Abstract
Background
Adding radiotherapy (RT) to systemic therapy improves progression-free survival (PFS) and overall survival (OS) in oligometastatic non-small cell lung cancer (NSCLC). Whether these findings translate to epidermal growth factor receptor (EGFR)–mutated NSCLC remains unknown. The SINDAS trial (NCT02893332) evaluated first-line tyrosine kinase inhibitor (TKI) therapy for EGFR-mutated synchronous oligometastatic NSCLC and randomized to upfront RT vs no RT; we now report the prespecified interim analysis at 68% accrual.
Methods
Inclusion criteria were biopsy-proven EGFR-mutated adenocarcinoma (per amplification refractory mutation system or next generation sequencing), with synchronous (newly diagnosed, treatment naïve) oligometastatic (≤5 metastases; ≤2 lesions in any one organ) NSCLC without brain metastases. All patients received a first-generation TKI (gefitinib, erlotinib, or icotinib), and randomization was between no RT vs RT (25-40 Gy in 5 fractions depending on tumor size and location) to all metastases and the primary tumor/involved regional lymphatics. The primary endpoint (intention to treat) was PFS. Secondary endpoints included OS and toxicities. All statistical tests were 2-sided.
Results
A total of 133 patients (n = 65 TKI only, n = 68 TKI with RT) were enrolled (2016-2019). The median follow-up was 23.6 months. The respective median PFS was 12.5 months vs 20.2 months (P < .001), and the median OS was 17.4 months vs 25.5 months (P < .001) for TKI only vs TKI with RT. Treatment yielded no grade 5 events and a 6% rate of symptomatic grade 3-4 pneumonitis in the TKI with RT arm. Based on the efficacy results of this prespecified interim analysis, the ethics committee recommended premature cessation of this trial.
Conclusions
As compared with a first-line TKI alone, addition of upfront local therapy using RT statistically significantly improved PFS and OS for EGFR-mutated NSCLC.
The management of metastatic non-small cell lung cancer (NSCLC) with a limited number of metastatic deposits (oligometastases) continues to rapidly evolve. Whereas this condition was once thought to be incurable, contemporary trials of aggressive local therapy (most commonly with radiation therapy [RT]) to all existing areas of disease have called this dogma into question. Two randomized trials in NSCLC (1,2) as well as a randomized basket trial (3), have illustrated improvements in progression-free survival (PFS) and overall survival (OS) with this approach.
A salient limitation of the aforementioned randomized trials has been the stark underrepresentation of NSCLC cases harboring mutations in the epidermal growth factor receptor (EGFRm). EGFRm NSCLC represents a unique population with considerably different demographics, biological characteristics, treatment approaches, and prognoses as compared with the general NSCLC population. Thus, the extrapolation of general NSCLC data to EGFRm NSCLC is not reliable. For instance, unlike all other metastatic NSCLC cases, the standard of care for metastatic EGFRm NSCLC is tyrosine kinase inhibitor (TKI) therapy (4,5). However, it is known that, similar to EGFR-unmutated metastatic NSCLC, EGFRm metastatic NSCLC tends to recur at the sites of original disease (6,7).
Despite the success of RT for oligometastatic NSCLC, it remains unclear whether RT benefits patients with oligometastatic EGFRm NSCLC. To address this knowledge gap, we conducted the phase III SINDAS trial to examine outcomes of TKI therapy with or without upfront RT to all areas of disease for EGFRm synchronous oligometastatic (≤5 metastases) NSCLC.
Methods
Ethical Approval
This open-label, parallel-group, phase III clinical trial (NCT02893332) was approved by the institutional review boards of each of the 5 participating centers, with all patients having provided written informed consent before enrollment. This trial was conducted according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice. Complete information regarding this trial can be found in the study protocol (see the Supplementary Materials, available online).
Patients
Inclusion criteria for this randomized trial were patients aged 18 years and older and 75 years or younger with a Zubrod performance status of 0-2, an estimated life expectancy of at least 6 months, and the ability to sign informed consent. All patients were required to have biopsy-proven EGFRm adenocarcinoma (defined as any deletion in exon 19 or any mutation in exon 21, by means of either an amplification refractory mutation system or next generation sequencing) as well as synchronous (newly diagnosed, treatment-naïve) oligometastatic disease. Oligometastatic disease was defined as 5 or less discrete distant metastases with no more than 2 discrete areas of metastatic disease in any one organ (as confirmed by multidisciplinary review). The involved regional lymph nodes (regardless of nodal number) were not counted in the definition of metastatic disease and were grouped with the primary tumor. Involved nonregional lymph nodes were categorized as metastatic disease.
