Abstract
Rice grain size is a key determinant of both grain yield and quality. In this study, we conducted QTL mapping on grain size using a recombinant inbred line (RIL) population derived from a cross between japonica variety Beilu130 (BL130) and indica variety Jin23B (J23B). A total of twenty-two QTL related to grain length (GL), grain width (GW), grain length-to-width ratio (LWR), grain thickness (GT), and thousand grain weight (TGW) were detected under two environments, and 14 of them were repeatedly detected. Two minor QTL, qTGW2b and qGL9, were validated and further delimited to regions of 631 kb and 272 kb, respectively. Parental sequence comparison of genes expressed in inflorescence in corresponding candidate regions identified frameshifts in the exons of LOC_Os02g38690 and LOC_Os02g38780, both of which encode protein phosphatase 2C-containing protein, and LOC_Os09g29930, which encodes a BIM2 protein. Scanning electron microscopy (SEM) analysis revealed that the increase of cell size rather than cell number caused the differences in grain size between NILs of qTGW2b and qGL9. Quantitative RT-PCR analysis showed that the expression levels of EXPA4, EXPA5, EXPA6, EXPB3, EXPB4, and EXPB7 were significantly different in both qTGW2b NILs and qGL9 NILs. Our results lay the foundation for the cloning of qTGW2b and qGL9, and provide genetic materials for the improvement of rice yield and quality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-022-01328-2.
Keywords: Rice, RIL, Grain size-related traits, QTL, qTGW2b, qGL9
Background
Rice (Oryza sativa L.) is a direct source of calories for more people than other crops, and it serves as the main food for about 560 million chronically hungry people (Seck et al. 2012; WFP et al. 2017). Thus, increasing rice yield is of great necessity to ensure food security, and has been the primary goal of scientists and breeders in the past few decades. Grain weight, a major component of rice yield, is directly influenced by grain size, which is further charactered by grain length (GL), grain width (GW), length-to-width ratio (LWR), and grain thickness (GT) (Tan et al. 2000). Lots of previous studies demonstrated that the four factors of grain size are quantitative traits and are controlled by many quantitative trait loci (QTL) or genes. Therefore, mapping and cloning novel QTL for grain size is of great significance to have a comprehensive understanding of grain size regulation, and could provide valuable gene resource to facilitate improvement of grain size and yield in rice breeding.
Till now, large numbers of QTL for grain size have been mapped, and some major QTL or genes have been successfully cloned and charactered. Among those, genes encoding subunits of G protein have been proved to have effects on grain size, such as GS3, DEP1, OsGGC2, RGG1, RGG2, OsRGB1, and RGA1 (Fan et al. 2006; Fujisawa et al. 1999; Miao et al. 2019; Sun et al. 2018; Tao et al. 2020; Zhang et al. 2021a; Zhou et al. 2009). Some components of the MAPK pathway have been shown to play important roles in regulating grain size. OsMAPK6, OsMAPKK4 (SMG1), and OsMAPKKK10 (SMG2) form a cascade to positively regulate grain size in rice (Duan et al. 2014; Liu et al. 2015b; Xu et al. 2018), and OsMAPK1 (SNG1) decreases the effect of the cascade on grain size by deactivating OsMAPK6 (Guo et al. 2018; Xu et al. 2018). The ubiquitin–proteasome degradation pathway is also involved in grain size regulation in rice. Both GW2 and CLG1 encode RING-type E3 ubiquitin ligases, but have opposite effects on regulation of grain size (Song et al. 2007; Yang et al. 2021). Phytohormone signaling-related genes regulate grain size by controlling cell proliferation and expansion. TGW3 and TGW6 negatively affect grain size by controlling cell size and number through the auxin signaling pathway (Hu et al. 2015; Ishimaru et al. 2013). GW6 encodes a GA-regulated GAST family protein that positively regulates grain width by controlling cell elongation (Shi et al. 2020). GS5, GW5, and GS9 affect the BR signaling pathway and consequently result in grain size changes (Li et al. 2011; Liu et al. 2017; Zhao et al. 2018). Besides, two major transcription factor families have been shown to regulate grain size. One is the basic helix-loop-helix (bHLH) family (An-1, OsbHLH079, and OsbHLH107) (Seo et al. 2020; Yang et al. 2018), and the other is the SQUAMOSA promotor binding protein-like (SPL) family (GLW7/OsSPL13, GW8/OsSPL16, and OsSPL18) (Si et al. 2016; Wang et al. 2012; Yuan et al. 2019).
Improving grain size is an efficient way to increase grain weight. As previously reported, GL3.3, GS2, BG1, SMG1, and SMG2 positively regulate grain length and grain width to increase grain size and grain weight (Che et al. 2015; Duan et al. 2014; Liu et al. 2015a; Xia et al. 2018; Xu et al. 2018). In contrast, GS3 and GSN1 act as negative regulators of grain length and width, resulting in decreased grain size and weight (Fan et al. 2006; Guo et al. 2018). GL3.1, qTGW2, and GW2 negatively regulate grain width to reduce grain weight (Qi et al. 2012; Ruan et al. 2020; Song et al. 2007), whereas GS5 and OsSPL18 positively regulate grain width, and thereby led to the increase in grain weight (Li et al. 2011; Yuan et al. 2019). GW5 showed increased length-to-width ratios, but with opposite effects on grain weight (Liu et al. 2017).
In this study, QTL mapping of rice grain size-related traits was performed in two years using a RIL population derived from a cross between japonica rice variety BL130 and indica rice variety J23B. Two minor QTL, qTGW2b and qGL9, were repeatedly detected under 2 years and fine mapped by recombinant progeny testing. To identify the candidate gene underlying two QTL, parental sequence comparison of genes expressed in inflorescence within candidate intervals was performed. Additionally, the cell size and cell number of spikelet lemma and the expression levels of cell expansion-related genes in both qTGW2b NILs and qGL9 NILs were examined. The results of this study will lay a foundation for cloning of qTGW2b and qGL9, and will be helpful for breeding applications.
Materials and methods
Plant materials and field experiment
The RIL population consisting of 184 lines was derived from a cross between BL130 as the donor parent and J23B as the recipient parent. BL130 is a japonica variety, which has short and round grains and large TGW. J23B is a widely used maintainer indica variety of three-line hybrid rice, with slender grain shape and small TGW. The parents and RILs of F6 and F7 generations were planted at the Lingshui experimental station in Hainan Province from December 2011 to April 2012 and at the Wuhan experimental station in Hubei Province from May to September 2012, respectively.
In order to validate the genetic effect of qGL9 and qTGW2b, RILs that carry the corresponding homozygous region from BL130 were backcrossed with the recurrent parent J23B for 4 times, and each QTL was selected by two flanking markers at every generation. The BC4F2 population, which was defined as the NIL-F2 population, was generated from a self-pollinated BC4F1 plant with heterozygous fragment in the QTL mapping interval. On May 25, 2016, the NIL-F2 populations of qTGW2b and qGL9 were planted at the Wuhan experimental station in Hubei Province with each population consisting of 192 individuals.
Recombinants were screened and used for progeny testing. For qTGW2b, a total of 450 recombinants were screened between markers RM262 and RM263 in a BC4F2 population consisting of 2000 individuals. In terms of qGL9, 184 recombinants were screened between markers RM566 and RM288 from a BC4F2 population consisting of 2000 individuals. Fine mapping of qTGW2b and qGL9, a small population containing 96 individuals was developed from each recombinant planted at the Wuhan experimental station in Hubei Province from May to September 2017.
