Abstract
Introduction:
The prognosis for patients with metastatic and recurrent pediatric rhabdomyosarcoma (RMS) remains poor. The availability of preclinical models is essential to identify promising treatments We established a series of pediatric RMS patient derived xenografts (PDXs), all faithfully mirroring primary tumor characteristics and representing a unique tool for clarifying the biological processes underlying RMS progression and relapse.
Methods:
Fresh tumor samples from 12 RMS patients were implanted subcutaneously in both flanks of immunocompromised mice. PDXs were considered as grafted after accomplishing three passages in mice. Characterization of tumor tissues and models was performed by comparing both morphology and immunoistochemical and fluorescence in situ hybridization (FISH) characteristics.
Results:
Six PDXs were established, with a successful take rate of 50%. All models closely mirrored parental tumor characteristics. An increased grafting rate for tumors derived from patients with worse outcome (p = 0.006) was detected. For 50% PDXs grafting occurred when the corresponding patient was still alive.
Conclusion:
Our findings increase the number of available RMS PDX models and strengthen the role of PDXs as useful preclinical tools for patients with unmet medical needs and to develop personalized therapies.
Keywords: Rhabdomyosarcoma, patient-derived xenografts, personalized therapy
Introduction
Rhabdomyosarcoma (RMS) is the most frequent soft tissue sarcoma of childhood and adolescence: it is a high-grade tumor characterized by local invasiveness and strong propensity to metastasize. RMS shows varying degrees of skeletal muscle differentiation. 1 Two main subtypes are observed in pediatric age: the embryonal RMS (ERMS), characterized by round and spindle cells in a frequent myxoid stroma, often presenting a molecular loss of heterozygosity at 11p15 along with the acquisition of chromosome 8 and several other mutations; and the more aggressive alveolar RMS (ARMS), characterized by a solid growth of round cells, with PAX3/7-FOXO1 fusion genes in the large majority of cases.2,3 In addition, also sclerosing/spindle cell (Sc/Sp) RMS is reported in pediatric ages, characterized by a variable cellularity, an abundant collagenous stroma and/or spindle cells, and MYOD1 mutations. 1
Although a large proportion of pediatric RMS patients can currently be cured with intensive multimodal treatments, about 25% patients do not survive due to unresectable, metastatic, or refractory disease. Thus, understanding the biological process of tumorigenesis and exploring the efficacy of new biological drugs still remains an important clinical need in RMS.
Limited appropriate and molecularly well characterized RMS patient-derived pre-clinical models are available. This makes proof-of-concepts preclinical testing of novel molecular targeted compounds a challenge, limiting evidence for in vivo preclinical efficacy before going into clinical studies.
Patient-derived xenografts (PDXs) represent in vivo models that closely recapitulate many important features of patients’ tumors.4,5 These models have been deeply exploited for studying the biology of the disease, and for developing new treatments. In the last few years, generation of PDXs collections have been reported for many different types of solid tumors.6–11 PDXs have been widely used to predict therapeutic approaches efficacy,12–14 and to identify new therapeutic markers15,16 in adult neoplasia. Their preclinical value has also been consolidated for pediatric malignancies as a panel of 261 PDX models (from 37 distinct tumor entities) have been generated. 17 Moreover in Europe, public-private partnership such as the ITCC-P4 (www.itccp4.eu) project has been launched.
Particularly for RMS PDXs, platforms have been developed and used to predict response for specific treatments.18–20 Ideally, a PDXs collection should be large enough to encompass heterogeneity of a specific tumor type. Unfortunately, as RMS is a rare tumor, this is hard to achieve and every new model established is considered of great importance as it may offer the possibility to add new critical information necessary to lay foundation for the comprehension of biological processes underlying RMS growth.
For all these reasons, our study was aimed at generating a collection of pediatric RMS of different subtype (ERMS, ARMS, Sc/Sp RMS), clinical presentation, site of onset and stage of disease. We here describe the successful establishment of six RMS PDX derived from patients with different RMS subtypes which recapitulate patients’ tumor characteristics.
Materials and methods
Patient selection
All samples were collected from July 2014 to January 2020, with a sterile procedure in the operating room. A diagnosis of RMS and subtyping had been performed on the tumor tissue of the patients following the 2019 WHO Classification of soft tissue and bone tumors (WHO). All ARMS were characterized for the occurrence and type of gene fusion. Sc/Sp RMS was diagnosed on the basis of morphology coupled with the strong and diffuse expression of MyoD1. 1
Tissue specimens were obtained according to the Internal Review and the Ethics Boards of the Fondazione IRCCS Istituto Nazionale Tumori of Milan. Written informed consent and age-appropriate assent were obtained for all patients. All analyses of clinical and biological data were planned and executed according to the guidelines of the revised Declaration of Helsinki.
