Abstract
Proton Pump Inhibitors are used widely to manage many gastric acid-related conditions such as gastroesophageal disease, gastritis, esophagitis, Barrett’s esophagus, Zollinger-Ellison syndrome, peptic ulcer disease, nonsteroidal anti-inflammatory drug-associated ulcers, and Helicobacter pylori eradication, around the globe. This review article focuses on adverse effects associated with the long-term use of proton pump inhibitors. Various observational studies, clinical trials, and meta-analyses have established the adverse effects associated with the long-term use of proton pump inhibitors including renal disorders (acute interstitial nephritis, acute kidney injury, chronic kidney disease, and end-stage renal disease), cardiovascular risks (major adverse cardiovascular events, myocardial infarction, stent thrombosis, and stroke), fractures, infections (Clostridium difficile infection, community-acquired pneumonia, and Coronavirus disease 2019), micronutrient deficiencies (hypomagnesemia, anemia, vitamin B12 deficiency, hypocalcemia, hypokalemia), hypergastrinemia, cancers (gastric cancer, pancreatic cancer, colorectal cancer, hepatic cancer), hepatic encephalopathy, and dementia. Clinicians including prescribers and pharmacists should be aware of the adverse effects of taking proton pump inhibitors for an extended period of time. In addition, the patients taking proton pump inhibitors for long-term should be monitored for the listed adverse effects. The American Gastroenterological association recommends a few non-pharmacological measures and the use of histamine 2 blockers to lessen gastrointestinal symptoms of gastroesophageal reflex disease and the utilization of proton pump inhibitors treatment if there is a definitive indication. Additionally, the American Gastroenterological association’s Best Practice Advice statements emphasize deprescribing when there is no clear indication for proton pump inhibitors therapy.
Keywords: Proton Pump Inhibitors, Cardiovascular Diseases, Risk Factors
INTRODUCTION
Proton Pump Inhibitors (PPIs) are antisecretory agents that are used widely to diminish acid secretion. PPIs are prescribed commonly to manage gastric acid-related conditions such as gastroesophageal reflex disease (GERD), gastritis, esophagitis, Barrett esophagus, Zollinger-Ellison syndrome, peptic ulcer disease, nonsteroidal anti-inflammatory drug-associated ulcers, and Helicobacter pylori (H.pylori) eradication, around the globe.1 Current PPIs may include omeprazole, esomeprazole, lansoprazole, dexlansoprazole, pantoprazole and rabeprazole.2 PPIs diminish acid secretion by binding covalently to sulfhydryl groups of cysteines of proton pump in parietal cells of stomach, thereby inactivating H+/K+-ATPase (Proton pump).3
The most common side effects of PPIs may include headache, constipation, diarrhea, nausea and vomiting.4 In addition, long-term use of PPIs found to be associated with some serious and rare adverse effects including kidney diseases (acute kidney injury, acute interstitial nephritis, chronic kidney disease, end stage renal disease), cardiovascular disease (myocardial infarction, stroke), liver disease (hepatocellular carcinoma), fractures, infections (Clostridioides difficile infection, Community-acquired pneumonia, COVID-19), micronutrient deficiencies (hypomagnesemia, anemia, vitamin B12 deficiency, hypocalcemia), dementia, and gastric cancer.5
Inappropriate use (overuse or misuse) of PPIs enhances the healthcare cost as well as the risk of polypharmacy and numerous PPI-associated adverse effects. The use of PPIs is increased exponentially in recent decades. Approximately half of the PPI prescriptions found to be with inappropriate indications.6,7 PPIs are the most widely used drugs around the globe and they are considered one of the top ten most used drugs. Generally, PPIs are misused to prevent gastro-duodenal ulcers in patients without risk factors, overtreatment to manage functional dyspepsia, treatment with antiplatelets or anticoagulants without the risk of gastric injury, stress ulcer prophylaxis in patients not admitted in intensive care units, and steroid alone therapy.8
As per previous studies, PPIs are prescribed for up to 70% of cases without any clear indication. According to a prospective observational cross-sectional study conducted in the emergency department, almost one-third of PPI prescriptions were determined to be inappropriate.9 Similar findings were made by another prospective observational cross-sectional investigation of PPI-using hospitalized patients, which found that almost half of the patients had received their prescriptions for erroneous conditions.10
The GI symptoms could be managed non-pharmacologically by various measures including avoidance of meals within 2-3 hours of bedtime, elevation of head of bed, weight loss, cessation of smoking or tobacco products, and avoidance of dietary triggers.11
Online databases such as Medline/Pubmed/PMC, Google Scholar, Science Direct, Ebsco, Scopus, Web of science, Embase, and reference lists, were searched using keywords like proton pump inhibitors, renal diseases, acute kidney injury, acute interstitial nephritis, chronic kidney disease, end stage renal disease, cardiovascular disease, myocardial infarction, stroke, liver disease, hepatocellular carcinoma, fractures, infections, Clostridioides difficile infection, Community-acquired pneumonia, COVID-19, micronutrient deficiencies, hypomagnesemia, anemia, vitamin B12 deficiency, hypocalcemia, dementia, and gastric cancer to identify relevant publications. The articles published in English are included in this review while discarding the duplicates.
ADVERSE EFFECTS-ASSOCIATED WITH LONG-TERM PPI THERAPY
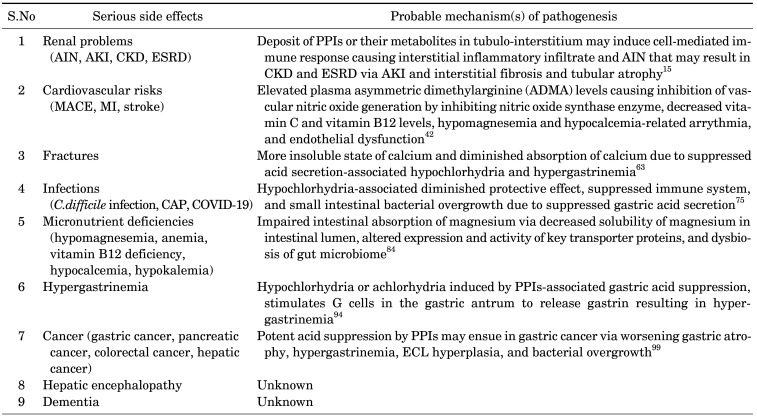
The long-term use of PPIs is associated with some serious and rare adverse effects including renal diseases (acute interstitial nephritis, acute kidney injury, chronic kidney disease, end stage renal disease), cardiovascular disease (myocardial infarction, stroke), hepatic disease (hepatocellular carcinoma), fractures, infections (Clostridioides difficile infection, Community-acquired pneumonia, COVID-19), micronutrient deficiencies (hypomagnesemia, anemia, vitamin B12 deficiency, hypocalcemia), dementia, and gastric cancer (Table 1).
TABLE 1. PPI therapy-associated adverse effects.
There are no clear definitions of long-term therapy of PPIs. However, prolonged (more than 4-8 weeks) use of PPIs for the management of heartburn, non-erosive esophagitis, mild or moderate GERD could be considered as long-term use of PPIs.12
That said, the patients with Barrett’s esophagus, severe esophagitis, and history of bleeding gastrointestinal ulcers and the users of long-term non-steroidal anti-inflammatory drugs (NSAIDs) may need long-term therapy of PPIs.7,11 Consequently, such patients should be monitored for adverse effects associated with the use of long-term PPI therapy.
To avoid inappropriate use of PPIs, the patients who initiated PPI therapy for uninvestigated dyspepsia should be evaluated within 2-4 weeks. The deprescribing of PPI therapy should be considered for the patients who took 4-8 weeks of PPIs and resolved their symptoms and for the patients who have no definitive indication.7
The risk of PPI-associated adverse effects is higher among the patients with advanced age, comorbid conditions, concomitant medications, and others. The elderly population is already at risk of developing many complications that could be aggravated by PPI therapy. Regular monitoring is indicated in elderly PPI users to determine the need of PPI therapy continuation.13
Few concomitant medications such as metformin, diuretics, etc. may enhance PPI-associated adverse effects. Concurrent use of PPI and metformin may lead to diminished absorption of vitamin B12 resulting in vitamin B12 deficiency.14 Similarly, the risk of hypomagnesemia found to be higher among the patients using a PPI and a diuretic concomitantly.
