Highlights
-
•
Reclamation of surface water using Advanced Oxidation Processes.
-
•
Hybrid Hydrodynamic Cavitation(HC) technique for lake water treatment.
-
•
Optimized operating parameters i.e. pH, inlet pressure, H2O2 loading.
-
•
Achieved ∼64% removal of COD and BOD and ∼95% removal of microbes using hybrid HC + H2O2 Technique.
Keywords: Advanced oxidation process, Disinfection, Hydrodynamic cavitation, Reactive oxygen species, Surface water
Abstract
Water reclamation from lakes needs to be accomplished efficiently and affordably to ensure the availability of clean, disinfected water for society. Previous treatment techniques, such as coagulation, adsorption, photolysis, ultraviolet light, and ozonation, are not economically feasible on a large scale. This study investigated the effectiveness of standalone HC and hybrid HC + H2O2 treatment techniques for treating lake water. The effect of pH (3 to 9), inlet pressure (4 to 6 bar), and H2O2 loading (1 to 5 g/L) were examined. At pH = 3, inlet pressure of 5 bar and H2O2 loadings of 3 g/L, maximum COD and BOD removal were achieved·H2O2 was observed to significantly improve the performance of the HC when used as a chemical oxidant. In an optimal operating condition, a COD removal of 54.5 % and a BOD removal of 51.5 % using HC alone for 1 h is observed. HC combined with H2O2 removed 64 % of both COD and BOD. The hybrid HC + H2O2 treatment technique resulted in a nearly 100% removal of pathogens. The results of this study indicate that the HC-based technique is an effective method for removing contaminants and disinfection of the lake water.
1. Introduction
The use of surface water bodies, such as ponds, lakes, rivers, etc., has been a major source of domestic water. As a result of sewage discharges directly into water bodies without proper treatment and several anthropogenic activities, many water bodies have become unusable. Ingestion of this polluted water containing several pathogens can result in serious health problems such as diarrhoea, cholera, dysentery, and typhoid. Some of the most significant pollutants in surface water bodies include personal care products, surfactants, pharmaceuticals, pesticides, and household chemicals [1], [2]. Researchers are increasingly interested in emerging pollutants found in water. Since they are present in trace amounts (ppb), monitoring and removing them becomes more challenging [3]. Due to the current water scarcity problem, practical and economical methods must be developed to ensure the availability of clean surface water for household use. Water supply is one of the largest challenge for the rural areas in the developing countries, especially in areas where most of the population relies directly on rivers, lakes, dams, and ponds for their daily needs. Conventional technologies include chlorination, coagulation [4], biological techniques [5], [6], membrane processes [7], [8], [9], photocatalysis [10], adsorption [11], [12], ozonation [13], etc. Several challenges face the development of these technologies, including economic viability, scalability, production of harmful by-products, and higher operational costs.
Alakaparampil et al. [14] used moringa seed powder as a coagulant and copper as a disinfectant to reduce turbidity and pathogens in contaminated river water. In treated water samples, the turbidity ranged from 3.3 to 5.0 NTU, and there was no sign of E. coli contamination. According to their estimates, the treatment costs approximately 0.5 USD per litre, making it an affordable and effective option. Contaminated water bodies may also contain a variety of pathogenic microbes capable of transmitting several fatal water-borne diseases. For this reason, it is imperative to kill the pathogens in the water bodies so that they can be consumed [15]. It has been demonstrated that water can be disinfected using various physical methods, including filtration, coagulation, adsorption, UV irradiation, and chemical methods, including chlorination, ozonation, etc. [16], [17]. A successful disinfection technique requires the inactivation or killing of microorganisms by damaging their cell structure or disrupting their metabolic activities.
In treating complex wastewater containing recalcitrant pollutants, AOPs are very effective [18]. Several AOPs have been investigated for surface water treatment to effectively remove micro-pollutants and disinfect them. The techniques described above are considered green and efficient [19]. The effectiveness of ozonation for removing carbamazepine and diclofenac was studied by Sui et al. An ozone dosage of 5 mg/L removed more than 95% of pollutants [20].
The use of cavitation has been a promising alternative for treating and disinfection of complex wastewater in recent years. The cavitation phenomenon involves the nucleation, growth, and implosion of vaporous cavities in a reactor at numerous locations. It is a localised phenomenon resulting in extreme temperatures (5000–6000 K) and critical pressure conditions (4000–5000 atm.) within the collapsing bubble[21], [22]. Bubble collapse results in chemical and mechanical effects, and both contribute to the contaminant degradation[23], [24]. On the basis of medium used for the radical generation, cavitation is classified as acoustic, hydrodynamic, optical, and particle cavitation. The most significant of them are; acoustic and hydrodynamic cavitation.
In acoustic cavitation, high-energy ultrasound transducers generate radicals, making the process more energy intensive [21]. The hydrodynamic cavitation method involves the passing the liquid through a constriction device at a high velocity resulting in a sudden fall in the pressure below the vapour pressure of liquid and the formation of vaporous cavities takes place [25], [26]. Different type of constriction device used are orifices, vortex diodes, venturi etc. It is a highly energy-efficient technique with a greater scale-up potential [27], [28]. Recent research has indicated that HC-based hybrid AOPs offer a lot of promise for addressing persistent organic pollutants. The efficacy and cost-effectiveness of various hybrid treatment technologies have been demonstrated in real-life applications [29]. Numerous studies demonstrated that the use of HC in combination with other chemical oxidants, such as H2O2, Ozone, Persulfates, Fenton's Reagent, peracids etc. significantly improved the efficiency of mineralization [30], [31]. The oxidant itself is oxidised to produce reactive OH. radicals. In additional to radical degradation, pyrolysis inside the cavitation bubble can cause contaminants to dissociate, as illustrated in equation (1). The system's oxidants and these radicals engage in a series of chain reactions resulting in the mineralization of contaminants [32], [33] Therefore, the issues with the separate processes can be resolved by combining HC with these oxidants.
| (1) |
Persulfates radicals having relatively higher oxidation potential (2.6 V) and longer lifetime than hydroxyl radicals have also been used effectively with HC for treatment of groundwater and industrial wastewaters [34]. It demands effective activation techniques due to symmetric structure and higher bonding energy of persulfate anion. Techniques such as UV. Ultrasound, Heat, Catalysts such as transition metals, bases, carbonaceous materials have been studied for generation of sulphate anion [35]. HC is also one of the effective activation method for generation of persulfate anion. Under HC, SO4•− radicals are formed under extreme conditions existing inside cavities which further reacts with water molecules to form HO• radicals. All these generated radical further result in mineralisation of the contaminants. Kirill Fedorov et. al. used HC-PS technique for minerallisation of BTEX in the water and achieved degradation of 91.51%, 95.50%, 94.65%, 94.95% of benzene, toluene, ethylbenzene and o-xylene respectively in 240 min [36].
