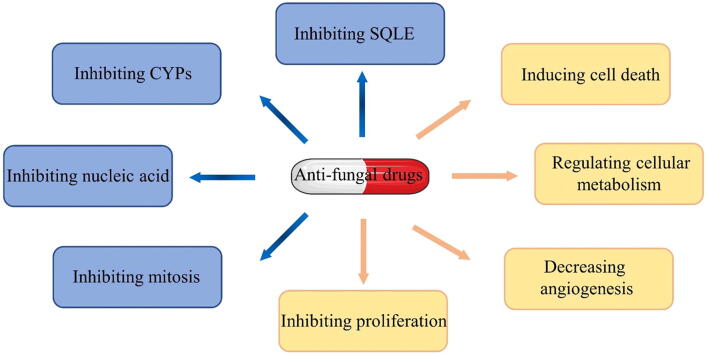
Graphical abstract
Keywords: Drug repurposing, Drug discovery, Cancer therapy, Antifungal drugs, Drug targets
Abstract
Background
Repurposing antifungal drugs in cancer therapy has attracted unprecedented attention in both preclinical and clinical research due to specific advantages, such as safety, high-cost effectiveness and time savings compared with cancer drug discovery. The surprising and encouraging efficacy of antifungal drugs in cancer therapy, mechanistically, is attributed to the overlapping targets or molecular pathways between fungal and cancer pathogenesis. Advancements in omics, informatics and analytical technology have led to the discovery of increasing “off-site“ targets from antifungal drugs involved in cancerogenesis, such as smoothened (D477G) inhibition from itraconazole in basal cell carcinoma.
Aim of review
This review illustrates several antifungal drugs repurposed for cancer therapy and reveals the underlying mechanism based on their original target and “off-site” target. Furthermore, the challenges and perspectives for the future development and clinical applications of antifungal drugs for cancer therapy are also discussed, providing a refresh understanding of drug repurposing.
Key scientific concepts of review
This review may provide a basic understanding of repurposed antifungal drugs for clinical cancer management, thereby helping antifungal drugs broaden new indications and promote clinical translation.
Introduction
Cancer was estimated to be responsible for 10 million deaths in 2020 [1], emerging as a severe global health and financial burden. Although effective therapeutic strategies are continuously being developed, cancer-related deaths have increased by 25.4% over the past decade [2]. Due to the limited availability of routine screening and the lack of specific symptoms for early-stage cancer, the majority of patients with cancer are diagnosed at an advanced stage at their initial clinical consultation and few treatment agents are available. Furthermore, acquired drug resistance is likely to occur. These current bottlenecks of clinical cancer treatment emphasize the necessity for developing alternative strategies for cancer therapy [3], [4]. Drug repurposing, which refers to evaluating existing drugs for their possible new indication outside the original scope, significantly reduces the cost and shortens the cycle of drug research and development compared with de novo drug discovery. Although there are some legitimate objections to the concept of drug repurposing, including concerns about insufficient single-drug activity and the emergence of adverse outcomes based on retrospective observational studies [5]. Nevertheless, enthusiasm for drug repurposing remains high. This is because drug repurposing may be the last hope for patients with advanced cancer who have no drugs available after treatment failure. In addition, non-oncology drugs obtained by drug repurposing can contribute to cancer patients through drug combination. The most successful examples of drug reposition include arsenic trioxide, which is previously considered as a highly toxic substance and now as an FDA-approved drug for the treatment of acute promyelocytic leukemia [6], [7]. In addition to serendipity, recent advances in genomics and proteomics have facilitated more non-oncology drugs to find their new targets in cancer therapy, providing excellent opportunities for drug repurposing in precision medicine [8]. Notably, these existing agents have undergone at least phase II clinical trials, which systematically investigate the pharmacokinetics and pharmacodynamics of candidate drugs, guaranteeing safety even in a different disease context [9]. In recent years, a variety of non-oncology drugs have been discovered for their anticancer activity based on a drug repurposing strategy, among which antifungal agents have attracted great attention [9].
In general, antifungal drugs mainly exert their therapeutic effects via four mechanisms of action: blocking the formation of fungal cell membranes and inducing transmembrane pores by inhibiting the synthesis of ergosterol; preventing the synthesis of fungal cell wall components such as 3-β-D-glucan; interfering with intracellular microtubule assembly and inhibiting mitosis; and reducing thymidylate synthase, resulting in DNA and RNA damage [10], [11] (Fig. 1). Except for fungal cell wall components, the remaining biological structure and activities in fungi are also ubiquitous in cancer cells. Several common or similar targets and pathways are shared between fungal infections and malignancies, providing a reliable foundation for repurposing antifungal drugs in cancer therapy. For example, squalene epoxidase (SQLE) catalyses a rate-limiting step in fungal ergosterol biosynthesis and promotes cancer progression in the human body as the second rate-limiting enzyme of cholesterol synthesis. Moreover, the activities of thymidylate synthesis and mitosis are ubiquitous in fungal and cancer cells. In addition to the long-accepted understanding of the antifungal mechanism based on the original targets, recent investigations with the application of advanced multi-omics and analytical technologies have revealed that some antifungal drugs exert multiple effects on fungal or host cells. Amphotericin B, which inhibits fungal cells by directly binding ergosterol, modulates oxidative damage and the immune system in the human body [12]. Several antifungal agents suppress a broad spectrum of fungal organisms, such as Cryptococcus, Candida, and Aspergillus, further implying that antifungal drugs may have multiple targets. For example, itraconazole, a commonly used triazole, exerts anticancer activities by inhibiting numerous molecular mechanisms, including smoothened (SMO) D477G mutations, sterol carrier protein 2 (SCP2), voltage-dependent anion channel 1 (VDAC1), and Niemann-Pick Type C 1 (NPC1) (Fig. 2).
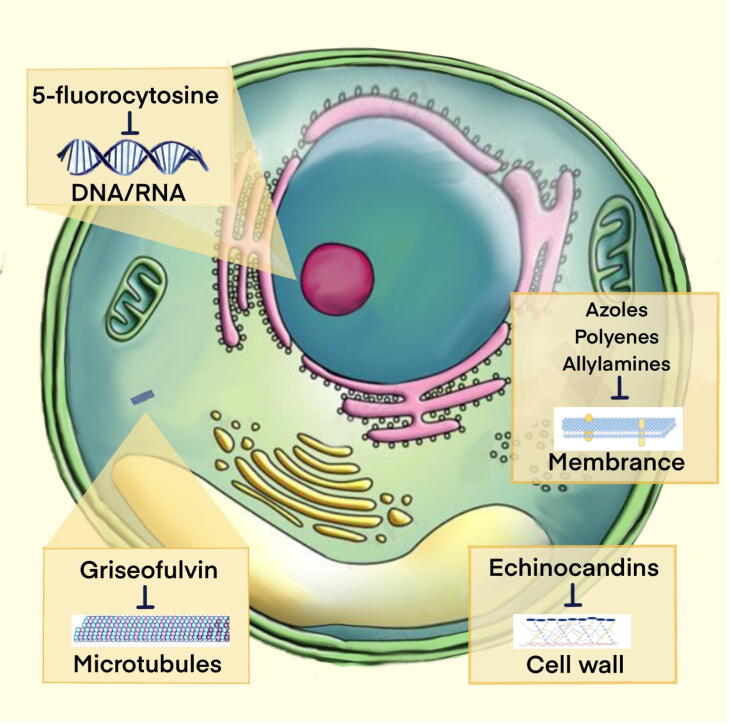
Fig. 1.
Schematic of mechanistic sites for common antifungal drugs There are four main antifungal agent classes approved for human fungal infection: azoles, polyenes, and allylamines interfere with the synthesis of fungal cell membranes; echinocandins prevent the synthesis of fungal cell walls; griseofulvin interferes with intracellular microtubule assembly and inhibits mitosis; and 5-fluorocytosine reduces thymidylate synthase, resulting in DNA and RNA damage.
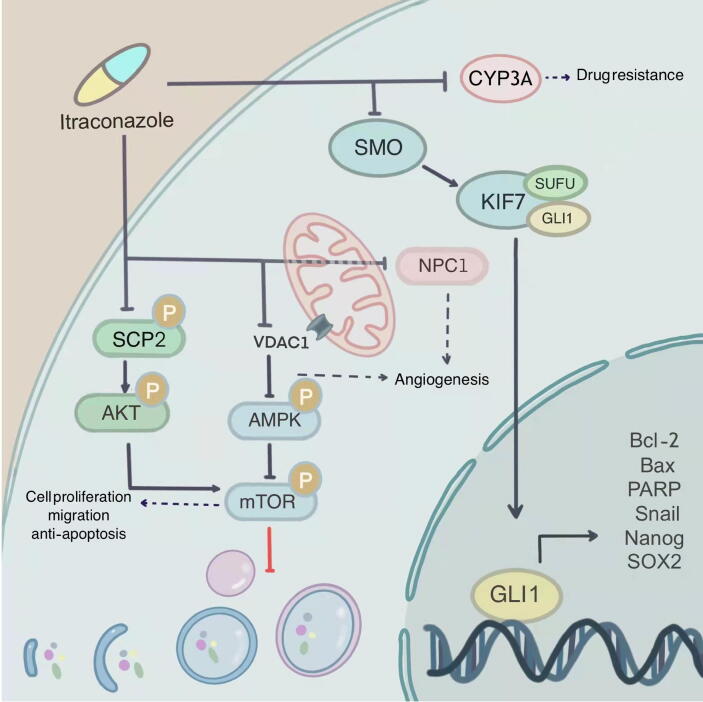
Fig. 2.
Signaling pathways mediated by itraconazole Itraconazole influences diverse targets of cancer including CYP3A4, smoothened D477G mutations, sterol carrier protein 2 (SCP2), voltage-dependent anion channel 1 (VDAC1), and Niemann-Pick Type C 1 (NPC1).
In this review, the recent progress of repurposing antifungal drugs for cancer therapy based on their original targets and off-site targets is summarized, highlighting a new perspective for the clinical application of antifungal drugs in cancer prevention and therapy (Table 1). In addition, the current limitations of this strategy, as well as future directions for optimizing antifungal drugs as ideal candidates for cancer in the clinic, are discussed. The topic of overcoming bottlenecks in cancer treatment through drug repurposing has been widely debated. However, these papers have mostly focused on the discussion of specific single drugs for drug repurposing or on a comprehensive review of all classes of non-oncology drugs based on their biology against cancer features [9], [13], [14], [15], [16], [17], [18]. As far as we know, this is the first reviews on systematic discussion about the anti-tumor activities exerted by anti-fungal agents. This review may provide a basic understanding of repurposed antifungal drugs for clinical cancer management, thereby helping antifungal drugs broaden new indications and promote clinical translation.
Table 1.
Repurposing antifungal drugs for cancer therapy.
