Abstract
Hypercalcemia is a vital laboratory marker because it can show underlying severe diseases like cancer and infections. Of all the causes of hypercalcemia, primary hyperparathyroidism, and malignancies are the most common, but granulomatous diseases, such as certain fungal infections, can also be the cause. Here we describe the case of a 29-year-old woman, an insulin-dependent diabetic, found unconscious and tachypneic at home. In the emergency room, the medical team diagnosed diabetic ketoacidosis (DKA) and acute kidney injury (AKI). During hospitalization, despite resolving acidemia, persistent hypercalcemia attracted attention. Laboratory tests showed decreased parathyroid hormone (PTH) levels, confirming non-PTH-dependent hypercalcemia. Computed tomography (CT) of the chest and abdomen demonstrated no alterations, but an upper digestive endoscopy revealed an ulcerated and infiltrative lesion in the stomach. A biopsy showed a granulomatous infiltrate due to mucormycosis infection. The patient received liposomal amphotericin B for 30 days and isavuconazonium for two months. Serum calcium levels improved during treatment. Inquiry of the etiology of hypercalcemia should begin with the PTH assay; high levels are consistent with hyperparathyroidism; low levels, with calcium or vitamin D intoxication, malignancies, prolonged immobilization, and granulomatous diseases. In the latter cases, the overproduction of 1-alpha-hydroxylase by the granulomatous tissue increases the conversion of 25(OH)vitamin D into 1–25(OH)vitamin D, which causes the intestinal absorption of calcium. We have described the first hypercalcemia related to mucormycosis infection in a young diabetic patient, although case presentations associate other fungal infections with elevated serum calcium.
Keywords: Hypercalcemia, Acute kidney injury, Diabetic ketoacidosis, Mucormycosis
Introduction
Hypercalcemia is a typical metabolic abnormality well described in primary hyperparathyroidism and malignancy with paraneoplastic production of parathyroid hormone-related protein (PTHrP) [1]. Therefore, in the investigation, it is vital to rule out factitious hypercalcemia and measure intact PTH levels [2]. Furthermore, PTH-independent etiologies include some fungal disorders, probably because of granulomatous tissue 1-α-hydroxylase production, which increases 25(OH)vitamin D to 1–25(OH)vitamin D conversion [1, 3].
Mucormycosis is a rare and severe fungal infection that most commonly affects the face, but in rare cases, it can also involve the gastrointestinal system [4]. In addition, it usually occurs in patients with compromised immune systems, such as poorly controlled diabetics [5]. To our knowledge, there is no publication of hypercalcemia related to mucormycosis infection.
We present a case of a woman with diabetic ketoacidosis (DBK) who developed acute kidney injury (AKI) and hypercalcemia that persisted despite treatment for DBK and hemodialysis. Further investigation revealed the diagnosis of gastrointestinal mucormycosis as the probable cause of hypercalcemia.
Case report
A 29-year-old diabetic woman, unconscious and tachypneic, was admitted to the emergency department. Her medical history included poorly controlled type 1 diabetes mellitus (DM). Initial physical examination showed a young patient dehydrated, with BP 100 × 50 mmHg, HR 120 bpm, RR 32 rpm, pulmonary and cardiac auscultation without alterations, and blood glucose test 312 mg/dL.
After stabilization measure and airway protection, admission laboratory tests showed leukocytosis with neutrophilia, blood glucose of 338 mg/dL, metabolic acidosis (pH 7.08, HCO3 8.6, and PCO2 29), Urea 56 mg/dL (BUN 26.1 mg/dL), Creatinine 1.9 mg/dL, CRP 76.7 mg/L, Na 146 mmol/ L, K 2.9 mmol/L, Cl 103 mmol/L, Lactate 16 mg/dL, Ionic Ca 1.51 mmol/L and urinalysis with ketonuria (2 +), proteinuria (1 +), hemoglobin (2 +) and glycosuria (3 +). The SARS-CoV-2 PCR test (nasopharyngeal swab) was negative.
According to the patient’s clinical condition, hyperglycemia, ketonuria, and metabolic acidosis, a diagnosis of DBK was made. Our team started treatment with intravenous fluid therapy, KCl replacement, and insulin therapy. However, she evolved into an oliguric AKI requiring hemodialysis. Symptomatic hypercalcemia persisted despite acidosis correction (total Ca 11.2 mg/dL, albumin 3.2 g/dL, ionic Ca 1.41 mmol/L, and PTH 3.54 pg/mL). Faced with severe and symptomatic hypercalcemia, we administered pamidronate 90 mg until the etiology was clarified.
