Abstract
The vascular system in living tissues is a highly organized system that consists of vessels with various diameters for nutrient delivery and waste transport. In recent years, many vessel construction methods have been developed for building vascularized on-chip tissue models. These methods usually focused on constructing vessels at a single scale. In this work, a method that can build a hierarchical and perfusable vessel networks was developed. By providing flow stimuli and proper HUVEC concentration, spontaneous anastomosis between endothelialized lumens and the self-assembled capillary network was induced; thus, a perfusable network containing vessels at different scales was achieved. With this simple method, an in vivo-like hierarchical vessel-supported tumor model was prepared and its application in anticancer drug testing was demonstrated. The tumor growth rate was predicted by combining computational fluid dynamics simulation and a tumor growth mathematical model to understand the vessel perfusability effect on tumor growth rate in the hierarchical vessel network. Compared to the tumor model without capillary vessels, the hierarchical vessel-supported tumor shows a significantly higher growth rate and drug delivery efficiency.
Keywords: hierarchical vessel network, perfusable, anastomosis, vessel-on-a-chip, HUVECs, tumor model
Introduction
In the last two decades, with the development of microfluidics and 3D cell culture technologies, various organ-on-chip models have been developed for biological and pharmaceutical studies. Through microfabrication and integration with the 3D cell culture system, various biomimetic environments and functional structures can be recapitulated on the organ-on-chip models.1−3 To build more realistic models and broaden their applications, blood vessels have been integrated into these models.4−7 The integrated vascular system not only distributes oxygen and nutrients in large tissue models but also makes it possible to recapitulate the paracrine signaling, which promotes the tissue model maturation.8,9 More importantly, with the vascular network, various hemodynamics influenced biological and drug delivery processes, such as white blood cells and circulating tumor cells (CTCs) adhesion,10,11 and nano-carrier transport,12,13 can be investigated with these on-chip vascularized tissue models.
Various methods have been reported on vessel construction for in vitro vascularized tissue models. According to the dimensions of the resultant vessels, these methods can be generally grouped into two categories:14,15 pre-made channel endothelialization and endothelial cell self-assembly-based vasculogenesis. The first method builds blood vessels using endothelial cells to line the constructed channels with desired geometries in the hydrogel. With this method, vessels with diameters ranging from a few hundred micrometers to a few millimeters can be achieved.16−20 For example, Kinstlinger et al. used sacrificial materials to build millimeter-scale channels in the hydrogel with 3D printing technology. After seeding the human umbilical vein endothelial cells (HUVECs) to fully cover the channel inner surface, a perfusable dendritic vascular network was constructed in vitro.(20) Likewise, Virumbrales-Muñoz et al. used PDMS rods as templates to cast channels in the hydrogel. After template removal and cell seeding, the patient-derived vessel model could be rapidly reconstituted in vitro for personalized medicine study.16 These models not only realized 3D vessel–tissue interface but also make it possible to study the vessel–tissue interaction at the cellular level.19,21−23 However, due to the limited resolution of the fabrication technology and the difficulty of perfusing cells into thin channels, it is difficult to construct capillary vessels with complicated structures and diameters below 100 μm.24
To construct vessels with a diameter smaller than 100 μm, the second method, based on endothelial cell self-assembly in hydrogels, has been reported.25−30 After being seeded into hydrogels at appropriate concentrations, endothelial cells can deform and self-assemble into interconnected and perfusable capillary networks.25,26,28,31 This approach has been broadly used to build vascularized tissue models on-chip in the last decades.25,29 For example, Offeddu et al. reported their work on building a breast cancer metastasis model with this approach and evaluated the role of endothelial cell glycocalyx in tumor cell metastatic extravasation.29 Furthermore, by providing mechanical and biochemical cues in the hydrogels on such platforms, endothelial cells can migrate and sprout in the hydrogel to form new vessels.26,28,30,32 Kim et al. demonstrated that the endothelial cells could sense both interstitial flow and growth factor gradient, thus sprout to form new vessels against the flow direction and the growth factor gradient in hydrogel.26,28 Nashimoto et al. show that the vascularized tumor model can be constructed by inducing angiogenesis with the spheroids consisting of fibroblasts and tumor cells in hydrogel.30
To date, these two approaches have been predominately used to construct vascularized on-chip tissue models. However, vessel networks in real tissue usually have a hierarchical structure that includes vessels with different scales and structures.33 Hemodynamic conditions diversified by such hierarchical structure significantly influence the physiological and pathological processes, such as cell adhesion and thrombus formation in vascular network.11,12,34 Therefore, a hierarchical vessel network consisting of vessels at different scales is desired for constructing more realistic models. By inducing anastomosis between the vessels of different scales, such a hierarchical network can be built. Recently, a few studies have reported their works on hierarchical vessel network construction. Kim et al. and Nashimoto et al. both reported that the tissue models integrated with hierarchical vessels network can be constructed by inducing angiogenesis under proper mechanical and biochemical environments.26,28,30 Zohar et al. and Szklanny et al. reported another 3D microfabrication-based approach for building hierarchical networks at a large scale. In their work, a fenestrated vessel-like scaffold was fabricated and embedded in hydrogels containing endothelial cells and stromal cells. Then, the constructs were cultured to mature the tissues and the vessel perfusability was validated by microsphere perfusion.35,36 These works proved that the perfusable hierarchical network can be constructed by inducing the anastomotic fusion between the vessels at different scales. The anastomotic fusion reported in these works was induced with the support and guidance of microposts and microchannels. Salameh et al. reported that anastomotic fusion of endothelialized lumens and capillaries in hydrogel can be built without the extra physical supports;37 however, the perfusability of the anastomosis sites was not demonstrated.
