Abstract
Objectives
To examine the impact of COVID-19 vaccination on hospital admissions in England in 2021.
Design
Observational study of emergency admissions for COVID-19 by vaccination status in people 16 years and over in England.
Setting
Hospitals in England.
Participants
A total of 48.1 million people registered with an English GP, aged ≥16 years with a recent NHS contact.
Main outcome measures
Emergency hospital admissions with a primary diagnosis of COVID-19 between 1 January and 31 December 2021. Monthly admission rates were directly standardised for age, sex, risk category and vaccination dose to estimate vaccine effectiveness (VE) over time, between vaccine doses, age groups and risk groups.
Results
A total of 192,047 hospital admissions were included. The unvaccinated admission rate was higher in December 2021 (6.1 admissions per 100,000 person-days; 95% CI: 5.9 to 6.3) than January 2021 (4.9; 95% CI: 4.9 to 5.0). Vaccinated admission rates were ≤1 per 100,000 from February to December. Doses 1 and 2 VE waned over time, particularly in older and clinically vulnerable groups (although this may reflect that they were vaccinated earlier). Dose 3 VE remained above 93%.
Conclusions
COVID-19 hospitalisations were consistently highest in the unvaccinated. Despite high case rates at the end of 2021, overall admission rates remained stable, driven by low admission rates among vaccinated people. There is population-level waning in VE, recovering after subsequent doses, potentially more marked in older and at-risk groups. The findings support JCVI (Joint Committee on Vaccination and Immunisation) guidance for an ongoing booster programme, especially in older people and higher clinical risk groups.
Keywords: Infectious diseases, public health, vaccination programmes
Introduction
The UK COVID-19 pandemic placed huge pressure on the National Health Service (NHS) with peak daily admissions of over 4000 patients per day. 1 COVID-19 vaccination was a key intervention to reduce hospitalisations as part of the wider public health approach. Following early clinical trials, the Pfizer BioNTech (BNT162b2) COVID-19 vaccine (Comirnaty®) was the first vaccine licensed in the UK (2 December 2020).2–5 Vaccination was offered in priority groups, first to those in residential homes and healthcare workers, followed by the most vulnerable. By the end of December 2021, 90% of the English population aged 12+ had received at least one dose of a COVID-19 vaccine, and 59% had received at least three doses. 6
COVID-19 vaccination reduced hospital admissions, with a Scottish study estimating efficacy in preventing admission in adults to be 91% and 88% following one dose of Comirnaty® or AstraZeneca ChAdOx1-S/nCoV-19 [recombinant] (Vaxzevria®), respectively. 7 Another study showed a slightly lower efficacy of 80% in those aged over 70 years. 8 A further study in Northwest London observed very low rates of admission (0.07%) among its vaccinated population. 9 These studies were conducted from December 2020 to February 2021. Similar findings have been replicated around the world.10–13
Much of this research was conducted over limited time frames or near the beginning of vaccination programmes, and therefore is not reflective of the multiple waves and variants seen during the pandemic. 14 Furthermore, immunological waning may reduce vaccine efficacy over time, a phenomenon which may not have been captured in earlier studies, or those completed over a shorter time.15,16 This study adds to previous research by measuring vaccine effectiveness (VE) over a longer time period and in the context of multiple variants, using population-level data and an improved denominator dataset to allow further sub-analyses.
Materials and methods
Data sources
We used NHS England (NHSE) data for the entire English population of general practitioner (GP)-registered patients aged 16 years and over. To counter for known list inflation within GP-register data, the population was filtered to only include those with known NHS contacts since 2017. 17 This estimate of the English population is higher than the latest available Office for National Statistics (ONS) estimates (mid-2020 at the time of the analysis), but lower than the full GP-register population, 18 therefore more representative of the national population. For further details on this adjustment, see Supplementary Table 1.
Data sources (listed below) were used to produce population-level estimates of admission rates and VE by vaccination status. Pseudonymised datasets were linked at the patient level and prepared within an NHSE data warehouse (Foundry, Palantir Technologies Inc.).
Hospital Episode Statistics/Secondary Uses Service;
Case numbers for the English population aged 16+ published by UK Health Security Agency 6 ;
GP-registered population (National Health Application and Infrastructure Services);
Vaccination events (National Immunisation Management Service);
Recent contact with NHS services was determined using vaccination and National Booking System data; Test and Trace data and inpatient, outpatient and A&E attendances.
Individual patients were already categorised to risk groups based on data collated from GP registers and existing national data collections (e.g. the Cardiovascular Disease Register and NHS Electronic Staff Register).
