Key Points
Question
Do the new National Comprehensive Cancer Network (NCCN) guidelines identify cutaneous squamous cell carcinomas (CSCCs) at risk for poor outcomes?
Findings
In this cohort study of 8727 patients with 10 196 primary CSCCs, the NCCN high- and very-high-risk groups identified CSCCs at highest risk for developing poor outcomes, including local recurrence, metastasis, and disease-specific death. The study also highlights significant reduction in risk of poor outcomes with Mohs micrographic surgery or peripheral and deep en face margin assessment.
Meaning
These findings suggest that the new NCCN guidelines allow for risk stratification of CSCCs in a clinically meaningful way.
This cohort study compares outcomes in very high-, high-, and low-risk National Comprehensive Cancer Network groups of cutaneous squamous cell carcinoma and compares outcomes stratified by Mohs micrographic surgery and wide local excision.
Abstract
Importance
The 2022 National Comprehensive Cancer Network (NCCN) reclassified cutaneous squamous cell carcinoma (CSCC) into low-, high-, and very high-risk groups to better risk stratify tumors. Mohs micrographic surgery (Mohs) or peripheral and deep en face margin assessment (PDEMA) became preferred surgical modalities for high- and very high-risk tumors. This new risk stratification and the recommendation for Mohs or PDEMA in high- and very high-risk groups have not been validated.
Objective
To compare outcomes in very high-, high-, and low-risk NCCN groups of CSCCs and in CSCCs treated with Mohs or PDEMA compared with wide local excision (WLE).
Design, Setting, and Participants
This retrospective cohort study of CSCCs was performed in 2 tertiary care academic medical centers. Patients 18 years or older and diagnosed between January 1, 1996, and December 31, 2019, at Brigham and Women’s Hospital and Cleveland Clinic Foundation were included. Data were analyzed from October 20, 2021, to March 29, 2023.
Exposures
NCCN risk group, Mohs or PDEMA, and WLE.
Main Outcomes and Measures
Local recurrence (LR), nodal metastasis (NM), distant metastasis (DM), and disease-specific death (DSD).
Results
A total of 10 196 tumors from 8727 patients were stratified by NCCN guidelines into low-, high-, and very high-risk groups (6003 [59.0%] men; mean [SD] age, 72.4 [11.8] years). Compared with the low-risk group, the high- and very high-risk groups demonstrated a greater risk of LR (high-risk subhazard ratio [SHR], 1.99 [95% CI, 1.21-3.27; P = .007]; very high-risk SHR, 12.66 [95% CI, 7.86-20.39; P < .001]), NM (high-risk SHR, 4.26 [95% CI, 1.28-14.23; P = .02]; very high-risk SHR, 62.98 [95% CI, 19.24-206.17; P < .001]), DM (high-risk SHR, 2.2 × 107 [95% CI, 4.7 × 103-1.1 × 1011; P < .001]; very high-risk SHR, 6.3 × 108 [95% CI, 1.4 × 105-2.9 × 1012; P < .001]), and DSD (high-risk SHR, 4.02 [95% CI, 1.18-13.71; P = .03]; very high-risk SHR, 93.87 [95% CI, 29.19-301.85; P < .001]). Adjusted 5-year cumulative incidence was significantly higher in very high- vs high- and low-risk groups for LR (9.4% [95% CI, 9.2%-14.0%] vs 1.5% [95% CI, 1.4%-2.1%] and 0.8% [95% CI, 0.5%-1.2%], respectively), NM (7.3% [95% CI, 6.8%-10.9%] vs 0.5% [95% CI, 0.4%-0.8%] and 0.1% [95% CI, 0.03%-0.3%], respectively), DM (3.9% [95% CI, 2.6%-5.6%] vs 0.1% [95% CI, 0.04%-0.2%] and 0.01% [95% CI, not applicable], respectively), and DSD (10.5% [95% CI, 10.3%-15.4%] vs 0.5% [95% CI, 0.4%-0.8%] and 0.1% [95% CI, 0.04%-0.3%], respectively). Compared with CSCCs treated with WLE, those treated with Mohs or PDEMA had lower risk of LR (SHR, 0.65 [95% CI, 0.46-0.90]; P = .009), DM (SHR, 0.38 [95% CI, 0.18-0.83]; P = .02), and DSD (SHR, 0.55 [95% CI, 0.36-0.84]; P = .006).
Conclusions and Relevance
The findings of this cohort study suggest that the NCCN high- and very high-risk groups identify CSCCs at greatest risk for developing poor outcomes. Further, Mohs or PDEMA resulted in lower LR, DM, and DSD compared with WLE.
Introduction
Cutaneous squamous cell carcinoma (CSCC) is the second most common keratinocyte carcinoma in the US, with an estimated 700 000 to 1 000 000 cases reported annually.1,2 While most patients with CSCC are cured with Mohs micrographic surgery (Mohs) or wide local excision (WLE) alone, a subset of patients go on to develop poor outcomes, including local recurrence (LR), nodal metastasis (NM), distant metastasis (DM), and disease-specific death (DSD).3,4,5,6,7 Current staging systems such as the American Joint Committee on Cancer’s AJCC Cancer Staging Manual, 8th edition (AJCC-8) and the Brigham and Women’s Hospital (BWH) staging system are based on tumor characteristics that have been shown to impact prognosis.8,9,10 Such factors include large tumor diameter, poorly differentiated histologic findings, perineural invasion of large-caliber nerves, and deep tumor invasion.5,6,7,8,9,10,11,12,13,14 While useful for prognostication, current staging systems do not incorporate patient factors or other high-risk tumor features that influence outcomes.
