Abstract
Background
Over the past decades, the therapeutic landscape has markedly changed for patients with metastatic solid cancer, yet few studies have evaluated its effect on population-based survival. The objective of this study was to evaluate the change in survival of patients with de novo metastatic solid cancers during the last 30 years.
Methods
For this retrospective study, data from almost 2 million patients diagnosed with a solid cancer between January 1, 1989, and December 31, 2018, were obtained from the Netherlands Cancer Registry, with follow-up until January 31, 2021. We classified patients as with or without de novo metastatic disease (M1 or M0, respectively) at diagnosis and determined the proportion with M1 disease over time. Changes in age-standardized net survival were calculated as the difference in the 1- and 5-year survival rates of patients diagnosed in 1989-1993 and 2014-2018.
Results
Different cancers showed divergent trends in the proportion of M1 disease and increases in net survival for M1 disease (approximately 0-50 percentage points at both 1 and 5 years). Patients with gastrointestinal stromal tumors saw the largest increases in 5-year survival, but we also observed substantial 5-year survival increases for patients with neuroendocrine tumors, melanoma, prostate cancer, and breast cancer.
Conclusion
Over 30 years, the survival of patients with de novo M1 disease modestly and unevenly increased among cancers. Metastatic cancer still remains a very lethal disease. Next to better treatment options, we call for better preventive measures and early detection to reduce the incidence of metastatic disease.
Solid cancers are a major public health problem worldwide, with approximately 16 million new patients and 8.5 million deaths reported in 2020 (1). Approximately 4%-65% of cancers are diagnosed as metastatic disease, which is often associated with a poor prognosis (2,3). Although the approval of over 80 novel systemic therapies since 1990 has expanded the treatment options for most metastatic tumors (4), improving survival for these patients remains a challenge (5).
Population-based cancer survival statistics, including 5-year survival rates, are important metrics for evaluating and prioritizing cancer control policy (6). Many prior studies have compared survival rates between countries or have assessed the differences in survival between periods irrespective of cancer stage (6,7). However, nationwide studies focusing on changes in the survival of patients with distant metastases are scarce because many existing cancer registries have no or incomplete data on stage at diagnosis (8).
Systemic therapy constitutes the backbone of treatment for most metastatic cancers. Historically, this primarily included cytotoxic chemotherapy and hormonal therapy, but in recent decades, the therapeutic landscape has rapidly changed because of the approval of several targeted and immune therapies. Although randomized controlled trials (RCT) have demonstrated the safety and efficacy of each of these new medicines, the impact on survival in unselected population-based samples has been inadequately studied (9). This is an important knowledge gap because clinical trial results may not be representative of the general patient population (10). Moreover, RCTs do not usually include the cumulative benefit of sequential therapies. Analysis of the changes in population-based survival over several decades while considering the systemic therapies that have been introduced might offer valuable insights into the overall impact of new medicines for metastatic cancer.
In this study, we investigate the survival trends of patients with metastatic cancers at the time of diagnosis (de novo metastatic cancer [M1]) using data from the nationwide Netherlands Cancer Registry (NCR). We evaluate changes in the survival of patients presenting with a solid cancer between 1989-1993 and 2014-2018, and we discuss this in light of the systemic therapies introduced. Our aim is to determine whether M1 cancer survival has improved during a period in which many novel medicines have been approved.
Methods
Study design
We performed a retrospective cohort study based on data from the NCR between 1989 and 2018. The primary outcomes of this study were the changes in the 1-year and 5-year net survival of patients with distant M1 disease. We divided the data into 2 cohorts (1989-1993 and 2014-2018) to ensure comparison of periods before and after the implementation of novel medicines. Additionally, we report on the trends in the proportion of patients with M1 disease (from 1989 to 2018).
We included adult patients aged 18 years and older, diagnosed with a solid primary cancer between 1989 and 2018. Information on patients’ vital status was available until January 31, 2021. We excluded patients diagnosed on the date of death. Patients with multiple primary tumors were grouped by their first tumor only and were excluded if the first tumor was diagnosed before 1989. This study was approved by the NCR’s Ethics Committee (written informed consent was not required).
Data source
The NCR reached national coverage in 1989 and has an estimated completeness of more than 95% of all malignancies in the Netherlands (11). Data managers record data on patient, tumor, and initial treatments directly from patient files. The International Classification of Diseases for Oncology is used for coding topography and morphology. Staging methods are considered when determining the stage at diagnosis, but no information is recorded on the modalities used or the outcomes. In most tumors, the stage is coded according to the Union for International Cancer Control tumor, node, metastasis (TNM) classification (4th-8th edition). Otherwise, tumors are classified by the extent of the disease (EoD) as local, regional, or distant. The NCR is linked each year on January 31 to the Municipal Personal Records Database to obtain information on vital status. Patients who emigrate are censored at the date of emigration.
