0.11 |
| Major IVC diameter D2 | 1.38 (1.0–1.7) | 1.35 (1.09–1.68) | 1.41 (0.95–1.73) | 0.98 |
| Fluid responsiveness D2 | 21 (46.7) | 11 (57.9) | 10 (38.5) | 0.23 |
| Crystalloids-12 h, mL | 1000 (500–2000) | 1500 (750–2250) | 1000(375–1500) | 0.02 |
| Crystalloids-24 h, mL | 1000 (0–1500) | 1000 (500–2000) | 500 (0–1000) | 0.008 |
| Accumulated fluid balance-ICU stay (IQR) | 3320 (196–5330) | 5010 (3776–6645) | 104 (− 887 to 3066) | < 0.001 |
Data are presented as n (%) or median (Q1–Q3)
LUS lung ultrasound at inclusion, D1 at inclusion, D2 12–24 h after the first exam, IVC inferior vena cava, ICU intensive care unit
Discussion
In this study, adding bedside ultrasound assessment to the parameters currently used in ICU to guide the hemodynamic management of patients in the POP of major surgeries did not modify the incidence of acute kidney injury defined by the presence of any KDIGO level. Likewise, our results showed no impact on the accumulated fluid balance, amount of volume administered during ICU stay, and the ICU mortality or hospital mortality in both groups.
Hypovolemia during the perioperative period is one of the main mechanisms of AKI in this group of patients [19]. However, the amount of volume needed in each patient is controversial, with some studies suggesting that a restrictive fluid strategy during the procedure and over the subsequent 24 h increases the incidence of AKI [20]. The use of goal-directed clinical protocols during the preoperative period to guide fluid administration seems to be associated with a reduction in the incidence of AKI, as well as shorter periods of hospitalization and lower number of re-hospitalizations [21, 22].
Studies encompassing the use of different goals over the perioperative period had controversial results. In a metanalysis with more than 3000 high-risk surgery patients included, guiding fluid therapy using cardiac output optimization and oxygen delivery entailed a reduction in the incidence of AKI [23]. On the other hand, using a similar strategy, Ripollés et al. did not demonstrate a reduction in the incidence of complications in non-cardiac postoperative patients, including AKI. However, they observed lower mortality rates in the subgroup of treatment guided by objective parameters obtained by pulmonary artery catheter and LidCO [24]. Xu et al. evaluated the use of fluid therapy based on central venous oxygen saturation and esophageal Doppler parameters in patients submitted to colorectal surgeries. They did not note between-group differences regarding 30 day-mortality, length of hospital stay, and recovery after surgery. However, these authors did not measure the occurrence of AKI in the studied population [25]. As mentioned above, the heterogeneity of the tested protocols, and the different AKI definitions adopted in these studies may partially explain the variability in their results.
Our study did not show a reduction in the frequency of AKI using the ultrasound among patients in POP of high-risk surgeries, which might be partly explained by the gap—as much as 12 h—between ICU admission and the first ultrasound exam. This interval might have limited the impact of the ultrasound findings on the hemodynamic interventions, notably over fluid therapy. Nevertheless, an analysis restricted to the subgroup of patients (n = 33) screened in the first 6 h after ICU admission did not show differences between the groups. Even though our protocol had not predicted guided interventions during the intraoperative phase, we did not observe difference between the two groups studied in the amount of volume administered during this period.
The rationale of our study came from some literature data suggesting a potential benefit of using ultrasound to guide fluid therapy in different clinical scenarios. The RUSH (rapid ultrasound in shock) ultrasound protocol [7] was efficient in the diagnosis of shock causes in critical patients [26]. Specifically, lung ultrasound has been suggested as a helpful tool to guide the management of patients with circulatory shock because this method provides dynamic physiologic information regarding air and water ratio [27]. Another point is the evaluation of IVC variation, whose benefit is controversial in postoperative patients. Murthi et al. evaluated the role of this variable to determine fluid responsiveness and predict AKI in the immediate postoperative period, with negative results [28]. In our study, the analysis restricted to the US group showed that the IVC variation was higher in the subgroup that developed AKI. Paradoxically, these patients developed AKI despite receiving a higher amount of fluids.
Interestingly, we noticed higher amounts of positive fluid balance in patients with negative KDIGO in the control group, despite no difference in the mortality, showing that the use of ultrasound might be useful to avoid excessive volume administration when it is not necessary.
Some strengths of this study are worth mentioning. Firstly, it was a randomized study with a pre-specified protocol. Despite being an open-labeled study, which was inherent to the study design, the assistant physicians adopted research team's recommendations in all but one of the patients allocated to the intervention group (data not shown). Furthermore, AKI characterization by the KDIGO score was accurate, which could be demonstrated by the higher mortality rate observed among patients who developed AKI and by the gradual worsening of this outcome according to the injury level, i.e., rising from KDIGO stage 1 to KDIGO stage 3. Finally, patients were selected rigorously utilizing criteria for identifying those with increased risk of developing AKI and, therefore, with a high likelihood of benefitting from the protocol tested.
This study has several limitations that must be taken into account. It was a single-center study encompassing different types of surgeries. Each one of them has different risks of AKI development. Also, the calculation of the KDIGO score was based only on the creatinine levels in patients with inaccurate diuresis volume measurement, mainly at 24 h, and 7th day of follow up. We did not use other sonography technics that can be valuable to detect the hemodynamic changes in the perioperative period, such as the measure of the kidney size, the echogenicity changes, and the perfusion renal index since we looked forward to apply the existing knowledge of intensive care physicians in our center. The sample size predicted in the initial protocol could not be reached. Although an interim analysis had not been predicted in the initial protocol, after the addition of 111 patients, a partial data analysis did not show a difference between the groups. Therefore, due to futility reasons, the study was interrupted.