Seedlings of 25 ~ 30 days after sowing were transplanted with a single plant spacing of 16.5 cm and 26.4 cm between rows in the field. Field management followed local practices.
Phenotyping
For each RIL line, eight individuals with a similar plant architecture were harvested in the middle of each row for measuring grain size-related traits. All plants were genotyped and harvested for QTL validation in the NIL-F2 populations of qTGW2b and qGL9. In order to fine map qTGW2b and qGL9, each plant of recombinant progeny line was genotyped and harvested.
Before measurement, harvested grains were air dried at room temperature for at least 3 months to maintain a consistent water balance. To analyze the performance of grain size-related traits in the RIL population, GL, GW, and GT of 10 full seeds were measured by vernier calipers, LWR was obtained from GL divided by GW, and the weight of 200 full grains was measured for calculating TGW. The GL, GW, and TGW performance of NIL-F2 populations and recombinant progeny lines were measured by high-throughput rice phenotyping facility (HRPF) according to the method reported previously (Yang et al. 2014).
Genotyping and sequence analysis
A total of 201 evenly distributed polymorphic markers were used to genotype 184 RILs as previously report with slight modifications (Yun et al. 2016). Genotype of the NIL-F2 populations and recombinant progeny lines of qTGW2b and qGL9 were identified by two flanking markers of QTL mapping interval. Other 9 and 3 InDel markers, which were named as M1–M9 and M21–M23, were developed to genotype the recombinants of qTGW2b and qGL9, respectively, and the relevant primers can be found in Table S1. The genotyping was performed with 4% polyacrylamide gel (PAGE) migration as previously reported (Panaud et al. 1996).
Sequencing of parental varieties BL130 and J23B was performed using Illumina HiSeq2000 (Illumina, San Diego, CA, USA), and the sequencing data were compared and assembled based on the rice reference genome (Rice Genome Annotation Project, http://rice.uga.edu/, 06/02/2022) (Li et al. 2009). Parental sequence variation analysis of genes expressed in inflorescence was performed according to the sequencing data of two parents.
Progeny testing analysis
Progeny testing was conducted in the progeny segregation population of each recombinant. In the progeny line of each recombinant, Student’s t test was performed to compare the grain size differences between plants with homozygous J23B allele and homozygous BL130 allele. If the p value was less than 0.05, the candidate gene was considered to be located in the heterozygous fragment; otherwise, it was considered to be located in the homozygous fragment.
Genetic map construction and QTL analysis
The genetic map was constructed using MapMaker/Exp3.0 software with the Kosambi mapping function (Lander et al. 1987). QTL detection of RILs was performed by composite interval mapping (CIM) method using Windows QTL cartographer 2.5 software (Wang et al. 2005) with a threshold of LOD score 2.4 (selected by permutation test based on 1000 runs, P = 0.05), and the genetic linkage map was generated using the LinkageMapView package of R software.
RNA extraction, reverse transcription, and quantitative RT-PCR
Total RNA was extracted from 8-cm-young panicles using RNA extraction kit (TRIzol, Invitrogen, Carlsbad, CA, USA). Approximately 2 μg of total RNA was used as a template for reverse transcription to generate cDNA, total RNA was pretreated with DNase I (Invitrogen), and first-strand cDNA was synthesized using oligo (dT)18 as primer (Promega, Madison, WI, USA). Real-time PCR was carried out using Bio-Rad T100™ real-time PCR system (Bio-Rad, Hercules, CA, USA) with the SYBR Green I mix (TaKaRa, Shiga, Japan) on the QuantStudio6Flex instrument (Applied Biosystems, Carlsbad, CA, USA). All tests were performed at least three biological repeats. OsActin1 was used as an internal control, and relative expression level was calculated by 2−ΔΔCt. The relevant primers for this analysis are listed in Table S2.
Scanning electron microscopy (SEM) analysis
For scanning electron microscopy, lemmas of pre-headed spikelet (4 days before heading) were collected and coated with gold under vacuum conditions. The morphology of lemma cell was observed by scanning electron microscope (JSM-6390LV, Jeol Ltd., Tokyo, Japan) under 10-kV acceleration voltage and a 30-nm spot size. Cell number and cell size were calculated at 40 × and 65 × magnification, respectively. Scanning electron microscopy analysis was based on at least three biological replications of mounted specimens.
Results
Phenotypic variation of grain size-related traits in RILs
The performance of GL, GW, LWR, GT, and TGW of parents and RILs was analyzed in 2 years. Compared with J23B, BL130 showed larger values in GL, GW, GT, and TGW, but smaller values in LWR (Fig. S1a-f). In the RIL population, all these traits showed transgressive segregation and followed normal distribution in both years (Fig. S1b-f). A similar grain size performance of parents and RILs was observed in two years.
Correlation analysis was conducted for these traits. The correlation coefficients ranged from − 0.88 to 0.87 in year 2011 and − 0.88 to 0.88 in year 2012 (Fig. S2). TGW displayed significantly positive correlation with GL, GW, and GT, but negative correlation with LWR. GW displayed significantly positive correlation with GT but negative correlation with LWR. LWR displayed significantly negative correlation with GT. In addition, highest correlation coefficients were detected among GW, GT, and TGW.
QTL mapping of grain size-related traits
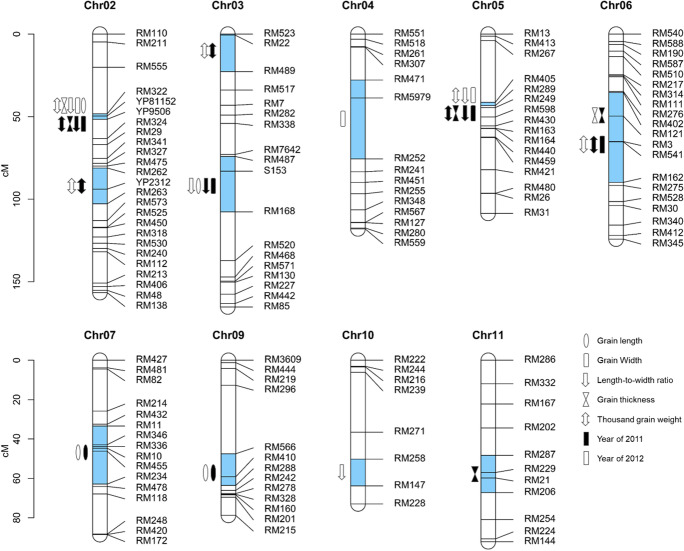
A total of 22 QTL related to grain size were detected on 9 chromosomes in 2 years, and the phenotypic variation explained by each QTL ranged from 3.8 to 57.1% (Fig. 1, Table 1).
Fig. 1.
Genetic linkage map of grain size-related traits in RILs. The blue boxes on the chromosome columns mean intervals of corresponding QTL. The ruler on the left side showed the genetic distance of chromosomes
Table 1.