1. Renal problems
A number of studies have found a connection between PPIs and the onset of renal diseases like acute interstitial nephritis (AIN), acute kidney injury (AKI), chronic kidney disease (CKD), and end-stage renal disease (ESRD).7
Some studies have hypothesized that the accumulation of PPIs or their metabolites in the tubule-interstitium could trigger a cell-mediated immune response, resulting in an inflammatory infiltrate and AIN, which could cause AKI, interstitial fibrosis, or tubular atrophy, all of which could result in CKD and ESRD. Moreover, long-term use of PPIs is associated with hypomagnesemia that could result in endothelial cell dysfunction, accelerated endothelial senescence, enhanced oxidative stress, hyperinflammation, and vascular senescence and subsequent progression of kidney disease.15
1) Acute interstitial nephritis (AIN): An inflammatory infiltration in the renal interstitium is the defining feature of AIN, and patients may have symptoms such as oliguria, lethargy, anorexia, weight loss, nausea, and vomiting. According to a nested case-control study from New Zealand, current PPI users had a significantly higher chance of developing AIN.16 Similar to this, a number of retrospective analyses showed a link between the onset of AIN and PPIs like lansoprazole and omeprazole.17,18,19,20 Moreover, a number of cases of PPI-related AIN have been documented.
2) Acute kidney injury (AKI): Acute renal failure (ARF), often known as AKI, is the sudden or rapid loss of a kidney’s filtration ability. Reduced urine production, fluid retention, breathlessness, weakness, an irregular pulse, confusion, and nausea are some of the signs and symptoms of AKI. PPI users had a greater risk of AKI, according to many nested case-control studies.21,22 Moreover, a population-based investigation showed that starting PPI in individuals 66 years of age or older increased the incidence of AKI.23 Similar to this, many cohort studies have demonstrated the elevated risk of AKI in PPI-using individuals.24,25 In addition, a meta-analysis of cohort studies and case-control studies emphasizes that PPI users have shown a greater prevalence of AKI.26
The number of deaths, life-threatening events, hospitalizations, and disability events related to PPI use were higher due to PPI-associated AKI than PPI-associated CKD, according to analyses of adverse reports of AKI and CKD associated with the use of PPIs in the United States (US) Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database from 2004 to 2019.27,28,29
3) Chronic kidney disease (CKD): CKD is a progressive loss of renal function over time that is characterized by increased or decreased urination, fatigue, dry skin, appetite loss, anemia, muscular cramps, nausea, headaches, shortness of breath, weight loss and other symptoms.30 According to two distinct retrospective case-control designs, PPI users had an increased risk of incident CKD and death.31 Also, a long-running population-based cohort study (Atherosclerosis Risk in Communities (ARIC) study) discovered that PPI users had a greater risk of developing incident CKD.32 The use of PPIs was also linked to an increased risk of incident CKD and a drop in estimated glomerular filtration rate (eGFR), according to an analysis of national datasets from the Department of Veterans Affairs.33
Furthermore, a retrospective analysis of the Stockholm creatinine measurements database showed that the start of PPI therapy and cumulative PPI exposure increased the risk of CKD progression.34 PPIs are also linked to an increased risk of CKD in diabetic patients, according to a population-based retrospective cohort study of diabetic database data from the National Health Insurance Research Database.35 Moreover, a retrospective cohort study found that PPI use increased the risk of incident CKD.36 Also, two population-based retrospective cohort studies found that PPI users had a greater risk of incident CKD.37,38
4) End stage renal disease (ESRD): The fifth and final stage of CKD, known as ESRD, occurs when the eGFR drops below 15 ml/min. ESRD symptoms include exhaustion, itchy skin, constipation, anorexia, discomfort, disturbed sleep, anxiety, dyspnea, nausea, depression, and others.39 Among individuals with renal disorders, PPI consumption increased the incidence of ESRD, as was seen in a case-control research using the Taiwan National Health Insurance Research Database.40 Also, it was discovered through a study of national databases maintained by the Department of Veterans Affairs that PPI use increased the risk of incident CKD, CKD progression, and ESRD.41
2. Cardiovascular risks
Several studies have demonstrated a link between PPI exposure and the prevalence of cardiovascular disorders such as major adverse cardiovascular events (MACE), myocardial infarction, stent thrombosis, and stroke. PPI-associated enhanced risk of adverse cardiovascular outcomes may occur via various mechanisms including elevated plasma asymmetric dimethylarginine (ADMA) levels causing inhibition of vascular nitric oxide generation by inhibiting nitric oxide synthase enzyme, decreased vitamin C and vitamin B12 levels, hypomagnesemia and hypocalcemia-related arrythmia, and endothelial dysfunction.42
1) Major adverse cardiovascular events (MACE): Patients who took PPIs for GERD had a 70% higher risk of serious cardiovascular events, according to a meta-analysis of seventeen randomized controlled clinical trials.43 The risk of major adverse cardiovascular events (MACE), myocardial infarction, stent thrombosis, and target vessel revascularization was also raised in PPI users, according to a meta-analysis of eleven studies.44 A meta-analysis of seven observational studies also revealed a link between PPI use and an increased risk of adverse cardiovascular events.45 Another meta-analysis of fifteen randomized controlled clinical trials found that PPI users had a significantly greater risk of MACE, myocardial infarction recurrence, stent thrombosis, target vessel revascularization, and stroke.46 Also, results from a meta-analysis of 33 observational studies revealed that the risk of adverse clinical outcomes was increased in users of PPIs.47
On the other hand, a meta-analysis of sixty-six studies failed to identify a link between the use of PPIs and the emergence of MACE.48 Another meta-analysis of 19 randomized controlled clinical trials found no evidence of a significant increase in the incidence of major adverse cardiovascular and cerebrovascular events (MACCE), all-cause death, cardiovascular death, myocardial infarction, stent thrombosis, or gastroduodenal ulcer.49 Nevertheless, no discernible rise in cardiovascular adverse events linked to PPI usage was found in a meta-analysis of eleven observational studies.50
2) Myocardial infarction (MI): Increased levels of asymmetrical dimethylarginine (ADMA) caused by the use of PPIs is hypothesized to increase the risk of myocardial infarction by blocking the enzyme dimethylarginine dimerhylaminohydrolase (DDAH). Nitric oxide synthase is blocked by ADMA, which leads to a decrease in NO production, increased vascular contraction, and decreased vascular relaxation.51
An increased risk of myocardial infarction was seen in new PPI users, according to a nested case-control study from the United Kingdom (UK).52 Long-term or high-dose PPI use increased the risk of new-onset acute myocardial infarction in patients who did not have a history of ischemic heart disease, according to another nested case-control research involving 27,624 patients.53
Additionally, a meta-analysis of six randomized controlled clinical trials, two post-hoc analysis, and two observational studies showed a positive correlation between the use of PPIs and the incidence of myocardial infarction and other cardiovascular issues.54
A self-controlled case series from Hong Kong, on the other hand, found no correlation between PPI use and the occurrence of myocardial infarction.55 Moreover, a German cohort of new PPI users did not uncover any proof that PPI usage increased the risk of myocardial infarction.56
3) Stroke: The use of PPIs raises the risk of stroke by raising plasma levels of ADMA and lowering NO levels.57 Furthermore, a multivariate Cox regression analysis showed that long-term PPI usage is linked to the development of cerebral small vascular disease (SVD) and deep white matter hyperintensities (WMH), which can lead to stroke or cognitive decline.58
The risk of first-time ischemic stroke and myocardial infarction increased in long-term users of PPI, according to a retrospective Danish nationwide cohort study of 214, 998 individuals.59 The use of PPIs was found to be positively associated with an increased risk of hospitalization for ischemic stroke in another retrospective nationwide cohort study from Taiwan.60 Also, a prospective analysis of participants from the UK Biobank (492,479) revealed that the regular use of PPIs was associated with a 16% increased risk of stroke compared to non-users of PPIs.61
PPI use increases the incidence of stroke, according to a meta-analysis of 13 observational cohort studies and a case-control study.62 Also, a meta-analysis of nine high-quality randomized controlled clinical trials revealed a positive correlation between the usage of PPIs and the incidence of stroke.61
3. Fractures
The long-term use of PPIs is also linked to an increased risk of hip, spine, and wrist fractures, according to numerous earlier studies. The increased risk of fractures may be caused by more insoluble calcium and decreased calcium absorption as a result of hypochlorhydria and hypergastrinemia caused by inhibited acid secretion.63
The use of PPIs was found to slightly increase the incidence of hip, spine, and any-site fractures in a meta-analysis of eleven case-control/cohort studies.64 In a similar vein, a second meta-analysis of 18 studies, including 9 case-control studies and 9 prospective observational studies, found that PPI use increased the risk of fractures (hip, spine, and any-site fractures). PPI use has been linked to an increased incidence of hip fracture in fifteen of these studies.65 Moreover, in May 2010, the U.S. Food and Drug Administration (FDA) has issued a safety alert regarding an association between the use of PPI and elevated risk of fractures of hip, wrist and spine.66
4. Infections
Long-term PPI use has been linked to an increase in the incidence of infections such as Clostridium difficile infection, community-acquired pneumonia (CAP), and Coronavirus disease 2019 (COVID-19). PPI use affects the gut microbiota, which is necessary for reducing bacterial growth or boosting the immune system.
