Abstract
BACKGROUND
The recent heart failure (HF) guideline recommends the inclusion of cardiac biomarkers in defining Stage B HF.
OBJECTIVES
The authors evaluated the impact of incorporating cardiac biomarkers to reclassify HF in 5,324 participants (mean age: 75.8 years) without prevalent HF enrolled in the ARIC (Atherosclerosis Risk In Communities) study and assessed prognosis of Stage B using cardiac biomarkers.
METHODS
Using N-terminal pro-B-type natriuretic peptide (<125 pg/mL or ≥125 pg/mL), high-sensitivity troponin T (<14 ng/L or ≥14 ng/L), and abnormal cardiac structure/function by echocardiography, individuals were classified as Stage Anew and Stage Bnew HF, respectively. Stage Bnew was further evaluated as elevated biomarker only, abnormal echocardiogram only, and abnormalities in both (echo + biomarker). The authors assessed risk for incident HF and all-cause death using Cox regression.
RESULTS
Overall, 4,326 (81.3%) individuals were classified as Stage Bnew with 1,123 (21.1%) meeting criteria for elevated biomarkers only. Compared with Stage Anew, Stage Bnew was associated with increased risk for incident HF (HR: 3.70 [95% CI: 2.58-5.30]) and death (HR: 1.94 [95% CI: 1.53-2.46]). Stage Bbiomarkers only and Stage Becho only were associated with increased HF risk, whereas Stage Bbiomarkers only was also associated with increased death. Stage Becho+biomarker had the highest risk for HF (HR: 6.34 [95% CI: 4.37-9.19]) and death (HR: 2.53 [95% CI: 1.98-3.23]).
CONCLUSIONS
Incorporating biomarkers based on the new HF guideline reclassified approximately 1 in 5 older adults without prevalent HF to Stage B. The routine measurement of biomarkers can help to identify individuals at higher HF risk who may benefit most from HF prevention efforts.
Keywords: heart failure stages, pre-heart failure, cardiovascular risk, heart failure prevention, Stage B heart failure
The recently published 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America Guideline for the Management of Heart Failure proposed a more standardized classification of heart failure (HF).1 This approach aims to improve provider and patient understanding of the disease as well as facilitate the adoption of guideline-directed diagnosis, prognostic assessment, and management of HF. Individuals are classified into stages that describe the development and progression of HF, with the goal of identifying those at greatest risk of HF and guiding preventive and therapeutic efforts.
The stages of HF from the 2013 American College of Cardiology/American Heart Association guidelines define the spectrum of the disease as: Stage A, at high risk for HF but without structural heart disease or symptoms of HF; Stage B, structural heart disease but without signs or symptoms of HF; Stage C, structural heart disease with prior or current symptoms of HF; and Stage D, refractory HF requiring specialized interventions.2 One important revision to this classification of HF stages, proposed in a recent consensus document on the universal definition of HF and now adopted in the 2022 HF guideline, was the inclusion of natriuretic peptides and cardiac troponin in defining Stage B HF.1,3 The guideline now recommends that an individual with a B-type natriuretic peptide (BNP) ≥35 pg/mL or N-terminal pro-B–type natriuretic peptide (NT-proBNP) of ≥125 pg/mL in an ambulatory setting, or with persistently elevated levels of high-sensitivity cardiac troponins should be considered as having Stage B HF. The use of biomarkers to define Stage B HF has important clinical implications because more individuals would likely be identified as being at risk for progression to clinical HF. Moreover, this change may prompt consideration for cardiac biomarkers in routine screening strategies for HF.4,5 The identification of individuals at risk for HF is becoming more impactful, given that strategies such as the implementation of intensive blood pressure control and initiation of sodium-glucose cotransporter 2 (SGLT2) inhibitors among high-risk patients with diabetes may prevent disease progression.6 It is not known how the reclassification of individuals with abnormal biomarkers from Stage A to Stage B HF impacts risk assessment in older adults. The aim of our study was to leverage data from the ARIC (Atherosclerosis Risk In Communities) study to assess the proportion of individuals from a community-dwelling population who will now be identified as Stage B HF based on the addition of biomarkers (NT-proBNP and troponin) as well as to explore how the new Stage B classification will perform compared with the previous classification with respect to prediction of future risk for clinical HF and death.
METHODS
STUDY POPULATION.
The ARIC study is a prospective population-based study of cardiovascular disease incidence in adults aged 45-64 years when recruited from 4 U.S. communities between 1987 and 1989 (visit 1). The study protocol was approved by the institutional review boards of all participating centers, and all participants provided written informed consent. ARIC visit 5 (2011-2013) was the index visit for our current analyses.
Of the 6,538 participants at visit 5, we excluded individuals with prevalent HF, race other than White or Black, as well as Black participants from Minnesota and Washington county due to small numbers, participants missing echocardiography data, and those missing information on NT-proBNP or high-sensitivity troponin T (hs-TnT). We further excluded participants without cardiac risk factors, cardiac structural abnormalities, or elevated biomarkers, because they do not fit into the Stage A to D definitions. Prevalent HF was defined as having a HF event that occurred at or before visit 5, which was determined by diagnosis code (International Classification of Diseases-9th edition, code 428) or self-reported HF before 2005 or via adjudication by an expert panel if the event occurred from 2005 onward.7 Risk factors in our study included hypertension, diabetes, metabolic syndrome, obesity, and prevalent coronary heart disease (CHD). After exclusions, 5,324 participants were included for the primary analyses.
CARDIAC BIOMARKER QUANTIFICATION.
