Highlights
-
•
Aggressive angiomyxoma has a unique potential to invade locally, with a rare incidence of metastasis.
-
•
While simple excision is sufficient for superficial angiomyxoma, wide local excision is warranted for the aggressive type.
-
•
Early preoperative diagnosis of aggressive angiomyxoma aids in optimization of surgical management.
-
•
Aggressive angiomyxoma can respond to adjuvant hormonal therapies.
Keywords: Vulva, Superficial angiomyxoma, Aggressive angiomyxoma, Lower genital tract disease
Abstract
Vulvar angiomyxomas are rare benign mesenchymal neoplasms. Superficial and Aggressive angiomyxomas are two distinct phenotypes that present similarly to other more common vulva-perineal pathologies. Albeit both angiomyxomas carry a risk of recurrence, especially in the setting of incomplete resection, simple excision is insufficient for Aggressive angiomyxoma. It requires wide local excision because of its unique potential for local invasion, infiltration of the paravaginal and pararectal tissue, and more distant metastasis. Here, we present a case of Superficial angiomyxoma and a case of Aggressive angiomyxoma to highlight the diagnostic challenges and management strategies of each tumor. In both cases, angiomyxomas were initially misdiagnosed because of their rarity and nonspecific presentation. Magnetic resonance imaging is the modality of choice for evaluation due to inherent higher spatial resolution of soft tissue anatomical details. Early diagnosis of Aggressive angiomyxoma can prevent incomplete excision and recurrence, spare additional surgery, and offer hormonal therapy options.
1. Introduction
Angiomyxomas are rare benign neoplasms originating from mesenchymal cells and are characterized by significant myxoid matrix and numerous blood vessels. (Goyal et al., 2022) Two phenotypes of angiomyxomas have been identified: Superficial (or Cutaneous) and Aggressive (or Deep). Superficial angiomyxoma is a noninvasive cutaneous tumor, first described in association with the Carney complex, a rare multiple neoplasia syndrome; although, it more often arises as a sporadic tumor (Mehrotra et al., 2021, O’Flynn O’Brien et al., 2020) Conversely, Aggressive angiomyxoma is a locally invasive soft tissue neoplasm. (Steeper and Rosai, 1983).
Both Superficial and Aggressive phenotypes can involve the vulva. Vulvar angiomyxomas are challenging to diagnose given their rarity and nonspecific presentation. Surgical resection is a mainstay of treatment for both. Here, we present two distinct cases of vulvar angiomyxoma, Superficial and Aggressive, with the primary goal of comparing these two vulvar tumor entities, highlighting diagnostic challenges, and discussing management strategies for each tumor.
2. Case 1
A 28-year-old nulliparous woman presented for evaluation of a vulvar mass, which was slowly growing for the past three years. The patient denied any associated symptoms besides mass engorgement upon arousal. On exam, the patient had a non-tender 4 cm pendulous mass on the right labia majora without signs of fluid tracking. Initial computerized tomography (CT) of the pelvis showed a 6.3 × 2.8 cm hypo-enhancing right vulvar mass for which magnetic resonance imaging (MRI) was performed for better characterization. MRI demonstrated a well-circumscribed exophytic T2 hyperintense mass arising from the right labia majora (Fig. 1).