Percarbonates have also been studied as a safer and cheaper alternative to H2O2 based AOPs. Most commonly studied material is Sodium Percarbonate (Na2CO3⋅1·.5H2O2). It has several advantages; application over wide range of pH and stops acidification of treated media unlike H2O2.[37] Several techniques were used for SPC activation e.g. Ultrasound [37], Ferrous ions (Fe2+) [38], UV irradiation [39] etc. Action of Percarbonates is based on generation reactive oxygen species (ROS) including superoxide (O2•−−) and carbonate (CO3•−−) In spite of having lower oxidation potential (1.78 V) carbonate radicals react with electron rich organic contaminants e.g. phenols and amines etc. These Techniques are thus bet suitable for samples containing electron rich pollutants. Kirill Fedorov et. al. (2022) studied HC activation of SPC and Ozone for degradation of 1,4 dioxane. They observed More than 99% removal efficiency in 120 min at optimum operating conditions of Cv-0.27, pH-5, operating temperature 25±2 °C, Ozone input 0.86 g/h, initial pollutant concentration 100 ppm [40].
Apart from reactive •OH radicals, SO4•−, O2•− and HO2• are several other ROS that are involved in the pollutant degradation [41]. Thus presence of multiple ROS can result occurrence of several simultaneous reactions during AOPs treatments. Many of this reactions apart from main reaction can result into the formation of different by-products which may be more toxic than primary contaminants. Some of the intermediate products formed can dimerise or crosslink, thereby spoiling the effectiveness of degradation by the treatment. Several studies reported the formation of nitro compounds during degradation of contaminants either in presence or in absence of nitrite ions [42]. These compounds have been reported as carcinogenic and should be monitored during the treatment. Moreover, nitrate and nitrite ions reacts with hydroxyl radicals to form RNs as secondary radicals with lower oxidation potential [43].
| (2) |
| (3) |
For water samples that contain pathogenic microbes, cavitation may be a viable alternative to conventional disinfection methods [44]. A possible explanation for the mechanism of cavitation disinfection is oxidative damage and mechanical disruption of the cell wall. It has been reported that hybrid hydrodynamic cavitation technology provides nearly 100% disinfection [45]. With hybrid HC + H2O2, disinfection rates were increased several times, drastically reducing treatment time [44]. Dong and Zhao evaluated the disinfection of Shengli River using HC at an initial microbial concentration of 3.76×10−4 CFU/mL. They observed that almost 100 % of the microbes were killed within 20 min [46]. The present study examined the percentage reduction of COD and BOD and the microbial disinfection of real lake water samples using a standalone HC as well as hybrid HC + H2O2 techniques. A comparison of the performance of both techniques under optimum operating conditions is presented here based on different optimisation parameters. The energy requirements and treatment costs of both the treatment methods are also studied.
2. Materials and methods
2.1. Chemicals and reagents
Analytical grade H2O2 (30% w/v) and Sulphuric Acid (98% w/v) were obtained from S.D. Fine Chemicals Limited, Baroda, India. Sodium hydroxide pellets were received from Sisco Research Laboratories Pvt. Ltd. Taloja, India. pH was adjusted using H2SO4 (1 M) and NaOH (1 M). In all cases, the chemicals used were analytical grade and utilised as received from the suppliers. Fresh distilled water was obtained from the laboratory's distilled water plant for preparing solutions. For COD and BOD analysis, all reagents were prepared freshly and stored according to standard procedures.
2.2. Description of the site and sampling
Surface water samples were collected at Padmakshi Lake, Warangal, Telangana State, India. Padmakshi Lake is located near a residential area and the Padmakshi Temple. Religious activities and the discharge of domestic waste primarily cause pollution in the Padmakshi lake. In order to collect the samples, sterile glass bottles were used. They were then transported to the laboratory for subsequent analysis. After collection, the experiments were conducted within 1 to 3 h, and the treated samples were stored in the refrigerator for further analysis. Initial analysis of the collected sample was done and is reported in Table 1.
Table 1.
Parameter analysis of collected lake water sample.
| Sr. No. | Water Analysis Parameter | Unit | Collected Samples |
|---|---|---|---|
| 1 | BOD | mg O2/L | 18–23 |
| 2 | COD | mg O2/L | 50–61 |
| 3 | Dissolved Oxygen | mg O2/L | 2.5–5 |
| 4 | TOC | ppm | 33–38 |
| 5 | Turbidity | NTU | 65–75 |
| 6 | Nitrates | ppm | 2.5–3.4 |
| 7 | Phosphates | ppm | 7–9 |
| 8 | TDS | ppm | 280–320 |
| 9 | pH | 6.5–8.5 |
2.3. Experimental
2.3.1. Setup of the hydrodynamic cavitation system
Fig. 1(a) illustrates the experimental setup for HC, which includes a 5 L holding tank, a pump, manual control ball valves, pressure gauges and cavitating device. The positive displacement pump of capacity 2HP (Model No. LS-30N) manufactured by L Shyong Machinery Industry Co. Ltd. (Taiwan) is used. The stainless steel (SS 316) material was used for fabrication of the setup. The pump inlet is connected with the bottom of the holding tank. An outlet of the pump is divided into two lines, a bypass line and a mainline. The control valve on the bypass line controls water flow through the mainline. The mainline is equipped with a cavitating device, and pressure gauges P1 and P2 measures pressure and downstream recovered pressure, respectively. There is a submersion of the mainline and bypass line below the solution level within the holding tank to prevent air entrapment, which may lead to a variation in the results.
Fig. 1.
(a) Schematic diagram of a hydrodynamic cavitation system (b) Schematic of an orifice plate.
As shown in Fig. 1(b), the cavitating device used in our setup is an orifice plate made from SS 316. Single hole 0.5 mm thick orifice plate with 2 mm diameter hole in the centre is used as cavitating device and is fitted on the mainline. The setup was cleaned by circulating water for 5 min before each experiment. The orifice was detached and cleaned properly with detergent during the cleaning process. Before conducting any experiment, all water was removed to prevent sample contamination and dilution.
2.4. Experimental methodology
2.4.1. Treatment using HC alone
HC was used first to treat the collected lake water samples. HC technology offers several advantages in wastewater treatment, including robust equipment setups, shorter treatment times, high efficiency, and greater scale-up potential [47], [48]. The performance of the HC is determined by several operating parameters, including the inlet pressure, pH, cavitation number, orifice geometry, and physicochemical properties of the liquid, including surface tension, density, viscosity, temperature, etc. [47]. The present study investigates the effect of operating parameters, operating time, inlet pressure, pH, oxidant dosages, etc., on degradation performance. COD and BOD analyses were conducted on samples collected at regular intervals of 10 min.
2.4.2. Treatment using hybrid HC + H2O2
The combination of HC with other AOPs, such as UV, ozone, Fenton, etc. showed significant improvement in the mineralisation of pollutants [49]. HC can, for instance, be significantly improved by combining it with a strong chemical oxidant, such as ozone or H2O2·H2O2 is an additional source of hydroxyl radicals, a strong oxidant. This study utilised HC and H2O2 as a hybrid treatment for lake water treatment, thus intensifying the HC process [50]. It has been reported that pH and H2O2 dosage significantly impact the oxidative performance of H2O2. A pH range of 3 to 9 and a range of H2O2 dosages (1 to 5 g/L) were used. At the beginning of the experiment, H2O2 was added to the holding tank. H2SO4 (1 M) and NaOH (1 M) were used to adjust the pH. After every 10 min, samples were collected, and COD and BOD were analysed.
