Abstract
In order to study the role of GmXTH1 gene in alleviating drought stress, soybean seeds with GmXTH1 gene were transferred by T4 treated with PEG6000 concentration of 0%, 5%, 10%, and 15% respectively. The germination potential, germination rate, germination index, and other indicators were measured. The results showed that the germination potential, germination rate, and germination index of OEA1 and OEA2 strains overexpressed in T4 generation were significantly higher than those of the control material M18. After 0-day, 7-day, and 15-day drought stress, the analysis of seedling phenotypes and root-shoot of different T4 generation transgenic soybean lines showed that under stress conditions, the growth of GmXTH1 overexpression material was generally better than that of the control material M18. The growth of GmXTH1 interference expression material was generally worse than that of the control material M18, with significant differences in plant phenotypes. The root system of GmXTH1 overexpressed material was significantly developed compared with that of the control material M18. The analysis of physiological and biochemical indexes showed that the relative water content and the activity of antioxidant enzymes (superoxide dismutase and peroxidase) of GmXTH1 transgenic soybean material were significantly higher than those of the control material M18, and the accumulation of malondialdehyde was lower under the same stress conditions at seedling stage. Fluorescence quantitative PCR assay showed that the relative expression of GmXTH1 gene in transgenic soybean was significantly increased after drought stress. The results showed that the overexpression of GmXTH1 could increase the total root length, surface area, total projection area, root volume, average diameter, total cross number, and total root tip number, thereby increasing the water intake and reducing the transpiration of water content in leaves, thus reducing the accumulation of MDA and producing more protective enzymes in a more effective and prompt way, reducing cell membrane damage to improve drought resistance of soybean.
Keywords: Drought stress, GmXTH1 gene, Germination index, Soybean, Physiology and biochemistry
Introduction
In the face of severe climate change, abiotic stresses such as salinization, drought, extreme temperature, and waterlogging are intensified. A series of changes in plant organ morphology, physiological, and biochemical levels occur accordingly. These include mechanical damage of protoplasts and cell walls, stomatal closure of leaves, decreased photosynthetic efficiency and cell dehydration, impairment of biofilm system, and changes in membrane permeability, resulting in metabolic disorders (Osmond et al. 1995). In response to drought stress, plants avoided drought by developing plasticity and shortening life cycle, increasing water intake, and reducing water loss. At the same time, stress tolerance can also be enhanced by increasing osmotic regulation, antioxidant capacity, and dehydration tolerance (Zhang et al. 2007). Drought stress can lead to dehydration of plant cells and directly affect cell turgor pressure and then the extension of cell wall. Cells maintain cell turgor pressure by adjusting cell size and cell wall extensibility, thus contributing to plant water loss adaptation (Mohammadi et al. 2012). Minimizing water loss and cell dehydration is crucial for drought tolerance and salt tolerance of plants and recovery of growth.
Studies have shown that xyloglucantransglycosidase/hydrolase, XTH by catalytic plant cell walls of xylan chain cutting and reconnect to modify and restructuring of cellulose, xylan skeleton, so as to change and adjust the ductility of cell wall. This in turn affects plant growth and development and stress response (Rose et al. 2002; Vissenberg et al. 2005; Miedes et al. 2011).
According to its sequence characteristics, XTHs are divided into I, II, and III (Rose et al. 2002), and III into IIIA and IIIB subclasses (Baumann et al. 2007). Among them, class IIIA showed XEH activity and specifically hydrolyzed the β-1,4 glycosidic bond of xyloglucan. Class IIIB, class I, and class II XTHs can internally cut xyloglucan molecules to produce a reducing terminal, which is linked to another xyloglucan chain and has significant XET activity (Baumann et al. 2007; Saladie et al. 2006; Kallas et al. 2005). The characteristic sequence of XTH enzyme is DEIDFEFLG, which contains amino acid residues that can mediate catalytic activity. The threonine or serine residues near this catalytic site are modified by N-glycosylation (Kallas et al. 2005), which is significantly associated with enzyme activity. N-Glycation sites conserved have not been found in subclass IIIa (Baumann et al. 2007), but have been found in class I/II of XTH proteins. The C-terminal of XTH protein can form disulfide bonds that stabilize the protein structure because it usually contains highly conserved cysteine. Proteomic studies have shown that certain enzymes related to cell wall polysaccharide synthesis/hydrolysis, lignin biosynthesis, and cell wall porosition are involved in the response to drought stress (Aranjuelo et al. 2011; Lee et al. 2007; Amor et al. 1995; Dong et al. 2011; Ashoub et al. 2013).
In addition, XTHs play an extremely important role in various growth and differentiation processes, participating in the regulation of primary root elongation, hypocotyl growth, vascular differentiation, flowering, fruit ripening, petal shedding, and wood formation (Osato et al. 2006; Wu et al. 2005; Matsui et al. 2005; Harada et al. 2011; Saladie et al. 2006; Singh et al. 2011; Nishikubo et al. 2011). AtXTH is very sensitive to metal aluminum ion stress, and Arabidopsis xth15, xth17, and xth31 mutants have improved A13+ stress tolerance compared with wild-type plants (Zhu et al. 2012, 2013, 2014). Excessive expression of CaXTH3 in Arabidopsis thaliana and tomato can improve drought and salt tolerance of transgenic plants (Cho et al. 2006; Choi et al. 2011). Overexpression of BcXTH1 promotes the growth of flowering shoots and thus increases plant height (Shin et al. 2006). Atxth15, 19, 16, and 17 can promote petiole elongation by regulating cell wall ductility (Sasidharan et al. 2010). PC-XET1 may be involved in cell wall degradation during the ripening and softening process of pear (Hiwasa et al. 2003).
Drought affects more than 10% of arable land and reduces the average yield of major crops by 50%. Soybean (Glycine max) is an important grain and oil crop, which occupies an extremely important position in the world agricultural production. However, due to high transpiration coefficient, large water demand, and relatively weak drought resistance of soybean, drought has a great impact on its growth and development, yield, and quality (Farooq et al. 2017). The growth and development of soybean require the unity of leaf photosynthesis and underground root group absorption, and the developed root system can absorb more water to promote photosynthesis of the above-ground leaves (Hallmark et al. 1987). With the intensification of global climate change, the drought problem will be more prominent, which puts forward new requirements for the drought resistance of soybean varieties (Louren et al. 2011). It is an effective way to improve the drought resistance of soybean by discovering high-quality drought-resistant genes.
The gene GmXTH1 used in this study was isolated and cloned from the root of JN18 mutant soybean by RACE technology. Agrobacteria-mediated method was used to obtain OEA1 and OEA2 transgenic strains with overexpression of GmXTH1 gene and IEA1 and IEA2 transgenic strains with interference of GmXTH1 gene. Under drought stress, the transgenic strain plants germinated in response to non-growth stress, and the phenotypes at seedling stage and physiological and biochemical indexes were measured. Under drought stress, the relative expression level of GmXTH1 gene in different transgenic lines was compared with that of endogenous E3 link-enzyme gene GMPLR-2 (GenBank: EU362626.1) and WRKY transcription factor gene GmWRKY35 (GenBank: KM587699.1) and the relative expression levels of transcription factor JCVI-FLGM-14H24 (GenBank: BT095106.1) were analyzed.
Materials and methods
Experimental materials
The plant materials were provided by the Plant Biotechnology Center of Jilin Agricultural University. The GmXTH1 gene was transferred into the control material M18 by Agrobacterium-mediated method. Having tested and planted, the transgenic soybean lines OEA1 and OEA2 with GmXTH1 gene overexpressed in high generation. The transgenic soybean lines IEA1 and IEA2 with GmXTH1 gene interfered with the expression, as well as control material M18. The reference material M18 was derived from JN18 mutants with developed roots obtained under drought stress.
Experimental design
This experiment was carried out in the Plant Biotechnology Center Laboratory of Jilin Agricultural University, and the materials were tested and strictly selected. After removing impurities, the healthy and plump soybean seeds were selected.
