Abstract
Background
There is growing evidence that patients with COVID-19 are at increased risk of new-onset diabetes. The limited preliminary studies do not provide strong evidence. To assess the association of the SARS-CoV-2 virus with new-onset diabetes and to characterize the population.
Methods
Search PubMed, Embase, Cochrane Library, and Web of Science electronic databases for a limited period from December 2019 to July 2022. Two independent reviewers conducted a thorough review of eligible articles and extracted relevant information. Pooled proportions, risk ratios (RR), and 95% confidence intervals (95% CI) indicated the incidence and risk ratios of events.
Results
The incidence of new-onset diabetes and hyperglycemia in patients with COVID-19 was 5% (P < 0.001) (3 and 30% for new-onset diabetes and hyperglycemia, respectively), with age, ethnicity, time of diagnosis, and study type all having an impact on the incidence (P < 0.05). New-onset diabetes and hyperglycemia were 1.75 times higher in COVID-19 patients than in non-COVID-19 patients. In new-onset diabetes and hyperglycemia population, the percentage of men is 60% (40% for women), with a mortality rate of 17%. The proportion of new-onset diabetes and hyperglycemia after infection with COVID-19 was 25% in men and 14% in women.
Conclusions
The incidence and relative risk of new-onset diabetes and hyperglycemia are elevated after COVID-19 infection, especially in the early COVID-19 and male populations.
Systemic review registration
PROSPERO registration no.: CRD42022382989 https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=382989.
Keywords: COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), new-onset, secondary diabetes, hyperglycemia
Introduction
Since the outbreak of the epidemic at the end of 2019, COVID-19 is still spreading globally. As of November 2022, 629 million confirmed cases and 6.5 million deaths have been reported worldwide (due to the decline in confirmed cases worldwide, the actual number may be underestimated), which has a major impact on the global economy, society, and human health. The multiple factors of public health policy, viral mutations, and COVID-19 vaccine development and vaccination have resulted in a decline in new patients and mortality. While healthy people may be asymptomatic or recover within weeks of developing symptoms, they are also at risk for long-term COVID-19 disease (organ damage that makes it difficult to return to a healthy state, or increased risk of disease) (1).
Numerous investigations have demonstrated that the SARS-CoV-2 virus invades the respiratory system as well as a number of human tissues and organs, impairing their activities. In the study by Al-Aly et al. (2) the effects of COVID-19, which include neurological illnesses, mental health disorders, metabolic disorders, cardiovascular disease, gastrointestinal disorders, weariness, muscle-skeletal discomfort, and anemia, were thoroughly described. Similarly, it has been reported that the human pancreas is a target of the SARS-CoV-2 viral attack (3).
A growing number of clinical observations have shown that COVID-19 positive patients are at greater risk of developing diabetes than negative patients. One study highlighted that infection with the SARS-CoV-2 virus increases the risk of diabetes by ~40%, affecting ~2 in 100 patients (4). A recent meta-analysis by Zhang et al. (5) reported the risk of new-onset diabetes after COVID-19, but the study only included cohort studies. Moreover, this inconsistency also hinders our understanding of the causal relationship between them. As new data become available, there is a need to reassess the relationship between COVID-19 and new-onset diabetes. Therefore, more literature was included in this meta-analysis to assess the incidence of new-onset diabetes after COVID-19.
Method
Search strategy
The current systematic review and meta-analysis follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Appendix S1) (6) and the study is registered with PROSPERO (Registration number: CRD42022382989). Two independent reviewers (JJL and YPL) systematically searched electronic databases PubMed, Embase, Cochrane Library, and Web of Science for relevant literature from December 2019 to July 2022. The search strategy combined three constructs: COVID-19 or SARS-CoV-2, diabetes or hyperglycemia, and new start or second or new diagnosis. We searched for relevant literature regardless of country, language, or article type. See Appendix S2 for detailed search strategies.
Inclusion and exclusion criteria
The inclusion criteria of this study followed the PICOS framework: (1) Participant (P): non-diabetic patients or blood glucose levels within the normal range; (2) Intervention (I): infected with COVID-19; (3) Comparison (C): matched to the experimental group and not infected with COVID-19 (case-control study) or no control group (cross-sectional study); (4) Outcome (O): the number of people with new-onset diabetes or blood glucose values exceeding the normal range; (5) Study design (S): cross-sectional studies, cohort studies, or case-control studies. Data given in the relevant literature were excluded if they could not be extracted or the full text could not be found.
Data extraction and literature quality assessment
Based on the above inclusion and exclusion criteria, two independent reviewers (JJL and YPL) conducted a thorough review of eligible articles and extracted the following information: author, year, country, type of study, participants (sample size, age, number of males [calculated when not directly stated], number of newly diagnosed diabetes), diagnostic criteria for diabetes, time of diabetes diagnosis, type of diabetes, and quality of literature. The quality of the literature for cross-sectional studies, cohort studies, and case-control studies was assessed using the Agency for Health care Research and Quality (AHRQ) (11 points total; low quality 0–3; moderate quality 4–7; high quality 8–11) (7) and the Newcastle-Ottawa Score (NOS) (nine stars; ≤ six stars for poor quality; seven and eight stars for moderate quality; nine stars for high quality) (8). If opinions diverged, they were resolved by consulting a third author.
Statistical analysis
Statistical software R (version 4.1.1) was used for statistical analysis. We used the metaprop package to calculate the pooled risk ratio (RR) and 95% confidence intervals (95% CI). A random-effects model was selected if I2 ≥ 50% and a fixed-effects model was selected if the opposite was true. Subgroup analysis was used to explore potential sources of heterogeneity. Subsequently, the publication bias of the included literature was analyzed by Egger's and Begg's tests. We also constructed funnel plots as a visualization to assess the possibility of publication bias. Furthermore, we tested the robustness of the results by sequentially removing and accumulating each study.