Statistical analyses
Emergency admissions with a primary diagnosis of suspected or confirmed COVID-19 from January to December 2021 were included (ICD-10 codes U07.1 and U07.2). The population was divided into cohorts according to vaccination dose – Dose 1, Dose 2 (received two doses in total, both doses 1 and 2), Dose 3 (≥3 doses received) or unvaccinated. An individual was considered vaccinated ≥14 days after vaccination, in line with the NHSE definition. 19 The number of days each individual was alive and vaccinated for a particular dose (or unvaccinated) within each month was calculated. Person-days is the person-time unit for the incidence rate: the total number of days alive for the specified population in which the event of admissions could have occurred.
The population was divided into age groups (16–17 years, 18–24 years, five-year age bands from 25 to 89 years, ≥90 years), sex (female or male) and aggregated risk groups (Clinically Vulnerable, Exposure Risk, Close Contacts, Lower Risk).
‘Clinically Vulnerable’ included individuals with high-risk co-morbidities identified in the ‘Green Book’: immunosuppression, on the learning disability or severe mental illness registers, care home residents and those otherwise ‘at risk’ or with a high QCovid score. 5 ‘Exposure Risk’ included frontline healthcare workers with higher risk of exposure. ‘Close Contacts’ included carers and household contacts of immunosuppressed people. ‘Lower Risk’ included individuals not in the groups mentioned above.
Directly standardised admission rates (DSARs), which standardised for age, sex, risk group and vaccination dose, were calculated for each month and 95% gamma confidence intervals (CI) calculated. 20 If there were <10 admissions for a cohort in a month, DSARs and VE were not calculated and the admissions for that cohort excluded. Trends in DSARs over time were compared to published case data for COVID-19. 6
DSARs were compared between the unvaccinated (DSARU) population and each vaccinated (DSARV) population (Dose 1, Dose 2, Dose 3) to calculate VE for each dose, expressed as a percentage
DSARs were also compared between the unvaccinated and the entire vaccinated (‘All Vaccinated’) population. Trends in VE were examined for January to December 2021. Estimates of VE in December were calculated to demonstrate variation in VE with age and risk-group status by comparing DSARs between vaccinated and equivalent unvaccinated cohorts.
Statistical analysis was conducted in R studio version 4.1.2. 21
Patient and public involvement
The paper was reviewed by the Patient and Public Voice (PPV) representative for the NHSE COVID-19 Vaccination Programme. They commented that the paper addresses several important issues that need to be clearly communicated to the general public, for example that the vaccine programme protects against severe disease and reduces hospital admissions, and that booster vaccinations are particularly important in the elderly and clinically at-risk groups.
The PPV representative commented that the paper is clear and well presented, particularly the various figures. They identified ways to simplify data presentation and highlighted topics for future exploration: variation in uptake across risk groups, why waning may be higher in the elderly and clinical vulnerable and re-analysis of data to understand how efficacy changes over time.
Results
Population
The study population included those who were 16 years and over, registered with an English GP and with recent NHS contact, a total of 48.1 million individuals in June and July 2021 and 17.6 billion person-days across the 12 months. The median age was 48.3 years (interquartile range 32 to 64); 23.2% of the person-days were for those aged 65 years and over. Women accounted for 51.3% of the person-days at risk, and White British 70.8%.
Vaccine coverage
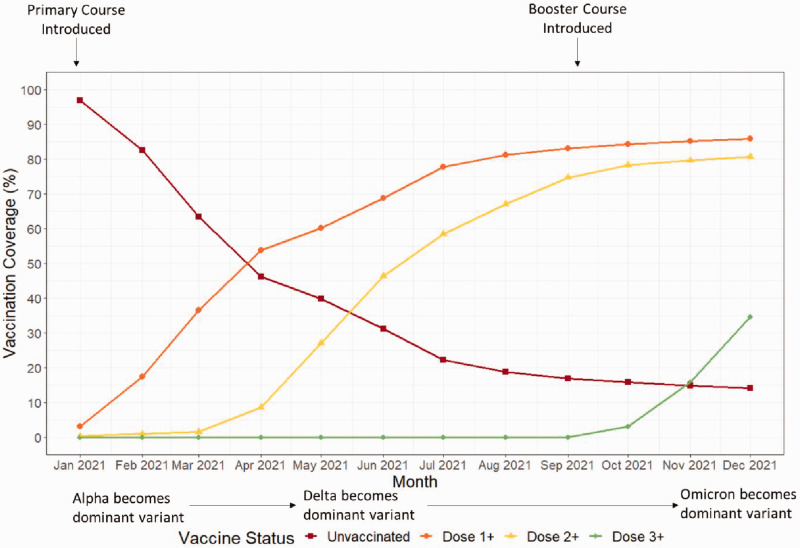
Figure 1 depicts the vaccination coverage for the study population in 2021.
