Abstract
Anthocyanin makes snap bean (Phaseolus vulgaris L.) pods purple, which helps seed dispersal and protects against environmental stress. In this study, we characterised the snap bean purple mutant pv-pur, which has purple cotyledon, hypocotyl, stem, leaf vein, flower and pod tissues. Total anthocyanin, delphinidin and malvidin levels in mutant pods were significantly higher than in wild-type plants. We constructed two populations for fine mapping of the PV-PUR purple mutation gene, located in the 243.9-kb region of chromosome 06. We identified Phvul.006g018800.3, encoding F3’5’H, as a candidate gene for PV-PUR. Six single-base mutations occurred in the coding region of this gene, altering protein structure. PV-PUR and pv-pur genes were transferred into Arabidopsis, respectively. Compared with the wild-type, the leaf base and internode of T-PV-PUR plant were purple, and the phenotype of T-pv-pur plant remained unchanged, which verified the function of the mutant gene. The results demonstrated that PV-PUR is a crucial gene for anthocyanin biosynthesis in snap bean, resulting in purple colouration. The findings lay a foundation for future breeding and improvement of snap bean.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-023-01362-8.
Keywords: Snap bean, Mutant, PV-PUR, Anthocyanin, Fine mapping
Introduction
Snap bean (Phaseolus vulgaris L.) is a leguminous vegetable, and seed pods are widely eaten. It is native to America and is widely planted in China, especially in the north. With improvements in living standards, people put forward higher requirements for the appearance of high-quality vegetables. Therefore, the colour of bean pods is an important characteristic of snap bean. However, snap bean cultivation is facing challenges including a narrow genetic background and lack of germplasm resources. The creation of mutants through artificial mutagenesis can provide important materials for bean breeding (Lunde et al. 2003). The purple pv-pur mutant was obtained after treating snap bean A18-1 seeds with 60Co-γ radiation. Therefore, gene mapping of the purple mutation and clarifying the molecular mechanism of purple pod colour formation can accelerate the genetic improvement and appearance traits of snap bean.
Studies on colour changes in fruits, leaves and petals of horticultural crops showed that they may be caused by changes in chlorophyll, anthocyanin and carotenoid content. Purple is mainly caused by anthocyanin, synthesised from flavonoids, and the site of action is the endoplasmic reticulum (Grotewold 2006; Jørgensen et al. 2005). Under the action of a variety of enzymes, phenylalanine is first synthesised into colourless anthocyanins, then forms coloured anthocyanins through a series of complex reactions (such as methylation and substituent reactions) and finally transported to vacuoles for enrichment (Grotewold 2006). In-depth studies have been carried out on some model plants (Irain et al. 2003; Koes et al. 2005; Zheng et al. 2021).
The first step of flavonoid synthesis is catalysed by chalcone synthase (CHS), a member of the polyketide synthase (PKS) family. CHS is usually co-regulated with chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H) and flavonol synthase (FLS) at the key branching step in flavonol synthesis (Hartmann et al. 2005; Albert et al. 2009). Flavonols bestow UV protection, form nectar ducts visible to insects and can be used as co-pigments of anthocyanins (Davies et al. 2012). Dihydroflavonol 4-reductase (DFR) catalyses the synthesis of colourless anthocyanins in a key regulatory step of anthocyanin synthesis. Under the action of anthocyanin synthase (ANS), chromophores are then synthesised containing coloured anthocyanins, which are glycosylated to form different anthocyanins (Tanaka 2010). Structural genes, most of which encode enzymes, play a key role in this synthesis process, including CHS, CHI, F3H, F3’H and F3’5’H, DFR, ANS and UFGT (Koes et al. 2005; Springob et al. 2003; Holton and Cornish 1995). F3’H and F3’5’H play a key role in the synthesis of various colourless basic anthocyanins, and all belong to the cytochrome P450 family. F3’H converts dihydroflavanol (DHK) to colourless cyanidin anthocyanin. F3’5’H converts dihydroflavanol (DHK) and dihydroquercetin (DHQ) to dihydromyricetin (DHM), a precursor of delphinidin (Seitz et al. 2007). F3’H is responsible for the synthesis of anthocyanins from the cyanidin branch, while F3’5’H is responsible for the synthesis of anthocyanins from the delphinidin branch. In plants, changes in the value of F3’5’H/F3’H will lead to changes in the appearance colour. The higher the F3’5’H/F3’H ratio, the more intense the purple colour (Zeng et al. 2014).
In our previous study, the pv-pur purple snap bean mutant was generated using 60Co-γ radiation. Genetic analysis of the purple phenotype showed that the mutant character was controlled by a single dominant nuclear gene, and it was named PV-PUR. Transcriptome and metabolome analysis of mutant and wild-type (WT) plants showed that the differential abundance in the content of the metabolite delphinidin may be related to differential expression of three genes, namely, phvul.006G024700, Phvul.002g152700 and phvul.006g018800 (Liu et al. 2022). However, the results of this previous study did not unclear show which gene mutation caused the purple phenotype. Therefore, in the present work, we mapped the PV-PUR purple mutant, screened and cloned candidate genes and explored their functions to investigate the mechanism for the change in anthocyanin synthesis in the PV-PUR mutant. The findings not only lay a foundation for further study of the molecular mechanism of anthocyanin synthesis in snap bean, but also provide theoretical support for the breeding of cultivars with different colours.
Materials and methods
Materials
Dried seeds of A18-1 were treated with 150Gy 60Co-γ radiation to construct a snap bean mutant library. M3 generation plants were grown at the horticulture research base of Hulan campus, Heilongjiang University, Harbin, Heilongjiang, China. The purple phenotype was observed in the pv-pur mutant. In order to locate the PV-PUR mutant gene, we constructed the A18-1pv-pur F2 hybrid population for preliminary mapping and the Golden hookxpv-pur F2 hybrid population for fine mapping. These F2 isolated populations were grown in the field at the horticulture research base in Hulan campus, Heilongjiang University. Columbia ecotype A. thaliana plants used for transgenic experiments were grown in a light incubator at the horticulture laboratory of Heilongjiang University, provided by BioRun Biotechnology Company (Wuhan, China).