The 2022 edition of the National Comprehensive Cancer Network (NCCN) reclassified CSCC into low-, high-, and very high-risk groups to improve prognostication and guide treatment recommendations (Table 1).11 The very high-risk group was added to identify tumors at greatest risk for poor outcomes. Based on the new risk stratification groups, the NCCN guidelines also made a new recommendation that Mohs or peripheral and deep en face margin assessment (PDEMA) be the preferred method for tissue processing for high- and very high-risk tumors.11 Neither change to the NCCN guidelines has been validated. The purpose of this study was 2-fold: to compare outcomes in very high-, high-, and low-risk NCCN group CSCCs and to compare outcomes of CSCCs stratified by Mohs and WLE.
Table 1. NCCN Risk Stratificationa.
| Characteristic | NCCN risk group | ||
|---|---|---|---|
| Low | High | Very high | |
| History and physical | |||
| Location and size | Trunk, extremities <2 cm | Trunk, extremities 2 to <4 cm; head, neck, hands, feet, pretibial, and anogenital (any size) | ≥4 cm (any location) |
| Borders | Well-defined | Poorly defined | NA |
| Primary vs recurrent | Primary | Recurrent | NA |
| Immunosuppression | Negative | Positive | NA |
| Site of prior RT or chronic inflammatory process | Negative | Positive | NA |
| Rapidly growing tumor | Negative | Positive | NA |
| Neurological symptoms | Negative | Positive | NA |
| Pathological findings | |||
| Degree of differentiation | Well or moderately differentiated | NA | Poor differentiation |
| Histologic features: acantholytic (adenoid), adenosquamous (showing mucin production), or metaplastic (carcinosarcomatous) subtypes | Negative | Positive | Desmoplastic SCC |
| Depth: thickness or level of invasion | ≤6 mm and no invasion beyond subcutaneous fat | NA | >6 mm or invasion beyond subcutaneous fat |
| Perineural involvement | Negative | Positive | Tumor cells within the nerve sheath of a nerve lying deeper than the dermis or measuring ≥0.1 mm |
| Lymphatic or vascular involvement | Negative | Negative | Positive |
Abbreviations: NA, not applicable; NCCN, National Comprehensive Cancer Network; RT, radiotherapy; SCC, squamous cell carcinoma.
Adapted from NCCN 2022 guidelines. Any tumor with 1 or more high- or very high-risk features was categorized as high risk or very high risk, respectively.
Methods
Data Source and Study Population
This retrospective cohort study was approved by the Mass General Brigham Human Research Committee and the Cleveland Clinic Foundation (CCF) institutional review boards, which determined that obtaining informed consent from all patients would be prohibitive. However, the patient consent form and process for procedures and treatments included consent that materials or patient information may be accessed and used for research purposes including presentations, clinical meetings, and publications in scientific journals. Patients 18 years or older with CSCC diagnosed between January 1, 2000, and December 31, 2017, at BWH and between January 1, 1996, and December 31, 2019, at CCF were retrospectively included in the analysis. The methods for data collection have been published previously.6,10 Tumors that were noncutaneous, in situ, or metastatic at presentation were excluded from analysis. Tumors occurring at an anogenital site or that were recurrent were included in the analysis, as these factors are included in the 2022 NCCN risk stratification guidelines. This study used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
NCCN Risk Groups and Outcome Definition
Data on race and ethnicity were collected, and CSCC predominantly occurred in White patients (96.2% of the cohort). Tumors were classified by NCCN risk group per the 2022 guidelines and analyzed by low-, high-, and very high-risk characteristics; any tumor with 1 or more high- or very high-risk features was categorized as high risk or very high risk, respectively (Table 1 and Table 2). The outcomes analyzed included LR, NM, DM, and DSD.6 Briefly, LR was considered to have occurred if pathology documentation of recurrent invasive CSCC in the same location was confirmed by documentation by the treating physician. Nodal metastasis was considered to occur if there was pathologically confirmed CSCC in a draining nodal basin of the primary tumor. Distant metastasis was considered to occur if histologic and/or radiological evidence was documented. Disease-specific death was considered to occur if the patient died of CSCC or direct complications of CSCC attributed to that index tumor by the treating physician. Poor outcomes were further evaluated stratified by Mohs compared with WLE.