To further analyze the PTH-independent hypercalcemia, we perform a CT scan of the chest and abdomen and immunofixation of serum proteins. All of them were normal. Nevertheless, an esophagogastroduodenoscopy (EGD) showed a large ulcerated and infiltrative lesion on the stomach, with imprecise limits, friable, with a whitish and amorphous structure adhered in its central portion (Fig. 1). The histopathological analysis confirmed the diagnosis of mucormycosis (Fig. 2).
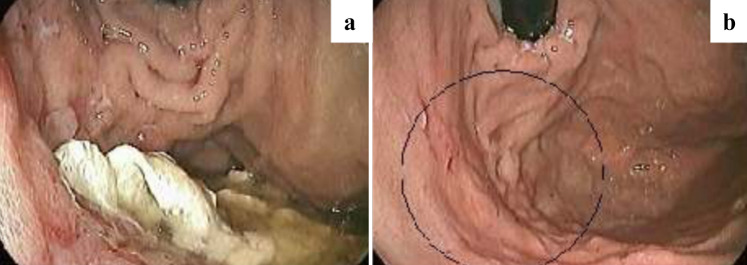
Fig. 1.
The esophagogastroduodenoscopy (EGD) findings. a Infiltrative ulcer with a whitish and amorphous structure adhered in its central portion in the fornix of the stomach. b Control EGD after 30 days of treatment with liposomal amphotericin B
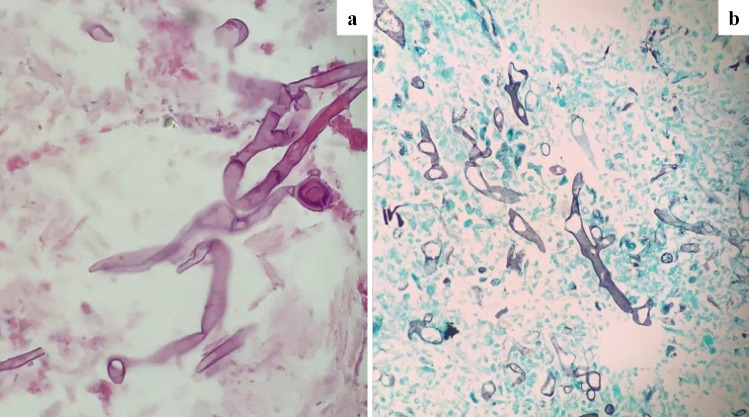
Fig. 2.
Histologic analysis of gastric mucosal biopsy. a Mucormycosis organisms are easily identified on hematoxylin and eosin sections. b Special staining with GMS (Grocott’s methenamine silver) can highlight the organisms and allow a more precise assessment of morphology [4, 8]
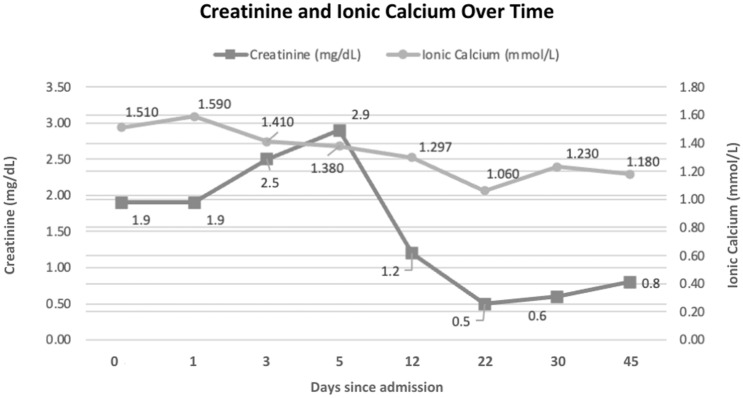
The patient was treated with liposomal amphotericin B for 30 days, followed by isavuconazole for two months. She evolved with clinical improvement, normalizing serum calcium and kidney recovery (Fig. 3).
Fig. 3.
Calcium and creatinine evaluation over time
Discussion
Hypercalcemia is high serum calcium levels (total Ca > 10.5 mg/dL or ionized Ca2 + > 5.25 mg/dL). The leading causes include primary hyperparathyroidism and neoplasms [1]. In most cases, treatment involves the correction of underlying diseases, and thereby, precise identification of the underlying etiology is crucial. The hypercalcemia diagnostic approach excludes factitious hypercalcemia (ionized calcium or total calcium corrected by albumin) and the measurement of intact PTH. This step is essential to differentiate between PTH-mediated hypercalcemia and non-PTH-mediated hypercalcemia [2].
