Abstract
Advances in cell-based regenerative therapy create new opportunities for the treatment of bone-related disorders and injuries, by improving the reparative phase of bone healing. Apart from the classical approach of bone grafting, the application of cell-based therapies, particularly stem cells (SCs), has gained a lot of attention in recent years. SCs play an important role in regenerative therapy due to their excellent ability to differentiate into bone-forming cells. Regeneration of new bone is regulated by a wide variety of signalling molecules and intracellular networks, which are responsible for coordinating cellular processes. The activated signalling cascade is significantly involved in cell survival, proliferation, apoptosis, and interaction with the microenvironment and other types of cells within the healing site. Despite the increasing evidence from studies conducted on signalling pathways associated with bone formation, the exact mechanism involved in controlling the differentiation stage of transplanted cells is not well understood. Identifying the key activated pathways involved in bone regeneration may allow for precise manipulation of the relevant signalling molecules within the progenitor cell population to accelerate the healing process. The in-depth knowledge of molecular mechanisms would be advantageous in improving the efficiency of personalised medicine and targeted therapy in regenerative medicine. In this review, we briefly introduce the theory of bone repair mechanism and bone tissue engineering followed by an overview of relevant signalling pathways that have been identified to play an important role in cell-based bone regenerative therapy.
Keywords: Bone, Cell-based therapy, Regeneration, Signalling pathway, Stem cells
المخلص
إن التقدم في العلاج التجديدي القائم على الخلايا يخلق فرصا جديدة في علاج الاضطرابات والإصابات المرتبطة بالعظام، من خلال تحسين المرحلة التعويضية لشفاء العظام. بصرف النظر عن النهج الكلاسيكي لتطعيم العظام، اكتسب تطبيق العلاجات القائمة على الخلايا، وخاصة الخلايا الجذعية، الكثير من الاهتمام في السنوات الأخيرة. تلعب الخلايا الجذعية دورا مهما في العلاج التجديدي، نظرا لخصائصها الممتازة للتمايز إلى خلايا مكونة للعظام. يتم تنظيم تجديد العظام الجديدة من خلال مجموعة متنوعة من جزيئات الإشارات والشبكات داخل الخلايا المسؤولة عن تنسيق العمليات الخلوية. تشارك سلسلة الإشارات المنشطة بشكل كبير في بقاء الخلايا وانتشارها وموت الخلايا المبرمج والتفاعل مع البيئة المحيطة وأنواع الخلايا الأخرى داخل موقع الشفاء. على الرغم من الأدلة المتزايدة التي تم جمعها من الدراسات التي أجريت على مسارات الإشارات المرتبطة بتكوين العظام، فإن الآلية الدقيقة التي ينطوي عليها التحكم في مرحلة التمايز للخلايا المزروعة ليست مفهومة جيدا. قد يسمح تحديد المسار الرئيسي المنشط المتضمن في تجديد العظام بالتلاعب الدقيق بجزيئات الإشارة ذات الصلة داخل مجموعة الخلايا السلفية لتسريع عملية الشفاء. ستكون المعرفة المتعمقة بالآليات الجزيئية مفيدة في تحسين كفاءة الطب الشخصي والعلاج المستهدف في الطب التجديدي. في هذه المراجعة، نقدم بإيجاز نظرية آلية إصلاح العظام وهندسة أنسجة العظام تليها نظرة عامة على مسارات الإشارات ذات الصلة التي تم تحديدها لتلعب دورا مهما في العلاج التجديدي للعظام القائم على الخلايا.
الكلمات المفتاحية: العظام, التجديد, مسار الإشارات, العلاج القائم على الخلايا, الخلايا الجذعية
Introduction
Regenerative therapy for bone tissue has great potential for individuals suffering from bone defects, particularly for a bone defect that cannot heal normally or fails to recover after a long period without any sign of healing. Although the natural healing process is able to restore the defective tissue to its preinjury state, there are cases of impaired bone healing where surgical procedure and bone regeneration are required as clinical intervention.1 The main objective of bone regenerative therapy is to restore bone function by reinstating its former properties with the newly formed bone, which will be identical to the adjacent healthy bone. The procedure involves the stimulation of new bone formation during the fracture healing period followed by continuous bone remodelling throughout adulthood.2 Fracture repair is the most prevalent form of bone regeneration in the clinical context, where depending on the degree of injury, healing mechanisms can be different.
Bone grafting is a widely used method in clinical practice where the transplanted bone is utilised to augment bone regeneration. However, the downside of this approach includes the additional time for surgery, donor site morbidity, and limited bone availability. To address these limitations, cell-based therapy is regarded as an alternative, which allows stem cells (SCs), biomaterials, and growth factors to facilitate bone repair and regeneration.3 Theoretically, cell therapy plays an important part in bone tissue engineering by stimulating the migration of bone progenitor cells to the injury site to promote bone repair and undergo further differentiation into bone-forming cells and tissue.4 The process also involves the regulation of various intracellular signalling molecules with the incorporation of growth factors and cytokines that are responsible for bone regeneration.
Studies on signalling pathways in cell-based technology allow the development of a new treatment approach by targeting one or two specific pathways, which regulate the bone-forming capacity of SCs. Understanding the fundamentals of the signalling cascade, and the activated genes at the molecular level are advantageous for bone reconstruction. This paper provides an overview of the basic biology of bone repair and different modalities for regenerative therapy with the focus of reviewing the relevant signalling pathways in the osteogenesis of SCs during the bone reparative phase.
Overview of bone repair and regeneration
Notably, the bone healing mechanisms can be categorised into primary bone healing, which involves restoration of the cortex without callus formation; and secondary bone healing, which involves callus formation prior to the bone remodelling stage.5 Primary or direct fracture healing rarely occurs since it needs a stable complex, as well as appropriate anatomical reduction of the fracture ends without any gap formation.6 In contrast to the primary process, secondary or indirect fracture healing is more prevalent, which involves an inflammatory response of the periosteum and the neighbouring soft tissue at the fracture area.7 The repair process also involves recapitulation of both endochondral and intramembranous bone formation aided by the homing of mesenchymal cells and osteoprogenitor cells. In secondary bone healing, the process undergoes an anabolic phase at the beginning, which then overlaps with the catabolic phase when callus volume is lessened.7 A deeper understanding of basic bone biology is very important to formulate a specific and personalised bone reparative therapy, which effectively targets the key biomolecules within the repair site, at an optimal dose and within a precise timeframe.
To date, the bone grafting technique remains one of the most common approaches in clinical settings to repair and regenerate bone loss or defects. The method can be further classified into autograft, allograft, and xenograft. Autologous bone grafts are the gold standard in bone regeneration as it provides an osteoinductive, osteoconductive, and osteogenic potential for bone formation while maintaining the native cells’ osteogenic potential.2 In addition, it also carries minimal risk of disease transfer, lower risk of graft rejection, and lower cost of procedures.9 However, the downside of autograft includes the limitation in graft supply, requiring additional surgery due to shape restrictions, as well as donor site morbidity.8 Hence, the use of allograft can be beneficial, by eliminating the need for surgical procedures to harvest the graft. However, patients have increased risk of immunologic reaction and transmission of disease.9,10 Similarly, the use of xenografts or heterologous grafts from other species may have biological limitations concerning the low osteogenic and osteoinductive capacity of the material.11 Considering the advantages and limitations of each technique, an alternative strategy must be applied to reduce the existing risk and increase the treatment efficiency.
SC-based bone regenerative therapy
Bone tissue engineering aims to restore bone function by stimulating regeneration capacity via the use of SCs, growth factors, and scaffolds for mechanical support and transplantation.12 The approach can either be a cell-free or cell-based procedure. Cell-free approaches refer to the utilisation of constructs to support osteogenesis, osteoconductivity, and osteoinductivity while maintaining mechanical stability such as scaffold and biomimetic materials.13 Meanwhile, cell-based approaches focus on manipulating the differentiation of SCs or progenitor cells derived from various tissues into osteoblasts, the bone-forming cells that support new bone tissue formation.
Application of cell-based therapy to bone regenerative strategy incorporates the use of multipotent cells that are able to differentiate into osteogenic cells to restore the damaged tissues.14 Osteoblasts are among the potential cell types that can be employed for bone repair. Other types of cells that could be used for bone regeneration are SCs, namely embryonic SCs (ESCs), induced pluripotent SCs (iPSCs), and mesenchymal SCs (MSCs).15 Osteoblasts are commonly employed in the bone regeneration procedure as they are naturally produced within the bone environment. Even so, they are limited in number and exhibit low proliferative capacity.16 Although ESCs are pluripotent, the use of these cells in cell-based therapy is still controversial due to the ethical concerns associated with their primary source of isolation, and the risk of teratoma formation along with the immunologic incompatibility.17 Hence, the use of ESCs for bone tissue engineering is not favourable. Hence, in recent years, an increasing number of studies have been dedicated to exploring the potential benefit of IPSCs in bone regenerative therapy. Nevertheless, the genetic manipulation approach used to develop iPSCs is the main concern in applying these cells to clinical applications.