Table 2. Patient and Tumor Characteristics for NCCN groups.
| Characteristic | NCCN risk groupa | P valueb | ||
|---|---|---|---|---|
| Low (n = 3054) | High (n = 6269) | Very high (n = 873) | ||
| Median follow-up time (range), mo | 37 (18-63) | 35 (13-62) | 27 (11-54) | <.001 |
| Hospital | ||||
| BWH | 2208 (72.3) | 3892 (62.1) | 714 (81.8) | <.001 |
| CCF | 846 (27.7) | 2377 (37.9) | 159 (18.2) | |
| Sex | ||||
| Men | 1287 (42.1) | 4083 (65.1) | 633 (72.5) | <.001 |
| Women | 1767 (57.9) | 2186 (34.9) | 240 (27.5) | |
| Age, mean (SD), y | 73.6 (11.3) | 71.8 (12.0) | 72.6 (12.2) | |
| Tumor location | ||||
| Head or neck | 6 (0.2) | 3962 (63.2) | 575 (65.9) | <.001 |
| Trunk or extremities | 3048 (99.8) | 2286 (36.5) | 280 (32.1) | |
| Anogenital | 0 | 21 (0.3) | 18 (2.1) | |
| Surgical treatment | ||||
| WLE | 1591 (52.1) | 1456 (23.2) | 423 (48.5) | <.001 |
| Mohs | 939 (30.7) | 3932 (62.7) | 369 (42.3) | |
| Other | 524 (17.2) | 881 (14.1) | 81 (9.3) | |
| Perineural invasion | ||||
| None or unknown | 3054 (100) | 6147 (98.1) | 623 (71.4) | <.001 |
| Small caliber | 0 | 122 (1.9) | 133 (15.2) | |
| Large caliber | 0 | 0 | 117 (13.4) | |
| Immune status | ||||
| Immunocompetent | 3054 (100) | 4140 (66.0) | 612 (70.1) | <.001 |
| Immunosuppressedc | 0 | 2129 (34.0) | 261 (29.9) | |
| Depth | ||||
| Dermis or subcutaneous fat | 3000 (98.2) | 6145 (98.0) | 575 (65.9) | <.001 |
| Beyond subcutaneous fat | 0 | 0 | 175 (20.0) | |
| Bone | 0 | 0 | 51 (5.8) | |
| Other or unknown | 54 (1.8) | 124 (2.0) | 72 (8.2) | |
| Differentiation | ||||
| Well | 2722 (89.1) | 4973 (79.3) | 170 (19.5) | <.001 |
| Moderate | 154 (5.0) | 723 (11.5) | 117 (13.4) | |
| Poor | 0 | 0 | 562 (64.4) | |
| Undifferentiated or anaplastic | 0 | 0 | 1 (0.1) | |
| Unknown | 178 (5.8) | 573 (9.1) | 23 (2.6) | |
| Lymphovascular invasion | ||||
| No | 3052 (99.9) | 6247 (99.6) | 816 (93.5) | <.001 |
| Yes | 0 | 0 | 56 (6.4) | |
| Unknown | 2 (0.1) | 22 (0.4) | 1 (0.1) | |
| Tumor size, cm | ||||
| <2.0 | 2850 (93.3) | 5181 (82.6) | 356 (40.8) | <.001 |
| 2.0-3.9 | 0 | 707 (11.3) | 260 (29.8) | |
| ≥4.0 | 0 | 0 | 204 (23.4) | |
| Unknown | 204 (6.7) | 381 (6.1) | 53 (6.1) | |
| BWH stage | ||||
| T1 | 2738 (89.7) | 4767 (76.0) | 3 (0.3) | <.001 |
| T2a | 0 | 814 (13.0) | 341 (39.1) | |
| T2b | 0 | 28 (0.4) | 359 (41.1) | |
| T3 | 0 | 0 | 57 (6.5) | |
| Unknown | 316 (10.3) | 660 (10.5) | 113 (12.9) | |
| AJCC-8 stage | ||||
| T1 | 9 (0.3) | 3287 (52.4) | 132 (15.1) | <.001 |
| T2 | 0 | 280 (4.5) | 52 (6.0) | |
| T3 | 0 | 66 (1.1) | 265 (30.4) | |
| T4a | 0 | 5 (0.1) | 37 (4.2) | |
| T4b | 0 | 0 | 3 (0.3) | |
| Unknown | 3045 (99.7) | 2631 (42.0) | 384 (44.0) | |
| Local recurrence | ||||
| No | 3033 (99.3) | 6176 (98.5) | 786 (90.0) | <.001 |
| Yes | 21 (0.7) | 93 (1.5) | 87 (10.0) | |
| Nodal metastasis | ||||
| No | 3051 (99.9) | 6239 (99.5) | 806 (92.3) | <.001 |
| Yes | 3 (0.1) | 30 (0.5) | 67 (7.7) | |
| Distant metastasis | ||||
| No | 3054 (100) | 6264 (99.9) | 845 (96.8) | <.001 |
| Yes | 0 | 5 (0.1) | 28 (3.2) | |
| Disease-specific death | ||||
| No | 3051 (99.9) | 6240 (99.5) | 776 (88.9) | <.001 |
| Yes | 3 (0.1) | 29 (0.5) | 97 (11.1) | |
Abbreviations: AJCC-8, American Joint Committee on Cancer Manual, 8th edition; BWH, Brigham and Women’s Hospital; CCF, Cleveland Clinic Foundation; Mohs, Mohs micrographic surgery; NCCN, National Comprehensive Cancer Network; WLE, wide local excision.
Unless otherwise indicated, data are expressed as No. (%) of tumors. Percentages have been rounded and may not total 100.
