Abstract
Many citrus fruits have polyembryonic traits, and their seeds contain many nucellar embryos along with a single zygotic embryo, affecting the crossbreeding process. Generally, nucellar embryos are considered to have more vigorous growth than zygotic embryos. Therefore, the in vitro method using an embryo rescue culture is often chosen to obtain zygotic embryo-derived individuals. Nevertheless, hybrids can be obtained with a certain probability from the seeds sown in the soil. The in-soil method, which sows seeds in the soil, has distinct advantages over the in vitro method, including lower cost and simpler technology. However, the efficiency of obtaining hybrids from these methods has not been compared in detail. The current study evaluates the effectiveness of these methods for obtaining hybrids using polyembryonic Satsuma mandarin as the female parent. The number of mature embryos per seed using the in-soil method was less than one-third of that produced using the in vitro method. Although the in vitro method produced more hybrids than the in-soil method, the ratio of the hybrids to the resulting population was significantly higher in the in-soil method. Thus, the in-soil method was more efficient and practical than the in vitro method for selecting hybrids from polyembryonic Satsuma mandarin seeds. The observations of the individuals obtained using the in-soil method suggest that zygotic embryos were not poorer in growth than nucellar embryos when using our selected parental combinations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-022-01324-6.
Keywords: Satsuma mandarin, Citrus, Polyembryonic, Crossbreeding, Zygotic, InDel marker
Introduction
Citrus hybrid generation is critical in crossbreeding programs, but this has not been widely achieved when the female parent is polyembryonic. Citrus varieties and cultivars (cultivated varieties among the varieties) developed in Japan are traceable, allowing us to view the current status of citrus breeding in the country. As of July 2021, the Japanese plant variety registration system shows that there are 288 registered citrus varieties with diverse traits which have been developed (http://www.hinshu2.maff.go.jp/vips/cmm/apCMM110.aspx). Cultivars can be developed with new traits by flexibly crossing these varieties; however, many of the varieties are polyembryonic, which makes crossbreeding such varieties challenging. More than 50% (149 varieties) of the total varieties were bred by mutations such as bud sport, whereas crossbred varieties account for approximately 30% (87 varieties) of the total varieties. Additionally, the female parents of the crossbred varieties are often a rare monoembryonic type such as ‘Kiyomi’, which was registered in 1979 (Omura and Shimada 2016) and accounts for approximately 25% (21/87) of all crossbred varieties. Thus, many hybrids have been bred through limited crosses between varieties.
Generating citrus hybrids with a polyembryonic female parent results in seeds with a zygotic embryo (hybrid embryo) as well as multiple nucellar embryos. Therefore, many of the progeny will be clonal individuals derived from nucellar embryos that share the same genetic information as the female parent. To obtain hybrid individuals, zygotic embryo-derived individuals must be selected from a population containing many nucellar-embryo-derived plants. In contrast, when a monoembryonic variety is used as the female parent, only a zygotic embryo is formed in the seed, making selection unnecessary. For these reasons, monoembryonic varieties such as ‘Kiyomi’ are often used as the female parent in citrus crossbreeding.
Only a limited number of embryos formed in polyembryonic seeds develop during the embryo maturation process due to spatial or nutritional competition with numerous coexisting nucellar embryos (Frost and Soost 1968). Moreover, nucellar embryos are more vigorous and inhibit the full development of the zygotic embryo (Soost and Roose 1996; Xu et al. 2013). In seeds with mature nucellar embryos, immature zygotic and nucellar embryos are also often present but do not germinate (Ueno et al. 1967; Esen and Soost 1977; Koltunow et al. 1995). Therefore, an in vitro method using an embryo rescue culture appears to be an effective method for the efficient acquisition of zygotic embryo-derived individuals (Ohta and Frusato 1957; Carimi et al. 1998; Pérez-Tornero and Porras 2008; Shen et al. 2011).
The two main issues with crossing polyembryonic female parents are: (1) the necessity to select zygotic embryos-derived individuals from a population containing numerous nucellar-embryo-derived individuals, and (2) the equipment, advanced techniques, and expenses that accompany the in vitro method. To resolve the first issue, we have developed insertion–deletion (InDel) markers that enable clear and inexpensive identification of hybridization using simple agarose gel electrophoresis (Noda et al. 2020,. 2021). Compared to simple sequence repeat (SSR) markers, which have been widely used to identify hybrids in recent years (Ferrante et al. 2010; Yildiz et al. 2013; Woo et al. 2019; Singh et al. 2020), the InDel markers allow for rapid identification at a lower cost. Methods using these markers do not require advanced or expensive equipment, such as a capillary DNA sequencer (Ferrante et al. 2010; Woo et al. 2019), or less complicated but time-consuming procedures, such as polyacrylamide gel electrophoresis (Yildiz et al. 2013; Singh et al. 2020).
The purpose of this study was to address the second issue. Previous in vitro culture experiments (Noda et al. 2020), which used polyembryonic female Satsuma mandarin parent, provided important insights for combatting this challenge. To our knowledge, embryo competition causes a decrease in the number of embryos in a seed, and if nucellar embryos are more vigorous than zygotic embryo, seeds with lower embryo numbers are likely to contain no zygotic embryo. However, previous analysis (Noda et al. 2020) of the individuals obtained from in vitro method did not show this tendency; there was no significant difference in the number of embryos between seeds containing zygotic embryos and those without zygotic embryos, and the zygotic embryos could be obtained even when there was only one embryo in the seed. This finding suggests that among many embryos formed in a polyembryonic seed, the zygotic embryo was not necessarily less vigorous than its nucellar counterparts. Therefore, the in-soil method, a method of obtaining hybrids from polyembryonic seeds sown in the soil, may be effective. Indeed, previous studies have shown that hybrid individuals can be obtained from in-soil methods using polyembryonic female parents (Nishiura and Iwashita 1964; Okudai et al. 1981). Additionally, there are several cultivars such as ‘Beauty Maple’ (Shikano et al. 1982), which have been registered as being bred from in-soil methods using polyembryonic female parents. The in-soil method has significant practical advantages over the in vitro method, such as lower cost, simpler technology, and less need for advanced equipment. However, the efficiency of these two methods has not yet been compared in detail.
Now that a rapid and inexpensive technique for selecting hybrids using InDel markers is available, it is easy to identify hybrids with a large number of samples. The current study compared the two methods, the in vitro and in-soil method, in detail and evaluated which method is the most practical for obtaining hybrids using polyembryonic Satsuma mandarin as the female parent.
Materials and methods
Plant materials
For polyembryonic inter-variety crosses, ‘Imamura’ (a sport of Satsuma) was used as the female parent, and ‘Kumamoto EC12’ (Mihara et al. 2018) and ‘AK13’ (female parent ‘Kumamoto EC10’ (Kitamura et al. 2014) × male parent ‘Kanpei’ (Shigematsu et al. 2008)) were used as the male parents. Artificial cross-pollination experiments were performed in 2020 when the plants were in full bloom (early May). The plants were preserved and grown at the Kumamoto Prefectural Fruit Tree Research Institute (32°38ʹ N, 130°42ʹ E; altitude: 49 m). Methods of pollen preparation and artificial pollination have been described in our previous study (Noda et al. 2020).
