Key Points
Question
Is reduced dosing of direct oral anticoagulants not recommended by the Food and Drug Administration (FDA) associated with longitudinal adherence to anticoagulation treatment among patients with nonvalvular atrial fibrillation?
Findings
In this cohort analysis of 86 919 patients with nonvalvular atrial fibrillation, 10 964 (12.6%) were underdosed not according to FDA recommendations. Receiving a not-recommended dose occurred more frequently in patients with worse renal function and was associated with a lower likelihood of adherence and higher risk of anticoagulation discontinuation by 1 year.
Meaning
These findings suggest that there is a need for efforts to improve the quality of direct oral anticoagulant use and dosing.
This cohort study examines whether underdosing of direct oral anticoagulants not recommended by the Food and Drug Administration is associated with longitudinal adherence to anticoagulation treatment among patients with nonvalvular atrial fibrillation (NVAF).
Abstract
Importance
Although reduced doses of direct oral anticoagulants (DOACs) are approved for patients with nonvalvular atrial fibrillation (NVAF) at high risk of bleeding, little is known about dosing accuracy, particularly in patients with renal dysfunction.
Objective
To determine whether underdosing of DOACs is associated with longitudinal adherence to anticoagulation.
Design, Setting, and Participants
This retrospective cohort analysis used data from the Symphony Health claims data set. This national medical and prescription data set comprises 280 million patients and 1.8 million prescribers in the US. Patients included had at least 2 claims for NVAF between January 2015 and December 2017. The dates of analysis for this article were from February 2021 to July 2022.
Exposures
This study included patients with CHA2DS2-VASc scores of 2 or higher who were treated with a dose of DOACs who did and did not meet label-specified criteria for dose reduction.
Main Outcomes and Measures
Logistic regression models examined factors associated with off-label dosing (ie, dosing not recommended by US Food and Drug Administration [FDA] labeling), the association of creatinine clearance with recommended DOAC dosing, and the association of DOAC underdosing and excess dosing with 1-year adherence.
Results
Among the 86 919 patients included (median [IQR] age, 74 [67-80] years; 43 724 men [50.3%]; 82 389 White patients [94.8%]), 7335 (8.4%) received an appropriately reduced dose, and 10 964 (12.6%) received an underdose not consistent with FDA recommendations, meaning that 59.9% (10 964 of 18 299) of those who received a reduced dose received an inappropriate dose. Patients who received off-label doses of DOACs were older (median [IQR] age, 79 [73-85] vs 73 [66-79] years) and had higher CHA2DS2-VASc scores (median [IQR], 5 [4-6] vs 4 [3-6]) compared with patients who received appropriate doses (as recommended by FDA labeling). Renal dysfunction, age, heart failure, and the prescribing clinician being in a surgical specialty were associated with dosing not recommended by FDA labeling. Almost one-third of patients (9792 patients [31.9%]) with creatinine clearance less than 60 mL per minute taking DOACs were either underdosed or excess-dosed not consistent with FDA recommendations. For every 10-unit decrease in creatinine clearance, the odds of the patient receiving an appropriately dosed DOAC was lower by 21%. Treatment with underdosed DOACs was associated with a lower likelihood of adherence (adjusted odds ratio, 0.88; 95% CI, 0.83-0.94) and higher risk of anticoagulation discontinuation (adjusted odds ratio, 1.20; 95% CI, 1.13-1.28) by 1 year.
Conclusions and Relevance
In this study of oral anticoagulant dosing, DOAC dosing that did not follow FDA label recommendations was observed in a substantial number of patients with NVAF, occurred more frequently in patients with worse renal function, and was associated with less-consistent long-term anticoagulation. These results suggest a need for efforts to improve the quality of DOAC use and dosing.
Introduction
Previous work1 observed an approximately 4-fold higher use of reduced-dose direct oral anticoagulants (DOACs) in US cardiology practices compared with DOAC use in nonvalvular atrial fibrillation (NVAF) registration trials. In a Danish registry study,2 reduced-dose apixaban use was associated with higher risk of ischemic stroke or systemic embolism and no significant difference in bleeding compared with warfarin. However, that study,2 like many others, lacked data on renal function or weight and, thus, was unable to determine whether reduced dosing was inappropriate or appropriate according to US Food and Drug Administration (FDA) dose labeling.3 In the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF II),3 patients who received off-label oral anticoagulation doses had higher CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke, vascular disease, age 65-74 years, and female sex) scores, and underdosing (not recommended per FDA recommendations) was associated with significantly higher risk of cardiovascular hospitalization. A recent trial4 of older Japanese patients with NVAF who were not candidates for standard doses of oral anticoagulants found that a once-daily dose of 15 mg of edoxaban vs placebo was superior in preventing stroke while not increasing major bleeding risk. There are few data on the rationale behind off-label, reduced-dosed DOAC prescription, but this practice likely reflects both patient and clinician desire to have the patient stay anticoagulated while avoiding bleeding complications.5
This study leveraged a large, national medical and prescription claims data set linked to clinical data, including patients’ age, weight, and renal function, to determine the prevalence of patients with NVAF treated with a DOAC dose not recommended by FDA labeling (hereafter referred to as off-label dosing). We analyzed patient-level and prescribing practitioner factors associated with off-label underdosing or excess dosing. Finally, we examined prescription fill data to assess whether off-label underdosing of DOACs was associated with greater longitudinal adherence to anticoagulation in patients with CHA2DS2-VASc score of 2 or higher who are at high risk for thromboembolism. We hypothesized that off-label dosing would be associated with increased risks of nonadherence and discontinuation of DOACs. We hypothesized that an increased risk of nonadherence and discontinuation may be a component of the mechanism for higher clinical risks (ie, stroke and bleeding) in patients prescribed an off-label DOAC dose.