NT-proBNP was measured in EDTA plasma (collected at visit 5; stored at −70 °C) using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics).8 The lower limit of detection for this assay is 5 pg/mL, with an interassay coefficient of variance of 7.4% for a mean control level of 134 pg/mL.9 Hs-cTnT was measured in EDTA plasma (collected at visit 5; stored at −70 °C) using a highly sensitive assay (Elecsys Troponin T Gen 5 STAT, Roche Diagnostics). The limit of detection of this assay per the manufacturer package insert is 5 ng/L with an interassay coefficient of variance of 6.4% for a mean control level of 29 ng/L.9,10 We defined elevated cardiac biomarkers as an NT-proBNP ≥125 pg/mL and/or an hs-TnT ≥14 ng/L, which represented the 99th percentile of a healthy reference population for the assay.5
ECHOCARDIOGRAPHY ASSESSMENT OF CARDIAC STRUCTURE AND FUNCTION.
Cardiac structure and function was assessed using comprehensive echocardiography, which included 2-dimensional, Doppler, tissue Doppler, and speckle-tracking echocardiography performed at visit 5.11 A predefined imaging protocol with uniform imaging hardware and software was used for echocardiographic acquisition and processing. Quantitative measures were assessed according to American Society of Echocardiography guidelines. We defined abnormal cardiac structure and function as having any of the following: left ventricular ejection fraction (LVEF) <50%, global longitudinal strain <16%, regional wall motion abnormality, left ventricular mass index >116 g/m2 in men and >95 g/m2 in women, left ventricular end diastolic volume index ≥75 mL/m2 in men and ≥62 mL/m2 in women, left atrial volume index ≥29 mL/m2, average E/e’ ≥15, tricuspid regurgitant velocity >280 cm/s, or having a valvular abnormality.1 In our study, we defined valvular abnormality as peak aortic valve velocity ≥300 cm/s, mitral regurgitation jet area ≥4 cm2, the presence of moderate or greater aortic insufficiency, and the presence of moderate or greater mitral stenosis.12,13
MODELING OF HF STAGES.
The primary exposure variable for our study was HF stages categorized as Stage A and Stage B, which we modeled in several ways. First, we sought to emulate the Stage A and B classifications from the former guideline, which classified individuals based on structural heart disease, irrespective of cardiac biomarkers.2 Thus, individuals with risk factors but without abnormal cardiac structure or function as noted on echocardiography (irrespective of biomarker) were defined as Stage Aformer and those with cardiac structural/functional abnormalities were defined as Stage Bformer.
Next, we defined a Stage A and Stage B categories that reflected the new guideline. Stage Anew was defined as individuals with risk factors but without echo abnormalities or cardiac biomarker elevation. Meanwhile, Stage Bnew included individuals with echo abnormalities and/or elevated cardiac biomarkers (in the presence of risk factors). Acknowledging that there was significant overlap between individuals with abnormal cardiac structure or function by echocardiography and those with elevated cardiac biomarkers, we further characterized participants as follows: individuals with elevated cardiac biomarkers but no structural or function abnormalities on echocardiography (Stage Bbiomarker only), individuals with cardiac structural or function abnormalities on echocardiography but without elevated cardiac biomarkers (Stage Becho only), and individuals with both echo abnormalities and elevated biomarkers (Stage Becho+biomarker).
COVARIATES.
Sex, race, age, smoking, education level and use of medications were self-reported. Blood pressure was measured with an automatic sphygmomanometer at visit 5 by a certified trained technician using an appropriately sized cuff.9 Total cholesterol and high-density lipoprotein cholesterol (HDL-C) were measured using an enzymatic assay.14 Diabetes was defined as self-reported diabetes diagnosed by a physician, use of hypoglycemic medications, nonfasting serum glucose levels ≥200 mg/dL or a fasting serum glucose level ≥126 mg/dL. The estimated glomerular filtration rate (eGFR) was calculated based on the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.15 Height and weight were measured by trained personnel and used to calculate body mass index (BMI). Obesity was defined as a BMI ≥30 kg/m2. The metabolic syndrome was defined as having at least 3 of the 5 components of waist circumference ≥102 cm for men or ≥88 cm for women, elevated triglycerides >150 mg/dL, HDL-C <40 mg/dL for men or <50 mg/dL for women, a systolic blood pressure >130 mm Hg or a diastolic blood pressure >85 mm Hg, and a fasting glucose >100 mg/dL. Prevalent CHD was defined as self-reported myocardial infarction before visit 1 and ARIC-adjudicated myocardial infarction, silent myocardial infarction identified by electrocardiography changes, or coronary revascularization between visits 1 to 5.16 Education level was defined as high (lifetime educational attainment of college, graduate or professional degree) vs a non-high level.
OUTCOME MEASURES.
The outcome measures were incident HF events and all-cause mortality. Incident HF events were adjudicated by an expert panel as a definite or probable hospitalization for acute decompensated HF. HF events were further adjudicated as heart failure with preserved ejection fraction (HFpEF) (LVEF ≥50%) or heart failure with reduced ejection fraction (HFrEF) (LVEF <50%) if LVEF information was available at or around time of hospitalization.17 Deaths were ascertained by diagnostic codes from hospital discharge records and from death certificates. The cutoff date for administrative censoring for those without events was December 31, 2019, except for participants from the Jackson field center, where the cutoff date was December 31, 2017.
STATISTICAL ANALYSES.
We determined the proportion of individuals reclassified from Stage A to Stage B with the incorporation of cardiac biomarkers. Clinical characteristics were compared across categories using analysis of variance for continuous variables or by the chi-squared test for categorical variables. Incidence rates were calculated as events per 1,000 person-years.
We used Cox regression models to calculate HRs and 95% CIs for the association between HF stages with risk for incident HF events and all-cause death. Given the relatively high rate of death in an older population sample, we accounted for the competing risk of death for the overall HF outcome using the Fine and Gray method.18 For analysis of the HF subtypes (HFrEF and HFpEF), we performed a competing risk analysis accounting for death, the other HF subtype, and unclassified HF (due to a lack of data of ejection fraction). Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for model 1 plus systolic blood pressure, diastolic blood pressure, heart rate, antihypertensive medication use, diabetes, total cholesterol, HDL-C, cholesterol-lowering medication use, BMI, eGFR, current smoking, prevalent CHD, and education level. Proportionality assumption was verified graphically using log-log plots.
