Abstract
Purpose
Sodium-glucose co-transporter-2 inhibitor (SGLT-2i) administration is associated with some concerns in regard to the increased risk of genital and urinary tract infections (UTI) in kidney transplant recipients (KTR). In this study, we present the results of SGLT-2i use in KTR, including the early post-transplant period.
Methods
Participants were divided into two groups: SGLT-2i-free diabetic KTR (Group 1, n = 21) and diabetic KTR using SGLT-2i (Group 2, n = 36). Group 2 was further divided into two subgroups according to the posttransplant prescription day of SGLT-2i; < 3 months (Group 2a) and ≥ 3 months (Group 2b). Groups were compared for development of genital and urinary tract infections, glycated hemoglobin a1c (HgbA1c), estimated glomerular filtration rate (eGFR), proteinuria, weight change, and acute rejection rate during 12-month follow-up.
Results
Urinary tract infections prevalence was 21.1% and UTI-related hospitalization rate was 10.5% in our cohort. Prevalence of UTI and UTI-related hospitalization, eGFR, HgbA1c levels, and weight gain were similar between the SGLT-2i group and SGLT-2i-free group, at the 12-month follow-up. UTI prevalence was similar between groups 2a and 2b (p = 0.871). No case of genital infection was recorded. Significant proteinuria reduction was observed in Group 2 (p = 0.008). Acute rejection rate was higher in the SGLT-2i-free group (p = 0.040) and had an impact on 12-month follow-up eGFR (p = 0.003).
Conclusion
SGLT-2i in KTR is not associated with an increased risk of genital infection and UTI in diabetic KTR, even in the early posttransplant period. The use of SGLT-2i reduces proteinuria in KTR and has no adverse effects on allograft function at the 12-month follow-up.
Keywords: SGLT-2i, Kidney transplantation, Urinary tract infection, Diabetes, NODAT
Introduction
Nephrology is, unfortunately, unlucky in regards to available drug variety and the chance of using a novel drug immediately soon after its introduction. Experience with the effects of some drugs on the kidneys only becomes indirectly known over time. This is not surprising because individuals with chronic kidney disease (CKD), especially those with advanced renal disease, are not generally enrolled in drug development studies due to safety concerns.
Sodium-glucose cotransporter-2 (SGLT-2), a member of the “solute carrier” group of proteins, is encoded in humans by the SLC5A2 gene [1]. This protein facilitates the transport of glucose in a sodium-dependent manner and is the major pathway in the kidney responsible for glucose reabsorption [2, 3]. SGLT-2 inhibitor (SGLT-2i), namely gliflozin, induces glucosuria and lower blood glucose [3, 4]. However, the benefit of SGLT-2i goes beyond lowering blood glucose [5, 6]. The unique mechanism of SGLT-2i allows for (i) lowering blood glucose, (ii) weight loss through enhanced diuresis via the glucose-induced osmotic effect, and (iii) lowering proteinuria by restoring the impaired tubuloglomerular feedback mechanism [5, 6]. Given all this, SGLT-2i(s) are now considered first-line agents for the treatment of patients with type 2 diabetes mellitus (DM), heart failure with low ejection fraction, and diabetic nephropathy [7–9].
Diabetes mellitus is the leading cause of end-stage renal disease (ESRD) worldwide, but experience with the use of SGLT-2i in diabetic ESRD patients is limited. In addition, the use of SGLT-2i in diabetic kidney transplant recipients (KTR) is an important issue that remains to be clarified given the potential benefits in weight changes and proteinuria after transplantation. Recently, some reports on the use of SGLT-2i in renal transplant recipients have been published, encouraging clinicians to consider the use of SGLT-2i in renal transplant recipients [9]. However, de novo use of SGLT-2i immediately after renal transplantation is associated with several concerns, including increased risk of genital and urinary tract infections (UTI), unpredictable hemodynamic effects, and volume loss, all of which are risk factors for worse allograft function. The available evidence on the safety and efficacy of SGLT-2i therapy in KTR is very limited, with only 9 published studies as of October 2021, consisting of 8 manuscripts and 1 abstract involving 182 patients from 8 countries [10].
In this study, we aim to share our experience in a small sample-sized cohort with de novo use of SGLT-2i in KTR, including the early post-transplant period.
Materials and methods
This retrospective study was conducted between January 2021 and July 2022 in the organ transplant departments of Istanbul Yeni Yuzyil University, Gaziosmanpasa Private Hospital, and Medicana International Ankara Hospital. The study design was approved by the local Human Research Ethics Committee (date: 11/04/2022, number: 2022/14). In the first step, the hospital’s automation software systems were scanned with E.10 and E.14 codes of the International Classification of Diseases (ICD), and a group of recipients who received an SGLT-2i continuously for at least six months were labeled. In addition, to confirm the diagnosis of DM, these codes were compared with patients’ prescriptions and data from the National Health Surveillance System (E-Nabiz; https://enabiz.gov.tr/). Thus, diagnosis of previous DM and posttransplant blood glucose courses were assessed in two ways. KTR with DM and individuals who developed new-onset diabetes mellitus after transplantation (NODAT) were included in the study. Baseline characteristics of participants (age, sex, underlying disease, duration of renal replacement therapy, donor and transplant type, cardiovascular disease, diagnosis of previous diabetes, hospitalizations, etc.) were recorded. Recipients with hyperglycemia detected during a hospitalization or outpatient visit were initially instructed to a low-calorie diet, and patients with persistent hyperglycemia were intensified on antiglycemic treatment. Patients with NODAT initially received antihyperglycemic treatment without SGLT-2i, however, if this approach failed to reduce hyperglycemia, SGLT-2i was added to the therapy. Study participants were divided into two main groups and two subgroups as follows;
Group 1 (SGLT-2i-free) = Diabetic KTR (long-standing DM or NODAT) who received antiglycemic therapy, not including an SGLT-2i.