In vitro method using embryo rescue culture
Immature citrus seeds obtained from artificial hybridization were collected approximately 100 days after pollination (Deng et al. 1996; Tusa et al. 1996). The embryo-like tissues, including small globules of less than 1 mm in size, were excised from immature seeds under a stereomicroscope (Horiuchi et al. 1990; Takayanagi et al. 1991; Pan et al. 2009). The excised tissue was transferred to a test tube with media and sealed with aluminum foil. It was then incubated in a clean room under the following conditions: at 25 °C, under 16 h of continuous fluorescent light (5000 lx), followed by an 8-h dark period. Tissue that could be differentiated as plantlets were counted as the embryo number. The methods for the preparation of seeds from the resultant fruits, excision of embryos from the resultant seeds, and the culture of embryos have been described in our previous study (Noda et al. 2020). This method is referred to as the ‘in vitro method’ in this study.
In-soil method using mature seed-derived seedling
Mature citrus seeds obtained from artificial hybridization were collected approximately 210 days after pollination. The cut surface of the harvested fruit was exposed as in the in vitro method using an embryo rescue culture, and the seeds were removed from the cut surface of the fruit using a stainless-steel spoon. The pulp adhering to the seeds was removed under running water, and the seeds were placed in paper bags in cold storage (5 °C) overnight to dry the seed coat, which was then removed. The seeds, with the coat removed, were placed individually in Petri dishes lined with wet filter paper and kept in the dark at 28 °C for several days to allow rooting. Rooted seeds were then transplanted into horticultural soil No. 5 (Minami Kyushu Chemical Industry, Miyazaki, Japan) and grown in a greenhouse at 25 °C. The number of sprouts that germinated on the soil was counted as the embryo number of seed. This method is referred to as the ‘in-soil method’ in this study.
Assay to identify hybrid plants
The method of designing InDel markers has been described in our previous study (Noda et al. 2020). InDel markers, developed based on Satsuma mandarin genomic information, are DNA markers that facilitate genotyping of the zygosity of single-locus regions. These InDel markers classified the single-locus region into three genotypes based on the combination of two different allelic loci (loci with inserted sequences and those with deletion sequences). When heterozygous loci were analyzed using PCR, a longer fragment with an insertion sequence (homoduplex DNA molecule) and a shorter fragment with a deletion sequence (homoduplex DNA molecule) were amplified, and a heteroduplex DNA molecule derived from these DNA fragments of different length was formed (Lichten and Fox 1983; Delwart et al. 1993; Ota et al, 2013; Noda et al. 2020, 2021). Despite the presence of two alleles at one locus, three bands were detected by normal (non-denaturing) agarose gel electrophoresis (the lower two bands are the allelic homoduplex DNA molecules). When homozygous loci were analyzed using PCR, either the longer or shorter fragment derived from the allele locus was detected as a single band.
Genotypic change described above was used as an indicator to determine whether each individual was a hybrid plant. In Satsuma mandarin, the female parent used in this study, the genotypes of all InDel markers were heterozygous. Therefore, if the genotype of the progeny was homozygous, they were determined to be hybrid individuals. Since the probability of a change from heterozygous to homozygous genotype at each locus is 50%, the detection probability could be increased by using multiple InDel markers. For example, the use of four independent InDel markers, each derived from a different chromosome, results in a detection probability of 93.75%. Therefore, we used four independent InDel markers (LG1-7, LG2-3, LG3-1, and LG4-1) developed from different chromosomes for this assay. Plants that showed genotypic changes in one or more of the four markers were considered hybrids. Marker information, such as nucleotide positions, primer sequences, and sizes of fragments amplified by PCR, is listed in Table 1.
Table 1.
InDel markers used in this study
| Chromosome number | Marker | Nucleotide position numbera | InDel size (bp) | PCR primers | PCR product sizes (bp) (theoretical value of homoduplex DNA) | ||
|---|---|---|---|---|---|---|---|
| Tagged forward primer | Tailed reverse primer | Longer sequence allele with an insertion | Shorter sequence allele with a deletion | ||||
| 1 | LG1-7 | 16,218,933 | 23 | TGGCTTTCATGACTCTCATACC | AGCCAAAAATGCTGGATACG | 238 | 215 |
| 2 | LG2-3 | 5,629,260 | 25 | CCAATTTCATAAGATCCAGCAA | TGCTTCTCTTGTCCATCCTTC | 208 | 183 |
| 3 | LG3-1 | 4,063,617 | 20 | TGCTAAACCATAAGATCAAGCAAG | TCCCTTGGATGATTTTGTATCC | 233 | 213 |
| 4 | LG4-1 | 2,960,013 | 19 | TGCAAAAATGAAAGAGGTGATG | CCCAAGAGGTAAGGACACCA | 241 | 222 |
a The locus information for each InDel marker can be viewed in the Satsuma mandarin genome (Satsuma Pseudomolecules ver. 1.0) using the Satsuma mandarin genome browser (http://citrusgenome.db.naro.go.jp/). For example, for the InDel marker LG1-7 (the number after LG indicates the chromosome number), locus information can be obtained by searching for the nucleotide position number 16218933 of chromosome 1 (chr1)
Since the Satsuma mandarin female parent genotypes used in this study were heterozygous for all InDel markers, multiple InDel markers were needed to improve the detection probability of hybridization. InDel markers used in this study are transferrable to other citrus and related species (Noda et al. 2020). Hence, only one marker can be used to identify hybrids when crosses between varieties with different types of homozygous genotypes occur.
DNA extraction and PCR amplification
Total genomic DNA was purified using the DNAs-ici-P DNA extraction kit (RIZO Inc., Ibaraki, Japan). The DNA concentration was assessed using a Nanodrop 1000 spectrophotometer (Invitrogen, MA, USA) at 260 nm. PCR amplification was performed in 10-μL reaction volumes consisting of 0.1 μL of Blend Taq® (Toyobo, Osaka, Japan) (2.5 units μL−1), 1.0 μL of 10 × buffer for Blend Taq®, 1.0 μL dNTP (2 mM each), 0.2 μL of the forward and reverse primers each (10 μM), and 1.0 μL of template DNA (40 ng μL−1). InDel markers were amplified using a TP-600 PCR thermal cycler (Takara Bio Inc., Shiga, Japan), using a three-step PCR protocol with the following cycling conditions: denaturation at 95 °C for 5 min; and 30 cycles of denaturation at 95 °C for 30 s, primer annealing at 55 °C for 30 s, and primer extension at 72 °C for 30 s.
The resulting PCR products were subjected to electrophoretic gel analysis on 4% agarose. The agarose used was Kanto HC (Kanto Chemical Co., Tokyo, Japan), which is suitable for the separation of low molecular weight DNA fragments. The amplified size was compared with that of a 100 bp DNA ladder marker.
