Abstract
Citrullination, the post-translational modification of arginine residues, is catalyzed by the four catalytically active peptidylarginine deiminase (PAD or PADI) isozymes and alters charge to affect target protein structure and function. PADs were initially characterized in rodent uteri and, since then, have been described in other female tissues including ovaries, breast, and the lactotrope and gonadotrope cells of the anterior pituitary gland. In these tissues and cells, estrogen robustly stimulates PAD expression resulting in changes in levels over the course of the female reproductive cycle. The best-characterized targets for PADs are arginine residues in histone tails, which, when citrullinated, alter chromatin structure and gene expression. Methodological advances have allowed for the identification of tissue-specific citrullinomes, which reveal that PADs citrullinate a wide range of enzymes and structural proteins to alter cell function. In contrast to their important physiological roles, PADs and citrullinated proteins are also involved in several female-specific diseases including autoimmune disorders and reproductive cancers. Herein, we review current knowledge regarding PAD expression and function and highlight the role of protein citrullination in both normal female reproductive tissues and associated diseases.
Keywords: peptidylarginine deiminase, citrullination, anterior pituitary, uterus, mammary gland, ovary
Peptidylarginine deiminase enzymes are expressed in female reproductive tissues and post-translationally convert arginine residues in target proteins to citrulline.
Graphical Abstract
Graphical Abstract.
Introduction
Citrullination is the post-translational modification of arginine to the non-coded residue citrulline. Citrullinated proteins were first discovered in hair follicles in 1962, which subsequently led to the identification of a family of calcium-dependent enzymes named peptidylarginine deiminases (PADs or PADIs) [1–3]. Since their initial characterization, five PAD isozymes have been identified: PAD1, 2, 3, 4, and 6. The PAD isozymes are well conserved at the nucleotide and amino acid level, and PAD2 is thought to be the ancestral homolog of the gene family with the remaining members arising through a series of gene duplications [4, 5]. PAD enzymes are widely expressed across vertebrates, but absent in yeast, worms, and flies. Phylogenetic analysis suggests that PADs were introduced from cyanobacteria into animals by horizontal gene transfer indicating an unusual evolution of the gene responsible for this protein modification [6]. The PAD genes are clustered on the human chromosome 1p36.1 with PAD2 oriented in the opposite direction of PAD1, 3, 4, and 6 and this unique genomic arrangement is conserved across species [4, 5].
In this article, we first provide biochemical details regarding PAD enzyme structure and function followed by a discussion of what is currently known about PAD tissue distribution and targets for protein citrullination. Next reviewed are PAD expression and function in the anterior pituitary, uterus, ovary, and mammary gland and involvement in female reproduction. Lastly, we provide an overview of our current understanding of the function of PADs and citrullinated proteins in autoimmunity and female reproductive cancers.
Biochemistry of protein citrullination
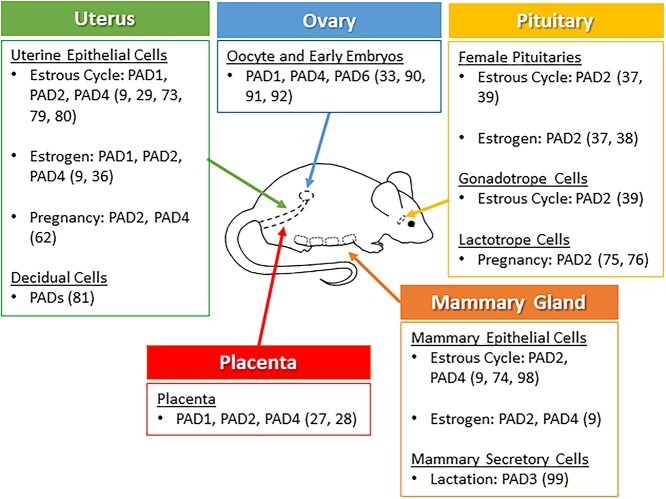
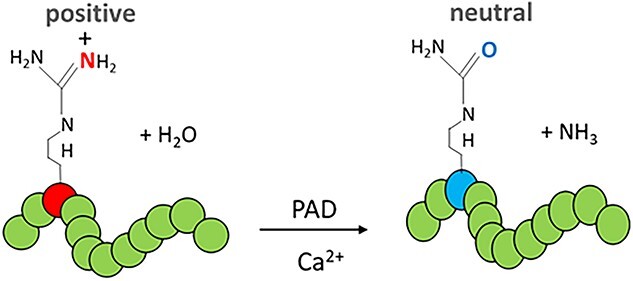
PADs catalyze the hydrolytic conversion of positive peptidylarginine into neutral peptidylcitrulline in substrate proteins (Figure 1). Citrullination is also referred to as deimination because the imine group on peptidylarginine is exchanged for a carbonyl group, which leads to a 1 Da decrease with each modification [7, 8]. Because an arginine’s guanidinium group has one of the highest pKa values (pKa = 12.5), at physiological pH the residues are protonated and carry a positive charge. This positive charge allows for electrostatic interactions between an arginine and negatively charged moieties including the R-groups of acidic amino acids and the phosphate backbone of DNA. Citrullination elicits a net loss in positive charge, which alters the target’s inter- and/or intramolecular electrostatic interactions to affect protein structure and function (Figure 2) [4, 9, 10].
Figure 1.
PAD enzymes remove the positively charged imine group from arginine (red) resulting in the neutrally charged, non-coded, residue citrulline (blue).
Figure 2.
Conversion of a positive arginine (red) to neutral citrulline (blue) by PAD enzymes can alter intra- and inter-molecular interactions of citrullinated proteins, mediate proteolysis and immunogenicity, change the active site, and regulate chromatin structure.
The PADs require calcium (Ca2+) binding for full catalytic activity, and this is an essential property defining the enzyme family [10, 11]. Each PAD monomer can bind up to six calcium ions, which transforms a disordered cavity into a stabilized, catalytically competent active site [12, 13]. Calcium binding is thought to occur in a sequential fashion resulting in a conformational change that positions a critical nucleophilic cysteine residue into the active site [14]. As a result, PAD activity increases up to 10 000-fold compared to that of the calcium-free enzyme [12]. Optimal catalytic activity of PADs in vitro occurs at calcium concentrations in the 0.1–2 mM range, which is 10- to 10 000-fold higher than typical cellular physiologic concentrations that vary from 0.1 to 10 μM [13, 15, 16]. It has been proposed that PADs may temporarily localize near intracellular ion channels where calcium concentrations can reach millimolar levels allowing for maximal activation [13, 17, 18]. Work by Mechin et al. found that the calcium concentration required for optimal PAD activity is substrate dependent. Calcium requirements for PAD1, 2, and 3 activity varied for synthetic arginine substrates and filaggrin, suggesting that the structure of the target can modulate catalytic activity [19].
Interestingly, the binding of calcium also alters PADs in ways other than just mediating catalytic activity. Zheng et al. found that PAD2 interacts with cytoplasmic Annexin A5 in the absence of calcium, but that in the presence of calcium this interaction is weakened thereby allowing PAD2 to preferentially associate with ras-related nuclear proteins (RAN), which is well documented to facilitate nuclear transport [20]. As it does not contain a nuclear localization sequence, this unique calcium-mediated mechanism facilitates PAD2 nuclear translocation [21]. PAD calcium-binding sites are structurally conserved across isozymes, and this region is thought to act as a molecular switch that can be regulated by protein binding [13]. In support of this, antibodies that bind to PAD4 near this region decrease the requirement of calcium for catalytic activity [22].
Crystallographic studies demonstrate that PAD2, 3, and 4 can form homodimers in which two monomers bind in a head-to-tail fashion [23–25]. The catalytic activity and target specificity of PAD monomers as compared to homodimers remain unknown. This observation coupled with the high levels of homology between the isoforms advances another question for future research: Can PADs form heterodimers and, if so, how does this affect catalytic activity and substrate specificity?
PAD tissue distribution
PADs are expressed in a wide range of tissues and cells. PAD1 and 3 predominantly localize in the epidermis, hair follicles, and keratinocytes [4, 26–28]. PAD1 mRNA is present in all layers of the epidermis as well as the prostate, testis, placenta, spleen, and thymus [28, 29]. PAD2 is the most ubiquitously expressed and found in brain, skeletal muscle, skin, spleen, and leukocytes [4, 30]. PAD4 is expressed in the spleen, neutrophils, granulocytes, monocytes, and eosinophils [31–33]. PAD6 is the only catalytically inactive PAD isozyme, and it localizes in mouse oocyte cytoplasm and early embryos as well as in testis and peripheral blood leukocytes [5, 34, 35].
Some of the earliest work in the field characterized PAD expression patterns in female reproductive tissues [36–38]. Senshu et al. discovered that prepubescent female and male rat pituitaries have negligible PAD activity, whereas sexually mature females show markedly higher activity that correlates with age and stage of the estrous cycle [38]. Additionally, PAD mRNA transcript levels are 50-fold higher in female rat pituitaries as compared to the male [39, 40]. This sexual dimorphic pattern is most likely because PAD expression is strongly stimulated by 17β-estradiol (E2) but suppressed by androgens [36–38, 41]. In uteri, mammary glands, and the anterior pituitary gland, PAD expression fluctuates across the estrous cycle, with highest expression during estrus following elevated serum E2 in proestrus [9, 37, 40]. Many studies now support that PADs are expressed in female reproductive tissues across the lifecycle (Figure 3) [38, 42].
Figure 3.
PAD expression in female reproductive tissues.
Hormonal regulation of PAD expression
Estrogen regulation of PAD expression in female reproductive tissues is well documented. Takahara et al. was the first to show that PAD expression is low in mouse uteri following ovariectomy; however, expression is rescued in uterine luminal and glandular epithelial cells in a time and dose-dependent manner following treatment with exogenous estrogens [37]. Subsequent ovariectomy experiments show that treatment with exogenous E2 stimulates PAD expression in the uterus and mammary gland as compared to vehicle-treated controls [9]. Following exogenous E2 treatment, PAD expression is absent in uteri of estrogen receptor α (ESR1) knockout (KO) and ESR1 mutant mice, which contain an ESR1 that cannot bind estrogen response elements (EREs), but retains ligand binding, dimerization, and interactions with other transcription factors. These findings suggest that ESR1 mediates PAD expression [43]. Supporting this idea, chromatin conformation capture experiments investigating uterine development show that the Pad gene locus contains an ESR1 super-enhancer [44]. At the transcriptional level, the Pad 1, 3, and 4 gene promoters contain putative EREs [45]. Although its expression is highly E2 dependent in vivo, the Pad2 gene promoter lacks an ERE. The promoter does contain multiple binding sites for the transcription factor SP1, which may interact with ESR1 to promote PAD2 expression following E2 stimulation [46, 47].
While E2 stimulates PAD expression, other hormones such as progesterone can alter PAD activity. Ovariectomized mice treated for 30 h with exogenous progesterone do not show increases in PAD activity in uterine lysates compared to vehicle controls; however, progesterone does dampen PAD activity following E2 priming [37]. More recently, studies found that hormones can induce rapid calcium influx that promotes PAD2 nuclear translocation resulting in increased histone citrullination. In the gonadotrope-derived LβT2 cell line, treatment with buserelin, a gonadotrope releasing hormone (GnRH) agonist, induces a rapid calcium influx resulting in the translocation of PAD2 to the nucleus [40] with a similar finding following progesterone treatment of an ovine uterine luminal epithelial (OLE) cell line [48]. Further, progesterone stimulation of human neutrophils does not increase PAD4 expression compared to vehicle-treated cells, but does increase levels of citrullinated histone 3 [49]. Taken together, these studies suggest that E2 stimulates PAD expression but that progesterone treatment may increase intracellular calcium, allowing PADs to translocate to the nucleus and citrullinate histones.
