Abstract
Objectives
This study compared the epidemiological and clinical manifestations of patients hospitalized with respiratory syncytial virus (RSV) infection before and during the coronavirus disease 2019 (COVID-19) pandemic at a tertiary care hospital in Chiang Mai Province, Thailand.
Methods
This retrospective observational study utilized data from all cases of laboratory-confirmed RSV infection at Maharaj Nakorn Chiang Mai Hospital from January 2016 to December 2021. Differences in the clinical presentation of RSV infection before (2016 to 2019) and during (2020 to 2021) the COVID-19 pandemic were analyzed and compared.
Results
In total, 358 patients hospitalized with RSV infections were reported from January 2016 to December 2021. During the COVID-19 pandemic, only 74 cases of hospitalized RSV infection were reported. Compared to pre-pandemic levels, the clinical presentations of RSV infection showed statistically significant decreases in fever on admission (p=0.004), productive cough (p=0.004), sputum (p=0.003), nausea (p=0.03), cyanosis (p=0.004), pallor (p<0.001), diarrhea (p<0.001), and chest pain (p<0.001). Furthermore, vigilant measures to prevent the spread of COVID-19, including lockdowns, also interrupted the RSV season in Thailand from 2020 to 2021.
Conclusions
The incidence of RSV infection was affected by the COVID-19 pandemic in Chiang Mai Province, Thailand, which also changed the clinical presentation and seasonal pattern of RSV infection in children.
Keywords: Epidemiology, Respiratory syncytial virus, COVID-19, Retrospective study
GRAPHICAL ABSTRACT
INTRODUCTION
Respiratory syncytial virus (RSV) infection is a cause of hospitalization for infants and children with acute respiratory tract infections (ARTIs) [1,2]. Outbreaks of RSV in Thailand follow distinct spatiotemporal patterns with predictable seasonal timing and length [3,4]. During an RSV season, 60-70% of all children admitted with RSV infection are under the age of 12 months, while 80% are under the age of 2 years [5-7]. The clinical presentation of RSV infection usually begins with a runny nose, nasal congestion, and cough. Approximately 20% of patients have severe symptoms, including difficulty breathing, wheezing, bronchiolitis, and pneumonia [8]. However, the outbreak of coronavirus disease 2019 (COVID-19) affected the pattern of RSV infection [9,10] and decreased the incidence of RSV in many regions of Thailand from 2020 to 2021 [11]. The use of broadspectrum antibiotics during the COVID-19 pandemic in Korea dropped by 14-30% due to a decrease in ARTI-related hospitalizations related to stringent public health interventions and social distancing measures [12].
After the World Health Organization declared the COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [9], public health measures such as lockdowns proved effective in limiting the transmission of SARSCoV-2 as well as other respiratory diseases like RSV [10]. In Thailand, COVID-19 control measures were implemented to prevent the spread of airborne infections. Consequently, children had less exposure to all viruses that cause respiratory tract infections.
The transmission of respiratory viruses can be rapid within pediatric populations. Therefore, strict measures were taken in Thailand to restrict the spread of SARS-CoV-2. A strategy with the acronym DMHTT (“distancing, mask wearing, hand washing, testing, and the Thai Chana application”) was implemented to manage the spread of COVID-19 [13]. This method resulted in a significant decrease in the risk of respiratory infections in Thailand [11]. Although some measures to prevent COVID-19 have continued, when the DMHTT restrictions were lifted, the incidence of RSV infections increased in children [14].
A previous study found differences between the clinical manifestations of RSV before and during COVID-19, but these RSV patterns were limited [15]. Moreover, the epidemiological and clinical data on RSV infection in children during the COVID-19 pandemic lockdown is limited due to the lack of studies in Thailand. The purpose of this study was to compare the epidemiological and clinical manifestations of patients hospitalized with RSV infection before and during the COVID-19 pandemic at a tertiary care hospital in Chiang Mai from 2016 to 2021.
METHODS
Study Design
This retrospective descriptive study was conducted at Maharaj Nakorn Chiang Mai Hospital, a tertiary care hospital in northern Thailand. We included patients aged 0-15 years who were admitted to the hospital from January 1, 2016, to December 31, 2021, and diagnosed with RSV-associated lower respiratory tract infection (LRTI).