Further exclusion criteria were the presence of brain metastases as detected on contrast-enhanced magnetic resonance imaging (MRI), prior irradiation to the thorax or metastatic sites (or other contraindications to receiving RT, such as tumor within 5 mm of the spinal cord), history of previous malignancies, prior receipt of any test drugs or investigational compounds within 4 weeks, inadequate bone marrow or hepatorenal function, severe or uncontrolled cardiovascular comorbidities, any contraindications to receiving TKI therapy, mental illness or psychotropic substance abuse, and pregnant or breastfeeding women.
Patients were screened for the criteria mentioned above, and initial evaluation began with a complete history and physical examination performed by a multidisciplinary team. Routine bloodwork was performed, along with systemic imaging for purposes of tumor staging (performed with the American Joint Committee on Cancer cancer staging manual, 7th edition). Imaging included brain MRI in all patients; additionally, whole-body positron emission tomography (PET) was encouraged, but contrast-enhanced computed tomography (CT) with a 99Tc bone scan was acceptable as well.
Randomization and Masking
Patients were randomly assigned (1:1) to receive either TKI therapy alone or upfront RT prior to TKI, using the methods of Pocock and Simon (8) to dynamically balance for 7 prognostic covariates (age 63 years or younger vs older than 63 years, male vs female sex, Zubrod performance status of 0 vs 1-2, number of metastases of 1-2 vs 3-5, T stage 1-2 vs 3-4, N stage 1 vs 2-3, and EGFR exon 19 vs exon 21 mutation). Owing to the nature of the clinical question and study design, the randomization was not masked.
Study Procedures
All patients received a first-generation TKI (gefitinib 250 mg once daily, erlotinib 150 mg once daily, or icotinib 125 mg thrice daily) based on the discretion of the treating oncologist. TKI dose adjustment or interruption was allowed after grade 3-4 adverse events and was performed individually per the treating oncologist. TKI interruption was allowed for a maximum of 3 weeks; if greater, the patient was placed off study (but still analyzed for the primary and secondary endpoints). Of note, osimertinib was not allowed on this study, as randomized data supporting osimertinib were published toward the end of study accrual (9).
RT was directed to all metastases plus the primary tumor/involved regional nodes on imaging; it was performed in 5 fractions using well-recognized principles, such as 3-dimensional CT simulation, custom immobilization techniques, and daily image guidance. Because the total prescribed dose is highly dependent on tumor location and/or size, we allowed for a dose of 25-40 Gy (10-12), generally using the maximum dose that did not exceed 5-fraction dose tolerances to adjacent organs at risk. The primary tumor was also treated in 5 fractions using constraints of a mean dose less than 16.5 Gy for the trachea and large bronchus and a V30 less than 5 cc for the heart, ribs, and esophagus. The prescription dose was to cover at least 95% of the planning target volume (PTV); the protocol allowed for 120% of the prescription dose to be received by not more than 2 cc only within the PTV, as well as 110% or more of the prescription dose to at most 1 cc within the PTV. RT was delivered to all areas concurrently, and all RT was to be completed within 2 weeks.
In both arms, standard-of-care chemotherapy (specific agents as per oncologist judgment) was recommended upon disease progression; neither second- or third-generation TKIs nor immune checkpoint inhibitors were allowed because chemotherapy was the most recognized second-line option for these patients when the trial was initially designed. No radiotherapy was allowed in the TKI-only arm unless symptomatology dictated a need for palliative radiation.
Patients were evaluated for toxicities (per the Common Terminology Criteria for Adverse Events, v4.0) regularly during therapy. Posttreatment follow-up with a history and physical examination along with systemic imaging (contrast-enhanced CT, PET, and/or MRI) was performed every 6 weeks for the first year, every 3 months for the following 2 years, and every 6 months thereafter.
Endpoints
The primary endpoint was PFS, defined from the time of randomization to the time of disease progression (defined by response evaluation criteria in solid tumor criteria) or death; secondary endpoints were OS (from randomization to death from any cause) and safety. It was hypothesized that the addition of RT would increase the 6-month PFS from 75% with TKI only (5) to 90%. As a result, the trial would require 200 patients to achieve a power of 80% with a 2-sided α of .05. The protocol prespecified an interim analysis when 68% of accrual was reached, to either continue enrollment or prematurely close if any statistically significant difference in outcomes was detected (so as to avoid continuation of a therapy paradigm with known inferior outcomes).