Protein targets for citrullination
Each PAD enzyme preferentially citrullinates specific arginine residues in multiple proteins (i.e., the citrullinome), though target specificity can overlap between isozymes [10, 19, 23, 50–52]. Mass spectrometry (MS) has identified the citrullinome in a number of tissues [53–55], which shows that targets include metabolic enzymes, clotting factors, cytoskeletal proteins, transcription factors, and blood proteins [53, 54, 56–59]. While the functional consequence of citrullination on protein function is known for some targets such as histones and filaggrin, for most proteins the impacts of citrullination are still unclear. For example, citrullinated tubulin has been detected by MS, yet how this post-translational modification alters cytoskeletal dynamics and architecture is still not well defined. By far the best-characterized functional consequence of protein citrullination is the decreased affinity of citrullinated histones for DNA, which leads to changes in chromatin organization and gene expression [48, 60–65]. Although histone citrullination has clear physiologic importance, aberrant protein citrullination is increasingly associated with diseases. Hyper-citrullination of histones, as occurs during neutrophil extracellular trap (NET) formation, leads to large webs of disorganized chromatin that are extruded from the cell and function as part of the intrinsic immune response [66, 67]; however, NETs are also implicated in both autoimmune disorders and cancer progression [68–72]. Another characterized citrullinated protein is filaggrin, which helps aggregate keratin filaments in the epidermis. Citrullinated filaggrin is a component of the cornified envelope formed during terminal differentiation of keratinocytes and participates in epidermal barrier formation [27, 73, 74]. While numerous proteins are citrullinated, the effect of this post-translational modification at the protein and cellular level and consequences on reproductive function is still unresolved. The remainder of this article will comprehensively review the role of PADs and citrullinated proteins in female reproductive tissue.
PADs and citrullinated proteins in female reproductive physiology
PADs were originally characterized in the rodent uterus and pituitary gland in the late 1980s and early 1990s and have subsequently been discovered in reproductive tissues of several female mammals including humans, rodents, cats, dogs, and sheep [9, 61, 62, 75, 76]. Since the original characterization of expression patterns, work has shifted to focus on determining the function of PADs and citrullinated proteins in female reproductive tissues. In E2 regulated female tissues and cells, increased PAD expression results in elevated levels of protein citrullination, suggesting an important role for this post-translation modification in reproductive physiology. The sections to follow detail our current understanding of PADs and protein citrullination in female reproductive tissues.
Anterior pituitary gland
Seminal studies by Senshu et al. characterizing PAD expression and activity in the pituitary laid the groundwork for ongoing studies investigating the function of PADs and citrullinated proteins in this gland [38]. Of the five hormone secreting anterior pituitary gland cell types, PADs are best characterized in gonadotrope and lactotrope cells, which are both highly E2 regulated.
In response to hormonal inputs, gonadotrope cells synthesize and secrete luteinizing hormone (LH) and follicle stimulating hormone (FSH), which are required for follicle development and ovulation in all female mammals. PAD2 expression is highest in mouse gonadotrope cells during estrus, with lower but detectable levels of PAD4 [40]. PAD2 is also expressed in the gonadotrope-derived LβT2 cell line, and 30 min of GnRH agonist treatment results in the formation of distinct puncta of PAD2 in the nucleus. This nuclear translocation is dependent on GnRH-induced calcium influx through L-type voltage-gated calcium channels [21, 40]. Within 30 min of GnRH stimulation, PADs citrullinate histone H3 tail arginine residues at sites 2, 8, and 17, which increases transcription of the Lhb and Fshb genes [40]. Despite these results, many questions remain regarding PAD function in gonadotrope cells such as the full cohort of genes regulated by histone citrullination as well as the identity of other citrullinated proteins.
Lactotrope cells synthesize prolactin and undergo controlled hypertrophy and hyperplasia during pregnancy in response to increasing serum E2. This hormone-dependent transformation of the lactotrope population is vital to maximize prolactin synthesis, initiate lactation, and stimulate milk production by mammary secretory cells. Akiyama et al. first showed that PADs localize to lactotrope cells and that catalytic activity in rat pituitaries increases over 8-fold from pregnancy day 12 to 21, paralleling a 4-fold increase in serum E2 across the same period [77, 78]. PADs have also been studied in the lactotrope-derived MtT/S and GH3 cell lines, which were derived from lactotropes and somatolactotropes, respectively. In MtT/S cells, insulin increases PAD expression and activity prior to induction of prolactin synthesis [79]. In GH3 cells, PAD2 catalyzed histone citrullination suppresses the expression of miRNAs Let-7c, miR-23b, and miR-29c [61]. These miRNAs bind the 3′untranslated regions of important target mRNAs involved in cell proliferation and growth and decrease their expression. This histone citrullination/miRNA expression mechanism may regulate lactotrope hypertrophy and hyperplasia during pregnancy to support the increased production of prolactin needed to initiate lactation. In support of this idea, inhibition of PADs with the pan-PAD inhibitor biphenyl-benzimidazole-Cl-amidine (BB-ClA) results in a significant decrease in GH3 cell growth and proliferation. Further work is clearly warranted to elucidate the mechanism by which PAD catalyzed citrullination regulates lactotrope function during pregnancy and lactation.
Uterus
Initial genomic analysis revealed that PAD1, 2, and 4 mRNA levels are highest in mouse uterine tissue as compared to 50 other tissue types examined [80]. Single-cell RNA sequencing confirms this earlier work showing that PAD1, 2, and 4 are expressed in mouse uterine epithelia while PAD2 and 4 are the major isoforms in humans [81, 82]. PAD1, 2, and 4 are expressed in luminal and glandular uterine epithelial cells and levels change over the course of the estrous cycle [9, 30, 75, 83, 84]. Expression of both PAD2 and 4 peaks in the estrus phase in rodents while PAD1 is elevated in proestrus. Estrogen-dependent PAD expression was first observed in mouse uterine luminal and glandular epithelia and has been confirmed by additional studies [9, 37]. Work by Ledee et al. found that endometrial biopsies collected during the mid-luteal phase overexpress PAD1 in women with implantation failure following IVF/ICSI compared to fertile controls [85]. While this finding supports an important role for endometrial PAD1 in implantation, the mechanism underlying this defect is unknown.
Uterine PAD expression also fluctuates during pregnancy and parturition [86]. PAD2 and 4 are expressed in ewe uterine luminal and glandular epithelia at day 25 of gestation [62]. PADs most likely function to epigenetically regulate gene expression as ewe gestation day 25 uterine lysates and the OLE cell line both display high levels of citrullinated histone H3 tail residues at positions 2, 8, 17, and 26. Our previous work shows that progesterone stimulation of OLE cells induces citrullination of histones associated with ovine IGFBP1, which is an important component of the secreted histotroph fluid that is critical during early pregnancy. Mechanistically, progesterone stimulation triggers rapid calcium influx through L-type calcium channels, required for PAD2 nuclear translocation and, ultimately, histone citrullination [48].
In mice, PADs are expressed in decidual cells as they differentiate from endometrial stromal cells surrounding the implantation site at day 9, while at day 10 strong expression is detected in the decidua capsularis and along the cytotrophoblast-labyrinth structure [86]. PAD enzymatic activity in uterine lysates increases dramatically in mid-pregnancy (~days 9–11) before gradually decreasing near term [86]. Single-cell RNA sequencing studies found that during implantation, PAD1 is expressed in mouse glandular epithelial cells while PAD2 expression is detected in epithelial, plasmacytoid dendritic, and natural killer cells [87–89]. In humans, PAD1 and 2 are expressed in placental cytotrophoblast cells [90, 91]. To date, no work has examined PAD2 protein expression during placentation [28, 29]. To address this gap in knowledge, we examined PAD2 localization in different placental types across species (Figure 4). Immunofluorescence staining reveals that PAD2 localizes to fetal trophoblast cells in cotyledonary (sheep), diffuse (horse), zonary (dog), and hemochorial (Guinea pig) placentas, suggesting PAD2 plays a conserved role in placental cell differentiation. Clearly, additional work is necessary to determine the functional role of PAD2 in placentation.
Figure 4.
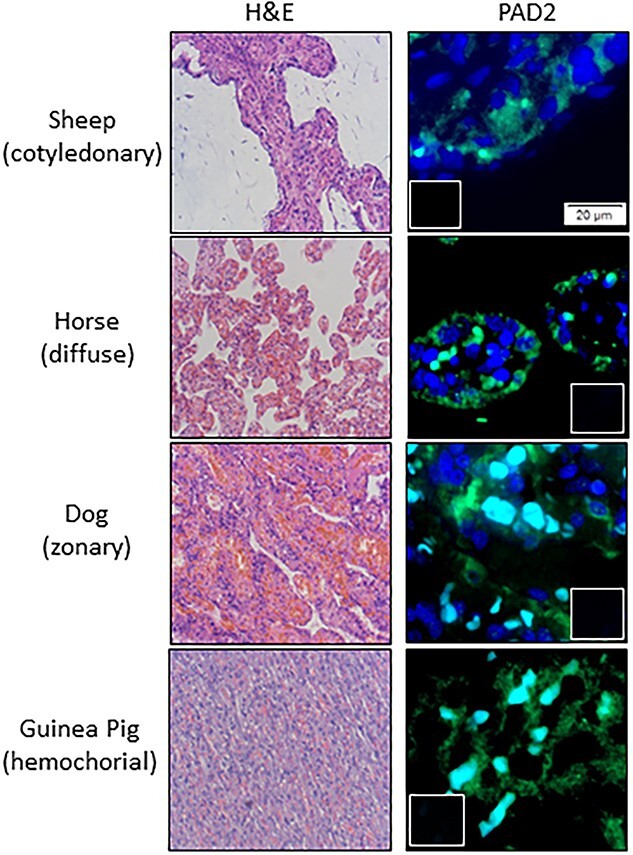
PAD2 (green) is expressed in placentas from different species. Placental tissue samples were collected under approval of the Colorado State University IACUC committee (protocol number 1303). Individual sheep, horse, dog, and guinea pig placentas were obtained at gestational days 75 (sheep) and at term (horse, dog, guinea pig) then fixed in 4% paraformaldehyde and paraffin embedded. Five micron sections were processed for H&E staining or routine immunofluorescence using an anti-PAD2 antibody (1:100 ProteinTech, 12110-1-AP) and imaged using Olympus BX63 fluorescence microscope. Blue nuclear staining is DAPI, small inserts are no primary antibody negative controls, and scale bar represents 20 μm. Light blue in zonary and hemochorial is autofluorescence from red blood cells.
