Abstract
Oilseed rape (B. napus) is the main oil crop in China as well as in the world. Nitrogen (N) deficiency significantly reduces the seed yield of B. napus. However, a very few studies involved in the genetic mechanism of seed yield and SY-related traits of B. napus in response to N deficiency. In this study, plant height (PH), branch number per plant (BN), pod number per plant (PN), seed number per pod (SN), 1000-seed weight (SW), and seed yield per plant (SY) were investigated using a B. napus double haploid (BnaTNDH) population derived from a cross between cultivars “Tapidor” and “Ningyou7” grown at an optimal N (ON) and a low N (LN) supplies in three-year field trials. Great variations of SY and related traits were observed in BnaTNDH population under contrasting N supplies. A total of 106 and 110 significant quantitative trait loci (QTLs) were detected for six traits at ON and LN in three field trials, respectively. All of these significant QTLs for the same trait identified in two or three trials were integrated into 20 stable QTLs. A total of 50 consensus QTLs and 53 unique QTLs were obtained from 172 significant QTLs and 20 stable QTLs, including 35 ON-specific QTLs, 29 LN-specific QTLs and 39 constitutive QTLs detected at both ON and LN. cqA3l was integrated from four QTLs for PN, PH, SN, SY at LN, cqC9c was integrated from QTLs for BN, SY, PN at ON and LN. Both cqA3l and cqC9c were detected in three trials. In addition, a total of 194 epistatic interactions, inculding 15 pleiotropic epistatic interactions, were identified. Eight of the 15 pleiotropic epistatic interactions were detected to affect SY. This result may help to better understand the genetic mechanism of yield traits in response to low N and promote the breeding of N-efficient varieties.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-022-01281-0.
Keywords: QTL, Seed yield, Optimal nitrogen, Low nitrogen, Brassica napus
Introduction
Nitrogen (N) is a component of nucleic acids, proteins, chlorophyll, alkaloids, vitamins, and hormones, which is essential for plant growth and development (Marschners et al. 2012). Oilseed rape (Brassica napus L.; B. napus) is one of the most important oil crops worldwide, which acquires nitrate and ammonium and recycles organic nitrogen (Masclaux-Daubresse et al. 2010). The application rate of N fertilizer in B. napus ranged from 65 to 325 kg/hm2 in China, which depends on the field fertility, SY target, varieties, and other factors (Zhang et al. 2020). Rational application of N fertilizer can significantly promote the SY, oil production, protein content, and polyunsaturated fatty acid content of B. napus (Gao et al. 2019). On the contrary, irrational fertilization not only decreases the crop yield and quality, but also causes soil acidification and eutrophication (Liu et al. 2013b; Guo et al. 2010; Hirel et al. 2011). Breeding N-efficient B. napus cultivars is an important strategy to improve the SY in a sub-optimal N supply and reduce the application of N fertilizers.
SY is a complex trait, which is mainly related to the potential of B. napus for growth and branching after flowering which enable the crop to use one yield component to compensate for limitations in another one (Bouchet et al. 2014). SY of B. napus is directly related to PN, SN, and SW, and also indirectly associated with PH and BN (Ding et al. 2012). It has previously been shown that PH negatively correlates with PN, owing to the greater lodging risk of taller plants and BN positively correlates with PN (Qiu et al. 2006; Chen et al. 2014). N deficiency significantly decreased SY components such as plant density, BN, PN, SN, except for SW (Cong et al. 2020).
Quantitative trait loci (QTL) analysis based on high-density genetic linkage map can provide basic information on the genetic architecture of quantitative traits (Agrama 2006). Bouchet et al. (2016) map 17 low- N–specific QTLs, 18 optimal-N–specific QTLs for flowering days, seed protein content, SY, SN, SW, oil content, and oil/protein in a double haploid (DH) population of B. napus through three-year field trials, and homologous QTLs for SY were found on A3/C3, A5/C5, and A9/C9 chromosomes. Wang et al. (2017) find that all major QTLs and some stable QTLs for N use efficiency are associated with root morphology traits in B. napus at ON and/or LN. At present, many QTLs have been mapped for SY or N use efficiency in B. napus at ON, but limited QTLs were detected at LN.
In this study, a B. napus DH population derived from a cross between N-efficient cultivar Ningyou7 and N-inefficient cultivar Tapidor (BnaTNDH population) was employed to conduct field trials at ON and LN for three years. The QTLs for SY and SY-related traits of B. npaus under contrasting N supplies were identified. Some major QTLs in response to low N were obtained.
Materials and methods
Plant materials and field trials
A BnaTNDH population with 182 lines was used in this study, which was derived from a cross between a European winter type cultivar “Tapidor” and a Chinese semi-winter type cultivar “Ningyou7” by microspore culture (Qiu et al. 2006). Ningyou7 was characterized as a N-efficient cultivar with better growth than Tapidor under low N (LN) and optimal N (ON) conditions in pot culture (Shi et al. 2010).
Three field trials were conducted in sandy paddy soil in Qichun County, Hubei Province, China (115° 45′ N latitude, 30° 19′ E longitude), during B. napus growing seasons from 2008 to 2009 (Tri.1), from 2009 to 2010 (Tri.2), and from 2010 to 2011 (Tri.3). Soil properties were as follows: pH (1:1 H2O) 4.8, organic matter 34.9 g·kg−1, total N 0.22 g·kg−1, available N 0.074 g·kg−1, Olsen-phosphorus 3.32 mg·kg−1, available potassium 42 mg·kg−1, and available boron 0.09 mg·kg−1. The basal fertilizers included P 38.7 kg·ha−1, K 124.5 kg·ha−1, ZnSO4·7H2O 45 kg·ha−1, and Borax (Na2B4O7·10H2O) 15 kg·ha−1. Sixty percent of 120 and 40 kg·ha−1 N were applied to create ON and LN conditions before transplantation, and the rest of the urea was applied before the overwinter stage. Three replications for 182 BnaTNDH lines and their parents were planted in a randomized complete-plot design with each plot comprising 18 plants, separated by a distance of 0.20 m between plants and 0.28 m between rows. Seeds were sown in a nursery bed in the field in middle September and seedlings were transplanted 30 days after sowing. Plants were harvested in the following middle May. Standard agricultural practices were followed for field management.
Measurement of phenotypic traits
In each plot, six individuals from the middle row were used to determine PH measured from ground level to the tip of the main inflorescence, BN measured as the number of primary branches arising from main shoot, and SN measured as the average number of well-filled seeds from 100 well-developed pods sampled from the primary branch in the middle of each plant studied. All representative individuals from each plot were harvested by hand at maturity stage to investigate SY) and SW. PN was calculated using the following formula: PN = (SY × 1000) / (SW × SN).
Statistical analysis and QTL detection
Data analysis was conducted using SPSS 20.0 (IBM, USA) and Microsoft Excel 2019 (Microsoft, USA). Duncan multiple-range test was used for multiple comparison of different traits between two parents and between two N supplies in three trials. Three-way ANOVA with F test was used at P < 0.05 level. Different growth environments (years) and N treatments were treated as fixed factors, and genotypes were treated as random factor. Correlation analysis was conducted to determine the relationship between the tested traits. The broad-sense heritability (h2) for each trait was calculated at both N levels as follows: h2 = σg2/(σg2 + σge2 /n + σe2/nr), where σg2 is the genotypic variance, σge2 is the interaction variance of genotype with environment, σe2 is the error variance, n is the number of environments, and r is the number of replicates.
The BnaTNDH linkage map contained a total of 2041 molecular marker and the average marker density was from 0.39 to 0.97 per cM (Zhang et al. 2016). QTLs were detected by composite interval mapping (CIM) using WinQTL cartographer 2.5 software (http://statgen.ncsu.edu/qtlcar/WQTLCart.htm) (Wang et al. 2006). For each trait, QTL threshold (P < 0.05) was estimated from 1000 permutations (Silva et al. 2012). Phenotypic variation explained (PVE) was calculated as follows: PVE = (VG/Vp) × 100%, where VG is genetic variance of QTL, Vp is phenotypic variance, VG = 4fof1a2, in which f0 and f1 is the frequency of QTL (genotype = XX) and QTL (genotype = xx), respectively, and a is the additive effect (Li et al. 2010). The epistasis QTLs were identified by the inclusive composite interval mapping (ICIM) method in MET module using IciMapping V4.2 software (Meng et al. 2015). The walk speed was 5 cM, and PIN were set as 0.0001. The LOD thresholds was set to 5.0 as the default manual input value (Wang et al. 2020). Biomercator v4.2 was used to integrate stable QTL, consensus QTL and unique QTL (Arcade et al. 2004). The significant QTLs for the same trait identified in the different trials were integrated into stable QTLs by meta-analysis. Then, the stable and significant QTLs for different traits that overlapped were integrated into consensus QTLs (pleiotropic QTL). The QTLs corresponding to a single trait were called unique QTLs. Each significant QTL was denominated as ‘‘q’’ (abbreviation of QTL) + trait name + trial number + chromosome name + the serial letter (a,b,c...). For example, qPHON3-A3b denoted the second QTL for plant height on chromosome A3 at ON in Tri.3. Each stable QTL was denominated as “sq” (abbreviation of stable QTL) + trait name + chromosome name + the serial letter. For example, sqSWON—A4a indicated the first QTL for SW at ON located on A4. Each consensus QTL was denominated as “cq” (abbreviation of consensus QTL) + chromosome name + the serial letter. For example, cqA2b indicated the second consensus QTL on A2. Each unique QTL was denominated as “uq” (abbreviation of unique QTL) + chromosome name + the serial letter. For example, uqA3f indicated the sixth unique QTL located on A3.
