Abstract
Urological complications in diabetes mellitus are very common; in fact, genitourinary complications are more common than diabetic neuropathy or nephropathy. These complications consist of sexual and urinary dysfunction, greatly impact quality of life, and result in increased morbidity. Diabetic autonomic neuropathy affects the entire autonomic nervous system and can lead to dysfunction of the cardiovascular, gastrointestinal, and genitourinary organ systems. Genitourinary dysfunction associated with diabetic autonomic neuropathy includes diabetic bladder dysfunction, sexual dysfunction, and recurrent urinary tract infections. While several studies have reported on genitourinary dysfunction in individuals with diabetes, UroEDIC, an ancillary study to the Diabetes Control and Complications Trial (DCCT) and its observational follow up, the Epidemiology of Diabetes Interventions and Complications study (EDIC), comprehensively characterized urologic complications in the cohort and examined the association between cardiovascular autonomic neuropathy and sexual and urinary dysfunction. UroEDIC demonstrated significant associations between autonomic neuropathy and urologic complications in type 1 diabetes, specifically erectile dysfunction, female sexual dysfunction, and lower urinary tract symptoms. In this narrative review, we review the current literature on urological complications in diabetes.
Keywords: Diabetes, Autonomic Neuropathy, Bladder Dysfunction, Erectile Dysfunction, Sexual Dysfunction
1. Introduction
Diabetic autonomic neuropathy (DAN), is a serious and common complication often identified in patients with type 1 diabetes mellitus (T1DM) (Pop-Busui et al., 2017a). Autonomic neuropathy in diabetes mellitus (DM) has a significant impact on morbidity, mortality, and quality of life. DAN often involves and affects the entire autonomic nervous system, and dysfunction can present in the major organ systems including cardiovascular, gastrointestinal, and genitourinary (Pop-Busui et al., 2017a). DAN may be isolated or coexist with other peripheral neuropathies and other diabetic complications. In isolation, it frequently precedes the detection of other complications (Thompson et al., 2005, Gandaglia et al., 2013).
The genitourinary complications associated with DAN contributes to various disorders including bladder and sexual dysfunction (Pop-Busui et al., 2017a). These urologic complications occur frequently in both men and women living with T1DM (Wessells, 2013, Brown et al., 2005) and type 2 diabetes (T2DM) (Sayyid and Fleshner, 2016, Kouidrat et al., 2017) and are associated with significant reductions in health related quality of life above and beyond other diabetic complications (Jacobson et al., 2015). In this article, we discuss the epidemiology, clinical presentation, risk factors and gaps in knowledge based on a detailed review of peer reviewed publications of autonomic dysfunction in diabetes impacting the genitourinary system. We also present a summary of current findings from UroEDIC, an ancillary study examining urologic complications of diabetes among participants from the Diabetes Control and Complications Trial (DCCT) and its observational follow up, the Epidemiology of Diabetes Interventions and Complications study (EDIC) (Wessells et al., 2018). Given the unparalleled, comprehensive phenotyping of this large T1DM cohort for all complications and risk factors, UroEDIC provides the best insight into the association between DAN and urological complications.
2. Autonomic Dysfunction in Diabetes
The autonomic nervous system controls several organ systems in the body, including the cardiovascular, gastrointestinal, and urogenital organ systems. Chronic hyperglycemia associated with diabetes is largely responsible for damage to small nerve fibers, resulting in diabetic autonomic neuropathy (DAN). DAN is a subtype of the peripheral polyneuropathies that accompany diabetes (Pop-Busui et al., 2017a).
Major clinical manifestations of DAN include resting tachycardia, exercise intolerance, orthostatic hypotension, constipation, gastroparesis, erectile dysfunction, sudomotor dysfunction, impaired neurovascular function, and hypoglycemic autonomic failure (Pop-Busui et al., 2017a). Signs and symptoms related to DAN typically do not occur until long after the onset of diabetes and vary greatly from asymptomatic to severe, and relate to the specific affected end organ systems. Subclinical DAN can occur within a year of diagnosis of diabetes (Pfeifer et al., 1984). Given the association of DAN with adverse cardiovascular outcomes, such as cardiovascular deaths, cardiovascular autonomic neuropathy (CAN) is the most clinically important and well-studied form of DAN (Pop-Busui et al., 2010, Pop-Busui et al., 2017a, Pop-Busui et al., 2017b). Noninvasive testing of CAN allows for extensive clinical and epidemiologic investigation. CAN has widespread early effects in the progression of DAN (Ziegler, 1994). In many studies, including those from UroEDIC, CAN is the surrogate measure of DAN. Reduced heart rate variation is the earliest indicator of CAN (Ziegler, 1999), and is an integral measure used to characterize CAN in UroEDIC participants.
3. Genitourinary Dysfunction in Diabetes
The World Health Organization (WHO) estimated that in 2014, of 422 million people worldwide living with diabetes (Roglic, 2016), 25-90% had diabetic uropathy, a complication that is more common that neuropathy or nephropathy (Panigrahy et al., 2017, Daneshgari and Moore, 2006). Diabetic uropathy has been recognized since 1935. The spectrum of diabetic uropathy consists of diabetic bladder dysfunction, sexual dysfunction, and recurrent urinary tract infections. Dysfunctional nerves in the lower spinal cord, from DAN, can cause urinary dysfunction. This dysfunction can present as decreased bladder sensation, incomplete emptying, urinary urgency, and urinary incontinence (Vinik et al., 2003) which can then lead to urinary tract infections. The impact of neuropathy on vascular tone and sympathetic autonomic response can also lead to erectile dysfunction and female sexual dysfunction (Thorve et al., 2011, Enzlin et al., 1998, Pop-Busui et al., 2015, Hotaling et al., 2016). Though not life- threatening, these symptoms have a major impact on quality of life and can result in increased morbidity (Hill et al., 2008).
4. DCCT/EDIC and UroEDIC
The DCCT and EDIC studies have been described in detail previously (Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, 1999, Molitch et al., 1993, Nathan et al., 2003). Briefly, the DCCT included 1,441 subjects with T1DM for 1-15 years with no (primary prevention cohort) or minimal diabetic retinopathy (secondary intervention cohort). Subjects were randomly assigned to either intensive or conventional treatment and were followed for 3-9 years (mean 6.5 years) (Molitch et al., 1993). The trial was terminated early in 1993 when intensive therapy was recommended for all subjects. In 1994, 96% of the original DCCT cohort agreed to participate in EDIC, which included annual examinations for complication status. Annual EDIC examinations began in 1994, with 1,375 (96%) DCCT subjects consenting to participate in EDIC. The mean age of the participants at EDIC baseline was 33.6 years with a mean duration of diabetes of 12.2 years. All men and women enrolled in EDIC were invited to participate in UroEDIC, an ancillary study designed to examine urologic complications of diabetes.
4.1. Urological Complications Evaluations in UroEDIC
This included the first standardized and validated assessments of ED, FSD, LUTS and UI in 2003 (EDIC year 10, UroEDIC baseline), 2010 (EDIC year 17, UroEDIC II), and annually thereafter. (Describe the measurements here…) in detail then remove from later paragraphs where you can discuss just findings
4.2. Cardiovascular Autonomic Neuropathy Evaluations in DCCT/EDIC (Braffett et al., 2016)
Standardized and rigorous CAN evaluations were established as part of DCCT.(put above ref here?) These included cardiovascular autonomic reflex tests, which assessed R-R response to paced breathing (R-R variation), Valsalva maneuver, and postural changes in blood pressure measured at baseline, biennially during DCCT, and at years 13/14 and 16/17 during EDIC (The Diabetes Control and Complications Research Trial Group, 1998, Pop-Busui et al., 2009). These cardiovascular reflex tests are objective, highly reproducible and recommended by consensus in the field as the gold-standard (Spallone et al., 2011). The standardized cut points for CAN measures used in DCCT included R-R variation<15 and Valsalva ratio≤1·5. Abnormal CAN function was defined as: either R-R variation<15 or R-R variation between 15-19·9 plus either a Valsalva ratio ≤1·5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure (Pop-Busui et al., 2009).