PDXs establishment
PDXs were established as previously described.8,21 PDXs models were propagated for three rounds in mice (P1–P3) before being considered stabilized, and then frozen in a solution of 90% FBS and 10% DMSO and stored in liquid nitrogen. Animal studies were conducted according to the guidelines of the Ethics Committee for Animal Experimentation (OPBA) of the Fondazione IRCCS Istituto Nazionale dei Tumori. 22 All experiments were approved by the OPBA and by the Italian Ministry of Health.
Immunohistochemistry
All tumor samples/tissues and PDX were immunostained for desmin, myogenin, and MyoD1. 2.5/3 micron-thick sections were cut from paraffin blocks, dried, de-waxed, rehydrated, and unmasked (with Dako PT-link, EnVision™ FLEX Target Retrieval Solution, High pH 96°C). Antibodies agains desmin (clone D33, Dako - Agilent, dilution 1:400), myogenin (clone F5D, Dako - Agilent, Ready-to-Use) and MyoD1 (clone 5.8A, Dako - Agilent, dilution 1:50) were incubated with a commercially available detection kit (EnVision™ FLEX+, Dako, Agilent) in an automated Immunostainer (Dako Autostainer Link 48 - Agilent)
PAX3/7-FOXO1 assessment by fluorescence in situ hybridization (FISH) analysis
FISH analysis was performed on selected areas on 2–4 μm-thick paraffin sections of all Formalin-Fixed Paraffin-Embedded (FFPE) ARMS human and PDX’s tissues by counting at least 100 tumor cells, Briefly, a commercial available dual color single fusion probe (Abbott Molecular, ZytoLight® SPEC FOXO1/PAX7 Dual Color Single Fusion Probe), specifically designed to detect the translocation t(1;13) (p36.1;q14.1) was used to corroborate the diagnosis of PAX7/FOXO1 fusion transcript. The commercial probe was used according to manufacturer’s instructions. Interpretation of FISH results in a normal situation without a translocation involving the respective gene regions, revealed two separate green (distal to the PAX7 breakpoint region) and orange signals appear (proximal to the FOXO1 breakpoint region). Whereas, in presence of a rearrangement, the gene fusion was indicated by one orange/green fusion signal, together with one separate orange signal, one separate green signal.
Statistical analysis
Analyses were performed using GraphPad Prism (GraphPad Software, La Jolla California USA) and R software i386 3.6.3 (packages: ggplot2, ggpubr, ggsci, survival and survminer) and RStudio Version 1.2.5033.
Results
Establishment of a pediatric patient derived xenografts perpetual bank
Tumor samples from 12 RMS patients (five ARMS, five ERMS, and two Sc/Sp RMS; 1-23 years old) have been implanted in both flanks of SCID mice. PDXs showed variable growth features during the first three passages (P1–P3). In particular in the P1-P3 period latency time was generally higher and variable, with tumor volume increasing and decreasing, whereas at P3 PDXs grew with more homogeneous characteristics (Suppl. Figure 1A-C). PDXs were considered as grafted after reaching P3. We successfully generated six RMS PDXs (two ARMS, three ERMS, and one Sc/Sp RMS), reaching 50% (6/12) take rate (40%, 60%, and 50% within ARMS, ERMS, Sc/Sp RMS, respectively). We reached 67% (2/3) take rate for fusion-positive ARMS, whereas fusion-negative ARMS, both derived from paratesticular tumors, did not graft as PDXs (Table 1).
Table 1.
Patients and tumors characteristics, overall and according to graft.