Result
Search results and article selection
We retrieved a total of 3,254 relevant literature from the four databases, and there were still 2,057 pieces of literature after removing duplicate entries. Subsequent scans of titles and abstracts left 111 articles after removing reviews, conference abstracts, case reports, experimental registers, and studies whose content was not relevant to the purpose of this study. The remaining literature was further screened by reviewing the full text according to the inclusion and exclusion criteria formulated above, and 27 articles that met the criteria were eventually included, while 84 were excluded for reasons such as reviews, case reports, or irrelevance to the study content, or unavailability of data. The detailed literature search process is shown in Figure 1.
Figure 1.
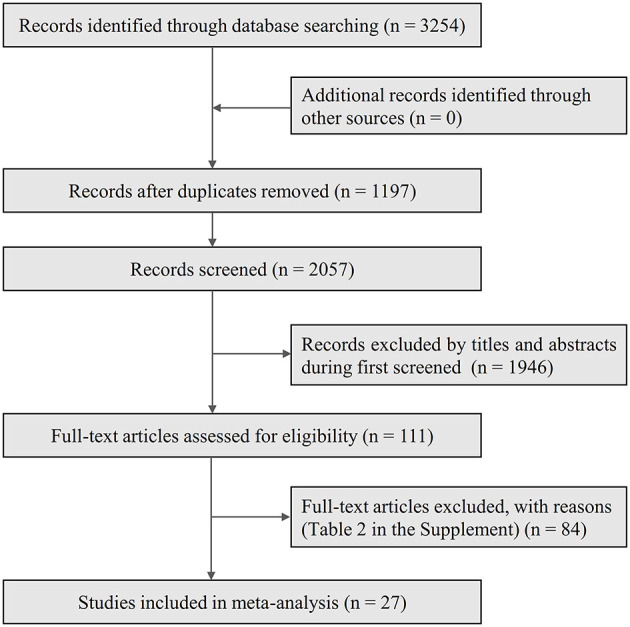
Flow chart of literature screening.
Characteristics of the included studies
The 27 included studies included 14 cross-sectional studies, 11 cohort studies, and 2 case-control studies, including a total of 3,976,089 COVID-19 patients and 33,069,542 non-COVID-19 patients. Nine of the studies were from China (9–17), eight from the United States (4, 18–24), three each from India (25–27) and Italy (28–30), and one each from Bangladesh (31), Egypt (32), the United Kingdom (33), and South Africa (34). Sample sizes ranged from 66 to 2,489,266. The time of diagnosis of new-onset diabetes or hyperglycemia was either newly identified during hospitalization or within an established follow-up period. Four of the studies reported both new-onset diabetes and hyperglycemia (9, 11, 12, 30), three studies reported only new-onset hyperglycemia (16, 17, 25), while the remaining studies reported only new-onset diabetes. Notably, two studies were younger than 18 years (18, 21), four studies had no age limit (22, 26, 31, 33), and five studies did not report age (11, 14, 15, 27, 28), and the remaining 16 studies had ages ≥18 years. The basic characteristics of the included studies are summarized in Tables 1, 2.
Table 1.
The main characteristics of the study of new-onset diabetes and hyperglycemia in the COVID-19 population.
| References | Country | Study type | Ethnicity | Study period | COVID-19 | Age | Male, % | Event | Definition of NDD | Time of diagnosis | Type of diabetes | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akter et al. (31) | Bangladesh | Cross-sectional study | Asian | April 1 and June 30, 2020 | 598 | No restrictions | NR | 10 | HbA1c ≥ 6.5% or a random glucose level ≥ 11.1 mmol/l | During hospitalization | NR | 3 |
| Cromer et al. (20) | USA | Cross-sectional study | Caucasian | March and September 2020 | 1,385 | ≥18 | NR | 77 | No prior history of diabetes, HbA1c ≥ 6.5%, or 2 International Disease Classification codes for any form of diabetes, insulin use, or severe hyperglycemia (≥16.7 mmol/L) at admission | During hospitalization | T2D: 62; The rest did not report | 5 |
| Fadini et al. (28) | Italy | Cross-sectional study | Caucasian | February and April 2020 | 327 | Unclear | 189 (57.80%) | 21 | HbA1c ≥ 6.5% or a random glucose level ≥ 11.1 mmol/l, accompanied by signs and symptoms of hyperglycemia | During hospitalization | NR | 4 |
| Farag et al. (32) | Egypt | Cross-sectional study | Caucasian | 1 April 2020 to 31 May 2020 | 558 | ≥18 | 310 (55.56%) | 65 | No preceding history of DM with FPG ≥ 126 mg/dL or RBG ≥ 200 mg/dL and HbA1c <6.5% or previously undiagnosed DM (FPG ≥ 126 mg/dL or RBG ≥ 200 mg/dL and HbA1c ≥ 6.5% or HbA1c ≥ 6.5% only) | During hospitalization | T1D: 7; T2D:58 | 4 |
| Lampasona et al. (29) | Italy | Cross-sectional study | Caucasian | 25 February and 19 April 2020 | 419 | ≥18 | 272 (64.92%) | 49 | If patients without a diagnosis of diabetes had a mean FPG ≥ 7.0 mmol/l during the hospitalization for COVID-19 pneumonia | During hospitalization | NR | 4 |