Ovaries
In the ovary, PAD6 is predominantly found in maturing oocytes and early embryos [34, 92]. Although PAD6 is not catalytically active, female PAD6 KO mice are infertile, while males are fertile [93]. A similar phenotype occurs in women who have PAD6 mutations, which result in premature embryonic arrest and infertility [94, 95]. PAD6 expression is regulated by the oocyte-specific transcription factor NOBOX (newborn ovary homeobox) and downregulated in NOBOX KO mouse ovaries [96]. Mutations in PAD6 result in abnormal early embryo cleavage events [97]. PAD6 KO mouse oocytes display reduced content of acetylated microtubules, abnormal microtubule structure, diffuse distribution of endoplasmic reticulum and mitochondria, and abnormal distribution of organelles during maturation as compared to wild-type oocytes [98]. PAD6 is a component of the subcortical maternal complex in the oocyte cytoplasm, which is required for early embryo development. Studies also indicate that PAD6 is involved in genomic imprinting disorders such as Beckwith-Wiedemann syndrome [99].
Citrullinated histones are present in mouse oocytes and preimplantation embryos, suggesting that catalytically active members of the PAD family, beyond PAD6, function in oocyte development and embryogenesis [100]. When PAD activity is inhibited in pronuclear phase zygotes with a pan-PAD inhibitor, Cl-amidine, histone tail citrullination is reduced and early cleavage blocked. PAD inhibition and PAD1 depletion by morpholinos lead to a decrease in citrullination in 2 and 4 cell embryos, the loss of phosphorylation of RNA polymerase II at serine residue 2, and a decrease in overall transcription. The authors conclude that PAD1 catalyzed citrullination of histone tails facilitates early embryo gene transactivation [100]. PAD4 is also present in oocytes and preimplantation embryos, and PAD4 knockdown delays embryonic development, while PAD inhibition arrests embryos at the first cleavage [101, 102]. It should be noted that Cl-amidine irreversibly inhibits all PADs, so results obtained using this inhibitor cannot be attributed to a specific isozyme. Isozyme-specific PAD inhibitors are under development, which will undoubtedly be valuable in parsing out the relative roles of different PADs in all tissues including the ovary [103–107].
Mammary gland
The canine, feline, rodent, and human mammary glands all express PAD enzymes. During canine anestrus and estrus, PAD2 protein is sparse in mammary epithelial cells; however, during late diestrus PAD2 is widely expressed throughout the canine mammary epithelia [108]. Levels of citrullinated histones are also highest in the canine mammary gland during diestrus. In mice, PAD2 is expressed in mammary epithelial cells throughout the estrous cycle, peaking in estrus, while PAD4 expression is lower but detectible during this phase [9]. PAD2 is also expressed in human breast epithelial cells [76]. E2 strongly stimulates PAD2 and 4 expression in mammary epithelial cells of ovariectomized mice compared to placebo-treated controls [9]. PAD3 is present in the lactation day 9 mouse mammary gland [109], and studies show that prolactin stimulates PAD3 expression in the CID-9 cell line derived from mid-pregnant mouse mammary epithelial cells. In CID-9 cells, prolactin activates the JAK2/STAT5 signaling pathway to upregulate PAD3 mRNA and protein expression. Using mass spectrometry, we identified the lactation day 9 mouse mammary gland citrullinome, which contains 107 citrullinated proteins including histone H2A, α-tubulin, and β-casein. Interestingly, in vitro citrullination of purified tubulin appears to alter the overall polymerization rate of microtubules, suggesting that tubulin citrullination may play a role in vesicle trafficking or milk secretion by mammary secretory cells [53].
The most characterized target for PAD-catalyzed citrullination in mammary gland cells is histone arginine residues; however, the majority of this information stems from investigations using the breast cancer cell lines [60, 110, 111]. In MCF7 cells, E2 stimulates PAD2-catalyzed citrullination of histone H3 arginine residue 26 (H3R26). This histone modification subsequently colocalizes with ESR1 at decondensed chromatin loci surrounding EREs of target gene promoters [112]. Interestingly, the citrullination of H3R26, which upregulates gene expression, reduces the methylation of H3K27 that normally represses gene expression [63]. These findings indicate that there are both direct and indirect consequences of PAD activity on epigenetic gene expression. Li et al. detected citrullinated histone H2A in the lactating mouse mammary gland and investigated the functional significance of this observation using CID-9 cells [53]. Stimulation of the CID-9 cells with prolactin increases not only histone H2A citrullination but also the expression of the lactation-related β-casein (Csn2) and butyrophilin (Btn1a1) genes. The full cohort of genes regulated by the histone citrullination mechanism is unknown as are the functions of the additional citrullinated proteins (e.g., the major milk protein β-casein) detected in the lactating mouse mammary gland.
PADs and citrullinated proteins in disease
Citrullination and autoimmunity
PAD enzymes and citrullinated proteins are increasingly associated with the etiology of female-biased autoimmune diseases such as systemic lupus erythematosus, Sjogren syndrome, multiple sclerosis, and rheumatoid arthritis (RA); yet, citrullinated proteins are also implicated in non-sex-biased autoimmune disorders including type 1 diabetes and psoriasis [113–124]. A mechanistic role for citrullinated proteins in female-biased autoimmune disorders is potentially due to estrogen-mediated PAD expression combined with the fact that citrullinated proteins can act as antigens to stimulate autoantibody production.
Anti-citrullinated protein antibodies in female rheumatoid arthritis
Women have a 3-fold higher incidence of RA, develop the disease at a younger age, and have worse clinical outcomes as compared to men [125, 126]. RA can be clinically diagnosed by the presence of anti-citrullinated protein antibodies (ACPAs) in blood. ACPAs can be present 3–5 years before onset of clinical symptoms and are highly predictive of disease severity [127–131]. Strengthening this connection is the fact that genetic mutations that upregulate PAD expression are associated with increased RA disease risk [132–134]. A woman’s RA risk is correlated with the frequency and duration of exposure to female reproductive hormones. Moreover, RA flares are associated with specific phases of the menstrual cycle and the incidence of RA is higher in pre- versus post-menopausal women [125, 135, 136]. Additional RA risk factors include parity, the postpartum period, birth control type, and early age of menarche [137–139]. Given that PAD expression is stimulated by E2 in female reproductive tissues and suppressed by androgens, it follows that this would likely result in higher levels of citrullinated proteins and potentially increase exposure to autoantigens. Unfortunately, our understanding of the role of sex hormones in RA has been inconclusive and has not substantially improved our understanding of disease etiology. For instance, the synovial joint citrullinome from RA patients contains non-sex-specific citrullinated protein antigens such as fibrinogen, vimentin, and α-enolase [51, 54, 140, 141]. Therefore, identifying novel, sex-specific citrullinated protein antigens and determining their relative levels in key populations, such as women, is a key goal in the ongoing search for diagnostic tests and therapies to improve clinical outcomes for women with RA.
PADs in cancer
PADs are expressed in numerous cancers including those of the brain, colon, breast, liver, lung, esophagus, and kidney [142–144]. Alterations in PAD expression result in aberrant changes in the level of citrullinated proteins including histones and transcription factors that have a wide range of oncogenic effects including epigenetically regulating tumor gene expression, promoting epithelial-to-mesenchymal transition (EMT), and progression to metastatic disease [145–147]. Among others, extracellular matrix (ECM) proteins, cytoskeletal filaments, and enzymes such as α-enolase are citrullinated in tumor cell lines [148, 149]. In addition to α-enolase, the glycolytic enzyme pyruvate kinase M2 is citrullinated by PAD1 and PAD3 resulting in increased glycolysis during proliferation of multiple types of cancer cell lines [150, 151]. The following section will focus on our current understanding of PADs and citrullinated proteins in cancers of the female reproductive tissues.
Uterine and ovarian cancers
Just as the physiological role of PADs in uterine tissues is not well defined, neither is the role of citrullination in uterine cancer. Analysis of mRNA profiles from endometrial cancer samples show high levels of PAD2 as compared to other isoforms, while PAD4 is present in uterine cervicitis [144, 152]. To increase our knowledge of PAD expression in uterine cancers, we examined a US Biomax T094 human uterine tumor array by immunohistochemistry. While PAD2 and 4 are present in normal endometrial tissue, only 2 of 14 grade 2 malignant endometrial adenocarcinoma samples had detectable expression (Figure 5). To our knowledge, the only mechanistic study of PAD function in endometrial cancer shows that PAD2 citrullinates MEK1, which activates ERK1/2 to promote insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1) mediated SOX2 mRNA stability in the human endometrial cancer Ishikawa cell line [152].
Figure 5.
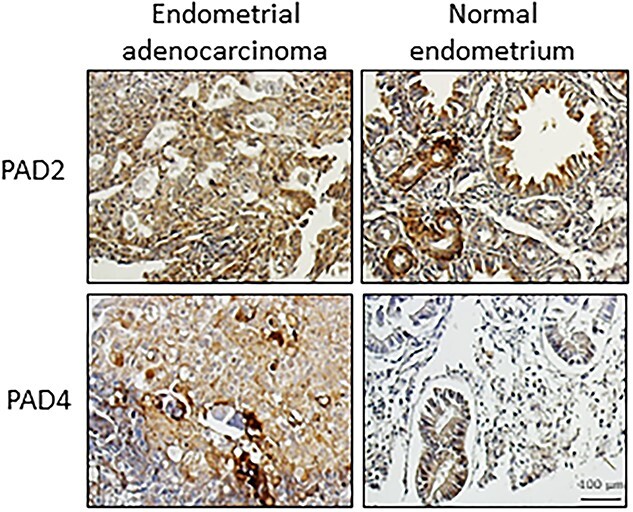
PAD2 and 4 are expressed in normal human endometrial tissue (n = 2/ out of 4 cores) and a subset of endometrial adenocarcinomas (n = 2/ out of 14 cores). Serial uterine tumor array slides were purchased from US Biomax (T094) and contain 12 cases with 24 cores and include associated pathology grade. Tumor array slides were examined by immunohistochemistry using an anti-PAD2 antibody (1:100 ProteinTech, 12110-1-AP), anti-PAD4 (1:100 Millipore Sigma, P4874) or an equal mass of non-specific IgG (data not shown). Images were taken with a Zeiss AxioVert.A1 with AxioCam MRc and scale bar represents 100 μm.
Slightly more is known about PAD expression and function in ovarian cancers. Ovarian tumors cultured in the presence of E2 express PAD4 in cystadenocarcinomas and dysgerminomas; however, PAD4 expression is low or non-detectable in other ovarian cancers such as granulosa cell tumors and malignant thecomas [153]. In PAD4 expressing ovarian cancer subtypes, the full role of PAD4 in tumorigenesis remains unclear, but it appears to function in the p53 pathway and contributes to tumor metastasis by regulating the expression of IGF1 and WAS/WASL-interacting protein family member 1 (WIPF1) genes [154]. As for other isozymes, PAD2 expression is associated with poor prognosis for epithelial ovarian cancer, and knockdown of endogenous PAD2 in A2780 and SKOV3 ovarian cancer cell lines suppresses colony formation, proliferation, migration, and invasion [155]. Given the link between hormonal regulation of these cancers and PAD expression, understanding the function of PADs in uterine and ovarian cancers remains an intriguing area for future research.