Grain size-related QTL detected in the RIL population
| Traits | QTL | Interval | 2011 | 2012 | ||||
|---|---|---|---|---|---|---|---|---|
| LOD | A | V (%) | LOD | A | V (%) | |||
| GL | qGL2 | RM322-YP9506 | 5.7 | 0.19 | 13.9 | |||
| GL | qGL3 | RM487-RM168 | 5.2 | − 0.18 | 11.1 | |||
| GL | qGL7 | RM11-RM234 | 4.7 | − 0.20 | 11.8 | 3.4 | − 0.15 | 7.2 |
| GL | qGL9 | RM566-RM288 | 3.0 | − 0.14 | 6.1 | 3.6 | − 0.15 | 6.4 |
| GW | qGW2 | RM322-YP9506 | 32.7 | 0.25 | 49.0 | 27.3 | 0.26 | 44.5 |
| GW | qGW3 | RM487-RM168 | 5.1 | 0.09 | 5.8 | |||
| GW | qGW4 | RM471-RM252 | 4.2 | 0.09 | 6.8 | |||
| GW | qGW5 | RM405-RM289 | 13.1 | 0.17 | 21.5 | 10 | 0.19 | 23.1 |
| GW | qGW6 | RM121-RM162 | 3.7 | 0.08 | 4.8 | |||
| LWR | qLWR2 | RM322-YP9506 | 16.3 | − 0.19 | 26.8 | 15.2 | − 0.18 | 24.7 |
| LWR | qLWR3 | RM487-RM168 | 5.7 | − 0.12 | 8.9 | 4.7 | − 0.10 | 8.0 |
| LWR | qLWR5 | RM405-RM289 | 9.5 | − 0.18 | 22.9 | 10.3 | − 0.19 | 23.2 |
| LWR | qLWR10 | RM258-RM147 | 4.3 | − 0.10 | 6.9 | |||
| GT | qGT2 | RM322-YP9506 | 33.8 | 0.11 | 57.1 | 26.9 | 0.10 | 44.5 |
| GT | qGT5 | RM405-RM289 | 3.9 | 0.03 | 5.7 | |||
| GT | qGT6 | RM402-RM3 | 4.9 | 0.04 | 6.7 | 4.2 | 0.03 | 5.8 |
| GT | qGT11 | RM287-RM206 | 2.9 | − 0.03 | 4.0 | |||
| TGW | qTGW2a | RM322-YP9506 | 25.3 | 3.10 | 41.9 | 28.2 | 3.41 | 45.2 |
| TGW | qTGW2b | RM262-RM263 | 4.5 | 1.27 | 8.7 | 4.0 | 1.18 | 6.3 |
| TGW | qTGW3 | RM523-RM489 | 3.2 | − 1.00 | 3.9 | 3.5 | − 1.04 | 3.8 |
| TGW | qTGW5 | RM405-RM289 | 4.4 | 1.24 | 9.0 | 2.8 | 1.22 | 6.1 |
| TGW | qTGW6 | RM121-RM162 | 3.1 | 1.00 | 3.9 | 3.4 | 1.12 | 4.7 |
2011 and 2012 means 2011 Hainan and 2012 Wuhan, respectively
GL grain length, GW grain width, LWR length-to-width ratio, GT grain thickness, TGW thousand-grain weight, QTL quantitative trait locus, LOD logarithms of odds, A additive effect (the negative value means that J23B allele increases the trait value), V variance (phenotypic variation explained by the QTL)
Four QTL for GL were detected, of which two were repeatedly detected in 2 years. qGL7 had an additive effect of 0.20 mm in 2011 and 0.15 mm in 2012, and explained 11.8% and 7.2% of the phenotypic variation, respectively. qGL9 had an additive effect of 0.14 mm in 2011 and 0.15 mm in 2012, and explained 6.1% and 6.4% of the phenotypic variation, respectively. The enhanced alleles of both qGL7 and qGL9 were derived from J23B. The remaining two QTL were only detected in year 2012.
Five QTL for GW were detected, and two major QTL, qGW2 and qGW5, were repeatedly detected in 2 years. qGW2 had an additive effect of 0.25 mm in 2011 and 0.25 mm in 2012, and qGW5 had an additive effect of 0.17 mm in 2011 and 0.19 mm in 2012, respectively. Among the remaining three QTL, qGW3 and qGW6 were detected in year 2011, and qGW4 was detected in year 2012. The enhanced alleles of all five QTL were derived from BL130.
Four QTL for LWR were detected. Among those, two major QTL, qLWR2 and qLWR5, and a minor QTL qLWR3 were repeatedly detected in 2 years. qLWR2 and qLWR5 had additive effects of 0.19 and 0.18 in 2011 and 0.18 and 0.19 in 2012, respectively. qLWR3 had an additive effect of 0.12 in 2011 and 0.10 in 2012, respectively. qLWR10, the minor QTL detected in year 2012, had an additive effect of 0.10. The enhanced alleles of all four QTL were derived from J23B.
Four QTL for GT were detected, of which two were repeatedly detected in 2 years. The major QTL qGT2 had an additive effect of 0.11 mm in 2011 and 0.10 mm in 2012, and explained 57.1% and 44.5% of the phenotypic variation, respectively. qGT6, a minor QTL, had an additive effect of 0.04 mm in 2011 and 0.03 mm in 2012, and explained 6.7% and 5.8% of the phenotypic variation, respectively. The enhanced alleles of both qGT2 and qGT6 were derived from BL130. The remaining two minor QTL were only detected in year 2011.
Five QTL for TGW were detected, of which all were repeatedly detected in 2 years. The major QTL qTGW2a had an additive effect of 3.10 g in 2011 and 3.41 g in 2012, and explained 41.9% and 45.2% of the phenotypic variation, respectively. The other four had minor effects on TGW. The enhanced alleles of qTGW2a, qTGW2b, qTGW5, and qTGW6 were derived from BL130, whereas that of qTGW3 was derived from J23B.
Among the QTL detected above, many were co-located to four intervals conferring grain size and weight. The RM322–YP9506 interval on chromosome 2 and RM405-RM289 interval on chromosome 5 showed consistent effects on GW, LWR, GT, and TGW in 2 years. The RM487-RM168 interval on chromosome 3 exhibited significant effect on GW and LWR in 2011 and on GL and LWR in 2012, respectively. The RM121-RM162 interval on chromosome 6 had significant effect on GW and TGW in 2011 and on TGW only in 2012, respectively. Since many cloned genes related to grain size were found located to the interval of major QTL identified in this study (Ishimaru et al. 2013; Li et al. 2011; Liu et al. 2017; Song et al. 2007; Wang et al. 2015; Xia et al. 2018), two minor QTL, qTGW2b and qGL9, were selected for further fine mapping.
Validation and fine mapping of qTGW2b
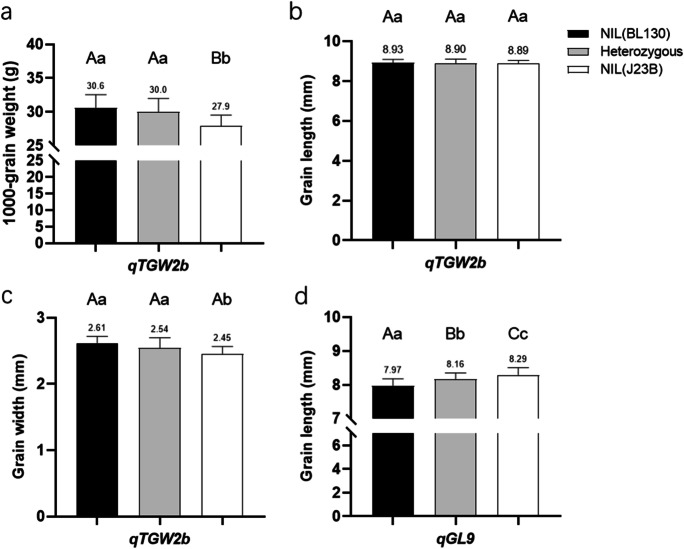
In order to validate the genetic effect of qTGW2b, we developed a NIL-F2 population for qTGW2b and reanalyzed its effect on grain size-related traits. A QTL analysis showed that qTGW2b explained about 12.3% of the TGW variation and 8% of the GW variation, and the allele from BL130 increased both TGW and GW (Table 2). Significant differences were found in TGW and GW between NIL(J23BqTGW2b) and NIL(BL130qTGW2b) while no difference was detected in GL and GT (Fig. 2a-c).
