Abstract
Grain hardness (HI) is a key trait for wheat milling and end-use quality. Puroindoline genes (PINs) are the major genes responsible for grain hardness, but other QTLs also contribute to the trait. Therefore, it is essential to identify loci associated with the HI and allelic variations of PINs in wheat. In the present study, 287 accessions from Shanxi province representing 70 years of wheat breeding were grown in one rainfed and two irrigated conditions to study grain hardness. Genome-wide association analysis (GWAS) was performed using the 15 K array, and the variability of PIN alleles was investigated. Among the accessions, hard wheat was most common. The broad-sense heritability (H2) among the three environments was 99.5%, suggesting HI was mainly affected by heredity. GWAS identified nine significant marker–trait associations (MTAs), including that PINs, which explained 7.03% to 17.70% of phenotypic variation. Four MTAs on chromosome 2A, 2B, 5A, and 7A were novel loci. As for diversity of PINs, a total of 11 PINs haplotypes were detected, composed of 12 allelic variations of the PIN gene. The most frequent haplotypes were Pina-D1a/Pinb-D1b (43.9%) and Pina-Dla/Pinb-D1p (18.8%), and both the frequency of Pina-D1a/Pinb-D1b and the HI value increased with breeding years were related to local dietary habits probably. A novel double deletion allele of the PINs haplotype was found in Donghei1206. These results will be useful not only in understanding of the genetics of the HI but also in breeding for improved grain texture.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-022-01303-x.
Keywords: Wheat, Grain hardness, PIN gene alleles, GWAS, MATs
Introduction
Wheat (Triticum aestivum L.) is the most widely cultivated major food crop, providing staple food for approximately 40% of the world’s population in 2019 (FAO; http://faostat.fao.org/). Grain hardness is a key trait contributing to wheat processing quality and often correlates with other important traits, such as thousand-grain weight and grain bulk density (Morris et al. 2013; Wang et al. 2012, 2014). It is affected by certain physical and chemical properties of the grain, which determines the flour yield and milling quality, and influences end-use quality parameters. Grain hardness was often caused by varying degrees of interaction between starch grains and the protein matrix within the endosperm (Chichti et al. 2015). Hard wheat exhibits high resistance to crushing, as a result of strong adhesion between starch particles and the protein matrix. Flour from these hard grains is coarse-textured with more damaged starch, and it is can absorb more water than flour from soft grains. Hard wheat is usually suited for making breads and noodles. In contrast, soft wheat is less resistant to crushing, and the flour has a finer texture with less damaged starch, making it better for cookies, cakes, and pastries. Thus, the HI has been an important breeding target for end-uses. For example, soft and waxy pasta is preferred in Southern China, and these products require soft grains (Liu et al. 2003). People in the north of China prefer products that need stronger gluten flour, and consequently, cultivars from the north have higher the HI value (Chen et al. 2006a).
The genetic basis of kernel hardness has been studied widely for several decades. Many studies have demonstrated that grain hardness is mainly controlled by multiple genes and is less affected by environment and other grain characteristics (Tranquilli et al. 1999; Wang et al. 2014). Notably, amilestone work of Mr 15,000 protein Friabilin was discovered on the surface of water-washed starch granules by Philip Greenwell and J. David Schofield (1986). Subsequently, a series of biochemical separation and amino acid sequencing studies found that Friabilin was composed of the two major proteins, puroindoline a and b (PINA and PINB, respectively), and a minor protein, grain softness protein-1(Gsp-1) (Turner et al. 1999). The Pina-D1, Pinb-D1, and Gsp-D1 genes are linked at the Ha site on chromosome 5DS (Tranquilli et al. 1999). A PIN-like gene nearly identical to the Pinb gene sequence was first discovered in 2008 (Wilkinson et al. 2008). Pina and Pinb play a key role in the HI. Studies have been focused on identifying allelic variations for Pin and the resulting phenotypes. Till now, 26 alleles of Pina and 33 alleles of Pinb, as well as a few double null alleles were identified, only co-presence of Pina-D1a and Pinb-D1a (wild-type alleles) usually lead to softness of grain. (Chen et al. 2006b, 2012, 2013; Kumar et al. 2015; Li et al. 2019; Tu and Li 2020). Although most of alleles related to the HI are not widely used, a few have shown good utility for breeding. For example, Pinb-D1p is a rare allele but is found in the cultivar Jinmai 47(Pina-D1a/Pinb-D1p) which has been unusually successful and grown on 1.33 × 107 ha since its approval in 1995 (Li et al. 2019).
In addition to the Puroindoline genes, quantitative trait loci (QTL) for the HI–related traits have been reported on all wheat chromosomes by using bi-parental mapping populations (Sun et al. 2010; Wang et al. 2012; Li et al. 2013, 2016; Tu and Li 2020). Using recombinant inbred lines (RILs), the Pinb gene was found to explain 76.8% of phenotypic variation, and four QTLs were found to explain only 2.8% to 6.5% (Sun et al. 2010). Two loci controlling soft wheat grain hardness, qkha.Orr4B and Qkha.OR4D explaining 20 ~ 34% of the phenotypic variation, were found by using ultra-soft RILs (Wang et al. 2012). By using soft RILs, a major QTL (Q.HI.scau-7D) was detected that accounted for ~ 30% of the phenotypic variation in kernel hardness (Li et al. 2013).