| Li et al. (9) | China | Case-control study | Asian | January 22, 2020 to March 17, 2020 | 355 | ≥18 | 180 (50.70%) | NDD: 94 NOH: 129 Total: 223 | Hyperglycaemia: fasting glucose 5.6–6.9 mmol/L and/or HbA1c 5.7–6.4%; NDD: fasting glucose ≥ 7 mmol/L and/or HbA1c ≥ 6.5% | During hospitalization | NR | 6 |
| Lin et al. (10) | China | Cohort study | Asian | December 30th, 2019 and April 12th, 2020 | 3,114 | 18–75 | 1,390 (44.64%) | 351 | At least two FPG readings ≥ 7 mmol/L | During hospitalization | NR | 6 |
| Mithal et al. (25) | India | Cross-sectional study | Asian | July 9, 2020, to August 8, 2020 | 212 | 18–92 | 135 (63.68%) | NOH: 21 | Those who did not meet the criteria for diabetes but required insulin to maintain normoglycemia were classified as NOH | During hospitalization | NR | 4 |
| Nesan et al. (26) | India | Cross-sectional study | Asian | June to November 2020 | 1,222 | No restrictions | NR | 10 | HbA1c ≥ 6.5% | After discharge | NR | 3 |
| Rajueni et al. (27) | India | Cross-sectional study | Asian | December 2020 to April 2021 | 629 | Unclear | NR | 12 | HbA1c ≥ 6.5% | After discharge | NR | 3 |
| Smith et al. (23) | USA | Cross-sectional study | Caucasian | 16 March-2 May 2020 | 70 | 21–100 | NR | 29 | NDD was defined by persistently elevated FBG > 125 mg/dL and requiring insulin therapy. | During hospitalization | NR | 4 |
| Van der Westhuizen et al. (34) | South Africa | Cross-sectional study | Caucasian | 8 June 2020–18 August 2020 | 897 | ≥18 | NR | 125 | HbA1c ≥ 6.5% | During hospitalization | NR | 4 |
| Wang S. et al. (11) | China | Cohort study | Asian | 24 January 2020 to 10 February 2020 | 605 | Unclear | 322 (53.22%) | NDD: 176 NOH: 100 Total: 276 | According to WHO guidelines in terms of admission FBG (<6.1, 6.1–6.9, and ≥7.0 mmol/l) | During hospitalization | NR | 6 |
| Wang Z. et al. (12) | China | Cross-sectional study | Asian | February 9–28, 2020 | 101 | 24–88 | NR | NDD: 16 NOH: 44 Total: 60 | WHO guidelines on medicines for diabetes treatment intensification | During hospitalization | NR | 5 |
| Yang et al. (13) | China | Cross-sectional study | Asian | January 29, 2020, to March 20, 2020 | 69 | ≥18 | 34 (49.28%) | 21 | FBG ≥ 7.0 mmol/L for two times during hospitalization, without glucocorticoid treatment, and without a history of diabetes in COVID-19 patients were defined as NDD | During hospitalization | NR | 6 |
| Yi et al. (14) | China | Cross-sectional study | Asian | January to February 2020 | 470 | Unclear | NR | 3 | HbA1c ≥ 6.5% | During hospitalization | NR | 3 |
| Yuan et al. (15) | China | Cross-sectional study | Asian | 10 January 2020 and 30 March 2020 | 740 | Unclear | 361 (48.78%) | 187 | HbA1c ≥ 6.5% | During hospitalization | NR | 5 |
| Zhang et al. (16) | China | Cohort study | Asian | February 8 to March 21, 2020 | 105 | ≥18 | 52 (49.52%) | NOH: 21 | FPG levels of ≥7.0 mmol/L once and HbA1c levels of <6.5% | During hospitalization | NR | 6 |
| Zhou et al. (17) | China | Case-control study | Asian | January to March 2020 | 66 | ≥18 | 38 (57.58%) | NOH: 22 | No past histories of diabetes, HbA1c <6.5%, RBG > 11.1 mmol/L during hospitalization, and normal blood glucose after discharge from the hospital | During hospitalization | NR | 5 |
DM, diabetes mellitus; FBG, fasting blood glucose; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; NDD, Newly diagnosed diabetes; NOH, New onset hyperglycemia; NR, Not reported; RBG, random blood glucose; T1D, type 1 diabetes; T2D, type 2 diabetes; WHO, World health organization.
Table 2.
The main characteristics of the study of new-onset diabetes and hyperglycemia in the COVID-19 population versus the non-COVID-19 population.