Figure 1.
Coverage of COVID-19 vaccination doses over time in the English population aged 16 years and over. Dose 1+ equals total number of first doses administered, Dose 2+ equals total number of second doses administered, Dose 3+ equals total number of third doses administered.
Dose 1 coverage rapidly increased in the first half of 2021, followed by a more gradual uptake in the last few months of the year. Dose 2 coverage demonstrated a similar pattern of uptake from April 2021 and Dose 3 from October 2021. By December, 34.7% of the study population had received ≥3 doses, 46.0% two doses only and 5.1% one dose only; 14.2% were unvaccinated. There is a slight difference between these figures and published UKHSA statistics, as our analysis only recognises vaccination 14 days after the event and uses a different denominator based on patient days at risk.
As vaccine rollout was staggered by age group, with older ages prioritised for primary and booster doses, the age composition of vaccinated and unvaccinated populations varied over time (Supplementary Figure 1). Uptake of vaccine doses also varied by risk group (Supplementary Figure 2). By the end of 2021, 28.8% of the Lower Risk, 32.0% of the Close Contacts, 48.9% of the Clinically Vulnerable and 54.4% of the Exposure Risk groups had received ≥3 COVID-19 vaccination doses; 3.3% of the Exposure Risk and 9.9% of the Clinically Vulnerable were unvaccinated.
Hospital admission rates by vaccination status
In 2021, 192,047 patients were admitted with a primary diagnosis of COVID-19 (67.3% unvaccinated). Full data on admissions per vaccine status per month can be found in Supplementary Table 2.
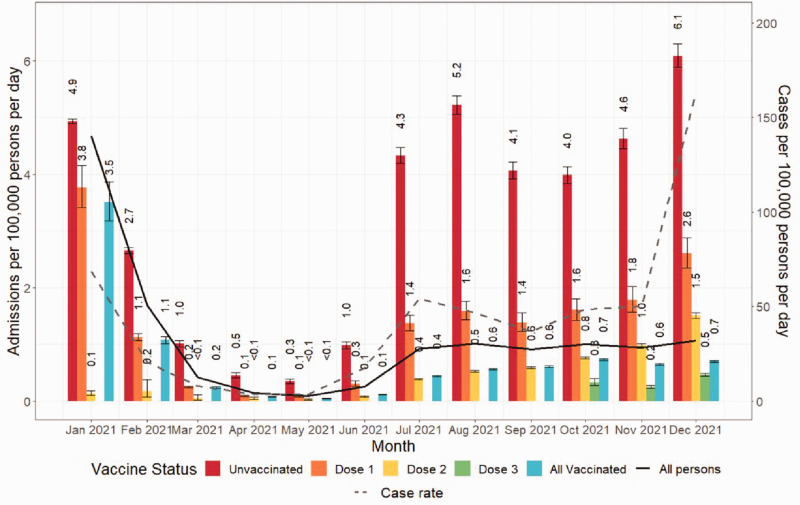
Figure 2 summarises the admission rates over the course of the 2021.
Figure 2. Directly standardised admission rates per 100,000 person-days by COVID-19 vaccine status. Rates were standardised for month, vaccination status, age, sex and risk group. The COVID-19 case rate is shown as a dashed line. Bars are shown with 95% confidence intervals. Dose 1 equals those who received only one dose, Dose 2 equals those who received only two doses, Dose 3 equals those who received three or more doses.
Until Autumn 2021, changes in DSARs for the population closely followed overall case rates, with 4.7 admissions per 100,000 person-days in January falling to 0.1 in May, rising to 0.9 in June and July. After this, admission rates remained stable despite sharply increasing case numbers from November.
When analysed by vaccination status, DSARs increased for both unvaccinated and vaccinated between September and December, although to differing degrees by number of doses. Hospitalisations were lower as the number of vaccine doses increased. DSARs for the vaccinated population remained stable (95% CI: 0.1 to 0.1) compared to an increase of 1.5 (95% CI: 1.4 to 1.5) admissions per 100,000 person-days among the unvaccinated.