5. Diabetic Bladder Dysfunction
Diabetic bladder dysfunction (DBD) is the most common genitourinary complication of diabetes (Daneshgari et al., 2009). Afferent nerve impulses of bladder sensation and reflex bladder contraction are carried by sympathetic, parasympathetic, and somatic afferent and efferent nerves of the spinal cord. Bladder autonomic dysfunctions, therefore include sensory abnormalities resulting in insensate bladder, which leads to an elevated threshold (post void residual) to initiate the micturition reflex, then leading to increased bladder capacity and urinary retention (Blaivas, 1982). Damage to efferent parasympathetic fibers from DAN can cause symptoms including weak steam and dribbling, with detrusor areflexia. DBD is an umbrella description representing progressive clinical symptoms of storage and voiding bladder problems (Liu and Daneshgari, 2014). The presentation of diabetic bladder dysfunction varies based on gender, age, concurrent voiding problems, and diabetes duration (Esteghamati et al., 2007). Early stages include storage problems, such as urinary frequency, urgency, and urge incontinence (Daneshgari et al., 2009), and symptoms consistent with overactive bladder (OAB). This can then progress to insensate, decompensated bladder characterized by overflow incontinence, urinary retention, and increased post-void residual volumes also known as “diabetic cystopathy” (Yuan et al., 2015).
Diabetic cystopathy is also referred to as neurogenic bladder and is presumed to represents later stage bladder dysfunction attributed to DAN (Gomez et al., 2011). Changes in detrusor physiology, neuronal impairment, and urothelial dysfunction are the major factors which contribute to diabetic cystopathy (Gomez et al., 2011). Unlike other sequelae of DAN, the pathogenesis of diabetic bladder dysfunction results from the impact of both hyperglycemia and polyuria. Hyperglycemia induces oxidative stress (Rolo and Palmeira, 2006), which can damage smooth muscle cells and induce cell apoptosis leading to accelerated neurodegeneration in the diabetic bladder (Kanika et al., 2011, Whitmire et al., 2011). Polyuria leads to an adaptation to diuresis including bladder wall remodeling, thereby altering bladder function in diabetic persons (Liu and Daneshgari, 2006, Daneshgari et al., 2006).
The most common, classic urodynamic findings in individuals with diabetic bladder dysfunction are insensate bladder, increased post void residual volume, and decreased detrusor contractility (Wittig et al., 2019). Recent clinical studies have demonstrated these classic findings and emphasized that patients can often present with a mixed clinical picture. Table 1 reviews studies describing associations between neuropathic complications and bladder dysfunction in both men and women. A study by Ueda et al. evaluated asymptomatic diabetic patients. In this study, increased bladder volume at first sensation to void, decreased detrusor contractility, and increased post void residual volumes were observed in asymptomatic diabetic patients. This study, however, also noted a 25% incidence of detrusor overactivity, consistent with early stages of diabetic bladder dysfunction (Ueda et al., 2000). Large scale studies of urinary incontinence have demonstrated a 30-70% increased risk of overall incontinence with diabetes, with a 50% increase risk of urge incontinence in women (Brown et al., 1996). A study evaluating 1359 patients with diabetes, demonstrated that 23% of diabetic patients had overactive bladder; of these 48% had urinary incontinence (Liu et al., 2011).
Table 1.
Studies of bladder dysfunction in diabetes
| Authors (Year) | Overall Population (Diabetes type) |
Definition of Neuropathy |
Definition of Bladder Dysfunction |
Findings |
|---|---|---|---|---|
| Men and Women | ||||
| Wessells et al (2018) | 1059 T1DM DCCT/EDIC study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | LUTS, UTI | In men, age associated with LUTS, and persistence of LUTS. HbA1C in women associated with emergence of LUTS and persistent UI. |
| Wilke et al (2015) | 456,586 T2DM | n/a | UTI | Highest UTI event rates in those aged >89 years. Most important factors in UTI risk were older age, female gender, UTIs in the previous 2 years, number of comorbidities, and at |
| Pavy-Le Traon et al (2010) | 684 T1DM | CAN severity Ewing Score (0-5): deep breathing, Valsalva, stand test, HRV, SBS | Bladder dysfunction symptoms | Bladder dysfunction independently associated with CAN |
| Liu et al (2011) | 1359 T2DM | Detailed interview | OAB symptom score | The prevalence of OAB and OAB wet was 2.4-fold and 4.2-fold greater, in patients with diabetes duration>10 years and age>50 years. Age and male sex were independent risk factors for OAB, age and waist circumference were independent risk factors for OAB wet. |
| Esteghamati et al. (2007) | 66 | Neurological consultation for the presence of peripheral somatic neuropathy (sensory, motor). | IPSS | Female sex was associated with increased bladder capacity. Male sex was associated with decreased bladder compliance and bladder outlet obstruction. Old age associated with low flow rate and outlet obstruction. Detrusor instability associated with shorter duration of diabetes. Peripheral somatic neuripathy associated with low flow rate. |
| Kebapci et al (2007) | 54 T2DM 27 males 27 females | CAN: deep breathing, Valsalva, stand test | LUTS: IPSS, Urinary Incontinence, Urodynamic studies | QT prolongation associated with with increased Post void residual urine OR 2.33 (0.16-34.89) |
| Low et al (2004) | 231 T1DM/T2DM | Autonomic Symptom Profile (ASP) Composite Autonomic Severity Score (CASS) | ASP urinary domain: Bladder Dysfunction, Sexual dysfunction (males only) | Significant correlations between ASP urinary domain and overall CASS and domain scores |
| Ueda et al (2000) | 3500 n/a | 23 item urinary incontinence questionnaire | Urinary incontinence | Women with history of diabetes mellitus had increased risk for UI |
| Ueda et al (1997) | 63 Diabetes* | Sympathetic skin response: Mystro plus MS20 | Volume at first desire to void Max bladder capacity Bladder pressure Residual urine | Mean Vol. at first desire to void, Max bladder capacity lower for Sympathetic Skin response absent. Mean Bladder pressure and Residual urine greater for Sympathetic Skin response absent. |
| Men | ||||
| Pop-Busui et al (2015) | 635 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | LUTS: AUASI 8-35 | LUTS prevalence: 158 (25%) Odds of ED+LUTS: 2.65 (1.47-4.79) |
| Sarma et al (2012) | 186 | n/a | LUTS: AUASI | Men with diabetes had higher odds of moderate/severe LUTS. Those not taking medications had higher odds of worse LUTS than those taking medications. |
| Bansal et al (2011) | 52 Diabetes* | Sympathetic skin response: Medtronic electromyographic system | LUTS: IPSS 8-35 Urodynamic studies | Diabetic cystopathy correlated with abnormal motor and sensory nerve conduction velocity studies and abnormal sympathetic skin responses |
| Joseph et al (2003) | 708 n/a | n/a | LUTS: AUASI | History of diabetes was positively associated with LUTS |
| Michel et al (2000) | 1290 Diabetes* | n/a | LUTS: IPSS | Older age and IPSS independently associated with increased odds of having diabetes. Diabetics had significantly greater IPSS and smaller maximum flow rate than non-diabetic patients. |
| Women | ||||
| Tai et al. (2016) | 400 T2DM | Medical history of peripheral neuropathy | LUTS: AUASI | Women with poor glycemic control more likely to develop urinary retention. Diabetic neuropathy significant predicted LUTS. |
| Hotaling et al (2016) | 571 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | UI: Sandvik Severity Index 3-12 | UI prevalence: 172 (30%) |
| Lenherr et al (2016) | 64 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | UI: Sandvik Severity index | 15.3% of women with T1DM reported incident UI. Mean HbA1c was associated with increased odds of incident UI. |
| Lenherr et al (2016) | 572 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | Self-report | 15% of women reported at least one UTI in 12 months. Higher HbA1C associated with number of UTIs. |
| Boyko et al (2005) | 218 Diabetes* | n/a | UTI, asymptomatic bacteriuria, PVR | Increased risk of UTI in women with diabetes, specifically women taking insulin and women with longer diabetes duration. Increased asymptomatic bacteriuria in women with diabetes. |
| Lee et al (2004) | 194 | Detailed questioning about symptoms of paresthesia, dulled sensation, pain in legs and feet, measurement of sensory threshold (vibratory and thermal) on the feet. | LUTS: AUASI, PVR | Women with diabetes had higher nocturia scores, weaker urinary streams, less voided volumes, and lower maximal flow rates. Diabetes significant associated with decrease in baseline maximum flow. Peripheral neuropathy indepently associated with decrease in emptying efficiency. |
| Geerlings et al (2000) | 589 T1DM and T2DM | n/a | UTI | 14% of women with T1DM developed UTI, 23% of women with T2DM developed UTI. Risk factors for UTI development was presence of asymptomatic bacteriuria |
Type of diabetes not indicated
Note: AUASI, American Urological Association Symptom Index; CAN, cardiovascular autonomic neuropathy; DBP, diastolic blood pressure; DCCT/EDIC, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; ED, erectile dysfunction; HRV, heart rate variability; IIEF, International Index of Erectile Dysfunction; IPPS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; SBS, spontaneous baroreflex slope; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; OAB, overactive bladder; UI, urinary incontinence
Bladder dysfunction in diabetes is an important complication of diabetes and can start at an early stage; neuropathy is an explanation for the asymptomatic presentation of many patients as it can lead to an insensate bladder. The urothelium has an important sensor controlling bladder function. The bladder urothelium may also contribute to the decreased sensation in those with diabetic bladder dysfunction owing to reactive oxidative damage (Fedele, 2005).