Group 2 (SGLT-2i) = Diabetic KTR (long-standing DM or NODAT) who received antiglycemic therapy, including an SGLT-2i.
Group 2a = Diabetic KTR who received an SGLT-2i in the early posttransplant period (< 3 months).
Group 2b = Diabetic KTR who received an SGLT-2i posttransplant ≥ 3 months.
Case selection and exclusion
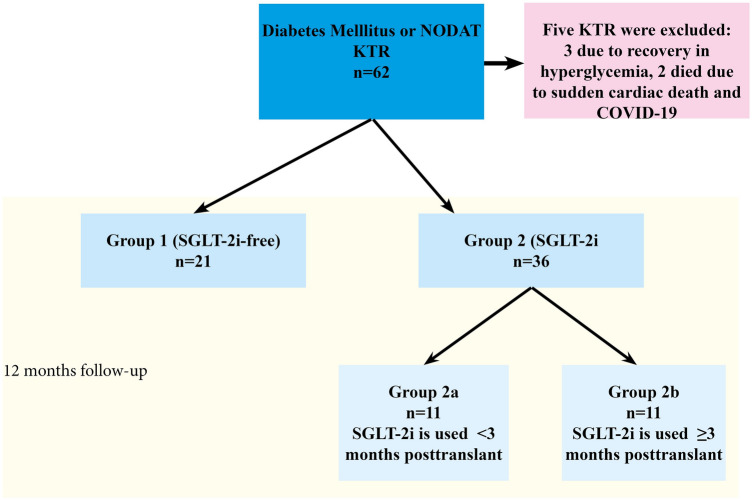
Non-diabetic recipients were excluded. Data before 2021 were not analyzed because there were few cases of SGLT-2i users. KTR who had to discontinue SGLT-2i during follow-up periods because of attenuation of hyperglycemia were also excluded (< 6 months SGLT-2i users were excluded and > 6 months SGLT-2i users were included in the study). Empagliflozin and dapagliflozin were used in hyperglycemic patients randomly. The initial doses were 10 mg/day and when necessary, doses were titrated to 25 mg/day. The data of KTR during an active infection or rejection were not used and the next data in a stable period of at least one month (eGFR, proteinuria, glycolized hemoglobin A1c [HgbA1c], glucose, etc.) were considered for evaluation. In collecting the study data, we noticed that previous or recent UTI was not considered as an exclusion criterion by the transplant team, because some cases had received an SGLT-2i soon after UTI recovery, especially in the early posttransplant period. The study design is shown in Fig. 1.
Fig. 1.
Flow-chart of the included population. NODAT new-onset diabetes mellitus after transplantation, KTR kidney transplant recipient, SGLT-2i sodium-glucose cotransporter-2 inhibitor
Diagnosis of the urinary tract and genital infections
Routine physical examination of the genitalia was not performed if the patient did not report specific symptoms such as swollen labia or changes in color, odor, or amount of vaginal discharge. UTI was diagnosed if > 105 colony forming unit/ml was detected in urine culture and symptoms included dysuria, frequency, urinary urgency, fever, or pain were suggestive of the diagnosis [11]. Symptoms and findings such as vaginitis, and vulvitis in women and balanitis, orchitis, epididymitis, and balanoposthitis in men were used in the diagnosis of genital infections [12].
NODAT definition
In this study, we tried to adhere to the American Diabetes Association Professional Practice Committee for the diagnosis criteria of NODAT (2022) which are; HgbA1c value ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dL, plasma glucose ≥ 200 mg/dL 2 h after an oral glucose load, and random plasma glucose ≥ 200 mg/dL in a patient with traditional symptoms of hyperglycemia. However, in this study, in hospitalized patients, the diagnosis of NODAT mostly depended on random plasma glucose levels. All recipients with multiple random plasma glucose levels ≥ 200 mg/dL and a few individuals with multiple fasting plasma glucose levels ≥ 126 mg/dL were classified as NODAT. Posttransplant hyperglycemia resolving within the first weeks after transplantation was excluded from the study. An oral glucose tolerance test was not performed in any case. HgbA1c level was used to diagnose NODAT only if fasting or postmeal plasma glucose levels were abnormal but did not correspond to the diagnosis of NODAT, and if the posttransplant period was more than 1 month [13].
Data collection
Initial enrollment data obtained from KTR in polyclinics between January 2021 and July 2022, were designated as “baseline.” Follow-up intervals of KTR were different depending on KTR’s clinical status (immunologic risk, assessment of recent infection, etc.). During the 12-month follow-up, data were gathered at three-month intervals, retrospectively (if the interval superposed to an acute infection or rejection, the data were gathered from the closest stable period). Some KTR had applied to the closest hospitals to their residence for follow-up. Therefore, data were also taken from E-Nabiz when data were missing in-center software at the follow-up intervals of the study. If there was more than one measurement for fasting glucose, HgbA1c, serum creatinine, tacrolimus trough levels, etc., the mean values were recorded. In Turkey, medications may be prescribed all at once for a period of 3 months and can be monitored on E-Nabiz. Therefore, we were able to confirm that all oral antidiabetic agents and insulins, including SGLT-2i, were prescribed at least twice within a 12-month follow-up.