The DSAR for the unvaccinated population was higher in December at 6.1 (95% CI: 5.9 to 6.3) admissions per 100,000 person-days, than in January at 4.9 (95% CI: 4.9 to 5.0; relative risk 1.23 (95% CI: 1.19 to 1.28)). In contrast, the DSAR for vaccinated people in December (0.7; 95% CI: 0.7 to 0.7) was lower than in January (3.5; 95% CI: 3.2 to 3.9), relative risk 0.2 (95% CI: 0.2 to 0.2)).
By December 2021, the rate of COVID-19-related emergency admissions for unvaccinated people (14.2% of the population) was 8.7 (95% CI: 8.3 to 9.0) times higher than for the overall vaccinated population.
Vaccine effectiveness over time
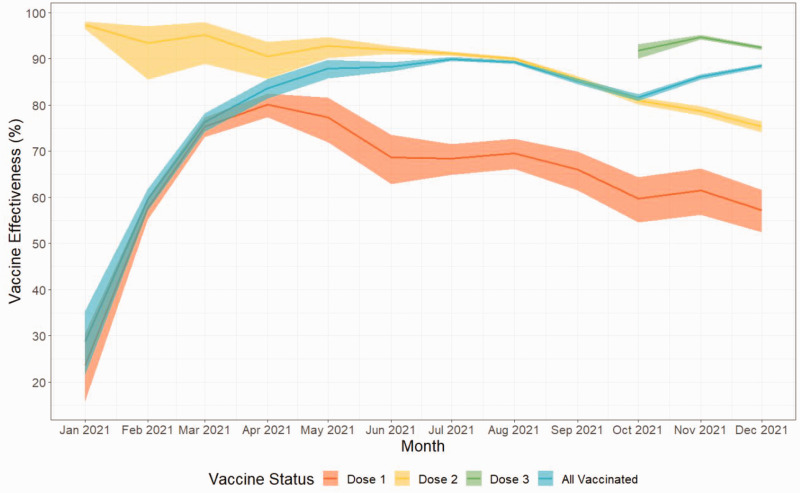
VE for Doses 1 and 2 steadily declined over the course of 2021 but continued to confer protective effects (Figure 3 and Table 1).
Figure 3.
Vaccine effectiveness by vaccination dose and month, January 2021 to December 2021. Shaded regions indicated 95% confidence intervals. Dose 1 equals those who received only one dose, Dose 2 equals those who received only two doses, Dose 3 equals those who received three or more doses.
Table 1.
Vaccine effectiveness (VE) by vaccination dose and month, January 2021 to December 2021.
| Dose 1 |
Dose 2 |
Dose 3 |
All vaccinated |
|||||
|---|---|---|---|---|---|---|---|---|
| Month | VE | 95% CI | VE | 95% CI | VE | 95% CI | VE | 95% CI |
| January 2021 | 23.7 | 15.9–30.7 | 97.3 | 96.4–98.0 | – | – | 28.9 | 21.6–35.5 |
| February 2021 | 57.7 | 55.2–60.1 | 93.4 | 85.5–97.0 | – | – | 59.5 | 57.1–61.8 |
| March 2021 | 75.3 | 73.1–77.3 | 95.2 | 88.9–97.9 | – | – | 76.3 | 74.2–78.3 |
| April 2021 | 80.1 | 77.4–82.5 | 90.5 | 85.6–93.7 | – | – | 83.6 | 81.4–85.6 |
| May 2021 | 77.3 | 71.9–81.7 | 92.7 | 90.1–94.7 | – | – | 87.9 | 85.8–89.8 |
| June 2021 | 68.7 | 62.9–73.6 | 92.0 | 91.0–92.8 | – | – | 88.3 | 87.2–89.3 |
| July 2021 | 68.4 | 64.9–71.6 | 91.1 | 90.7–91.5 | – | – | 90.0 | 89.5–90.4 |
| August 2021 | 69.6 | 66.1–72.7 | 90.0 | 89.6–90.4 | – | – | 89.3 | 88.8–89.7 |
| September 2021 | 66.0 | 61.5–69.9 | 85.6 | 85.0–86.2 | – | – | 85.1 | 84.4–85.7 |
| October 2021 | 59.8 | 54.5–64.4 | 81.0 | 80.1–81.8 | 91.8 | 90.0–93.2 | 81.6 | 80.8–82.4 |
| November 2021 | 61.5 | 56.2–66.2 | 78.7 | 77.7–79.7 | 94.7 | 94.1–95.2 | 86.1 | 85.4–86.7 |
| December 2021 | 57.3 | 52.5–61.6 | 75.3 | 74.1–76.5 | 92.4 | 91.9–92.8 | 88.5 | 88.0–88.9 |
Dose 1 equals those who received only one dose, Dose 2 equals those who received only two doses, Dose 3 equals those who received three or more doses.
