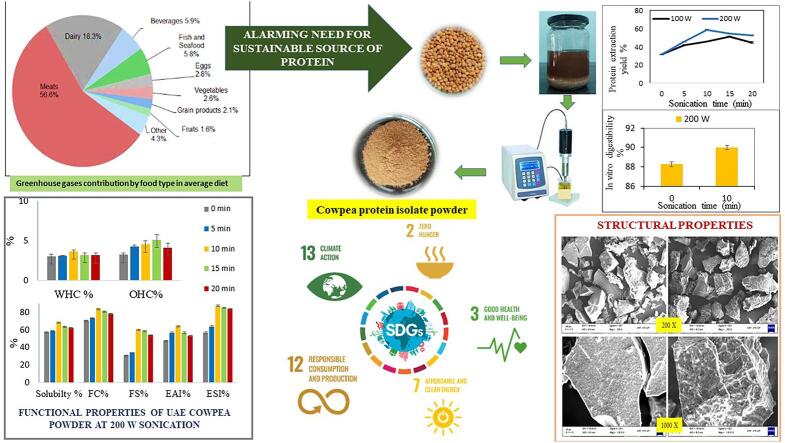
Graphical abstract
Keywords: Cowpea protein, Ultrasound, Novel extraction technologies, Sustainable protein source, Functional and structural properties
Highlights
-
•
A move to sustainable plant proteins is vital to fulfilling future protein demand.
-
•
Ultrasound-assisted extraction (UAE) boosts cowpea protein yield and performance.
-
•
By disrupting structural cells, sonication enhances protein properties.
-
•
Consuming cowpea plant protein promotes sustainable energy, health, and nutrition.
Abstract
Natural resource depletion, negative environmental effects and the challenge to secure global food security led to the establishment of the Sustainable Development Goals (SDGs). In need to explore underutilized sustainable protein sources, this study aims at isolating protein from cowpea by ultrasound-assisted extraction (UAE), where the techno-functional characteristics of the protein isolates were studied at different sonication conditions i.e., 100 W and 200 W at processing times ranging from 5 to 20 min. The US at 200 W-10 min produced the optimal results for all properties. In this process combination, there was an increase in protein yield, solubility, water-holding capacity, foaming capacity and stability, emulsion activity and stability, zeta-potential, and in-vitro protein digestibility from 31.78% to 58.96%, 57.26% to 68.85%, 3.06 g/g to 3.68 g/g 70.64% to 83.74%, 30.76% to 60.01%, 47.48% to 64.26%, 56.59% to 87.71%, –32.9 mV to −44.2 mV and 88.27% to 89.99%, respectively and particle size dropped from 763 nm to 559 nm in comparison to control. The microstructure and secondary-structure alterations of proteins caused by sonication were validated by SEM images, SDS-PAGE, and FTIR analyses. Sonication leads to acoustic cavitation and penetrate the cell walls, improving extraction from the solid to liquid phase. After sonication, the hydrophobic protein groups were exposed and proteins were partially denatured which increased its functionality. The findings demonstrated that UAE of cowpea protein improved yield, modify characteristics to fit the needs of the food industry, and contribute to achieving SDGs 2, 3, 7, 12, and 13.
1. Introduction
Plant protein has been extensively isolated using leguminous plants including kidney beans, chickpeas, lentils, and peas [1]. A legume plant’s edible seed is known as a “pulse”. Pulses have several advantages, including high protein varying between 15 and 30 percent of dry weight, fiber, mineral content, and bioactive substances as well as reducing diabetes and cholesterolemia thereby contributing to better health [2]. In addition, pulses are affordable and shelf-stable.
Pulses are cultivated everywhere across the globe, with Asia producing roughly half of the total. With an output of 26.96 million metric tonnes in 2021–22 (25% of overall production), India is the world’s biggest producer, customer (27% of total consumption), and importer (14%) [3]. Pulses have the potential to dramatically improve the sustainability of our diets by assisting agriculture and the food industry in becoming more environmentally friendly, as they minimize greenhouse gas emissions and consume animal-based goods [4]. These factors are also critical in accomplishing Sustainable Development Goal (SDG) 2, which is to improve nutrition as well as encourage sustainable farming. The Sustainable Development Goals (SDGs) consist of 17 objectives agreed upon by the United Nations’ 193 member countries to end poverty and create a society that is equal, just, and secure for people, the planet, and prosperity.
Plant-derived foods and commodities have lately gotten a huge focus in the pharmaceutical and food industries owing to their environmental sustainability, reduced deforestation and climate change [5], reduced risk of infection and contamination, the ability to target vegetarian consumers, as well as their versatility and lower cost encouraging sustainable energy [6]. These features address SDG 12 (sustainable consumption), SDG 13 (combating climate change), SDG 3 (promoting wellbeing), and SDG 7 (ensuring sustainable and modern energy). However, comparatively plant proteins have low functionality, as indicated by poor solubility, foaming, emulsifying, and gelling capabilities, and the presence of anti-nutritional substances with a noticeable off-taste, restricting their usage in food preparations. This is intensified by the rigorous conventional protein extraction conditions, which promote denaturation, aggregation, and loss of functioning in some proteins [7]. The crucial step in isolating and recovering proteins is the extraction process. Plant proteins have been extracted using a variety of techniques, including conventional alkaline, organic solvent, salt, reverse micelle, and enzyme extraction [8]. Traditional extraction procedures, on the other hand, are time-consuming, energy-intensive, and ecologically unfriendly related to the application of acids, alkalis, and organic solvents, which contradict SDG 7. Because of its ease and inexpensive, alkaline precipitation extraction (APE) is the most popular approach for protein extraction [9]. However, currently, scientists are exploring various modern methods including microwave-assisted extraction [10], pulsed-electric field [11], enzymatic extraction [12], gamma irradiation [13], high hydrostatic pressure [14], ultrasound-assisted extraction [15], ultrafiltration [16], cold atmospheric plasma [17], etc. to extract protein with less time-consumption, low use of energy and in an environmentally friendly way to achieve SDG 7. Ultrasound-assisted extraction (UAE), is one such novel technologies. It is claimed to be an efficient extraction technique that can significantly cut down on solvent usage and extraction time, when compared to conventional approaches which are economical, eco-friendly, repeatable, and thereby promoting sustainable energy thus favoring SDG 7 [18]. UAE is formed on the dispersion of pressure oscillations at the speed of sound in a liquid media, resulting in acoustic cavitation and related physical effects including shockwaves, turbulence, as well as shear forces. The energy produced as a result of cavitation causes poration and disintegrates the plant cell, increases hydration, and increases the surface area by reducing the particle size, making the extraction easier [8]. The reduction in particle size increases the surface area of the cell’s cytoplasm for extracting liquid as well as the accumulation of protein bodies [19]. In addition, Mccarthy et al. [20] have reported ultrasonication has improved proteins’ functionality. Studies have reported that solubility [21], gelling ability, foaming properties, and water/oil holding capacity [22] were enhanced in sonicated pea proteins. Likewise, UAE on soy protein was also found to improve yields of protein, oil [23], improved zeta-potential, and smaller particle size [24], and similar observations were reported on faba bean protein [25], sprouted green gram [26], lupine protein [27], and black gram mill protein [28]. Furthermore, in vitro digestibility was reported to be improved in sonication to the buckwheat protein [29] and chickpea protein [30]. Hence it is noted that UAE while extracting the protein can modify its properties like nutritional/biological, functional, and physicochemical properties. Modified pulse-based plant proteins can be used as food additive to add value and improve the functional quality of packaged foodstuffs. These proteins have better application in the manufacture of various packaged foodstuffs such as dairy analogs, beverages, plant meat, desserts, nutribar, nutritional supplements, etc. [31].
To lessen the detrimental effects of the current nutrition on human and global health, people are being advised to eat supplementary vegan diets. According to recent findings, substituting plant-based diets for animal-based ones would significantly improve both world and human health [32]. To create meat substitutes, different plant proteins have been used. These include lupine steak, which is made from pulses (pea, lentil, lupine, and chickpea) [33], textured vegetable protein-based patties, which are made from a soybean protein isolate and gluten or soybean protein concentrate mixture [34]. Minimally processed components were also employed to create soybean emulsions as well as gels in sausages and to structure products that resemble muscles [33]. Increased consumption of heme, cholesterol, and other nutrients from animal origin, particularly red meat, has been linked to metabolic disorders, cardiovascular disease, type II diabetes, strokes, and cancer. Contrarily, plant protein, from any source, is consistently linked to nutrient sufficiency [33]. Adding more plant-based protein to adolescent diets in place of animal-based protein can help prevent obesity and may have good effects on cardio-metabolic variables. In human diets, rather than animal-based foods, plant-based proteins are being employed more and more as a cost-effective and health-promoting substitute source [35]. In addition to these drawbacks for animal-based diets, more than half of the greenhouse gas emission production is accounted for by meat consumption alone with a production of 56.6%, and has larger carbon footprints per calorie than grain or vegetable products. Consumption of plant-based diets significantly reduces greenhouse gas emissions and carbon footprints [36]. Thus, the consumption of plant-based products can thereby ensure healthy lifestyles and enhance wellbeing at all ages, thereby addressing SDG 3.
Among the pulse crops, cowpea is the least explored for extraction of protein and functional modification of the same for usage in the preparation of supplements or plant-based processed foods. Cowpea, Vigna unguiculata L. Walp. is indeed a low-cost pulse crop that contains proteins (23–32%), carbohydrates (50–60%), relatively lesser fat concentration (1%), vitamins, fibers, and bioactive substances including phenols and polyamines, making it a popular choice in developing nations [37]. Cowpea production worldwide reached 8.9 million metric tonnes in 2019 [3] with a 2.7 fold increase since 2000. Due to its drought-resistant and warm-weather tolerant, it is a desirable forage and food crop in a common tropical lowland region, promoting healthy living (SDG 3) and achieving food security (SDG 2). Cowpea is normally contemplated as a complete food owing to their substantial crude protein content and great distribution of amino acids, which favors both SDG 3 and 7, i.e., promoting health and lowering the relative incidence of energy and protein malnutrition [38].
Cowpea also has medicinal properties including anti-hypertensive, anti-cancer, anti-inflammatory, anti-hyperlipidemia, and anti-diabetic characteristics [39], which protect against a variety of chronic illnesses like cancer [40], intestinal disorders [41], coronary heart disease, high cholesterol, and overweight [42]. Additionally, in the agroecosystem, cowpea cultivation can fix atmospheric nitrogen; as a result, it improves soil qualities and thrives even in inferior soils. Cowpeas also can restrict weed growth. Thus it addresses SDG 2 which promotes sustainable agriculture and food production strategy which can be formulated to accomplish food safety and sufficient nourishment. Unlike other pulses, cowpea protein hasn’t been explored much. Only a few isolation techniques, such as salt assisted extraction [43], micellization precipitate [44], isoelectric precipitate [45], etc., have been used to extract protein from cowpea. Earlier studies claimed that cowpea protein could be used as a protein supplement for newborns, young children, and the elderly [46]. Unfortunately, there is still a shortage of literature on the functional and structural features of cowpea proteins. The extraction of cowpea protein employing ultrasound is another unexplored area of research. A variety of legume proteins have been extracted using innovative technologies, including UAE, and studies have revealed improvements in both functional and biological aspects of the protein. Extraction and modification of plant protein using green technologies such as ultrasound can aid in the achievement of numerous SDGs. Table 1 summarises some examples of research that contributes to the SDGs. Thus, we envisage a huge opportunity for research for improving the yield as well as the quality of cowpea protein using ultrasound since it is not explored in the literature. Hence, throughout this study, UAE and APE were performed to isolate cowpea protein, and the structural, functional, and biological attributes of cowpea proteins were analyzed and compared to provide the best quality protein that meets the demands of the processed food sector as well as the SDGs 2, 3, 7, 12, and 13.