The two groups (Groups 1 and 2) were compared in terms of the development of UTI and genital infections, allograft function, proteinuria, acute rejection, and weight changes. Fasting glucose and HgbA1c levels were also compared between the groups. Arterial/venous blood testing was not a standard follow-up parameter in this cohort, KTR were queried for a diagnosis of euglycemic diabetic ketoacidosis using ICD code E.11.
Immunosuppression
A similar standard immunosuppression protocol has been used in two centers for years. Antithymocyte globulin (ATG) 1.5 mg/kg/day (ATG-Fresenius-Grafalon), the dosing interval, and count are determined in the pre-transplant period according to the immunological risk status by transplant nephrologists. In addition, 3 doses of methylprednisone 250–500 mg/day are administered to all recipients in induction therapy. For maintenance immunosuppression, a combination of prednisolone + mycophenolic acid + calcineurin inhibitor (most commonly tacrolimus) is used.
Statistical analysis
A statistical package program was used for data analysis (SPSS for Windows, version 15.0, Chicago, SPSS Inc). Histograms and Shapiro–Wilk tests were used to check the agreement of continuous variables with the normal distribution. Normally distributed continuous variables (parametric variables) were presented as mean ± standard deviation (SD), and nonnormally distributed (nonparametric) continuous variables were presented as median and minimum–maximum ranges. Parametric variables were compared with the independent-sample t test and nonparametric variables with the Mann–Whitney U test. Univariate and multivariate logistic and linear regression analyses were performed to determine factors affecting allograft function. The changes in repeated measures were compared by using a general linear model. All p values reported in the study are two-tailed, and p < 0.05 was considered statistically significant.
Results
A total of 57 diabetic KTR, 21 in Group 1 and 36 in Group 2, were evaluated. 45.6% of recipients had a previous diagnosis of DM and 54.4% of recipients developed NODAT. One recipient in Group 1 died of sudden cardiac death and one in Group 2 died of COVID-19. No allograft loss was observed in this cohort except for deceased-censored allograft loss. Three cases with NODAT on SGLT-2i therapy recovered from hyperglycemia within 6 months (two KTR in Group 2a and one in Group 2b) and did not require an antiglycemic agent, so they were excluded from the study (Fig. 1). No case of euglycemic diabetic ketoacidosis was observed. The demographic and etiologic characteristics of the subjects are shown in Table 1.
Table 1.
The clinical and demographic features of the participants
| Age, years | 51.30 ± 10.98 |
| BMI, kg/m2 | 28.49 ± 4.48 |
| Sex; male/female, N, % | 36 (63.2%) and 21 (36.8%) |
| Preemptive, N | 19 (33.3%) |
| Donor type, N | |
| Living | 54 (94.7%) |
| Cadaveric | 3 (5.3%) |
| UTI prevalence, N, % | 12/57 (21.1%) |
| Hospitalization (UTI related), n, % | 6/51 (10.5%) |
| CVD history, N | 27 (47.4%) |
| RRT type, N | |
| HD | 34 (89.5%) |
| PD | 4 (10.5%) |
| Rejection rates, N, % | 11 (19,3%) (Clinical suspicion + biopsy proven) |
| NODAT, N | 31 (54.4%) |
| Previous DM, N | 26 (45.6%) |
| Primary disease in ESRD etiology, N | |
| 24 (42.1%) DM | |
| 11 (19.3%) HT | |
| 12 (21.1%) Unknown | |
| 5 (8.8%) GN | |
| 3 (5.3%) Rare diseases | |
| 2 (3.5%) PCKD | |
| SGLT-2i users, N, % (SGLT-2i was given as add-on therapy) | 36 (63.2%) |
| 20 (35.1%) only insulin | |
| 9 (15, 8) insulin + OAD (14%) | |
| 7 (12.3%) OAD | |
| SGLT-2i related complications | Euglycemic diabetic ketoacidosis: NO |
| GFR decreases > 30% (within 2–4 weeks): NO | |
| Hypoglycemia: NO | |
| Urogenital fungal infection: NO | |
| Induction protocol | |
| rATG | 1.5 mg/kg rabbit anti-thymocyte globulin, 1–5 times (according to immunological risk assessment) |
| Methylprednisolone | 500 mg/day 3 days |
| Maintenance immunosuppression | Prednisolon + mycophenolic acid + tacrolimus |
BMI body mass index, UTI urinary tract infection, CVD cardiovascular disease, RRT renal replacement therapy, HD hemodialysis, PD peritoneal dialysis, NODAT new-onset diabetes mellitus after transplantation, DM diabetes mellitus, ESRD end-stage renal disease, SGLT-2i sodium-glucose cotransporter-2 inhibitor, rATG rabbit anti-thymocyte globulin, HT hypertension, GN glomerulonephritis, PCKD polycystic kidney disease, OAD oral antidiabetic drug(s), GFR glomerular filtration rate
UTI prevalence was 21.1% in our cohort and was female-dominated (75% vs. 25%, p = 0.002). The prevalence and UTI-related hospitalization rates were similar in both groups (28.57% in Group 1 and 16.67% in Group 2 and p = 0.327, and 23.8% in Group 1 and 8.3% in Group 2, p = 0.105, respectively) (Table 2). No case of genital infection was observed in this cohort, so we did not perform statistical analysis in this regard.
Table 2.