Breast cancer
Breast cancer is by far the most-characterized female reproductive cancer associated with PADs and citrullinated proteins. PAD4 is expressed in breast carcinomas and the original groundbreaking work that characterized PAD4 as an epigenetic enzyme utilized the MCF7 breast cancer cell line [60, 144]. In these cells, citrullination of histone H3 arginine residues is directly associated with E2-responsive gene expression [60]. Studies using these cells also demonstrate that PAD4 interacts with Elk1 to activate c-Fos gene expression and is predictive of actively transcribed genes [156, 157]. PAD4 can also downregulate the expression of the transcription factors NANOG and Oct4, thereby regulating breast cancer stem cells [158]. As for other citrullinated proteins, studies suggest that they have a functional role in cancer metastases. Shi et al. found that in breast cancer 4T1 cells high levels of PAD4 facilitate cancer extracellular chromatin networks, which promote lung metastasis in a xenograft model [110].
PAD2 is expressed in human, feline, and canine mammary cancers and is the most highly expressed isoform in primary breast tumors and luminal breast cancer cell lines [76, 159, 160]. In MCF7 cells, knockdown of endogenous PAD2 regulates the expression of multiple genes implicated in tumorigenesis including pleiotrophin (PTN) and melanoma antigen A12 (MAGEA12) [111]. PAD2 also promotes EMT and abnormal cell migration during tumorigenesis [161, 162]. Elegant work by Sharma et al. discovered that PAD2 citrullinates RNA polymerase II, which is required to overcome pausing and initiate transcription of genes important for cell cycle regulation and proliferation in the T47D breast cancer cell line [163]. The single-nucleotide polymorphism rs10788656 in PAD2 confers susceptibility to breast cancer, and PAD2 is hypothesized as a potential breast cancer biomarker and therapeutic target [161, 164]. Taken together, these studies indicate that citrullination is a component of the etiology and progression of breast cancer, but further work is needed to discover all the mechanisms involved to develop effective citrullination-specific strategies for detection and treatment of breast cancer.
Pituitary tumors
PAD2 and 4 and citrullinated histones are present in human prolactinomas and somatoprolactinomas [61]. Expanding upon this, we examined additional pituitary tumor subtypes for PAD2 expression by immunohistochemistry and found that PAD2 is present in non-functioning, corticotrope-, gonadotrope-, and somatotrope-derived tumors (Figure 6). Mechanistically, PAD function has only been investigated in the somatoprolactinoma-derived GH3 cell line. As discussed above, DeVore et al. discovered that PAD inhibition induces expression of miRNAs let-7c, miR-23b, and miR-29c, suggesting that PAD-catalyzed histone citrullination normally represses the expression of these pri-miRNA genes. Functionally, let-7c and miR-23b suppress the expression of the oncogene high mobility group AT-hook 1 (HMGA1), which is highly implicated in the pathogenesis and proliferation of human prolactinomas and somatoprolactinomas [61, 165]. Directly connecting PADs and miRNAs, HMGA1 mRNA and protein expression are suppressed following PAD inhibition but are restored by transfection of GH3 cells with let-7c and miR-23b antagomirs. Collectively, these studies suggest that histone citrullination suppresses let-7c and miR-23b expression, leading to increased HMGA1. Further work is clearly needed to elucidate the mechanism by which PAD-catalyzed citrullination regulates proliferation in pituitary adenomas.
Figure 6.
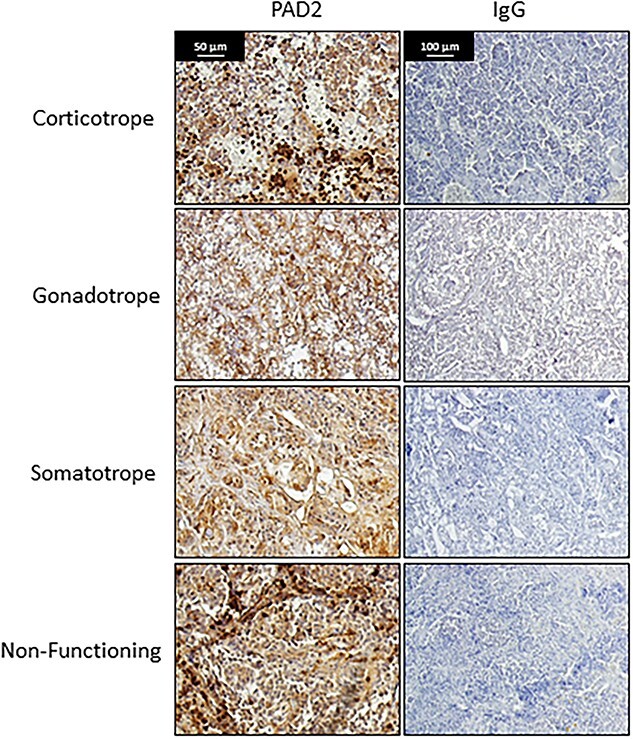
PAD2 expression is present in multiple subtypes of pituitary tumors. Pituitary tumor samples including subtype pathology were obtained from Institut D’Investigacions Biomediques August Pi i Sunyer Biobanc (Barcelona, Spain). Samples were de-identified and thus were exempt from University of Wyoming institutional review board (IRB) approval (protocol number 20140814BC00496). Tumors (n = 4 /tumor subtype) were examined by immunohistochemistry using an anti-PAD2 antibody (1:100 ProteinTech, 12110-1-AP) or an equal mass of non-specific IgG. Images were taken with a Zeiss AxioVert.A1 with AxioCam MRc and scale bar represents 50 or 100 μm.
PAD inhibitors as therapeutic modalities
Since PADs function in autoimmune diseases and the pathogenesis, invasion, and metastasis of tumors, PAD inhibitors have generated interest as potential therapeutic modalities [164, 166, 167]. To date, the most well-characterized pan-PAD inhibitor is BB-ClA, having been investigated in a number of different disease models [166, 168, 169]. In the human breast cancer MCF10DCIS cell line, BB-ClA treatment suppresses tumor spheroid growth by altering expression of p21, GADD45α, and Ki67, which are implicated in tumor progression [164]. In tamoxifen-resistant MCF7 cells, there is a dramatic upregulation of PAD2 expression; however, PAD2 depletion or BB-ClA treatment increase sensitivity to tamoxifen and efficacy of docetaxel [166, 170]. While the treatment of feline and canine mammary cancer cells with BB-ClA activated the endoplasmic reticulum stress pathway and reduced tumor viability in vitro, treatment of mouse xenografts with BB-ClA did not significantly reduce tumor size [166].
Since PAD inhibitors may represent adjuvant therapeutic options for treatment of breast and a range of other cancers, there is a strong push to develop and characterize new reversible and irreversible PAD inhibitors such as streptonigrin, NSC 95397, streptomycin, minocycline, and chlortetracycline [23, 164, 166]. Of these, streptonigrin, NSC 95397, and chlortetracycline already have documented anticancer/antitumor activity [171–175]. For example, streptonigrin is an antitumor antibiotic with function in lymphomas and carcinomas (reviewed in [176]) and was recently shown to be effective against renal carcinoma cells in an in vitro pre-clinical model [177].
PAD inhibition also disrupts NET formation, which is implicated in inflammatory events, tumorigenesis progression, and metastasis [69, 168, 178]. Tumors produce the chemokine receptor agonists CXCR1 and CXCR2, which stimulate NETosis and interfere with immune cytotoxic attack, while inhibition of NETosis sensitizes tumors to PD-1 and CTLA-4 dual checkpoint blockade [179]. NETs can interact with tumor cells to promote metastatic progression at secondary sites [180], and PAD4 KO mice, which are NET deficient, have decreased levels of spontaneous liver tumor metastasis compared to wild-type mice [181]. Collectively, these findings underscore the therapeutic potential of PAD4-specific inhibitors with acceptable treatment safety profiles for inhibition of NETosis.
It is highly likely that PAD inhibitors will also prove beneficial for the treatment of autoimmune disorders, given the well-documented relationship between citrullination, inflammation, and autoimmunity [4, 9, 182]. Specifically, PAD inhibitors can modulate the immune response and show promise in several animal models of autoimmunity. For instance, in lupus MRL/lpr mice, BB-ClA and Cl-amidine protect against lupus-associated kidney, skin, and vascular disease [168]. BB-ClA also prevents the development of diabetes in non-obese diabetic (NOD) mice, which are a model for autoimmune type I diabetes [183]. In a mouse collagen-induced arthritis (CIA) model, Cl-amidine treatment reduces the levels of synovial citrullinated proteins while decreasing clinical disease activity by approximately half [169]. Finally, Cl-amidine, given prophylactically and after disease onset, reduces disease symptoms in a mouse model of colitis [184]. Thus, there is a growing body of data to support the use of PAD inhibitors with acceptable safety profiles in clinical trials as adjuvant therapeutics for the treatment of cancer and autoimmune disorders.
Citrullinated protein cancer vaccines
A novel cancer therapy under development is vaccination with citrullinated proteins frequently found in tumors. The intermediate filament vimentin is citrullinated during EMT in many epidermal-derived tumors. When injected, citrullinated vimentin peptides are presented by major histocompatibility complex II (MHC-II) molecules and stimulate a CD4 T-cell-specific response. This therapy provides a survival benefit up to 14 days after tumor implantation in HLA-DR4 mice, which contain a MHC-II molecule with a humanized peptide-binding domain [185]. Injection with citrullinated enolase also generates an antitumor response against melanoma cells in C57Bl/6J and HLA-DR4 transgenic mice, with similar results using pancreatic and lung tumor cells. Importantly, non-citrullinated enolase does not induce antitumor response [149]. A potent CD4 T-cell-mediated antitumor response is stimulated in HLA transgenic mice after injection with combined citrullinated vimentin and citrullinated enolase [186]. This is an exciting area of research and work is under way to identify other citrullinated proteins that may provide therapeutic benefit for a variety of cancers [187, 188].
Conclusions and future studies
PADs are expressed in female reproductive tissues, yet the function of protein citrullination and the identity of the citrullinome in many tissues is still an open question. Of particular importance to reproductive biology is the role of E2 in stimulating PAD expression, which is followed by citrullination of numerous target proteins. With increased PADs, there is a subsequent increase in histone citrullination, which gives rise to epigenetic changes in gene expression in gonadotropes, lactotropes, mammary epithelial, and uterine cells [40, 48, 53, 61]. Clearly, further studies are required to identify other targets for PAD-catalyzed citrullination in these cell types. In non-reproductive models, the citrullination of cytoskeletal filaments regulate secretory mechanisms [189]. The uterus, mammary gland, and anterior pituitary gland are highly secretory and synthesize and secrete a wide range of molecules critical at all levels of reproduction. Given this, it is intriguing to speculate that citrullinated cytoskeletal proteins may contribute to secretory mechanisms and clearly warrant further investigation given the importance of this process to reproductive function. Additionally, there is a compelling need for the development and analysis of PAD KO mice. While independent global PAD2 and PAD4 KO lines exist, neither display any major reproductive phenotype, most likely due to compensation by other isoforms [190, 191]. Future studies would greatly benefit from the creation of tissue-specific single and PAD2/4 double KO mice lines to evaluate reproductive phenotypes.
In contrast to reproductive physiology, aberrant PAD expression and protein citrullination are associated with poorer outcomes in both autoimmune disorders and female reproductive cancers. Defining the mechanisms by which PADs and citrullinated proteins stimulate autoimmune initiation and progression and tumorigenesis remains to be elucidated. However, recent advances support the need for the continued development of selective PAD inhibitors for the treatment of autoimmune disorders and cancers, while simultaneously studying off target side effects and toxicities of PAD and citrullination directed therapies. In conclusion, determining the role of PADs and protein citrullination is critical not only to improve our understanding of reproductive biology, but also how their dysfunction leads to disease.