| References | Country | Study type | Ethnicity | Study period | COVID-19 patients | Non-COVID-19 patients | Definition of NDD | Time of diagnosis | Type of diabetes | Study quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age | Male, % | Event | N | Age | Male, % | Event | |||||||||
| Ayoubkhani et al. (33) | UK | Cohort study | Caucasian | 1 January to 31 August 2020 | 36,100 | No restrictions | NR | 400 | 36,100 | No restrictions | NR | 125 | Primary and secondary ICD-10 codes (codes U07.1 and U07.2) | After discharge | T1D or T2D | 7 |
| Barrett et al. (18) (HealthVerity) | USA | Cohort study | Caucasian | March 1, 2020–June 28, 2021 | 439,439 | <18 | 219,427 (49.93%) | 1,120 | 439,439 | <18 | 219,427 (49.93%) | 853 | One or more health care claims with a diabetes diagnosis (ICD-10-CM codes E08–E13) occurring >30 days after the index date (excluding cases of transient, resolved hyperglycemia) | After discharge | T1D or T2D (94.0%) | 6 |
| Barrett et al. (18) (IQVIA) | USA | Cohort study | Caucasian | March 1, 2020–June 28, 2021 | 80,893 | <18 | 40,376 (49.91%) | 68 | 404,465 | <18 | 201,880 (49.91%) | 132 | One or more health care claims with a diabetes diagnosis (ICD-10-CM codes E08–E13) occurring >30 days after the index date (excluding cases of transient, resolved hyperglycemia) | After discharge | T1D or T2D (94.1%) | 6 |
| N | Age | Male, % | Event | N | Age | Male, % | Event | |||||||||
| Birabaharan et al. (19) | USA | Cohort study | Caucasian | 20 January 2020 to 20 January 2021 | 324,360 | ≥18 | NR | 3,934 | 330,734 | ≥18 | NR | 2,632 | One or more ICD-10 E11 | After discharge | T2D | 6 |
| Kendall et al. (21) | USA | Cohort study | Caucasian | March 2020 and December 2021 | 285,628 | <18 | 143,289 (50.17%) | 123 | 285,628 | <18 | 144,029 (50.43%) | 72 | ICD-10 code U07.1 | After discharge | T1D | 5 |
| Laurenzi et al. (30) | Italy | Cohort study | Caucasian | February 25 to May 15, 2020 | 471 | ≥18 | NR | NDD: 39 NOH: 256 Total: 295 | 64 | ≥18 | NR | NDD: 7 NOH: 15 Total: 22 | (1) They had a negative history of diabetes, no prescription of diabetes medications, and a FBG during hospitalization, in the absence of infusions of dextrose, of 7.0 mmol/L or higher (ADA criteria); (2) Hyperglycemia not in the diabetes range if they had random blood glucose levels between 100 and 199 mg/dL or 2 FBG >100 and <126 mg/dL; | During hospitalization | NR | 4 |
| Qeadan et al. (22) | USA | Cohort study | Caucasian | December 1, 2019 through July 31, 2021 | 2,489,266 | No restrictions | 1,081,608 (43.45%) | 5,163 | 24,803,613 | No restrictions | 10,579,475 (42.65%) | 36,348 | T1D associated ICD-10 codes | During hospitalization | T1D | 7 |
| N | Age | Male, % | Event | N | Age | Male, % | Event | |||||||||
| Wander et al. (24) | USA | Cohort study | Caucasian | 1 March 2020 and 10 March 2021 | 126,710 | ≥18 | 109,693 (86.57%) | 748 | 2,651,058 | ≥18 | 2,291,801 (86.45%) | 8,402 | (1) Two or more abnormal laboratory values from plasma or serum (random glucose ≥ 200 mg/dL, fasting glucose ≥ 126 mg/dL, 2-h glucose from an oral glucose tolerance test ≥ 200 mg/dL) or whole blood (A1c ≥ 6.5%); (2) Two outpatient or one inpatient ICD-10 codes of E08–E13; or (3) receipt of an initial and one refill prescription of a glucose-lowering medication. | After discharge | T1D, T2D or other | 7 |
| Xie et al. (4) | USA | Cohort study | Caucasian | March 1, 2020, and Sept 30, 2021 | 181,280 | ≥18 | 159,666 (88.08%) | 7,396 | 4,118,441 | ≥18 | 3,655,034 (88.75%) | 127,858 | The ICD-10 codes (E08.X to E13.X) or a HbA1c measurement of more than 6.4% (46 mmol/mol) | After discharge | Mostly T2D | 7 |
ADA, American Diabetes Association; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; ICD-10, the International Classification of Diseases, 10th revision; NDD, Newly diagnosed diabetes; NOH, New onset hyperglycemia; NR, Not reported; T1D, type 1 diabetes; T2D, type 2 diabetes.
Quality assessment
The AHRQ was used to assess the quality of the literature for cross-sectional studies, of which four were rated as low quality (14, 26, 27, 31) and the remaining 10 studies were rated as moderate quality. The mean score was 4.07 and was judged to be of moderate quality overall. NOS was used to assess the quality of the literature for both cohort and case-control studies. Four of the cohort studies were rated as moderate quality (4, 22, 24, 33), and the remaining seven were rated as low quality. The average score was 6.1, and the comprehensive quality is medium. Both case-control studies were of low quality, with an average score of 5.5 (Tables 1, 2). Subgroup analysis based on the quality level of the literature showed a higher proportion of new-onset diabetes and hyperglycemia in the medium-quality literature (RR = 0.07; 95% CI, 0.03–0.17). However, there was no significant difference between the two groups (P = 0.27) (Table 3).
Table 3.
Subgroup analysis of new-onset diabetes and hyperglycemia.