2.5. Analytical methods
The COD digester (B.S. Scientific Co., Ambala 133001, Punjab, India) is an open-reflux method for analysing organic pollutants found in lake water samples. BOD of the lake water sample was determined using APHA (American Public Health Association) Standard Methods for the Examination of Water and Wastewater, 2005. BOD5 technique is used to determine BOD values of the sample. D.O. of the blank, initial, final sample was determined using Wrinkler’s Iodometric titration method. Using the serial dilution method, the microbial count of the samples was performed. Turbidity, pH, and TDS were determined respectively using a nephelometric turbidity meter, pH meter, and TDS meter manufactured and supplied by Hanna Instruments, USA. In order to improve the accuracy of the results, all analyses were carried out in triplicate using real water samples.
3. Results and discussion
3.1. Standalone HC technique
3.1.1. Effect of operating time on the degradation of organic pollutants
An important factor influencing the efficiency of the HC process is the operating time. The amount of water that passes through the cavitating device increases with increased treatment time. As a result, the sample will be exposed to the cavitation zones for longer, leading to higher degradation. Initial experiments in this study showed that COD and BOD values did not significantly decrease after a treatment time of 1 h. Therefore, all further experiments were conducted with a treatment time of 1 h.
3.1.2. Inlet pressure
The degradation characteristics of the HC process are greatly influenced by the pressure at the entrance to the cavitating device [51]. Cavity collapse is intensified by higher inlet pressures, resulting in a greater cavitational active volume and greater mineralisation. However, there is an optimum inlet pressure beyond which degradation does not increase significantly due to super cavitation (choked cavitation). By forming larger vapour cavities, microbubbles implode, reducing the generation of free radicals [47]. A study by Suresh Kumar et al. [52] examined the degradation of dye wastewater at varying pressures of 2–8 bar. At an optimum pressure value of 6 bar, the maximum level of decolourisation was observed to be 31.23 %, after which there was no significant improvement in the level of decolourisation. Wang et al. [53] also observed a similar trend when using HC for the degradation of Alachlor.
This study treated lake water samples using inlet pressures of 3 to 6 bar. Since the pollutants in lake water are complex, all results are expressed as a percentage reduction in COD and BOD of samples collected at regular intervals. With an increase in pressure from 3 to 5 bar, the percentage reduction in BOD and COD increased from 27 to 39 and 29.7 to 48.9 % respectively. As reported in the literature, the increased implosion intensity in cavities may explain this phenomenon. Furthermore, increased pressure to 6 bar reduced 35.2 and 46 %, respectively, in BOD and COD. In addition, Boczkaj et al. [48] reported a maximum reduction in COD of 13% at an optimum pressure of 8 bar when inlet pressures ranged from 6 to 10 bar. A similar pattern of degradation of dichlorvos due to super cavitation beyond the optimum inlet pressure has been observed by Joshi and Gogate [54].
Cavitation number (Cv) is the dimesnsionles number that correlates inlet pressure and velocity of fluid at the throat of cavitating device[55]. It is very important parameter for determining intensity of cavitation phenomenon and needs to be optimized. As reported from the literature, with an increasing pressure the velocity of the liquid through cavitating device reduces and thereby reducing the value of Cv. The cavitation inception number (Cvi) is the value of Cv at which first cavity is formed and it is affected by geometry of the cavitational device.[56] It has been reported that lower Cv generates higher number of cavities, thereby generating greater number of •OH radicals. Additionally, increases velocity at lower Cv values increases the number of passes through the cavitating device resulting increased exposure time of pollutants to the cavitational zone.[47] All these events result in greater extent of degradation of pollutants at lower Cv values. Ideally, Cv <1 is preferred for effective pollutant degradation. Similar trend in Cv values was observed in the present study as shown in the Table 2 and maximum COD removal of 48.9% was achieved at 5 bar with optimum Cv = 0.19.
Table 2.
Effect of inlet pressure on % COD removal (pH: 6±1, Orifice diameter: 2 mm, Time: 60 min).
| Sr. No. | Inlet Pressure (bar) | Vol. Flow rate (LPH) | Velocity (m/s) |
Cavitation Number (Cv) | % COD removal |
|---|---|---|---|---|---|
| 1 | 3 | 260 | 24.35 | 0.37 | 29.7 |
| 2 | 4 | 310 | 27.10 | 0.26 | 38 |
| 3 | 5 | 360 | 31.85 | 0.19 | 48.9 |
| 4 | 6 | 400 | 44.64 | 0.08 | 46 |
In this study, it was observed that the BOD and COD levels of the treated samples decreased following similar trend. According to Fig. 2, Fig. 3, the maximum reduction in BOD and COD was observed at an inlet pressure of 5 bar with optimum Cv value of 0.19. Therefore, all subsequent experiments were conducted at this inlet pressure.Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Fig. 2.
Effect of inlet pressure on the degradation of BOD in lake water samples (Operating conditions: pH 6±2(original), Treatment time 1 h).
Fig. 3.
Effect of inlet pressure on the degradation of COD in lake water samples (Operating conditions: pH 6±1.5 (original), Treatment time 1 h).
Fig. 4.
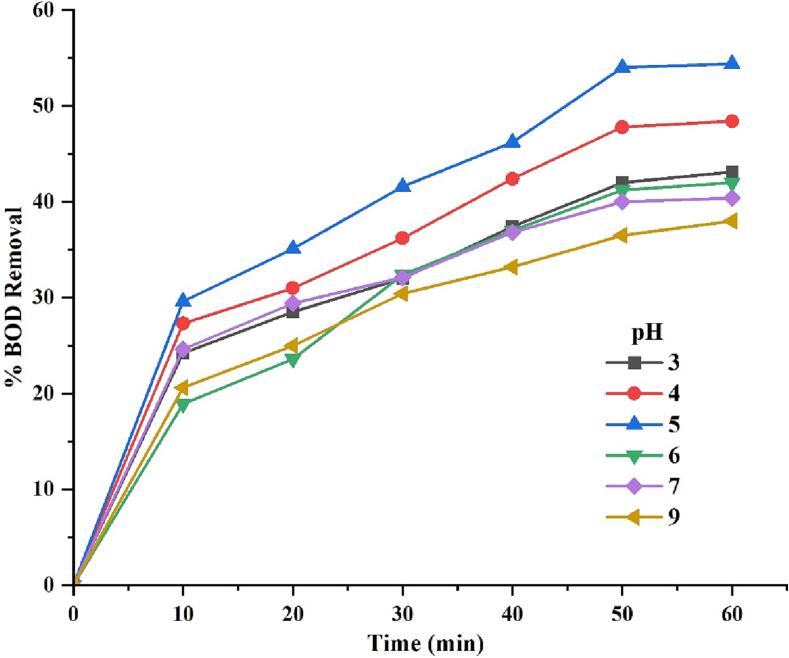
Effect of pH on the degradation of BOD in lake water samples (Inlet pressure 5 bar, H2O2 dosage 4 g/L, Treatment time 1 h).