Case definitions
Patients were classified as having M1 (de novo metastatic cancer) based on 1 of 3 criteria: 1) M1 disease according to the TNM classification (clinically and/or pathologically proven); or 2) distant disease according to the EoD classification system (clinical EoD and/or pathologically proven EoD = 6; used in the NCR for only a minority of cancers); or 3) the presence of metastasis with an unknown primary tumor (C80.9) (Supplementary Table 1, available online). All other patients were classified as without de novo metastatic cance (M0), including those with unknown stage. In the Netherlands, clinically diagnosed metastases are typically based on radiological evaluation rather than clinical examination alone.
We defined 28 cancers based on the histology (carcinoma) and the primary site (most cancers) or the histology only (gastrointestinal stromal tumors [GIST], neuroendocrine tumors [NET], melanoma), including a group of “other” cancers and a group of cancers with an unknown primary site (C80.9) (Supplementary Table 2, available online). Approximately 75% of patients with metastatic melanoma had melanoma of unknown primary. These often present with lymph node metastases only, so they do not fit within our definition of M1 disease (distant metastases). Still, we included them in our analyses because they share similar treatment strategies (12).
Lung cancer was divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) subgroups based on their clinical and treatment differences and into “lung other” (mostly cases without pathological confirmation). All patients with central nervous system tumors (ie, meninges C70, brain C71, other parts of central nervous system C72, pituitary gland C75.1, and pineal gland C75.3) were excluded because these patients usually die from the local tumor burden rather than metastasis. Patients with Kaposi’s sarcoma (C46) were excluded because most do not die from metastatic disease but from the underlying disease (mostly HIV/AIDS), and patients with mesothelioma (C45) were excluded because TNM data were unavailable for this cancer between 1989 and 1992.
Statistical analyses
The proportion of patients with M1 disease was calculated by dividing the annual number of patients with M1 disease per cancer by the total annual number of patients with and without distant metastases at diagnosis (M0 + M1). Trends over time of the proportion of M1 were plotted, and by visual inspection of the graphs we grouped the cancer types into a constant, increasing, decreasing, or a combination of an increasing and decreasing trend. We also reported changes over time with the number, and mean age, proportion of men, of patients diagnosed between 1989-1993 and 2014-2018. The 1- and 5-year net survival for patients with M1 disease were estimated for the 2 defined time periods, considering net survival to be the survival observed if cancer was the only possible cause of death (13). net survival can be interpreted as cancer-specific survival. The Pohar-Perme estimator served to estimate the age-standardized net survival with 95% confidence intervals (14). Age standardization was conducted to account for changes in the distribution of age over time, which is important because the age distribution has a significant impact on the population’s survival. The International Cancer Survival Standard was used for most cancer types and Dutch weightings for prostate cancer (find the weights in Supplementary Table 2, available online). We used the same age distribution for all time periods. Age-standardized net survival analyses were not possible for certain cancers due to the limited number of diagnoses and events in 1 or more age strata. For these cancers, weighting groups were adjusted (eg, testicular cancer) or the diagnostic cohorts were changed (eg, vulva or vagina, hepatocellular cancer); additionally, analyses not standardized by age were performed for cancers with 20 or less patients in 1 or more age strata (see Supplementary Table 3, available online).
We used the cohort approach to estimate patient net survival. This approach can estimate the 5-year net survival of a cohort even in the absence of complete follow-up data (such as in our study for the cohort 2014-2018) by using the survival information of all patients until censoring (eg, like the Kaplan-Meier and life table methods) (13). Survival changes over time were calculated as the arithmetic gain by subtracting the estimated survival rate of the 1989-1993 cohort from that of the 2014-2018 cohort. The statistical significance changes were assessed with the Z-test (P < .01) (15).
In supplemental analyses, we also evaluated the changes in median overall survival, and we reported trends in M1 survival over time by estimating the net survival of the intermediate cohorts.
Results
Patient population
In total, almost 2 million adult patients were diagnosed with an eligible solid cancer between 1989 and 2018. We included 52 263 and 84 383 diagnosed with de novo metastases (M1) in the survival analyses for 1989-1993 and 2014-2018, respectively. Information on M stage at diagnosis was based on clinical or pathological classification in most patients (69% in 1989-1993 and 92% in 2014-2018). The number with M1 disease increased between 1989-1993 and 2014-2018 for all cancers except for stomach cancer, whereas the number of patients with an unknown primary cancer more than halved (Table 1; Supplementary Table 4, available online).
Table 1.