Table 1.
Some examples of research that contributes to the Sustainable Development Goals (SDGs).
| The focus of the study | Key points | SDGs and targets | Countries of affiliation (authors) | Reference |
|---|---|---|---|---|
| Investigated the impact of treatment on the functional and structural attributes of soy protein isolate using ultrasound. |
|
|
China and Canada | [58] |
| The influence of various treatment techniques (ultrasonication, homogenization, and micro fluidization) on the emulsifying properties of pea protein isolate was compared. |
|
|
Ireland | [20] |
| UAE was utilized to intensify the extraction yield of soy slurry and okara |
|
|
United Kingdom and The Netherlands | [23] |
| The impact of ultrasonic on the technological-functional and structural characteristics of jackfruit seed protein isolate was evaluated. |
|
|
Mexico | [66] |
| Investigated the impact of high-power US on the functional characteristics of millet protein concentrates. |
|
|
Iran and Denmark | [68] |
| The functional characteristics and morphology of a pea protein isolate were studied using a pH-shifting and ultrasonic hybrid method. |
|
|
United States, Jordan and Saudi Arabia | [21] |
| Examined several novel protein extraction methods (including ultrasound) from plant sources. |
|
|
Serbia and Ireland | [18] |
| The morphological alterations underlying the effects of sonication on functional characteristics and hydrolysis levels in plum seed protein isolate were examined. |
|
|
China | [67] |
| The impact of sonication on the functional characteristics of faba bean protein isolate was explored. |
|
|
Mexico | [25] |
| The influence of physical (including ultrasound) and biological alteration on the quality of food proteins was demonstrated, and insight into the process causing structural changes were presented. |
|
|
China, Spain and Sweden | [72] |
| Research was conducted into how different walnut proteins’ yields and physicochemical characteristics were affected by UAE. |
|
|
China and Egypt | [59] |
| Research was conducted on how ultrasonic treatment affected the properties of quinoa seed protein isolates. |
|
|
India | [62] |
| The impacts of the UAE process on the protein molecule, functionality, and bioactivities of a pea protein isolate were evaluated. |
|
|
China | [22] |
| Investigated the impact of US application on protein yield and alkaloid outcome during the lupin alkali extraction method. |
|
|
Mexico | [27] |
| Described the latest developments in protein extraction from plant sources employing typical and innovative green extraction technologies (including ultrasound). |
|
|
India, China, Poland, UK and USA. | [8] |
| The effects of sonication on the in vitro digestibility of buckwheat protein isolates were studied. |
|
|
China, Canada and Saudi Arabia | [29] |
| The influence of US and micronization extraction methods on protein yield and functionality from black gram by-product were evaluated. |
|
|
India. | [28] |
| Highlighted the core aspects for producing meat analogues from plant-based protein sources, as well as how different methods of extraction influence protein performance. |
|
|
The Netherlands | [34] |
| The influence of ultrasound extraction and alteration of plant proteins on protein structural alterations and corresponding physicochemical, functional, and nutritional characteristics have been addressed. |
|
|
United States | [19] |
| The extraction of soybean protein has been evaluated using both alkaline phosphate buffer and UAE. |
|
|
United Kingdom | [24] |
2. Materials and methods
2.1. Raw materials
Cowpea was procured at Thanjavur’s local market, in India. Before starting preliminary trials, cowpea grains were cleaned with distilled water, and other unwanted materials like damaged grains, other edible grains, infested grains, and foreign matter were removed from them. The cleaned grains were left to dry in a tray dryer (Everflow, Tamil Nadu, India) at 55 to 60 °C for 8 to 12 h.
2.2. Extraction of protein isolate from cowpea.
2.2.1. Preparation of cowpea flour
Whole dried cowpea grains were pulverized (KK Lifesciences, Tamil Nadu, India) until powder and pre-treated in a soxhlet apparatus for 4 h using hexane to remove the fat.
2.2.2. Conventional extraction
Cowpea protein isolates have been extracted employing Khalid and Balla’s technique [47], with minimal changes. A 1:10 (w/v) solution of distilled water and defatted cowpea flour was prepared. The solution’s pH level was corrected to 9.0 by introducing 1 N NaOH. Upon blending for half an hour at room temperature, in a refrigerated centrifuge, the solution was separated at 5000g/30 min at 4 °C for 30 min. Following the collection of supernatant, the pH was reduced to 4.5 with the introduction of 1.0 N HCl before mixing for another half an hour at room temperature, then centrifuged at 5000g/30 min at 4 °C in a refrigerated centrifuge. The supernatant was thrown away after centrifugation, and the deposit (precipitate) was retrieved. The deposit was rinsed twice with distilled water and its pH was recalibrated to 7.0 with 1 N NaOH. The neutralized precipitate was allowed to stand in the refrigerator overnight (4 °C). The cowpea protein isolate was freeze-dried and then kept in a closed container at a low temperature until suited for evaluation. The conventional extraction method demands a total extraction time of around 120 min.
2.2.3. Ultrasound-assisted extraction
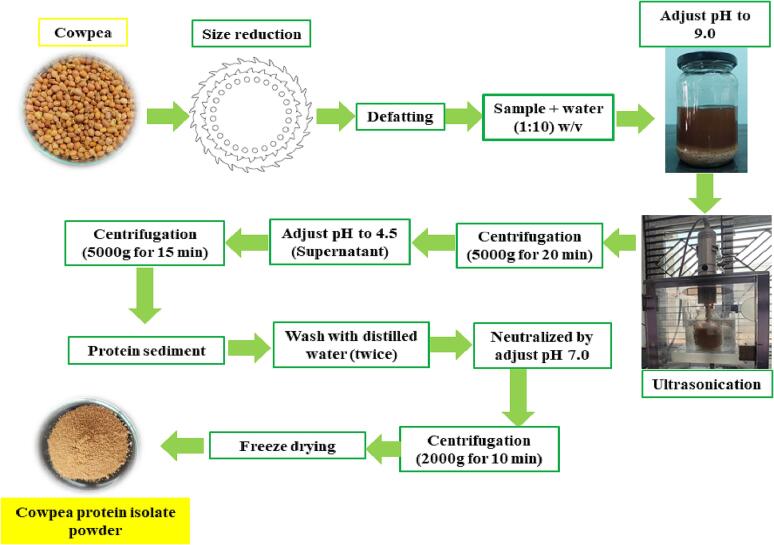
As illustrated in Fig. 1, cowpea protein isolate powder (CPIP) was obtained from cowpea seed flour.
Fig. 1.
Preparation of cowpea protein isolates by ultrasound-assisted extraction.
The defatted cowpea flour was added to distilled water in a 1:10 (w/v) ratio, and the pH of the cowpea powder-distilled water mixture was corrected to 9.0 using 1 N NaOH. The combined solution was treated ranging from 5 to 20 min by a probe ultrasonic processor (Sonic CV334) with a 14 mm probe size at power levels of 100 and 200 W. Sonication had a working and stopping time of 5 s. The entire sonication procedure was carried out in an ice bath at 25 °C. Secondly, the sample solution quantity for the sonication procedure was kept constant. Sonication was followed by centrifugation at 4 °C at 5000g/15 min. The supernatant’s pH level was reduced to 4.5 with 1 N HCl before centrifugation (5000g/15 min). The supernatant was disposed of and the precipitate that remained was rinsed twice with distilled water before being calibrated to a pH of 7.0 with 1 N NaOH. The neutralized precipitate was allowed to stand in the refrigerator overnight (4 °C). The cowpea protein isolate was freeze-dried and then kept at a low temperature in a sealed container till ready for analysis. Only roughly 65 min of total extraction time is needed for the UAE.
The CPIP extraction level was assessed using the Kjeldahl technique and the equation (2.1) below.
| (2.1) |
where b1 is the amount of protein in cowpea,
b2 is the amount of protein in cowpea flour,
a1 is the weight of cowpea protein, and
a2 is the weight of cowpea flour.
2.3. Proximate composition
The proximate of the CPIP was calculated following the AOAC method [48]. The hot air oven method was used to determine the moisture content. The empty dish was dried for 3 h at 105 °C and then kept desiccator to cool completely to remove any moisture in the dish. The Soxhlet apparatus unit was used to determine the fat content. A sample of about 2 g was weighed and placed in a clean thimble lined with cotton wool. Then thimble was placed in the extractor. Hexane was used to extract fat over 8 h. The protein content was determined by using the Kjeldahl method and to the estimated total nitrogen, 6.25 was multiplied to get the total protein percentage. The sample’s ash content was also determined by burning in a muffle furnace at 550 °C for 3 h or longer until light grey ash was formed.
To obtain the total carbohydrates, the amount of moisture, fat, protein, and ash content was subtracted from 100 [49].
2.4. Functional properties
2.4.1. Solubility
With very few adjustments, the approach used by Wang et al. [22] to evaluate protein solubility was used. The pH of the CPIP solution (10 g/L) was altered to pH 7.0. The protein dispersion was stirred using a vortex for 20 min and followed by centrifugation at 5000g for 10 min. The supernatant was taken and the solubility of cowpea protein was assessed employing the Biuret method in accordance with the equation (2.2):
| (2.2) |
2.4.2. Water holding capacity (WHC) and oil holding capacity (OHC).
With minor alterations, the CPIP’s water-holding capacity (WHC) and oil-holding capacity (OHC) were assessed using the approach provided by Wani et al. [50]. The CPIP sample (100 mg) was combined with water or 3 mL of soybean oil and then agitated for 5 min at ambient temperature in a vortex shaker. After centrifuging the mixture for 20 min at 5000 rpm, the supernatant was cautiously drained without disturbing the residues. The residue was then weighed and WHC and OHC were estimated by the equation below (2.3):
| (2.3) |
Where x is the mass of the tube containing the protein isolate plus absorbed water or oil,y is the mass of the tube and protein isolate, andz is the mass of the protein isolate.
2.4.3. Foam properties
The following approach was used to calculate the foaming capacity (FC) and foaming stability (FS) of the CPIP samples. Using a homogenizer, 15 mL of CPIP solutions (10 g/L, pH 7.0) were blended (IKA Ultra turrax, Bangalore, India). The amount of the foam was assessed to calculate the FC, and then again after 30 min to estimate the FS [22]. The FC and FS were evaluated by applying the equations shown underneath:
| (2.4) |
| (2.5) |
2.4.4. Emulsifying properties
The CPIP’s emulsion activity index (EAI) and emulsion stability index (ESI) were determined using the approach described by Malik et al. [51], with some adjustments. With a homogenizer (IKA Ultra Turrax, Bangalore, India) at 12,000 rpm for 2.5 min at room temperature, 5 mL of CPIP solution (2%) was homogenized with 5 mL soybean oil. The emulsions were then centrifuged at 1000g for 5 min. The emulsified layer and the tube’s entire contents’ heights were estimated.
EAI and ESI were assessed using the following formula:
| (2.6) |
The emulsions were tested for stability by heating them at 80 °C for half an hour prior to centrifuging them at 1000g for 5 min.
| (2.7) |
2.5. Structural and biological properties
2.5.1. Fourier transform infrared spectroscopy (FTIR)
An FTIR spectrophotometer (SHIMADZU & IR Affinity −1S) was utilized to produce CPIP FTIR spectra between wavelengths 400–4000 cm−1. CPIP was blended with KBr and crushed into pellets.
2.5.2. SDS-PAGE (SDS-polyacrylamide gel electrophoresis)
The analysis was executed following the Kamani et al. [28] method with few variations. At 95 °C for 5 min, 100 µl of sample buffer diluted with 10 mg of CPIP was fully denatured. For 10 min, the fluid was centrifuged at 8000g. 30 µl of supernatant was injected into the polyacrylamide gel. The samples were run through the stacking gel at 90 V before being passed through the separating gel at 120 V. The coomassie brilliant blue stained the gel, and destaining was performed in the absence of dye using the same solvent. 10–250 kDa protein marker (Bio-Rad) was chosen as a reference to determine the molecular weight.