Fig. 5.
Effect of pH on the degradation of COD in lake water samples (Inlet pressure 5 bar, H2O2 dosage 4 g/L, Treatment time 1 h).
Fig. 6.
Effect of varying H2O2 dosage on the degradation of BOD in lake water samples (pH 5, Inlet pressure 5 bar, Treatment time 1 h).
Fig. 7.
Effect of varying H2O2 dosage on the degradation of COD in lake water samples (pH 5, Inlet pressure 5 bar, Treatment time 1 h).
Fig. 8.
Microbial counts of lake water (a) Initial (b) After HC treatment (c) After HC + H2O2 treatment.
3.2. Hybrid HC + H2O2 technique
3.2.1. Effect of pH
HC-based techniques are also significantly affected by the pH of the water sample. Additionally, the nature and state of the pollutants under cavitating conditions, i.e. ionic or molecular state, affects their degradation significantly. Pollutants are degraded by the action of hydroxyl radicals generated during the degradation process [57] It is, therefore, very important to know the molecular structure and type of functional groups present in the pollutants. A study by Patil et al. [58] indicated that imidacloprid degrades more rapidly under acidic conditions. At pH 3.0, they found the highest degradation of 23.85%. In addition, Barik and Gogate [59] examined the degradation of 2, 4, and 6-trichlorophenol at pH ranges between 3 and 11. At an optimum pH of 5, a maximum degradation of 32.13% was observed. Acidic conditions promote generation of hydroxyl radicals with greater oxidation potential. It was also reported that rated of rate of radical recombination is lower under acidic conditions [60].The degradation efficiency decreases with increasing pH. This is attributed to the fact that under alkaline pH environment H2O2 is unstable and dissociates into to oxygen and water as shown in equation (4)[61]. Moreover, HO2– ions formed in equation (5) reacts rapidly with hydroxyl radical (k = 7.5×109M−1s−1) producing superoxide anions having lower oxidizing power compared to hydroxyl radical [62]. Thus degradation efficiency of the H2O2 reduces in alkaline environment.
| (4) |
| (5) |
| HO2− + H2O2 → H2O + O2 + OH− | (6) |
| OH• + HO2− → H2O + •O2–− | (7) |
Although most studies reported better degradation performance under acidic conditions, there are few studies that showed better degradation performance under alkaline conditions [63]. A hybrid process (HC + H2O2 + O3) was investigated by Rajoriya et al. [64] for the degradation of Rhodamine 6G at varying pH levels from 2 to 12. At pH 10, the highest degradation was observed. According to them, Rhodamine 6G molecules became hydrophobic under alkaline conditions due to the transfer of charge from the amine nitrogen to the aromatic rings of the molecule. It is, therefore, necessary to optimise the pH of the sample in order to maximise the efficiency of HC and H2O2. Also, the cost of chemicals used to adjust pH contributes to the operating costs of the process. Moreover, a subsequent neutralisation of the discharge is necessary since it is expected to have a pH of approximately 7.
By varying pH in the range of 3–9, the effect of pH on hybrid HC + H2O2 process performance was investigated. Performance was evaluated by determining the percentage reduction in COD and BOD values based on samples collected at regular intervals. It was observed that the maximum degradation of 54.4 % in BOD and 56.2 % in COD occurred at a pH value of 5. The results indicate that acidic conditions resulted in the degradation of COD and BOD, possibly due to the chemical nature of the contaminants favouring the mineralisation under acidic pH conditions, thereby showing higher COD removal under acidic conditions. Therefore, all subsequent experiments were conducted at pH 5.
3.2.2. Effect of H2O2 dosage
Chemical oxidants such as H2O2, ozone, etc., can significantly improve the performance of the HC process. Under cavitation, H2O2 is susceptible to dissociation, increasing the count of hydroxyl radicals available in the system. Moreover, different operating parameters e.g. pH, H2O2 dosage, initial concentration of pollutant etc. significantly affect the performance of this hybrid technique. A significant increase in degradation was reported when H2O2 concentrations were increased, possibly due to the generation of more radicals. A hybrid HC and H2O2 method was used by Thanekar et al. [65] to degrade Naproxen (NAP). An increase in the molar ratio of H2O2 to NAP from 50 to 1000 resulted in an increase in degradation from 34.4% to 80%. The improvement in degradation is seen, however, only up to a certain optimum concentration of H2O2, above which there is only a marginal improvement.
It may be due to the scavenging effect of H2O2 at higher concentrations. As shown in equation (8), the hydroxyl radicals recombine with an excess of H2O2 present in the system and don’t take part in the main degradation reaction [45].
| H2O2 + OH·→H2O + HO2 | (8) |
Therefore, it is necessary to optimise the optimum dosage of H2O2. This study investigated the effect of different H2O2 loadings on the reduction of BOD and COD values. The concentration of oxidant, i.e·H2O2, was optimised by varying the H2O2 dosage over a range of 1–5 g/L. Other operating parameters, such as inlet pressure, pH, were maintained at 5 bar, 5 respectively. As H2O2 loading increased from 1 to 3 g/L, the degree of BOD degradation increased from 43.7 to 61.6 %, and COD degradation increased from 46.1 to 65.5%. As H2O2 dosage was further increased to 5 g/L, the degradation of BOD fell to 48.3 and the degradation of COD to 62.7%. In 3 g/L of H2O2, the maximum degradation of BOD and COD was 61.6% and 65.5%, respectively. Likely, the initial increase in degradation by increasing H2O2 loading from 1 g/L to 3 g/L, is because of the formation of extra hydroxyl radicals from dissociation of additional H2O2 available in the system. As a result of the scavenging action of excess H2O2 in the system, there is a reduced degradation beyond the loading of 3 g/L of H2O2. As a result, the HC standalone and HC + H2O2 techniques were compared at optimum conditions using 3 g/L of H2O2.
3.3. HC disinfection of microorganisms
Several water-borne fatal diseases can be transmitted to humans through water contaminated with pathogens. Cavitation has proven to be a competent alternative to conventional disinfection methods. With hybrid hydrodynamic cavitation technology, nearly 100% disinfection was observed. Jyoti et al. [66] investigated using HC to disinfect bore well water. Studies have found that chemical oxidants such as H2O2 result in a multiple-fold increase in disinfection. As a disinfection method, cavitation has been successfully used with chlorine and H2O2 [67].
This study assessed the effectiveness of HC alone and HC combined with H2O2 in controlling microbial contamination. All experiments were performed at the optimum parameter values. The killing of pathogens by cavitation results from oxidative damage to bacterial cell structure caused by generated radicals. Additionally, shock waves formed during cavity implosion disrupt microbes mechanically. The oxidative damage of the cell structure is rapidly reduced as the presence of H2O2 increases in the system, as illustrated in Fig. 9.
Fig. 9.
Comparison between HC and HC + H2O2 techniques for the treatment of lake water samples.