Per cancer type and time period, the number of newly diagnosed patients with de novo metastatic disease and the mean age
| Cancera | 1989-1993, No. | Mean age (95% CI), y | Male, No. (%) | 2014-2018, No. | Mean age (95% CI), y | Male, No. (%) | Increase or decrease |
|---|---|---|---|---|---|---|---|
| Prostate | 5477 | 74 (73 to 74) | 5477 (100) | 8491 | 73 (73 to 73) | 8491 (100) | ↓ |
| Testicular | 210 | 32 (30 to 33) | 210 (100) | 293 | 35 (34 to 36) | 293 (100) | ↑ |
| Thyroid | 182 | 67 (65 to 69) | 64 (35) | 259 | 67 (66 to 69) | 116 (45) | ≠ |
| Breast | 2808 | 64 (63 to 64) | 12 (0) | 3826 | 63 (62 to 63) | 42 (1) | ↓ |
| NET | 304 | 64 (63 to 65) | 158 (52) | 840 | 64 (63 to 65) | 466 (55) | ≠ |
| Vulva/vagina | 27 | 70 (65 to 76) | 0 (0) | 85 | 71 (69 to 74) | 0 (0) | ↑ |
| Melanoma | 440 | 57 (55 to 58) | 249 (57) | 1041 | 63 (62 to 64) | 639 (61) | ↑ |
| Ovarian | 1060 | 66 (65 to 67) | 0 (0) | 1517 | 68 (68 to 69) | 0 (0) | ↑ |
| Corpus uteri | 248 | 69 (68 to 70) | 0 (0) | 672 | 70 (69 to 71) | 0 (0) | |
| GIST | 50 | 59 (56 to 63) | 21 (42) | 160 | 66 (64 to 68) | 101 (63) | ↑ |
| Rectum | 1956 | 67 (67 to 68) | 1159 (59) | 3644 | 66 (66 to 66) | 2254 (62) | ↓ |
| Cervix uteri | 144 | 59 (57 to 62) | 0 (0) | 373 | 58 (57 to 60) | 0 (0) | ↓ |
| Sarcoma | 390 | 57 (55 to 59) | 214 (55) | 545 | 60 (59 to 62) | 329 (60) | ↑ |
| Colon | 4753 | 68 (68 to 69) | 2259 (48) | 9332 | 68 (68 to 68) | 4961 (53) | ≠ |
| Kidney | 1489 | 65 (64 to 65) | 918 (62) | 2205 | 67 (67 to 68) | 1467 (67) | ↑ |
| HNSCC | 97 | 62 (60 to 64) | 77 (79) | 238 | 66 (64 to 67) | 178 (75) | ↑ |
| Bladder | 549 | 69 (68 to 70) | 384 (70) | 1768 | 70 (70 to 71) | 1164 (66) | ↑ |
| Other solid cancers | 811 | 65 (64 to 66) | 364 (45) | 1187 | 65 (64 to 65) | 586 (49) | ≠ |
| Cancers with an unknown primary site | 11 659 | 69 (69 to 69) | 6406 (55) | 5280 | 73 (72 to 73) | 2661 (50) | ↑ |
| SCLC | 3575 | 66 (65 to 66) | 2871 (80) | 4873 | 67 (67 to 68) | 2564 (53) | ↑ |
| Esophagus/cardia | 1646 | 65 (64 to 65) | 1229 (75) | 4387 | 67 (67 to 67) | 3464 (79) | ↑ |
| Stomach | 2720 | 69 (68 to 69) | 1697 (62) | 2148 | 69 (68 to 69) | 1329 (62) | ≠ |
| NSCLC | 7510 | 65 (65 to 65) | 6055 (81) | 19 868 | 67 (67 to 67) | 11 262 (57) | ↑ |
| Lung other | 1045 | 70 (70 to 71) | 874 (84) | 2890 | 76 (75 to 76) | 1706 (59) | ↑ |
| Bile ducts | 289 | 69 (67 to 70) | 133 (46) | 1347 | 67 (66 to 67) | 665 (49) | ↓ |
| Hepatocellular | 114 | 62 (59 to 65) | 69 (61) | 508 | 68 (67 to 69) | 395 (78) | ↑ |
| Gallbladder | 339 | 71 (70 to 72) | 73 (22) | 383 | 70 (69 to 71) | 111 (29) | ↓ |
| Pancreas | 2371 | 67 (66 to 67) | 1288 (54) | 6223 | 69 (69 to 69) | 3275 (53) | ↑ |
| Total | 52 263 | 67 (67 to 67) | 20 002 (62) | 84 383 | 68 (68 to 68) | 48 519 (57) | ↑ |
See Supplementary Table 2 (available online) for a detailed description of the cancer types; ↑ = increase; ↓ = decrease; ≠ = remained the same. CI = confidence interval; GIST = gastrointestinal stromal tumors; HNSCC = head and neck squamous cell carcinoma; NET = neuroendocrine tumor; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
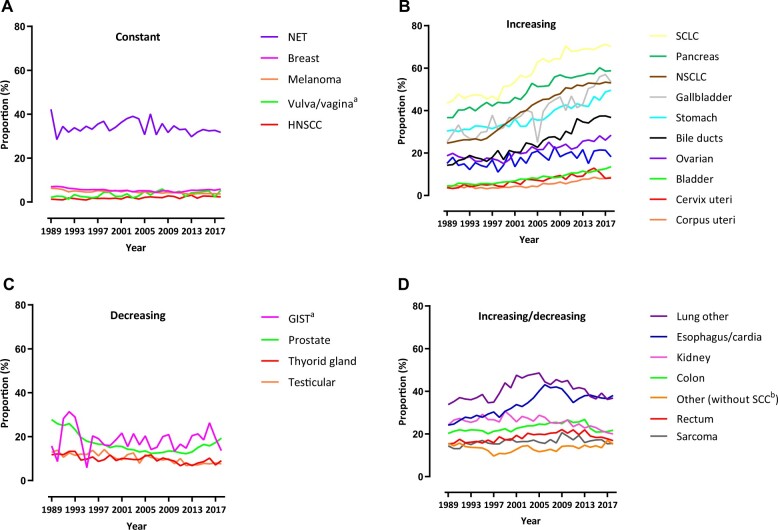
The proportion of patients with M1 disease at diagnosis and disease evolution differed between cancers. For instance, almost one-half of the patients with pancreatic cancer had distant metastases at diagnosis as opposed to approximately 1% of patients with head and neck squamous cell carcinoma. Furthermore, certain cancers (eg, NSCLC or SCLC) showed a clear increasing trend over time, whereas others clearly decreased (eg, prostate) (see Figure 1, A–D; Supplementary Table 4, available online).