2.5.3. Scanning electron microscopy (SEM)
Under high vacuum conditions, the morphology of the protein powders was examined using a ZEISS EVO 60 scanning electron microscope with an oxford EDS detector (SEM: Carl ZEISS SMT, Germany). The acceleration voltage remained constant at 20 kV. The work spacing was kept between 8 and 10 mm, which is appropriate for a 20 kV acceleration voltage. For higher resolution and high-resolution imaging, the mid-column aperture was kept at 3 mm [52]. The samples were gold sputter coated and affixed to a carbon-coated SEM stub using double-sided adhesive tapes even before the examination (40–50 nm). The samples were then put in the SEM chamber and the morphology of the encapsulated powders was examined at various magnifications.
2.5.4. Zeta potential (ζ-potential) and particle size
Zetasizer was employed to examine the CPIP’s zeta potential. The distribution of particle sizes of the CPIP was assessed employing dynamic light scattering technology and the Zetasizer model NanoZS (Malvern Instruments, UK). The protein sample has a refractive index (RI) of 1.33.
2.5.5. In vitro digestibility
With a few adjustments, the in vitro protein digestibility was evaluated by adopting Almeida et al’s approach [53]. A protein solution of 250 mg of every sample or 250 µl of distilled water (taken as the blank sample) was mixed in 15 mL of 0.1 N HCl incorporating 1.5 mg/mL pepsin and kept in a water bath for 3 h at 37 °C. The pancreatic digestion was then initiated by adding 10 mL of 0.2 N phosphate buffer (pH 8.0) including 10 mg of pancreatin and kept overnight at 37 °C. Following the pancreatic degradation, 1 mL of 10 g/100 mL trichloroacetic acid was introduced, succeeded by 20 min of centrifugation at 2100 rpm. The supernatant was obtained and the concentration of total protein was assessed using the Kjeldahl AOAC technique [48] relying on the nitrogen content. The ratio of protein concentration present in the sample solution prior to and after digesting was used to calculate in vitro digestibility.
2.6. Statistics analysis
Every measurement was conducted three times. Minitab software (Version 18, Minitab Statistical Software, United States) was applied to conduct a variance analysis (ANOVA). At a confidence level of 0.05, Tukey’s test was employed to assess mean value differences.
3. Result and discussion
3.1. Extraction and optimization of protein isolate from cowpea
3.1.1. Proximate analysis
Table 2 provides the approximate composition of defatted raw cowpea flour and its protein isolates, which were isolated using the traditional approach. The cowpea is a remarkable source of proteins needed for human tissue production as well as repair [54]. Except for a small difference in the number of carbohydrates (61.72%), the proximate composition of defatted cowpea flour in this research is comparable to that described by Adetutu Adeyoju et al. [46] and Affrifah et al. [54]. This difference could be attributed to the defatting as well as processing conditions for preparations of cowpea flour as well as the cultivar type. The samples with greater reported carbohydrate levels did not distinguish between soluble and insoluble fractions. The moisture, protein, fat, ash, fiber, and carbohydrate contents of defatted raw cowpea flour were 5.5 ± 0.01, 26.4 ± 0.45, 1.82 ± 0.06, 3.46 ± 0.05, 1.1 ± 0.06, and 67.17 ± 0.45% respectively. The proximate composition of cowpea protein isolates extracted by alkaline precipitation showed 77.3% of protein and carbohydrate of 17.6% (Table 2). Crude fiber and fat were found in traces. These results are found in the range reported by Adetutu Adeyoju et al. [46] with slight variation, which can be contributed due to differences in cowpea variety and the isolation methods involved.
Table 2.
Proximate composition of defatted flour of cowpea and cowpea protein isolate powder (CPIP).
| Chemical constituents | Defatted cowpea flour | CPIP |
|---|---|---|
| Moisture | 5.5 ± 0.01a | 2.9 ± 0.05b |
| Protein | 26.4 ± 0.45b | 77.3 ± 0.45a |
| Fat | 1.82 ± 0.06 | Traces |
| Fibre | 1.1 ± 0.06 | Traces |
| Ash | 3.46 ± 0.05a | 2.2 ± 0.05b |
| Carbohydrates (by difference) |
61.72 ± 0.41a | 17.6 ± 0.53b |
Different superscripts within same column are statistically significantly different at p < 0.05.
3.1.2. Protein extraction yield by conventional process vs UAE
The extraction variables such as solvent ratio, sonication time, power level, etc. have a huge effect on the yield of protein throughout the extraction procedure. Moulton and Wang [55] demonstrated that protein yield was highest in soybean flakes-to-water ratios with 1:30 when compared with 1:10 and 1:20 owing to more effects of cavitation towards less-density slurry. Another study also reported that more extraction happens during ultrasonication as the solvent amount increases because the solutes experienced more cavitational effects when there was more solvent water available [19]. However, from an industrial standpoint, using less water is preferable. Keeping in view to reducing the usage of resources and a more practical approach for protein extraction and supporting the sustainable management of water which is SDG 6, we took the cowpea flour to water ratio of 1:10.
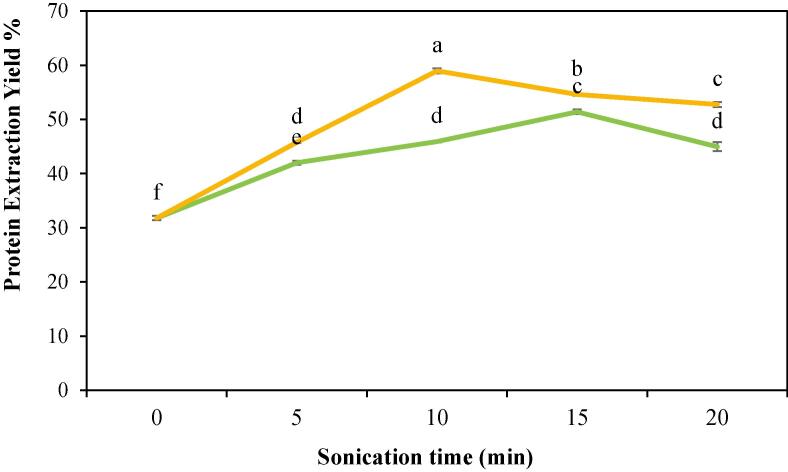
Fig. 2 demonstrates the influence of ultrasonic power at 100 and 200 W on the extraction yield of CPIPs with a time ranging between 0 min (untreated or control) and 20 min. When compared to untreated cowpea flour, protein extraction yield was higher when exposed to high-power sonication (31.78%). In 15 min of UAE at 100 W, a maximum yield of 51.41% was achieved. Furthermore, increasing the ultrasound power to 200 W led to a maximum yield of 58.96% in 10 min of sonication time. The extraction yield was enhanced by raising the ultrasonic power from 100 to 200 W. This could be due to the increased ultrasound power generating more acoustic bubbles, which collapse rapidly through the extraction solution [15]. This similar increase was noted for UAE of protein in pea protein isolate [22]. Increasing the protein yield with cheap sources by sustainable means will help in combating global malnutrition and promoting food security (SDG 2) and thereby improving well-being and ensuring better health for all, irrespective of age (SDG 3).
Fig. 2.
Protein extraction yield of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
However, after long sonication, there is a decreasing trend in the protein yield observed in both the power level. At 100 W, after 15 min of sonication, the extraction yield was reduced to 44.96% (20 min). Similarly, for 200 W, there was a significant reduction in the yield from 15 min (54.61%) to 20 min (52.76 %). A similar pattern was noticed in bitter melon seed protein, where high power level and long sonication time significantly reduce the protein extraction yield. This reduction could be attributed to the fact that US treatment causes aggregation of partially denatured protein to form macromolecular insoluble aggregates. Proteins aggregates to form bigger molecule which is resistant to penetration of the solvent for extraction [15]. Another study also revealed that the stable macro-aggregates generated are fragmented into smaller aggregates due to the strong mechanical action. Because these small aggregates revealed more hydrophobic clusters, they became unstable and more likely to re-associate, leading to formation of stable and large insoluble aggregates [56]. Considering the processing time and extraction yield, the UAE is capable of extracting protein efficiently. The conventional approach requires a total extraction time of about 120 min, while the UAE only requires 65 min (taking 20 min for sonication). Thus, if UAE is used instead of conventional extraction, protein extraction time can be reduced by half. Furthermore, UAE will also play a vital role in attaining energy efficiency in support of SDG 7.
3.2. Functional properties
Because of their building, texturizing, emulsification, foaming, wetting, and nutritional capabilities, proteins are the most important functional elements in a food system. Given their sustainable origin and low cost of production, ease of availability, and advantages over their animal-based equivalents in terms of health, plant-based proteins provide a feasible alternative to our nutritional needs (SDG 3). In food formulations, plant-based proteins play a variety of roles, such as thickening and gelling agents, emulsion and foam stabilizers, and binding agents for fat and water. However, because of their poor water solubility, complexity, and sensitivity to pH, ionic strength, and temperature, the majority of plant-based proteins are insoluble, which restricts their applicability. Therefore, it is imperative that their attributes be changed to increase their functionality [6].
3.2.1. Solubility
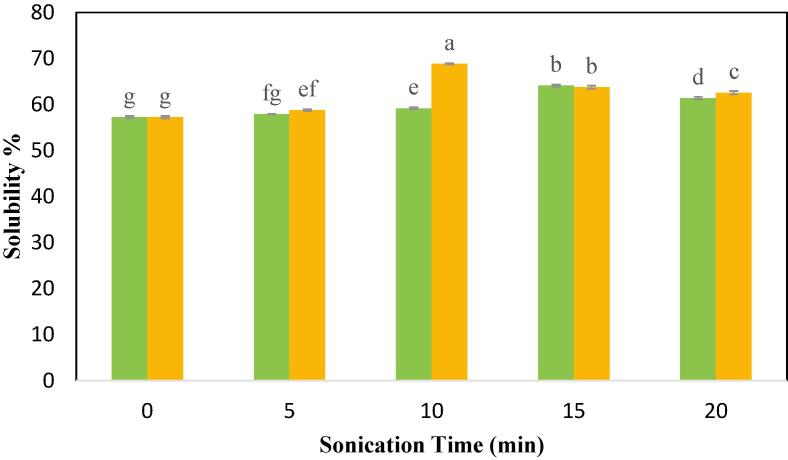
A fundamental index for the hydration of protein is solubility, which has a substantial impact on protein functional qualities including emulsification and gelation, thus determining its end-product usage. The solubility of CPIPs achieved by UAE was much greater compared to the control (57.26 %), as depicted in Fig. 3. The highest solubility of 68.85 % of CPIP was observed in US-10 min-200 W. At US-100 W, the highest solubility was observed at 15 min (63.24%). However, there was no significant improvement in the solubility until it reach the sonication time of 10 min. The solubility of CPIP at 100 W for 5 min and 10 min was observed to be 57.93% and 59.20% respectively. Similarly, at US-200 W, there was no significant improvement till 5 min (58.78%). Similar results with improvement in solubility after ultrasound treatment were described for meat protein [57], and protein isolates of chickpea [30], soy [58], faba bean [25], walnut [59], and pea [22]. These researchers concurred that the improvement in solubility is due to the downsize in protein particles and an improvement in protein-water interactions in the US. The development of soluble protein aggregates via exposure of internal hydrophilic as well as structural alteration also might account for the improvement in solubility [56]. According to Yu et al. [60], ultrasonic treatment reduced the α-helix and raised the random coils contents in proteins, resulting in enhanced protein solubility.
Fig. 3.