Results
Differences in the six tested traits between cultivars Tapidor and Ningyou 7, and among BnaTNDH population
Compared with at ON, BN and SY of Ningyou7 were significantly decreased at LN (Fig. 1; Table 1). PH of Tapidor at LN was lower than that at ON in Tri.1 and Tri.3, and PH of Ningyou7 at LN was lower than that at ON in Tri.3. PN of the two parents is lower at LN than ON in Tri1 and Tri3. There were no significant difference in SW and SN of Ningyou7 and Tapidor under contrasting N supplies. At ON, SY of Tapidor was significantly lower than that of Ningyou7 in the three trials; BN of Tapidor was lower than that of Ningyou7 in Tri.1 and Tri.2; PN of Tapidor was obviously more than that of Ningyou7 in Tri.1; SN of Tapidor was lower than that of Ningyou7 in Tri.3 (Fig. 1; Table 1). At LN, BN of Tapidor was less than that of Ningyou7 in Tri.1 and Tri.2, and SY of Tapidor was less than that of Ningyou7 in Tri.3 (Fig. 1; Table 1). SW of Tapidor was significantly less than that of Ningyou7 at both ON and LN in the three trials. There was no significant difference in PH between Tapidor and Ningyou7 at two nitrogen supplies in three trials.
Fig. 1.

Phenotyping of Brassica napus cultivars Tapidor (left) and Ningyou7 (right) grown at an optimal N (up) and a low N (down) supply
Table 1.
Means and ranges of the seed yield (SY) and SY-related traits in the parental lines and the BnaTNDH population grown at an optimal N (ON) and a low N supply (LN) in three field trials
| Parental lines | BnaTNDH line | |||||||
|---|---|---|---|---|---|---|---|---|
| Trait | N treatment | Trial | Tapidor | Ningyou7 | Mean | Range | CV (%)a | h2b |
| PHc (cm) | ONi | Tri.1 | 129.6 ± 5.6a | 127.6 ± 3.9a | 140.2a | 109.3–169.2 | 8.5 | 0.75 |
| Tri.2 | 103.1 ± 5.9d | 109.6 ± 3.4 cd | 122.8c | 94.1–153.9 | 10.4 | |||
| Tri.3 | 124.2 ± 1.8ab | 130.6 ± 5.1a | 136.5b | 84.0–174.5 | 9.4 | |||
| LNj | Tri.1 | 116.4 ± 9.4bc | 123.5 ± 8.1ab | 122.4c | 97.0–154.3 | 9.2 | 0.56 | |
| Tri.2 | 104.2 ± 3.7d | 105.1 ± 1.3d | 117.7d | 84.4–154.3 | 10.5 | |||
| Tri.3 | 105.3 ± 12.1d | 113.0 ± 7.3 cd | 114.5e | 87.0–135.9 | 9.0 | |||
| BNd (N) | ON | Tri.1 | 4.5 ± 0.3c | 6.9 ± 0.4a | 5.9b | 4.3–7.6 | 12.4 | 0.59 |
| Tri.2 | 4.5 ± 0.8c | 6.5 ± 0.4a | 5.7c | 2.8–8.8 | 18.0 | |||
| Tri.3 | 6.3 ± 0.3ab | 7.1 ± 0.3a | 6.7a | 2.5–9.5 | 15.3 | |||
| LN | Tri.1 | 4.0 ± 0.7c | 5.5 ± 0.2b | 4.7e | 2.0–6.2 | 15.9 | 0.51 | |
| Tri.2 | 4.4 ± 0.2c | 5.5 ± 0.5b | 5.1d | 2.7–7.7 | 16.9 | |||
| Tri.3 | 4.4 ± 1.1c | 4.4 ± 0.4c | 4.4f | 1.9–6.7 | 18.0 | |||
| PNe (N) | ON | Tri.1 | 179.7 ± 58.0a | 124.8 ± 11.0bc | 164.3a | 54.4–406.1 | 27.3 | 0.59 |
| Tri.2 | 100.4 ± 13.8bcde | 118.5 ± 17.0bcd | 140.5b | 10.0–312.9 | 36.7 | |||
| Tri.3 | 105.4 ± 19.8bcd | 132.2 ± 9.5b | 124.0c | 18.5–244.4 | 38.2 | |||
| LN | Tri.1 | 82.7 ± 34.3cdef | 63.0 ± 11.8ef | 88.8d | 31.7–174.5 | 26.2 | 0.55 | |
| Tri.2 | 86.8 ± 6.0cdef | 81.0 ± 3.8def | 95.1d | 25.5–222.4 | 33.3 | |||
| Tri.3 | 56.5 ± 6.7f | 57.4 ± 13.1f | 46.8e | 5.8–106.3 | 38.6 | |||
| SNf (N) | ON | Tri.1 | 15.7 ± 2.0ab | 16.3 ± 0.9a | 17.7a | 11.0–25.0 | 17.4 | 0.69 |
| Tri.2 | 14.0 ± 1.0abcd | 15.4 ± 1.2abc | 14.1c | 4.5–21.6 | 20.9 | |||
| Tri.3 | 12.7 ± 0.8de | 15.5 ± 0.8abc | 15.0b | 2.2–22.2 | 24.4 | |||
| LN | Tri.1 | 14.3 ± 0.3abcd | 13.9 ± 1.0abcd | 14.8b | 7.1–22.1 | 22.9 | 0.67 | |
| Tri.2 | 13.1 ± 1.5cde | 13.1 ± 1.4cde | 13.4d | 6.6–23.1 | 19.5 | |||
| Tri.3 | 11.0 ± 2.0e | 13.2 ± 1.7bcde | 12.3e | 4.2–22.8 | 29.0 | |||
| SWg (g/1000 seeds) | ON | Tri.1 | 2.3 ± 0.1e | 3.8 ± 0.1b | 2.9c | 2.1–4.1 | 13.3 | 0.84 |
| Tri.2 | 2.5 ± 0.1d | 4.0 ± 0.1ab | 3.1b | 2.0–4.8 | 14.2 | |||
| Tri.3 | 2.1 ± 0.1f | 3.6 ± 0.2c | 2.7d | 1.4–4.0 | 15.8 | |||
| LN | Tri.1 | 2.3 ± 0.1e | 3.9 ± 0.1b | 2.9c | 1.9–4.1 | 15.0 | 0.80 | |
| Tri.2 | 2.5 ± 0.1d | 4.1 ± 0.2a | 3.3a | 2.1–5.0 | 14.7 | |||
| Tri.3 | 2.1 ± 0.1f | 3.5 ± 0.1c | 2.7d | 1.1–4.7 | 20.8 | |||
| SYh (kg/ha) | ON | Tri.1 | 1194.8 ± 308.5b | 1465.7 ± 167.9a | 1509.8a | 455.7–2297.4 | 24.7 | 0.76 |
| Tri.2 | 603.1 ± 140.0 cd | 1355.3 ± 79.6ab | 1120.0b | 263.1–2210.0 | 36.9 | |||
| Tri.3 | 470.1 ± 73.0d | 1332.8 ± 168.5ab | 974.8c | 50.4–2029.6 | 49.2 | |||
| LN | Tri.1 | 489.9 ± 183.0d | 651.6 ± 167.5 cd | 703.0d | 168.3–1353.7 | 32.4 | 0.73 | |
| Tri.2 | 535.3 ± 79.1 cd | 807.1 ± 108.8c | 747.7d | 153.4–1708.6 | 36.8 | |||
| Tri.3 | 208 ± 35.9e | 501.4 ± 92.1d | 303.5e | 13.1–979.9 | 57.1 | |||
Different letters indicated the significant difference at P = 0.05 level. Data are mean ± SD, n = 3. aCV, coefficient of variation; bh2, broad-sense heritability; cPH, plant height; dBN, branch number; ePN, pod number per plant; fSN, seed number per pod; gSW, seed weight of 1000 seeds; hSY, seed yield; iON, optimal nitrogen; jLN, low nitrogen
Compared with at ON, SY, PH, BN, PN, and SN of the BnaTNDH population at LN were significantly decreased. Among them, SY at LN in Tri.1, Tri.2, and Tri.3 decreased by 53.4%, 33.2%, and 68.9%, respectively; SN decreased by 45.9%, 32.3%, and 62.2%, respectively (Table 1). The h2 observed in the BnaTNDH population for the six traits ranged from 0.51 for BN at LN to 0.84 for SY at ON. In general, a higher h2 was observed at ON than at LN (Table 1).
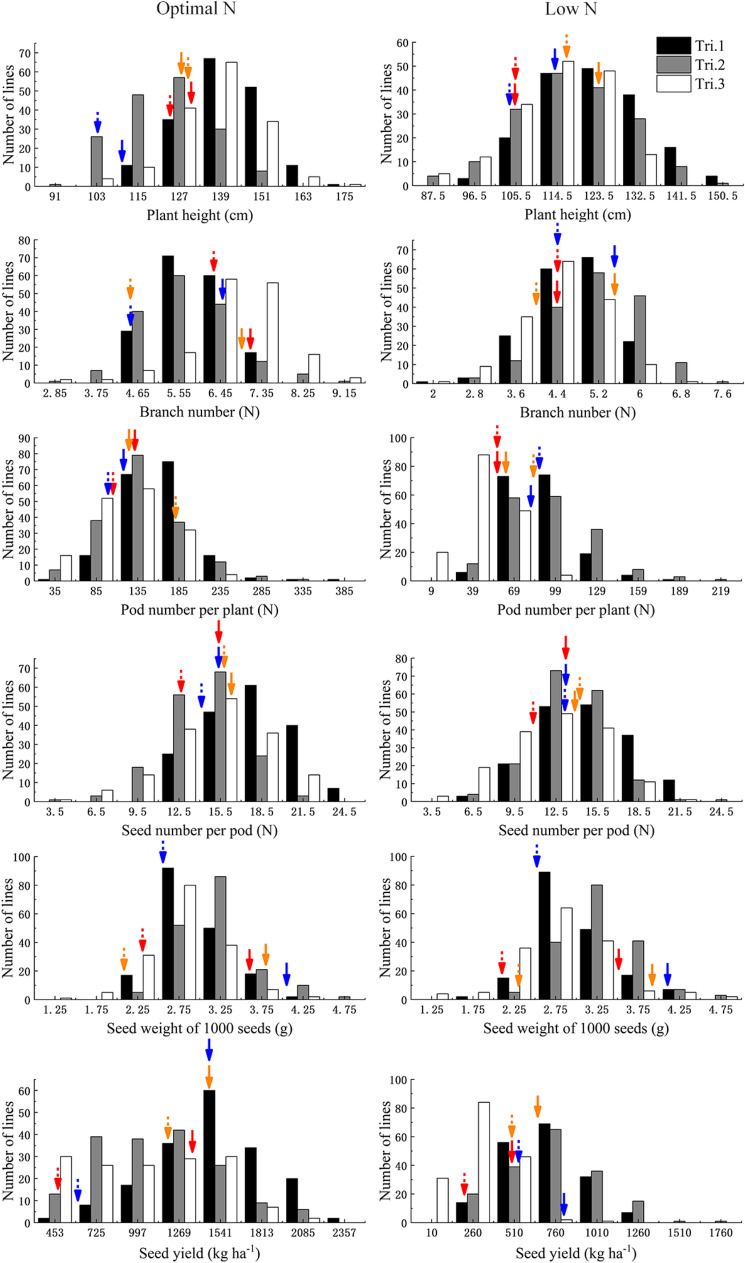
In the three trials, all traits showed approximately normal distributions and transgressive segregations at two N supplies (Table 1; Fig. 2). The results of ANOVA showed that environment, N treatment, genotype, and their interactions had significant effects on all the tested traits (Table 2). SY was highly positively correlated with PN and SN at both ON and LN across three field trials (Table 3). There was a weak correlation between PH and BN at both ON and LN across three field trials, while no significant correlation and weak correlation was observed between SY and SW at both ON and LN in Tri.1 and Tri.2, and Tri.3, respectively (Table 3). ANOVA showed that environment, N treatment, genotype,
Fig. 2.