Calculated using a paired t test (for age), χ2 test (for sex, surgical modality, tumor size, local recurrence, distant metastasis, and disease-specific death), and Fisher exact test (for location, immunocompromised status, perineural invasion, depth of invasion, differentiation, lymphovascular invasion, BWH, AJCC-8, and nodal metastasis).
Includes patients with immune deficiency, organ transplant, immunosuppressive medication use, prolonged corticosteroid use, HIV, or bone marrow transplant.
Statistical Analysis
Data were analyzed from October 20, 2021, to March 29, 2023. Baseline characteristics of primary CSCC tumors stratified by low-, high-, and very high-risk NCCN group were analyzed using descriptive statistics and frequency tabulation, with χ2 test, paired t test, and Fisher exact test used as appropriate to evaluate for differences between groups. Univariate competing risk regression was used to assess factors including age, sex, NCCN risk group, and surgical approach, and variables with P < .20 on univariate analysis were included in the multivariable model (eTable in Supplement 1). Only variables with a P < .05 were considered statistically significant in final models. Patients who did not develop an outcome of interest were censored on their date of death or at last follow-up by a clinician if the patient was alive at time of data collection. Patients with missing diagnosis dates for outcome events were excluded. Fine and Gray competing risk regression modeling was used to evaluate end points of LR, NM, DM, and DSD.6 The Fine and Gray competing risk regression model is a subdistribution hazards regression model, yielding a subhazard ratio (SHR) that can be interpreted similarly to a hazard ratio as in Cox proportional hazards regression, with values greater than 1.00 associated with higher risk or hazard. Regression analysis excludes tumors with missing data; thus, if there were missing data for a specific analysis being performed, that tumor would not contribute data (Table 3). Five-year cumulative incidence function curves were generated for low-, high-, and very high-risk tumors for competing end points of LR, NM, DM, and DSD. For LR, NM, and DM, any death was considered a competing risk; for DSD, non–CSCC-related death was considered a competing risk. Five-year cumulative incidence function curves were adjusted for covariates included in the multivariable analysis, and cumulative incidence and 95% CIs were tabulated using stcompet and stcomlist in Stata, version 17.0 (StataCorp LLC). All statistical tests were performed using a 2-sided test, and P < .05 was considered statistically significant.
Table 3. Multivariable Analysis of Poor Outcomes for NCCN Risk Groups and for Mohs vs WLEa.
| Variable | SHR (95% CI) | P value |
|---|---|---|
| Local recurrence (n = 188) | ||
| Low risk | 1 [Reference] | NA |
| High risk | 1.99 (1.21-3.27) | .007 |
| Very high risk | 12.66 (7.86-20.39) | <.001 |
| WLE | 1 [Reference] | NA |
| Mohs | 0.65 (0.46-0.90) | .009 |
| Nodal metastasis (n = 96) | ||
| Low risk | 1 [Reference] | NA |
| High risk | 4.26 (1.28-14.23) | .02 |
| Very high risk | 62.98 (19.24-206.17) | <.001 |
| WLE | 1 [Reference] | NA |
| Mohs | 0.83 (0.54-1.28) | .40 |
| Distant metastasis (n = 32) | ||
| Low/high risk | 1 [Reference] | NA |
| High risk | 2.2 × 107 (4.7 × 103-1.1 × 1011) | <.001 |
| Very high risk | 6.3 × 108 (1.4 × 105-2.9 × 1012) | <.001 |
| WLE | 1 [Reference] | NA |
| Mohs | 0.38 (0.18-0.83) | .02 |
| Disease-specific death (n = 114) | ||
| Low/high risk | 1 [Reference] | NA |
| High risk | 4.02 (1.18-13.71) | .03 |
| Very high risk | 93.87 (29.19-301.85) | <.001 |
| WLE | 1 [Reference] | NA |
| Mohs | 0.55 (0.36-0.84) | .006 |
Abbreviations: Mohs, Mohs micrographic surgery; NCCN, National Comprehensive Cancer Network; SHR, subhazard ratio; WLE, wide local excision.
The number of tumors included in the model and the number of tumors excluded (censored because outside of analysis time or missing event time) were 10 009 and 187, respectively, for local recurrence; 10 004 and 192, respectively, for nodal metastasis; 10 008 and 188, respectively, for distant metastasis; and 10 008 and 188, respectively, for disease-specific death. Based on univariate analysis, the covariates included in the model were risk group, surgical modality, sex, and age for local recurrence; risk group and surgical modality for distant metastasis; and risk group, surgical modality, and sex for nodal metastasis and disease-specific death.
Results
A total of 10 196 tumors from 8727 patients were stratified by NCCN guidelines into low-risk (3054 tumors [30.0%]), high-risk (6269 tumors [61.5%]), and very high-risk (873 tumors [8.6%) groups (6003 [59.0%] from men and 4193 from women [41.1%]); mean [SD] age, 72.4 [11.8] years) (Table 2). Median follow-up time was 37 (range, 18-63) months for low-risk, 35 (range, 13-62) months for high-risk, and 27 (range, 11-54) months for very high-risk tumors. In the high-risk and very high-risk groups, there was a male predominance, with 4083 (65.1%) of the high- and 633 (72.5%) of the very high-risk tumors being from male patients (P < .001). Very high-risk tumors were more likely to have high-risk-tumor and histologic features, such as large-caliber perineural invasion, large tumor diameter, invasion beyond the subcutaneous fat or bone, poor differentiation, and lymphovascular invasion. These features resulted in more very high-risk tumors being high BWH (T3, 57 [6.5%] vs 0) and AJCC-8 (T4b, 3 [0.3%] vs 0; P < .001) stages.