| Drug | Cancer | Study type | Dose of administration | Main mechanism | Ref |
|---|---|---|---|---|---|
| Terbinafine | hepatocellular carcinoma |
In vitro In vivo |
0–50 μM 80 mg/kg |
Inhibiting SQLE | [34] |
| colorectal cancer |
In vitro In vivo |
0–50 μM 50 mg/kg |
Inhibiting SQLE | [35] | |
| promyelocytic leukemia |
In vitro |
0–30 μM | Inducing mitochondrial dysfunction and apoptosis | [142] | |
| oral squamous cell carcinoma | In vitro | 0–60 μM | Inducing G0/G1 cell-cycle arrest | [169] | |
| hepatocellular carcinoma |
In vitro In vivo |
0–80 μM 100 mg/kg |
Regulating AMPK -mTORC1 signaling | [204] | |
| oral squamous cell carcinoma |
In vitro |
0–150 μg/μL | Suppressing Raf-MEK-ERK signaling | [205] | |
| colon cancer |
In vitro In vivo |
0–120 μM 50 mg/kg |
Inducing G0/G1 cell cycle arrest | [206] | |
| Natamycin | hepatocellular carcinoma |
In vitro In vivo |
0–40 μM 50 mg/kg |
Inducing ROS accumulation and subsequent apoptosis | [145] |
| Itraconazole | medulloblastoma and basal cell carcinoma |
In vitro In vivo |
0–0.5 μM 75 mg/kg |
Inhibiting Hedgehog pathway | [123] |
| endometrial cancer | In vitro | 0–10 μM | Inhibiting Hedgehog pathway | [127] | |
| melanoma |
In vitro In vivo |
0–4 μM 0–100 mg/kg |
Suppressing Hedgehog, Wnt, and PI3K/mTOR pathways | [128] | |
| glioblastoma |
In vitro In vivo |
0–4 μM 75 mg/kg |
Inducing autophagic cell death | [129] | |
| endometrial cancer | In vitro | 0–10 μM | Inhibiting AKT/mTOR signaling | [130] | |
| cutaneous squamous cell carcinoma |
In vitro In vivo |
0–4 μM 0–80 mg/kg |
Targeting HMGCS1/ACSL4 axis | [131] | |
| hepatocellular carcinoma | In vitro | 0–8 μg/mL | Regulating Wnt, PI3K/AKT/mTOR, and ROS pathways | [132] | |
| colon cancer |
In vitro In vivo |
0–10 μM 75 mg/kg |
Inhibiting the Hedgehog pathway | [133] | |
| oral squamous cell carcinoma |
In vitro In vivo |
0–5 μM 50 mg/kg |
Inhibiting the Hedgehog pathway | [134] | |
| pancreatic cancer | In vitro | 0–80 μM | Activation of Bak-1 | [135] | |
| breast cancer |
In vitro In vivo |
0–20 μg/mL 30 mg/kg |
Inhibiting Hedgehog pathway | [136] | |
| gastric cancer | In vitro | 0–10 μM | Inhibiting Hedgehog pathway | [143] | |
| nasopharyngeal carcinoma | In vitro | 0–20 nM | Triggering ferroptosis | [159] | |
| lung cancer |
In vitro In vivo |
0–3 μM 100 mg/kg |
Inhibiting angiogenesis and tumor growth | [164] | |
| Ketoconazole | colon and breast cancer | In vitro | 0–30 μM | Inducing cell cycle arrest | [107] |
| colorectal and hepatocellular carcinoma | In vitro | 0–20 μM | Inducing G0/G1 cell cycle arrest | [108] | |
| hepatocellular carcinoma |
In vitro In vivo |
0–20 μM 50 mg/kg |
Inducing mitophagy and apoptosis | [156] | |
| glioblastoma |
In vitro In vivo |
0–10 μM 25 mg/kg |
Targeting HK2 | [203] | |
| Miconazole | colon carcinoma |
In vitro In vivo |
0–50 μM 50 mg/kg |
Inducing G0/G1 cell cycle arrest and apoptosis | [109] |
| bladder cancer | In vitro | 0–100 μM | Inducing apoptosis | [112] | |
| osteosarcoma | In vitro | 0–100 μM | Inducing intracellular Ca2+ rises | [115] | |
| breast cancer |
In vitro |
0–50 μM | Inducing intracellular Ca2+ rises | [116] | |
| lung cancer |
In vitro In vivo |
0–20 μM 50 mg/kg |
Suppressing STAT3 activation | [147] | |
| bladder cancer | In vitro | 0–50 μM | Inducing apoptosis | [154] | |
| Econazole | lung cancer |
In vitro In vivo |
0–20 μM 50 mg/kg |
Inhibiting PI3K activity and promoting apoptosis | [144] |
| colon cancer |
In vitro In vivo |
0–60 μM 50 mg/kg |
Inducing G0/G1 cell cycle arrest and apoptosis | [148] | |
| gastric cancer |
In vitro |
0–20 μM | Inducing p53-dependent apoptosis | [149] | |
| pancreatic cancer |
In vitro In vivo |
0–40 μM 50 mg/kg |
Inducing autophagy arrest and apoptosis | [158] | |
| Clotrimazole | breast cancer |
In vitro |
0–100 μM | Inducing apoptosis and G1 arrest | [110] |
| lung, colon cancer and melanoma |
In vitro In vivo |
0–10 μM 120 mg/kg |
Depleting the intracellular Ca2+ stores | [113] | |
| endometrial cancer |
In vitro In vivo |
0–20 μM 20 μM |
Blocking IKCa1 channels | [114] | |
| lung carcinoma and colon adenocarcinoma |
In vitro |
0–50 μM | Decreasing glycolysis and the viability |
[173] | |
| breast cancer |
In vitro |
0–50 μM | Disrupting glycolysis | [174] | |
| breast cancer |
In vitro |
0–100 μM | Disrupting glycolysis | [175] | |
| melanoma |
In vitro |
0–50 μM | Reducing glycolysis and ATP level | [177] | |
| Sertaconazole | lung cancer |
In vitro In vivo |
0–40 μM 75 mg/kg |
Inducing proapoptotic autophagy | [157] |
| 5-fluorocytosine | glioblastoma | In vivo | NA | Gene-directed enzyme prodrug therapy (GDEPT) | [89] |
| Griseofulvin | ovarian cancer |
In vitro |
0–120 μM | Suppressing spindle microtubule dynamics | [95] |
| colorectal cancer |
In vitro In vivo |
0–60 μM 50 mg/kg |
Inducing apoptosis and G2/M cell cycle arrest | [99] | |
| GF-15 | colon cancer and multiple myeloma |
In vitro In vivo |
0–1.25 μM 0–100 mg/kg |
Inhibiting of centrosomal clustering | [100] |
Repurposing antifungal drugs for cancer therapy based on original targets
Although successful repurposing of antifungal drugs in cancer therapy is mainly based on opportunistic and serendipitous discovery, further investigations reveal that some underlying molecular mechanisms of antifungal drugs that have anticancer properties are associated with the original targets of the drugs. These drugs have been approved for antifungal uses as inhibitors of SQLE, sterol 14α−demethylase (CYP51), mitosis, and nucleic acid biosynthesis, and their biological activities may be effective cancer treatments with sufficient potency.
Targeting SQLE
SQLE catalyses the epoxidation of squalene and is then converted into 2,3-epoxy squalene, which is an important rate-limiting step in the fungal ergosterol biosynthesis pathway. Allylamine and its derivatives exert inhibitory effects on SQLE by binding to its lipophilic site and further changing its conformation. Allylamines are a class of antifungal agents most preferably used in mycosis caused by dermatophytes. The most common allylamine antifungal agents in clinical practice include natifan, terbinafine, and butenafine, which display considerable efficacy and safety. Natifan and butenafine are available as a topical cream, gel, or spray, while the oral form of terbinafine can be used to treat systematic fungal infections [19]. SQLE is also the secondary rate-controlling enzyme in human cholesterol biosynthesis, preceded only by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) [20], [21], [22]. Cholesterol is a fundamental structural component of human cell membranes and a precursor for steroid hormones, fat-soluble vitamins, and bile acids. Cholesterol biosynthesis mainly occurs in the liver and is a complex process involving nearly 30 cascades of enzymatic reactions [23]. Sterol regulatory element binding protein (SREBP) transcription factors are the main regulators of cholesterol biosynthesis, and the activities of SQLE and HMGCR are controlled by SREBP and feedback regulation loops. Low cholesterol levels promote the translocation of SREBP into the nucleus and SQLE expression, further activating cholesterol synthesis. High cholesterol levels induce SQLE degradation via the ubiquitin–proteasome system and subsequently reduce cholesterol levels.
Cholesterol homeostasis is associated with multiple physiological and pathological conditions, such as well-known cardiovascular diseases. In recent years, the relationship between cholesterol dysregulation and cancer progression has been demonstrated in various malignancies, including renal, breast and colorectal cancers (CRC) [24]. The high level of cholesterol either in plasma or tissue may contribute to the risk of cancer, supporting the oncogenic role of cholesterol in tumours. In addition, cholesterol-lowering drugs, such as statins, have preventive and therapeutic properties against cancer [18], [25], [26]. As the second rate-limiting enzyme of cholesterol synthesis, SQLE is positively correlated with a high cancer occurrence and poor prognosis in numerous tumours [27], [28], [29], [30], [31]. Moreover, increasing evidence demonstrates that SQLE contributes to cancer cell growth and migration [32], [33]. Due to their actions as SQLE inhibitors, allylamines have been demonstrated to be potential candidates for cancer therapy. Liu et al. identified SQLE as an oncogene in non-alcoholic fatty liver disease (NAFLD)-induced hepatocellular carcinoma (HCC) via RNA sequencing analyses of 17 paired NAFLD-HCC tissues and adjacent tissues. SQLE is upregulated in NAFLD-HCC tissues and positively correlated with a poor prognosis. Further mechanistic research revealed that SQLE promotes carcinogenesis via cholesteryl ester and nicotinamide adenine dinucleotide phosphate (NADP+), which epigenetically silence PTEN via the ROS-DNA methyltransferase 3A axis and trigger the PTEN/PI3K/AKT/mTOR signaling cascade in HCC. Additionally, the antitumor effect of terbinafine in subcutaneous xenografts and orthotopic xenografts was evaluated in this previous study. Terbinafine significantly reduced free cholesterol and cholesteryl ester, prolonged the survival time, and suppressed tumour growth in xenograft mice [34]. He et al. reported that SQLE is highly upregulated in CRC and correlated with a poor prognosis. SQLE promotes CRC proliferation by accumulating calcitriol and activating CYP24A1-mediated MAPK signaling. Terbinafine significantly suppresses CRC growth in CRC organoids and xenograft mice [35].
The importance of cholesterol metabolism in cancer occurrence and development has been recognized recently. SQLE catalyses the second rate-limiting step in cholesterol synthesis and is described as a driver for tumour metabolic shift and a key player in maintaining cell survival in hypoxic conditions [36]. As SQLE can be considered molecular target in cancer therapy, the repurposing of allylamine and its derivatives, namely SQLE inhibitors, as anti-cancer agents should be investigated.
Targeting cytochrome P450 monooxygenase family members
CYP51 mediates the conversion of lanosterol into ergosterol by demethylating the 14-a position, which is another essential step in ergosterol synthesis. Azoles are the most widely used broad-spectrum antifungal drugs in clinical practice and exert their inhibitory effect on CYP51 by engaging with the heme pocket [37], [38], [39]. Azoles are a group of heterocyclic compounds and are divided into two categories: triazoles and imidazoles. In general, triazoles and their derivatives (such as such as itraconazole and fluconazole) can be administered systemically, while imidazoles (such as such as ketoconazole, miconazole, and clotrimazole) are often used topically due to their toxicity [10].
CYP51 belongs to the cytochrome P450 monooxygenase (CYP) superfamily, which is an abundant superfamily of heme-thiolate proteins with more than 6,000 members [40]. CYPs ubiquitously exist in all life cells and participate extensively in biological activities. CYPs mediate the conversion from environmental lipophilic xenobiotics to human endogenous compounds, particularly in drug metabolism and ultimately in carcinogen formation [38], [41], [42], [43]. As omnipresent enzymes, CYPs catalyse a variety of reactions, including epoxidation, reduction, hydroxylation, dealkylation, and deamination. Although expressed in numerous human tissues, CYPs are predominantly enriched in the liver, which is the main location for the biotransformation of xenobiotics and drugs. Despite the variability of CYPs between species, there is a certain degree of conservation between humans and fungi, leading to the potential influence of azoles on the human body and cancer cells [38].
Due to their actions as metabolite enzymes, CYPs exert at least two important effects on cancer cells: they mediate the conversion from procarcinogens to active carcinogenic compounds [44], [45], [46], and they catalyse antitumor drug reactions. Approximately 90% of human malignancy formation is caused by nongenotoxic carcinogens including nitrosamine, azo-aromatic amine, and alkyl benzene. Some CYPs, especially CYP1 isoforms, are responsible for the conversion of these procarcinogens to carcinogens [47], [48], [49]. CYPs are also involved in chemotherapeutic compound activation and inactivation [50]. Upon the metabolism of CYPs, inactive antitumor drugs are converted into cytotoxic compounds in cancer cells, and CYP2 and CYP3 isoforms are the most common subfamily responsible for anticancer agent catalysis [51]. In contrast, some CYPs are involved in the inactivation of antitumor agents, as suggested by the observation that the increased expression of CYPs in tumour tissue is positively associated with chemotherapy resistance, and the inhibition of specific CYPs improves the response to therapy [52]. In addition, the expression of some CYPs is reported to be upregulated and associated with poor prognosis in a variety of cancer types [53], [54], [55]. Further studies suggest that CYPs play an important role in cancer initiation, progression, and therapeutic response and that they may serve as prognostic biomarkers and therapeutic targets. Kumarakulasingham et al. investigated the expression of 23 CYPs in 264 patients with CRC using immunohistochemistry and found that CYP51, CYP1B1, CYP2S1, CYP2U1, and CYP3A5 are overexpressed in CRC; among them, CYP51 is an independent prognostic biomarker for CRC [56]. In addition, CYPs may regulate cancer proliferation, invasion, angiogenesis, apoptosis, or differentiation as dominant upstream molecules of signaling pathways in cancer, including MAPK, PI3K/Akt, and NF-κB [57].
Azole antifungal agents are potent inhibitors of CYP activity. In addition to CYP51, azole antifungals have been found to inhibit other CYP family members. Ketoconazole is a broad-spectrum antifungal used in superficial fungal infections and is derived from imidazole, whose systemic use is limited due to potential side effects, such as hepatotoxicity [58], [59]. Ketoconazole has been identified as an inhibitor of 17α-hydroxylase/17, 20 lyase (CYP17A1), and CYP3A4 [13], [60], [61]. CYP17A1 possesses two main catalytic activities, among which 17α-hydroxylase mediates the conversion from pregnenolone to 17α-hydroxypregnenolone, while 17α-lyase catalyses the reactions from 17α-hydroxylase products into dehydroepiandrosterone and androstenedione [13]. Considering the essential role of CYP17A1 in steroidogenesis and prostate cancer, ketoconazole is suggested as a second-line therapy for the clinical treatment of castration-resistant prostate cancer (CRPC), as a CYP17A1 inhibitor [62], [63], [64], [65], [66]. Several clinical studies have reported that ketoconazole treatment can induce a decrease in prostate-specific antigen (PSA), relieve clinical symptoms, and prolong progression time in prostate cancer [67], [68]. Interestingly, azole antifungals show some degree of substrate overlap for CYP family member inhibition. For example, ketoconazole, itraconazole, and posaconazole inhibit CYP3A4 activity, though with different potencies. CYP3A may be the most well-known CYP member involved in tumour chemotherapy resistance. CYP3A4 is responsible for the detoxification of various common antitumor drugs in the clinic, including docetaxel, irinotecan, gefitinib, cisplatin, paclitaxel, tamoxifen, and vinorelbine [69], [70], [71], [72], [73], [74], [75], [76]. The overexpression of CYP3A4 limits the chemotherapeutic response; therefore, downregulating CYP3A4 expression may improve the therapeutic response [57]. Subsequently, ketoconazole is used in combination with standard treatments when administered as a CYP3A4 inhibitor, due to its function in catalysing antitumor drugs into inactive derivatives. Outcomes from several clinical studies suggest that ketoconazole may increase drug exposure or reduce the drug clearance of lapatinib, docetaxel, and irinotecan [52], [77], [78], [79]. Itraconazole is a broad-spectrum triazole antifungal drug, unlike imidazole, which displays significant hepatotoxicity, and the systemic triazole exerts relatively rare side effects. Itraconazole has emerged as a ketoconazole alternative in CYP3A inhibition, with comparable potency [80], [81], [82].