Patients and Definitions
All patient data were collected from medical records. Patients with confirmed RSV infections were identified from data provided by the virology laboratory. The inclusion criteria were: (1) patients diagnosed according to the International Classification of Diseases with Clinical Modification-10th edition (ICD-10-CM) with respiratory syncytial virus pneumonia (J12.1), acute bronchitis due to respiratory syncytial virus (J20.5), or acute bronchiolitis due to the respiratory syncytial virus (J21.0); (2) patients aged 0-15 years; (3) patients hospitalized for at least 1 day; and (4) patients who tested negative for COVID-19.
The medical record of each patient with a positive RSV test was examined to evaluate whether they met the inclusion criteria and determine if clinical manifestation data had been collected. RSV-associated LRTI was defined by a diagnosis of pneumonia, bronchiolitis, or bronchitis, with concomitant detection of RSV in upper respiratory tract samples using a realtime polymerase chain reaction (RT-PCR) test or the immunochromatography screening test kit (Quick NaviTM Flu+RSV®, Denka Seiken Co., Ltd., Tokyo, Japan) (a rapid antigen test) to confirm RSV infection. Data collection from January 2016 to December 2019 was designated as before the COVID-19 pandemic and from January 2020 to December 2021 as during the COVID-19 pandemic.
Clinical Manifestation Data
The patient characteristics included age, sex, length of hospital stay, type of diagnosis (primary diagnosis of RSV infection, RSV infection with comorbidity, or a complication of RSV infection), and Charlson comorbidity index (CCI) score (divided into 3 groups: mild [scores 1-2], moderate [scores 3-4], and severe [scores ≥5]) [16]. Clinical findings included: fever on admission, productive cough, sputum, dyspnea, sneezing, rhinorrhea, hemoptysis, wheezing, sore throat, nausea, cyanosis, pallor, diarrhea, chest pain, seizures, and decreased breath sounds.
Statistical Analysis
Demographic characteristics were analyzed and compared between the groups. The normality of the distribution of continuous variables was tested. Medians and interquartile ranges (IQRs) were used to describe non-normally distributed variables, which were further analyzed using the Mann-Whitney U test. Categorical variables were cross-tabulated using the chisquare test, while the Fischer exact test was used if the number of expected cases was <5. The data were then tabulated with Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA) and analyzed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The threshold for statistical significance in all analyses was set at p-value<0.05.
Ethics Statement
Ethical review and approval were provided by the Institutional Review Board of the Faculty of Medicine of Chiang Mai University (IRB No. 511/2021).
RESULTS
In total, 358 patients with positive RSV tests were admitted to Maharaj Nakorn Chiang Mai Hospital between 2016 and 2021. The median age of patients with RSV infection was 1 year (IQR, 0-2), and 284 (79.3%) and 74 (20.7%) were admitted before and during the COVID-19 pandemic, respectively. The median hospital stay was 6 days (IQR, 3-10). The ICD-10 codes showed RSV pneumonia in 332 cases (92.7%), acute bronchitis due to RSV in 15 cases (4.2%), and acute bronchiolitis due to RSV in 11 cases (3.1%). Most patients were diagnosed with RSV infection as a primary diagnosis. A smaller proportion of patients with RSV infection were diagnosed with a comorbidity (9.2%) or a complication (7.8%).
The baseline demographic data are presented in Table 1. Although there was an overall decrease in the number of patients hospitalized with RSV during the COVID-19 pandemic, the age distribution of patients remained stable. The diagnostic characteristics and the hospitalization rates in our tertiary hospital also remained stable over the study period. We stratified hospitalized patients into 2 age groups; 0-5 years old (n=342, 95.5%) and 6-15 years old (n=16, 4.5%). RSV infection was most common in children <5 years. Of the 33 cases with comorbidities, the mean and maximum CCI scores were 1.67 and 1.00, respectively. Most patients were in the mild comorbidity range, with a CCI score of 1.00 (90.9%). The common comorbidities were pulmonary disease (61.0%), congenital heart disease (21.0%), and cerebral disease (18.0%). Of the 358 confirmed RSV patients, 284 (79.3%) were diagnosed during the RSV seasons of 2016 to 2019 (i.e., before COVID-19), while 74 cases (21.7%) were diagnosed during the RSV seasons of 2020 and 2021 (i.e., during COVID-19).