Table 2.
Genetic effect of qTGW2b and qGL9 in NIL-F2 population
| QTL | Trait | Interval | LOD | A | D | V (%) |
|---|---|---|---|---|---|---|
| qTGW2b | TGW | RM262-RM263 | 6.80 | 0.99 | 0.86 | 12.3 |
| qTGW2b | GW | RM262-RM263 | 3.35 | 0.06 | 0.03 | 8.0 |
| qGL9 | GL | RM288-RM257 | 11.24 | − 0.18 | 0.03 | 32.0 |
TGW thousand-grain weight, GL grain length, QTL quantitative trait locus, LOD logarithms of odds, A additive effect (the negative value means that J23B allele increases the trait value), V variance (phenotypic variation explained by the QTL)
Fig. 2.
Grain size difference among three haplotypes in the NIL-F2 population of qTGW2b and qGL9. a Thousand-grain weight difference of qTGW2b. b Grain length difference of qTGW2b. c Grain width difference of qTGW2b. d Grain length difference of qGL9. Numbers above bars represent the mean value. Different capital letters and small letters mean significant differences at P < 0.01 and P < 0.05 level, respectively, Duncan’s multiple range test
Furthermore, the TGW and GW of heterozygous lines were similar to that of NIL(BL130qTGW2b), indicating the complete dominant effect on GW and TGW of qTGW2b.
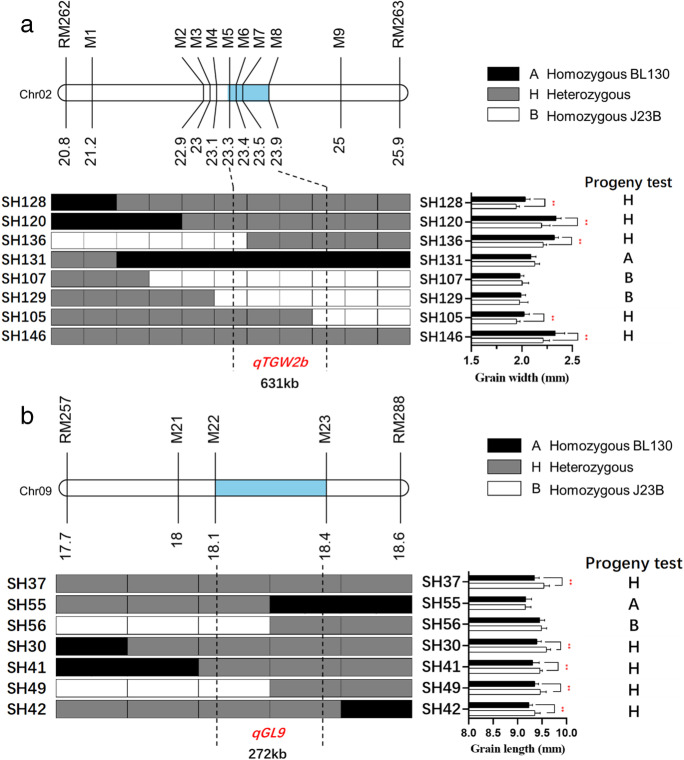
In order to fine map qTGW2b, we developed nine new polymorphic InDel markers (M1 to M9) in the region flanked by markers RM262 and RM263, which divided the target region into 10 subregions. Five recombinants of each subregion were selected for progeny testing (Fig. 3a). TGW is a complex trait influenced by grain size-related traits (Che et al. 2015; Duan et al. 2014; Fan et al. 2006; Guo et al. 2018; Qi et al. 2012). Due to the significant difference in GW but not GL between NILs of qTGW2b, we used GW as the target trait to conduct fine mapping of qTGW2b. Results of progeny testing showed that recombinants SH120, SH128, and SH136 carried heterozygous genotype of qTGW2b, meaning that qTGW2b should be located in the region downstream of marker M5 (Fig. 3a). SH105 showed heterozygous genotype of qTGW2b, indicating that qTGW2b should be located in the region upstream M8. The homozygous genotypes of SH107, SH129, and SH131 confirm these results. Therefore, qTGW2b was finally delimited into the 631-kb region between markers M5 and M8.
Fig. 3.
Fine mapping of qTGW2b (a) and qGL9 (b). The progeny test results represented the genotypes reflected by the phenomenon differences in recombinant progeny; if the p value was less than 0.05, the candidate gene was considered to be located in the heterozygous fragment; otherwise, it was considered to be located in the homozygous fragment. Black, white, and grey blocks represent the genotypes of homozygous BL130, homozygous J23B, and heterozygote, respectively. The blue boxes on the chromosome columns mean the QTL mapping interval of corresponding QTL
According to the annotation information and RNA-seq data of Rice Genome Annotation Project website (RGAP, Rice Genome Annotation Project (uga.edu)), 44 genes were found to be expressed in inflorescence within the qTGW2b region. SNPs and InDels existing in the qTGW2b interval were screened by whole-genome sequencing results, and the genes at the loci causing frameshift were preferentially selected as candidate genes. There was a deletion (2 bp) and two insertions (7 bp and 13 bp) in the J23B genome of LOC_Os02g38690 and LOC_Os02g38780, respectively (Table S3). Both the two genes encode protein phosphatase 2C (PP2C) containing proteins.
Validation and fine mapping of qGL9
In order to validate the genetic effect of qGL9, we developed a NIL-F2 population, which was used to reanalyze its effect on GL. The qGL9 allele from J23B had an additive effect of 0.18 mm and explained 32% of the phenotypic variation (Table 2). Significant GL differences were observed among lines carrying different genotypes of qGL9, suggesting an incomplete dominant effect on GL (Fig. 2d).
To conduct fine mapping of qGL9, we developed three new polymorphic markers (M21 to M23) in the region flanked by markers RM257 and RM288, which divided the target region into 4 subregions. Five recombinants of each subregion were selected for progeny testing (Fig. 3b). Results of progeny testing showed that recombinants SH37, SH30, SH41, SH49, and SH42 carried heterozygous genotype of qGL9, meaning that qGL9 should be located in the region downstream of marker M22. SH55 and SH56 carried homozygous genotype, indicating that qGL9 should be located in the region upstream M23. Therefore, qGL9 should be located in the 272-kb region between markers M22 and M23.