Methods
Data Source and Study Population
Symphony Health data contain demographic, claims, laboratory, and prescription fill data for 280 million patients, as well as prescriber information for 1.8 million prescribers in the US. A patient’s race is self-reported and extracted from electronic health record data. Data on race are included in this study to provide a complete demographic picture of the cohort. All data were deidentified and linked via a compliant, anonymous unique identifier. Symphony includes claims submitted to all payer types, including commercial plans, Medicare, and Medicaid. These are adjudicated claims collected from major US clearing houses, as well as large national retail, mail order, and specialty pharmacy chains.6,7 This study received approval from the Duke University institutional review board, and informed consent was not needed because the data are anonymous, in accordance with 45 CFR §46. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.8 The dates of analysis for this article were from February 2021 to July 2022.
We defined atrial fibrillation (AF) as having at least 2 claims for AF (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes I48.0, I48.1, I48.2, and I48.91 or International Classification of Diseases, Ninth Revision [ICD-9] code 427.31) between January 2015 and December 2017 (4 959 101 patients). The index date started when the second claim for AF was filed. We excluded 334 patients without available information on sex and 6830 patients who were younger than 18 years. According to the 2019 American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines for the management of patients with AF, NVAF is defined as AF in the absence of moderate-to-severe mitral stenosis or a mechanical heart valve.9 Because diagnosis codes do not allow for ascertainment of mitral stenosis severity, we excluded all 72 615 patients with a history of mitral stenosis (ICD-10 codes I05.0 or I05.2; ICD-9 codes 394.0, 396.0, or 396.1). We also excluded 276 534 patients with a history of mechanical or bioprosthetic mitral valve replacement (ICD-10 codes Z95.2, Z95.3, or Z95.4; ICD-9 codes V42.2 or V43.3), as well as 25 397 patients with a history of mitral valve repair (ICD-9 code V15.1). We then excluded 1 260 921 patients without a qualifying prescription of any medication captured by the Symphony Health Dataverse during the period between the first NVAF claim and the index date and 942 405 patients without a qualifying prescription more than 12 months after the index date; these measures allowed us to focus on patients with complete capture of 1-year prescriptions. This resulted in an analysis study population of 2 374 065 patients with NVAF between January 2015 and December 2017. We then excluded 1 522 097 patients without electronic medical record data linkage within 1 year of the index date, 59 953 patients without weight information within 1 year of the index date, 485 423 patients without creatinine data within 1 year of the index date, and 219 673 patients with a CHA2DS2-VASc score less than 2.10 This resulted in a population of patients with NVAF with CHA2DS2-VASc scores of 2 or higher and the requisite information to assess for on-label vs off-label anticoagulant dosing. The eFigure in Supplement 1 shows the enrollment flowchart for the final study population.
Definitions
We examined 4 categories of oral anticoagulant dosing according to drug package labeling, including (1) off-label underdosing, (2) off-label excess dosing, (3) appropriate reduced dose according to package labeling, and (4) appropriate full dose according to package labeling. eTable 1 in Supplement 1 describes the dosing definitions for each medication. The DOAC drugs of interest include apixaban, rivaroxaban, dabigatran, and edoxaban.
Outcomes of interest within 1 year included DOAC adherence (primary), DOAC discontinuation (primary), hospitalization for bleeding (secondary), and stroke (secondary). We defined DOAC adherence as the proportion of days covered greater than 80%, calculated by the number of days any anticoagulant was available divided by the number of days in the follow-up period (12 months).11 We defined DOAC discontinuation as a gap in prescription fill longer than 30 days following the run-out date of the last observed claim without initiation of another anticoagulant. Hospitalization for bleeding was defined as hospitalization involving at least 1 overnight stay with a diagnosis code for bleeding (codes are listed in eTable 2 in Supplement 1). Stroke or transient ischemic attack (TIA) end point was defined as hospitalization involving at least 1 overnight stay with a diagnosis code for acute stroke or TIA.
Statistical Analysis
We described anticoagulant use at or within 30 days after the index date among patients with NVAF stratified by CHA2DS2-VASc score. Subsequent analyses focused on patients with NVAF and CHA2DS2-VASc score of 2 or higher treated with a DOAC. We described the percentage of patients who received a package-labeled dose of a DOAC per FDA recommendations (ie, appropriate dose) vs those who were underdosed or treated with an excess dose of a DOAC (according to package labeling), stratified by DOAC type.