Bulked segregant analysis coupled to whole-genome sequencing (BSA-seq) analysis
An F2 generation mapping population was constructed by crossing the purple mutant pv-pur with WT A18-1. In the F2 population, 50 plants with the same phenotype as WT plants were selected for DNA extraction, and the 50 DNA extracts were mixed in equal amounts to construct a mixed pool, which was denoted W-type. Fifty plants with the same phenotype as the mutant were screened from the F2 population, and the 50 DNA extracts were mixed in equal amounts to construct a mixed pool, which was denoted M-type. DNA was extracted from leaves of one mutant and one WT parent, and samples were denoted P_ mutant and P_ wild, respectively.
The library was constructed and sequenced by Hangzhou Lianchuan Biotechnology Co., Ltd (Hangzhou, China). After the sequencing data were acquired, quality control was carried out. After removing low-quality sequences and sequences with connectors, clean data were obtained. Clean data were compared with the reference genome, and SNPs and indels were detected and annotated according to the comparison results. The SNP index and difference value of hybrid pools were calculated, regions with significant differences in SNP index between the two offspring were selected, and target trait regions were located on chromosomes.
PV-PUR gene fine mapping
There is a construction of the F2 fine-mapping population by hybridisation between ‘Golden Hook’ and purple mutant pv-pur plants. ‘Golden Hook’ is a dwarf bean variety. Its phenotype is similar to that of ‘A18-1’, with green stems and leaves and yellow pods, but its genetic relationship with ‘a18-1’ is far. There will be more SSR markers available between the two parents using ‘Golden Hook’ and mutants ‘pv-pur’ to construct a fine-mapping population. In total, 588 F2 plants segregating for the recessive phenotype were selected for fine mapping. According to the preliminary candidate regions identified by BSA-seq, SSR markers were designed for fine mapping of the PV-PUR gene. We downloaded candidate region sequences from the P. vulgaris v2.1 reference genome database. Primer Premier 6.0 software (Premier Biosoft International, Palo Alto, CA, USA) was used to design primers, which were sent to Genewiz Biotechnology Co., Ltd. for synthesis (Supplement Table 1). Primers were screened against the two parents for polymorphisms, and the selected primers were used to screen the F2 population. The number of recombinant bands was recorded for fine mapping. We performed PCR amplification in a 10-μL reaction comprising 1 μL (50 ng) of template DNA, 0.5 μL of 10 μM forward and reverse SSR primers, 5 μL of 2 × Rapid Taq Master mix and 3 μL of DEPC (diethypyrocarbonate)-ddH2O. PCR was carried out on an iCycler thermocycler (Bio-Rad, Hercules, CA, USA) using the following reaction conditions: an initial denaturation at 95°C for 3 min, followed by 35 cycles at 95°C for 15 s, 60°C for 15 s and 72°C for 15 s and a final extension at 72°C for 5 min. A 5% denaturing polyacrylamide gel was then used to separate PCR products, followed by silver staining.
Table 1.
Prediction of candidate genes within the gene-mapped region on Chr 06
| Gene ID | Start | End | Gene annotations |
|---|---|---|---|
| Phvul.006G018800.3 | 4328039 | 4334826 | K13083 - flavonoid 3′,5′-hydroxylase (CYP75A) |
| Phvul.006G018911.1 | 4347887 | 4349636 | PTHR23155//PTHR23155:SF560 - LEUCINE-RICH REPEAT-CONTAINING PROTEIN // SUBFAMILY NOT NAMED |
| Phvul.006G019022.1 | 4349689 | 4351601 | PTHR23155//PTHR23155:SF560 - LEUCINE-RICH REPEAT-CONTAINING PROTEIN // SUBFAMILY NOT NAMED |
| Phvul.006G018600.1 | 4382741 | 4385405 | PTHR11413//PTHR11413:SF50 - CYSTATIN FAMILY MEMBER // SUBFAMILY NOT NAMED |
| Phvul.006G018500.1 | 4403804 | 4407407 | PTHR32468:SF17 - CATION/H(+) ANTIPORTER 10-RELATED |
| Phvul.006G018400.1 | 4430330 | 4431271 | Unknown protein |
| Phvul.006G018300.1 | 4442500 | 4447162 | PTHR32468:SF17 - CATION/H(+) ANTIPORTER 10-RELATED |
| Phvul.006G018200.1 | 4476622 | 4478137 | PF00646//PF01344 - F-box domain (F-box) // Kelch motif (Kelch_1) |
| Phvul.006G018100.1 | 4517068 | 4520052 | PF03168 - Late embryogenesis abundant protein (LEA_2) |
Linkage analysis data for mutant genes in the F2 population were integrated, and linkage relationships between SSR markers and mutant genes were analysed by MapChart software (Wageningen, Netherlands). Finally, TBtools software (Nanjing, China) was used to construct a genetic linkage map from polymorphic SSR markers and segregation data linked to genes.
Anthocyanin content determination
Separate 0.5 g samples of A18-1 and pv-pur pod walls were used for extraction of total anthocyanins using a mixture of methanol:hydrochloric acid:purified water (16:1:3), the absorbance was measured at 657 nm and 530 nm using a UV spectrophotometer, and the relative content of total anthocyanins was calculated according to the formula:
Relative content of anthocyanin = (A530 − 0.25 × A657)/fresh weight. (Frank et al. 2005)
Levels of six anthocyanins (delphinidin, cyanidin, petunia, pelargonidin, peonidin and malvidin) in pod walls of A18-1 and pv-pur plants were determined by HPLC using the analytical method referred to in the agricultural industry standard of the People’s Republic of China (ny/t2640-2014).