Because kidney disease can impact cardiac biomarker levels, we performed a sensitivity analysis excluding participants (n = 1,259) with elevated biomarkers in the setting of renal dysfunction, which we defined as an eGFR <60 mL/min/1.73 m2. We further performed secondary analysis redefining the elevated biomarker group by NT-proBNP and hs-TnT separately and repeated assessments of association with risk. Additionally, to evaluate improvement in risk prognostication using echocardiography and biomarkers, area under the receiver-operating characteristic curve (AUC) was calculated. The base model was derived from cardiovascular risk factors including age, sex, race, systolic blood pressure, antihypertensive medication use, diabetes, BMI, eGFR, smoking, heart rate, and prevalent CHD based on a previously validated ARIC HF risk score.19 Extended models added echocardiography abnormalities and biomarker parameters.
Given the older age of individuals at ARIC visit 5, we further assessed distribution of NT-proBNP and hs-TnT ≥125 pg/mL and ≥14 ng/L, respectively, at ARIC visits 2 and 4 among participants without prevalent HF to help understand the prevalence of abnormal biomarkers (and hence Stage B HF) at younger and middle ages. All statistical analysis was performed using STATA software version 16.1 (Stata-Corp LLC).
RESULTS
The mean age of the study population (n = 5,324) was 75.8 ± 5.2 years and 58.6% were women. The median (25th percentile, 75th percentile) values of NT-proBNP and hs-TnT at visit 5 were 130.1 pg/mL (67.6, 253.9 pg/mL) and 10 ng/L (7.16 ng/L), respectively. Overall, 63.4% of the population had either NT-proBNP ≥125 pg/mL and/or hs-TnT ≥14 ng/L. Overall, 51.6% had elevated natriuretic peptides and 33.3% had elevated troponins. In all, there were 998 (18.7%) participants who had normal biomarkers and echo (Stage A new), whereas 4,326 (81.3%) individuals had either cardiac structural or functional abnormalities on echocardiography or elevated cardiac biomarkers (Stage Bnew). Of these, 3,203 participants would have been identified as Stage B HF based on the prior definition of cardiac structural or functional abnormalities (Stage Bformer). There were 1,123 participants comprising 21.1% of the study population who had elevated cardiac biomarkers without echocardiography abnormalities (Stage Bbiomarker only) and were reclassified from Stage Aformer to Stage Bnew HF. Finally, among those with abnormal echocardiography, 852 did not have elevated biomarkers (Stage Becho only) and 2,351 had both echocardiography abnormality as well as elevated biomarkers (Stage Becho+biomarker) (Central Illustration). Baseline characteristics across categories are displayed in Table 1 and Supplemental Table 1. Those in the Stage Bbiomarker only group were older, more likely to be male, White, have prevalent CHD, and have a lower BMI and eGFR compared with those in the Stage Anew and Stage Becho only groups. The Stage Becho+biomarker group had the highest age, systolic blood pressure, rate of hypertension medication use, diabetes, cholesterol medication use, and prevalent CHD, as well as the lowest diastolic blood pressure, total cholesterol, and HDL-C.
CENTRAL ILLUSTRATION. Reclassification of HF Stages Using Cardiac Biomarkers and Association With Risk for Incident HF Events and All-Cause Death.
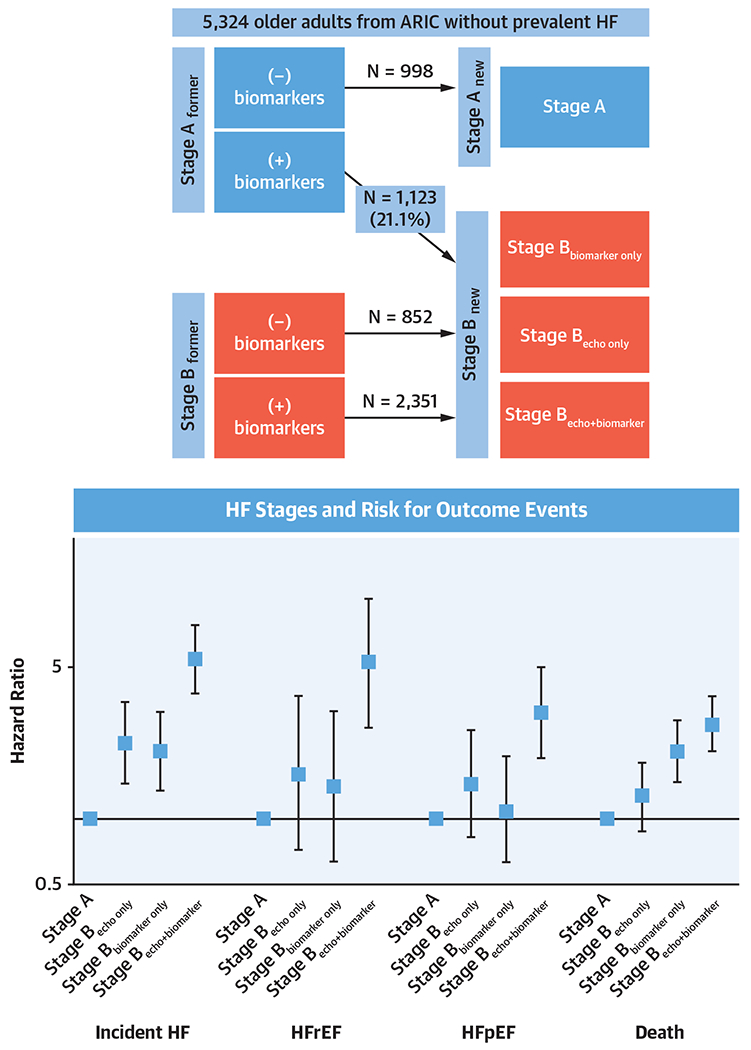
Adjustment for systolic blood pressure, diastolic blood pressure, hypertension medication use, heart rate, diabetes, low-density lipoprotein cholesterol, triglycerides, cholesterol medication, body mass index, estimated glomerular filtration rate, current smoking, prevalent coronary heart disease. HR and 95% CIs are plotted in log-scale. ARIC = Atherosclerosis Risk in Communities; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
TABLE 1.