Statistical analysis
To compare the means of the two groups, a t test was performed using Welch’s method for a two-tailed test. To compare the proportions of categorical variables between the two groups, the χ-square test was performed. Pearson’s correlation coefficients were calculated to examine the relationships between the continuous variables. Statistical significance was set at the 5% level (p < 0.05). Numerical data are presented as the mean ± standard deviation (SD), unless otherwise noted. Statistical analysis was performed using BellCurve for Excel version 3.21 (Social Survey Research Information Co., Tokyo, Japan).
Results
Hybrid selection using InDel markers
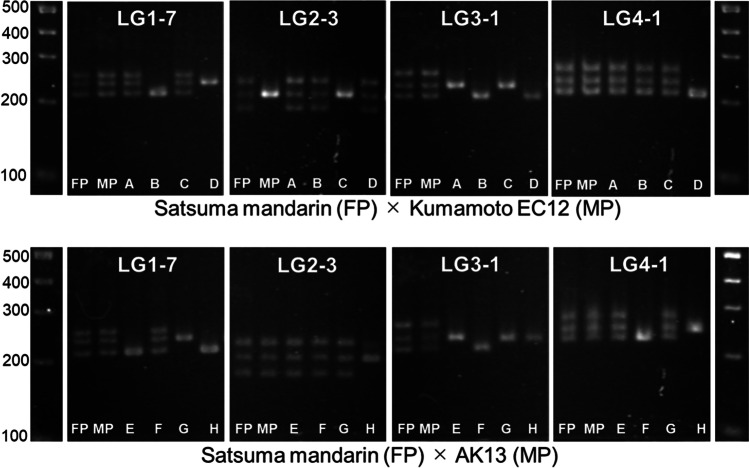
Figure 1 exhibits an example assays to identify hybrids using InDel markers. With at least one of the four InDel markers, the heterozygous genotype of the female parent was found to be homozygous in the hybrid plant. Moreover, the genotypic changes in all InDel markers between the parents and progeny were consistent.
Fig. 1.
An example of an assay to identify hybrids using four different InDel markers. Lane FP: female parent; lane MP: male parent; lanes A–D: hybrid plants from Satsuma mandarin (female parent) and ‘Kumamoto EC12’ (male parent); lanes E–F: hybrid plants from Satsuma mandarin (female parent) and ‘AK13’ (male parent). Electrophoresis was performed using a 4% agarose gel. The number after LG in the marker name indicates the chromosome number. In the assay to identify hybrids, InDel markers located on four independent chromosomes were used
Comparison between the in vitro and in-soil method
The in vitro and in-soil methods were compared using two cross combinations with ‘Satsuma mandarin’ as the female parent and ‘Kumamoto EC12’ and ‘AK13’ as male parents (Table 2).
Table 2.
The acquisition process of hybrid plants obtained from in vitro and in-soil methods
| Male parent Male parent |
Processes Processes |
Methods | Significant difference testa Significant difference test a |
||
|---|---|---|---|---|---|
| In vitro | In-soil | ||||
| Kumamoto EC12 | Number (no.) of flowers pollinated | 350 | |||
| No. of fruits set | 52 | 62 | |||
| No. of fruits containing seeds | 40 | 41 | |||
| Percentage (%) of fruits containing seeds | 76.9 | 66.1 | n.s | χ2 (1) = 1.6020, p = 0.2056 | |
| No. of seeds per fruit (mean ± SD) | 2.35 ± 1.48 (n = 40) | 2.95 ± 1.96 (n = 41) | n.s | t test, p = 0.1228 | |
| Total no. of seeds | 94 | 121 | |||
| No. of seeds containing embryos | 88 | 112 | |||
| % of seeds containing embryos | 93.6 | 92.6 | n.s | χ2 (1) = 0.0907, p = 0.7632 | |
| No. of embryo-like tissues placed on the culture medium | 668 | ‒ | |||
| Total no. of embryosb | 616 | 188 | |||
| No. of embryos per seed (mean ± SD) | 7.00 ± 4.07 (n = 88) | 1.68 ± 0.77 (n = 112) | * | t test, p = 1.0915 × 10−20 | |
| No. of individuals derived from zygotic embryos | 36 | 28 | |||
| No. of embryos per seed with zygotic embryo (mean ± SD) | 7.53 ± 4.37 (n = 36) | 1.29 ± 0.54 (n = 28) | * | t test, p = 3.8169 × 10−10 | |
| No. of individuals derived from nucellar embryos | 580 | 160 | |||
| No. of embryos per seed without zygotic embryos (mean ± SD) | 6.64 ± 3.86 (n = 52) | 1.81 ± 0.80 (n = 84) | * | t test, p = 3.6417 × 10−12 | |
| % of seeds containing zygotic embryo in total seeds | 40.9 | 25.0 | * | χ2 (1) = 5.7320, p = 0.0167 | |
| % of hybrids in total individuals | 5.8 | 14.9 | * | χ2 (1) = 16.1001, p = 6.0081 × 10−5 | |
| AK13 | No. of flowers pollinated | 354 | |||
| No. of fruits set | 54 | 60 | |||
| No. of fruits containing seeds | 40 | 47 | |||
| % of fruits containing seeds | 74.1 | 78.3 | n.s | χ2 (1) = 0.2853, p = 0.5933 | |
| No. of seeds per fruit (mean ± SD) | 2.98 ± 1.89 (n = 40) | 2.72 ± 1.80 (n = 47) | n.s | t test, p = 0.5288 | |
| Total no. of seeds | 119 | 128 | |||
| No. of seeds containing embryos | 106 | 105 | |||
| % of seeds containing embryos | 89.1 | 82.0 | n.s | χ2 (1) = 2.4578, p = 0.1169 | |
| No. of embryo-like tissues placed on the culture medium | 634 | ‒ | |||
| Total no. of embryosb | 577 | 157 | |||
| No. of embryos per seed (mean ± SD) | 5.44 ± 4.06 (n = 106) | 1.50 ± 0.68 (n = 105) | * | t test, p = 7.2097 × 10−17 | |
| No. of individuals derived from zygotic embryos | 54 | 35 | |||
| No. of embryos per seed with zygotic embryo (mean ± SD) | 5.28 ± 4.11 (n = 54) | 1.23 ± 0.49 (n = 35) | * | t test, p = 2.0393 × 10−9 | |
| No. of individuals derived from nucellar embryos | 516 | 122 | |||
| No. of embryos per seed without zygotic embryo (mean ± SD) | 5.62 ± 4.04 (n = 52) | 1.63 ± 0.73 (n = 70) | * | t test, p = 3.8314 × 10−9 | |
| % of seeds containing zygotic embryo in total seeds | 50.9 | 33.3 | * | χ2 (1) = 6.7073, p = 0.0096 | |
| % of hybrids in total individuals | 9.4 | 22.3 | * | χ2 (1) = 18.8290, p = 1.430 × 10−5 | |
aIn the significance test, data sets deemed not to be significantly different are shown as n.s. (not significant, p > 0.05), and data sets deemed to be significantly different are shown as * (p < 0.05). The t test was performed using Welch’s method with a two-tailed test
bThe number of embryos in the in vitro method is the number of immature embryos that have differentiated into plantlets, while the number of embryos in the in-soil method is the number of mature embryos that have germinated in the soil
Using ‘Kumamoto EC12’, 616 progenies from 52 immature fruits were studied for the in vitro method, and 188 progenies from 62 mature fruits were studied for the in-soil method. There were no significant differences in the mean number of seeds per fruit and the percentage of seeds containing embryos between the two methods. However, there were significant differences (p < 0.05) between the in vitro and in-soil methods in the mean number of embryos per seed, mean number of embryos per seed with zygotic embryos, mean number of embryos per seed without zygotic embryos, percentage of seeds with zygotic embryos, and percentage of hybrids among the total individuals obtained.