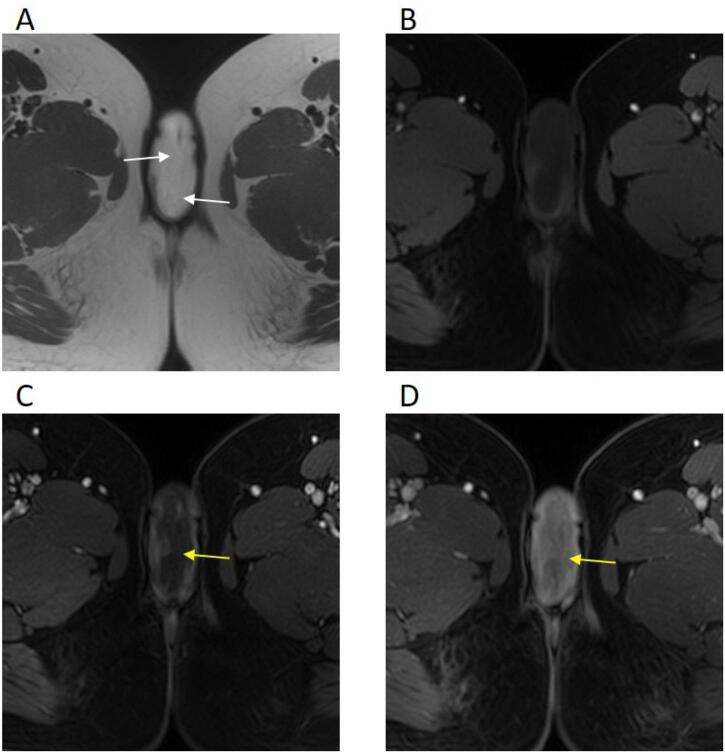
Fig. 1.
A 28-year-old female with a right labia majora tumor. Magnetic resonance imaging A: Axial T2 weighted image of the vulvar tumor demonstrates a relatively homogenous high signal intensity related to the myxoid matrix with low signal bands (white arrows) giving its characteristic swirling/laminated appearance. B-D: Axial T1 weighted images before (B) and after contrast administration (C and D) showing iso- to hypointense signal relative to skeletal muscles, and progressive enhancement, more intense in late post-contrast images (yellow arrows).
A right simple vulvectomy was performed. The pathology of the specimen demonstrated a predominance of small spindled to stellate cells embedded in a myxomatous stroma, numerous blood vessels ranging in size from capillaries to thick-walled, hyalinized larger vessels, with occasional perivascular smooth muscle proliferation (Fig. 2-A). Features described were consistent with a myxoid neoplasm, favoring Deep Aggressive angiomyxoma, extending to the margins of resection. On immunohistochemical staining, the stromal cells were positive for desmin and estrogen receptors, negative for CD34 and smooth muscle actin (SMA).
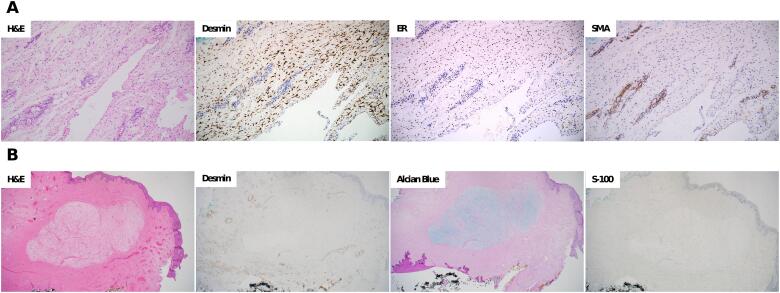
Fig. 2.
Pathology and immunohistochemical staining A. Deep Aggressive angiomyxoma: (H&E) Hematoxylin and eosin stain showing the lesion with dilated lymphatics and entrapped fat; (Desmin) Desmin staining diffusely positive in stromal cells; (ER) Estrogen receptor is diffusely positive in the stromal cells; (SMA) Smooth muscle actin highlights the perivascular smooth muscle while negative in the stromal cells. B. Superficial angiomyxoma: (H&E) Hematoxylin and eosin stain demonstrating a well-circumscribed spindle cell proliferation with a prominent myxoid stroma, numerous small blood vessels, and clear margins of excision; (Desmin) Desmin staining negative in stromal cells; (Alcian Blue) Alcian blue PAS, Periodic Acid Schiff, strongly positive for mucin; (S-100) S-100 staining negative. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A subsequent right radical vulvectomy was performed with a V-Y advancement flap given limited subcutaneous tissue. No residual tumor was found on final pathology, and additional treatment was not indicated. The patient has been monitored with no local recurrence eight months after surgery.
