Abstract
Purpose of Review
The objective of this review was to provide an update on recent malaria epidemiology, both globally and in non-endemic areas, to identify the current distribution and repercussions of genetically diverse Plasmodium species and summarize recently implemented intervention and prevention tools.
Recent Findings
Notable changes in malaria epidemiology have occurred in recent years, with an increase in the number of total cases and deaths globally during 2020–2021, in part attributed to the COVID-19 pandemic. The emergence of artemisinin-resistant species in new areas and the expanding distribution of parasites harbouring deletions of the pfhrp2/3 genes have been concerning. New strategies to curb the burden of this infection, such as vaccination, have been implemented in certain endemic areas and their performance is currently being evaluated.
Summary
Inadequate control of malaria in endemic regions may have an effect on imported malaria and measures to prevent re-establishment of transmission in malaria-free areas are essential. Enhanced surveillance and investigation of Plasmodium spp. genetic variations will contribute to the successful diagnosis and treatment of malaria in future. Novel strategies for an integrated One Health approach to malaria control should also be strengthened.
Keywords: Plasmodium spp., Malaria, COVID-19, Artemisinin resistance, Pfhrp2/3 gene deletion
Introduction
Recent World Health Organization (WHO) data provide insight into the evolving epidemiology of malaria globally. According to the World Malaria Report 2022, the number of total malaria cases increased again in 2021 (to 247 million), although case incidence remained stable following an increase from the preceding year [1••]. Similarly, deaths due to malaria increased in 2020 with respect to 2019, but then declined slightly in 2021. The excess in cases and deaths in these years have been attributed mainly to the disruption to prevention and control strategies attributed to the COVID-19 pandemic.
The epidemiology of imported malaria is also changing. Mirroring the decrease in population movements worldwide during the pandemic due to travel restrictions, the incidence of travel-related infections also decreased [2]. Despite this, several reports during the pandemic years alerted to a possible increase in severe malaria among persons returning from endemic areas and the possible causes are under investigation [3]. Travel is expected to gradually return to pre-pandemic levels so the possible spread of Plasmodium spp. following importation into non-endemic areas should continue to be monitored given the reports of competent Anopheles spp. expansion into new geographical areas, the appearance of autochthonous malaria cases in regions where malaria never occurred or which had interrupted malaria transmission, and the reporting of several cases of malaria following nosocomial transmission [4].
Genetic variability of Plasmodium species worldwide, including the emergence of artemisinin (ART) partially resistant species in new areas and the expanding distribution of parasites harbouring deletions of the pfhrp2/3 genes which may complicate diagnosis, is a cause for concern. Other factors impacting malaria epidemiology, not only direct human-related elements, should also be considered, such as the effect of climate change on the distribution of arthropod-borne diseases. Malaria may be emerging in temperate regions due to the increase in mobile populations, the deficiencies in public health systems and increased environmental temperatures [5, 6•].
All these interlinking factors contribute to the complexity of malaria dynamics and a profound knowledge of these interplaying aspects is necessary to maintain prevention strategies which may effectively tackle the worldwide malaria burden. The objective of this review was to provide an update on recent malaria epidemiology, both globally and in non-endemic areas, to identify the current distribution and repercussions of genetically diverse Plasmodium species and to focus on recent intervention and prevention tools such as vaccine development and implementation and possible benefits of other strategies such as mass drug administration (MDA) [7].
Epidemiology
Global Malaria Epidemiology
The number of total malaria cases globally increased in 2021 (from 245 million in 2020 to 247 million in 2021), with most of the increase occurring in Africa. However, case incidence remained stable from 2020 to 2021 (59 cases per 1000 population at risk) following an increase from 2019 (57 cases/1000 population) [1••]. The increase in 2020 was associated with disruption to prevention and control strategies attributed to the COVID-19 pandemic. Similarly, deaths due to malaria increased in 2020 with respect to 2019 by 10%, but then declined to 619,000 in 2021 [1••]. According to data from the Global Fund’s malaria programme, at the start of the pandemic use of insecticide-treated bed nets decreased in certain areas, although home delivery of nets to avoid crowding managed to increase the number of nets distributed overall. However, the number of people with suspected malaria tested decreased by around 4%, with a consequent decline in treatment [8]. Overall, an estimated 63,000 malaria deaths between 2019 and 2021 were attributed to interruption of malaria control strategies in some areas due to COVID-19 [1••].
Trends in Imported Malaria
A meta-analysis of national statistics on imported malaria from 40 non-endemic countries covering data on more than 50,000 individual cases over a 10-year period, performed in pre-pandemic years, identified several favoured routes for importation, with the West Africa region accounting for 56% of all cases [9]. France and the UK were the top receiving countries with an average 4000 malaria cases reported annually. Malaria surveillance data from the USA for 2018 also found a majority of imported cases were acquired in Africa (85%, out of over 1800 cases), and most of these were from West Africa (70% of those acquired in Africa) [10]. Plasmodium falciparum accounted for the majority of imported infections overall.