Germination test
M18 and OEA1, OEA2, IEA1, and IEA2 were soaked in 75% ethanol and 5% NaClO for 120 s respectively and washed with distilled water for 3 times. Then, they placed in 9-cm Petri dishes. Two layers of sterile filter paper were placed on each side and repeated for 3 times, with a total of 60 pans. Based on previous research results, PEG-6000 solutions with different mass concentrations were set to simulate different water potential as follows: 0% (CK), 5%, 10%, 15%, corresponding water potential of 0 (CK), −0.1, −0.2, −0.4 MPa (Michael et al. 1973). Each concentration was added with 20 mL to simulate drought stress treatment, and the same amount of distilled water was added to the control group. The seeds were germinated in an incubator with artificial climate. The seeds were placed in an artificial climate incubator with 14 h of light and 10 h of darkness. The germination was accelerated at a constant temperature of 25 ℃ and relative humidity of 70%. The germinating standard was that the radicle broke through the seed coat by 1 mm, and the germ was half the length of the seed. The number of germinated seeds was recorded on a regular basis day by day, and the germinating test was ended 6 days later. After the experiment, five representative seedlings were selected from each dish to measure root length and seedling height.
Seedling test
The selected seeds were sown in a plastic basin with a height of 20 cm, a width of 20 cm, and a length of 50 cm, and each basin was filled with 10 kg of sand soil. The experimental design was completely random design with two factors of different strains and drought treatment. The watering conditions were set at three levels, which were normal, dry for 7 days, and dry for 15 days respectively. In five lines, OEA1 and OEA2 were transgenic soybean lines with overexpression of GmXTH1 gene. The transgenic GmXTH1 gene interfered with the expression of soybean lines IEA1, IEA2, and control material M18. Each line and each treatment were repeated three times, with a total of 27 basins, and 5 seedlings were left in each basin. In potted matrix for sand, sand was washed three times before use and put the good experimental material in artificial climate chamber. Before the first three ternate fully expanded, normal irrigation and soil moist basic state were maintained. After the first three ternate fully expanded soybean dry processing, drought treatment was carried out 15 days after germination. Under normal circumstances, drought 7 days, the physiological and biochemical indexes of drought for 15 days were determined.
Determination of germination stage indexes of different soybean lines
The germination rate (GR), germination potential (GE), germination index (GI), and vigor index (VI) were calculated according to the number of germination seeds. The germination drought tolerance index and stress index were calculated as described (Bouslama et al. 1984) and the formulas were as follows:
Among them, the , where Nd2, Nd4, and Nd6 are the germination rates of seeds on the second, fourth, and sixth days respectively, and 1.00, 0.75, and 0.50 are the drought tolerance coefficients given by the corresponding germination days respectively.
Phenotypic identification and physiological and biochemical index determination of transgenic GmXTH1 soybean at seedling stage
The root scanner scans the total root length, surface area, total root projected area, root volume, average root diameter, total root crossing number, and total root tip number.
All the leaves of each treated plant were cut off, and they were quickly put into an aluminum box with known weight, and weigh fresh weight (WF). Then, the samples were taken out and immersed in distilled water for 6~8 h. After that, the samples were taken out and the water on the surface of the samples with absorbent paper was wiped. The saturated fresh weight (Wt) of the sample was obtained until the saturated weight of the sample was approximately obtained. Then, the sample was put into an aluminum box with known weight, and put into a 105 ℃ oven for 15 min, which, after drying, then turned to 80 ℃ for constant weight. Then, the dry weight (WD) was weighed. RWC = (WF−WD) / (WT−WD), and relative water content (RWC) refers to the percentage of water content of plant leaves in saturated water content.
The activities of SOD and POD were measured using 0.3-g leaf samples. The leaf tissue was ground in 1.5 mL of 0.1 M phosphate buffer (pH 7.4) using a tissue homogenizer in an ice bath, and the resulting homogenate was centrifuged at 3500 rpm for 10 min. The supernatant was used for enzyme activity measurements following the instructions in the relevant detection kits (superoxide dismutase assay kit and peroxidase assay kit) (Nanjing Jiancheng Institute of Biological Engineering). We measured malondialdehyde (MDA) content using a spectrophotometer (UV-160a; Shimadzu Scientific Instruments, Japan) as described in Du and Bramlage (1992).
Expression of drought stress response gene in transgenic GmXTH1 soybean at seedling stage
When soybean seedlings grew to the first triple compound leaves, drought lasted for 7 days, and the control group was irrigated normally. Three soybean plants were selected from each strain, and total RNA was extracted from leaves and roots: Trizol method was used to extract total RNA from leaves and roots of soybean. TaKaRa reagent (TaKaRa Company) instructions were followed for specific experimental procedures. Purity was detected by nucleic acid protein detector. Reverse transcription: a total of 2.0 μg RNA was taken for reverse transcription. The Transcriptor 1st Strand cDNA Synthesis Kit was purchased from Roche company.
Using β-actin as the reference gene, the relative expression level of GmXTH1 (Schedule sequence 1) gene in different transgenic strains was correlated with the endogenous E3 ligase gene GMPLR-2 (Schedule sequence 2), WRKY transcription factor, and gene GmWRKY35 (Schedule sequence 3). The transcription factor JCVI-FLGM-14H24 (Schedule sequence 4) was analyzed by qRT-PCR using a fluorescence quantitative PCR instrument. Primers are shown in Schedule 1.
The PCR reaction system was as follows: 2x All-in-One™qPCR Mix 10 μL, upstream and downstream primers (10 μmol/L) 2 μL each, cDNA (< 100 ng) 2 μL, and sterilized ddH2O 4 μL. The relative expression of the target gene was calculated by formula 2-ΔΔCT.
Data processing method
DPS V17.5 and Excel 2010 were used for all statistical analyses, and the Dunnet control method was used for single factor test to compare and analyze the significance of the difference between the control material M18 and transgenic lines (*P < 0.05, **P < 0.01). Data are expressed as mean ± standard deviation for three replicates.
Results
Identification of drought tolerance of transgenic GmXTH1 soybean at germination stage
After 6 days, under the condition of clear water (CK), the root lengths of M18, OEA1, OEA2, IEA1, and IEA2 seeds showed no significant differences (Fig. 1). However, the number of lateral roots of OEA1 and OEA2 transgenic materials with overexpression of GmXTH1 gene was significantly more than that of IEA1 and IEA2 transgenic materials with interfering expression of GmXTH1 gene (Fig. 1). After 5% PEG-6000 treatment, the root length of OEA1 and OEA2 was significantly longer than that of M18, the root length of IEA1 and IEA2 was significantly shorter than that of M18, the root length of OEA1 and OEA2 was 1.7 times of that of IEA1 and IEA2, and the number of lateral roots was also significantly more than that of IEA1 and IEA2 (Fig. 1). After 10% PEG-6000 treatment, the root length of OEA1 and OEA2 was significantly longer than that of M18, and the root length of IEA1 and IEA2 was significantly shorter than that of M18. The root length of OEA1 and OEA2 was 2.1 times of that of IEA1 and IEA2. After 15% PEG-6000 treatment, the root length of OEA1 and OEA2 was significantly longer than that of M18, and the root length of IEA1 and IEA2 was significantly shorter than that of M18 (Fig. 1). The root length of OEA1 and OEA2 was 3.1 times of that of IEA1 and IEA2. It can be seen from the phenotype that the overexpression of gene is beneficial to the generation of tested roots and the elongation of taproot during seed germination.
Fig. 1.
Phenotype analysis of germination of M18 and GmXTH1 transgenic soybean under different concentrations of PEG-6000 stress after 6 days. Phenotypes of root growth of M18 and OEA1, OEA2, IEA1, and IEA2 under 0%, 5%, 10%, and 15% PEG concentration stress
Germination potential, germination rate, and germination index of soybean seeds were significantly decreased with the increase of PEG concentration, and decreased with the increase of PEG concentration. Under the condition of clear water (CK), the germination potential and germination rate of M18, OEA1, OEA2, IEA1, and IEA2 seeds were the largest and had no significant difference, indicating that all strains germinated well under normal conditions. With the increase of PEG concentration, the germinating state of OEA1 and OEA2 transgenic materials with GmXTH1 gene overexpression was significantly stronger than that of the control group M18, and the germinating state of materials with GmXTH1 gene interference expression was significantly weaker than that of the control group M18. After 5% PEG-6000 treatment, the relative germination potential of OEA1 and OEA2 was significantly higher than that of the control group M18, and the relative germination potential of IEA1 and IEA2 was significantly lower than that of the control group M18. Compared with water treatment, the relative germination rate of M18, OEA1, and IEA2 had no significant changes. OEA2 and IEA1 were slightly decreased. The germination index and vigor index of the GmXTH1 overexpressed materials OEA1 and OEA2 were extremely significantly higher than those of the control material M18, and the germination index and vigor index of the GmXTH1 interfered expression materials IEA1 and IEA2 were extremely significantly lower than those of the control material M18 (Table 1). After 10% and 15% PEG-6000 treatments, the relative germination potential, relative germination rate, germination index, and vigor index of GmXTH1 gene overexpression materials OEA1 and OEA2 were extremely significantly higher than those of the control material M18, and the GmXTH1 gene interference expression materials IEA1 and IEA2 were extremely significantly lower than that of the control material M18 (Table 1). The results showed that the overexpression of GmXTH1 gene could significantly increase the germination potential, germination rate, and germination index of soybean seeds under drought stress.