Among the potential above-mentioned cells, MSCs appear more suitable for bone engineering due to their high proliferation rate and differentiation capacity, multipotency, and distinct immunological quality.18 MSCs are capable of differentiating into bone-forming osteoblasts, chondrocytes, and myocytes in response to different conditions (transcription factors, signalling molecules, molecular pathways), which makes them good cellular candidates for bone tissue engineering.15 Bone marrow (BM) has been the main source of MSCs since they were first isolated from this tissue in 1976.19 However, obtaining MSCs from BM sources is considered invasive, requires general anaesthesia, and is a painful harvesting procedure. Hence, the research and clinical applications of BM-MSCs are rather limited.20
Nevertheless, the discovery of MSCs from noninvasive sources such as adipose tissue, dental pulp, periosteum, peripheral blood, and umbilical cord provide alternative options for the isolation of MSCs.14,21,22 There are also a few potential sites for the isolation of MSCs within the craniofacial region. Accordingly, various types of dental SCs have been isolated, characterised, and studied such as dental pulp SCs (DPSCs),23 SCs from exfoliated deciduous teeth (SHED),24 periodontal ligament SCs,25 SCs from apical papilla,26 and dental follicle progenitor cells.27
Among the dental SCs, DPSCs and SHEDs are among the most studied in bone regeneration, including craniofacial bone defects. Regarding DPSCs alone, their osteogenic capacity has been frequently utilised for scaffold development to improve the osteogenic inductivity of cells embedded in scaffold.28, 29, 30 DPSC differentiation can be induced either in vitro or in vivo by incorporating the use of several growth factors such as bone morphogenetic proteins (BMPs),31,32 transforming growth factor beta (TGF-β),33 vascular endothelial growth factor,34,35 and fibroblast growth factor.36 Similarly, SHED has also been extensively studied in the context of bone regeneration.37, 38, 39 Although SHED is almost identical to DPSCs morphologically, which exhibit fibroblast-like cells and the ability to differentiate into osteogenic lineage, they differ in certain factors such as the expression level of growth factors.59 Practically, with a suitable microenvironment and appropriate delivery of growth factors, osteogenic signalling molecules can be transmitted to produce a desirable result. Given the information gathered from relevant studies, it is crucial to investigate the key signalling pathways that contribute to the osteogenic differentiation of these osteoprogenitor cells, to further comprehend their characteristics for bone repair therapy.
Signalling pathways in bone regeneration
Although significant progress has been made in bone regenerative therapy in recent years, understanding the fundamentals behind the cellular and molecular mechanisms is important for successfully implementing this approach. Depending on the signals or ligands that bind to the cell-surface receptor, activation of the pathway can stimulate different downstream signalling cascades, resulting in different gene expression and SC fate. It further allows the regulation of targeted pathways in osteoblast formation. Activation of pathways that stimulate osteoblast recruitment can lead to bone formation, while stimulation of pathways involving osteoclast formation will lead to bone loss unless there is interference from other factors.40
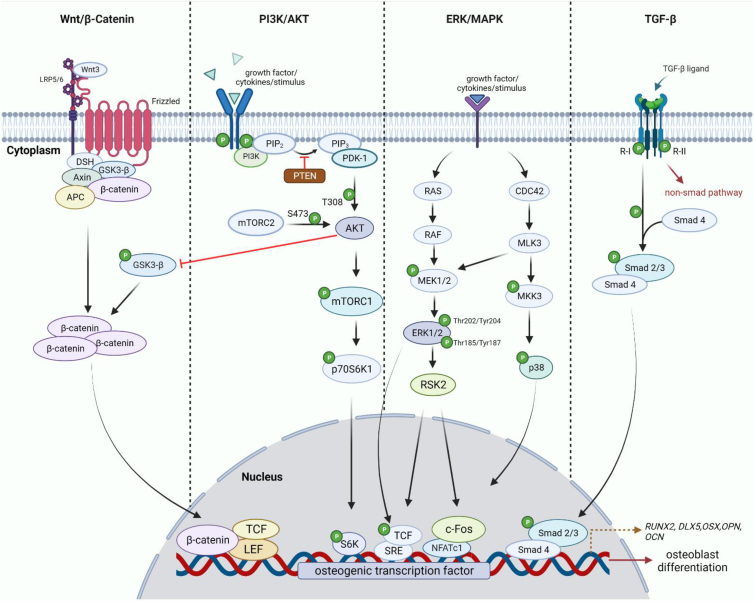
Differentiation of MSCs to osteoblast progenitors in bone formation is regulated by several signalling pathways such as BMP, Hedgehog, Wnt/β-catenin, Notch, and extracellular signal-regulated kinase (ERK), as shown in Figure 1.41,42 Table 1 depicts each of the signalling molecules’ functions in bone repair and regeneration. Despite meaningful and successfully collected experimental data from both in vitro and in vivo methods, the knowledge of bone signalling pathways has not yet been applied to the current cell-based regenerative treatment.
Figure 1.
Schematic illustration of the identified signalling pathways for craniofacial bone regeneration. The downstream of each pathway differs depending on the ligand binding to the receptor. The activated molecule then triggers the cascade reaction of the downstream molecules which will be transported into the nucleus to stimulate the osteogenic transcription factor associated with the osteoblast formation.
Table 1.
Key signalling molecules involved in bone repair and regeneration.
| Signalling molecules | Features | Function in bone regeneration | References |
|---|---|---|---|
| Extracellular messengers | |||
| BMP-2 | Osteogenic regulation factor | Involved in enhancement of ALP activity, regulation of Dlx5 in osteogenic differentiation (specifically target) | 68 |
| BMP-4 | Osteogenic and chondrogenic regulation factor | Increases in BMP4 expression activates the phosphorylation of Smad1/5/8 in Smad signalling. BMP4 acts as an early stimulator of endochondral ossification | 69,70 |
| BMP-6 | Osteogenic regulation factor | Regulation of BMP6 via Smad1/5/9 induces osteogenic differentiation in hMSCs. | 71 |
| BMP-7 | Osteoblast differentiation factor | Regulates osteoblast lineage determination by inducing Osf2/Cbfa1 expression before any other osteoblast-specific gene | 72 |
| BMP-9 | Osteogenic regulation factor | Stimulates early and late osteogenic differentiation and enhances the osteogenic marker expression in MSCs. Activation of Smad1/5/8 also depends on BMP-9-induced osteogenic differentiation of MSCs. | 73,74 |
| TGF-β | Osteoblast differentiation factor; bone mineralisation regulator | Induces osteoblast differentiation, proliferation, and migration with the support of various signalling molecules such as Smad, and AKT. It also regulates mineralisation of the bone matrix. | 75,76 |
| p38MAPK | Osteoblast differentiation regulator | An important regulator of early bone formation and development. p38 bound to BMP2/7 contributes to Runx2 and Osx transcriptional activity | 77,78 |
| ERK1/2 | Osteoblast differentiation factor | Regulates lineage specification into osteoblast and osteoclast differentiation | 79 |
| Intracellular messengers | |||
| Runx2 | Osteogenic transcription factor | Also known as core-binding factor subunit alpha-1 (Cbfa1), an important transcription factor that regulates the expression of major bone matrix protein genes through osteoblast-specific cis-acting element (OSE2), a direct binding site in the promoter of several osteoblast-specific genes. | 80 |
| Osterix (Osx) | Osteoblast-specific transcription factor | Also known as transcription factor Sp7. Downstream of Runx2 and is particularly expressed in osteoblast lineage cells. Osx is only present in mature osteoblasts; however, it acts as a negative regulator as it inhibits osteoblast proliferation via the Wnt/β-catenin pathway. | 81 |
| Dlx5 | Positive regulator of osteoblastogenesis | Promotes osteogenic differentiation in MSCs in cooperation of BMP-2 | 68 |
| Osteocalcin (OCN) | Bone formation marker; bone turnover marker | Vitamin K-dependent protein that is secreted only by mature osteoblasts into the bone microenvironment, which then induce the formation of hydroxyapatite crystals. Production of OCN only occurs at the mineralisation phase of bone formation. | 82,83 |
| Alkaline phosphatase (ALP) | Bone mineralisation regulator; bone turnover marker | Resides in osteoblast membranes and is then released into circulation. It also assists in hydroxyapatite production by providing inorganic phosphate. ALP can be utilised as a biochemical parameter for osteoblast formation and rate of fracture healing. | 84, 85, 86 |
| Bone sialoprotein (Bsp) | Bone development regulator, mineralisation regulator | Produced by osteoblasts and assists in cell attachment by promoting adherence of osteoblasts and osteoclasts to the matrix. It stimulates hydroxyapatite crystal formation in bones and teeth. | 87,88 |
| Msx1 | Craniofacial bone formation regulator | Involved in morphoregulation of craniofacial development as well as teeth and alveolar bone formation. Expression of Msx1 is also interrelated with other genes that regulate osteogenic cell lines such as Runx2,and BMP2. | 89,90 |
| Msx2 | Craniofacial bone formation regulator | Mainly involved in cranial bone formation. Msx2 stimulates MSCs into osteoblast lineage along in a Runx2-independent pathway and is induced by BMPs. Msx2 also supresses PPARγ and C/EBP transcriptional activity from progressing into adipocyte lineage. | 91 |
| β-catenin | Osteoblast differentiation factor | Its activation regulates bone-forming osteoblast activity and osteoblast lineage determination via the canonical Wnt pathway. | 92 |
In addition, several approaches within in vitro settings have been tested to study cellular signalling pathways for bone tissue engineering, comprising genomics and proteomics that can further be divided into different methodological approaches.43 Table 2 summarises the different experimental works involving in vitro techniques for signalling studies in bone repair. The inhibition assay has been widely used in targeting a specific molecule in the pathway to identify the molecule's effect on cell activity, which further leads to discovering the mechanisms of the signalling pathway itself.44, 45, 46, 47 Typically, the inhibition assay and Western blotting are performed consecutively. Western blotting used to evaluate the protein expressed after the inhibition assay. The cytotoxic effect on the cell determined by the inhibition assay depends on the doses of inhibitor used following a specified parameters consisting of the half maximal effective concentration (EC50) and the half maximal inhibitory concentration (IC50), which vary for different inhibitors.46,48 Due to the nature of the inhibitor used to repress the signalling molecules, performing an inhibition assay on the cells may affect the cell's activity such as proliferation, survival, and osteogenesis, depending on the pathway. targeted.