Figure 1.
Trends in the proportion of patients with metastatic cancer at diagnosis M1/(M0+M1)) per cancer type from 1989 to 2018. A) Constant trend. B) Increasing trend. C) Decreasing trend. D) Increasing and decreasing trend. aFluctuations because of small patient numbers in each year. bPatients with SCC excluded. Note: The increases in the proportion of M1 disease may be caused by various reasons (eg, changes in the proportion of M1/(M0_M1) or changes primarily in the incident of M1 or M0). Therefore, the absolute patient numbers with M0 and M1 are also provided in Supplementary Table 4 (available online). GIST = gastrointestinal stromal tumors; HNSCC = head and neck squamous cell carcinoma; M0 = patients with locoregional cancer; M1 = patients with de novo metastatic disease; NET = neuroendocrine tumor; NSCLC = non-small cell lung cancer; SCC = squamous cell carcinoma; SCLC = small cell lung cancer.
Changes in M1 survival
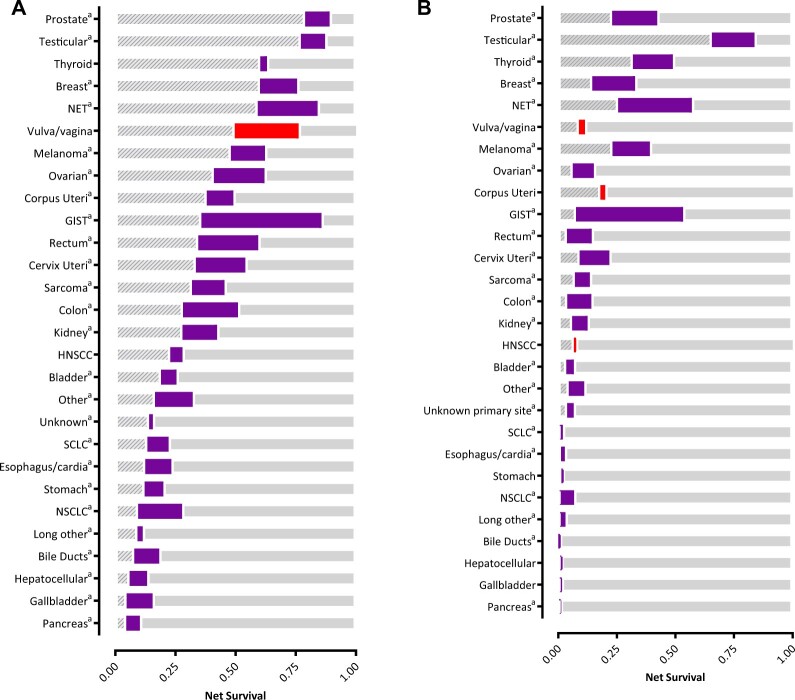
The 1- and 5-year age-standardized net survival rates increased over time for most cancers (Figure 2; Supplementary Figure 1; Supplementary Tables 5-7, available online). There remains a large gap in survival between cancers with the most and the least favorable survival. The percentage point increases in 1-year net survival ranged from 0 to 50, and that for 5-year survival ranged from 0 to 46. The largest gains in 1-year net survival were observed for GIST, NET, and cancers of the rectum, colon, and ovaries. The largest increases in 5-year net survival were seen in patients with GIST, NET, and cancers of the prostate, breast, testicles, and thyroid (all subtypes). The increases were mainly seen from 1994-1998 onward for GIST, during the entire study period for NET, from 2004-2008 onward for prostate cancer, from 1999-2003 onward for breast cancer, in the earliest period for testicular cancer (ie, 1989-1993 and 1994-1998), and from 1994-1998 onward for thyroid cancer (see Supplementary Figure 1, available online). Changes in median overall survival were also largest in GIST, NET, breast, and prostate cancer (see Supplementary Table 8, available online).
Figure 2.