4. Comparative analysis of hybrid treatment approaches
Various complex wastewaters can be effectively treated with hydrodynamic cavitation. Combining HC with other AOPs can significantly increase its efficacy. This study examined HC standalone and hybrid HC + H2O2 methods for treating lake water. The optimal operating conditions for the standalone HC and hybrid HC + H2O2 treatments were (Inlet pressure 5 bar, Treatment time 1 h, pH 5) and (Inlet pressure 5 bar, H2O2 dosage 3 g/L, Treatment time 1 h, pH 5), respectively. We studied different parameters to assess lake water quality, such as BOD, COD, TDS, turbidity, etc., before and after treatment. Furthermore, the efficiency of both techniques was examined in terms of microbial disinfection. A 60 min treatment time led to changes in the parameters, and Fig. 9 illustrates the efficacy of both techniques.
After 1 h of treatment, 51.5% of BOD was reduced by standalone HC and 64.1% by HC + H2O2. Moreover, the COD reduction increased from 54.5% for the standalone HC technique to 64 % for the HC + H2O2 technique. Both techniques only marginally reduced the TDS values of the sample. The turbidity of the water (in NTU units) is also an important parameter to assess its clarity. A significant removal of turbidity was observed in treated samples. Although, with the hybrid technique greater turbidity removal was observed. High turbidity indicates that the water contains many suspended particles, making it appear hazy. After a 1 h treatment time, the percentage TDS reduction in this study increased from 29% for standalone HC to 48.4% for HC + H2O2 (3 g/L). On comparing the results achieved in both methods, it can be concluded that using strong chemical oxidants such as H2O2 intensifies the degradation process by generating more reactive radicals.
An analysis of the effect of treatment on various parameters used to monitor lake water quality has been conducted. The results are presented in Fig. 9.In the present study H2O2 (0.5 M) was used to study the effective COD removal using H2O2 alone and 36% COD removal was observed after 1 h. For this H2O2 was added in the holding tank and water was passed through by pass line and closing the main line. As shown in Table 3, the removal efficiencies of H2O2, HC and hybrid HC + H2O2 were 36, 49, 65 % respectively. It clearly confirms the highest COD removal of 65% obtained using hybrid technique. Synergistic index of the hybrid technique can be calculated by using equation:
f= =1.1
Table 3.
Effect of H2O2 dosage on % COD removal (Inlet Pressure = 5 bar, pH = 3, Orifice Diameter = 2 mm) Table: Molar ratio of Oxidant to COD (moles H2O2 to moles O2) for hybrid treatment.
| Sr. No. | Conc. of oxidant H2O2 |
COD removed after 60 min (mg O2/ml) | COD removed after 60 min (moles O2/l) | Molar ratio of Oxidant to COD (moles H2O2 to moles O2) | |
|---|---|---|---|---|---|
| g/L | Molar conc. (M) | ||||
| 1 | 1 | 0.03 | 26.8 | 0.83 | 27.66 |
| 2 | 2 | 0.06 | 32.06 | 1.00 | 16.67 |
| 3 | 3 | 0.09 | 37.99 | 1.19 | 13.22 |
| 4 | 4 | 0.12 | 36.5 | 1.14 | 9.5 |
| 5 | 5 | 0.15 | 35.6 | 1.11 | 7.4 |
The obtained synergistic index value of 1.1 shows the improved efficiency of the hybrid process at optimum operating conditions as compared to individual techniques. Table 4.
Table 4.
Extent of COD reduction and rate constants for different treatment techniques.
| Sr. No. | Treatment Technique | % COD removal | COD value after 60 min | k* 103 min−1 |
|---|---|---|---|---|
| 1 | HC | 49 | 29.58 | 11.2 |
| 2 | H2O2 | 36 | 42.8 | 5.0 |
| 3 | HC + H2O2 | 65 | 20.3 | 17.5 |
5. Energy calculations
5.1. Hydrodynamic cavitation
The holding tank of the HC setup has a working volume of 5 L. For HC, a plunger pump was utilised (power 1.1 kW); An orifice with a diameter of 2 mm serves as a cavitation device.
-
I.
Total volume of liquid = Dead volume + System volume
In this case, the total volume of liquid is 5 L.
Considering the reactor size, pipe sizing, and liquid accumulated in the pump chamber, the system volume is estimated to be 4 L[68].
-
II.
Hence, dead volume = total volume – system volume = 5–4 = 1 L.
-
III.
Total amount of energy dissipated in HC:
Energy dissipated (ED) = dP × Q × T.
Q = Volumetric flow rate of the sample through orifice = 360 LPH
T = Total circulation time through the orifice = 60 min
dP = Pressure drop across the orifice nozzle = 5 bar = 4.9×105 Pa.
Flow rate (Q) at 5 kg/cm2 = 360 LPH = 360×10−3/3600 m3/sec = 1×10−4m3/s.
Energy dissipated into the system (ED) = (4.9×105) (1×10−4) (60×60) = 176400 J = 176.4 kJ.
-
IV.
Cavitation number, C for an inlet pressure of 5 kg/cm2:
where, Inlet fluid pressure = 5 kg/cm2 = 4.9×105 Pa
Downstream recovered pressure (P2) = 101325 Pa
Absolute pressure = Gauge pressure + Atmospheric pressure
Gauge pressure (P1) = 5 kg/cm2 = 4.9×105 Pa.
Atmospheric pressure = 1 atm = 101325 Pa.
Thus, absolute inlet pressure = 4.9×105 Pa + 101325 Pa = 591325 Pa.
Vapour pressure (Pv) of water at 30 °C = 4242.14 Pa.
Volumetric flow rate (Q) = 360 LPH = 1 *10−4 m3/s.
Diameter (d) of orifice = 2 mm.
Flow Area (a) =
a = 3.14×10−6 m2.
Velocity at the throat of the orifice (V0) = (Q/a) = 31.85 m/s.
Therefore, Cavitation number (CV) = (101325–4242.14)/[0.5×1000 × (31.85)2] = 0.191.
-
V.
Number of passes through the orifice:
Flow rate (Q) through the orifice at an inlet pressure of 5 kg/cm2 = 360 LPH = 1×10−4 m3/s.
Total liquid volume = 5 L = 5×10−3 m3.
Treatment time = 60 min = 3600 s.
Number of passes = (1×10−4/5×10−3) (3600) = 72 Passes.
5.2. Treatment costs
Treatment costs were calculated based on a 1 m3 capacity. Using the HC technique only involves the cost of the electricity used by the pump. Hybrid HC + H2O2, however, requires the consideration of the cost of peroxide as well as the cost of chemicals used to adjust pH.
5.2.1. Hydrodynamic cavitation
Treatment costs include the cost of electrical energy used for pump operation.
Treatment Time = 60 min (1 h).
Treatment Volume = 5 L.
Electrical power required for pump = 1.1 kW.
In Telangana State, the average cost of electricity for the industrial sector is 0.086 $/kWh
= 1.1×1/(5×10−3) = 220 kWh/m3.
Electricity cost of treatment = 220×0.086 = 18.92 $/m3.
5.2.2. Hydrodynamic cavitation + H2O2
In this case, the cost of adding H2O2 and the electrical energy required to treat HC must be considered.
Obtained optimum dosage of H2O2 = 3 g/L = 3000 g/m3.