Candidate gene cloning
The DNA reference sequences of candidate genes were searched against the database P. vulgaris v2.1 database (http://brassicadb.org/brad/downloadOverview.php), and specific primers were designed by Primer Premier 6.0 software (Supplement Table 2). DNA from A18-1 and pv-pur plants was used as template, and candidate gene-encoding regions were amplified by PCR. After the PCR products were separated by 1.5% agarose gel electrophoresis, target bands were excised using a UV Gel cutter, and fragments were recovered with gel recovery reagent (Vazyme Biotech Co., Ltd). The recovered products were sent to Genewiz Biotechnology Co., Ltd. for sequencing. DNAMAN (LynnonBiosoft, USA) was used for sequence alignment. The tertiary structure of PV-PUR protein was predicted by SWISS-MODEL software (Basel, Switzerland).
Construction of PV-PUR and pv-pur gene expression vectors
Total RNA from tender leaves of pv-pur and WT plants was extracted using a Plant Total RNA Extraction Kit (Tiangen, Beijing, China), respectively. First-strand cDNA was synthesised using a reverse transcription kit (Novozan, Nanjing, China). According to the above primer design (Supplement Table 3), PCR amplification was carried out with cDNA as template in 50-μL reactions. The target band was recovered by agarose gel electrophoresis and named rDNAg1 and rDNAg2. The pBWA (V) HS-ccdb GLosgfp vector and rDNAg1/rDNAg2 were combined and purified with the PCR purification kit. Purified product is used for the next step of the ligation reaction. The vector map is shown in Supplementary Fig 1, and the vector was named PV-PUR-pBWA-GFP and pv-pur-pBWA-GFP. The vector could be used as an overexpression vector for Arabidopsis genetic transformation.
Gene expression analysis by qRT-PCR
To explore the mode of action of candidate genes, the cDNA samples from stems, leaves, flowers, pods, hypocotyls and cotyledons of A18-1 and pv-pur plants were obtained as described above, and specific primers for F3’5’H (Phvul.006G018800.3) and F3’H (Phvul.004G021200) were designed by Primer Premier 6.0 (Supplement Table 4). A MY17295272 fluorescence quantitative PCR instrument (Agilent Technologies Inc. CA., USA) was used for qPCR experiments. Actin was used as the internal reference gene, the 2−ΔΔCT method was used to calculate relative expression levels of genes (Hongjian et al. 2009), the least significant difference (LSD) test was used for single factor difference analysis, and GraphPad software (https://www.graphpad.com/scientific-software/prism/) was used for mapping. The reaction system for qRT-PCR was 10 µL of 2×Fast qPCR Mix, 0.4 µL of forward and reverse primer (10 μM), 2 µL of cDNA and 7.2 µL of DEPC-ddH2O. The reaction procedure is initial denaturation at 95°C for 30 s and then 40 cycles at 95°C for 5 s and 60°C for 15 s. Three technical replicates and three biological replicates were performed.
Genetic transformation of PV-PUR candidate genes
The PV-PUR-pBWA-GFP and pv-pur-pBWA-GFP overexpression vector was transformed into Agrobacterium tumefaciens GV3101 by electric shock, respectively. Colonies were collected and grown to OD600 = 0.8–1.2 in the medium. Silwet-77 was then added to a final concentration of 0.02%. Arabidopsis inflorescences were dipped in the suspension for 2–3 s and then sealed with film. We keep the humidity >90% and incubate at 25°C for 24 h. The soaking period was 7 days, and three replicates were included. The genetic transformation of WT A. thaliana was performed by dipping flowers using the method of Zhang et al (2006). Hygromycin was used as a screening marker for T1 generation screening, and Arabidopsis genomic DNA was extracted by the CTAB (cetyltrimethylammonium bromide) method for PCR detection. The phenotypes of transgenic plants and wild-type plants were observed, and their total anthocyanin content and the expression levels of PV-PUR, pv-pur, CHS, PAL, ANS, CHI, F3H and F3’H genes were detected. The testing method is the same as seen in ‘Anthocyanin content determination’ and ‘Gene expression analysis by qRT-PCR’; for primers, see Supplement Table 4.
Statistical analysis
The average value of three repetitions was taken for all measurement indexes. The significance of difference was tested by IBM SPSS 23.0 (http://www.ibm.com/cnzh/products/spss-statistics, accessed on 10 December 2021).
Results
Phenotypic characteristics of the pv-pur purple mutant
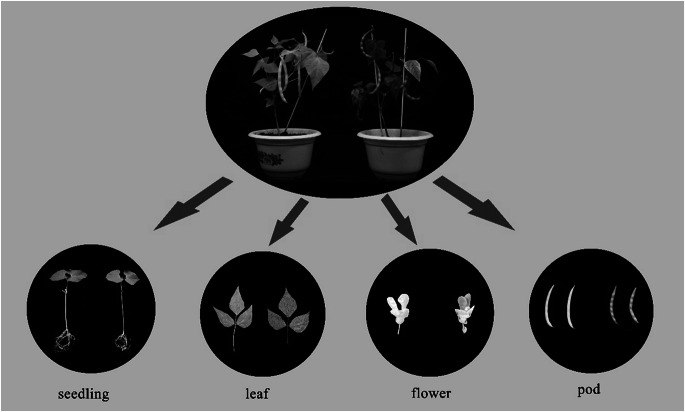
Unlike WT A18-1 plants, the pv-pur purple mutant has dark purple cotyledons, light purple hypocotyls, purple leaf veins, petioles and stems, obvious purple vexils and wing petals of flowers and purple pod walls after pod maturity (Fig. 1). In previous studies, we analysed the genetic underpinnings of the purple mutant phenotype, and the results showed that the mutant phenotype is a quality trait controlled by a single dominant nuclear gene (Liu et al. 2022), which we named PV-PUR.
Fig. 1.