Demographic and clinical characteristics among patients were compared between the following groups: underdosed (not recommended by FDA dose labeling) vs appropriate dose (reference, appropriate dose), excess dose (not recommended by FDA dose labeling) vs appropriate dose (reference, appropriate dose), and appropriate full dose vs appropriate reduced dose. A Pearson χ2 test was used to compare the categorical characteristics of the aforementioned DOAC dosing strategies, whereas a Welch 2-sample t test was used for continuous variables. We also performed multivariable logistic regression models examining factors associated with underdosed and excess-dosed DOACs, adjusting for a priori covariates chosen for clinical relevance. These covariates included age, sex, race, weight, ethnicity, median income, payer (commercial, Medicare or Medicaid, and uninsured), hypertension, coronary artery disease, peripheral artery disease, prior congestive heart failure, recent percutaneous coronary intervention within 6 months, prior coronary artery bypass graft, recent thromboembolism within 6 months, liver disease, dementia, diabetes, prior cerebral vascular accident or TIA, hospitalization for bleeding, hospitalization for fall, prior intracranial hemorrhage, anemia, dialysis, and prescriber specialty (eTable 3 in Supplement 1). Factors were ranked by χ2 test, and associations were quantified with adjusted odds ratios (aORs) and 95% CIs. Two-sided P < .05 was considered statistically significant.
Because renal impairment is a dose-reduction criterion for all currently available DOACs, we examined the percentage of patients treated with appropriately full, appropriately reduced, off-label underdosed, and off-label excess-dosed DOAC, stratified by DOAC type and renal function. To examine the association of renal function with off-label underdosing and excess dosing of DOACs, we performed multivariable logistic regression models with creatinine clearance, calculated using the Cockcroft-Gault equation,12 as a continuous independent variable and the outcome the odds of appropriate anticoagulant dosing among patients with CHA2DS2-VASc score 2 or higher and taking a DOAC. Variables included in the model are listed in the eTable 3 in Supplement 1.
We performed multivariable logistic regression models to compare rates of anticoagulant adherence and discontinuation of all anticoagulants over a 12-month follow-up period between the following groups: off-label underdosed vs appropriately dosed, off-label excess dosed vs appropriately dosed, and appropriately full dose vs appropriately reduced dose. We adjusted for the aforementioned clinical covariates using inverse probability–weighted, propensity-adjusted methods. We also performed multivariable Cox regression models to examine rates of various clinical outcomes, including bleeding and stroke or TIA over the 12-month follow-up period using the same groups as above for comparison. We adjusted for clinical covariates using inverse probability–weighted, propensity-adjusted methods. All statistical analyses were performed using R statistical software version 3.61 (R Project for Statistical Computing).
Results
Between January 2015 and December 2017, there were 86 919 patients (median [IQR] age, 74 [67-80] years; 43 724 men [50.3%]; 82 389 White patients [94.8%]) with NVAF with CHA2DS2-VASc scores of 2 or higher and the requisite information to assess for on-label vs off-label anticoagulant dosing (final study population). Patients who were treated with an off-label DOAC dose, regardless of whether they received an excess dose or underdose of DOACs, were older (median [IQR] age, 79 [73-85] vs 73 [66-79] years) and had both higher estimated stroke risk (median [IQR] CHA2DS2-VASc score, 5 [4-6] vs 4 [3-6]) and high ORBIT bleeding risk score compared with patients who were treated with an appropriately dosed DOAC (Table 1). Patients with off-label underdosed DOACs were more likely to have established cardiovascular disease (prior coronary artery disease, stroke, or heart failure), whereas patients who were treated with off-label excess-dosed DOACs were more likely to have prior thromboembolic disease compared with those treated with appropriately dosed DOACs. Both the underdosing and excess dosing groups had a higher likelihood of prior anemia compared with the appropriately dosed DOAC group.
Table 1. Demographic and Clinical Characteristics of Patients With Nonvalvular Atrial Fibrillation by Direct Oral Anticoagulant Dosing Strategy.
| Characteristic | Patients, No. (%) | P value | ||||
|---|---|---|---|---|---|---|
| Overall (N = 86 919) | Appropriate dose (n = 71 636) | Underdose (n = 10 964) | Excess dose (n = 4319) | Underdose vs appropriate | Excess dose vs appropriate | |
| Demographics | ||||||
| Age, median (IQR), y | 74 (67-80) | 73 (66-79) | 79 (73-85) | 81 (76-85) | <.001 | <.001 |
| Sex | ||||||
| Female | 43 195 (49.7) | 34 524 (48.2) | 5952 (54.3) | 2719 (63.0) | <.001 | <.001 |
| Male | 43 724 (50.3) | 37 112 (51.8) | 5012 (45.7) | 1600 (37.0) | <.001 | <.001 |
| Race | ||||||