Table 2.
Application of in vitro techniques in signalling pathway studies.
| In vitro technique | Type of method | Principle | Samples Analysed | Key Findings | References |
|---|---|---|---|---|---|
| Genomics | Microarray analysis | Identification of gene expression profiles involved in osteogenic differentiation | Study of gene expression profiling microarray data during osteogenic differentiation of BM-MSCs, focusing on several signalling pathways based on KEGG pathway analysis: LPS-mediated, PI3K/AKT, and Wnt. | Microarray data from database repository revealed several upregulated differentially expressed genes (DEGs) related to the biological processes and pathways. | 99 |
| Real-time PCR (qRT-PCR) | Assessment of expression of bone-specific markers or gene expression of the signalling molecules in the osteogenic differentiated cells | Study of the effect of the PI3K inhibitor, LY3023414, on osteogenesis and osteoclastogenesis for bone remodelling using a preosteoblast cell line and bone marrow-derived macrophage cells (BMMs). | Treatment with a PI3K inhibitor led to reduced mRNA expression of osteoblast-specific genes in the preosteoblast cell line as well as expression of AKT in the preosteoblast cell during osteoclastogenesis in the BMMs. | 96 | |
| Proteomics | Specific inhibition assay | Assessment of the effect of pathway knockdown | Preosteoblast cell line MC3T3-E1 was subjected to a specific inhibitor targeting the PI3K pathway | Effect of inhibitor treatment decreased the mRNA expression of osteoblast-specific genes (Runx2, ALP, OCN), and expression of phosphorylated AKT related to the downstream process of the pathway. | 96,100 |
| Western blotting | Evaluation of protein expression and signalling molecules after cells were treated with pathway inhibitors. . |
MC3T3-E1 cells treated with C3G to enhance osteoblast regulation were subjected to ERK1/2 inhibition to study the involvement of the ERK/MAPK pathway in osteoblast proliferation. | Inhibition resulted in decreased phosphorylated ERK1/2 and OCN protein expression. Although Runx2 and ALP protein were not inhibited, it can be concluded that ERK was partially responsible for osteoblast differentiation due to the inhibitor effect on OCN. | 101 | |
| Immunostaining | Analysis of protein expression, distribution, and localisation of osteogenic differentiated cells | Immunofluorescence staining to examine the expression level of type I collagen in β-ecdysterone-treated MC3T3-E1 cells to study the osteogenic effect. | Expression of type I collagen in the treated group was significantly increased compared to the control group, which shows that β-ecdysterone can be implemented for bone regeneration due to its osteogenic properties. | 102 | |
| Mass spectrometry (MS) | Analysis of intercellular signalling proteins and the regulation of cellular posttranslational modifications | Comparative proteomics of gingival tissues and alveolar bone after tooth extraction to study the protein interaction and molecular mechanisms involved in periodontal bone tissue healing. | Several proteins and canonical pathways in both soft tissue and bone are interconnected for cellular organization and maintenance. This shows that both soft tissue and hard tissue are involved in bone repair and regeneration. | 103 |
In the next section, the role of the relevant signalling pathways in bone formation will be elaborated upon in further detail. In studies performed between 2017 and 2022, four major pathways have been extensively studied for in vitro bone regeneration, namely the Wnt, phosphoinositide 3-kinase (PI3K)/AKT, ERK/mitogen-activated protein kinase (MAPK), and TGF-β pathways.
Wnt pathway
The Wnt signalling pathway plays a significant role in cell regulation such as cell fate determination and proliferation during embryonic development while regulating cell maintenance, differentiation, and pluripotency in adult SCs.49 The Wnt/β-catenin pathway comprises canonical and non-canonical branches consisting of Wnt/Ca2+ and Wnt/planar cells polarity (PCP) pathways.50 The canonical Wnt signalling cascade is activated by the attachment of Wnt family proteins to the receptor. Notably, the canonical pathway is the most widely studied Wnt signalling pathway, specifically in bone homeostasis and development. The canonical Wnt pathway is associated with bone formation whereby β-catenin upregulation stimulates downstream gene expression.51
The role of β-catenin in the Wnt pathway includes regulating cell adhesion and mediating signalling activities, depending on the protein structural composition.52 Wnt/β-catenin induces bone formation through β-catenin/T-cell factor 1-mediated activation of the transcription factor Runt-related transcription factor 2 (Runx2), which is responsible for the osteogenic lineage of MSCs.53 The Wnt pathway plays a role in regulating osteoblast and osteoclast formation by upregulating the transcription of osteoprotegerin (OPG) while suppressing the level of receptor activator of nuclear factor kappa B ligand (RANKL), leading to a decrease in the RANKL/OPG ratio.54 Higher OPG expression in the RANKL/OPG ratio also inhibits the differentiation of osteoclasts while promoting osteoblast differentiation.55,56
Theoretically, the Wnt pathway is regulated by various effectors that either act as agonists or antagonists. A study of the Wnt pathway is demonstrated by targeting the signaling molecules and further analysis of gene and protein associated with mature bone formation. Recent studies on a Wnt inhibitor, Dikkopf 2 (DKK2), which binds to low-density lipoprotein receptor-related proteins 5 and 6, demonstrated that overexpression of DKK2 significantly affects cell viability and DNA synthesis.56,57 Overexpression of DKK2 also results in lower mRNA levels of the osteogenic-related factors, ALP, Runx, and OCN, as well as lower protein levels of β-catenin and Wnt1.56,57
Meanwhile, inhibition of pathways using DKK1 and DKK2 leads to OPG expression in osteoblasts, showing that inhibiting the Wnt pathway affects the ability of SCs to produce osteogenic-related factors, which are crucial for bone formation.58 Figure 2 depicts the mechanism of Wnt signalling in osteoblast formation. Another notable component of the Wnt pathway, glycogen synthase kinase 3 (GSK-3), regulates various signalling pathways that modulate cellular activities including cell signalling, proliferation, and cell fate determination.59,60 One of the GSK-3 isoforms, GSK-3β, negatively regulates the canonical Wnt/β-catenin whereby GSK-β by binding to adenomatous polyposis coli and axon, forming a protein complex that degrades β-catenin.61 A previous study showed that GSK-3β inhibition enhanced bone formation through Runx2-dependent transcription activity.62 Another study confirmed the role of GSK-3 in cell fate determination by demonstrating that inhibition of GSK-3β enhanced the mesenchymal progenitors with osteogenic potential by increasing the osteoblast number within 14 days.63 Despite the great potential for activating the Wnt pathway, inhibition of GSK-3β affects cell viability if the inhibitors are used at high concentrations.64
Figure 2.