3. Case 2
A 42-year-old G3P3 woman presented to an outside hospital with a complaint of a subdermal mass involving the left vulva. The patient underwent a left simple partial vulvectomy for excision of the lesion with benign gynecology. Her recovery was complicated by multiple wound debridements. The external pathology report of a 1.5 × 1.5 × 1.0 cm specimen was suggestive of benign angiomyofibroblastoma described as cytologically bland nodular fibromyxoid spindle cell proliferation with positive margins. Immunohistochemistry was positive for vimentin, estrogen receptor, progesterone receptor, and periodic mucin.
The patient was referred for a second opinion. Upon examination, she had a residual cystic subdermal mass on the left labia at the site of previous vulvectomy. Initial MRI of the pelvis showed a 2 × 1.3 cm left vulvar mass with hyperintense signal on T2 weighted images and mild peripheral progressive enhancement on T1 weighted postcontrast images, which could be due to postsurgical changes. Subsequently, the patient underwent repeat left simple partial vulvectomy. A well-circumscribed cystic lesion was noted in the subdermal space. Pathology was consistent with Superficial angiomyxoma, demonstrating a well-circumscribed spindle cell proliferation with a low mitotic rate and a prominent myxoid stroma, numerous small blood vessels, and stromal neutrophils, with clear margins of excision (Fig. 2-B). Immunohistochemical staining was strongly positive for mucin and negative for desmin and S100.
After nine months of surveillance, the patient presented with a new vulvar lesion. Exam was notable for a 1.0 cm pink polyp on left vulva at a 12o’clock position between clitoris and urethra and a small scar from prior vulvectomy at 3o’clock position without underlying abnormalities. A repeat MRI of the pelvis demonstrated a corresponding 1.2 × 1.7 cm left vulvar mass with MR imaging characteristics resembling the primary tumor, concerning for a recurrence. Biopsy was deferred due to the lesion being deep to the mucosa. Patient opted for subsequent excision, and pathology demonstrated benign squamous mucosa and submucosa. No recurrence occurred after ten months of follow-up since the last surgery.
4. Discussion
Vulvar angiomyxomas are rare and frequently misdiagnosed due to their nonspecific clinical, radiographic, and histologic features (Mehrotra et al., 2021, O’Flynn O’Brien et al., 2020, Steeper and Rosai, 1983). Both Superficial and Aggressive phenotypes should be included in the differential diagnosis for a painless, slowly growing vulvar mass. Angiomyxomas can recur, especially in the setting of residual disease. Aggressive angiomyxoma has a propensity to invade locally and to rarely metastasize, thus complete excision with negative margins and close monitoring are imperative.
Superficial angiomyxomas are cutaneous tumors that can grow to be quite large (Mehrotra et al., 2021). They can appear on the trunk, head, neck, and extremities, frequently in middle-aged men (Kim et al., 2010, Mehrotra et al., 2021, O’Flynn O’Brien et al., 2020). Less commonly, they occur in genital regions in women of reproductive age (Lee et al., 2016, Mehrotra et al., 2021). The most common presentation of vulvar Superficial angiomyxoma is a slowly growing painless mass, frequently involving the labia majora or minora (Kim et al., 2010, Lee et al., 2016). It is thought to be derived from fibroblast-like cells that can be modulated by cytokines (Mehrotra et al., 2021).
