Abstract
Objective:
With legislative changes to cannabis legalization and increasing prevalence of use, cannabis is the most commonly used federally illicit drug in pregnancy. Our study aims to assess the perinatal outcomes associated with prenatal cannabis use disorder.
Methods:
We conducted a retrospective cohort study using California linked hospital discharge-vital statistics data and included singleton, non-anomalous births occurring between 23 to 42 weeks gestational age. Chi-squared and multivariable logistic regression were utilized for statistical analyses.
Results:
A total of 2,380,446 patients were included and 9,144 (0.38%) were identified as using cannabis during pregnancy. There was a significantly increased risk for adverse birthing person outcomes, including gestational hypertension (aOR 1.19, 95% CI 1.06–1.34; p = 0.004), preeclampsia (aOR 1.16, 95% CI 1.0–1.28; p=0.006), preterm delivery (aOR 1.45, 95% CI 1.35–1.55; p<0.001), and severe maternal morbidity (aOR 1.22, 95% CI 1.02–1.47; p=0.033). Prenatal cannabis use disorder was also associated with an increased risk of neonatal outcomes including respiratory distress syndrome (aOR 1.16, 95% CI 1.07–1.27; p<0.001), small for gestational age (aOR 1.47, 95% CI 1.38–1.56; p<0.001), neonatal intensive care unit admission (aOR 1.24, 95% CI 1.16–1.33; p<0.001), and infant death (aOR 1.86, 95% CI 1.44–2.41; p<0.001). There was no statistically significant difference in stillbirth (aOR 0.96, 95% CI 0.69–1.34; p=0.80) and hypoglycemia (aOR 1.22, 95% CI 1.00–1.49; p=0.045)
Conclusion:
Our study suggests that prenatal cannabis use disorder is associated with increased maternal and neonatal morbidity and mortality. As cannabis use disorder in pregnancy is becoming more prevalent, our findings can help guide preconception and prenatal counseling.
Keywords: cannabis, marijuana, neonatal, fetal, pregnancy
INTRODUCTION
Recreational and medicinal cannabis legislation in the United States (US) has expanded the accessibility and acceptability of cannabis, with the prevalence of use in pregnancy rapidly rising the last decade.1 Studies have reported that the prevalence of prenatal cannabis use ranges widely from 3% up to 35% in the US, largely dependent on age.2–5 Cannabis is the number one used federally illicit substance in pregnancy.5,6 The American College of Obstetricians and Gynecologists6 and other national society guidelines7,8 have advised pregnant and lactating individuals to abstain from cannabis. However, approximately half of pregnant individuals who use cannabis continue to use throughout pregnancy, particularly in the first trimester during organogenesis when the fetus is most sensitive to adversity.1,9–13 This trend is concerning given the existing literature suggests an adverse effect on pregnancy and fetal outcomes, and offspring health and developmental trajectories (Figure 1).14–23
Figure 1:
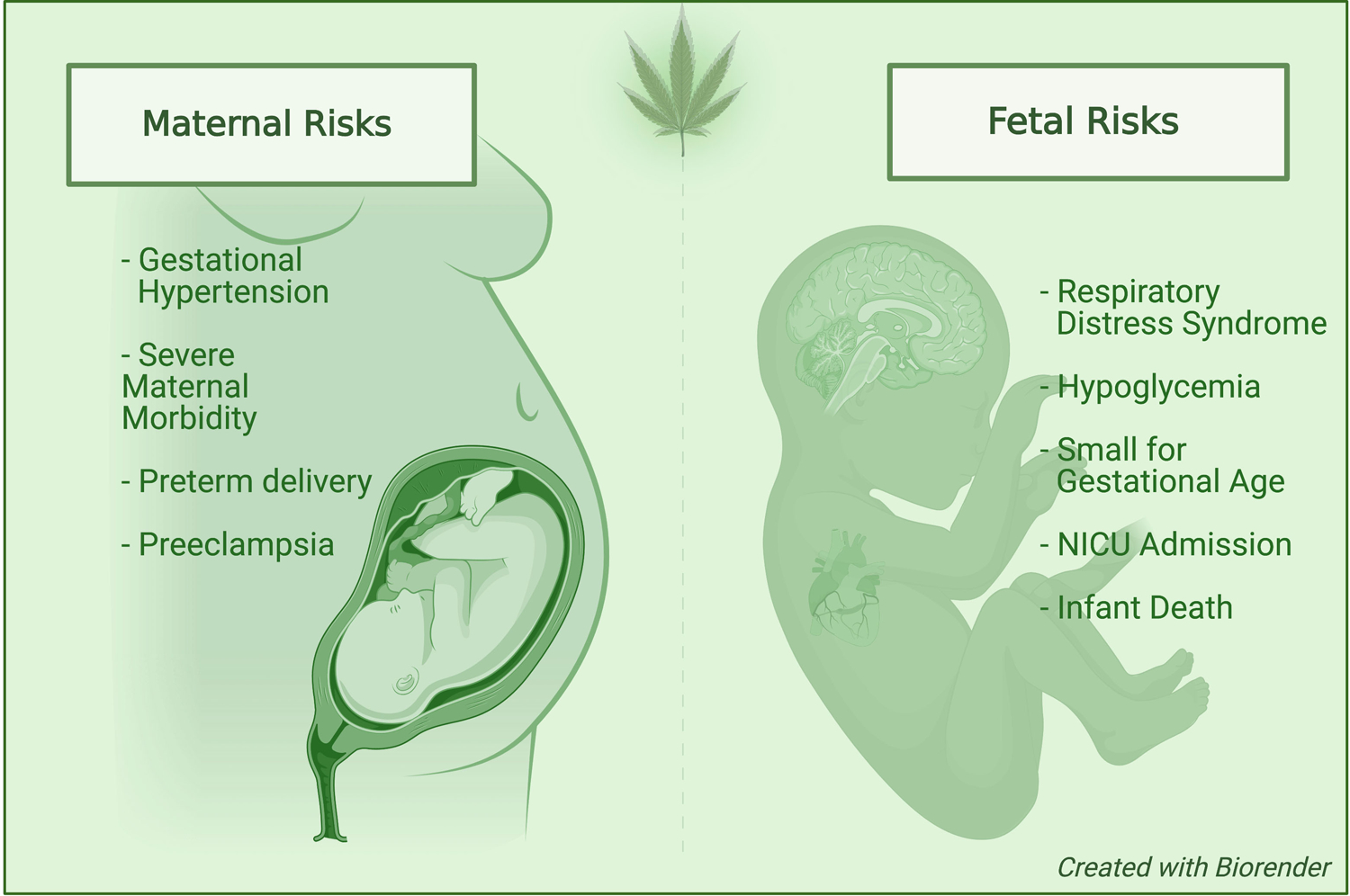
Perinatal complications associated with prenatal cannabis use disorder.