5.1. Diabetic Bladder Dysfunction in Women
Women with diabetes suffer from a high rate of urinary incontinence. A recent survey demonstrated that 43% of women aged 50-64 and 51% of women aged 65-80 report urinary incontinence. Of these, 31% of women reported daily leakage episodes (Swenson C, November 2018). The Nurses’ Health Study, which examined 14,286 nurses, reported that women with DM were at significantly greater risk of prevalent incontinence, which was more marked for larger volumes of leakage. Greater risk of incontinence was associated with longer duration of DM. Women with type 2DM for over 10 years had an almost 50% risk of incontinence (Lifford et al., 2005). A study of 7949 women over the age of 65, demonstrated that those with DM were at greater risk of daily incontinence. The Diabetes Control and Complications Trial (DCCT) and its observational follow up, the Epidemiology of Diabetes Interventions and Complications study (EDIC) (Brown et al., 1999). In addition, a study of 1500 women aged 70-79 demonstrated a risk of urge incontinence in women with DM on insulin, demonstrating an association of DM severity and incontinence (Diokno et al., 1990). These studies are based on self-reported symptoms and may in effect, underestimate the risk of incontinence associated with DM.
Women also demonstrate other manifestations of diabetic bladder dysfunction. Lee et al. evaluated the effects of diabetes on voiding behavior in a cross-sectional study of 194 female patients with diabetes; a comparison with 162 nondiabetic controls was done. Confounding factors, such as neurological disorders and aging were eliminated. Voiding was evaluated with the American Urological Association Symptom-Index (AUA-SI) questionnaire, in addition to uroflowmetry and post void residual urine volume (Lee et al., 2004). Compared to controls, women with diabetes had more nocturia, weaker urinary streams, less voided volumes, and lower maximal flow rates. High residual urine (≥100ml) was demonstrated in 13.9% of participants, compared to 1.8% of controls. Female gender has also been associated with increased bladder capacity (Esteghamati et al., 2007). A study of 400 women with type 2 Diabetes demonstrated that women with poor glycemic control (as measured by Hemoglobin A1C greater than 8.4) are more likely to develop urinary retention than those with proper glycemic control (Tai et al., 2016). This study also evaluated the impact of diabetic neuropathy on lower urinary tract symptoms in women, and showed that peripheral diabetic neuropathy, not Hemoglobin A1C, was a significant predictor of lower urinary tract symptoms in women with DM.
5.2. Diabetic Bladder Dysfunction in Men
Male gender is associated with decreased bladder compliance and bladder outlet obstruction. Moreover, although benign prostatic hyperplasia (BPH) and diabetes have significant overlap in voiding symptoms, there is evidence that diabetes promotes the disease process of BPH (Gomez et al., 2011). The proposed mechanism is through the increased sympathetic tone of the prostate through high insulin levels. High insulin levels then increase sympathetic nerve activity and stimulate prostate growth (Parsons et al., 2006, Rohrmann et al., 2005, Sarma et al., 2009).
In men, lower urinary tract symptoms (LUTS), including straining, intermittency, postvoid dribbling, and weak stream can be attributed to benign prostatic hyperplasia (BPH) and diabetes. There is significant overlap, which is demonstrated by Kaplan et al, who studied diabetic men and showed that 57% of men with diabetes and LUTS had bladder outlet obstruction on urodynamics (Kaplan et al., 1995). Similar symptoms can be seen from urethral obstruction from BPH, and may also result from bladder dysfunction due to denervation and poor detrusor contractility. It is important to differentiate the clinical overlap of BPH and OAB. Men with diabetes may also have detrusor overactivity secondary to microvascular complications, which can also cause symptoms consistent with OAB including urinary urgency, frequency and nocturia. Several studies suggest that men with diabetes report increased frequency of LUTS with an estimated 25% to nearly twofold-increased risk of LUTS in men with diabetes (Sarma AV, Joseph et al., 2003, Michel et al., 2000a). Michel et al demonstrated in a large cohort of men with clinically diagnosed BPH, 13% of men with diabetes had worse LUTS and lower flow rate (Michel et al., 2000b). In addition, among men with BPH, diabetes is associated with increased LUTS compared to nondiabetic men (Michel et al., 2000a).
5.3. Diabetes and Urinary Tract Infections
Compared to individuals without diabetes, those with diabetes have an increased risk of UTI (Boyko et al., 2005, Chen et al., 2009). Epidemiological studies suggest that both asymptomatic bacteriuria and symptomatic UTIs may occur with more frequency in women with DM (Stapleton, 2002, Zhanel et al., 1995, Geerlings et al., 2000b). Women with DM have a 2-3 fold higher prevalence of asymptomatic bacteriuria and are at higher risk of symptomatic infection. While woman with type 2 DM and asymptomatic bacteriuria are at increased risk of symptomatic UTI (Geerlings et al., 2000a), those with T1DM and asymptomatic bacteriuria are at increased risk of pyelonephritis and impaired renal function (Geerlings et al., 2001). The increased risk of asymptomatic bacteriuria and symptomatic UTI is due to several mechanisms including glucosuria which can promote bacterial growth, immunosuppression and elevated postvoid residual or incomplete emptying (Hill et al., 2008, Chen et al., 2009). UTI risk increases with disease duration and severity (Gomez et al., 2011). In addition, Escherichia coli expressing type 1 fimbriae have increased adherence to the urothelium of diabetic patients. An analysis of 456,586 patients with diabetes demonstrated that UTI risk was associated with high Hemoglobin A1C values in the previous year and poor kidney function (Wilke et al., 2015). Older women with diabetes and previous UTI are also at greater risk of UTI.
5.4. Findings from UroEDIC
Bladder Dysfunction Evaluations in UroEDIC (Braffett et al., 2016)
LUTS severity in men was determined with the American Urological Association Symptom Index (AUASI), a standardized seven-item questionnaire (Barry et al., 1992). Scores range from 0 to 35 with 8-35 indicating the presence of LUTS (8-19 moderate, 20-25 severe) (Barry et al., 1992). Urinary incontinence in women was determined based on incontinence frequency and amount of urine lost per episode (drops, small splashes, more), using the validated Sandvik Severity Index (Sandvik et al., 1995). The Sandvik Severity Index is calculated from frequency and amount of urine loss on a scale of 0 to 12 (dry/mild – 0 to 2, moderate – 3 to 6, severe – 8 to 9, very severe – 12) with scores 3 to 12 indicating moderate/very severe UI.
UroEDIC has evaluated the effect of glycemic control on urologic complications, including urinary incontinence and urinary tract infections.
A UroEDIC study of 64 women with Type 1 Diabetes demonstrated that mean EDIC HbA1C was associated with increased odds of urinary incontinence (Lenherr et al., 2016b). Poor glycemic control was also associated with higher frequency of urinary tract infections (Lenherr et al., 2016a). In examining urologic complications at EDIC year 10 (UroEDIC I) and EDIC year 17/18 (UroEDIC II) (Wessells et al., 2018), most participants who had a urological complication at UroEDIC I had persistence of the same complication at UroEDIC II. The one exception was UTI in females, which was noted to have lower prevalence at UroEDIC II (29% who reported UTI at UroEDIC 1 also had UI at UroEDIC II).
6. Sexual Dysfunction in Diabetes
Impaired sexual function is a common complication of diabetes in men and women. Many studies have focused on erectile dysfunction (ED) in men, but it is important to note that women can also present with sexual dysfunction (Tamas and Kempler, 2014). Sexual dysfunction in both sexes is associated with depression and diminished quality of life. Table 2 reviews studies describing associations between neuropathic complications and sexual dysfunction in both men and women.
Table 2.