Genome-wide association study (GWAS) is another most widely used approach, which can reduce the limitations of bi-parental QTL. However, only a few studies have used GWAS to identify novel loci for the HI. Two studies used GWAS for the HI identified four (Lou et al. 2021) and nine (Navrotskyi et al. 2020) single-nucleotide polymorphisms (SNPs) associated with the trait. The phenotypic variation explanation rate (R2) ranged from 2.6 to 12.68%, and the R2 of SNPs on the PIN gene were the highest. The Pinb gene on chromosome 5D has been located in association analysis of 372 European varieties in up to eight environments (Muqaddasi et al. 2020). These studies all located SNPs in the Pinb gene with the highest R2. Due to some populations have low phenotypic variation and low PIN gene locus variability, they did not have the SNPs on the Pin gene with the highest R2, and even not identify the PIN gene. For example, 400 lines developed by crossing and backcrossing the Japanese cultivar Norin61 were used to identify 47 significant hardness-related MTAs explaining 8.3 ~ 22.6% of the phenotypic variation, yet the R2 of the MTA in the PIN gene was only 13% (Elhadi et al. 2021). Twenty SNPs were located using 172 advanced soft white winter wheat breeding lines in a GWAS (Aoun et al. 2021). Among these, QSKhard.wql-3A and QSKhard.wql-5A had the highest R2. Similarly, Chen et al. (2019) selected 299 American hard winter wheats and located nine SNPs significantly related to using the 90 K SNP array. The R2 of barc154-7D and wms130 on chromosome 7D was higher than that for the two SNPs located on the PIN gene. These studies demonstrated that in the case of low variability of the Ha locus, other loci have been found. Studies on the HI using the GWAS and diverse populations were scarce, and therefore, it is likely that other loci for this trait have yet to be discovered.
Shanxi is located in the Loess Plateau and has been the main wheat producing area in China since ancient times, inside Huanghuai wheat zone, northern spring and winter wheat zone. Wheat is the most important crop, and there has a long history of wheat cultivation (Tang et al 2018). There are at least 300 kinds of pasta that have been invented in the province. Under long-term artificial and natural selection, Shanxi wheat is famous for its abundant resources, drought resistance, and high quality. For example, Yanda 1817 is the core parent of the northern winter wheat area. It was a selection from Pingyao white wheat, and 53 varieties have been bred using Yanda 1817 as a founder parent (Wu et al. 2015). Linfen 5064 is one of the three high-quality core parents in China and has derived more than 50 varieties distributed in different regions (Zheng et al. 2021). Jinmai 47 has been used as a trial reference in the dryland wheat regional trial in the northern part of Huanghuai wheat region since it was released in the 1990s (Zheng et al. 2021). Therefore, it offers an excellent platform to investigate important agronomic traits under artificial and natural selection and breeding. In the present study, Shanxi wheat germplasm and 15 K microarray were used for GWAS and identify the variability of PIN alleles. The experimental results provide usefull information for molecular marker-assisted breeding.
Materials and methods
Plant materials
A diverse hexaploid wheat collection of 287 accessions from Shanxi Province, China, was used in present study (Table S1). The test materials were released during various wheat breeding eras and have significant phenotypic differences.These accessions include 40 landraces from the Chinese wheat core collection from Shanxi Province (Hao et al. 2008). The other 247 modern cultivars, including 127 irrigated cultivars and 120 dryland cultivars, are 85% of the varieties bred in Shanxi Province since the founding of the People’s Republic of China (PRC).
Field experiments
The 287 hexaploid wheats were planted in the Yaodu district in Shanxi province of China, at Linfen (36°08′ N, 111°52′ E, altitude 450 m) in 2019–2020 and 2020–2021. The trial in 2019–2020 was grown under rainfed condition (E1). Two tails in 2020–2021 were grown under two irrigation methods, one trial E2 was irrigated once at the overwintering stage, and anotther trail E3 was irrigated at overwintering, jointing, and booting stages. The seed was sown in two 2-m rows per line spaced 0.3 m apart at 40 seeds per row. Field management practices were followed based on the commonly used wheat production guide in the region.
Grain hardness (HI) measurement
The HI of the 287 accessions was measured with a wheat grain hardness index tester (Wuxi Suibang Science and Technology Co., LTD., JYDB 100 40) by the standard method (GB1351-2008). Before testing, grain sample of the 287 accessions were placed under identical temperature and moisture conditions and dried for about 3 days to standardize the moisture content of test materials to about 12%. For each sample, 25 g of seed were placed into the HI tester, and the value was measured after automatic grinding for 50 s. The average of three samples was recorded. The accessions with kernel HI < 40 were classified as soft wheat, while the accessions with kernel HI > 60 were classified as hard wheat. The accessions with HI between 40 and 60 were mixed wheat.
Phenotypic data analysis
The best linear unbiased predictions (BLUPS) for the three environments were estimated by JMP Pro 16 used for GWAS. Broad-sense heritability (H2) was defined as H2 = VG/(VG + VE), where VG and VE are the genetic and environment estimations, respectively (Zheng et al. 2020). Correlation analyses were performed using SPSS 2.0 (BM SPSS Statistics; IBM Corp., Armonk, NY, USA).
Genome-wide association analysis
GWAS for kernel hardness was performed using a mixed linear model (MLM) with a total of 9,793 high-quality SNPs (Zheng et al. 2021) with version 5 of the TASSEL software. The MLM model was used to make association analyses between grain hardness and SNP markers. GWAS was conducted with the BLUP, E1, E2, and E3 datasets. The models described above were compared by plotting expected versus observed − log10(P value) in the form of a quantile–quantile (QQ) plot. The best model was determined by checking how well the observed − log10(P value) aligned with the expected. TASSEL MLM products were used to generate Manhattan plots using the R package. The linkage disequilibrium (LD) of each SNP marker was extended on each chromosome (Zheng et al. 2021). The extended region where the LD between nearby SNPs and the peak SNP decayed to R2 = 0.2 was defined as the local LD-based QTL interval (Zheng et al. 2019). The physical positions of SNP markers were obtained from Chinese Spring (RefSeq v1.0) at the International Wheat Genome Sequencing Consortium website (IWGSC, http://www.wheatgenome.org/).
PIN genotyping
Genomic DNA was extracted and preserved by Zheng et al. (2021) and used as a PCR template for the identification of Pina and Pinb genes of soft and hard wheat. Any mixed wheat cultivars were excluded from this study, as they possibly contained multiple haplotype (Chen et al. 2006b).
A stepwise approach was employed to characterize Pina and Pinb alleles (Chen et al. 2006b, 2012, 2013; Li et al. 2019). First, according to the method of Chen et al. (2013), potential null alleles of Pina and Pinb were identified, and the known alleles of the PIN gene were verified. The test materials for which the allelic variation type of the PIN gene could not be identified were sent for sequencing. Novel deletion alleles of the Pina and/or Pinb genes were detected with a primer walking strategy (Li et al. 2019).