According to the investigation, our patient falls into a non-PTH-mediated hypercalcemia group (Table 1). This issue opens several diagnostic possibilities, as shown in Table 2 [1–3]. We discarded the patient’s medication history as calcium or vitamin D supplementation in the investigation. Furthermore, thyrotoxicosis was excluded after normal TSH (3.95 mIU/L) and free T4 (1.17 ng/dL). The protein immunofixation test and CT imaging of the chest and abdomen were normal, which, adjunct with the patient's data, made lung cancer, multiple myeloma, tuberculosis, and sarcoidosis possibilities less likely. On further evaluation, EGD revealed an ulcerated and infiltrating lesion in the stomach. Biopsy showed granulomatous inflammation and large, non-septate hyphae with hyphae branching at a 90-degree angle, features of mucormycosis.
Table 1.
Biochemistry test results
| Days since admission | Blood Gas | Ionic Calcium (mmol/L) | Serum Calcium (mg/dL) | Albumin (g/dL) | Creatinine (mg/dL) | eGFR (ml/min/1.73m2) | PTH (pg/mL) |
|---|---|---|---|---|---|---|---|
| 0a | 7.08/29/8.6/247d | 1.510 | 9.2 | 2.6 | 1.9 | 35.02 | – |
| 1 | 7.35/22/12.1/203d | 1.590 | 11.2 | 3.2 | 1.9 | 35.02 | 3.54 |
| 3 | 7.40/22/34.6/194d | 1.410 | 10.7 | 2.6 | 2.5 | 25.13 | 4.10 |
| 5b | 7.42/29/18.8/177d | 1.380 | 9.5 | 2.7 | 2.9 | 21.01 | - |
| 12 | 7.38/31/18.3/65e | 1.297 | 9.1 | 2.4 | 1.2 | 61.05 | – |
| 22c | 7.33/44/23.2/47e | 1.060 | 8.5 | – | 0.5 | 139.77 | – |
| 30 | 7.31/41/20.6/116** | 1.230 | 8.8 | – | 0.6 | 124.50 | – |
| 45 | 7.32/44/22.7/80e | 1.180 | 9.4 | 4.6 | 0.8 | 98.93 | – |
| 2 yearsf | – | 1.225 | 9.8 | 4.6 | 0.4 | 135.60 | – |
| Other tests | |||||||
| Immunofixation of serum proteins (mg/dL) | IgG—882 | IgM—152 | IgA—139 | Kappa—723 | Lambda—345 | ||
| TSH (mIU/L) | 3.95 | Free T4 (ng/dL) | 1.17 | ||||
Reference values: Ionic calcium = 1,150 to 1,320 mmol/L; serum calcium = 8.4 to 10.2 mg/dL (for the patient's age group); albumin = 3.5 to 5.0 g/dL (for the patient's age group); GFR = estimated using CKD−EPI equation; PTH = 15.0 to 65.0 pg/mL; IgG = 640 to 1700 mg/dL (for the patient’s age group and sex); IgM = 50 to 300 mg/dL (for the patient's age group); IgG = 52 a 468 mg/dL (for the patient’s age group and sex); Kappa = 629 to 1350 mg/dL; Lambda = 313 to 723 mg/dL; TSH = 0,46 to 4,68 mIU/L (for the patient's age group); free T4 = 0,62 to 2,19 ng/dL
eGFR = estimated glomerular filtration rate; PTH = parathyroid hormone; TSH = thyroid stimulating hormone
aAdmission
bPamidronate infusion
cFirst day of antifungal therapy
dArterial blood gas
eVenous blood gas = pH / pCO2 / HCO3 / PaO2
fOutpatient follow-up
Table 2.