Studies of sexual dysfunction in diabetes
| Authors (Year) | Population | Definition of Autonomic Neuropathy |
Definition of Sexual Dysfunction |
Findings |
|---|---|---|---|---|
| Men and Women | ||||
| Wessells et al. (2018) | 1059 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | ED: IIEF single item FSD: FSFI-R | Majority with complication at UroEDIC I had persistence of the complication at UroEDIC II. In men, age associated with persistence of ED, OD, and LD. HbA1C associated with persistence of OD and ED. In women, age associated with with emergence of FSD, persistence of FSD. HbA1C in women associated with emergence of LUTS and persistent UI |
| Bak et al. (2017) | 215 T2DM | Medical history | ED: IIEF FSD: FSFI | Sexual dysfunction correlated with age and duration of diabetes. Sexual disorders correlated with occurrence of depression and acceptance of illness. |
| Bjerggaard et al. (2015) | 1170 T2DM | n/a | ED: IIEF FSD: FSFI-R | 54% of men and 12% of women had sexual dysfunction |
| Pavy-Le Traon et al. (2010) | 684 T1DM | CAN severity Ewing Score (0-5): deep breathing, Valsalva, stand test, HRV, SBS | Erectile dysfunction symptoms (erection frequency and maintenance) | Erectile dysfunction independently associated with CAN severity (p=0.0005) |
| Low et al. (2004) | 231 T1DM/T2DM | Autonomic Symptom Profile (ASP) Composite Autonomic Severity Score (CASS) | ASP: Sexual dysfunction | Men with T1DM and T2DM had significantly worse sexual function scores compared to controls (p<0.05 for both) |
| Men | ||||
| Corona et al. (2016) | 449 T2DM | Medical interviews | ED: IIEF | The combination of phosphodiesterase 5 inhibitor therapy and an integrated approach to achieving metabolic targets in men with T2DM can improve sexual function and depressive symptoms. |
| Ghafoor et al. (2015) | 200 Diabetes patients with ED* | Composite Autonomic Severity Score (CASS) | ED: IIEF | Autonomic Neuropathy prevalence: 86 (43%) |
| Pop-Busui et al. (2015) | 635 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | ED: IIEF single item | ED prevalence: 290 (46%) Odds of ED+LUTS: 2.65 (1.47-4.79) |
| Bansal et al (2011) | 52 Diabetes* | Sympathetic skin response: Medtronic electromyographic system | ED: 5-item score <21 | Diabetic neuropathy not associated with ED |
| Penson et al. (2009) | 713 T1DM | IIEF | ED, OD, DL | ED was present in 34%, OD in 20%, and DL in 55%. All cause bother, though ED causes more general sexual bother. |
| Hamdan et al. (2008) | 56 T2DM, 30 controls | R-R variation <10, Valsalva ratio ≤1.2 | Ultrasound penile vasculature assessment PSV≤30 cm/sec and EDV≥5 cm/sec | Diabetic ED group had higher HbA1c and oxidative stress levels (p=0.001), lower R-R ratio (p<0.002) and neurophysiological parameters compared to controls |
| Debono et al. (2008) | 22 T2DM | CAN: Age specific Inspiration Ratio (E/I from R-R variation), Valsalva ratio ≤1.2, standing 30:15 ratio ≤1.031 | ED: IIEF≤21 | No significant associations observed between CAN measures and ED |
| Burke et al. (2007) | 53 Diabetes* | n/a | Previously validated Male sexual function index | Men with diabetes at baseline has greater dysfunction in all 5 sexual domains (sexual drive, erectile function, ejaculatory function, sexual problem assessment, and sexual satisfaction). |
| Pegge et al. (2006) | 33 ED (20 T1DM/T2DM), 30 controls (15 T1DM/T2DM) | Inspiration Ratio (E/I from R-R variation), Valsalva ratio | ED: IIEF | E/I ratios of diabetic men significantly lower than controls (p<0.02). No difference in CAN measures by ED status. |
| Bleustein et al. (2002) | 73 (53 ED, 20 No ED) | Index finger and glans penis vibration, pressure, spatial perception, warm/cold thermal thresholds | ED: IIEF≤25 | Neuropathic measures of the glans penis significantly associated with ED |
| De Angelis et al. (2001) | 60 T2DM | CAN: Deep breathing, Squatting vagal test, Squatting sympathetic test, Heat-pain threshold, Warm threshold, Vibratory threshold | ED: IIEF≤25 | Heat-pain, warm perception thresholds, cardiovascular reflex tests abnormal in men with ED (p<0.05) |
| Sairam et al. (2001) | 129 n/a | n/a | ED: men were already diagnosed | The prevalence of undiagnosed DM was higher in men with ED than in the general population. |
| Hecht et al. (2001) | 49 ED | 15-item Autonomic Symptom questionnaire, Nerve Condition studies, Sphincter ani electromyography, Vibratory thresholds, Temperature Perception thresholds, CAN: Heart rate variability | ED: physician referral based on diagnosis | Frequency of abnormal nerve conduction studies, heart rate variability higher in men with diabetic ED |
| Fedele et al. (2001) | 1010 T1DM/T2DM | Ewing Score ≥2 positive responses | ED: failure to achieve and maintain erection sufficient for satisfactory sexual performance | Erectile dysfunction associated with autonomic neuropathy (RR=1.16) |
| Wellmer et al. (1999) | 79 T1DM/T2DM | Thermal thresholds, Vibration thresholds, Light touch thresholds, Axon reflex vasodilation, Axon reflec sweating, Sural and peroneal nerve conduction studies | ED: erection insufficient for intercourse and erection could not be sustained for duration of intercourse | Neuropathic pain (p<0.05), abnormal sensory axon-reflex vasodilation (p<0.001), and decreased sural nerve action potential (p<0.01) significantly greater in men with ED |
| Women | ||||
| Hotaling et al. (2016) | 371 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | FSD: FSFI-R | FSD prevalence: 153 (41%) |
| Maiorino et al. (2016) | 145 | Diabetic neuropathy index | FSD: FSFI Sexual activity-related distress: FSDS | Depression and mental health were independent predictors of FSD. Sexual function was significant impaired in women on multiple daily injection. |
| Elyasi et al. (2015) | 150 | Medical record history | FSD: FSFI | High prevalence of sexual dysfunction (79%), especially among those with depression. |
| Enzlin et al. (2009) | 424 T1DM DCCT/EDIC Study | CAN: R-R variation <15, or R-R variation 15-19.9 plus Valsalva ratio ≤1.5, 10 mm Hg drop in DBP | FSD: FSFI-R | 35% of women had FSD. Depression and marital status were significant predictors of FSD. |
| Fatemi et al. (2009) | 50 T2DM | n/a | FSD: Arizona Sexual Experience Scale (ASEX) form | Diabetes significantly impaired the sexual performance of diabetic women. Determinants of sexual function included age and duration of diabetes |
| Abu Ali et al. (2008) | 1137 | Medical record history of autonomic neuropathy | FSD: FSFI | No independent association between autonomic neuropathy and FSD |
Type of diabetes not indicated
Note: AUASI, American Urological Association Symptom Index; CAN, cardiovascular autonomic neuropathy; DBP, diastolic blood pressure; DCCT/EDIC, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; ED, erectile dysfunction; OD, orgasmic dysfunction; LD, low sexual desire; DL, decreased libido; FSD, female sexual dysfunction; FSFI-R, Female Sexual Function Index; FSDS, Female Sexual Distress Scale; IIEF, International Index of Erectile Dysfunction; LUTS, lower urinary tract symptoms; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus
6.1. Female Sexual Dysfunction
Female sexual dysfunction (FSD) encompasses dyspareunia, vaginal laxity, and decreased sexual desire, arousal, or orgasm (Basson et al., 2000, Haylen et al., 2010). These are also symptoms that have been reported with higher frequency in women with T1DM and T2DM (Aslan and Fynes, 2008, Enzlin et al., Fatemi and Taghavi, 2009). The Female Sexual Function Index (FSFI) was established to provide a valid, reliable instrument to assess key domains of female sexual function, including desire, arousal, lubrication, orgasm, satisfaction and pain, and is the scale used to evaluate female sexual function (Rosen et al., 2000).
In the DCCT cohort, depression and marital status were predictors of sexual dysfunction in women. Similar findings were observed in women with T2DM (Nowosielski et al., 2010). A recent study of sexual function in 145 women with T1DM and compared them to controls. In addition, this study evaluated the impact of insulin delivery method on the prevalence of sexual dysfunction. This study demonstrated that young women with T1DM and insulin pump had similar prevalence of sexual dysfunction compared to health age- matched women (Maiorino et al., 2017). Those on multiple daily injections, however, had significantly impaired sexual function compared to health age-matched women. In this study, similar to previous studies, depression and mental health status were independent predictors of FSD in diabetic women. Despite the higher prevalence of sexual dysfunction among diabetic women, in these studies, no strong association was found with diabetes related factors, such as glycemic control and complications of diabetes. With this, many conclude that female sexual dysfunction in diabetic patients, as in patients without diabetes (Laumann et al., 1999), are more psychogenic in nature (Giraldi and Kristensen, 2010). This would imply that women with diabetes have more psychological sequelae, potentially from their disease burden. Unlike research in male sexual dysfunction, research in female sexual dysfunction, however, is significantly limited leading to less conclusive than those of studies in men (Enzlin et al., 1998).