Schematic illustration of Wnt pathway mechanism in osteoblast differentiation. Wnt upregulates the expression of OPG in osteoblast, resulting in low RANKL/OPG ratio and inhibit the osteoclast formation which in turn increases the osteoblast differentiation.
PI3K/AKT pathway
PI3K has several downstream targets including phosphoinositide-dependent kinase-1 (PDK1), serine/threonine kinase AKT (also known as protein kinase B), and p70 ribosomal protein S6 kinase (p70S6K).65 PI3K/AKT plays an important role in regulating various physiological processes such as apoptosis, proliferation, and cell differentiation. PI3K catalyses the conversion of phosphatidylinositol (4,5)-biphosphate (PIP2) to phosphatidylinositol (3,4,5)-triphosphate (PIP3), which then activates PDK1 and AKT in sequence.66 The critical role of PI3K/AKT in bone regulation has been demonstrated through the induction of progenitor cells into mature osteoblasts, as observed in AKT-knockout mice, which exhibited lesser bone mass compared to control mice.67 PI3K inhibitors have also been utilised in studies on the PI3K/AKT pathway in order to observe changes in the bone healing process based on the expression level of bone marker genes. Inhibition of the PI3K inhibitor using LY294002 revealed diminished osteoblast differentiation in the osteoporosis group.68 Furthermore, the inhibition of PI3K/AKT led to a decrease in genes associated with bone markers such as ALP, OCN, osterix (Osx), and Runx2.68,69 Collectively, these findings suggest the potential role of the PI3K/AKT signalling pathway in bone repair and regeneration.
A recent study on the role of the PI3K/AKT pathway in bone regeneration showed that treating the samples with the LY294002 inhibitor resulted in failure of the bone tissue to fill in a gap of a fractured bone.70 However, the study postulated possible crosstalk between PI3K/AKT and the Wnt pathway, whereby activation of AKT inhibits GSK-3β activity, leading to the accumulation of β-catenin, further confirming that PI3K/AKT regulates osteoblast function and fracture healing via β-catenin as well. This finding is consistent with another study that focused on the inhibition of pre-osteoblast apoptosis and showed that AKT activation results in the phosphorylation of GSK-3β, which leads to β-catenin activation via glucagon-like peptide-1, which is associated with bone marrow-derived mesenchymal stem/stromal cell (BMSC) osteogenic differentiation.71,72 In addition, another study on the PI3K/AKT pathway targeting PDK1, which is an upstream regulator of AKT, revealed that inhibition of PDK1 suppressed the ability of BMSCs to differentiate into osteoblasts in vitro.73 PDK1 is partly responsible for AKT activation by phosphorylating AKT at T308 upon binding to PIP3.74 The inhibition of PDK1 affects BMSCs at both the mRNA and protein levels, causing the expression of PDK-1 and AKT to steadily decrease over time.
Meanwhile, the mammalian target of rapamycin complex 2 (mTORC2) is responsible for phosphorylating AKT at S473, leading to complete activation of AKT, and thus being indirectly involved in regulating osteoblast differentiation. Deletion of the mTORC2 component, Rictor, leads to a lower level of ALP activity as well as the expression of the other osteoblast markers, further suggesting that mTORC2 enhances osteoblast differentiation via the PI3K/AKT pathway.75,76 In addition, mTORC2-activated hypoxia-inducible factor 1 alpha (HIF-1) also induces BMSC to differentiate into osteoblasts. Another multiprotein complex of mTOR which is mTORC1 also involves in the regulation of protein synthesis in the cell through p70S6K1.77 Activation of mTORC1 modulates osteoblast differentiation by regulating Runx2 expression through augmentation of Runx2 enhancer activity.78 The only difference between mTORC1 and mTORC2 is the complex component where mTORC1 consists of Raptor and is sensitive to rapamycin, whereas mTORC2 comprises Rictor which is insensitive to rapamycin.
Phosphatase and tensin homolog (PTEN) is a natural inhibitor of the PI3K/AKT pathway that antagonizes the action of PI3K by dephosphorylating PIP3 to generate PIP2.79 Hence, the inactivation of PTEN may directly contribute to the activation of AKT signalling. Targeted PTEN inactivation has been investigated in several studies to understand the role of the PI3K/AKT pathway in bone formation.80, 81, 82 The suppression of PTEN leads to a higher expression level of osteogenic markers, including OCN, ALP, and Runx2, after treatment with a specific PTEN inhibitor.83 Treatment of BMSCs with a PTEN inhibitor further significantly enhances the osteogenic activity through the PI3K/AKT pathway, which proves that inhibition of this specific signalling component increases osteoblast cell proliferation.84,85
ERK/MAPK pathway
MAPK is a protein kinase family responsible for the activation of bone signalling pathways when stimulated by extracellular messengers, such as growth factors and cytokines. The three main pathways in mammalian cells are: ERK1/2, c-Jun N-terminal kinases (JNK 1–3), and p38 MAPKs (α, β, δ, and γ).86 Each pathway class responds to different extracellular stimuli. For instance, ERK1/2 is stimulated by growth factors, hormones, and proinflammatory stimuli; whereas JNK and p38 are activated by environmental stresses and proinflammatory mediators.87 Both ERK isoforms, ERK1 (MAPK3) and ERK2 (MAPK1), are involved in the differentiation of MSCs to osteoblast-lineage cells where activation of the pathway is activated via phosphorylation at Thr202/Tyr204 and Thr185/Tyr187 by MAPK kinase (MEK1) and MEK2.88 Cascade activation is initiated by the binding of ligand to a membrane receptor, followed by RAS activation, which further stimulates RAF protein kinases.88 Runx2 is an ERK substrate that is involved in osteoblast differentiation. Activation of ERK contributes to the transcriptional activity of Runx2 at the phosphorylation sites S301 and S319. Tandem mass spectrometry analysis of Runx2 phosphorylation revealed S319 to be a direct ERK substrate.89
Besides Runx2, ERK also has other substrates that modulate osteogenesis such as RSK2, which phosphorylates activating transcription factor 4 (ATF4) and plays an important role in late osteoblast differentiation as it regulates bone mineralisation.90,91 However, ATF4 deficiency does not alter Runx2 although it does reduce Osx expression as Osx is a downstream gene of Runx292. ATF4 and Runx2 are co-expressed in osteoblast differentiation to enhance the activation of several osteoblast-specific genes such as Osx and OCN.92, 93, 94 Another relevant substrate of ERK that is associated with osteoblast formation is the Fos family of subunits of the AP-1 transcriptional complex, comprising FOS, FOSL1, FOSL2, and FOSB.95 The mechanism of Fos and AP-1 stimulation in osteoblast formation by ERK activation remains unclear; however, FOS proteins via cGMP-mediated ERK are reportedly involved in bone formation by mechanical stimulation.95,96
The role of ERK in the osteogenic differentiation of SCs is demonstrated by increases in the phosphorylation of ERK1/2 and Runx2 during cell growth in osteogenic conditions.97 ERK/MAPK involvement in osteoblast formation has also been demonstrated via knockout of osteoblast-specific MEK 1/2, leading to decreases in the transcriptional activities of Runx2, β-catenin, and ATF4, which that are crucial for the proliferation and survival of osteoblasts.88,92 Also, inhibition of ERK1/2 by PD98059 specifically suppresses upstream activation of ERK1/2 which is MEK, affecting the osteogenic capacity of dental SCs by decreasing the mRNA expression levels of osteogenesis-associated genes such as ALP, OPN, Col-1, and Bsp.98 Treatment of SCs with the selective inhibitor ERK1/2 kinase, U0126, also led to decreases in Runx2 protein level as well as the mRNA expression level of ALP.99,100 Although U0126 significantly suppressed the activation of ERK1/2, the inhibitor somehow did not negatively affect the cell viability depending on the dose applied.