Basic Characteristics Among Individuals at Visit 5 Without Prevalent HF
| Overall (N = 5,324) | Stage Anew (n = 998) | Stage Bnew | P Value | |||
|---|---|---|---|---|---|---|
| Stage Becho only (n = 852) | Stage Bbiomarker only (n = 1,123) | Stage Becho+biomarker (n = 2,351) | ||||
| Age, y | 75.8 (5.2) | 73.3 (4.2) | 73.9 (4.7) | 75.9 (4.9) | 77.5 (5.3) | <0.001 |
| Women | 58.6 | 61.4 | 65.1 | 56.4 | 56.2 | <0.001 |
| Black | 23.7 | 26.6 | 32.7 | 18.2 | 21.9 | <0.001 |
| SBP, mm Hg | 131.6 (18.4) | 127.7 (15.8) | 130.6 (17.5) | 131.5 (17.1) | 133.8 (19.9) | <0.001 |
| DBP, mm Hg | 67.1 (10.7) | 68.0 (9.4) | 68.8 (10.3) | 66.9 (10.5) | 66.2 (11.4) | <0.001 |
| Hypertension medication use | 73.5 | 74.4 | 67.1 | 76.3 | 73.5 | <0.001 |
| Heart rate, beats/min | 62.6 (10.6) | 63.5 (9.9) | 63.3 (11.2) | 62.0 (9.6) | 62.2 (11.1) | 0.001 |
| Diabetes | 35.3 | 33.4 | 36.0 | 32.1 | 37.5 | 0.010 |
| Metabolic syndrome | 25.2 | 24.8 | 27.8 | 22.1 | 25.9 | 0.022 |
| TC, mg/dL | 180.5 (41.7) | 182.3 (40.1) | 186.2 (42.3) | 181.8 (42.7) | 177.0 (41.4) | <0.001 |
| HDL-C, mg/dL | 51.7 (13.9) | 51.8 (13.3) | 52.3 (13.4) | 52.5 (15.0) | 51.0 (13.7) | 0.017 |
| Cholesterol-lowering medication | 57.1 | 55.8 | 53.5 | 58.9 | 58.1 | 0.052 |
| BMI, kg/m2 | 29.0 (5.7) | 29.3 (5.2) | 29.6 (5.7) | 28.4 (5.3) | 29.0 (6.1) | <0.001 |
| eGFR, mL/min/1.73m2 | 69.3 (17.4) | 74.6 (14.5) | 76.9 (15.1) | 65.8 (16.6) | 66.0 (18.2) | <0.001 |
| Current cigarette smoking | 5.9 | 5.2 | 7.0 | 5.7 | 5.9 | 0.468 |
| Prevalent CHD | 15.5 | 9.2 | 7.8 | 15.7 | 20.9 | <0.001 |
| High education level | 43.0 | 46.3 | 46.6 | 40.9 | 41.2 | 0.001 |
Values are n (%) or mean ± SD compared using analysis of variance, unless otherwise indicated. Categorical variable presented as percentage compared using chi-squared test. Stage A = risk factors with no structural/functional abnormalities on echo and no elevated biomarker; Stage Becho only = Stage B heart failure (HF) defined by structural/functional abnormalities on echo without elevated biomarkers; Stage Bbiomarker only = elevated biomarkers in the presence of risk factors without structural/functional abnormalities on echo; Stage Becho+biomarker = Stage B HF defined by structural and functional abnormalities and biomarker elevation; Stage Bnew = Stage B defined by the new heart failure guideline which includes biomarker elevation (in the presence of risk factors) or cardiac structural/functional abnormalities.
BMI = body mass index; CHD = coronary heart disease; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; SBP = systolic blood pressure; TC = total cholesterol.
CARDIOVASCULAR RISK ASSOCIATED WITH THE NEW STAGE B HF DEFINITION.
Over a median follow-up period of 7.2 years, 730 incident HF events and 1,136 deaths occurred. The incidence rate per 1,000-person years for HF was 4.7 (95% CI: 3.3-6.7) in those categorized as Stage Anew, 26.4 (95% CI: 24.5-28.4) in those categorized as Stage Bnew, compared with 8.9 (95% CI: 7.5-10.6) in those categorized as Stage Aformer, and 31.5 (95% CI: 29.1-34.1) in those categorized as Stage Bformer. The incidence rate per 1,000 person-years for death was 12.5 (95% CI: 10.1-15.5) for Stage Anew, 20.1 (95% CI: 17.9-22.6) for Stage Aformer, 38.3 (95% CI: 36.0-40.7) for Stage Bnew, and 42.3 (95% CI: 39.5-45.3) for Stage Bformer. In our Cox regression models, Stage Bnew was associated with significant increased risk for incident HF (HR: 3.89 [95% CI: 2.71-5.58]), HFrEF (HR: 3.08 [95% CI: 1.59-5.95]), HFpEF (HR: 2.10 [95% CI: 1.33-3.30]), and all-cause death (HR: 1.95 [95% CI: 1.54-2.47]) compared with Stage Anew after model 2 adjustment. Meanwhile, the associations with risk for incident HF, HFrEF, HFpEF, and death for Stage Bformer compared with Stage Aformer were HR: 2.95 (95% CI: 2.42-3.61), HR: 3.28 (95% CI: 2.11-5.10), HR: 2.50 (95% CI: 1.82-3.45), and HR: 1.69 (95% CI: 1.46-1.95), respectively, after model 2 adjustment.