Solubility of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
However, when ultrasonic time increased, solubility elevated at first and subsequently dropped at the same ultrasonic power level. This trend was observed in both the power level. At 100 W, the solubility increased till 15 min and then decreased to 61.4% when sonicated till 20 min. Similarly, at 200 W, the solubility was found to decrease when sonicated at 15 min and 20 min. The solubility for 15 min and 20 min at 200 W was observed to be 63.77% and 62.58% respectively. As per Yan et al. [61], excessive sonication induces re-polymerization of the proteins resulting in aggregation, thus lowering its solubility.
3.2.2. Water holding capacity (WHC) and oil holding capacity (OHC)
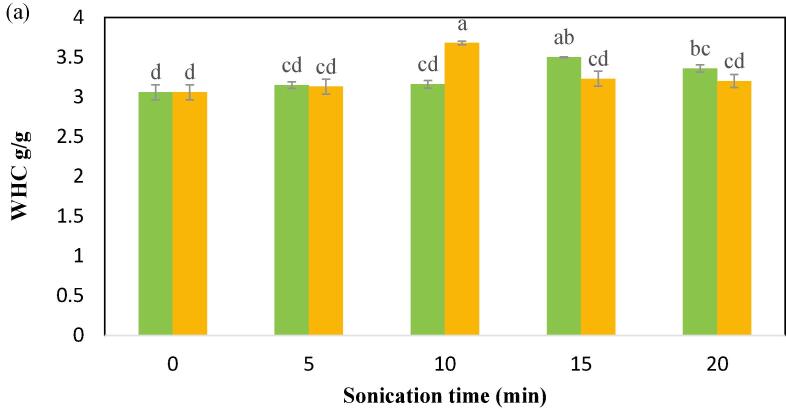
The WHC is referred to as the quantity of water absorbed per gram of prtein content. WHC denotes a product’s ability to interact with water in an environment where water is scarce. Protein WHC contributes significantly to the thickening, juiciness, and viscosity of many food products [19]. As shown in Fig. 4a, the control CPIP has the least WHC (3.06 g/g) in comparison to all the US treatments regardless of the variation in the power level and the sonication time. Upon sonication with 100 W, there was no significant (p < 0.05) improvement in WHC at different exposure times of 5 min, and 10 min except for the 15 min which has shown a significant increase and is 3.5 g/g. The WHC value for 5 and 10 min of 100 W is 3.15 g/g, and 3.16 g/g, respectively. Upon sonication with 200 W, there was significant (p < 0.05) improvement in WHC at different exposure times from 0 to 10 min. The maximum WHC (3.69 g/g) was observed at 10 min of US-200 W. The current results are comparable to that reported in sonicated black gram protein isolate, which was in the range of 3.12–3.26 g/g [28]. Similar findings were also reported in sonicated quinoa protein isolate [62]. These researchers reported that the improved WHC could be because of the downsize in particles and increased solubility. These assumptions are also consistent with our solubility results published in the preceding section. Secondly, particle size decrease is observed in this study and is discussed in the following sections. Similarly, Choudhary & Rawson [63], also reported that the increase in WHC in ultrasound-treated curd may be due to exposure of the thiol group and the hydrophobic regions of the amino acids toward the water. Protein isolate with a high WHC content can be used to make sausage, dough, soups, and baked goods, among other things. Protein isolate can be used for enriching food components to boost protein reserves.
Fig. 4a.
Water-holding capacity (WHC) of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
However, the treatment of US for prolonged time results in a reduced ability of WHC of the CPIP in both the power levels. At the power level 100 W, there was a significant reduction in WHC beyond 15 min treatment time, and a similar trend was observed after 10 min of US-200 W. Similar trend has also been found in sonicated protein isolates of safflower and quinoa [62], [64], which has been related to protein denaturation and enhanced hydrophobic surface [64]. Because amino acid hydrophobic-hydrophilic balance is a primary element in defining WHC, whatever variation in this balance results in a change in WHC in the protein molecule [65].
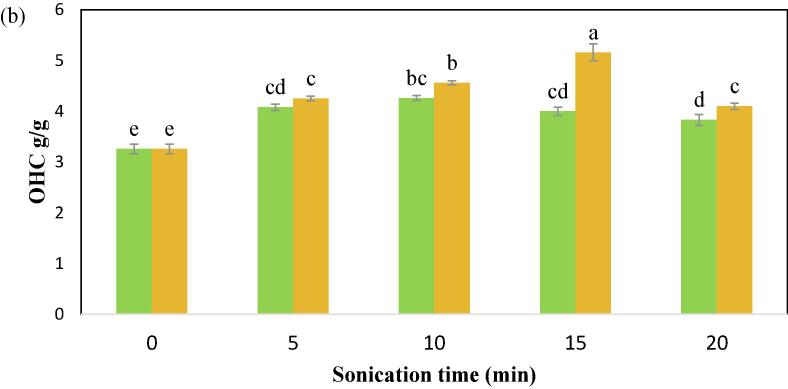
OHC is the ability to physically entrap oil, and higher OHC provides superior flavor retention and mouthfeel in food. As indicated in Fig. 4b the control CPIP has the least OHC (3.26 g/g) in comparison to all the US treatments. The maximum OHC (5.16 g/g) of the CPIP was observed at 15 min of US-200 W treatment. Ultrasonicated protein isolates of safflower, as well as sunflower meal, showed a similar result for OHC. This spike in OHC could be attributed to the proteins which are exposed to the external environment especially the hydrophobic groups after being subjected to the US, allowing for improved trapping of oil physically by the ones that are modified [62], [64]. Resendiz-Vazquez et al. [66] and Xue et al. [58] pointed out that the outcome of ultrasound on a protein’s technological features is greatly influenced by factors like ionic strength, pH, and the time, temperature, intensity, and frequency of the ultrasound as well as the intrinsic attributes of the protein being studied. In comparison to the unmodified soybean protein isolate, the fat absorption index of phosphorylated soybean protein isolates improved from 2.82 mL/g to 3.39 mL/g.
Fig. 4b.
Oil-holding capacity (OHC) of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
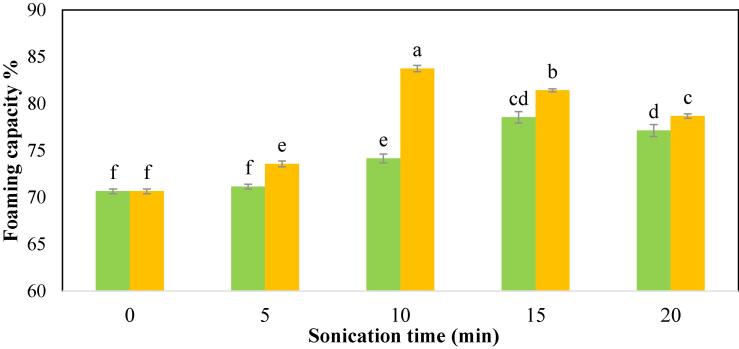
3.2.3. Foaming capacity (FC) and foaming stability (FS)
In comparison to all of the US treatments, the control CPIP has the least FC (70.64 %), as seen in Fig. 5a, regardless of the power level or sonication time. There was a gradual improvement in FC with increasing sonication duration after sonication. In 15 min and 10 min, respectively, the highest FC of the CPIP was found for 100 W (78.53 %) and 200 W (83.74 %). Similarly, chickpea protein isolate [30], black gram protein isolate [28], quinoa protein isolates [62], millet protein concentrate [68], and sunflower protein isolate [51].
Fig. 5a.
Foaming capacity of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
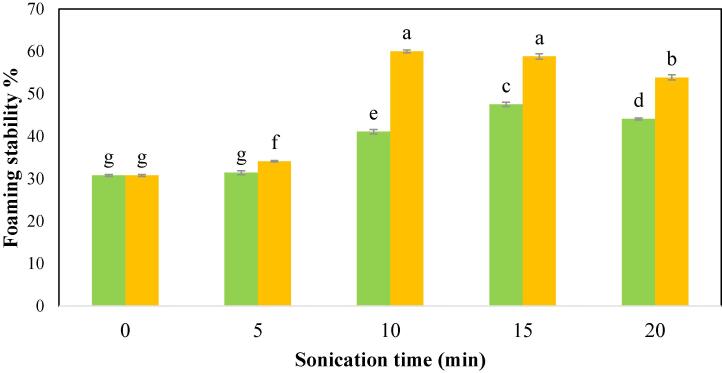
The increment in foaming properties was linked to protein partial denaturation, which leads to a larger air–water diffusion contact owing to the foam’s increased cohesiveness and flexibility [64], [67]. Upon US, the hydrophobic groups were exposed resulting in good protein activity at the air/water interface [69]. Malik [51] and Teliz [70] suggested that reduced particle size after ultrasound treatment may also explain the improvement in the FC. Fig. 5b depicts the FS of various CPIP samples (b). The FS of CPIP was improved significantly (p < 0.05) following ultrasonication at both power levels.
Fig. 5b.
Foaming stability of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
Corresponding to the result of the FC, the highest FS of the CPIP for 100 W (47.51%) and 200 W (60.01 %) was observed in 15 min and 10 min respectively. According to Lv et al. (46), the UAE’s shearing forces plus sonic cavitation, typically scatter particles of protein more uniformly and increase foaming capabilities, are most likely responsible for the improvement in foaming properties.
However, an extended US treatment led to a reduction in the FC and FS of the CPIP at both power levels. Beyond 15 min of treatment time, the foaming characteristics of the power level 100 W were significantly reduced, and a similar trend was found after US-10 min-200 W. Kang et al. [30] also reported a decrease in the foaming characteristics of chickpea protein isolate and suggested that the degree of protein denaturation increased with treatment time, resulting in the development of aggregates and the re-masked hydrophobic area of the protein. As a result, there was a reduction in the foaming capabilities owing to the weakening of the adsorption capacity at the air–water interface by the over-treated protein. Naik et al. [15] also testified that the foaming capabilities of the bitter melon protein isolate were reduced after prolonged treatment, possibly due to a decrease in intermolecular cohesion and elasticity.
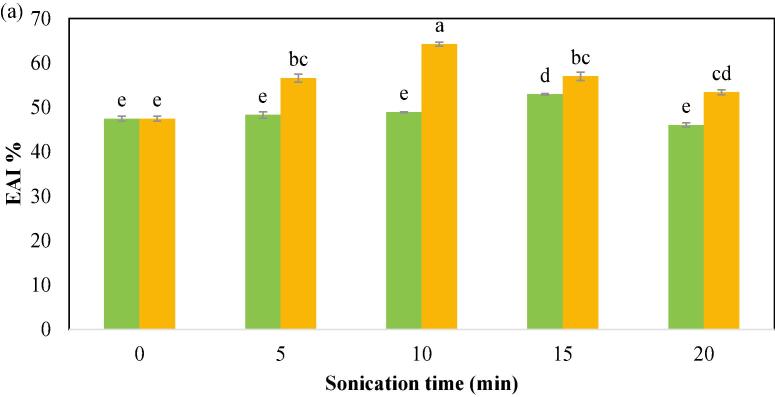
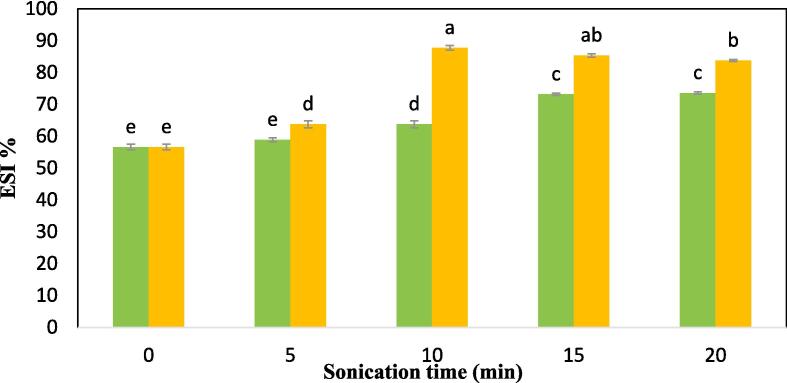
3.2.4. Emulsion activity index (EAI) and emulsion stability index (ESI)
In comparison to the control CPIP sample, the US-treated CPIP showed a significant enhancement in EAI and ESI (Fig. 6a and Fig. 6b). At 100 W power level, no significant improvements were seen in EAI except in the sonication time of 15 min (52.96%). The EAI value for 5 and 10 min of 100 W is 48.32%, and 48.93%, respectively. Notable enhancement (p < 0.05) in EAI was produced with 5 min US-200 W (56.59%) treatment in comparison to the control (47.48 %). The highest EAI was observed in the US-10 min-200 W (64.26%). The EAI value for 15 min and 20 min of 200 W is 57.01% and 53.41%. In the case of ESI, compared to the control (56.59%), US-5 min-200 W (63.77%) resulted in a significant rise in ESI. The US-10 min-200 W treatment had the highest ESI (87.71%). There was no notable improvement in the ESI of the CPIP at 100 W power level until it reached the sonication time of 10 min (63.77%). Data comparable to these have been made for the black gram protein isolate [28], quinoa protein isolates [62] millet protein concentrate [68], tamarind seed protein [65], and other leguminous crop proteins [71]. A protein’s increased emulsibility after ultrasound treatment could have been due to its surface charge, hydrophobicity, or solubility [62], [72]. The immediate pressure of sonication may alter the protein’s tertiary as well as quaternary configurations, improving its ability to absorb oil at the oil–water interface [51], [67]. The effect of ultrasonic cavitation and shearing force improved the emulsifying activity. With greater protein surface area contributing to the formation of an interfacial layer as a result of ultrasonication, EAI may improve [73]. Additionally, the thermal, chemical and mechanical impacts of sonication influence the size of protein aggregation, secondary structure, and hydrophobic properties, altering EAI [74]. Protein isolates with high emulsifying qualities can be used as a fat emulsion stabilizer in the manufacturing of sausage, broth, and desserts [75].