| No. of studies | Proportion/RR | 95%CI | P | I 2 | Q | Psubgroup(X2 test) | |
|---|---|---|---|---|---|---|---|
| Incidence of new-onset diabetes and hyperglycemia in the COVID-19 population | |||||||
| Age | |||||||
| No restrictions | 4 | 0.01 | 0.00–0.02 | <0.01 | 100% | 70.27 | <0.01 |
| <18 | 3 | 0.00 | 0.00–0.00 | <0.01 | 100% | ||
| ≥18 | 16 | 0.13 | 0.07–0.25 | 0 | 100% | ||
| Unclear | 5 | 0.07 | 0.01–0.30 | <0.01 | 99% | ||
| Ethnicity | |||||||
| Asian | 13 | 0.11 | 0.04–0.26 | <0.01 | 99% | 4.83 | 0.03 |
| Caucasian | 15 | 0.02 | 0.01–0.07 | 0 | 100% | ||
| Time of diagnosis | |||||||
| During hospitalization | 18 | 0.13 | 0.06–0.27 | 0 | 100% | 22.14 | <0.01 |
| After discharge | 10 | 0.01 | 0.00–0.02 | 0 | 100% | ||
| Study type | |||||||
| Cross-sectional study | 14 | 0.08 | 0.04–0.16 | <0.01 | 98% | 25.35 | <0.01 |
| Cohort study | 12 | 0.02 | 0.00–0.07 | 0 | 100% | ||
| Case-control study | 2 | 0.47 | 0.25–0.87 | <0.01 | 92% | ||
| Study quality | |||||||
| Low | 14 | 0.03 | 0.01–0.11 | 0 | 100% | 1.24 | 0.27 |
| Moderate | 14 | 0.07 | 0.03–0.17 | 0 | 100% | ||
| Sample size | |||||||
| <10,000 | 20 | 0.14 | 0.07–0.25 | 0 | 99% | 35.54 | <0.01 |
| >10,000 | 8 | 0.00 | 0.00–0.01 | 0 | 100% | ||
| The risk ratio for new-onset diabetes and hyperglycemia in COVID-19 vs. non-COVID-19 populations | |||||||
| Age | |||||||
| No restrictions | 2 | 2.11 | 0.95–4.70 | <0.01 | 98% | 0.68 | 0.71 |
| <18 | 3 | 1.76 | 1.19–2.60 | <0.01 | 90% | ||
| ≥18 | 4 | 1.58 | 1.33–1.88 | <0.01 | 97% | ||
| Time of diagnosis | |||||||
| During hospitalization | 2 | 1.51 | 1.22–1.88 | 0.15 | 51% | 1.12 | 0.29 |
| After discharge | 7 | 1.81 | 1.41–2.32 | <0.01 | 97% | ||
| Study quality | |||||||
| High | 4 | 1.81 | 1.23–2.66 | <0.01 | 98% | 0.09 | 0.77 |
| Moderate | 5 | 1.69 | 1.36–2.11 | <0.01 | 83% | ||
RR, risk ratio.
Main results of the meta-analysis
Incidence of new-onset diabetes and hyperglycemia after COVID-19 infection
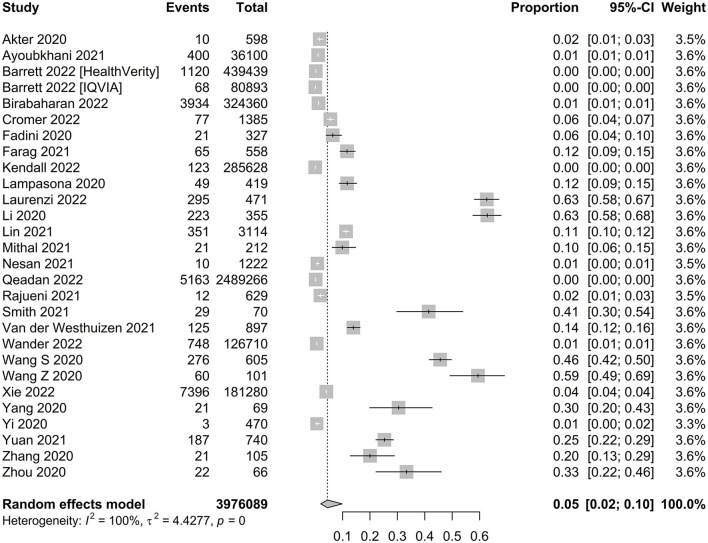
The pooled proportion of new-onset diabetes and hyperglycemia after a positive diagnosis of COVID-19 was 0.05 (95% CI, 0.02–0.10, P < 0.001, n = 3,976,089) (Figure 2). Age subgroup analysis showed a higher proportion of new-onset diabetes and hyperglycemia in patients aged ≥ 18 years compared with those aged <18 years (0.13 for ≥18 years old, 0.00 for <18 years old, P < 0.01). In a subgroup analysis by race, the prevalence of new-onset diabetes and hyperglycemia was significantly higher in Asian than in Caucasian populations (0.11 for Asian, 0.02 for Caucasian; P = 0.03). According to the subgroup analysis of diagnosis time, the proportion of new-onset diabetes and hyperglycemia diagnosed during hospitalization was significantly higher than after discharge (0.13 during hospitalization, 0.01 after discharge, P < 0.01). Likewise, subgroup analysis by study type showed the highest proportion of pooled case-control studies (0.47 for case-control studies, 0.08 for cross-sectional studies, and 0.02 for cohort studies, P < 0.01). In addition, subgroup analysis of study quality showed a higher proportion of studies combined with medium quality scores than with low quality scores, however, there was no significant difference between groups (0.07 for medium quality and 0.03 for low quality; P = 0.27). Analysis based on sample size subgroups showed that the prevalence of new-onset diabetes and hyperglycemia was significantly higher in studies with small sample sizes than in studies with large sample sizes (0.14 for sample sizes <10,000 and 0.00 for sample sizes >10,000; P < 0.01). We then performed a meta-analysis of new-onset diabetes and new-onset hyperglycemia, which showed that the proportion of new-onset diabetes was 0.03 (95% CI, 0.01–0.07, P = 0, n = 3,975,706) (Supplementary Figure S1), and the proportion of new-onset hyperglycemia was 0.30 (95% CI, 0.18–0.42, P < 0.01, n = 1,915) (Supplementary Figure S2).
Figure 2.
Forest plot of the incidence of new-onset diabetes and hyperglycemia.
New-onset diabetes and hyperglycemia in COVID-19 patients vs. non-COVID-19
Overall, the pooled RR values based on a randomized controlled model showed a significant difference in the proportion of COVID-19 (n = 3,964,147) and non-COVID-19 (n = 33,069,542) groups with new-onset diabetes and hyperglycemia (RR = 1.75, 95% CI, 1.43 to 2.14) and significant heterogeneity (I2 = 96%; P < 0.01) (Supplementary Figure S3). Subgroup analysis of age, study quality, and time of diagnosis all showed no significant differences between subgroups (all P > 0.05) (Table 3).