Table 1.
Comparison and analysis of traits of GmXTH1 transgenic soybeans with different PEG-6000 concentrations at germination stage
| Normal circumstances | 5% PGE-6000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | ||||||||||
| M18 | OEA1 | OEA2 | IEA1 | IEA2 | M18 | OEA1 | OEA2 | IEA1 | IEA2 | |
| Germination potential | 98.33 ± 1.67 | 100.00 ± 0.00* | 95.00 ± 2.89* | 98.33 ± 1.67 | 98.33 ± 1.67 | 65.00 ± 2.89 | 86.67 ± 1.67** | 86.67 ± 1.67** | 58.33 ± 1.67** | 55.00 ± 2.89** |
| Germination rate | 98.33 ± 1.67 | 100.00 ± 0.00* | 98.33 ± 1.67 | 98.33 ± 1.67 | 98.33 ± 1.67 | 98.33 ± 1.67 | 100.00 ± 0.00* | 95.00 ± 2.89* | 96.67 ± 1.67* | 98.33 ± 1.67 |
| Germination index | 9.44 ± 0.03 | 9.67 ± 0.10* | 9.39 ± 0.17* | 9.47 ± 0.10 | 9.28 ± 0.10 | 7.47 ± 0.07 | 8.56 ± 0.10** | 8.78 ± 0.18** | 6.69 ± 0.27** | 6.53 ± 0.10** |
| Vigor index | 6.77 ± 0.13 | 7.34 ± 0.08* | 7.07 ± 0.24* | 6.54 ± 0.17 | 6.34 ± 0.04* | 4.18 ± 0.12 | 5.76 ± 0.14** | 5.94 ± 0.14** | 3.55 ± 0.18** | 3.33 ± 0.08** |
| GDRI | 0.80 ± 0.01 | 0.88 ± 0.00** | 0.93 ± 0.02** | 0.70 ± 0.03** | 0.71 ± 0.02** | |||||
| GSI | 0.79 ± 0.01 | 0.89 ± 0.00** | 0.93 ± 0.01** | 0.71 ± 0.02** | 0.70 ± 0.02** | |||||
| 10% PGE-6000 | 15% PGE-6000 | |||||||||
| Genotype | ||||||||||
| M18 | OEA1 | OEA2 | IEA1 | IEA2 | M18 | OEA1 | OEA2 | IEA1 | IEA2 | |
| Germination potential | 51.67 ± 1.67 | 75.00 ± 0.00** | 81.67 ± 1.67** | 41.67 ± 1.67** | 36.67 ± 1.67** | 26.67 ± 4.41 | 41.67 ± 1.67** | 40.00 ± 2.89** | 16.67 ± 1.67** | 15.00 ± 2.89** |
| Germination rate | 91.67 ± 4.41 | 96.67 ± 1.6** | 93.33 ± 1.67** | 90.00 ± 2.89** | 88.33 ± 4.41** | 68.33 ± 6.01 | 86.67 ± 7.26** | 90.00 ± 5.77** | 71.67 ± 4.41** | 61.67 ± 9.28** |
| Germination index | 6.11 ± 0.15 | 7.36 ± 0.22** | 7.31 ± 0.15** | 5.64 ± 0.07** | 5.25 ± 0.34** | 3.78 ± 0.35 | 5.11 ± 0.16** | 5.14 ± 0.24** | 3.69 ± 0.07** | 3.17 ± 0.27** |
| Vigor index | 2.63 ± 0.09 | 4.19 ± 0.12** | 4.24 ± 0.13** | 2.11 ± 0.09** | 1.99 ± 0.10** | 1.43 ± 0.12 | 2.28 ± 0.17** | 2.31 ± 0.09** | 1.27 ± 0.05** | 1.01 ± 0.11** |
| GDRI | 0.70 ± 0.02 | 0.76 ± 0.03** | 0.76 ± 0.02** | 0.66 ± 0.02** | 0.64 ± 0.05** | 0.41 ± 0.01 | 0.52 ± 0.02** | 0.53 ± 0.03** | 0.40 ± 0.02 | 0.35 ± 0.04** |
| GSI | 0.65 ± 0.02 | 0.76 ± 0.03** | 0.78 ± 0.01** | 0.60 ± 0.01** | 0.57 ± 0.04** | 0.40 ± 0.03 | 0.53 ± 0.03** | 0.55 ± 0.03** | 0.39 ± 0.02 | 0.34 ± 0.04** |
Within a row and treatment (normally or drought), values followed by asterisks are significantly different from M18
*P < 0.05; **P < 0.01
Comparative analysis of plant types of different soybean strains under different drought stresses
Under normal water conditions, OEA1, OEA2, IEA1, and IEA2 showed good phenotypic performance and thick green stalks, which showed no significant difference compared with the control group M18 (Fig. 2a).
Fig. 2.
Seedling phenotypic analysis of M18 and GmXTH1 transgenic soybean under different drought stresses. (a After 30 days of germination, the phenotypes of M18 and OEA1, OEA2, IEA1, and IEA2 at seedling stage were analyzed under 0 day drought stress. b Phenotypic analysis of M18 and OEA1, OEA2, IEA1, and IEA2 at seedling stage under drought stress for 7 days after germination for 30 days. c Phenotypic analysis of M18 and OEA1, OEA2, IEA1, and IEA2 at seedling stage under drought stress for 15 days after germination for 30 days)
After 7 days of drought treatment, OEA1 and OEA2 lines with overexpression of GmXTH1 had slightly drooping and dark green leaves and strong and upright stalks. IEA1 was expressed by GmXTH1 interference. The plants were moderately wilting, the leaves were moderately drooping, curled and shriveled, and the stalks were bending due to mild drought stress. IEA2 plants with moderate wilting were more serious and drooping, and the stems also showed bending phenomenon. The control material M18 had slightly wilting leaves, slightly drooping leaves, slightly yellowing, and slightly curled and wrinkled edges. After 7 days of drought treatment, there were significant differences in the overall phenotypes among different strains. After rehydration for 2h, in OEA1 and OEA2, the leaves gradually returned to dark green and the stalks were strong and straight. In IEA1 and IEA2 after 24h, the plants gradually stood upright from wilting, and the leaves gradually recovered from drooping to rising dark green. After 12h, the M18 control material returned to strong and straight stems with upturned leaves, and there was significant difference in overall recovery (Fig. 2b). The results showed that the overexpression of GmXTH1 gene was beneficial for the improvement of drought tolerance and recovery of plants, and had a positive effect on the response of plants to drought stress.
After 15 days of drought, OEA1, the transgenic line with GmXTH1 overexpression, was more severely shriveled. But a small part of the leaves extended normally and the stalks were relatively erect. OEA2 plant leaves are seriously wrinkled, but a small part of them are normally extended, and the stalks are relatively erect. In the control group, the leaves of M18 plants were seriously wrinkled and the stems were seriously dehydrated and bent. Transforming GmXTH1 interferes with the expression of IEA1 and IEA2. The leaves of the plants are extremely seriously wrinkled, and the stalks are also dry and short due to extremely severe dehydration (Fig. 2c). After rehydration, OEA1 and OEA2 gradually returned to the normal growth state of dark green leaves and strong and straight stalks 24h later. IEA1 and IEA2 showed no recovery after rehydration, and the plants dried up and died. The control material M18 plants did not recover after rehydration, and the plants dried up and died (Fig. 2c). The results showed that the overexpression of GmXTH1 gene was beneficial to the improvement of drought tolerance and recovery of plants, and had a positive effect on the response of plants to drought stress
Comparative analysis of root systems of different soybean strains under different drought stresses
Under normal water conditions, the total root length, surface area, total root projection area, root volume, mean root diameter, total cross number of roots, and total root tip number of OEA1 transplants overexpressed with GmXTH1 were significantly higher than those of the control material M18. The total root length, root volume, average root diameter, total cross number, and total root tip number of OEA2 transgenic line with GmXTH1 over expression were significantly higher than those of the control group M18. The root surface area and total projected area were significantly higher than those of the control group M18. The total root length and mean root diameter of IEA1 transgenic lines were significantly lower than those of the control group M18, and the root volume was significantly lower than that of the control group M18 (Table 2). The root surface area, total projection area, total cross number, and total root tip number showed no difference with those of the control group M18. The total root length, total root projection area, mean root diameter, total root crossover number, and total root tip number of IEA2 transgenic lines with GmXTH1 overexpression were significantly lower than those of the control material M18. The root surface area and volume were significantly lower than those of the control material M18 (Table 2).