Table 1.
Baseline demographic characteristics of children hospitalized with RSV infection
| Characteristics | Total (n = 358) | COVID-19 pandemic |
χ2 | p-value | |
|---|---|---|---|---|---|
| Before (n=284) | During (n=74) | ||||
| Sex (female) | 162 (45.3) | 129 (45.5) | 33 (44.6) | 0.016 | 0.89 |
| Age (y) | 1 [0-2] | 1 [0-2] | 2 [1-3] | - | 0.011 |
| Length of hospital stay (day) | 6 [3-10] | 5 [3-10] | 5 [3-12] | - | 0.641 |
| ICD-10 diagnosis code | |||||
| J12.1: Respiratory syncytial virus pneumonia | 332 (92.7) | 264 (79.5) | 68 (20.5) | 0.753 | 0.09 |
| J20.5: Acute bronchitis due to respiratory syncytial virus | 15 (4.2) | 11 (73.3) | 4 (26.7) | 0.343 | 0.55 |
| J21.0: Acute bronchiolitis due to respiratory syncytial virus | 11 (3.1) | 9 (81.8) | 2 (18.2) | 0.043 | 0.83 |
| Primary diagnosis | 297 (83.0) | 235 (79.1) | 62 (20.9) | 0.023 | 0.87 |
| Comorbidity | 33 (9.2) | 26 (28.8) | 7 (21.2) | 0.005 | 0.94 |
| Complication | 28 (7.8) | 23 (81.5) | 5 (18.5) | 0.087 | 0.76 |
| CCI score | |||||
| 1-2 | 30 (90.9) | 23 (76.7) | 7 (23.3) | 0.888 | 0.34 |
| 3-4 | 1 (3.0) | 1 (100) | 0 (0.0) | 0.278 | 0.59 |
| >5 | 2 (6.1) | 2 (100) | 0 (0.0) | 0.573 | 0.44 |
Values are presented as number (%) or median [interquartile range].
RSV, respiratory syncytial virus; COVID-19, coronavirus disease 2019; CCI, Charlson comorbidity index; ICD-10, International Classification of Diseases, 10th edition.
Mann-Whitney U test.
Of the pre-pandemic patients (2016-2019), 264 were diagnosed with RSV pneumonia. Acute bronchitis due to RSV was less common (11 cases), as well as acute bronchiolitis due to RSV (9 cases). During the COVID-19 pandemic (2020-2021), 68 patients were diagnosed with RSV pneumonia, 4 patients with acute bronchitis due to RSV, and 2 patients with acute bronchiolitis due to RSV.
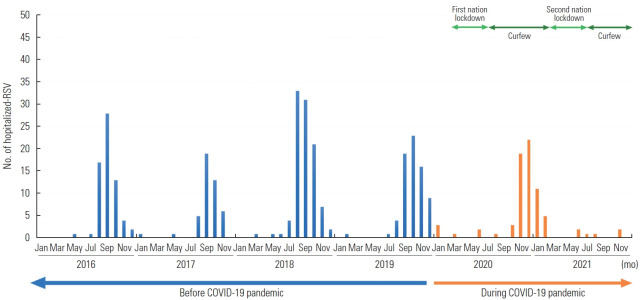
As shown in Figure 1, before the COVID-19 pandemic (2016 to 2019), RSV-associated LRTIs were seen from July to February in northern Thailand and peaked from August to November of each year, which is considered the early winter season. During the COVID-19 pandemic, the number of RSV infections declined during this period. The RSV cases peaked in October and then suddenly decreased until the following January. The RSV season from 2020 to 2021 was shorter and ended more rapidly than the previous 4 RSV seasons (2016 to 2019).
Figure. 1.
Monthly numbers of laboratory-confirmed cases of respiratory syncytial virus (RSV) in Chiang Mai. The blue arrow marks before coronavirus disease 2019 (COVID-19) pandemic period, orange arrow masks for during COVID-19 pandemic period, and green arrows mark the vigilant measure in Thailand. The dark green for nationwide state of emergency (curfew), which restrict movement at night from 20.00 to 4.00 the next, and the light green for lockdown stage, which is gatherings of more than five people are banned except for work, religious ceremonies, and traditional ceremonies.