PCR amplifications were performed in a Thermocycler (BioRad, C1000 Touch) and were conducted in 50 μL reactions using 50 ng of genomic DNA, 0.15 μM primers, and 10 μL 2 × EasyTaq PCR SuperMix (TransGen Biotech). The cycling conditions were 94 °C for 5 min following 33 cycles of 94 °C for 50 s, 50 °C to 65 °C for 50 s, and 72 °C for 1 min (see Table S2 for primer-specific annealing temperatures and extension time), following a 10-min final extension time at 72 °C. Each sequencing material was amplified four times, and 50-μL PCR amplification products were sent to Biomed Genetic Technology Co., Ltd., for forward or reverse sequencing to determine the type of allelic variation of the PIN gene.
Sequencing comparison of 145 resequenced materials
In the wheat union website (http://wheat.cau.edu.cn/WheatUnion/), resequencing results from 145 Chinese cultivars were available (Hao et al. 2020), and using MEGA6 software, the PIN gene sequences were compared. The 145 resequenced materials represent widely planted wheats and representative parental wheats in China, including 100 modern Chinese cultivars, 25 Chinese landraces, and 20 elite cultivars introduced from other national breeding programs (Hao et al. 2020).
Results
Overall variation of HI wheat sample
According to the international grading standard for determining grain hardness, the HI distributions of 287 accessions were basically similar under three environments. In E1, E2, and E3, there were 211, 203, and 192 entries were classified as hard wheats with mean HI values of 65.35, 63.87, and 63.68, respectively. Mixed wheat were identified 68, 79, and 92 accessions in the three environment with mean HI values of 53.52, 54.26, and 53.82, respectively. Soft wheats accounted for the fewest entries, with only seven, four, and three identified in the three environments, respectively (Fig. 1, Table S3).
Fig. 1.
Schematic diagram of grain hardness distribution under three environments. a The orange violin bar represents soft wheat, HI < 40, the red violin bar represents mixed wheat,40 < HI < 60, and the purple violin bar represents hard wheat, HI > 60. b Cross-sections of various grain hardness types
Across environments, the HI values ranged from 36.8 to 74.71 with average values of 61.88, 60.83, and 60.55, respectively, and the coefficients of variation ranged from 11.34 to 10.08%. The HI correlation coefficients among environments were highly significant and ranged from 0.889 to 0.934. The H2of HI in the three environments was 99.5%, indicating that the grain hardness was mainly influenced by heredity. In E2 and E3, which are different irrigation conditions, the correlation coefficient was 0.934, and the H2was 94.36%, indicating that water had little effect on HI (Table S4).
Grain hardness loci identified by GWAS
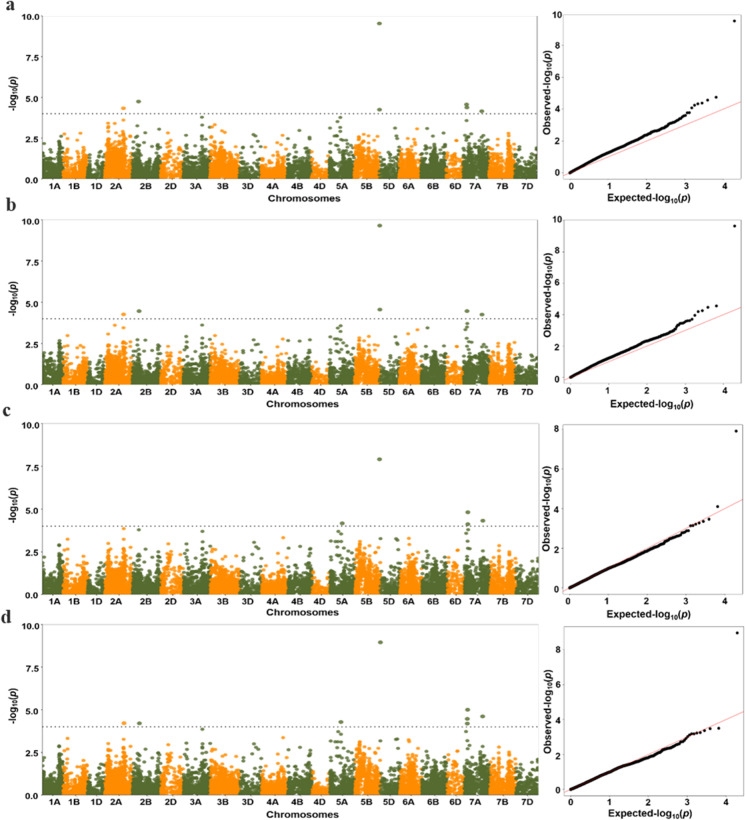
Association analysis between phenotypic traits and SNP was performed using the MLM model. GWAS was conducted on the E1, E2, E3, and BLUP datasets, and significant MTAs (− log10(P value) = 4.0) were identified. Five MTAs were identified on chromosome 2B, 5D, and 7A in E1(Fig. 2b), five MTAs on chromosomes 5A, 5D, and 7A in E2 (Fig. 2c), and six MTAs on chromosome 5A, 5D, and 7A in E3(Fig. 2d). With the BLUP dataset, seven MTAs were identified on chromosomes 2A, 2B, 5D, and 7A (Fig. 2a).
Fig. 2.