1) Clostridioides difficile infection: PPIs have been linked to an increased risk of Clostridioides difficile infection, according to a number of studies. As PPIs raise gastric pH, PPI-associated alkaline intestine pH may facilitate C. difficile sporulation.67
Long-term use of PPIs is positively associated with C. difficile infection, according to numerous systematic reviews and meta-analyses that examined a large number of observational studies, including case-control and cohort studies on thousands of patients.68 Additionally, a meta-analysis of ten case-control studies and six cohort studies revealed that PPI users had significantly higher rates of C. difficile recurrence.69 In addition, the FDA released a safety advisory regarding PPI-associated C. difficile infection in February 2012.70
2) Community-acquired pneumonia (CAP): Positive association of the use of PPI and a heightened risk of community-acquired pneumonia (CAP) has been established in several studies. PPI users may be at greater risk for developing CAP due to aspiration of acid-labile pathogenic bacteria and increased bacterial colonization of larynx, esophagus and lungs.71
PPI medication significantly increases the incidence of pneumonia, according to a meta-analysis of 10 randomized controlled clinical trials and 48 observational studies.72 Another meta-analysis of seven observational studies found a strong correlation between PPI use and the probability of developing CAP.73 Moreover, a meta-analysis of 13 studies, including 7 case-control studies, 4 cohort studies, and 2 observational studies, found that patients taking PPI had a higher chance of developing CAP than those who did not.74
3) Coronavirus disease 2019 (COVID-19): The use of PPIs raised the risk of the coronavirus disease 2019 (COVID-19) infection and its mortality, according to numerous research studies. Various reasons have been proposed for the association of PPIs and COVID-19 severity that include hypochlorhydria-associated diminished protective effect, enhanced survival of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) virus in stomach, suppressed immune system, and small intestinal bacterial overgrowth due to suppressed gastric acid secretion.75
According to a meta-analysis of six observational studies, current PPI users have a significantly higher risk of developing COVID-19 as well as dying from it.76 Similarly, in another meta-analysis, which included twelve observational retrospective cohort studies, the poor outcome in PPI-using patients was quantified.77 Also, a meta-analysis of four case-control studies and 10 cohort studies found a significantly enhanced risk of severity and mortality of COVID-19 infection in current users of PPIs.75 Furthermore, a meta-analysis of fifteen observational retrospective cohort studies determined a significantly higher risk of severe outcomes in patients with COVID-19 due to the current use of PPIs.78
5. Micronutrient deficiencies
The long-term use of PPIs has been linked to micronutrient deficiencies like hypomagnesemia, anemia, vitamin B12 deficiency, and hypocalcemia, according to previous studies.
1) Hypomagnesemia: There have been a number of reports regarding hypomagnesemia brought on by PPIs. Also, the U.S. Food and Drug Administration (FDA) released a safety advisory in March 2011 addressing hypomagnesemia linked to PPIs.79
A meta-analysis of nine retrospective observational studies found that using PPIs may increase the incidence of hypomagnesemia.80 Also, a meta-analysis of five cross-sectional studies, three cohort studies, and a case-control study revealed a link between using PPIs and an increased risk of hypomagnesemia.81 Furthermore, a meta-analysis of 16 observational studies, including 13 cross-sectional studies, 2 case-control studies, and 1 cohort study, discovered that patients taking PPIs significantly increased their risk of hypomagnesemia.82 On the other hand, a meta-analysis of fifteen research found a hazy relationship between PPI and the incidence of hypomagnesemia.83
PPI-associated hypomagnesemia has been linked to a number of different mechanisms, including impaired intestinal absorption of magnesium via decreased solubility of magnesium in intestinal lumen, altered expression and activity of key transporter proteins, and dysbiosis of gut microbiome.84
2) Anemia: PPI-associated anemia has been documented in numerous case reports and observational studies. Many different mechanisms, including iron malabsorption that may result from PPI-induced hypochlorhydria, have been hypothesized for the development of PPI-associated anemia.85 PPIs may restrict the absorption of iron by upregulating the peptide hormone hepcidin, which is known to regulate iron metabolism and block the cellular iron exporter duodenal ferroportin.86
Iron deficiency was found to have favorable dose-response and time-response correlations in patients taking PPIs, according to a population-based case-control research.87 Moreover, a meta-analysis of fourteen studies found that long-term PPI users have an elevated risk of iron deficiency anemia.88
3) Vitamin B12 deficiency: A positive association between Vitamin B12 deficiency and PPI use has been found in numerous cross sectional and cohort studies. For vitamin B12 insufficiency linked to PPIs, various mechanisms have been put forth. PPIs lessen the production of stomach acid, which is necessary for pepsinogen to turn into pepsin, which releases vitamin B12 from dietary proteins. Pepsinogen is converted into pepsin by the action of pepsinogen. Vitamin B12 absorption is ultimately decreased as pepsin production declines.89
Long-term PPI use has been linked to the emergence of vitamin B12 insufficiency, according to a single-center cohort study.90 Moreover, an observational study showed that PPI users had a greater prevalence of vitamin B12 deficiency.91 The use of PPIs is also linked to a rise of vitamin B12 deficiency, according to a meta-analysis of four case-control studies and one prospective cohort study.92
4) Hypocalcemia and hypokalemia: Hypocalcemia and hypokalemia are significantly associated with PPI users, according to an analysis of more than ten million reports from the FDA Adverse Event Reporting System (FAERS). PPI use over an extended period of time may reduce calcium absorption, resulting in hypocalcemia. Moreover, hypokalemia in PPI users may be facilitated by PPI-associated CKD.93
6. Hypergastrinemia
Hypochlorhydria or achlorhydria induced by PPIs-associated gastric acid suppression, stimulates G cells in the gastric antrum to release gastrin resulting in hypergastrinemia.94 Hypergastrinemia may induce rebound hypersecretion of acid once PPIs are discontinued. Hypergastrinemia-associated rebound hyperacidity may cause dyspeptic symptoms and reintroduction of PPIs.95 Patients taking PPIs for a prolonged period of time had moderate hypergastrinemia, according to a systematic review of 16 studies.96 Hypergastrinemia may also induce hyperplasia of enterochromaffin-like (ECL) cells in oxyntic mucosa, gastric cancer and other PPI-related adverse effects.97
7. Cancers
Long-term use of PPIs may enhance the incidence of cancers including gastric cancer, pancreatic cancer, colorectal cancer and hepatic cancer. An umbrella review of twenty-one meta-analyses that analyzed 65 observational studies concluded that PPI users had a significantly higher risk of developing malignancies like gastric, pancreatic, colorectal, and hepatic cancer.98
1) Gastric cancer: The extended use of PPIs is positively associated with the emergence of gastric cancer, according to several observational studies. Those taking PPIs for a prolonged period of time showed a more than two-fold increased risk of gastric cancer incidence. There have been several hypothesized mechanisms for PPI-associated gastric cancer. Potent acid suppression by PPIs may ensue in gastric cancer via increasing gastric atrophy, hypergastrinemia, ECL hyperplasia, and bacterial overgrowth.99
A positive association between long-term PPI use and increased risk of fundic gland polyps and gastric cancer was found by performing a meta-analysis of twelve studies, which included a randomized controlled trial, four case-control studies, and seven cohort studies.100 Corresponding to this, a meta-analysis of 926,386 patients found that long-term PPI users had a twofold greater chance of developing gastric cancer.101 Also, a meta-analysis of seven trials with a combined total of 943,070 individuals found that long-term PPI use was linked to an increased risk of gastric cancer.102 Moreover, a meta-analysis of two randomized clinical trials and twelve non-randomized studies involving more than 6 million patients revealed that the evidence of enhanced risk of gastric cancer associated with the long-term use of PPIs was minimal.103