Phenotypic characteristics of A18-1 and pv-pur plants. A18-1 and pv-pur are shown on the left and right, respectively, in all parts of the figure
The PV-PUR gene is located on chromosome 06
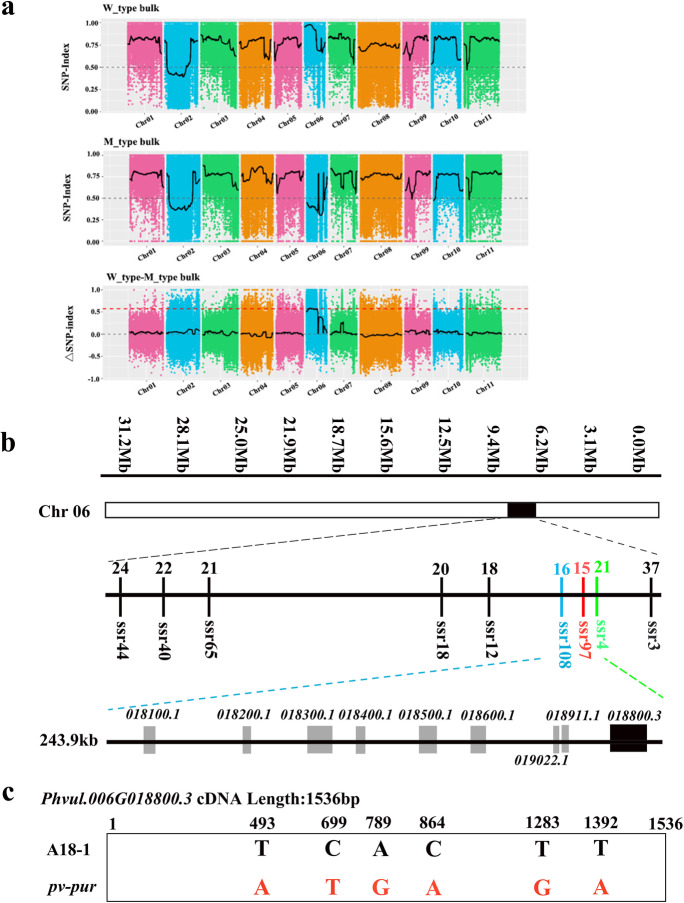
In total, 269,198,004 and 185,721,966 valid reads were obtained from M-type and W-type data. Bulk segregant analysis (BSA) was used to compare the reference genomic sequence between the reads and the P. vulgaris reference sequence. The sequencing results for each sample were compared with the reference genome, and mutation sites were analysed to obtain possible single-nucleotide polymorphisms (SNPs) and indel information. These SNPs were used to calculate SNP and ΔSNP indices (Fig. 2a). The closer the SNP index is to 1, the stronger is the association between SNPs and traits. According to the results of sliding window calculation, the SNP index value in the top 1% region was selected to identify the chromosome segment on which the purple mutant PV-PUR gene is located. According to the results, PV-PUR was predicted to be located in the range of 3,740,000−9,590,000 on chromosome 06. The confidence value of the ΔSNP index in the 3.74−9.59 Mb region of chromosome 06 was >0.5 (p <0.01), and no other minor effect sites of mutant genes were found (Fig. 2a). This result is consistent with the conclusion that the purple trait is controlled by a single dominant nuclear gene locus.
Fig. 2.
Map-based cloning of the PV-PUR locus. a Using BSA-seq, the PV-PUR locus was mapped to a 5.85 Mb region on chromosome 06. b Physical locations of fine-mapping markers and number of recombinants in the PV-PUR region. The PV-PUR gene was fine-mapped to the region between ssr4 and ssr108, in which nine genes were annotated. c Compared with WT, the mutant pv-pur has six single-base mutations in the Phvul.006G018800.3
Fine mapping of PV-PUR
A total of 124 pairs of simple sequence repeat (SSR) markers were developed in the 5.85 Mb preliminary location area for fine mapping of PV-PUR, of which nine pairs of SSR primers with stable and clear differential bands in the two parents were selected for linkage map construction (ssr3, ssr4, ssr12, ssr18, ssr40, ssr44, ssr65, ssr97 and ssr108). These nine pairs of primers were used to amplify 588 individuals with a recessive phenotype in the F2 population. The relative positions of these markers were obtained by statistical analysis and recombination rate calculation. The results showed that ssr3 and ssr4 were on one side of PV-PUR, while ssr108, ssr12, ssr18, ssr65, ssr40 and ssr44 were on the other side (Fig. 2b). Furthermore, ssr4 and ssr108 were closely linked to PV-PUR, and the PV-PUR gene is located in the 243.9 kb region between the two markers (Chr06:4295155−4539091).
Comparative analysis of anthocyanin content in pv-pur and A18-1 plants
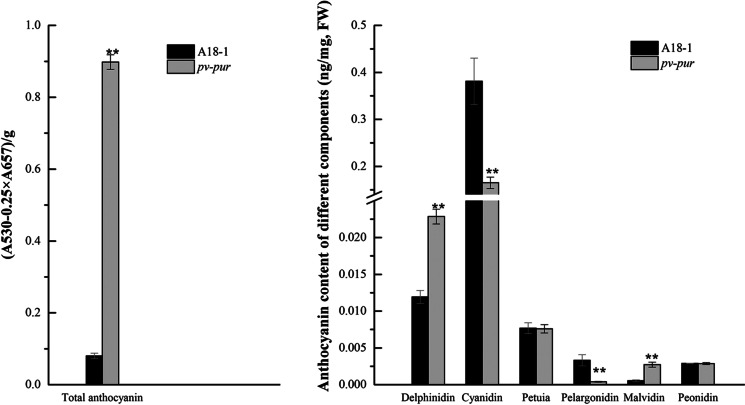
The contents of total anthocyanin, delphinidin, cyanidin, petunia, pelargonidin, peonidin and malvidin in the pod wall of pv-pur and A18-1 plants were determined. The results showed that the levels of total anthocyanin, delphinidin and malvidin in the pod wall of pv-pur plants were significantly higher than for A18-1, while cyanidin and pelargonidin levels were significantly lower than for A18-1, and petunia and peonidin were not significantly different between pv-pur mutant and WT plants (Fig. 3). This shows that from the three branches after DHK, the anthocyanin content synthesised by the F3’5’H branch increases, and the anthocyanin content synthesised by the other two branches decreases. Thus, we speculated that this content change may be due to changes in the three branch steps after DHK caused by the mutant PV-PUR gene.