By contrast, data from other studies have demonstrated that inhibition of the ERK pathway suggests possible crosstalk either within the MAPK pathway or with another pathway.101,102 Inhibition of ERK with U0126 in osteogenic-induced BMSCs resulted in no significant increase in Runx2 gene expression, but a noticeable upregulation occurred in the protein expression of ALP, OPN, and OCN.102 The crosstalk concept suggested that inhibition of ERK promotes p38 activity, which then modulates ALP expression and mineralisation. Thus, inhibition of the ERK pathway indicates mechanotransduction of the pathway by improvement in bone mineralisation and higher deposition of minerals into the collagenous matrix.103
Similarly, genetically deleted MEK1/2 in mature osteoblasts turned out to modulate bone formation by stimulating osteogenesis and the osteogenic factors, suggesting the involvement of another signalling pathway, specifically PI3K/AKT via mTORC2 molecules.101 Knockdown of MEK1/2 exhibits an increase in phosphorylation of mTORC2 downstream molecules, except mTORC1, which showed no changes in the downstream molecules. Therefore, it can be concluded that ERK may have a dual role in osteoblast differentiation and bone formation depending on various factors that remain unclear.
TGF-β pathway
The TGF-β superfamily consists of multiple subfamilies that are involved in growth factor secretion as regulatory peptides that control various cellular processes including bone resorption and formation. The known TGF-β subfamilies are TGF-β, BMPs, growth and differentiation factors, nodal, and activin. There are three isoforms of the TGF-β family in humans, namely TGF-β1, TGF-β2, and TGF-β3, which are located in different locations on various chromosomes.104 In the context of bone formation, TGF-β1 is the predominant growth factor in the bone matrix among the other TGF-β isoforms. Activation of TGF-β involves the binding of the ligands to the type II receptor (TGF-βr2) which recruits TGF-βr1 activation once phosphorylated.105 Hence, inducing signals through different TGF-β signalling can be divided into canonical and non-canonical pathways. The canonical TGF-β signalling pathway consists of TGF-β/activin/nodal activation, which requires the phosphorylation of Smad2/3 complexes joined with Smad4 followed by translocation into the nucleus where an additional co-factor is recruited to regulate target gene expression.106 The canonical pathway via Smad2/3 activation involves binding of the ligand to the type II activin receptor, which then recruits the type I receptor, activin receptor-like kinase (ALK4/5/7).107, 108, 109 Another canonical TGF-β pathway comprises the phosphorylation of Smad 1/5/8, whereby BMP signals are transduced by type I receptors consisting of ALK2/3/6, along with type II receptors.110
The BMPs involved in osteogenesis induction are BMP-2,4,6,7, and 9. In most cell types, TGF-β signals via TGF-βrII and ALK5. ALK5 induces cells into the osteoblast lineage by increasing the expression level of phosphorylated Smad2/3, which slowly decreases with osteoblast maturation.108 One study showed ALK5 inhibition by SB431542, which targets the activin/BMP/TGF-β pathway, resulting in the decreased expression of Runx2, RANKL, and OCN.111 Conversely, several other studies showed osteoblast induction when ALK5 was inhibited, leading to the elevated mRNA expression of osteoblast-specific markers such as Runx2, ALP, Osx, and OCN but decreased expression of phosphorylated Smad2/3.112,113 It is postulated that the crosstalk with various pathways such as ERK, p38, and BMP result in osteoblast differentiation even when TGF-β/Smad is inhibited.112,114
Meanwhile, non-canonical TGF-β signalling is stimulated independently of Smad2/3 activation. For instance, TGF-β1 via PI3K/AKT/mTOR/S6K1 induces osteogenic differentiation in human osteoblasts cell lines upon treatment with a PI3K/AKT inhibitor, leading to a reduction in ALP activity.115 The constitutively active AKT induces osteoblast induction when osteoblasts are inhibited by TGF-β1 treatment in the early stages when it exhibits increased OCN and ALP expression.116 The study also suggested that AKT plays a significant role in the TGF-β1-induced osteoblastic differentiation of pre-osteoblast MC3T3-E1 cells. Furthermore, the activation of TGF-β via p38 MAPK regulates osteoblast differentiation by upregulating the expression of Smad1/5/8, MKK3, p38 and several other osteoblast-specific markers such as Runx2, Osx, and OPN.117 The mechanism of p38 MAPK activation is suggested via phosphorylation at Thr and Tyr residue, which is stimulated by cytokines and in vivo environmental stress. Depending on the condition, TGF-β may also have dual roles where it is capable of regulating osteoblast differentiation in the early stages, but acts as an inhibitor in the late stages.118, 119, 120 In the late stages of osteoblast differentiation, TGF-β inhibits the expression of Runx2 and the terminal differentiation of osteoblasts, most likely via Smad3 that suppresses matrix mineralisation.121,122 Overall, it can be concluded that the TGF-β pathway plays a significant role in regulating molecular and cellular biological processes at the bone repair site, depending on the stage of bone cell formation.
Conclusion
Rapid development in SC research indicates huge potential for the utilisation of cells in regenerative medicine. Cell-based bone regenerative therapy serves as a great opportunity for the advancement of techniques used in bone repair and regeneration. This review emphasises the extreme necessity of understanding basic bone biology to identify the best strategies for a therapeutic approach. It is important for studies on bone tissue engineering, including biomaterials design and scaffold development, to understand the fundamental knowledge on cell growth and bone formation at the cellular and molecular levels to correctly assess the cell's responses. Apart from manipulating the external source, it is rather interesting to venture into manipulating the key players in bone formation itself, namely the bone progenitor cells. As we briefly discussed, the multipotent capacity of SCs to differentiate into various lineages offers vast opportunities for researchers to control the cell's commitment to osteoblast lineage, which is a crucial element for bone repair and regeneration. Despite the sufficient number of studies investigating the relevant pathways in bone regeneration, the lack of unanimous evidence on targeting a specific, identifiable predominant pathway remains a challenge. However, some of the studies reviewed within this paper demonstrated consistent beneficial outcomes for future therapy. Some of the reviewed signalling pathways should be explored in further detail, within in vitro and in vivo settings to obtain a wider picture of the different expression levels of osteoblastic markers at the gene, protein, cell, and tissue levels. The role of each signalling pathway could also be explored by targeting the downstream molecules and regulators within the signalling cascade. However, the crosstalk that occurs between different pathways and the system of the cellular activity when pathway alteration occurs needs to be further investigated. To date, only limited data exist on the efficacy of manipulating targeted signalling pathways for personalised cell-based bone regenerative treatment. The information provided in this review, coupled with an optimised biomaterials design, may potentially accelerate the rate of recovery, and improve treatment modalities for bone repair.
Source of funding
This research was supported by the Ministry of Higher Education (MOHE) Malaysia, through Fundamental Research Grant Scheme (FRGS/1/2020/SKK06/USM/03/3).