Changes in 1- and 5-year net survival of de novo metastatic cancer patients with primary cancer diagnosis over 2 time periods: 1989-1993 vs 2014-2018 (purple = increase, red = reduction). A) Changes in 1-year net survival. B) Changes in 5-year net survival. The striped gray bar on the left represents the net survival in the period 1989-1993; the thicker purple bar represents the survival change from 1989-1993; and transparent gray bar on the right represents the proportion of survival that is still to gain. aSignificant difference in net survival of patients in 1989-1993 vs those in 2014-2018 (α = .01). GIST = gastrointestinal stromal tumors; HNSCC = head and neck squamous cell carcinoma; NET = neuroendocrine tumor; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Overall, the net survival rate for M1 disease exceeded 50% at 1 year for 11 cancers and exceeded 20% at 5 years for 8 (2014-2018).
Discussion
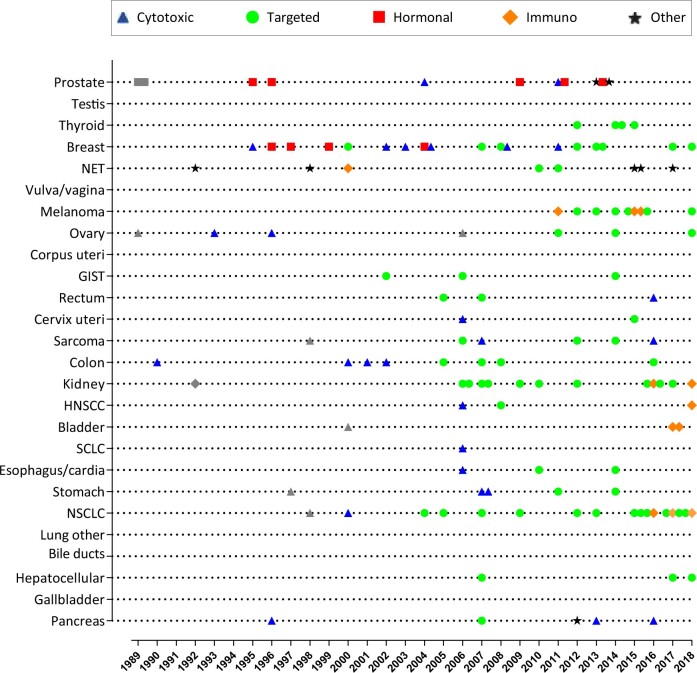
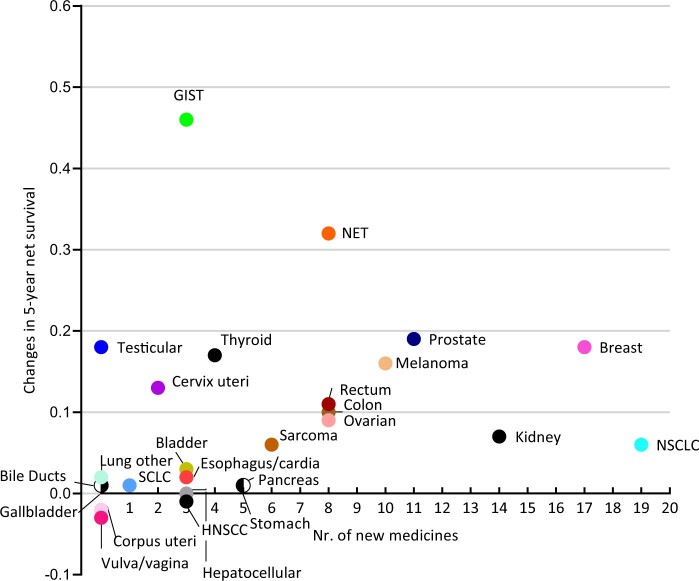
This study showed that the 1-year survival increased for most patients with M1 disease, but the magnitude of increase differed substantially between cancers. Additionally, we observed increases (>15%) in the 5-year survival for GIST, NET, melanoma, and cancer of the prostate, breast, thyroid, and testis. Approval was granted for more than 80 novel medicines and multiple new indications for existing medicines to treat metastatic disease (Figure 3; Supplementary Tables 9 and 10, available online). Although introducing new medicines probably contributed to some of the observed survival increases, the changes in survival did not appear to relate to the number of new medicines approved (Figure 4). Besides, we cannot assume causation because we do not know how many of the patients actually received the newly approved medicines. Moreover, a higher number of new treatment options does not always result in better survival at the population level.
Figure 3.
Timeline of newly approved medicines for patients with metastatic cancer per cancer type in the Netherlands. Gray dots, squares, and triangles show medicines for which the year of approval is uncertain (see Supplementary Table 10, available online). GIST = gastrointestinal stromal tumors; HNSCC = head and neck squamous cell carcinoma; NET = neuroendocrine tumor; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Figure 4.
Association between the number of new medicines approved during the period 1989-2018 and the 5-year net survival changes from 1989-1993 and 2014-2018 of patients with different solid cancers. GIST = gastrointestinal stromal tumors; HNSCC = head and neck squamous cell carcinoma; NET = neuroendocrine tumor; NSCLC = non-small cell lung cancer; SCC = squamous cell carcinoma; SCLC = small cell lung cancer.