Conclusion
The association of a specific treatment protocol guided by bedside ultrasound with lung examination, a variation of inferior vena cava, and subjective evaluation of cardiac contractility did not lead to a reduction in the incidence of AKI among patients in the POP admitted to the ICU after high-risk surgery. Multicenter studies involving early screening after admission to ICU coupled with systematic serial US assessments might bring new perspectives in this field.
Acknowledgements
This work was support in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author contributions
CGR and VN authors contributed to conception and design, data acquisition, data analysis, and writing of the manuscript. PFV, CCM, TBLSA, RDF, GCR, MRM, and FLB authors contributed to the data acquisition. PFV, CCM, IBN, APFN, AGGR, and ACSS contributed in the data analysis, and written the manuscript.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Availability of data and materials
All relevant data are within the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethics approval and consent to participate
The study was approved by the local Research Ethics Committee (COEP-UFMG), protocol number CAEE 82449417.3.0000.5149. All the patients included in the study or their next-of-kin signed the consent form.
Trial registration
The trial was registered at ClinicalTrials.gov under protocol NCT03509935 on April 27, 2018.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 2.Jämsä P, Jämsen E, Lyytikäinen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop. 2017;88(4):370–376. doi: 10.1080/17453674.2017.1301743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gameiro J, Neves JB, Rodrigues N, Bekerman C, Melo MJ, Pereira M, et al. Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: a cohort analysis. Clin Kidney J. 2016;9(2):192–200. doi: 10.1093/ckj/sfv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozrazgat-Baslanti T, Thottakkara P, Huber M, Berg K, Gravenstein N, Tighe P, et al. Acute and chronic kidney disease and cardiovascular mortality after major surgery. Ann Surg. 2016;264(6):987–996. doi: 10.1097/SLA.0000000000001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turan A, Cohen B, Adegboye J, Makarova N, Liu L, Mascha EJ, et al. Mild acute kidney injury after noncardiac surgery is associated with long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 2020. [DOI] [PubMed]
- 6.Arkiliç CF, Taguchi A, Sharma N, Ratnaraj J, Sessler DI, Read TE, et al. Supplemental perioperative fluid administration increases tissue oxygen pressure. Surgery. 2003;133(1):49–55. doi: 10.1067/msy.2003.80. [DOI] [PubMed] [Google Scholar]
- 7.Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010;28(1):29–56, vii. [DOI] [PubMed]
- 8.Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014–1020. doi: 10.1378/chest.09-0001. [DOI] [PubMed] [Google Scholar]
- 9.Calsavara AJC, Costa PA, Nobre V, Teixeira AL. Factors associated with short and long term cognitive changes in patients with sepsis. Sci Rep. 2018;8(1):4509. doi: 10.1038/s41598-018-22754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez Ravetti C, Ataíde TBLS, Barreto LM, Bastos FL, Gomes AGR, Bragança RD, et al. Lung ultrasound is useful in oncohematologic patients with respiratory dysfunction admitted to an Intensive Care Unit (ICU): a pilot study. 2020. [DOI] [PubMed]
- 12.Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40(7):2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 13.Haynes SR, Lawler PG. An assessment of the consistency of ASA physical status classification allocation. Anaesthesia. 1995;50(3):195–199. doi: 10.1111/j.1365-2044.1995.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. [DOI] [PubMed]
- 16.Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005;31(10):1336–44. [DOI] [PMC free article] [PubMed]
- 17.Kellum JA, Lameire N, Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medve L, Antek C, Paloczi B, Kocsi S, Gartner B, Marjanek Z, et al. Epidemiology of acute kidney injury in Hungarian intensive care units: a multicenter, prospective, observational study. BMC Nephrol. 2011;12:43. doi: 10.1186/1471-2369-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bundgaard-Nielsen M, Jørgensen CC, Secher NH, Kehlet H. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiol Scand. 2010;54(4):464–469. doi: 10.1111/j.1399-6576.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- 20.Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 21.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 22.Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt S, Hansen B, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109(2):191–199. doi: 10.1093/bja/aes163. [DOI] [PubMed] [Google Scholar]
- 23.Giglio M, Dalfino L, Puntillo F, Brienza N. Hemodynamic goal-directed therapy and postoperative kidney injury: an updated meta-analysis with trial sequential analysis. Crit Care. 2019;23(1):232. doi: 10.1186/s13054-019-2516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripollés-Melchor J, Espinosa Á, Martínez-Hurtado E, Abad-Gurumeta A, Casans-Francés R, Fernández-Pérez C, et al. Perioperative goal-directed hemodynamic therapy in noncardiac surgery: a systematic review and meta-analysis. J Clin Anesth. 2016;28:105–115. doi: 10.1016/j.jclinane.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Peng J, Liu S, Huang Y, Guo X, Xiao H, et al. Goal-directed fluid therapy versus conventional fluid therapy in colorectal surgery: a meta analysis of randomized controlled trials. Int J Surg. 2018;56:264–273. doi: 10.1016/j.ijsu.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Stickles SP, Carpenter CR, Gekle R, Kraus CK, Scoville C, Theodoro D, et al. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: a systematic review and meta-analysis. CJEM. 2019;21(3):406–417. doi: 10.1017/cem.2018.498. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein D, Karakitsos D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol) J Crit Care. 2012;27(5):533.e11–9. doi: 10.1016/j.jcrc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Murthi SB, Fatima S, Menne AR, Glaser JJ, Galvagno SM, Biederman S, et al. Ultrasound assessment of volume responsiveness in critically ill surgical patients: two measurements are better than one. J Trauma Acute Care Surg. 2017;82(3):505–511. doi: 10.1097/TA.0000000000001331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.