| Graft | ||||
|---|---|---|---|---|
| All subjects (n=12) | Yes (n=6) | No (n=6) | p-value | |
| Patients characteristics | ||||
| Sex | ||||
| Female | 3 (25.0) | 1 (33.3) | 2 (66.7) | 1.000 1 |
| Male | 9 (75.0) | 5 (55.5) | 4 (44.5) | |
| Age | ||||
| Mean (SD) – Median (IQR) | 14.0 (6.0) – 14.0 (11.8-17.0) | 12.9 (5.9) – 13.0 (11.0-16.0) | 15.8 (5.1) – 15.5 (13.5-20.3) | |
| Paediatric (0-14 years old) | 7 (58.3) | 5 (71.4) | 2 (28.6) | |
| Adolescent (15-25 years old) | 5 (41.7) | 1 (20.0) | 4 (80.0) | 0.242 1 |
| Outcome | ||||
| Mortality | ||||
| Alive | 7 (58.3) | 1 (14.3) | 6 (85.7) | 0.015 1 |
| Dead | 5 (41.7) | 5 (100) | 0 (0.0) | |
| Tumor characteristics | ||||
| IRS | ||||
| I/II | 4 (33.3) | 1 (25.0) | 3 (75.0) | |
| III/IV | 8 (66.7) | 5 (62.5) | 3 (37.5) | 0.546 1 |
| I/II/III | 10 (83.3) | 6 (60.0) | 4 (40.0) | |
| IV | 2 (16.7) | 0 (0.0) | 2 (100) | 0.455 1 |
| TNM | ||||
| T = 1 | 5 | 1 | 4 | |
| T > 1 | 7 | 5 | 2 | 0.242 |
| N = 0 | 10 | 4 | 6 | |
| N > 0 Subtype |
2 | 2 | 0 | 0.455 |
| ERMS | 7 (58.9) | 4 (57.1) | 3 (42.9) | 1.000 1 |
| ARMS | 5 (41.1) | 2 (40.0) | 3 (60.0) | |
| PAX3-7/FKHR+ | 3 (60.0) | 2 (66.7) | 1 (33.3) | 0.400 1 |
| PAX3-7/FKHR- | 2 (40.0) | 0 (0) | 2 (100) | |
| Tumor Site | ||||
| ERMS | 7 (58.9) | |||
| Paratesticular | 1 (14.3) | 0 (0.0) | 1 (100.0) | |
| Limb | 2 (28.6) | 1 (50.0) | 1 (50.0) | |
| Prostate | 1 (14.3) | 1 (100.0) | 0 (0.0) | |
| Trunk | 2 (28.6) | 1 (50.0) | 1 (50.0) | |
| Abdomen | 1 (14.3) | 1 (100.0) | 0 (0.0) | |
| ARMS | 5 (41.1) | |||
| Paratesticular 2 | 2 (40.0) | 0 (0.0) | 2 (100.0) | |
| Limb | 3 (60.0) | 2 (66.7) | 1 (33.3) | |
Two-tailed Fisher’s exact test; 2both fusion-negative. ARMS: alveolar rhabdomyosarcoma ERMS: embryonal rhabdomyosarcoma; IQR: interquartile range; IRS: intergroup rhabdomyosarcoma studies; SD: standard deviation.
PDXs mirror the most important human tumor features
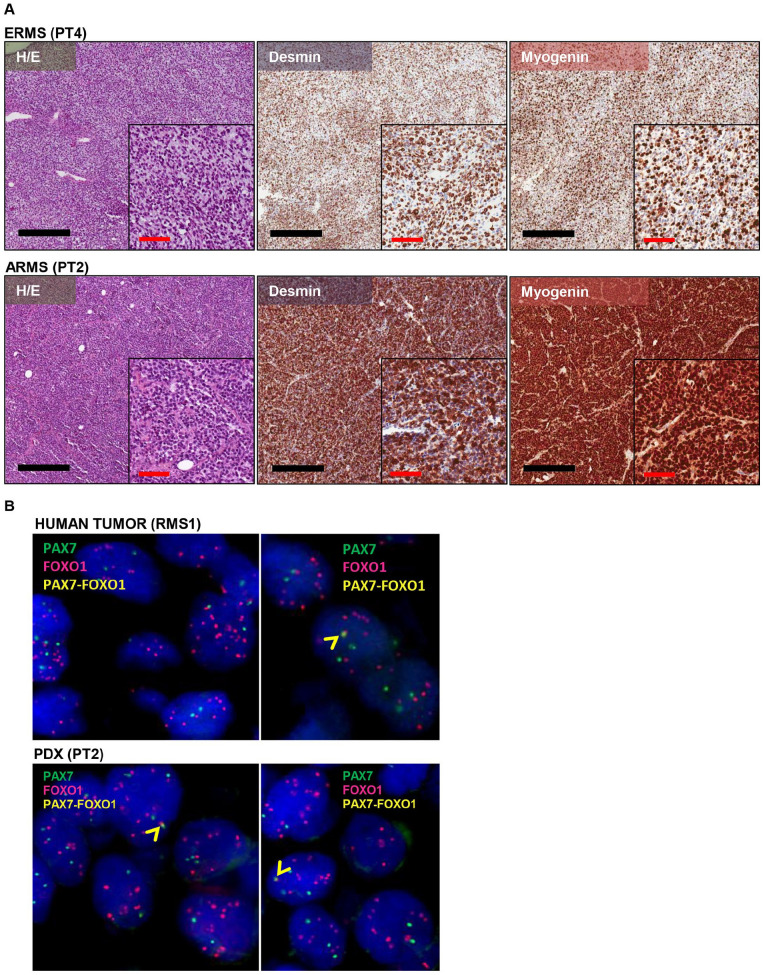
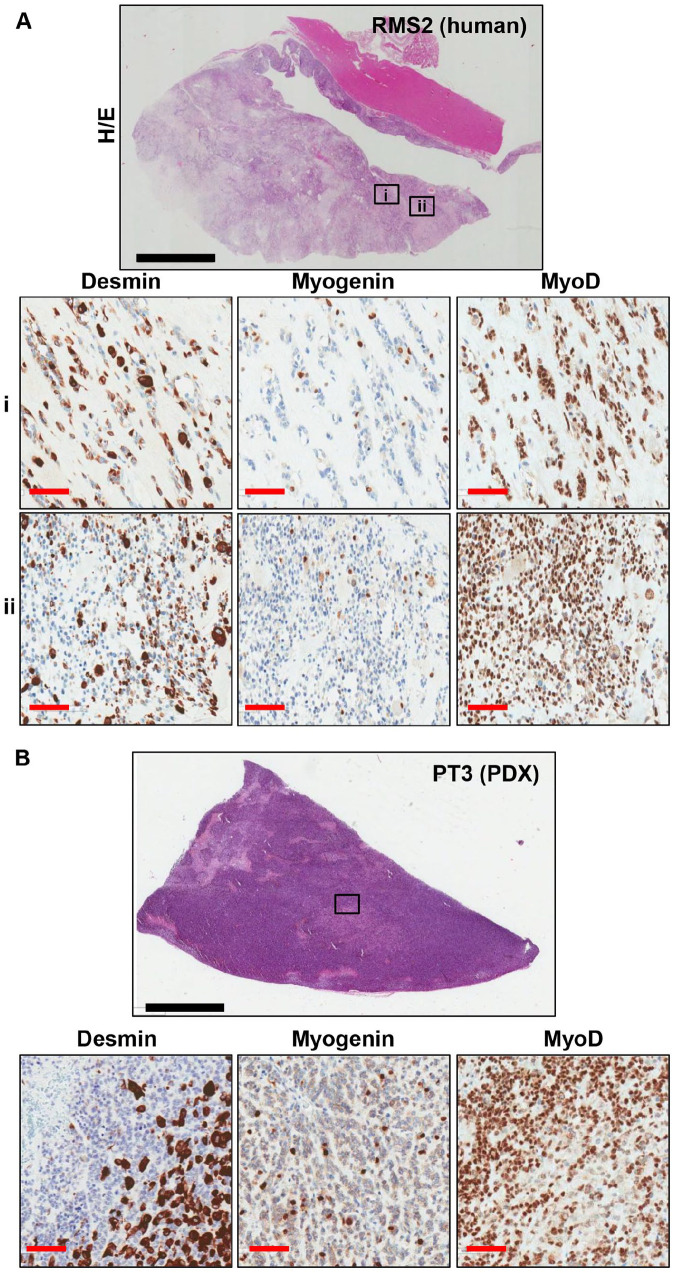