Fig. 6a.
Emulsion activity index (EAI) of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
Fig. 6b.
Emulsion stability index (ESI) of cowpea protein isolate powder treated with  100 W and
100 W and  200 W in 0, 5, 10, 15, and 20 min of sonication times.
200 W in 0, 5, 10, 15, and 20 min of sonication times.
However, the EAI was significantly (p < 0.05) reduced in US-200 W-15 min (57.01 %) and 20 min (53.41 %) treatment time of the CPIP. There was a similar drop in the EAI of the CPIP in the US −100 W for 20 min (46.06%) in comparison to the control (47.48%). In the context of the ESI of the CPIP, no decline was observed in the case of US-100 W after 15 min of treatment, and yet no significant improvement was also found. However, there was a gradual reduction in the ESI of the US-200 W after the treatment time of 10 min. These observations are likely to be related to protein structural breakdown and diminished oil/water interfaces [62] as well as the clustering of denatured protein molecules when proteins were sonicated for prolonged periods [51].
3.3. Structural properties
3.3.1. Fourier transform-infrared spectroscopy (FT-IR)
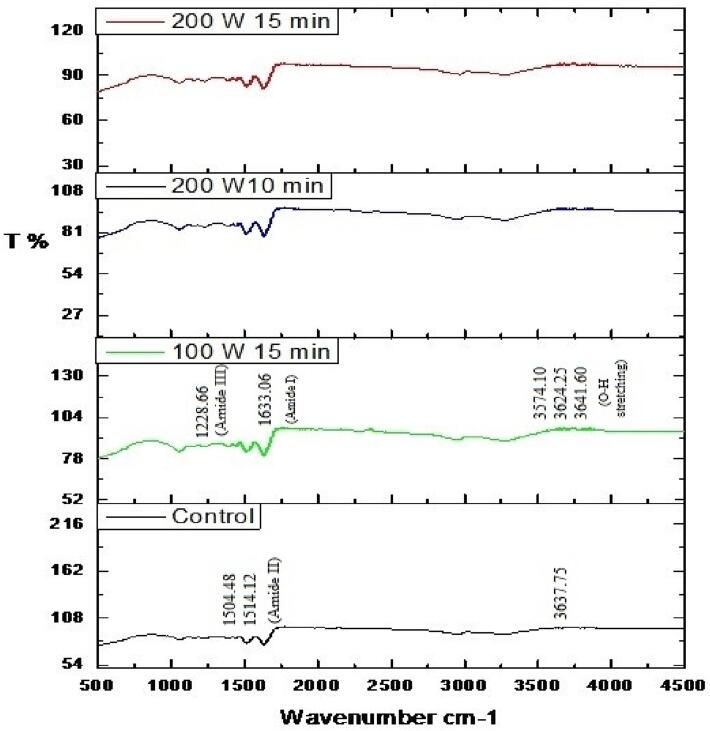
Since all the functional properties of CPIP showed a better value for US treatment of 200 W at 10 min, 15 min, and US 15 min – 100 W, these optimized US-treated samples were further tested for studies on FT-IR, SDS-PAGE, and SEM.
Fig. 7 depicts the FT-IR spectra of various CPIP samples in the 500–4500 cm−1 range. The peaks in the control CPIP sample ranged from 1220 to 1700 cm−1. The peaks for the amide II area was recorded at 1514.12 and 1504. 48 cm−1, meanwhile the amide III region’s peaks were measured in 1228.66 cm−1. The UAE CPIP also showed similar peaks to the control sample. The variation in the power level which is 100 W and 200 W with different sonication times showed no variations in the amide I, II, and III regions (Fig. 7). The peak observed at 1613.78 cm−1 (amide I) for all the treated and untreated sample of CPIP is similar to that of the peak at 1633.06 cm−1 of the native pulse protein as reported by Shevkani et al. [76], corresponding to β-sheet and β-strand structures. The same author has also mentioned that in pulses the β-strand, β-turns, and β-sheets are the major secondary configurations while α-helix appears in a comparatively lesser fraction. Likewise, Mir et al. [62] also found no substantial transformation in the amide II bands of US-treated protein isolates of quinoa, and Kamani et al. [28] also found no noticeable variations in any of the amide bands with varying sonication times during UAE of black gram protein isolate. Another notable finding was that the UAE CPIP showed additional peaks at 3641.60 cm−1, 3624.25 cm−1, and 3574.10 cm−1 in comparison to the control CPIP at 3637.75 cm−1. These peaks could be assigned to O–H stretching of alcoholic groups [77]. This increase in the number of peaks could be explained by the observation that sonication decreased the average size of protein and was described by Nazari et al. [68] in protein isolate of millet, where there is an increase in the number of peaks of US treated samples, such as 722.25 cm−1 and 872.89 cm−1 in comparison to control millet protein isolate with a peak at 723.34 cm−1.The FT-IR study revealed that sonicating a Bombay locust protein isolate for 20 min at 60% amplitude of 750 W resulted in the conversion of random coils from alpha-helical to a greater extent of disordered structure than the ordered structure [78].
Fig. 7.
Fourier transform-infrared spectroscopy of cowpea protein isolate powder samples of control (untreated), sonicated for 10 min, 15 min at 200 W, and sonicated for 15 min at 100 W.
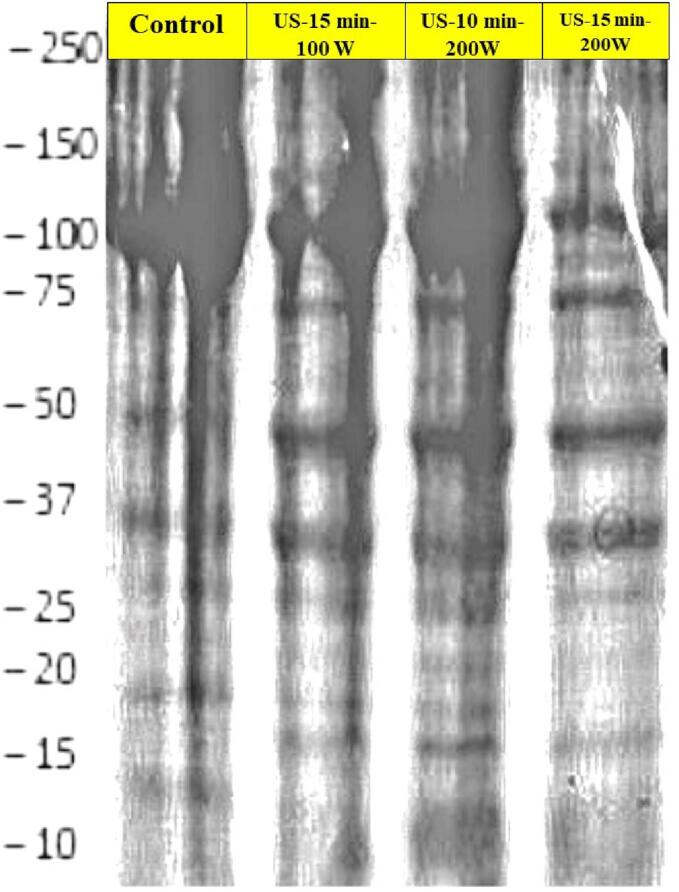
3.3.2. SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
Fig. 8 depicts the existence of a comprehensive array of molecules by weight in the control and modified CPIP samples. The CPIPs exhibited similar bands irrespective of whether ultrasound was involved. Major bands were detected at ∼12 kDa to 55 kDa in both the sonicated and unsonicated CPIP samples. In comparison to the sonicated CPIP samples, the untreated (control) had shown a lesser intensity with some of the bands, visually. In all the CPIPs samples, 55–60 kDa had the highest intensity that accounts for the vicilin α + β + γ fractions and legumin α + β fractions. Vicilin is the most abundant storage protein in cowpea, consisting of up to 88% of total globulins [39].
Fig. 8.
SDS images of cowpea protein isolate powder samples of control (untreated), sonicated for 10 min, 15 min at 200 W, and sonicated for 15 min at 100 W.
In the control untreated sample, under 15–35 kDa only fewer bands were observed but after sonication, the number of bands increased (vicilin, legumin fractions) indicating the high molecular weight proteins have reduced in molecular weight and have moved down the gel column, particularly in case of 15 min − 100 W and 10 min – 200 W. The intensity and the number of bands were highest in the US-10 min- 200 W when compared to US-15 min-200 W and US-10 min-100 W. Similarly, in a study by Malik et al. [51], a reduction in the molecular weight was also reported on protein isolates of sonicated sunflower seeds. Resendiz-Vazquez et al. [66] also found that ultrasonication altered the molecular weight of protein isolates of jackfruit seed. The reduction in the mass of sonicated protein molecules was due to the shears and physical turbulences, leading to the breaking up of structures, and further resulting in a reduction of sizes.
Also, more bands are seen above 35 kDa (vicilin α + β fractions) and very few bands were observed in the lower regions, i.e., <35 kDa for US-15 min-200 W. This may probably be due to the sonication process carried out at a higher power and a longer time leading to aggregation of protein molecules resulting in bigger molecular sizes that could not penetrate down the gel and thus not noted [79].