Clinical Manifestations Before and During the COVID-19 Pandemic
The clinical manifestations of children hospitalized with RSV infection before and during the COVID-19 pandemic showed statistically significant differences in 7 clinical symptoms. The pre-pandemic clinical manifestation data (284 cases) showed fever on admission (69.0%), productive cough (73.2%), increased or new sputum (77.5%), vomiting or nausea (35.9%), cyanosis (21.1%), pallor (29.2%), diarrhea (11.6%), and chest pain (21.5%), while the clinical data during the pandemic (74 cases) showed a decreased incidence of fever on admission (50.0%, p=0.004), productive cough (55.4%, p=0.004), increased or new sputum (59.5%), vomiting or nausea (23.0%, p=0.038), cyanosis (6.8%), and pallor (6.8%, p<0.001). Moreover, no cases of diarrhea or chest pain were reported from 2020 to 2021, as shown in Table 2.
Table 2.
Differences in the clinical manifestations of RSV infection before and during the COVID-19 pandemic
| Clinical manifestation | Total RSV positive (n=358) | COVID-19 pandemic |
χ2 | p-value | |
|---|---|---|---|---|---|
| Before (n = 284) | During (n = 74) | ||||
| Fever on admission | 233 (65.1) | 196 (69.0) | 37 (50.0) | 9.331 | 0.004 |
| Productive cough | 249 (69.6) | 208 (73.2) | 41 (55.4) | 8.811 | 0.004 |
| Increased/new sputum | 264 (73.7) | 220 (77.5) | 44 (59.5) | 9.82 | 0.003 |
| Increased/new dyspnea | 157 (43.9) | 130 (45.8) | 27 (36.5) | 2.051 | 0.188 |
| Sneezing | 195 (54.5) | 157 (55.3) | 38 (51.4) | 0.361 | 0.601 |
| Rhinorrhea | 288 (80.4) | 234 (82.4) | 54 (73.0) | 3.31 | 0.073 |
| Hemoptysis | 90 (25.1) | 71 (25.0) | 19 (25.7) | 0.01 | 0.882 |
| Wheezing | 153 (42.7) | 116 (40.8) | 37 (50.0) | 2.011 | 0.187 |
| Sore throat | 244 (68.2) | 193 (68.0) | 51 (68.9) | 0.021 | 1.000 |
| Vomiting/nausea | 119 (33.2) | 102 (35.9) | 17 (23.0) | 4.431 | 0.038 |
| Cyanosis | 65 (18.2) | 60 (21.1) | 5 (6.8) | 8.15 | 0.004 |
| Pallor | 88 (24.6) | 83 (29.2) | 5 (6.8) | 15.98 | <0.001 |
| Diarrhea | 33 (9.2) | 33 (11.6) | 0 (0.0) | 9.47 | <0.001 |
| Chest pain | 61 (17.0) | 61 (21.5) | 0 (0.0) | 19.15 | <0.001 |
| Seizures | 24 (6.7) | 22 (7.7) | 2 (2.7) | 2.38 | 0.189 |
| Decreased breath sounds | 188 (52.5) | 154 (54.2) | 34 (45.9) | 1.641 | 0.240 |
Values are presented as number (%).
RSV, respiratory syncytial virus; COVID-19, coronavirus disease 2019.
Fischer exact test.
DISCUSSION
In our retrospective study of children aged 0-15 years who were hospitalized with RSV-associated LRTIs, the number of RSV infections admitted to the tertiary care hospital in Chiang Mai, Thailand, from January 2016 to December 2021 showed an interruption of RSV infections during the COVID-19 pandemic.