Frequency distribution of seed yield (SY) and SY-related traits in the BnaTNDH population grown at an optimal (left) and a low (right) N supplies in three field trials. Tri.1, field trial conducted during 2008–2009; Tri.2, field trial conducted during 2009–2010; Tri.3, field trial conducted during 2010–2011. The dashed and solid arrows indicated the position of phenotypic values of cultivars Tapidor and Ningyou7, respectively. The orange, blue, and red arrows indicated trial 1, trial 2, and trial 3, respectively
Table 2.
Significance of three-way ANOVA analysis of the seed yield and yield-related traits among the BnaTNDH population grown at an optimal and a low N supply in three field trials
| PHa | BNb | PNc | SNd | SWe | SYf | ||
|---|---|---|---|---|---|---|---|
| Environment | d.f.g | 2 | 2 | 2 | 2 | 2 | 2 |
| sig.h | *** | *** | *** | *** | *** | *** | |
| N treatment | d.f | 1 | 1 | 1 | 1 | 1 | 1 |
| sig | *** | *** | *** | *** | ** | *** | |
| Genotype | d.f | 181 | 181 | 181 | 181 | 181 | 181 |
| sig | *** | *** | *** | *** | *** | *** | |
| Environment × N treatment | d.f | 2 | 2 | 2 | 2 | 2 | 2 |
| sig | *** | *** | *** | *** | *** | *** | |
| Environment × genotype | d.f | 337 | 337 | 344 | 341 | 341 | 344 |
| sig | *** | *** | *** | *** | *** | *** | |
| N treatment × genotype | d.f | 181 | 181 | 181 | 181 | 181 | 181 |
| sig | ** | ** | *** | *** | *** | *** |
aPH, plant height; bBN, branch number; cPN, pod number per plant; dSN, seed number per pod; eSW, seed weight of 1000 seeds; fSY, seed yield; gd;f;, degrees of freedom; hsig;, significance. **P < 0.01, ***P < 0.001
Table 3.
Correlation coefficients among seed yield (SY) and SY-related traits in the BnaTNDH population grown at an optimal (above diagonal) and a low N supply (below diagonal) in three field trials
| Environments | PHa | BNb | PNc | SNd | SWe | SYf | |
|---|---|---|---|---|---|---|---|
| Tri.1 | PH | 0.19* | 0.20** | 0.18* | − 0.24** | 0.26** | |
| BN | 0.13 | 0.34** | 0.17* | − 0.02 | 0.45** | ||
| PN | 0.42** | 0.15* | − 0.15* | − 0.43** | 0.61** | ||
| SN | 0.28** | 0.05 | 0.09 | − 0.07 | 0.55** | ||
| SW | − 0.26** | 0.07 | − 0.47** | 0.06 | − 0.02 | ||
| SY | 0.39** | 0.23** | 0.57** | 0.77** | 0.07 | ||
| Tri.2 | PH | 0.34** | 0.12 | 0.23** | − 0.17* | 0.26** | |
| BN | 0.26** | 0.57** | 0.07 | − 0.02 | 0.57** | ||
| PN | 0.25** | 0.36** | − 0.08 | − 0.18* | 0.68** | ||
| SN | 0.17* | − 0.03 | 0.06 | − 0.05 | 0.53** | ||
| SW | − 0.19* | − 0.06 | − 0.23** | − 0.01 | 0.06 | ||
| SY | 0.25** | 0.30** | 0.80** | 0.52** | 0.08 | ||
| Tri.3 | PH | 0.45** | 0.31** | 0.46** | 0.01 | 0.41** | |
| BN | 0.49** | 0.34** | 0.29** | 0.16* | 0.40** | ||
| PN | 0.29** | 0.32** | 0.43** | − 0.11 | 0.83** | ||
| SN | 0.29** | 0.13 | 0.34** | 0.08 | 0.73** | ||
| SW | 0.10 | 0.11 | 0.00 | 0.16* | 0.23** | ||
| SY | 0.38** | 0.32** | 0.77** | 0.75** | 0.34** |
aPH, plant height; bBN, branch number; cPN, pod number per plant; dSN, seed number per pod; eSW, seed weight of 1000 seeds; fSY, seed yield. **P < 0.01, *P < 0.05
QTL detection and stable QTL
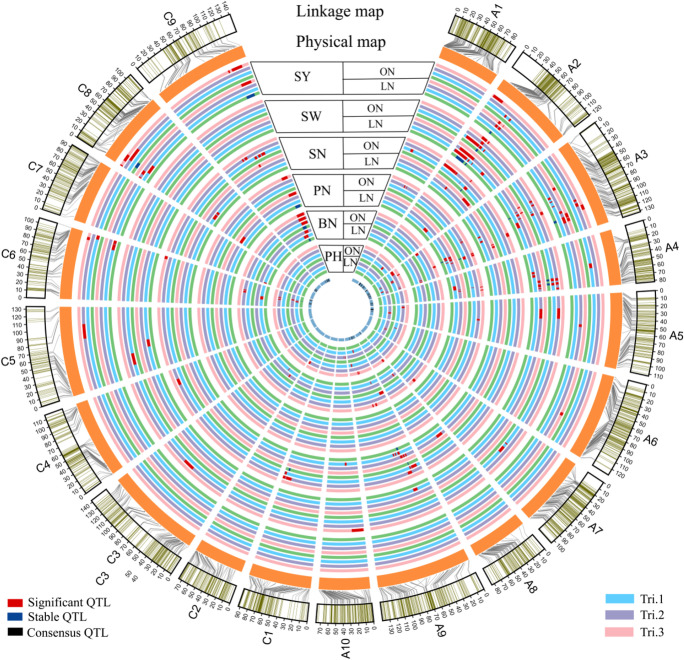
A total of 216 significant QTLs for SY and SY-related traits were detected at two N supplies in three trials (Fig. 2; Supplementary Table 1). These QTLs were mainly located on chromosomes A2, A3, A9, C6, and C9, and the PVE ranged from 4.0 to 23.5%. They were subjected to the first round of QTL meta-analysis trait-by-trait, and 20 stable QTLs detected in two or three trials were obtained (Table 4).
Table 4.