Characteristics of new-onset diabetes and hyperglycemia populations in COVID-19 patients
Notably, there were more men than women in the new-onset diabetes and hyperglycemia groups (60 and 40%, respectively, P < 0.01, n = 6,182) (Supplementary Figures S4, S5) Mortality in the new-onset diabetes and hyperglycemia group was 17% (95% CI, 0.11–0.25, P < 0.01, n = 1,161) (Supplementary Figure S6). In another analysis, men had higher rates of new-onset diabetes and hyperglycemia after COVID-19 infection than women (men: 25%, 95% CI, 0.11–0.38, P < 0.01, n = 1,084,297; women: 14%, 95% CI, 0.06–0.24, P < 0.01, n = 1,266,926) (Supplementary Figures S7, S8).
Sensitivity analysis
We used a removal-by-study and cumulative-by-study approach to evaluate the stability of the results of the above two meta-analyses. First, under the random-effects model, we sequentially deleted one literature and merged the remaining literature, and the results showed that none of the merged values were significantly different after deleting a study (Supplementary Figures S9, S10). Similarly, for the cumulative method, under the random-effects model, we used sequentially adding one literature and merging the results, and the results showed that none of the merged values were significantly different after adding a study (Supplementary Figures S11, S12) In conclusion, both methods showed robust results.
Publication bias
We performed a publication bias test for meta-analysis of the incidence of new-onset diabetes and hyperglycemia after infection with COVID-19 (n > 10). We found no publication bias by Egger test (intercept = −4.4375, SE = 0.4392, P = 0.1971). (Supplementary Figure S13A) Similarly, Begg's test results showed no publication bias (P = 0.1332) (Supplementary Figure S13B). Visual review of funnel plots revealed publication bias (Supplementary Figure S13C), and we used the cut-and-patch method to add nine studies to achieve symmetry (Supplementary Figure S13D).
Discussion
To the best of our knowledge, this study is the most recent meta-analysis evaluating the occurrence of new-onset diabetes and hyperglycemia after infection with COVID-19. Overall, the incidence of new-onset diabetes and hyperglycemia after COVID-19 infection was 5%, with age, ethnicity, time of diagnosis, and study type all having an impact on the incidence. Notably, the incidence was 3 and 30% when new-onset diabetes and new-onset hyperglycemia were proportionally combined separately. Further, new-onset diabetes and hyperglycemia were 1.75 times higher in COVID-19 patients than in non-COVID-19 patients. Statistical description of the population with new-onset diabetes and hyperglycemia revealed a higher proportion of males (60%) than females (40%) and a mortality rate of 17%. Based on the total sample size, a much higher proportion of male COVID-19 patients (25%) developed new-onset diabetes and hyperglycemia than females (14%).
According to the latest data released by the International Diabetes Federation, there are 537 million people with diabetes worldwide. This meta-analysis found an alarming 5% prevalence of new-onset diabetes and hyperglycemia after COVID-19 infection in people without prior diabetes. Even more worrisome is the reported higher risk of death from new-onset diabetes compared to known diabetes in patients hospitalized with COVID-19 (9). In a recent study on the association between COVID-19 and diabetes in children (under 18 years of age), an almost 72% increase in newly diagnosed T1D was seen in patients with COVID-19 compared to the non-COVID-19 respiratory population (21). However, the prevalence of new-onset diabetes and hyperglycemia was much lower in children than in adults in the age-based subgroup analysis. This result may be related to the fact that fewer studies are currently examining COVID-19 and childhood diabetes. Subgroup analysis for ethnicity showed an incidence of 11% in Asian populations and 2% in Caucasians, a result that may be related to genetic susceptibility (35). Further, we combined the incidence of new-onset diabetes and hyperglycemia during hospitalization and post-discharge separately, showing a 13% incidence in hospital and only a 1% incidence after discharge. This interesting phenomenon may be observed in the study by Kendall et al. (21), where there was a decreasing trend in the number of new T1D diagnoses at 1, 3, and 6 months, respectively. More interestingly, Cromer et al. reported that newly diagnosed diabetes was characterized by hospitalized hyperglycemia that usually subsided after the acute illness subsided, with the final data showing that only 7.8% of patients required insulin (20). Subgroup analysis by study type showed a high incidence of new-onset diabetes and hyperglycemia in the case-control group of 47%, which we interpreted as perhaps an error due to the small sample size. Similarly, subgroup analyses by study quality, which we attributed to the small number of low- and high-quality included literature, had a larger error. Interestingly, for subgroup analysis by sample size, we found that the small sample study prevalence (14%) was much higher than the large sample study prevalence (0.00%). We speculate that the possible reason for this is that the large sample data were derived from a web-based database, whereas the small sample studies were mostly from inpatients. In contrast, hospitalized patients either had the comorbid underlying disease or severe clinical symptoms after SARS-CoV-2 infection. When we combined the incidence of new-onset diabetes and new-onset hyperglycemia separately, we found that the incidence of new-onset hyperglycemia (30%) was much higher than that of new-onset diabetes (3%), which we explain as a possible reason for this phenomenon because the time of diagnosis in all studies of new-onset hyperglycemia was during hospitalization.