Manhattan and quantile–quantile plots for grain hardness loci as identified with BLUP (a), E1 (b), E2 (c), and E3 (d) datasets. In the Manhattan plots, the dashed black line represents the threshold − log10(P value) = 4.0
In total, nine MTAs with a phenotypic variation explanation rate (R2) ranging from 7.03% to 17.70% were identified across chromosomes 2A (1), 2B (1), 5D (2), 5A (1), and 7A (4) (Fig. 2, Table 1). Three MTAs (5D_3609894, 7A_93084120, and 7A_514977827) were significantly associated with HI in all four datasets explained phenotypic variation of 17.7%, 8.83%, and 8.11%, respectively. Among these, 5D_3609894 corresponded to the Pinb gene of the Ha locus and had the highest R2. The other six MTAs (2A_521944695, 2B_169782527, 5A_311126149, 5D_7029654, 7A_927 45,962, and 7A_101617032) showed significant associations in two datasets. These MTAs were on chromosomes 2A, 2B, 5A, 5D, and 7A and explained 7.49%, 8.96%, 7.09%, 8.05%, 7.47%, and 7.59% of phenotypic variation, respectively. According to the Zheng et al. (2021) analysis of LD attenuation distance, the MTAs 7A_92745962, 7A_101617032, and 7A_93084120 were the same QTL and explained 7.03 ~ 8.83% of the phenotypic variation. 7A_93084120 had the largest R2 as measured from the E3 dataset. The loci that significantly associated with 2A_521944695, 2B_169782527, 5A_311126149, and 7A_514977827 have not been reported previously (Table 1).
Table 1.
MTAs significantly associated with grain hardness in 287 wheat cultivars
| Chromosome | Position (Mb) | Marker | Dataset | P value | − log10P | R2 (%) |
|---|---|---|---|---|---|---|
| 5D | 3.61 | 5D_3609894 | BLUP | 5.66E-05 | 9.53 | 17.29% |
| E3 | 2.79E-05 | 8.96 | 16.37% | |||
| E2 | 2.95E-10 | 7.91 | 14.37% | |||
| E1 | 1.09E-09 | 9.65 | 17.70% | |||
| 7.03 | 5D_7029654 | BLUP | 1.23E-08 | 4.25 | 7.47% | |
| E1 | 2.24E-10 | 4.55 | 8.05% | |||
| 5A | 311.13 | 5A_311126149 | E3 | 8.91E-05 | 4.05 | 7.09% |
| E2 | 9.29E-05 | 4.03 | 7.08% | |||
| 7A | 92.75–101.62 | 7A_93084120 | BLUP | 2.69E-05 | 4.57 | 7.92% |
| E3 | 9.99E-06 | 5.00 | 8.83% | |||
| E2 | 1.53E-05 | 4.82 | 8.51% | |||
| E1 | 3.38E-05 | 4.47 | 7.80% | |||
| 7A_92745962 | E3 | 5.49E-05 | 4.26 | 7.47% | ||
| E2 | 7.62E-05 | 4.12 | 7.23% | |||
| 7A_101617032 | BLUP | 4.08E-05 | 4.39 | 7.59% | ||
| E3 | 9.57E-05 | 4.02 | 7.03% | |||
| 514.98 | 7A_514977827 | BLUP | 8.17E-05 | 4.09 | 7.05% | |
| E3 | 2.46E-05 | 4.61 | 8.11% | |||
| E2 | 4.77E-05 | 4.32 | 7.60% | |||
| E1 | 5.52E-05 | 4.26 | 7.42% | |||
| 2A | 521.94 | 2A_521944695 | BLUP | 4.66E-05 | 4.33 | 7.49% |
| 2B | 169.78 | 2B_169782527 | BLUP | 1.87E-05 | 4.73 | 8.96% |
| E1 | 6.66E-05 | 4.18 | 7.76% |
Variability of PINs in wheat samples
Previous studies demonstrated that grain hardness differences were primarily associated with PIN haplotypes. Therefore, the 217 hard wheats and seven soft wheats classified based on HI values from the three environments were assessed for PIN gene allelic variation using known molecular markers (Table S2) and sequencing methods. Among the 224 accessions, six Pina alleles (Table S1) were detected, including the wild-type allele Pina-D1a, a mutant allele Pina-D1n, and four deletion alleles (Pina-D1b, Pina-D1s, Pina-D1r, and Pina-D1u). Pina-D1a was observed in 199 accessions (87.34%), Pina-D1b in eight cultivars (3.93%), Pina-D1r in two landraces (0.87%), and Pina-D1s in three landraces (1.31%). The remaining known alleles of Pina, including Pina-D1n and Pina-D1u, were rare. Five known variants of Pinb (Table S1) were observed in the germplasm, of which Pinb-D1a, Pinb-D1b, and Pinb-D1p were common with allelic frequencies of 6.99%, 61.14%, and 25.33%, respectively. In total, 12 alleles of the PIN gene were found, ten in cultivars, and only four in the landraces.
The relationship between different PIN gene haplotypes and grain hardness was assessed. In the 224 accessions, combinations of Pina-D1 and Pinb-D1 alleles produced 11 haplotypes (Table S1). Among these, there were 16 wild-type hard wheats and a hard wheat with a double deletion of the Pin gene. In the remaining 207 materials, PIN haplotype combinations were only one of Pina and Pinb which had allelic variation. Therefore, the distribution of allelic variation combination of PIN gene is the same as Pina/Pinb allelic variation. In the landraces, Pina-D1a/Pinb-D1p was the main variant. In the cultivars, Pina-D1a/Pinb-D1b was the main variant, followed by Pina-D1a/Pinb-D1p.
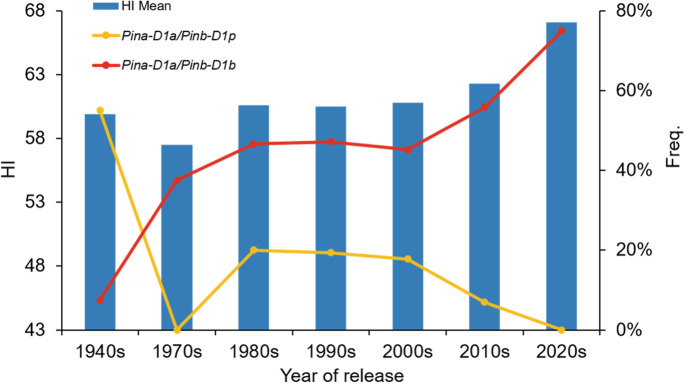
The average HI of Shanxi wheats increased from 59.87 in the accessions from the 1940s to 67.16 currently (Fig. 3). The frequency of Pina-D1a/Pinb-D1b among Shanxi wheats from the 1940s has increased from 7.5 to 75%, and the proportion of Pina-D1a/Pinb-D1p decreased from a high of 55% in the 1940s (Fig. 3). It is likely that the Pina-D1a/Pinb-D1b haplotype increased and Pina-D1a/Pinb-D1p haplotype decreased mainly due to artificial selection from breeding.