| White | 82 389 (94.8) | 68 019 (95.0) | 10 283 (93.8) | 4087 (94.6) | <.001 | .49 |
| Any other racea | 4530 (5.2) | 3617 (5.0) | 681 (6.2) | 232 (5.4) | ||
| Insurance | ||||||
| Commercial insurance | 28 854 (33.2) | 24 778 (34.6) | 2894 (26.4) | 1182 (27.4) | <.001 | <.001 |
| Medicare or Medicaid | 57 294 (65.9) | 46 300 (64.6) | 7914 (72.2) | 3080 (71.3) | <.001 | <.001 |
| Uninsured | 771 (0.8) | 558 (0.8) | 156 (1.4) | 57 (1.3) | <.001 | <.001 |
| Clinical history | ||||||
| Weight, median (IQR), lb | 193 (161-230) | 198 (165-235) | 182 (154-217) | 155 (129-187) | <.001 | <.001 |
| CHA2DS2-VASc score, median (IQR) | 4 (3-6) | 4 (3-6) | 5 (4-6) | 5 (4-6) | <.001 | <.001 |
| ORBIT-AF bleeding score, median (IQR) | 2 (1-3) | 2 (1-3) | 3 (1-4) | 3 (2-4) | <.001 | <.001 |
| Coronary artery disease | 30 459 (35.0) | 24 464 (34.2) | 4514 (41.2) | 1481 (34.3) | <.001 | .86 |
| Prior percutaneous coronary intervention | 4793 (5.5) | 3746 (5.2) | 848 (7.7) | 199 (4.6) | <.001 | .08 |
| Prior coronary artery bypass graft | 1890 (2.1) | 1533 (2.1) | 272 (2.5) | 85 (2.0) | .03 | .48 |
| Heart failure | 36 429 (41.9) | 28 850 (40.3) | 5631 (51.4) | 1948 (45.1) | <.001 | <.001 |
| Prior stroke or transient ischemic attack | 27 022 (31.1) | 21 405 (29.9) | 3986 (36.4) | 1631 (37.8) | <.001 | <.001 |
| Peripheral arterial disease | 14 581 (16.8) | 11 567 (16.1) | 2160 (19.7) | 854 (19.8) | <.001 | <.001 |
| Diabetes | 29 939 (34.4) | 24 592 (34.3) | 4064 (37.1) | 1283 (29.7) | <.001 | <.001 |
| Hypertension | 71 280 (82.0) | 58 641 (81.9) | 9111 (83.1) | 3528 (81.7) | .002 | .79 |
| Thromboembolism | 6065 (7.0) | 4626 (6.4) | 840 (7.7) | 599 (13.9) | <.001 | <.001 |
| Liver disease | 3937 (4.5) | 3272 (4.6) | 469 (4.3) | 196 (4.5) | .18 | .96 |
| Anemia | 35 012 (40.3) | 27 559 (38.5) | 5368 (49.0) | 2085 (48.3) | <.001 | <.001 |
| Prior warfarin use | 5586 (6.4) | 4375 (6.1) | 821 (7.5) | 390 (9.0) | <.001 | <.001 |
| Concomitant medications | ||||||
| Antiplatelet therapy | 11 182 (12.9) | 8846 (12.3) | 1780 (16.2) | 556 (12.9) | <.001 | .32 |
| Antiarrhythmic therapy | 32 966 (37.9) | 27 678 (38.6) | 3818 (34.8) | 1470 (34.0) | <.001 | <.001 |
| Prescribing practitioner specialty | ||||||
| Cardiology | 42 878 (49.3) | 35 940 (50.2) | 5083 (46.4) | 1855 (42.9) | <.001 | <.001 |
| Internal medicine | 20 793 (23.9) | 17 052 (23.8) | 2631 (24.0) | 1110 (25.7) | <.001 | <.001 |
| Family medicine | 16 938 (19.5) | 13 788 (19.2) | 2205 (20.1) | 945 (21.9) | <.001 | <.001 |
| Surgical specialty | 685 (0.8) | 400 (0.6) | 247 (2.2) | 38 (0.9) | <.001 | <.001 |
Abbreviations: CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes, stroke, vascular disease, age 65-74 years, and female sex; ORBIT-AF, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
SI conversion factor: To convert pounds to kilograms, multiply by 0.45.
Includes all patients who did not self-report White race.
eTable 4 in Supplement 1 illustrates the proportion of patients taking various anticoagulation regimens stratified by CHA2DS2-VASc score. There were 2 374 065 patients with at least 2 claims for NVAF. Among the 2 164 501 patients with NVAF and a CHA2DS2-VASc score of 2 or higher, 869 044 (40.1%) patients were not taking any oral anticoagulation, 637 091 (29.4%) were taking warfarin, and 658 366 (30.4%) were taking a DOAC. Table 2 describes the number and proportion of patients receiving appropriate, off-label underdosed, or off-label excess-dosed DOACs among patients with NVAF with a CHA2DS2-VASc score of 2 or higher, and linked to clinical data to determine dosing appropriateness. Among 86 919 patients in the final study population, 7335 (8.4%) received an appropriately reduced dose; 10 964 (12.6%) received an off-label underdose and 4319 (5.0%) were prescribed an off-label excess dose, for a total of 15 013 patients (17.3%) who received an inappropriate dose. Of 18 299 patients who received reduced-dose anticoagulation, 10 964 (59.9%) received inappropriate off-label doses. Apixaban and rivaroxaban were the mostly commonly used DOACs. Although rates of underdosing were similar across DOAC types, rivaroxaban users had higher rates of excess dosing (2693 patients [8.8%]) compared with apixaban (1476 patients [3.1%]) and dabigatran (120 patients [1.4%]).
Table 2. Proportion of Patients Who Received an Appropriate Dose, Underdose, or Excess Dose of a Direct Oral Anticoagulant.
| Dosing | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| Overall (N = 86 919) | Apixaban (n = 47 891) | Rivaroxaban (n = 30 448) | Dabigatran (n = 8278) | Edoxaban (n = 302) | |
| Appropriately full dose | 64 301 (74.0) | 35 897 (75.0) | 21 328 (70.0) | 6908 (83.4) | 168 (55.6) |
| Appropriately reduced dose | 7335 (8.4) | 4084 (8.5) | 3066 (10.1) | 124 (1.5) | 61 (20.2) |
| Underdose (not consistent with FDA recommendations) | 10 964 (12.6) | 6434 (13.4) | 3361 (11.0) | 1126 (13.6) | 43 (14.2) |
| Excess dose (not consistent with FDA recommendations) | 4319 (5.0) | 1476 (3.1) | 2693 (8.8) | 120 (1.4) | 30 (9.9) |
Abbreviation: FDA, US Food and Drug Administration.