The risk of gastric cancer was doubled in PPI users according to a meta-analysis of eight case-control studies and five retrospective cohort studies.104 In addition, a meta-analysis of eighteen studies involving 4,348,905 patients found that PPI users had an increased risk of stomach cancer.105 Moreover, a meta-analysis of thirteen observational studies determined a link between PPI use and the occurrence of gastric cancer.106 Furthermore, a meta-analysis of sixteen cohorts and case-control studies found that the risk of gastric cancer was significantly elevated in patients taking PPIs.107
2) Pancreatic cancer: Various observational studies including a nationwide case-control study based on the French National Health Data System (SNDS),108 a population-based nationwide Swedish cohort study,109 a nested case-control study and a retrospective cohort study in The Health Improvement Network (THIN),110 and a twelve-year longitudinal population-based study using the Korean National Health Insurance Corporation claims database111 found a link between PPI use and the incidence of pancreatic cancer. PPI-associated hypergastrinemia, hypochlorhydria-associated bacterial overgrowth, and other mechanisms may all increase the risk of pancreatic cancer.111
The risk of pancreatic cancer increased due to the use of PPIs, revealed by a meta-analysis of eight case-control and three cohort studies.112 Similarly, a meta-analysis of seven case-control studies and three cohort studies demonstrated that the risk of pancreatic cancer was significantly enhanced in PPI users.113 In addition, a meta-analysis of one randomized controlled clinical trial, two cohort studies and five nested case-control studies demonstrated that the use of PPIs was positively associated with enhanced risk of pancreatic cancer.114 Moreover, a nested case-control study determined that the risk of pancreatic cancer was elevated in PPI users.115 In contrast, no conclusive evidence of PPI-associated pancreatic cancer was observed in a meta-analysis of six case-control and cohort studies.116
3) Colorectal cancer: The long-term use of PPIs has increased the risk of colorectal cancer. Long-term PPI use was found to be linked to higher all-cause and colorectal cancer-specific mortality in a population-based cohort analysis conducted in Sweden.117 By causing hypergastrinemia, which can cause colonic and rectal cells to proliferate, prolonged PPI usage may increase the risk of colorectal cancer.118 Moreover, PPI-associated hypergastrinemia, activation of the Yes-associated protein (YAP), altered gut flora, and fecal alkalization may all contribute to the growth and spread of colorectal cancer.119
A substantial correlation between the risk of colorectal cancer and PPI usage was not found, according to the findings of three sizable prospective cohort studies.120 Also, a meta-analysis of three cohort studies and six case-control studies found a slight correlation between the risk of colorectal cancer and prolonged use of PPIs.121
4) Hepatic cancer: Hepatocellular carcinoma risk may rise with prolonged PPI use. A favorable correlation between the use of PPIs and a greater risk of hepatocellular carcinoma was found in a nested case-control study from Taiwan. PPI-associated hypergastrinemia may increase the risk of hepatocellular cancer.122 Among patients with chronic liver disease who took PPIs, there was an increased risk of hepatocellular carcinoma and mortality, according to a meta-analysis of eleven trials.123
On the other hand, a Korean observational study that examined data from the National Health Insurance Service revealed no increased risk of hepatocellular carcinoma among PPI users.124 Moreover, a meta-analysis of five studies found no evidence that taking PPIs increased the chance of developing hepatocellular carcinoma.125
8. Hepatic encephalopathy
Patients with chronic liver disease have seen a number of negative clinical consequences as a result of PPI use. The use of PPIs was linked to a number of unfavorable clinical outcomes in patients with chronic liver disease, according to a meta-analysis of 47 observational studies, including 35 cohort studies and 12 case-control studies.126 Furthermore, a meta-analysis of three case-control studies found a link between PPI usage and an increased risk of hepatic encephalopathy in individuals with acute liver dysfunction.127 A meta-analysis of nine observational studies, including five case-control studies and four cohort studies, found that the risk of hepatic encephalopathy was significantly elevated in patients with advanced liver disease who used PPIs.128 Likewise, a meta-analysis of four case-control studies and three cohort studies demonstrated that the use of PPIs in patients with liver cirrhosis increases the risk of hepatic encephalopathy.129 Furthermore, a meta-analysis of ten observational studies found a positive association between the use of PPIs and elevated risk of hepatic encephalopathy in patients with hepatic cirrhosis.130
9. Dementia
The likelihood of dementia in PPI users has been assessed by numerous observational studies. PPI users have a considerably higher risk of dementia, according to a meta-analysis of six cohort studies.131
In contrast, no statistically significant link between increased risk of dementia and PPI use was found in meta-analyses of six cohort studies,132 ten independent studies,133 four case-control studies and eight cohort studies,134 seventeen observational studies,135 eleven observational studies,136 one randomized controlled clinical trial, and five prospective observational studies137 and nine observational studies.138
The level of evidence of adverse effects-associated with long-term PPI therapy is low as most of the studies discussed here are observational in nature and most findings need to be confirmed by randomized controlled clinical trials. Inappropriate use of PPI, such as overuse, misuse, high-dose PPIs without definitive indication, and off-label prescriptions, should be curbed. To manage mild-moderate symptoms, PPIs should always be used for the shortest possible duration at the smallest effective dose. However, the patients with definitive indications should continue long-term use of PPIs with regular monitoring.
AMERICAN GASTROENTEROLOGICAL ASSOCIATION (AGA) RECOMMENDATIONS
The recommended duration of PPI therapy for the management of patients with GERD, H. pylori infection, and peptic ulcer disease is limited to 2-12 weeks, while indefinite PPI therapy may be indicated in the management of patients with Barrett’s esophagus and severe esophagitis.139 The American Gastroenterological association (AGA) offers various evidence-based recommendations (Table 2) to manage patients with GERD that include replacing PPIs with histamine 2 blockers, administration of PPIs 30-60 minutes before meal rather than at bedtime, indefinite PPI maintenance therapy to manage Los Angeles grade C or D esophagitis, no baclofen therapy without any objective evidence of GERD, avoidance of prokinetic agent use without any objective evidence of gastroparesis, no sucralfate therapy for GERD except during pregnancy, weight loss in overweight and obese patients, avoiding meals within 2-3 of bedtime, elevating the head of the bed, quitting smoking and tobacco products, avoidance of “trigger foods”, deprescribing of PPI if possible, use of lowest possible PPI maintenance dose, and avoidance of routine addition of medical therapies in PPI non-responders.140
TABLE 2. AGA recommendations on PPI therapy in the management of GERD140.
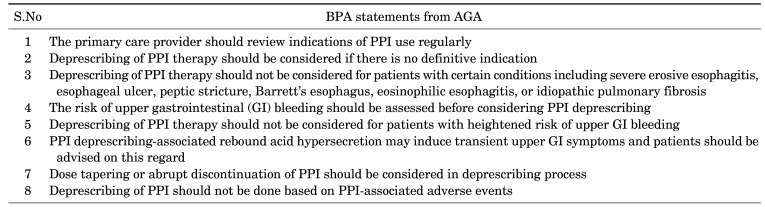
Moreover, Best Practice Advice (BPA) statements from the AGA on deprescribing PPIs therapy (Table 3) recommend that the primary care provider should review indications of PPI use on a regular basis, deprescribing of PPI therapy should be considered if there is no definitive indication, patients taking-twice daily doses of PPI for long-term should be considered for a once-daily dose, deprescribing of PPI therapy should not be considered for patients with certain conditions including severe erosive esophagitis, esophageal ulcer, peptic stricture, Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis, the risk of upper gastrointestinal (GI) bleeding should be assessed before PPI deprescribing consideration, deprescribing of PPI therapy should not be considered for patients with heightened risk of upper GI bleeding, PPI deprescribing-associated rebound acid hypersecretion may induce transient upper GI symptoms and patients should be advised on this regard, dose tapering or abrupt discontinuation of PPI should be considered in deprescribing process, and deprescribing of PPI should not be done based on PPI-associated adverse events.141
TABLE 3. Best Practice Advice (BPA) statements from the AGA on deprescribing PPI therapy141.