Fig. 3.
The proportion of Pina-D1a/Pinb-D1b and Pinb-D1b/Pinb-D1p alleles and the HI value trend over time
A novel allele of Puroindoline-D1
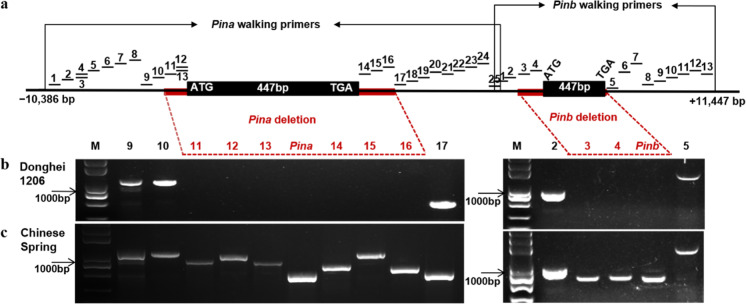
Our previous studies have illustrated that Donghei1206 had a double deletion of the PIN gene and greater HI of 68.56. To identify PIN gene allelic variation of Donghei1206, we used the primer step method. A series of primers were used to explore the double deletion sites of the Donghei1206 PIN gene. The eight pairs of primers from PinA-11 to PinA-16 and PinB-3 to PinB-4 did not generate corresponding PCR amplification products. PCR products were obtained after the amplification of other primers (Fig. 4). Donghei1206 had a ~ 4500 bp deletion in the Pina coding region and a ~ 1500 bp deletion in the Pinb coding region. This novel double deletion of the Pina and Pinb alleles was identified in Donghei1206 via comparison with step-primer results from various PIN gene deletion mutations (Table S5).
Fig. 4.
The alleles of Donghei1206 were identified using a PCR marker and primer walking strategy. a Schematic diagram of the primer walking strategy to character the Pin-null allele on the DNA level. The black boxes are the coding regions of Pina and Pinb, and the black bars are the gene sequences before and after PIN gene. The primers which did not generate products with the expected sizes are marked with red bars in the PIN-null alleles. b A PCR results from the walking markers for detection of Donghei1206. c A PCR results from the walking markers for detection of the allele in Chinese Spring
MTAs’ effect analysis within the wild PIN background
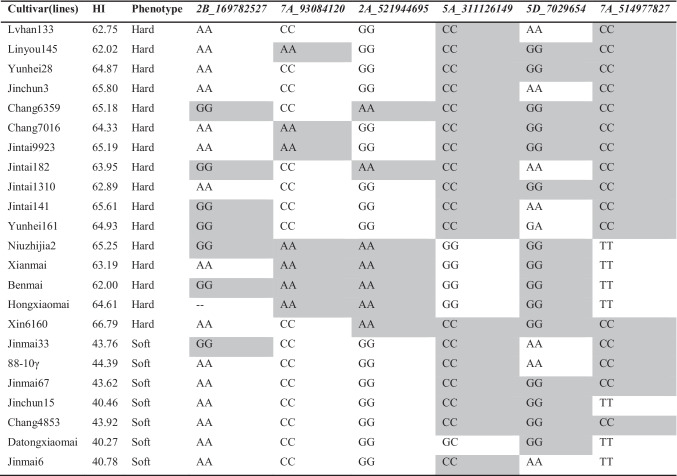
PIN genotypes found in previous study (16 hard wheat and 7 soft wheat) could evade the main effect of PIN gene on grain hardness. To study the effect of significant MTAs other than PIN genes on grain hardness, we used 23 varieties with wild-type. Within 23 test materials with the wild-type PIN haplotype, we examined the effects of MTAs identified via GWAS that were significantly associated with grain hardness. The analysis revealed that a combination of markers 2B_169782527, 7A_93084120, and 2A_521944695 differentiated the soft wheat group from the hard wheat group (Table 2). Except Lvhan133, Yunhei28, Jinchun3, and Jintai1310, the hard wheat group carried the GG allele of 2B_169782527, the AA allele of 7A_93084120, and the AA allele of 2A_521944695. Except for Jinmai33, all of the soft wheat group carried the AA allele of 2B_169782527, the CC allele of 7A_93084120, and the GG allele of 2A_521944695. Therefore, these three MTAs loci are associated with significant effects on grain hardness.
Table 2.
Alleles of the most significant MTAs per QTL in wheat variety with wild haplotypes (color table online)
The light gray-filled cells indicate haplotypes associated with grain hardness
Discussion
Novel loci and haplotypes related to grain hardness
Grain hardness is not only controlled by the main effect genes (Ha) on chromosome 5DS but also through minor genes on other chromosomes (Tu and Li 2020). In the present study, nine MTAs associated with grain hardness were located on 2A, 2B, 5A, 5D, and 7A chromosomes. Kumar et al. (2019) reported the wsnp_Ex_c42653_49180603 and Excalibur_c42993_561 markers for grain hardness on chromosome 7A and responsible for the HI by using populations of offspring from crossings between high-quality and non-adapted bread wheat. These markers coincide with the physical location of 7A_93084120 in the present study, which indicates that all three markers belong to the same locus. Similarly, a QTL constituted with SSR markers viz. Xbarc130, Xcfd18, Xgwm190, gpw326, and gwm190 were previously revealed by Sun et al. (2010), Tu and Li (2020), were associated with grain hardness; which is overlapped with the loci 5D_7029654 on chromosome 5D, identified in this study. On the other hand, loci associated with HI are located in 2A(521.94 Mb), 2B(169.78 Mb), 5A(311.13 Mb), and 7A(514.98 Mb) have not been reported yet and are likely novel loci (Table S6).