The comparison of SGLT-2i free and SGLT-2i groups for UTI and acute rejection development, proteinuria, and other clinical outcomes
| Group 1 (SGLT-2i free) N = 21 | Group 2 (SGLT-2i) N = 36 | p value | |
|---|---|---|---|
| Age, years | 55.00 ± 9.68 | 49.14 ± 11.17 | 0.049 |
| Sex, male/female, n | 12/9 | 24/12 | 0.472 |
| BMI, kg/m2 | 28.60 ± 4.52 | 28.41 ± 4.53 | 0.886 |
| RRT duration, month | 16 (0–125) | 9 (0–144) | 0.391 |
| Transplantation age, months | 18(0–108) | 59 (0–161) | 0.008 |
| rATG, mg | 330.00 ± 183.10 | 393.06 ± 246.52 | 0.331 |
| DM age, months | 170.63 ± 117.90 | 181.66 ± 139.48 | 0.788 |
| NODAT, n (yes/no) | 7/14 (33.3%) | 22/14 (61.1%) | 0.043 |
| UTI, n (yes/no) | 6/15 (28.57%) | 6/30 (16.67%) | 0.327 |
| Hospitalization, n, % | 5/21(23.8%) | 3/36 (8.3%) | 0.105 |
| SGLT-2i beginning time | – | 25 recipients (posttransplant > 3 months) | |
| 11 recipients (posttransplant 0–3 months) | |||
| All acute rejection episodes, yes/no, n | 7/14(33.3%) | 4/32(11.1%) | 0.040 |
| One acute rejection episode occurred after SGLT2-i use | |||
| HgbA1c, % | |||
| Baseline | 6.37 ± 1.03 | 9.06 ± 2.24 | < 0.001 |
| Month 3 | 7.29 ± 2.68 | 7.98 ± 1.44 | 0.375 |
| Month 6 | 6.86 ± 0.98 | 7.82 ± 1.65 | 0.133 |
| Month 12 | 7.14 ± 0.98 | 7.71 ± 1.25 | 0.256 |
| Creatinine, mg/dl | |||
| Baseline | 1.06 ± 0.26 | 1.14 ± 0.29 | 0.321 |
| Month 12 | 1.14 ± 0.40 | 1.17 ± 0.32 | 0.790 |
| eGFR, ml/min/1.73 m2 | |||
| Baseline | 73.20 ± 19.72 | 71.94 ± 18.17 | 0.811 |
| Month 3 | 67.85 ± 17.89 | 68.36 ± 19.98 | 0.925 |
| Month 6 | 63.75 ± 19.34 | 70.02 ± 18.92 | 0.243 |
| Month 12 | 65.36 ± 17.19 | 72.96 ± 16.62 | 0.123 |
| Proteinuria, mg/day | |||
| Baseline | 229 (63–909) | 321 (45–2565) | 0.704 |
| Month 12 | 156 (44–1355) | 195 (51–1905) | 0.372 |
Bold values indicate statistical significance at the p < 0.05 level
BMI body mass index, RRT renal replacement therapy, rATG rabbit anti-thymocyte globulin, DM diabetes mellitus, NODAT new-onset diabetes mellitus after transplantation, UTI urinary tract infection, SGLT-2i sodium-glucose cotransporter-2 inhibitor, eGFR estimated glomerular filtration rate
Median transplantation duration (from kidney transplantation to enrollment into the study) was higher in the SGLT-2i group. NODAT was more common in Group 2 (Table 2). 61.1% of the SGLT-2i users had NODAT. HgbA1c level was higher in the SGLT-2i group at the beginning of SGLT-2i therapy, and the difference between Group 1 and Group 2 in terms of HgbA1c level disappeared at the 12 months of follow-up (p = 0.256). Transplant duration and ATG dosages did not affect the UTI development risk (p = 0.890 and p = 0.290, respectively).
Eleven recipients in Group 1 received an SGLT-2i within three months after transplantation (Group 2a) (Table 3). One recipient in Group 2a developed a severe UTI requiring hospitalization, and SGLT-2i was discontinued because UTI persisted (Table 3; case 3). However, data demonstrated that this patient had several UTI episodes in the early post-transplant period previous to receiving SGLT-2i. The risk of UTI development was similar between Group 2a and Group 2b in the early and late posttransplant periods, despite more immunosuppression being administered in the early period in Group 2a (OR = 1.16 [95% CI 0.18–7.57], p = 0.871) (Table 4).
Table 3.
The summary of Group 2a
| Case | Posttransplant SGLT-2i beginning time | Therapy | UTI after SGLT-2 (1 year) | 1-year creatinine, mg/dl | 1-year HgbA1c, % (0, 3, 6, and 12 months) | Weight change, kg (0, 3, 6, and 12 months) |
|---|---|---|---|---|---|---|
| 1. 40-year-old, male | 2 weeks | SGLT-2i + previous OAD | No | 1.13 | 9.94, 6.09, 6.40, 6.70 | 94, 92, 91, 92 |
| 2. 48-year-old, female | 3 months | SGLT-2i + previous insulin | No | 1.08 | 7.40, 7.20, 6.30, 6.40 | 66, 65, 65, 65 |
| 3. 67-year-old, male | 6 weeks | SGLT-2i + previous OAD | Yes, (he had also UTI episodes priorly to SGLT-2i use) hospitalization was required and SGLT-2i was ceased at the 8th month of SGLT-2i use | 1.06 | 8.50, 8.20, 9.10, 7.20 | 68, 67, 67, 67 |
| 4. 58-year-old, male | 3 months | SGLT-2i + insulin | No | 1.00 | 8.80, 8.20, 7.40, 7.50 | 69, 67, 66, 67 |
| 5. 43-year-old, male | 3 months | SGLT-2i + previous insulin + previous OAD | No | 1.31 | 9.80, 9.10, 8.60, 8.78 | 108, 102, 101, 107 |
| 6. 56-year-old, male | 3 months | SGLT-2i + previous insulin | No | 1.01 | 8.70, 9.80, 8.20, 9.10 | 82, 78, 77, 81 |
| 7. 42-year-old, male | 1 week | SGLT-2i + previous insulin | No | 0.81 | 5.90, 10.60, 8.60, 9.60 | 64, 70, 68, 69 |
| 8. 58-year-old, male | 3 months | SGLT-2i + previous insulin + previous OAD | No | 1.19 | 10.30, 7.60, 7.40, 7,70 | 64, 64, 66, 65 |
| 9. 63-year-old, male | 6 weeks | SGLT-2i + previous insulin + previous OAD | Yes | 1.51 | 6.90, 7.20, 8.50, 7.70 | 70, 74, 76, 73 |
| 10. 43-year-old, male | 2 weeks | SGLT-2i + previous insulin | No | 1.20 | 7.0, 11.10, 8.80, 7,90 | 57, 67, 70, 71 |
| 11. 45-year-old, male | 7 weeks | SGLT-2i + previous OAD | No | 1.20 | 8.10, 13.30, 9.90, 7.40 | 84, 86, 85, 85 |
SGLT-2i sodium-glucose cotransporter-2 inhibitor, UTI urinary tract infection, HgbA1c glycated hemoglobin a1c, OAD oral antidiabetic drug(s)
Table 4.