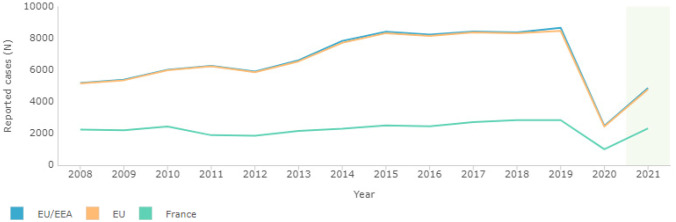
During the 2020–2021 period, as expected, registered cases of imported malaria were lower than for pre-pandemic years, although initial data were not homogeneous and detailed evidence was generally based on small series. The number of imported cases of malaria were higher in 2021 compared to 2020, coinciding with the easing of COVID-related travel restrictions. The European Centre for Disease Prevention and Control (ECDC), registered 2432 confirmed annual malaria cases in the European Union in 2020, and 4780 in 2021, following a peak of 8462 in 2019 (although this figure included over 1700 cases reported from the UK) [11]. Top reporting countries in 2020 and 2021 were France, Germany, Spain and Belgium (see Fig. 1 and Table 1).
Fig. 1.
ECDC. Surveillance Atlas of Infectious Diseases. Yearly reported confirmed cases of imported malaria [11]
Table 1.
ECDC. Yearly reported confirmed cases of imported malaria in the European Union and top reporting countries, 2019–2021 (adapted from [11])
| 2019 | 2020 | 2021 | |
|---|---|---|---|
| EU | 8462* | 2432 | 4780 |
| France | 2839 | 1007 | 2322 |
| Germany | 999 | 366 | 605 |
| Belgium | 417 | 241 | 365 |
| Italy | 811 | 181 | 443 |
| Spain | 783 | 210 | 430 |
*Data for UK included until 2019
As travel increases again, the number of imported malaria cases are rising. Of concern, several recent publications have highlighted an increase in severe malaria cases [3, 4, 12]. A report on malaria among personnel in the French Armed Forces noted an increase in severe cases in 2020 which was not attributed to an increased incidence in the cohort or decreased compliance with chemoprophylaxis [12]. A report from a Spanish national collaborative network also noted a significant increase in cases of severe malaria during 2020–2021 [3]. Another publication on imported P. falciparum found that parasitemia, rates of hyperparasitemia and severe malaria were significantly higher following relaxation of COVID-19 restrictions [4]. Although currently under investigation, some of the possible causes identified included delays between disease onset and diagnosis, due to fear of SARS-CoV-2 transmission in overcrowded hospital services, misdiagnosis of febrile illness as COVID-19 (cases of Plasmodium spp. and SARS-CoV-2 coinfection have been reported, complicating diagnosis further) and overburdening of laboratories during the pandemic [12–14].
Autochthonous Transmission in Non-Endemic Areas
Introduction or reintroduction and autochthonous transmission of Plasmodium spp. in certain geographical areas may alter disease dynamics.
In recent years, several cases of introduced malaria (mosquito-borne transmission from an imported case), airport malaria (due to an imported infected mosquito) and iatrogenic transmission have been noted in non-endemic areas [15, 16]. In the latter cases, parasite whole-genome and phylogenomic analyses may aid investigation of nosocomial malaria transmission, as demonstrated in a recent retrospective case report [17]. Importation of P. falciparum artemisinin-resistant lineages have already been reported in returning travellers highlighting further the relevance of clinical and genotypic surveillance of imported infections which may spread locally if host, environmental and vectorial conditions are favourable [18].
The potential role in transmission dynamics of submicroscopic malaria in recently arrived migrants should also be considered. Several studies have detected submicroscopic malaria in migrants using PCR (polymerase chain reaction), with prevalences of up to 14% in asymptomatic migrants from Sub-Saharan Africa [19]. The more widespread use of molecular techniques to detect Plasmodium spp. infections in various settings has contributed to the detection of many infections not diagnosed by conventional microscopy. Although, by definition, these correspond to low-density infections, the potential for transmission exists [20].
Various species of Anopheles mosquitoes, such as A. atroparvus, inhabit temperate regions of the world and though these may be refractory to African strains of P. falciparum, transmission of other Plasmodium species such as P. vivax may be possible [21, 22]. If other competent Anopheles species are introduced and become established in novel geographical areas due to climate changes, the risk of local transmission may be increased. Spread of insecticide-resistant species such as Anopheles stephensi in the African region may also challenge control efforts and initiatives have been launched to halt the further spread of this vector which may thrive in urban environments [23].
Plasmodium spp. Genetic Variability
The cornerstone of malaria control is effective and rapid diagnosis and access to appropriate and highly effective treatment. The World Health Organization (WHO) recommends that before treating a patient with malaria symptoms, the presence of the parasite must be confirmed by an appropriate diagnostic method (microscopy or rapid diagnostic test, RDT). Both microscopy and RDT diagnosis should be supported by a quality assurance programme [23]. This avoids clinical or presumptive diagnosis and therefore unnecessary treatment. However, there are currently two major problems in dealing with malaria cases: (a) false-negative RDT results and (b) the growing and worrying presence of antimalarial resistance, both for prevention and treatment.