Interpreting survival trends over several decades is complex because various factors can explain any observed changes. Together with better treatment, factors such as better staging due to improvements in imaging and changes in registration, coding practices and TNM classifications (both causing stage migration), and distribution of prognostic factors (eg, subtypes) could account for the survival improvements. Nevertheless, it appears plausible that at least part of the survival increases observed in our study represents true treatment progress, such as the substantial 18-46 percentage point increases in 5-year survival for breast cancer, NET, and GIST that coincided with the approval of novel medicines.
The tyrosine kinase inhibitor imatinib, for instance, was approved in 2002 for the treatment of metastatic GIST (see Figure 4). In RCTs, patients treated with this agent showed median survival times of approximately 50-60 months, increasing from historic medians of just 9 months (16,17). Approximately 84% of patients in our 2014-2018 cohort received a tyrosine kinase inhibitor as first-line therapy, suggesting that imatinib has been widely implemented in the Netherlands (Supplementary Table 11, available online). In addition, survival mainly increased from 1994-1998 to 1999-2003 (Supplementary Figure 1, available online). Hence, at least some of the observed survival increase likely reflects the adoption of effective treatment options for de novo metastatic GIST.
Concerning de novo metastatic breast cancer, the 5-year net survival in our study increased by 18 percentage points during a period when 17 new medicines were approved. Several of these, such as the targeted agents trastuzumab and pertuzumab, can improve survival in patients with HER2+ metastatic breast cancer. Although this subgroup accounts for only approximately 15% of patients, we consider it plausible that the successful implementation of these medicines accounts for some of the observed improvements in 5-year survival, especially given that most patients in the 2014-2018 cohort received one of these medicines as a first-line therapy (Supplementary Table 11, available online). Unfortunately, we could not separately evaluate the survival of this subgroup because HER2 status was not recorded in the NCR before 2005. Other medicines that probably contributed to the improved survival in our study include aromatase inhibitors and taxanes (18).
Octreotide represented an important breakthrough in the treatment of metastatic NET when implemented in the Netherlands in 1992 (19). Several other therapies were also granted regulatory approval since then. In our study, the number of patients diagnosed with NET markedly increased over time, likely reflecting improved pathology assessment and greater awareness of these uncommon cancers. Moreover, NETs are a heterogeneous group of tumors for which survival depends on multiple factors, including the histology and primary site, the distribution of which varied between the periods in this study (20,21). Therefore, the substantial survival increase of 32 percentage points is probably not only the effect of advances in treatment.
In addition to these cancers, our data uncovered substantial improvements of 16 percentage points in long-term survival from metastatic melanoma. These findings are relevant because the treatment landscape has dramatically changed with the advent of immune checkpoint inhibitors (ICIs) and multiple targeted agents over recent years. ICIs represent particularly important breakthroughs, improving the 5-year survival from metastatic melanoma by up to 52% in RCTs from historic levels less than 10% (22,23). Studies investigating the impact of ICIs in daily practice have also revealed an improved prognosis (24). The NCR regrettably lacks data on therapies not administered first line, and thus we do not know how many patients received ICI. However, previous data in the Netherlands suggest the rapid implementation of new systemic options for melanoma (25). The survival increases observed in our study may therefore reflect this trend of ICI application.
We found long-term survival increases of 6 percentage points in patients with NSCLC. Stage migration since implementation of fluorodeoxyglucose positron emission tomography in 1997 likely accounts for some of this increase in the Netherlands. Since then, the percentage of patients with M1 NSCLC increased from 29% to 53% (Figure 1), but the limited survival increases are disappointing given that it received most novel medicines (Figure 4). The minor changes in population-based long-term progress can be explained in several ways. First, some patients in the 2014-2018 cohort may not have received the newest and most effective agents because many of these had only recently been implemented (eg, ICIs in 2016). Second, we cannot expect long-term benefits for all patients (eg, 5-year survival of 16%-33% with ICIs in RCTs) (22,26). Third, our follow-up duration may not have been long enough to capture improvements in 5-year survival because of the latest medicines. Future studies should confirm whether survival from metastatic NSCLC improves over time.
Despite the breakthroughs in systemic treatment for metastatic solid cancers, debate persists regarding the effectiveness and rising costs of new cancer medicines (27). Some may bring few health benefits, with a recent study estimating that approximately 22% of all newly approved medicines for solid cancer between 2003 and 2013 offered no overall survival benefits compared with standard care (28). Although other medicines had a positive impact, with a mean 3-month improvement in survival, the magnitude was often modest (28). This may also explain why the 1- and 5-year survival rates of some cancers have changed little in the last 30 years. Nevertheless, even minor benefits in survival or other outcomes (eg, quality of life) may represent progress in treating patients with metastatic cancer.