Density of H2O2 solution (30% w/v) = 1.11 g/cm3.
Thus, weight of 500 mlLof H2O2 solution (30% w/v) = 1.11×500 = 555 g.
Cost of 500 mL (i.e., 555 g) of 30% H2O2 (w/v) = 9.15 $
i.e., cost/g of H2O2 = 9.15/555 = 0.02 $/g of H2O2 solution.
Cost of H2O2 addition required for 1 m3 lake water = 3000×0.02 = 60 $ /m3.
Total treatment cost = Cost of electrical energy used + cost of added H2O2.
= 18.92 $/m3 + 60 $/m3 = 78.92 $ /m3.
6. Conclusion
Hydrodynamic cavitation (HC) was used as a standalone method or in conjunction with other AOPs to maximise efficiency. A hybrid treatment strategy using HC alone and HC + H2O2 for the treatment of lake water was examined in this study. Our study is focused on optimising a few operating parameters that affect HC and HC-based techniques, namely pH (3 to 8), inlet pressure (4–6 bar), and H2O2 dosage (1–5 g/L). The highest BOD and COD degradation was observed at optimum values; pH 5, inlet P 5 bar, H2O2 dosage 3 g/L. A serial dilution method was also employed to study microbial disinfection, and it was found that HC + H2O2 produced nearly 100% removal of pathogens. Synergy index of hybrid technique was found to be 1.1 that confirms the efficacy of hybrid technique over individual techniques. Hybrid HC + H2O2 treatment for lake water seems to be a very promising approach and has a great deal of potential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Prof. Shirish H. Sonawane, and Mr. Yogesh Patil, wish to acknowledge the financial support of the DST-WTI Project [Grant No DST/TMD/EWO/WTI/2K19/EWFH/2019/111(C)] Dated 04/02/2021 of the Department of Science and Technology, Government of India. They would like to acknowledge the support from Kakatiya Urban Development Authority (KUDA), Warangal. Dr. Xun Sun gratefully acknowledges the financial support provided by the National Natural Science Foundation of China (Grant Nos. 52276032 and 51906125).
Contributor Information
Shirish H. Sonawane, Email: shirish@nitw.ac.in.
Xun Sun, Email: xunsun@sdu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Park J., Yamashita N., Park C., Shimono T., Takeuchi D.M., Tanaka H. Removal characteristics of pharmaceuticals and personal care products: Comparison between membrane bioreactor and various biological treatment processes. Chemosphere. 2017;179:347–358. doi: 10.1016/j.chemosphere.2017.03.135. [DOI] [PubMed] [Google Scholar]
- 2.Verlicchi P., Al Aukidy M., Zambello E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012;429:123–155. doi: 10.1016/j.scitotenv.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee A., Mullick A., Vadthya P., Moulik S., Roy A. Surfactant degradation using hydrodynamic cavitation based hybrid advanced oxidation technology: A techno economic feasibility study. Chem. Eng. J. 2020;398 doi: 10.1016/j.cej.2020.125599. [DOI] [Google Scholar]
- 4.Hussain S., Awad J., Sarkar B., Chow C.W.K., Duan J., van Leeuwen J. Coagulation of dissolved organic matter in surface water by novel titanium (III) chloride: Mechanistic surface chemical and spectroscopic characterisation. Sep. Purif. Technol. 2019;213:213–223. doi: 10.1016/j.seppur.2018.12.038. [DOI] [Google Scholar]
- 5.Maurya A., Singh M.K., Kumar S. Waterborne Pathogens. Elsevier; 2020. Biofiltration technique for removal of waterborne pathogens; pp. 123–141. [DOI] [Google Scholar]
- 6.Barra Caracciolo A., Grenni P., Rauseo J., Ademollo N., Cardoni M., Rolando L., Patrolecco L. Degradation of a fluoroquinolone antibiotic in an urbanized stretch of the River Tiber. Microchem. J. 2018;136:43–48. doi: 10.1016/j.microc.2016.12.008. [DOI] [Google Scholar]
- 7.Kamrani M., Akbari A., A. Yunessnia lehi Chitosan-modified acrylic nanofiltration membrane for efficient removal of pharmaceutical compounds. J. Environ. Chem. Eng. 2018;6:583–587. doi: 10.1016/j.jece.2017.12.044. [DOI] [Google Scholar]
- 8.Licona K.P.M., Geaquinto L.R.d.O., Nicolini J.V., Figueiredo N.G., Chiapetta S.C., Habert A.C., Yokoyama L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018;25:195–204. doi: 10.1016/j.jwpe.2018.08.002. [DOI] [Google Scholar]
- 9.Foureaux A.F.S., Reis E.O., Lebron Y., Moreira V., Santos L.V., Amaral M.S., Lange L.C. Rejection of pharmaceutical compounds from surface water by nanofiltration and reverse osmosis. Sep. Purif. Technol. 2019;212:171–179. doi: 10.1016/j.seppur.2018.11.018. [DOI] [Google Scholar]
- 10.Dalrymple O.K., Yeh D.H., Trotz M.A. Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J. Chem. Technol. Biotechnol. 2007;82:121–134. doi: 10.1002/jctb.1657. [DOI] [Google Scholar]
- 11.Jjagwe J., Olupot P.W., Menya E., Kalibbala H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021;6:292–322. doi: 10.1016/j.jobab.2021.03.003. [DOI] [Google Scholar]
- 12.Liu D., Xie Q., Huang X., Wan C., Deng F., Liang D., Liu J. Backwashing behavior and hydrodynamic performances of granular activated carbon blends. Environ. Res. 2020;184 doi: 10.1016/j.envres.2020.109302. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Bai Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017;312:79–98. doi: 10.1016/j.cej.2016.11.118. [DOI] [Google Scholar]
- 14.Varkey A.J. Purification of river water using Moringa Oleifera seed and copper for point-of-use household application. Sci. Afr. 2020;8:e00364. [Google Scholar]
- 15.Ranade V.V., Bhandari V.M. Industrial Wastewater Treatment, Recycling and Reuse. Elsevier; 2014. Industrial Wastewater Treatment, Recycling and Reuse; pp. 1–80. [DOI] [Google Scholar]
- 16.Richardson S., Plewa M., Wagner E., Schoeny R., Demarini D. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutation Research/Reviews in Mutation. Research. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Villanueva C.M., Cordier S., Font-Ribera L., Salas L.A., Levallois P. Overview of Disinfection By-products and Associated Health Effects. Curr. Environ. Health Rep. 2015;2:107–115. doi: 10.1007/s40572-014-0032-x. [DOI] [PubMed] [Google Scholar]
- 18.Boczkaj G., Fernandes A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017;320:608–633. doi: 10.1016/j.cej.2017.03.084. [DOI] [Google Scholar]
- 19.Fernandes A., Makoś P., Boczkaj G. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018;195:374–384. doi: 10.1016/j.jclepro.2018.05.207. [DOI] [Google Scholar]