Dose 1 VE peaked at 80.1% (95% CI: 77.4 to 82.5) in April and declined to 57.3% (95% CI: 52.5 to 61.6) by December. Dose 2 VE was over 90% until August and then declined to 75.3% (95% CI: 74.1 to 76.5) by December. As a result, overall VE declined from 90.0% (95% CI: 89.5 to 90.4) in July to 81.6% (95% CI: 80.8 to 82.4) in October. However, the introduction of a third dose (VE 91.8% (95% CI: 90.0 to 93.2)) to the wider public in October meant that overall VE increased to 88.5% (95% CI: 88.0 to 88.9) by the end of 2021.
Vaccine effectiveness by age and number of doses
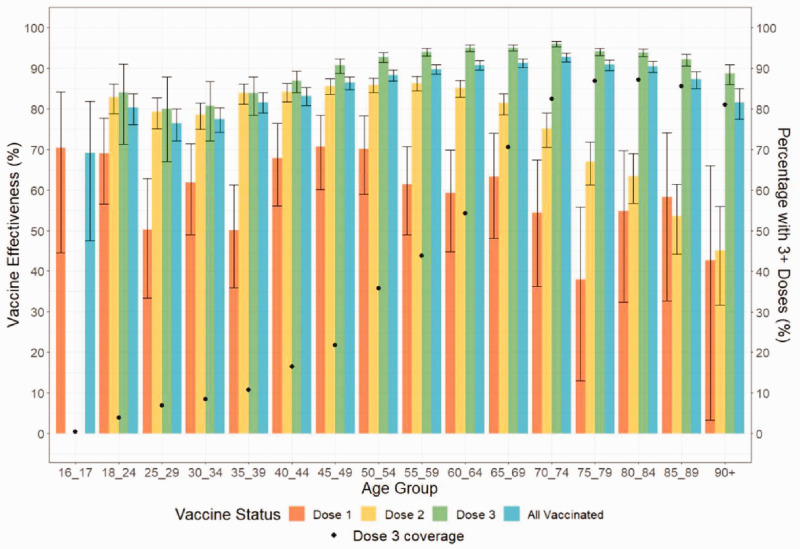
Figure 4 summarises VE by number of doses and age for December 2021.
Figure 4.
Vaccine effectiveness (VE) per age group and vaccination dose from 1 to 31 December 2021. Bars are shown with 95% confidence intervals. (Note: For the 16–17-year-old group, only 10.6% had two doses and 0.4% had 3+ doses, with <10 admissions so VE was not calculated). Dose 1 equals those who received only one dose, Dose 2 equals those who received only two doses, Dose 3 equals those who received three or more doses.
Dose 1 VE was broadly similar across all age groups (5.1% of the population received only one dose, giving wide CI). For Dose 2, there was a significant difference in VE between people aged 70 years and over (65.3%; 95% CI: 62.1 to 68.2) and those under 70 (79.8%; 95% CI: 78.8 to 80.7).
In contrast, Dose 3 VE remained high for all age groups (total: 92.4%; 95% CI: 91.9 to 92.8; range 80.0% (95% CI: 67.0 to 87.9) in 25- to 29-year-olds to 96.1% (95% CI: 95.3 to 96.7) in 70- to 74-year-olds). Dose 3 VE was significantly higher than Dose 2 VE in age groups >39 years. The disparity between Doses 2 and 3 VE was greatest in those aged 70 years or more, with 82.3% (95% CI: 81.2 to 83.4) fewer admissions per 100,000 person-days for those with ≥3 doses versus those with two.
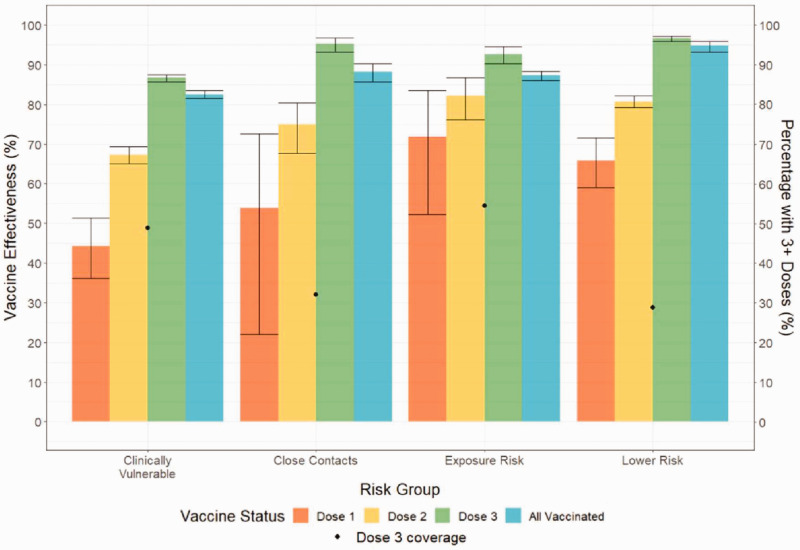
Vaccine effectiveness by risk group
Figure 5 summarises VE by risk group for December 2021.