According to the RGAP website, 20 genes were found to be expressed in the inflorescence within the 272-kb region. SNPs and InDels existing in the qGL9 interval were screened by whole-genome sequencing results, and the genes at the loci causing frameshift were preferentially selected as candidate genes. In J23B genome, a cytosine (C) to thymine (T) substitution was found in the exon of LOC_Os09g29800 leading to an early translation termination, and a cytosine (C) deletion in the exon of LOC_Os09g29930 resulted in a frameshift (Table S3). LOC_Os09g29800 encodes an expressed protein. LOC_Os09g29930 encodes a transcription factor BIM2 (BES1 Interacting Myc-like protein 2), which belongs to the bHLH protein family and has been shown to affect the grain size of rice (Seo et al. 2020; Yang et al. 2018).
qTGW2b affects grain width by influencing cell width of spikelet
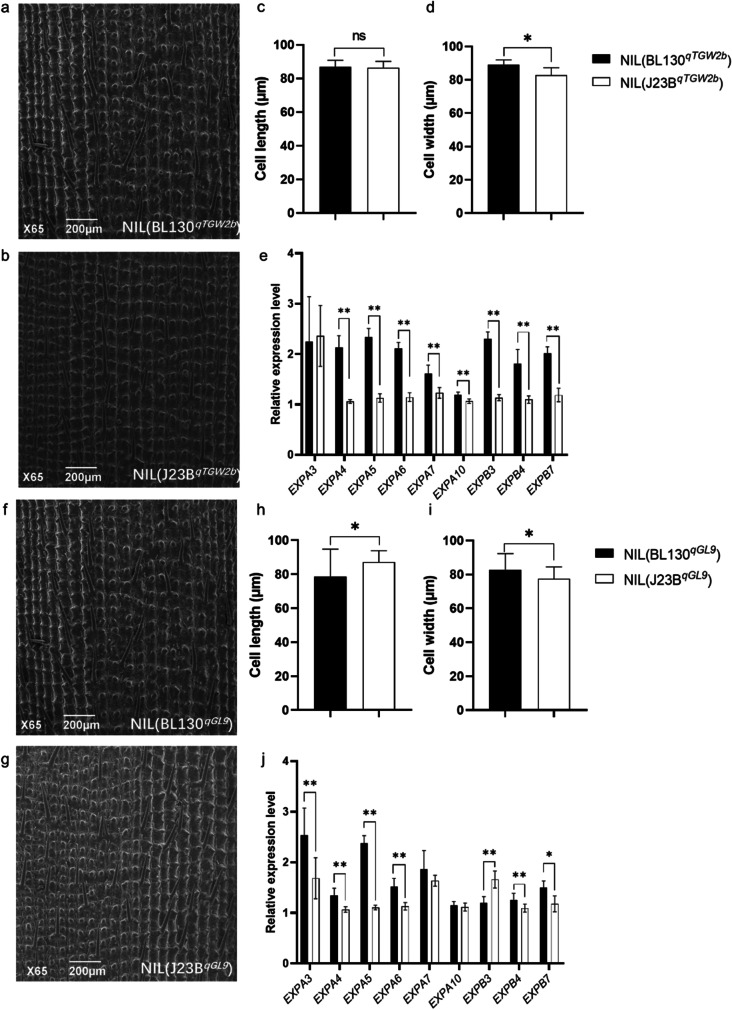
In general, changes in grain size are usually accompanied with differences in cell size and/or cell number. To investigate the reason of the significant difference in grain size between NILs of qTGW2b (Fig. 2), we conducted cytological observation of the spikelet lemma using scanning electron microscopy (SEM). The cell width of NIL(BL130qTGW2b) was significantly larger than that of NIL(J23BqTGW2b) while no difference was observed in cell length and cell number of the two NILs (Figs. 4a-d and S3a-b). As expansion proteins are the only ones that directly induce cell wall extension, many studies showed that the expression of expansion protein genes is associated to grain size (Che et al. 2015; Lizana et al. 2010; Wang et al. 2019). Therefore, we analyzed the expression of cell expansion protein genes in the 8-cm-young panicles of the two NILs by quantitative RT-PCR. A total of 9 cell expansion protein genes were analyzed (Table S2). Results showed that expression levels of eight genes, namely EXPA4, EXPA5, EXPA6, EXPA7, EXPA10, EXPB3, EXPB4, and EXPB7, were significantly higher in NIL(BL130qTGW2b) than in NIL(J23BqTGW2b) (Fig. 4e). These results suggest that the qTGW2b allele from BL130 upregulates the expression level of cell expansion-related genes, resulting in the increased cell width of spikelet and finally the increase in GW.
Fig. 4.
Difference in cell size and relative expression level of cell expansion-related genes between NILs of qTGW2b and qGL9 a SEM observation on the lemma of NIL(BL130qTGW2b). b SEM observation on the lemma of NIL (J23BqTGW2b). c Cell length difference between NILs of qTGW2b. d Cell width difference between NILs of qTGW2b. e qRT-PCR analysis of cell expansion-related genes in NILs of qTGW2b. f SEM observation on the lemma of NIL(BL130qGL9). g SEM observation on the lemma of NIL (J23BqGL9). h Cell length difference between NILs of qGL9. i Cell width difference between NILs of qGL9. j qRT-PCR analysis of cell expansion-related genes in NILs of qGL9. * and ** mean significant differences at P < 0.05 and P < 0.01, respectively, Student’s t tests
qGL9 affects grain length by influencing spikelet cell length
To uncover the reason underlying the significant difference in GL between NILs of qGL9 (Fig. 2), we analyzed cell size and cell number differences of spikelet lemma using scanning electron microscopy (SEM). NIL(J23BqGL9) had significantly longer and narrower cell size than NIL(BL130qGL9) (Fig. 4f-i) while no difference was observed in cell number (Fig. S3c-d). We then examined the expression level of cell expansion protein genes in the 8-cm-young panicles of two NILs by quantitative RT-PCR (Table S2). Among the 9 expansion protein genes analyzed, the expression levels of EXPA3, EXPA4, EXPA5, EXPA6, EXPB4, and EXPB7 were significantly higher in NIL(BL130qGL9) than in NIL(J23BqGL9), while that of EXPB3 acted in the opposite direction (Fig. 4j). These results indicate that qGL9 upregulates the expression levels of cell expansion-related genes, resulting in the increased cell length of spikelet and finally the increase in GL.
Discussion
Pyramiding superior alleles contribute to larger grain size and grain weight
It is well known that indica varieties usually show slender grains, while japonica varieties exhibit shorter and wider grains. Populations derived from crosses between indica and japonica showed vast variations on grain size, and many related QTL were identified (Chen et al. 2019; Yin et al. 2015; Zhou et al. 2019). In this study, QTL mapping for grain size-related traits was carried out using a RIL population derived from a cross between indica variety J23B and japonica variety BL130. A total of 22 QTL on 9 chromosomes were found to control five grain size-related traits, and 14 of them were detected in 2 years. Among those, most of the enhanced alleles of QTL for GL and LWR were derived from J23B that displayed a slender grain size, while those for GW, GT, and TGW were derived from BL130 that produced wider and heavier grains, suggesting that pyramiding of superior alleles plays a crucial role in controlling grain size and grain weight.
QTL mapping of grain size-related traits
Many cloned genes related to grain size were found in the same region with the QTL detected in this study. qGL2, qGW2, qLWR2, qGT2, and qTGW2a were co-mapped between markers RM322 and YP9506, and a cloned gene GW2 was found in this interval (Song et al. 2007). As previously report, a 1-bp deletion in the fourth exon of GW2 led to an increase of GW and TGW, which was also found in BL130 that had a larger GW and TGW. Another QTL cluster in the RM487-RM168 interval was found to contribute to GL, GW, and LWR, and the cloned GL3.1 was found to be located in this interval (Qi et al. 2012). The multi-effect interval between markers RM405 and RM289 was found to affect GW, LWR, GT, and TGW, and two cloned genes GS5 (Li et al. 2011) and GW5/qSW5 (Liu et al. 2017) located in this interval were found to affect grain size. Moreover, GS6 and GL7 were found to be located in the qGW6/qTGW6 and qGL7 intervals, respectively (Sun et al. 2013; Wang et al. 2015). Taken together, QTL mapping using RIL populations is a solid method for detecting QTL in rice. However, with the increase of the number of related genes cloned in the QTL mapping interval, the efficiency of QTL mapping is greatly reduced.