- 20.Sui Q., Huang J., Deng S., Yu G., Fan Q. Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res. 2010;44:417–426. doi: 10.1016/j.watres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Yusaf T. Experimental study of microorganism disruption using shear stress. Biochem. Eng. J. 2013;79:7–14. doi: 10.1016/j.bej.2013.07.001. [DOI] [Google Scholar]
- 22.Sun X., Liu S., Zhang X., Tao Y., Boczkaj G., Yoon J.Y., Xuan X. Recent advances in hydrodynamic cavitation-based pretreatments of lignocellulosic biomass for valorization. Bioresour. Technol. 2022;345:126251. doi: 10.1016/j.biortech.2021.126251. [DOI] [PubMed] [Google Scholar]
- 23.Mevada J., Devi S., Pandit A. Large scale microbial cell disruption using hydrodynamic cavitation: Energy saving options. Biochem. Eng. J. 2019;143:151–160. doi: 10.1016/j.bej.2018.12.010. [DOI] [Google Scholar]
- 24.Sun X., Liu S., Manickam S., Tao Y., Yoon J.Y., Xuan X. Intensification of biodiesel production by hydrodynamic cavitation: A critical review. Renew. Sustain. Energy Rev. 2023;179:113277. doi: 10.1016/j.rser.2023.113277. [DOI] [Google Scholar]
- 25.Mezule L., Tsyfansky S., Yakushevich V., Juhna T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination. 2009;248:152–159. doi: 10.1016/j.desal.2008.05.051. [DOI] [Google Scholar]
- 26.Xuan X., Wang M., You W., Manickam S., Tao Y., Yoon J.Y., Sun X. Hydrodynamic cavitation-assisted preparation of porous carbon from garlic peels for supercapacitors. Ultrason. Sonochem. 2023;94:106333. doi: 10.1016/j.ultsonch.2023.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loraine G., Chahine G., Hsiao C.-T., Choi J.-K., Aley P. Disinfection of gram-negative and gram-positive bacteria using DynaJets® hydrodynamic cavitating jets. Ultrason. Sonochem. 2012;19:710–717. doi: 10.1016/j.ultsonch.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Sun X., Yang Z., Wei X., Tao Y., Boczkaj G., Yoon J.Y., Xuan X., Chen S. Multi-objective Optimization of the Cavitation Generation Unit Structure of an Advanced Rotational Hydrodynamic Cavitation Reactor. Ultrason. Sonochem. 2021;80:105771. doi: 10.1016/j.ultsonch.2021.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gogate P.R., Patil P.N. Combined treatment technology based on synergism between hydrodynamic cavitation and advanced oxidation processes. Ultrason. Sonochem. 2015;25:60–69. doi: 10.1016/j.ultsonch.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Fedorov K., Dinesh K., Sun X., Darvishi Cheshmeh Soltani R., Wang Z., Sonawane S., Boczkaj G. Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon – A review. Chem. Eng. J. 2022;432 doi: 10.1016/j.cej.2021.134191. [DOI] [Google Scholar]
- 31.Gągol M., Cako E., Fedorov K., Soltani R.D.C., Przyjazny A., Boczkaj G. Hydrodynamic cavitation based advanced oxidation processes: Studies on specific effects of inorganic acids on the degradation effectiveness of organic pollutants. J. Mol. Liq. 2020;307 doi: 10.1016/j.molliq.2020.113002. [DOI] [Google Scholar]
- 32.Sun X., You W., Xuan X., Ji L., Xu X., Wang G., Zhao S., Boczkaj G., Yoon J.Y., Chen S. Effect of the cavitation generation unit structure on the performance of an advanced hydrodynamic cavitation reactor for process intensifications. Chem. Eng. J. 2021;412 doi: 10.1016/j.cej.2021.128600. [DOI] [Google Scholar]
- 33.Gągol M., Przyjazny A., Boczkaj G. Effective method of treatment of industrial effluents under basic pH conditions using acoustic cavitation – A comprehensive comparison with hydrodynamic cavitation processes. Chem. Eng. Process. 2018;128:103–113. doi: 10.1016/j.cep.2018.04.010. [DOI] [Google Scholar]
- 34.Watts R.J., Teel A.L. Treatment of Contaminated Soils and Groundwater Using ISCO, Practice Periodical of Hazardous, Toxic, and Radioactive. Waste Manage. 2006;10:2–9. doi: 10.1061/(ASCE)1090-025X(2006)10:1(2). [DOI] [Google Scholar]
- 35.Lei Y.-J., Tian Y., Fang C., Zhan W., Duan L.-C., Zhang J., Zuo W., Kong X.-W. Insights into the oxidation kinetics and mechanism of diesel hydrocarbons by ultrasound activated persulfate in a soil system. Chem. Eng. J. 2019;378 doi: 10.1016/j.cej.2019.122253. [DOI] [Google Scholar]
- 36.Fedorov K., Sun X., Boczkaj G. Combination of hydrodynamic cavitation and SR-AOPs for simultaneous degradation of BTEX in water. Chem. Eng. J. 2021;417 doi: 10.1016/j.cej.2020.128081. [DOI] [Google Scholar]
- 37.Eslami A., Mehdipour F., Lin K.-Y.-A., Sharifi Maleksari H., Mirzaei F., Ghanbari F. Sono-photo activation of percarbonate for the degradation of organic dye: The effect of water matrix and identification of by-products. J. Water Process Eng. 2020;33 doi: 10.1016/j.jwpe.2019.100998. [DOI] [Google Scholar]
- 38.Ling X., Deng J., Ye C., Cai A., Ruan S., Chen M., Li X. Fe(II)-activated sodium percarbonate for improving sludge dewaterability: Experimental and theoretical investigation combined with the evaluation of subsequent utilization. Sci. Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149382. [DOI] [PubMed] [Google Scholar]
- 39.Rivas J., Gimeno O., Borralho T., Beltrán F. Influence of oxygen and free radicals promoters on the UV-254nm photolysis of diclofenac. Chem. Eng. J. 2010;163:35–40. doi: 10.1016/j.cej.2010.07.027. [DOI] [Google Scholar]
- 40.Fedorov K., Rayaroth M.P., Shah N.S., Boczkaj G. Activated sodium percarbonate-ozone (SPC/O3) hybrid hydrodynamic cavitation system for advanced oxidation processes (AOPs) of 1,4-dioxane in water. Chem. Eng. J. 2023;456 doi: 10.1016/j.cej.2022.141027. [DOI] [Google Scholar]
- 41.Yuan R., Jiang Z., Wang Z., Gao S., Liu Z., Li M., Boczkaj G. Hierarchical MnO2 nanoflowers blooming on 3D nickel foam: A novel micro-macro catalyst for peroxymonosulfate activation. J. Colloid Interface Sci. 2020;571:142–154. doi: 10.1016/j.jcis.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y., Bu L., Duan X., Zhu S., Kong M., Zhu N., Zhou S. Mini review on the roles of nitrate/nitrite in advanced oxidation processes: Radicals transformation and products formation. J. Clean. Prod. 2020;273 doi: 10.1016/j.jclepro.2020.123065. [DOI] [Google Scholar]
- 43.Goldstein S., Rabani J. Mechanism of nitrite formation by nitrate photolysis in aqueous solutions: the role of peroxynitrite, nitrogen dioxide, and hydroxyl radical. J. Am. Chem. Soc. 2007;129:10597–10601. doi: 10.1021/ja073609+. [DOI] [PubMed] [Google Scholar]