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Authors contributions
NY designed the initial study, analysed the research material, and finalised the final manuscript. NJNN conducted the research, and collected, organised, and interpreted the data. NA and KBAAN proofread and critically reviewed the final draft. All authors approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Saul D., Menger M.M., Ehnert S., Nüssler A.K., Histing T., Laschke M.W. Bone healing gone wrong: pathological fracture healing and non-unions—overview of basic and clinical aspects and systematic review of risk factors. Bioeng. 2023;10:85. doi: 10.3390/BIOENGINEERING10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9 doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iaquinta M.R., Mazzoni E., Bononi I., Rotondo J.C., Mazziotta C., Montesi M., et al. Adult stem cells for bone regeneration and repair. Front Cell Dev Biol. 2019;7 doi: 10.3389/fcell.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosset P., Deschaseaux F., Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res. 2014;100:S107–S112. doi: 10.1016/j.otsr.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Sheen J.R., Garla V.V. StatPearls; 2022. Fracture healing overview. [PubMed] [Google Scholar]
- 6.Marsell R., Einhorn T.A. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigham-Sadegh A., Oryan A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int Wound J. 2014;12:238–247. doi: 10.1111/iwj.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrivats A.R., Alvarez P., Schutte L., Hollinger J.O. Princ. Tissue Eng. Elsevier; 2014. Bone regeneration; pp. 1201–1221. [DOI] [Google Scholar]
- 9.Betz R.R. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002;25 doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 10.Oryan A., Alidadi S., Moshiri A., Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9 doi: 10.1186/1749-799x-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offner D., de Grado G.F., Meisels I., Pijnenburg L., Fioretti F., Benkirane-Jessel N., et al. Bone grafts, bone substitutes and regenerative medicine acceptance for the management of bone defects among French population: issues about ethics, religion or fear? Cell Med. 2019;11 doi: 10.1177/2155179019857661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Isla N., Huseltein C., Jessel N., Pinzano A., Decot V., Magdalou J., et al. Introduction to tissue engineering and application for cartilage engineering. Bio Med Mater Eng. 2010;20:127–133. doi: 10.3233/bme-2010-0624. [DOI] [PubMed] [Google Scholar]
- 13.Bueno E.M., Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–697. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 14.Husch J.F.A., van den Beucken J.J.J.P. Dent. Implant. Bone grafts. Elsevier; 2020. Cell-based therapies in bone regeneration; pp. 217–250. [DOI] [Google Scholar]
- 15.Battafarano G., Rossi M., Martino V De, Marampon F., Borro L., Secinaro A., et al. Strategies for bone regeneration: from graft to tissue engineering. Int J Mol Sci. 2021;22:1128. doi: 10.3390/ijms22031128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karamzadeh R., Eslaminejad M.B., Aflatoonian R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J Vis Exp. 2012 doi: 10.3791/4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansouri V., Beheshtizadeh N., Gharibshahian M., Sabouri L., Varzandeh M., Rezaei N. Recent advances in regenerative medicine strategies for cancer treatment. Biomed Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111875. [DOI] [PubMed] [Google Scholar]
- 18.Musiał-Wysocka A., Kot M., Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28:801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedenstein A., Chailakhjan R., Lalykina K. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 20.Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry. 2017;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 21.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9 doi: 10.1186/1478-811x-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaikh M.S., Shahzad Z., Tash E.A., Janjua O.S., Khan M.I., Zafar M.S. Human umbilical cord mesenchymal stem cells: current literature and role in periodontal regeneration. Cells. 2022;11 doi: 10.3390/CELLS11071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/PNAS.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/PNAS.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 26.Sonoyama W., Liu Y., Fang D., Yamaza T., Seo B.-M. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morsczeck C., Götz W., Schierholz J., Zeilhofer F., Kühn U., Möhl C., et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/J.MATBIO.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Sevari S.P., Shahnazi F., Chen C., Mitchell J.C., Ansari S., Moshaverinia A. Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J Biomed Mater Res A. 2020;108:557–564. doi: 10.1002/JBM.A.36836. [DOI] [PubMed] [Google Scholar]
- 29.Aghali A., Arman H.E. Photoencapsulated-mesenchymal stromal cells in biodegradable thiol-acrylate hydrogels enhance regeneration of craniofacial bone tissue defects. Regen Med. 2020;15:2115–2127. doi: 10.2217/RME-2020-0061. [DOI] [PubMed] [Google Scholar]
- 30.Alipour M., Ghorbani M., Johari Khatoonabad M., Aghazadeh M. A novel injectable hydrogel containing polyetheretherketone for bone regeneration in the craniofacial region. Sci Rep. 2023;13:864. doi: 10.1038/S41598-022-23708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skogh A.C.D., Kihlström L., Neovius E., Persson C., Beckman M.O., Engstrand T. Variation in calvarial bone healing capacity: a clinical study on the effects of BMP-2-hydrogel or bone autograft treatments at different cranial locations. J Craniofac Surg. 2013;24:339–343. doi: 10.1097/SCS.0B013E31827FF2B6. [DOI] [PubMed] [Google Scholar]
- 32.Tóth F., Gáll J.M., Tőzsér J., Hegedűs C. Effect of inducible bone morphogenetic protein 2 expression on the osteogenic differentiation of dental pulp stem cells in vitro. Bone. 2020;132 doi: 10.1016/J.BONE.2019.115214. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W., Yang G., Wang X., Jiang L., Jiang F., Li G., et al. COMMUNICATION magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration tissue regeneration. Adv Mater. 2017;29:43. doi: 10.1002/adma.201703795. [DOI] [PubMed] [Google Scholar]
- 34.Pankajakshan D., Voytik-Harbin S.L., Nör J.E., Bottino M.C. Injectable highly tunable oligomeric collagen matrices for dental tissue regeneration. ACS Appl Bio Mater. 2020;3:859–868. doi: 10.1021/ACSABM.9B00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Divband B., Aghazadeh M., Al-qaim Z.H., Samiei M., Hussein F.H., Shaabani A., et al. Bioactive chitosan biguanidine-based injectable hydrogels as a novel BMP-2 and VEGF carrier for osteogenesis of dental pulp stem cells. Carbohydr Polym. 2021:273. doi: 10.1016/J.CARBPOL.2021.118589. [DOI] [PubMed] [Google Scholar]
- 36.Del Angel-Mosqueda C., Gutiérrez-Puente Y., Pricila López-Lozano A., Romero-Zavaleta R.E., Mendiola-Jiménez A., Medina-De La Garza C.E., et al. Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro. 2015. [DOI] [PMC free article] [PubMed]
- 37.Farea M., Husein A., Halim A.S., Abdullah N.A., Mokhtar K.I., Lim C.K., et al. Synergistic effects of chitosan scaffold and TGFβ1 on the proliferation and osteogenic differentiation of dental pulp stem cells derived from human exfoliated deciduous teeth. Arch Oral Biol. 2014;59:1400–1411. doi: 10.1016/J.ARCHORALBIO.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Kharaziha M., Fathi M.H., Edris H., Nourbakhsh N., Talebi A., Salmanizadeh S. PCL-forsterite nanocomposite fibrous membranes for controlled release of dexamethasone. J Mater Sci Mater Med. 2015;26:1–11. doi: 10.1007/S10856-014-5364-4. [DOI] [PubMed] [Google Scholar]
- 39.Hiraki T., Kunimatsu R., Nakajima K., Abe T., Yamada S., Rikitake K., et al. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020;26:381–390. doi: 10.1111/ODI.13244. [DOI] [PubMed] [Google Scholar]
- 40.Iñiguez-Ariza N.M., Clarke B.L. Bone biology, signaling pathways, and therapeutic targets for osteoporosis. Maturitas. 2015;82:245–255. doi: 10.1016/J.MATURITAS.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Hojo H., Ohba S., Chung U Il. Signaling pathways regulating the specification and differentiation of the osteoblast lineage. Regen Ther. 2015;1:57–62. doi: 10.1016/J.RETH.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khotib J., Gani M.A., Budiatin A.S., Lestari M.L.A.D., Rahadiansyah E., Ardianto C. Signaling pathway and transcriptional regulation in osteoblasts during bone healing: direct involvement of hydroxyapatite as a biomaterial. Pharmaceuticals (Basel) 2021;14 doi: 10.3390/PH14070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaiswal N. Phytochem an in-silico in-vitro update. 2019. In-vitro techniques to study cell signaling; pp. 267–277. [DOI] [Google Scholar]