Our study found that the rate of hospitalizations for RSV infection during the COVID-19 pandemic was lower than before the pandemic, especially in 2021 because of lockdown measures. One measure to control the spread of COVID-19 was to limit public gatherings, especially in poorly ventilated places such as schools. As a result, contact between children was limited, and the spread of respiratory viruses was reduced. Since RSV infections are commonly identified in schools and daycare centers [6,17], children who attended daycare before the COVID-19 pandemic had a higher risk of upper or lower respiratory tract infections than children who stayed at home [18,19]. Thus, the strict public health measures for COVID-19 resulted in a decrease in the rate of respiratory infections in Thailand [11]. According to a previous study, social separation at work helped prevent the spread of respiratory infections [20]. In January 2020, Singapore identified its first case of COVID-19 and responded with strict contact tracing and testing without imposing a lockdown. Following the start of Thailand’s national state of emergency and lockdown in March 2020, the overall number of pediatric admissions due to respiratory tract infections decreased dramatically [21]. During the COVID-19 pandemic, children lived in social isolation and did not attend school, extracurricular activities, and daycare centers. In addition to the changing infection rate, the age of children infected with RSV also increased from a median age of 1 year to a median age of 2 years during the COVID-19 pandemic (Table 1). A study from Australia in 2020 also found a higher median age (18.4 months) during the COVID-19 pandemic [22]. The length of the hospital stay and the primary differential diagnosis were similar both before and during the COVID-19 pandemic. Pneumonia remained the most common RSV-related diagnosis in children.
A 2016-2017 multi-country study by Thongpan et al. [23] showed that the onset of RSV season occurred consistently depending on rainfall, humidity, and temperature. Nonetheless, the vigilant measures employed to protect against the COVID-19 outbreak (i.e., statewide lockdown at the beginning of 2020) impacted the RSV season in Thailand. The season started late in October 2020 and produced a large gap in the seasonal RSV infection graph in 2021 [24]. Before the COVID-19 pandemic, declines in RSV infection rates had not been as dramatic. This was a strong indicator that the spread of RSV was slowed by the social restrictions. Past research has shown that the spread of RSV epidemics decreases when schools are closed and school holidays are timed accordingly [9,25]. Similar results were seen during the pandemic when the Thai government declared a state of emergency in all localities throughout the country, closing schools and other businesses (Figure 1). Only limited health services and grocery stores remained operational. As a result, the rate of respiratory illness dropped dramatically. Furthermore, the population in Thailand was already changing their behaviors in response to public health messaging about how to protect themselves from respiratory virus infection [26]. Although lockdown measures reduced the spread of the virus, transmission continued to occur when large gatherings were held. Therefore, the government of Thailand implemented a curfew to limit the movement of the population and reduce the spread of infection to other provinces. As a result of the curfew, the number of respiratory infections gradually decreased. A study in Finland also reported a shorter RSV season in 2020, shorter than the preceding 4 seasons [25]. In the Nordic regions and Germany, biannual epidemiologic fluctuation in RSV infections was common, with a high season following a low season [27]. In Korea, preventive measures to limit host susceptibility and extrinsic factors such as school closures (2 weeks only) were found to be effective in controlling respiratory viral transmission within the community [28]. The nationwide shutdown was most likely responsible for these results, which is supported by the fact that pandemic viruses spread more quickly than seasonal viruses. The results were similar to those found in studies of children in other countries.
In this study, almost half of the hospitalized RSV infections were considered severe. Infants were considered to be at high risk of RSV-associated LRTI since the majority of RSV hospitalizations were children 0-12 months old [29]. The common presentations were rhinorrhea, cough, dyspnea, sputum production, fever, wheezing, and sore throat, with possible residual inflammatory processes in severe cases [30,31]. In the present study, the occurrence of diarrhea, chest pain, seizures, and cyanosis was low (Table 2), suggesting predominant involvement of the lower respiratory tract, which has also been associated with disease severity [29].
According to available statistics on the clinical manifestations of RSV infection during the COVID-19 pandemic, children had a higher incidence of upper respiratory tract involvement than lower respiratory tract involvement [32]. This finding was similar to a previous study in Japan that found that the clinical manifestations of RSV infection, such as body temperature, wheezing, and consolidation, declined during the COVID-19 pandemic (p<0.05) [33]. Furthermore, Xie [34] found that children were less likely to be infected with the virus during the pandemic, in part because of fewer outdoor activities and less overseas travel. Underdiagnosis in children may also have resulted because they had milder symptoms and underwent fewer laboratory tests.