At a biochemical level, the primary psychoactive substance in cannabis, delta-9-tetrahydrocannabinol (THC), readily crosses the placental barrier and binds to cannabinoid receptors (CB1 and CB2) on the placenta and fetus.24 The ability of THC to disrupt endogenous cannabinoid signaling results in neurotoxicant effects at the level of glutamatergic, GABAergic, dopaminergic, serotonergic, and adrenergic neurotransmitter systems.25,26 Thus, it is biologically plausible that cannabis exposure in pregnancy can result in some degree of placental and offspring developmental disruption.
Despite increasing rates of prenatal cannabis use, there has been limited counseling by providers of obstetric patients regarding the potential health implications of the drug.27 This in part stems from providers’ limited understanding of cannabis, mostly due to difficulty synthesizing the available conflicting evidence on prenatal cannabis exposure and cannabis use disorder.28 As a result, patients often perceive this lack of communication as implicit endorsement of its safety in pregnancy.4,29
Human studies on cannabis in pregnancy have demonstrated inconsistent and mixed results, but overall suggest an association with adverse perinatal outcomes.30 Some studies have demonstrated an increased risk of maternal anemia, maternal hypertensive disorders, neonatal intensive care unit (NICU) admission,14,17 preterm birth,16,17 low birth weight,14–17 infant death,18 miscarriage,19 stillbirth,20 and neuropsychologic impairments.21–23 However, other studies have found no such associations, or attribute these associations to confounding from concomitant substance use or socioeconomic status.16,31–34
The existing literature on cannabis use disorder in pregnancy has methodologic limitations including small sample sizes, polysubstance use, and lack of statistical adjustment for important confounding factors, especially prenatal tobacco use.24,35 There may be also other contributions to the heterogeneity of these studies, such as the variation in potency and bioavailability of different strains of cannabis plants and the delivery route used.36 As a large portion of the prenatal cannabis literature is from the 1980s, prior to widespread cannabis legalization, some of this data may also be limited by reporting bias.
Due to the paucity and heterogeneity of data on prenatal cannabis use disorder on perinatal outcomes, more research is needed to elucidate the impact of cannabis on neonatal and maternal health. The purpose of this study is to add to the growing literature on cannabis exposure in pregnancy. By using a large population-based birth cohort, we add to the available data that are primarily derived from smaller sample sizes. Our study’s objective is to assess the association between reported prenatal cannabis use disorder and maternal and neonatal outcomes.
METHODS
We conducted a retrospective cohort study of singleton, non-anomalous births occurring between the gestational age of 23–42 weeks in California (2007–2011). We identified individuals through the California Vital Statistics Birth Certificate data, which is linked with California Patient Discharge Data by the California Office of Statewide Health Planning and Development Health Care Information Resource Center under the State of California Health and Human Services Agency. Linkage was performed by California Office of Statewide Health Planning and Development. We utilized data from 2007–2011, the most recent data currently available in California. Institutional Review Board approval was obtained from the Committee for the Protection of Human Subjects at California and the IRB at Oregon Health & Science University. Informed consent was not obtained as the linked dataset did not contain potential patient privacy and identification information.
The primary exposure was cannabis use disorder during pregnancy, which was identified using International Classification of Diseases, Ninth Revision (ICD-9) codes from hospital discharge data. The following ICD-9 codes were used: 304.30 (cannabis dependence, unspecified), 304.31 (cannabis dependence, continuous), 304.32 (cannabis dependence, episodic), 305.20 (cannabis abuse, unspecified), 305.21 (cannabis abuse, continuous), 305.22 (cannabis abuse, episodic). We excluded pregnancies with multiple gestations, congenital fetal anomalies, or a gestational age less than 23 weeks or greater than 42 weeks.