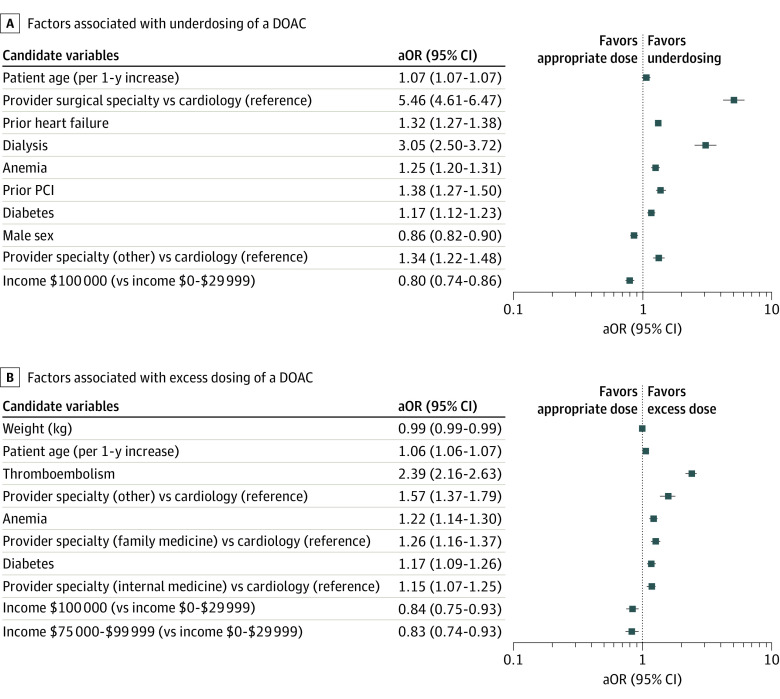
The top factors associated with off-label underdosing and excess dosing in multivariable models are shown in the Figure. The prescribing practitioner being in a surgical specialty (aOR, 5.46; 95% CI, 4.61-6.47), a history of heart failure (aOR, 1.32; 95% CI 1.27-1.38), the patient undergoing dialysis (aOR, 3.05; 95% CI, 2.50-3.72), and the patient’s age (aOR, 1.07; 95% CI, 1.07-1.07) were the factors most associated with underdosing of a DOAC. A history of thromboembolism (aOR, 2.39; 95% CI, 2.16-2.63), anemia (aOR, 1.22; 95% CI, 1.14-1.30), the practitioner’s specialty not being cardiology (aOR, 1.57; 95% CI, 1.37-1.79), and patient’s age (aOR, 1.06; 95% CI, 1.06-1.07) were most associated with excess dosing of a DOAC.
Figure. Factors Associated With Dosing of a Direct Oral Anticoagulant (DOAC).
Graphs show factors associated with underdosing (A) and excess dosing (B) of a DOAC. Reference groups for candidate variables are cardiac medicine for specialty and $0 to $29 999 for income. Weight is per 1-kg increase. aOR indicates adjusted odds ratio; and PCI, percutaneous coronary intervention.
Association of Renal Function With Underdosing and Excess Dosing of DOACs
The likelihood of receiving anticoagulation decreased with worsening renal function; among patients with CHA2DS2-VASc scores of 2 or higher, anticoagulation rates were 62.5% (85 676 of 136 988 patients) among patients with creatinine clearance greater than 60 mL per minute, 61.3% (45 180 of 73 717 patients) among patients with creatinine clearance 30 to 59 mL per minute, and 56.2% (7196 of 12 797 patients) among patients with creatinine clearance less than 30 mL per minute. Among anticoagulated patients with CHA2DS2-VASc scores of 2 or higher, DOACs were prescribed for 65.6% (56 245 of 85 676 patients) of patients with creatinine clearance greater than 60 mL per minute, 59.3% (26 744 of 45 180 patients) of patients with creatinine clearance 30 to 59 mL per minute, and 54.2% (3900 of 7196 patients) of patients with creatinine clearance less than 30 mL per minute.
Table 3 demonstrates the proportion of patients receiving appropriate, off-label underdosed, or off-label excess-dosed DOACs, stratified by creatinine clearance. Almost one-third of patients (9792 patients [31.9%]) with creatinine clearance less than 60 mL per minute and treated with a DOAC were either underdosed or excess dosed off-label. For every 10-unit decrease in creatinine clearance, the odds of the patient receiving an appropriately dosed DOAC was reduced by 21%. The incidence of underdosing was highest (5484 patients [20.5%]) among patients with creatinine clearance 30 to 59 mL per minute; excess dosing occurred most frequently (705 patients [18.1%]) in patients with creatinine clearance less than 30 mL per minute. Among patients with creatinine clearance greater than 60 mL per minute, underdosing occurred in 5068 patients (9.0%); rates of underdosing or excess dosing did not differ substantially among the more commonly used DOACs. Among patients with creatinine clearance of 30 to 59 mL per minute, underdosing rates were higher among patients treated with apixaban and dabigatran, whereas excess dosing was most frequent among rivaroxaban-treated patients. Excess dosing occurred in more than one-third of patients with creatinine clearance less than 30 mL per minute treated with rivaroxaban, dabigatran, or edoxaban.