Summary of stable QTL and their corresponding significant QTL
| Stable QTL | Significant QTL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | N Treatment | QTL | Chro.a | Position (cM) | CIb (cM) | QTL | LOD | PVEc (%) | Position (cM) | CI (cM) | AEd |
| PHe | LNk | sqPHLN–C6 | C6 | 42.6 | 41.6–43.5 | qPHLN2–C6c | 4.5 | 7.5 | 42.8 | 41.3–45.3 | + |
| qPHLN1–C6d | 3.3 | 5.2 | 42.5 | 41.3–43.5 | + | ||||||
| BNf | ONl | sqBNON–C9 | C9 | 116.7 | 108.1–125.3 | qBNON2–C9a | 3.3 | 7.4 | 114.0 | 106.0–137.6 | + |
| qBNON3–C9b | 3.7 | 7.4 | 117.3 | 107.2–137.2 | + | ||||||
| qBNON1–C9c | 4.8 | 9.4 | 118.3 | 110.2–138.6 | + | ||||||
| LN | sqBNLN–C9 | C9 | 114.6 | 110.6–118.6 | qBNLN2–C9b | 3.7 | 7.4 | 116.5 | 106.7–125.4 | + | |
| qBNLN1–C9c | 4.1 | 7.9 | 113.0 | 106.1–116.5 | + | ||||||
| PNg | ON | sqPNON–A3 | A3 | 97.7 | 96.3–99.0 | qPNON1–A3 | 5.6 | 11.2 | 98.7 | 95.3–104.4 | – |
| qPNON3–A3a | 6.6 | 12.7 | 95.7 | 95.3–98.8 | – | ||||||
| qPNON3–A3b | 4.5 | 8.9 | 100.9 | 100–104.7 | – | ||||||
| LN | sqPNLN–A3 | A3 | 83.5 | 82.0–84.9 | qPNLN1–A3a | 4.5 | 9.1 | 77.9 | 74.0–81.0 | – | |
| qPNLN2–A3b | 2.8 | 5.5 | 84.3 | 77.1–84.7 | – | ||||||
| qPNLN1–A3c | 7.5 | 14.7 | 84.7 | 82.2–85.7 | – | ||||||
| sqPNLN–C | C9 | 125.8 | 116.4–135.2 | qPNLN3–C9a | 7.1 | 15.2 | 124.3 | 111.8–137.8 | + | ||
| qPNLN2–C9b | 3.0 | 5.8 | 127.4 | 113.1–140.4 | + | ||||||
| SNh | ON | sqSNON–A2 | A2 | 48.1 | 47.1–49.1 | qSNON1–A2a | 5.5 | 10.4 | 46.2 | 39.9–48.6 | + |
| qSNON3–A2a | 3.7 | 7.2 | 48.2 | 46.5–48.6 | + | ||||||
| sqSNON–A9 | A9 | 26.3 | 25.0–26.7 | qSNON1–A9b | 3.3 | 6.4 | 26.1 | 23.9–26.7 | – | ||
| qSNON2–A9c | 3.2 | 5.4 | 28.9 | 25.4–35.1 | – | ||||||
| sqSNON–C1 | C1 | 2.6 | 0.6–4.5 | qSNON1–C1a | 2.8 | 5.3 | 3.6 | 1.1–6.1 | + | ||
| qSNON2–C1b | 4.3 | 8.2 | 1.0 | 0.0–6.1 | + | ||||||
| LN | sqSNLN–A9 | A9 | 37.4 | 35.3–39.5 | qSNLN2–A9c | 3.1 | 6.1 | 35.2 | 31.9–38.0 | – | |
| qSNLN1–A9d | 4.5 | 8.7 | 39.4 | 35.2–41.0 | – | ||||||
| SWi | ON | sqSWON–A2 | A2 | 82.9 | 81.0–84.9 | qSWON1–A2b | 5.9 | 8.5 | 80.8 | 78.3–83.8 | + |
| qSWON2–A2a | 3.7 | 6.8 | 84.7 | 80.8–86.9 | + | ||||||
| qSWON3–A2b | 5.1 | 10.1 | 84.0 | 79.9–91.2 | + | ||||||
| sqSWON–A4a | A4 | 12.7 | 10.8–14.6 | qSWON1–A4b | 4.1 | 5.7 | 12.7 | 9.8–14.8 | + | ||
| qSWON2–A4c | 4.9 | 9.0 | 10.8 | 10.4–14.8 | + | ||||||
| sqSWON–A4b | A4 | 20.6 | 18.8–22.4 | qSWON1–A4d | 4.7 | 6.5 | 20.7 | 17.8–23.4 | + | ||
| qSWON2–A4e | 4.5 | 8.5 | 20.7 | 18.1–22.7 | + | ||||||
| LN | sqSWLN–A2a | A2 | 81.4 | 80.1–82.7 | qSWLN2–A2b | 4.7 | 8.5 | 84.7 | 80.1–86.9 | + | |
| qSWLN1–A2c | 8.2 | 13.8 | 80.8 | 80.6–83.4 | + | ||||||
| sqSWLN–A2b | A2 | 97.8 | 87.9–107.7 | qSWLN1–A2d | 4.3 | 13.6 | 100.9 | 86.9–118.9 | + | ||
| qSWLN2–A2c | 4.2 | 11.9 | 95.9 | 86.9–112.1 | + | ||||||
| sqSWLN–A3 | A3 | 58.4 | 57.6–59.3 | qSWLN2–A3f | 5.5 | 10.1 | 59.5 | 58.6–62.7 | + | ||
| qSWLN3–A3g | 2.5 | 5.2 | 58.9 | 58.6–60.8 | + | ||||||
| SYj | ON | sqSYON–C8 | C8 | 15.3 | 10.7–19.9 | qSYON3–C8c | 2.7 | 4.7 | 9.8 | 9.4–20.7 | + |
| qSYON2–C8d | 4.4 | 8.6 | 25.7 | 15.1–30.7 | + | ||||||
| LN | sqSYLN–A2 | A2 | 84.8 | 83.3–86.4 | qSYLN3–A2c | 4.5 | 8.3 | 84.7 | 82.0–86.3 | + | |
| qSYLN2–A2d | 2.6 | 4.6 | 85.0 | 82.0–86.7 | + | ||||||
| sqSYLN–A3 | A3 | 102.6 | 100.5–104.7 | qSYLN3–A3f | 5.1 | 8.8 | 95.7 | 95.3–102.9 | – | ||
| qSYLN2–A3g | 3.7 | 7.1 | 101.9 | 99.8–110.8 | – | ||||||
| qSYLN3–A3h | 2.8 | 5.1 | 106.7 | 103.9–109.6 | – | ||||||
| sqSYLN–C9 | C9 | 124.6 | 117.0–132.1 | qSYLN2–C9a | 3.6 | 6.3 | 125.3 | 111.2–140.4 | + | ||
| qSYLN3–C9b | 8.2 | 15.4 | 124.3 | 117.2–134.9 | + | ||||||
aChro., chromosome; bCI, confidence interval; cPVE, phenotypic variation explained; dAE, additive effect; ePH, plant height; fBN, branch number; gPN, pod number per plant; hSN, seed number per pod; iSW, seed weight of 1000 seeds; jSY, seed yield; kLN, low nitrogen; lON, optimal nitrogen
A total of 38 significant QTLs for PH were detected at both N supplies, among which 20 QTLs had PVE of 4.0–16.6% at ON, and 18 QTLs had PVE of 4.4–11.9% at LN. sqPHLN–C6 was considered to be a stable QTL (Table 4). It was detected in both Tri.1 and Tri.2 at LN, with an average PVE of 6.4%.
A total of 23 significant QTLs for BN were detected at two N supplies, including 12 QTLs with PVE of 5.3–10.2% at ON, and 11 QTLs with PVE of 4.2–7.9% at LN. sqBNON–C9 detected in three trials and sqBNLN–C9 detected in Tri.1 and Tri.2 were considered to be stable QTLs (Table 4). The average PVE of sqBNON–C9 at ON in three trials was 8.1%, and that of sqBNLN–C9 at LN in Tri.1 and Tri.2 was 7.7%.
A total of 29 significant QTLs for PN were detected at both N supplies. Among them, 13 QTLs at ON accounted for 4.5–12.7% of PVE, while 16 QTLs at LN accounted for 4.5–15.2% of PVE. sqPNON–A3, sqPNLN–A3, and sqPNLN–C9 were considered to be stable QTLs (Table 4). sqPNON–A3 at ON was detected simultaneously in Tri.1 and Tri.3 with an average PVE of 10.9%; sqPNLN–A3 was identified in the Tri.1 and Tri.2 with an average PVE of 9.8%. sqPNLN–C9 at LN was detected in Tri.2 and Tri.3 with an average PVE of 15.5%.
A total of 41 significant QTLs for SN were detected at two N supplies. Among them, 21 QTLs at ON accounted for PVE of 4.3–14.4%, while 20 QTLs at LN accounted for PVE of 4.3–11.1%. sqSNON–A2, sqSNON–A9, sqSNON–C1, and sqSNLN–A9 were considered to be stable QTL (Table 4). sqSNON–A2 was detected simultaneously in Tri.1 and Tri.3, with an average PVE of 8.8%. sqSNON–A9 and sqSNON–C1 were both detected in Tri.1 and Tri.2 and the average PVE was 5.9% and 6.8%, respectively. sqSNLN–A9 was detected in Tri.1 and Tri.2, and the average PVE was 7.4%.
A total of 48 significant QTLs for SW were detected at both N supplies. The PVE of 24 QTLs at ON ranged from 4.0 to 23.5%, while that of 24 QTLs at LN ranged from 4.0 to 13.8%. Thirteen significant QTLs were integrated to six stable QTLs (Table 4). Among of them, three stable QTLs were identified at ON. sqSWON–A2 was detected in all the trials with PVE of 8.5%. sqSWON–A4a and sqSWON–A4b were identified in Tri.1 and Tri.2 with PVE of 7.4% and 7.5%, respectively. At LN, there were three QTLs for SW: sqSWLN–A2a, sqSWLN–A2b, sqSWLN–A3. sqSWLN–A2a and sqSWLN–A2b were detected in Tri.1 and Tri.2, with an average PVE of 11.2% and 12.8%, respectively. sqSWLN–A3 was detected simultaneously in Tri.2 and Tri.3, which accounted for an average PVE of 7.6%.
A total of 37 significant QTLs for SY were detected at two N supplies. Among of them, the PVE of 16 QTLs at ON ranged from 4.4% to 11.0%, and that of 21 QTLs at LN ranged from 4.1% to 15.3%.sqSYON–C8 was detected in Tri.2 and Tri.3, with an average PVE of 6.8%. sqSYLN–A2, sqSYLN-A3 and sqSYLN-C9 were detected in Tri.2 and Tri.3 and the average PVE was 6.5%, 7.0%, and 10.9%, respectively.
Unique QTL and consensus QTL identified at ON and LN
Most of the significant QTLs associated with various traits overlapped on A2, A3, C6, and C9 chromosomes. A total of 50 consensus QTLs and 53 unique QTLs were obtained from 172 significant QTLs and 20 stable QTLs, including 35 ON-specific QTLs, 29 LN-specific QTLs, and 39 constitutive QTLs detected at both ON and LN (Fig. 2; Supplementary Table 2).
Among 35 ON-specific QTLs, cqA2c, cqA3a, and cqA9a for two traits were clustered on A2, A3, and A9, respectively (Table 5). cqA2c for two traits of PH and SN was located in the interval of 47.1–49.0 cM on A2. cqA3a was integrated from two significant QTLs, qPHON2–A3a and qBNON1–A3, and was clustered in the interval of 0.0–8.3 cM on A3. cqA9a was obtained from two QTLs, qBNON1–A9b and qSNON1–A9a, and clustered in the interval of 10.9–20.4 cM on A9 (Table 5, Supplementary Table 3).
Table 5.