Studies on female sexual dysfunction in diabetes have been limited by small sample size, inadequate characterization of diabetes, particularly with regard to glycemic control, neurovascular complications, psychological adjustment to diabetes, and presence or absence of comorbid depression (Enzlin et al., 1998) A recent study evaluated the relationship of sexual dysfunction with depression and acceptance of illness in women and men with T2DM. There were 114 women with T2DM and 183 controls (Bak et al., 2017). The FSFI was used in women, this study found 68% prevalence of FSD compared to 17% in controls. Patients with DM had higher scores on the Beck Depression Inventory (BDI), which negatively correlated with point values on the FSFI. This study concluded that sexual dysfunction in diabetic women is correlated with depression and acceptance of their illness.
The contribution of DAN to female sexual dysfunction is less well-established. It is likely that disruption of the autonomic nervous system, which contributes to major components of sexual function, is a driver of dysfunctions in certain domains of female sexual function, such as arousal and orgasmic function.
6.2. Male Sexual Dysfunction
Male sexual function is often impaired in diabetes. The majority of studies of sexual function in men have focused on ED. However, male sexual dysfunction encompasses abnormalities of orgasmic, ejaculatory function, desire/libido and erectile function (Penson et al., 2009). Burke et al. demonstrated that the presence of diabetes was significantly associated with all aspects of sexual function and sexual satisfaction (Burke et al., 2007).
Erectile dysfunction is as a man’s consistent or recurrent inability to attain and/or maintain penile erection sufficient for sexual activity,(NIH Consensus Conference,1993) and has been found to be an age-related disease that affects 20% of men over the age of 40 years. ED is thought to be a surrogate marker for both diabetes and cardiovascular disease and has been shown to be the first sign of diabetes, diagnosed in 12-30% of men who initially present with ED (Lewis, 2001, Sairam et al., 2001, Gur et al., 2014)· Up to 75% of men with diabetes have ED, with diabetic men being affected at a younger age (Kouidrat et al., 2017). A meta-analysis of 145 studies, estimates the overall prevalence of ED at 52.5%, 37.5% for T1DM and 57.7% for T2DM. A study demonstrated that compared to controls without diabetes, those with diabetes were at increased odds of having ED (OR 3.56; 95 CI 2.54-5.16). In men with diabetes, ED not only occurs earlier than in the normal population, but it is also less responsive to oral pharmacological therapy (Feldman et al., 1994). ED has a multifactorial pathophysiology and can occur concurrently with vasculopathy, neuropathy, and depression (Lizza and Rosen, 1999, Kouidrat et al., 2017). DAN is an important factor in the development of diabetes associated ED and DAN affects neural systems at all levels of tumescence and rigidity. Mechanistically, diabetes can impair endothelial relaxation leading to ED. Studies have demonstrated impairment in the initial stage of tumescence, with impaired non-adrenergic-non-cholinergic (NANC) nerve endings and neuronal nitric oxide synthase (nNOS) activation and NOS release (Hidalgo-Tamola and Chitaley, 2009).
6.3. Findings from UroEDIC
Sexual Dysfunction Evaluations in UroEDIC (Braffett et al., 2016)
Presence of ED in men was initially assessed with the validated International Index of Erectile Function (IIEF) (Rosen et al., 1997). ED was then ascertained based on a single question from the IIEF: In the last 4 weeks, how would you rate your confidence to get and keep an erection? This single question had been shown in DCCT/EDIC studies to strongly correlate with the IIEF erectile function domain composite score. In addition, it was shown to correlate well with bother due to erectile problems and global sexual bother, and thus serves as a proxy for global sexual function and bother (Penson et al., 2009). A separate question queried use of oral medications and/or erectile aids/devices of all participants. Those who reported any use were categorized as having ED. FSD was evaluated by the abbreviated version of the Female Sexual Function Index (FSFI-R), a widely used, well-validated, multi-dimensional, self-report measure that assesses sexual function across six domains including sexual desire, arousal, lubrication, orgasm, satisfaction, and pain. Presence of FSD was defined by a score ≥22.75 on the FSFI-R (Rosen et al., 2000).
Female Sexual Dysfunction in UroEDIC
The overall prevalence of FSD at EDIC year 10 (UroEDIC I) was 35.4%. Though biivariate analysis demonstrated that women with FSD were more likely to have evidence of microvasculopathy, multivariable analysis demonstrated that only depression status and marital status were predictors of FSD (Enzlin et al., 2009). Women with depression had 2.08 higher odds of FSD than women who were not depressed, and married women had 2.49 higher odds of FSD than unmarried women. UroEDIC also evaluated the association between measures of CAN with FSD and UI among female UroEDIC participants (Hotaling et al., 2016). This study demonstrated that CAN was significantly more prevalent among women with FSD and/or UI; 41% and 44% of women with FSD and UI, respectively, had positive measures of CAN compared to 30% and 38% of women without FSD or UI. Similar associations were observed between CAN and UI at EDIC year 13/14. In multivariable analyses adjusting for known risk factors such as age, BMI, post-menopausal status, parity, smoking, alcohol consumption, HbA1c, SBP, duration of diabetes, and beta blocker use, lower R-R variation at EDIC year 16/17 were associated with significantly increased odds of FSD and Valsalva ratio ≤1.5 was associated with increased odds of UI at EDIC year 13/14. Although autonomic dysfunction has been considered to be an important factor in the etiology of many diabetic complications, this study is among the first to systematically demonstrate a link between CAN and FSD in a large cohort of well-characterized patients with T1DM (Vinik et al., 2003).
Erectile Dysfunction in UroEDIC
UroEDIC examined the association between CAN and ED and LUTS in a large cohort of male participants with T1DM in the DCCT/EDIC study and found that the prevalence of an abnormal composite CAN was higher in participants with ED or LUTS compared with those without ED or LUTS (p<0·0001) (Pop-Busui et al., 2015). In multivariable analysis, participants with CAN had 2.65 greater odds of ED and LUTS (95% CI=1.47,4.79). This strong association between CAN, ED and LUTS suggests that CAN may be a useful surrogate biomarker of not only more generalized autonomic neuropathy, but also may predict the development of ED and LUTS in men with long-standing T1DM. These findings are the first to systematically demonstrate a link between CAN and ED/LUTS in a large cohort of well-characterized men with T1DM. In evaluating other male sexual dysfunctions, we have demonstrated that participants with Orgasmic Dysfunction and ED had 2.89 higher odds of CAN and 2.28 higher odds of peripheral neuropathy as measured by the Michigan Neurological Screening Instrument (MNSI).
Persistence of urologic complications in UroEDIC
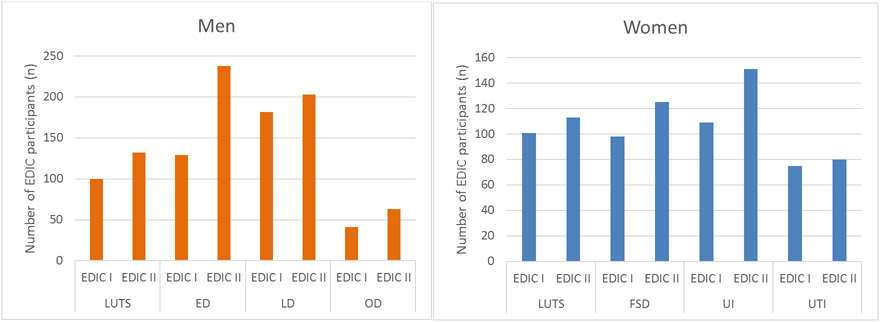
UroEDIC recently examined urologic complications at EDIC year 10 (UroEDIC I) and EDIC year 17/18 (UroEDIC II) and demonstrated that most participants who had a urological complication at UroEDIC I had persistence of the same complication at UroEDIC II (Wessells et al., 2018) (Figure 1). For women, the prevalence of FSD at UroEDIC II was highest at 42%, and for men, the prevalence of ED was highest at 45%. The important factor in this study was that though there was persistence of many urological complications, there was a subset of participants in which there was remission of symptoms. As demonstrated in Table 3, women with autonomic neuropathy at UroEDIC II had 1.67 higher odds of FSD (95%CI= 1.07,2.60) and 1.57 higher odds of UTI (95%CI= 1.00,2.47). In men, those with autonomic neuropathy at UroEDIC II had 2.07 higher odds of LUTS (95%CI=1.42, 3.01), 2.82 higher odds of ED (95%CI= 2.01, 3.94), and 2.40 higher odds of Orgasmic Dysfunction (95%CI=1.49, 3.88).