Table 3. Proportion of Patients Who Were Treated With Various Dosing Strategies by DOAC and Creatinine Clearance Rate.
| Dosing | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| Any DOAC | Apixaban | Rivaroxaban | Dabigatran | Edoxaban | |
| Creatinine clearance >60 mL/min | |||||
| Patients, No. | 56 245 | 29 093 | 21 299 | 5692 | 161 |
| Appropriately full dose | 50 647 (90.0) | 26 407 (90.8) | 18 895 (88.7) | 5213 (91.6) | 132 (82.0) |
| Appropriately reduced dose | 107 (0.2) | 107 (0.4) | NA | NA | NA |
| Underdose (not consistent with FDA recommendations) | 5068 (9.0) | 2353 (8.1) | 2217 (10.4) | 469 (8.2) | 29 (18.0) |
| Excess dose (not consistent with FDA recommendations) | 423 (0.8) | 226 (0.8) | 187 (0.9) | 19 (0.3) | NA |
| Creatinine clearance 30-59 mL/min | |||||
| Patients, No. | 26 774 | 16 072 | 8222 | 2355 | 125 |
| Appropriately full dose | 13 183 (49.2) | 9019 (56.1) | 2433 (29.6) | 1695 (72.0) | 36 (28.8) |
| Appropriately reduced dose | 4916 (18.4) | 2410 (26.7) | 2454 (29.8) | NA | 52 (41.6) |
| Underdose (not consistent with FDA recommendations) | 5484 (20.5) | 3669 (22.8) | 1144 (13.9) | 657 (27.9) | 14 (11.2) |
| Excess dose (not consistent with FDA recommendations) | 3191 (11.9) | 974 (6.1) | 2191 (26.6) | 3 (0.1) | 23 (18.4) |
| Creatinine clearance <30 mL/min | |||||
| Patients, No. | 3900 | 2726 | 927 | 231 | 16 |
| Appropriately full dose | 471 (12.1) | 471 (17.3) | NA | NA | NA |
| Appropriately reduced dose | 2312 (59.3) | 1567 (57.5) | 612 (66.0) | 124 (52.2) | 9 (56.3) |
| Underdose (not consistent with FDA recommendations) | 412 (10.6) | 412 (15.1) | NA | NA | NA |
| Excess dose (not consistent with FDA recommendations) | 705 (18.1) | 276 (10.1) | 315 (34.0) | 107 (46.3) | 7 (43.8) |
Abbreviations: DOAC, direct oral anticoagulant; FDA, US Food and Drug Administration; NA, not applicable.
Dosing Strategy and DOAC Adherence, Anticoagulation Persistence, and Outcomes
Compared with patients treated with an appropriate dose of a DOAC, patients who were underdosed inappropriately had a lower adjusted risk of DOAC adherence (adjusted odds ratio, 0.88; 95% CI, 0.83-0.94) and a higher likelihood of discontinuing anticoagulation (adjusted odds ratio, 1.20; 95% CI, 1.13-1.28) by 1 year (Table 4). Treatment with an excess-dosed DOAC was also associated with lower adjusted risk of DOAC adherence and higher likelihood of discontinuing anticoagulation (Table 4). In contrast, patients prescribed an appropriately reduced DOAC dose had no significant difference in DOAC adherence or anticoagulation persistence compared with patients treated appropriately with a full dose.
Table 4. Association of Underdosing and Excess Dosing of Direct Oral Anticoagulants (Not Consistent With US Food and Drug Administration Labeling) With Anticoagulant Adherence and Discontinuation.
| Outcome | Unadjusted event rates, % | Adjusted OR (95% CI) |
|---|---|---|
| Underdosing vs appropriate dosing | ||
| Adherence | 23.0 vs 25.2 | 0.88 (0.83-0.94) |
| Discontinuation of anticoagulation | 26.1 vs 21.4 | 1.20 (1.13-1.28) |
| Excess vs appropriate dosing | ||
| Adherence | 23.7 vs 25.2 | 0.87 (0.79-0.96) |
| Discontinuation of anticoagulation | 26.0 vs 21.3 | 1.16 (1.05-1.28) |
| Appropriately reduced vs appropriately full dosing | ||
| Adherence | 25.2 vs 25.3 | 0.92 (0.85-1.01) |
| Discontinuation of anticoagulation | 25.3 vs 20.9 | 1.06 (0.98-1.17) |
Abbreviation: OR, odds ratio.
Patients treated with an appropriately reduced dose of DOACs (according to FDA recommendations) were more likely to have a hospitalization involving bleeding compared with patients treated with appropriately full-dose DOACs, even after propensity adjustment (adjusted hazard ratio [HR], 1.21; 95% CI, 1.01-1.44), but there were no significant differences in the adjusted risk of stroke (adjusted HR, 0.94; 95% CI, 0.82-1.07). There were no significant differences in hospitalization involving bleeding (adjusted HR, 0.95; 95% CI, 0.84-1.08) or in stroke (adjusted HR, 0.99; 95% CI, 0.90-1.09) observed between off-label underdosed and appropriately dosed patients.