Adverse perinatal outcomes were assessed using ICD-9 codes and birth certificate data. Maternal outcomes of interest were gestational hypertension (642.0, 642.3), preeclampsia (642.4, 642.5, or birth certificate), and preterm delivery (gestational age of <37 weeks). We defined severe maternal morbidity (SMM) using a published list of ICD-9 codes from the Center of Disease Control and Prevention.37 These codes were previously validated using these data.38 Severe maternal morbidity indicators included are acute myocardial infarction, aneurysm, acute renal failure, acute respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, conversion of cardiac rhythm, disseminated intravascular coagulation, eclampsia, heart failure, puerperal cerebrovascular disorders, pulmonary edema/acute heart failure, severe anesthesia complications, sepsis, shock, sickle cell disease with crisis, air and thrombotic embolism, blood products transfusion, hysterectomy, temporary tracheostomy, and ventilation. Birthing patients with at least one of these indicators were classified as having SMM. Neonatal outcomes of interest were stillbirths (656.4, 768.0, V27.1 or birth certificate), infant death (798.x or birth certificate), NICU admission (birth certificate), respiratory distress syndrome (769.x, 770.x or birth certificate), and hypoglycemia (775.6, 251.0, 251.1, 251.2). Small for gestational age was captured using a published algorithm and defined as birthweight less than the tenth percentile for gestational age.39
Maternal characteristics, including pre-pregnancy body mass index (BMI), race/ethnicity, maternal age, education, parity, insurance, smoking status, and other substance use, were retrieved from either birth certificate or hospital discharge data. We categorized pre-pregnancy BMI as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30 kg/m2). Maternal race/ethnicity was self-identified as either non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic, Asian, American Indian, or Other/Multi-racial. Maternal age at time of delivery was grouped into three categories (<20 years, 20–35 years, or ≥35 years). We used these categories as teenagers (<20 years) and advanced maternal age (≥35 years) are at increased risk for adverse perinatal outcomes.40,41 Based on the previous literature,42 we have categorized prenatal visits into less than five or at least 5 total prenatal visits. Patients were identified as either nulliparous or multiparous. Maternal educational attainment was categorized as either having attended some college or attendance through high school or less. Insurance status was identified as public/self-pay/uninsured or private; while alcohol use was captured using ICD-9 codes (291.81, 303.0, 303.9, 305.0, 790.3, 980.x). ICD-9 codes were used to determine additional substance use of cocaine (304.2, 305.60, 305.61, 305.62, 970.81), opioids (304.00, 304.01, 304.02, 305.50, 305.51, 305.52), and/or methamphetamines (304.40, 304.41, 304.42). Smoking status was defined as cigarette use during any trimester of pregnancy. Birth certificate as well as ICD-9 codes were used to determine chronic hypertension (642.1, 642.2, 401.x, 402.x, 403.x, 404.x, 405.x) and pre-existing diabetes (250.x).
We performed all statistical calculations using Stata (version 17; Stata Corp, College Station, TX). We compared demographics (maternal race and ethnicity, age, education, insurance, pre-pregnancy BMI, parity, smoking status, prenatal visits, alcohol use, other substance use, chronic hypertension and pre-existing diabetes) and outcomes (gestational hypertension, preterm delivery, preeclampsia, severe maternal morbidity, still births, infant deaths, NICU admission, small for gestational age, respiratory distress syndrome and hypoglycemia) using chi-square tests for categorical variables and two-sample t-tests for continuous variables. Multivariable logistic regression models were used to assess the relationship between maternal cannabis use disorder and perinatal outcomes. Potential confounders were chosen based on published research and a priori assumptions of which confounders might affect the outcomes. Potential confounders identified included maternal race/ethnicity, age, education, body mass index, parity, prenatal visits, insurance, smoking, alcohol use, and other substance use.43 In addition, we adjusted for preterm birth in the neonatal outcomes. Because of multiple comparisons, statistical significance was set at 0.005 in order to decrease Type I error rate.44
RESULTS
A total of 2,380,446 pregnancies met inclusion criteria, and 9,144 (0.38%) of these individuals used cannabis during pregnancy. The majority of birthing persons with cannabis use disorders had unspecified cannabis abuse (7,793, 85.2%) followed by continuous cannabis abuse (656, 7.2%), unspecified cannabis dependence (375, 4.1%), episodic cannabis abuse (155, 1.7%), continuous cannabis dependence (148, 1.6%) and episodic cannabis dependence (17, 0.2%).
Compared with individuals with no cannabis use disorder, individuals who used cannabis were more likely to self-identify as non-Hispanic black (22.1% vs 5.0%; p<0.001), be less than 20 years old (16.8% vs 9.1%; p<0.001), and have no college education (73.3% vs 52.1%; p<0.001). They were also more likely to have public insurance (80.5% vs. 51.9%; p<0.001) and fewer than 5 prenatal visits during the course of their pregnancy (15.6% vs. 2.3%; p<0.001). Individuals with cannabis use disorder were more likely to consume alcohol (4.5% vs. 0.1%; p<0.001) and use other substances (6.5% vs 0.1%; p<0.001) (Table 1).