DISCUSSION
Clinicians, including prescribers and pharmacists, should be aware of the adverse effects associated with long-term use of PPIs such as renal disorders (AIN, AKI, CKD, ESRD), cardiovascular risks (MACE, MI, Stroke), fractures, infections (c,difficile infection, CAP, COVID-19), micronutrient deficiencies (hypomagnesemia, anemia, vitamin B12 deficiency, hypocalcemia, hypokalemia), hypergastrinemia, cancers (gastric cancer, pancreatic cancer, colorectal cancer, hepatic cancer), hepatic encephalopathy, and dementia. In addition, the patients taking PPIs for long-term should be monitored for the listed adverse effects. The AGA recommends some non-pharmacological measures and use of histamine 2 blockers to reduce GI symptoms of GERD and the use of PPI therapy if there is definitive indication. Moreover, BPA statements from the AGA emphasize on deprescribing if there is no definitive indication for PPI therapy.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Scarpignato C, Gatta L, Zullo A, Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases - a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yibirin M, De Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse effects associated with proton pump inhibitor use. Cureus. 2021;13:e12759. doi: 10.7759/cureus.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long-term proton pump inhibitor use. J Neurogastroenterol Motil. 2018;24:182–196. doi: 10.5056/jnm18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19–24. doi: 10.1016/j.ejim.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Al-Aly Z, Maddukuri G, Xie Y. Proton pump inhibitors and the kidney: implications of current evidence for clinical practice and when and how to deprescribe. Am J Kidney Dis. 2020;75:497–507. doi: 10.1053/j.ajkd.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Savarino V, Marabotto E, Zentilin P, Furnari M, Bodini G, De Maria C, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11:1123–1134. doi: 10.1080/17512433.2018.1531703. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen PV, Tamaz R. Inappropriate prescription of proton pump inhibitors in a community setting. Can J Hosp Pharm. 2018;71:267–271. [PMC free article] [PubMed] [Google Scholar]
- 10.Sattayalertyanyong O, Thitilertdecha P, Auesomwang C. The inappropriate use of proton pump inhibitors during admission and after discharge: a prospective cross-sectional study. Int J Clin Pharm. 2020;42:174–183. doi: 10.1007/s11096-019-00955-8. [DOI] [PubMed] [Google Scholar]
- 11.Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354–364. [PMC free article] [PubMed] [Google Scholar]
- 12.Haastrup PF, Jarbøl DE, Thompson W, Hansen JM, Søndergaard J, Rasmussen S. When does proton pump inhibitor treatment become long term? A scoping review. BMJ Open Gastroenterol. 2021;8:e000563. doi: 10.1136/bmjgast-2020-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf. 2017;8:273–297. doi: 10.1177/2042098617715381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakkir Maideen NM, Jumale A, Balasubramaniam R. Drug interactions of metformin involving drug transporter proteins. Adv Pharm Bull. 2017;7:501–505. doi: 10.15171/apb.2017.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:331–342. doi: 10.1093/ndt/gfw470. [DOI] [PubMed] [Google Scholar]
- 16.Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837–844. doi: 10.1038/ki.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torpey N, Barker T, Ross C. Drug-induced tubulo-interstitial nephritis secondary to proton pump inhibitors: experience from a single UK renal unit. Nephrol Dial Transplant. 2004;19:1441–1446. doi: 10.1093/ndt/gfh137. [DOI] [PubMed] [Google Scholar]
- 18.Geevasinga N, Coleman PL, Webster AC, Roger SD. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol. 2006;4:597–604. doi: 10.1016/j.cgh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Simpson IJ, Marshall MR, Pilmore H, Manley P, Williams L, Thein H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology (Carlton) 2006;11:381–385. doi: 10.1111/j.1440-1797.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 20.Härmark L, van der Wiel HE, de Groot MC, van Grootheest AC. Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol. 2007;64:819–823. doi: 10.1111/j.1365-2125.2007.02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150. doi: 10.1186/1471-2369-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikuta K, Nakagawa S, Momo K, Yonezawa A, Itohara K, Sato Y, et al. Association of proton pump inhibitors and concomitant drugs with risk of acute kidney injury: a nested case-control study. BMJ Open. 2021;11:e041543. doi: 10.1136/bmjopen-2020-041543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoniou T, Macdonald EM, Hollands S, Gomes T, Mamdani MM, Garg AX, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3:E166–E171. doi: 10.9778/cmajo.20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svanström H, Lund M, Melbye M, Pasternak B. Use of proton pump inhibitors and the risk of acute kidney injury among patients with rheumatoid arthritis: cohort study. Drug Saf. 2018;41:817–826. doi: 10.1007/s40264-018-0663-1. [DOI] [PubMed] [Google Scholar]
- 25.Sutton SS, Magagnoli J, Cummings TH, Hardin JW. Risk of acute kidney injury in patients with HIV receiving proton pump inhibitors. J Comp Eff Res. 2019;8:781–790. doi: 10.2217/cer-2019-0017. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, George KC, Shang WF, Zeng R, Ge SW, Xu G. Proton-pump inhibitors use, and risk of acute kidney injury: a meta-analysis of observational studies. Drug Des Devel Ther. 2017;11:1291–1299. doi: 10.2147/DDDT.S130568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch HK, Kellum JA, Kane-Gill SL. Drug-associated acute kidney injury identified in the United States Food and Drug Administration Adverse Event Reporting System database. Pharmacotherapy. 2018;38:785–793. doi: 10.1002/phar.2152. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Ning LJ, Qin Y, Zhao B, Mei D, Li XM. Acute kidney injury following the use of different proton pump inhibitor regimens: a real-world analysis of post-marketing surveillance data. J Gastroenterol Hepatol. 2021;36:156–162. doi: 10.1111/jgh.15151. [DOI] [PubMed] [Google Scholar]
- 29.Wu B, Li D, Xu T, Luo M, He Z, Li Y. Proton pump inhibitors associated acute kidney injury and chronic kidney disease: data mining of US FDA adverse event reporting system. Sci Rep. 2021;11:3690. doi: 10.1038/s41598-021-83099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 31.Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17:112. doi: 10.1186/s12882-016-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91:1482–1494. doi: 10.1016/j.kint.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Klatte DCF, Gasparini A, Xu H, de Deco P, Trevisan M, Johansson ALV, et al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153:702–710. doi: 10.1053/j.gastro.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Juang SY, Liao KF. Proton pump inhibitors use and risk of chronic kidney disease in diabetic patients. Diabetes Res Clin Pract. 2019;147:67–75. doi: 10.1016/j.diabres.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Hart E, Dunn TE, Feuerstein S, Jacobs DM. Proton pump inhibitors and risk of acute and chronic kidney disease: a retrospective cohort study. Pharmacotherapy. 2019;39:443–453. doi: 10.1002/phar.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez-Poncelas A, Barceló MA, Saez M, Coll-de-Tuero G. Duration and dosing of proton pump inhibitors associated with high incidence of chronic kidney disease in population-based cohort. PLoS One. 2018;13:e0204231. doi: 10.1371/journal.pone.0204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guedes JVM, Aquino JA, Castro TLB, Augusto de Morais F, Baldoni AO, Belo VS, et al. Omeprazole use and risk of chronic kidney disease evolution. PLoS One. 2020;15:e0229344. doi: 10.1371/journal.pone.0229344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14:82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Peng YC, Lin CL, Yeh HZ, Chang CS, Wu YL, Kao CH. Association between the use of proton pump inhibitors and the risk of ESRD in renal diseases: a population-based, case-control study. Medicine (Baltimore) 2016;95:e3363. doi: 10.1097/MD.0000000000003363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27:3153–3163. doi: 10.1681/ASN.2015121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ariel H, Cooke JP. Cardiovascular risk of proton pump inhibitors. Methodist Debakey Cardiovasc J. 2019;15:214–219. doi: 10.14797/mdcj-15-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun S, Cui Z, Zhou M, Li R, Li H, Zhang S, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926. doi: 10.1111/nmo.12926. [DOI] [PubMed] [Google Scholar]