COMPLEMENTARY EFFECT OF ECHOCARDIOGRAPHY AND CARDIAC BIOMARKERS ON CARDIOVASCULAR RISK PREDICTION.
By refining Stage B categories as Stage Bbiomarker only, Stage Becho only, and Stage Becho+biomarker, we found that Stage Bbiomarker only and Stage Becho only were both associated with higher risk for incident HF (HR: 2.07 [95% CI: 1.37-3.12] and HR: 2.24 [95% CI: 1.45-3.47], respectively) compared with the Stage Anew group after adjusting for demographic and cardiovascular risk factors. Stage Bbiomarker only but not Stage Becho only was also significantly associated with an increased risk for death. The association between Stage Bbiomarker only and Stage Becho only with HFrEF and HFpEF events, when assessed separately, did not achieve statistical significance. Notably, individuals with both abnormal echo findings and elevated cardiac biomarkers (Stage Becho+biomarker) had the highest risk for incident HF (HR: 5.44 [95% CI: 3.77-7.85]), HFrEF (HR: 5.20 [95% CI: 2.64-10.26]), HFpEF (HR: 3.08 [95% CI: 1.91-4.94]), and death (HR: 2.76 [95% CI: 2.06-3.69]) (Table 2, Central Illustration). Sensitivity analysis excluding participants with renal dysfunction yielded similar results (Supplemental Table 2). Additionally, secondary analysis defining biomarker elevation by NT-proBNP and hs-TnT separately demonstrated similar patterns of associations for each biomarker with risk (Supplemental Tables 3 and 4). The distribution of individual echocardiography parameters showed that the Stage Becho+biomarker group had higher proportions of individuals with parameters suggestive of chamber enlargement, decreased LVEF, regional wall motion abnormality, and diastolic dysfunction, as well as most valvular abnormalities compared with the Stage Becho only group. There were similar proportions of abnormal strain in the Stage Becho only group compared with the Stage Becho+biomarker group (Table 3).
Table 2.
Association of HF Stages With Incident HF Events and All-Cause Death
| Stage Anew (n = 998) | Stage Becho only (n = 852) | Stage Bbiomarker only (n = 1,123) | Stage Becho+biomarker (n = 2,351) | |
|---|---|---|---|---|
| HF events | ||||
| n | 32 | 63 | 96 | 539 |
| IR (95% CI) | 4.7 (3.3-6.7) | 11.2 (8.7-14.3) | 15.9 (10.7-15.9) | 40.0 (36.8-43.5) |
| IRR (95% CI) | Ref. | 2.4 (1.9-3.0) | 3.4 (2.3-3.4) | 8.5 (7.8-9.2) |
| Model 1 | Ref. | 2.20 (1.44-3.36) | 2.40 (1.61-3.57) | 6.77 (4.73-9.69) |
| Model 2 | Ref. | 2.24 (1.45-3.47) | 2.07 (1.37-3.12) | 5.44 (3.77-7.85) |
|
| ||||
| HFrEF | ||||
| n | 10 | 13 | 17 | 110 |
| IR (95% CI) | 1.8 (1.0-3.3) | 2.7 (1.6-4.7) | 3.0 (1.9-4.9) | 11.7 (9.7-14.1) |
| IRR (95% CI) | Ref. | 1.6 (0.9-2.7) | 1.7 (1.1-2.8) | 6.6 (5.5-8.0) |
| Model 1 | Ref. | 1.46 (0.65-3.32) | 1.49 (0.68-3.27) | 5.26 (2.70-10.24) |
| Model 2 | Ref. | 1.63 (0.72-3.67) | 1.42 (0.63-3.21) | 5.20 (2.64-10.26) |
|
| ||||
| HFpEF | ||||
| n | 22 | 29 | 31 | 162 |
| IR (95% CI) | 3.9 (2.6-5.9) | 6.2 (4.3-8.9) | 5.5 (3.9-7.9) | 17.4 (14.9-20.3) |
| IRR (95% CI) | Ref. | 1.6 (1.1-2.3) | 1.4 (1.0-2.0) | 4.5 (3.8-5.2) |
| Model 1 | Ref. | 1.50 (0.86-2.60) | 1.24 (0.72-2.14) | 3.57 (2.26-5.66) |
| Model 2 | Ref. | 1.44 (0.82-2.55) | 1.10 (0.63-1.95) | 3.08 (1.91-4.94) |
|
| ||||
| All-cause death | ||||
| n | 85 | 96 | 205 | 750 |
| IR (95% CI) | 12.5 (10.1-15.5) | 16.8 (13.8-20.6) | 27.5 (24.0-31.5) | 52.4 (48.8-56.3) |
| IRR (95% CI) | Ref. | 1.3 (1.1-1.7) | 2.2 (1.9-2.5) | 4.2 (3.9-4.5) |
| Model 1 | Ref. | 1.35 (0.97-1.89) | 1.93 (1.42-2.62) | 2.70 (2.05-3.54) |
| Model 2 | Ref. | 1.26 (0.87-1.82) | 2.06 (1.49-2.84) | 2.76 (2.06-3.69) |
Stage B by biomarker defined as NT-proBNP ≥125 pg/mL and/or hs-TnT ≥14 ng/L. IR expressed as per 1,000-person years and compared by IRR. Association expressed as HR (95% CI). Stages defined as in Table 1. Model 1 adjusted for age, sex, and race; model 2 adjusted for model 1 plus SBP, DBP, hypertension medication use, heart rate, diabetes, TC, HDL-C, cholesterol-lowering medication, BMI, eGFR, current smoking, prevalent CHD, and education level.
HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; IR = incidence rate; IRR = incidence rate ratio; NT-proBNP = N-terminal pro-B-type natriuretic peptide; Ref. = reference; other abbreviations as in Table 1.
Table 3.