The comparison of SGLT-2i use in the early and late periods of kidney transplantation
| Group 2a, n = 11 | Group 2b, n = 25 | p value | |
|---|---|---|---|
| Age, years | 51.18 ± 9.47 | 48.24 ± 11.91 | 0.475 |
| BMI, kg/m2 | 26.92 ± 4.26 | 29.13 ± 4.58 | 0.211 |
| RRT duration, month | 6 (0–114) | 12 (0–118) | 0.517 |
| Transplantation age, months | 17 (12–34) | 72 (27–161) | < 0.001 |
| rATG, mg | 318.18 ± 95.58 | 434.25 ± 293.46 | 0.215 |
| DM age, months | 72 (12–348) | 150 (64–432) | 0.361 |
| NODAT, n (yes/no) | 7/4 (63.6%) | 15 (60.0%) | 0.837 |
| Urinary tract infection, n (yes/no) | 2/9 (18.2%) | 4/21 (16.0%) | 0.871 |
| Hospitalization, n, % | 1/11 (9.1%) | 2/25 (8.0%) | 0.105 |
| Mean tacrolimus mg/dl; | |||
| 0 | 10.04 ± 2.57 | 6.73 ± 2.07 | < 0.001 |
| Month 12 | 7.92 ± 2.28 | 6.09 ± 1.54 | 0.076 |
| Fasting glucose, mg/dl | |||
| Baseline | 232.27 ± 100.63 | 148.93 ± 50.64 | 0.090 |
| Month 12 | 145.16 ± 57.56 | 147.90 ± 51.22 | 0.882 |
| HgbA1c, % | |||
| Baseline | 8.55 ± 2.24 | 9.29 ± 2.25 | 0.374 |
| Month 12 | 8.03 ± 1.19 | 7.51 ± 1.19 | 0.428 |
| Proteinuria, mg/day | |||
| Baseline | 243 ± 166 | 329 (45–1565) | 0.479 |
| Month 12 | 141 ± 54 | 205 (67–1750) | 0.999 |
Bold values indicate statistical significance at the p < 0.05 level
SGLT-2i sodium-glucose cotransporter-2 inhibitor, BMI body mass index, RRT renal replacement therapy, rATG rabbit anti-thymocyte globulin, DM diabetes mellitus, NODAT new-onset diabetes mellitus after transplantation, HgbA1c glycated hemoglobin a1c
Allograft functions were similar at baseline and 3, 6, and 12 months after transplantation between Group 1 and Group 2 (p > 0.05). In Group 2, eGFR initially (from baseline to 3rd-month post-transplant) decreased from 71.94 ± 18.17 ml/min/1.73 m2 to 68.36 ± 19.98 ml/min/1.73 m2, however, then increased to 72.96 ± 16.62 ml/min/1.73 m2 at 12 months post-transplant. In contrast, in Group 1, eGFR decreased from 73.20 ± 19.72 to 65.36 ± 17.19 ml/min/1.73 m2 from baseline to 12 months posttransplant. A general linear model for repeated measures showed that the comparison of the changes in both groups was not statistically significant (p = 0.078). The trough tacrolimus levels during the 12-month follow-up were similar between Group 1 and Group 2 (p > 0.05). In addition, eGFR levels were similar between Group 2a and Group 2b during the 12-month follow-up (p > 0.05). The acute rejection rate was lower in the SGLT-2i group (p = 0.040), and linear regression showed that acute rejection also had a strong influence on eGFR at 12 months posttransplant in Group 1 (p = 0.003). This influence was not observed in Group 2 (p = 0.129).
Proteinuria decreased in Group 2 from baseline to 12 months post-transplant from 321 (45–2565) mg/day to 195 (51–1905) mg/day (p = 0.008). In contrast, proteinuria did not change in Group 1 during the same period (229 [63–909] vs. 156 [44–1355], p = 0.210). Moreover, proteinuria decreased in Group 2a from 243 ± 166 mg/day to 141 ± 54 mg/day (p = 0.046), from baseline to 12 months posttransplant, and in Group 2b from 329 (45–1565) mg/day to 205 (67–1750) mg/day (p = 0.011).