RDTs and the Presence of False Negatives Due to Deletion in pfhrp2 and pfhrp3 Genes
The use, variety and quality of malaria rapid diagnostic tests (RDTs) have increased significantly during the last 10 years and they are currently the preferred field diagnostic test for malaria [24]. The majority of RDTs are based on detecting HRP2 (histidine-rich protein 2), a specific P. falciparum protein encoded by the pfhrp2 gene [25]. Testing using RDTs is a method with high sensitivity and specificity for the diagnosis of malaria; however, its performance has been threatened by the detection of parasites lacking the pfhrp2 and pfhrp3 genes [26]. Initially detected in Peru, the presence of deletions in both genes is currently widespread throughout the world, also being detected in Sub-Saharan Africa and Asia [27–29]. The presence of these mutations in Africa has a particularly critical role as the high prevalence of P. falciparum malaria in this continent increases the potential consequences of these deletions for malaria control and public health.
A deletion prevalence of 5% for these genes has been defined by the WHO as the minimum prevalence for changing the type of RDT used, as a prevalence higher than 5% could threaten the effectiveness of the test and affect public health guidelines for malaria control [29]. The increasing presence of parasites with deletions is complicating the diagnosis of P. falciparum not only in endemic areas, but also in non-endemic countries where migrant patients or travellers with malaria are seen. For this reason, a second confirmatory diagnostic test may be necessary when RDTs are negative. The use of exon 2 of pfhrp2 and pfhrp3 to detect deletions (non-amplification of exon 2 being associated with false-negative RDTs) could be performed by semi-nested PCR [30].
Resistance to Antimalarial Drugs
The emergence of drug resistance, particularly among P. falciparum parasites, the most prevalent species in the world, has been a major contributor to the global burden of malaria in the past 30 years [31]. Resistance has played a critical role in the recent doubling of malaria-related child deaths in Eastern and Southern Africa [32].
Resistance to Sulfadoxine/Pyrimethamine (SP)
The SP combination was withdrawn as a treatment for malaria years ago due to high resistance. Nowadays, although it is not useful as a treatment option in Africa, it is routinely administered as intermittent preventive treatment (IPT) for malaria, particularly to pregnant women (IPTp) and infants (IPTi, now called Perennial Malaria Chemoprophylaxis) [23].
Resistance to SP has been associated with a single nucleotide polymorphism (SNP) in two different genes, dihydrofolate reductase (pfdhfr) and dihydropteroate synthase (pfdhps), which encode for the enzymes PfDHFR and PfDHPS respectively, both of which are important in the folate synthesis pathway [33]. Three haplotypes with combinations of SNPs related to SP resistance have been described: partially resistant (quadruple mutant: pfdhfr 51I59R/108N + pfdhps 437G), IRNG haplotype; fully resistant (quintuple mutant: pfdhfr 51I/59R/108N + pfdhps 437G/540E), IRNGE haplotype; and super resistant (sextuple mutant: pfdhfr 51I/59R/108N + pfdhps 437G/540E/581G), IRNGEG haplotype [34, 35].
Artemisinin Combination Therapies (ACTs)
ACTs are the treatment of choice for uncomplicated malaria worldwide. The combination of an artemisinin derivative and an associated treatment with a longer half-life achieves a complete cure of the patient in the majority of cases. However, the recent gains in global malaria control as a result of ACT use are threatened by the emergence of artemisinin resistance in Southeast Asia (SEA) and the spread to other geographical areas [36]. Failure rates associated with specific ACT combinations have led to some provinces changing their first-line therapy to artesunate-pyronaridine combinations (another WHO recommended ACT for uncomplicated malaria) [1••]. Low-level ART resistance has been identified in Africa to date [37]. Ongoing worldwide surveillance is necessary due to the potential public health impact such resistance could have, especially in children under 5 years of age and pregnant women from Africa, as well as in non-immune travellers [37].
ART resistance in the SEA region has been linked to SNPs in the Kelch propeller domain on chromosome 13 (pfk13). Markers, validated by WHO, which have been associated with resistance to ART, are F446I, N458Y, M476I, Y493H, R539T, I543T, P553L, R561H, P574L and C580Y [37]. Only mutations C580Y, Y493H, R539T, I543T and N458Y have been detected in parasites with the slow-clearance phenotypic trait [37, 38]. These mutations can be detected by nested PCR, sequencing and sequence analysis using appropriate software [39].
Given the number of malaria-infected migrants and travellers arriving to non-endemic countries, epidemiological surveillance of these infections would be necessary to check for slow clearance, the first step in detecting resistance, or actual resistance. Molecular studies of parasites to determine their genetic profile by PCR and sequencing may be useful to understand the parasite’s behaviour in relation to the treatment used. Experts have raised concerns that a three-day treatment may be insufficient to achieve cure and that patients should be followed-up closely beyond the 3-day treatment period [40].