Interestingly, grain size-related traits seem to also have effect on rice quality. In present study, the RM322-YP9506 interval on chromosome 2, which had effect on GL, GW, GT, LWR, and TGW, was also found to affect white-belly rate and white-core rate of chalkiness in a previous study, and qTGW3, qGW5/qLWR5/qGT5/qTGW5, qGT6, qGW6/qTGW6, and qGL7 were co-mapped in same regions with qWCR3, qWBR5, qWCR6, qWBR6, and qWBR7 (Yun et al. 2016). Previous studies have shown that chalkiness is usually negatively correlated with grain length and positively correlated with grain width (Gong et al. 2017; Li et al. 2014; Xie et al. 2013). These findings provide evidences that grain size-related QTL may also be used in rice chalkiness improvement.
PP2C domain containing proteins and a putative BIM transcription factor could be the candidate gene underlying qTGW2b and qGL9, respectively
Many studies have shown that grain weight is influenced by grain length, grain width, and grain thickness (Che et al. 2015; Duan et al. 2014; Guo et al. 2018; Li et al. 2011; Liu et al. 2015a; Qi et al. 2012; Ruan et al. 2020; Song et al. 2007; Xia et al. 2018; Xu et al. 2018). In the present study, highest correlations were found between GW, GT, and TGW, and both TGW and GW differences were detected in the NIL-F2 population of qTGW2b, implying that both GW and GT were key traits affecting TGW, and the effect of qTGW2b on TGW was mediated by GW. qTGW2b was further fine mapped to 631-kb interval by recombinant progeny testing. By comparing the parental sequences of 44 genes expressed in inflorescences within this interval, frameshift mutations were found in exon of LOC_Os02g38690 and LOC_Os02g38780 in the J23B genome, both of which encoded protein phosphatase 2C (PP2C) containing protein. As previously reported, OsGL3.1/OsGL3 encoded a putative protein phosphatase with Kelch-like repeat domain (OsPPKL1) that have been shown to affect grain size and yield of rice (Qi et al. 2012). In soybean, PP2C-1 was associated with GmBZR1 to control grain weight and grain size (Lu et al. 2017). These indicated that LOC_Os02g38690 and LOC_Os02g38780 could be the candidate gene underlying qTGW2b to control GW and TGW. In the future, transgenic studies on these two genes will be carried out to verify the correctness of the candidate gene.
qGL9 was fine mapped to 272-kb interval by progeny testing. By comparing the parental sequence of 20 genes expressed in inflorescences within this interval, a premature translation termination and a frameshift were found in exon of LOC_Os09g29800 and LOC_Os09g29930 of the J23B genome, respectively. LOC_Os09g29800 encodes an expressed protein. LOC_Os09g29930 encodes a putative BIM2 transcription factor which belongs to the bHLH protein family. Previous studies have shown that overexpression of OsbHLH107 increases grain size (Yang et al. 2018), and OsbHLH079 acts as a positive regulator of BR signaling to determine leaf angle and grain size (Seo et al. 2020). BRs have been reported to play a key role in regulating cell expansion and division (Zhang et al. 2021b, 2022). Therefore, LOC_Os09g29930 may be the candidate gene controlling GL within the qGL9 interval. In the future, genetic transformation experiments will be used to verify the correctness of the candidate gene.
Cell size determinates grain size
During the early developmental stages, extensive cell division occurs in the grain hull to increase the number of cells. Subsequently, the cell division gradually slows down, and the cells start to expand to increase the cell size (Li et al. 2018). Cell expansion protein was identified to have the ability to induce wall extension (Yu et al. 2011; Zou et al. 2015). In present study, the qTGW2b allele from BL130 promoted the expression of cell expansion protein genes, which led to the increase of cell width, and finally led to the increase of grain width in NIL(BL130qTGW2b). In terms of qGL9, NIL(BL130qGL9) showed a longer and slender cell shape. Meanwhile, NIL(BL130qGL9) had higher expression levels of EXPA3, EXPA4, EXPA5, EXPA6, EXPB4, and EXPB7 and lower expression level of EXPB3 than that of NIL(J23BqGL9). These indicated that qGL9 regulates grain size by affecting the expression of cell expansion protein genes, but EXPB3 may play different roles in regulating cell length and width.
Conclusion
Rice grain size is a key determinant of quality and yield. Using a RIL population derived from a cross between BL130 and J23B, we identified 22 QTL affecting grain size-related traits in 2 years. Four QTL clusters were found to have multi-effects, which may explain the significant correlations among these traits. Most of the enhanced alleles of QTL for GL and LWR were derived from J23B, while those for GW, GT, and TGW were derived from BL130, suggesting that the pyramiding of superior alleles plays a crucial role in controlling grain size and grain weight. Moreover, a part of grain size-related QTL in this study was co-mapped with rice chalkiness QTL in a previous study, indicating that these QTL can be used in improving not only grain size and yield but also grain chalkiness. By progeny testing, two QTL, qTGW2b and qGL9, were finely mapped to 631-kb and 272-kb intervals, respectively. Parental sequence comparison of genes expressed in inflorescence within qTGW2b and qGL9 intervals showed that LOC_O02g38690 and LOC_O02g38780 may be the candidate gene for qTGW2b, while LOC_O09g29930 may be the candidate gene underlying qGL9. Scanning electron microscopy (SEM) and quantitative RT-PCR analysis showed that qTGW2b and qGL9 regulate grain size by influencing cell size. These findings will contribute to the improvement of rice yield and quality, and lay a foundation for cloning qTGW2b and qGL9.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their appreciation to the support from National Key Laboratory of Crop Genetic Improvement and Hubei Hongshan Laboratory, Huazhong Agricultural University.
Abbreviations
- QTL
Quantitative trait locus
- RIL
Recombinant inbred line
- GL
Grain length
- GW
Grain width
- LWR
Length-to-width ratio
- GT
Grain thickness
- TGW
Thousand grain weight
- SEM
Scanning electron microscopy
- NIL
Near isogenic line
- CIM
Composite interval mapping
- qRT-PCR
Quantitative real-time PCR
- SNP
Single nucleotide polymorphism
Author contribution
H. Shi, P. Yun, and Y. Zhu performed most of the experiments; L. Wang and Q.F. Feng participated in part of phenotyping, genotyping, and biochemical experiments; Q.L. Zhang participated in partial field experiments. H. Shi wrote the manuscript, and P.B. Li, D. Xia, and G.M Lou improved it. G.J. Gao and Y.Q. He designed and supervised this study. All authors discussed and commented on the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (U21A20211, 31901529), the Ministry of Science and Technology (2021YFF1000200), the Science and Technology Major Program of Hubei Province (2021ABA011), and the China Agriculture Research System (CARS-01–01).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All authors approved the submission.
Consent to participate
N/A
Consent for publication
Yes.
Conflict of interest
The authors declare no competing interests.