Weight changes in Group 2 from baseline to 12 months posttransplant were as follows: Baseline: 77.11 ± 14.90, 3 months: 74.62 ± 11.91, 6 months: 74.54 ± 12.13, and 12 months: 75.85 ± 12.42 (the change from baseline to 12 months post-transplant; p = 0.125). There was a slight weight loss in Group 2. However, in Group 1, minimal weight gain from baseline to 12 months post-transplant was observed (79.35 ± 13.25 vs. 80.53 ± 15.67), which was not statistically significant (p = 0.879). A striking finding was that while SGLT-2i users in Group 2a showed non-significant weight gain from baseline to 12 months post-transplant (baseline; 75.80 ± 15.92 vs. 12 months; 78.85 ± 15.60, p = 0.474), in Group 2b, SGLT-2i users showed weight loss from baseline to 12 months post-transplant (baseline; 79.80 ± 15.92 vs. 12 months; 74.82 ± 15.60, p = 0.056).
Discussion
SGLT-2i(s) have cardiac and renal benefits in diabetic patients regardless of diabetes status. SGLT-2i(s) exert their renoprotective effects by re-regulating the tubuloglomerular feedback mechanism by ultimately lowering intraglomerular pressure. However, genital and urinary tract infections are the main concerns in patients treated with SGLT-2i [14, 15]. This is an important concern for KTR and their physicians to consider the use of an SGLT-2i when administering immunosuppressants, especially in the early posttransplant period. In this study, we demonstrated that SGLT-2i should not be considered “out of option” for regulating hyperglycemia in KTR. In this small sample-sized cohort, 1-year posttransplant UTI development risk and allograft function were similar between SGLT-2i users and SGLT-2i-free individuals.
Diabetic and non-diabetic recipients are exposed to immunosuppression-induced hyperglycemia after renal transplantation, which is another problem in organ transplantation and increases cardiovascular morbidity and mortality [16, 17]. NODAT is observed with a prevalence of up to 50% in KTR, and up to 30% of them become diabetic for life long [18, 19]. In our cohort, 54.4% of hyperglycemic/diabetic recipients were diagnosed with NODAT. This finding gives clinicians an indication of how prevalent NODAT is and that it is a problem that should not be ignored in the transplant era.
SGLT-2i delivers its cardiac and renal protective effects independent of the glucose-lowering effect. The CREDENCE study showed a significant 30% reduction in the risk of CKD progression with canagliflozin in diabetic CKD patients, and the study was terminated early [20]. In the DECLARE-TIMI 58 study, 16,843 participants with type 2 DM and creatinine clearance > of 60 ml/min/1.73 m2 were randomized to dapagliflozin, and during 4-year follow-up, renoprotective effects were observed in all proteinuria levels, including normoalbuminuric patients [21]. The EMPA-KIDNEY study was stopped early due to strong evidences suggesting that SGLT-2i(s) may soon be indicated for patients with CKD without albuminuria [22]. In the DAPA-CKD study, the administration of 10 mg of dapagliflozin significantly reduced the risk of progression to CKD in patients without type 2 DM [23]. Further analysis of the DAPA-CKD study revealed that patients with an eGFR of 25–30 ml/min per 1.73 m2 had received benefits similar to patients with an eGFR ≥ 30 ml/min/1.73 m2. However, at lower eGFR values (< 30 ml/min/1.73 m2), starting SGLT-2i treatment is strongly discouraged because of limited evidence of safety and efficacy. In addition, almost no patients today undergo renal transplantation while on an SGLT-2i-involving antidiabetic regimen. Thus, questions have arisen whether an SGLT-2i could be an option for the treatment of hyperglycemia after renal transplantation and whether its use should be discontinued in the perioperative period if in the future it will be possible to be used in advanced stages of CKD. In this study, eGFR did not change from baseline to 12 months posttransplant (a slight decrease in eGFR occurred following starting SGLT-2i, however, it returned to the baseline level at follow-ups). In the SGLT-2i-free group, a slight decrease in eGFR was observed in this period (8.9%) which was not significant. Additionally, proteinuria decreased significantly following SGLT-2i therapy, especially in Group 2b (> 3 months posttransplant). Since proteinuria in the early posttransplant period may be due to native kidneys, the effect of SGLT-2i on proteinuria in allografts may be better interpreted in the late posttransplant period.
All published large randomized controlled trials that investigated the efficacy and safety of SGLT-2i have excluded CKD patients in advanced stages and KTR, as no study addresses a specific patient group in an efficacy and safety trial at baseline [24, 25]. Alkindi et al. reported the results of using SGLT-2i in 8 diabetic renal transplant patients and found a low risk of recurrence of UTI and no worsening of renal function during the 12-month follow-up period [26]. Recently, a brief review of the current literature on the use of SGLT-2i in KTR with diabetes (including 9 studies and 144 patients) demonstrated either a small or nonsignificant reduction in BP and overall stable renal function [9, 26]. In addition, this review found a modest improvement in glycemic control as well as weight reduction and a low incidence of adverse events as reported in clinical trials except for transplantation [9]. Lim et al. included in their study 2083 KTR with diabetes from 6 transplant centers in Korea and indicated that SGLT-2i improved all-cause mortality, death-censored graft failure, or doubling of serum creatinine [27].