Conflict of Interest
Our manuscript contains original research, but it is not published nor is it under editorial consideration elsewhere. All research was carried out following ethical guidelines including strict guidelines governing the use of human and animal tissue. None of the authors has anything to disclose.
Author Contributions
The manuscript was written by AOC, HMR, and BDC. GL, CHY, BS, SAK, SBD, and AMN all helped edit and revise the manuscript based on their contributions to the field. SE, GJB, BDC, and HMR conducted the experiments in Figures 4–6. Financial support for the project was to BDC.
Footnotes
† Grant Support: Research reported in this publication was supported by the National Institute of General Medical Sciences and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Numbers P20GM103432 and R21HD090541, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Amanda O Christensen, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
Guangyuan Li, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
Coleman H Young, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
Bryce Snow, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
Shaihla A Khan, Genus PLC, DeForest, WI 53532, USA.
Stanley B DeVore, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH 45267, USA.
Sydney Edwards, Animal Reproduction and Biotechnology Laboratory, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Gerrit J Bouma, Animal Reproduction and Biotechnology Laboratory, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Amy M Navratil, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
Brian D Cherrington, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
Heather M Rothfuss, Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071, USA.
References
- 1. Rogers GE. Occurrence of citrulline in proteins. Nature 1962; 194:1149–1151. [DOI] [PubMed] [Google Scholar]
- 2. Rogers GE, Harding HW, Llewellyn-Smith IJ. The origin of citrulline-containing proteins in the hair follicle and the chemical nature of trichohyalin, an intracellular precursor. Biochim Biophys Acta 1977; 495:159–175. [DOI] [PubMed] [Google Scholar]
- 3. Rogers GE, Taylor LD. The enzymic derivation of citrulline residues from arginine residues in situ during the biosynthesis of hair proteins that are cross-linked by isopeptide bonds. Adv Exp Med Biol 1977; 86A:283–294. [DOI] [PubMed] [Google Scholar]
- 4. Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003; 25:1106–1118. [DOI] [PubMed] [Google Scholar]
- 5. Chavanas S, Mechin MC, Takahara H, Kawada A, Nachat R, Serre G, Simon M. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene 2004; 330:19–27. [DOI] [PubMed] [Google Scholar]
- 6. Cummings TFM, Gori K, Sanchez-Pulido L, Gavriilidis G, Moi D, Wilson AR, Murchison E, Dessimoz C, Ponting CP, Christophorou MA. Citrullination was introduced into animals by horizontal gene transfer from cyanobacteria. Mol Biol Evol 2022; 39. 10.1093/molbev/msab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol 2006; 38:1662–1677. [DOI] [PubMed] [Google Scholar]
- 8. Witalison EE, Thompson PR, Hofseth LJ. Protein arginine deiminases and associated citrullination: physiological functions and diseases associated with dysregulation. Curr Drug Targets 2015; 16:700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horibata S, Coonrod SA, Cherrington BD. Role for peptidylarginine deiminase enzymes in disease and female reproduction. J Reprod Dev 2012; 58:274–282. [DOI] [PubMed] [Google Scholar]
- 10. Fuhrmann J, Clancy KW, Thompson PR. Chemical biology of protein arginine modifications in epigenetic regulation. Chem Rev 2015; 115:5413–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hidenari Takahara HOaKS . Calcium-dependent properties of peptidylarginine deiminase from rabbit skeletal muscle. Agric Biol Chem 1986; 50:2899–2904. [Google Scholar]
- 12. Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol 2004; 11:777–783. [DOI] [PubMed] [Google Scholar]
- 13. Slade DJ, Fang P, Dreyton CJ, Zhang Y, Fuhrmann J, Rempel D, Bax BD, Coonrod SA, Lewis HD, Guo M, Gross ML, Thompson PR. Protein arginine deiminase 2 binds calcium in an ordered fashion: implications for inhibitor design. ACS Chem Biol 2015; 10:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu YL, Lee CY, Huang YN, Chen HY, Liu GY, Hung HC. Probing the roles of calcium-binding sites during the folding of human peptidylarginine deiminase 4. Sci Rep 2017; 7:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, Takahara H, Thompson PR. Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry 2010; 49:4852–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dreyton CJ, Knuckley B, Jones JE, Lewallen DM, Thompson PR. Mechanistic studies of protein arginine deiminase 2: evidence for a substrate-assisted mechanism. Biochemistry 2014; 53:4426–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. Calcium green FlAsH as a genetically targeted small-molecule calcium indicator. Nat Chem Biol 2007; 3:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science 1992; 256:677–679. [DOI] [PubMed] [Google Scholar]
- 19. Mechin MC, Enji M, Nachat R, Chavanas S, Charveron M, Ishida-Yamamoto A, Serre G, Takahara H, Simon M. The peptidylarginine deiminases expressed in human epidermis differ in their substrate specificities and subcellular locations. Cell Mol Life Sci 2005; 62:1984–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore MS. Ran and nuclear transport. J Biol Chem 1998; 273:22857–22860. [DOI] [PubMed] [Google Scholar]
- 21. Zheng L, Nagar M, Maurais AJ, Slade DJ, Parelkar SS, Coonrod SA, Weerapana E, Thompson PR. Calcium regulates the nuclear localization of protein arginine deiminase 2. Biochemistry 2019; 58:3042–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med 2013; 5:186ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mondal S, Thompson PR. Protein arginine deiminases (PADs): biochemistry and chemical biology of protein citrullination. Acc Chem Res 2019; 52:818–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CY, Lin CC, Liu YL, Liu GY, Liu JH, Hung HC. Molecular interplay between the dimer interface and the substrate-binding site of human peptidylarginine deiminase 4. Sci Rep 2017; 7:42662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saijo S, Nagai A, Kinjo S, Mashimo R, Akimoto M, Kizawa K, Yabe-Wada T, Shimizu N, Takahara H, Unno M. Monomeric form of peptidylarginine deiminase type I revealed by X-ray crystallography and small-angle X-ray scattering. J Mol Biol 2016; 428:3058–3073. [DOI] [PubMed] [Google Scholar]
- 26. Rogers G, Winter B, McLaughlan C, Powell B, Nesci T. Peptidylarginine deiminase of the hair follicle: characterization, localization, and function in keratinizing tissues. J Invest Dermatol 1997; 108:700–707. [DOI] [PubMed] [Google Scholar]
- 27. Senshu T, Kan S, Ogawa H, Manabe M, Asaga H. Preferential deimination of keratin K1 and filaggrin during the terminal differentiation of human epidermis. Biochem Biophys Res Commun 1996; 225:712–719. [DOI] [PubMed] [Google Scholar]
- 28. Nachat R, Mechin MC, Takahara H, Chavanas S, Charveron M, Serre G, Simon M. Peptidylarginine deiminase isoforms 1-3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol 2005; 124:384–393. [DOI] [PubMed] [Google Scholar]
- 29. Guerrin M, Ishigami A, Mechin MC, Nachat R, Valmary S, Sebbag M, Simon M, Senshu T, Serre G. cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type I. Biochem J 2003; 370:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terakawa H, Takahara H, Sugawara K. Three types of mouse peptidylarginine deiminase: characterization and tissue distribution. J Biochem 1991; 110:661–666. [DOI] [PubMed] [Google Scholar]
- 31. Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem 2002; 277:49562–49568. [DOI] [PubMed] [Google Scholar]
- 32. Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, Barrera P, Zendman AJ, van Venrooij WJ. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 2004; 63:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wiersma VR, Clarke A, Pouwels SD, Perry E, Abdullah TM, Kelly C, Soyza A, Hutchinson D, Eggleton P, Bremer E. Galectin-9 is a possible promoter of immunopathology in rheumatoid arthritis by activation of peptidyl arginine deiminase 4 (PAD-4) in granulocytes. Int J Mol Sci 2019; 20. 10.3390/ijms20164046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wright PW, Bolling LC, Calvert ME, Sarmento OF, Berkeley EV, Shea MC, Hao Z, Jayes FC, Bush LA, Shetty J, Shore AN, Reddi PP et al. ePAD, an oocyte and early embryo-abundant peptidylarginine deiminase-like protein that localizes to egg cytoplasmic sheets. Dev Biol 2003; 256:73–88. [DOI] [PubMed] [Google Scholar]
- 35. Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta 2013; 1829:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagata S, Yamagiwa M, Inoue K, Senshu T. Estrogen regulates peptidylarginine deiminase levels in a rat pituitary cell line in culture. J Cell Physiol 1990; 145:333–339. [DOI] [PubMed] [Google Scholar]
- 37. Takahara H, Kusubata M, Tsuchida M, Kohsaka T, Tagami S, Sugawara K. Expression of peptidylarginine deiminase in the uterine epithelial cells of mouse is dependent on estrogen. J Biol Chem 1992; 267:520–525. [PubMed] [Google Scholar]
- 38. Senshu T, Akiyama K, Nagata S, Watanabe K, Hikichi K. Peptidylarginine deiminase in rat pituitary: sex difference, estrous cycle-related changes, and estrogen dependence. Endocrinology 1989; 124:2666–2670. [DOI] [PubMed] [Google Scholar]
- 39. Watanabe K, Hikichi K, Nagata S, Senshu T. Estrous cycle dependent regulation of peptidylarginine deiminase transcripts in female rat pituitary. Biochem Biophys Res Commun 1990; 172:28–34. [DOI] [PubMed] [Google Scholar]
- 40. Khan SA, Edwards BS, Muth A, Thompson PR, Cherrington BD, Navratil AM. GnRH stimulates peptidylarginine deiminase catalyzed histone citrullination in gonadotrope cells. Mol Endocrinol 2016; 30:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, Song G, Zhang X, Feng T, Pan J, Chen W, Yang M, Bai X, Pang Y, Yu J, Han J, Han B. PADI2-mediated citrullination promotes prostate cancer progression. Cancer Res 2017; 77:5755–5768. [DOI] [PubMed] [Google Scholar]
- 42. Schwartz CL, Vinggaard AM, Christiansen S, Darde TA, Chalmel F, Svingen T. Distinct transcriptional profiles of the female, male, and finasteride-induced feminized male anogenital region in rat fetuses. Toxicol Sci 2019; 169:303–311. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem 2006; 281:26683–26692. [DOI] [PubMed] [Google Scholar]
- 44. Jefferson WN, Kinyamu HK, Wang T, Miranda AX, Padilla-Banks E, Suen AA, Williams CJ. Widespread enhancer activation via ERalpha mediates estrogen response in vivo during uterine development. Nucleic Acids Res 2018; 46:5487–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol 2004; 18:1411–1427. [DOI] [PubMed] [Google Scholar]
- 46. Dong S, Kojima T, Shiraiwa M, Mechin MC, Chavanas S, Serre G, Simon M, Kawada A, Takahara H. Regulation of the expression of peptidylarginine deiminase type II gene (PADI2) in human keratinocytes involves Sp1 and Sp3 transcription factors. J Invest Dermatol 2005; 124:1026–1033. [DOI] [PubMed] [Google Scholar]