Figure 5.
Vaccine effectiveness per risk group and vaccination dose for the period of 1 to 31 December 2021. Bars are shown with 95% confidence intervals. NB: ‘Lower Risk’ refers to individuals who are not in a high-risk group. Dose 1 equals those who received only one dose, Dose 2 equals those who received only two doses, Dose 3 equals those who received three or more doses.
For the Clinically Vulnerable, Dose 1 VE was significantly lower than the Lower Risk group; Dose 2 VE was significantly lower compared to Lower Risk and Exposure Risk groups; Dose 3 VE was significantly lower than all other groups. However, vaccination with three or more doses still conferred a VE of 86.7% (95% CI: 85.8 to 87.6).
Except for the clinically vulnerable, Dose 3 VE exceeded 90% in all risk groups with 59.3% (95% CI: 56.7 to 61.7) fewer admissions per 100,000 person-days among the Clinically Vulnerable and 81.2% (95% CI: 72.6 to 87.2) fewer among the Close Contacts group relative to Dose 2.
Discussion
The UKHSA produces weekly COVID-surveillance reports that provide ‘real-time' assessments of COVID-19 VE. 22 These show vaccines can be over 95% effective at preventing hospital admissions but the level of protection varies substantially by population group, COVID-19 variant, vaccine manufacturer and time since vaccination. Our analysis differs in showing the effectiveness at monthly time points which when considered alongside vaccine coverage demonstrates likely direct vaccine impact at a point in time.
Hospital admission rates by vaccination status
Rates of COVID-19 hospital admissions in the unvaccinated greatly exceeded those of the vaccinated throughout 2021 (Figure 2). Despite sharply increasing case rates at the end of 2021, admission rates remained stable, driven by low admission rates in the vaccinated group.
Vaccine effectiveness over time
Both Doses 1 and 2 VE wanes with time (Figure 1). The introduction of Dose 3 led to a rise in overall VE but our analysis cannot determine if this reflects the increased number of doses, or having a more recent dose. By the end of December 2021, 59% of the English population aged 12+ had received a booster dose, compared to 87% for the primary course, leaving many without a recent vaccine. 6 This is concerning given the benefit of multiple doses and the risk of VE waning over time. 15
Vaccine effectiveness by age group and number of doses
DSARs were lower with more vaccine doses, suggesting a dose-dependent effect. Despite increasing prevalence and new variants, overall DSARs for the vaccinated remained steady from September to December 2021 (Figure 2), likely because more people had received a second and third dose. In people aged over 50 years, the addition of a third or later dose appears to be critical to providing effective protection. This is particularly important, as increased age is a significant risk factor for mortality from COVID-19. 23
Vaccine effectiveness by risk group
Vaccination protects against admission in all risk groups (Figure 5). VE for both Doses 1 and 2 was highest for the Exposure Risk group despite the prolonged time since initial vaccination, potentially due to reduced immunological waning in younger populations 24 (although may also reflect a greater degree of natural immunity from exposure). For the clinically vulnerable, although VE was lowest across all doses, vaccination with three or more doses still conferred substantial protection. Since the group includes the severely immunosuppressed, this could reflect reduced immunological response to vaccination or accelerated immunological waning. 25 Given that the Exposure Risk and Clinically Vulnerable were both vaccinated early, waning VE alone does not fully explain the reduced VE for the Clinically Vulnerable.
Strengths and limitations of the study
We used data over a whole calendar year, during the emergence of multiple variants, variable case rates and changing vaccine uptake. This provides a population-level overview of the impact of vaccination that is not possible from studies over a shorter period. Using primary diagnosis of COVID-19 as the inclusion criteria increases the specificity of our study by excluding those co-incidentally COVID-19 positive but admitted for another reason.