Aggressive angiomyxoma is a locally invasive soft tissue tumor that occurs almost exclusively in the pelvic-perineal region of premenopausal women, with vulva being the most common site of involvement (Goyal et al., 2014, York et al., 2022). These tumors frequently have estrogen and progesterone receptors, and thus affect women of reproductive age and can rapidly enlarge during pregnancy (Goyal et al., 2022). Pathogenesis of Aggressive angiomyxoma is not well understood. Frequent tumor expression of desmin and smooth muscle actin suggests mesenchymal or perivascular progenitor cell origin. Aggressive angiomyxoma can involve the deeper tissues of the pelvis and retroperitoneum; thus, the extent of the tumor may be underestimated on initial physical exam. While CT imaging is usually utilized due to its wide availability, MRI has inherent higher spatial resolution for better characterization of soft tissue and anatomical details. MRI is superior to CT for the evaluation of perineal/vulvar angiomyxomas in assessing deep tumor extent and the relation of the tumor to the pelvic floor, which may help to differentiate Superficial from Aggressive angiomyxoma. Hence, MRI is the imaging of choice for preoperative diagnosis and monitoring for recurrences. MRI features of Aggressive angiomyxoma include iso- or hypo-intensity on T1-weighted images, hyperintensity and characteristic swirling/laminated layering on T2-weighted images (due to myxoid matrix), and intense progressive enhancement on post-contrast T1-weighted images reflecting inherent vascularity (Table 1). Unlike Superficial angiomyxoma, Aggressive angiomyxoma tends to be larger in size with deep extension into the pelvic floor structures.
Table 1.
Clinical and histopathological features of vulvar angiomyxomas.
| Superficial angiomyxoma | Aggressive angiomyxoma | |
|---|---|---|
| Age | Mean: 23 years. Range: 15–60 years |
Most common: women of reproductive age. Range: 16–70 years |
| Incidence | ∼50–60 cases reported | ∼350–400 cases reported |
| Clinical presentation | Soft lobulated mass | Pedunculated mass, can be lobulated, soft to rubbery |
| Size | Varies, usually small (ranges from 0.9 to 4 cm) | Varies (ranges from 3 to 60 cm), usually larger (most > 10 cm) |
| Imaging | Preserved fat plane between the mass and levator muscles. MRI: Iso- to hypo-intense signal to muscle on T1WI, homogenous hyper-intense signal on T2WI with heterogeneous enhancement on postcontrast T1WI. |
Tends to involve levator muscles and/or deep pelvic structures. MRI: Iso- to hypo-intense signal to muscle on T1WI, homogenous hyper-intense signal on T2WI with swirl/laminated appearance, and progressive avid enhancement on postcontrast T1WI. |
| Gross pathology | Variably circumscribed, unencapsulated, superficial dermal nodule with multinodular gelatinous cut surface with thin fibrous septa, may contain cysts with keratin debris | Variably circumscribed, unencapsulated, deep-seated lobulated or polypoid mass, myxoid, gelatinous, or rubbery cut surface |
| Cell origin | Histogenesis is uncertain, may arise from fibroblast-like mesenchymal cells | Histogenesis is uncertain, may arise from specialized mesenchymal cells and/or multipotent perivascular progenitor cells |
| Histology | Hypocellular dermal nodule of spindled to stellate cells in an abundant myxoid background (neutrophils present in a subset of cases), numerous arborizing small thin-walled blood vessels. Entrapped benign epithelial elements may be present. | Hypocellular lesion of small spindled to stellate cells with an infiltrative growth pattern (entraps fat and nerves), abundant myxomatous stroma with collagen fibers, numerous variably-sized blood vessels with occasional perivascular smooth muscle proliferation, stromal mast cells and extravasated red blood cells common |
| Nuclear atypia | Absent | Absent |
| Mitotic index | Low | Low |
| Immunohistochemistry | Vimentin + S100 –Mucin (via Alcian blue PAS) + Desmin – or focal ER/PR – SMA – or focal CD34 + HMGA2 – |
Vimentin + S100 –Mucin (via Alcian blue PAS) + Desmin + ER/PR + Variable SMA CD34 – HMGA2 overexpressed |
| Genetic testing | If associated with Carney complex, may have autosomal dominant inactivating mutations in PRKAR1A (17q22-24) | Various rearrangements involving HMGA2 (12q13-15) in approximately 1/3 of cases |
| Treatment | Simple excision | Wide local excision +/- Adjuvant hormonal therapy |
| Recurrence rate | 30–40% (∼with inadequate resection) | 50–70% (∼with inadequate resection) |
| Metastasis | No | Extremely rare |
MRI: Magnetic resonance imaging; T1WI: T1 weighted image; T2WI: T2 weighted image; PAS: Periodic Acid Schiff; ER: Estrogen receptor; PR: Progesterone receptor; SMA: Smooth muscle actin; HMGA2: High mobility group A2 protein; PRKAR1A: Protein kinase CAMP-dependent type I regulatory subunit alpha.