Table 1:
Demographics of birthing patients who use cannabis vs who did not use cannabis in California (2007–2011)
| Total | Birthing patients with cannabis use disorder | Birthing patients who do not use cannabis | P* | |
|---|---|---|---|---|
| N=2,380,446 | N=9,144 | N=2,371,302 | ||
| Race and ethnicity | ||||
| Non-Hispanic white | 622,308 (26.4%) | 3,543 (39.3%) | 618,765 (26.4%) | <0.001 |
| Non-Hispanic Black | 120,247 (5.1%) | 1,990 (22.1%) | 118,257 (5.0%) | |
| Hispanic | 1,279,780 (54.4%) | 2,754 (30.5%) | 1,277,026 (54.5%) | |
| Asian | 280,279 (11.9%) | 118 (1.3%) | 280,161 (11.9%) | |
| AIAN | 7,089 (0.3%) | 132 (1.5%) | 6,957 (0.3%) | |
| Other/Multiracial | 44,368 (1.9%) | 484 (5.4%) | 43,884 (1.9%) | |
| Maternal age | ||||
| <20 years | 216,398 (9.1%) | 1,539 (16.8%) | 214,859 (9.1%) | <0.001 |
| 20–34 years | 1,751,538 (73.6%) | 6,969 (76.2%) | 1,744,569 (73.6%) | |
| >=35 years | 412,444 (17.3%) | 636 (7.0%) | 411,808 (17.4%) | |
| No college education | 1,200,296 (52.2%) | 6,459 (73.3%) | 1,193,837 (52.1%) | <0.001 |
| Pre-pregnancy BMI | ||||
| Mean (Range) | 25.8 (10.5–167.5) | 25.8 (13.2–71.8) | 25.8 (10.5–167.5) | 0.50 |
| Underweight | 89,925 (4.1%) | 547 (6.5%) | 89,378 (4.1%) | <0.001 |
| Normal | 1,092,829 (50.1%) | 4,025 (48.1%) | 1,088,804 (50.1%) | |
| Overweight | 560,723 (25.7%) | 1,985 (23.7%) | 558,738 (25.7%) | |
| Obese | 437,627 (20.1%) | 1,804 (21.6%) | 435,823 (20.1%) | |
| Public insurance | 1,204,790 (52.0%) | 7,357 (80.5%) | 1,197,433 (51.9%) | <0.001 |
| Nulliparous | 943,659 (39.7%) | 3,889 (42.8%) | 939,770 (39.7%) | <0.001 |
| Cigarette smoker during pregnancy | 73,588 (3.1%) | 2,382 (26.0%) | 71,206 (3.0%) | <0.001 |
| Prenatal visits (less than five total visits) | 55,371 (2.4%) | 1,365 (15.6%) | 54,006 (2.3%) | <0.001 |
| Alcohol use | 2,487 (0.1%) | 412 (4.5%) | 2,075 (0.1%) | <0.001 |
| Other Substance use | 4,087 (0.2%) | 597 (6.5%) | 3,490 (0.1%) | <0.001 |
| Chronic hypertension | 21,072 (0.9%) | 121 (1.3%) | 20,951 (0.9%) | <0.001 |
| Pre-existing diabetes | 22,771 (1.0%) | 80 (0.9%) | 22,691 (1.0%) | 0.42 |
Chi-square test/two-sample t-test
BMI-Body mass index, AIAN- American-Indian Alaskan-Native
All the demographics were captured using vital statistics data, but alcohol use, other substance use, chronic hypertension and pre-existing diabetes were determined using ICD-9 codes.
With regards to birthing patient outcomes (Table 2), cannabis use disorder was associated with higher proportions of gestational hypertension (3.8% vs. 2.6%; p<0.001), preeclampsia (5.2% vs 3.8%; p<0.001), preterm delivery (14.2% vs 6.7%; p<0.001), and severe maternal morbidity (1.7% vs 0.9%; p<0.001). Individuals who had cannabis use disorder also had a higher proportion of adverse neonatal outcomes, infant death (0.9% vs 0.2%; p<0.001), NICU admission (18.2% vs 8.9%; p<0.001), respiratory distress syndrome (9.9% vs 5.5%; p<0.001), hypoglycemia (1.4% vs 0.8%; p<0.001), and small for gestational age (16.0% vs 8.5%; p<0.001).
Table 2:
Perinatal outcomes in cannabis use disorder vs no cannabis use in California
| Total | Cannabis use disorders | No Cannabis Use | P* | |
|---|---|---|---|---|
| N=2,380,446 | N=9,144 | N=2,371,302 | ||
| Birthing patient outcomes | ||||
| Gestational hypertension | 62,601 (2.6%) | 350 (3.8%) | 62,251 (2.6%) | <0.001 |
| Preterm delivery (<37 weeks) | 159,229 (6.7%) | 1,299 (14.2%) | 157,930 (6.7%) | <0.001 |
| Pre-eclampsia | 90,487 (3.8%) | 474 (5.2%) | 90,013 (3.8%) | <0.001 |
| Severe maternal morbidity | 21,798 (0.9%) | 158 (1.7%) | 21,640 (0.9%) | <0.001 |
| Neonatal outcomes | ||||
| Intrauterine fetal death | 9,187 (0.4%) | 91 (1.0%) | 9,096 (0.4%) | <0.001 |
| Infant death | 4,426 (0.2%) | 86 (0.9%) | 4,340 (0.2%) | <0.001 |
| NICU admission | 205,757 (9.0%) | 1,631 (18.2%) | 204,126 (8.9%) | <0.001 |
| Small for gestational age | 202,611 (8.5%) | 1,459 (16.0%) | 201,152 (8.5%) | <0.001 |
| Respiratory distress syndrome | 130,659 (5.5%) | 906 (9.9%) | 129,753 (5.5%) | <0.001 |
| Hypoglycemia | 19,022 (0.8%) | 126 (1.4%) | 18,896 (0.8%) | <0.001 |
Chi-square test
Adjusted odds ratios (adjusted for maternal race, age, education, pre-pregnancy BMI, insurance, prenatal care, parity, substance use, smoking and alcohol use) for the association between cannabis use disorder and adverse perinatal outcomes is reported in Table 3 and 4. Compared with individuals with no cannabis use in pregnancy, individuals who used cannabis had higher risks of preterm delivery (aOR=1.45, 95%CI: 1.35–1.55; p<0.001), preeclampsia (aOR=1.16, 95% CI: 1.04–1.28; p=0.006), gestational hypertension (aOR=1.19, 95% CI: 1.06–1.34; p=0.004), and severe maternal morbidity (aOR=1.22, 95% CI: 1.02–1.47; p=0.03). Neonates exposed to in utero cannabis exposure had higher risks of respiratory distress syndrome (aOR 1.16, 95% CI 1.07–1.27), small for gestational age (aOR 1.47, 95% CI 1.38–1.56), NICU admission (aOR 1.24, 95% CI 1.16–1.33), and infant death (aOR 1.86, 95% 1.44–2.41). We did not identify a statistically significant difference in risk of stillbirth (aOR 0.96, 95% CI 0.69–1.34) and hypoglycemia (aOR 1.22, 95% CI 1.00–1.49)