Statistical Analyses
All statistical analyses were conducted in an intention-to-treat manner, used a statistical significance level of .05, and were performed with SPSS software (v.22.0, Chicago, IL, USA). PFS and OS were calculated by Kaplan-Meier methodology and compared using the log-rank test, although the protocol-specified analysis was a comparison of the 6-month PFS rates using the χ2 or Fisher exact test. A post hoc multivariable Cox regression analysis was conducted to adjust for statistically significant covariates, using backward stepwise selection and selecting all variables with a P value less than .05 on univariable analysis. Efron approximation was used for tie handling. The proportional hazards assumption was checked with log-log plots, and any missing data were censored. Interaction terms were analyzed using the Spearman relative analysis system, and the model adequacy was assessed using goodness-of-fit evaluation.
Results
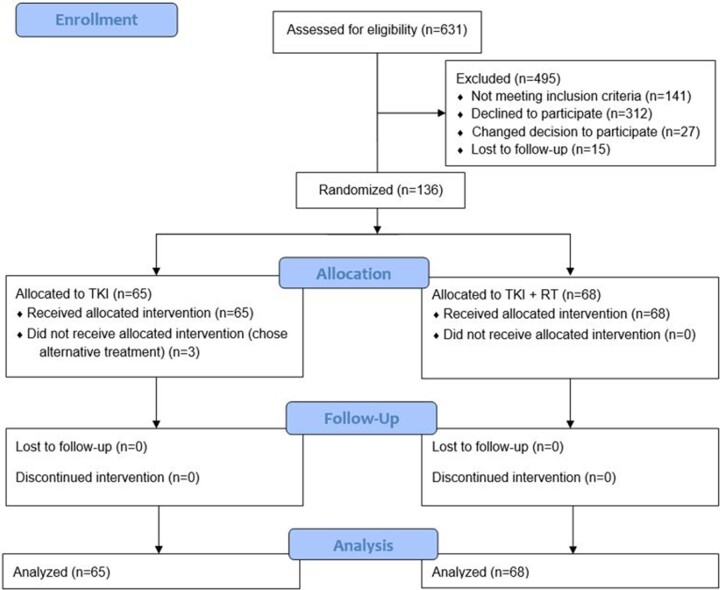
From January 15, 2016, to June 28, 2019, a total of 631 patients from 5 centers were screened for enrollment, of whom 136 participants met criteria and were randomly assigned; 3 patients chose to receive alternate therapies, leaving 133 patients (n = 65 TKI only, n = 68 TKI with RT; Figure 1). Based on the positive efficacy results of this prespecified interim analysis, the ethics committee did not recommend further recruitment of patients onto this trial. The cutoff for patient follow-up was 1 year from the assessment of efficacy results. The median follow-up was 23.6 months (interquartile range = 9.4-41.0 months).
Figure 1.
CONSORT diagram for the trial. RT = radiation therapy; TKI = tyrosine kinase inhibitor.
Baseline characteristics of the study population is presented in Table 1. Of note, the distribution of oligometastatic lesions was comparable in both arms; the majority of all lesions were bone metastases (117 of 160 [73.1%] for TKI only; 115 of 167 [68.9%] for TKI with RT). Other areas included the abdomen (30 of 160 [18.8%] for TKI only; 30 of 167 [18.0%] for TKI with RT) and contralateral lung (11 of 160 [6.9%] for TKI only; 16 of 167 [9.6%] for TKI with RT). The most common EGFR abnormality was in exon 19 (92 of 133 [69.2%]). Gefitinib (70 of 133 [52.6%]) and erlotinib (53 of 133 [39.8%]) were used more often than icotinib. The most common RT doses were 30 Gy (121 of 226 fields [53.5%]) and 25 Gy (70 of 226 fields [31.0%]).
Table 1.