On multivariable stepwise competing risk regression analysis, the very high-risk and high-risk NCCN groups demonstrated significantly worse outcomes compared with low-risk NCCN group for LR (high-risk SHR, 1.99 [95% CI, 1.21-3.27; P = .007]; very high-risk SHR, 12.66 [95% CI, 7.86-20.39; P < .001]), NM (high-risk SHR, 4.26 [95% CI, 1.28-14.23; P = .02]; very high-risk SHR, 62.98 [95% CI, 19.24-206.17; P < .001]), DM (high-risk SHR, 2.2 × 107 [95% CI, 4.7 × 103-1.1 × 1011; P < .001]; very high-risk SHR, 6.3 × 108 [95% CI, 1.4 × 105-2.9 × 1012; P < .001]), and DSD (high-risk SHR, 4.02 [95% CI, 1.18-13.71; P = .03]; very high-risk SHR, 93.87 [95% CI, 29.19-301.85; P < .001]) (Table 3). For very high-risk groups compared with high- and low-risk groups, adjusted 5-year cumulative incidence was 9.4% (95% CI, 9.2%-14.0%) vs 1.5% (95% CI, 1.4%-2.1%) and 0.8% (95% CI, 0.5%-1.2%), respectively, for LR; 7.3% (95% CI, 6.8%-10.9%) vs 0.5% (95% CI, 0.4%-0.8%) and 0.1% (95% CI, 0.03%-0.3%), respectively, for NM; 3.9% (95% CI, 2.6%-5.6%) vs 0.1% (95% CI, 0.04%-0.2%) and 0.01% (95% CI, not applicable), respectively, for DM; and 10.5% (95% CI, 10.3%-15.4%) vs 0.5% (95% CI, 0.4%-0.8%) and 0.1% (95% CI, 0.04%-0.3%), respectively, for DSD (Figure and Table 4). Mohs or PDEMA had a 35% lower risk of LR (SHR, 0.65 [95% CI, 0.46-0.90]; P = .009), nearly 60% lower risk of DM (SHR, 0.38 [95% CI, 0.18-0.83]; P = .02), and a 45% lower risk of DSD (SHR, 0.55 [95% CI, 0.36-0.84]; P = .006) compared with WLE (Table 3).
Figure. Five-year Cumulative Incidence Curves for Poor Outcomes in Low-, High-, and Very High-Risk National Comprehensive Cancer Network Groups.
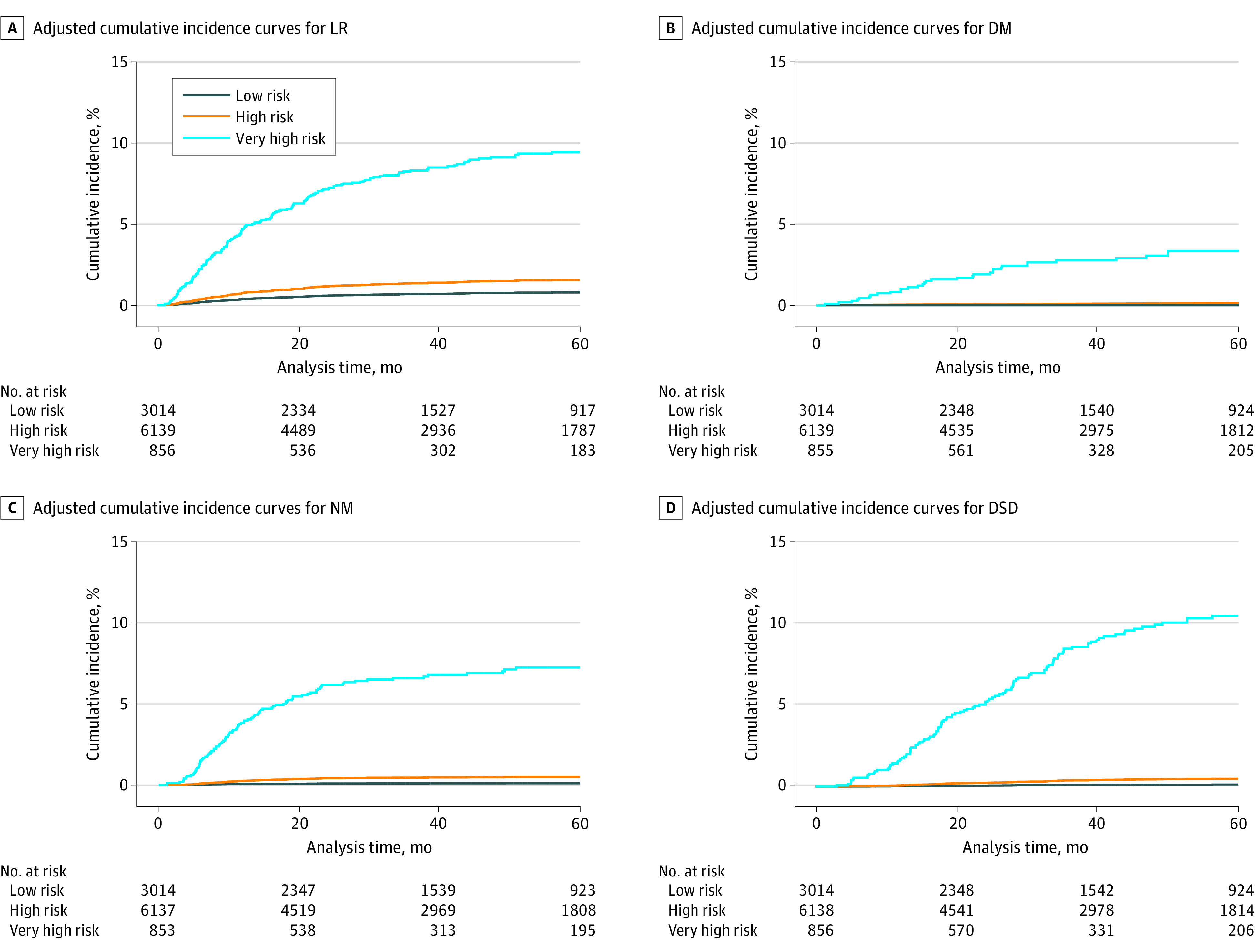
DM indicates distant metastasis; DSD, disease-specific death; LR, local recurrence; NM, nodal metastasis.