The results of this meta-analysis showed that the risk of new-onset diabetes and hyperglycemia was significantly higher in the COVID-19 population than in the non-COVID-19 population (RR = 1.75). The current study suggested a bidirectional relationship between COVID-19 and diabetes (36). In other words, COVID-19 not only aggravates the condition in diabetic patients, but also may induce new-onset diabetes in normal individuals. Cromer et al. (20) suggested that COVID-19 infection may not directly cause diabetes, but may promote further progression in patients with prediabetes or in those with undiagnosed diabetes; the authors also suggested that new-onset diabetes and hyperglycemia may be a transitional disease related to COVID-19. In conclusion, we suggest that the significantly higher incidence of new-onset diabetes and hyperglycemia in the COVID-19 population compared with the non-COVID-19 population may be the result of the combined effect of “a bidirectional relationship between COVID-19 and diabetes”.
The current meta-analysis found a higher proportion of men than women with new-onset diabetes and hyperglycemia (60 vs. 40%). Based on the total sample size, the probability of new-onset diabetes and hyperglycemia after infection with COVID-19 was similarly higher in men than in women (25 vs. 14%). According to the International Diabetes Federation 2021 Global Diabetes Map (10th edition), the prevalence of diabetes was slightly higher in men than in women among adults aged 20–79 years (10.8 vs. 10.2%). Therefore, we suggested that males are more likely to develop new-onset diabetes when SARS-CoV-2 virus induces new-onset diabetes in healthy population compared to females. Further, it has been suggested that the interaction between COVID-19 and diabetes increases serum inflammatory cytokine levels as an important cause of death in heavy and critically ill COVID-19 patients (37).
Currently, some scholars believed that the new-onset diabetes caused by SARS-CoV-2 might be a new type of diabetes (36) and perhaps a transient hyperglycemia (20). Laboratory studies have shown that pancreatic islet cells are highly sensitive to SARS-CoV-2 virus. SARS-CoV-2 infection causes islet cell stress response and high expression of chemokines (3). Wu et al. (38) found that SARS-CoV-2 receptors (ACE2 and related entry factors, such as TMPRSS2, NRP1, and TRFC) were expressed in β-cells after infection with the virus, which in turn attenuated insulin expression levels and induced β-cell apoptosis. However, Accili (39) argued that diabetic ketoacidosis after COVID-19 should require conventional insulin therapy if the virus causes permanent loss of β-cell function, but the challenge in clinical practice is mainly extrinsic to the β-cells. In conclusion, the mechanism between COVID-19 and diabetes mellitus needs to be further demonstrated.
There are some limitations of this study. First, most of the studies lacked baseline data prior to COVID-19 infection. According to the theory of the “bidirectional relationship between COVID-19 and diabetes”, new-onset diabetes and hyperglycemia may be the result of pre-diabetes or further progression in patients with undiagnosed diabetes. Second, according to Cromer et al. (20), who observed that patients with COVID-19 have “early onset hyperglycemia and a large part of it subsides later”, the incidence of new-onset diabetes and hyperglycemia varies considerably by the time of diagnosis. Therefore, the inconsistent follow-up time after discharge had a degree of influence on the judgment of the outcome. Third, corticosteroids (glucocorticoids) as a routine drug for the treatment of patients with COVID-19 also caused hyperglycemia in patients (40), and we could not determine whether new-onset diabetes and hyperglycemia were caused by SARS-CoV-2 or corticosteroids. Finally, risk factors for new-onset diabetes (age, obesity, pregnancy, mental status and family history of diabetes, etc.) were not assessed in groups, which does not facilitate a precise understanding of COVID-19-induced new-onset diabetes and hyperglycemia. In conclusion, our knowledge of SARS-CoV-2 is still limited.
Conclusion
The incidence and relative risk of new-onset diabetes and hyperglycemia are elevated after COVID-19 infection, especially in the early COVID-19 and male populations. We hypothesize that COVID-19-related hyperglycemia may be a transient phenomenon, with most patients returning to normal blood glucose ranges over time. Future researchers should work on the potential mechanisms of the relationship between COVID-19 and diabetes to provide effective preventive measures and treatments for the development of diabetes in the context of COVID-19.
Author contributions
JL, ZW, and YL participated in the literature searches, abstracts and full-text reviews, data extraction, synthesis and interpretation of data, and drafting of manuscripts. HZ and LH contributed to evaluating the quality of the literature studies and critically revised the manuscript. NL and LH initiated and designed the study, helped to explain the data, and modified the manuscript. All authors read and approved the final manuscript and are responsible for the content.