ON- and LN-specific consensus QTLs associated with at least two traits and ON&LN constitutive consensus QTLs associated with at least three traits
| Consensus QTL | Significant QTL and stable QTL | ||||||
|---|---|---|---|---|---|---|---|
| Chro.a | N treatment | Position (cM) | CIb (cM) | Position (cM) | CI (cM) | ||
| A2 | cqA2a | LNc | 29.9 | 28.7–31.0 | qSNLN3–A2c | 29.4 | 27.8–30.4 |
| qPNLN1–A2a | 31.6 | 27.8–32.9 | |||||
| cqA2b | ON&LN | 42.6 | 40.6–44.6 | qPNLN1–A2b | 41.0 | 32.9–44.3 | |
| qPHON1–A2a | 42.0 | 39.8–45.2 | |||||
| qSWLN1–A2a | 44.4 | 37.8–45.2 | |||||
| cqA2c | ONd | 48 | 47.1–49.0 | qPHON1–A2b | 47.2 | 45.4–52.8 | |
| sqSNON–A2 | 48.1 | 47.1–49.1 | |||||
| cqA2g | LN | 77.6 | 76.2–78.8 | qSYLN2–A2a | 76.0 | 75.6–79.9 | |
| qSWLN2–A2a | 76.5 | 75.1–80.1 | |||||
| qSNLN2–A2 | 80.1 | 77.5–82.0 | |||||
| cqA2h | ON&LN | 83 | 82.3–83.7 | sqSWLN–A2a | 81.4 | 80.1–82.7 | |
| sqSWON–A2 | 82.9 | 81.0–84.9 | |||||
| qSYON1–A2b | 83.0 | 82.0–85.0 | |||||
| qSNON2–A2a | 84.0 | 82.0–86.0 | |||||
| sqSYLN–A2 | 84.8 | 83.3–86.4 | |||||
| A3 | cqA3a | ON | 3.3 | 0.0–8.3 | qPHON2–A3a | 20.4 | 0.6–27.8 |
| qBNON1–A3 | 0.6 | 0.0–10.8 | |||||
| cqA3d | ON&LN | 59.4 | 59.1–59.7 | sqSWLN–A3 | 58.4 | 57.6–59.3 | |
| qPHLN1–A3b | 59.4 | 58.8–59.5 | |||||
| qSYLN1–A3a | 59.5 | 58.9–62.7 | |||||
| qPHON1–A3b | 62.7 | 58.8–65.6 | |||||
| cqA3e | LN | 65.6 | 65.2–66.0 | qSNLN1–A3a | 65.5 | 62.7–66.5 | |
| qPHLN1–A3c | 65.6 | 65.5–66.3 | |||||
| cqA3h | ON&LN | 76.7 | 76.0–77.4 | qSYLN1–A3c | 76.7 | 75.4–76.9 | |
| qSWON3–A3a | 76.9 | 75.4–81.0 | |||||
| qSNLN1–A3b | 76.4 | 75.4–80.0 | |||||
| qSWLN1–A3a | 76.4 | 73.8–84.0 | |||||
| cqA3i | ON&LN | 84.9 | 84.4–85.5 | qSYON2–A3a | 81.6 | 81.0–84.3 | |
| sqPNLN–A3 | 83.5 | 82.0–84.9 | |||||
| qSWLN1–A3b | 85.7 | 84.0–85.7 | |||||
| qSNON3–A3a | 86.3 | 84.7–87.2 | |||||
| cqA3j | ON&LN | 92 | 91.2–92.9 | qSYON2–A3b | 92.0 | 87.2–92.8 | |
| qSYLN2–A3a | 92.0 | 88.7–92.8 | |||||
| qSNON3–A3b | 91.8 | 87.2–93.1 | |||||
| qPNLN2–A3b | 92.0 | 86.6–92.1 | |||||
| qBNON3–A3 | 84.7 | 84.3–91.5 | |||||
| qPNLN1–A3c | 92.8 | 92.3–94.6 | |||||
| cqA3k | ON&LN | 97.5 | 96.8–98.3 | qSYON3–A3c | 95.7 | 92.8–100.9 | |
| qSNLN3–A3a | 95.7 | 95.3–97.5 | |||||
| sqPNON–A3 | 97.7 | 96.3–99.0 | |||||
| cqA3l | LN | 101.8 | 100.6–103.1 | qPNLN2–A3c | 100.9 | 99.7–102.2 | |
| qPHLN1–A3e | 104.7 | 100.0–108.8 | |||||
| qSNLN3–A3b | 100.9 | 100.0–104.7 | |||||
| sqSYLN–A3 | 102.6 | 100.5–104.7 | |||||
| A4 | cqA4d | ON&LN | 31 | 30.4–31.7 | qSWLN1–A4a | 31.0 | 30.4–35.8 |
| qPNLN1–A4a | 31.0 | 30.4–31.7 | |||||
| qSYON1–A4 | 31.7 | 31.0–38.6 | |||||
| cqA4e | LN | 39.3 | 37.1–41.6 | qPNLN1–A4b | 37.0 | 31.7–42.8 | |
| qSWLN1–A4b | 39.8 | 37.4–42.4 | |||||
| A5 | cqA5c | ON&LN | 54.5 | 52.9–56.0 | qSYLN2–A5 | 53.3 | 50.6–55.0 |
| qPHLN2–A5 | 53.5 | 53.3–63.6 | |||||
| qPNON2–A5 | 56.0 | 54.3–59.0 | |||||
| A8 | cqA8 | ON&LN | 69.9 | 68.0–71.8 | qPHON3–A8 | 76.6 | 68.5–79.3 |
| qBNLN2–A8 | 68.0 | 66.0–70.9 | |||||
| qSNLN2–A8b | 70.9 | 68.0–74.9 | |||||
| A9 | cqA9a | ON | 15.6 | 10.9–20.4 | qBNON1–A9b | 10.8 | 6.6–19.8 |
| qSNON1–A9a | 20.8 | 8.6–22.3 | |||||
| cqA9c | LN | 37.2 | 36.0–38.4 | qSWLN1–A9 | 29.9 | 28.9–38.0 | |
| sqSNLN–A9 | 37.4 | 35.3–39.5 | |||||
| qBNLN1–A9 | 38.0 | 35.2–38.4 | |||||
| C8 | cqC8b | ON&LN | 18.7 | 15.5–22.0 | qPNLN2–C8 | 9.3 | 1.9–26.1 |
| sqSYON–C8 | 15.3 | 10.7–19.9 | |||||
| qSNLN1–C8a | 24.2 | 15.2–28.5 | |||||
| qSYLN1–C8a | 25.0 | 15.2–30.7 | |||||
| C9 | cqC9b | ON&LN | 102.2 | 98.5–105.9 | qBNLN1–C9a | 102.9 | 93.4–105.3 |
| qSNON1–C9c | 101.7 | 93.7–103.1 | |||||
| qSYON3–C9a | 107.2 | 107.1–109.5 | |||||
| C9 | cqC9c | ON&LN | 122.42 | 117.8–127.0 | sqBNLN–C9 | 114.6 | 110.6–118.6 |
| sqBNON–C9 | 116.7 | 108.1–125.3 | |||||
| qSYON3–C9b | 117.3 | 113.3–130.5 | |||||
| qPNON3–C9 | 118.3 | 113.5–130.8 | |||||
| sqSYLN–C9 | 124.6 | 117.0–132.1 | |||||
| sqPNLN–C9 | 125.8 | 116.4–135.2 | |||||
aChro., chromosome; bCI, confidence interval; cLN, low nitrogen; dON, optimal nitrogen
Among 29 LN-specific QTLs, 6 LN-specific QTLs for more than two traits were clustered on A2, A3, A4, and A9 chromosomes (Table 5). cqA3l for four traits of PH, PN, SN, and SY was located in the interval of 100.6–103.1 cM on A3. cqA9c for three traits of BN, SN, and SW was integrated from three significant QTLs of qSWLN1–A9, sqSNLN–A9, and qBNLN1–A9, and located in the interval of 36.0–38.4 cM on A9. cqA2g for SY, SW and SN was located in the interval of 76.2–78.8 cM on A2.
Among 39 constitutive QTLs, 13 consensus QTLs detected for more than three traits were located on A2, A3, A4, A5, A8, A9, C8, and C9, respectively (Table 5). Among of them, cqA3j for four traits of BN, PN, SN, and SY at ON and LN was integrated from qSYON2–A3b, qSYLN2–A3a, qSNON3–A3b, qPNLN2–A3b, qBNON3–A3, and qPNLN1–A3c and located in the interval of 91.2–92.9 cM on A3.
Epistatic interaction for seed yield and yield related traits at ON and LN
A total of 194 epistatic interactions, including 90 at ON and 104 at LN, were detected for PH, BN, PN, SN, SW and SY (Supplementary Table 3). There were 56 epistatic interactions on the A genome, 54 on the C genome and 84 between A and C genomes of B. napus. The phenotypic contributions of these epistatic interactions for six traits varied from 0.40% to 5.04% (Supplementary Table 3). Fifteen epistaitc interactions associated with different traits or one trait at ON and LN were called pleiotropic epistatic interactions (Table 6). Two pleiotropic epistatic interactions for PN and SY at LN were identified on chromosome C1 and C8, respectively. One for PN and SY at ON was detected on chromosome A9 and C8 (Table 6). A pleiotropic epistatic interaction affecting SN and SY at ON was found on chromosome A9 and C5, and the other one for SN at LN and SY at ON and LN was identified on chromosome A3 and A6 (Table 6). There were two epistatic interactions associated with the same trait at both ON and LN. The epistatic interaction for SY was identified on chromosome A5 and A10, and that for SN was detected on chromosome A3 and C3 (Table 6). Eight epistatic interactions were identified to affect SY. SY was closely associated with SN and PN so consensus QTLs associated with PN, SN and SY were focused.
Table 6.