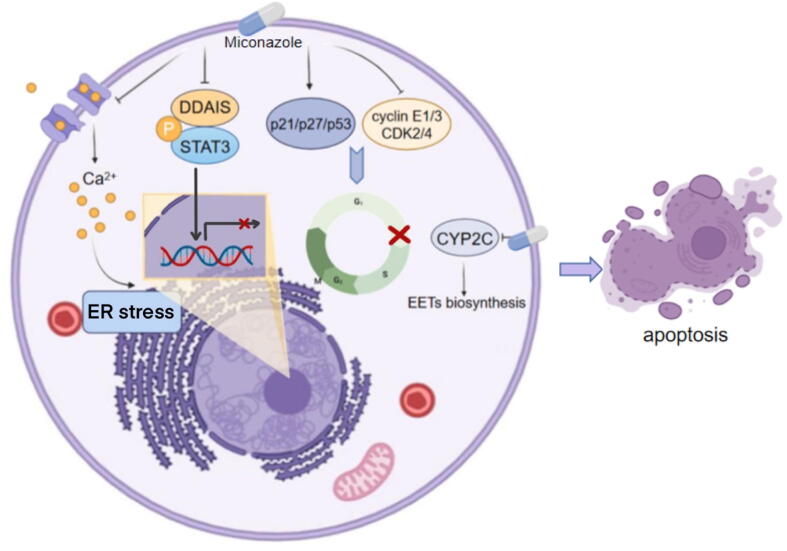
Miconazole is a common topical antifungal agent that belongs to the imidazole class. Studies investigating the antitumor properties of miconazole were reported in 1991 [83]. Miconazole is a potent inhibitor of CYP2C, which is involved in cancer formation and progression. CYP2C9 oxidizes arachidonic acid (ARA) to epoxyeicosatrienoic acids (EETs) and promotes mitogenesis and angiogenesis. Inhibition of CYP2C9 suppresses EET biosynthesis, subsequently reducing the cell proliferation and migration of human endothelial cells [84], [85] (Fig. 3).
Fig. 3.
Signaling pathways mediated by miconazole Miconazole influences diverse hallmarks of cancer including drug resistance, nutrition metabolism, and cell proliferation.
Targeting nucleic acids
One of the oldest antifungal drugs synthesized and commercially marketed in the 1960s, 5-Fluorocytosine (5-FC) is used for treating Candida and Cryptococcus infections [86]. Recently, 5-FC was suggested as a pillar in gene-directed enzyme prodrug therapy (GDEPT), which is a promising cancer therapeutic strategy characterized by the selective conversion of pro-drugs into cytotoxic metabolites within malignant cells, resulting in maximized concentrations of cytotoxic drugs in the cancer area and minimized toxicity in normal tissues [87]. The GDEPT system is composed of three basic components: the prodrug, intended suicide gene, and vector. The vectors are a number of delivery systems that are responsible for the transduction of the intended gene into tumour cells, and the intended gene encodes an enzyme that can convert the prodrug into cytotoxic metabolites. As the intended gene is located after the tumour-specific promoter, the expression of enzymes and the conversion of prodrugs occur only in cancer cells. Moreover, the enzymes used in the GDEPT system are typically not found in human cells, which further ensures specific toxicity to malignant cells. In addition to selective toxicity, another advantage of GDEPT is the bystander effect. The cytotoxic effect induced by GDEPT can spread to surrounding cancer cells, leading to an extensive killing zone [87], [88]. The cytosine deaminase (CD)/5-FC system is one of the most commonly used GDEPT systems. CD is an enzyme expressed in bacterial or fungal cells that is not found in human cells and is responsible for converting 5-FU to 5-FC intracellularly. For over four decades, 5-FU has been one of the most commonly used chemotherapeutic drugs. In human cells, 5-FU is converted into several metabolites, including 5FURNA and 5FU-DNA, which inhibit thymidylate synthesis and induce apoptosis. CD/5-FC research is often applied in glioblastoma due to the ability of 5-FC to cross the blood–brain barrier. In recent years, the development of targeted vectors to enhance the transduction efficiency and tissue specificity of the CD/5-FC system has been a common research topic. Toca 511 (Vocimagene amiretrorepvec) is among the most widely studied retroviral replicating vectors for intended genes that can encode CD. The Toca 511+5-FC system has achieved significant success in preclinical models [89] and is reported to be well-tolerated by patients in clinical trials [90]. Cloughesy et al. performed a phase I trial including 56 patients with recurrent, high-grade glioma and found that Toca 511+FC therapy improved survival and durable complete therapeutic responses (NCT01470794) [91]. In addition, DNA/RNA sequencing and multiplex digital enzyme-linked immunoassay results suggest that the clinical outcomes are related to molecular and immunological signatures [92]. Recently, Toca 511+5-FC therapy was rated as a breakthrough therapy by the Food and Drug Administration (FDA) while it received support from the European Medicines Agency for the treatment of high-grade glioma. In addition, various clinical trials focusing on the CD/5-FC system based on novel vectors, including APS001F and TG6002, are ongoing.
Targeting mitosis
Griseofulvin is an antifungal agent that has been used to treat dermatophytic infections for more than 60 years. Griseofulvin inhibits fungal growth by blocking mitosis [93], [94]. However, the inhibitory effect of griseofulvin on mitosis involves various mechanisms, such as inhibiting centrosome clustering and inducing mitotic aneuploidy [95], [96], [97]. As uncontrolled proliferation is the basic hallmark of cancer and mitosis is the most common method of cell division in the human body, drugs targeting mitosis by suppressing microtubule dynamics, such as paclitaxel and vinca alkaloids, are effective chemotherapies [98]. Studies focusing on the anticancer activity of griseofulvin based on its inhibitory function on mitosis and low toxicity have attracted much attention. Ho et al. reported that griseofulvin inhibits the growth of CRC by inducing G2/M cell cycle arrest and apoptosis and that griseofulvin exhibits synergistic effects with nocodazole, a classical microtubule inhibitor [99]. Panda et al. reported that griseofulvin inhibits the proliferation of ovarian cancer cells by blocking the cell cycle at prometaphase in anaphase of mitosis and inducing apoptosis. Further mechanistic research has revealed that griseofulvin disturbs microtubule polymerization and organization in HeLa cells, suggesting its potential as an antimitotic drug in cancer treatment [95]. In addition, derivatives or analogues of griseofulvin inhibit centrosome clustering in cancer cells. GF-15, a derivative of griseofulvin, induces cancer apoptosis by reducing spindle tension and spindle multipolarity [96], [100]. Griseofulvin has emerged as a promising antitumor drug or adjuvant in combination with other therapies based on its use as a systemic antifungal with few side effects and rare reports of toxicity.
Indeed, the fact that some antifungal drugs have the same therapeutic targets as those driving malignant tumorigenesis is enough to suggest that these antifungal drugs have great potential for the treatment of human cancers [101]. This strategy relies heavily on ever-refining bioinformatics and computer technology. For instance, the therapeutic role effect of terbinafine was proposed after identifying the oncogenic activity of SQLE [33], [34], [35]. With the advancements in multiple-omics and analytical tools, increasing new cancer targets and driving mechanisms are emerging and identified, large numbers of antifungal drugs against the particular targets could be translated into cancer therapy.
Repurposing antifungal drugs for cancer therapy based on off-site targets
In addition to the administration of antifungal drugs in cancer therapy based on common biological pathways and targets shared by fungal and tumour cells, another successful approach is based on the fact that approximately all approved drugs have numerous targets [102]. The off-site target effects, which are traditionally considered to cause toxic side effects, result in unexpected efficacy for the treatment of cancer [103], [104]. Increasing experimental and preclinical observations have demonstrated that antifungal drugs exert their antitumor properties via off-site effects regardless of the original targets.
Inhibiting cancer cell proliferation
Excessive proliferation is among the most remarkable biological capabilities of cancer cells. The sustainability of proliferation is dependent upon the activation of growth factor signaling pathways. In general, growth factors bind to cell-surface ligands and activate intracellular kinases. The signals involved in cell growth and division are conveyed via downstream intracellular pathways [105]. A high proliferation rate is characterized by a quick cell cycle, which is an evolutionarily conserved process that constitutes two distinct phases. Genomic DNA replication occurs in interphase, while replicated DNA segregation occurs during the M phase. Recent evidence suggests that continuous and excessive division of cancer cells mainly results from failure to exit the cell cycle rather than uncontrolled cell division [106]. The crucial decision of whether to enter a new cycle or exit is made during a window at the metaphase–anaphase transition, the G0-G1 phase. An increasing number of studies suggest that some antifungal drugs inhibit cancer cell proliferation by inducing cell cycle arrest. Forgue-Lafitte et al. reported that ketoconazole suppresses cell proliferation and the incorporation of 3H-thymidine in colon and breast cancer cells. Further studies showed that ketoconazole increases the proportion of cells in the G0-G1 phase of the cell cycle and reduces the proportion of cells in the S phase [107]. Chen et al. also demonstrated that ketoconazole induces cell growth inhibition and G0/G1 arrest in CRC and HCC [108]. In addition, other imidazoles, such as clotrimazole and miconazole, inhibit cancer cell cycle progression and proliferation [109], [110]. Clotrimazole preferentially reduces the expression of cyclin A, E, and D1, leading to cell cycle arrest in the G1 phase [111]. A similar outcome of clotrimazole-induced G0/G1 arrest was observed in endometrial cancer. In addition, miconazole increases the levels of p53, p21, and p27 and decreases the levels of cyclin E1/D3, CDK2, and CDK4, resulting in G0/G1 cell cycle arrest [109], [112] (Fig. 3). The intracellular ion balance plays a critical role in cell proliferation. Benzaquen et al. first reported that clotrimazole suppresses ionic mitogenic signals by reducing intracellular Ca2+ stores in 1995 [113]. Another study reported that clotrimazole inhibits cancer cell growth by blocking translation, which is associated with intracellular Ca2+ dysregulation [111]. A mechanistic study revealed that clotrimazole depletes intracellular Ca2+ stores, which activates PKR and eIF2α, inhibiting the initiation of protein translation. Wang et al. suggested that clotrimazole blocks the cell cycle and suppresses proliferation via the inhibition of intermediate-conductance Ca2+-activated K+ channels [114]. In addition, miconazole increases the intracellular Ca2+ concentration in human osteosarcoma and breast cancer cells, which subsequently regulates proliferation [115], [116] (Fig. 3).
Recently, the Hedgehog (Hh) signaling pathway has emerged as a crucial regulator in cancer cell proliferation. Aberrant overactivation of the Hh signaling pathway leads to carcinogenesis in various cancers; therefore, its inhibitors have been proposed as therapeutic targets, including SMO and glioma-associated oncogene (GLI) [117], [118], [119], [120]. The importance of the Hh signaling pathway in basal cell carcinoma (BCC) has been explicitly recognized in recent years. Vismodegib and sonidegiba, two Hh signaling pathway inhibitors targeting the SMO receptor, have been approved by the FDA for the treatment of locally advanced or metastatic BCC [121]. However, acquired SMO mutations contribute to drug resistance to SMO antagonists and limit the clinical benefits in patients. To overcome this obstacle, Kim et al. screened a library of 2,400 drugs and identified itraconazole as a potent Hh signaling pathway inhibitor (Fig. 2). Interestingly, a mechanism distinct from other existing SMO antagonists is involved in the inhibitory effect of itraconazole on the Hh signaling pathway [121]. Itraconazole inhibits the Hh pathway with an efficacy of 65%, while vismodegib reduces 90% of Hh target gene expression [122]. However, itraconazole remains a promising second-line therapy as it acts on acquired SMO mutations, including SMO D477G [123]. Kim et al. performed an exploratory phase II trial of itraconazole in 29 patients with BCC and found that a common daily dose of itraconazole reduces tumour proliferation after at least one month [124]. In addition, itraconazole inhibits tumour growth by suppressing the Hh pathway in malignant pleural mesothelioma, endometrial cancer, and medulloblastoma [123], [125], [126], [127]. Several studies have also suggested that the antitumor effects of itraconazole are due to the inhibition of other proliferative pathways, including the mTOR and wnt/β-catenin signaling pathways [128], [129], [130], [131], [132], [133], [134], [135], [136].