Distribution of Abnormal Echo Parameters by Stage Becho only and Stage Becho+biomarker
| Stage Becho only (n = 852) | Stage Becho+biomarker (n = 2,351) | |
|---|---|---|
| LVEF <50% | 0.7 | 4.5 |
| Longitudinal strain <16% | 33.4 | 30.4 |
| Regional wall motion abnormality | 0.2 | 1.7 |
| LV mass index >116 g/m2 in men and >95 g/m2 in women | 14.1 | 19.4 |
| LV end diastolic volume index ≥75 mL/m2 in men and ≥62 mL/m2 in women | 14.8 | 19.5 |
| Left atrial volume index ≥29 mL/m2 | 36.3 | 53.6 |
| Average E/e′ ≥15 | 25.9 | 35.0 |
| Peak aortic valve velocity >300 cm/s | 0.5 | 1.27 |
| Mitral regurgitation jet area >4 cm2 | 1.1 | 3.8 |
| Moderate or greater aortic insufficiency | 0.5 | 0.8 |
| Moderate or greater mitral stenosis | 0 | 0 |
| Tricuspid regurgitation velocity >280 cm/s | 5.3 | 8.8 |
Values are n (%).
LV = left ventricular; LVEF = left ventricular ejection fraction.
The addition of echocardiography or biomarker parameters significantly improved discrimination of HF and death by Harrell’s C-statistic when added to the base model of cardiovascular risk factors. Importantly, the addition of biomarkers to a model that included risk factors plus echocardiography parameters further enhanced discrimination for future adverse events (Table 4). When analyzed individually, both NT-proBNP and hs-TnT provided prognostic value when added to the base model. However, the addition of NT-proBNP to a model that included risk factors plus hs-TnT further improved risk prognostication, whereas the addition of hs-TnT to a model that included NT-proBNP did not (Supplemental Table 5).
Table 4.
Prognostication of Future HF Events and Death by AUC Analysis
| C-Statistic Primary Model (95% CI) | C-Statistic Comparison Model (95% CI) | Difference (95% CI) | P Value | |
|---|---|---|---|---|
| HF events | ||||
|
| ||||
| Model A vs model B1 | 0.717 (0.698-0.737) | 0.758 (0.740-0.776) | 0.040 (0.029-0.052) | <0.001 |
| Model A vs model B2 | 0.717 (0.698-0.737) | 0.753 (0.735-0.770) | 0.035 (0.025-0.045) | <0.001 |
| Model A vs model B3 | 0.717 (0.698-0.737) | 0.778 (0.762-0.795) | 0.061 (0.048-0.074) | <0.001 |
| Model B1 vs model B3 | 0.758 (0.740-0.776) | 0.778 (0.762-0.795) | 0.020 (0.014-0.027) | <0.001 |
|
| ||||
| Death | ||||
|
| ||||
| Model A vs model B1 | 0.683 (0.666-0.699) | 0.693 (0.677-0.709) | 0.010 (0.004-0.016) | 0.001 |
| Model A vs model B2 | 0.683 (0.666-0.699) | 0.701 (0.685-0.717) | 0.018 (0.011-0.025) | <0.001 |
| Model A vs model B3 | 0.683 (0.666-0.699) | 0.706 (0.690-0.722) | 0.023 (0.015-0.031) | <0.001 |
| Model B1 vs model B3 | 0.693 (0.677-0.709) | 0.706 (0.690-0.722) | 0.013 (0.008-0.019) | <0.001 |
Model A = risk factors model; model B1 = risk factors + cardiac structural/functional abnormality on echo; model B2 = risk factors + elevated biomarkers; model B3 = risk factors + structural/functional abnormality on echo + elevated biomarkers.
AUC = area under the curve; other abbreviation as in Table 1.
DISTRIBUTION OF CARDIAC BIOMARKERS AT MIDDLE AGE.
Given the older population at visit 5, we further conducted exploratory analysis to assess distribution in these biomarkers at earlier ARIC study visits. At visit 2 (mean age: 57 ± 6 years), 18.8% of 12,714 participants without prevalent HF had elevated biomarkers of whom 16.0% had an NT-proBNP ≥125 pg/mL and 4.6% had an hs-TnT ≥14 ng/L. At visit 4 (mean age: 63 ± 6 years), 32.9% of the included individuals had elevated biomarkers, of whom 28.5% had increased NT-proBNP and 8.5% had increased hs-TnT. Distributions of biomarkers by age categories for visits 2, 4, and 5 are shown in Figure 1.
FIGURE 1. Distribution of Elevated Cardiac Biomarkers by Age Categories.