Due to the rarity of angiomyxomas and the lack of distinct signs and symptoms, both Superficial and Aggressive phenotypes are often diagnosed only on histological examination following surgical resection (Haldar et al., 2010, Mehrotra et al., 2021, O’Flynn O’Brien et al., 2020, Steeper and Rosai, 1983). A different vascular pattern and involvement of the deeper tissues differentiate Aggressive angiomyxoma from the Superficial phenotype (Table 1). Given the predilection for pelvic and perineal regions, misdiagnosis as skin tag, polyp, labial inclusion cyst, Bartholin cyst, Gartner’s duct cyst, lipoma, perineal herniation, neurofibroma, desmoid tumor, or sarcoma are common (Goyal et al., 2014, Mehrotra et al., 2021). Furthermore, Superficial and Aggressive angiomyxomas should be distinguished from other myxoid and vascular tumors, such as angiomyofibroblastoma, cellular angiofibroma, myxoid liposarcoma, myxofibrosarcoma, myxoid desmoid fibromatosis, and myxoid dermatofibrosarcoma protuberans (DFSP), among others. Although these tumors entities may present similarly, they can be diagnosed based on characteristic clinicopathologic, immunohistochemical, and molecular findings (Angelico et al., 2022, Baheti et al., 2015, Ganeshan et al., 2019, Mentzel et al., 2007). Similarly to Aggressive angiomyxoma, angiomyofibroblastoma and cellular angiofibroma also have a predilection to the lower female genital tract. One should be suspicious of Aggressive angiomyxoma in a premenopausal woman with a large vulvovaginal lesion that shows a swirling pattern on T2-weighted images. Both myxoid desmoid fibromatosis and myxoid DFSP are very rare, locally aggressive, and can clinically resemble angiomyxoma, with the former being a deep-seated connective tissue tumor developing in musculoaponeurotic tissues and the latter a mesenchymal tumor of skin and subcutis (Ganeshan et al., 2019, Mentzel et al., 2007). Malignant myxoid neoplasms that can rarely involve the pelvic-perineal region include myxofibrosarcoma and myxoid liposarcoma. Myxofibrosarcoma typically presents in the sixth decade of life as a heterogeneous lesion with ill-defined infiltrative margins and inhomogeneous contrast enhancement; occasionally, a T2-hyperintense enhancing curvilinear projection extending into adjacent soft tissues may be seen, which represents a tail sign (Baheti et al., 2015). Myxoid liposarcoma presents as a well-defined multilobulated or septate heterogeneous mass with varying components on MRI.
Surgical excision is the first-line treatment and can be curative for both Superficial and Aggressive phenotypes (Mehrotra et al., 2021, Steeper and Rosai, 1983). While simple excision is usually sufficient for the removal of Superficial angiomyxoma, wide local excision with negative margins of at least 1 cm is warranted for the treatment of Aggressive angiomyxoma because of its higher rate of recurrence and unique potential for local invasion, infiltrating the paravaginal and pararectal tissue, and a rare incidence of metastasis (York et al., 2022). Thus, preoperative diagnosis of Aggressive angiomyxoma is beneficial for optimizing surgical management. Yet, biopsy results usually lead to a differential instead of a definitive diagnosis given limited tissue, tumor heterogeneity, lack of sensitive diagnostic features and infiltration. Distinguishing between Superficial and Aggressive angiomyxoma intraoperatively on frozen section is extremely difficult as both are hypocellular myxoid lesions without nuclear atypia, and best practice is to defer final characterization to the permanent section results including ancillary immunohistochemistry. In our first case, Aggressive angiomyxoma was initially misdiagnosed as an epidermal inclusion cyst based on physical exam and imaging. Simple excision was performed, which resulted in resection with positive margins, thus additional surgery was warranted.