Table 3:
Multivariable logistic regression analyses showing association of cannabis use disorder with birthing patient’s outcomes. Logistic regression results for covariates also shown
| Birthing patient outcomes | ||||
|---|---|---|---|---|
| Gestational hypertension | Preterm delivery | Preeclampsia | Severe maternal morbidity | |
| Cannabis use disorders | 1.19 (1.06–1.34)* | 1.45 (1.35–1.55)* | 1.16 (1.04–1.28) | 1.22 (1.02–1.47) |
| Race and ethnicity (Ref: Non-Hispanic white) | ||||
| Non-Hispanic Black | 1.38 (1.33–1.43) | 1.58 (1.54–1.62) | 1.38 (1.34–1.42) | 2.07 (1.94–2.21) |
| Hispanic | 0.77 (0.75–0.79) | 1.15 (1.13–1.16) | 0.97 (0.95–0.99) | 1.38 (1.32–1.43) |
| Asian | 0.85 (0.83–0.88) | 1.21 (1.19–1.24) | 0.84 (0.82–0.86) | 1.42 (1.34–1.49) |
| AIAN | 0.95 (0.82–1.10) | 1.18 (1.06–1.30) | 1.25 (1.11–1.41) | 1.59 (1.24–2.04) |
| Other/Multiracial | 1.09 (1.03–1.16) | 1.18 (1.13–1.23) | 1.10 (1.05–1.16) | 1.32 (1.18–1.47) |
| Maternal age (Ref: 20–34 years) | ||||
| <20 years | 0.99 (0.96–1.03) | 1.00 (0.98–1.02) | 1.05 (1.03–1.08) | 1.04 (0.99–1.10) |
| >=35 years | 1.58 (1.55–1.62) | 1.34 (1.32–1.36) | 1.41 (1.38–1.44) | 1.61 (1.55–1.68) |
| Education (Ref: No college education) | ||||
| Some college education | 0.98 (0.96–1.01) | 0.95 (0.93–0.96) | 0.98 (0.97–1.00) | 0.88 (0.85–0.91) |
| BMI (Ref: Normal weight) | ||||
| Underweight | 0.65 (0.61–0.69) | 1.19 (1.16–1.23) | 0.77 (0.73–0.81) | 1.12 (1.04–1.20) |
| Overweight | 1.90 (1.86–1.95) | 1.04 (1.03–1.06) | 1.67 (1.63–1.70) | 1.01 (0.97–1.04) |
| Obese | 3.71 (3.63–3.79) | 1.20 (1.18–1.22) | 2.74 (2.69–2.79) | 1.04 (1.00–1.08) |
| Insurance (Ref: Private) | ||||
| Public insurance | 0.94 (0.92–0.96) | 1.02 (1.01–1.04) | 1.01 (0.99–1.03) | 1.18 (1.14–1.22) |
| Prenatal visits (Ref: >=5 visits) | ||||
| <5 visits | 0.94 (0.88–0.99) | 3.28 (3.20–3.37) | 1.27 (1.21–1.33) | 2.16 (2.02–2.32) |
| Parity (Ref: Multiparous) | ||||
| Nulliparous | 1.99 (1.94–2.02) | 1.09 (1.08–1.10) | 2.55 (2.51–2.59) | 1.42 (1.37–1.46) |
| Other substance use | 1.87 (1.59–2.20) | 2.68 (2.45–2.92) | 1.43 (1.22–1.67) | 2.08 (1.67–2.59) |
| Cigarrete smoker | 1.10 (1.04–1.16) | 1.28 (1.24–1.32) | 0.95 (0.91–1.00) | 1.19 (1.09–1.30) |
| Alcohol use | 1.25 (1.01–1.56) | 1.41 (1.23–1.62) | 1.23 (1.02–1.49) | 1.49 (1.08–2.07) |
p<0.005, Adjusted odds ratio (95% I) reported
Table 4:
Multivariable logistic regression analyses showing association of cannabis use disorder with infant outcomes
| Infant outcomes | ||||||
|---|---|---|---|---|---|---|
| Stillbirth | Infant death | NICU admission | Small for gestational age | Respiratory distress syndrome | Hypoglycemia | |
| Cannabis use disorders | 0.96 (0.69–1.34) | 1.86 (1.44–2.41)* | 1.24 (1.16–1.33)* | 1.47 (1.38–1.56)* | 1.16 (1.07–1.27)* | 1.22 (1.00–1.49) |
| Race and ethnicity (Ref: Non-Hispanic white) | ||||||
| Non-Hispanic Black | 1.41 (1.25–1.59) | 1.46 (1.29–1.66) | 1.15 (1.12–1.18) | 2.38 (2.33–2.43) | 0.95 (0.93–0.98) | 1.02 (0.95–1.09) |
| Hispanic | 1.06 (0.98–1.15) | 0.75 (0.69–0.83) | 1.04 (1.03–1.05) | 1.31 (1.29–1.33) | 0.77 (0.76–0.78) | 0.75 (0.72–0.78) |
| Asian | 0.86 (0.76–0.96) | 1.01 (0.89–1.14) | 1.14 (1.12–1.16) | 1.93 (1.89–1.96) | 0.72 (0.71–0.74) | 0.80 (0.76–0.84) |
| AIAN | 0.97 (0.58–1.63) | 1.15 (0.71–1.87) | 1.04 (0.95–1.15) | 1.18 (1.07–1.31) | 0.93 (0.83–1.03) | 1.19 (0.94–1.53) |
| Other/Multiracial | 0.59 (0.44–0.79) | 1.15 (0.92–1.45) | 1.15 (1.10–1.19) | 1.36 (1.31–1.42) | 0.96 (0.91–0.99) | 1.02 (0.92–1.13) |
| Maternal age (Ref: 20–34 years) | ||||||
| <20 years | 0.32 (0.28–0.38) | 1.48 (1.33–1.65) | 0.93 (0.92–0.95) | 1.04 (1.02–1.06) | 0.93 (0.91–0.95) | 0.87 (0.82–0.93) |
| >=35 years | 1.77 (1.65–1.90) | 0.85 (0.76–0.94) | 1.09 (1.07–1.11) | 1.05 (1.03–1.06) | 1.12 (1.10–1.14) | 1.18 (1.14–1.23) |
| Some college education | 0.76 (0.71–0.82) | 0.86 (0.79–0.94) | 0.92 (0.91–0.94) | 0.89 (0.88–0.91) | 0.99 (0.98–1.01) | 1.08 (1.04–1.12) |
| BMI (Ref: Normal weight) | ||||||
| Underweight | 0.79 (0.65–0.95) | 1.05 (0.88–1.24) | 0.95 (0.93–0.98) | 1.57 (1.54–1.60) | 0.89 (0.87–0.93) | 1.00 (0.92–1.08) |
| Overweight | 1.35 (1.25–1.45) | 1.20 (1.10–1.31) | 1.10 (1.09–1.12) | 0.79 (0.78–0.80) | 1.16 (1.14–1.17) | 1.18 (1.14–1.23) |