Table 2.
Comparison and analysis of root traits of transgenic GmXTH1 soybean at seedling stage under different drought conditions
| M18 | OEA1 | OEA2 | IEA1 | IEA2 | ||
|---|---|---|---|---|---|---|
| Normal irrigation | TL (cm) | 130.17 ± 0.28 | 176.81 ± 2.41** | 159.03 ± 0.89** | 106.32 ± 3.01** | 100.96 ± 4.45** |
| SA (cm2) | 20.14 ± 2.18 | 34.18 ± 3.56** | 31.37 ± 4.15* | 20.59 ± 1.71 | 15.19 ± 0.44* | |
| PA (cm2) | 6.41 ± 0.69 | 10.88 ± 1.13** | 9.99 ± 1.32* | 6.55 ± 0.55 | 4.83 ± 0.14** | |
| Vol (cm3) | 0.25 ± 0.05 | 0.53 ± 0.10** | 0.51 ± 0.14** | 0.32 ± 0.06* | 0.18 ± 0.02* | |
| AvgD (mm) | 0.49 ± 0.05 | 0.61 ± 0.06** | 0.63 ± 0.08** | 0.62 ± 0.07** | 0.48 ± 0.03 | |
| TNT | 892.33 ± 32.20 | 1293.00 ± 139.08** | 1151.30 ± 168.00** | 854.67 ± 76.22 | 790.00 ± 42.06** | |
| TNF | 897.33 ± 149.63 | 1564.00 ± 242.09** | 1559.00 ± 13.80** | 909.67 ± 24.22 | 791.67 ± 78.25** | |
| TNC | 60.33 ± 9.21 | 127.67 ± 6.84** | 101.00 ± 7.77** | 56.00 ± 10.69 | 47.67 ± 7.42* | |
| Drought 7 days | TL (cm) | 337.66 ± 10.31 | 508.61 ± 36.97** | 392.67 ± 17.74* | 242.28 ± 2.56** | 239.18 ± 3.10** |
| SA (cm2) | 91.87 ± 12.20 | 134.47 ± 5.51** | 107.74 ± 28.40* | 52.08 ± 1.69** | 51.47 ± 7.56** | |
| PA (cm2) | 29.24 ± 3.88 | 42.80 ± 1.75** | 34.30 ± 9.04* | 16.58 ± 0.54** | 16.38 ± 2.41** | |
| Vol (cm3) | 2.05 ± 0.54 | 2.84 ± 0.16** | 2.59 ± 1.01* | 0.89 ± 0.07** | 0.92 ± 0.25** | |
| AvgD (mm) | 0.86 ± 0.10 | 0.84 ± 0.04 | 0.85 ± 0.20 | 0.68 ± 0.03* | 0.69 ± 0.10* | |
| TNT | 2751.67 ± 237.17 | 3608.00 ± 87.50** | 2376.33 ± 269.22* | 1637.67 ± 156.33** | 2012.67 ± 160.32* | |
| TNF | 5871.33 ± 259.68 | 9039.33 ± 147.54** | 5782.00 ± 1231.13 | 2400.33 ± 38.67** | 3211.33 ± 494.89** | |
| TNC | 434.33 ± 23.38 | 671.00 ± 36.59** | 404.33 ± 57.91 | 150.00 ± 3.00** | 222.00 ± 43.11** | |
| Drought 15 days | TL (cm) | 243.86 ± 13.06 | 295.09 ± 6.74** | 280.27 ± 3.22** | 206.55 ± 10.91** | 199.16 ± 6.44** |
| SA (cm2) | 50.33 ± 14.08 | 80.15 ± 9.88** | 58.12 ± 5.55* | 51.65 ± 9.92 | 38.63 ± 2.39** | |
| PA (cm2) | 16.02 ± 4.48 | 25.51 ± 3.14** | 18.50 ± 1.77 | 15.09 ± 2.10 | 12.30 ± 0.76* | |
| Vol (cm3) | 0.91 ± 0.45 | 1.78 ± 0.38** | 0.98 ± 0.17 | 0.83 ± 0.16 | 0.60 ± 0.06** | |
| AvgD (mm) | 0.64 ± 0.14 | 0.87 ± 0.09** | 0.66 ± 0.06 | 0.70 ± 0.05 | 0.62 ± 0.03 | |
| TNT | 1847 ± 132.22 | 2342.33 ± 111.44** | 1859 ± 160.07 | 1292.67 ± 99.34** | 1380 ± 17.39** | |
| TNF | 2986.67 ± 785.96 | 5311.33 ± 444.03** | 3661.33 ± 727.30** | 2196.33 ± 371.68** | 2102 ± 129.19** | |
| TNC | 238.67 ± 20.51 | 379 ± 9.07** | 283.33 ± 63.60** | 136.67 ± 31.00** | 145 ± 1.00** |
Within a row and treatment (normally or drought), values followed by asterisks are significantly different from M18. *P < 0.05; **P < 0.01
In the case of 7 days of drought, the total root length, surface area, total root projection area, root volume, total cross number, and total root tip number of OEA1 transgenic lines overexpressed with GmXTH1 were significantly higher than those of the control group M18, and the average root diameter showed no difference with that of the control group M18. The total length, surface area, total projection area, root volume, and total cross number of roots of OEA2 transgenic lines overexpressed with GmXTH1 were significantly higher than those of the control group M18. There was no difference between the total projection area, average diameter, and total number of root tips of OEA2 transgenic lines overexpressed with GmXTH1 and the control group M18 (Table 2). The total root length, surface area, total root projection area, root volume, total cross number, and total root tip number of IEA1 transgenic lines were significantly lower than those of the control material M18, and the mean root diameter was significantly lower than that of the control material M18 (Table 2). The total root length, surface area, total root projection area, root volume, total root crossover number, and total root tip number of IEA2 transgenic lines were significantly lower than those of the control material M18, and the average root diameter was significantly lower than that of the control material M18 (Table 2).
The total root length, surface area, total root projection area, root volume, mean root diameter, total cross number, and total root tip number of OEA1 transgenic lines overexpressed by GmXTH1 were significantly higher than those of the control group M18 under the condition of drought for 15 days. The total root length and total root tip number of OEA2 transgenic lines overexpressed with GmXTH1 were significantly higher than those of the control material M18. The root surface area was significantly higher than that of the control material M18. There were no differences in the total root projection area, root volume, average root diameter, and total cross number of roots of the control material M18. The total root length, total cross number, and total root tip number of IEA1 transgenic lines were significantly lower than those of the control material M18. The average diameter surface area, total root projection area, and root volume of IEA1 transgenic lines were not different from those of the control material M18 (Table 2). The total root length, surface area, total root projection area, root volume, total cross number, and total root tip number of IEA2 transgenic lines were significantly lower than those of the control group M18, and the mean root diameter had no difference with that of the control group M18 (Table 2).
The total root length, surface area, total root projection area, root volume, mean root diameter, total cross number, and total root tip number of all strains increased significantly after 7 days of drought compared with normal conditions. But OEA1 and OEA2 strains overexpressed by GmXTH1 were more significant (Table 2). The total root length, surface area, total root projection area, root volume, mean diameter, total cross number, and total root tip number of each strain decreased significantly after 15 days of drought compared with 7 days of drought, but the decrease amplitude of OEA1 and OEA2 in GmXTH1 over expression lines was small (Table 2).
The above results indicated that the overexpression of GmXTH1 gene could significantly increase the total root length, surface area, total projection area, root volume, mean root diameter, total cross number, and total root tip number of the plant root system, which promoted the more developed root system and was more conducive to the absorption of water and minerals.