Using ‘AK13’, 577 progenies from 54 immature fruits were studied for the in vitro method, and 157 progenies from 60 mature fruits were studied for the in-soil method. Results showed the items with and without significant differences were the same as those when using ‘Kumamoto EC12’ as the male parent.
Effects of different male parents
The effects of two male parents on the efficiency of acquiring hybrid individuals were compared (Table S1). In the in vitro method, there were no significant differences (p > 0.05) between the two male parents in the percentage of fruits containing seeds, mean number of seeds per fruit, percentage of seeds containing embryos, percentage of cultured tissues that differentiated into plantlets, mean number of embryos per seed without zygotic embryos, and percentage of seeds containing zygotic embryos. Significant differences (p < 0.05) between the two male parents were observed in the mean number of embryos per seed, mean number of embryos in seeds containing zygotic embryos, and percentage of hybrid individuals among the total individuals obtained.
In the in-soil method, the percentage of germinated seeds was significantly higher with the male parent, ‘Kumamoto EC12’, than with ‘AK13’, but there were no significant differences in the other comparisons.
Embryo number distribution in seeds with and without zygotic embryos
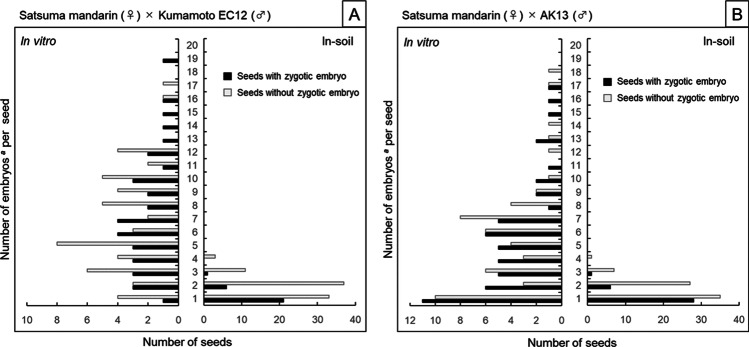
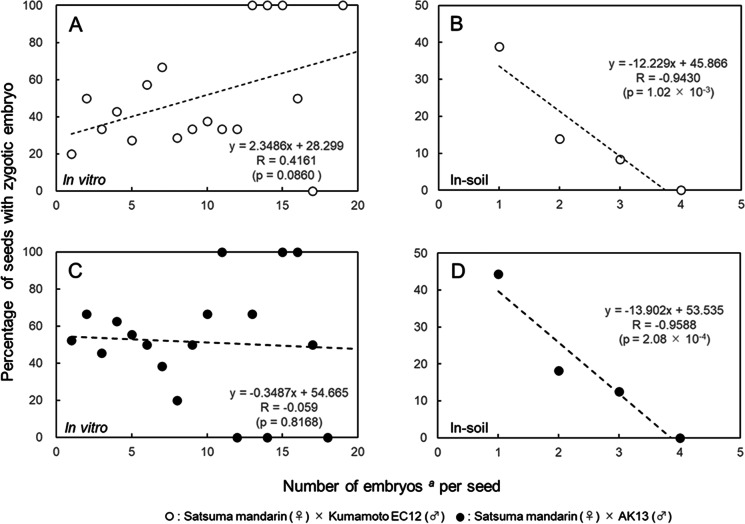
The embryo number distribution in seeds with and without zygotic embryos was compared (Fig. 2). In the in vitro method using ‘Kumamoto EC12’ as the male parent (Fig. 2A), the embryo number distributions in seeds with and without zygotic embryos ranged from 1 to 19 (median 7) and from 1 to 17 (median 6), respectively. There was no significant difference in the proportion of seeds with and without zygotic embryos in relation to the embryo number in the seeds (χ2 (17) = 11.9639, p = 0.8023). In contrast, the in-soil method showed a distribution of embryo number with and without zygotic embryos ranging from 1 to 3 (median 1) and from 1 to 4 (median 2), respectively. There was a significant difference in the proportion of seeds with and without zygotic embryos based on the embryo number in the seeds (χ2 (3) = 11.1318, p = 0.0110). Pearson’s correlation coefficients between the embryos number per seed and the percentage of seeds with zygotic embryo were statistically not significant (0.4161, p = 0.0860) in the in vitro method (Fig. 3A) and statistically significant (− 0.9430, p = 1.02 × 10−3) in the in-soil method (Fig. 3B), supporting this result.
Fig. 2.
Embryo number distribution in seeds with and without zygotic embryos. Seeds for the in vitro method were prepared from immature fruit harvested approximately 100 days after pollination. Seeds for the in-soil method were prepared from mature fruit harvested approximately 210 days after pollination. aThe number of embryos in the in vitro method is the number of immature embryos that have differentiated into plantlets, while the number of embryos in the in-soil method is the number of mature embryos that have germinated in the soil. In the in vitro method, both A and B parental combinations showed no significant differences in the percentage of seeds with and without zygotic embryos in relation to the number of embryos in the seeds (χ2 (17) = 11.9639, p = 0.8023 and χ2 (17) = 10.8747, p = 0.8630, respectively). In the in-soil method, both A and B parental combinations showed significant differences in the percentage of seeds with and without zygotic embryos in relation to the number of embryos in the seeds (χ2 (3) = 11.1318, p = 0.0110, p = 0.8023 and χ.2 (3) = 8.9716, p = 0.0297, respectively)
Fig. 3.