Clinicopathologic characteristics of the study populationa
| Parameter | TKI only (n = 65) |
TKI + RT (n = 68) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 63 (11) | 67 (10) |
| Sex, No. (%) | ||
| Male | 26 (40.0) | 25 (36.8) |
| Female | 39 (60.0) | 43 (63.2) |
| Zubrod performance status, No. (%) | ||
| 0 | 31 (47.7) | 36 (52.9) |
| 1 | 33 (50.8) | 32 (47.1) |
| 2 | 1 (1.5) | 0 (0.0) |
| Clinical T classification, No. (%) | ||
| 1 | 9 (13.8) | 5 (7.4) |
| 2 | 16 (24.6) | 17 (25.0) |
| 3 | 22 (33.8) | 20 (29.4) |
| 4 | 17 (26.2) | 23 (33.8) |
| Unknown | 1 (1.5) | 3 (4.4) |
| Clinical N classification, No. (%) | ||
| 0 | 8 (12.3) | 8 (11.8) |
| 1 | 23 (35.4) | 19 (27.9) |
| 2 | 24 (36.9) | 27 (39.7) |
| 3 | 10 (15.4) | 13 (19.1) |
| Unknown | 0 (0.0) | 1 (1.5) |
| EGFR mutation, No. (%) | ||
| Exon 19 | 47 (72.3) | 45 (66.2) |
| Exon 21 | 18 (28.7) | 23 (33.8) |
| Number of metastases, No. (%) | ||
| 1-2 | 38 (58.5) | 32 (47.1) |
| 3-4 | 23 (35.4) | 30 (44.1) |
| 5 | 4 (6.2) | 6 (8.8) |
| TKI, No. (%) | ||
| Gefitinib | 38 (58.5) | 32 (47.1) |
| Erlotinib | 23 (35.4) | 30 (44.1) |
| Icotinib | 4 (6.2) | 6 (8.8) |
EGFR = epidermal growth factor receptor; RT = radiation therapy; TKI = tyrosine kinase inhibitor.
At the time of last follow-up, local control of both the primary tumor and metastases was maintained in 36 of 65 (55.4%) patients in the TKI-only arm, compared with 62 of 68 (91.2%) patients in the TKI with RT arm (P < .001). Figures 2 and 3 illustrate the analysis for PFS and OS. The median PFS in the TKI-only and TKI with RT arms was 12.5 months (95% confidence interval [CI] = 11.6 to 13.4 months) vs 20.2 months (95% CI = 17.9-22.5 months; P < .001, hazard ratio [HR] = 0.22, 95% CI 0.17 to 0.46). The respective median OS times were 17.6 months (95% CI = 15.4 to 19.8 months) vs 25.5 months (95% CI = 23.2 to 27.8 months; P < .001, HR = 0.44, 95% CI = 0.28 to 0.68). The respective 6-month PFS was 95.2% (95% CI = 93.6% to 96.9%) vs 99.1% (95% CI = 98.7% to 99.5%; P > .05). At the time of analysis, 93 of the 133 patients had died (n = 47 TKI only, n = 46 TKI with RT); in the TKI-only arm, 38 patients had received subsequent palliative radiotherapy for symptomatology, with 6 patients having undergone radiofrequency ablation and 1 each of surgery and hyperthermia.
Figure 2.
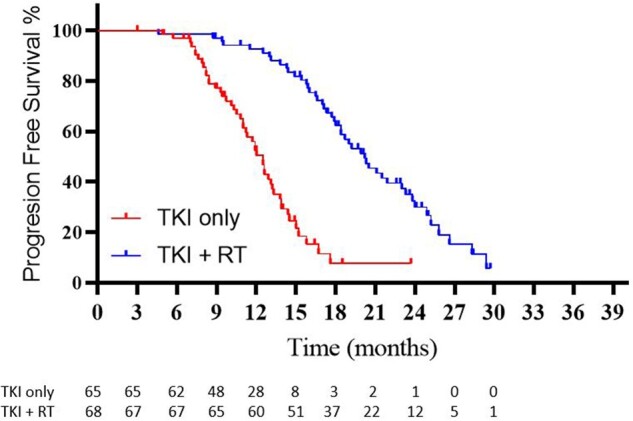
Kaplan-Meier curves illustrating progression-free survival between arms. RT = radiation therapy; TKI = tyrosine kinase inhibitor.
Figure 3.
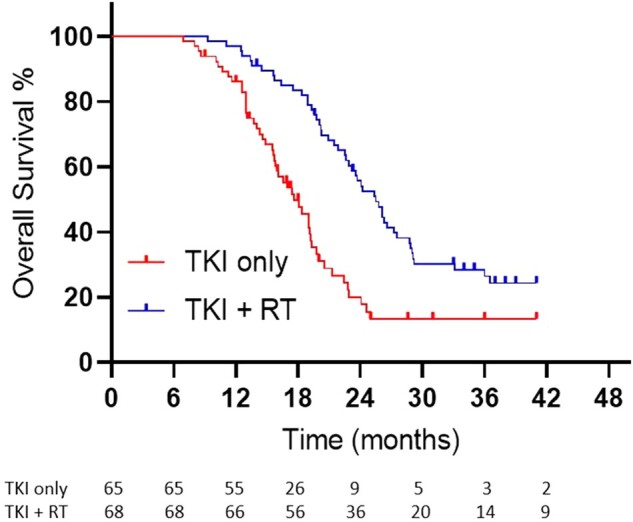
Kaplan-Meier curves illustrating overall survival between arms. RT = radiation therapy; TKI = tyrosine kinase inhibitor.