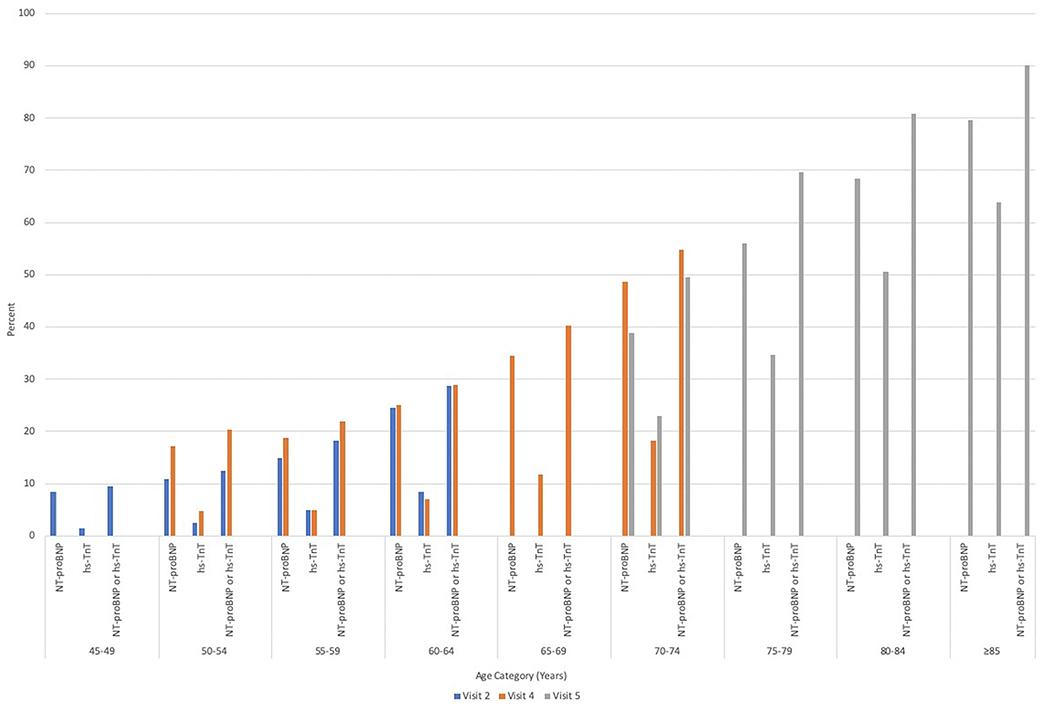
Distribution of biomarkers above respective thresholds (NT-proBNP ≥125 pg/mL, hs-TnT ≥14 pg/mL) among participants without prevalent heart failure at ARIC visit 2, visit 4, and visit 5 by age categories. ARIC = Atherosclerosis Risk in Communities; hs-TnT = high-sensitivity troponin T; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
DISCUSSION
In our study of older community-dwelling individuals without prevalent HF, we found that a significant proportion of older adults (>1 in 5) were reclassified from Stage A to Stage B HF when incorporating cardiac biomarkers (NT-proBNP and hs-TnT). We demonstrated that these reclassified individuals have an elevated risk for future HF events as well as an increased risk for all-cause death. Simultaneously, the use of biomarkers improved the de-risking of participants categorized as Stage A HF. When further refining risk assessment of individuals with abnormal echocardiography parameters, delineating between those with elevated biomarkers and those without, we found that echocardiography abnormalities alone without elevation in biomarkers were associated with increased risk for HF, but not death, and echocardiography abnormalities with an elevation in biomarkers portended to the highest risk for future HF events and death among all subgroups. Moreover, the use of biomarkers complemented echocardiography and provided modest but significant improvement in discrimination of those at risk for HF and death.
Clinical HF carries significant morbidity and mortality especially in older adults, despite advances in management.20 With this in mind, the inclusion of cardiac biomarkers to define Stage B HF helps to better identify a high-risk population for HF prevention who would have otherwise been missed by the prior definition of cardiac structural or functional abnormalities alone. Recent studies have shown that cardiac biomarkers can help to further risk stratify subjects across blood pressure ranges.21 Furthermore, data are beginning to emerge that individuals with elevated biomarker levels may derive the most benefit with risk factor modification in prevention of HF. An analysis from SPRINT (Systolic Blood Pressure Intervention Trial) recently demonstrated that intensive blood pressure control among those with elevated NT-proBNP and/or hs-TnT resulted in the large absolute risk reduction in HF and mortality.22 Likewise, analysis from CANVAS (Canagliflozin Cardiovascular Assessment Study) found that treatment with the SGLT2 inhibitor canagliflozin attenuated increase of natriuretic peptides and cardiac troponins among older adults with type 2 diabetes.23
We also found that cardiac biomarkers, when used in conjunction with echocardiography, can enhance the risk stratification of individuals with Stage B HF. Risks associated with different structural and functional abnormalities seen on echocardiography are likely heterogeneous. Although HF and death were associated with a combination of abnormalities, isolated structural or functional abnormalities may not be associated with an increased risk.24 The ability of biomarkers to refine risk associated with structural cardiovascular phenotypes has been reported previously. For example, abnormal cardiac troponin and NT-proBNP have been associated with fibrosis and changes in left ventricular structure, as well as risk for subsequent HF.25,26 Here, we extend that concept to a much broader range of underlying cardiac structural and functional abnormalities. Our results are also in line with a prior analysis from CHS (Cardiovascular Health Study), which showed that the use of NT-proBNP together with echocardiography significantly improved HF risk reclassification over a clinical prediction model.27 We build on these findings by demonstrating the distinct risk profile for incident HF that corresponds with echocardiography abnormalities with and without biomarker elevation. We further show that the complementary role of biomarker and echocardiography extends to risk stratification for death. Importantly, individuals with both biomarker elevation and abnormalities on echocardiography have the highest risk for events compared with individuals with either echo or biomarker abnormalities alone, representing a subgroup that may benefit the most from intensive prevention efforts.
The incorporation of natriuretic peptides and high-sensitivity troponins in defining Stage B HF by the recent HF guideline and our results should stimulate reexamination of the broader use of cardiac biomarker as a screening tool among ambulatory patients with clinical risk factors. We observed ~19% of participants at visit 2 and ~33% of participants at visit 4 who had elevated cardiac biomarkers, suggesting that broader testing in a younger population with cardiovascular risk factors can also yield a significant number of individuals who may benefit from HF prevention efforts. Similar to efforts using biomarkers such hemoglobin A1C and prostate-specific antigen, natriuretic peptides and troponin can be used to identify high-risk individuals. The clinical manifestation of HF, after all, can have a prognosis that is worse than some malignancies.28
STUDY LIMITATIONS.
First, echocardiography data were only available at ARIC visit 5; thus, our analysis primarily focused on older adults the and prevalence of Stage B HF, irrespective of definition used will likely be lower in a younger population. Second, the guideline definition of cardiac structural and functional abnormalities is broad, encompassing not only additional echocardiography parameters, but also include those that may be derived from cardiac magnetic resonance or invasive catheterization assessment, which were not available in our study. For practical purposes, we included a select number of echocardiography parameters that reflected cardiac structure and functional status when modeling Stage B HF in our analysis. Third, because incident HF in our study was defined as hospitalization for HF, we were not able to account for possible HF decompensation that may have occurred without hospitalization.