| Obese | 1.59 (1.48–1.72) | 1.47 (1.35–1.60) | 1.23 (1.22–1.26) | 0.71 (0.69–0.72) | 1.34 (1.32–1.37) | 1.44 (1.38–1.49) |
| Public insurance | 1.24 (1.15–1.33) | 1.30 (1.19–1.41) | 1.20 (1.19–1.22) | 1.16 (1.14–1.17) | 1.04 (1.02–1.05) | 0.92 (0.89–0.95) |
| <5 total prenatal visits | 3.87 (3.53–4.24) | 3.42 (3.08–3.81) | 1.57 (1.53–1.62) | 1.21 (1.17–1.24) | 1.22 (1.18–1.26) | 0.98 (0.89 −1.07) |
| Nulliparous | 1.47 (1.38–1.57) | 0.97 (0.89–1.05) | 1.36 (1.34–1.38) | 1.65 (1.63–1.67) | 1.35 (1.33–1.37) | 1.19 (1.15–1.24) |
| Other substance use | 0.82 (0.55–1.22) | 1.43 (1.00–2.04) | 6.72 (6.20–7.27) | 1.83 (1.67–2.01) | 2.19 (1.97–2.42) | 1.92 (1.53–2.39) |
| Cigarrete smoker | 1.09 (0.93–1.27) | 1.40 (1.20–1.63) | 1.30 (1.26–1.34) | 1.69 (1.65–1.75) | 1.02 (0.97–1.06) | 0.99 (0.89–1.09) |
| Alcohol use | 0.42 (0.17–1.02) | 0.62(0.27–1.40) | 1.46 (1.29–1.67) | 1.28 (1.12–1.45) | 1.24 (1.06–1.45) | 1.19 (0.83–1.72) |
| Preterm birth | 22.08(20.73–23.52) | 9.92 (9.25–10.64) | 11.40 (11.26–11.54) | 1.06 (1.04–1.08) | 10.26 (10.11–10.41) | 8.28 (8.02–8.56) |
p<0.005, Adjusted odds ratio (95% I) reported
Chi-square test.
DISCUSSION
In this large retrospective cohort study, prenatal cannabis use disorder was associated with increased odds of perinatal complications (Tables 3 and 4), which persisted despite controlling for synergistic variables such as polysubstance use and demographic factors. Birthing persons with prenatal cannabis use disorder were more likely to be younger consistent with the existing literature.2 Adverse maternal health associated with cannabis use disorder in pregnancy included a higher magnitude of hypertensive disorders of pregnancy, preterm birth and overall morbidity. Neonatal effects included increased rates of respiratory distress, small for gestational age, NICU admission, and infant death. Our findings are consistent with other existing studies, Shi et al. also found that prenatal cannabis use disorder was associated with a greater odds of being small for gestational age, preterm birth, low birth weight, and death within 1 year of birth.18 Similarly, other recent large systematic reviews have found significant increases in adverse neonatal outcomes among women who used cannabis in pregnancy including small for gestational age, low birth weight, preterm birth, and NICU admissions14,16,17. Other studies have reported an association of comorbid behavioral conditions with prenatal cannabis use including increased depression and anxiety, but this was not assessed in our study.45
Compared to other studies focused on prenatal cannabis use disorder, our study included a sizeable and diverse patient population and also adjusted for confounders including tobacco, alcohol and substance use. Additionally, our study assessed many clinically relevant prenatal and neonatal outcomes, including infant death which has not been consistently reported. However, our study has some limitations. Our data relied on ICD-9 codes for categorizing outcomes of interest and is retrospective in nature, thus limited by a potential for selection bias and unmeasured confounding. The prevalence of cannabis use disorder was found to be 0.38%, which is similar to a prior study that noted prenatal cannabis use disorder in 0.4% of mothers identified by ICD-9 codes recorded at delivery.18 These rates are lower than published rates of self-reported prenatal cannabis use (3.38%) and adjusted prevalence of daily use (0.69%) in 2017.2 Reliance on self-reported measures of cannabis use likely underestimates the prevalence due to nondisclosure,46,47 particularly since our dataset is derived from pre-legalization of recreational cannabis in California. Additionally, incidental cannabis identification on toxicology reports due to provider selection bias can result in outcomes we attribute to cannabis use disorder rather than social determinants of health. Our inability to limit all confounders can also skew our results towards associations that are secondary to structural factors, such as racism, rather than cannabis use disorder.48 We were also not able to differentiate the dose, frequency or mode of cannabis administration and given smoked cannabis is the most common form of use in pregnancy,49 it is possible that the adverse impact on neonatal outcomes observed may be partly impacted by the harmful effects of inhaled smoke. Additionally, inaccuracies in coding may exist and can lead to misclassification bias. Such bias is towards the null and Type II error; thus our findings may represent lower bound estimates of effect sizes or underestimate the impact of cannabis on perinatal outcomes.