ARMS and ERMS subgroups were maintained in all the successfully grafted PDXs (Table 2). ERMS PDXs were characterized by mostly spindle-shaped cells in a fibrous or fibromyxoid stroma, with reactivity for both desmin and myogenin, and ARMS models were characterized by mostly round cells with a high reactivity for both markers (Figure 1). One of the established PDX (PT2) was derived from a tumor diagnosed as a translocated ARMS with PAX7/FOXO1 fusion by RT-qPCR analysis. FISH analysis performed on tumor and corresponding PDX samples showed a similar FOXO1 amplification (FOXO1X8~>15, and FOXO1X3>15 in tumor and PDX, respectively), and few signals indicating PAX7/FOXO1 fusion (12 and 10% of cells with one fusion signal in tumor and PDX, respectively, Figure 1B). PAX7/FOXO1 translocation was confirmed both in the human tumor and in the PDXs by NGS analysis (data not shown). Of note, PT3 was derived from a tumor (RMS2) diagnosed as Sc/Sp RMS, and characterized by areas with different density of cells (Figure 2). In the PDX, a central core of rhabdomyoblastic cells was surrounded by densely cellular spindle cells. Both human tumor and the corresponding PDX showed a strong and diffuse nuclear immunoreactivity for MyoD1 (Figure 2).
Table 2.
Comparison between human tumor and PDX diagnosis.
| Human | PDX | ||
|---|---|---|---|
| ID | Diagnosis | Diagnosis | ID |
| RMS1 | ARMS | ARMS | PT2 |
| RMS2 | ERMS | ERMS | PT3 |
| RMS3 | ERMS | ERMS | PT4 |
| RMS6 | ERMS | ERMS | PT9 |
| RMS9 | ARMS | ARMS | PT13 |
| RMS10 | ERMS | ERMS | PT14 |
Figure 1.
(A) Representative images of RMS PDX morphology and immunohistochemical analysis. PDXs morphological and immunophenotypical features of ERMS and ARMS are depicted in H/E staining. Black line: 400 µm; red line= 100 µm; (B) FISH analysis showing amplification of FOXO1 gene and PAX7/FOXO1 fusion product in RMS1 human sample and in the corresponding PDX (PT2). Blue: nuclei, red: FOXO1 gene, green: PAX7 gene, yellow: PAX7/FOXO1 fusion gene.
Figure 2.