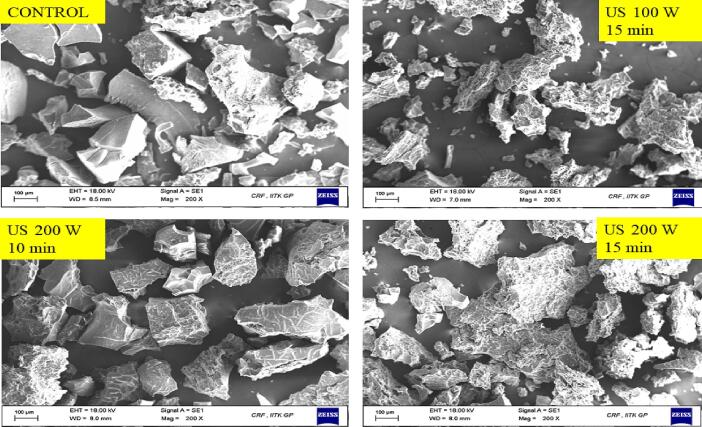
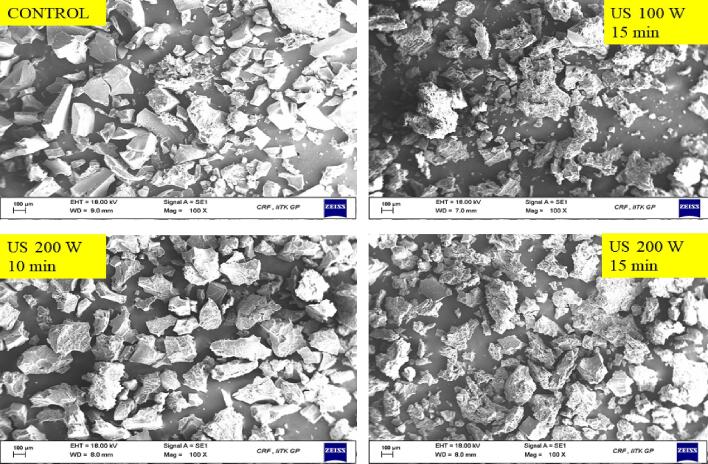
3.3.3. Scanning electron microscopy (SEM)
The structural properties of the CPIP were witnessed using microscopic observation, as illustrated in Fig. 9. The surface morphology of untreated CPIP (control) and treated CPIP of US 15 min – 100 W, US 10 min – 200 W, and US 15 min – 200 W were observed at different magnification levels at 100x and 200x. The untreated CPI showed an open structure like flakes (Fig. 9a), which was similar to that Rudra et al. [43], had observed. Fig. 9(a) depicts a collection of SEM pictures at a magnification of 100x. Various sizes were observed with structures like flakes/sheets in every CPIP sample. Every CPIP sample upon sonication treatment showed relatively smaller particles in comparison to control CPIP, suggesting that the US shear forces and physical effects might have broken down the extracted and dried matrix which is made primarily of 89–90% proteins.
Fig. 9.
Scanning electron microscopy images of cowpea protein isolate powder samples of control (untreated), sonicated for 10 min, 15 min at 200 W, and sonicated for 15 min at 100 W (a) 100X (b) 200X.
Fig. 9(b) depicts a collection of SEM pictures at a magnification of 200x. As compared to the control CPIP, the treated CPIP had shown rougher and more irregular surfaces which can be well observed in Fig. 9(b). Similar observations were reported in black bean protein isolates [80] and quinoa protein isolates [62]. Mir et al. [62] described that the roughness and the geometry of the quinoa protein isolates are influenced by the sonication time, i.e., an increase in sonication time showed more irregular and rough surfaces in comparison to the lower processing time.
Comparably, Jiang et al. [80] also observed a comparable outcome with the black bean protein isolates and related it to acoustic cavitations generated by the probe through sonication and the further physical effects like microstreaming and turbulent forces in liquids. Morphology of CPIP of US of 10 min- 200 W was found to be more even compared to other samples, implying that moderate sonication duration was able to create uniform configurations than prolonged sonication duration. This alteration is most likely caused by an increase in surface hydrophobic groups and the unfolding of proteins of the CPIP molecules.
Secondly, in Fig. 9(b) a few aggregated structures of larger size were microscopically observed in the sample with 15 min sonication treatment in both the power level i.e., 100 W and 200 W. The sonication beyond the US 10 min – 200 W correspondingly altered the geometry of protein however, it leads to protein fragments of larger size. Even at the lower power level at 100 W with sonication treatment of 15 min showed aggregation in the CPIP. Jiang et al. [80], also observed a similar change where sonication for a prolonged time resulted in an incomplete unfolding of black-bean proteins which led to the exposure of functional groups (such as hydrophobic groups) which then interacted with one another, resulting in protein aggregation and network development. Mir et al. (50) discovered that the geometry of quinoa protein isolates changed depending on the length of sonication action.
These modifications might have perhaps taken place as a result of the turbulence force generated throughout sonication, which exposed hydrophobic and free sulfhydryl groups onto the stretched protein surface resulting in the creation of a bigger protein aggregate. Another investigation on the extraction of walnut protein isolate by sonication by Lv et al. observed that the globulin proteins had bigger dimensions than the untreated ones [59]. The globulins being the major content in pulse-based plant proteins, UAE promoted protein unfolding leading to exposure of hydrophobic regions and free sulfhydryl, followed by the development of aggregates. The above-mentioned modifications may have an impact on the protein’s functionality, which is observed in this study.
3.3.4. Zeta potential (ζ-potential) and particle size
Zeta potential is the imbalance that occurs across the solid particle’s surface and the liquid in which it is submerged. If an emulsion is farther from the zero point and has a high zeta potential (positive or negative), it has the potential to remain electrically stable. It may be unstable if it has a lower zeta potential (prone to easily coagulating or flocculating) [19]. The zeta potential of the control CPIP sample and US- 10 min- 200 W was determined. The magnitude of the CPIP sample’s zeta potential after ultrasonication was greater compared to the sample before ultrasonication, as depicted in Table 3.
Table 3.
Zeta potential and Particle Size of cowpea protein isolate powder of control (untreated) and ultrasound assisted extraction (200 W/10 min).
| CPIP sample | Zeta potential | Particle size |
|---|---|---|
| Control | –32.9 ± 0.08b mV | 763 ± 0.81a nm |
| Ultrasound treated | −44.2 ± 0.16a mV | 559 ± 0.40b nm |
Different superscripts within same column are statistically significantly different at p < 0.05.
Ultrasonication enhanced the zeta potential of the CPIP from –32.9 mV (attained with alkaline extraction) to −44.2 mV. Similar findings were reported in chickpea protein isolate [30], black gram protein isolate [28], and millet protein concentrate [68]. Kang et al. [30] suggested that the improvement in zeta potential following ultrasonication could be attributed to more negatively charged protein groups being exposed.
According to Sun et al. [81], ultrasound may cause charged groups previously concealed in protein structures to be exposed, increasing the amplitude of zeta potential. The same author also stated that because of the enhanced zeta potential, ultrasonicated protein isolates may generate emulsions and scatter with improved stability, which is advantageous in mayonnaise, beverages, and salad dressing compositions. Furthermore, the particle size of the samples (Table 3) correlates with the above theories. The particle size of CPIP (763 nm) was substantially different (p < 0.05) from that of ultrasonicated CPIP (559 nm), and this attribute may contribute to the reduced zeta potential of CPIP samples. Similarly, ultrasonic processing reduced the particle size of protein isolates of peanut [82], soy [83], and milk protein concentrate [81]. Sun et al. [81], suggested that this may be related to turbulent forces, micro streamings, and cavitations during ultrasound treatment. Despite this, Zhao et al. [84] claimed that higher ultrasonic treatment power and long processing times cause the particle size of protein to rise, implying the development of tiny aggregates. Additionally, extended sonication (>40 min) drastically expanded the size of bovine serum albumin particles, indicating the production of tiny aggregates [85]. Proteins with smaller particle sizes and higher zeta potential are preferred because they generate more stable emulsions and prevent coagulation. Sonication of pea protein isolates resulted in reduced particle size and boosted antioxidant potential, especially DPPH as well as hydroxyl free radical scavenging capabilities [86].
3.4. In vitro digestibility
Since all the functional properties of protein showed a better value for US treatment of 200 W at different processing times in comparison to 100 W sonicated samples, the US-treated 200 W samples were further tested for studies on in vitro digestibility. The results of the in vitro digestibility of CPIPs treated with US of 200 W treatment are shown in Table 4. The control CPIP sample’s in vitro digestibility in the present research was 88.27%, which was within the range reported by Elharadallou et al. [44], which is 87%, and Affrifah et al. [54], which is 89%. In the US 5 min – 200 W, the digestibility of treated CPIP has not shown a significant change from that of the control sample. This observation may be owing to the shorter sonication period, which is insufficient to produce the required conformational change in the surface hydrophobicity of proteins and thus the improved digestibility. The sonication duration was extended from 5 min to 10 min, resulting in a significant (p < 0.05) improvement in digestibility from 88.68% to 89.99%.
Table 4.
In vitro digestibility of different cowpea protein isolate powder of control (untreated) and ultrasound assisted extraction at 200 W.
| Process time at US 200 W (min) | In vitro digestibility % |
|---|---|
| 0 (Control) | 88.27 ± 0.27b |
| 5 | 88.68 ± 0.23b |
| 10 | 89.99 ± 0.15a |
| 15 | 87.02 ± 0.03c |
| 20 | 86.18 ± 0.24d |
Different superscripts within same column are statistically significantly different at p < 0.05.
A comparable statement was noted by Jin et al, on buckwheat protein isolate upon sonication at 60% amplitude for 10 min, and was inferred that the change was probably due to the change in the protein conformation upon sonication leading to the exposure of the protein’s cleavage sites during digestion by the digestive enzymes [29]. According to the same author, this enhancement might be linked to a modification in the protein isolates’ molecular shape and microstructure, making it more accessible to the digesting enzyme. Based on the earlier SEM analysis, the sonicated CPIP had rougher and irregular surfaces with more homogeneous configurations, which may have influenced the protein’s in vitro digestibility. Kang et al. also observed a similar under the condition of sonication of 15 min at 600 W which showed the highest digestibility [30]. Modification to proteins brought by the sonication increased it’s in vitro digestibility, benefiting everyone’s well-being and health and contributing to the achievement of SDG 3.
From Table 4, it is been well shown that the prolonged sonication process between 15 and 20 min has shown a significant decrease in digestibility which is 87.02 % and 86.18 % respectively. Jin et al. [29] found that extending sonication beyond 10 min decreases digestion due to denaturation and renaturation of protein molecules, which reduces digestibility. Although denaturation helps digestion, the successive renaturation makes the protein resistant to breakdown. Because the protein denaturation-renaturation cycle is in equilibrium, sonication can trigger denaturation.
Furthermore, prolonged sonication can cause protein aggregation development [19]. The digestibility of buckwheat protein isolate was dramatically lowered after a 20 min sonication treatment, according to the same author [19]. Similarly, Wang et al. [87] also witnessed that increasing the sonication time from 20 to 50 min with a 10 min interval reduced the degree of oat protein hydrolysis caused by the refolding and concealment of protein’s cleavage sites in the core, which lowers digestibility. Thus, it is very clear that the in vitro digestibility data show an increasing trend until 10 min of processing time followed by a decreasing trend until 20 min at 200 W ultrasound treatment.
4. Conclusion
A plant protein was isolated from cowpea pulse as an alternative to animal-based protein using ultrasound technology in varied power and time (100 W and 200 W for 5, 10, 15, and 20 min), and its techno-functional qualities were studied. When the CPIP was subjected to sonication for 10 min at 200 W and 15 min at 100 W, respectively, in comparison with the control sample, the extraction yield was increased by 85.53% and 61.77%. High ultrasound power generates more sonic bubbles, which at the same time collapse more quickly through the extraction solvent. Additionally, the acoustic cavitations produced by this process result in the penetration of solvent into the cell matrix, disturbing the bonds between molecules and speeding up the rate of mass transfer rate and extraction. Being an underutilized and cheap protein source, cowpeas have a lot of potential for protein extraction through the US, which may then be further investigated to combat protein-energy malnutrition. With the UAE, time and energy may be saved, and also addresses SDG 7 which includes being environmentally friendly, decreasing energy use, and encouraging sustainable energy. The UAE CPIP sample treated with 200 W-10 min exhibited the best functional characteristics. Again, sonication for 15 min was recorded to show the highest functional properties among the CPIP samples when treated at 100 W. The unfolding of the protein shape and the more even distribution of protein particles resulting from the sonic cavitation and shearing forces contribute to the increased functional characteristics of the protein. Additionally, the UAE CPIP showed better zeta potential and decreased particle size, which might have helped the protein isolate function more efficiently. Results from SDS PAGE also support the reduced protein particle size. Contrarily, excessive sonication lowered functional properties due to protein re-polymerization, which causes aggregation and re-masks the hydrophobic part of the protein. Thus, compared to the traditional alkaline extraction approach, cowpea protein may be optimally extracted by UAE, yielding a greater protein yield and superior functional properties promoting health and nutrition (SDG 2 and 3). Consumption of pulses contributes to combating climate change (SDG 13) since it accounts for a much smaller environmental footprint than sources of animal protein. Legume cultivation has great potential to support soil fertility in cropping systems with high levels of productivity supporting sustainable agriculture (SDG 2). Promoting the use and potential of cowpea protein can therefore lead to more widespread use of plant proteins as a partial replacement for animal proteins when all the aforementioned factors are taken into consideration.