Figure 1a and 1b. Increased Prevalence of Urologic complications in men (a) and women (b) from Uro EDIC year I and II.
Data from Wessels, H. et al., 2018, Diabetes Care
Increased prevalence of urologic complications from EDIC I to EDIC II in men and women.
Cohort totals: Women n=508, Men n=551
*EDIC: Epidemiology of Diabetes Interventions and Complications study
*LUTS: Lower Urinary Tract Symptoms
*FSD: Female Sexual Dysfunction
*UI: Urinary Incontinence
*UTI: Urinary Tract Infection
*ED: Erectile Dysfunction
*LD: Low Desire
*OD: Orgasmic Dysfunction
Table 3.
Women with autonomic neuropathy have higher odds of FSD and UTI; Men with autonomic neuropathy have higher odds of LUTS, ED and OD.
| Women | ||||
|---|---|---|---|---|
| LUTS | FSD | UI | UTI | |
| N total respondents | 579 | 371 | 571 | 555 |
| N (%) | 128 (22) | 153 (41) | 172 (30) | 95 (17) |
| Autonomic Neuropathy | 1.28 (0.85-1.92) | 1.67 (1.07-2.60) | 1.28 (0.88-1.85) | 1.57 (1.00-2.47) |
| Men | ||||
| LUTS | ED | LD | OD | |
| N total respondents | 643 | 635 | 598 | 564 |
| N(%) | 158 (25) | 290 (46) | 243 (41) | 83 (15) |
| Autonomic Neuropathy | 2.07 (1.42-3.01) | 2.82 (2.01-3.94) | 1.28 (0.91-1.80) | 2.40 (1.49-3.88) |
Data from Wessels, H. et al., 2018, Diabetes Care
Note: Data are odds ratios (95% CI) unless otherwise specified. Significant values are in boldface type. Autonomic neuropathy defined at EDIC year 16/17 as an R-R variation <15 or R-R variation <20 in combination with a Valsalva ratio ≤1.5 or a decrease of >10 mmHg in diastolic blood pressure upon standing. DCCT/EDIC, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; LUTS, lower Urinary tract symptoms; UI, urinary incontinence; UTI urinary tract infection; ED, erectile dysfunction; OD, orgasmic dysfunction; LD, low sexual desire; FSD, female sexual dysfunction
7. Conclusions
Urological complications of diabetes appear to be strongly associated with diabetic autonomic neuropathy. The influence of glycemic control in DM on these complications is important given the link of occurrence of these complications with increasing diabetes severity. In men and women with long-standing diabetes, CAN may be a predictor of ED and LUTS and FSD and UI respectively. Though it is not possible to reverse neuropathy once it occurs, understanding long term, downstream effects of autonomic neuropathy on organ systems including the genitourinary system is important. An increased understanding can potentially lead to patients taking control of their diabetes to prevent or delay further nerve damage. Additional large-sample longitudinal studies are needed to evaluate the association and progression of urogenital complications as a function of autonomic dysfunction in diabetes and to identify treatment strategies to reduce the burden and psychosocial consequences of CAN on genitourinary complications.
Acknowledgments
This work was supported by National Health Institute (NIH) grant K12DK111011 (National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1993. NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. Jama, 270, 83–90. [PubMed] [Google Scholar]
- 1998. The Diabetes Control and Complications Research Trial Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia, 41, 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1999. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care, 22, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASLAN E & FYNES M 2008. Female sexual dysfunction. Int Urogynecol J Pelvic Floor Dysfunct, 19, 293–305. [DOI] [PubMed] [Google Scholar]
- BAK E, MARCISZ C, KRZEMINSKA S, DOBRZYN-MATUSIAK D, FOLTYN A & DROSDZOL-COP A 2017. Relationships of Sexual Dysfunction with Depression and Acceptance of Illness in Women and Men with Type 2 Diabetes Mellitus. Int J Environ Res Public Health, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY MJ, FOWLER FJ JR., O'LEARY MP, BRUSKEWITZ RC, HOLTGREWE HL, MEBUST WK & COCKETT AT 1992. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Journal of Urology, 148, 1549–57; discussion 1564. [DOI] [PubMed] [Google Scholar]
- BASSON R, BERMAN J, BURNETT A, DEROGATIS L, FERGUSON D, FOURCROY J, GOLDSTEIN I, GRAZIOTTIN A, HEIMAN J, LAAN E, LEIBLUM S, PADMA-NATHAN H, ROSEN R, SEGRAVES K, SEGRAVES RT, SHABSIGH R, SIPSKI M, WAGNER G & WHIPPLE B 2000. Report of the international consensus development conference on female sexual dysfunction: definitions and classifications. J Urol, 163, 888–93. [PubMed] [Google Scholar]
- BLAIVAS JG 1982. The neurophysiology of micturition: a clinical study of 550 patients. J Urol, 127, 958–63. [DOI] [PubMed] [Google Scholar]
- BOYKO EJ, FIHN SD, SCHOLES D, ABRAHAM L & MONSEY B 2005. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol, 161, 557–64. [DOI] [PubMed] [Google Scholar]
- BRAFFETT BH, WESSELLS H & SARMA AV 2016. Urogenital Autonomic Dysfunction in Diabetes. Curr Diab Rep, 16, 119. [DOI] [PubMed] [Google Scholar]
- BROWN JS, GRADY D, OUSLANDER JG, HERZOG AR, VARNER RE & POSNER SF 1999. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol, 94, 66–70. [DOI] [PubMed] [Google Scholar]
- BROWN JS, SEELEY DG, FONG J, BLACK DM, ENSRUD KE & GRADY D 1996. Urinary incontinence in older women: who is at risk? Study of Osteoporotic Fractures Research Group. Obstet Gynecol, 87, 715–21. [DOI] [PubMed] [Google Scholar]
- BROWN JS, WESSELLS H, CHANCELLOR MB, HOWARDS SS, STAMM WE, STAPLETON AE, STEERS WD, VAN DEN EEDEN SK & MCVARY KT 2005. Urologic complications of diabetes. Diabetes Care, 28, 177–85. [DOI] [PubMed] [Google Scholar]
- BURKE JP, JACOBSON DJ, MCGREE ME, NEHRA A, ROBERTS RO, GIRMAN CJ, LIEBER MM & JACOBSEN SJ 2007. Diabetes and sexual dysfunction: results from the Olmsted County study of urinary symptoms and health status among men. J Urol, 177, 1438–42. [DOI] [PubMed] [Google Scholar]
- CHEN SL, JACKSON SL & BOYKO EJ 2009. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol, 182, S51–6. [DOI] [PubMed] [Google Scholar]
- DANESHGARI F, HUANG X, LIU G, BENA J, SAFFORE L & POWELL CT 2006. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol, 290, R1728–35. [DOI] [PubMed] [Google Scholar]
- DANESHGARI F, LIU G, BIRDER L, HANNA-MITCHELL AT & CHACKO S 2009. Diabetic bladder dysfunction: current translational knowledge. J Urol, 182, S18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANESHGARI F & MOORE C 2006. Diabetic uropathy. Semin Nephrol, 26, 182–5. [DOI] [PubMed] [Google Scholar]
- DIOKNO AC, BROCK BM, HERZOG AR & BROMBERG J 1990. Medical correlates of urinary incontinence in the elderly. Urology, 36, 129–38. [DOI] [PubMed] [Google Scholar]
- ENZLIN P, MATHIEU C, VANDERSCHUEREN D & DEMYTTENAERE K 1998. Diabetes mellitus and female sexuality: a review of 25 years' research. Diabet Med, 15, 809–15. [DOI] [PubMed] [Google Scholar]
- ENZLIN P, ROSEN R, WIEGEL M, BROWN J, WESSELLS H, GATCOMB P, RUTLEDGE B, CHAN KL & CLEARY PA 2009. Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/ EDIC study cohort. Diabetes Care, 32, 780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTEGHAMATI A, RASHIDI A, NIKFALLAH A & YOUSEFIZADEH A 2007. The association between urodynamic findings and microvascular complications in patients with long-term type 2 diabetes but without voiding symptoms. Diabetes Res Clin Pract, 78, 42–50. [DOI] [PubMed] [Google Scholar]