CONCLUSION:
While decriminalization and growing social acceptance of cannabis helps to reduce inequities in the legal system,50 the impact of its use during pregnancy on both birthing person and fetal outcomes poses a public health concern. Dysregulated cannabis use disorder could widen gaps in health and social equity for pregnant patients, new parents, and their children51. Ultimately, national cannabis legislation is evolving quickly, but our research is regionally limited. Further studies are needed to understand the magnitude of cannabis exposure on short- and long-term maternal and offspring health outcomes in order to better gauge the scope and dimensions of its potential harm. These future studies need to be conducted through the lens of social determinants of health, expounding on the nexus between racism, criminalization of cannabis, and perinatal outcomes. In adding to the existing literature on the effects of prenatal cannabis use disorder on pregnancy and offspring outcomes, we hope our study can help guide clinical conversations and improve provider comfort with counseling on cannabis exposure in pregnancy.
Footnotes
Presented at the 53rd American Society of Addiction Medicine Meeting March 30-April 3rd, 2022 in Hollywood, Florida and won the Best Overall Abstract Submission Award.
Conflict of Interest Statement: None
BIBLIOGRAPHY
- 1.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. Jama. 2017;317(2):207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young-Wolff KC, Tucker L-Y, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009–2016. Jama. 2017;318(24):2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young-Wolff KC, Ray GT, Alexeeff SE, et al. Rates of Prenatal Cannabis Use Among Pregnant Women Before and During the COVID-19 Pandemic. Jama. Nov 2 2021;326(17):1745–1747. doi: 10.1001/jama.2021.16328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark K, Gryczynski J, Axenfeld E, Schwartz RP, Terplan M. Pregnant women’s current and intended cannabis use in relation to their views toward legalization and knowledge of potential harm. Journal of addiction medicine. 2017;11(3):211–216. [DOI] [PubMed] [Google Scholar]
- 5.Martin CE, Longinaker N, Mark K, Chisolm MS, Terplan M. Recent trends in treatment admissions for marijuana use during pregnancy. J Addict Med. Mar-Apr 2015;9(2):99–104. doi: 10.1097/adm.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 6.Braillon A, Bewley S. Committee opinion no. 722: marijuana use during pregnancy and lactation. Obstetrics & Gynecology. 2018;131(1):164. [DOI] [PubMed] [Google Scholar]
- 7.Ryan S, Ammerman S, O’Connor M. Committee on Substance Use and Prevention; Section on Breastfeeding. Marijuana use during pregnancy and breastfeeding: implications for neonatal and childhood outcomes. Pediatrics. 2018;142(3):e20181889. [DOI] [PubMed] [Google Scholar]
- 8.US Surgeon General’s Advisory: Marijuana Use and the Developing Brain.; 2019.
- 9.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. Jama. 2019;322(2):167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abuse S, Administration MHS. Results from the 2013 National Survey on Drug Use and Health: Summary of national findings. NSDUH Series H-48, HHS Publication No(SMA) 14–4863. 2014:1–143. [Google Scholar]
- 11.Beatty JR, Svikis DS, Ondersma SJ. Prevalence and perceived financial costs of marijuana versus tobacco use among urban low-income pregnant women. Journal of addiction research & therapy. 2012;3(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DG, Turner JD, Parrott AC, et al. During pregnancy, recreational drug-using women stop taking ecstasy (3, 4-methylenedioxy-N-methylamphetamine) and reduce alcohol consumption, but continue to smoke tobacco and cannabis: initial findings from the Development and Infancy Study. Journal of Psychopharmacology. 2010;24(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passey ME, Sanson-Fisher RW, D’Este CA, Stirling JM. Tobacco, alcohol and cannabis use during pregnancy: clustering of risks. Drug and Alcohol Dependence. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 14.Gunn J, Rosales C, Center K, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ open. 2016;6(4):e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen VH, Harley KG. Prenatal cannabis use and infant birth outcomes in the Pregnancy Risk Assessment Monitoring System. The Journal of Pediatrics. 2022;240:87–93. [DOI] [PubMed] [Google Scholar]
- 16.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Meta-Analysis Review. Obstetrics & Gynecology. October 2016;128(4):713–23. Abstrackr Priority Dual-screen batch. doi: 10.1097/AOG.0000000000001649 [DOI] [PubMed] [Google Scholar]
- 17.Marchand G, Masoud AT, Govindan M, et al. Birth Outcomes of Neonates Exposed to Marijuana in Utero: A Systematic Review and Meta-analysis. JAMA Network Open. 2022;5(1):e2145653–e2145653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Zhu B, Liang D. The associations between prenatal cannabis use disorder and neonatal outcomes. Addiction. Nov 2021;116(11):3069–3079. doi: 10.1111/add.15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman-Cowger VH, Oga EA, Peters EN, Mark K. Prevalence and associated birth outcomes of co-use of Cannabis and tobacco cigarettes during pregnancy. Neurotoxicology and teratology. 2018;68:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varner MW, Silver RM, Hogue CJR, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstetrics and gynecology. 2014;123(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daha SK, Sharma P, Sah PK, Karn A, Poudel A, Pokhrel B. Effects of prenatal cannabis use on fetal and neonatal development and its association with neuropsychiatric disorders: A systematic review. Neurology, Psychiatry and Brain Research. 2020;38:20–26. [Google Scholar]
- 22.Paul SE, Hatoum AS, Fine JD, et al. Associations between prenatal cannabis exposure and childhood outcomes: results from the ABCD study. JAMA psychiatry. 2021;78(1):64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsi DJ, Donelle J, Sucha E, et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Research Support, Non-U.S. Gov’t. Nat Med Oct 2020;26(10):1536–1540. doi: 10.1038/s41591-020-1002-5 [DOI] [PubMed] [Google Scholar]