Fig. 3.
Determination of anthocyanin levels in WT and mutant bean pods. Error bars donate S.D. (std. deviation)
Candidate gene screening and sequence analysis
The 243.9-kb interval of the fine-mapping region was compared with the P. vulgaris v2.1 database (https://genome.jgi.doe.gov/portal/). The analysis found that this region contains eight genes with annotation or prediction information and one gene of unknown function (Table 1). Phvul.006G018800.3 is a gene-encoding flavonoid 3′-5′-hydroxylase, belonging to the cytochrome P450 family and participating in the biosynthesis of flavonoids and flavonols. F3’5’H is one of the most critical enzymes in the synthesis of delphinidin glycosides in the anthocyanin synthesis pathway. Its main function is to convert DHK and DHQ into DHM, the precursor for the synthesis of delphinidin, and malvidin can be catalysed by delphinidin. This is consistent with the results of physiological experiments; hence, we speculate that Phvul.006G018800.3 is a candidate gene for PV-PUR.
The coding sequence of PV-PUR was amplified and sequenced for pv-pur and A18-1. Sequence analysis showed that the coding sequence (CDS) was 1536 bp with single-base mutations at positions 493, 699, 798, 864, 1283 and 1392 (Fig. 2c). Among them, three SNPs are nonsynonymous mutations, which lead to changes in amino acids, namely, C493S, H864Q and M1392R. Analysis of the configuration changes of mutation sites in the tertiary structure of the PV-PUR protein for A18-1 and pv-pur showed changes in protein structure for mutation sites C493S and H864Q (Supplementary Fig. 2). Meanwhile, functional domain analysis showed that the PV-PUR protein contained a P450 domain, located at position 36-498. The base mutation at C493S is located in the protein domain, which will lead to changes in protein structure and affect protein function.
Analysis of PV-PUR gene expression patterns
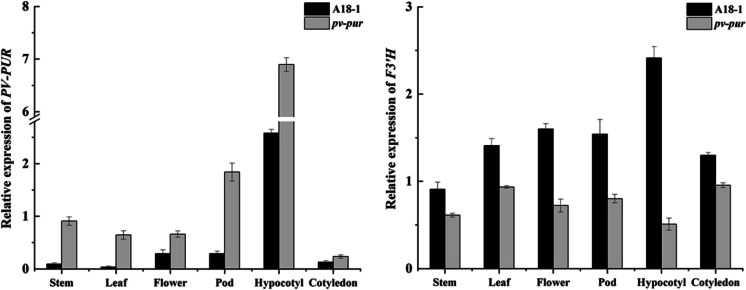
Expression patterns of PV-PUR candidate genes in stems, leaves, flowers, pods and other parts of pv-pur and A18-1 plants were analysed by quantitative real-time PCR (qRT-PCR). The results showed that PV-PUR was expressed in all tissues in both plants, but there was great variation in expression levels (Fig. 4). In leaves, flowers, stems, hypocotyls, pods and cotyledons, the expression of the PV-PUR gene was significantly higher than in A18-1 plants, by 18.5-, 9.5-, 6.4-, 2.8-, 2.5- and 1.7-fold, respectively. At the same time, we also detected the expression of another branch of DHK synthetase gene F3’H in various parts of mutant and WT. The results showed that the expression of F3’H in A18-1 was significantly higher than that in the pv-pur. The change in the expression of F3’5’H and F3’H may be due to the substrate competition mechanism.
Fig.4.
Expression of PV-PUR and F3’H in different parts of pv-pur and A18-1 plants. Error bars donate S.D. (std. deviation)
Generation and characterisation of transgenic Arabidopsis
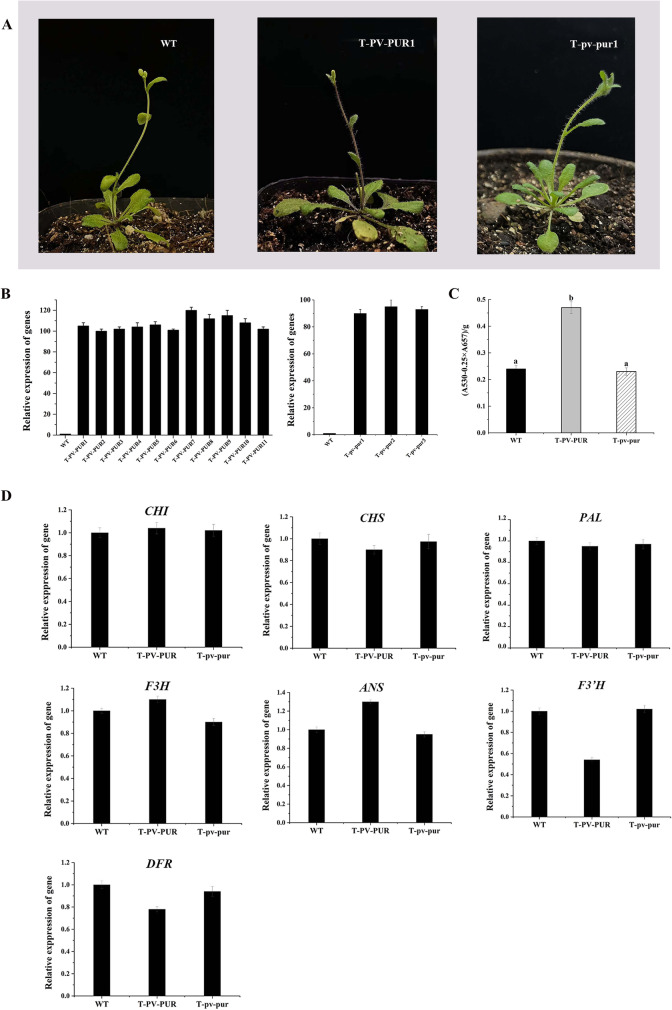
Through hygromycin and PCR screening of positive PV-PUR and pv-pur transgenic Arabidopsis plants, 11 and 3 transgenic lines with hygromycin resistance were obtained, namely, it T-PV-PUR1-11 and T-pv-pur1-3. qRT-PCR was used to detect the expression of PV-PUR and pv-pur genes in transgenic Arabidopsis plants. The expression of PV-PUR and pv-pur genes in overexpressed Arabidopsis lines was significantly higher than that in WT Arabidopsis plants. It was confirmed that the genes were overexpressed in Arabidopsis plants (Fig. 5B). We selected one of the identified T-PV-PUR and T-pv-pur transgenic lines for phenotype and subsequent identification (Fig. 5A), and the phenotype of other transgenic lines is shown in Supplement Fig 3. Phenotypic identification of T-PV-PUR showed that unlike WT Columbia Arabidopsis and T-pv-pur, the stem base and internodes of transgenic Arabidopsis were purple and no change in the colour of flowers and pods (Fig. 5A).