- FATEMI SS & TAGHAVI SM 2009. Evaluation of sexual function in women with type 2 diabetes mellitus. Diab Vasc Dis Res, 6, 38–9. [DOI] [PubMed] [Google Scholar]
- FEDELE D 2005. Therapy Insight: sexual and bladder dysfunction associated with diabetes mellitus. Nat Clin Pract Urol, 2, 282–90; quiz 309. [DOI] [PubMed] [Google Scholar]
- FELDMAN HA, GOLDSTEIN I, HATZICHRISTOU DG, KRANE RJ & MCKINLAY JB 1994. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J.Urol, 151, 54–61. [DOI] [PubMed] [Google Scholar]
- GANDAGLIA G, SALONIA A, PASSONI N, MONTORSI P, BRIGANTI A & MONTORSI F 2013. Erectile dysfunction as a cardiovascular risk factor in patients with diabetes. Endocrine, 43, 285–92. [DOI] [PubMed] [Google Scholar]
- GEERLINGS SE, STOLK RP, CAMPS MJ, NETTEN PM, COLLET JT, SCHNEEBERGER PM & HOEPELMAN AI 2001. Consequences of asymptomatic bacteriuria in women with diabetes mellitus. Arch Intern Med, 161, 1421–7. [DOI] [PubMed] [Google Scholar]
- GEERLINGS SE, STOLK RP, CAMPS MJ, NETTEN PM, COLLET TJ & HOEPELMAN AI 2000a. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care, 23, 1737–41. [DOI] [PubMed] [Google Scholar]
- GEERLINGS SE, STOLK RP, CAMPS MJ, NETTEN PM, HOEKSTRA JB, BOUTER KP, BRAVENBOER B, COLLET JT, JANSZ AR & HOEPELMAN AI 2000b. Asymptomatic bacteriuria may be considered a complication in women with diabetes. Diabetes Mellitus Women Asymptomatic Bacteriuria Utrecht Study Group. Diabetes Care, 23, 744–9. [DOI] [PubMed] [Google Scholar]
- GIRALDI A & KRISTENSEN E 2010. Sexual dysfunction in women with diabetes mellitus. J Sex Res, 47, 199–211. [DOI] [PubMed] [Google Scholar]
- GOMEZ CS, KANAGARAJAH P & GOUSSE AE 2011. Bladder dysfunction in patients with diabetes. Curr Urol Rep, 12, 419–26. [DOI] [PubMed] [Google Scholar]
- GUR S, PEAK TC, KADOWITZ PJ, SIKKA SC & HELLSTROM WJ 2014. Review of erectile dysfunction in diabetic animal models. Curr Diabetes Rev, 10, 61–73. [DOI] [PubMed] [Google Scholar]
- HAYLEN BT, DE RIDDER D, FREEMAN RM, SWIFT SE, BERGHMANS B, LEE J, MONGA A, PETRI E, RIZK DE, SAND PK & SCHAER GN 2010. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn, 29, 4–20. [DOI] [PubMed] [Google Scholar]
- HIDALGO-TAMOLA J & CHITALEY K 2009. Review type 2 diabetes mellitus and erectile dysfunction. J Sex Med, 6, 916–926. [DOI] [PubMed] [Google Scholar]
- HILL SR, FAYYAD AM & JONES GR 2008. Diabetes mellitus and female lower urinary tract symptoms: a review. Neurourol Urodyn, 27, 362–7. [DOI] [PubMed] [Google Scholar]
- HOTALING JM, SARMA AV, PATEL DP, BRAFFETT BH, CLEARY PA, FELDMAN E, HERMAN WH, MARTIN CL, JACOBSON AM, WESSELLS H & POP-BUSUI R 2016. Cardiovascular Autonomic Neuropathy, Sexual Dysfunction, and Urinary Incontinence in Women With Type 1 Diabetes. Diabetes Care, 39, 1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON AM, BRAFFETT BH, CLEARY PA, DUNN RL, LARKIN ME, WESSELLS H, SARMA AV & GROUP, D. E. R. 2015. Relationship of urologic complications with health-related quality of life and perceived value of health in men and women with type 1 diabetes: the Diabetes Control and Complications Trial/Epidemiology of Interventions and Complications (DCCT/EDIC) cohort. Diabetes care, 38, 1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPH MA, HARLOW SD, WEI JT, SARMA AV, DUNN RL, TAYLOR JMG, JAMES SA, COONEY KA, DOERR KM, MONTIE JE & SCHOTTENFELD D 2003. Risk Factors for Lower Urinary Tract Symptoms in a Population-based Sample of African-American Men. Am.J.Epidemiol, 157, 906–914. [DOI] [PubMed] [Google Scholar]
- KANIKA ND, CHANG J, TONG Y, TIPLITSKY S, LIN J, YOHANNES E, TAR M, CHANCE M, CHRIST GJ, MELMAN A & DAVIES KD 2011. Oxidative stress status accompanying diabetic bladder cystopathy results in the activation of protein degradation pathways. BJU Int, 107, 1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN SA, TE AE & BLAIVAS JG 1995. Urodynamic findings in patients with diabetic cystopathy. J Urol, 153, 342–4. [DOI] [PubMed] [Google Scholar]
- KOUIDRAT Y, PIZZOL D, COSCO T, THOMPSON T, CARNAGHI M, BERTOLDO A, SOLMI M, STUBBS B & VERONESE N 2017. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med, 34, 1185–1192. [DOI] [PubMed] [Google Scholar]
- LAUMANN EO, PAIK A & ROSEN RC 1999. Sexual dysfunction in the United States: prevalence and predictors. Jama, 281, 537–44. [DOI] [PubMed] [Google Scholar]
- LEE WC, WU HP, TAI TY, LIU SP, CHEN J & YU HJ 2004. Effects of diabetes on female voiding behavior. J Urol, 172, 989–92. [DOI] [PubMed] [Google Scholar]
- LENHERR SM, CLEMENS JQ, BRAFFETT BH, CLEARY PA, DUNN RL, HOTALING JM, JACOBSON AM, KIM C, HERMAN W, BROWN JS, WESSELLS H & SARMA AV 2016a. Glycemic Control and Urinary Tract Infections in Women with Type 1 Diabetes: Results from the DCCT/EDIC. J Urol, 196, 1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENHERR SM, CLEMENS JQ, BRAFFETT BH, DUNN RL, CLEARY PA, KIM C, HERMAN WH, HOTALING JM, JACOBSON AM, BROWN JS, WESSELLS H & SARMA AV 2016b. Glycaemic control and risk of incident urinary incontinence in women with Type 1 diabetes: results from the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabet Med, 33, 1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS RW 2001. Epidemiology of erectile dysfunction. Urol Clin North Am, 28, 209–16, vii. [DOI] [PubMed] [Google Scholar]
- LIFFORD KL, CURHAN GC, HU FB, BARBIERI RL & GRODSTEIN F 2005. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc, 53, 1851–7. [DOI] [PubMed] [Google Scholar]
- LIU G & DANESHGARI F 2006. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol, 291, R837–43. [DOI] [PubMed] [Google Scholar]
- LIU G & DANESHGARI F 2014. Diabetic bladder dysfunction. Chin Med J (Engl), 127, 1357–64. [PMC free article] [PubMed] [Google Scholar]
- LIU RT, CHUNG MS, LEE WC, CHANG SW, HUANG ST, YANG KD, CHANCELLOR MB & CHUANG YC 2011. Prevalence of overactive bladder and associated risk factors in 1359 patients with type 2 diabetes. Urology, 78, 1040–5. [DOI] [PubMed] [Google Scholar]
- LIZZA EF & ROSEN RC 1999. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res, 11, 141–3. [DOI] [PubMed] [Google Scholar]
- MAIORINO MI, BELLASTELLA G, CASTALDO F, PETRIZZO M, GIUGLIANO D & ESPOSITO K 2017. Sexual function in young women with type 1 diabetes: the METRO study. J Endocrinol Invest, 40, 169–177. [DOI] [PubMed] [Google Scholar]
- MICHEL MC, MEHLBURGER L, SCHUMACHER H, BRESSEL HU & GOEPEL M 2000a. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J.Urol, 163, 1725–1729. [PubMed] [Google Scholar]
- MICHEL MC, MEHLBURGER L, SCHUMACHER H, BRESSEL HU & GOEPEL M 2000b. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol, 163, 1725–9. [PubMed] [Google Scholar]
- MOLITCH ME, STEFFES MW, CLEARY PA & NATHAN DM 1993. Baseline analysis of renal function in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group [corrected]. Kidney Int, 43, 668–74. [DOI] [PubMed] [Google Scholar]
- NATHAN DM, LACHIN J, CLEARY P, ORCHARD T, BRILLON DJ, BACKLUND JY, O'LEARY DH & GENUTH S 2003. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med, 348, 2294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWOSIELSKI K, DROSDZOL A, SIPINSKI A, KOWALCZYK R & SKRZYPULEC V 2010. Diabetes mellitus and sexuality--does it really matter? J Sex Med, 7, 723–35. [DOI] [PubMed] [Google Scholar]