Physiological and biochemical analysis of GmXTH1 transgenic soybean at seedling stage
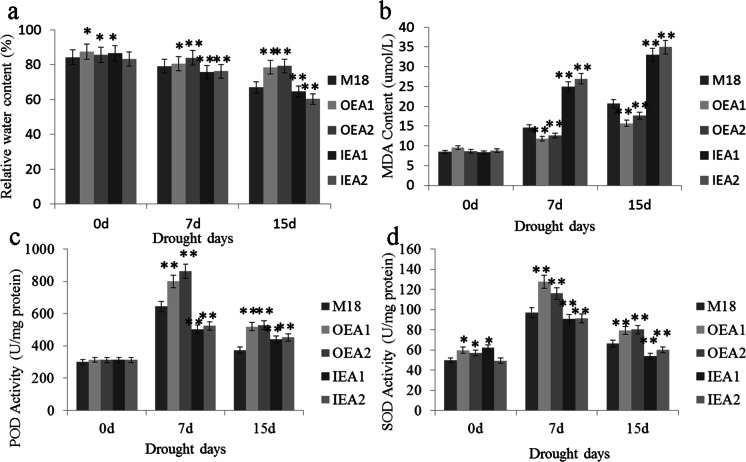
Without drought treatment, RWC of leaves of different soybean strains had significant differences as a whole. After 7 days of drought treatment, the RWC of OEA1 and OEA2 leaves was significantly higher than that of the M18 control group (79.14%, 80.56%, and 83.97%, respectively). The RWC of IEA1 and IEA2 leaves was significantly lower than that of the M18 control group (79.14%, 75.68%, and 76.25%, respectively). There were significant differences in RWC among leaves after 7 days of drought. After 15 days of drought treatment, the RWC of OEA2 and OEA1 leaves was significantly higher than that of M18 in the control group (66.93%, 79.24%, and 78.54%, respectively) (Fig. 3a). The RWC of IEA2 and IEA1 leaves was significantly lower than that of M18 in the control group (66.93%, 60.35%, and 61.77%, respectively) (Fig. 3a). The RWC of leaves was significantly different after 15 days of drought. The results indicated that the overexpression of GmXTH1 gene could significantly delay the decrease of RWC and reduce transpiration of water in leaves.
Fig. 3.
Physiological and biochemical indexes at seedling stage of different GmXTH1 transgenic soybean lines under different drought conditions. (a Leaf relative water content. b Malondialdehyde content. c Peroxidase activity. d Superoxide dismutase activity. *P < 0.05; **P < 0.01)
The MDA content of different strains showed no significant difference without drought treatment. (Fig. 3b). After 7 days of drought treatment, the MDA content of OEA2 and OEA1 was significantly lower than that of M18, and the MDA content of IEA2 and IEA1 was significantly higher than that of M18 while the growth rates of MDA content of OEA2 and OEA1 were 46.57% and 37.50%, respectively. And it was significantly lower than that of the control M18 73.67%. The MDA content of IEA2 and IEA1 increased by 200.24% and 206.95%, respectively (Fig. 3b). After 15 days of drought treatment, the MDA content of OEA2 and OEA1 was significantly lower than that of M18. The MDA content of IEA2 and IEA1 was significantly lower than that of M18, and the MDA content of OEA2 and OEA1 increased by 104.30% and 82.94%, respectively. They were significantly lower than those of the control M18 (144.84%). The MDA content of IEA2 and IEA1 increased by 297.72% and 296.99%, respectively. The results indicated that the overexpression of GmXTH1 gene could slow down the peroxidation degree of membrane lipid.
There was no significant difference observed in POD activity between different strains before drought treatment (0 days) (Fig. 3c). After 7 days of drought treatment, the POD activity of OEA2 and OEA1 was significantly higher than that of M18, and the POD activity of IEA2 and IEA1 was significantly lower than that of M18. The growth rates of POD activity of OEA2 and OEA1 were 176.45% and 155.24% respectively, which were significantly higher than those of the control M18 (113.54%). The POD activity of IEA2 and IEA1 increased by 67.66% and 61.27%, respectively. After 15 days of drought treatment, the POD activity of OEA2 and OEA1 was significantly higher than that of M18, and the POD activity of IEA2 and IEA1 was significantly lower than that of M18. The growth rates of POD activity of OEA2 and OEA1 were 69.12% and 65.59% respectively, which were significantly higher than those of the control M18 (57.00%). The POD activity of IEA2 and IEA1 increased by 44.65% and 40.94%, respectively (Fig. 3c). The results showed that the effective removal of harmful substances during the seedling stage of soybean transgenic GmXTH1 gene overexpression could produce more protective enzymes and resist the damage caused by drought.
Without drought treatment, SOD activity of different strains showed no significant difference. After 7 days of drought treatment, the SOD activities of OEA2 and OEA1 were significantly higher than those of M18, and the SOD activities of IEA2 and IEA1 were significantly lower than those of M18, whereas the SOD activity growth rates of OEA2 and OEA1 were 126.89% and 156.57%, respectively. Higher significant difference was observed as compared to that of the control M18 (94.65%). The SOD activity of IEA2 and IEA1 increased by 84.98% and 74.63%, respectively. After 15 days of drought treatment, the SOD activities of OEA2 and OEA1 were significantly higher than those of M18, and the SOD activities of IEA2 and IEA1 were significantly lower than those of M18. The SOD activity growth rates of OEA2 and OEA1 were 56.63% and 59.50% respectively, which were significantly higher than those of the control M18 (33.55%) (Fig. 3d). The SOD activity of IEA2 and IEA1 increased by 21.88% and 22.88%, respectively (Fig. 3d). The results demonstrated that the effective removal of harmful substances during the seedling stage of soybean transgenic GmXTH1 gene overexpression could produce more protective enzymes and resist the damage caused by drought.
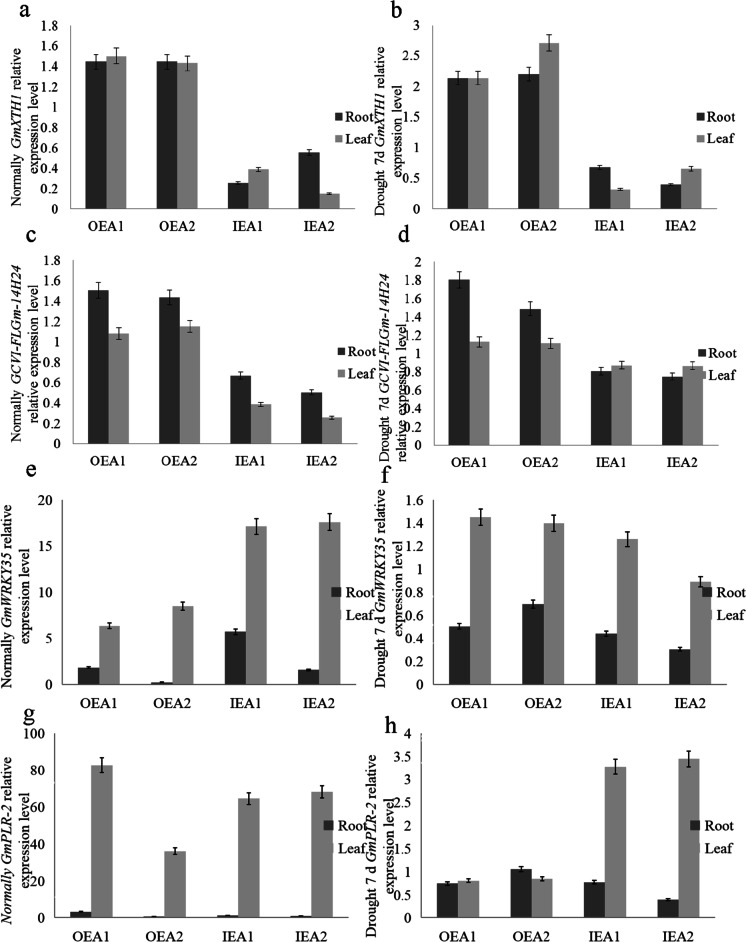
Relative expression levels of target gene and other endogenous genes in transgenic GmXTH1 soybean at seedling stage
Under normal water conditions, the expression level of OEA1 GmXTH1 in soybean roots and leaves was increased by 44.39% and 50.00% respectively. The expression level of OEA2 strain GmXTH1 in soybean roots and leaves was increased by 56.37% and 42.90% respectively. The expression level of GmXTH1 of IEA1 strain was decreased by 74.74% in soybean root and 61.31% in leaf. The expression level of GmXTH1 of IEA1 strain was decreased by 44.71% in soybean root and 85.39% in leaf (Fig. 4a).
Fig. 4.