Fig. 5.
Screening and identification of transgenic Arabidopsis. A Phenotypes of wild-type and transgenic Arabidopsis plant. WT, Arabidopsis Columbia wild-type; T-PV-PUR1, transgenic Arabidopsis lines overexpressing PV-PUR; transgenic Arabidopsis lines overexpressing pv-pur. B qRT-PCR screening of transgenic Arabidopsis lines overexpressing PV-PUR and pv-pur. C Analysis of total anthocyanin content in wild-type, T-PV-PUR and T-pv-pur transgenic Arabidopsis. D Expression level of structural genes related to anthocyanin synthesis in Arabidopsis. Error bars donate S.D. (std. deviation)
The total anthocyanin content of transgenic T-PV-PUR, T-pv-pur and WT Arabidopsis was determined, respectively. The aboveground parts of plants growing for about 20 days were collected for the determination of total anthocyanin content, as shown in Fig. 5C. The total anthocyanin content in T-PV-PUR plants increased significantly compared with WT, while the total anthocyanin content in T-pv-pur plants did not differ significantly compared with WT, which was consistent with the observed phenotype. Preliminary analysis showed that PV-PUR led to the increase of anthocyanin content.
In order to prove that PV-PUR is involved in the regulation of anthocyanin biosynthesis in Arabidopsis, the expression of PV-PUR and anthocyanin synthesis-related genes was analysed by qRT-PCR. Figure 5D shows that, compared with WT, the expression levels of CHI, CHS, F3H and PAL structural genes in T-PV-PUR plants have no significant difference. The expression of ANS gene was significantly increased, and the expression of DFR, F3’H was decreased. Therefore, it is speculated that PV-PUR participates in anthocyanin synthesis and is positively correlated with anthocyanin accumulation.
Discussion
Anthocyanins are one of the three major pigments. It can improve the nutritional value of fruits by participating in the coloration of plant tissues, mediating plant development and reproduction and resisting pathogens and ultraviolet rays (Dixon and Paiva 1995; Gandikota et al. 2001; Chen et al. 2019). Therefore, anthocyanins play an important role in plants. Purple mutants have facilitated the study of plant gene function, helped to clarify mutation mechanisms and assisted exploration of key genes regulated by anthocyanins. Sung et al. (2013) used artificial mutations to turn white chrysanthemums purple, and the anthocyanin content in mutant petals was 3.5 times higher than in white petals. This is similar to the results of our current study showing that the anthocyanin content in all tissues of the snap bean purple mutant obtained by physical radiation mutation was significantly higher than in WT tissues. The determination of six major anthocyanins in pods of A18-1 and pv-pur plants showed that the increase in the accumulation of delphinidin and malvidin was the reason for the purple phenotype of pv-pur plants, consistent with results for transgenic tobacco (Okinaka et al. 2003). Therefore, we speculated that the purple mutant phenotype of pv-pur was caused by mutation of genes regulating the synthesis of delphinidin and malvidin.
In previous studies, we determined that the purple phenotype of pv-pur is inherited through a single dominant nuclear gene. In the present study, after preliminary mapping using the method of segregation population grouping analysis, specific SSR primers were designed for fine mapping within the 5.85 Mb preliminary mapping interval using the principle of linkage genetics. This positioning method has been widely used in corn (Tang et al. 2014), wheat (Wang et al. 2018), rape (Sundqvist and Dahlin 1997) and snap bean (Yang et al. 2021). Eventually, the gene was mapped to the 243.9-kb interval, in which there are nine genes, including phvul 006g018800.3 (F3’5’H) related to anthocyanin synthesis; hence, we speculated that it is a candidate gene for PV-PUR. Previous clones of F3’5’H in anthocyanin-free tomato mutants revealed that the gene synthesised a frameshift mutation, and the forward shift of the termination codon led to loss of gene function and inability to synthesise coloured anthocyanins (Jong et al. 2004). Herein, we cloned and sequenced the F3’5’H gene from A18-1 and pv-pur and found that there were six single-base mutations in the CDS, of which a nonsynonymous mutation located in the protein domain likely alters the protein structure.