- 44.Zhuang L.F., Jiang H.H., Qiao S.C., Appert C., Si M.S., Gu Y.X., et al. The roles of extracellular signal-regulated kinase 1/2 pathway in regulating osteogenic differentiation of murine preosteoblasts MC3T3-E1 cells on roughened titanium surfaces. J Biomed Mater Res A. 2012;100:125–133. doi: 10.1002/JBM.A.33247. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q., Ren X., Bischoff D., Weisgerber D.W., Yamaguchi D.T., Miller T.A., et al. Nonmineralized and mineralized collagen scaffolds induce differential osteogenic signaling pathways in human mesenchymal stem cells. Adv Healthc Mater. 2017;6 doi: 10.1002/ADHM.201700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X., Chen W., Aung Z.M., Han W., Zhang Y., Chai G. LY3023414 inhibits both osteogenesis and osteoclastogenesis through the PI3K/Akt/GSK3 signalling pathway. Bone Joint Res. 2021;10:237–249. doi: 10.1302/2046-3758.104.BJR-2020-0255.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F., Chen G.D., Fan L.K. Knockdown of PDX1 enhances the osteogenic differentiation of ADSCs partly via activation of the PI3K/Akt signaling pathway. J Orthop Surg Res. 2022;17 doi: 10.1186/S13018-021-02825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai M., Feng M., Ye Y., Wu X., Liu D., Liao M., et al. Exogenous avian leukosis virus-induced activation of the ERK/AP1 pathway is required for virus replication and correlates with virus-induced tumorigenesis. Sci Rep. 2016;6 doi: 10.1038/SREP19226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Camp J.K., Beckers S., Zegers D., Van Hul W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev Reports. 2013 102 2013;10:207–229. doi: 10.1007/S12015-013-9486-8. [DOI] [PubMed] [Google Scholar]
- 50.Lojk J., Marc J. Roles of non-canonical wnt signalling pathways in bone biology. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Zhang X., Shao J., Liu H., Liu X., Luo E. Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/β-catenin pathway. Sci Rep. 2017;7 doi: 10.1038/S41598-017-03899-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valenta T., Hausmann G., Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714. doi: 10.1038/EMBOJ.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaur T., Lengner C.J., Hovhannisyan H., Bhat R.A., Bodine P.V.N., Komm B.S., et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/JBC.M500608200. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi Y., Maeda K., Takahashi N. Roles of Wnt signaling in bone formation and resorption. Jpn Dent Sci Rev. 2008;44:76–82. doi: 10.1016/J.JDSR.2007.11.002. [DOI] [Google Scholar]
- 55.Naoyuki T., Kazuhiro M., Akihiro I., Shunsuke U., Yasuhiro K. Regulatory mechanism of osteoclastogenesis by RANKL and wnt signals. Front Biosci (Landmark Ed) 2011;16:21–30. doi: 10.1109/leoswt.2008.4444364. [DOI] [PubMed] [Google Scholar]
- 56.Zhou B., Peng K., Wang G., Chen W., Liu P., Chen F., et al. miR-483-3p promotes the osteogenesis of human osteoblasts by targeting dikkopf 2 (DKK2) and the Wnt signaling pathway. Int J Mol Med. 2020;46:1571–1581. doi: 10.3892/ijmm.2020.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C., Qiao X., Zhang Z., Li C. MiR-128 promotes osteogenic differentiation of bone marrow mesenchymal stem cells in rat by targeting DKK2. Biosci Rep. 2020;40 doi: 10.1042/BSR20182121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujita K.-I., Janz S. Attenuation of WNT signaling by DKK-1 and-2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. 2007. [DOI] [PMC free article] [PubMed]
- 59.Cohen P., Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. 210 2001. [DOI] [PubMed] [Google Scholar]
- 60.Kapadia R.M., Guntur A.R., Reinhold M.I., Naski M.C. Glycogen synthase kinase 3 controls endochondral bone development: contribution of fibroblast growth factor 18. Dev Biol. 2005;285:496–507. doi: 10.1016/J.YDBIO.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Metcalfe C., Bienz M. Inhibition of GSK3 by Wnt signalling – two contrasting models. J Cell Sci. 2011;124:3537–3544. doi: 10.1242/JCS.091991. [DOI] [PubMed] [Google Scholar]
- 62.Kugimiya F, Kawaguchi H, Ohba S, Kawamura N, Hirata M, Chikuda H, et al. GSK-3b controls osteogenesis through regulating Runx2 activity n.d. 10.1371/journal.pone.0000837. [DOI] [PMC free article] [PubMed]
- 63.Gambardella A., Nagaraju C.K., O'Shea P.J., Mohanty S.T., Kottam L., Pilling J., et al. Glycogen synthase kinase-3α/β inhibition promotes in vivo amplification of endogenous mesenchymal progenitors with osteogenic and adipogenic potential and their differentiation to the osteogenic lineage. J Bone Miner Res. 2011;26:811–821. doi: 10.1002/JBMR.266. [DOI] [PubMed] [Google Scholar]
- 64.Naujok O., Lentes J., Diekmann U., Davenport C., Lenzen S. Cytotoxicity and activation of the Wnt/beta-catenin pathway in mouse embryonic stem cells treated with four GSK3 inhibitors. BMC Res Notes. 2014;7:1–8. doi: 10.1186/1756-0500-7-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dugourd C., Gervais M., Corvol P., Monnot C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertens (Dallas, Tex 1979) 2003;41:882–890. doi: 10.1161/01.HYP.0000060821.62417.35. [DOI] [PubMed] [Google Scholar]
- 66.Ramazzotti G., Ratti S., Fiume R., Follo M.Y., Billi A.M., Rusciano I., et al. Phosphoinositide 3 kinase signaling in human stem cells from reprogramming to differentiation: a tale in cytoplasmic and nuclear compartments. Int J Mol Sci. 2019;20:2026. doi: 10.3390/IJMS20082026. 2019;20:2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng X., Xu P.-Z., Chen M.-L., Hahn-Windgassen A., Skeen J., Jacobs J., et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xi J.-C., Zang H.-Y., Guo L.-X., Xue H.-B., Liu X.-D., Bai Y.-B., et al. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J Recept Signal Transduction. 2015;35:640–645. doi: 10.3109/10799893.2015.1041647. [DOI] [PubMed] [Google Scholar]
- 69.Chen L.L., Huang M., Tan J.Y., Chen X.T., Lei L.H., Wu Y.M., et al. PI3K/AKT pathway involvement in the osteogenic effects of osteoclast culture supernatants on preosteoblast cells. Tissue Eng Part A. 2013;19:2226. doi: 10.1089/TEN.TEA.2012.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong J., Xu X., Zhang Q., Yuan Z., Tan B. The PI3K/AKT pathway promotes fracture healing through its crosstalk with Wnt/β-catenin. Exp Cell Res. 2020;394 doi: 10.1016/j.yexcr.2020.112137. [DOI] [PubMed] [Google Scholar]
- 71.Wu X., Li S., Xue P., Li Y. Liraglutide inhibits the apoptosis of MC3T3-E1 cells induced by serum deprivation through cAMP/PKA/β-catenin and PI3K/AKT/GSK3β signaling pathways. Mol Cell. 2018;41:234–243. doi: 10.14348/molcells.2018.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meng J., Ma X., Wang N., Jia M., Bi L., Wang Y., et al. Activation of GLP-1 receptor promotes bone marrow stromal cell osteogenic differentiation through β-catenin. Stem Cell Rep. 2016;6:579–591. doi: 10.1016/J.STEMCR.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai Y., Zhang Q., Zhou Q., Zhang Y., Nong H., Liu M., et al. Effects of inhibiting PDK-1 expression in bone marrow mesenchymal stem cells on osteoblast differentiation in vitro. Mol Med Rep. 2021;23 doi: 10.3892/MMR.2020.11757/HTML. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemmings B.A., Restuccia D.F. The PI3K-PKB/Akt pathway. Cold Spring Harbor Perspect Biol. 2015;7:a026609. doi: 10.1101/cshperspect.a026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J., Holguin N., Shi Y., Silva M.J., Long F. mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res. 2015;30:369. doi: 10.1002/JBMR.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J., Long F. mTOR signaling in skeletal development and disease. Bone Res. 2018 61 2018;6:1–6. doi: 10.1038/s41413-017-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen W., Wu P., Yu F., Luo G., Qing L., Tang J. HIF-1α regulates bone homeostasis and angiogenesis, participating in the occurrence of bone metabolic diseases. Cells. 2022;11:1–23. doi: 10.3390/cells11223552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai Q., Xu Z., Ma X., Niu N., Zhou S., Xie F., et al. MTOR/Raptor signaling is critical for skeletogenesis in mice through the regulation of Runx2 expression. Cell Death Differ. 2017;24:1886–1899. doi: 10.1038/CDD.2017.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pulido R. PTEN inhibition in human disease therapy. Molecules. 2018;23 doi: 10.3390/molecules23020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chai J., Xu L., Liu N. miR-23b-3p regulates differentiation of osteoclasts by targeting PTEN via the PI3k/AKT pathway. Arch Med Sci. 2022;18:1542–1557. doi: 10.5114/AOMS.2019.87520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgers T.A., Hoffmann M.F., Collins C.J., Zahatnansky J., Alvarado M.A. Mice lacking pten in osteoblasts have improved intramembranous and late endochondral fracture healing. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jang H.D., Noh J.Y., Shin J.H., Lin J.J., Lee S.Y. PTEN regulation by the Akt/GSK-3β axis during RANKL signaling. Bone. 2013;55:126–131. doi: 10.1016/J.BONE.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Chen T., Wu Y., Tang Z. Role and mechanism of PTEN in adiponectin-induced osteogenesis in human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2017;483:712–717. doi: 10.1016/J.BBRC.2016.12.076. [DOI] [PubMed] [Google Scholar]