Inducing cell death
The induction of cell death is a main mechanism by which drugs exert antitumor activities. The antineoplastic properties of antifungal drugs against a wide range of malignancies have been reported as the regulation of apoptosis, autophagy, or other cell death processes. The evasion of apoptosis is another remarkable biological trait of cancer cells. Although cancer cells have developed multiple approaches to circumvent apoptosis, other regulated cell death forms, such as autophagy, ferroptosis, and pyroptosis, have emerged as alternative strategies to kill cancer cells [137], [138].
Apoptosis is the most studied type of regulated cell death since its introduction to cancer therapy in 1972 [139]. Apoptosis is an evolutionarily conserved death process that is characterized by morphological features of chromatin condensation, cell shrinkage, and apoptotic body formation. Apoptosis includes the extrinsic pathway, which involves cell death receptors such as FAS ligands, and the intrinsic pathway, which involves toxic BH3 domain proteins and mitochondria [140], [141]. Numerous antifungal drugs regulate the apoptotic levels in cancer cells, such as terbinafine, natamycin, itraconazole, econazole, and miconazole [142], [143], [144], [145]. Sobecks et al. reported that miconazole induces apoptosis in human T-cell leukaemia cells, which may be attributed to intracellular Ca2+ changes [146]. Other researchers subsequently obtained similar conclusions on the role of miconazole in apoptosis in bladder cancer, colon carcinoma, and lung cancer [109], [112], [147]. In addition, Yuan et al. suggested that miconazole induces both extrinsic and intrinsic apoptosis in bladder cancer, attributing to DR5-dependent and mitochondrial-mediated pathways [112]. Terbinafine, a broad-spectrum antifungal drug, has anticancer activity against promyelocytic leukaemia cells via the induction of apoptosis involving mitochondrial dysfunction and proapoptotic Bcl-2 family members [142]. Other studies have reported that econazole, a traditional imidazole antifungal drug, induces caspase 8-independent apoptosis in colon cancer cells [148] and p53-dependent apoptosis in gastric cancer cells [149].
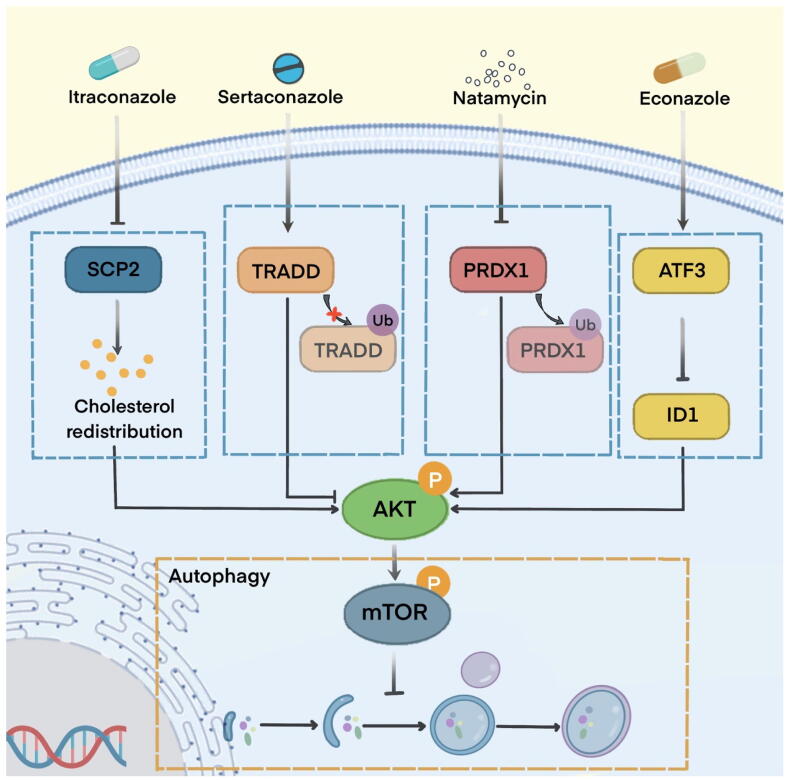
Autophagy is a lysosomal-dependent degradative process and plays a context-dependent role in tumorigenesis. The functions of autophagy can be divided into four categories based on therapeutic intervention: cytoprotective autophagy, cytotoxic autophagy, cytostatic autophagy, and nonprotective autophagy [150], [151], [152]. Autophagy is a compensatory mechanism for recovery from chemotherapeutic toxicity, conferring acquired drug resistance [153]. For example, Ho et al. found that miconazole triggers apoptosis and protective autophagy in bladder cancer cells, and the inhibition of autophagy enhances the apoptotic level of miconazole [154]. Similar outcomes have been reported for natamycin, as An et al. reported that natamycin induces apoptosis and protective autophagy in HCC [145]. In contrast, excessive autophagy may provoke apoptosis-independent or apoptosis-dependent signaling pathways that exert tumour-suppressive effects; this autophagy is identified as cytostatic and cytotoxic autophagy [155], [156]. For example, Liu et al. reported that itraconazole exerts significant anticancer activities in glioblastoma cells via autophagic cell death, which is independent of apoptosis. Mechanistically, itraconazole reduces the expression of sterol carrier protein 2, increasing cholesterol redistribution and repressing the AKT/mTOR pathway that activates autophagy [129]. Another study reported that ketoconazole induces cytotoxic autophagy in HCC by triggering mitophagy-dependent apoptosis and inhibiting mitophagy relieved mitochondrial dysfunction and apoptosis. Mechanistically, ketoconazole decreases COX-2 expression, promoting PINK1 accumulation, resulting in the mitochondrial translocation of Parkin, which subsequently activates mitophagy [156]. Zhang et al. reported that sertaconazole induces proapoptotic autophagy in non-small cell lung cancer cells, stabilizes the TNF receptor type 1-associated death domain (TRADD) protein from ubiquitination‐mediated degradation, and further suppresses the AKT/mTOR pathway to trigger autophagy [157] (Fig. 4). Intact autophagy flux is involved in cell death and growth inhibition, and impaired autophagy also contributes to the anticancer properties of this cellular process. Weng et al. reported that econazole induces autophagy arrest, leading to autophagosome accumulation and endoplasmic reticulum stress-mediated apoptosis in pancreatic cancer cells [158].
Fig. 4.
Partially repurposed antifungal drugs in autophagy modulation Itraconazole suppresses the growth of glioblastoma through autophagy involved in sterol carrier protein 2 (SCP2) suppression. Sertaconazole provokes proapoptotic autophagy by stabilizing TNF receptor type 1-associated death domain (TRADD) in non-small cell lung cancer (NSCLC). Natamycin triggers apoptosis and protective autophagy in hepatocellular carcinoma (HCC). Econazole inhibits pancreatic cancer via autophagy regulated by the ATF3/ID1 pathway.
Other forms of cell death, such as ferroptosis and pyroptosis, have received increased attention in recent years. For example, itraconazole induces ferroptosis by sequestering iron in lysosomes and subsequently reducing the radio-resistance in nasopharyngeal carcinoma cells [159]. With a deeper understanding of cancer cell death, the detailed mechanisms involved in the anticancer activity of antifungal drugs can be elucidated further.
Decreasing angiogenesis
Malignancies generate neo-vasculature to provide supplemental metabolites and evacuate metabolic wastes. To facilitate tumour growth, the angiogenic switch is activated, including the genesis of new endothelial cells, tube assembly, and vessel sprouting. Angiogenesis is mainly driven by growth factors secreted from tumour cells, including vascular endothelial growth factor-A (VEGF-A), which is the primary target of antiangiogenic therapy. Several studies have reported the anti-angiogenic effect of itraconazole, mediated by the inhibition of the VEGF pathway, the basic fibroblast growth factor (bFGF) pathway, or the platelet-derived growth factor pathway [160], [161], [162], [163], [164]. Aftab et al. reported the potential antiangiogenic activity of itraconazole in non-small cell lung cancer. Itraconazole disrupts the proliferation, migration, and tube formation of endothelial cells via both VEGF- and bFGF-mediated angiogenic stimulation [164]. Additional mechanisms are involved in the antiangiogenetic properties of itraconazole. Head et al. reported that itraconazole suppresses angiogenesis by directly binding VDAC1, disrupting its function in mitochondrial metabolism. The dysregulation of cellular metabolism activates AMPK and inhibits mTOR, subsequently suppressing endothelial cell proliferation and angiogenesis [165]. In addition to VDAC1, itraconazole exerts its inhibitory function on mTOR signaling and angiogenesis by targeting NPC1, highlighting the multiple targets of itraconazole for the inhibition of angiogenesis [166].
Terbinafine, another oral antifungal agent, also has antiangiogenic activities, such as cell cycle arrest and the inhibition of vascular endothelial cell migration [167], [168]. In addition, terbinafine exerts anticancer and antiangiogenic effects in oral squamous cell carcinoma [169].
Regulating cellular metabolism
Reprogrammed metabolism is a well-substantiated hallmark of cancer. Tumour cells adjust their metabolic pathways autonomously to satisfy the vigorous demand for bioenergetics and biosynthesis. The Warburg effect is a well-known cancer metabolic reprogramming phenomenon that was first observed in the 1930s. Tumour cells prefer aerobic glycolysis rather than oxidative phosphorylation, even with sufficient oxygen. Hexokinase is an essential enzyme involved in glycolytic flux and plays an important role in tumour growth [170]. Hexokinase binds to the mitochondrial outer membrane via transmembrane voltage-dependent anion channels and stabilizes cytochrome c, preventing apoptosis [171], [172]. Considering the importance of glycolysis in malignancies, the inhibition of hexokinase is a potential anti-cancer therapeutic strategy. Most studies regarding on the antitumor properties of clotrimazole are related to glycolysis disruption. Penso et al. reported that clotrimazole induces the detachment of glycolytic enzymes from the cytoskeleton in lung and colon cancer cells [171], [173]. Other studies reported that clotrimazole disrupts glycolysis in breast cancer [174], [175], [176] and melanoma cells [177].
Existing research has focused on the repurposing of antifungal drugs in cancer that exert their antitumor effects by modulating the hallmarks of cancer [105]. Currently, with the increasing studies in tumor molecular biology, this strategy will have a broader use. For example, the recent revelation of the polymorphic microbiome as a new tumor hallmark means that certain antifungal drugs may be identified as new antitumor candidates.
Antifungal therapy for malignancies
It is estimated that approximately 15–20% of malignancies are attributable to infectious agents [178], [179]. Well-known carcinogenic microorganisms include Helicobacter pylori, which causes gastric cancer; human papilloma virus, which causes cervical cancer; and hepatitis B and hepatitis C viruses, which cause liver cancer [180]. In addition, significant advances in metagenomic sequencing approaches and computational analysis have occurred in the past decade, providing remarkable progress regarding the knowledge of human microbiota, including fungi. Fungi reside within the human body, including on the skin and mucosa and within the respiratory and digestive tracts. These are mutualistic agents in the healthy body but become pathogenic microorganisms in immunodeficiency disorders. Fungi are ubiquitous and influence human health as well as carcinogenesis via metabolism, immunoregulation, and inflammation [181], [182]. Candida is the most common and opportunistic fungus residing in humans [183], [184]. Although research focusing on the relationship between fungi and malignancies is limited, the importance of fungi in tumorigenesis and progression has attracted much attention recently, and fungi may be a promising biomarker and therapeutic target. Coker et al. compared fungal communities between 184 patients with CRC and 204 healthy controls via faecal shotgun metagenomic sequencing and found that Basidiomycota/Ascomycota and Malasseziomycetes were increased while Saccharomycetes and Pneumocystidomycetes were decreased in patients with CRC. Faecal fungal signatures display excellent sensitivity and specificity for the diagnosis of CRC [185]. Several specific fungi, such as Candida, Malassezia, and Trichosporon, and decreased total fungal richness were recently reported in the carcinogenesis process [186], [187], [188], [189], [190], [191], [192]. Aykut et al. reported that Malassezia is significantly increased in pancreatic cancer tissues and that fungal ablation can effectively slow proliferation, while the repopulation of Malassezia accelerates tumour growth [189]. Further mechanistic studies have suggested that Malassezia promotes pancreatic cancer by driving the complement cascade through mannose-binding lectin activation. The administration of amphotericin B, the most common systemic antifungal drug, can effectively inhibit cancer progression in invasive pancreatic cancer models [189]. In addition, Hu et al. reported that post-diagnostic administration of terbinafine is associated with a decreased risk of death and metastasis in patients with CRC in a population-based study from Sweden. Further mechanistic research revealed that terbinafine can inhibit fungus-induced myeloid-derived suppressor cell infiltration and restore antitumor immune response in CRC [193]. Therefore, antifungal drugs can be used as preventive and therapeutic agents for specific malignant tumours that are initiated and promoted by fungi.