Table 4. Five-Year Cumulative Incidence for Poor Outcomes in NCCN Risk Groups.
| Outcome | NCCN risk group, 5-y cumulative incidence (95% CI), % | ||
|---|---|---|---|
| Low | High | Very high | |
| Local recurrence | 0.8 (0.5-1.2) | 1.5 (1.4-2.1) | 9.4 (9.2-14.0) |
| Nodal metastasis | 0.1 (0.03-0.3) | 0.5 (0.4-0.8) | 7.3 (6.8-10.9) |
| Distant metastasis | 0.01 (NA)a | 0.1 (0.04-0.2) | 3.9 (2.6-5.6) |
| Disease specific death | 0.1 (0.04-0.3) | 0.5 (0.4-0.8) | 10.5 (10.3-15.4) |
Abbreviations: NA, not applicable; NCCN, National Comprehensive Cancer Network.
The sample size was too small to generate a 95% CI.
Discussion
This dual-institute cohort study demonstrates that NCCN high- and very high-risk group CSCCs have a significantly increased risk of developing LR, NM, or DM and of dying from their disease. Further, regardless of NCCN risk group, Mohs or PDEMA confers lower risk of developing LR, DM, and DSD. These findings support the modifications made to the 2022 NCCN risk stratification guidelines.
Tumor staging and risk stratification are essential to prognostication and clinical decision-making. While current staging systems including BWH and AJCC-8 help to estimate CSCC outcomes, the recent NCCN risk stratification guidelines are unique in that they incorporate clinical in addition to pathological features. Clinical features incorporated in NCCN risk stratification, including patient immune status (immunosuppressed vs immunocompetent), whether the tumor arises in a site of prior radiotherapy and/or chronic inflammation, recurrent disease, and whether there are rapid growth or neurological symptoms, account for characteristics that have been shown to be important for prognosis and impact treatment choices.5,9,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 Further, the NCCN risk groups also include pathological features not incorporated into the BWH or AJCC-8 staging systems, including lymphovascular invasion, which has recently been shown to be an important variable associated with poor outcomes.39,40 This study demonstrates that the recent NCCN risk-stratification guidelines for CSCCs identify tumors at higher risk of developing poor outcomes.
The 2022 NCCN guidelines are the first to incorporate the recommendation for Mohs or PDEMA for high- and very high-risk tumors. In WLE, a standard margin around tumor is excised and processed with horizonal sectioning. Typically, less than 2% of the margins are evaluated for residual tumor by a pathologist following excision. Mohs or PDEMA examines 100% of the margins for residual tumor, which aids in identifying infiltrative growth patterns and perineural invasion. Mohs has been shown to lead to low rates of LR, NM, and DSD.41 Further, studies conducted with cohorts undergoing Mohs only compared with cohorts with patients who received Mohs or WLE demonstrate that Mohs-only cohorts have a lower risk of NM.41 In this study, Mohs or PDEMA resulted in a 35% lower risk of developing LR, nearly 60% lower risk of developing DM, and a 45% lower risk of developing DSD. This study demonstrates that Mohs or PDEMA provides benefit, specifically in terms of poor outcomes, including LR, DM, and DSD.