Wide local excision with clear margins is often required for the treatment of Aggressive angiomyxoma given its locally infiltrative potential. In the settings of deep invasion or positive margins with wide local excision, radical surgery might be warranted which is associated with high operative morbidity (e.g., persistent discomfort, groin pain, urinary complications, and sexual dysfunction). If the tumor is estrogen and progesterone receptor positive, medical management and adjuvant therapy can be considered. These include gonadotropin-releasing hormone (GnRH) agonists (such as leuprolide acetate and goserelin), raloxifene, or tamoxifen (Fine et al., 2001). Hormonal therapy is associated with long-term side effects such as menopausal symptoms and bone loss. Given the generally young age of the population affected by Aggressive angiomyxoma, hormonal therapy is not recommended as a first-line treatment. A short course can be considered as adjuvant therapy for residual disease in the setting of positive margins on resection or recurrence (Aguilar-Frasco et al., 2018, Goyal et al., 2022). Radiation and chemotherapy are not indicated due to the low mitotic index of the tumor. There is a paucity of literature on the utilization of adjuvant treatments given the rarity of Aggressive angiomyxoma. Overall, the risks and benefits of surgical and medical management should be discussed with the patient to promote shared decision-making.
Both Superficial and Aggressive angiomyxomas are known for local recurrences, which are usually associated with incomplete excision (Goyal et al., 2022, Mehrotra et al., 2021). Superficial angiomyxoma is not known to be malignant or to metastasize. On the other hand, rare events of metastatic Aggressive angiomyxoma have been reported in the literature (Goyal et al., 2022). Thus, long-term follow-up up to 15 years after the primary resection is advised for surveillance of Aggressive angiomyxoma, although this is not evidence-based given the rarity of the tumor and limited data availability.
In summary, we describe two distinct cases of vulvar angiomyxomas that presented similarly as a slow-growing mass. We provide clinically relevant points that can help in prompt workup, diagnosis, and management of these tumors by gynecologic oncologists. Superficial and Aggressive angiomyxomas are frequently misdiagnosed due to their vague clinical findings, but it is imperative to keep these diagnoses in the differential to ensure proper treatment with local excision and, particularly with Aggressive angiomyxoma, wide surgical margins and close surveillance due to the high risk of recurrence. Awareness of characteristic features on MRI in both primary and recurrent cases may assist in achieving correct diagnosis. Aggressive angiomyxomas, including those that are not completely resected or that recur, can respond to adjuvant hormonal treatments, although evidence is still lacking due to the rarity of the condition.
CRediT authorship contribution statement
Anastasia Navitski: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Leslie Adams: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Bogna N. Brzezinska: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Jessa Suhner: Writing – review & editing. Taylor Sliker: Writing – review & editing. Pramila Moideen: Writing – review & editing. Amanda Barrett: Writing – review & editing. Abdul R. Abualruz: Writing – review & editing. Marian S. Johnson: Writing – review & editing. Bunja Rungruang: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Informed Consent
Informed consent was obtained from the patients for publication of the case reports and accompanying images. A copy of the consent documentation is available for review by the Editor-in-Chief of this journal on request.
Funding Statement
No funding required.