Relative expression levels of GmXTH1 and JCVI-FLGM-14H24 in different transgenic soybean lines under different drought conditions at seedling stage. (a Under normal circumstances, the relative expression of GmXTH1 in OEA1, OEA2, IEA1, and IEA2 roots and leaves. b Relative expression levels of GmXTH1 in OEA1,OEA2, IEA1, and IEA2 roots and leaves after 7 days of drought. c Under normal conditions, the relative expression levels of JCVI-FLGM-14H24 in OEA1, OEA2, IEA1, and IEA2 roots and leaves. d JCVI-FLGM-14H24 in OEA1, OEA2, IEA1, and IEA2 roots and leaves. e Relative expression levels of GmWRKY35 in roots and leaves of OEA1, OEA2, IEA1, and IEA2. f The relative expression levels of GmWRKY35 in OEA1, OEA2, IEA1, and IEA2 roots and leaves. g The relative expression levels of GmPLR-2 in OEA1, OEA2, IEA1, and IEA2 roots and leaves. h The relative expression levels of GmPLR-2 in OEA1, OEA2, IEA1, and IEA2 roots and leaves)
After 7 days of drought, the expression level of OEA1 GmXTH1 in soybean roots and leaves increased by 113.61%. The expression level of OEA2 strain GmXTH1 in soybean roots and leaves was increased by 120.38% and 171.32% respectively. The expression of IEA1 strain GmXTH1 in soybean roots and leaves was reduced by 32.40% and 68.00% respectively. The expression level of GmXTH1 of IEA2 strain was decreased by 60.50% in soybean roots and 34.48% in soybean leave (Fig. 4b).
Under normal water conditions, the expression level of OEA1 strain JCVI-FLGM-14H24 in soybean roots and leaves was increased by 50.52% and 7.92% respectively. The expression level of OEA2 strain JCVI-FLGM-14H24 in soybean roots and leaves was increased by 43.40% and 14.87% respectively. The expression level of IEA1 strain JCVI-FLGM-14H24 was decreased by 33.10% in soybean root and 61.31% in soybean leaf. The expression level of IEA2 strain JCVI-FLGM-14H24 was decreased by 49.65% in soybean roots and 74.30% in soybean leaves. The above results indicated that the overexpression of GmXTH1 promoted the expression of JCVI-FLGM-14H24 under normal water conditions, while the interference of GmXTH1 inhibited the expression of JCVI-FLGM-14H24 (Fig. 4c).
After 7 days of drought, the expression level of OEA1 strain JCVI-FLGM-14H24 in soybean roots and leaves increased by 80.25% and 12.51%. The expression level of OEA2 strain JCVI-FLGM-14H24 was increased by 48.45% in soybean roots and 10.96% in leaves. The expression level of IEA1 strain JCVI-FLGM-14H24 was decreased by 19.34% in soybean roots and 12.95% in soybean leaves. The expression level of IEA2 strain JCVI-FLGM-14H24 was decreased by 25.26% in soybean roots and 13.55% in soybean leaves. The above results indicated that the overexpression of GmXTH1 promoted the expression of JCVI-FLGM-14H24, while the interference expression of GmXTH1 inhibited the expression of JCVI-FLGM-14H24 under drought for 7 days (Fig. 4d).
Under normal water conditions, the expression level of OEA1 strain GmWRKY35 in soybean roots and leaves increased by 80.25% and 536.43% (Fig. 4e). The expression level of OEA2 strain GmWRKY35 was decreased by 78.61% in soybean roots and increased by 748.55% in leaves. The expression level of IEA1 strain GmWRKY35 in soybean roots and leaves was increased by 471.60% and 1608.90% respectively. The expression level of IEA2 strain GmWRKY35 in soybean roots and leaves was increased by 56.37% and 1656.95% respectively. The above results indicated that the low expression level of GmXTH1 under normal water condition was conducive to the expression of GmWRKY35.
After 7 days of drought, the expression of OEA1 strain GmWRKY35 in soybean roots decreased by 49.48% and increased by 44.89% in leaves. The expression level of OEA2 strain GmWRKY35 was decreased by 30.27% in soybean roots and increased by 39.47% in leaves. The expression level of IEA1 strain GmWRKY35 was decreased by 56.02% in soybean roots and increased by 25.70% in soybean leaves. The expression level of IEA2 strain GmWRKY35 was decreased by 69.75% in soybean roots and 11.12% in soybean leaves. The above results indicated that the overexpression of the target gene GmXTH1 was beneficial to the expression of GmWRKY35 in soybean leaves under drought for 7 days, while the expression of the target gene GmXTH1 inhibited the expression of GmWRKY35 in soybean roots, and the higher the expression level, the weaker the inhibition (Fig. 4f).
Under normal water conditions, the expression level of OEA1 strain GmPLR-2 in soybean roots and leaves was increased by 217.11% and 8171.06% respectively (Fig. 4g). The expression level of OEA2 strain GmPLR-2 was decreased by 95.00% in soybean roots and increased by 3512.69% in leaves. The expression level of GmPLR-2 of IEA1 strain was decreased by 44.91% in soybean root and increased by 6344.52% in soybean leaf. The expression level of GmPLR-2 of IEA2 strain was decreased by 85.39% in soybean roots and increased by 6711.97% in soybean leaves. The results showed that the expression of target gene GmXTH1 inhibited the expression of GmPLR-2 in soybean roots and promoted the expression of GmPLR-2 in soybean leaves under normal water conditions.
After 7 days of drought, the expression level of OEA1 strain GmPLR-2 in soybean roots and leaves decreased by 25.26% and 19.34%. The expression level of OEA2 strain GmPLR-2 in soybean roots was increased by 5.70% and decreased by 15.03% in leaves. The expression level of GmPLR-2 of IEA1 strain was decreased by 22.62% in soybean roots and increased by 227.16% in soybean leaves. The expression of IEA2 strain GmPLR-2 was decreased by 60.09% in soybean roots and increased by 244.62% in soybean leaves. The above results indicated that the target gene GmXTH1 was overexpressed and inhibited the expression of GmPLR-2 in soybean leaves under drought for 7 days, while the target gene GmXTH1 interfered with the expression and promoted the expression of GmPLR-2 in soybean leaves (Fig. 4h)
Discussion
Germination and phenotypic data analysis of GmXTH1 transgenic soybean
Seed germination stage is a relatively important stage for the study of drought resistance of plants, which can be used for early identification of drought tolerance of plants (Lai et al. 2009). Crop varieties with strong drought resistance have a fast water absorption rate and can sprout quickly under drought stress, with better germination indexes such as relative germination rate, relative germination potential, and drought resistance coefficient of germination. The test results showed that when the water content of seeds was normal at germination stage, the root number of transgenic OEA1 and OEA2 strains with over expression of GmXTH1 was significantly higher than that of the control material M18. The root number of transgenic IEA1 and IEA2 strains with interference expression of GmXTH1 was significantly lower than that of the control material M18, indicating that GmXTH1 promoted lateral root meristem of soybean root. With the increase of PEG-6000 concentration, the germination potential, germination rate, and germination index of OEA1 and OEA2 strains transgenic with GmXTH1 overexpression were significantly higher than those of the control group M18. The germination potential, germination rate, and germination index of IEA1 and IEA2 expressed by GmXTH1 interference were significantly lower than those of the control group M18. Moreover, the transgenic lines OEA1 and OEA2 with overexpression of GmXTH1 had better growth and longer soybean roots under the same conditions. It was inferred that the overexpression of GmXTH1 promoted lateral root meristem and root elongation of soybean roots, and improved drought tolerance of soybean seeds.
The root system is the first organ that crops feel soil drought. The characteristics and activities of root system are closely related to drought resistance. As an important part of crop drought tolerance research and improvement, root system is attracting the attention of researchers (Liu et al. 2004). Once compared the root traits of two soybean varieties with similar agronomic traits but different drought resistance, with found that the root quantity, root volume, and root surface area of the varieties with strong drought resistance were much more than those with poor drought resistance (Hudak and Patterson 1995, 1996). The total root length, surface area, total projection area, root volume, mean diameter, total cross number, and total root tip number of GmXTH1 transgenic materials OEA1 and OEA2 were significantly higher than those of the control material M18. The transgenic GmXTH1 gene interference expression materials IEA1 and IEA2 were significantly lower than the control material M18. The results showed that the overexpression of GmXTH1 gene could significantly improve the root condition of the plant. There were significant differences in plant type performance of different soybean strains at seedling stage under drought stress. With the increase of drought time, the plant type performance of OEA1 and OEA2 transgenic materials with GmXTH1 gene overexpression was significantly better than that of the control group. The plant type performance of IEA1 and IEA2 transgenic materials with GmXTH1 gene interference expression was significantly worse than that of the control group. The results showed that the overexpression of GmXTH1 gene can significantly improve the drought resistance of plants.