Footnotes
Huan Shi and Peng Yun are co-first authors of the article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Che RH, Tong HN, Shi BH, Liu YQ, Fang SR, Liu DP, Xiao YH, Hu B, Liu LC, Wang HR. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat Plants. 2015;2:1–8. doi: 10.1038/nplants.2015.195. [DOI] [PubMed] [Google Scholar]
- Chen JX, Zhou H, Gu Y, Xia D, Wu B, Gao GJ, Zhang QL, He YQ. Mapping and verification of grain shape QTLs based on high-throughput SNP markers in rice. Mol Breed. 2019;39:42. doi: 10.1007/s11032-019-0955-x. [DOI] [Google Scholar]
- Duan PG, Rao YC, Zeng DL, Yang YL, Xu R, Zhang BL, Dong GJ, Qian Q, Li YH. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J. 2014;77:547–557. doi: 10.1111/tpj.12405. [DOI] [PubMed] [Google Scholar]
- FAO, IFAD, UNICEF, WFP & WHO (2017) The state of food security and nutrition in the world 2017: Building resilience for peace and food security. Rome. http://www.fao.org/3/a-I7695e.pdf
- Fan CC, Xing YZ, Mao HL, Lu TT, Han B, Xu CG, Li XH, Zhang QF. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet. 2006;112:1164–1171. doi: 10.1007/s00122-006-0218-1. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci U S A. 1999;96:7575–7580. doi: 10.1073/pnas.96.13.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JY, Miao JS, Zhao Y, Zhao Q, Feng Q, Zhan QL, Cheng BY, Xia JH, Huang XH, Yang SH. Dissecting the genetic basis of grain shape and chalkiness traits in hybrid rice using multiple collaborative populations. Mol Plant. 2017;10:1353–1356. doi: 10.1016/j.molp.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Guo T, Chen K, Dong NQ, Shi CL, Ye WW, Gao JP, Shan JX, Lin HX. GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell. 2018;30:871–888. doi: 10.1105/tpc.17.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wang YX, Fang YX, Zeng LJ, Xu J, Yu HP, Shi ZY, Pan JJ, Zhang D, Kang SJ. A rare allele of GS2 enhances grain size and grain yield in rice. Mol Plant. 2015;8:1455–1465. doi: 10.1016/j.molp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B-i, Onishi A. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat Genet. 2013;45:707–711. doi: 10.1038/ng.2612. [DOI] [PubMed] [Google Scholar]
- Lander E, Green P, Abrahamson J, Barlow A, Daly M, Lincoln S, Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Fan CC, Xing YZ, Jiang YH, Luo LJ, Sun L, Shao D, Xu CJ, Li XH, Xiao JH, He YQ, Zhang QF. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet. 2011;43:1266–1269. doi: 10.1038/ng.977. [DOI] [PubMed] [Google Scholar]
- Li YB, Fan CC, Xing YZ, Yun P, Luo LJ, Yan B, Peng B, Xie WB, Wang GW, Li XH, Xiao JH, Xu CG, He YQ. Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat Genet. 2014;46:398–404. doi: 10.1038/ng.2923. [DOI] [PubMed] [Google Scholar]
- Li N, Xu R, Duan P, Li Y. Control of grain size in rice. Plant Reprod. 2018;31:237–251. doi: 10.1007/s00497-018-0333-6. [DOI] [PubMed] [Google Scholar]
- Liu LC, Tong HN, Xiao YH, Che RH, Xu F, Hu B, Liang CZ, Chu JF, Li JY, Chu CC. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc Natl Acad Sci U S A. 2015;112:11102–11107. doi: 10.1073/pnas.1512748112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, Hua L, Dong SJ, Chen HQ, Zhu XD, Jiang JE, Zhang F, Li YH, Fang XH, Chen F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015;84:672–681. doi: 10.1111/tpj.13025. [DOI] [PubMed] [Google Scholar]
- Liu JF, Chen J, Zheng XM, Wu FQ, Lin QB, Heng YQ, Tian P, Cheng ZJ, Yu XW, Zhou KN. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants. 2017;3:1–7. doi: 10.1038/nplants.2017.43. [DOI] [PubMed] [Google Scholar]
- Lizana XC, Riegel R, Gomez LD, Herrera J, Isla A, McQueen-Mason SJ, Calderini DF. Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.) J Exp Bot. 2010;61:1147–1157. doi: 10.1093/jxb/erp380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Xiong Q, Cheng T, Li QT, Liu XL, Bi YD, Li W, Zhang WK, Ma B, Lai YC, Du WG, Man WQ, Chen SY, Zhang JS. A PP2C-1 allele underlying a quantitative trait locus enhances soybean 100-seed weight. Mol Plant. 2017;10:670–684. doi: 10.1016/j.molp.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Miao J, Yang ZF, Zhang DP, Wang YZ, Xu MB, Zhou LH, Wang J, Wu SJ, Yao YL, Du X. Mutation of RGG2, which encodes a type B heterotrimeric G protein γ subunit, increases grain size and yield production in rice. Plant Biotechnol J. 2019;17:650–664. doi: 10.1111/pbi.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaud O, Chen X, McCouch S. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.) Mol Gen Genet. 1996;252:597–607. doi: 10.1007/BF02172406. [DOI] [PubMed] [Google Scholar]
- Qi P, Lin YS, Song XJ, Shen JB, Huang W, Shan JX, Zhu MZ, Jiang LW, Gao JP, Lin HX. The novel quantitative trait locus GL3. 1 controls rice grain size and yield by regulating Cyclin-T1; 3. Cell Res. 2012;22:1666–1680. doi: 10.1038/cr.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan BP, Shang LG, Zhang B, Hu J, Wang YX, Lin H, Zhang AP, Liu CL, Peng YL, Zhu L. Natural variation in the promoter of TGW2 determines grain width and weight in rice. New Phytol. 2020;227:629–640. doi: 10.1111/nph.16540. [DOI] [PubMed] [Google Scholar]
- Seck PA, Diagne A, Mohanty S, Wopereis M. Crops that feed the world 7: Rice. Food Security. 2012;4:7–24. doi: 10.1007/s12571-012-0168-1. [DOI] [Google Scholar]
- Seo H, Kim SH, Lee BD, Lim JH, Lee SJ, An G, Paek NC. The rice basic Helix–Loop–Helix 79 (OsbHLH079) determines leaf angle and grain shape. Int J Mol Sci. 2020;21:2090. doi: 10.3390/ijms21062090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CL, Dong NQ, Guo T, Ye WW, Shan JX, Lin HX. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 2020;103:1174–1188. doi: 10.1111/tpj.14793. [DOI] [PubMed] [Google Scholar]
- Si LZ, Chen JY, Huang XH, Gong H, Luo JH, Hou QQ, Zhou TY, Lu TT, Zhu JJ, ShangGuan YY, Chen EW, Gong CX, Zhao Q, Jing YF, Zhao Y, Li Y, Cui LL, Fan DL, Lu Y, Weng QJ, Wang YC, Zhan QL, Liu KY, Wei XH, An K, An G, Han B. OsSPL13 controls grain size in cultivated rice. Nat Genet. 2016;48:447–456. doi: 10.1038/ng.3518. [DOI] [PubMed] [Google Scholar]
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet. 2007;39:623–630. doi: 10.1038/ng2014. [DOI] [PubMed] [Google Scholar]