References
- Aguilar-Frasco J., Ruben-Castillo C., Rodríguez-Quintero J.H., Medina-Franco H. Aggressive angiomyxoma: giant recurrence successfully treated with wide excision and adjuvant therapy with GnRH analogue. BMJ. Case. Rep. 2018;11 doi: 10.1136/bcr-2018-226973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelico G., Marletta S., Broggi G., Vigneri P., Vecchio G.M., Salvatorelli L., Magro G. Practical Approach to the Diagnosis of the Vulvo-Vaginal Stromal Tumors: An Overview. Diagnostics. (Basel) 2022;12 doi: 10.3390/diagnostics12020357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baheti A.D., Tirumani S.H., Rosenthal M.H., Howard S.A., Shinagare A.B., Ramaiya N.H., Jagannathan J.P. Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR. Am. J. Roentgenol. 2015;204:374–385. doi: 10.2214/AJR.14.12888. [DOI] [PubMed] [Google Scholar]
- Fine B.A., Munoz A.K., Litz C.E., Gershenson D.M. Primary medical management of recurrent aggressive angiomyxoma of the vulva with a gonadotropin-releasing hormone agonist. Gynecol. Oncol. 2001;81:120–122. doi: 10.1006/gyno.2000.6119. [DOI] [PubMed] [Google Scholar]
- Ganeshan D., Amini B., Nikolaidis P., Assing M., Vikram R. Current Update on Desmoid Fibromatosis. J. Comput. Assist. Tomogr. 2019;43:29–38. doi: 10.1097/RCT.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L.D., Garg P., Badyal R., Bhalla S. Aggressive (deep) angiomyxoma of the vulva: a case report. J. Med. Case. Rep. 2022;16:71. doi: 10.1186/s13256-022-03284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P., Agrawal D., Sehgal S., Ghosh S., Kumar A., Singh S. Aggressive angiomyxoma in pregnancy. Rare. Tumors. 2014;6:5362. doi: 10.4081/rt.2014.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K., Martinek I.E., Kehoe S. Aggressive angiomyxoma: a case series and literature review. Eur. J. Surg. Oncol. 2010;36:335–339. doi: 10.1016/j.ejso.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Kim H.-S., Kim G.Y., Lim S.-J., Ki K.-D., Kim H.C. Giant superficial angiomyxoma of the vulva: a case report and review of the literature. J. Cutan. Pathol. 2010;37:672–677. doi: 10.1111/j.1600-0560.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Cho Y.J., Han M., Bae J.W., Park J.-W., Oh S.R., Kim S. Superficial Angiomyxoma of the Vulva in a Postmenopausal Woman: A Case Report and Review of Literature. J. Menopausal. Med. 2016;22:180–183. doi: 10.6118/jmm.2016.22.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra K., Bhandari M., Khullar G., Sharma S. Large Superficial Angiomyxoma of the Vulva-report of Two Cases with Varied Clinical Presentation. Indian. Dermatol. Online. J. 2021;12:605–607. doi: 10.4103/idoj.IDOJ_489_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel T., Schärer L., Kazakov D.V., Michal M. Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases. Am. J. Dermatopathol. 2007;29:443–448. doi: 10.1097/DAD.0b013e318145413c. [DOI] [PubMed] [Google Scholar]
- O’Flynn O’Brien K.L., Cortes-Santiago N., Patil N.M., Bercaw-Pratt J.L. A Rare Case of Vulvar Superficial Angiomyxoma in a Pediatric Patient. J. Pediatr. Adolesc. Gynecol. 2020;33:727–729. doi: 10.1016/j.jpag.2020.07.015. [DOI] [PubMed] [Google Scholar]
- Steeper T.A., Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am. J. Surg. Pathol. 1983;7:463–475. doi: 10.1097/00000478-198307000-00009. [DOI] [PubMed] [Google Scholar]
- York D., Saikumar S., Patel P., Edwards C., Garcia G., Naqvi H. A Paraurethral Aggressive (Deep) Angiomyxoma. Case. Rep. Obstet. Gynecol. 2022;2022:5604460. doi: 10.1155/2022/5604460. [DOI] [PMC free article] [PubMed] [Google Scholar]