- 84.Yang S., Tian Y.S., Lee Y.J., Yu F.H., Kim H.M. Mechanisms by which the inhibition of specific intracellular signaling pathways increase osteoblast proliferation on apatite surfaces. Biomaterials. 2011;32:2851–2861. doi: 10.1016/J.BIOMATERIALS.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Dong J., Xu X., Zhang Q., Yuan Z., Tan B. Critical implication of the PTEN/PI3K/AKT pathway during BMP2-induced heterotopic ossification. Mol Med Rep. 2021;23 doi: 10.3892/MMR.2021.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keyse S.M. Handb. Cell signal. Elsevier; 2010. MAP kinase phosphatases; pp. 755–769. [DOI] [Google Scholar]
- 87.Kyriakis J.M., Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 88.Kim J.M., Yang Y.S., Park K.H., Oh H., Greenblatt M.B., Shim J.H. The ERK MAPK pathway is essential for skeletal development and homeostasis. Int J Mol Sci. 2019;20 doi: 10.3390/IJMS20081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ge C., Xiao G., Jiang D., Yang Q., Hatch N.E., Roca H., et al. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009;284:32533–32543. doi: 10.1074/JBC.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X., Matsuda K., Bialek P., Jacquot S., Masuoka H.C., Schinke T., et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: implication for Coffin-Lowry syndrome. Cell. 2004;117:387–398. doi: 10.1016/S0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 91.Chen C., Koh A.J., Datta N.S., Zhang J., Keller E.T., Xiao G., et al. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem. 2004;279:29121–29129. doi: 10.1074/JBC.M313000200. [DOI] [PubMed] [Google Scholar]
- 92.Yu S., Franceschi R.T., Luo M., Fan J., Jiang D. Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PLoS One. 2009;4:7583. doi: 10.1371/journal.pone.0007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao G., Jiang D., Ge C., Zhao Z., Lai Y., Boules H., et al. Cooperative interactions between activating transcription factor 4 and runx2/cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. 2005;280:30689–30696. doi: 10.1074/JBC.M500750200. [DOI] [PubMed] [Google Scholar]
- 94.Tominaga H., Maeda S., Hayashi M., Takeda S., Akira S., Komiya S., et al. CCAAT/Enhancer-binding protein promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell. 2008;19:5373–5386. doi: 10.1091/mbc.E08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greenblatt M.B., Shim J.H., Bok S., Kim J.M. The extracellular signal-regulated kinase mitogen-activated protein kinase pathway in osteoblasts. J Bone Metab. 2022;29:1–15. doi: 10.11005/JBM.2022.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rangaswami H., Marathe N., Zhuang S., Chen Y., Yeh J.C., Frangos J.A., et al. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem. 2009;284:14796–14808. doi: 10.1074/JBC.M806486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ge C., Cawthorn W.P., Li Y., Zhao G., Macdougald O.A., Franceschi R.T. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARγ transcription factors. J Cell Physiol. 2016;231:587–596. doi: 10.1002/jcp.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang L., Tang Z. Expression and regulation of the ERK1/2 and p38 MAPK signaling pathways in periodontal tissue remodeling of orthodontic tooth movement. Mol Med Rep. 2018;17:1499–1506. doi: 10.3892/mmr.2017.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang P., Wu Y., Jiang Z., Jiang L., Fang B. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. Int J Mol Med. 2012;29:1083–1089. doi: 10.3892/ijmm.2012.934. [DOI] [PubMed] [Google Scholar]
- 100.Ge C., Yang Q., Zhao G., Yu H., Kirkwood K.L., Franceschi R.T. Interactions between extracellular signal-regulated kinase 1/2 and P38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res. 2012;27:538. doi: 10.1002/JBMR.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J.-M., Yang Y.-S., Hong J., Chaugule S., Chun H., van der Meulen M.C., et al. Biphasic regulation of osteoblast development via the ERK MAPK–mTOR pathway. Elife. 2022;11 doi: 10.7554/eLife.78069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doan T.K.P., Park K.S., Kim H.K., Park D.S., Kim J.H., Yoon T.R. Inhibition of JNK and ERK pathways by SP600125- and U0126-enhanced osteogenic differentiation of bone marrow stromal cells. Tissue Eng Regen Med. 2012 96 2012;9:283–294. doi: 10.1007/S13770-012-0352-6. [DOI] [Google Scholar]
- 103.Lund A.W., Stegemann J.P., Plopper G.E. Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three-dimensional collagen I culture. Stem Cell Dev. 2009;18:331. doi: 10.1089/SCD.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poniatowski L.A., Wojdasiewicz P., Gasik R., Szukiewicz D. Transforming growth factor beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat Inflamm. 2015;2015 doi: 10.1155/2015/137823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clayton S.W., Ban G.I., Liu C., Serra R. Canonical and noncanonical TGF-β signaling regulate fibrous tissue differentiation in the axial skeleton. Sci Rep. 2020 101 2020;10:1–11. doi: 10.1038/s41598-020-78206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu M., Chen G., Li Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4 doi: 10.1038/BONERES.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pearsall R.S., Canalis E., Cornwall-Brady M., Underwood K.W., Haigis B., Ucran J., et al. A soluble activin Type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci U S A. 2008;105:7082–7087. doi: 10.1073/PNAS.0711263105/SUPPL_FILE/0711263105SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsunobu T., Torigoe K., Ishikawa M., de Vega S., Kulkarni A.B., Iwamoto Y., et al. Critical roles of the TGF-β type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev Biol. 2009;332:325–338. doi: 10.1016/J.YDBIO.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang M., Ma T., Hu B., Xiang W. FOXP1 promotes osteoblast differentiation via regulation of TGF-β/ALK-5 pathway. Sci Asia. 2022;48:423–428. doi: 10.2306/SCIENCEASIA1513-1874.2022.071. [DOI] [Google Scholar]
- 110.Xu X., Zheng L., Yuan Q., Zhen G., Crane J.L., Zhou X., et al. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018;6 doi: 10.1038/s41413-017-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang C., Wang Y., Xu H. Fluoride regulate osteoblastic transforming growth factor-β1 signaling by mediating recycling of the type I receptor ALK5. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0170674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamamoto K., Kishida T., Nakai K., Sato Y., Kotani S.I., Nishizawa Y., et al. Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal. Sci Rep. 2018;8 doi: 10.1038/S41598-018-26745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hedayati S., Parvaneh Tafreshi A., Moradi N., Zeynali B. Inhibition of transforming growth factor-β signaling pathway enhances the osteogenic differentiation of unrestricted somatic stem cells. J Cell Biochem. 2018;119:9327–9333. doi: 10.1002/JCB.27209. [DOI] [PubMed] [Google Scholar]
- 114.Maeda S., Hayashi M., Komiya S., Imamura T., Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23:552–563. doi: 10.1038/SJ.EMBOJ.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Zhang X., Zhao D., Liu B., Wang B., Yu W., et al. TGF-β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol Med Rep. 2019;19:3505–3518. doi: 10.3892/MMR.2019.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki E., Ochiai-Shino H., Aoki H., Onodera S., Saito A., Saito A., et al. Akt activation is required for TGF-β1-induced osteoblast differentiation of MC3T3-E1 pre-osteoblasts. PLoS One. 2014;9 doi: 10.1371/JOURNAL.PONE.0112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y., Zheng W.-K., Gao W.-S., Shen Y., Ding W.-Y. Function of TGF-beta and p38 MAKP signaling pathway in osteoblast differentiation from rat adipose-derived stem cells. Eur Rev Med Pharmacol Sci. 2013;17(12):1611–1619. [PubMed] [Google Scholar]
- 118.De Gorter D.J.J., Van Dinther M., Korchynskyi O., Ten Dijke P. Biphasic effects of transforming growth factor β on bone morphogenetic protein–induced osteoblast differentiation. J Bone Miner Res. 2011;26:1178–1187. doi: 10.1002/JBMR.313. [DOI] [PubMed] [Google Scholar]
- 119.Wang M.-K., Sun H.-Q., Xiang Y.-C., Jiang F., Su Y.-P., Zou Z.-M. Different roles of TGF-β in the multi-lineage differentiation of stem cells. World J Stem Cell. 2012;4:28. doi: 10.4252/WJSC.V4.I5.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nam B., Park H., Lee Y.L., Oh Y., Park J. TGF β 1 suppressed matrix mineralization of osteoblasts differentiation by regulating SMURF1 – C/EBP β – DKK1 Axis. Int J Mol Sci. 2020;21:9771. doi: 10.3390/ijms21249771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohyama Y., Tanaka T., Shimizu T., Matsui H., Sato H., Koitabashi N., et al. Runx2/Smad3 complex negatively regulates TGF-induced connective tissue growth factor gene expression in vascular smooth muscle cells. J Atherosclerosis Thromb. 2012;19(1):23–35. doi: 10.5551/jat.9753. [DOI] [PubMed] [Google Scholar]
- 122.Iwasaki Y., Yamato H., Fukagawa M. TGF-beta signaling in bone with chronic kidney disease. Int J Mol Sci. 2018;19:2352. doi: 10.3390/IJMS19082352. [DOI] [PMC free article] [PubMed] [Google Scholar]