UTI is the most common infection in KTR and its incidence ranges from 6 to 86% [28, 29]. UTI is also responsible for approximately 50% of all infectious complications in KTR [30, 31]. In this study, the incidence of UTI in diabetic KTR was 21.1%, and the incidence was similar in the SGLT-2i group compared with the SGLT-2i-free group during the 12-month follow-ups. We also compared the incidence of SGLT-2i users in the early (> 3 months) and late (> 3 months) renal transplantation periods for UTI. Previous studies, in contrast to our study, included KTR after transplantation > 3 months (in a relatively stable period of renal transplantation) to show the effects of SGLT-2i use on allograft function and blood glucose levels to avoid confounding factors, and they did not specifically address UTI risk assessment [27, 32]. We demonstrated that the use of SGLT-2i even in the early post-transplant period was not associated with an increased risk of UTI and allograft dysfunction. In Group 2a, one recipient developed urosepsis, and SGLT-2i was discontinued. However, he had UTI episodes in his past history, and we are not sure whether UTI was directly related to the use of SGLT-2i in this recipient (Group 2a: case 3) (Table 3). Allograft functions were similar at the 12-month follow-ups among all groups (group 1 vs. group 2 and group 2a vs. group 2b), and surprisingly, the incidence of acute rejection was slightly higher in the SGLT-2i-free group. A limitation of the study is that we did not examine the immunologic risk status of the patients, which may explain this difference in acute rejection rate between SGLT-2i and SGLT-2i-free groups. Additionally, the acute rejection rate had an impact on the SGLT-2i-free group. We did not observe any case of genital infection in our cohort, which could be due to the relatively small number of women.
SGLT-2i is associated with weight loss in diabetics and non-diabetics. Because the majority of KTR gain weight after renal transplantation, the use of SGLT-2i may be beneficial in this regard [33]. In our study, whereas SGLT-2i users lost weight slightly, SGLT-2i-free recipients gained weight slightly, and this difference was not statistically significant. In the SGLT-2i group, KTR who received an SGLT-2i < 3 months after transplantation slightly gained weight. This result was likely due to the improvement in metabolism immediately after transplantation and was related to the potential gain in muscle mass and adipose tissue. In contrast, KTR who received an SGLT-2i ≥ 3 months after transplantation reduced weight (p = 0.056 weak importance probably due to low sample size).
Previous studies with SGLT-2i revealed those agents are very effective in reducing HgbA1c, with an average reduction of HgbA1c by 0.5–1% depending on the baseline level [34]. Monami et al. reported that reduction in HgbA1c in comparison to placebo reaches its maximum at approximately 6 months and is maintained for up to 1 year [35]. In this study, SGLT-2i reduced HgbA1c by 1.35% during a 12-month follow-up. In the SGLT-2i group, the initial HgbA1c level was higher compared to the SGLT-2i-free group since an SGLT-2i was added on current therapy if the hyperglycemia did not restore by first-line approach (diet, oral antiglycemic agents, insulin, etc.). Also, NODAT was more common in the SGLT-2i group. Additionally, in the SGLT-2i-free group, HgbA1c increased due to the worsening of glycemia after transplantation, and NODAT cases.
The small sample size of this cohort is the major limitation of the study. Therefore, a comprehensive analysis of the allograft function relevant to the use of SGLT-2i is not available. A detailed urologic risk assessment for genital and urinary tract infections is not possible due to a lack of data. Because acute rejection rates were somewhat lower in the SGLT-2i group, rejection treatment protocols may also have an impact on infection development and allograft functions. The absence of an assessment of the impact of other anti-proteinuric drugs on proteinuria and eGFR is another lack of the study. Moreover, due to the low sample size, the impact of oral antiglycemic drugs and insulin could not be assessed in detail. Additionally, the primary etiological factors of ESRD (DM, glomerulopathies, hypertensive nephropathy, etc.) may be the cause of proteinuria in the early posttransplant period (< 3 months) and the impact of those could not be studied due to limited sample size and data.
In conclusion, the use of SGLT-2i in renal transplant recipients appears to be safe and likely provides additional benefits in lowering HgbA1c. UTI development risk should not discourage clinicians from considering SGLT-2i treatment for hyperglycemia even in the early period of kidney transplantation. UTI developmental risk in KTR under SGLT-2i therapy is likely similar to that in SGLT-2-free KTR. Despite all the negative aspects, we consider the results of our study important because there are limited data on the use of SGLT-2i in KTR.
Acknowledgements
Special thanks to Deren Gurbuz, MD, and Ceren Melissa Akkaya, MD.
Author contributions
All authors declare that they participated in the conception, execution, and analysis of the work and approved the final version.