The anthocyanin biosynthesis pathway is regulated by various factors including the environment, signal transduction, key enzyme genes and transcription factors (Holton and Cornish 1995). At present, the corresponding genes have been cloned from Arabidopsis (Deluc et al. 2008), Petunia (Stracke et al. 2001), some other crops and goldfish (Bachem et al. 1996), and their expression and regulation have been studied in depth. F3’H and F3’5’H belong to the total branch chain of cytochrome P450 and are the key enzymes controlling anthocyanin biosynthesis in some species. When F3’H and F3’5’H are both expressed, F3’5’H is dominant. In other words, F3’5’H not only promotes the synthesis of anthocyanins by flavanone-3′-5′-hydroxyanthocyanins, but also blocks the biosynthetic pathway of flavanone-3′-hydroxyanthocyanins (Holton and Cornish 1995; Castellarin and Di 2007). This can also explain why, in our study, after F3’5’H mutation, the content of delphinidin and malvidin synthesised by the F3’5’H branch was increased in the pv-pur mutant, whereas the levels of cyanidin and geranium synthesised by the other two branches were decreased, compared with WT A18-1 plants. Mazza found that blue/purple components of anthocyanins are mainly malvidin, petunia and delphinidin, while previous studies found that delphinid can be further modified by flavonoid 3-o-glucosyltransferase (UFGT) and methyltransferase (MET) to produce blue/purple malvidin (Fang et al. 2018; Holton and Cornish 1995). This is consistent with our results showing that a significant increase in the contents of delphinidin and malvidin leads to the coloured phenotype of the purple mutant.
We predicted the candidate genes of PV-PUR based on gene mapping and physiological results and then confirmed them via transgenic verification. There have been some reports on genetic transformation systems in Phaseolus vulgaris, but most involve infection wounds or calli (Zhang et al. 1997; Estrada-navarrete et al. 2006; Amugune et al. 2011). Even successful cases cannot be replicated in subsequent reports, and a stable genetic transformation system has not been reported (Collado et al. 2016; Mohamed et al. 2006). Therefore, we chose Arabidopsis thaliana as the model plant for functional verification of candidate genes. Since there is no homolog of phvul.006g018800.3 in Arabidopsis, we believe that the phenotypic changes of transgenic plants after are due to the overexpression of the phvul 006g018800.3 gene in Arabidopsis. Specifically, the purple leaf base and internodes of homozygous T3 transgenic Arabidopsis plants are due to the function of the protein encoded by the expressed phvul.006g018800.3 gene. This further confirmed that phvul.006g018800.3 is the PV-PUR gene.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
CL and XY conceived, designed and performed the experiments and wrote the manuscript; YH and QC conducted partial gene mapping experiments; YH participated in the transgenic Arabidopsis experiments; ZY contributed to the experimental materials and field planting; DL and GF revised the manuscript and performed data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 32002031); the Basic Scientific Research Operating Expenses of Provincial College in Heilongjiang Province, China (grant numbers 2020-KYYWF-1026 and 2020-KYYWF-1027); and the Heilongjiang Provincial Natural Science Foundation of China (grant number LH2020C090).
Data availability
All data and materials generated or analysed during this study are included in this article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
On behalf of all authors, the corresponding author provides the consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chang Liu and Xiaoxu Yang contributed equally to this work and share the first authorship.
Contributor Information
Dajun Liu, Email: jianlongedu@163.com.
Guojun Feng, Email: feng998@126.com.
References
- Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, Davies KM. Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot. 2009;60(7):2191–2202. doi: 10.1093/jxb/erp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amugune NO, Anyango B, Mukiama TK. Arobacterium-mediated transformation of common bean. Afr Crop Sci J. 2011;19(3):137–147. doi: 10.4314/acsj.v19i3. [DOI] [Google Scholar]
- Bachem CW, van der Hoeven RS, de Bruijn SM, Vreugdenhil D, Zabeau M, Visser RG. Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: analysis of gene expression during potato tuber development. Plant J. 1996;9(5):745–53. doi: 10.1046/j.1365-313x.1996.9050745.x. [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Di Gaspero G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007;7(1):46–46. doi: 10.1186/1471-2229-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Hu B, Qin YH, Hu GB, Zhao JT. Advance of the negative regulation of anthocyanin biosynthesis by MYB transcription factors. Plant Physiol Biochem. 2019;136:178–187. doi: 10.1016/j.plaphy.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Collado R, Bermúdez-Caraballoso I, Veitía N, Torres D, Romero C, Angenon G. Epicotyl sections as targets for plant regeneration and transient transformation of common bean using Agrobacterium tumefaciens. In Vitro Cell Dev Biol-Plant. 2016;52(5):1–12. doi: 10.1007/s11627-016-9769-2. [DOI] [Google Scholar]
- Davies KM, Albert NW, Schwinn KE. From landing lights to mimicry: the molecular regulation of flower colouration and mechanisms for pigmentation patterning. Funct Plant Biol. 2012;39(8):619. doi: 10.1071/fp12195. [DOI] [PubMed] [Google Scholar]
- Deluc L, Bogs J, Walker AR, Ferrier T, Decendit A, Merillon JM, Robinson SP, Barrieu F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008;147(4):2041–2053. doi: 10.1104/pp.108.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7(7):1085. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Navarrete G, Alvarado-Affantranger X, Olivares JE, Díaz-Camino C, Santana O, Murillo E, Guillén G, Sánchez-Guevara N, Acosta J, Quinto C, Li DL, Gresshoff PM, Sánchez F. Agrobacterium rhizogenes transformation of the Phaseolus spp: a tool for functional genomics. MPMI. 2006;19(12):1385–1393. doi: 10.1094/mpmi-19-1385. [DOI] [PubMed] [Google Scholar]