Perspectives
As laureate of the 1988 Nobel Prize in Physiology or Medicine James Black said, “The most fruitful basis for the discovery of a new drug is to start with an old drug,” [194]. The repurposing of effective, safe, and inexpensive non-oncology drugs for antineoplastic therapy has attracted great interest. Nearly all classes of antifungal drugs have shown anticancer activity in preclinical studies. However, only a few of these drugs, especially those used in a systemic manner, are approved for the treatment of patients with cancer or are currently undergoing clinical trials including patients with cancer. As shown in Table 2, two antifungal agents (itraconazole and ketoconazole) are in or have completed clinical trials that include patients with cancer [62], [124], [195], [196], [197], [198], [199], [200], [201]. The rapid advances in multi-omics screening and bioinformatics technologies and the growing understanding of the biological features of cancer have facilitated the successful repurposing of oral antifungal agents such as itraconazole for the treatment of cancer. Once the antitumor effects of a drug have been confirmed, the probability of failure due to adverse toxicology is minimal. However, even as new targets of antifungal drugs are identified, much research is required before these drugs are used in cancer therapy. For example, itraconazole has a significant inhibitory effect on SMO, but sonidegib and vismodegib remain the only two Hh inhibitors approved by the FDA for the treatment of BCC [202]. Nevertheless, itraconazole has a high potential to be used as a second-line treatment or as combination therapy for cancer. Ketoconazole, which has been used in clinical trials to treat metastatic CRPC that is refractory to chemotherapy and other standard therapies also requires more research before it is a standard anti-cancer therapy [203]. Accordingly, antifungal drugs for which specific targets have been identified in preclinical studies and therapeutic doses have passed phase I toxicity trials in humans, such as itraconazole and ketoconazole, require more clinical results to determine their specific application in standardized and individualized therapy. The antitumor activity of other antifungal agents, such as terbinafine and griseofulvin, has been observed only in preclinical studies thus far [204], [205], [206]. Whether their antitumor properties can be translated into the clinic, at what effective dose, and with what safety profile must be determined in additional clinical trials. In conclusion, translating an approved antifungal drug with a known molecular target and toxicity as a clinical therapeutic agent for the treatment of cancer has a low probability of failure, especially when artificial intelligence techniques are widely used in clinical oncology research. In addition, the optimization or remodeling of the original structure and changes in the mode of administration can modulate the pharmacokinetic and pharmacodynamic profiles of antifungal drugs, contributing to their efficacy and safety.
Table 2.
Repurposing anti-fungal drugs under clinical trials.
| Drug | Cancer | Clinical trial identifier | Main finding | Ref |
|---|---|---|---|---|
| Itraconazole | basal cell carcinoma | NCT01108094 (phase II) | Itraconazole reduced cell proliferation by 45%, Hedgehog pathway activity by 65%. | [124] |
| esophageal cancer | NCT02749513 (phase I) | Itraconazole blockade of HER2/AKT signaling. | [195] | |
| lung cancer | NCT00769600 (phase II) | Overall survival was longer in patients receiving itraconazole (median 32 months) versus control (8 months). | [196] | |
| lung cancer | NCT02157883 (phase 1) | Itraconazole can be co-administered with Osimertinib. | [197] | |
| Ketoconazole | prostate cancer | NCT00673127 (phase II) | The response proportion to ketoconazole, hydrocortisone, and dutasteride was at least comparable with previous studies of ketoconazole alone, whereas time to progression was substantially longer. | [62] |
| breast cancer | NCT00544804 (phase I) | Ketoconazole was able to increase lapatinib exposure. | [77] | |
| breast cancer | NCT00212082 NCT00212095 (phase II) | Ketoconazole-modulated docetaxel resulted in reduced docetaxel clearance. | [78] | |
| prostate cancer | NCT00460031 (phase II) | The combination of ketoconazole and lenalidomide was well tolerated. | [198] | |
| prostate cancer | NCT00298155 (phase 1) | Combination with ketoconazole show larger number of complete and near-complete responses than bicalutamide and dutasteride alone. | [199] | |
| prostate cancer | NCT01199146 (phase II) | Abiraterone demonstrates modest clinical efficacy in prostate cancer patients previously treated with ketoconazole. | [200] | |
| 5-fluorocytosine | glioma | NCT02414165 (phase I) | Toca 511 and Toca FC showed promising survival, excellent tolerability in recurrent high-grade glioma. | [90] |
| glioma | NCT01470794 (phase I) | Toca 511 + Toca FC shown multiyear durable response. | [91] |
Conclusions
Repurposing antifungal agents in cancer therapy may be a safer, faster, and less expensive way to overcome bottlenecks in the development of antitumour therapeutic agents. Studies exploring the exact antitumor properties and their underlying mechanisms are accumulating rapidly. However, several limitations remain. First, except for some systematic antifungal compounds, many antifungal drugs with antitumour activity have been reported to possess considerable toxicity and side effects. Efforts should be made to balance the original toxicity and antitumor properties of these drugs and maximize the clinical benefits. Moreover, malignancy is very different than fungal infections, and the optimal plasma concentration and tolerated dose require more exploration before the final clinical application of antifungal drugs for patients with cancer. Technology to help overcome these barriers has been developed. High-throughput screening drug technology and preclinical models, such as patient-derived xenografts or organoids, have greatly facilitated the process of screening and comparing the anticancer activities of antifungal drugs. Modifications of the drug structure and delivery systems help reduce drug toxicity and strengthen the antitumor properties. Mechanistic investigations based on transcriptome analyses and molecular techniques provide an underlying foundation for the use of antifungal drugs as cancer treatments. Finally, clinical trials must be conducted to allow for the successful clinical application of antifungal drugs as cancer treatments.
Funding
This work was funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21042); Sichuan Science and Technology Programme (2019YFS0042).
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Ningna Weng has obtained her MD and PhD degrees from Sichuan University, Chengdu, China. Her research focuses on the development novel agents for gastrointestinal malignancies including stomach cancer, hepatocellular carcinoma, pancreatic cancer and colorectal cancer.

Zhe Zhang is currently a PhD student in State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University. He focuses on the investigation of drug reposition, immune therapies for tumors, as well as tumor metabolism, microenvironment and redox biology. Zhe Zhang has published more than 10 articles in journals such as EMBO Mol Med, Signal Transduct Target Ther, Mol Cancer, Theranostics, and Biochim Biophys Acta Rev Cancer et al. Nowadays, his current interest is focused on molecular mechanisms of epigenetic modification and metabolic enzymes in cancer.
Yunhan Tan is an undergraduate student at Sichuan University.
Xiaoyue Zhang is a post-graduate student at Sichuan University.

Xiawei Wei, professor of the State Key Lab of Biotherapy, West China Hospital, Sichuan University, deputy director of the National Clinical Research Center for Geriatrics. She has published over 40 SCI papers in international academic journals, including EMBO Mol Med, Signal Transduct Target Ther, Cell Res, Nano Lett, Mol Cancer.

Qing Zhu, professor and doctoral supervisor at West China Hospital of Sichuan University, Chengdu, China. Her research is supported by ‘‘National Natural Science Foundation of China (No.81874223 and No.81272200)”, ‘‘Sichuan Science and Technology Programme (2019YFS0042)” and ‘‘1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21042)”. She has published over 50 SCI papers in international academic journals, including Nat Commun, Oncogene, Am J Cancer Res and Front Oncol. Her h-index is 18.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736-88. [DOI] [PMC free article] [PubMed]
- 3.Qin S., Jiang J., Lu Y., Nice E.C., Huang C., Zhang J. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct Target Ther. 2020;5:228. doi: 10.1038/s41392-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z., Qin S., Chen Y., Zhou L., Yang M., Tang Y. Inhibition of NPC1L1 disrupts adaptive responses of drug-tolerant persister cells to chemotherapy. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202114903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran A.A., Prasad V. Drug repurposing for cancer treatments: a well-intentioned, but misguided strategy. Lancet Oncol. 2020;21:1134–1136. doi: 10.1016/S1470-2045(20)30424-1. [DOI] [PubMed] [Google Scholar]
- 6.Sanz M.A., Fenaux P., Tallman M.S., Estey E.H., Löwenberg B., Naoe T. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133:1630–1643. doi: 10.1182/blood-2019-01-894980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abaza Y., Kantarjian H., Garcia-Manero G., Estey E., Borthakur G., Jabbour E. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129:1275–1283. doi: 10.1182/blood-2016-09-736686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollak M. Overcoming Drug Development Bottlenecks With Repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat Med. 2014;20:591–593. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Zhou L., Xie N., Nice E.C., Zhang T., Cui Y. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther. 2020;5:113. doi: 10.1038/s41392-020-00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y., Betts H., Keller S., Cariou K., Gasser G. Recent developments of metal-based compounds against fungal pathogens. Chem Soc Rev. 2021 doi: 10.1039/d0cs00945h. [DOI] [PubMed] [Google Scholar]
- 11.Wiederhold N.P. The antifungal arsenal: alternative drugs and future targets. Int J Antimicrob Agents. 2018;51:333–339. doi: 10.1016/j.ijantimicag.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Mesa-Arango A.C., Scorzoni L., Zaragoza O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front Microbiol. 2012;3:286. doi: 10.3389/fmicb.2012.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel V., Liaw B., Oh W. The role of ketoconazole in current prostate cancer care. Nat Rev Urol. 2018;15:643–651. doi: 10.1038/s41585-018-0077-y. [DOI] [PubMed] [Google Scholar]
- 14.Pounds R., Leonard S., Dawson C., Kehoe S. Repurposing itraconazole for the treatment of cancer. Oncol Lett. 2017;14:2587–2597. doi: 10.3892/ol.2017.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlos-Escalante J.A., de Jesús-Sánchez M., Rivas-Castro A., Pichardo-Rojas P.S., Arce C., Wegman-Ostrosky T. The Use of Antihypertensive Drugs as Coadjuvant Therapy in Cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.660943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfab C., Schnobrich L., Eldnasoury S., Gessner A., El-Najjar N. Repurposing of Antimicrobial Agents for Cancer Therapy: What Do We Know? Cancers (Basel) 2021;13 doi: 10.3390/cancers13133193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu L., Jin W., Zhang J., Zhu L., Lu J., Zhen Y. Repurposing non-oncology small-molecule drugs to improve cancer therapy: Current situation and future directions. Acta Pharm Sin B. 2022;12:532–557. doi: 10.1016/j.apsb.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W., Hu J.W., He X.R., Jin W.L., He X.Y. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res. 2021;40:241. doi: 10.1186/s13046-021-02041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petranyi G., Ryder N.S., Stütz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984;224:1239–1241. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka H., Coates H.W., Chua N.K., Hashimoto Y., Brown A.J., Ohgane K. A key mammalian cholesterol synthesis enzyme, squalene monooxygenase, is allosterically stabilized by its substrate. Proc Natl Acad Sci U S A. 2020;117:7150–7158. doi: 10.1073/pnas.1915923117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Zhang H., Zhang M., Lin C., Wang H., Yao J. OSBPL2 deficiency upregulate SQLE expression increasing intracellular cholesterol and cholesteryl ester by AMPK/SP1 and SREBF2 signalling pathway. Exp Cell Res. 2019;383 doi: 10.1016/j.yexcr.2019.111512. [DOI] [PubMed] [Google Scholar]
- 22.Mullen P.J., Yu R., Longo J., Archer M.C., Penn L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 23.Huang B., Song B.L., Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 24.Llaverias G., Danilo C., Mercier I., Daumer K., Capozza F., Williams T.M. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Q.W., Yu S.Y., Teng T.K., Li X., Cheung K.S., Wu M.Z. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur Heart J. 2021;42:3049–3059. doi: 10.1093/eurheartj/ehab325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murtola T.J., Siltari A. Statins for Prostate Cancer: When and How Much? Clin Cancer Res. 2021;27:4947–4949. doi: 10.1158/1078-0432.CCR-21-1891. [DOI] [PubMed] [Google Scholar]