According to the CCI scores in our study, the rate of comorbidity was mild both before and during the COVID-19 pandemic. In contrast, Aikphaibul et al. [32], who studied the risk factors of RSV infection in children aged 0-5 years, showed that the number of RSV infections with comorbidity was high (51%, 219/427), primarily in children with chronic lung disease (19%) and congenital heart disease (16%). The present study found that the clinical manifestations and severity of symptoms in older children infected with RSV declined during the COVID-19 pandemic. Recent evidence suggests that older children have a mechanism that regulates the interplay of their immune and respiratory systems, which may contribute to milder illness [35]. Another possible reason for the significant decrease in clinical manifestations during the COVID-19 pandemic may be that parents changed their hygiene habits to prevent respiratory infections. Therefore, some reduction was due to what may have been avoidable hospital visits for minor respiratory illnesses during previous RSV seasons. This conclusion was supported by Ryu et al. [36], who found that the strict social distancing measures, telephone consultations, and changes in health-seeking behaviors reduced the use of medical services and resulted in fewer prescriptions to treat ARTIs.
The simplicity, straightforward assumptions, and rapid impact of this study, as well as the inclusion of data from hospitalized children in Chiang Mai, Thailand from January 2016 to December 2021, were its major advantages. However, there were limitations to this study. RSV infection may not have been screened in all patients with ARTIs , and this study may have understated the true burden of RSV infection at our facility. This underestimation may also be more significant in patients who had milder symptoms and were therefore less frequently screened for RSV than patients with severe infections.
As lockdown measures are eased, the RSV infection rate may increase. Therefore, health personnel should be vigilant and have a prevention plan in place to reduce the incidence of RSV infections. An increase in children hospitalized with RSV infection following the relaxation of COVID-19 restrictions should be an important lesson for health providers dealing with future pandemics that impact young children. Ali et al. [12] estimated that the easing of the COVID-19 pandemic and the subsequent relaxation of social distancing measures could lead to a 1-fold to 5-fold rise in the peak magnitude of the upcoming 2022-2023 influenza season for 11 countries in both the southern and northern hemispheres. The financial and healthcare burden of RSV infections highlights the need for continued surveillance to prevent severe respiratory infections and ultimately decrease the mortality rate in children.
Acknowledgments
We would like to thank the Medical Records and Statistics Section of Maharaj Nakorn Chiang Mai Hospital, for their important cooperation in establishing the pediatric preparedness register.
The funding sources did not influence the study design, the collection or interpretation of data, the preparation or approval of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
FUNDING
The study was funded by the Faculty of Medicine, Chiang Mai University, Thailand, grant No. 86-2565.
AUTHOR CONTRIBUTIONS
Conceptualization: Chaiut W, Sapbamrer R, Dacha S, Malasao R. Data curation: Chaiut W, Malasao R. Formal analysis: Chaiut W, Sapbamrer R, Dacha S, Malasao R. Funding acquisition: Chaiut W, Sapbamrer R, Dacha S, Sudjaritruk T, Malasao R. Methodology: Chaiut W, Sapbamrer R, Dacha S, Malasao R. Project administration: Chaiut W, Sapbamrer R, Dacha S, Malasao R. Visualization: Chaiut W, Sapbamrer R, Dacha S, Parwati I, Sumarpo A, Malasao R. Writing – original draft: Chaiut W, Sapbamrer R, Dacha S, Malasao R. Writing – review & editing: Chaiut W, Sapbamrer R, Dacha S, Sudjaritruk T, Parwati I, Sumarpo A, Malasao R.