According to the comparative results of six significant haplotype loci by using 27 wheat samples with the wild-type PIN gene, the combination markers of 2B_169782527, 7A_93084120, and 2A_521944695 greatly influenced HI. Further development of markers can be used to select hard and soft wheat in the future. For instance, when the PIN gene is in the wild-type background, the haplotype composed of the G allele of 2B_169782527, the A allele of 7A_93084120, and the A allele of 2A_521944695 could be chosen to improve HI. As well, the haplotype with the A allele of 2B_169782527, the G allele of 7A_93084120, and G allele of 2A_521944695 could be used for soft wheat breeding.
Diversity of PINs in Shanxi wheat and novel double deletion of PIN haplotype
Shanxi Province is a historical and favorable growing region for wheat due to its unique geographical location and abundant germplasm resources, which germplasm has been effectively protected in China. In the present study, 287 materials accounted for 85% of Shanxi Province germplasm resources from the past 70 years. A total of 12 alleles of PIN gene consisting of 11 PIN gene haplotypes were found. However, in the 145 resequenced materials originating from various regions of China, Pinb-D1b allelic variation arose in only 57 materials, including NanDai2419, ZhongNong28, and other excellent imported cultivars. These results indicated that the diversity of PINs allelic variation in Shanxi wheat was richer than that in other parts of China probably.
The PIN gene is essential for HI, and the identification of any new allele is of interest to wheat breeders (Chen et al. 2019; Muqaddasi et al. 2020; Tu and Li 2020; Lou et al. 2021). The cultivar Donghei1206 was found to have double deletion alleles of ~ 4.5 Kb Pina-null and ~ 1.5 Kb Pinb-null for the PIN gene using the stepwise primer approach in this study, which alleles are rare in Chinese germplasm. Only two double deletion alleles of the PIN gene, located at ~ 33 Kb and ~ 29 Kb distance, has been reported previously (Chen et al. 2013; Li et al. 2019). It is uncertain if these deletions represent either the same or different mutations. By comparing the results of the stepwise method, Pina-null and Pinb-null on Donghei1206 differ from the previously described double deletions, which have a pedigree of (Heixiaomai76// Tai633 / Zi6)/Wheike962. The HI value of Heixiaomai76 is 66.41, and its PIN haplotype is Pina-D1a/Pinb-D1b (Table S1), which does not have a gene deletion. Zi6 and Wheike962 are descendants of a wide hybridization between Thinopyrum intermedium and wheat. The double deletion of the PIN gene in Donghei1206 may have been derived from either Zi6 or Wheike962, and this hypothesis could be tested by using step primers in future experiments.
Relationship between breeding for grain hardness and dietary habit
The selection of grain quality attributes is influenced by the pasta quality characteristics preferred by various groups of consumers. The texture and smoothness of noodles are connected to grain hardness, and a HI value of 60–70 is needed to ensure high-quality noodles and steamed bread (Chen et al. 2006a; Zhao 2009; Murray et al. 2018). The majority of Shanxi wheat was hard wheat with an average HI score of about 60. This indicated that wheat breeding for grain hardness may have been influenced by local dietary preferences for noodles.
In the late 1940s, the influence of dietary preference was also visible during breeding. The Pina-D1a/Pinb-D1p were the most common landrace of PIN haplotypes until the 1940s. In the early 1950s, Chinese wheat was improved mainly by introducing cultivars such as NanDai2419, ZhongNong28, and other quality cultivars. Hao et al. (2020) reported that introducing these cultivars helped the breeders select the novel alleles such as Pinb-D1b.. Cultivars with Pinb-D1b provide outstanding processing qualities for flour, noodles, and steamed bread (Chen et al. 2006b; Liu et al. 2003). Therefore, since grain hardness was required for better quality, the Pina-D1a/Pinb-D1b haplotype combination was strongly selected by the local wheat breeders, resulting in a decrease in the proportion of the Pina-D1a/Pinb-D1p haplotype in the Shanxi region.
Conclusions
In the present study, 287 hexaploid wheat representing 70 years of Shanxi breeding history were used to identify GWAS loci associated with grain hardness. Nine significant MTAs were identified on chromosomes 2A, 2B, 5A, 5D, and 7D, respectively. Among them, one MTA with the highest R2 was located at PIN locus, and four MTAs were novel loci on 2A(521.94 Mb), 2B(169.78 Mb), 5A(311.13 Mb), and 7A(514.98 Mb). The marker combinations composed of 2B_169782527, 7A_93084120, and 2A_521944695 had a great influence on grain hardness of varieties with the wild-type PIN gene. A total of 11 haplotypes of PIN gene alleles were identified; one of PIN haplotypes was a novel double deletion allele with a ~ 4500 bp deletion in the Pina coding region and a ~ 1500 bp deletion in the Pinb coding region. Pina-D1a/Pinb-D1b (43.9%) and Pina-D1a/Pinb-D1p (18.8%) are the main variant haplotypes. The distribution frequency and HI values of germplasm with Pina-D1a/Pinb-D1b increased during breeding, indicating selection to fit local quality requirements. These results from the present study provide useful information for further genetic analysis and molecular breeding for grain hardness.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Junyou Wang, investigation, data curation, and writing—original draft. Chenkang Yang, investigation, data curation, and visualization. Ying Wang, investigation, resources, and data curation. Wenjia Zhao, investigation and formal analysis. Bangbang Wu, validation and software. Jiajia Zhao and Ling Qiao, validation. Jun Zheng, conceptualization, methodology, project administration, and writing—original draft. Xingwei Zheng, resources, supervision, and funding acquisition. Juanling Wang, supervised the study and data interpretation.
Funding
This work was supported by the State Key Laboratory of Sustainable Dryland Agriculture (in preparation), Shanxi Agricultural University [No. 202102–1], and Shanxi Key Laboratory of Crop Genetics and Molecular Improvement [KFJJ2019-02].
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juanling Wang, Email: 13994267508@163.com.
Jun Zheng, Email: sxnkyzj@126.com.