- 27.Ge H., Zhao Y., Shi X., Tan Z., Chi X., He M. Squalene epoxidase promotes the proliferation and metastasis of lung squamous cell carcinoma cells though extracellular signal-regulated kinase signaling. Thorac Cancer. 2019;10:428–436. doi: 10.1111/1759-7714.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Fang L., Liu W. High SQLE Expression and Gene Amplification Correlates with Poor Prognosis in Head and Neck Squamous Cell Carcinoma. Cancer Manag Res. 2021;13:4709–4723. doi: 10.2147/CMAR.S305719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown D.N., Caffa I., Cirmena G., Piras D., Garuti A., Gallo M. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci Rep. 2016;6:19435. doi: 10.1038/srep19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S., Kon M., DeLisi C. Pathway-based classification of cancer subtypes. Biol Direct. 2012;7:21. doi: 10.1186/1745-6150-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Arcy M., Fleming J., Robinson W.R., Kirk E.L., Perou C.M., Troester M.A. Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res Treat. 2015;152:437–448. doi: 10.1007/s10549-015-3474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui Z., Zhou J., Cheng Z., Lu P. Squalene epoxidase (SQLE) promotes the growth and migration of the hepatocellular carcinoma cells. Tumour Biol. 2015;36:6173–6179. doi: 10.1007/s13277-015-3301-x. [DOI] [PubMed] [Google Scholar]
- 33.Cirmena G., Franceschelli P., Isnaldi E., Ferrando L., De Mariano M., Ballestrero A. Squalene epoxidase as a promising metabolic target in cancer treatment. Cancer Lett. 2018;425:13–20. doi: 10.1016/j.canlet.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Liu D., Wong C.C., Fu L., Chen H., Zhao L., Li C. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aap9840. [DOI] [PubMed] [Google Scholar]
- 35.He L., Li H., Pan C., Hua Y., Peng J., Zhou Z. Squalene epoxidase promotes colorectal cancer cell proliferation through accumulating calcitriol and activating CYP24A1-mediated MAPK signaling. Cancer Commun (Lond) 2021;41:726–746. doi: 10.1002/cac2.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haider S., McIntyre A., van Stiphout R.G., Winchester L.M., Wigfield S., Harris A.L. Genomic alterations underlie a pan-cancer metabolic shift associated with tumour hypoxia. Genome Biol. 2016;17:140. doi: 10.1186/s13059-016-0999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405–2419. doi: 10.2165/11596540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Li L., Lv Q., Yan L., Wang Y., Jiang Y. The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front Microbiol. 2019;10:691. doi: 10.3389/fmicb.2019.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daum G., Lees N.D., Bard M., Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Bièche I., Narjoz C., Asselah T., Vacher S., Marcellin P., Lidereau R. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17:731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- 41.Li S., Du L., Bernhardt R. Redox Partners: Function Modulators of Bacterial P450 Enzymes. Trends Microbiol. 2020;28:445–454. doi: 10.1016/j.tim.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Tamási V., Monostory K., Prough R.A., Falus A. Role of xenobiotic metabolism in cancer: involvement of transcriptional and miRNA regulation of P450s. Cell Mol Life Sci. 2011;68:1131–1146. doi: 10.1007/s00018-010-0600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Antona C., Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 44.Badawi A.F., Cavalieri E.L., Rogan E.G. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50:1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 45.Hecht S.S., Stepanov I., Carmella S.G. Exposure and Metabolic Activation Biomarkers of Carcinogenic Tobacco-Specific Nitrosamines. Acc Chem Res. 2016;49:106–114. doi: 10.1021/acs.accounts.5b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nebert D.W., Dalton T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 47.Haque M.W., Pattanayak S.P. Taxifolin Inhibits 7,12-Dimethylbenz(a)anthracene-induced Breast Carcinogenesis by Regulating AhR/CYP1A1 Signaling Pathway. Pharmacogn Mag. 2018;13:S749–S755. doi: 10.4103/pm.pm_315_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang W., Wang L., Kondraganti S.R., Fazili I.S., Couroucli X.I., Felix E.A. Disruption of the gene for CYP1A2, which is expressed primarily in liver, leads to differential regulation of hepatic and pulmonary mouse CYP1A1 expression and augmented human CYP1A1 transcriptional activation in response to 3-methylcholanthrene in vivo. J Pharmacol Exp Ther. 2010;335:369–379. doi: 10.1124/jpet.110.171173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nock N.L., Tang D., Rundle A., Neslund-Dudas C., Savera A.T., Bock C.H. Associations between smoking, polymorphisms in polycyclic aromatic hydrocarbon (PAH) metabolism and conjugation genes and PAH-DNA adducts in prostate tumors differ by race. Cancer Epidemiol Biomarkers Prev. 2007;16:1236–1245. doi: 10.1158/1055-9965.EPI-06-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molina-Ortiz D., Camacho-Carranza R., González-Zamora J.F., Shalkow-Kalincovstein J., Cárdenas-Cardós R., Ností-Palacios R. Differential expression of cytochrome P450 enzymes in normal and tumor tissues from childhood rhabdomyosarcoma. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0093261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishida C.R., Lee M., de Montellano P.R. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol Pharmacol. 2010;78:497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathijssen R.H., de Jong F.A., van Schaik R.H., Lepper E.R., Friberg L.E., Rietveld T. Prediction of irinotecan pharmacokinetics by use of cytochrome P450 3A4 phenotyping probes. J Natl Cancer Inst. 2004;96:1585–1592. doi: 10.1093/jnci/djh298. [DOI] [PubMed] [Google Scholar]
- 53.Murray G.I., Patimalla S., Stewart K.N., Miller I.D., Heys S.D. Profiling the expression of cytochrome P450 in breast cancer. Histopathology. 2010;57:202–211. doi: 10.1111/j.1365-2559.2010.03606.x. [DOI] [PubMed] [Google Scholar]
- 54.Downie D., McFadyen M.C., Rooney P.H., Cruickshank M.E., Parkin D.E., Miller I.D. Profiling cytochrome P450 expression in ovarian cancer: identification of prognostic markers. Clin Cancer Res. 2005;11:7369–7375. doi: 10.1158/1078-0432.CCR-05-0466. [DOI] [PubMed] [Google Scholar]
- 55.McFadyen M.C., Melvin W.T., Murray G.I. Cytochrome P450 CYP1B1 activity in renal cell carcinoma. Br J Cancer. 2004;91:966–971. doi: 10.1038/sj.bjc.6602053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumarakulasingham M., Rooney P.H., Dundas S.R., Telfer C., Melvin W.T., Curran S. Cytochrome p450 profile of colorectal cancer: identification of markers of prognosis. Clin Cancer Res. 2005;11:3758–3765. doi: 10.1158/1078-0432.CCR-04-1848. [DOI] [PubMed] [Google Scholar]
- 57.Alzahrani A.M., Rajendran P. The Multifarious Link between Cytochrome P450s and Cancer. Oxid Med Cell Longev. 2020;2020:3028387. doi: 10.1155/2020/3028387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heeres J., Backx L.J., Mostmans J.H., Van Cutsem J. Antimycotic imidazoles. part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J Med Chem. 1979;22:1003–1005. doi: 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- 59.Borgers M., Van den Bossche H., De Brabander M. The mechanism of action of the new antimycotic ketoconazole. Am J Med. 1983;74:2–8. doi: 10.1016/0002-9343(83)90507-7. [DOI] [PubMed] [Google Scholar]
- 60.Ketoconazole as an inhibitor of steroid production. N Engl J Med 1988; 318: 710-1. [PubMed]
- 61.Basseville A., Preisser L., de Carné T.S., Boisdron-Celle M., Gamelin E., Coqueret O. Irinotecan induces steroid and xenobiotic receptor (SXR) signaling to detoxification pathway in colon cancer cells. Mol Cancer. 2011;10:80. doi: 10.1186/1476-4598-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taplin M.E., Regan M.M., Ko Y.J., Bubley G.J., Duggan S.E., Werner L. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7099–7105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small E.J., Halabi S., Dawson N.A., Stadler W.M., Rini B.I., Picus J. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 64.Ryan C.J., Weinberg V., Rosenberg J., Fong L., Lin A., Kim J. Phase II study of ketoconazole plus granulocyte-macrophage colony-stimulating factor for prostate cancer: effect of extent of disease on outcome. J Urol. 2007;178:2372–2376. doi: 10.1016/j.juro.2007.08.011. discussion 7. [DOI] [PubMed] [Google Scholar]
- 65.Scholz M., Jennrich R., Strum S., Brosman S., Johnson H., Lam R. Long-term outcome for men with androgen independent prostate cancer treated with ketoconazole and hydrocortisone. J Urol. 2005;173:1947–1952. doi: 10.1097/01.ju.0000158449.83022.40. [DOI] [PubMed] [Google Scholar]
- 66.Keizman D., Huang P., Carducci M.A., Eisenberger M.A. Contemporary experience with ketoconazole in patients with metastatic castration-resistant prostate cancer: clinical factors associated with PSA response and disease progression. Prostate. 2012;72:461–467. doi: 10.1002/pros.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kucuk O., Fisher E., Moinpour C.M., Coleman D., Hussain M.H., Sartor A.O. Phase II trial of bicalutamide in patients with advanced prostate cancer in whom conventional hormonal therapy failed: a Southwest Oncology Group study (SWOG 9235) Urology. 2001;58:53–58. doi: 10.1016/s0090-4295(01)01010-x. [DOI] [PubMed] [Google Scholar]
- 68.Trachtenberg J., Pont A. Ketoconazole therapy for advanced prostate cancer. Lancet. 1984;2:433–435. doi: 10.1016/s0140-6736(84)92909-x. [DOI] [PubMed] [Google Scholar]
- 69.Quintanilha J.C.F., de Sousa V.M., Visacri M.B., Amaral L.S., Santos R.M.M., Zambrano T. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol. 2017;80:223–233. doi: 10.1007/s00280-017-3358-x. [DOI] [PubMed] [Google Scholar]
- 70.Preissner S., Simmaco M., Gentile G., Preissner R. Personalized Cancer Therapy Considering Cytochrome P450 Variability. Adv Pharmacol. 2015;74:113–130. doi: 10.1016/bs.apha.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Norden A.D., Raizer J.J., Abrey L.E., Lamborn K.R., Lassman A.B., Chang S.M. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96:211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santos A., Zanetta S., Cresteil T., Deroussent A., Pein F., Raymond E. Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans. Clin Cancer Res. 2000;6:2012–2020. [PubMed] [Google Scholar]
- 73.Rahman A., Korzekwa K.R., Grogan J., Gonzalez F.J., Harris J.W. Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res. 1994;54:5543–5546. [PubMed] [Google Scholar]
- 74.Chu W., Fyles A., Sellers E.M., McCready D.R., Murphy J., Pal T. Association between CYP3A4 genotype and risk of endometrial cancer following tamoxifen use. Carcinogenesis. 2007;28:2139–2142. doi: 10.1093/carcin/bgm087. [DOI] [PubMed] [Google Scholar]
- 75.Henningsson A., Marsh S., Loos W.J., Karlsson M.O., Garsa A., Mross K. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11:8097–8104. doi: 10.1158/1078-0432.CCR-05-1152. [DOI] [PubMed] [Google Scholar]
- 76.Kajita J., Kuwabara T., Kobayashi H., Kobayashi S. CYP3A4 is mainly responsibile for the metabolism of a new vinca alkaloid, vinorelbine, in human liver microsomes. Drug Metab Dispos. 2000;28:1121–1127. [PubMed] [Google Scholar]
- 77.Chien A.J., Munster P.N., Melisko M.E., Rugo H.S., Park J.W., Goga A. Phase I dose-escalation study of 5-day intermittent oral lapatinib therapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. J Clin Oncol. 2014;32:1472–1479. doi: 10.1200/JCO.2013.52.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim Y.W., Goh B.C., Wang L.Z., Tan S.H., Chuah B.Y.S., Lim S.E. Pharmacokinetics and pharmacodynamics of docetaxel with or without ketoconazole modulation in chemonaive breast cancer patients. Ann Oncol. 2010;21:2175–2182. doi: 10.1093/annonc/mdq230. [DOI] [PubMed] [Google Scholar]
- 79.Kehrer D.F., Mathijssen R.H., Verweij J., de Bruijn P., Sparreboom A. Modulation of irinotecan metabolism by ketoconazole. J Clin Oncol. 2002;20:3122–3129. doi: 10.1200/JCO.2002.08.177. [DOI] [PubMed] [Google Scholar]
- 80.Ke A.B., Zamek-Gliszczynski M.J., Higgins J.W., Hall S.D. Itraconazole and clarithromycin as ketoconazole alternatives for clinical CYP3A inhibition studies. Clin Pharmacol Ther. 2014;95:473–476. doi: 10.1038/clpt.2014.41. [DOI] [PubMed] [Google Scholar]
- 81.Templeton I., Peng C.C., Thummel K.E., Davis C., Kunze K.L., Isoherranen N. Accurate prediction of dose-dependent CYP3A4 inhibition by itraconazole and its metabolites from in vitro inhibition data. Clin Pharmacol Ther. 2010;88:499–505. doi: 10.1038/clpt.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Budha N.R., Ji T., Musib L., Eppler S., Dresser M., Chen Y. Evaluation of Cytochrome P450 3A4-Mediated Drug-Drug Interaction Potential for Cobimetinib Using Physiologically Based Pharmacokinetic Modeling and Simulation. Clin Pharmacokinet. 2016;55:1435–1445. doi: 10.1007/s40262-016-0412-5. [DOI] [PubMed] [Google Scholar]
- 83.Jones A.L., Dowsett M.J., Powles T.J., Gallagher C.J., Coombes R.C. Treatment of advanced breast cancer with miconazole: a potential inhibitor of peripheral oestrogen synthesis. Eur J Cancer. 1991;27:301. doi: 10.1016/0277-5379(91)90530-q. [DOI] [PubMed] [Google Scholar]