Relationship between the number of embryos per seed and the percentage of seeds containing zygotic embryos. A: Embryos rescued from immature seeds (approximately 100 days after pollination) crossed with the male parent ‘Kumamoto EC12’. B: Embryos obtained from mature seeds (approximately 210 days after pollination) crossed with the male parent ‘Kumamoto EC12’. C: Embryos rescued from immature seeds (approximately 100 d after pollination) crossed with the male parent ‘AK13’. D: Embryos obtained from mature seeds (about 210 days after pollination) crossed with the male parent ‘AK13’. aThe number of embryos per seed in vitro rescue culture is the number of immature embryos that have differentiated into plantlets, while the number of embryos per seed sown in the soil is the number of mature embryos that have germinated in the soil
In the in vitro method using ‘AK13’ as the male parent (Fig. 2B), the embryo number distribution in seeds with and without zygotic embryos ranged from 1 to 17 (median 4.5) and from 1 to 18 (median 5.5), respectively. There was no significant difference in the proportion of seeds with and without zygotic embryos, in relation to the embryo number in the seeds (χ2 (17) = 10.8747, p = 0.8630). In contrast, in the in-soil method, the distribution of the embryo number in seeds with and without zygotic embryos ranged from 1 to 3 (median 1) and from 1 to 4 (median 1.5), respectively. There was a significant difference in the proportion of seeds with and without zygotic embryos depending on the embryo number in the seeds (χ2 (3) = 8.9716, p = 0.0297). Pearson’s correlation coefficients between the embryo number per seed and the percentage of seeds with zygotic embryo were statistically not significant (− 0.059, p = 0.8168) in the in vitro method (Fig. 3C) and statistically significant (− 0.9588, p = 2.08 × 10−4) in the in-soil method (Fig. 3D), supporting this result.
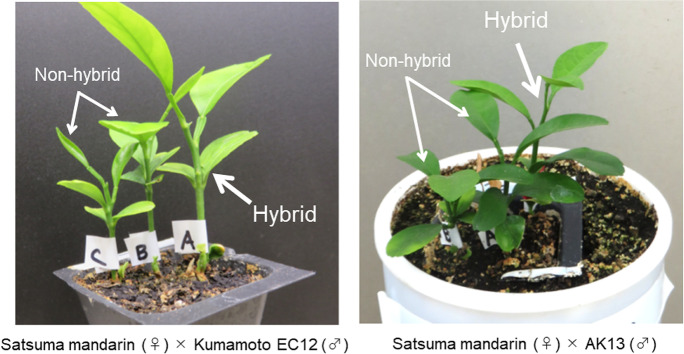
These results indicate that with the in vitro method, there was a minor relationship between the immature embryo number in a seed and the possibility of obtaining hybrid individuals, whereas in the in-soil method, a smaller number of mature embryos in a seed related to a greater probability of obtaining hybrid individuals. Furthermore, of the seeds from which hybrid plants were obtained by the in-soil method, there were seven seeds from each parental combination that germinated two to three sprouts per seed (Fig. 2). In most of these seeds (7/7 and 5/7 from female parents ‘Kumamoto EC12’ and ‘AK13’, respectively), the most vigorous sprouts were the hybrids (Fig. 4).
Fig. 4.
Hybrid individuals derived from polyembryonic seeds by the in-soil method. Of the seeds from which hybrids were obtained by the in-soil method, several seeds germinated two to three sprouts per seed (Fig. 2). In most of these seeds (7/7 and 5/7 from male parents ‘Kumamoto EC12’ and ‘AK13’, respectively), the most vigorous sprouts were the hybrids
Discussion
The purpose of this study was to examine whether the in vitro or in-soil approach was more practical for obtaining zygotic embryo-derived individuals when crossbreeding polyembryonic Satsuma mandarin as the female parent. We found that the in-soil method was superior to the in vitro method in several aspects and discuss the advantages of each method based on the obtained data below.
The in-soil method provides the advantage of higher rate of zygotic embryo acquisition relative to the total assay number. The percentage in the in-soil method was twofold higher than that in the in vitro method (14.9/5.8 or 22.3/9.5), indicating higher selection efficiency. However, an advantage of the in vitro method is that the maximum number of zygotic embryos can be obtained from the cross-experiment seeds. The acquisition rate of seeds containing zygotic embryos was 40.9% and 50.9% with the male parents ‘Kumamoto EC12’ and ‘AK13’, respectively, but decreased to 25.0% and 33.3%, respectively, when the in-soil method was used. This result implies that a certain proportion of zygotic embryos failed to develop fully along with nucellar embryos during the seed maturation process.
Thus, the only disadvantage of the in-soil method compared to the in vitro method was the small number of hybrid individuals obtained from the resulting seeds. However, individuals obtained by the in vitro method using an embryo rescue culture were plantlets cultured in vitro; therefore, acclimatization treatment was necessary to enable growth in the field. The success rate of acclimatization of culture plants in vitro was approximately 20% in previous experiments (Noda et al. 2020). Therefore, we predict that the number of acclimatized plants will be much lower than 40.9% or 50.9% after the acclimatization treatment of in vitro cultured plants. Plants can likely be obtained more efficiently from in vitro cultured individuals through micro-grafting techniques or by improving the acclimatization techniques.
In contrast, individuals derived from zygotic embryos selected from in-soil methods were plants that can be grown in the field without the need for an acclimatization process. The availability of hybrid plants from the in-soil method was greater than half the availability of hybrids plants from the in vitro culture (40.9% in the in vitro method and 25.0% in the in-soil method for the male parent ‘Kumamoto EC12’; 50.9% in the in vitro method and 33.3% in the in-soil method for the male parent ‘AK13’). When considering the acclimatization rate, using the in-soil method may be superior to using the in vitro method in terms of the number of hybrid plants that can be grown in the field; however, this depends on the skill level when implementing the acclimatization technique.
In summary, in our parental combinations, selection using the in vitro method was effective for obtaining the greatest number of hybrid plants from the obtained seeds, whereas selection using the in-soil method was more practical for efficiently obtaining hybrid plants that can be used for breeding.
The hybrids obtained using the methods reported here are only a subset of the population with the potential of possessing useful traits. To develop new varieties, individuals with useful traits can be selected from this population. Even with grafting techniques, it can take a long time to evaluate fruit traits. In citrus, genomic selection technology has the potential to predict key characteristics based on DNA marker information (Minamikawa et al. 2017). If this technology for evaluating traits prior to grafting is improved, it will likely enable early selection from the crossbred population in combination with DNA marker selection of the hybrids.
In the in vitro method, there was no significant difference in the number of embryos per seed between the cases with and without zygotic embryos, and the former could be obtained when there was only one embryo in the seed. These results are similar to previous reports (Noda et al. 2020), although the varieties of the male parent used were different. However, using the in-soil method, the two cross combinations showed a strong negative correlation between the number of mature embryos in the seed and the percentage of seeds containing zygotic embryo (Fig. 3B, D). If the growth of the nucellar embryos inhibited the development of the zygotic embryo, mature seeds with lower embryo numbers were likely to contain no zygotic embryos. Therefore, the results suggest that zygotic embryos are not easily outcompeted by nucellar embryos in our parental combinations. These results contradict the previously proposed viewpoint that it is difficult to obtain hybrid plants from mature seeds because of poorer zygotic embryos growth rates when compared to nucellar embryos growth rates (Frost and Soost 1968; Furusato et al. 1957; Horiuchi et al. 1990; Shimada et al. 2018). The relationship between zygotic and nucellar embryos in immature seeds was not as clear (Fig. 3A, C) as that in mature seeds (Fig. 3B, D), possibly because they were subjected to selection pressure for a shorter period. Further evaluation is necessary to determine the influence of the period on embryo rescue.