- 44.Li W.-X., Tang C.-D., Wu Z.-L., Wang W.-M., Zhang Y.-F., Zhao Y., Cravotto G. Eutrophic water purification efficiency using a combination of hydrodynamic cavitation and ozonation on a pilot scale. Environ. Sci. Pollut. Res. 2015;22:6298–6307. doi: 10.1007/s11356-014-3889-1. [DOI] [PubMed] [Google Scholar]
- 45.Jain P., Bhandari V.M., Balapure K., Jena J., Ranade V.V., Killedar D.J. Hydrodynamic cavitation using vortex diode: An efficient approach for elimination of pathogenic bacteria from water. J. Environ. Manage. 2019;242:210–219. doi: 10.1016/j.jenvman.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 46.Dong Z., Zhao W. Killing rate of colony count by hydrodynamic cavitation due to square multi-orifice plates. IOP Conf. Ser. Earth Environ. Sci. 2018;121 doi: 10.1088/1755-1315/121/2/022004. [DOI] [Google Scholar]
- 47.Gągol M., Przyjazny A., Boczkaj G. Wastewater treatment by means of advanced oxidation processes based on cavitation – A review. Chem. Eng. J. 2018;338:599–627. doi: 10.1016/j.cej.2018.01.049. [DOI] [Google Scholar]
- 48.Boczkaj G., Gągol M., Klein M., Przyjazny A. Effective method of treatment of effluents from production of bitumens under basic pH conditions using hydrodynamic cavitation aided by external oxidants. Ultrason. Sonochem. 2018;40:969–979. doi: 10.1016/j.ultsonch.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 49.Sun X., Xuan X., Ji L., Chen S., Liu J., Zhao S., Park S., Yoon J.Y., Om A.S. A novel continuous hydrodynamic cavitation technology for the inactivation of pathogens in milk. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raut-Jadhav S., Saharan V.K., Pinjari D., Sonawane S., Saini D., Pandit A. Synergetic effect of combination of AOP’s (hydrodynamic cavitation and H2O2) on the degradation of neonicotinoid class of insecticide. J. Hazard. Mater. 2013;261:139–147. doi: 10.1016/j.jhazmat.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Saharan V.K., Rizwani M.A., Malani A.A., Pandit A.B. Effect of geometry of hydrodynamically cavitating device on degradation of orange-G. Ultrason. Sonochem. 2013;20:345–353. doi: 10.1016/j.ultsonch.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Kumar M.S., Sonawane S.H., Bhanvase B.A., Bethi B. Treatment of ternary dye wastewater by hydrodynamic cavitation combined with other advanced oxidation processes (AOP’s) J. Water Process Eng. 23. 2018:250–256. doi: 10.1016/j.jwpe.2018.04.004. [DOI] [Google Scholar]
- 53.Wang X., Zhang Y. Degradation of alachlor in aqueous solution by using hydrodynamic cavitation. J. Hazard. Mater. 2009;161:202–207. doi: 10.1016/j.jhazmat.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 54.Joshi R.K., Gogate P.R. Degradation of dichlorvos using hydrodynamic cavitation based treatment strategies. Ultrason. Sonochem. 2012;19:532–539. doi: 10.1016/j.ultsonch.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Gogate P.R., Pandit A.B. HYDRODYNAMIC CAVITATION REACTORS: A STATE OF THE ART REVIEW. Rev. Chem. Eng. 2001;17:1–85. doi: 10.1515/REVCE.2001.17.1.1. [DOI] [Google Scholar]
- 56.Wang B., Su H., Zhang B. Hydrodynamic cavitation as a promising route for wastewater treatment – A review. Chem. Eng. J. 2021;412 doi: 10.1016/j.cej.2021.128685. [DOI] [Google Scholar]
- 57.Chakinala A.G., Gogate P.R., Burgess A.E., Bremner D.H. Treatment of industrial wastewater effluents using hydrodynamic cavitation and the advanced Fenton process. Ultrason. Sonochem. 2008;15:49–54. doi: 10.1016/j.ultsonch.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Patil A.L., Patil P.N., Gogate P.R. Degradation of imidacloprid containing wastewaters using ultrasound based treatment strategies. Ultrason. Sonochem. 2014;21:1778–1786. doi: 10.1016/j.ultsonch.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 59.Barik A.J., Gogate P.R. Hybrid treatment strategies for 2,4,6-trichlorophenol degradation based on combination of hydrodynamic cavitation and AOPs. Ultrason. Sonochem. 2018;40:383–394. doi: 10.1016/j.ultsonch.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 60.Dhanke P.B., Wagh S.M. Intensification of the degradation of Acid RED-18 using hydrodynamic cavitation. Emerg. Contam. 2020;6:20–32. doi: 10.1016/j.emcon.2019.12.001. [DOI] [Google Scholar]
- 61.Wei H., Hu D., Su J., Li K. Intensification of levofloxacin sono-degradation in a US/H2O2 system with Fe3O4 magnetic nanoparticles. Chin. J. Chem. Eng. 2015;23:296–302. doi: 10.1016/j.cjche.2014.11.011. [DOI] [Google Scholar]
- 62.Ben Hammouda S., Adhoum N., Monser L. Synthesis of magnetic alginate beads based on Fe3O4 nanoparticles for the removal of 3-methylindole from aqueous solution using Fenton process. J. Hazard. Mater. 2015;294:128–136. doi: 10.1016/j.jhazmat.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 63.Rajoriya S., Bargole S., Saharan V.K. Degradation of a cationic dye (Rhodamine 6G) using hydrodynamic cavitation coupled with other oxidative agents: Reaction mechanism and pathway. Ultrason. Sonochem. 2017;34:183–194. doi: 10.1016/j.ultsonch.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 64.Rajoriya S., Bargole S., Saharan V.K. Degradation of reactive blue 13 using hydrodynamic cavitation: Effect of geometrical parameters and different oxidizing additives. Ultrason. Sonochem. 2017;37:192–202. doi: 10.1016/j.ultsonch.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Thanekar P., Gogate P.R. Improved processes involving hydrodynamic cavitation and oxidants for treatment of real industrial effluent. Sep. Purif. Technol. 2020;239 doi: 10.1016/j.seppur.2020.116563. [DOI] [Google Scholar]
- 66.Jyoti K.K., Pandit A.B. Water disinfection by acoustic and hydrodynamic cavitation. Biochem. Eng. J. 2001;7:201–212. doi: 10.1016/S1369-703X(00)00128-5. [DOI] [Google Scholar]
- 67.Alexandre E.M.C., Brandão T.R.S., Silva C.L.M. Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innov. Food Sci. Emerg. Technol. 2013;17:99–105. doi: 10.1016/j.ifset.2012.11.009. [DOI] [Google Scholar]
- 68.Korpe S., Bethi B., Sonawane S.H., Jayakumar K.V. Tannery wastewater treatment by cavitation combined with advanced oxidation process (AOP) Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.