Prevention Strategies
Malaria prevention includes several strategies such as vector control, preventive chemotherapies, mass drug administration (MDA), vaccines and more recently, the use of monoclonal antibodies.
Preventive Chemotherapy
In the past, preventive chemotherapy in moderate to high transmission areas was usually linked to other health interactions; intermittent preventive treatment of malaria in pregnancy (IPTp) was delivered through antenatal clinics and perennial malaria chemoprophylaxis (PMC), together with expanded immunization programmes (EPI) [23]. A shift towards decentralisation is taking place, seasonal malaria chemoprevention (SMC), recommended for under 5s in areas of moderate to high seasonal transmission, is benefitting from a door to door community-based approach to obtain high coverage [41]. The most recent guidelines are more flexible regarding age of administration and number of cycles required as well as adding several new recommendations [23]. PMC has been expanded to children up to 24 months of age [42]. When the needs of the population at higher risk, under 5s, are covered, intermittent preventive treatment of malaria in school-aged children (IPTsc) can be considered. The latter targets children between 5 and 15 years old, and can be delivered through schools [43]. Finally, post-discharge malaria chemoprevention (PDMC) consists of administering full courses of antimalarials at predetermined intervals to children admitted with severe anaemia in endemic settings to reduce re-admission and mortality, a community-based delivery system is often preferred [44].
MDA
Mass drug administration (MDA) consists of administering a full course of antimalarial drugs to the whole population at the same time and often at repeated intervals. MDA is recommended together with other malaria control strategies to reduce the burden of disease in areas with moderate to high transmission of P falciparum, including during emergency settings. Its use is also recommended to reduce transmission in areas with very low to low transmission of P. falciparum or P. vivax [7, 45].
Several reviews suggest that MDA has a limited impact on prevalence of disease in moderate to high transmission settings and a more important effect on both prevalence and incidence in low transmission settings. This impact is limited in time, lasting from 1 to 3 months, and the strategies should be tailored to the setting and used as part of the overall package of malaria prevention strategies. The need for further research to better ascertain the long term impact of MDA malaria campaigns has been identified [7, 46–48].
The effect on malaria prevalence in areas of moderate to high transmission seems to be greater in emergency settings when access to healthcare facilities is limited. During the recent Ebola epidemic in West Africa, MDA administration reduced malaria morbidity but moreover reduced the number of febrile cases presenting to a weakened health system and reducing the risk of nosocomial transmission of Ebola [49, 50].
Vaccines
Two malaria vaccines, RTS,S/AS01 and R21/MM, are currently available.
RTS,S/AS01 is a recombinant protein based on repeat T epitopes from an antigen on the surface of the P. falciparum sporozoite, S antigen, derived from the hepatitis B surface antigen (HBsAg) and AS01, a proprietary adjuvant [51–54].
In a phase III trial, the RTS,S/AS01 vaccine demonstrated vaccine-induced partial protection against clinical malaria in children between 5 to 17 months old receiving 3 doses plus a booster, with added benefit when a booster was administered at 20 months. Vaccine efficacy was 36% (95% CI 32–40) [55]. The effect of the vaccine wanes with time with a reduction in one of the trials for children ages 5 to 17 months who had received three doses of RTS,S/AS01 from 44% (95% CI 16–62) to zero efficacy at years 1 and 4 of follow-up [52].
Since 2019, a pilot vaccine implementation programme has been ongoing in areas of Ghana, Kenya and Malawi [56]. Based on the initial results from this initiative, RTS,S was approved by WHO in 2021 and recommended in the 2022 guidelines for children at risk in areas of moderate to high transmission of P. falciparum in Sub-Saharan Africa [23, 57, 58•]. The implementation of RTS,S in these areas is underway. Similar to other malaria prevention strategy, RTS,S vaccine shows greater efficacy when combined with other interventions such as SMC [59].
The R21/MM vaccine is a virus-like particle based on the circumsporozoite from P. falciparum fused to the N terminus HBsAg and M-matrix as a proprietary adjuvant. In a phase II trial, with 3 doses, the vaccine efficacy was 74% (95% CI 63–82%) with low-dose adjuvant MM and 77% (95% CI 67–84%) when combined with high-dose adjuvant MM. The trial was implemented in addition to passive and active case detection, bednet use, SMC and indoor residual spraying [60]. However, there are limited data at this time on durability of protection and this vaccine has not been prequalified yet. Data comparing R21/MM and RTS,S/AS01 use are currently lacking.
There are other vaccines which are currently under investigation, such as a whole attenuated sporozoite vaccine PfSPZ [61], protein-based vaccines targeting other stages of the life cycle (Rh5, Pfs35, Pfs230) [62, 63], as well as DNA- and mRNA-based vaccines [64].
Monoclonal Antibodies
A novel approach to control malaria is the use of monoclonal antibodies (mAbs), and two are currently under development.