References
- Aoun M, Carter AH, Ward BP, Morris CF. Genome-wide association mapping of the ‘super-soft’ kernel texture in white winter wheat. Theor Appl Genet. 2021;134(8):2547–2559. doi: 10.1007/s00122-021-03841-y. [DOI] [PubMed] [Google Scholar]
- Chen F, Chen DS, Qian SH. Influence of puroindoline gene on milling performance, steamed bread and noodle qualities in spring wheat. Acta Agron Sin. 2006;32:980–986. [Google Scholar]
- Chen F, He ZH, Xia XC, Xia LQ, Zhang XY, Lillemo M, Morris CF. Molecular and biochemical characterization of puroindoline a and b alleles in Chinese landraces and historical cultivars. Theor Appl Genet. 2006;112(3):400–409. doi: 10.1007/s00122-005-0095-z. [DOI] [PubMed] [Google Scholar]
- Chen F, Zhang FY, Xia XC, Dong ZD, Cui DQ. Distribution of puroindoline alleles in bread wheat cultivars of the Yellow and Huai valley of China and discovery of a novel puroindoline a allele without PINA protein. Mol Breeding. 2012;29(2):371–378. doi: 10.1007/s11032-011-9553-2. [DOI] [Google Scholar]
- Chen F, Li H, Cui D. Discovery, distribution and diversity of Puroindoline-D1 genes in bread wheat from five countries (Triticum aestivum L.) BMC Plant Biology. 2013;13(1):1–13. doi: 10.1186/1471-2229-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Zhang FY, Zhao CJ, Lv GG, Sun CW, Pan YB, Guo XY, Chen F. Genome wide association study of six quality traits reveals the association of the TaRPP13L1 gene with flour colour in Chinese bread wheat. Plant Biotechnol J. 2019;17(11):2106–2122. doi: 10.1111/pbi.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichti E, George M, Delenne JY, Lullien-Pellerin V. Changes in the starch-protein interface depending on common wheat grain hardness revealed using Atomic Force Microscopy. Plant Sci. 2015;239:1–8. doi: 10.1016/j.plantsci.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Elhadi GMI, Kamal NM, Gorafi YSA, Yamasaki Y, Ban Y, Kato K, Tahir ISA, Ishii T, Tanaka H, Tsujimoto H. Novel loci for kernel hardness appeared as a response to heat and combined heat-drought conditions in wheat harboring Aegilops tauschii diversity. Agronomy. 2021;11(6):1061. doi: 10.3390/agronomy11061061. [DOI] [Google Scholar]
- GB1351-2008. Hardness index method for wheat hardness determination, Wheat.
- Greenwell P, Schofield JD. A starch granule protein associated with endosperm softness in wheat. Cereal Chem. 1986;63:379–380. [Google Scholar]
- Hao CY, Dong YC, Wang LF, You GX, Zhang HN, Gai HM, Jia JZ, Zhang XY. The construction and genetic diversity analysis of the core species of wheat in our country. Chin Sci Bull. 2008;53(8):908–915. [Google Scholar]
- Hao CY, Jiao CZ, Hou J, Li T, Liu HX, Wang YQ, Zheng J, Liu H, Bi ZH, Xu FF, Zhao J, Ma L, Wang YM, Majeed U, Liu X, Appels R, Maccaferri M, Tuberosa R, Lu HF, Zhang XY. Resequencing of 145 landmark cultivars reveals asymmetric sub-genome selection and strong founder genotype effects on wheat breeding in China. Mol Plant. 2020;13(12):1733–1751. doi: 10.1016/j.molp.2020.09.001. [DOI] [PubMed] [Google Scholar]
- Kumar R, Arora S, Singh K, Garg M. Puroindoline allelic diversity in Indian wheat germplasm and identification of new allelic variants. Breed Sci. 2015;65(4):319–326. doi: 10.1270/jsbbs.65.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Mantovani EE, Simsek S, Jain S, Elias EM, Mergoum M. Genome wide genetic dissection of wheat quality and yield related traits and their relationship with grain shape and size traits in an elite × non-adapted bread wheat cross. PLoS ONE. 2019;14(9):e0221826. doi: 10.1371/journal.pone.0221826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Liang H, Tang ZX, Zhang HQ, Yan BJ, Ren ZL. QTL analysis for grain pentosans and hardness index in a Chinese 1RS. 1BL × non-1RS. 1BL wheat cross. Plant molecular biology reporter. 2013;31(2):477–484. doi: 10.1007/s11105-012-0517-4. [DOI] [Google Scholar]
- Li CL, Bai GH, Chao SM, Carver B, Wang ZH. Single nucleotide polymorphisms linked to quantitative trait loci for grain quality traits in wheat. The Crop Journal. 2016;4(1):1–11. doi: 10.1016/j.cj.2015.10.002. [DOI] [Google Scholar]
- Li XY, Li Y, Zhang M, Yu XF, Hu R, Chang JL, Yang GX, Wang YS, He GY. Diversity of Puroindoline genes and their association with kernel hardness in Chinese wheat cultivars and landraces. Mol Breeding. 2019;39(4):1–13. doi: 10.1007/s11032-019-0967-6. [DOI] [Google Scholar]
- Liu JJ, He ZH, Zhao ZD, Pena RJ, Rajaram S. Wheat quality traits and quality parameters of cooked dry white Chinese noodles. Euphytica. 2003;131(2):147–154. doi: 10.1023/A:1023972032592. [DOI] [Google Scholar]
- Lou HY, Zhang RQ, Liu YT, Guo DD, Zhai SS, Chen AY, Zhang YF, Xie CJ, You MS, Peng HR, Liang RQ, Ni ZF, Sun QX, Li BY. Genome-wide association study of six quality-related traits in common wheat (Triticum aestivum L.) under two sowing conditions. Theoretical and Applied Genetics. 2021;134(1):399–418. doi: 10.1007/s00122-020-03704-y. [DOI] [PubMed] [Google Scholar]