- Sun LJ, Li XJ, Fu YC, Zhu ZF, Tan LB, Liu FX, Sun XY, Sun XW, Sun CQ. GS6, a member of the GRAS gene family, negatively regulates grain size in rice. J Integr Plant Biol. 2013;55:938–949. doi: 10.1111/jipb.12062. [DOI] [PubMed] [Google Scholar]
- Sun SY, Wang L, Mao HL, Shao L, Li XH, Xiao JH, Ouyang YD, Zhang QF. A G-protein pathway determines grain size in rice. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-018-03141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YF, Xing YZ, Li JX, Yu SB, Xu CG, Zhang QF. Genetic bases of appearance quality of rice grains in Shanyou 63, an elite rice hybrid. Theor Appl Genet. 2000;101:823–829. doi: 10.1007/s001220051549. [DOI] [PubMed] [Google Scholar]
- Tao YJ, Miao J, Wang J, Li WQ, Xu Y, Wang FQ, Jiang YJ, Chen ZH, Fan FJ, Xu MB. RGG1, involved in the cytokinin regulatory pathway, controls grain size in rice. Rice. 2020;13:1–13. doi: 10.1186/s12284-020-00436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Basten CJ, Zeng ZB (2005) Windows QTL cartographer version 2.5. Stat Genet North Carolina State University, Raleigh
- Wang SK, Wu K, Yuan QB, Liu XY, Liu ZB, Lin XY, Zeng RX, Zhu HT, Dong GJ, Qian Q, Zhang GQ, Fu XD. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2012;44:950–954. doi: 10.1038/ng.2327. [DOI] [PubMed] [Google Scholar]
- Wang SG, Yang HL, Mei JS, Liu XL, Wen Z, Zhang LJ, Xu ZP, Zhang BC, Zhou YH. Rice homeobox protein KNAT7 integrates the pathways regulating cell expansion and wall stiffness. Plant Physiol. 2019;181:669–682. doi: 10.1104/pp.19.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Xiong GS, Hu J, Jiang L, Yu H, Xu J, Fang YX, Zeng LJ, Xu EB, Xu J, Ye WJ, Meng XB, Liu RF, Chen HQ, Jing YH, Wang YH, Zhu XD, Li JY, Qian Q. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet. 2015;47:944–948. doi: 10.1038/ng.3346. [DOI] [PubMed] [Google Scholar]
- Xia D, Zhou H, Liu RJ, Dan WH, Li PB, Wu B, Chen JX, Wang LQ, Gao GJ, Zhang QL, He YQ. GL3.3, a novel QTL encoding a GSK3/SHAGGY-like kinase, epistatically interacts with GS3 to produce extra-long grains in rice. Mol Plant. 2018;11:754–756. doi: 10.1016/j.molp.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Xie LH, Tang SQ, Chen N, Luo J, Jiao GA, Shao GN, Wei XJ, Hu PS. Rice grain morphological characteristics correlate with grain weight and milling quality. Cereal Chem. 2013;90:587–593. doi: 10.1094/CCHEM-03-13-0055-R. [DOI] [Google Scholar]
- Xu R, Duan PG, Yu HY, Zhou ZK, Zhang BL, Wang RC, Li J, Zhang GZ, Zhuang SS, Lyu J. Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol Plant. 2018;11:860–873. doi: 10.1016/j.molp.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Yang WN, Guo ZL, Huang CL, Duan LF, Chen GX, Jiang N, Fang W, Feng H, Xie WB, Lian XM, Wang GW, Luo QM, Zhang QF, Liu Q, Xiong LZ. Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat Commun. 2014;5:1–9. doi: 10.1038/ncomms6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Ren YL, Cai Y, Niu M, Feng ZM, Jing RN, Mou CL, Liu X, Xiao LJ, Zhang X. Overexpression of OsbHLH107, a member of the basic helix-loop-helix transcription factor family, enhances grain size in rice (Oryza sativa L.) Rice. 2018;11:1–12. doi: 10.1186/s12284-018-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Wu K, Wang B, Liu HH, Guo SY, Guo XY, Luo W, Sun SY, Ouyang YD, Fu XD. The RING E3 ligase CLG1 targets GS3 for degradation via the endosome pathway to determine grain size in rice. Mol Plant. 2021;14:1699–1713. doi: 10.1016/j.molp.2021.06.027. [DOI] [PubMed] [Google Scholar]
- Yin CB, Li HH, Li SS, Xu LD, Zhao ZG, Wang JK. Genetic dissection on rice grain shape by the two-dimensional image analysis in one japonica × indica population consisting of recombinant inbred lines. Theor Appl Genet. 2015;128:1969–1986. doi: 10.1007/s00122-015-2560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZM, Kang B, He XW, Lv S, Bai L, YH, Ding WN, Chen M, Cho HT, Wu P, Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011;66:725–734. doi: 10.1111/j.1365-313X.2011.04533.x. [DOI] [PubMed] [Google Scholar]
- Yuan H, Qin P, Hu L, Zhan SJ, Wang SF, Gao P, Li J, Jin MY, Xu ZY, Gao Q. OsSPL18 controls grain weight and grain number in rice. J Genet Genomics. 2019;46:41–51. doi: 10.1016/j.jgg.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Yun P, Zhu Y, Wu B, Gao GJ, Sun P, Zhang QL, He YQ. Genetic mapping and confirmation of quantitative trait loci for grain chalkiness in rice. Mol Breed. 2016;36:1–8. doi: 10.1007/s11032-016-0600-x. [DOI] [Google Scholar]
- Zhang DP, Zhang MY, Liang JS. RGB1 regulates grain development and starch accumulation through its effect on OsYUC11-mediated auxin biosynthesis in rice endosperm cells. Front Plant Sci. 2021;12:224. doi: 10.3389/fpls.2021.585174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JQ, Gao XY, Cai G, Wang YJ, Li JB, Du HY, Wang RQ, Zhang HS, Huang J (2021b) An adenylate kinase OsAK3 involves brassinosteroid signaling and grain length in rice (Oryza sativa L.). Rice 14:105. 10.1186/s12284-021-00546-0 [DOI] [PMC free article] [PubMed]
- Zhang Q, Liu G, Jin J, Liang J, Zhang J, Peng H, Wang W, Zhang Z. RIP2 interacts with REL1 to control leaf architecture by modulating brassinosteroid signaling in rice. Theor Appl Genet. 2022 doi: 10.1007/s00122-021-04011-w. [DOI] [PubMed] [Google Scholar]
- Zhao DS, Li QF, Zhang CQ, Zhang C, Yang QQ, Pan LX, Ren XY, Lu J, Gu MH, Liu QQ. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat Commun. 2018;9:1–14. doi: 10.1038/s41467-018-03616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhu JY, Li ZY, Yi CD, Liu J, Zhang HG, Tang SZ, Gu MH, Liang GH. Deletion in a quantitative trait gene qPE9-1 associated with panicle erectness improves plant architecture during rice domestication. Genetics. 2009;183:315–324. doi: 10.1534/genetics.109.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hou J, Li PB, Yang HY, Xia D, Zhou H, Alam M, Gao GJ, Zhang QL, He YQ. Genetic dissection and validation of QTLs for grain shape and weight in rice and fine mapping of qGL13, a major QTL for grain length and weight. Mol Breed. 2019;39:170. doi: 10.1007/s11032-019-1079-z. [DOI] [Google Scholar]
- Zou HY, Wenwen Y, Zang G, Kang Z, Zhang Z, Huang J, Wang G. OsEXPB2, a β-expansin gene, is involved in rice root system architecture. Mol Breed. 2015;35:1–14. doi: 10.1007/s11032-015-0203-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.