L9LS, a mAb targeting the circumsporozoite protein, achieved 88% protection against malaria infection in a small number of healthy individuals in a phase I trial following a single dose [65]. Phase II clinical trials are currently ongoing. CIS43LS is a mAb against P. falciparum sporozoites. In a phase II trial, this mAb showed a degree of protection against malaria infection after one dose of 88% (adjusted 95% CI 79–93) [66•].
Further research on the use of mAbs against malaria is needed and should be performed in endemic areas and include at risk groups such as under 5s and pregnant women. The future role of mAbs for malaria prevention will depend on accessibility and the ultimate overall cost of implementation.
Future Perspective
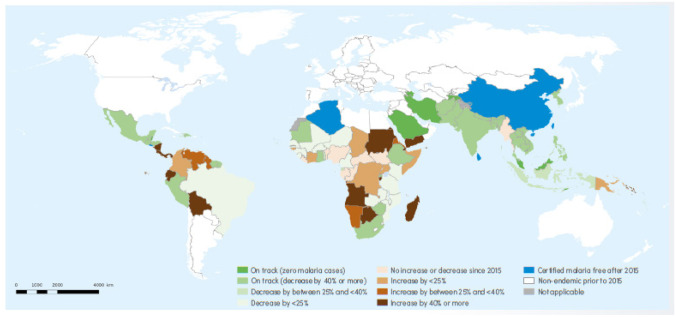
An unexpected pandemic maimed the progress towards the global control of malaria but gradually prevention strategies are regaining impetus to build on the achievements of the last decades. There may be room for some optimism as several endemic countries have shown progress in achieving milestones set in the Global Technical Strategy 2016–2030 (see Fig. 2) [1••]. Control and elimination in endemic regions may have repercussions on the prevalence of imported malaria and measures to prevent re-establishment of transmission in malaria-free areas are also essential. Enhanced surveillance and research of Plasmodium spp. genetic variations will contribute to the successful diagnosis and treatment of malaria in future, both in endemic and non-endemic countries.
Fig. 2.
World Health Organization. Map of malaria endemic countries showing progress towards the GTS (Global Technical Strategy 2016–2030) 2020 malaria case incidence milestone of at least 40% reduction from a 2015 baseline [1]
Although key factors contributing to prevention have been highlighted (vector control strategies, surveillance of Plasmodium spp. genetic variability, use of preventive chemotherapy, vaccines and monoclonal antibodies), control of disease will not be achieved if only one aspect is appraised in an isolated manner and in this sense, an integrated One Health approach to malaria control should be considered. Novel strategies such as using insecticide-treated livestock to eliminate zoophagic mosquitos are under investigation (partial zoophagic behavior contributes to maintenance of vector populations even though certain animals are not a natural reservoir for the parasite) [67]. Adaptation of technological advances and social networking to strengthen control may also have a role. Web-based mobile phone apps may reduce the burden of reporting RDT results in low-resource settings and assist with surveillance strategies [68]. Smartphone applications have been used for automated malaria screening of peripheral blood smear images making the process faster and less human dependent [69•]. For international travellers, telemedicine provided through a mobile application may also allow earlier diagnosis and prompt treatment of malaria [70]. A profound knowledge of all interlinking aspects of malaria epidemiology is necessary to maintain prevention strategies which may effectively tackle the worldwide malaria burden.
Funding
This research was supported by CIBER -Consorcio Centro de Investigación Biomédica en Red- (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea – NextGenerationEU.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.••World Health Organization. World malaria report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. (accessed 3–1–2023). https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Essential comprehensive up-to-date assessment of trends in global malaria control and elimination published by the World Health Organization.
- 2.Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic—impact on notified infectious diseases in Switzerland. J Travel Med. 2021;27:1–3. doi: 10.1093/JTM/TAAA180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman FF, Treviño-Maruri B, Giardín JMR, Gullón-Peña B, Salvador F, Serre N, et al. Trends in imported malaria during the COVID-19 pandemic, Spain (+Redivi Collaborative Network) J Travel Med. 2022;29:1–6. doi: 10.1093/jtm/taac083. [DOI] [PubMed] [Google Scholar]
- 4.Choy B, Bristowe H, Khozoee B, Lampejo T. Increased imported severe Plasmodium falciparum malaria involving hyperparasitaemia (>10%) in a UK hospital following relaxation of COVID-19 restrictions compared to the pre-pandemic period. J Travel Med. 2022;29:1–3. doi: 10.1093/jtm/taac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mironova VA, Shartova NV, Beljaev AE, Varentsov MI, Korennoy FI, Grishchenko MY. Re-introduction of vivax malaria in a temperate area (Moscow region, Russia): a geographic investigation. Malar J. 2020;19:1–20. doi: 10.1186/s12936-020-03187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.•Thomson MC, Stanberry LR. Climate Change and Vectorborne Diseases. N Engl J Med 2022;387:1969–78. 10.1056/nejmra2200092. Excellent review on possible effects of climate change on the epidemiology of vectorborne infections. [DOI] [PubMed]
- 7.Shah MP, Hwang J, Choi L, Lindblade KA, Kachur SP, Desai M. Mass drug administration for malaria. Cochrane Database Syst Rev 2021;2021. 10.1002/14651858.CD008846.pub3. [DOI] [PMC free article] [PubMed]
- 8.The Global Fund. Results Report 2021. (accessed 5–1–23). https://www.theglobalfund.org/media/11304/corporate_2021resultsreport_report_en.pdf.