A polyembryonic female parent can produce several different seed types (Wakana and Uemoto 1988; Horiuchi et al. 1990): Type I seeds with one zygotic embryo, Type II seeds with one nucellar embryo, Type III seeds with one zygotic and one or more nucellar embryos, and Type IV seeds with multiple nucellar embryos. There were significant differences in the proportion of seed types between the in vitro and in-soil methods, and the percentage of seeds with one embryo was significantly higher in the in-soil method than in the in vitro method for both parental combinations (Table 3). Therefore, the seed maturation process supports the survival of a single full-developed embryo, especially in the in-soil method. Despite the results that zygotic embryo growth obtained from the in-soil method was more vigorous than nucellar embryo growth in our parental combinations, the number of seeds with a single nucellar embryo was higher than the number of seeds with a single zygotic embryo in the in-soil method. A possible reason for this seemingly contradictory result is that many of the seeds with only one nucellar embryo may have been Type II or Type IV embryos. Thus, when seeds contain one mature nucellar embryo, the zygotic embryo was not outcompeted, but rather the zygotic embryo may not have originally been formed.
Table 3.
Seed type distribution in in vitro and in-soil methods
| Male parent | Methods | Seed typesa | ||||
|---|---|---|---|---|---|---|
| Type I | Type II | Type III | Type IV | |||
| Kumamoto EC12 | In vitro | No. of seeds | 1 | 4 | 35 | 48 |
| % of seeds | 1.1 | 4.5 | 39.8 | 54.5 | ||
| In-soil | No. of seeds | 21 | 33 | 7 | 51 | |
| % of seeds | 18.8 | 29.5 | 6.3 | 45.5 | ||
| AK13 | In vitro | No. of seeds | 11 | 10 | 43 | 42 |
| % of seeds | 10.4 | 9.4 | 40.6 | 39.6 | ||
| In-soil | No. of seeds | 28 | 35 | 7 | 35 | |
| % of seeds | 26.7 | 33.3 | 6.7 | 33.3 | ||
aSeed types are classified as follows: (I) seeds with one zygotic embryo; (II) seeds with one nucellar embryo; (III) seeds with one zygotic and one or more nucellar embryos; and (IV) seeds with multiple nucellar embryos. There were significant differences in the percentage of seed type distribution between the in vitro and in-soil methods in both female parents (χ2(3) = 57.6188, p = 1.8958 × 10−12 and χ2(3) = 47.8518, p = 2.2898 × 10−10 for female parents ‘KumamotoEC12’ and ‘AK13’, respectively)
The difficulty of obtaining hybrids from polyembryonic seeds by the in-soil method is widely known; however, polyembryony is a useful trait to generate large numbers of uniform rootstocks from a seed. Zhu et al (2013) compared the in vitro and in-soil methods using the rootstock cultivars and reported that no hybrids were obtained by the in-soil method. In contrast, a certain percentage of hybrid individuals can be obtained using the in-soil method with the female parent Satsuma mandarins used in this study (Nishiura and Iwashita 1964; Okudai et al. 1981). The hybrid progeny percentages depend on the parental combination (Spiegel-Roy et al. 1977; Tan et al 2007; Caruso et al. 2014), pollen origin (Cameron and Soost 1980; Soares-Filho et al. 1995), and environmental influences (Khan and Roose 1988; Moore and Castle 1988). Using an embryo rescue culture in the in vitro method, plant recovery from immature embryos is also affected by embryo stage and culture medium (Pérez-Tornero and Porras 2008; Shen et al.2011; Singh et al. 2020). In this study, there was a significant difference in the hybrid generation between the two parental combinations (Table S1), suggesting that the male parents affected the percentage of recovered zygotic plants. The results of this study were obtained in a single year, and it is necessary to examine the effects of the diverse factors mentioned above on the efficiency of the hybrid embryo generation.
Although the crossbreeding combinations performed in this study were limited to two, we demonstrated the effectiveness of the in-soil method for selecting hybrid plants for crossbreeding between polyembryonic varieties. It is particularly noteworthy that among many embryos formed in a polyembryonic seed, zygotic embryos were more vigorous than their nucellar competitors in our parental combinations. These results provide evidence that the in-soil method is more efficient than the in vitro method. The phenomena observed in this study, which do not follow the established theory on the relationship between zygotic and nucellar embryos, have the potential to further develop existing knowledge in this area. It will be interesting to see if phenomena such as those observed in this study are limited to our parental combinations or can be observed in various parental combinations.
Citrus crosses are not only used to obtain diploid hybrid individuals by crossing diploid varieties but also used to obtain triploid hybrids. These are mainly used for breeding seedless varieties. Two main cross methods have been successfully applied to recover triploid citrus hybrids: diploid and tetraploid sexual hybridization (Esen and Soost 1973; Xie et al 2019) and inter-diploid sexual hybridizations (Esen and Soost 1971; Navarro et al 2015). In both cases, an embryo rescue culture is a decisive technique, especially for recovering triploid individuals from embryos formed in small undeveloped seeds. Triploid individuals can be obtained from fully developed seed-derived embryos in interploid sexual hybridization, even when polyembryonic varieties are used as female parents (Kaneyoshi et al. 1997; Viloria and Grosser 2005; Aleza et al. 2012). This suggests that triploid individuals may be obtained from the in-soil method, even if the female parent is polyembryonic. Although the transferability of the InDel markers to polyploid citrus varieties has not been tested, it is likely possible. Findings from the current study highlight the selection efficiency of the in-soil method to obtain diploid hybrids from diploid intercrosses. However, further research is needed to examine whether a similar phenomenon can be observed in interploid sexual hybridization.
Although crossbreeding between polyembryonic citrus varieties is possible, technical difficulties such as the in vitro method and hybrid detection techniques, have created a substantial challenge. However, the straightforward method of selecting hybrid plants from the in-soil method in combination with inexpensive genotyping technology using InDel markers can reduce the cost and technical issues. This method can be implemented by smaller research institutions that do not have extensive facilities.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Naomi Matsuda and Keiko Otaguro for their technical assistance. We would like to thank Editage (www.editage.com) for the English editing services.
Author contribution
TN, KD, TM, HM, and YN conceived and designed the experiments. TM prepared the citrus genetic resources. TN and KD performed the experiments. The manuscript was drafted by TN and edited by YN, and was subsequently reviewed by all authors.