- 44.Bundhun PK, Teeluck AR, Bhurtu A, Huang WQ. Is the concomitant use of clopidogrel and proton pump inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012 - 2016) BMC Cardiovasc Disord. 2017;17:3. doi: 10.1186/s12872-016-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batchelor R, Kumar R, Gilmartin-Thomas JFM, Hopper I, Kemp W, Liew D. Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther. 2018;48:780–796. doi: 10.1111/apt.14955. [DOI] [PubMed] [Google Scholar]
- 46.Pang J, Wu Q, Zhang Z, Zheng TZ, Xiang Q, Zhang P, et al. Efficacy and safety of clopidogrel only vs. clopidogrel added proton pump inhibitors in the treatment of patients with coronary heart disease after percutaneous coronary intervention: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2019;23:100317. doi: 10.1016/j.ijcha.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Ren X, Fang Z. Systematic review and meta-analysis: the effects of prophylactic proton pump inhibitor treatment in patients with coronary heart disease receiving dual antiplatelet therapy. J Cardiovasc Pharmacol. 2021;77:835–861. doi: 10.1097/FJC.0000000000001014. [DOI] [PubMed] [Google Scholar]
- 48.Farhat N, Fortin Y, Haddad N, Birkett N, Mattison DR, Momoli F, et al. Systematic review and meta-analysis of adverse cardiovascular events associated with proton pump inhibitors used alone or in combination with antiplatelet agents. Crit Rev Toxicol. 2019;49:215–261. doi: 10.1080/10408444.2019.1583167. [DOI] [PubMed] [Google Scholar]
- 49.Shang YS, Zhong PY, Ma Y, Bai N, Niu Y, Wang ZL. Efficacy and safety of proton pump inhibitors in patients with coronary artery diseases receiving oral antiplatelet agents and/or anticoagulants: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2022;80:1–12. doi: 10.1097/FJC.0000000000001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolde M, Ahn N, Dreischulte T, Krause E, Güntner F, Günter A, et al. Proton pump inhibitors and the risk of cardiovascular events and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2022;106:80–89. doi: 10.1016/j.ejim.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Freedberg DE, Yang YX, Abrams JA. Proton pump inhibitors and myocardial infarction. Gastroenterology. 2015;149:830–833. doi: 10.1053/j.gastro.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y, Jick S. Proton-pump inhibitor use and myocardial infarction: a nested case-control study in the UK Clinical Practice Research Datalink. Epidemiology. 2020;31:423–431. doi: 10.1097/EDE.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 53.Tseng HJ, Cheng CM, Tsai SJ, Lin WC, Bai YM, Tsai CF, et al. Proton pump inhibitor exposure and acute myocardial infarction risk: a nested cohort study. Cardiovasc Toxicol. 2021;21:444–450. doi: 10.1007/s12012-021-09637-2. [DOI] [PubMed] [Google Scholar]
- 54.Jeridi D, Pellat A, Ginestet C, Assaf A, Hallit R, Corre F, et al. The safety of long-term proton pump inhibitor use on cardiovascular health: a meta-analysis. J Clin Med. 2022;11:4096. doi: 10.3390/jcm11144096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chui CSL, Cheung KS, Brown JP, Douglas IJ, Wong ICK, Chan EW, et al. Proton pump inhibitors and myocardial infarction: an application of active comparators in a self-controlled case series. Int J Epidemiol. 2022 doi: 10.1093/ije/dyac196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nolde M, Ahn N, Dreischulte T, Rückert-Eheberg IM, Güntner F, Günter A, et al. The long-term risk for myocardial infarction or stroke after proton pump inhibitor therapy (2008-2018) Aliment Pharmacol Ther. 2021;54:1033–1040. doi: 10.1111/apt.16565. [DOI] [PubMed] [Google Scholar]
- 57.Ghebremariam YT, LePendu P, Lee JC, Erlanson DA, Slaviero A, Shah NH, et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. 2013;128:845–853. doi: 10.1161/CIRCULATIONAHA.113.003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang MK, Shin JH, Kim TJ, Lee JS, Yoon BW, Ko SB. Use of proton pump inhibitor may be associated with progression of cerebral small vessel disease. PLoS One. 2022;17:e0279257. doi: 10.1371/journal.pone.0279257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sehested TSG, Gerds TA, Fosbøl EL, Hansen PW, Charlot MG, Carlson N, et al. Long-term use of proton pump inhibitors, dose-response relationship and associated risk of ischemic stroke and myocardial infarction. J Intern Med. 2018;283:268–281. doi: 10.1111/joim.12698. [DOI] [PubMed] [Google Scholar]
- 60.Wang YF, Chen YT, Luo JC, Chen TJ, Wu JC, Wang SJ. Proton-pump inhibitor use and the risk of first-time ischemic stroke in the general population: a nationwide population-based study. Am J Gastroenterol. 2017;112:1084–1093. doi: 10.1038/ajg.2017.101. [DOI] [PubMed] [Google Scholar]
- 61.Yang M, He Q, Gao F, Nirantharakumar K, Veenith T, Qin X, et al. Regular use of proton-pump inhibitors and risk of stroke: a population-based cohort study and meta-analysis of randomized-controlled trials. BMC Med. 2021;19:316. doi: 10.1186/s12916-021-02180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S, Liu F, Chen C, Zhu W, Ma J, Hu J, et al. Real-world relationship between proton pump inhibitors and cerebro-cardiovascular outcomes independent of clopidogrel. Int Heart J. 2019;60:910–918. doi: 10.1536/ihj.18-584. [DOI] [PubMed] [Google Scholar]
- 63.Thong BKS, Ima-Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health. 2019;16:1571. doi: 10.3390/ijerph16091571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou B, Huang Y, Li H, Sun W, Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 66.Sugiyama T. Proton pump inhibitor use and fracture risk: an update of drug safety communication needed? Am J Gastroenterol. 2019;114:360–361. doi: 10.14309/ajg.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 67.Wetzel D, McBride SM. The impact of pH on Clostridioides difficile sporulation and physiology. Appl Environ Microbiol. 2020;86:e02706–e02719. doi: 10.1128/AEM.02706-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tawam D, Baladi M, Jungsuwadee P, Earl G, Han J. The positive association between proton pump inhibitors and clostridium difficile infection. Innov Pharm. 2021;12:10.24926/iip.v12i1.343. doi: 10.24926/iip.v12i1.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Silva KM, Mehta R, Mitchell M, Lee TC, Singhal V, Wilson MG, et al. Proton pump inhibitor use and risk for recurrent Clostridioides difficile infection: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:697–703. doi: 10.1016/j.cmi.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Goyal H, Katner H. Proton pump inhibitors and Clostridium difficile infection. Am J Gastroenterol. 2017;112:805. doi: 10.1038/ajg.2016.600. [DOI] [PubMed] [Google Scholar]
- 71.Fohl AL, Regal RE. Proton pump inhibitor-associated pneumonia: not a breath of fresh air after all? World J Gastrointest Pharmacol Ther. 2011;2:17–26. doi: 10.4292/wjgpt.v2.i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, et al. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18:163–172. doi: 10.1080/14740338.2019.1577820. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen PA, Islam M, Galvin CJ, Chang CC, An SY, Yang HC, et al. Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia. Int J Qual Health Care. 2020;32:292–299. doi: 10.1093/intqhc/mzaa041. [DOI] [PubMed] [Google Scholar]
- 74.Xun X, Yin Q, Fu Y, He X, Dong Z. Proton pump inhibitors and the risk of community-acquired pneumonia: an updated meta-analysis. Ann Pharmacother. 2022;56:524–532. doi: 10.1177/10600280211039240. [DOI] [PubMed] [Google Scholar]