Representative IHC images of Desmin, Myogenin and MyoD1 staining on human (A) and PDX (B) tissue sections. (A) In the human tumor, two differently cellulated areas are appreciable, the first poorly cellulated and the second densely cellulated (ii). (B) In the PDX only the densely cellulated component was detected. Black line: 6mm; red line= 100 µm.
PDX grafting is associated with tumor aggressiveness
As shown in Table 1, tumor samples of patients with dismal clinical outcome revealed increased graft capability compared to those derived from patients with a longer survival. We experienced a successful PDX graft in 100% (5/5) of tumors derived from patients with fatal prognosis, and 14.3% (1/7) of tumors derived from patients who were still alive at the time of the analysis (p = 0.015, Table 1). The Kaplan-Meier estimate showed a poorer prognosis for patients whose tumor successfully grafted in mice (log rank p = 0.006, Figure 3). A worse prognosis was observed for tumors with IRS IV (log rank p = 0.01, Suppl. Figure 3A), whereas no significant difference in overall survival (OS) were observed between patients in pediatric (0-14 years old) and adolescent (15-23 years old) age (log rank p = 0.38, Suppl. Figure 3B). PDXs take remained a negative prognostic marker also within tumors categorized as IRS group I-III (log rank p = 0.02, Suppl. Figure 3C).
Figure 3.
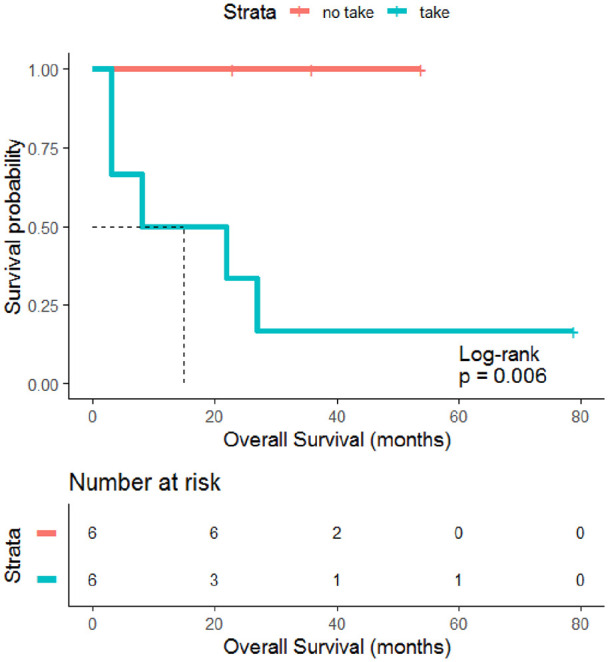
Prognostic significance of PDX graft. Kaplan Meier estimate shows that patients whose tumor successfully grafted in mice had a significantly lower OS compared to those whose tumors did not grow as PDX (p=0.006).
PDXs as avatars to develop personalized therapies
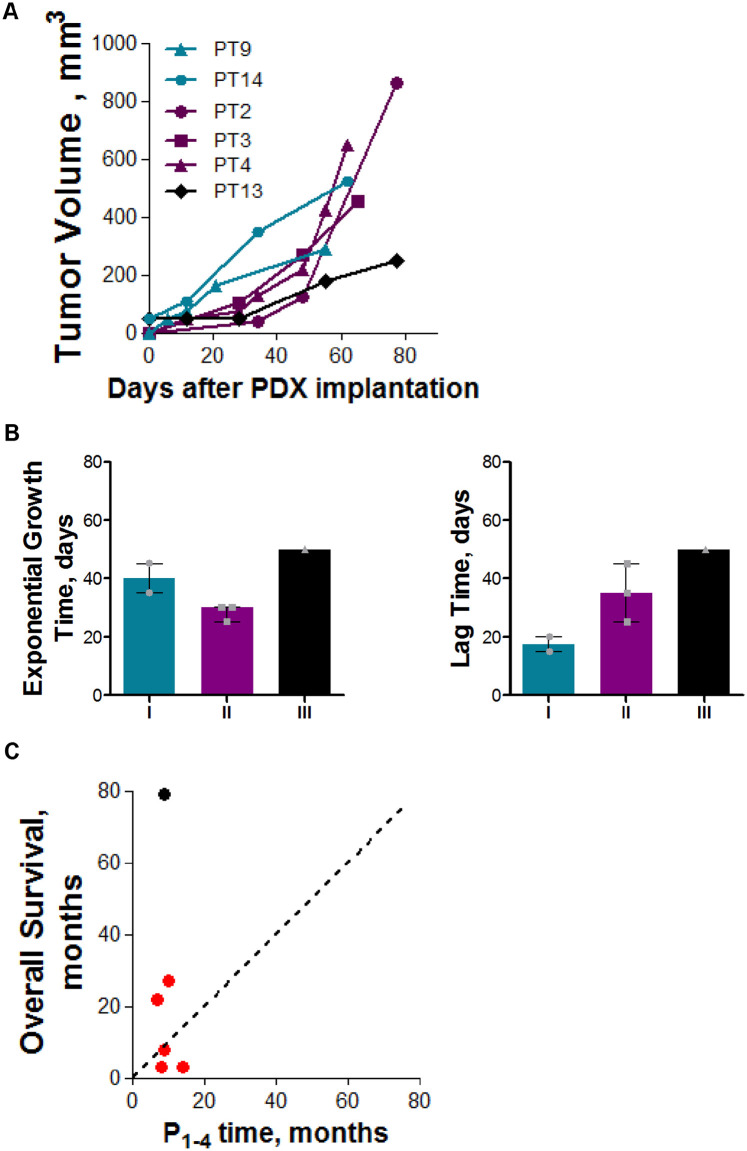
The median grafting time (days between tumor injection and PDX graft) was 195 days, while the fastest and the slowest PDXs had a grafting time of 165 and 315 days, respectively (Suppl. Table 1). RMS PDXs were subdived into three groups according to their growth characteristics at P3 (lag time – LT, and exponential growth time - EGT, Suppl. Table 1). Group I PDXs (PT9 and PT14) were characterized by a short (15-20 days) LT and a long (35-45 days) EGT. PDXs belonging to Group II (PT2, PT3, and PT4) showed a long LT (35-45 days) and a shorter EGT (25-30 days). PT13 was characterized by a slow growth with long LT (55 days) and long EGT (50 days, Figure 4A, B and Suppl. Table 1). Changes in growth characteristics were mainly ascribed to a different lag time (Figure 4B). No significant correlation between growth characteristics in mouse and tumor subtype or patients OS was observed (Suppl. Figure 4). Of note, patients OS was higher than P1-4 time for 50% of grafted PDXs (Figure 4C).
Figure 4.
(A) PDX growth curves. Graph shows the growth of all successfully implanted PDX at P3. In blue are indicated models belonging to group I (low latency period and slow growth), in violet those belonging to group II (high latency period and fast growth), and in black the PDX belonging to group III (high latency period and slow growth); (B) different growth characteristic in terms of latency period and exponential growth time of the three identified PDXs groups; (C) Comparison between the time needed for the PDX to accomplish four passages in mice and the OS of the corresponding patient. Dotted line indicate x=y line, red = dead; black = no evidence of disease.