Analysis by stage requires appropriate data registration and few unknown or missing data. The NCR provided accurate stage information for the entire period, which facilitated the analysis of changes in M1 survival in a comprehensive list of cancers. By providing an overview of survival changes for all M1 solid cancers, our study supplements existing knowledge. Nevertheless, further research is needed to improve our understanding of the population impact of new medicines for metastatic cancer. As a prerequisite, databases should include data beyond the first-line treatment together with confounding variables (ie, co-morbidity) and other relevant outcomes (ie, quality of life, adverse events). A complete overview will also require further study in patients with metachronous metastatic disease. Unfortunately, it is not possible to easily obtain such data.
Our results show that the survival of patients with de novo metastatic cancer improved slowly over 30 years but that these gains were typically modest and unevenly distributed among cancers. Unfortunately, metastatic cancer remains a very lethal disease for almost all cancer types. Next to better treatment options to improve survival in patients with metastatic cancer, we call for better preventive measures and early detection to reduce the incidence of metastatic disease.
Supplementary Material
Acknowledgements
We thank Dr Robert Sykes for providing editorial assistance in the final drafts of this manuscript, Professor Adri Steenhoek for providing us a document with all newly approved cancer medicines in the Netherlands, and Heidi Fransen for providing feedback on the final drafts of this manuscript.
Poster presentation at the European Network of Cancer Registries (ENCR) conference (November 16-18, 2021).
Role of the funder: N/A
Contributor Information
Marianne Luyendijk, Department of Research and Development, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht, the Netherlands; Erasmus School of Health Policy & Management, Erasmus University, Rotterdam, the Netherlands.
Otto Visser, Department of Registration, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht, the Netherlands.
Hedwig M Blommestein, Erasmus School of Health Policy & Management, Erasmus University, Rotterdam, the Netherlands.
Ignace H J T de Hingh, Department of Surgery, Catharina Hospital, Eindhoven, the Netherlands.
Frank J P Hoebers, Department of Radiation Oncology (MAASTRO), Research Institute GROW, Maastricht University, Maastricht, the Netherlands.
Agnes Jager, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Gabe S Sonke, Department of Medical Oncology, The Netherlands Cancer Institute, Amsterdam, the Netherlands.
Elisabeth G E de Vries, Department of Medical Oncology, University Medical Centre Groningen, University of Groningen, Groningen, the Netherlands.
Carin A Uyl-de Groot, Erasmus School of Health Policy & Management, Erasmus University, Rotterdam, the Netherlands.
Sabine Siesling, Department of Research and Development, Netherlands Comprehensive Cancer Organization (IKNL), Utrecht, the Netherlands; Department of Health Technology and Services Research, Technical Medical Centre, University of Twente, Enschede, the Netherlands.
Data availability
The individual patient-level data that support the results of this research are available at the Netherlands Comprehensive Cancer Organization and can be obtained upon request using the data request form on the website: https://iknl.nl/nkr/cijfers-op-maat/gegevensaanvraag.
Author contributions
Gabe S Sonke, PhD (Writing — review & editing), Agnes Jager, PhD (Conceptualization; Supervision; Writing — review & editing), Elisabeth G E de Vries, PhD (Writing — original draft; Writing — review & editing), Sabine Siesling, PhD (Conceptualization; Investigation; Project administration; Supervision; Writing — original draft; Writing — review & editing), Carin A Uyl de Groot, PhD (Writing — review & editing), Otto Visser, PhD (Conceptualization; Data curation; Formal analysis; Software; Supervision; Writing — original draft; Writing — review & editing), Marianne Luyendijk, MSc (Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Software; Visualization; Writing — original draft; Writing — review & editing), Hedwig M Blommestein, PhD (Conceptualization; Investigation; Supervision; Visualization; Writing — original draft; Writing — review & editing), Frank J P Hoebers, PhD (Writing — review & editing), Ignace H J T de Hingh, PhD (Writing — review & editing).
Funding
Not applicable.