- 24.Thompson R, DeJong K, Lo J. Marijuana use in pregnancy: a review. Obstetrical & gynecological survey. 2019;74(7):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacology & therapeutics. 2018;182:133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinky PD, Bloemer J, Smith WD, et al. Prenatal cannabinoid exposure and altered neurotransmission. Neuropharmacology. 2019;149:181–194. [DOI] [PubMed] [Google Scholar]
- 27.Bayrampour H, Zahradnik M, Lisonkova S, Janssen P. Women’s perspectives about cannabis use during pregnancy and the postpartum period: An integrative review. Prev Med. Feb 2019;119:17–23. doi: 10.1016/j.ypmed.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 28.Panday J, Taneja S, Popoola A, et al. Clinician responses to cannabis use during pregnancy and lactation: a systematic review and integrative mixed-methods research synthesis. Family practice. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisbeck SJ, Bright KS, Ginn CS, Smith JM, Hayden KA, Ringham C. Perceptions about cannabis use during pregnancy: a rapid best-framework qualitative synthesis. Canadian Journal of Public Health. 2021;112(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Health Effects of Marijuana: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press (US); 2017: Jan 12. 4, Therapeutic Effects of Cannabis and Cannabinoids. Available at: https://www.ncbi.nlm.nih.gov/books/NBK425767/. Accessed October 20, 2022. [Google Scholar]
- 31.Fergusson D, Horwood L, Northstone K. Avon Longitudinal Study of Pregnancy and Childhood. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109(1):21–7. [DOI] [PubMed] [Google Scholar]
- 32.Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. Oct 2016;215(4):506.e1–7. doi: 10.1016/j.ajog.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 33.Torres CA, Medina-Kirchner C, O’Malley KY, Hart CL. Totality of the Evidence Suggests Prenatal Cannabis Exposure Does Not Lead to Cognitive Impairments: A Systematic and Critical Review. Systematic Review. Front Psychol. 2020;11:816. doi: 10.3389/fpsyg.2020.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metz TD, Allshouse AA, Hogue CJ, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. American journal of obstetrics and gynecology. 2017;217(4):478. e1–478. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan KS, Bash JC, Hanna CB, Hedges JC, Lo JO. Effects of marijuana on reproductive health: preconception and gestational effects. Current opinion in endocrinology, diabetes, and obesity. 2021;28(6):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biological psychiatry. 2016;79(7):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Center for Disease Control and Prevention: Severe Morbidity Indicators and Corresponding ICD-9-CM/ICD-10-CM/PCS Codes during Delivery Hospitalizations. Accessed May 1, 2022, https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm
- 38.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. American journal of obstetrics and gynecology. 2016;214(5):643. e1–643. e10. [DOI] [PubMed] [Google Scholar]
- 39.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133(5):844–853. [DOI] [PubMed] [Google Scholar]
- 40.Cnattingius S, Forman MR, Berendes HW, Isotalo L. Delayed childbearing and risk of adverse perinatal outcome. A population-based study. Jama. Aug 19 1992;268(7):886–90. [PubMed] [Google Scholar]
- 41.Zhang T, Wang H, Wang X, et al. The adverse maternal and perinatal outcomes of adolescent pregnancy: a cross sectional study in Hebei, China. BMC Pregnancy Childbirth. Jun 1 2020;20(1):339. doi: 10.1186/s12884-020-03022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghafari-Saravi A, Chaiken SR, Packer CH, Davitt CC, Garg B, Caughey AB. Cesarean delivery rates by hospital type among nulliparous and multiparous patients. J Matern Fetal Neonatal Med. Oct 19 2021:1–9. doi: 10.1080/14767058.2021.1990884 [DOI] [PubMed] [Google Scholar]
- 43.Gabrhelík R, Mahic M, Lund IO, et al. Cannabis Use during Pregnancy and Risk of Adverse Birth Outcomes: A Longitudinal Cohort Study. Eur Addict Res. 2021;27(2):131–141. doi: 10.1159/000510821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. Bmj. Jan 21 1995;310(6973):170. doi: 10.1136/bmj.310.6973.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meinhofer A, Hinde JM, Keyes KM, Lugo-Candelas C. Association of Comorbid Behavioral and Medical Conditions With Cannabis Use Disorder in Pregnancy. JAMA Psychiatry. Jan 1 2022;79(1):50–58. doi: 10.1001/jamapsychiatry.2021.3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Gelder MM, Donders ART, Devine O, Roeleveld N, Reefhuis J, Study NBDP. Using Bayesian Models to Assess the Effects of Under‐reporting of Cannabis Use on the Association with Birth Defects, National Birth Defects Prevention Study, 1997–2005. Paediatric and perinatal epidemiology. 2014;28(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg M, Garrison L, Leeman L, et al. Validity of self-reported drug use information among pregnant women. Maternal and child health journal. 2016;20(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiff DM, Work EC, Foley B, et al. Perinatal Opioid Use Disorder Research, Race, and Racism: A Scoping Review. Pediatrics. Mar 1 2022;149(3)doi: 10.1542/peds.2021-052368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young-Wolff KC, Adams SR, Wi S, Weisner C, Conway A. Routes of cannabis administration among females in the year before and during pregnancy: Results from a pilot project. Addict Behav. Jan 2020;100:106125. doi: 10.1016/j.addbeh.2019.106125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farley EJ, Orchowsky S. Measuring the criminal justice system impacts of marijuana legalization and decriminalization using state data. JRSA, Justice Research and Statistics Association; 2019. [Google Scholar]
- 51.Solomon R. Racism and its effect on cannabis research. Cannabis and cannabinoid research. 2020;5(1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