Limitations
This study has some limitations. While it includes tumors from 2 large tertiary referral-center cohorts, it did not include community-based CSCCs, which may behave differently. Additionally, if tumors initially evaluated at one of the tertiary referral centers were later lost to follow-up or followed up at an outside center, certain information may be missing. As this relates to DSD, DSD was determined through extensive review of medical records that typically state whether the patient died of the tumor or complications of the tumor. Most patients are followed up longitudinally in the electronic medical record at both tertiary care centers, especially those with advanced cases who typically do not return to community clinicians. Unfortunately, to our knowledge CSCC is not captured in any national registries to help identify cases that may be lost to follow-up. For all cases of DSD, the certainty of whether the tumor or complications of the tumor caused a DSD was ranked, and only tumors for which the cause of death was confirmed were used in the analysis. There are limitations inherent to a retrospective study design, including the possibility of underreporting of certain risk factors. An independent review of histologic risk factors was not performed for each tumor; therefore, if medical record review of the pathology and/or Mohs reports did not indicate the presence or absence of a risk factor, the default assumption was that the risk factor was not present, a standard of practice in the field.
Conclusions
The findings of this cohort study support the new risk stratification system in the 2022 NCCN guidelines for CSCC. This study highlights that these guidelines allow for risk stratification of CSCC tumors in a clinically meaningful way. High- and very high-risk CSCC tumors are at a much greater risk for developing poor outcomes, including LR, NM, DM, and DSD. These tumors benefit from Mohs or PDEMA, as both lead to a lower risk of LR, DM, and DSD. Additional studies are needed that further validate the NCCN guidelines, compare these guidelines with current BWH and AJCC-8 staging systems, and determine whether very high-risk tumors benefit from closer surveillance and other therapies.
eTable. Univariate Analysis
Data Sharing Statement
References
- 1.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957-966. doi: 10.1016/j.jaad.2012.11.037 [DOI] [PubMed] [Google Scholar]
- 2.Smile TD, Ruiz ES, Kus KJB, et al. Implications of satellitosis or in-transit metastasis in cutaneous squamous cell carcinoma: a prognostic omission in cancer staging systems. JAMA Dermatol. 2022;158(4):390-394. doi: 10.1001/jamadermatol.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975-983. doi: 10.1056/NEJM200103293441306 [DOI] [PubMed] [Google Scholar]
- 4.Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35(4):574-585. doi: 10.1111/j.1524-4725.2009.01095.x [DOI] [PubMed] [Google Scholar]
- 5.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. doi: 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 6.Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149(5):541-547. doi: 10.1001/jamadermatol.2013.2139 [DOI] [PubMed] [Google Scholar]
- 7.Mullen JT, Feng L, Xing Y, et al. Invasive squamous cell carcinoma of the skin: defining a high-risk group. Ann Surg Oncol. 2006;13(7):902-909. doi: 10.1245/ASO.2006.07.022 [DOI] [PubMed] [Google Scholar]
- 8.Amin MB, Edge S, Greene F, et al. , eds; American Joint Commission on Cancer . AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017. [Google Scholar]
- 9.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402-410. doi: 10.1001/jamadermatol.2013.2456 [DOI] [PubMed] [Google Scholar]
- 10.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327-334. doi: 10.1200/JCO.2012.48.5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines® insights: squamous cell skin cancer, version 1.2022. J Natl Compr Canc Netw. 2021;19(12):1382-1394. doi: 10.6004/jnccn.2021.0059 [DOI] [PubMed] [Google Scholar]
- 12.Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990. doi: 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 13.de Lima Vazquez V, Sachetto T, Perpetuo NM, Carvalho AL. Prognostic factors for lymph node metastasis from advanced squamous cell carcinoma of the skin of the trunk and extremities. World J Surg Oncol. 2008;6:73. doi: 10.1186/1477-7819-6-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eroğlu A, Berberoğlu U, Berreroğlu S. Risk factors related to locoregional recurrence in squamous cell carcinoma of the skin. J Surg Oncol. 1996;61(2):124-130. doi: [DOI] [PubMed] [Google Scholar]
- 15.Edwards MJ, Hirsch RM, Broadwater JR, Netscher DT, Ames FC. Squamous cell carcinoma arising in previously burned or irradiated skin. Arch Surg. 1989;124(1):115-117. doi: 10.1001/archsurg.1989.01410010125024 [DOI] [PubMed] [Google Scholar]
- 16.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. doi: 10.1001/jamadermatol.2015.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28(3):268-273. [DOI] [PubMed] [Google Scholar]
- 18.Wermker K, Kluwig J, Schipmann S, Klein M, Schulze HJ, Hallermann C. Prediction score for lymph node metastasis from cutaneous squamous cell carcinoma of the external ear. Eur J Surg Oncol. 2015;41(1):128-135. doi: 10.1016/j.ejso.2014.07.039 [DOI] [PubMed] [Google Scholar]