Discussion
An established collection of six pediatric RMS PDXs, with a 50% graft rate was here reported and described. The grafted models recapitulate the main clinical and pathological features of the tumor of origin. Indeed, morphology, and immunophenotype were maintained in the mouse models. Thus, RMS PDXs could represent in vivo models valuable for studying RMS biology, and potentially useful for testing new therapies. The analysis of clinico-pathological characteristics indicated a strong correlation between PDX establishment and patients OS, as already reported in another pediatric sarcomas platform. 23 Indeed, grafted PDXs were derived mainly from patients with very aggressive tumors. We observed a higher take rate for fusion-positive ARMS compared to ERMS, further suggesting that a more aggressive RMS histology is more likely to successfully grow in mice. Moreover, two fusion-negative paratesticular ARMS, already proposed as a RMS with favorable prognosis,24,25 did not give rise to PDXs. Interestingly, the prognostic role of PDX graft was maintained also for IRS group I-III tumors, indicating that PDX graft may potentially be considered as prognostic also for low stage RMS. These insights further highlight the importance for RMS PDX as a pre-clinical model for testing new therapies, particularly so in case of aggressive RMS, with no effective therapy.
Tumor aggressiveness is often associated to the capacity of cancer cells to disseminate and to grow as metastasis in distant organs. RMS PDXs described in our study are human tumors that grow subcutaneously in mice. Thus, the correlation between OS and PDX grafting may indicate that only RMS more proficient in developing metastasis are able to grow successfully in the “distant organ” represented by the mice subcutaneous region. Moreover, differences in PDXs growth rate are mainly due to increased lag time, which reflects the capability of tumor cells to establish a pro-tumorigenic cross-talk with murine tumor microenvironment (TME), reported to influence tumor growth as well as metastasis formation.26–28 Based on this consideration, RMS PDXs may also represent a useful model to study mechanisms underlying metastasis development.
Interestingly, we reported histological differences in one PDX (PT3) compared to the corresponding human tumor (RMS2). Indeed, in PDX only the most cellular component was observed. Of note, MyoD1-positivity of cells was maintained in the murine model. MyoD1 expression was reported as associated with a dismal prognosis in RMS. 29 We recently reported the correlation between tumor aggressiveness and PDX graft, and the loss of less aggressive histological subtypes in lung cancer PDXs. 8 Similarly, in this study we encounter the same association of tumor aggressiveness in graft rate of pediatric RMS PDXs. Thus, another hypothesis to explain morphological differences between RMS2 and PT3 may be that only the more aggressive cells survived implantation in mice.