Our results are expressed in relation to calendar months rather than time since vaccination, making it difficult to accurately estimate waning of VE for each dose. There are unmeasured confounding factors, including exposure to COVID-19, adherence to social distancing and use of personal protective equipment. Such health-seeking behaviours may reflect individual propensity for vaccination, further biasing the analysis.
The study relies on routinely collected NHS data sources with some data-quality issues and misclassification. For the risk group analyses, there may be cross-over with individuals included in multiple groups. Additionally, changes in patient’s clinical status and risk group definitions mean that those included in these groups varied during 2021.
DSARs were the only outcome measured. Although decisions to admit would be based on clinical severity, we cannot exclude that vaccine status may have been a consideration in individual cases. In addition, thresholds for admission vary geographically, limiting the comparability of results with other countries. Future research into the effectiveness of subsequent boosters and analyses of impact on other indicators of severe disease (e.g. length of stay, critical care admissions, mortality) would be useful although may be harder to undertake, as testing for COVID in both the community and hospitals is now more restricted.
Comparison with other studies
We found a dose-dependent relationship between vaccination and reduced hospitalisations on a national-level and substantial VE waning of the primary course over time, mirrored in other international research,13,15,26,27 including one study that demonstrated a waning antibody response to a fourth booster vaccine. 28 Primary course VE waning was most notable in older people, suggesting that the primary course alone may not provide sufficient protection in older age groups. Other research in the UK and USA has also shown lower rates of protection in the elderly.11,29 Although a large cohort study in Italy found a larger risk reduction in hospitalisations in older populations compared to younger, this only included first doses. 30 We found differences between risk groups, with the Clinically Vulnerable group generally exhibiting a lower VE than other groups. A meta-analysis of vaccine efficacy in the immunocompromised noted reduced seroconversion after their first and second vaccines (except for those with HIV). 25 They concluded that there was a significant additional benefit of receiving a third dose for this group, in line with our findings. A limitation of both studies is the heterogenicity within groups and the difficulty defining and identifying the immunocompromised.
Conclusion
Our analysis shows population level waning in VE over time, improving with subsequent doses. We demonstrated a disparity in VE between age and risk groups, with greater protection after a third dose in older people and some risk groups. If this trend of VE waning after each dose continues, our analysis supports an ongoing programme of regular booster vaccinations for the elderly and those at increased risk. This is in line with recent JCVI announcements regarding future booster programmes. Further research could include analyses for different sub-groups (e.g. ethnicity and deprivation, vaccine type/manufacturer and time since vaccination). The use of other outcomes, such as mortality, length of stay or critical care admissions, would add further insight into protection against severe disease.
What is already known on this topic
Vaccination against COVID-19 is effective at preventing hospitalisation, but much of the available research is conducted over limited time periods, mainly near the start of vaccination programmes or in relation to a specific variant, dose or type of vaccine.
What this study adds
Our study adds to existing literature by using a whole calendar year of linked data. Our research shows that the pandemic is not over for people who are unvaccinated, and that relatively low admission rates in vaccinated people drove the stable overall admission rates seen at the end of 2021 despite sharply increasing COVID-19 case numbers.
We demonstrate a dose-dependent effect of vaccination, as well as apparent waning of the effectiveness of each vaccination dose, potentially more marked in elderly and specific clinical risk groups, highlighting the value of booster vaccinations. Based on this pattern of dose-dependent effectiveness and waning, our analysis supports an ongoing programme of booster vaccinations, especially in the elderly and risk groups.
Supplemental Material
Supplemental material, sj-pdf-1-jrs-10.1177_01410768231157017 for Impact of COVID-19 vaccination on COVID-19 hospital admissions in England during 2021: an observational study by Felicity Cornforth, Lucie Webber, Gabriele Kerr, Hywell Dinsdale, Azeem Majeed and Peter Greengross in Journal of the Royal Society of Medicine
Footnotes
ORCID iDs: Lucie Webber https://orcid.org/0000-0001-9480-2335
Azeem Majeed https://orcid.org/0000-0002-2357-9858
Declarations
Competing Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PG is employed by NHS England as Joint Medical Director for the National COVID-19 Vaccination Programme. FC and LW have both been attached to the programme as part of their Foundation Year training attachments. HD is employed by Integral Health Solutions and contracted to NHS England Vaccination Programmes. The other authors declare no competing interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GK and AM are supported by the National Institute for Health Research (NIHR) under the Applied Research Collaboration (ARC) programme for Northwest London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health and Social Care. All researchers were independent from funders and all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. There were no study sponsors.