Funding
This work was partly supported by a Grant-in-Aid for Scientific Research (18K05623) from the Japan Society for the Promotion of Science to Yukio Nagano.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
On behalf of all authors, the corresponding author states that the experimental research on plants were performed in accordance with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reference
- Aleza P, Juárez J, Cuenca J, Ollitrault P, Navarro L. Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent-effect on the length of the juvenile phase. Plant Cell Rep. 2012;31:1723–1735. doi: 10.1007/s00299-012-1286-0. [DOI] [PubMed] [Google Scholar]
- Cameron JW, Soost RK. Mono and poly embryony among tetraploid Citrus hybrids. Hortic Sci. 1980;15:730–731. [Google Scholar]
- Carimi F, de Pasquale F, Puglia AM. In vitro rescue of zygotic embryos of sour orange, Citrus aurantium L., and their detection based on RFLP analysis. Plant Breed. 1998;117:261–266. doi: 10.1111/j.1439-0523.1998.tb01936.x. [DOI] [Google Scholar]
- Caruso M, Distefano G, Pietro PD, La Malfa S, Russo G, Gentile A, Recupero GR. High-resolution melting analysis for early identification of citrus hybrids: a reliable tool to overcome the limitations of morphological markers and assist rootstock breeding. Sci Hortic. 2014;180:199206. doi: 10.1016/j.scienta.2014.10.024. [DOI] [Google Scholar]
- Delwart EL, Shpaer EG, Louwagie J, McCutchan FE, Grez M, Rübsamen-Waigmann H, Mullins JI. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993;262:1257–1261. doi: 10.1126/science.8235655,Pubmed:8235655. [DOI] [PubMed] [Google Scholar]
- Deng XX, Yi HL, Li F, Guo WW. Triploid plants regenerated from crossing diploid pummelo and tangerine with allotetraploid somatic hybrid of Citrus. Proc Intl Soc Citricult. 1996;1:189–192. [Google Scholar]
- Esen A, Soost RK. Unexpected triploids in citrus: their origin, identification and possible use. J Hered. 1971;62:329–333. doi: 10.1093/oxfordjournals.jhered.a108186. [DOI] [Google Scholar]
- Esen A, Soost RK. Seed development in citrus with special reference to 2x X 4x crosses. Am J Bot. 1973;60:448–462. doi: 10.1002/j.1537-2197.1973.tb05945.x. [DOI] [Google Scholar]
- Esen A, Soost RK. Adventive embryogenesis in citrus and its relation to pollination and fertilization. Am J Bot. 1977;64:607–614. doi: 10.2307/2441710. [DOI] [Google Scholar]
- Ferrante SP, Lucretti S, De Patrizio A, Abbate L, Tusa N, Scarano M. Assessment of the origin of new citrus tetraploid hybrids (2n = 4x) by means of SSR markers and PCR based dosage effects. Euphytica. 2010;173:223–233. doi: 10.1007/s10681-009-0093-3. [DOI] [Google Scholar]
- Frost HB, Soost RK. Seed reproduction. Development of gametes and embryos. In: Reuther W, Batchelor LD, Webber HJ, editors. The citrus industry. Berkeley: University of California Press; 1968. pp. 290–324. [Google Scholar]
- Furusato K, Ohta Y, Ishibashi K. Studies on polyembryony in citrus. Seiken Ziho. 1957;8:40–48. [Google Scholar]
- Horiuchi S, Yuda E, Nakagawa S, Morimoto J, Gato Y. Shapes of seed including well-developed zygotic embryo in polyembryonic citrus species/cultivars. J Jpn Soc Hortic Sci. 1990;59:225–235. doi: 10.2503/jjshs.59.225. [DOI] [Google Scholar]
- Kaneyoshi J, Kanou T, Kuwata Y, Hirao A, Nakatani S, Kobayashi S. Breeding of triploid citrus cultivars I: Production of triploids from Satsuma mandarin (Citrus unshiu Marc.) × Tetraploid Ponkan Mandarin (Citrus reticulata Blanco) crosses. Engei Gakkai zasshi. 1997;66:9–14. doi: 10.2503/jjshs.66.9. [DOI] [Google Scholar]
- Khan IA, Roose ML. Frequency and characteristics of nucellar and zygotic seedlings in three cultivars of trifoliate orange. J Am Soc Hortic Sci. 1988;113:105–110. doi: 10.21273/JASHS.113.1.105. [DOI] [Google Scholar]
- Kitamura M, Sakaki H, Sakanishi M, Fujita K, Kitazono K, Fukunaga Y, Mitsuta M, Isobe A. Characteristic of early citrus cultivar ‘Kumamoto EC10’. Kumamoto Prefectural Agric Res Cent. 2014;21:42–46. [Google Scholar]
- Koltunow AM, Soltys K, Nito N, McClure S. Anther, ovule, seed, and nucellar embryo development in Citrus sinensis cv. Valencia Can J Bot. 1995;73:1567–1582. doi: 10.1139/b95-170. [DOI] [Google Scholar]
- Lichten MJ, Fox MS. Detection of non-homology-containing heteroduplex molecules. Nucl Acids Res. 1983;11:3959–3971. doi: 10.1093/nar/11.12.3959,Pubmed:6223275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara T, Kitamura M, Sakanishi M, Kitazono K. New citrus cultivar ‘Kumamoto EC12’ can be shipped in December, with high quality. Research Kumamoto Prefectural Agric Res Cent. 2018;26:19–24. [Google Scholar]
- Minamikawa MF, Nonaka K, Kaminuma E, Kajiya-Kanegae H, Onogi A, Goto S, Yoshioka T, Imai A, Hamada H, Hayashi T, Matsumoto S, Katayose Y, Toyoda A, Fujiyama A, Nakamura Y, Shimizu T, Iwata H. Genome-wide association study and genomic prediction in citrus: potential of genomics-assisted breeding for fruit quality traits. Sci Rep. 2017;7:4721. doi: 10.1038/s41598-017-05100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, Castle WS. Morphological and isozymic analysis of open-pollinated Citrus rootstock populations. J Hered. 1988;79:59–63. doi: 10.1093/oxfordjournals.jhered.a110448. [DOI] [Google Scholar]
- Navarro L, Aleza P, Cuenca J, Juárez J, Pina JA, Ortega C, Navarro A, Ortega V. The mandarin triploid breeding program in Spain. Acta Horticult. 2015;1605:389–395. doi: 10.17660/ActaHortic.2015.1065.48. [DOI] [Google Scholar]
- Nishiura M, Iwashita T. Studies on the citrus breeding. II: Number of seedlings per seed and number of gametic and nucellar seedlings from Satsuma mandarin by cross-pollination. Bull Hort Res Sta Ser B. 1964;3:1–9. [Google Scholar]
- Noda T, Daiou K, Mihara T, Nagano Y. Development of indel markers for the selection of Satsuma mandarin (Citrus unshiu Marc.) hybrids that can be used for low-cost genotyping with agarose gels. Euphytica. 2020;216:115. doi: 10.1007/s10681-020-02654-2. [DOI] [Google Scholar]