Results of the post hoc multivariable analyses are shown in Table 2 (univariable analyses in Supplementary Table 1, available online). Independent predictors of PFS included Zubrod performance status (P = .02) and the number of metastases (P = .004), along with RT (P = .005). Independent predictors of OS were Zubrod performance status (P = .02), T stage (P = .02), number of metastases (P = .004), mutation type (P = .001), and RT (P = .004).
Table 2.
Multivariable analyses of progression-free and overall survival
| Variablea | Progression-free survival |
Overall survival |
||
|---|---|---|---|---|
| HR (95% CI) | P b | HR (95% CI) | P b | |
| Zubrod performance status (0 vs 1-2) | 0.50 (0.22 to 0.75) | .02 | 0.01 (0.01 to 0.44) | .02 |
| Clinical T classification (T3-4 vs.T1-2) | 1.10 (0.99 to 1.22) | .09 | 2.06 (1.08 to 5.54) | .02 |
| Clinical N classification (N2-3 vs N0-1) | — | — | 1.56 (1.19 to 3.69) | .06 |
| Number of metastases (3-5 vs 1-2) | 1.96 (1.30 to 4.70) | .004 | 1.93 (1.21 to 3.07) | .004 |
| EGFR mutation (exon 19 deletion vs exon 21 mutation) | 0.94 (0.61 to 1.43) | .09 | 0.09 (0.02 to 0.38) | .001 |
| Randomization arm (TKI only vs TKI + RT) | 1.39 (1.07 to 1.95) | .005 | 2.11 (1.31 to 5.97) | .004 |
Only variables included in the final multivariable model are displayed. The notation in parentheses refers to comparator group vs reference group. — = N/A; CI = confidence interval; EGFR = epidermal growth factor receptor; HR = hazard ratio; RT = radiation therapy; TKI = tyrosine kinase inhibitor.
All tests were 2-sided.
Altogether, treatment was tolerated well (Table 3). The most frequent TKI-related adverse events were skin reactions in both treatment arms. Dose reductions were required in 3 of 65 (4.6%) patients in the TKI-only arm and 5 of 68 (7.4%) in the TKI with RT arm; discontinuations occurred in 1 of 65 (1.5%) and 2 of 68 (2.9%), respectively. No patient experienced grade 5 events. In the TKI-only arm, 10 of 65 (15.4%) patients developed grade 3-4 skin toxicity, and 8 of 65 (12.3%) had grade 3-4 pruritus. In the TKI with RT arm, 10 of 68 (14.7%) patients developed a grade 3-4 skin rash and 5 of 68 (7.4%) experienced grade 3-4 pneumonitis. One (1.5%) patient developed a rib fracture likely related to chest radiotherapy. Two (2.9%) participants required long-term pain management after RT; 1 was attributed to nephrolithiasis and the other to herpes zoster, both of which were unlikely related to RT.
Table 3.
Toxicities possibly, probably, or definitely related to protocol treatmenta
| Toxicity | TKI only, No. (%) |
TKI + RT, No. (%) |
||||
|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3 | Grade 4 | Grade 1/2 | Grade 3 | Grade 4 | |
| Skin rash | 47 (72.3) | 5 (7.7) | 4 (6.2) | 46 (67.6) | 8 (11.8) | 2 (2.9) |
| Pruritus | 20 (30.8) | 7 (10.8) | 1 (1.5) | 22 (32.4) | 5 (7.4) | 0 (0.0) |
| Fatigue | 47 (72.3) | 0 (0.0) | 1 (1.5) | 46 (67.6) | 0 (0.0) | 0 (0.0) |
| Neutropenia | 20 (30.8) | 0 (0.0) | 0 (0.0) | 16 (23.5) | 0 (0.0) | 0 (0.0) |
| Anemia | 18 (27.7) | 0 (0.0) | 0 (0.0) | 22 (32.4) | 0 (0.0) | 0 (0.0) |
| Thrombocytopenia | 3 (4.6) | 0 (0.0) | 0 (0.0) | 3 (4.4) | 0 (0.0) | 0 (0.0) |
| Transaminitis | 11 (16.9) | 0 (0.0) | 1 (1.5) | 8 (11.8) | 0 (0.0) | 1 (1.5) |
| Diarrhea/nausea | 43 (66.2) | 0 (0.0) | 0 (0.0) | 38 (55.9) | 0 (0.0) | 0 (0.0) |
| Esophagitis | 18 (27.7) | 2 (3.1) | 0 (0.0) | 24 (35.3) | 2 (2.9) | 1 (1.5) |
| Pericarditis | 0 (0.0) | 1 (1.5) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pleural effusion | 0 (0.0) | 0 (0.0) | 2 (3.1) | 2 (2.9) | 0 (0.0) | 0 (0.0) |
| Pneumonitis (symptomatic) | 1 (1.5) | 1 (1.5) | 0 (0.0) | 3 (4.4) | 3 (4.4) | 1 (1.5) |
| Pneumonitis (asymptomatic) | 14 (21.5) | 1 (1.5) | 0 (0.0) | 19 (27.9) | 1 (1.5) | 0 (0.0) |
RT = radiation therapy; TKI = tyrosine kinase inhibitor.