- Fang L, Yang YJ, Gao JW, Ma CL, Bi YP. A comparative transcriptome analysis of a wild purple potato and its red mutant provides insight into the mechanism of anthocyanin transformation. Plos One. 2018;13(1):0191406. doi: 10.1371/journal.pone.0191406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Harald K, Pawel B, Bernd W. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 2005;138:1083–1096. doi: 10.1104/pp.104.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandikota M, Kochko A, Chen L, Ithal N, Fauquet C, Reddy A. Development of transgenic rice plants expressing maize anthocyanin genes and increased blast resistance. Mol Breed. 2001;7:73–83. doi: 10.1023/A:1009657923408. [DOI] [Google Scholar]
- Grotewold E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B. Differential combinatorial interactions ofcis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol Biol. 2005;57(2):155–171. doi: 10.1007/s11103-004-6910-0. [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7(7):1071–1083. doi: 10.1105/tpc.7.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongjian W, Zhao ZG, Qian CT, Sui YH, Malik AA, Chen JF. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem. 2009;399(2):257–261. doi: 10.1016/j.ab.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Irain NG, Hernandez JM, Grotewold E. Chapter three regulation of anthocyanin pigmentation. Recent Adv Phytochem. 2003;37:59–78. doi: 10.1016/S0079-9920(03)80018-7. [DOI] [Google Scholar]
- Jong WSD, Eannetta NT, Jong DMD, Bodis M (2004) Candidate gene analysis of anthocyanin pigmentation loci in the Solanaceae. Theor Appl Genet 108(3):423–432. 10.1007/s00122-003-1455-1 [DOI] [PubMed]
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol. 2005;8(3):280–291. doi: 10.1016/j.pbi.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends in Plant Sci. 2005;10(5):236–242. doi: 10.1016/j.tplants.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Li XH, Uddin MR, Parka WT, Kima YB, Seo JM, Kim S, Nou I, Lee J, Kim H, Parka SU. Accumulation of anthocyanin and related genes expression during the development of cabbage seedlings. Proc Biochem. 2014;49(7):1084–1091. doi: 10.1016/j.procbio.2014.03.008. [DOI] [Google Scholar]
- Liu C, Yang XX, Yan ZS, Liu DJ, Feng GJ. Identification and characterization of a mutant PV-PUR gene responsible for the purple phenotype of snap bean (Phaseolus vulgaris L.) Int J Mol Sci. 2022;23:1265. doi: 10.3390/ijms23031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde CF, Morrow DJ, Roy LM, Walbot V. Progress in maize gene discovery: a project update. Funct Integr Genom. 2003;3(1):25–32. doi: 10.1007/s10142-002-0078-y. [DOI] [PubMed] [Google Scholar]
- Mohamed MF, Cao J, Earle ED. Toward production of genetically modified common bean via Agrobacterium-mediated transformation. Annu REP-BEAN Improv Coop. 2006;49:147. [Google Scholar]
- Okinaka Y, Shimada Y, Nakano-Shimada R, Ohbayashi M, Kiyokawa S, Kikuchi Y. Selective accumulation of delphinidin derivatives in tobacco using a putative flavonoid 3′,5′-hydroxylase cDNA from Campanula medium. Biosci Biotechnol Biochem. 2003;67(1):161–5. doi: 10.1271/bbb.67.161. [DOI] [PubMed] [Google Scholar]
- Seitz C, Ameres S, Forkmann G. Identification of the molecular basis for the functional difference between flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase. FEBS Lett. 2007;581(18):3429–3434. doi: 10.1016/j.febslet.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Springob K, Nakajima J, Yamazaki M, Saito K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat Prod Rep. 2003;20(3):288–303. doi: 10.1039/b109542k. [DOI] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis Thaliana Ralf Stracke, Martin Werber and Bernd Weisshaar. Curr Opin Plant Biol. 2001;4(5):447–456. doi: 10.1016/s1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- Sundqvist C, Dahlin C (1997) With chlorophyll pigments from prolamellar bodies to light-harvesting complexes. Physiologia Plantarum. 100(4). 10.1111/j.1399-3054.1997.tb00002.x
- Sung SY, Kim SH, Velusamy V, Lee YM, Ha BK, Kim JB, Kang SK, Kim HG, Kim DS. Comparative gene expression analysis in a highly anthocyanin pigmented mutant of colorless chrysanthemum. Mol Biol Rep. 2013;40(8):5177–5189. doi: 10.1007/s11033-013-2620-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008;54(4):733–749. doi: 10.1111/j.1365-313x.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- Tang HM, Liu SZ, Hill-Skinner S, Wu W, Reed D, Yeh CT, Nettleton D, Schnable PS. The maize brown midrib2 (bm2) gene encodes a methylenetetrahydrofolate reductase that contributes to lignin accumulation. Plant J. 2014;77(3):380–92. doi: 10.1111/tpj.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Liu ZY, Zhang Y, Li CY, Feng HY. Identification and fine mapping of a stay-green gene (Brnye1) In Pakchoi (Brassica Campestris L Ssp. Chinensis. Theor Appl Genet. 2018;131(3):673–684. doi: 10.1007/s00122-017-3028-8. [DOI] [PubMed] [Google Scholar]
- Yang XX, Liu C, Li YM, Yan ZS, Liu DJ, Feng GJ. Identification and fine genetic mapping of the golden pod gene (pv-ye) from the snap bean (Phaseolus vulgaris L.) Theor Appl Genet. 2021;134:3773–3784. doi: 10.1007/s00122-021-03928-6. [DOI] [PubMed] [Google Scholar]
- Zeng SH, Wu M, Zou CY, Liu XM, Shen XF, Hayward A, Liu CZ, Wang Y. Comparative analysis of anthocyanin biosynthesis during fruit development in two Lycium species. Physiol Plant. 2014;150(4):505–516. doi: 10.1111/ppl.12131. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Coyne DP, Mitra A. Factors affecting Agrobacterium mediated transformation of common bean. J Am Soc Hortic Sci. 1997;122(3):300–305. doi: 10.21273/JASHS.122.3.300. [DOI] [Google Scholar]
- Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1:641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wu H, Zhao M, Yang Z, Zhou Z, Guo Y, Lin Y, Chen H. OsMYB3 is a R2R3-MYB gene responsible for anthocyanin biosynthesis in black rice. Mol Breed. 2021;41:51. doi: 10.1007/s11032-021-01244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials generated or analysed during this study are included in this article and its supplementary information files.