- 75.Fatima K, Almas T, Lakhani S, Jahangir A, Ahmed A, Siddiqui A, et al. The use of proton pump inhibitors and COVID-19: a systematic review and meta-analysis. Trop Med Infect Dis. 2022;7:37. doi: 10.3390/tropicalmed7030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toubasi AA, AbuAnzeh RB, Khraisat BR, Al-Sayegh TN, AlRyalat SA. Proton pump inhibitors: current use and the risk of Coronavirus Infectious Disease 2019 development and its related mortality. Meta-analysis. Arch Med Res. 2021;52:656–659. doi: 10.1016/j.arcmed.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pranata R, Huang I, Lawrensia S, Henrina J, Lim MA, Lukito AA, et al. Proton pump inhibitor on susceptibility to COVID-19 and its severity: a systematic review and meta-analysis. Pharmacol Rep. 2021;73:1642–1649. doi: 10.1007/s43440-021-00263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim HB, Kim JH, Wolf BJ. Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78:383–391. doi: 10.1007/s00228-021-03255-1. Erratum in: Eur J Clin Pharmacol 2022;78:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47:773–780. doi: 10.1345/aph.1R556. [DOI] [PubMed] [Google Scholar]
- 80.Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558. doi: 10.1371/journal.pone.0112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, Edmonds PJ, Ungprasert P, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237–1241. doi: 10.3109/0886022X.2015.1057800. [DOI] [PubMed] [Google Scholar]
- 82.Srinutta T, Chewcharat A, Takkavatakarn K, Praditpornsilpa K, Eiam-Ong S, Jaber BL, et al. Proton pump inhibitors and hypomagnesemia: a meta-analysis of observational studies. Medicine (Baltimore) 2019;98:e17788. doi: 10.1097/MD.0000000000017788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao S, Gan L, Mei Z. Does the use of proton pump inhibitors increase the risk of hypomagnesemia: an updated systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15011. doi: 10.1097/MD.0000000000015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gommers LMM, Hoenderop JGJ, de Baaij JHF. Mechanisms of proton pump inhibitor-induced hypomagnesemia. Acta Physiol (Oxf) 2022;235:e13846. doi: 10.1111/apha.13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boxer M. Iron deficiency anemia from iron malabsorption caused by proton pump inhibitors. EJHaem. 2020;1:548–551. doi: 10.1002/jha2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamano H, Niimura T, Horinouchi Y, Zamami Y, Takechi K, Goda M, et al. Proton pump inhibitors block iron absorption through direct regulation of hepcidin via the aryl hydrocarbon receptor-mediated pathway. Toxicol Lett. 2020;318:86–91. doi: 10.1016/j.toxlet.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Tran-Duy A, Connell NJ, Vanmolkot FH, Souverein PC, de Wit NJ, Stehouwer CDA, et al. Use of proton pump inhibitors and risk of iron deficiency: a population-based case-control study. J Intern Med. 2019;285:205–214. doi: 10.1111/joim.12826. [DOI] [PubMed] [Google Scholar]
- 88.Ali MD. Proton pump inhibitors’ use and risk of iron deficiency anaemia: a systematic review and meta-analysis. Curr Rev Clin Exp Pharmacol. 2023;18:158–166. doi: 10.2174/2772432817666220307121220. [DOI] [PubMed] [Google Scholar]
- 89.Swarnakari KM, Bai M, Manoharan MP, Raja R, Jamil A, Csendes D, et al. The effects of proton pump inhibitors in acid hypersecretion-induced vitamin B12 deficiency: a systematic review (2022) Cureus. 2022;14:e31672. doi: 10.7759/cureus.31672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mumtaz H, Ghafoor B, Saghir H, Tariq M, Dahar K, Ali SH, et al. Association of vitamin B12 deficiency with long-term PPIs use: a cohort study. Ann Med Surg (Lond) 2022;82:104762. doi: 10.1016/j.amsu.2022.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter KM, Hoey L, Hughes CF, Ward M, Clements M, Strain J, et al. Associations of atrophic gastritis and proton-pump inhibitor drug use with vitamin B-12 status, and the impact of fortified foods, in older adults. Am J Clin Nutr. 2021;114:1286–1294. doi: 10.1093/ajcn/nqab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jung SB, Nagaraja V, Kapur A, Eslick GD. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis. Intern Med J. 2015;45:409–416. doi: 10.1111/imj.12697. [DOI] [PubMed] [Google Scholar]
- 93.Makunts T, Cohen IV, Awdishu L, Abagyan R. Analysis of postmarketing safety data for proton-pump inhibitors reveals increased propensity for renal injury, electrolyte abnormalities, and nephrolithiasis. Sci Rep. 2019;9:2282. doi: 10.1038/s41598-019-39335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee L, Ramos-Alvarez I, Ito T, Jensen RT. Insights into effects/risks of chronic hypergastrinemia and lifelong PPI treatment in man based on studies of patients with Zollinger-Ellison syndrome. Int J Mol Sci. 2019;20:5128. doi: 10.3390/ijms20205128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Helgadottir H, Bjornsson ES. Problems associated with deprescribing of proton pump inhibitors. Int J Mol Sci. 2019;20:5469. doi: 10.3390/ijms20215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 97.McCarthy DM. Proton pump inhibitor use, hypergastrinemia, and gastric carcinoids-what is the relationship? Int J Mol Sci. 2020;21:662. doi: 10.3390/ijms21020662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang ML, Fan YX, Meng R, Cai WK, Yin SJ, Zhou T, et al. Proton pump inhibitors and cancer risk: an umbrella review and meta-analysis of observational studies. Am J Clin Oncol. 2022;45:475–485. doi: 10.1097/COC.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 99.Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 2019;12:1756284819834511. doi: 10.1177/1756284819834511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–1719.e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 101.Wan QY, Wu XT, Li N, Du L, Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: a meta-analysis of 926 386 participants. Gut. 2019;68:762–764. doi: 10.1136/gutjnl-2018-316416. [DOI] [PubMed] [Google Scholar]
- 102.Jiang K, Jiang X, Wen Y, Liao L, Liu FB. Relationship between long-term use of proton pump inhibitors and risk of gastric cancer: a systematic analysis. J Gastroenterol Hepatol. 2019;34:1898–1905. doi: 10.1111/jgh.14759. [DOI] [PubMed] [Google Scholar]
- 103.Piovani D, Tsantes AG, Schünemann HJ, Bonovas S. Meta-analysis: use of proton pump inhibitors and risk of gastric cancer in patients requiring gastric acid suppression. Aliment Pharmacol Ther. 2023;57:653–665. doi: 10.1111/apt.17360. [DOI] [PubMed] [Google Scholar]
- 104.Segna D, Brusselaers N, Glaus D, Krupka N, Misselwitz B. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review with meta-analysis. Therap Adv Gastroenterol. 2021;14:17562848211051463. doi: 10.1177/17562848211051463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao H, Li L, Geng K, Teng C, Chen Y, Chu F, et al. Use of proton pump inhibitors for the risk of gastric cancer. Medicine (Baltimore) 2022;101:e32228. doi: 10.1097/MD.0000000000032228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poly TN, Lin MC, Syed-Abdul S, Huang CW, Yang HC, Li YJ. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers (Basel) 2022;14:3052. doi: 10.3390/cancers14133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng TR, Wu TW, Li CH. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review and meta-analysis. Int J Clin Oncol. 2023;28:99–109. doi: 10.1007/s10147-022-02253-2. [DOI] [PubMed] [Google Scholar]