Discussion
In this large cohort study, off-label DOAC dosing occurred in 17.3% of patients with NVAF and a CHA2DS2-VASc score of 2 or higher. Patients who were either underdosed or excess dosed were older and had higher CHA2DS2-VASc and ORBIT bleeding risk scores compared with patients who received appropriate doses. For every 10-unit decrease in creatinine clearance, the odds of the patient receiving an appropriately dosed DOAC was reduced by 21%, with 20.5% of patients with creatinine clearance 30 to 59 mL per minute underdosed and 18.1% of patients with a creatinine clearance less than 30 mL per minute treated with an excess dose. Off-label dosing of a DOAC not according to FDA recommendations was associated with decreased anticoagulant adherence and increased likelihood of anticoagulant discontinuation, whereas on-label reduced dosing was not associated with either. Off-label reduced dosing was not associated with lower bleeding risk compared with on-label dosing.
Our analysis highlights an important and ongoing trend in patients being prescribed off-label doses of DOACs. A previous analysis13 using data from 2010 to 2015 for approximately 14 000 patients with NVAF demonstrated that among patients with no renal indication for a reduced dose of a DOAC, 13% were prescribed an off-label underdose of DOAC. Our data examine a contemporary population of nearly 3 million patients with NVAF, yet still demonstrate that more than 17% of such patients are prescribed a dose of DOAC that is either too low or too high. Although the percentage of patients in our analysis who received a dose of DOAC that was either too low or too high is not as high as the 37% published using the Korean nationwide claims database,14 it is higher than what was published by Steinberg and colleagues3 in 2016 in the ORBIT II study (13%). Several other observational studies15,16,17 have demonstrated a significant proportion of patients being prescribed an off-label DOAC dose, with only the VA Health System describing low rates of off-label DOAC dosing.18 Although it may seem likely that clinicians would be conservative when dosing and prescribing new therapies, we found more underdosing, not less, even among contemporary patients with AF. In addition, as expected, our data showed that patients taking antiplatelet therapy were significantly more likely to be underdosed off-label compared with patients taking an appropriately dosed DOAC, likely from the clinician’s desire to reduce bleeding risk with concomitant antiplatelet therapy.
In an examination of patients with NVAF stratified by degree of renal dysfunction, we found that more than 1 in 5 patients with creatinine clearance between 30 and 59 mL per minute were receiving off-label underdosed DOACs, and just under 1 in 5 patients with a creatinine clearance less than 30 mL per minute were being prescribed an off-label excess dose. Yao and colleagues13 found that among patients with NVAF with a renal indication for a reduced dose of a DOAC, 43% still received a standard dose of a DOAC (thereby giving them an excess dose). In a single-center, retrospective analysis of 207 patients with moderate-to-severe renal impairment, Ting and colleagues19 demonstrated that almost one-third of these patients were either underdosed or received an excess dose of a DOAC.
Issues of off-label dosing not adhering to FDA dose recommendations in patients with NVAF arise out of a concern for a potentially increased risk of stroke (underdosing) and bleeding (excess dosing). Clinicians likely prescribe underdosed DOAC therapy per FDA dose recommendations in an attempt to get patients to take anticoagulant therapy, avoid bleeding complications, and prevent discontinuation or nonadherence. In addition, as shown in a recent analysis,5 only 35% of clinicians dosed DOACs according to the label-indicated dose in hypothetical survey scenarios. To our knowledge, examination of the association of DOAC dosing strategy with anticoagulant adherence and discontinuation has not been previously studied. However, as our data demonstrate, off-label dosing was not associated with improved adherence or lower risk of discontinuation of DOAC therapy. In addition, off-label dosing was not associated with reduced risk of bleeding while taking DOAC therapy. Interestingly, we did demonstrate a higher bleeding risk in patients with appropriately reduced doses of DOAC compared with appropriately full doses, suggesting that the developed dose criteria are appropriately discriminating patients who are at higher risk of bleeding. Further work is needed to better understand why DOAC dosing strategies may increase risk among certain patient populations for increased rates of discontinuation and nonadherence.
The present analysis suggests that opportunities remain to improve DOAC dosing through quality improvement initiatives and potentially through best practice alerts in the electronic health record that can detect off-label DOAC dosing. In addition, for a pharmacist to detect an off-label DOAC dose before filling a prescription, data on weight and/or renal function may need to be available. Therefore, successful electronic health record–based programs will need to both (1) alert the practitioner at the time of DOAC order and (2) provide the pharmacist with pertinent dosing information at the time of prescription fill. Health systems should use quality improvement initiatives to assist in detecting patients who are receiving off-label DOAC dosing.