Ethics approval
Not applicable. The sharing of data with Imperial College was approved by the Information Governance Team at NHS England without the need for further ethics approval.
Guarantors
FC, AM, PG.
Contributorship
All authors contributed to the study design and interpretation of data, and critical revision of the manuscript for important intellectual content. FC and LW drafted the manuscript. HD and GK were responsible for the preparation and analysis of the data. FC, AM and PG are the guarantors.
Provenance
Not commissioned; peer reviewed by Neil Snowise, Niamh Spence, and Julie Morris.
Data availability and sharing statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.UKHSA. Healthcare in England | Coronavirus in the UK. See https://coronavirus.data.gov.uk/details/healthcare?areaType=nation&areaName=England (last checked 29 April 2022).
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majeed A, Pollock K, Hodes S, Papaluca M.Implementation of Covid-19 vaccination in the United Kingdom. BMJ 2022; 378: e070344. [DOI] [PubMed] [Google Scholar]
- 5.GOV.uk. COVID-19: the green book, chapter 14a. GOV.UK. See www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a (last checked 3 May 2022).
- 6.England Summary | Coronavirus (COVID-19) in the UK. See https://coronavirus.data.gov.uk (last checked 9 May 2022).
- 7.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021; 397: 1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on Covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021; 373:n1088: bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glampson B, Brittain J, Kaura A, Mulla A, Mercuri L, Brett SJ, et al. Assessing COVID-19 vaccine uptake and effectiveness through the North West London Vaccination Program: retrospective cohort study. JMIR Public Health Surveill 2021; 7: e30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.León T, Dorabawila V, Nelson L.COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis – California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med 2022; 386: 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas EJ, McLaughlin JM, Khan F, Angulo FJ, Anis E, Lipsitch M, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis 2022; 22: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384(15): 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ONS. Coronavirus (COVID-19) latest insights – Office for National Statistics. See www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/overview (last checked 3 May 2022).
- 15.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021. Dec 9;385(24):e84. NEJMoa2114583. Epub 2021 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majeed A, Papaluca M, Molokhia M.Assessing the long-term safety and efficacy of COVID-19 vaccines. J R Soc Med 2021; 114: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHS England. Tackling list inflation for primary medical services. See www.england.nhs.uk/wp-content/uploads/2013/10/tack-infla.pdf (last checked 3 May 2022).
- 18.ONS. Population estimates for the UK, England and Wales, Scotland and Northern Ireland – Office for National Statistics. See www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2020 (last checked 6 June 2022).
- 19.NHS England. Public Definitions. See www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2020/12/Publication-definitions-1.pdf (last checked 3 May 2022).
- 20.Dobson AJ, Kuulasmaa K, Eberle E, Scherer J.Confidence intervals for weighted sums of Poisson parameters. Stat Med 1991; 10: 457–462. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; 2021. See www.R-project.org (last checked 3 May 2022).
- 22.UKHSA. COVID-19 vaccine monthly surveillance reports. GOV.UK. See www.gov.uk/government/publications/covid-19-vaccine-weekly-surveillance-reports (last checked 26 August 2022).
- 23.Romero Starke K, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A.The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health 2021; 6: e006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis 2022; 22: 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of Covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022; 376: e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 398: 2093–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 2022; 386(4): 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canetti M, Barda N, Gilboa M, Indenbaum V, Asraf K, Gonen T, et al. Six-month follow-up after a fourth BNT162b2 vaccine dose. N Engl J Med 2022; 387: 2092–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan C, Turtle L, Thorpe M, Harrison EM, Semple MG, Docherty AB.Hospital admission for symptomatic COVID‐19 and impact of vaccination: analysis of linked data from the Coronavirus Clinical Information Network and the National Immunisation Management Service. Anaesthesia 2022; 77: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mateo-Urdiales A, Spila Alegiani S, Fabiani M, Pezzotti, Filia A, Massari M, et al. Risk of SARS-CoV-2 infection and subsequent hospital admission and death at different time intervals since first dose of COVID-19 vaccine administration, Italy, 27 December 2020 to mid-April 2021. Euro Surveill: Eur Commun Dis Bull 2021; 26: 2100507. ES.2021.26.25.2100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jrs-10.1177_01410768231157017 for Impact of COVID-19 vaccination on COVID-19 hospital admissions in England during 2021: an observational study by Felicity Cornforth, Lucie Webber, Gabriele Kerr, Hywell Dinsdale, Azeem Majeed and Peter Greengross in Journal of the Royal Society of Medicine
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.