- 108.Lassalle M, Le Tri T, Afchain P, Camus M, Kirchgesner J, Zureik M, et al. Use of proton pump inhibitors and risk of pancreatic cancer: a nationwide case-control study based on the French National Health Data System (SNDS) Cancer Epidemiol Biomarkers Prev. 2022;31:662–669. doi: 10.1158/1055-9965.EPI-21-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brusselaers N, Sadr-Azodi O, Engstrand L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J Gastroenterol. 2020;55:453–461. doi: 10.1007/s00535-019-01652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kearns MD, Boursi B, Yang YX. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiol. 2017;46:80–84. doi: 10.1016/j.canep.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hwang IC, Chang J, Park SM. Association between proton pump inhibitor use and the risk of pancreatic cancer: a Korean nationwide cohort study. PLoS One. 2018;13:e0203918. doi: 10.1371/journal.pone.0203918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poly TN, Islam MM, Walther BA, Lin MC, Li YJ. Proton pump inhibitors use and the risk of pancreatic cancer: evidence from eleven epidemiological studies, comprising 1.5 million individuals. Cancers (Basel) 2022;14:5357. doi: 10.3390/cancers14215357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong HE, Kim AS, Kim MR, Ko HJ, Jung MK. Does the use of proton pump inhibitors increase the risk of pancreatic cancer? A systematic review and meta-analysis of epidemiologic studies. Cancers (Basel) 2020;12:2220. doi: 10.3390/cancers12082220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alkhushaym N, Almutairi AR, Althagafi A, Fallatah SB, Oh M, Martin JR, et al. Exposure to proton pump inhibitors and risk of pancreatic cancer: a meta-analysis. Expert Opin Drug Saf. 2020;19:327–334. doi: 10.1080/14740338.2020.1715939. [DOI] [PubMed] [Google Scholar]
- 115.Peng YC, Lin CL, Hsu WY, Lu IT, Yeh HZ, Chang CS, et al. Proton pump inhibitor use is associated with risk of pancreatic cancer: a nested case-control study. Dose Response. 2018;16:1559325818803283. doi: 10.1177/1559325818803283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laoveeravat P, Thavaraputta S, Vutthikraivit W, Suchartlikitwong S, Mingbunjerdsuk T, Motes A, et al. Proton pump inhibitors and histamine-2 receptor antagonists on the risk of pancreatic cancer: a systematic review and meta-analysis. QJM. 2020;113:100–107. doi: 10.1093/qjmed/hcz234. [DOI] [PubMed] [Google Scholar]
- 117.Wang X, Liu Q, Halfdanarson ÓÖ, Zoega H, Sadr-Azodi O, Engstrand L, et al. Proton pump inhibitors and survival in patients with colorectal cancer: a Swedish population-based cohort study. Br J Cancer. 2021;125:893–900. doi: 10.1038/s41416-021-01480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abrahami D, McDonald EG, Schnitzer ME, Barkun AN, Suissa S, Azoulay L. Proton pump inhibitors and risk of colorectal cancer. Gut. 2022;71:111–118. doi: 10.1136/gutjnl-2021-325096. [DOI] [PubMed] [Google Scholar]
- 119.Sasaki T, Mori S, Kishi S, Fujiwara-Tani R, Ohmori H, Nishiguchi Y, et al. Effect of proton pump inhibitors on colorectal cancer. Int J Mol Sci. 2020;21:3877. doi: 10.3390/ijms21113877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Babic A, Zhang X, Morales-Oyarvide V, Yuan C, Khalaf N, Khalili H, et al. Acid-suppressive medications and risk of colorectal cancer: results from three large prospective cohort studies. Br J Cancer. 2020;123:844–851. doi: 10.1038/s41416-020-0939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma T, Wu M, Jia S, Yang L. Proton pump inhibitors and the risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Int J Colorectal Dis. 2020;35:2157–2169. doi: 10.1007/s00384-020-03717-5. [DOI] [PubMed] [Google Scholar]
- 122.Shao YJ, Chan TS, Tsai K, Wu SY. Association between proton pump inhibitors and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;48:460–468. doi: 10.1111/apt.14835. [DOI] [PubMed] [Google Scholar]
- 123.Song HJ, Jiang X, Henry L, Nguyen MH, Park H. Proton pump inhibitors and risk of liver cancer and mortality in patients with chronic liver disease: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:851–866. doi: 10.1007/s00228-020-02854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim S, Jeong S, Park SJ, Chang J, Choi S, Cho Y, et al. Association between proton pump inhibitor use and risk of hepatocellular carcinoma: a Korean nationally representative cohort study. J Clin Med. 2022;11:2865. doi: 10.3390/jcm11102865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang TE, Huang YS, Perng CL, Huang YH, Hou MC. Use of proton pump inhibitors and the risk of hepatocellular carcinoma: a systematic review and meta-analysis. J Chin Med Assoc. 2019;82:756–761. doi: 10.1097/JCMA.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 126.Wang J, Wu Y, Bi Q, Zheng X, Zhang J, Huang W. Adverse outcomes of proton pump inhibitors in chronic liver disease: a systematic review and meta-analysis. Hepatol Int. 2020;14:385–398. doi: 10.1007/s12072-019-10010-3. [DOI] [PubMed] [Google Scholar]
- 127.Bian J, Wang A, Lin J, Wu L, Huang H, Wang S, et al. Association between proton pump inhibitors and hepatic encephalopathy: a meta-analysis. Medicine (Baltimore) 2017;96:e6723. doi: 10.1097/MD.0000000000006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tantai XX, Yang LB, Wei ZC, Xiao CL, Chen LR, Wang JH, et al. Association of proton pump inhibitors with risk of hepatic encephalopathy in advanced liver disease: a meta-analysis. World J Gastroenterol. 2019;25:2683–2698. doi: 10.3748/wjg.v25.i21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma YJ, Cao ZX, Li Y, Feng SY. Proton pump inhibitor use increases hepatic encephalopathy risk: a systematic review and meta-analysis. World J Gastroenterol. 2019;25:2675–2682. doi: 10.3748/wjg.v25.i21.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi D, Zhou Z, Dai Y, Pan X, Cao Q. Proton pump inhibitor therapy and hepatic encephalopathy risk in cirrhotic patients: a systematic review with meta-analysis. Clin Drug Investig. 2019;39:847–856. doi: 10.1007/s40261-019-00810-8. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Liang M, Sun C, Song EJ, Cheng C, Shi T, et al. Proton pump inhibitors use and dementia risk: a meta-analysis of cohort studies. Eur J Clin Pharmacol. 2020;76:139–147. doi: 10.1007/s00228-019-02753-7. [DOI] [PubMed] [Google Scholar]
- 132.Li M, Luo Z, Yu S, Tang Z. Proton pump inhibitor use and risk of dementia: systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14422. doi: 10.1097/MD.0000000000014422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song YQ, Li Y, Zhang SL, Gao J, Feng SY. Proton pump inhibitor use does not increase dementia and Alzheimer’s disease risk: an updated meta-analysis of published studies involving 642305 patients. PLoS One. 2019;14:e0219213. doi: 10.1371/journal.pone.0219213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hussain S, Singh A, Zameer S, Jamali MC, Baxi H, Rahman SO, et al. No association between proton pump inhibitor use and risk of dementia: evidence from a meta-analysis. J Gastroenterol Hepatol. 2020;35:19–28. doi: 10.1111/jgh.14789. [DOI] [PubMed] [Google Scholar]
- 135.Wang H, Tian L, Yan X. No association between acid suppressant use and risk of dementia: an updated meta-analysis. Eur J Clin Pharmacol. 2022;78:375–382. doi: 10.1007/s00228-021-03248-0. [DOI] [PubMed] [Google Scholar]
- 136.Khan MA, Yuan Y, Iqbal U, Kamal S, Khan M, Khan Z, et al. No association linking short-term proton pump inhibitor use to dementia: systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2020;115:671–678. doi: 10.14309/ajg.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 137.Desai M, Nutalapati V, Srinivasan S, Fathallah J, Dasari C, Chandrasekhar VT, et al. Proton pump inhibitors do not increase the risk of dementia: a systematic review and meta-analysis of prospective studies. Dis Esophagus. 2020;33:doaa041. doi: 10.1093/dote/doaa041. [DOI] [PubMed] [Google Scholar]
- 138.Ahn N, Nolde M, Krause E, Güntner F, Günter A, Tauscher M, et al. Do proton pump inhibitors increase the risk of dementia? A systematic review, meta-analysis and bias analysis. Br J Clin Pharmacol. 2023;89:602–616. doi: 10.1111/bcp.15583. [DOI] [PubMed] [Google Scholar]
- 139.Boster J, Lowry LE, Bezzant ML, Kuiper B, Surry L. Reducing the inappropriate use of proton pump inhibitors in an internal medicine residency clinic. Cureus. 2020;12:e6609. doi: 10.7759/cureus.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117:27–56. doi: 10.14309/ajg.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on de-prescribing of proton pump inhibitors: expert review. Gastroenterology. 2022;162:1334–1342. doi: 10.1053/j.gastro.2021.12.247. [DOI] [PubMed] [Google Scholar]