- PANIGRAHY R, SINGH B & DAS SK 2017. Diabetic uropathy and bladder dysfunctions. Diabetes Metab Syndr, 11, 81–82. [DOI] [PubMed] [Google Scholar]
- PARSONS JK, CARTER HB, PARTIN AW, WINDHAM BG, METTER EJ, FERRUCCI L, LANDIS P & PLATZ EA 2006. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab, 91, 2562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENSON DF, WESSELLS H, CLEARY P & RUTLEDGE BN 2009. Sexual dysfunction and symptom impact in men with long-standing type 1 diabetes in the DCCT/EDIC cohort. J Sex Med, 6, 1969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFER MA, WEINBERG CR, COOK DL, REENAN A, HALTER JB, ENSINCK JW & PORTE D JR. 1984. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care, 7, 447–53. [DOI] [PubMed] [Google Scholar]
- POP-BUSUI R, BOULTON AJ, FELDMAN EL, BRIL V, FREEMAN R, MALIK RA, SOSENKO JM & ZIEGLER D 2017a. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care, 40, 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POP-BUSUI R, BRAFFETT BH, ZINMAN B, MARTIN C, WHITE NH, HERMAN WH, GENUTH S & GUBITOSI-KLUG R 2017b. Cardiovascular Autonomic Neuropathy and Cardiovascular Outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care, 40, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POP-BUSUI R, EVANS GW, GERSTEIN HC, FONSECA V, FLEG JL, HOOGWERF BJ, GENUTH S, GRIMM RH, CORSON MA & PRINEAS R 2010. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care, 33, 1578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POP-BUSUI R, HOTALING J, BRAFFETT BH, CLEARY PA, DUNN RL, MARTIN CL, JACOBSON AM, WESSELLS H & SARMA AV 2015. Cardiovascular autonomic neuropathy, erectile dysfunction and lower urinary tract symptoms in men with type 1 diabetes: findings from the DCCT/EDIC. J Urol, 193, 2045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POP-BUSUI R, LOW P, WABERSKI B, MARTIN C, ALBERTS J, FELDMAN E, SOMMER C, CLEARY P, LACHIN J, HERMAN W & DCCT/EDIC RESEARCH GROUP 2009. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the DCCT/EDIC Study. Circulation, 119, 2886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGLIC G 2016. WHO Global report on diabetes: A summary. 1, 3–8. [Google Scholar]
- ROHRMANN S, PLATZ EA & GIOVANNUCCI E 2005. Lifestyle and benign prostatic hyperplasia in older men: what do we know? The Journal of Men's Health and Gender, 2, 230–235. [Google Scholar]
- ROLO AP & PALMEIRA CM 2006. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol, 212, 167–78. [DOI] [PubMed] [Google Scholar]
- ROSEN R, BROWN C, HEIMAN J, LEIBLUM S, MESTON C, SHABSIGH R, FERGUSON D & D'AGOSTINO R JR. 2000. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther, 26, 191–208. [DOI] [PubMed] [Google Scholar]
- ROSEN RC, RILEY A, WAGNER G, OSTERLOH IH, KIRKPATRICK J & MISHRA A 1997. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology, 49, 822–30. [DOI] [PubMed] [Google Scholar]
- SAIRAM K, KULINSKAYA E, BOUSTEAD GB, HANBURY DC & MCNICHOLAS TA 2001. Prevalence of undiagnosed diabetes mellitus in male erectile dysfunction. BJU Int, 88, 68–71. [DOI] [PubMed] [Google Scholar]
- SANDVIK H, HUNSKAAR S, VANVIK A, BRATT H, SEIM A & HERMSTAD R 1995. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol, 48, 339–43. [DOI] [PubMed] [Google Scholar]
- SARMA AV, PARSONS JK, MCVARY K & WEI JT 2009. Diabetes and benign prostatic hyperplasia/lower urinary tract symptoms--what do we know? J Urol, 182, S32–7. [DOI] [PubMed] [Google Scholar]
- SARMA AV, S. S. J., HOLLINGSWORTH JM, JACOBSON DJ, MCGREE ME, DUNN RL, LIEBER MM, JACOBSEN SJ AND THE UROLOGIC DISEASES IN AMERICA PROJECTS Diabetes Treatment and Progression of Benign Prostatic Hyperplasia in Community Dwelling Black and White Men. J Urol, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAYYID RK & FLESHNER NE 2016. Diabetes Mellitus Type 2: A Driving Force for Urological Complications. Trends Endocrinol Metab, 27, 249–261. [DOI] [PubMed] [Google Scholar]
- SPALLONE V, ZIEGLER D, FREEMAN R, BERNARDI L, FRONTONI S, POP-BUSUI R, STEVENS M, KEMPLER P, HILSTED J & TESFAYE S 2011. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes/metabolism research and reviews, 27, 639–653. [DOI] [PubMed] [Google Scholar]
- STAPLETON A 2002. Urinary tract infections in patients with diabetes. Am J Med, 113 Suppl 1A, 80s–84s. [DOI] [PubMed] [Google Scholar]
- SWENSON C, S. E., SINGER D, KIRCH M, KULLGREN J, MALANI P November 2018. Urinary incontinence: An inevitable part of aging? University of Michigan National Poll on Healthy Aging. . [Google Scholar]
- TAI HC, TAI TY, YANG WS, WANG SW & YU HJ 2016. Associations between lower urinary tract dysfunction and glycemic control in women with type 2 diabetes: A cross-sectional study. J Diabetes Complications, 30, 415–9. [DOI] [PubMed] [Google Scholar]
- TAMAS V & KEMPLER P 2014. Sexual dysfunction in diabetes. Handb Clin Neurol, 126, 223–32. [DOI] [PubMed] [Google Scholar]
- THOMPSON IM, TANGEN CM, GOODMAN PJ, PROBSTFIELD JL, MOINPOUR CM & COLTMAN CA 2005. Erectile dysfunction and subsequent cardiovascular disease. Jama, 294, 2996–3002. [DOI] [PubMed] [Google Scholar]
- THORVE VS, KSHIRSAGAR AD, VYAWAHARE NS, JOSHI VS, INGALE KG & MOHITE RJ 2011. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications, 25, 129–36. [DOI] [PubMed] [Google Scholar]
- UEDA T, TAMAKI M, KAGEYAMA S, YOSHIMURA N & YOSHIDA O 2000. Urinary incontinence among community-dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self-perception. Int J Urol, 7, 95–103. [DOI] [PubMed] [Google Scholar]
- VINIK AI, MASER RE, MITCHELL BD & FREEMAN R 2003. Diabetic autonomic neuropathy. Diabetes Care, 26, 1553–79. [DOI] [PubMed] [Google Scholar]
- WESSELLS H 2013. Insights and interventions in diabetes associated erectile dysfunction. The Journal of urology, 190, 15–16. [DOI] [PubMed] [Google Scholar]
- WESSELLS H, BRAFFETT BH, HOLT SK, JACOBSON AM, KUSEK JW, COWIE C, DUNN RL & SARMA AV 2018. Burden of Urological Complications in Men and Women With Long-standing Type 1 Diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Cohort. Diabetes Care, 41, 2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITMIRE W, AL-GAYYAR MM, ABDELSAID M, YOUSUFZAI BK & EL-REMESSY AB 2011. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis, 17, 300–8. [PMC free article] [PubMed] [Google Scholar]
- WILKE T, BOETTGER B, BERG B, GROTH A, MUELLER S, BOTTEMAN M, YU S, FUCHS A & MAYWALD U 2015. Epidemiology of urinary tract infections in type 2 diabetes mellitus patients: An analysis based on a large sample of 456,586 German T2DM patients. J Diabetes Complications, 29, 1015–23. [DOI] [PubMed] [Google Scholar]
- WITTIG L, CARLSON KV, ANDREWS JM, CRUMP RT & BAVERSTOCK RJ 2019. Diabetic Bladder Dysfunction:A Review. Urology, 123, 1–6. [DOI] [PubMed] [Google Scholar]
- YUAN Z, TANG Z, HE C & TANG W 2015. Diabetic cystopathy: A review. J Diabetes, 7, 442–7. [DOI] [PubMed] [Google Scholar]
- ZHANEL GG, NICOLLE LE & HARDING GK 1995. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis, 21, 316–22. [DOI] [PubMed] [Google Scholar]
- ZIEGLER D 1994. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev, 10, 339–83. [DOI] [PubMed] [Google Scholar]
- ZIEGLER D 1999. Cardiovascular autonomic neuropathy: Clinical manifestations and measurement. [Google Scholar]