Acknowledgments
We thank all authors for their contributions to the systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1170156/full#supplementary-material
References
- 1.Group P-CC. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. (2022) 10:761–75. 10.1016/S2213-2600(22)00127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. (2021) 594:259–64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 3.Tang X, Uhl S, Zhang T, Xue D, Li B, Vandana JJ, et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. (2022) 33:1577–1591.e7. 10.1016/j.cmet.2021.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. (2022) 10:311–21. 10.1016/S2213-8587(22)00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Mei Q, Zhang Z, Walline JH, Liu Y, Zhu H, et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med. (2022) 20:444. 10.1186/s12916-022-02656-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wei L, He L, Sun J, Liu N. Interferon-induced transmembrane protein 3 gene polymorphisms are associated with COVID-19 susceptibility and severity: a meta-analysis. J Infect. (2022) 84:825–33. 10.1016/j.jinf.2022.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. (2020) 35:49–60. 10.1007/s10654-019-00576-5 [DOI] [PubMed] [Google Scholar]
- 9.Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. (2020) 22:1897–906. 10.1111/dom.14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Chen Z, Ding T, Liu H, Zhou F, Huang X, et al. Newly-diagnosed diabetes and sustained hyperglycemia are associated with poorer outcomes in COVID-19 inpatients without pre-existing diabetes. Diabetes Metab Syndr Obes. (2021) 14:4469–82. 10.2147/DMSO.S332819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. (2020) 63:2102–11. 10.1007/s00125-020-05209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. (2020) 164:108214. 10.1016/j.diabres.2020.108214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JK, Zhao MM, Jin JM, Liu S, Bai P, He W, et al. New-onset COVID-19-related diabetes: an early indicator of multi-organ injury and mortally of SARS-CoV-2 infection. Curr Med. (2022) 1:6. 10.1007/s44194-022-00006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi H, Lu F, Jin X, Chen R, Liu B, Dong X, et al. Clinical characteristics and outcomes of coronavirus disease 2019 infections among diabetics: a retrospective and multicenter study in China. J Diabetes. (2020) 12:919–28. 10.1111/1753-0407.13098 [DOI] [PubMed] [Google Scholar]
- 15.Yuan S, Li H, Chen C, Wang F, Wang DW. Association of glycosylated haemoglobin HbA1c levels with outcome in patients with COVID-19: a retrospective study. J Cell Mol Med. (2021) 25:3484–97. 10.1111/jcmm.16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Li H, Zhang J, Cao Y, Zhao X, Yu N, et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab. (2020) 22:1443–54. 10.1111/dom.14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Ye S, Wang W, Li S, Hu Q. Clinical features of COVID-19 patients with diabetes and secondary hyperglycemia. J Diabetes Res. (2020) 2020:3918723. 10.1155/2020/3918723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years - United States, March 1, 2020-June 28, 2021. Morb Mortal Wkly Rep. (2022) 71:59–65. 10.15585/mmwr.mm7102e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birabaharan M, Kaelber DC, Pettus JH, Smith DM. Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: A cohort study. Diabetes Obes Metab. (2022) 24:1176–9. 10.1111/dom.14659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cromer SJ, Colling C, Schatoff D, Leary M, Stamou MI, Selen DJ, et al. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Comp. (2022) 36:108145. 10.1016/j.jdiacomp.2022.108145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw Open. (2022) 5:e2233014. 10.1001/jamanetworkopen.2022.33014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qeadan F, Tingey B, Egbert J, Pezzolesi MG, Burge MR, Peterson KA, et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner Real-World Data. PLoS ONE. (2022) 17:e0266809. 10.1371/journal.pone.0266809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. (2021) 93:409–15. 10.1002/jmv.26227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wander PL, Lowy E, Beste LA, Tulloch-Palomino L, Korpak A, Peterson AC, et al. The incidence of diabetes among 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care. (2022) 45:782–8. 10.2337/dc21-1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mithal A, Jevalikar G, Sharma R, Singh A, Farooqui KJ, Mahendru S, et al. High prevalence of diabetes and other comorbidities in hospitalized patients with COVID-19 in Delhi, India, and their association with outcomes. Diabetes Metab Syndr. (2021) 15:169–75. 10.1016/j.dsx.2020.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesan GSCQ, Keerthana D, Yamini R, Jain T, Kumar D, Eashwer A, et al. 3-Month symptom-based ambidirectional follow-up study among recovered COVID-19 patients from a tertiary care hospital using telehealth in Chennai, India. Inquiry. (2021) 58:469580211060165. 10.1177/00469580211060165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajueni K, Ambekar R, Solanki H, Momin AA, Pawar S. Assessment of the possible causes of diabetes mellitus developed in patients post covid-19 treatment in a tertiary care hospital. Int J Pharm Pharm Sci. (2021) 13:11–5. 10.22159/ijpps.2021v13i9.42508 [DOI] [Google Scholar]
- 28.Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. (2020) 168:108374. 10.1016/j.diabres.2020.108374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. (2020) 63:2548–58. 10.1007/s00125-020-05284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurenzi A, Caretto A, Molinari C, Mercalli A, Melzi R, Nano R, et al. No evidence of long-term disruption of glycometabolic control after SARS-CoV-2 infection. J Clin Endocrinol Metab. (2022) 107:e1009–e19. 10.1210/clinem/dgab792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akter F, Mannan A, Mehedi HMH, Rob MA, Ahmed S, Salauddin A, et al. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab Syndr. (2020) 14:2031–8. 10.1016/j.dsx.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farag AA, Hassanin HM, Soliman HH, Sallam A, Sediq AM, Abd Elbaser ES, et al. Newly diagnosed diabetes in patients with COVID-19: different types and short-term outcomes. Trop Med Infect Dis. (2021) 6:142. 10.3390/tropicalmed6030142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. (2021) 372:n693. 10.1136/bmj.n693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Westhuizen JN, Hussey N, Zietsman M, Salduker N, Manning K, Dave JA, et al. Low mortality of people living with diabetes mellitus diagnosed with COVID-19 and managed at a field hospital in Western Cape Province, South Africa. South Afr Med J. (2021) 111:961–7. 10.7196/SAMJ.2021.v111i10.15779 [DOI] [PubMed] [Google Scholar]
- 35.Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. (2020) 582:240–5. 10.1038/s41586-020-2263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catriona C, Paolo P. SARS-CoV-2 induced post-translational protein modifications: a trigger for developing autoimmune diabetes? Diabetes Metab Res Rev. (2022) 38:e3508. 10.1002/dmrr.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, et al. Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2 (COVID-19) infection. Diabetes Obes Metab. (2020) 22:1907–14. 10.1111/dom.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accili D. Can COVID-19 cause diabetes? Nat Metab. (2021) 3:123–5. 10.1038/s42255-020-00339-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fetters KB, Judge SP, Daar ES, Hatlen TJ. Burden of hyperglycemia in patients receiving corticosteroids for severe COVID-19. Mayo Clin Proc Innov Qual Outcomes. (2022) 6:484–7. 10.1016/j.mayocpiqo.2022.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.