CRediT authorship contribution statement
Geetarani Loushigam: Writing – original draft. Akalya Shanmugam: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank the National Institute of Food Technology, Entrepreneurship, and Management - Thanjavur, Ministry of Food Processing Industries, Government of India for providing knowledge and support.
Data availability
The data that has been used is confidential.
References
- 1.Boye J., Zare F., Pletch A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010;43:414–431. doi: 10.1016/J.FOODRES.2009.09.003. [DOI] [Google Scholar]
- 2.Hall C., Hillen C., Robinson J.G. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017;94:11–31. doi: 10.1094/CCHEM-03-16-0069-FI. [DOI] [Google Scholar]
- 3.F. Food and Agriculture Organization, Crop Production and Trade Data., (2021). https://www.fao.org/faostat/en/#data/QI/visualize (accessed April 2, 2022).
- 4.Magrini M.B., Anton M., Chardigny J.M., Duc G., Duru M., Jeuffroy M.H., Meynard J.M., Micard V., Walrand S. Pulses for sustainability: breaking agriculture and food sectors out of lock-in. Front. Sustain. Food Syst. 2018;2:1–17. doi: 10.3389/fsufs.2018.00064. [DOI] [Google Scholar]
- 5.Poore J., Nemecek T. Reducing food’s environmental impacts through producers and consumers. Science. 2018;80:987–992. doi: 10.1126/SCIENCE.AAQ0216. [DOI] [PubMed] [Google Scholar]
- 6.Nikbakht Nasrabadi M., Sedaghat Doost A., Mezzenga R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021;118 doi: 10.1016/J.FOODHYD.2021.106789. [DOI] [Google Scholar]
- 7.Moll P., Salminen H., Schmitt C., Weiss J. Impact of microfluidization on colloidal properties of insoluble pea protein fractions. Eur. Food Res. Technol. 2021;247:545–554. doi: 10.1007/S00217-020-03629-2/FIGURES/7. [DOI] [Google Scholar]
- 8.Kumar M., Tomar M., Potkule J., Verma R., Punia S., Satankar V., Bhoite A.G., Amarowicz R., Kaur C., Kennedy J.F. Advances in the plant protein extraction: mechanism and recommendations. Food Hydrocoll. 2021;115 doi: 10.1016/j.foodhyd.2021.106595. [DOI] [Google Scholar]
- 9.Jebitta S.R., Durga Devi P.R., Deva Dharshini L., Harika T.N.S., Vignesh K. A comprehensive review on protein isolates from legumes. Int. J. Recent Technol. Eng. 2021;9(6):215–222. doi: 10.35940/ijrte.f5523.039621. [DOI] [Google Scholar]
- 10.Choi I., Cho S.J., Chun J.K., Moon T.W. Extraction yield of soluble protein and microstructure of soybean affected by microwave heating. J. Food Process. Preserv. 2006;30:407–419. doi: 10.1111/J.1745-4549.2006.00075.X. [DOI] [Google Scholar]
- 11.Liang R., Cheng S., Wang X. Secondary structure changes induced by pulsed electric field affect antioxidant activity of pentapeptides from pine nut (Pinus koraiensis) protein. Food Chem. 2018;254:170–184. doi: 10.1016/j.foodchem.2018.01.090. [DOI] [PubMed] [Google Scholar]
- 12.Lu W., Chen X.W., Wang J.M., Yang X.Q., Qi J.R. Enzyme-assisted subcritical water extraction and characterization of soy protein from heat-denatured meal. J. Food Eng. 2016;169:250–258. doi: 10.1016/J.JFOODENG.2015.09.006. [DOI] [Google Scholar]
- 13.Malik M.A., Saini C.S. Gamma irradiation of alkali extracted protein isolate from dephenolized sunflower meal. LWT - Food Sci. Technol. 2017;84:204–211. doi: 10.1016/J.LWT.2017.05.067. [DOI] [Google Scholar]
- 14.Jung S., Mahfuz A.A. Low temperature dry extrusion and high-pressure processing prior to enzyme-assisted aqueous extraction of full fat soybean flakes. Food Chem. 2009;114:947–954. doi: 10.1016/J.FOODCHEM.2008.10.044. [DOI] [Google Scholar]
- 15.Naik M., Natarajan V., Modupalli N., Thangaraj S., Rawson A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: kinetics and quality measurements. LWT - Food Sci. Technol. 2022;155 [Google Scholar]
- 16.Eckert E., Han J., Swallow K., Tian Z., Jarpa‐Parra M., Chen L. Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. 2019;96(4):725–741. doi: 10.1002/cche.10169. [DOI] [Google Scholar]
- 17.Ji H., Dong S., Han F., Li Y., Chen G., Li L., Chen Y. Effects of dielectric barrier discharge (DBD) cold plasma treatment on physicochemical and functional properties of peanut protein. Food Bioprocess Technol. 2018;11:344–354. doi: 10.1007/s11947-017-2015-z. [DOI] [Google Scholar]
- 18.Pojić M., Mišan A., Tiwari B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018;75:93–104. doi: 10.1016/J.TIFS.2018.03.010. [DOI] [Google Scholar]
- 19.Rahman M.M., Lamsal B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021;20:1457–1480. doi: 10.1111/1541-4337.12709. [DOI] [PubMed] [Google Scholar]
- 20.Mccarthy N.A., Kennedy D., Sean I.N.S., Ins A.S.A., Kelly P.M., Thapa K., Murphy K.M., Fenelon M.A. Emulsification properties of pea protein isolate using homogenization, microfluidization and ultrasonicat. Food Res. Int. 2016 doi: 10.1016/j.foodres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Jiang S., Ding J., Andrade J., Rababah T.M., Almajwal A., Abulmeaty M.M., Feng H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017;38:835–842. doi: 10.1016/J.ULTSONCH.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Wang F., Zhang Y., Xu L., Ma H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT - Food Sci. Technol. 2020;127 doi: 10.1016/J.LWT.2020.109348. [DOI] [Google Scholar]
- 23.Preece K.E., Hooshyar N., Krijgsman A., Fryer P.J., Zuidam N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. 2017;113:94–101. doi: 10.1016/j.cep.2016.09.003. [DOI] [Google Scholar]
- 24.Eze O.F., Chatzifragkou A., Charalampopoulos D. Properties of protein isolates extracted by ultrasonication from soybean residue (okara) Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130837. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Velasco A., Lobato-Calleros C., Hernández-Rodríguez B.E., Román-Guerrero A., Alvarez-Ramirez J., Vernon-Carter E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018;44:97–105. doi: 10.1016/j.ultsonch.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Dattatray T.R., Surendra Babu A., Jagan Mohan R. Effect of pre-treatments on sprouting rate and nutritional quality of green gram (Vigna radiata L.) malt. J. Food Process. Preserv. 2020;44:1–12. doi: 10.1111/jfpp.14354. [DOI] [Google Scholar]
- 27.Aguilar-Acosta L., Serna-Saldivar S., Rodríguez-Rodríguez J., Escalante-Aburto A., Chuck-Hernández C. Effect of ultrasound application on protein yield and fate of alkaloids during lupin alkaline extraction process. Biomolecules. 2020;10(2):292. doi: 10.3390/biom10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamani M.H., Semwal J., Meera M.S. Functional modification of protein extracted from black gram by-product: effect of ultrasonication and micronization techniques. LWT - Food Sci. Technol. 2021;144 doi: 10.1016/j.lwt.2021.111193. [DOI] [Google Scholar]
- 29.Jin J., Okagu O.D., Yagoub A.E.A., Udenigwe C.C. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrason. Sonochem. 2021;70 doi: 10.1016/J.ULTSONCH.2020.105348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang S., Zhang J., Guo X., Lei Y., Yang M. Effects of ultrasonic treatment on the structure, functional properties of chickpea protein isolate and its digestibility in vitro. Foods. 2022;11:880. doi: 10.3390/FOODS11060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singhal A., Karaca A.C., Tyler R., Nickerson M. Pulse Proteins: From Processing to Structure-Function Relationships. Grain Legum. 2012;13:55–78. doi: 10.5772/64020. [DOI] [Google Scholar]
- 32.McClements D.J., Grossmann L. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci. Food. 2021;17:1–10. doi: 10.1038/s41538-021-00099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S. Kumar, V. Kumar, R. Sharma, A.A. Paul, P. Suthar, R. Saini, S. Kumar, V. Kumar, R. Sharma, A.A. Paul, P. Suthar, R. Saini, Plant Proteins as Healthy, Sustainable and Integrative Meat Alternates, Veganism - a Fash. Trend or Food as a Med. (2020) 1-19. https://doi.org/10.5772/INTECHOPEN.94094.
- 34.Kyriakopoulou K., Keppler J.K., van der Goot A.J. Functionality of ingredients and additives in plant-based meat analogues. Foods. 2021;10:600. doi: 10.3390/FOODS10030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hertzler S.R., Lieblein-Boff J.C., Weiler M., Allgeier C. Plant proteins: assessing their nutritional quality and effects on health and physical function. Nutrients. 2020;12:1–27. doi: 10.3390/NU12123704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Indicators S. Carbon Footprint Factsheet. Cent. Sustain. Syst. Univ. Michigan. 2018:19–20. [Google Scholar]
- 37.E. da S. Alves, L.A. da Silva, B.H.F. Saqueti, C.A.F. Artilha, D. de M.B. da Silva, L.C.S. de Sousa, M.R. da S. Scapim, J.V. Visentainer, Vegetable proteins as functional foods – review, Brazilian J. Dev. 6 (2020) 5869–5879. https://doi.org/10.34117/BJDV6N2-043.