- 9.Tatem AJ, Jia P, Ordanovich D, Falkner M, Huang Z, Howes R, et al. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect Dis. 2017;17:98–107. doi: 10.1016/S1473-3099(16)30326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mace KE, Lucchi NW, Tan KR. Malaria Surveillance — United States, 2018. MMWR Surveill Summ 2022;71:1–29. 10.15585/mmwr.ss7108a1. [DOI] [PMC free article] [PubMed]
- 11.European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. Malaria (accessed 4–1–23). https://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=3.
- 12.De Laval F, Maugey N, Bonet D’Oleon A, Pommier De Santi V, Ficko C. Increased risk of severe malaria in travellers during the COVID-19 pandemic. J Travel Med 2021;28:1–2. 10.1093/jtm/taab106. [DOI] [PMC free article] [PubMed]
- 13.Schubert L, Thurnher PMM, Machold PK, Tobudic PS, Winkler PS. Pandemic-related delay of falciparum malaria diagnosis in a traveller leading to cerebral malaria. J Travel Med. 2021;28:1–2. doi: 10.1093/jtm/taab159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilairatana P, Masangkay FR, Kotepui KU, Milanez GDJ, Kotepui M. Prevalence and characteristics of malaria among covid-19 individuals: a systematic review, meta-analysis, and analysis of case reports. PLoS Negl Trop Dis. 2021;15:1–18. doi: 10.1371/journal.pntd.0009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control. Multiple reports of locally-acquired malaria infections in the EU – 20 September 2017. Stockholm: ECDC; 2017. (accessed 5–1–23). https://www.ecdc.europa.eu/sites/default/files/documents/RRA-Malaria-EU-revised-September-2017_0.pdf.
- 16.Van Bortel W, Van Den Poel B, Hermans G, Driessche M Vanden, Molzahn H, Deblauwe I, et al. Two fatal autochthonous cases of airport malaria, Belgium, 2020. Eurosurveillance 2022;27. 10.2807/1560-7917.ES.2022.27.17.2100411. [DOI] [PMC free article] [PubMed]
- 17.Coppée R, Sarrasin V, Zaffaroulah R, Bouzayene A, Thellier M, Noël H, et al. Nosocomial malaria transmissions resolved by genomic analyses—a retrospective case report study in France: 2007–2021. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppée R, Bailly J, Sarrasin V, Vianou B, Zinsou BE, Mazars E, et al. Circulation of an artemisinin-resistant malaria lineage in a traveler returning from East Africa to France. Clin Infect Dis. 2022;75:1242–1244. doi: 10.1093/cid/ciac162. [DOI] [PubMed] [Google Scholar]
- 19.Corbacho-Loarte MD, Crespillo-Andújar C, Chamorro-Tojeiro S, Norman F, Pérez-Molina JA, Martín O, et al. Screening of imported malaria infection in asymptomatic migrants from Sub-Saharan Africa: a retrospective analysis of a 2010–2019 cohort. Travel Med Infect Dis 2022;49:102411. 10.1016/j.tmaid.2022.102411. [DOI] [PubMed]
- 20.Whittaker C, Slater H, Nash R, Bousema T, Drakeley C, Ghani AC, et al. Global patterns of submicroscopic Plasmodium falciparum malaria infection: insights from a systematic review and meta-analysis of population surveys. The Lancet Microbe. 2021;2:e366–e374. doi: 10.1016/S2666-5247(21)00055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrado L, Ezpeleta C, Rubio JM, Martín C, Azcona JM, Arteaga M, et al. Source identification of autochthonous-introduced Plasmodium vivax Malaria. Spain Infection. 2017;45:111–114. doi: 10.1007/s15010-016-0941-8. [DOI] [PubMed] [Google Scholar]
- 22.Santa-Olalla Peralta P, Vazquez-Torres MC, Latorre-Fandos E, Mairal-Claver P, Cortina-Solano P, Puy-Azón A, et al. First autochthonous malaria case due to Plasmodium vivax since eradication, Spain, October 2010. Euro Surveill. 2010;15:19684. doi: 10.2807/ese.15.41.19684-en. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization WHO Guidelines for malaria - Who. 2022;2022:1–396. [Google Scholar]
- 24.Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. 2020;36:112–126. doi: 10.1016/j.pt.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Marquart L, Butterworth A, McCarthy JS, Gatton ML. Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance. Malar J. 2012;11:1–9. doi: 10.1186/1475-2875-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 2010;5. 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed]
- 27.Berzosa P, González V, Taravillo L, Mayor A, Romay-Barja M, Garciá L, et al. First evidence of the deletion in the pfhrp2 and pfhrp3 genes in Plasmodium falciparum from Equatorial Guinea. Malar J. 2020;19:1–9. doi: 10.1186/s12936-020-03178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta H, Matambisso G, Galatas B, Cisteró P, Nhamussua L, Simone W, et al. Molecular surveillance of pfhrp2 and pfhrp3 deletions in Plasmodium falciparum isolates from Mozambique. Malar J. 2017;16:1–7. doi: 10.1186/s12936-017-2061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. From malaria control to malaria elimination: a manual for elimination scenario planning. Who 2014;52:67.