CONCLUSIONS
Among older, community-dwelling adults without prevalent HF, the use of elevated cardiac biomarkers reclassified a significant number of individuals to Stage B HF. Furthermore, biomarkers complimented echocardiography in risk stratification of HF and death with concomitant biomarker elevation and echocardiographic abnormality associated with the highest risk for future events. Our findings advocate for the use of both cardiac biomarker and echocardiography in at risk populations to identify the subgroup of individuals who can derive the most benefit from HF prevention efforts.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS 1:
Among older, community-dwelling adults without prevalent HF, the inclusion of elevated NT-proBNP or hs-TnT to the definition of HF staging reclassified almost 1 in 5 individuals to Stage B HF.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS 2:
Cardiac biomarkers and echocardiography are complementary in defining risk for incident HF and death among individuals with Stage B HF with those having abnormalities in both associated with the highest risk.
TRANSLATIONAL OUTLOOK 1:
Although the current study focused on an older population, studies that evaluate the complementary effect of cardiac biomarkers and echocardiology in risk stratification of patients with Stage B HF at earlier ages are warranted.
TRANSLATIONAL OUTLOOK 2:
The use of cardiac biomarkers and echocardiography should help to guide recruitment of at-risk patients in future clinical trials focused on HF prevention.
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The ARIC study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). Dr Bozkurt has received consulting fees from Bristol Myers Squibb, scPharmaceuticals, Baxter Healthcare Corporation, Sanofi-Aventis, Relypsa, Amgen; serves on the Clinical Event Committee for GUIDE HF Trial sponsored by Abbott Vascular, and Data Safety Monitoring Committee of ANTHEM trial (Autonomic REGULATION Therapy to Enhance Myocardial Function and Reduce progression of Heart Failure with reduced ejection fraction) sponsored by Liva Nova. Dr Ballantyne has received research support from National Institutes of Health grant R01HL134320 (relevant to study), Abbott Diagnostic, Akcea, Amgen, Arrowhead, Esperion, Ionis, Novartis, Regeneron, Roche Diagnostic, National Institutes of Health, AHA, and ADA; is a consultant for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Matinas BioPharma Inc, Merck-New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic, and Sanofi-Synthelabo. Dr Hoogeveen has received research grants from Denka Seiken and serves as a consultant to Denka Seiken. Dr de Lemos has received grant support from Abbott Diagnostics and Roche Diagnostics; and consulting income from Siemen’s Health Care Diagnostics, Quidel, Beckman Coulter, and Ortho Clinical Diagnostics; and is the co-inventor on a patent awarded to University of Maryland using high sensitivity cardiac troponin T and left ventricular hypertrophy as markers of heart failure risk (patent number: 61990386). Dr Nambi has stock ownership in Abbott labs. Dr Shah has received research support from Novartis and Philips Ultrasound; and consulting fees from Philips Ultrasound and Janssen. Dr Selvin has received research funding support from the National Institutes of Health grant R01HL134320 (relevant to study). Dr Virani has received grant support from the U.S. Department of Veterans Affairs, National Institutes of Health, Tahir, and the Jooma Family; and has received honorarium from the American College of Cardiology (Associate Editor for Innovations, acc.org). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- BNP
B-type natriuretic peptide
- CHD
coronary heart disease
- eGFR
estimated glomerular filtration rate
- HDL-C
high-density lipoprotein cholesterol
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- hs-TnT
high-sensitivity troponin T
- LVEF
left ventricular ejection fraction
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- SGLT2
sodium-glucose cotransporter 2
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):387–413. [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 5.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Januzzi JL Jr, Xu J, Li J, et al. Effects of canagliflozin on amino-terminal pro-b-type natriuretic peptide: implications for cardiovascular risk reduction. J Am Coll Cardiol. 2020;76:2076–2085. [DOI] [PubMed] [Google Scholar]
- 7.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 8.Ndumele CE, Matsushita K, Sang Y, et al. N-terminal pro-brain natriuretic peptide and heart failure risk among individuals with and without obesity: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016;133:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madan N, Lee AK, Matsushita K, et al. Relation of isolated systolic hypertension and pulse pressure to high-sensitivity cardiac troponin-T and N-terminal pro-B-type natriuretic peptide in older adults (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2019;124:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed A, Nambi V, Sun W, et al. Short-Term Global Cardiovascular Disease Risk Prediction in Older Adults. J Am Coll Cardiol. 2018;71:2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. [DOI] [PubMed] [Google Scholar]
- 13.Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation. 2012;126:2005–2017. [DOI] [PubMed] [Google Scholar]
- 14.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14:1098–1104. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conrad N, Judge A, Canoy D, et al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol. 2019;4:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain A, Sun W, Deswal A, et al. Association of NT-ProBNP, blood pressure, and cardiovascular events: the ARIC study. J Am Coll Cardiol. 2021;77: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry JD, Nambi V, Ambrosius WT, et al. Associations of high-sensitivity troponin and natriuretic peptide levels with outcomes after intensive blood pressure lowering: findings from the SPRINT randomized clinical trial. JAMA Cardiol. 2021;6:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Januzzi JL Jr, Butler J, Jarolim P, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. 2017;70:704–712. [DOI] [PubMed] [Google Scholar]
- 24.Shah AM, Claggett B, Loehr LR, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities study. Circulation. 2017;135:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey A, Keshvani N, Ayers C, et al. Association of cardiac injury and malignant left ventricular hypertrophy with risk of heart failure in African Americans: the Jackson Heart study. JAMA Cardiol. 2019;4:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seliger SL, Hong SN, Christenson RH, et al. High-sensitive cardiac Troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation. 2017;135:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalogeropoulos AP, Georgiopoulou VV, deFilippi CR, Gottdiener JS, Butler J, Cardiovascular Health Study. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: the Cardiovascular Health Study. J Am Coll Cardiol Img. 2012;5:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamas MA, Sperrin M, Watson MC, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail. 2017;19:1095–1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