Conflicts of interest
IHJTH reports research grants or contracts paid to the institute from Roche and RanD Biotechnologies. GSS reports grants or contracts paid to the institute from Agendia, AstraZeneca, Merck, Novartis, Roche, and Seagen; consulting fees from Biovica and Seagen also paid to the institute. EGEV reports grants or contracts paid to the institute for the financial support of clinical trials or contracted research from Amgen, Genentech, Roche, CytomX, G1 Therapeutics, Bayer, Synthon, Servier, Regeneron, and Crescendo; consulting fees paid to the institute (institutional financial support for advisory boards/consultancy) from NSABP, Daiichi Sankyo, and Crescendo Biologics; different roles without financial interests (including no-renumerated activities and public positions): member of the ESMO-MCBS, Chair ESMO Cancer Medicines Working Group, Co-chair RECIST committee, member expert panel for selection of Essential Medicine List WHO. CAU reports grants from Boehringer Ingelheim, Astellas, Celgene, Sanofi, Janssen-Cilag, Bayer, Amgen, Genzyme, Merck, Gilead, Novartis, and Astra Zeneca Roche (outside the submitted work). SS reports a role on the Supervisory Board of PALGA (national Pathology laboratory Archive) and received a personal fee for attending the meetings. Authors ML, OV, HMB, FHPH, and AJ had no conflicts of interest to declare.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2. Verhoeven RH, Karim-Kos HE, Coebergh JW, et al. Markedly increased incidence and improved survival of testicular cancer in the Netherlands. Acta Oncol. 2014;53(3):342-350. [DOI] [PubMed] [Google Scholar]
- 3. Pavlik T, Majek O, Buchler T, et al. Trends in stage-specific population-based survival of cancer patients in the Czech Republic in the period 2000–2008. Cancer Epidemiol. 2014;38(1):28-34. [DOI] [PubMed] [Google Scholar]
- 4. Lythgoe MP, Krell J, Mahmoud S, Mills E, Vasudevan A, Savage P.. Development and economic trends in anticancer drugs licensed in the UK from 2015 to 2019. Drug Discov Today. 2021;26(2):301-307. [DOI] [PubMed] [Google Scholar]
- 5. Gapstur SM, Thun MJ.. Progress in the war on cancer. JAMA. 2010;303(11):1084-1085. [DOI] [PubMed] [Google Scholar]
- 6. Allemani C, Matsuda T, Di Carlo V, et al. ; CONCORD Working Group. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with 1 of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siesling S, Kwast A, Gavin A, Baili P, Otter R; EUROCHIP-3 Workpackage 5. Availability of stage at diagnosis, cancer treatment delay and compliance with cancer guidelines as cancer registry indicators for cancer care in Europe: results of EUROCHIP-3 survey. Int J Cancer. 2013;132(12):2910-2917. [DOI] [PubMed] [Google Scholar]
- 9. Boyle JM, Hegarty G, Frampton C, et al. Real-world outcomes associated with new cancer medicines approved by the Food and Drug Administration and European Medicines Agency: a retrospective cohort study. Eur J Cancer. 2021;155:136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westgeest HM, Uyl-de Groot CA, van Moorselaar RJ, et al. Differences in trial and real-world populations in the Dutch Castration-resistant Prostate Cancer Registry. Eur Urol Focus. 2018;4(5):694-701. [DOI] [PubMed] [Google Scholar]
- 11. Schouten LJ, Hoppener P, van den Brandt PA, Knotterus JA, Jager JJ.. Completeness of cancer registration in Limburg, the Netherlands. Int J Epidemiol. 1993;22(3):369-376. [DOI] [PubMed] [Google Scholar]
- 12. Verver D, Grünhagen DJ, van Akkooi AC, et al. Clinical outcome of patients with metastatic melanoma of unknown primary in the era of novel therapy. Cancer Immunol Immunother. 2021;70(11):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickman PW. Estimating and modeling relative survival. Stata J. 2015;15(1):186-215. [Google Scholar]
- 14. Perme MP, Stare J, Estève J.. On estimation in relative survival. Biometrics. 2012;68(1):113-120. [DOI] [PubMed] [Google Scholar]
- 15. Jensen OP, MacLenna R, Muir CS, Skeet RG. Cancer registration: principles and methods: International Agency for Research on Cancer. 1991. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-Registration-Principles-And-Methods-1991. Accessed November 10, 2021.
- 16. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127-1134. [DOI] [PubMed] [Google Scholar]
- 17. Patel S. Long-term efficacy of imatinib for treatment of metastatic GIST. Cancer Chemother Pharmacol. 2013;72(2):277-286. [DOI] [PubMed] [Google Scholar]
- 18. Chia SK, Speers CH, D'yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population‐based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973-979. [DOI] [PubMed] [Google Scholar]
- 19. Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen M, Taal BG.. Epidemiology and survival in patients with carcinoid disease in the Netherlands: an epidemiological study with 2391 patients. Ann Oncol. 2001;12(9):1295-1300. [DOI] [PubMed] [Google Scholar]
- 20. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063-3072. [DOI] [PubMed] [Google Scholar]
- 22. Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5(10):1411-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546. [DOI] [PubMed] [Google Scholar]
- 24. Asher N, Ben-Betzalel G, Lev-Ari S, et al. Real world outcomes of ipilimumab and nivolumab in patients with metastatic melanoma. Cancers (Basel). 2020;12(8):2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franken MG, Leeneman B, Aarts MJB, et al. Trends in survival and costs in metastatic melanoma in the era of novel targeted and immunotherapeutic drugs. ESMO Open. 2021;6(6):100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score≥ 50. J Clin Oncol. 2021;39(21):2339-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vokinger KN, Hwang TJ, Grischott T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol. 2020;21(5):664-670. [DOI] [PubMed] [Google Scholar]
- 28. Salas-Vega S, Iliopoulos O, Mossialos E.. Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol. 2017;3(3):382-390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual patient-level data that support the results of this research are available at the Netherlands Comprehensive Cancer Organization and can be obtained upon request using the data request form on the website: https://iknl.nl/nkr/cijfers-op-maat/gegevensaanvraag.