- 38.Kebede E., Bekeko Z., Tejada Moral M. Expounding the production and importance of cowpea (Vigna unguiculata (L.) Walp.) in Ethiopia. Cogent Food Agric. 2020;6(1):1769805. doi: 10.1080/23311932.2020.1769805. [DOI] [Google Scholar]
- 39.Jayathilake C., Visvanathan R., Deen A., Bangamuwage R., Jayawardana B.C., Nammi S., Liyanage R. Cowpea: an overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018;98:4793–4806. doi: 10.1002/JSFA.9074. [DOI] [PubMed] [Google Scholar]
- 40.Cicero A.F.G., Fogacci F., Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br. J. Pharmacol. 2017;174(11):1378–1394. doi: 10.1111/BPH.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trehan I., Benzoni N.S., Wang A.Z., Bollinger L.B., Ngoma T.N., Chimimba U.K., Stephenson K.B., Agapova S.E., Maleta K.M., Manary M.J. Common beans and cowpeas as complementary foods to reduce environmental enteric dysfunction and stunting in Malawian children: Study protocol for two randomized controlled trials. Trials. 2015;16:1–12. doi: 10.1186/S13063-015-1027-0/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frota K.de M.G., dos Santos R.D., Ribeiro V.Q., Arêas J.A.G. Cowpea protein reduces LDL-cholesterol and apolipoprotein B concentrations, but does not improve biomarkers of inflammation or endothelial dysfunction in adults with moderate hypercholesterolemia. Nutr. Hosp. 2015;31:1611–1619. doi: 10.3305/NH.2015.31.4.8457. [DOI] [PubMed] [Google Scholar]
- 43.Rudra S.G., Sethi S., Jha S.K., Kumar R. Physico-chemical and functional properties of cowpea protein isolate as affected by the dehydration technique. Legum. Res. 2016;39:370–378. doi: 10.18805/LR.V0IOF.9441. [DOI] [Google Scholar]
- 44.Elharadallou S.B., Khalid I.I., Gobouri A.A., Abdel-Hafez S.H. Amino acid composition of cowpea (Vigna ungiculata L. Walp) flour and its protein isolates. Food Nutr. Sci. 2015;6:790–797. doi: 10.4236/FNS.2015.69082. [DOI] [Google Scholar]
- 45.Mune Mune M.A., Minka S.R. Production and characterization of cowpea protein hydrolysate with optimum nitrogen solubility by enzymatic hydrolysis using pepsin. J. Sci. Food Agric. 2017;97:2561–2568. doi: 10.1002/JSFA.8076. [DOI] [PubMed] [Google Scholar]
- 46.Adetutu Adeyoju O., Oyebode Adebowale K., Iromidayo olu-owolabi B., Okwudili Chibudike H., Chibudike E.C. Nutritional and functional properties of flour and protein isolates from germinated Solojo Cowpea Vigna unguiculata (L.) Walp. J. Food Sci. Nutr. Res. 2021;4(2) doi: 10.26502/JFSNR.2642-11000069. [DOI] [Google Scholar]
- 47.Khalid I.I., Balla E.S. Functional properties of cowpea (Vigna ungiculata L. Walp), and Lupin (Lupinus Termis) flour and protein isolates. J. Nutr. Food Sci. 2013;3 doi: 10.4172/2155-9600.1000234. [DOI] [Google Scholar]
- 48.C. AOAC, Official methods of analysis of the Association of Analytical Chemists International., Off. Methods Gaithersburg, MD, USA. (2005). https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Official+methods+of+analysis+of+the+Association+of+Analytical+Chemists+International&btnG= (accessed May 29, 2022).
- 49.Rincon L., Braz Assunção Botelho R., de Alencar E.R. Development of novel plant-based milk based on chickpea and coconut. LWT - Food Sci. Technol. 2020;128 doi: 10.1016/J.LWT.2020.109479. [DOI] [Google Scholar]
- 50.Wani I.A., Sogi D.S., Gill B.S. Physico-chemical and functional properties of native and hydrolysed protein isolates from Indian black gram (Phaseolus mungo L.) cultivars. LWT - Food Sci. Technol. 2015;60:848–854. doi: 10.1016/J.LWT.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malik M.A., Sharma H.K., Saini C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: effect on physicochemical and functional properties. Ultrason. Sonochem. 2017;39:511–519. doi: 10.1016/J.ULTSONCH.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 52.González-Ortega R., Faieta M., Di Mattia C.D., Valbonetti L., Pittia P. Microencapsulation of olive leaf extract by freeze-drying: effect of carrier composition on process efficiency and technological properties of the powders. J. Food Eng. 2020;285 doi: 10.1016/J.JFOODENG.2020.110089. [DOI] [Google Scholar]
- 53.Almeida C.C., Lúcia M., Monteiro G., Reis B., Alvares T.S., Conte-junior C.A. In vitro digestibility of commercial whey protein supplements. LWT - Food Sci. Technol. 2014 doi: 10.1016/j.lwt.2014.11.038. [DOI] [Google Scholar]
- 54.Affrifah N.S., Phillips R.D., Saalia F.K. Cowpeas: nutritional profile, processing methods and products—A review. Legum. Sci. 2021:131. doi: 10.1002/LEG3.131. [DOI] [Google Scholar]
- 55.Moulton K.J., Wang L.C. A pilot-plant study of continuous ultrasonic extraction of soybean protein. J. Food Sci. 1982;47:1127–1129. doi: 10.1111/J.1365-2621.1982.TB07632.X. [DOI] [Google Scholar]
- 56.Tang C.H., Wang X.Y., Yang X.Q., Li L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food Eng. 2009;92:432–437. doi: 10.1016/J.JFOODENG.2008.12.017. [DOI] [Google Scholar]
- 57.Wang J.-y., Yang Y.-L., Tang X.-Z., Ni W.-x., Zhou L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrason. Sonochem. 2017;38:225–233. doi: 10.1016/J.ULTSONCH.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Hu H., Wu J., Li-Chan E.C.Y., Zhu L., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. doi: 10.1016/J.FOODHYD.2012.08.001. [DOI] [Google Scholar]
- 59.Lv S., Taha A., Hu H., Lu Q., Pan S. Effects of ultrasonic-assisted extraction on the physicochemical properties of different walnut proteins. Molecules. 2019;24(23):4260. doi: 10.3390/MOLECULES24234260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu C., Wu F., Cha Y., Zou H., Guo Y., Piao H., Du M. Structural and functional changes in ultrasonicated oyster protein isolates. Int. J. Food Eng. 2019;15 doi: 10.1515/IJFE-2018-0190. [DOI] [Google Scholar]
- 61.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: ultrasound-treated soybean protein isolate. LWT - Food Sci. Technol. 2021;142 doi: 10.1016/J.LWT.2021.110881. [DOI] [Google Scholar]
- 62.Mir N.A., Riar C.S., Singh S. Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochem. 2019;58 doi: 10.1016/J.ULTSONCH.2019.104700. [DOI] [PubMed] [Google Scholar]
- 63.Choudhary P., Rawson A. Impact of power ultrasound on the quality attributes of curd and its fermentation/gelation kinetics. J. Food Process Eng. 2021;44:13698. doi: 10.1111/JFPE.13698. [DOI] [Google Scholar]
- 64.Zuñiga-Salcedo M.R., Ulloa J.A., Bautista-Rosales P.U., Rosas-Ulloa P., Ramírez-Ramírez J.C., Silva-Carrillo Y., Gutiérrez-Leyva R., Hernández C. Effect of ultrasound treatment on physicochemical, functional and nutritional properties of a safflower (Carthamus tinctorius L.) protein isolate. Ital. J. Food Sci. 2019;31:591–603. doi: 10.14674/IJFS-1440. [DOI] [Google Scholar]
- 65.Biswas B., Sit N. Effect of ultrasonication on functional properties of tamarind seed protein isolates. J. Food Sci. Technol. 2020;57:2070–2078. doi: 10.1007/S13197-020-04241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Resendiz-Vazquez J.A., Ulloa J.A., Urías-Silvas J.E., Bautista-Rosales P.U., Ramírez-Ramírez J.C., Rosas-Ulloa P., González-Torres L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017;37:436–444. doi: 10.1016/J.ULTSONCH.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 67.Xue F., Zhu C., Liu F., Wang S., Liu H., Li C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. Food Agric. 2018;98:5690–5699. doi: 10.1002/JSFA.9116. [DOI] [PubMed] [Google Scholar]
- 68.Nazari B., Mohammadifar M.A., Shojaee-Aliabadi S., Feizollahi E., Mirmoghtadaie L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018;41:382–388. doi: 10.1016/J.ULTSONCH.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Mirmoghtadaie L., Shojaee Aliabadi S., Hosseini S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016;199:619–627. doi: 10.1016/J.FOODCHEM.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 70.Téllez-Morales J.A., Hernández-Santo B., Rodríguez-Miranda J. Effect of ultrasound on the techno-functional properties of food components/ingredients: a review. Ultrason. Sonochem. 2020;61 doi: 10.1016/J.ULTSONCH.2019.104787. [DOI] [PubMed] [Google Scholar]
- 71.Gharibzahedi S.M.T., Smith B. The functional modification of legume proteins by ultrasonication: a review. Trends Food Sci. Technol. 2020;98:107–116. doi: 10.1016/J.TIFS.2020.02.002. [DOI] [Google Scholar]
- 72.Wu D.i., Tu M., Wang Z., Wu C., Yu C., Battino M., El-Seedi H.R., Du M. Biological and conventional food processing modifications on food proteins: Structure, functionality, and bioactivity. Biotechnol. Adv. 2020;40 doi: 10.1016/J.BIOTECHADV.2019.107491. [DOI] [PubMed] [Google Scholar]
- 73.Lafarga T., Álvarez C., Bobo G., Aguiló-Aguayo I. Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT - Food Sci. Technol. 2018;98:106–112. doi: 10.1016/J.LWT.2018.08.033. [DOI] [Google Scholar]
- 74.Sun X., Zhang W., Zhang L., Tian S., Chen F. Effect of ultrasound-assisted extraction on the structure and emulsifying properties of peanut protein isolate. J. Sci. Food Agric. 2021;101:1150–1160. doi: 10.1002/JSFA.10726. [DOI] [PubMed] [Google Scholar]
- 75.Adenekan M.K., Fadimu G.J., Odunmbaku L.A., Oke E.K. Effect of isolation techniques on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci. Nutr. 2017;6:146–152. doi: 10.1002/FSN3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shevkani K., Singh N., Chen Y., Kaur A., Yu L. Pulse proteins: secondary structure, functionality and applications. J. Food Sci. Technol. 2019;56 doi: 10.1007/S13197-019-03723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.SigmaAldrich, IR Spectrum Table & Chart, Sigma Aldrich. (2021) 18. https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html.
- 78.Kingwascharapong P., Chaijan M., Karnjanapratum S. Ultrasound - assisted extraction of protein from Bombay locusts and its impact on functional and antioxidative properties. Sci. Rep. 2021:1–14. doi: 10.1038/s41598-021-96694-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shanmugam A., Chandrapala J., Ashokkumar M. The effect of ultrasound on the physical and functional properties of skim milk. Innov. Food Sci. Emerg. Technol. 2012;16:251–258. doi: 10.1016/J.IFSET.2012.06.005. [DOI] [Google Scholar]
- 80.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. doi: 10.1016/J.FOODRES.2014.04.022. [DOI] [Google Scholar]
- 81.Sun Y., Chen J., Zhang S., Li H., Lu J., Liu L., Uluko H., Su Y., Cui W., Ge W., Jiaping L. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014;124:11–18. doi: 10.1016/J.JFOODENG.2013.09.013. [DOI] [Google Scholar]
- 82.Zhang Q.T., Tu Z.C., Xiao H., Wang H., Huang X.Q., Liu G.X., Liu C.M., Shi Y., Fan L.L., Lin D.R. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process. 2014;92:30–37. doi: 10.1016/J.FBP.2013.07.006. [DOI] [Google Scholar]
- 83.Arzeni C., Martínez K., Zema P., Arias A., Pérez O.E., Pilosof A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012;108:463–472. doi: 10.1016/J.JFOODENG.2011.08.018. [DOI] [Google Scholar]
- 84.Zhao H., Shen C., Wu Z., Zhang Z., Xu C. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020;44:e13157. doi: 10.1111/JFBC.13157. [DOI] [PubMed] [Google Scholar]
- 85.Gülseren I., Güzey D., Bruce B.D., Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007;14:173–183. doi: 10.1016/J.ULTSONCH.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 86.Ge J., Sun C.X., Corke H., Gul K., Gan R.Y., Fang Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020;19:1835–1876. doi: 10.1111/1541-4337.12573. [DOI] [PubMed] [Google Scholar]
- 87.Wang B., Atungulu G.G., Khir R., Geng J., Ma H., Li Y., Wu B. Ultrasonic treatment effect on enzymolysis kinetics and activities of ACE-inhibitory peptides from oat-isolated protein. Food Biophys. 2014;10:244–252. doi: 10.1007/S11483-014-9375-Y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.