Pleiotropic epistatic loci for two traits or one trait at ON and LN supplies
| Traits | N treatment | Chro.1a | Position1(cM) | LeftMarker1 | RightMarker1 | Chro.2 | Position2 | LeftMarker2 | RightMarker2 | LOD | PVEb(AAc) | PVE(AAbyEd) | Add1 | Add2 | AddbyAdd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNe | LN | A3 | 85 | C3M89 | C3M90 | A6 | 10 | C6M4 | C6M5 | 5.91 | 2.72 | 0.17 | − 0.02 | − 0.33 | − 0.53 |
| SYf | ON | A3 | 85 | C3M89 | C3M90 | A6 | 10 | C6M4 | C6M5 | 6.81 | 3.33 | 0.35 | 27.77 | − 19.69 | − 77.72 |
| LN | A3 | 85 | C3M89 | C3M90 | A6 | 10 | C6M4 | C6M5 | 5.94 | 2.80 | 0.19 | − 6.94 | − 14.95 | − 38.62 | |
| SN | ON | A3 | 110 | C3M121 | C3M122 | C3 | 95 | C13M82 | C13M83 | 5.98 | 2.97 | 0.41 | − 0.19 | 0.13 | − 0.57 |
| LN | A3 | 110 | C3M121 | C3M122 | C3 | 95 | C13M82 | C13M83 | 7.76 | 3.69 | 0.31 | 0.03 | 0.05 | − 0.63 | |
| SY | ON | A5 | 85 | C5M87 | C5M88 | A10 | 70 | C10M95 | C10M96 | 5.71 | 3.34 | 0.04 | 51.99 | 8.11 | 90.82 |
| LN | A5 | 85 | C5M87 | C5M88 | A10 | 70 | C10M95 | C10M96 | 5.81 | 2.52 | 0.57 | 25.33 | − 2.11 | 43.04 | |
| SN | ON | A9 | 35 | C9M18 | C9M19 | C5 | 20 | C15M12 | C15M13 | 5.13 | 2.56 | 0.36 | 0.29 | − 0.09 | − 0.53 |
| SY | ON | A9 | 35 | C9M18 | C9M19 | C5 | 20 | C15M12 | C15M13 | 7.96 | 4.47 | 0.15 | 30.04 | − 42.11 | − 90.77 |
| PNg | ON | A9 | 75 | C9M71 | C9M72 | C8 | 40 | C18M26 | C18M27 | 5.10 | 2.80 | 0.00 | − 2.57 | − 1.83 | − 8.02 |
| SY | ON | A9 | 75 | C9M71 | C9M72 | C8 | 40 | C18M26 | C18M27 | 7.85 | 3.94 | 0.58 | − 22.34 | − 2.82 | − 84.25 |
| PN | LN | C1 | 30 | C11M54 | C11M55 | C1 | 85 | C11M91 | C11M92 | 5.27 | 2.03 | 1.50 | − 0.18 | − 2.19 | − 3.67 |
| SY | LN | C1 | 30 | C11M54 | C11M55 | C1 | 85 | C11M91 | C11M92 | 5.63 | 1.97 | 1.06 | − 8.20 | − 9.61 | − 33.03 |
| PN | LN | C8 | 10 | C18M15 | C18M16 | C8 | 15 | C18M17 | C18M18 | 5.17 | 0.79 | 0.00 | − 7.73 | 4.84 | − 8.79 |
| SY | LN | C8 | 10 | C18M15 | C18M16 | C8 | 15 | C18M17 | C18M18 | 7.22 | 0.40 | 0.00 | − 74.08 | 42.87 | − 57.08 |
aChr.1, chromosome; bPVE, phenotypic variation explained; cAA, additive effect; dAAbyE, epistatic effect (additive × additive); eSN, seed number per pod; fSY, seed yield; gPN, pod number per plant
Candidate genes underlying stable QTLs at LN and two consensus QTLs
sqSYLN-C9 was considered to be the stable QTL among QTLs associated with SY due to the PVE of qSYLN3–C9b was 15.4% (Table 4, Supplementary Table 1). Three candidate genes, BnaC09g46700D, BnaC09g47860D and BnaC09g49790D were identified in the confidence regions of sqSYLN-C9. BnaC09g49790D was also identified in the interval of sqPNLN-C9 and cqC9c (Table 7). BnaC06.NPF3.1 (BnaC06g25780D) and BnaC06.NRT1.7 (BnaC06g25140D) were located in interval of sqPHLN–C6. The orthologue of AtNRT2.7 in B. napus, BnaA02g02200D, was located in the genomic region of sqSWON–A2, sqSWLN–A2a, sqSWLN–A2b and sqSYLN–A2.
Table 7.
Candidate genes within the intervals of some stable QTL and cqA3l, cqC9c, and their nucleotide variations in the promoter and coding sequence between cultivars Tapidor and Ningyou7 and gene annotations
| QTL name | Gene | Promoter (2 Kb upstream of TSS) | Coding sequence | Homologs in A. thaliana | Gene annotations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of SNPs | Number of InDels | Number of SNPs | Number of InDels | |||||||||
| Stop-gain | Stop-loss | Non-synonymous | Stop-gain | Stop-loss | Frameshift | Non-frameshift | ||||||
| sqSYLN–C9 | BnaC09g46700D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G10000(FD4) | Ferredoxin 4 |
| BnaC09g49790D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G05690(CYP90) | Cytochrome P450 superfamily protein | |
| BnaC09g47860D | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G07440(GDH2) | Flutamate dehydrogenase 2 | |
| sqPHLN–C6 | BnaC06g25780D | 4 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | AT1G68570(NPF3) | Nitrate transporter1/peptide transporter 3.1 |
| BnaC06g25140D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT1G69870(NRT1.7) | Nitrate transporter 1.7 | |
| sqSWON–A2 | ||||||||||||
| sqSWLN–A2a | BnaA02g02200D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G14570(NRT2.7) | Nitrate transporter 2.7 |
| sqSWLN–A2b | ||||||||||||
| sqSYLN–A2 | ||||||||||||
| sqPNLN-C9 | BnaC09g49790D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G05690(CYP90) | Cytochrome P450 superfamily protein |
| cqA3l | BnaA03g40850D | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT3G50500(SnRK2) | SNF1-related protein kinase 2.2 |
| BnaA03g40660D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT3G49940(LBD38) | LOB domain-containing protein 38 | |
| BnaA03g41350D | 22 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT3G51520(DGAT2) | Diacylglycerol acyltransferase family | |
| BnaA03g41250D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT3G51240(F3H) | Flavanone 3-hydroxylase | |
| cqC9c | BnaC09g47360D | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G08640(FLS1) | Flavonol synthase 1 |
| BnaC09g49790D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G05690(CYP90) | Cytochrome P450 superfamily protein | |
| BnaC09g47230D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G09220(AAP2) | Amino acid permease 2 | |
| BnaC09g50680D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | AT5G04590(SIR) | Sulfite reductase | |
Among consensus QTLs, cqA3l and cqC9c were detected in three trials and associated with four traits and three traits, respectively. Among the QTL region of cqA3l, BnaA03g40850D, BnaA03g40660D, BnaA03g41250D and BnaA03g41350D were predicted to be candidate genes as their orthologue genes in Arabidopsis are reported to respond to N deficiency. BnaC09g47360D (BnaC09.FLS1), BnaC09g47230D (BnaC09.AAP2), BnaC09g50680D (BnaC09.SIR) and BnaC09g49790D (BnaC09.CYP90) located in the interval of cqC9c were also predicted to be candidate genes. There were no SNPs and InDels differences in coding sequence except for BnaC06g25780D identified in sqPHLN–C6, which had three non-synonymous SNPs in coding sequence (Table 7). One or two SNPs or InDels were identified in promoter region of BnaC09g47860D, BnaA03g40850D, BnaC09g47360D. There were four SNPs and one InDels found in promoter of BnaC06g25780D. In the promoter region of BnaA03g41350D, 22 SNPs and 15 InDels differences were identified, indicating BnaA03.DGAT2 displayed more variations between Tapidor and Ningyou7 (Table 7).
Discussion
Pot culture experiments showed that SY of Ningyou7 was significantly higher than that of Tapidor at both ON and LN (Shi et al. 2010). In this study, at ON, SY of Ningyou7 was considerably higher than that of Tapidor in three trials, and at LN, SY of Ningyou7 was higher than that of Tapidor in Tri.3 (Table 1). The performance of Ningyou7 and Tapidor in field trials were similar to pot culture experiments. Seventy nine percent of the 216 significant QTLs for SY and SY-related traits were detected only in one trial (Supplementary Table 1). A large number of environment-specific QTLs for SY and its related traits are identified, indicating the growth environments have important effects on the function of the genes associated with these traits (Shi et al. 2009). There were three stable QTLs for SN and SW at ON, and SW and SY at LN. The stable QTLs for SN at ON and SW at ON and LN mainly detected in Tri.1 and Tri.2 (Table 4). These were consistent with the significance of the correlation coefficient of the investigated traits between each two trials (Supplementary Table 4). Twenty stable QTLs were detected simultaneously in at least two trials (Table 4), and the corresponding significant QTLs have similar additive phenotypic effects. At ON, Luo et al. (2017) also identified the QTLs for SY and SY related traits, such as sqBNON–C9, sqPNON–A3, sqSNON–A2, sqSNON–A9, sqSNON–C1, sqSWON–A4a, sqSWON–A4b and sqSYON–C8, and the intervals of these QTLs overlapped with the QTLs for the same traits at ON in this study. Bouchet et al. (2014) detected 40 QTLs for SY, SN, SW of B. napus DH population derived from a cross between cultivars Aviso and Montego at two contrasting N conditions in three grown environments. The genomic region of sqSWON–A4a and sqSWON–A4b were identified associated with SW under optimal N condition (Bouchet et al. 2014). These robust QTLs identified across different populations and/or environments could be become accessible to ongoing breeding programs.
A total of 106 and 110 QTLs for SY and its related traits were identified at ON and LN supplies, respectively (Supplementary Table 1). The QTLs detected at ON were different from that at LN for each trait but some of them were common to the two N supplies (Table 4; Fig. 3; Supplementary Table 1). For example, a total of 22 (59.4%) significant QTLs for SY at ON and LN were co-located; and a total of 28 (58.3%) and 16 (42.2%) significant QTLs for SW and PH, respectively, were found to be co-located under contrasting N supplies. The average proportion of these common QTLs in our study was less than previous studies by Bouchet et al. (2014). This could be due to the N stress in this study is more severe than Bouchets’ studies (2014) at LN supplies.
Fig. 3.
Summary of significant QTLs, stable QTLs, and consensus QTLs for seed yield (SY) and SY-related trait in the BnaTNDH population grown at an optimal and a low N supply. All significant QTLs in two or three trials of all the traits detected were integrated into stable QTLs, and then significant QTLs and stable QTLs were integrated into consensus QTLs. The outermost circle and the second circle represented the genetic map and physical map, respectively. All SNP markers on each chromosome were corresponded to the physical position. From the third circle to the sixth circle, each color of circle stands for one trial, except green ones, which showed the positions of stable QTLs of each trait at an optimal N or a low N supply. The innermost circle showed the positions of consensus QTLs on chromosomes. PH, plant height; BN, branch number; PN, pod number per plant; SN, seed number per pod; SW, seed weight of 1000 seeds; SY, seed yield; ON, optimal nitrogen; LN, low nitrogen
ON- and LN-specific QTL were reported for flowering days and SY (Bouchet et al. 2016), and root dry weight (Liu et al. 2009) in B. napus. In this study, there were 50 consensus QTLs and 53 unique QTLs, including 29 LN -specific QTLs, 35 ON-specific QTLs, and 39 constitutive QTLs for SY and its related traits (Supplementary Table 2). cqA2f, a constitutive QTL, was associated with SY and SW at ON and SY at LN (Supplementary Table 2). Its interval was overlapped the interval of es.A2-30 (Luo et al. 2017), which contained SY and SW at ON.