- 47. Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm 2001; 62:231–252. [DOI] [PubMed] [Google Scholar]
- 48. Young CH, Snow B, DeVore SB, Mohandass A, Nemmara VV, Thompson PR, Thyagarajan B, Navratil AM, Cherrington BD. Progesterone stimulates histone citrullination to increase IGFBP1 expression in uterine cells. Reproduction 2021; 162:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giaglis S, Stoikou M, Sur Chowdhury C, Schaefer G, Grimolizzi F, Rossi SW, Hoesli IM, Lapaire O, Hasler P, Hahn S. Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the pro-NETotic effect of estrogen and G-CSF. Front Immunol 2016; 7:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin Z, Fu Z, Yang J, Troncosco J, Everett AD, Van Eyk JE. Identification and characterization of citrulline-modified brain proteins by combining HCD and CID fragmentation. Proteomics 2013; 13:2682–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma M, Damgaard D, Senolt L, Svensson B, Bay-Jensen AC, Nielsen CH, Hagglund P. Expanding the citrullinome of synovial fibrinogen from rheumatoid arthritis patients. J Proteomics 2019; 208:103484. [DOI] [PubMed] [Google Scholar]
- 52. Assohou-Luty C, Raijmakers R, Benckhuijsen WE, Stammen-Vogelzangs J, de Ru A, van Veelen PA, Franken KL, Drijfhout JW, Pruijn GJ. The human peptidylarginine deiminases type 2 and type 4 have distinct substrate specificities. Biochim Biophys Acta 1844(4); 829–836. [DOI] [PubMed] [Google Scholar]
- 53. Li G, Young CH, Snow B, Christensen AO, Demoruelle MK, Nemmara VV, Thompson PR, Rothfuss HM, Cherrington BD. Identification and characterization of the lactating mouse mammary gland citrullinome. Int J Mol Sci 2020; 21. 10.3390/ijms21072634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tilvawala R, Nguyen SH, Maurais AJ, Nemmara VV, Nagar M, Salinger AJ, Nagpal S, Weerapana E, Thompson PR. The rheumatoid arthritis-associated citrullinome. Cell Chem Biol 2018; 25:691–704.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Swensen AC, Zhang T, Piehowski PD, Gaffrey MJ, Monroe ME, Zhu Y, Dong H, Qian WJ. Accurate identification of deamidation and citrullination from global shotgun proteomics data using a dual-search delta score strategy. J Proteome Res 2020; 19:1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Magnadottir B, Kraev I, Dodds AW, Lange S. The proteome and citrullinome of Hippoglossus hippoglossus extracellular vesicles-novel insights into roles of the serum secretome in immune, gene regulatory and metabolic pathways. Int J Mol Sci 2021; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tutturen AE, Fleckenstein B, de Souza GA. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J Proteome Res 2014; 13:2867–2873. [DOI] [PubMed] [Google Scholar]
- 58. van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, Oosterink E, Bozic B, Pruijn GJ. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum 2013; 65:69–80. [DOI] [PubMed] [Google Scholar]
- 59. Lee CY, Wang D, Wilhelm M, Zolg DP, Schmidt T, Schnatbaum K, Reimer U, Ponten F, Uhlen M, Hahne H, Kuster B. Mining the human tissue proteome for protein citrullination. Mol Cell Proteomics 2018; 17:1378–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004; 306:279–283. [DOI] [PubMed] [Google Scholar]
- 61. DeVore SB, Young CH, Li G, Sundararajan A, Ramaraj T, Mudge J, Schilkey F, Muth A, Thompson PR, Cherrington BD. Histone citrullination represses microRNA expression, resulting in increased oncogene mRNAs in somatolactotrope cells. Mol Cell Biol 2018; 38. 10.1128/MCB.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Young CH, Rothfuss HM, Gard PF, Muth A, Thompson PR, Ashley RL, Cherrington BD. Citrullination regulates the expression of insulin-like growth factor-binding protein 1 (IGFBP1) in ovine uterine luminal epithelial cells. Reproduction 2017; 153:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Clancy KW, Russell AM, Subramanian V, Nguyen H, Qian Y, Campbell RM, Thompson PR. Citrullination/methylation crosstalk on histone H3 regulates ER-target gene transcription. ACS Chem Biol 2017; 12:1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guertin MJ, Zhang X, Anguish L, Kim S, Varticovski L, Lis JT, Hager GL, Coonrod SA. Targeted H3R26 deimination specifically facilitates estrogen receptor binding by modifying nucleosome structure. PLoS Genet 2014; 10:e1004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cherrington B, Mohanan S, Coonrod S. PAD enzymes in female reproductive tissues and cancer pathogenesis. In: Nicholas AP, Bhattacharya SK (eds.), Protein Deimination in Human Health and Disease. New York: Springer; 2014: 305–326. [Google Scholar]
- 66. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 67. Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sorensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest 2016; 126:1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, Chung CW, Craggs PD et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015; 11:189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Neeli I, Radic M. Knotting the NETs: analyzing histone modifications in neutrophil extracellular traps. Arthritis Res Ther 2012; 14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu D, Zhang Y, Wang S. Histone citrullination: a new target for tumors. Mol Cancer 2021; 20:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, Weisman MH, Solomon JJ, Fischer A, Okamoto Y, Kelmenson LB, Parish MC et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol 2017; 69:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, Schmitt AM, Paul C, Poumay Y, Serre G, Simon M. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol 2014; 134:2938–2946. [DOI] [PubMed] [Google Scholar]
- 74. Mechin MC, Takahara H, Simon M. Deimination and peptidylarginine deiminases in skin physiology and diseases. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Takahara H, Tsuchida M, Kusubata M, Akutsu K, Tagami S, Sugawara K. Peptidylarginine deiminase of the mouse. Distribution, properties, and immunocytochemical localization. J Biol Chem 1989; 264:13361–13368. [PubMed] [Google Scholar]
- 76. Cherrington BD, Mohanan S, Diep AN, Fleiss R, Sudilovsky D, Anguish LJ, Coonrod SA, Wakshlag JJ. Comparative analysis of peptidylarginine deiminase-2 expression in canine, feline and human mammary tumours. J Comp Pathol 2012; 147:139–146. [DOI] [PubMed] [Google Scholar]
- 77. Akiyama K, Nagata S, Tanaka S, Inoue K, Watanabe K, Senshu T. Search for functional significance of peptidylarginine deiminase in rat pituitaries: variation during pregnancy and ultrastructural localization in prolactin cells. Cell Biol Int 1993; 17:487–494. [DOI] [PubMed] [Google Scholar]
- 78. Akiyama K, Inoue K, Senshu T. Immunocytochemical study of peptidylarginine deiminase: localization of its immunoreactivity in prolactin cells of female rat pituitaries. Endocrinology 1989; 125:1121–1127. [DOI] [PubMed] [Google Scholar]
- 79. Nagata S, Uehara T, Inoue K, Senshu T. Increased peptidylarginine deiminase expression during induction of prolactin biosynthesis in a growth-hormone-producing rat pituitary cell line, MtT/S. J Cell Physiol 1992; 150:426–432. [DOI] [PubMed] [Google Scholar]
- 80. Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM et al. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res 2009; 37:D885–D890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang L, Long W, Xu W, Chen X, Zhao X, Wu B. Digital cell atlas of mouse uterus: from regenerative stage to maturational stage. Front Genet 2022; 13:847646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Garcia-Alonso L, Handfield LF, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, Woodhams B, Arutyunyan A, Polanski K, Hoo R, Sancho-Serra C, Li T et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet 2021; 53:1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsuchida M, Takahara H, Minami N, Arai T, Kobayashi Y, Tsujimoto H, Fukazawa C, Sugawara K. cDNA nucleotide sequence and primary structure of mouse uterine peptidylarginine deiminase. Detection of a 3′-untranslated nucleotide sequence common to the mRNA of transiently expressed genes and rapid turnover of this enzyme's mRNA in the estrous cycle. Eur J Biochem 1993; 215:677–685. [DOI] [PubMed] [Google Scholar]
- 84. Rus'd AA, Ikejiri Y, Ono H, Yonekawa T, Shiraiwa M, Kawada A, Takahara H. Molecular cloning of cDNAs of mouse peptidylarginine deiminase type I, type III and type IV, and the expression pattern of type I in mouse. Eur J Biochem 1999; 259:660–669. [DOI] [PubMed] [Google Scholar]
- 85. Ledee N, Munaut C, Aubert J, Serazin V, Rahmati M, Chaouat G, Sandra O, Foidart JM. Specific and extensive endometrial deregulation is present before conception in IVF/ICSI repeated implantation failures (IF) or recurrent miscarriages. J Pathol 2011; 225:554–564. [DOI] [PubMed] [Google Scholar]
- 86. Arai T, Kusubata M, Kohsaka T, Shiraiwa M, Sugawara K, Takahara H. Mouse uterus peptidylarginine deiminase is expressed in decidual cells during pregnancy. J Cell Biochem 1995; 58:269–278. [DOI] [PubMed] [Google Scholar]
- 87. Yang Y, Zhu QY, Liu JL. Deciphering mouse uterine receptivity for embryo implantation at single-cell resolution. Cell Prolif 2021; 54:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang Y, He JP, Liu JL. Cell-cell communication at the embryo implantation site of mouse uterus revealed by single-cell analysis. Int J Mol Sci 2021; 22. 10.3390/ijms22105177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. He JP, Tian Q, Zhu QY, Liu JL. Single-cell analysis of mouse uterus at the invasion phase of embryo implantation. Cell Biosci 2022; 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A, Kustagi M, Tuschl T, Williams Z. A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 2018; 4:eaau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, Gomez-Lopez N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 2019; 8. 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang J, Dai J, Zhao E, Lin Y, Zeng L, Chen J, Zheng H, Wang Y, Li X, Ying K, Xie Y, Mao Y. cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type VI. Acta Biochim Pol 2004; 51:1051–1058. [PubMed] [Google Scholar]
- 93. Esposito G, Vitale AM, Leijten FP, Strik AM, Koonen-Reemst AM, Yurttas P, Robben TJ, Coonrod S, Gossen JA. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol Cell Endocrinol 2007; 273:25–31. [DOI] [PubMed] [Google Scholar]
- 94. Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B, Yan Z, Mao X et al. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet 2016; 99:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu J, Tan Z, He J, Jin T, Han Y, Hu L, Huang S. Two novel mutations in PADI6 and TLE6 genes cause female infertility due to arrest in embryonic development. J Assist Reprod Genet 2021; 38:1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Choi M, Lee OH, Jeon S, Park M, Lee DR, Ko JJ, Yoon TK, Rajkovic A, Choi Y. The oocyte-specific transcription factor, Nobox, regulates the expression of Pad6, a peptidylarginine deiminase in the oocyte. FEBS Lett 2010; 584:3629–3634. [DOI] [PubMed] [Google Scholar]