Discussion
Despite the noted success of RT for oligometastatic NSCLC in multiple randomized trials, it has been heretofore unknown whether these results apply to EGFRm NSCLC, owing to the clear underrepresentation of these patients in existing randomized trials. The randomized SINDAS trial aimed to address this knowledge gap and revealed that local therapy using RT is safe and improved PFS and OS in the EGFRm oligometastatic population.
The results of this trial corroborate the utility of local therapy for limited metastatic NSCLC in a population with markedly distinct tumor biology, treatment approaches, and prognosis. TKIs generally display high efficacy for EGFRm NSCLC, and it is therefore noteworthy that additional local therapy still proved to be efficacious. This implies that even targeted therapy may not be able to substitute for dedicated local therapy for limited metastatic EGFRm disease. It is also relatively intuitive that the addition of RT resulted in similar toxicity profiles as TKI alone, although this trial did not aim to evaluate concurrent TKI-RT.
This trial implemented upfront RT for treatment-naïve disease, which is somewhat different from 2 randomized trials of RT for oligometastatic NSCLC1-2, which used first-line chemotherapy (to perform patient selection based on tumor biology) and delivered local therapy if there was no progression. This paradigm is a useful approach and is also reflected in the designs of the ongoing NRG LU002 and SARON trials. In the EGFRm population, however, there could be a relatively lower necessity to administer upfront systemic therapy, in part because patient selection by tumor biology (ie, EGFR status) had already been performed before randomization. However, it is acknowledged that delivering upfront systemic therapy may better allow for reducing the number of irradiated sites because lesions with a clinical complete response may not require RT (1).
This trial did not implement high-dose ablative RT in the majority of patients for several reasons. First, there is no robust evidence in the literature to date (especially when this trial was designed) that higher doses cause more robust tumor control in the oligometastatic setting. Second, EGFR-mutant cases represent unique biology for which targeted therapy is much more locally effective than chemotherapy for nonmutated cases. Third, a wide variety of nonablative (including palliative) doses has been used in prior trials of local therapy for oligometastatic NSCLC (1). Although local control in the RT arm of this study was quite satisfactory, this study was not designed to evaluate whether higher RT doses would impact PFS as compared with the doses used herein.
The primary endpoint of this trial was 6-month PFS, but there were too few progression or death events at this short-term time point [which has been observed elsewhere for favorable oligometastatic subsets of EGFR-mutant NSCLC (13)]. However, we strongly believe that this issue should not be a deterrent to interpretation of this trial given the relatively large PFS and OS differences. Moreover, similar situations as the aforementioned are not uncommon in the literature. For instance, the randomized AMAROS trial in breast cancer overestimated the number of events for the primary comparison; because of a low event rate, the data monitoring committee of that trial allowed conducting the final analysis even though the primary comparison was underpowered (14). This issue did not hinder interpretation and widespread acceptance of that trial’s results.
This trial was conceived and constructed and commenced enrollment years before the FLAURA trial illustrated improvements in PFS and OS with osimertinib as compared with gefitinib or erlotinib in treatment-naïve NSCLC with deletions in EGFR exon 19 or mutations in exon 21 (9). The decision to continue as per protocol in light of this finding was important, because amending the protocol and allowing enrollment of osimertinib could have resulted in the introduction of a major confounding factor for the outcomes of the remainder of the trial. The trial could not have been repowered for an osimertinib subgroup, and performing post hoc power calculations for this purpose would not be statistically indicated or accurate. Nevertheless, it remains unclear whether RT would show additional outcome benefits when added to first-line osimertinib; this question is being addressed by the randomized phase II NORTHSTAR trial (NCT03410043). Although it is possible that the improved efficacy of osimertinib may dampen the potential benefits of additional RT, it is also possible that the increased systemic control afforded by osimertinib may increase the importance of preventing local progression. Additionally, the toxicity results herein cannot be extrapolated to the setting of combined lung RT and osimertinib, which could result in markedly higher rates of pneumonitis as compared with those reported herein (15).