Root traits of different soybean strains at seedling stage were significantly different under drought stress. The total root length, surface area, total root projection area, root volume, average root diameter, total root crossing number, and total root tip number of GmXTH1 transgenic materials OEA1 and OEA2 were significantly higher than those of the control material M18. The GmXTH1 gene interference expression materials IEA1 and IEA2 were significantly lower than the control material M18. The results showed that the overexpression of GmXTH1 gene could significantly improve the root meristem.
Analysis of physiological and biochemical indexes of GmXTH1 transgenic soybean
Leaf RWC and other indicators of leaf are sensitive to water deficit and are often used as important indicators for drought resistance identification. Water loss rate or retention rate reflects the conditions of free water and bound water in plant cells. Researchers generally believe that lower in vitro flag leaf RWL is a type of physiological mechanism of drought resistance that does not reduce yield (Clarke et al. 1989, 1991). The results of this study showed that leaf RWC decreased with the increase of drought time. The RWC of OEA1 and OEA2 transgenic materials with overexpression of GmXTH1 gene was significantly higher than that of the control material at the same period, and the RWC of IEA1 and IEA2 transgenic materials with interference expression of GmXTH1 gene was significantly lower than that of the control material at the same period, indicating that the overexpression of GmXTH1 gene could significantly delay the water loss of leaves.
Under drought stress, a large number of reactive oxygen radicals will be produced in plants, which will cause membrane lipid peroxidation and damage the cell membrane, thereby harming plant tissues, accelerating plant senescence, and even causing plant death (Cutler et al. 1980; Chaves et al. 2003). The antioxidant enzymes produced by plant stress can scour free radicals, reduce cell membrane damage, and enhance drought resistance of varieties. This study showed that the SOD and POD of OEA1 and OEA2 transgenic materials with overexpression of GmXTH1 gene responded to drought more quickly and had higher activity than those of the control variety M18. Compared with the control variety M18, the response of SOD and POD of GmXTH1 transgenic interference expression materials IEA1 and IEA2 to drought was slower and the activity was lower, which indicated that GmXTH1 transgenic overexpression soybean material could produce more protective enzymes timely in the effective removal of harmful substances in the seedling stage of soybean and resist the damage caused by drought. The activity of SOD and POD increased first and then decreased with the increase of drought time.
MDA is the product of membrane lipid peroxidation and reflects the strength of plant response to stress conditions that showed that MDA content increased with the extension of drought time, and similar results were also shown in this study. The increase of MDA content in the GmXTH1 overexpression materials OEA1 and OEA2 was significantly lower than that in the control materials of the same period, and the increase of MDA content in the GmXTH1 interference expression materials IEA1 and IEA2 was significantly higher than that in the control materials of the same period, indicating that the overexpression of GmXTH1 can slow down the peroxidation degree of membrane lipid.
Analysis of target gene and its endogenous gene expression in transgenic GmXTH1 soybean
Transcription factor (TF), also known as trans-acting factor, is a regulation product of gene coding, which can specifically bind with cis-acting elements in gene promoter region, so as to ensure the combination of protein molecules expressed by target gene at a specific intensity and at a specific time and space (Latchman et al. 1997). Transcription factors can activate the expression of multiple signaling pathway genes in plants and participate in almost all life activities such as growth and development, morphogenesis, and stress response (Baillo et al. 2019). In plant stress response, it has the function of signal transduction and gene expression regulation, such as CDPK, MAPK, and other protein kinases that sense and transact stress signals, as well as transcription factors that regulate gene expression such as bZIP, bHLH, NAC, DREB, ERF, RAV, WRKY, and MYB (Riechmann et al. 2000). The target gene of GmXTH1 transgenic soybean was expressed in both roots and leaves, and there was no difference in the expression between roots and leaves. The expression level of this gene increased with the degree of drought. Under normal water conditions, the overexpression of GmXTH1 promoted the expression of JCVI-FLGM-14H24, while the interference expression of GmXTH1 inhibited the expression of JCVI-FLGM-14H24, and the low expression level of GmXTH1 was conducive to the expression of GmWRKY35. The expression of target gene GmXTH1 inhibits the expression of GmPLR-2 in soybean roots, while the expression of target gene GmXTH1 promotes the expression of GmPLR-2 in soybean leaves. Under drought for 7 days, overexpression of GmXTH1 promoted the expression of JCVI-FLGM-14H24, while interference with GmXTH1 inhibited the expression of JCVI-FLGM-14H24. The overexpression of the target gene GmXTH1 is beneficial to the expression of GmWRKY35 in soybean leaves, while the expression of the target gene GmXTH1 inhibits the expression of GmWRKY35 in soybean roots. E3 ligases play an important role in the UPS (ubiquitin-proteasome) system, which is responsible for specific recognition, recruitment, and transport of target proteins (Moon et al. 2004), and then ubiquitination modification to regulate different physiological processes of plants. A large number of studies have shown that RING-H2-type E3 ligase is related to plant resistance to abiotic stress, especially drought stress. The higher the expression level, the weaker the inhibition. The overexpression of target gene GmXTH1 inhibited the expression of GmPLR-2 in soybean leaves, while the interference of the expression of target gene GmXTH1 promoted the expression of GmPLR-2 in soybean leaves.
Conclusions
Under drought stress, the overexpression of GmXTH1 gene could improve seed germination rate, promote root development, reduce cell membrane damage, and thus improve drought resistance of soybean. This study laid a theoretical foundation for further understanding the biological function and molecular mechanism of GmXTH1 in soybean under stress, and also provided a reference for further study of soybean breeding under stress.
Author contribution
Wrote first draft: YZ, YS, and PW. Designed experimental work: YZ, YS, HZ, JY, YD, JQ, and PW. Investigation: YZ. Provided experimental materials: YS, PW, JQ. Analyzed data: YZ. Wrote original manuscript: YZ, HZ. Wrote and edit review: YZ, HZ, YS. Visualization: YZ. Supervised the whole work: YD. Project administration: JQ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jilin Province Science and Technology Development Plan Project (grant number 20190103120JH) and National Natural Science Foundation of China Projects (grant numbers 31771817, 31801381).
Data availability
Data are available, upon any reasonable request, from the authors.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Supplementary information
The following are available online at www.mdpi.com/xxx/s1, Table S1: title.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yang Song, Email: songyangjlnd@163.com.
Pi-wu Wang, Email: peiwuw@163.com.