In conclusion, we here report the establishment of a small RMS PDXs platform, that closely recapitulates primary tumors features in terms of morphology, and immunphenotype. Despite the limited number of PDXs, we showed that tumor graft was directly related to RMS aggressiveness, further strengthening the importance of these models. Our data are the proof-of-concept that the effort to develop in vivo model is a fruitful investment also in the case of rare tumors such as RMS. However, the limited size of RMS cases available each year in a single institute makes it almost impossible to establish a complete PDX platform, comprehensive of all genetic alterations involved in RMS development. 30 For this reason, it is of critical important to achieve a multicentric effort to join resources and expertise like that by the European Innovative Medicine Initiative (IMI2) to develop a pediatric preclinical platform (ITCCP4 - https://www.itccp4.eu/). Nowadays, great efforts are focused at the generation of representative and robust preclinical models (PDXs, genetically engineered mouse models –GEMMs- and organoids) accurately reflecting human disease and providing efficient platforms for in vivo functional new drugs that would significantly accelerate the development of more precise and efficacious drugs for children with malignant solid tumors. Children have been given drugs designed for adults although malignancies in children are fundamentally different from their adult equivalents. In particular, the PDX platforms represent an unprecedented effort for an extensive molecular characterization and preclinical utilization of pediatric solid tumor models aiming to overcome the gaps in drugs provided to children and to deliver more precisely tailored therapies for children.
Supplemental Material
Supplemental material, sj-pdf-1-tmj-10.1177_03008916221110266 for Establishment of 6 pediatric rhabdomyosarcoma patient’s derived xenograft models closely recapitulating patients’ tumor characteristics by Patrizia Gasparini, Michela Casanova, Giovanni Centonze, Cristina Borzi, Luca Bergamaschi, Paola Collini, Adele Testi, Stefano Chiaravalli, Maura Massimino, Gabriella Sozzi, Andrea Ferrari and Massimo Moro in Tumori Journal
Supplemental material, sj-pdf-2-tmj-10.1177_03008916221110266 for Establishment of 6 pediatric rhabdomyosarcoma patient’s derived xenograft models closely recapitulating patients’ tumor characteristics by Patrizia Gasparini, Michela Casanova, Giovanni Centonze, Cristina Borzi, Luca Bergamaschi, Paola Collini, Adele Testi, Stefano Chiaravalli, Maura Massimino, Gabriella Sozzi, Andrea Ferrari and Massimo Moro in Tumori Journal
Footnotes
Author’s contributions: PG, MC, LB, SC, AF, and MMo created the experimental design; PG, GC, CB, PC, AT, and M.Mo acquired and analysed the data. PG, MC, GC, CB, LB, PC, AT, SC, MMa, GS, AF, MMo participated in data interpretation, as well as in writing and editing the manuscript. All authors approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Associazione Bianca Garavaglia: A/15/01N, A/18/01A,
ORCID iDs: Patrizia Gasparini  https://orcid.org/0000-0002-9548-3724
https://orcid.org/0000-0002-9548-3724
Cristina Borzi  https://orcid.org/0000-0003-2850-6559
https://orcid.org/0000-0003-2850-6559
Andrea Ferrari  https://orcid.org/0000-0002-4724-0517
https://orcid.org/0000-0002-4724-0517
Massimo Moro  https://orcid.org/0000-0003-2562-4007
https://orcid.org/0000-0003-2562-4007
Supplemental material: Supplemental material for this article is available online.
References
- 1.World Health Organization. WHO Classification of Tumors – 5th edition – Soft tissue and bone tumors. Book, edited by WHO Classification of Tumors Editorial Board, 2020, p.472–474.