REFERENCES
- 1.Fry AM, Chittaganpitch M, Baggett HC, Peret TC, Dare RK, Sawatwong P, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One. 2010;5(11):e15098. doi: 10.1371/journal.pone.0015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastula ST, Hackett J, Coalson J, Jiang X, Villafana T, Ambrose C, et al. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997-2012. Open Forum Infect Dis. 2017;4(1):ofw270. doi: 10.1093/ofid/ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horthongkham N, Athipanyasilp N, Sirijatuphat R, Assanasen S, Sutthent R. Prevalence and molecular characterization of human metapneumovirus in influenza a negative sample in Thailand. J Clin Lab Anal. 2014;28(5):398–404. doi: 10.1002/jcla.21700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piralam B, Tomczyk SM, Rhodes JC, Thamthitiwat S, Gregory CJ, Olsen SJ, et al. Incidence of pneumococcal pneumonia among adults in rural Thailand, 2006-2011: implications for pneumococcal vaccine considerations. Am J Trop Med Hyg. 2015;93(6):1140–1147. doi: 10.4269/ajtmh.15-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aikphaibul P, Theerawit T, Sophonphan J, Wacharachaisurapol N, Jitrungruengnij N, Puthanakit T. Risk factors of severe hospitalized respiratory syncytial virus infection in tertiary care center in Thailand. Influenza Other Respir Viruses. 2021;15(1):64–71. doi: 10.1111/irv.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naorat S, Chittaganpitch M, Thamthitiwat S, Henchaichon S, Sawatwong P, Srisaengchai P, et al. Hospitalizations for acute lower respiratory tract infection due to respiratory syncytial virus in Thailand, 2008-2011. J Infect Dis. 2013;208 Suppl 3:S238–S245. doi: 10.1093/infdis/jit456. [DOI] [PubMed] [Google Scholar]
- 7.Praphasiri P, Shrestha M, Patumanond J, Nakphook S, Chawalchitiporn S, Ditsungnoen D, et al. Underlying cardiopulmonary conditions as a risk factor for influenza and respiratory syncytial virus infection among community-dwelling adults aged ≥65 years in Thailand: findings from a two-year prospective cohort study. Influenza Other Respir Viruses. 2021;15(5):634–640. doi: 10.1111/irv.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195(7):1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delestrain C, Danis K, Hau I, Behillil S, Billard MN, Krajten L, et al. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr Pulmonol. 2021;56(12):3669–3673. doi: 10.1002/ppul.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Mattia G, Nenna R, Mancino E, Rizzo V, Pierangeli A, Villani A, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. 2021;56(10):3106–3109. doi: 10.1002/ppul.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaewpan W, Rojpaisarnkit K, Pengpid S, Peltzer K. Factors affecting face mask-wearing behaviors to prevent COVID-19 among Thai people: a binary logistic regression model. Front Psychol. 2022;13:996189. doi: 10.3389/fpsyg.2022.996189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali ST, Lau YC, Shan S, Ryu S, Du Z, Wang L, et al. Prediction of upcoming global infection burden of influenza seasons after relaxation of public health and social measures during the COVID-19 pandemic: a modelling study. Lancet Glob Health. 2022;10(11):e1612–e1622. doi: 10.1016/S2214-109X(22)00358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doung-Ngern P, Suphanchaimat R, Panjangampatthana A, Janekrongtham C, Ruampoom D, Daochaeng N, et al. Casecontrol study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis. 2020;26(11):2607–2616. doi: 10.3201/eid2611.203003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R, Fathima P, Strunk T, de Klerk N, Snelling TL, Richmond PC, et al. RSV prophylaxis use in high-risk infants in Western Australia, 2002-2013: a record linkage cohort study. BMC Pediatr. 2020;20(1):490. doi: 10.1186/s12887-020-02390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu W, Zheng C, Huang S, Zhang Y, Chen Z. Epidemiological trend of RSV infection before and during COVID-19 pandemic: a three-year consecutive study in China. Infect Drug Resist. 2022;15:6829–6837. doi: 10.2147/IDR.S388231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Althouse BM, Flasche S, Minh LN, Thiem VD, Hashizume M, Ariyoshi K, et al. Seasonality of respiratory viruses causing hospitalizations for acute respiratory infections in children in Nha Trang, Vietnam. Int J Infect Dis. 2018;75:18–25. doi: 10.1016/j.ijid.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dotan M, Ashkenazi-Hoffnung L, Samra Z, Livni G, Yarden-Bilavsky H, Amir J, et al. Hospitalization for respiratory syncytial virus bronchiolitis and disease severity in twins. Isr Med Assoc J. 2013;15(11):701–704. [PubMed] [Google Scholar]