References
- Aranjuelo I, Molero G, Erice G, et al. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.) J Exp Bot. 2011;62(1):111–123. doi: 10.1093/jxb/erq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor Y, Haigler CH, Johnson S, et al. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92(20):9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoub A, Beckhaus T, Berberich T. Comparative analysis of barley leaf proteome as affected by drought stress. Planta. 2013;237(3):771–781. doi: 10.1007/s00425-012-1798-4. [DOI] [PubMed] [Google Scholar]
- Baillo EH, Kimotho RN, Zhang Z, et al. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes. 2019;10(10):771–793. doi: 10.3390/genes10100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MJ, Eklof JM, Michel G, et al. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: biological implications for cell wall metabolism. Plant Cell. 2007;19(6):1947–1963. doi: 10.1105/tpc.107.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouslama M, Schapaugh WT. Stress tolerance in soybeans. i. evaluation of three screening techniques for heat and drought tolerance. Crop Science. 1984;24(5):933–937. doi: 10.2135/cropsci1984.0011183X002400050026x. [DOI] [Google Scholar]
- Campbell P, Braam J. Xyloglucan endotransglycosylases: diversity of genes, enzymes and potential wall -modifying functions. Trends Plant Sci. 1999;4(9):361–366. doi: 10.1016/S1360-1385(99)01468-5. [DOI] [PubMed] [Google Scholar]
- Cho SK, Kim JE, Park JA, et al. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006;580:3136–3144. doi: 10.1016/j.febslet.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Choi JY, Seo YS, Kim SJ, et al. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang) Plant Cell Rep. 2011;30:867–877. doi: 10.1007/s00299-010-0989-3. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Romagosa I, Jana S, et al. Relationship of excised leaf water loss rate and yield of durum in diverse environments. Can J Plant Sci. 1989;69(4):1075–1081. doi: 10.4141/cjps89-130. [DOI] [Google Scholar]
- Clarke JM, Richards RA, Condon AG. Effect of drought stress on residual transpiration and its relationship with water use of wheat. Can J Plant Sci. 1991;71:695–702. doi: 10.4141/cjps91-102. [DOI] [Google Scholar]
- Cutler JM. Influence of water deficits and osmotic adjustment on leaf elongation in rice. Crop Sci. 1980;20:314–318. doi: 10.2135/cropsci1980.0011183X002000030006x. [DOI] [Google Scholar]
- Chaves MM, Pereira JS, Maroco J. Understanding plant response to drought from genes to the whole plant. Funct Plant Biol. 2003;89:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Dong JL, Jiang YY, Chen RJ, et al. Isolation of a novel xyloglucan endotransglucosylase (OsXET9) gene from rice and analysis of the response of this gene to abiotic stresses. Afr J Biotech. 2011;10(76):17424–17434. [Google Scholar]
- Du ZY, Bramlage WJ. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem. 1992;40:1566–1570. doi: 10.1021/jf00021a018. [DOI] [Google Scholar]
- Latchman DS (1997) Transcription factor: An overview. Int J Biochem Cell Biol 29(12):1305–1312 [DOI] [PubMed]
- Farooq M, Gogoi N, Barthakur S, et al. Drought stress in grain legumes during reproduction and grain filling. J Agron Crop Sci. 2017;203(2):81–102. doi: 10.1111/jac.12169. [DOI] [Google Scholar]
- Harada T, Torii Y, Morita S, et al. Cloning, characterization and expression of xyloglucan endotransglucosylase/hydrolase and expansin genes associated with petal growth and development during carnation flower opening. J Exp Bot. 2011;62(2):815–823. doi: 10.1093/jxb/erq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwasa K, Nakano R, Inaba A, et al. Expression analysis of genes encoding xyloglucan endotransglycosylase during ripening in pear fruit. Acta Hortic. 2003;628:549–553. doi: 10.17660/ActaHortic.2003.628.69. [DOI] [Google Scholar]
- Hallmark WB, Barber SA. Root growth and morphology, nutrient uptake, and nutrient status of early growth of soybeans as affected by soil P and K1. Agron J. 1984;76(2):209–212. doi: 10.2134/agronj1984.00021962007600020010x. [DOI] [Google Scholar]
- Hudak CM, Patterson R. P1Vegetative growth analysis of a drought-resistant soybean plant introduction. Crop Sci. 1995;35(2):464–4711. doi: 10.2135/cropsci1995.0011183X003500020031x. [DOI] [Google Scholar]
- Hudak CM, Patterson RP (1996) Root distribution and soil moisture depletion pattern of a drought-resistant soybean plant introduction. Agron Journal (88):478–486
- Kallas AM, Piens K, Denman SE, et al. Enzymatic properties of native and deglycosylated hybrid aspen (Populus tremula×tremuloides) xyloglucan endotransglycosylase 16A expressed in Pichia pastoris. Biochem J. 2005;390(1):105–113. doi: 10.1042/BJ20041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louren T, Saibo N, Oliveira MM, et al. Inducible and constitutive expression of Hv CBF4 in rice leads to differential gene expression and drought tolerance. Biol Plant. 2011;55(4):653–663. [Google Scholar]
- Lai YP, Li J, Zhang ZQ, et al. Grey correlation ananlysis of morphological traits related to drught tolerance of wheat at seedling stage. Journal of Triticeae Crops. 2009;29(6):1005–1059. [Google Scholar]
- Liu FL, Andersen MN, Jensen CR. Root signal controls pod growth in drought-stressed soybean during the critical, abortion-sensitive phase of pod development. Field Crop Res. 2004;85:159–1661. doi: 10.1016/S0378-4290(03)00164-3. [DOI] [Google Scholar]
- Lee BR, Kim KY, Jung WJ, et al. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.) J Exp Bot. 2007;58(6):1271–1279. doi: 10.1093/jxb/erl280. [DOI] [PubMed] [Google Scholar]
- Moon J, Parry G, Estelle M. The ubiquitin-proteasome pathway and plant development. Plant Cell. 2004;16:3181–3195. doi: 10.1105/tpc.104.161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael BE, Kaufmann MR. The osmotic potential of polyethy lent glycol 6000. Plant Physiol. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedes E, Lorences EP. Xyloglucan endotransglucosylase / hydrolases (XTHs) during tomato fruit growth and ripening. J Plant Physiol. 2009;166(5):489–498. doi: 10.1016/j.jplph.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Matsui A, Yokoyama R, Seki M, et al. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. Plant J. 2005;42(4):525–534. doi: 10.1111/j.1365-313X.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- Miedes E, Zarra I, Hoson T, et al. Xyloglucan endotransglucosylase and cell wall extensibility. J Plant Physiol. 2011;168(3):196–203. doi: 10.1016/j.jplph.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Mohammadi PP, Moieni A, Hiraga S, et al. Organ-specific proteomic analysis of drought- stressed soybean seedlings. J Proteomics. 2012;75(6):1906–1923. doi: 10.1016/j.jprot.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Nishikubo N, Takahashi J, Roos AA, et al. Xyloglucan endotransglycosylase-mediated xyloglucan rearrangements in developing wood of hybrid aspen. Plant Physiol. 2011;155(1):399–413. doi: 10.1104/pp.110.166934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB, Grace SC. Perspectives on photoinhibition and photorespiration in the field: quintessentialin efficiencies of the light and dark reactions of photosynthesis. J Exp Bot. 1995;46:1351–1362. doi: 10.1093/jxb/46.special_issue.1351. [DOI] [Google Scholar]
- Osato Y, Yokoyama R, Nishitani K. A principal role for AtXTH18 in Arabidopsis thaliana root growth: a functional analysis using RNAi plants. J Plant Res. 2006;119(2):153–162. doi: 10.1007/s10265-006-0262-6. [DOI] [PubMed] [Google Scholar]
- Rose JK, Braam J, Fry SC, et al. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002;43(12):1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Riechmann JR, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000;3(5):423–434. doi: 10.1016/S1369-5266(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Saladie M, Rose JK, Cosgrove DJ, et al. Characterization of a new xyloglucan endotransglucosylase / hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 2006;47(2):282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- Singh AP, Tripathi SK, Nath P, et al. Petal abscission in rose is associated with the differential expression of two ethylene-responsive xyloglucan endotransglucosylase/hydrolase genes, RbXTH1 and RbXTH2. J Exp Bot. 2011;62(14):5091–5103. doi: 10.1093/jxb/err209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Yum H, Kim ES, et al. BcXTH1, a Brassica campestrishomologue of ArabidopsisXTH9, is associated with cell expansion. Planta. 2006;224(1):32–41. doi: 10.1007/s00425-005-0189-5. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Chinnappa CC, Staal M, et al. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol. 2010;154(2):978–990. doi: 10.1104/pp.110.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Fry SC, Pauly M, et al. XTH acts at the microfibril-matrix interface during cell elongation. J Exp Bot. 2005;56(412):673–683. doi: 10.1093/jxb/eri048. [DOI] [PubMed] [Google Scholar]
- Van Sandt VST, Guisez Y, Verbelen JP, et al. Analysis of a xyloglucan endotransglycosylase/ hydrolase (XTH) from the lycopodiophyte Selaginella kraussiana suggests that XTH sequence characteristics and function are highly conserved during the evolution of vascular plants. J Exp Bot. 2006;57(12):2909–2922. doi: 10.1093/jxb/erl064. [DOI] [PubMed] [Google Scholar]
- Wu Y, Jeong BR, Fry SC, et al. Change in XET activities, cell wall extensibility and hypocotyl elongation of soybean seedlings at low water potential. Planta. 2005;220(4):593–601. doi: 10.1007/s00425-004-1369-4. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Strategies for developing green super rice. Proc Natl Acad Sci USA. 2007;104(42):16402–16409. doi: 10.1073/pnas.0708013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Shi YZ, Lei GJ, et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell. 2012;24:4731–4747. doi: 10.1105/tpc.112.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Lei GJ, Wang ZW, et al. Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol. 2013;162:1947–1955. doi: 10.1104/pp.113.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Wan JX, Sun Y, et al. Xyloglucan endotransglucosylasehydrolase17 interacts with xyloglucan endotransglucosylasehydrolase31 to confer xyloglucan endotrans-glucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol. 2014;165:1566–1574. doi: 10.1104/pp.114.243790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available, upon any reasonable request, from the authors.
Not applicable.