- 97. Zheng W, Chen L, Dai J, Dai C, Guo J, Lu C, Gong F, Lu G, Lin G. New biallelic mutations in PADI6 cause recurrent preimplantation embryonic arrest characterized by direct cleavage. J Assist Reprod Genet 2020; 37:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kan R, Yurttas P, Kim B, Jin M, Wo L, Lee B, Gosden R, Coonrod SA. Regulation of mouse oocyte microtubule and organelle dynamics by PADI6 and the cytoplasmic lattices. Dev Biol 2011; 350:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cubellis MV, Pignata L, Verma A, Sparago A, Del Prete R, Monticelli M, Calzari L, Antona V, Melis D, Tenconi R, Russo S, Cerrato F et al. Loss-of-function maternal-effect mutations of PADI6 are associated with familial and sporadic Beckwith-Wiedemann syndrome with multi-locus imprinting disturbance. Clin Epigenetics 2020; 12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang X, Liu X, Zhang M, Li T, Muth A, Thompson PR, Coonrod SA, Zhang X. Peptidylarginine deiminase 1-catalyzed histone citrullination is essential for early embryo development. Sci Rep 2016; 6:38727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brahmajosyula M, Miyake M. Localization and expression of peptidylarginine deiminase 4 (PAD4) in mammalian oocytes and preimplantation embryos. Zygote 2013; 21:314–324. [DOI] [PubMed] [Google Scholar]
- 102. Brahmajosyula M, Miyake M. Role of peptidylarginine deiminase 4 (PAD4) in pig parthenogenetic preimplantation embryonic development. Zygote 2013; 21:385–393. [DOI] [PubMed] [Google Scholar]
- 103. Jamali H, Khan HA, Tjin CC, Ellman JA. Cellular activity of new small molecule protein arginine deiminase 3 (PAD3) inhibitors. ACS Med Chem Lett 2016; 7:847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aliko A, Kaminska M, Falkowski K, Bielecka E, Benedyk-Machaczka M, Malicki S, Koziel J, Wong A, Bryzek D, Kantyka T, Mydel P. Discovery of novel potential reversible peptidyl arginine deiminase inhibitor. Int J Mol Sci 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, Sato M, Thompson PR. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry 2006; 45:11727–11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, Knuckley B, Ebrahimi P, Chumanevich AA, Luo Y, Hashimoto H, Sato M et al. The development of N-alpha-(2-carboxyl)benzoyl-N(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-F-amidine) and N-alpha-(2-carboxyl)benzoyl-N(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J Med Chem 2011; 54:6919–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Muth A, Subramanian V, Beaumont E, Nagar M, Kerry P, McEwan P, Srinath H, Clancy K, Parelkar S, Thompson PR. Development of a selective inhibitor of protein arginine deiminase 2. J Med Chem 2017; 60:3198–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cherrington BD, Morency E, Struble AM, Coonrod SA, Wakshlag JJ. Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones. PLoS One 2010; 5:e11768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li G, Hayward IN, Jenkins BR, Rothfuss HM, Young CH, Nevalainen MT, Muth A, Thompson PR, Navratil AM, Cherrington BD. Peptidylarginine deiminase 3 (PAD3) is upregulated by prolactin stimulation of CID-9 cells and expressed in the lactating mouse mammary gland. PLoS One 2016; 11:e0147503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shi L, Yao H, Liu Z, Xu M, Tsung A, Wang Y. Endogenous PAD4 in breast cancer cells mediates cancer extracellular chromatin network formation and promotes lung metastasis. Mol Cancer Res 2020; 18:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cherrington BD, Zhang X, McElwee JL, Morency E, Anguish LJ, Coonrod SA. Potential role for PAD2 in gene regulation in breast cancer cells. PLoS One 2012; 7:e41242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang X, Bolt M, Guertin MJ, Chen W, Zhang S, Cherrington BD, Slade DJ, Dreyton CJ, Subramanian V, Bicker KL, Thompson PR, Mancini MA et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proc Natl Acad Sci U S A 2012; 109:13331–13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alghamdi M, Alasmari D, Assiri A, Mattar E, Aljaddawi AA, Alattas SG, Redwan EM. An overview of the intrinsic role of citrullination in autoimmune disorders. J Immunol Res 2019; 2019:7592851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Billi AC, Kahlenberg JM, Gudjonsson JE. Sex bias in autoimmunity. Curr Opin Rheumatol 2019; 31:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kakumanu P, Sobel ES, Narain S, Li Y, Akaogi J, Yamasaki Y, Segal MS, Hahn PC, Chan EK, Reeves WH, Satoh M. Citrulline dependence of anti-cyclic citrullinated peptide antibodies in systemic lupus erythematosus as a marker of deforming/erosive arthritis. J Rheumatol 2009; 36:2682–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lande R, Palazzo R, Gestermann N, Jandus C, Falchi M, Spadaro F, Riccieri V, James EA, Butera A, Boirivant M, Feldmeyer L, Surbeck I et al. Native/citrullinated LL37-specific T-cells help autoantibody production in systemic lupus erythematosus. Sci Rep 2020; 10:5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Olivares-Martinez E, Hernandez-Ramirez DF, Nunez-Alvarez CA, Llorente L, Hernandez-Molina G. Alpha-enolase is an antigenic target in primary Sjogren's syndrome. Clin Exp Rheumatol 2019; 37 Suppl 118:29–35. [PubMed] [Google Scholar]
- 118. Nezos A, Cinoku I, Mavragani CP, Moutsopoulos HM. Antibodies against citrullinated alpha enolase peptides in primary Sjogren’s syndrome. Clin Immunol 2017; 183:300–303. [DOI] [PubMed] [Google Scholar]
- 119. Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res 2007; 32:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nicholas AP, Sambandam T, Echols JD, Tourtellotte WW. Increased citrullinated glial fibrillary acidic protein in secondary progressive multiple sclerosis. J Comp Neurol 2004; 473:128–136. [DOI] [PubMed] [Google Scholar]
- 121. Atzeni F, Talotta R, Masala IF, Bongiovanni S, Boccassini L, Sarzi-Puttini P. Biomarkers in rheumatoid arthritis. Isr Med Assoc J 2017; 19:512–516. [PubMed] [Google Scholar]
- 122. Yang ML, Sodre FMC, Mamula MJ, Overbergh L. Citrullination and PAD enzyme biology in type 1 diabetes - regulators of inflammation, autoimmunity, and pathology. Front Immunol 2021; 12:678953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ishida-Yamamoto A, Senshu T, Takahashi H, Akiyama K, Nomura K, Iizuka H. Decreased deiminated keratin K1 in psoriatic hyperproliferative epidermis. J Invest Dermatol 2000; 114:701–705. [DOI] [PubMed] [Google Scholar]
- 124. Padhi A, Srivastava A, Ramesh A, Ehrstrom M, Simon M, Sonkoly E, Eidsmo L, Bergman P, Lysell J. IL-22 downregulates peptidylarginine deiminase-1 in human keratinocytes: adding another piece to the IL-22 puzzle in epidermal barrier formation. J Invest Dermatol 2022; 142:333–342.e6. [DOI] [PubMed] [Google Scholar]
- 125. Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum 2010; 62:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Greenlee RT. Measuring disease frequency in the Marshfield epidemiologic study area (MESA). Clin Med Res 2003; 1:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50:380–386. [DOI] [PubMed] [Google Scholar]
- 128. Brink M, Hansson M, Mathsson L, Jakobsson PJ, Holmdahl R, Hallmans G, Stenlund H, Ronnelid J, Klareskog L, Rantapaa-Dahlqvist S. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum 2013; 65:899–910. [DOI] [PubMed] [Google Scholar]
- 129. Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003; 48:2741–2749. [DOI] [PubMed] [Google Scholar]
- 130. Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, Weisman MH, Norris JM, Holers VM, Deane KD. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013; 65:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ramos-Remus C, Castillo-Ortiz JD, Aguilar-Lozano L, Padilla-Ibarra J, Sandoval-Castro C, Vargas-Serafin CO, de la Mora-Molina H, Ramos-Gomez A, Sanchez-Ortiz A, Avila-Armengol H, Aceves-Avila FJ. Autoantibodies in prediction of the development of rheumatoid arthritis among healthy relatives of patients with the disease. Arthritis Rheumatol 2015; 67:2837–2844. [DOI] [PubMed] [Google Scholar]
- 132. Farago B, Talian GC, Maasz A, Magyari L, Horvatovich K, Kovacs B, Cserep V, Kisfali P, Kiss CG, Czirjak L, Melegh B. Prevalence of functional haplotypes of the peptidylarginine deiminase citrullinating enzyme gene in patients with rheumatoid arthritis: no influence of the presence of anti-citrullinated peptide antibodies. Clin Exp Rheumatol 2007; 25:523–528. [PubMed] [Google Scholar]
- 133. Hung HC, Lin CY, Liao YF, Hsu PC, Tsay GJ, Liu GY. The functional haplotype of peptidylarginine deiminase IV (S55G, A82V and A112G) associated with susceptibility to rheumatoid arthritis dominates apoptosis of acute T leukemia Jurkat cells. Apoptosis 2007; 12:475–487. [DOI] [PubMed] [Google Scholar]
- 134. Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono M, Ohtsuki M, Furukawa H et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 2003; 34:395–402. [DOI] [PubMed] [Google Scholar]
- 135. Latman NS. Relation of menstrual cycle phase to symptoms of rheumatoid arthritis. Am J Med 1983; 74:957–960. [DOI] [PubMed] [Google Scholar]
- 136. Kvien TK, Uhlig T, Odegard S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci 2006; 1069:212–222. [DOI] [PubMed] [Google Scholar]
- 137. Berglin E, Kokkonen H, Einarsdottir E, Agren A, Rantapaa Dahlqvist S. Influence of female hormonal factors, in relation to autoantibodies and genetic markers, on the development of rheumatoid arthritis in northern Sweden: a case-control study. Scand J Rheumatol 2010; 39:454–460. [DOI] [PubMed] [Google Scholar]
- 138. Orellana C, Wedren S, Kallberg H, Holmqvist M, Karlson EW, Alfredsson L, Bengtsson C, Group ES . Parity and the risk of developing rheumatoid arthritis: results from the Swedish epidemiological investigation of rheumatoid arthritis study. Ann Rheum Dis 2014; 73:752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Orellana C, Saevarsdottir S, Klareskog L, Karlson EW, Alfredsson L, Bengtsson C. Oral contraceptives, breastfeeding and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis 2017; 76:1845–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. van Delft MAM, Huizinga TWJ. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun 2020; 110:102392. [DOI] [PubMed] [Google Scholar]
- 141. Wang F, Chen FF, Gao WB, Wang HY, Zhao NW, Xu M, Gao DY, Yu W, Yan XL, Zhao JN, Li XJ. Identification of citrullinated peptides in the synovial fluid of patients with rheumatoid arthritis using LC-MALDI-TOF/TOF. Clin Rheumatol 2016; 35:2185–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Uysal-Onganer P, MacLatchy A, Mahmoud R, Kraev I, Thompson PR, Inal JM, Lange S. Peptidylarginine deiminase isozyme-specific PAD2, PAD3 and PAD4 inhibitors differentially modulate extracellular vesicle signatures and cell invasion in two glioblastoma multiforme cell lines. Int J Mol Sci 2020; 21. 10.3390/ijms21041495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Loktionov A, Soubieres A, Bandaletova T, Mathur J, Poullis A. Colorectal cancer detection by biomarker quantification in noninvasively collected colorectal mucus: preliminary comparison of 24 protein biomarkers. Eur J Gastroenterol Hepatol 2019; 31:1220–1227. [DOI] [PubMed] [Google Scholar]