- Noda T, Daiou K, Mihara T, Nagano Y. Potential application of simple easy-to-use insertion-deletion (Indel) markers in citrus cultivar identification. Breed Sci. 2021;71:601–608. doi: 10.1270/jsbbs.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudai N, Oiyama I, Takahara T. Studies on the improvement of zygotic seedling yield of the polyebnryonic citrus. I: Differences in embryo number per seed and zygotic seedling yield among its varieties and strains. Bull Fruit Tree Res Stn Ser D. 1981;3:9–21. [Google Scholar]
- Omura M, Shimada T. Citrus breeding, genetics and genomics in Japan. Breed Sci. 2016;66:3–17. doi: 10.1270/jsbbs.66.3,Pubmed:27069387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Furusato K. Embryo culture in citrus. Seiken Zilho Rpt Kihara Inst Biol Res. 1957;23:49–54. [Google Scholar]
- Ota S, Hisano Y, Muraki M, Hoshijima K, Dahlem TJ, Grunwald DJ, Okada Y, Kawahara A. Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells. 2013;18:450–458. doi: 10.1111/gtc.12050,Pubmed:23573916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Guan R, Zhu S, Deng X. Proteomic analysis of somatic embryogenesis in Valencia sweet orange (Citrus sinensis Osbeck) Plant Cell Rep. 2009;28:281–289. doi: 10.1007/s00299-008-0633-7. [DOI] [PubMed] [Google Scholar]
- Pérez-Tornero O, Porras I. Assessment of polyembryony in lemon: rescue and in vitro culture of immature embryos. Plant Cell Tiss Organ Cult. 2008;93:173–180. doi: 10.1007/s11240-008-9358-0. [DOI] [Google Scholar]
- Shen X, Gmitter FG, Grosser JW. Immature embryo rescue and culture. Methods Mol Biol. 2011;710:75–92. doi: 10.1007/978-1-61737-988-8_7,Pubmed:21207263. [DOI] [PubMed] [Google Scholar]
- Shikano E, Hara T, Tachikawa T, Tanaka Y. On the characters of the citrus variety ‘Beauty maple tangor’. Bull Shizuoka Citrus Exp Sta. 1982;18:11–16. [Google Scholar]
- Shimada T, Endo T, Fujii H, Nakano M, Sugiyama A, Daido G, Ohta S, Yoshioka T, Omura M. MITE insertion-dependent expression of CitRKD1 with a RWP-RK domain regulates somatic embryogenesis in citrus nucellar tissues. BMC Plant Biol. 2018;18:166. doi: 10.1186/s12870-018-1369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu Y, Kita K, Yakushiji H, Ishikawa K, Inoue H, Nakataet H. The new citrus cultivar ‘Kanpei’. Bull Ehime Fruit Tree Exp Stn. 2008;22:1–4. [Google Scholar]
- Singh J, Dhaliwal HS, Thakur A, Sidhu GS, Chhuneja P, Gmitter FG. Optimizing recovery of hybrid embryos from interspecific citrus crosses of polyembryonic rough lemon (Citrus jambhiri lush) Agronomy. 2020;10:1940. doi: 10.3390/agronomy10121940. [DOI] [Google Scholar]
- Soares-Filho WdS, Lee LM, da Cunha Sobinho AP. Influence of pollinators on polyembryony in Citrus. Acta Hortic. 1995;403:256–265. doi: 10.17660/ActaHortic.1995.403.45. [DOI] [Google Scholar]
- Soost RK, Roose ML. Citrus. In: Janick J, Moore JN, editors. Fruit breeding. New York: Wiley; 1996. pp. 257–323. [Google Scholar]
- Spiegel-Roy P, Bardi A, Shani A. Peroxidase isozymes as a tool for early separation of nucellar and zygotic citrus seedlings. Fla-U S A. Second Meeting of the International Society of Citriculture. 1977;2:619–624. [Google Scholar]
- Takayanagi R, Miura Y, Kitanura T, Hidaka T (1991) Breeding of new citrus cultivar by embryo culture (I) Research bulletin of the Kanagawa prefectural agricultural research center 133: 75–81. (in Japanese with English abstract)
- Tan ML, Song JK, Deng XX. Production of two mandarin × trifoliate orange hybrid populations via embryo rescue with verification by SSR analysis. Euphytica. 2007;157:155–160. doi: 10.1007/s10681-007-9407-5. [DOI] [Google Scholar]
- Tusa N, Fatta DF, Nardi L, Lucretti S. Obtaining triploid plants by crossing Citrus Lemon cv ‘Femminello’ 2n × 4N allotetraploid somatic hybrids. Proc Int Soc Citricult. 1996;1:133–136. [Google Scholar]
- Ueno I, Iwamasa M, Nishiura M. Embryo number of various varieties of Citrus and its relatives. Bull Hort Res Sta Jpn. 1967;7:11–22. [Google Scholar]
- Viloria Z, Grosser JW. Acid citrus fruit improvement via interploid hybridization using allotetraploid somatic hybrid and autotetraploid breeding parents. J Amer Soc Hort Sci. 2005;130(3):392–402. doi: 10.21273/JASHS.130.3.392. [DOI] [Google Scholar]
- Wakana A, Uemoto S. Adventive embryogenesis in Citrus (Rutaceae) II Post-Fertilization Development Am J Bot. 1988;75:1033–1047. doi: 10.1002/J.1537-2197.1988.TB08810.X. [DOI] [Google Scholar]
- Woo JK, Park YC, Lee JW, Yun SH, Kim M, Park S, Lee Y, Song KJ, Kim HB. Evaluation of polyembryony for genetic resources and efficacy of simple sequence repeat markers for the identification of nucellar and zygotic embryo-derived individuals in citrus. Appl Biol Chem. 2019;62:30. doi: 10.1186/s13765-019-0437-1. [DOI] [Google Scholar]
- Xie KD, Yuan DY, Wang W, Xia QM, Wu XM, Chen CW, Chen CL, Grosser JW, Guo WW. Citrus triploid recovery based on 2x × 4x crosses via an optimized embryo rescue approach. Sci Hortic. 2019;252:104–109. doi: 10.1016/j.scienta.2019.03.038. [DOI] [Google Scholar]
- Xu Q, Chen L, Ruan X, Chen D, Zhu A, Chen C, Bertrand D. The draft genome of sweet orange (Citrus sinensis) Nature Genet. 2013;45:59–66. doi: 10.1038/ng.2472. [DOI] [PubMed] [Google Scholar]
- Yildiz E, Kaplankiran M, Demİrkeser TH, Uzun A, Toplu C. Identification of zygotic and nucellar individuals produced from several Citrus crosses using SSRs markers. Not Bot Hort Agrobot Cluj. 2013;41:478–484. doi: 10.15835/nbha4129037. [DOI] [Google Scholar]
- Zhu S, Wu B, Ma Y, Chen J, Zhong G. Obtaining citrus hybrids by in vitro culture of embryos from mature seeds and early identification of hybrid seedlings by allele-specific PCR. Sci Hortic. 2013;161:300–305. doi: 10.1016/j.scienta.2013.07.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.