- 30.Berhane A, Anderson K, Mihreteab S, Gresty K, Rogier E, Mohamed S, et al. Major threat to malaria control programs by plasmodium falciparum lacking histidine-rich protein 2. Eritrea Emerg Infect Dis. 2018;24:462–470. doi: 10.3201/eid2403.171723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measurement of trends in childhood malaria mortality in Africa: an assessment of progress toward targets based on verbal autopsy. Lancet Infect Dis. 2003;3:349–358. doi: 10.1016/S1473-3099(03)00657-1. [DOI] [PubMed] [Google Scholar]
- 32.Kavishe RA, Paulo P, Kaaya RD, Kalinga A, Van Zwetselaar M, Chilongola J, et al. Surveillance of artemether-lumefantrine associated Plasmodium falciparum multidrug resistance protein-1 gene polymorphisms in Tanzania. Malar J. 2014;13:1–6. doi: 10.1186/1475-2875-13-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandoko PN, Rouvier F, Kakina LM, Mbongi DM, Latour C, Likwela JL, et al. Prevalence of plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in the democratic republic of the congo: emergence of highly resistant PfdHFR/PfdHps alleles. J Antimicrob Chemother. 2018;73:2704–2715. doi: 10.1093/jac/dky258. [DOI] [PubMed] [Google Scholar]
- 34.Naidoo I, Roper C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013;29:505–515. doi: 10.1016/j.pt.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Berzosa P, Molina de la Fuente I, Ta-Tang TH, González V, García L, Rodríguez-Galet A, et al. Temporal evolution of the resistance genotypes of Plasmodium falciparum in isolates from Equatorial Guinea during 20 years (1999 to 2019). Malar J 2021;20:1–17. 10.1186/s12936-021-04000-w. [DOI] [PMC free article] [PubMed]
- 36.WHO. Strategy for malaria elimination in the Greater Mekong Subregion : 2015–2030 [Internet]. Manila : WHO Regional Ofce for the Western Pacif2015. https://apps.who.int/iris/handle/10665/208203. Accessed 16 Jun 2021.
- 37.World Health Organization. Status report on artemisinin resistance and ACT efficacy (August 2018). World Heal Organ 2018:10.
- 38.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menard D, Ariey F. PCR_sequencing for genotyping SNPs PF3D7_1343700 Kelch protein propeller domain. Protocol Exchange. 2013; Accessed 16 February 2023. https://protocols.scienceexchange.com/protocols/pcr_sequencing-for-genotyping-snps-pf3d7_1343700-kelch-protein-propeller-domain.
- 40.Silva-Pinto A, Domingos J, Cardoso M, Reis A, Benavente ED, Caldas JP, et al. Artemether-lumefantrine treatment failure of uncomplicated Plasmodium falciparum malaria in travellers coming from Angola and Mozambique. Int J Infect Dis. 2021;110:151–154. doi: 10.1016/j.ijid.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry A, Issiaka D, Traore T, Mahamar A, Diarra B, Sagara I, et al. Optimal mode for delivery of seasonal malaria chemoprevention in Ouelessebougou, Mali: A cluster randomized trial. PLoS ONE. 2018;13:1–11. doi: 10.1371/journal.pone.0193296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 2015;11. 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed]
- 43.Clarke SE, Jukes MC, Njagi JK, Khasakhala L, Cundill B, Otido J, et al. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwambai TK, Dhabangi A, Idro R, Opoka R, Watson V, Kariuki S, et al. Malaria chemoprevention in the postdischarge management of severe anemia. N Engl J Med. 2020;383:2242–2254. doi: 10.1056/nejmoa2002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Mass drug administration for falciparum malaria. 2017.
- 46.Nadia J, Lu F. Historical experiences on mass drug administration for malaria control and elimination, its challenges and China’s experience: a narrative review. Acta Trop 2022;225:106209. 10.1016/j.actatropica.2021.106209. [DOI] [PubMed]
- 47.Newby G, Hwang J, Koita K, Chen I, Greenwood B, Von Seidlein L, et al. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg. 2015;93:125–134. doi: 10.4269/ajtmh.14-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisele TP. Mass drug administration can be a valuable addition to the malaria elimination toolbox. Malar J. 2019;18:1–5. doi: 10.1186/s12936-019-2906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aregawi M, Smith SJ, Sillah-Kanu M, Seppeh J, Kamara ARY, Williams RO, et al. Impact of the mass drug administration for malaria in response to the Ebola outbreak in Sierra Leone. Malar J. 2016;15:1–13. doi: 10.1186/s12936-016-1493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuehne A, Tiffany A, Lasry E, Janssens M, Besse C, Okonta C, et al. Impact and lessons learned from mass drug administrations of malaria chemoprevention during the ebola outbreak in Monrovia, Liberia, 2014. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0161311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med 2012;367:2284–95. 10.1056/nejmoa1208394. [DOI] [PMC free article] [PubMed]
- 52.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, et al. Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368:1111–1120. doi: 10.1056/nejmoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bejon P, White MT, Olotu A, Bojang K, Lusingu JPA, Salim N, et al. Efficacy of RTS, S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13:319–327. doi: 10.1016/S1473-3099(13)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Efficacy and safety of RTS S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011;365:1863–75. 10.1056/NEJMoa1102287. [DOI] [PubMed]
- 56.Malaria vaccine implementation programme. Accessed 21 February 2023. https://www.who.int/initiatives/malaria-vaccine-implementation-programme.