Limitations
There are several limitations of this analysis. This is an observational analysis, and there may be residual confounding even after adjustment for the primary and secondary outcomes. Although we are able to examine the prescription fills of patients with NVAF, we are not able to determine whether the patient was actually taking the medication. However, because we base examination of adherence on timing of the patient filling prescription refills, this information is a good surrogate for determining whether the patient was taking the medication. In addition, a patient’s DOAC dosing strategy was characterized by what he or she was prescribed during the index period. This strategy may have changed in subsequent prescriptions to either an off-label prescription or to a labeled dose. In addition, we used claims data to define the population of patients with NVAF and CHA2DS2-VASc score of 2 or higher. There are limitations to claims data, and patients may have either been undercoded or overcoded for various diagnoses that could have changed their CHA2DS2-VASc score. Moreover, as we defined, there was missingness present for creatinine clearance and weight, in particular, so it is possible that this could have biased or impacted the generalizability of the results. In addition, we do note that there was low representation of racial diversity in the study (with only approximately 5% representation of patients who are not White). This could impact generalizability. Furthermore, we recognize that there may be patients with very high risks of stroke or bleeding where the clinician in discussion with the patient may decide to use an off-label dose in certain clinical scenarios. This analysis cannot capture the reasons for why clinicians opted to prescribe a particular dose. Off-label dosing may have a therapeutic role particularly in older populations as shown in ELDER-AF.4
Conclusions
Off-label DOAC dosing that did not adhere to FDA dose recommendations was observed in a substantial number of patients with NVAF in this large national cohort study. Dosing not according to FDA labeling occurred more frequently in patients with lower renal function. Off-label reduced dosing was not associated with lower bleeding risk or with long-term anticoagulant adherence. These results suggest the need for additional efforts to improve effective and safe dosing of DOACs.
eFigure. Consort Diagram Describing the Final Analytic Population
eTable 1. Dosing Definitions Used for Various Oral Anticoagulants
eTable 2. Diagnosis Codes Used for Bleeding and Stroke/TIA Outcomes
eTable 3. Covariates Adjusted for During Statistical Analysis
eTable 4. Proportion of Patients on Various Oral Anticoagulant Medications by CHA2DS2-VASc Score
Data Sharing Statement
References
- 1.Nguyen E, White CM, Patel MR, et al. Doses of apixaban and rivaroxaban prescribed in real-world United States cardiology practices compared to registration trials. Curr Med Res Opin. 2016;32(7):1277-1279. doi: 10.1185/03007995.2016.1170672 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. doi: 10.1136/bmj.j510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg BA, Shrader P, Thomas L, et al. ; ORBIT-AF Investigators and Patients . Off-label dosing of non-vitamin K antagonist oral anticoagulant and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68(24):2597-2604. doi: 10.1016/j.jacc.2016.09.966 [DOI] [PubMed] [Google Scholar]
- 4.Okumura K, Akao M, Yoshida T, et al. ; ELDERCARE-AF Committees and Investigators . Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med. 2020;383(18):1735-1745. doi: 10.1056/NEJMoa2012883 [DOI] [PubMed] [Google Scholar]
- 5.Rymer JA, Webb L, McCall D, Hills MT, Wang TY. Differences in preferences between clinicians and patients for the use and dosing of direct oral anticoagulants for atrial fibrillation. J Am Heart Assoc. 2021;10(11):e020697. doi: 10.1161/JAHA.120.020697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rymer JA, Fonseca E, Bhandary DD, Kumar D, Khan ND, Wang TY. Difference in medication adherence between patients prescribed a 30-day versus 90-day supply after acute myocardial infarction. J Am Heart Assoc. 2021;10(1):e016215. doi: 10.1161/JAHA.119.016215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert J, Sandhu H, Kean E, et al. A strategy to identify event specific hospitalizations in large health claims databases. BMC Health Serv Res. 2022;22(1):705. doi: 10.1186/s12913-022-08107-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 9.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 11.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. doi: 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54:33-42. doi: 10.1053/j.ajkd.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69(23):2779-2790. doi: 10.1016/j.jacc.2017.03.600 [DOI] [PubMed] [Google Scholar]
- 14.Lee SR, Choi EK, Park SH, et al. Off-label underdosed apixaban use in Asian patients with non-valvular atrial fibrillation. Eur Heart J Cardiovasc Pharmacother. 2021;7(5):415-423. doi: 10.1093/ehjcvp/pvab004 [DOI] [PubMed] [Google Scholar]
- 15.Ruiz Ortiz M, Muñiz J, Raña Míguez P, et al. ; FANTASIIA study investigators . Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors—a subanalysis of the FANTASIIA Registry. Europace. 2018;20(10):1577-1583. doi: 10.1093/europace/eux316 [DOI] [PubMed] [Google Scholar]
- 16.Sanghai S, Wong C, Wang Z, et al. Rates of potentially inappropriate dosing of direct-acting oral anticoagulants and associations with geriatric conditions among older patients with atrial fibrillation: the SAGE-AF Study. J Am Heart Assoc. 2020;9(6):e014108. doi: 10.1161/JAHA.119.014108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugrue A, Sanborn D, Amin M, et al. Inappropriate dosing of direct oral anticoagulants in patients with atrial fibrillation. Am J Cardiol. 2021;144:52-59. doi: 10.1016/j.amjcard.2020.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: insights from the Veterans Health Administration. J Pharm Pract. 2020;33(5):647-653. doi: 10.1177/0897190019828270 [DOI] [PubMed] [Google Scholar]
- 19.Ting C, Rhoten M, Dempsey J, Nichols H, Fanikos J, Ruff CT. Evaluation of direct oral anticoagulant prescribing in patients with moderate to severe renal impairment. Clin Appl Thromb Hemost. Published online January 31, 2021. doi: 10.1177/1076029620987900 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Consort Diagram Describing the Final Analytic Population
eTable 1. Dosing Definitions Used for Various Oral Anticoagulants
eTable 2. Diagnosis Codes Used for Bleeding and Stroke/TIA Outcomes
eTable 3. Covariates Adjusted for During Statistical Analysis
eTable 4. Proportion of Patients on Various Oral Anticoagulant Medications by CHA2DS2-VASc Score
Data Sharing Statement