- 84.Pozzi A., Popescu V., Yang S., Mei S., Shi M., Puolitaival S.M. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor alpha are arachidonic acid epoxygenase-mediated. J Biol Chem. 2010;285:12840–12850. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sausville L.N., Gangadhariah M.H., Chiusa M., Mei S., Wei S., Zent R. The Cytochrome P450 Slow Metabolizers CYP2C9*2 and CYP2C9*3 Directly Regulate Tumorigenesis via Reduced Epoxyeicosatrienoic Acid Production. Cancer Res. 2018;78:4865–4877. doi: 10.1158/0008-5472.CAN-17-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Espinel-Ingroff A., Shadomy S. In vitro and in vivo evaluation of antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;8:352–361. doi: 10.1007/BF01963469. [DOI] [PubMed] [Google Scholar]
- 87.Karjoo Z., Chen X., Hatefi A. Progress and problems with the use of suicide genes for targeted cancer therapy. Adv Drug Deliv Rev. 2016;99:113–128. doi: 10.1016/j.addr.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorer D.E., Nettelbeck D.M. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 89.Yagiz K., Huang T.T., Lopez Espinoza F., Mendoza D., Ibañez C.E., Gruber H.E. Toca 511 plus 5-fluorocytosine in combination with lomustine shows chemotoxic and immunotherapeutic activity with no additive toxicity in rodent glioblastoma models. Neuro Oncol. 2016;18:1390–1401. doi: 10.1093/neuonc/now089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cloughesy T.F., Landolfi J., Hogan D.J., Bloomfield S., Carter B., Chen C.C. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8:341ra75. doi: 10.1126/scitranslmed.aad9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cloughesy T.F., Landolfi J., Vogelbaum M.A., Ostertag D., Elder J.B., Bloomfield S. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018;20:1383–1392. doi: 10.1093/neuonc/noy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Accomando W.P., Rao A.R., Hogan D.J., Newman A.M., Nakao A., Alizadeh A.A. Molecular and Immunologic Signatures are Related to Clinical Benefit from Treatment with Vocimagene Amiretrorepvec (Toca 511) and 5-Fluorocytosine (Toca FC) in Patients with Glioma. Clin Cancer Res. 2020;26:6176–6186. doi: 10.1158/1078-0432.CCR-20-0536. [DOI] [PubMed] [Google Scholar]
- 93.Loo D.S. Systemic antifungal agents: an update of established and new therapies. Adv Dermatol. 2006;22:101–124. doi: 10.1016/j.yadr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 94.Gull K., Trinci A.P. Griseofulvin inhibits fungal mitosis. Nature. 1973;244:292–294. doi: 10.1038/244292a0. [DOI] [PubMed] [Google Scholar]
- 95.Panda D., Rathinasamy K., Santra M.K., Wilson L. Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci U S A. 2005;102:9878–9883. doi: 10.1073/pnas.0501821102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rønnest M.H., Rebacz B., Markworth L., Terp A.H., Larsen T.O., Krämer A. Synthesis and structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J Med Chem. 2009;52:3342–3347. doi: 10.1021/jm801517j. [DOI] [PubMed] [Google Scholar]
- 97.Pacchierotti F., Bassani B., Marchetti F., Tiveron C. Griseofulvin induces mitotic delay and aneuploidy in bone marrow cells of orally treated mice. Mutagenesis. 2002;17:219–222. doi: 10.1093/mutage/17.3.219. [DOI] [PubMed] [Google Scholar]
- 98.Steinmetz M.O., Prota A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018;28:776–792. doi: 10.1016/j.tcb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Ho Y.S., Duh J.S., Jeng J.H., Wang Y.J., Liang Y.C., Lin C.H. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer. 2001;91:393–401. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1070>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 100.Raab M.S., Breitkreutz I., Anderhub S., Rønnest M.H., Leber B., Larsen T.O. GF-15, a novel inhibitor of centrosomal clustering, suppresses tumor cell growth in vitro and in vivo. Cancer Res. 2012;72:5374–5385. doi: 10.1158/0008-5472.CAN-12-2026. [DOI] [PubMed] [Google Scholar]
- 101.Dinić J., Efferth T., García-Sosa A.T., Grahovac J., Padrón J.M., Pajeva I. Repurposing old drugs to fight multidrug resistant cancers. Drug Resist Updat. 2020;52 doi: 10.1016/j.drup.2020.100713. [DOI] [PubMed] [Google Scholar]
- 102.Gupta S.C., Sung B., Prasad S., Webb L.J., Aggarwal B.B. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 103.Palve V., Liao Y., Remsing Rix L.L., Rix U. Turning liabilities into opportunities: Off-target based drug repurposing in cancer. Semin Cancer Biol. 2021;68:209–229. doi: 10.1016/j.semcancer.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Overington J.P., Al-Lazikani B., Hopkins A.L. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 105.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 106.Matthews H.K., Bertoli C., de Bruin R.A.M. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2021 doi: 10.1038/s41580-021-00404-3. [DOI] [PubMed] [Google Scholar]
- 107.Forgue-Lafitte M.E., Coudray A.M., Fagot D., Mester J. Effects of ketoconazole on the proliferation and cell cycle of human cancer cell lines. Cancer Res. 1992;52:6827–6831. [PubMed] [Google Scholar]
- 108.Chen R.J., Lee W.S., Liang Y.C., Lin J.K., Wang Y.J., Lin C.H. Ketoconazole induces G0/G1 arrest in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Pharmacol. 2000;169:132–141. doi: 10.1006/taap.2000.9062. [DOI] [PubMed] [Google Scholar]
- 109.Wu C.H., Jeng J.H., Wang Y.J., Tseng C.J., Liang Y.C., Chen C.H. Antitumor effects of miconazole on human colon carcinoma xenografts in nude mice through induction of apoptosis and G0/G1 cell cycle arrest. Toxicol Appl Pharmacol. 2002;180:22–35. doi: 10.1006/taap.2002.9352. [DOI] [PubMed] [Google Scholar]
- 110.Bae S.H., Park J.H., Choi H.G., Kim H., Kim S.H. Imidazole Antifungal Drugs Inhibit the Cell Proliferation and Invasion of Human Breast Cancer Cells. Biomol Ther (Seoul) 2018;26:494–502. doi: 10.4062/biomolther.2018.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aktas H., Flückiger R., Acosta J.A., Savage J.M., Palakurthi S.S., Halperin J.A. Depletion of intracellular Ca2+ stores, phosphorylation of eIF2alpha, and sustained inhibition of translation initiation mediate the anticancer effects of clotrimazole. Proc Natl Acad Sci U S A. 1998;95:8280–8285. doi: 10.1073/pnas.95.14.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuan S.Y., Shiau M.Y., Ou Y.C., Huang Y.C., Chen C.C., Cheng C.L. Miconazole induces apoptosis via the death receptor 5-dependent and mitochondrial-mediated pathways in human bladder cancer cells. Oncol Rep. 2017;37:3606–3616. doi: 10.3892/or.2017.5608. [DOI] [PubMed] [Google Scholar]
- 113.Benzaquen L.R., Brugnara C., Byers H.R., Gatton-Celli S., Halperin J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- 114.Wang Z.H., Shen B., Yao H.L., Jia Y.C., Ren J., Feng Y.J. Blockage of intermediate-conductance-Ca(2+) -activated K(+) channels inhibits progression of human endometrial cancer. Oncogene. 2007;26:5107–5114. doi: 10.1038/sj.onc.1210308. [DOI] [PubMed] [Google Scholar]
- 115.Chang H.T., Chen W.C., Chen J.S., Lu Y.C., Hsu S.S., Wang J.L. Effect of miconazole on intracellular Ca2+ levels and proliferation in human osteosarcoma cells. Life Sci. 2005;76:2091–2101. doi: 10.1016/j.lfs.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 116.Roan C.J., Chou C.T., Liang W.Z., Chang H.T., Kuo D.H., Kuo C.C. Effect of Miconazole on [Ca2+]i and Cytotoxicity in ZR-75-1 Human Breast Cancer Cells. Chin J Physiol. 2015;58:377–384. doi: 10.4077/CJP.2015.BAD347. [DOI] [PubMed] [Google Scholar]
- 117.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 2013; 14: 416-29. [DOI] [PubMed]
- 118.Taipale J., Beachy P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 119.Zhang J., Fan J., Zeng X., Nie M., Luan J., Wang Y. Hedgehog signaling in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment. Acta Pharm Sin B. 2021;11:609–620. doi: 10.1016/j.apsb.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gorojankina T. Hedgehog signaling pathway: a novel model and molecular mechanisms of signal transduction. Cell Mol Life Sci. 2016;73:1317–1332. doi: 10.1007/s00018-015-2127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xin M., Ji X., De La Cruz L.K., Thareja S., Wang B. Strategies to target the Hedgehog signaling pathway for cancer therapy. Med Res Rev. 2018;38:870–913. doi: 10.1002/med.21482. [DOI] [PubMed] [Google Scholar]
- 122.Tang J.Y., Mackay-Wiggan J.M., Aszterbaum M., Yauch R.L., Lindgren J., Chang K. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim J., Aftab B.T., Tang J.Y., Kim D., Lee A.H., Rezaee M. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim D.J., Kim J., Spaunhurst K., Montoya J., Khodosh R., Chandra K. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 125.Pace J.R., DeBerardinis A.M., Sail V., Tacheva-Grigorova S.K., Chan K.A., Tran R. Repurposing the Clinically Efficacious Antifungal Agent Itraconazole as an Anticancer Chemotherapeutic. J Med Chem. 2016;59:3635–3649. doi: 10.1021/acs.jmedchem.5b01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.You M., Varona-Santos J., Singh S., Robbins D.J., Savaraj N., Nguyen D.M. Targeting of the Hedgehog signal transduction pathway suppresses survival of malignant pleural mesothelioma cells in vitro. J Thorac Cardiovasc Surg. 2014;147:508–516. doi: 10.1016/j.jtcvs.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 127.Inoue K., Tsubamoto H., Sakata K., Sakane R., Hao H., Hirota S. Expression of Hedgehog Signals and Growth Inhibition by Itraconazole in Endometrial Cancer. Anticancer Res. 2016;36:149–153. [PubMed] [Google Scholar]
- 128.Liang G., Liu M., Wang Q., Shen Y., Mei H., Li D. Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget. 2017;8:28510–28525. doi: 10.18632/oncotarget.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu R., Li J., Zhang T., Zou L., Chen Y., Wang K. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking. Autophagy. 2014;10:1241–1255. doi: 10.4161/auto.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsubamoto H., Inoue K., Sakata K., Ueda T., Takeyama R., Shibahara H. Itraconazole Inhibits AKT/mTOR Signaling and Proliferation in Endometrial Cancer Cells. Anticancer Res. 2017;37:515–519. doi: 10.21873/anticanres.11343. [DOI] [PubMed] [Google Scholar]
- 131.Xu C., Zhuo Y., Liu Y., Chen H. Itraconazole Inhibits the Growth of Cutaneous Squamous Cell Carcinoma by Targeting HMGCS1/ACSL4 Axis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.828983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W., Dong X., Liu Y., Ni B., Sai N., You L. Itraconazole exerts anti-liver cancer potential through the Wnt, PI3K/AKT/mTOR, and ROS pathways. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110661. [DOI] [PubMed] [Google Scholar]
- 133.Deng H., Huang L., Liao Z., Liu M., Li Q., Xu R. Itraconazole inhibits the Hedgehog signaling pathway thereby inducing autophagy-mediated apoptosis of colon cancer cells. Cell Death Dis. 2020;11:539. doi: 10.1038/s41419-020-02742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ban L., Mei T., Su Q., Li W., Huang Z., Liu L. Anti-fungal drug itraconazole exerts anti-cancer effects in oral squamous cell carcinoma via suppressing Hedgehog pathway. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117695. [DOI] [PubMed] [Google Scholar]
- 135.Jiang F., Xing H.S., Chen W.Y., Du J., Ruan Y.L., Lin A.Y. Itraconazole inhibits proliferation of pancreatic cancer cells through activation of Bak-1. J Cell Biochem. 2019;120:4333–4341. doi: 10.1002/jcb.27719. [DOI] [PubMed] [Google Scholar]
- 136.Wang X., Wei S., Zhao Y., Shi C., Liu P., Zhang C. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017;385:128–136. doi: 10.1016/j.canlet.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 137.Sui S., Xu S., Pang D. Emerging role of ferroptosis in breast cancer: New dawn for overcoming tumor progression. Pharmacol Ther. 2021 doi: 10.1016/j.pharmthera.2021.107992. [DOI] [PubMed] [Google Scholar]
- 138.Chen X., Zeh H.J., Kang R., Kroemer G., Tang D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol. 2021;18:804–823. doi: 10.1038/s41575-021-00486-6. [DOI] [PubMed] [Google Scholar]
- 139.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cosentino K., García-Sáez A.J. Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol. 2017;27:266–275. doi: 10.1016/j.tcb.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Booth L.A., Roberts J.L., Dent P. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin Cancer Biol. 2020;66:129–139. doi: 10.1016/j.semcancer.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang K.C., Wu C.C., Wu C.H., Chen J.H., Chu C.H., Chen C.H. Involvement of proapoptotic Bcl-2 family members in terbinafine-induced mitochondrial dysfunction and apoptosis in HL60 cells. Food Chem Toxicol. 2006;44:214–226. doi: 10.1016/j.fct.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 143.Hu Q., Hou Y.C., Huang J., Fang J.Y., Xiong H. Itraconazole induces apoptosis and cell cycle arrest via inhibiting Hedgehog signaling in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:50. doi: 10.1186/s13046-017-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dong C., Yang R., Li H., Ke K., Luo C., Yang F. Econazole nitrate inhibits PI3K activity and promotes apoptosis in lung cancer cells. Sci Rep. 2017;7:17987. doi: 10.1038/s41598-017-18178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.An Y., Jiang J., Zhou L., Shi J., Jin P., Li L. Peroxiredoxin 1 is essential for natamycin-triggered apoptosis and protective autophagy in hepatocellular carcinoma. Cancer Lett. 2021;521:210–223. doi: 10.1016/j.canlet.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 146.Sobecks R., McCormick T.S., Distelhorst C.W. Imidazole antifungals Miconazole and Econazole induce apoptosis in mouse lymphoma and human T cell leukemia cells: regulation by Bcl-2 and potential role of calcium. Cell Death Differ. 1996;3:331–337. [PubMed] [Google Scholar]