- 19.Fanos V, Scarcella A, Puddu M, Gallini F, Tuminelli F, Bragetti P, et al. Respiratory disorders and hospitalization rates during the second RSV season in preterm infants who received palivizumab prophylaxis during their first RSV season. J Chemother. 2009;21(3):302–310. doi: 10.1179/joc.2009.21.3.302. [DOI] [PubMed] [Google Scholar]
- 20.Sinaei R, Pezeshki S, Parvaresh S, Sinaei R. Why COVID-19 is less frequent and severe in children: a narrative review. World J Pediatr. 2021;17(1):10–20. doi: 10.1007/s12519-020-00392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uppala R, Sitthikarnkha P, Niamsanit S, Sutra S, Thepsuthammarat K, Techasatian L, et al. Effect of the COVID-19 pandemic on lower respiratory tract infection determinants in Thai hospitalized children: national data analysis 2015-2020. Trop Med Infect Dis. 2022;7(8):151. doi: 10.3390/tropicalmed7080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foley DA, Yeoh DK, Minney-Smith CA, Martin AC, Mace AO, Sikazwe CT, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin Infect Dis. 2021;73(9):e2829–e2830. doi: 10.1093/cid/ciaa1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thongpan I, Suntronwong N, Vichaiwattana P, Wanlapakorn N, Vongpunsawad S, Poovorawan Y. Respiratory syncytial virus, human metapneumovirus, and influenza virus infection in Bangkok, 2016-2017. PeerJ. 2019;7:e6748. doi: 10.7717/peerj.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39(12):e423–e427. doi: 10.1097/INF.0000000000002845. [DOI] [PubMed] [Google Scholar]
- 26.Chan HC, Tambyah PA, Tee NW, Somani J. Return of other respiratory viruses despite the disappearance of influenza during COVID-19 control measures in Singapore. J Clin Virol. 2021;144:104992. doi: 10.1016/j.jcv.2021.104992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huppertz HI, Kopp MV, Hübner J. Prevention of infections by influenza virus and respiratory syncytial virus after removal of the lockdown measures. Monatsschr Kinderheilkd. 2021;169(11):1072–1074. doi: 10.1007/s00112-021-01278-7. (German) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu S, Han C, Ali ST, Achangwa C, Yang B, Pei S. Impact of public health and social measures on hand-foot-mouth disease transmission and prediction of upcoming season after relaxation of COVID-19 control measures. Res Sq [Preprint] doi: 10.21203/rs.3.rs-1999622/v1. 2022 [cited 2023 Feb 21]. Available from: [DOI] [Google Scholar]
- 29.Chuaychoo B, Rattanasaengloet K, Banlengchit R, Horthongkham N, Athipanyasilp N, Totanarungroj K, et al. Characteristics, complications, and mortality of respiratory syncytial virus compared with influenza infections in hospitalized adult patients in Thailand. Int J Infect Dis. 2021;110:237–246. doi: 10.1016/j.ijid.2021.07.045. [DOI] [PubMed] [Google Scholar]
- 30.Chuaychoo B, Ngamwongwan S, Kaewnaphan B, Athipanyasilp N, Horthongkham N, Kantakamalakul W, et al. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J Clin Virol. 2019;117:103–108. doi: 10.1016/j.jcv.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niyatiwatchanchai N, Deesomchok A, Chaiwong W, Duangjit P, Pothirat C, Liwsrisakun C, et al. Comparative study of early impacts of post-COVID-19 pneumonia on clinical manifestations, pulmonary function, and chest radiographs. Medicina (Kaunas) 2022;58(2):216. doi: 10.3390/medicina58020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aikphaibul P, Theerawit T, Sophonphan J, Wacharachaisurapol N, Jitrungruengnij N, Puthanakit T. Risk factors of severe hospitalized respiratory syncytial virus infection in tertiary care center in Thailand. Influenza Other Respir Viruses. 2021;15(1):64–71. doi: 10.1111/irv.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozeki S, Kawada JI, Yamashita D, Yasufuku C, Akano T, Kato M, et al. Impact of the coronavirus disease 2019 pandemic on the clinical features of pediatric respiratory syncytial virus infection in Japan. Open Forum Infect Dis. 2022;9(11):ofac562. doi: 10.1093/ofid/ofac562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Z. Pay attention to SARS-CoV-2 infection in children. Pediatr Investig. 2020;4(1):1–4. doi: 10.1002/ped4.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu S, Hwang Y, Ali ST, Kim DS, Klein EY, Lau EH, et al. Decreased use of broad-spectrum antibiotics during the coronavirus disease 2019 epidemic in South Korea. J Infect Dis. 2021;224(6):949–955. doi: 10.1093/infdis/jiab208. [DOI] [PMC free article] [PubMed] [Google Scholar]