Although brain metastases are more common in EGFRm NSCLC (16), there remains a knowledge gap regarding the role of local therapy for oligometastatic EGFRm NSCLC with brain metastases. Although these patients were excluded from our trial, retrospective data suggest that performing brain radiation to address limited metastases in the brain may offer an additional benefit over TKI therapy alone (13,17). This is an especially important notion because newer generations of TKIs such as osimertinib have improved central nervous system penetration. Therefore, future trials such as NORTHSTAR (which allows for limited brain metastases) will be essential to extend the data presented herein to patients with limited brain disease at presentation.
Despite the randomized nature of this study, several shortcomings merit discussion. First, this study was not designed to evaluate upfront RT vs RT at the time of oligoprogression, nor was it powered for a stratified analysis based on the particular type of EGFR mutation. Second, radiotherapy in this trial was not standardized and allowed for a wide variety of radiotherapy doses, PTV margins, and planning techniques based on the discretion of the treating radiation oncologist. This was necessary because the location and/or size of the target lesion(s) dictates these parameters, and a one-size-fits-all approach is not recommended. Third, there was also no standardization of the type of required imaging modality during follow-up, which was also left to the discretion of the managing physician; this could have generated false-negative findings and affected the PFS figures herein. However, it is well recognized that a variety of imaging modalities are acceptable for surveillance of these patients (eg, CT, PET or CT, MRI, bone scans, or a combination thereof). Last, there was also suboptimal management of subsequent-line therapy after progression, including the lack of second- or third-generation TKIs, the lack of salvage RT (only palliative RT allowed), and the particular chemotherapy regimen decided in an individualized manner. These known limitations could potentially influence OS but would not impact the PFS findings herein. In spite of the lack of many protocol-specified options for salvage management, the presence of a “tail” on the OS curve implies that a proportion of patients still experience long-term survival despite the lack of the same tail on the PFS curve. This finding could have implications on the utility of aggressive management for oligorecurrent disease in the EGFR-mutant population, a concept for which randomized assessments are welcomed.
In summary, the phase III SINDAS trial demonstrated that the addition of upfront local therapy with RT was well tolerated and improved PFS and OS as compared with first-line TKI therapy alone for synchronous EGFRm oligometastatic NSCLC. Therefore, the known benefit of aggressive local therapy to all existing metastases (plus the primary tumor) in oligometastatic NSCLC may be applied to the EGFRm population as well. Further randomized data are required to confirm these results, especially in the setting of first-line osimertinib as well as cases with brain metastases.
Funding
This study was supported in part by the National Science and Technology Foundation (No. 3035031263), Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital (No. 30305031017P), the Clinical Research and Transformation Fund of Sichuan Provincial People’s Hospital (2021LY25), the Sichuan Science and Technology Office (No. 3050410336), and the Chengdu Science and Technology Innovation Research and Development Project (No. 2021-YF05-02143-SN).
Notes
Role of the funders: The funders had no role in the study design, data collection, analysis, and interpretation of data; in the writing of the report; or the decision to submit for publication.
Disclosures: All authors declare that conflicts of interest do not exist.
Author contributions: Conceptualization (XW, YB, MZ), data curation (XW, YB, MZ), formal analysis (XW, YB, VV, YG, MZ), funding acquisition (MZ), investigation (XW, YB, RY, WT, RA, YD, XZ, HL, HP, LY, HB, XL, MZ, YS, ZZ, SL, XC, BT, LH, YL, KZ, FZ, LJ, XH, YW, GF, KX, YL, MZ), methodology (XW, YB, YG, MZ), project administration (RY, WT, RA, YD, XZ, HL, HP, LY, HB, XL, MZ, YS, ZZ, SL, XC, BT, LH, YL, KZ, FZ, LJ, XH, YW, GF, KX, YL, MZ), resources (MZ), software (MZ), supervision (RY, WT, RA, YD, XZ, HL, HP, LY, HB, XL, MZ, YS, ZZ, SL, XC, BT, LH, YL, KZ, FZ, LJ, XH, YW, GF, KX, YL, MZ), validation (all authors), writing—original draft (VV, MZ), writing—review & editing (all authors).