- 19.Moore BA, Weber RS, Prieto V, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115(9):1561-1567. doi: 10.1097/01.mlg.0000173202.56739.9f [DOI] [PubMed] [Google Scholar]
- 20.Palme CE, O’Brien CJ, Veness MJ, McNeil EB, Bron LP, Morgan GJ. Extent of parotid disease influences outcome in patients with metastatic cutaneous squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129(7):750-753. doi: 10.1001/archotol.129.7.750 [DOI] [PubMed] [Google Scholar]
- 21.Oddone N, Morgan GJ, Palme CE, et al. Metastatic cutaneous squamous cell carcinoma of the head and neck: the Immunosuppression, Treatment, Extranodal Spread, and Margin Status (ITEM) prognostic score to predict outcome and the need to improve survival. Cancer. 2009;115(9):1883-1891. doi: 10.1002/cncr.24208 [DOI] [PubMed] [Google Scholar]
- 22.Wang JT, Palme CE, Wang AY, Morgan GJ, Gebski V, Veness MJ. In patients with metastatic cutaneous head and neck squamous cell carcinoma to cervical lymph nodes, the extent of neck dissection does not influence outcome. J Laryngol Otol. 2013;127(suppl 1):S2-S7. doi: 10.1017/S0022215112002101 [DOI] [PubMed] [Google Scholar]
- 23.McDowell LJ, Tan T-J, Bressel M, et al. Outcomes of cutaneous squamous cell carcinoma of the head and neck with parotid metastases. J Med Imaging Radiat Oncol. 2016;60(5):668-676. doi: 10.1111/1754-9485.12484 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt C, Martin JM, Khoo E, Plank A, Grigg R. Outcomes of nodal metastatic cutaneous squamous cell carcinoma of the head and neck treated in a regional center. Head Neck. 2015;37(12):1808-1815. doi: 10.1002/hed.23843 [DOI] [PubMed] [Google Scholar]
- 25.Southwell KE, Chaplin JM, Eisenberg RL, McIvor NP, Morton RP. Effect of immunocompromise on metastatic cutaneous squamous cell carcinoma in the parotid and neck. Head Neck. 2006;28(3):244-248. doi: 10.1002/hed.20321 [DOI] [PubMed] [Google Scholar]
- 26.Ch’ng S, Maitra A, Allison RS, et al. Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J Surg Oncol. 2008;98(2):101-105. doi: 10.1002/jso.21092 [DOI] [PubMed] [Google Scholar]
- 27.Shao A, Wong DKC, McIvor NP, et al. Parotid metastatic disease from cutaneous squamous cell carcinoma: prognostic role of facial nerve sacrifice, lateral temporal bone resection, immune status and P-stage. Head Neck. 2014;36(4):545-550. doi: 10.1002/hed.23323 [DOI] [PubMed] [Google Scholar]
- 28.Manyam BV, Gastman B, Zhang AY, et al. Inferior outcomes in immunosuppressed patients with high-risk cutaneous squamous cell carcinoma of the head and neck treated with surgery and radiation therapy. J Am Acad Dermatol. 2015;73(2):221-227. doi: 10.1016/j.jaad.2015.04.037 [DOI] [PubMed] [Google Scholar]
- 29.Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation. 2010;90(6):683-687. doi: 10.1097/TP.0b013e3181ec7228 [DOI] [PubMed] [Google Scholar]
- 30.Manyam BV, Garsa AA, Chin RI, et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer. 2017;123(11):2054-2060. doi: 10.1002/cncr.30601 [DOI] [PubMed] [Google Scholar]
- 31.Martin H, Strong E, Spiro RH. Radiation-induced skin cancer of the head and neck. Cancer. 1970;25(1):61-71. doi: [DOI] [PubMed] [Google Scholar]
- 32.Gül U, Kiliç A. Squamous cell carcinoma developing on burn scar. Ann Plast Surg. 2006;56(4):406-408. doi: 10.1097/01.sap.0000200734.74303.d5 [DOI] [PubMed] [Google Scholar]
- 33.Kowal-Vern A, Criswell BK. Burn scar neoplasms: a literature review and statistical analysis. Burns. 2005;31(4):403-413. doi: 10.1016/j.burns.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 34.Kyrgidis A, Tzellos TG, Kechagias N, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46(9):1563-1572. doi: 10.1016/j.ejca.2010.02.046 [DOI] [PubMed] [Google Scholar]
- 35.Han A, Ratner D. What is the role of adjuvant radiotherapy in the treatment of cutaneous squamous cell carcinoma with perineural invasion? Cancer. 2007;109(6):1053-1059. doi: 10.1002/cncr.22509 [DOI] [PubMed] [Google Scholar]
- 36.Galloway TJ, Morris CG, Mancuso AA, Amdur RJ, Mendenhall WM. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103(6):1254-1257. doi: 10.1002/cncr.20913 [DOI] [PubMed] [Google Scholar]
- 37.Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2010;3(4):39-48. [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Chin RI, Gastman B, et al. Association of disease recurrence with survival outcomes in patients with cutaneous squamous cell carcinoma of the head and neck treated with multimodality therapy. JAMA Dermatol. 2019;155(4):442-447. doi: 10.1001/jamadermatol.2018.5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levoska MA, Murad F, Schmults CD, Ruiz ES. A matched-pair study of cutaneous squamous cell carcinomas with and without lymphovascular invasion. J Am Acad Dermatol. 2020;82(4):1001-1003. doi: 10.1016/j.jaad.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 40.Kus KJB, Murad F, Smile TD, et al. Higher metastasis and death rates in cutaneous squamous cell carcinomas with lymphovascular invasion. J Am Acad Dermatol. 2022;86(4):766-773. doi: 10.1016/j.jaad.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 41.Marrazzo G, Zitelli JA, Brodland D. Clinical outcomes in high-risk squamous cell carcinoma patients treated with Mohs micrographic surgery alone. J Am Acad Dermatol. 2019;80(3):633-638. doi: 10.1016/j.jaad.2018.09.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Univariate Analysis
Data Sharing Statement