Differential diagnosis of hypercalcemia
| Types | Etiology | Pathophysiology |
|---|---|---|
| PTH-mediated hypercalcemia | Primary hyperparathyroidism | Hyperplasia or adenoma of the parathyroid glands. |
| Increased PTH → calcium resorption by renal tubule cells and bone resorption by osteoclastic hyperactivity | ||
| Persistent hyperparathyroidism | Autonomous parathyroid glands that remain hyperfunctioning after a kidney transplant. | |
| Familial hypocalciuric hypercalcemia | A genetic disorder characterized by decreased urinary calcium excretion and hypercalcemia. | |
| Caused by an autosomal dominant mutation of the CaSr gene. | ||
| Non-PTH-mediated hypercalcemia | Malignancy | Paraneoplastic production of PTHrP (e.g., squamous cell carcinomas of the lung, head, and neck; breast, ovarian, bladder, and kidney cancers; lymphoma; and leukemia). |
| Osteolytic lesions (e.g., multiple myeloma, breast cancer, renal cancer). | ||
| Paraneoplastic production of 1,25-dihydroxyvitamin D (e.g., lymphoma, ovarian dysgerminoma). | ||
| Granulomatous disorders | Hydroxylase activity in activated mononuclear cells produces 1,25-dihydroxyvitamin D outside the kidneys. | |
| Found in sarcoidosis, tuberculosis, deep mycosis, leprosy. | ||
| Medications | Thiazide diuretics decrease renal calcium excretion. | |
| Excess vitamin D increases intestinal calcium absorption. | ||
| Calcium supplements and antacids. | ||
| Vitamin A and vitamin A derivatives (e.g., isotretinoin) directly stimulate bone resorption. | ||
| Lithium reduces renal calcium excretion and alters the set point of PTH secretion. | ||
| Thyrotoxicosis | ↑ thyroid hormone → ↑ osteoclastic activity → ↑ bone resorption. | |
| Immobilization | Lack of weight-bearing activities → osteoclast activation → bone demineralization → hypercalcemia. | |
| Milk-alkali syndrome | Ingestion of large amounts of calcium and absorbable alkali. | |
| Adrenal insufficiency | The exact pathophysiology is unknown; several mechanisms have been proposed. |
Mucormycosis is a group of invasive and potentially fatal infections caused by fungi of the order Mucorales. This opportunistic infection is associated with DBK, neoplasms, and other immunodeficiency diseases [4]. In diabetic patients like ours, histological studies have shown that these fungi adhere to endothelial cells to start their angioinvasion [5], and glucose regulates its receptor on endothelial cells [6]. According to the site of infection and patient comorbidities, the mortality in patients with mucormycosis can be as high as 80% [4, 5]. Diagnosis depends on the identification of the agent in tissue or culture.
This fungus has gained notoriety lately, both because of the increase in immunosuppressed people (e.g., a more remarkable survival of transplanted patients and an increase in the number of diabetics) and after the COVID-19 pandemic, in which there are reports of patients infected by Sars-Cov-2 presenting severe forms of mucormycosis [4, 5, 7, 8]. However, due to its rarity, there are no previously published reports of hypercalcemia due to mucormycosis and few about this fungal infection being a cause of granuloma formation [7].
This case report suggests mucormycosis may be an infectious cause of non-PTH-dependent hypercalcemia. Therefore, we should consider this cause when investigating the etiology of hypercalcemia in susceptible patients whose most common causes have been ruled out.
Funding
None.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
Ethical approval was not required for our case publication. We got written informed consent from the patient to publish his anonymized information in this case report and accompanying images. Written permission was also given for its appearance in print, online, and licensed versions of the journal; for elements to be included in social media posting in journal staff to promote the article; and for the journal and its publisher to grant permission to third parties to publish this material. It is also her understanding that while her name will not be published, even with the best efforts of the authors to maintain anonymity and even with the journal’s best practices in place, complete anonymity cannot be guaranteed. Moreover, consenting to the publication does not remove his privacy rights, and the consent can be revoked before the publication. Still, once the information has been committed to publication (“gone to press”), it cannot revoke consent. Payments or royalties were not given for his consent.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(Suppl 2):S51–S61. doi: 10.1002/jbmr.5650061413. [DOI] [PubMed] [Google Scholar]
- 2.Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959–1966. [PubMed] [Google Scholar]
- 3.Spindel SJ, Hamill RJ, Georghiou PR, Lacke CE, Green LK, Mallette LE. Case report: vitamin D-mediated hypercalcemia in fungal infections. Am J Med Sci. 1995;310(2):71–76. doi: 10.1097/00000441-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.- Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. clinical infectious diseases: an official publication of the infectious diseases society of America [Internet]. 2012 Feb 1;54(Suppl 1):S16–22. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3286196/ [DOI] [PMC free article] [PubMed]
- 6.Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31(12):1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- 7.Binesh F, Nakafabadi PM, Mirhosseini S, Gheybi K. Granulomatous mucormycosis with excellent response to treatment, report of a case and review of literature. Int J Clin Stud Med Case Reports. 2020;8(2):1–4. doi: 10.46998/IJCMCR.2020.08.000184. [DOI] [Google Scholar]
- 8.Pal R, Singh B, Bhadada SK, et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64(12):1452–1459. doi: 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]