Among ON-specific QTLs, there were no QTLs co-located with the QTLs for SY. There were four consensus QTLs (cqA2g, cqA3h, cqA3l, cqC8b) for SY overlapped with QTLs for SN at LN (Table 5). At ON, the overlapped QTLs between for SY and for SN on A5 and C8 have also been reported in Brassica napus (Bouchet et al. 2014). Moreover, there was a high positive correlation between SY and SN at LN (Table 3).
Pleiotropic QTLs have also been reported for PH and spike length of wheat (Chai et al. 2019), PH and heading date of rice (Liu et al. 2013a). Xu et al. (2014) identified two genes in wheat, Rht8 on chromosome 2D and Rht-B1b on chromosome 4B, which had pleiotropic effects for PH, spike length, harvest index, and N utilization efficiency. QTLs for SY directly accounted for a small proportion of all identified QTLs (Chen et al. 2010; Peng et al. 2011; Luo et al. 2017). The QTLs for some plant canopy architecture traits, such as BN and PH, are co-located with QTLs for SY (Cai et al. 2016; Miersch et al. 2016). Canopy architecture traits strongly affect light interception and photosynthesis, and play an important role in total yield and harvest index (Sarlikioti et al. 2011). Among consensus QTLs, cqA3l, a LN-specific QTL, and cqC9c, a constitutive QTL, were associated with the overlapped QTLs for PH, SN, and SY at LN, and the overlapped QTLs for BN and SY at LN and ON, respectively (Table 5). Most of pleiotropic epistatic interaction also affected SY and canopy architecture traits, such as SN and PN (Table 6; Supplementary Table 3).
Fifteen genes located in the confidence regions of stable QTL and two consensus QTL (cqA3l and cqC9c) were predicted to be candidate genes. BnaC09g46700D, BnaC09g47860D and BnaC09g49790D were identified in the confidence regions of sqSYLN-C9. The orthologues of BnaC09g46700D and BnaC09g47860D in Arabidopsis have been reported in association with glutamate synthase and affecting N assimilation (Hanke et al. 2005; Fontaine et al. 2012). BnaC09g46700D encodes ferredoxin, which is involved in glutamate synthase (GOGAT). The ferredoxin-dependent GOGAT is important in N-carbon metabolomes in rice (Yang et al. 2016). BnaC09g47860D encodes glutamate dehydrogenase. The orthologue of BnaC09g49790D (AtCYP90) in Arabidopsis participates in root foraging response to N deficiency, which were also located in the interval of sqPNLN–C9 and cqC9c (Jia et al. 2020). AtNPF3.1 (NITRATE TRANSPORTER1/PEPTIDE TRANSPORTER 3.1), a gene that transport gibberellins (GAs) and its precursors, plays an important role in GA transport under low-nitrate conditions in Arabidopsis (David et al. 2016). AtNRT1.7 (NITRATE TRANSPORTER 1.7) is involved in source-to-sink remobilization of nitrate (Liu et al. 2016). BnaC06.NPF3.1 (BnaC06g25780D) and BnaC06.NRT1.7 (BnaC06g25140D) were located in interval of sqPHLN–C6. AtNRT2.7 is another nitrate transporter that controlled nitrate content in seeds (Chopin et al. 2007). BnaA02.NRT2.7 (BnaA02g02200D) was identified in the genomic region of sqSWON–A2, sqSWLN–A2a, sqSWLN–A2b and sqSYLN–A2. There were four candidate genes identified in the confidence region of cqA3l. AtSnRK2.2 (SNF1-RELATED PROTEIN KINASE 2.2) regulates nitrate uptake via phosphorylation of NRT1.1 (Su et al. 2020). AtLBD38 (LATERAL ORGAN BOUNDARY DOMAIN 38), a transcription factor, acts as negative regulator of anthocyanin biosynthesis in Arabidopsis in the absence of N (Rubin et al. 2009). Moreover, AtF3H (FLAVANONE 3-HYDROXYLASE) participates in anthocyanin biosynthesis positively (Zhang et al. 2017). BnaA03g41350D, whose orthologue gene in Arabidopsis affects triacylglycerol (TAG) biosynthesis in response N deficiency (Yang et al. 2011). BnaC09g47360D, BnaC09g49790D, BnaC09g47230D and BnaC09g50680D were located in the interval of cqC9c. Their homologous genes in Arabidopsis were also associated with low N responses. Among them, AtFLS1 (FLAVONOL SYNTHASE 1) is induced response to N deficiency to improve anthocyanin content (Feyissa et al. 2009); AtAAP2 (AMINO ACID PERMEASE 2) is negatively regulate N allocation to leaves to influence carbon fixation and photosynthesis N use efficiency (Perchlik and Tegeder, 2018); AtSIR (SULFITE REDUCTASE) is involved in enhance of photosynthesis under N and sulfur deficiency regulated by uroporphyrinogen III methyltransferase (UPM1) (Garai and Tripathy, 2018).
In conclusion, considerable variations of SY and SY-related traits were observed among the BnaTNDH population. N deficiency reduced SY and SY-related traits except for SW. Only 21% significant QTLs were detected in more than two trials, indicating that different genetic determinants were involved in regulating SY and its related traits at ON and LN. The overlaps of the QTLs for PH and SN with SY were detected in different trials, suggesting that canopy architecture had a significant effect on SY. Near-isogenic lines should be developed to fine map the major QTLs identified in this study such as sqSYLN–C9 and cqA3l. These will be helpful for a better understanding of the molecular mechanism of SY of B. napus under N deficiency and promote the molecular breeding of N-efficient cultivars.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- PH
Plant height
- BN
Branch number per plant
- PN
Pod number per plant
- SN
Seed number per pod
- SW
Seed weight of 1000 seeds
- SY
Seed yield per plant
- ON
Optimal nitrogen
- LN
Low nitrogen
- Tri.1
Field trial conducted during 2008 − 2009
- Tri.2
Field trial conducted during 2009 − 2010
- Tri.3
Field trial conducted during 2010 − 2011
- DH
Double haploid
- PVE
Phenotypic variation
Author contribution
MZ analyzed the data and wrote the main manuscript. TS designed and managed 3-year trials. WW analyzed the data. GD and FX taught TS and MZ to complete trials and data analysis and modified the main manuscript. LS taught TS and MZ to complete trials and data analysis, reviewed the manuscript.
Funding
This research was supported by the National Nature Science Foundation of China (Grant No. 31972498) and Natural and Fundamental Research Funds for the Central Universities of China (Grant No. 2662019PY013).
Data availability
The data sets supporting the results of this article are included within the article and its additional files.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrama HA. Application of molecular markers in breeding for nitrogen use efficiency. J Crop Improv. 2006;15:175–211. doi: 10.1300/J411v15n02_06. [DOI] [Google Scholar]
- Arcade A, Labourdette A, Falque M, Mangin B, Chardon F, Charcosset A, Joets J. Biomercator: integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics. 2004;20:2324–2326. doi: 10.1093/bioinformatics/bth230. [DOI] [PubMed] [Google Scholar]
- Bouchet AS, Nesi N, Bissuel C, Bregeon M, Lariepe A, Navier H, Ribiere N, Orsel M, Grezes-Besset B, Renard M, Laperche A. Genetic control of yield and yield components in winter oilseed rape (Brassica napus L) grown under nitrogen limitation. Euphytica. 2014;199:183–205. doi: 10.1007/s10681-014-1130-4. [DOI] [Google Scholar]
- Bouchet AS, Laperche A, Bissuel-Belaygue C, Baron C, Morice J, Rousseau-Gueutin M, Dheu JE, George P, Pinochet X, Foubert T, Maes O, Dugue D, Guinot F, Nesi N. Genetic basis of nitrogen use efficiency and yield stability across environments in winter rapeseed. BMC Genet. 2016;17:21. doi: 10.1186/s12863-016-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai GQ, Yang QY, Chen H, Yang Q, Zhang CY, Fan CC, Zhou YM (2016) Genetic dissection of plant architecture and yield-related traits in Brassica napus. Sci Rep 6. 10.1038/srep21625 [DOI] [PMC free article] [PubMed]
- Chai LL, Chen ZY, Bian RL, Zhai HJ, Cheng XJ, Peng HR, Yao YY, Hu ZR, Xin MM, Guo WL, Sun QX, Zhao AJ, Ni ZF. Dissection of two quantitative trait loci with pleiotropic effects on plant height and spike length linked in coupling phase on the short arm of chromosome 2D of common wheat (Triticum aestivum L) Theor Appl Genet. 2019;132:3223–3223. doi: 10.1007/s00122-019-03420-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Geng JF, Rahman M, Liu XP, Tu JX, Fu TD, Li GY, McVetty PBE, Tahir M. Identification of QTL for oil content seed yield and flowering time in oilseed rape (Brassica napus) Euphytica. 2010;175:161–174. doi: 10.1007/s10681-010-0144-9. [DOI] [Google Scholar]
- Chen BY, Xu K, Li J, Li F, Qiao JW, Li H, Gao GZ, Yan GX, Wu XM (2014) Evaluation of yield and agronomic traits and their genetic variation in 488 global collections of Brassica napus L. Genet Resour Crop Evol 61: 979–999. 10.1007/s10722-014-0091-8
- Cong RH, Wang Y, Li XK, Ren T, Lu JW. Differential responses of seed yield and yield components to nutrient deficiency between direct sown and transplanted winter oilseed rape. Int J Plant Prod. 2020;14:77–92. doi: 10.1007/s42106-019-00069-1. [DOI] [Google Scholar]
- Chopin F, Orsel M, Dorbe MF, Chardon F,Truong HN, Miller AJ, Krapp A,Vedele FD (2007) The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 19: 1590–1602. 10.1105/tpc.107.050542 [DOI] [PMC free article] [PubMed]
- David LC, Berquin P, Kanno Y, Seo M, Daniel-Vedele F, Ferrario-Me´ry S, N availability modulates the role of NPF3.1, a gibberellin transporter, in GA-mediated phenotypes in Arabidopsis. Planta. 2016;244:1315–1328. doi: 10.1007/s00425-016-2588-1. [DOI] [PubMed] [Google Scholar]