Funding
The author declared that this study has received no financial support.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The author has no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.SLC5A2 solute carrier family 5 member 2 [Homo sapiens (human)] (2022). https://www.ncbi.nlm.nih.gov/gene/6524. Accessed 1 Dec 2022
- 2.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93(1):397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyimesi G, Pujol-Giménez J, Kanai Y, Hediger MA. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflugers Arch. 2020;472(9):1177–1206. doi: 10.1007/s00424-020-02433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright EM. SGLT2 inhibitors: physiology and pharmacology. Kidney360. 2021;2(12):2027–2037. doi: 10.34067/KID.0002772021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo YH, Teo YN, Syn NL, Kow CS, Yoong CSY, Tan BYQ, Yeo TC, Lee CH, Lin W, Sia CH. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc. 2021;10(5):e019463. doi: 10.1161/JAHA.120.019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesař V. SGLT2 inhibitors in non-diabetic kidney disease. Adv Clin Exp Med. 2022;31(2):105–107. doi: 10.17219/acem/145734. [DOI] [PubMed] [Google Scholar]
- 7.Sajja AP, Dey AK, Guha A, Elnabawi Y, Joshi AA, Kalra A. SGLT-2 inhibitors and GLP-1 agonists: first-line therapy for diabetes with established cardiovascular disease. J Cardiovasc Pharmacol Ther. 2019;24(5):422–427. doi: 10.1177/1074248419838511. [DOI] [PubMed] [Google Scholar]
- 8.Keller DM, Ahmed N, Tariq H, Walgamage M, Walgamage T, Mohammed A, Chou JT, Kałużna-Oleksy M, Lesiak M, Straburzyńska-Migaj E. SGLT2 inhibitors in type 2 diabetes mellitus and heart failure-a concise review. J Clin Med. 2022;11(6):1470. doi: 10.3390/jcm11061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuster S, Al-Hadhrami Z, Moore S, Awad S, Shamseddin MK. Use of sodium-glucose cotransporter-2 inhibitors in renal transplant patients with diabetes: a brief review of the current literature. Can J Diabetes. 2022;46(2):207–212. doi: 10.1016/j.jcjd.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Ujjawal A, Schreiber B, Verma A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: what is the evidence? Ther Adv Endocrinol Metab. 2022;13:20420188221090001. doi: 10.1177/20420188221090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino M, Pesce F, Schena A, Simone S, Castellano G, Gesualdo L. Updates on urinary tract infections in kidney transplantation. J Nephrol. 2019;32(5):751–761. doi: 10.1007/s40620-019-00585-3. [DOI] [PubMed] [Google Scholar]
- 12.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27(5):479–484. doi: 10.1016/j.jdiacomp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Professional Practice Committee 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 14.Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103(3):373–381. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 16.Jindal RM, Sidner RA, Milgrom ML. Post-transplant diabetes mellitus. The role of immunosuppression. Drug Saf. 1997;16(4):242–257. doi: 10.2165/00002018-199716040-00002. [DOI] [PubMed] [Google Scholar]
- 17.Wauters RP, Cosio FG, Suarez Fernandez ML, Kudva Y, Shah P, Torres VE. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation. 2012;94(4):377–382. doi: 10.1097/TP.0b013e3182584831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinangil A, Celik V, Barlas S, Koc Y, Basturk T, Sakaci T, Akin EB, Ecder T. The incidence of new onset diabetes after transplantation and related factors: single center experience. Nefrologia. 2017;37(2):181–188. doi: 10.1016/j.nefro.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Zielińska K, Kukulski L, Wróbel M, Przybyłowski P, Zakliczyński M, Strojek K. Prevalence and risk factors of new-onset diabetes after transplantation (NODAT) Ann Transplant. 2020;25:e926556. doi: 10.12659/AOT.926556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, CREDENCE Trial Investigators Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 21.Mosenzon O, Wiviott SD, Heerspink HJL, Dwyer JP, Cahn A, Goodrich EL, Rozenberg A, Schechter M, Yanuv I, Murphy SA, Zelniker TA, Gause-Nilsson IAM, Langkilde AM, Fredriksson M, Johansson PA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS, Raz I. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021;44(8):1805–1815. doi: 10.2337/dc21-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardiance Phase III EMPA-KIDNEY trial will stop early due to clear positive efficacy in people with chronic kidney disease. Eli Lilly. https://investor.lilly.com/news-releases/news-release-details/jardiancer-phase-iii-empa-kidney-trial-will-stop-early-due-clear. Published 2022
- 23.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC, DAPA-CKD Trial Committees and Investigators Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 24.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 25.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, EMPA-REGOUTCOME Investigators Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 26.AlKindi F, Al-Omary HL, Hussain Q, Al Hakim M, Chaaban A, Boobes Y. Outcomes of SGLT2 Inhibitors use in diabetic renal transplant patients. Transplant Proc. 2020;52(1):175–178. doi: 10.1016/j.transproceed.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Lim JH, Kwon S, Jeon Y, Kim YH, Kwon H, Kim YS, Lee H, Kim YL, Kim CD, Park SH, Lee JS, Yoo KD, Son HE, Jeong JC, Lee J, Lee JP, Cho JH. The efficacy and safety of SGLT2 ınhibitor in diabetic kidney transplant recipients. Transplantation. 2022;106(9):e404–e412. doi: 10.1097/TP.0000000000004228. [DOI] [PubMed] [Google Scholar]
- 28.Chuang P, Parikh CR, Langone A. Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin Transplant. 2005;19(2):230–235. doi: 10.1111/j.1399-0012.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 29.Säemann M, Hörl WH. Urinary tract infection in renal transplant recipients. Eur J Clin Invest. 2008;38(Suppl 2):58–65. doi: 10.1111/j.1365-2362.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 30.Chan PC, Cheng IK, Wong KK, Li MK, Chan MK. Urinary tract infections in post-renal transplant patients. Int Urol Nephrol. 1990;22(4):389–396. doi: 10.1007/BF02549801. [DOI] [PubMed] [Google Scholar]
- 31.Rabkin DG, Stifelman MD, Birkhoff J, Richardson KA, Cohen D, Nowygrod R, Benvenisty AI, Hardy MA. Early catheter removal decreases incidence of urinary tract infections in renal transplant recipients. Transplant Proc. 1998;30(8):4314–4316. doi: 10.1016/s0041-1345(98)01423-7. [DOI] [PubMed] [Google Scholar]
- 32.Chewcharat A, Prasitlumkum N, Thongprayoon C, Bathini T, Medaura J, Vallabhajosyula S, Cheungpasitporn W. Efficacy and safety of SGLT-2 inhibitors for treatment of diabetes mellitus among kidney transplant patients: a systematic review and meta-analysis. Med Sci (Basel) 2020;8(4):47. doi: 10.3390/medsci8040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forte CC, Pedrollo EF, Nicoletto BB, et al. Risk factors associated with weight gain after kidney transplantation: a cohort study. PloS One. 2020;15(12):e0243394. doi: 10.1371/journal.pone.0243394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73–79. doi: 10.1097/MED.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(5):457–466. doi: 10.1111/dom.12244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.