- 57.WHO recommends groundbreaking malaria vaccine for children at risk. Accessed 21 February 2023. https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk.
- 58.•Malaria vaccine: WHO position paper - March 2022. Weekly Epidemiological Record,Vol. 97, No. 09, pp. 61–80. 4 March 2022. Geneva: World Health Organization 2022 2022:61–80. https://apps.who.int/iris/bitstream/handle/10665/352332/WER9709-eng-fre.pdf. Includes the rationale and updated WHO recommendations for the use of the RTS,S/AS01 vaccine to reduce malaria morbidity and mortality in children living in areas of moderate to high malaria transmission.
- 59.Chandramohan D, Zongo I, Sagara I, Cairns M, Yerbanga R-S, Diarra M, et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med. 2021;385:1005–1017. doi: 10.1056/nejmoa2026330. [DOI] [PubMed] [Google Scholar]
- 60.Datoo MS, Natama HM, Somé A, Bellamy D, Traoré O, Rouamba T, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22:1728–1736. doi: 10.1016/S1473-3099(22)00442-X. [DOI] [PubMed] [Google Scholar]
- 61.Butler D. Promising malaria vaccine to be tested in first large field trial. Nature. 2019 doi: 10.1038/d41586-019-01232-4. [DOI] [PubMed] [Google Scholar]
- 62.Minassian AM, Silk SE, Barrett JR, Nielsen CM, Miura K, Diouf A, et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med. 2021;2:701–719.e19. doi: 10.1016/j.medj.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Draper SJ, Angov E, Horii T, Miller LH, Srinivasan P, Theisen M, et al. Recent advances in recombinant protein-based malaria vaccines. Vaccine. 2015;33:7433–7443. doi: 10.1016/j.vaccine.2015.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mallory KL, Taylor JA, Zou X, Waghela IN, Schneider CG, Sibilo MQ, et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. Npj Vaccines. 2021;6:84. doi: 10.1038/s41541-021-00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu RL, Idris AH, Berkowitz NM, Happe M, Gaudinski MR, Buettner C, et al. Low-dose subcutaneous or intravenous monoclonal antibody to prevent malaria. N Engl J Med. 2022;387:397–407. doi: 10.1056/NEJMoa2203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.•Kayentao K, Ongoiba A, Preston AC, Healy SA, Doumbo S, Doumtabe D, et al. Safety and efficacy of a monoclonal antibody against malaria in Mali. N Engl J Med 2022;387:1833–42. 10.1056/NEJMoa2206966. Recently published Phase 2 trial found a single infusion of the antimalarial monoclonal antibody CIS43LS protected healthy adults against P. falciparum infection during a 6-month malaria season in Mali. [DOI] [PMC free article] [PubMed]
- 67.Ruiz-Castillo P, Rist C, Rabinovich R, Chaccour C. Insecticide-treated livestock: a potential One Health approach to malaria control in Africa. Trends Parasitol. 2022;38:112–123. doi: 10.1016/j.pt.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Moore C, Scherr T, Matoba J, Sing C, Lubinda M, Thuma P, et al. mHAT app for automated malaria rapid test result analysis and aggregation : a pilot study. Malar J 2021:1–11. 10.1186/s12936-021-03772-5. [DOI] [PMC free article] [PubMed]
- 69.•Yu H, Yang F, Rajaraman S, Ersoy I, Moallem G, Poostchi M, et al. Malaria Screener : a smartphone application for automated malaria screening. BMC Infect Dis 2020;20(1):825. doi: 10.1186/s12879-020-05453-1. Interesting study reflecting how novel technologies may be applied to improve the diagnosis of malaria. The mobile app utilizes high-resolution cameras and computing used by smartphones to screen blood smear images for P. falciparum parasites. [DOI] [PMC free article] [PubMed]
- 70.Rodriguez-Valero N, Carbayo ML, Camprubí-Ferrer D, Martí-Soler H, Sanchez DC, Vladimirov A, et al. Telemedicine for international travelers through a Smartphone-based monitoring platform (Trip Doctor®). Travel Med Infect Dis 2022;49:102356. 10.1016/j.tmaid.2022.102356. [DOI] [PubMed]