- Ding GD, Zhao ZK, Liao Y, Hu YF, Shi L, Long Y, Xu FS. Quantitative trait loci for seed yield and yield-related traits and their responses to reduced phosphorus supply in Brassica napus. Ann Bot. 2012;109:747–759. doi: 10.1093/aob/mcr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa DN, Løvdal T, Olsen KM, Slimestad R, Lillo C (2009) The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta 230:747–754. 10.1007/s00425-009-0978-3 [DOI] [PubMed]
- Fontaine JX, Tercé-Laforgue T, Armengaud P, Clément G, Renou JP, Pelletier S, Catterou M, Azzopardi M, Gibon Y, Lea PJ, Hirel B, Duboise F (2012) Characterization of a NADH-dependent glutamate dehydrogenase mutant of Arabidopsis demonstrates the key role of this enzyme in root carbon and nitrogen metabolism. Plant Cell 24:4044–4065. 10.1105/tpc.112.103689 [DOI] [PMC free article] [PubMed]
- Garai S, Tripathy B (2018) Alleviation of nitrogen and sulfur deficiency and enhancement of photosynthesis in Arabidopsis thaliana by overexpression of uroporphyrinogen iii methyltransferase (UPM1). Front Plant Sci 8:2265. 10.3389/fpls.2017.02265 [DOI] [PMC free article] [PubMed]
- Gao JQ, Pu HM, Long WH, Hu ML, Zhang JF, Chen S, Qi CK (2019) Effects of nitrogen application rate on seed yield and its quality parameters of Brassica napus L. Jiangsu J Agric Sci 35:602–611. 10.3969/j.issn.1000-4440.2019.03.014
- Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327. 10.1126/science1182570 [DOI] [PubMed]
- Hanke GT, Okutani S, Satomi Y, Takao T, Suzuki A, Hase T. Multiple iso-proteins of FNR in Arabidopsis: evidence for different contributions to chloroplast function and nitrogen assimilation. Plant Cell Environ. 2005;9:1146–1157. doi: 10.1111/j1365-3040200501352x. [DOI] [Google Scholar]
- Hirel B, Tetu T, Lea PJ, Dubois F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability. 2011;3:1452–1485. doi: 10.3390/su3091452. [DOI] [Google Scholar]
- Jia ZT, Giehl RFH, von Wiren N (2020) The root foraging response under low nitrogen depends ondwarf1-mediated brassinosteroid biosynthesis. Plant Physiol 183:998–1010. 10.1104/pp.20.00440 [DOI] [PMC free article] [PubMed]
- Li HH, Zhang LY, Wang JK (2010) analysis and answers to frequently asked questions in quantitative trait locus mapping. ACTA AGRONOMICA SINICA 36: 918–931. 10.3724/SP.J.1006.2010.00918
- Liu TM, Liu HY, Zhang H, Xing YZ, Xing YZ. Validation and characterization of GHD71 a major quantitative trait locus with pleiotropic effects on spikelets per panicle plant height and heading date in rice (Oryza sativa L) J Integr Plant Biol. 2013;55:917–927. doi: 10.1111/jipb12070. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Zhang Y, Han WX, Tang AH, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang FS. Enhanced nitrogen deposition over China. Nature. 2013;494:459–462. doi: 10.1038/nature11917. [DOI] [PubMed] [Google Scholar]
- Liu WW, Sun Q, Wang K, Du QG, Li WX (2016) Nitrogen Limitation Adaptation (NLA) is involved in source-tosink remobilization of nitrate by mediating the degradation of NRT1.7 in Arabidopsis. New Phytolo 214:734–744. 10.1111/nph.14396 [DOI] [PubMed]
- Luo ZL, Wang M, Long Y, Huang YJ, Shi L, Zhang CY, Liu X, Fitt BDL, Xiang JX, Mason AS. Incorporating pleiotropic quantitative trait loci in dissection of complex traits: seed yield in rapeseed as an example. Theor Appl Genet. 2017;130:1–17. doi: 10.1007/s00122-017-3005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschners P. Marschners mineral nutrition of higher plants. 3. London: Academic Press; 2012. [Google Scholar]
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105:1141–1157. doi: 10.1093/aob/mcq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Lei, Li Huihui, Zhang Luyan, Wang Jiankang. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. The Crop Journal. 2015;3(3):269–283. doi: 10.1016/j.cj.2015.01.001. [DOI] [Google Scholar]
- Miersch S, Gertz A, Breuer F, Schierholt A, Becker HC. Influence of the semi-dwarf growth type on seed yield and agronomic parameters at low and high nitrogen fertilization in winter oilseed rape. Crop Sci. 2016;56:1573–1585. doi: 10.2135/cropsci2015090554. [DOI] [Google Scholar]
- Peng B, Li YX, Wang Y, Liu C, Liu ZZ, Tan WW, Zhang Y, Wang D, Shi YS, Sun BC, Song YC, Wang TY, Li Y. QTL analysis for yield components and kernel-related traits in maize across multi-environments. Theor Appl Genet. 2011;122:1305–1320. doi: 10.1007/s00122-011-1532-9. [DOI] [PubMed] [Google Scholar]
- Perchlik M, Tegeder M (2018) Leaf amino acid supply affects photosynthetic and plant nitrogen use efficiency under nitrogen stress. Plant Physiol 178:174–188. 10.1104/pp.18.00597 [DOI] [PMC free article] [PubMed]
- Qiu D, Morgan C, Shi J, Long Y, Liu J, Li R, Zhuang X, Wang Y, Dietrich E, Weihmann T, Everett C, Vanstraelen S, Beckett P, Fraser F, Trick M, Barnes S, Wilmer J, Schmidt R, Li J, Li D, Meng J, Bancroft I. A comparative linkage map of oilseed rape and its use for QTL analysis of seed oil and erucic acid content. Theor Appl Genet. 2006;114:67–80. doi: 10.1007/s00122-006-0411-2. [DOI] [PubMed] [Google Scholar]
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR (2009) Members of the lbd family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21:3567–3584. 10.1105/tpc.109.067041 [DOI] [PMC free article] [PubMed]
- Sarlikioti V, Visser PHB, Buck-Sorlin GH, Marcelis LFM. How plant architecture affects light absorption and photosynthesis in tomato: towards an ideotype for plant architecture using a functional-structural plant model. Ann Bot. 2011;108:1065–1073. doi: 10.1093/aob/mcr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L, Wang S, Zeng ZB. Composite interval mapping and multiple interval mapping: procedures and guidelines for using Windows QTL Cartographer. Methods Mol Biol. 2012;871:75–119. doi: 10.1007/978-1-61779-785-9_6. [DOI] [PubMed] [Google Scholar]
- Shi JQ, Li RY, Qiu D, Jiang CC, Long Y, Morgan C, Bancroft I, Zhao JY, Meng JL. Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics. 2009;182:851–861. doi: 10.1534/genetics109101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi TX, Wang SX, Shi L, Meng JL, Xu FS (2010) Effects of different nitrogen and phosphorus levels on seed yield and quality parameters of double high and double low Brasssica napus. J Plant Nutr Fertilizers 16:959–964.
- Su H, Wang T, Ju CF, Deng JP, Zhang TQ, Li MT, Tian H, Wang C (2020) Abscisic acid signaling negatively regulates nitrate uptake via phosphorylation of NRT1.1 by SnRK2s in Arabidopsis. J Integr Plant Biol 63:597–610. 10.1111/jipb.13057 [DOI] [PubMed]
- Wang SC, Bastern J, Zeng ZB. Windows QTL Cartographer 25 Department of Statistics. Raleigh: North Carolina State University; 2006. [Google Scholar]
- Wang J, Dun XL, Shi JQ, Wang XF, Liu GH, Wang HZ. Genetic dissection of root m-orphological traits related to nitrogen use efficiency in Brassica napus L under two contrasting nitrogen conditions. Front Plant Sci. 2017;8:1709. doi: 10.3389/fpls201701709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ding GD, White PJ, Wang M, Zou J, Xu FS, Hammond JP, Shi L (2020) Genetic dissection of the shoot and root ionomes of Brassica napus grown with contrasting phosphate supplies. Ann Bot 126:119–140. 10.1093/aob/mcaa055 [DOI] [PMC free article] [PubMed]
- Xu YF, Wang RF, Tong YP, Zhao HT, Xie QG, Liu DC, Zhang AM, Li B, Xu HX, An DG. Mapping QTLs for yield and nitrogen-related traits in wheat: influence of nitrogen and phosphorus fertilization on QTL expression. Theor Appl Genet. 2014;127:59–72. doi: 10.1007/s00122-013-2201-y. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yu XC, Song LF, An CC. ABI4 Activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol. 2011;156:873–883. doi: 10.1104/pp111175950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Nian JQ, Xie QJ, Feng J, Zhang FX, Jing HW, Zhang J, Dong GJ, Liang Y, Peng JL, Wang GD, Qian Q, Zuo JR (2016) Rice ferredoxin-dependent glutamate synthase regulates nitrogen–carbon metabolomes and is genetically differentiated between japonica and indica subspecies. Mol Plant 9:1520–1534. 10.1016/j.molp.2016.09.004 [DOI] [PubMed]
- Zhang Y, Thomas CL, Xiang J, Long Y, Wang X, Zou J, Luo ZL, Ding GD, Cai HM, Graham NS, Hammond JP, King GJ, White PJ, Xu FS, Broadley MR, Shi L, Meng JL (2016) QTL meta-analysis of root traits in Brassica napus under contrasting phosphorus supply in two growth systems. Sci Rep 6. 10.1038/srep33 [DOI] [PMC free article] [PubMed]
- Zhang YQ, Liu ZJ, Liu JP, Lin S, Wang JF, Lin WX , Xu WF (2017) GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep 36:557–569. 10.1007/s00299-017-2102-7 [DOI] [PubMed]
- Zhang Z, Qiao Y, Liu DH, Chen YF, Hu C, Li SL (2020) Effects of high boron stress on the absorption and distribution of trace elements in Newhall navel orange plants. Soils and Fertilizers Sciences in China 2:140–145. 10.11838/sfsc.1673-6257.19185
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets supporting the results of this article are included within the article and its additional files.
Not applicable.