- 147.Yoon S.H., Kim B.K., Kang M.J., Im J.Y., Won M. Miconazole inhibits signal transducer and activator of transcription 3 signaling by preventing its interaction with DNA damage-induced apoptosis suppressor. Cancer Sci. 2020;111:2499–2507. doi: 10.1111/cas.14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ho Y.S., Wu C.H., Chou H.M., Wang Y.J., Tseng H., Chen C.H. Molecular mechanisms of econazole-induced toxicity on human colon cancer cells: G0/G1 cell cycle arrest and caspase 8-independent apoptotic signaling pathways. Food Chem Toxicol. 2005;43:1483–1495. doi: 10.1016/j.fct.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 149.Choi E.K., Park E.J., Phan T.T., Kim H.D., Hoe K.L., Kim D.U. Econazole Induces p53-Dependent Apoptosis and Decreases Metastasis Ability in Gastric Cancer Cells. Biomol Ther (Seoul) 2020;28:370–379. doi: 10.4062/biomolther.2019.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Galluzzi L., Bravo-San Pedro J.M., Levine B., Green D.R., Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gewirtz D.A. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74:647–651. doi: 10.1158/0008-5472.CAN-13-2966. [DOI] [PubMed] [Google Scholar]
- 153.Xiao M., Benoit A., Hasmim M., Duhem C., Vogin G., Berchem G. Targeting Cytoprotective Autophagy to Enhance Anticancer Therapies. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.626309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ho C.Y., Chang A.C., Hsu C.H., Tsai T.F., Lin Y.C., Chou K.Y. Miconazole induces protective autophagy in bladder cancer cells. Environ Toxicol. 2021;36:185–193. doi: 10.1002/tox.23024. [DOI] [PubMed] [Google Scholar]
- 155.Dou Q., Chen H.N., Wang K., Yuan K., Lei Y., Li K. Ivermectin Induces Cytostatic Autophagy by Blocking the PAK1/Akt Axis in Breast Cancer. Cancer Res. 2016;76:4457–4469. doi: 10.1158/0008-5472.CAN-15-2887. [DOI] [PubMed] [Google Scholar]
- 156.Chen Y., Chen H.N., Wang K., Zhang L., Huang Z., Liu J. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 2019;70:66–77. doi: 10.1016/j.jhep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 157.Zhang W., Zhou L., Qin S., Jiang J., Huang Z., Zhang Z. Sertaconazole provokes proapoptotic autophagy via stabilizing TRADD in nonsmall cell lung cancer cells. MedComm. 2020;2021(2):821–837. doi: 10.1002/mco2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weng N., Qin S., Liu J., Huang X., Jiang J., Zhou L. Repurposing econazole as a pharmacological autophagy inhibitor to treat pancreatic ductal adenocarcinoma. Acta Pharmaceutica Sinica B. 2022 doi: 10.1016/j.apsb.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Y., Wang Q., Li X., Chen Y., Xu G. Itraconazole attenuates the stemness of nasopharyngeal carcinoma cells via triggering ferroptosis. Environ Toxicol. 2021;36:257–266. doi: 10.1002/tox.23031. [DOI] [PubMed] [Google Scholar]
- 160.Nacev B.A., Grassi P., Dell A., Haslam S.M., Liu J.O. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J Biol Chem. 2011;286:44045–44056. doi: 10.1074/jbc.M111.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chong C.R., Xu J., Lu J., Bhat S., Sullivan D.J., Jr., Liu J.O. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2:263–270. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 162.Wang Y., Yao Y., Liu H., Ma X., Lv T., Yuan D. Itraconazole can inhibit malignant pleural effusion by suppressing lymphangiogenesis in mice. Transl Lung Cancer Res. 2015;4:27–35. doi: 10.3978/j.issn.2218-6751.2014.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen S., Zhuang K., Sun K., Yang Q., Ran X., Xu X. Itraconazole Induces Regression of Infantile Hemangioma via Downregulation of the Platelet-Derived Growth Factor-D/PI3K/Akt/mTOR Pathway. J Invest Dermatol. 2019;139:1574–1582. doi: 10.1016/j.jid.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 164.Aftab B.T., Dobromilskaya I., Liu J.O., Rudin C.M. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764–6772. doi: 10.1158/0008-5472.CAN-11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Head S.A., Shi W., Zhao L., Gorshkov K., Pasunooti K., Chen Y. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc Natl Acad Sci U S A. 2015;112:E7276–E7285. doi: 10.1073/pnas.1512867112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Head S.A., Shi W.Q., Yang E.J., Nacev B.A., Hong S.Y., Pasunooti K.K. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem Biol. 2017;12:174–182. doi: 10.1021/acschembio.6b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ho P.Y., Liang Y.C., Ho Y.S., Chen C.T., Lee W.S. Inhibition of human vascular endothelial cells proliferation by terbinafine. Int J Cancer. 2004;111:51–59. doi: 10.1002/ijc.20039. [DOI] [PubMed] [Google Scholar]
- 168.Ho P.Y., Zhong W.B., Ho Y.S., Lee W.S. Terbinafine inhibits endothelial cell migration through suppression of the Rho-mediated pathway. Mol Cancer Ther. 2006;5:3130–3138. doi: 10.1158/1535-7163.MCT-06-0457. [DOI] [PubMed] [Google Scholar]
- 169.Chien M.H., Lee T.S., Kao C., Yang S.F., Lee W.S. Terbinafine inhibits oral squamous cell carcinoma growth through anti-cancer cell proliferation and anti-angiogenesis. Mol Carcinog. 2012;51:389–399. doi: 10.1002/mc.20800. [DOI] [PubMed] [Google Scholar]
- 170.Wolf A., Agnihotri S., Micallef J., Mukherjee J., Sabha N., Cairns R. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Penso J., Beitner R. Detachment of glycolytic enzymes from cytoskeleton of Lewis lung carcinoma and colon adenocarcinoma cells induced by clotrimazole and its correlation to cell viability and morphology. Mol Genet Metab. 2002;76:181–188. doi: 10.1016/s1096-7192(02)00046-x. [DOI] [PubMed] [Google Scholar]
- 172.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Penso J., Beitner R. Clotrimazole decreases glycolysis and the viability of lung carcinoma and colon adenocarcinoma cells. Eur J Pharmacol. 2002;451:227–235. doi: 10.1016/s0014-2999(02)02103-9. [DOI] [PubMed] [Google Scholar]
- 174.Coelho R.G., Calaça Ide C., Celestrini Dde M., Correia A.H., Costa M.A., Sola-Penna M. Clotrimazole disrupts glycolysis in human breast cancer without affecting non-tumoral tissues. Mol Genet Metab. 2011;103:394–398. doi: 10.1016/j.ymgme.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 175.Furtado C.M., Marcondes M.C., Sola-Penna M., de Souza M.L., Zancan P. Clotrimazole preferentially inhibits human breast cancer cell proliferation, viability and glycolysis. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meira D.D., Marinho-Carvalho M.M., Teixeira C.A., Veiga V.F., Da Poian A.T., Holandino C. Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol Genet Metab. 2005;84:354–362. doi: 10.1016/j.ymgme.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 177.Penso J., Beitner R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur J Pharmacol. 1998;342:113–117. doi: 10.1016/s0014-2999(97)01507-0. [DOI] [PubMed] [Google Scholar]
- 178.IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 179.Bhatt A.P., Redinbo M.R., Bultman S.J. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67:326–344. doi: 10.3322/caac.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 181.Richard M.L., Sokol H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol. 2019;16:331–345. doi: 10.1038/s41575-019-0121-2. [DOI] [PubMed] [Google Scholar]
- 182.Paterson M.J., Oh S., Underhill D.M. Host-microbe interactions: commensal fungi in the gut. Curr Opin Microbiol. 2017;40:131–137. doi: 10.1016/j.mib.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Iliev I.D., Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017;17:635–646. doi: 10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ghannoum M.A., Jurevic R.J., Mukherjee P.K., Cui F., Sikaroodi M., Naqvi A. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Coker O.O., Nakatsu G., Dai R.Z., Wu W.K.K., Wong S.H., Ng S.C. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019;68:654–662. doi: 10.1136/gutjnl-2018-317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao R., Kong C., Li H., Huang L., Qu X., Qin N. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36:2457–2468. doi: 10.1007/s10096-017-3085-6. [DOI] [PubMed] [Google Scholar]
- 187.Luan C., Xie L., Yang X., Miao H., Lv N., Zhang R. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci Rep. 2015;5:7980. doi: 10.1038/srep07980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alnuaimi A.D., Ramdzan A.N., Wiesenfeld D., O'Brien-Simpson N.M., Kolev S.D., Reynolds E.C. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016;22:805–814. doi: 10.1111/odi.12565. [DOI] [PubMed] [Google Scholar]
- 189.Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J.I. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Selander C., Engblom C., Nilsson G., Scheynius A., Andersson C.L. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J Immunol. 2009;182:4208–4216. doi: 10.4049/jimmunol.0800885. [DOI] [PubMed] [Google Scholar]
- 191.Montoya A.M., González G.M., Martinez-Castilla A.M., Aguilar S.A., Franco-Molina M.A., Coronado-Cerda E. Cytokines profile in immunocompetent mice during Trichosporon asahii infection. Med Mycol. 2018;56:103–109. doi: 10.1093/mmy/myx018. [DOI] [PubMed] [Google Scholar]
- 192.Mukherjee P.K., Wang H., Retuerto M., Zhang H., Burkey B., Ghannoum M.A. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget. 2017;8:97273–97289. doi: 10.18632/oncotarget.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu L.P., Huang W., Wang X., Xu C., Qin W.T., Li D. Terbinafine prevents colorectal cancer growth by inducing dNTP starvation and reducing immune suppression. Mol Ther. 2022 doi: 10.1016/j.ymthe.2022.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Raju TN. The Nobel chronicles. 1988: James Whyte Black, (b 1924), Gertrude Elion (1918-99), and George H Hitchings (1905-98). Lancet 2000; 355: 1022. [DOI] [PubMed]
- 195.Zhang W., Bhagwath A.S., Ramzan Z., Williams T.A., Subramaniyan I., Edpuganti V. Itraconazole Exerts Its Antitumor Effect in Esophageal Cancer By Suppressing the HER2/AKT Signaling Pathway. Mol Cancer Ther. 2021;20:1904–1915. doi: 10.1158/1535-7163.MCT-20-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rudin C.M., Brahmer J.R., Juergens R.A., Hann C.L., Ettinger D.S., Sebree R. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2013;8:619–623. doi: 10.1097/JTO.0b013e31828c3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vishwanathan K., Dickinson P.A., So K., Thomas K., Chen Y.M., De Castro C.J. The effect of itraconazole and rifampicin on the pharmacokinetics of osimertinib. Br J Clin Pharmacol. 2018;84:1156–1169. doi: 10.1111/bcp.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barata P.C., Cooney M., Mendiratta P., Tyler A., Dreicer R., Garcia J.A. Ketoconazole plus Lenalidomide in patients with Castration-Resistant Prostate Cancer (CRPC): results of an open-label phase II study. Invest New Drugs. 2018;36:1085–1092. doi: 10.1007/s10637-018-0660-3. [DOI] [PubMed] [Google Scholar]
- 199.Mostaghel E.A., Nelson P.S., Lange P., Lin D.W., Taplin M.E., Balk S. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J Clin Oncol. 2014;32:229–237. doi: 10.1200/JCO.2012.48.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim W., Zhang L., Wilton J.H., Fetterly G., Mohler J.L., Weinberg V. Sequential use of the androgen synthesis inhibitors ketoconazole and abiraterone acetate in castration-resistant prostate cancer and the predictive value of circulating androgens. Clin Cancer Res. 2014;20:6269–6276. doi: 10.1158/1078-0432.CCR-14-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Figg W.D., Woo S., Zhu W., Chen X., Ajiboye A.S., Steinberg S.M. A phase I clinical study of high dose ketoconazole plus weekly docetaxel for metastatic castration resistant prostate cancer. J Urol. 2010;183:2219–2226. doi: 10.1016/j.juro.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhu H., Lewis D.J. Topical hedgehog inhibitors for basal cell carcinoma: how far away are we? Expert Opin Pharmacother. 2022;23:739–740. doi: 10.1080/14656566.2022.2050215. [DOI] [PubMed] [Google Scholar]
- 203.Agnihotri S., Mansouri S., Burrell K., Li M., Mamatjan Y., Liu J. Ketoconazole and Posaconazole Selectively Target HK2-expressing Glioblastoma Cells. Clin Cancer Res. 2019;25:844–855. doi: 10.1158/1078-0432.CCR-18-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang E.B., Zhang X., Wang K., Zhang F., Chen T.W., Ma N. Antifungal agent Terbinafine restrains tumor growth in preclinical models of hepatocellular carcinoma via AMPK-mTOR axis. Oncogene. 2021;40:5302–5313. doi: 10.1038/s41388-021-01934-y. [DOI] [PubMed] [Google Scholar]
- 205.Li B., Lu L., Zhong M., Tan X.X., Liu C.Y., Guo Y. Terbinafine inhibits KSR1 and suppresses Raf-MEK-ERK signaling in oral squamous cell carcinoma cells. Neoplasma. 2013;60:406–412. doi: 10.4149/neo_2013_052. [DOI] [PubMed] [Google Scholar]
- 206.Lee W.S., Chen R.J., Wang Y.J., Tseng H., Jeng J.H., Lin S.Y. In vitro and in vivo studies of the anticancer action of terbinafine in human cancer cell lines: G0/G1 p53-associated cell cycle arrest. Int J Cancer. 2003;106:125–137. doi: 10.1002/ijc.11194. [DOI] [PubMed] [Google Scholar]