- 144. Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer 2009; 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yuzhalin AE. Citrullination in cancer. Cancer Res 2019; 79:1274–1284. [DOI] [PubMed] [Google Scholar]
- 146. Zhang Y, Yang Y, Hu X, Wang Z, Li L, Chen P. PADs in cancer: current and future. Biochim Biophys Acta Rev Cancer 2021; 1875:188492. [DOI] [PubMed] [Google Scholar]
- 147. Yuzhalin AE, Gordon-Weeks AN, Tognoli ML, Jones K, Markelc B, Konietzny R, Fischer R, Muth A, O'Neill E, Thompson PR, Venables PJ, Kessler BM et al. Colorectal cancer liver metastatic growth depends on PAD4-driven citrullination of the extracellular matrix. Nat Commun 2018; 9:4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jiang Z, Cui Y, Wang L, Zhao Y, Yan S, Chang X. Investigating citrullinated proteins in tumour cell lines. World J Surg Oncol 2013; 11:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cook K, Daniels I, Symonds P, Pitt T, Gijon M, Xue W, Metheringham R, Durrant L, Brentville V. Citrullinated alpha-enolase is an effective target for anti-cancer immunity. Onco Targets Ther 2018; 7:e1390642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Coassolo S, Davidson G, Negroni L, Gambi G, Daujat S, Romier C, Davidson I. Citrullination of pyruvate kinase M2 by PADI1 and PADI3 regulates glycolysis and cancer cell proliferation. Nat Commun 2021; 12:1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Coassolo S, Davidson I. Regulation of glycolysis and cancer cell proliferation by PKM2 citrullination. Mol Cell Oncol 2021; 8:1927446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Xue T, Liu X, Zhang M, E Q, Liu S, Zou M, Li Y, Ma Z, Han Y, Thompson P, Zhang X. PADI2-catalyzed MEK1 citrullination activates ERK1/2 and promotes IGF2BP1-mediated SOX2 mRNA stability in endometrial cancer. Adv Sci (Weinh) 2021; 8:2002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang L, Chang X, Yuan G, Zhao Y, Wang P. Expression of peptidylarginine deiminase type 4 in ovarian tumors. Int J Biol Sci 2010; 6:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cui YY, Yan L, Zhou J, Zhao S, Zheng YB, Sun BH, Lv HT, Rong FN, Chang XT. The role of peptidylarginine deiminase 4 in ovarian cancer cell tumorigenesis and invasion. Tumour Biol 2016; 37:5375–5383. [DOI] [PubMed] [Google Scholar]
- 155. Liu L, Zhang Z, Zhang G, Wang T, Ma Y, Guo W. Down-regulation of PADI2 prevents proliferation and epithelial-mesenchymal transition in ovarian cancer through inhibiting JAK2/STAT3 pathway in vitro and in vivo, alone or in combination with Olaparib. J Transl Med 2020; 18:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhang X, Gamble MJ, Stadler S, Cherrington BD, Causey CP, Thompson PR, Roberson MS, Kraus WL, Coonrod SA. Genome-wide analysis reveals PADI4 cooperates with Elk-1 to activate c-Fos expression in breast cancer cells. PLoS Genet 2011; 7:e1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang X, Gamble MJ, Stadler S, Cherrington BD, Causey CP, Thompson PR, Allis CD, Kraus WL, Coonrod SA. Genome wide analysis reveals PADI4 to be predictive of subsets of actively transcribed genes in breast cancer cells. PLoS Genet 2011; 7:e1002112. 10.1371/journal.pgen.1002112 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Moshkovich N, Ochoa HJ, Tang B, Yang HH, Yang Y, Huang J, Lee MP, Wakefield LM. Peptidylarginine deiminase IV regulates breast cancer stem cells via a novel tumor cell-autonomous suppressor role. Cancer Res 2020; 80:2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/−) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 2010; 15:235–252. [DOI] [PubMed] [Google Scholar]
- 160. Mackay A, Tamber N, Fenwick K, Iravani M, Grigoriadis A, Dexter T, Lord CJ, Reis-Filho JS, Ashworth A. A high-resolution integrated analysis of genetic and expression profiles of breast cancer cell lines. Breast Cancer Res Treat 2009; 118:481–498. [DOI] [PubMed] [Google Scholar]
- 161. Wang H, Xu B, Zhang X, Zheng Y, Zhao Y, Chang X. PADI2 gene confers susceptibility to breast cancer and plays tumorigenic role via ACSL4, BINC3 and CA9 signaling. Cancer Cell Int 2016; 16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Horibata S, Rogers KE, Sadegh D, Anguish LJ, McElwee JL, Shah P, Thompson PR, Coonrod SA. Role of peptidylarginine deiminase 2 (PAD2) in mammary carcinoma cell migration. BMC Cancer 2017; 17:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sharma P, Lioutas A, Fernandez-Fuentes N, Quilez J, Carbonell-Caballero J, Wright RHG, Di Vona C, Le Dily F, Schuller R, Eick D, Oliva B, Beato M. Arginine citrullination at the C-terminal domain controls RNA polymerase II transcription. Mol Cell 2019; 73:84–96 e87. [DOI] [PubMed] [Google Scholar]
- 164. McElwee JL, Mohanan S, Griffith OL, Breuer HC, Anguish LJ, Cherrington BD, Palmer AM, Howe LR, Subramanian V, Causey CP, Thompson PR, Gray JW et al. Identification of PADI2 as a potential breast cancer biomarker and therapeutic target. BMC Cancer 2012; 12:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Palmieri D, D'Angelo D, Valentino T, De Martino I, Ferraro A, Wierinckx A, Fedele M, Trouillas J, Fusco A. Downregulation of HMGA-targeting microRNAs has a critical role in human pituitary tumorigenesis. Oncogene 2012; 31:3857–3865. [DOI] [PubMed] [Google Scholar]
- 166. Ledet MM, Anderson R, Harman R, Muth A, Thompson PR, Coonrod SA, Van de Walle GR. BB-cl-Amidine as a novel therapeutic for canine and feline mammary cancer via activation of the endoplasmic reticulum stress pathway. BMC Cancer 2018; 18:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Brentville VA, Vankemmelbeke M, Metheringham RL, Durrant LG. Post-translational modifications such as citrullination are excellent targets for cancer therapy. Semin Immunol 2020; 47:101393. [DOI] [PubMed] [Google Scholar]
- 168. Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, Hodgin JB, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis 2015; 74:2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol 2011; 186:4396–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Li F, Miao L, Xue T, Qin H, Mondal S, Thompson PR, Coonrod SA, Liu X, Zhang X. Inhibiting PAD2 enhances the anti-tumor effect of docetaxel in tamoxifen-resistant breast cancer cells. J Exp Clin Cancer Res 2019; 38:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Von Hoff DD, Rozencweig M, Soper WT, Helman LJ, Penta JS, Davis HL, Muggia FM. Whatever happened to NSC--? An analysis of clinical results of discontinued anticancer agents. Cancer Treat Rep 1977; 61:759–768. [PubMed] [Google Scholar]
- 172. Dubey NK, Peng BY, Lin CM, Wang PD, Wang JR, Chan CH, Wei HJ, Deng WP. NSC 95397 suppresses proliferation and induces apoptosis in colon cancer cells through MKP-1 and the ERK1/2 pathway. Int J Mol Sci 2018; 19. 10.3390/ijms19061625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jemaa M, Mischitelli M, Fezai M, Almasry M, Faggio C, Lang F. Stimulation of suicidal erythrocyte death by the CDC25 inhibitor NSC-95397. Cell Physiol Biochem 2016; 40:597–607. [DOI] [PubMed] [Google Scholar]
- 174. Larsson DE, Wickstrom M, Hassan S, Oberg K, Granberg D. The cytotoxic agents NSC-95397, brefeldin a, bortezomib and sanguinarine induce apoptosis in neuroendocrine tumors in vitro. Anticancer Res 2010; 30:149–156. [PubMed] [Google Scholar]
- 175. Macia E, Vazquez-Rojas M, Robiolo A, Fayad R, Abelanet S, Mus-Veteau I, Fontaine-Vive F, Mehiri M, Luton F, Franco M. Chlortetracycline, a novel Arf inhibitor that decreases the Arf6-dependent invasive properties of breast cancer cells. Molecules 2021; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wright JC. Cancer chemotherapy: past, present, and future--part I. J Natl Med Assoc 1984; 76:773–784. [PMC free article] [PubMed] [Google Scholar]
- 177. Lee SH, Lee WK, Kim N, Kang JH, Kim KH, Kim SG, Lee JS, Lee S, Lee J, Joo J, Kwon WS, Rha SY et al. Renal cell carcinoma is abrogated by p53 stabilization through transglutaminase 2 inhibition. Cancers (Basel) 2018; 10. 10.3390/cancers10110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol 2020; 11:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, Andueza MP, Nieto CP et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 2020; 52:856–871.e8. [DOI] [PubMed] [Google Scholar]
- 180. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013; 123:3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N, Cools-Lartigue J, Ferri LE et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight 2019; 4. 10.1172/jci.insight.128008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wu Z, Li P, Tian Y, Ouyang W, Ho JW, Alam HB, Li Y. Peptidylarginine deiminase 2 in host immunity: current insights and perspectives. Front Immunol 2021; 12:761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Sodre FMC, Bissenova S, Bruggeman Y, Tilvawala R, Cook DP, Berthault C, Mondal S, Callebaut A, You S, Scharfmann R, Mallone R, Thompson PR et al. Peptidylarginine deiminase inhibition prevents diabetes development in NOD mice. Diabetes 2021; 70:516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ. Suppression of colitis in mice by cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol 2011; 300:G929–G938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Brentville VA, Metheringham RL, Gunn B, Symonds P, Daniels I, Gijon M, Cook K, Xue W, Durrant LG. Citrullinated vimentin presented on MHC-II in tumor cells is a target for CD4+ T-cell-mediated antitumor immunity. Cancer Res 2016; 76:548–560. [DOI] [PubMed] [Google Scholar]
- 186. Brentville VA, Metheringham RL, Daniels I, Atabani S, Symonds P, Cook KW, Vankemmelbeke M, Choudhury R, Vaghela P, Gijon M, Meiners G, Krebber WJ et al. Combination vaccine based on citrullinated vimentin and enolase peptides induces potent CD4-mediated anti-tumor responses. J Immunother Cancer 2020; 8:e000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Symonds P, Marcu A, Cook KW, Metheringham RL, Durrant LG, Brentville VA. Citrullinated epitopes identified on tumour MHC class II by peptide elution stimulate both regulatory and Th1 responses and require careful selection for optimal anti-tumour responses. Front Immunol 2021; 12:764462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Katayama H, Kobayashi M, Irajizad E, Sevillarno A, Patel N, Mao X, Rusling L, Vykoukal J, Cai Y, Hsiao F, Yu CY, Long J et al. Protein citrullination as a source of cancer neoantigens. J Immunother Cancer 2021; 9:e002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lange S, Gogel S, Leung KY, Vernay B, Nicholas AP, Causey CP, Thompson PR, Greene ND, Ferretti P. Protein deiminases: new players in the developmentally regulated loss of neural regenerative ability. Dev Biol 2011; 355:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. van Beers JJ, Zendman AJ, Raijmakers R, Stammen-Vogelzangs J, Pruijn GJ. Peptidylarginine deiminase expression and activity in PAD2 knock-out and PAD4-low mice. Biochimie 2013; 95:299–308. [DOI] [PubMed] [Google Scholar]
- 191. Liu Y, Lightfoot YL, Seto N, Carmona-Rivera C, Moore E, Goel R, O'Neil L, Mistry P, Hoffmann V, Mondal S, Premnath PN, Gribbons K et al. Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]