Acknowledgements: The Journal notes Dr. Bang-Xian Tan, MD, could not be reached to verify authorship or their author contributions for this article prior to publication. The corresponding author and all coauthors have confirmed Dr. Tan's authorship and author contributions on their behalf. We thank the patients and their families, clinical researchers, and their teams and hospitals that participated in this study. We thank Deying Kang for assistance with the statistical analyses; Yan Feng, Juan Xu, Weiwei Zhu, Jinyan Xiong, Mei Zhang, and Shengming Xiang for their research support on the trial; and Feng Zhao for publication support. We would also like to acknowledge the support of the generous research grants as mentioned above. MZ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior presentations: This work was presented in part at the American Society of Clinical Oncology 2020 Annual Meeting in May 2020 as well as the American Society for Radiation Oncology 2020 Annual Meeting in October 2020.
Supplementary Material
Contributor Information
Xiao-Shan Wang, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Yi-Feng Bai, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Vivek Verma, Department of Radiation Oncology, University of Nebraska Medical Center, Omaha, NE, USA.
Rui-Lian Yu, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Wei Tian, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Rui Ao, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Ying Deng, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Xue-Qiang Zhu, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Hao Liu, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Hai-Xia Pan, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Lan Yang, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Han-Song Bai, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
Xing Luo, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
Yan Guo, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
Ming-Xiu Zhou, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
Yue-Mei Sun, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan Province, China.
Zi-Can Zhang, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan Province, China.
Si-Min Li, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China; Southwest Medical University, Luzhou City, Sichuan Province, China.
Xue Cheng, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
Bang-Xian Tan, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
Liang-Fu Han, Boao Evergrande International Hospital, Qionghai, Hainan Province, China.
Ying-Yi Liu, Cancer Center, Sichuan Friendship Hospital, Chengdu, Sichuan Province, China.
Kai Zhang, Cancer Center, Ziyang People’s Hospital, Ziyang, Sichuan Province, China.
Fan-Xin Zeng, Dazhou Central Hospital, Dazhou, Sichuan Province, China.
Lin Jia, Third Hospital of Mianyang (Sichuan Mental Health Center), Mianyang, Sichuan Province, China.
Xin-Bao Hao, Cancer Center, First Affiliated Hospital of Hainan Medical College, National Drug Clinical Trial Institute, Haikou, Hainan Province, China.
You-Yu Wang, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Gang Feng, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Ke Xie, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
You Lu, Cancer Center, West China Hospital, Chengdu, Sichuan Province, China.
Ming Zeng, Cancer Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Data Availability
Data collected for this study, including deidentified individual participant data and a data dictionary defining each field in the set can be made available to others on acceptance of an official request. The study protocol and other related documents can also be made available to others on request. The corresponding time frame shall be with publication and can be shared as an electronic or physical file after explicit approval of the study investigators and a signed data usage agreement between the participating institutions.
References
- 1. Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy Vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 6. Al-Halabi H, Sayegh K, Digamurthy SR, et al. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10(11):1601–1607. [DOI] [PubMed] [Google Scholar]
- 7. Patel SH, Rimner A, Foster A, et al. Patterns of initial and intracranial failure in metastatic EGFR-mutant non-small cell lung cancer treated with erlotinib. Lung Cancer. 2017;108:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pocock SJ, Simon R.. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 9. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. [DOI] [PubMed] [Google Scholar]
- 10. Hasselle M, Haraf D, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7(2):379–381. [DOI] [PubMed] [Google Scholar]
- 11. Milano MT, Katz AW, Zhang H, Okunieff P.. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83(3):878–886. [DOI] [PubMed] [Google Scholar]
- 12. Engels B, Everaert H, Gevaert T, et al. Phase II study of helical tomotherapy for oligometastatic colorectal cancer. Ann Oncol. 2011;22(2):362–368. [DOI] [PubMed] [Google Scholar]
- 13. Xu Q, Zhou F, Liu H, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018;13(9):1383–1392. [DOI] [PubMed] [Google Scholar]
- 14. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia W, Guo H, Jing W, et al. An especially high rate of radiation pneumonitis observed in patients treated with thoracic radiotherapy and simultaneous osimertinib. Radiother Oncol. 2020;152:96–100. [DOI] [PubMed] [Google Scholar]
- 16. Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH.. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9(2):195–199. [DOI] [PubMed] [Google Scholar]
- 17. Magnuson WJ, Lester-Coll NH, Wu A, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for this study, including deidentified individual participant data and a data dictionary defining each field in the set can be made available to others on acceptance of an official request. The study protocol and other related documents can also be made available to others on request. The corresponding time frame shall be with publication and can be shared as an electronic or physical file after explicit approval of the study investigators and a signed data usage agreement between the participating institutions.