- Morris CF, Geng H, Beecher BS, Ma D. A review of the occurrence of Grain softness protein-1 genes in wheat (Triticum aestivum L) Plant molecular biology. 2013;83(6):507–521. doi: 10.1007/s11103-013-0110-8. [DOI] [PubMed] [Google Scholar]
- Muqaddasi QH, Brassac J, Ebmeyer E, Kollers S, Korzun V, Argillier O, Stiewe G, Plieske J, Ganal MW, Röder MS. Prospects of GWAS and predictive breeding for European winter wheat’s grain protein content, grain starch content, and grain hardness. Sci Rep. 2020;10(1):1–17. doi: 10.1038/s41598-020-69381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JC, Kiszonas AM, Morris CF. Influence of soft kernel texture on fresh durum pasta. J Food Sci. 2018;83(11):2812–2818. doi: 10.1111/1750-3841.14363. [DOI] [PubMed] [Google Scholar]
- Navrotskyi S, Belamkar V, Baenziger PS, Rose DJ. Insights into the genetic architecture of bran friability and water retention capacity, two important traits for whole grain end-use quality in winter wheat. Genes. 2020;11(8):838. doi: 10.3390/genes11080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Marza F, Ma H, Carver BF, Bai G. Mapping quantitative trait loci for quality factors in an inter-class cross of US and Chinese wheat. Theor Appl Genet. 2010;120(5):1041–1051. doi: 10.1007/s00122-009-1232-x. [DOI] [PubMed] [Google Scholar]
- Tang M, Wang XY, Hou K, Hou LL. Carbon and nitrogen stable isotope of the human bones from the Xiaonanzhuang cemetery, Jinzhong, Shanxi: a preliminary study on the expansion of wheat in ancient Shanxi, China. Acta Anthropol Sin. 2018;37:318–330. [Google Scholar]
- Tranquilli G, Lijavetzky D, Muzzi G, Dubcovsky J. Genetic and physical characterization of grain texture-related loci in diploid wheat. Mol Gen Genet. 1999;262(4–5):846–850. doi: 10.1007/s004380051149. [DOI] [PubMed] [Google Scholar]
- Tu M, Li Y. Toward the genetic basis and multiple QTLs of kernel hardness in wheat. Plants. 2020;9(12):1631. doi: 10.3390/plants9121631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Mukai Y, Leroy P, Charef B, Appels R, Rahman S. The Ha locus of wheat: identification of a polymorphic region for tracing grain hardness in crosses. Genome. 1999;42(6):1242–1250. doi: 10.1139/g99-075. [DOI] [PubMed] [Google Scholar]
- Wang G, Leonard JM, Ross AS, Peterson CJ, Zemetra RS, Garland Campbell K, Riera-Lizarazu O. Identification of genetic factors controlling kernel hardness and related traits in a recombinant inbred population derived from a soft × ‘extra-soft’ wheat (Triticum aestivum L.) cross. Theoretical and Applied genetics. 2012;124(1):207–221. doi: 10.1007/s00122-011-1699-0. [DOI] [PubMed] [Google Scholar]
- Wang G, Leonard JM, Zitzewitz JV, Peterson CJ, Ross AS, Riera-Lizarazu O. Marker-trait association analysis of kernel hardness and related agronomic traits in a core collection of wheat lines. Mol Breeding. 2014;34(1):177–184. [Google Scholar]
- Wilkinson M, Wan Y, Tosi P, Leverington M, Snape J, Mitchell RA, Shewry PR. Identification and genetic mapping of variant forms of puroindoline b expressed in developing wheat grain. J Cereal Sci. 2008;48(3):722–728. doi: 10.1016/j.jcs.2008.03.007. [DOI] [Google Scholar]
- Wu Qiu-Hong, Chen Yong-Xing, Zhou Sheng-Hui, Fu Lin, Chen Jiao-Jiao, Xiao Yao, Zhang Dong, Ouyang Shu-Hong, Zhao Xiao-Jie, Cui Yu, Zhang De-Yun, Liang Yong, Wang Zhen-Zhong, Xie Jing-Zhong, Qin Jin-Xia, Wang Guo-Xin, Li De-Lin, Huang Yin-Lian, Yu Mei-Hua, Lu Ping, Wang Li-Li, Wang Ling, Wang Hao, Dang Chen, Li Jie, Zhang Yan, Peng Hui-Ru, Yuan Cheng-Guo, You Ming-Shan, Sun Qi-Xin, Wang Ji-Rui, Wang Li-Xin, Luo Ming-Cheng, Han Jun, Liu Zhi-Yong, Yin Tongming. High-Density Genetic Linkage Map Construction and QTL Mapping of Grain Shape and Size in the Wheat Population Yanda1817 × Beinong6. PLOS ONE. 2015;10(2):e0118144. doi: 10.1371/journal.pone.0118144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WH. The relationship between wheat hardness a quality of wheat [J] Chinese Food Processing. 2009 doi: 10.3969/j.issn.1007-6395.2009.04.004. [DOI] [Google Scholar]
- Zheng XW, Wen XJ, Qiao L, Zhao JJ, Zhang XJ, Li X, Zhang SW, Yang ZJ, Chang ZJ, Chen JL, Zheng J. A novel QTL QTrl.saw-2D.2 associated with the total root length identified by linkage and association analyses in wheat (Triticum aestivum L.) Planta. 2019;250(1):129–143. doi: 10.1007/s00425-019-03154-x. [DOI] [PubMed] [Google Scholar]
- Zheng XW, Liu C, Qiao L, Zhao JJ, Han R, Wang XL, Ge C, Zhang WY, Zhang SW, Qiao L, Zheng J, Hao CY. The MYB transcription factor TaPHR3-A1 is involved in phosphate signaling and governs yield-related traits in bread wheat. J Exp Bot. 2020;71(19):5808–5822. doi: 10.1093/jxb/eraa355. [DOI] [PubMed] [Google Scholar]
- Zheng XW, Qiao L, Liu Y, Wei N, Zhao J, Wu BB, Yang B, Wang J, Zheng J. Genome-wide association study of grain number in common wheat from Shanxi under different water regimes. Frontiers in Plant Science. 2021;12:806295–806295. doi: 10.3389/fpls.2021.806295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.