- 2.Chen X, Stewart E, Shelat AA, et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell 2013; 24: 710–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shern JF, Chen L, Chmielecki J, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov 2014; 4: 216–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo X, Giordano J, Srivastava A, et al. Author Correction: Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat Genet 2021; 53: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo G, Bertotti A, Leto S, et al. Patient-derived tumor models: a more suitable tool for pre-clinical studies in colorectal cancer. J Exp Clin Cancer Res 2021; 40: 178–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res 2008; 14: 6456–6468. [DOI] [PubMed] [Google Scholar]
- 7.Rivera M, Fichtner I, Wulf-Goldenberg A, et al. Patient-derived xenograft (PDX) models of colorectal carcinoma (CRC) as a platform for chemosensitivity and biomarker analysis in personalized medicine. Neoplasia 2021; 23: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moro M, Bertolini G, Caserini R, et al. Establishment of patient derived xenografts as functional testing of lung cancer aggressiveness. Sci Rep 2017; 7: 6689–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Wang J, Zheng X, et al. Establishment and preclinical therapy of patient-derived hepatocellular carcinoma xenograft model. Immunol Lett 2020; 223: 33–43. [DOI] [PubMed] [Google Scholar]
- 10.Coussy F, de Koning L, Lavigne M, et al. A large collection of integrated genomically characterized patient-derived xenografts highlighting the heterogeneity of triple-negative breast cancer. Int J Cancer 2019; 145: 1902–1912. [DOI] [PubMed] [Google Scholar]
- 11.Conte N, Mason J, Halmagyi C, et al. PDX Finder: A portal for patient-derived tumor xenograft model discovery. Nucleic Acids Res 2019; 47: D1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro M, Caiola E, Ganzinelli M, et al. Metformin enhances cisplatin-induced apoptosis and prevents resistance to cisplatin in co-mutated KRAS/LKB1 non-small cell lung cancer (NSCLC). J Thorac Oncol 2018; 13: 1692–1704. [DOI] [PubMed] [Google Scholar]
- 13.Hunter A, Newman H, DeZern A, et al. Integrated human and murine clinical study establishes clinical efficacy of ruxolitinib in chronic myelomonocytic leukemia. Clin Cancer Res 2021; Epub 2021 July 12 doi: 10.1158/1078-0432.CCR-21-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vangala D, Ladigan S, Liffers S, et al. Secondary resistance to anti-EGFR therapy by transcriptional reprogramming in patient-derived colorectal cancer models. Genome Med 2021; 13: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient- derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011; 1: 508–523. [DOI] [PubMed] [Google Scholar]
- 16.Renuse S, Madamsetty V, Mun D, et al. Tyrosine phosphoproteomics of patient-derived xenografts reveals ephrin type-b receptor 4 tyrosine kinase as a therapeutic target in pancreatic cancer. Cancers (Basel) 2021; 13: 3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rokita JL, Rathi KS, Cardenas MF, et al. genomic profiling of childhood tumor patient-derived xenograft models to enable rational clinical trial design. Cell Rep 2019; 29: 1675-1689.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzella G, Schreck LD, Breunis WB, et al. Phenotypic profiling with a living biobank of primary rhabdomyosarcoma unravels disease heterogeneity and AKT sensitivity. Nat Commun 2020; 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper JE, Cantor EL, Ehlen MS, et al. A patient-derived xenograft model of parameningeal embryonal rhabdomyosarcoma for preclinical studies. Sarcoma 2015; 2015: Article ID 826124, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cramer S, Miller A, Pressey J, et al. Pediatric anaplastic embryonal rhabdomyosarcoma: targeted therapy guided by genetic analysis and a patient-derived xenograft study. Front Oncol 2018; 7: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moro M, Bertolini G, Tortoreto M, et al. Patient-derived xenografts of non small cell lung cancer: Resurgence of an old model for investigation of modern concepts of tailored therapy and cancer stem cells. J Biomed Biotechnol 2012; 2012: Article ID 568567, 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Workman P, Aboagye EO, Balkwill F, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer 2010; 102: 1555–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo-Ecija H, Pascual-Pasto G, Perez-Jaume S, et al. Prognostic value of patient-derived xenograft engraftment in pediatric sarcomas. J Pathol Clin Res 2021; 7: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dantonello TM, Vokuhl C, Scheer M, et al. Paratesticular alveolar rhabdomyosarcomas do not harbor typical translocations: a distinct entity with favorable prognosis? Virchows Arch 2018; 472: 441–449. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari A, Bisogno G, Casanova M, et al. Is alveolar histotype a prognostic factor in paratesticular rhabdomyosarcoma? The experience of Italian and German Soft Tissue Sarcoma Cooperative Group. Pediatr Blood Cancer 2004; 42: 134–138. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016, p. 582–98. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 28.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shern JF, Selfe J, Izquierdo E, et al. Genomic classification and clinical outcome in rhabdomyosarcoma: A report From an International Consortium. J Clin Oncol 2021; 39: 2859–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari A, Gasparini P, Casanova M. A home run for rhabdomyosarcoma after 30 years: What now? Tumori 2020; 106: 5–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tmj-10.1177_03008916221110266 for Establishment of 6 pediatric rhabdomyosarcoma patient’s derived xenograft models closely recapitulating patients’ tumor characteristics by Patrizia Gasparini, Michela Casanova, Giovanni Centonze, Cristina Borzi, Luca Bergamaschi, Paola Collini, Adele Testi, Stefano Chiaravalli, Maura Massimino, Gabriella Sozzi, Andrea Ferrari and Massimo Moro in Tumori Journal
Supplemental material, sj-pdf-2-tmj-10.1177_03008916221110266 for Establishment of 6 pediatric rhabdomyosarcoma patient’s derived xenograft models closely recapitulating patients’ tumor characteristics by Patrizia Gasparini, Michela Casanova, Giovanni Centonze, Cristina Borzi, Luca Bergamaschi, Paola Collini, Adele Testi, Stefano Chiaravalli, Maura Massimino, Gabriella Sozzi, Andrea Ferrari and Massimo Moro in Tumori Journal