Here, we report spontaneous anastomosis sites between the primary vessels made of endothelialized lumens and HUVEC self-assembled capillary networks can be induced without the physical supports; thus, a hierarchical and perfusable vascular network can be constructed in an intact gel within 5-day incubation. The different factors which influence anastomosis were studied. It was found that both flow stimuli and HUVEC concentration contribute to induced anastomosis. As a demonstration, the network construction method was used to build an on-chip hierarchical vessel-supported tumor model for drug testing. Paclitaxel was used to test the drug response of the tumor model. By interfering with the microtubular disassembly, paclitaxel inhibits the mitosis of proliferating cells, thus triggering apoptosis.38,39 Compared to the tumor model without capillary support, which is referred to as a nonhierarchical vessel-supported tumor model, the hierarchical vessel-supported tumor model shows a significantly higher tumor growth rate and lower drug resistance. With the help of simulation analysis, the higher tumor growth rate in the hierarchical vessel-supported tumor model was explained by the higher mass flow rate of the medium provided by the hierarchical vessels. It is believed that this work may inspire future vascularized organ-on-a-chip model development and facilitate drug screening.
Materials and Methods
Device Fabrication
The microfluidic devices were fabricated using standard soft lithography techniques.16 In brief, a 20k DPI mask was fabricated on a flexible material from an AutoCAD file (CAD/Art Services, Inc.). The device pattern on the photomask (shown in Figure S1) was transferred to a layer of photoresist. Photoresist SU8 2150 was spin-coated on a clean silicon wafer at 1600 rpm to achieve a thickness of 400 μm. Then, the coated wafer was subjected to soft baking. To achieve the desired thickness of 800 μm, the spin-coat was repeated with the same parameter once. Next, UV exposure was performed using a Karl Suss MA6/BA6 aligner at the cleanroom of Lehigh University. After exposure, the wafer was post-baked and developed by following the SU8 processing guidelines.40 To fabricate the device, degassed polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning) was used to cast out the patterns from the mold on the silicon wafer. The ports on devices were made with ⌀1.5 and ⌀3 mm biopsy punches. Finally, the prepared PDMS pieces were bound to glass slides following oxygen plasma treatment.
The assembled devices were autoclaved and functionalized with APTES (440140, Sigma) and glutaraldehyde (G6257, Sigma) to enhance the adhesion between the hydrogel and the device surface. The functionalization procedure reported by Leivo et al. was adapted for this work.41 In brief, the assembled devices were treated with 10% APTES ethanol solution for 2 min at room temperature. Then, the APTES solution was removed and the devices were rinsed with 100% ethanol three times. Next, 3% glutaraldehyde water solution was used to treat the rinsed devices for 20 min at room temperature. After the incubation, the glutaraldehyde solution was removed, and the functionalized devices were rinsed with deionized water five times. Finally, the functionalized devices were dried in a 90 °C oven for the following use.
Cell Culture
Pooled HUVECs (Lonza) were cultured in EGM-2 MV medium (CC-3202, Lonza) and used for network formation between passages 3 and 7. Normal human lung fibroblasts (NHLFs) (Lonza) were cultured in FBM-2 medium (CC-3132, Lonza) and used for the experiments between passages 5 and 12. The NHLFs were used here as Margolis et al. reported that NHLFs can facilitate vessel network formation.42 To construct the hierarchical vessel-supported tumor model, the HCT116 cells (ATCC) were cultured in high-glucose DMEM (Gibco) with 10% fetal bovine serum (R&D systems) and 1% penicillin–streptomycin solution (Cytiva). All of the cells were proliferated in 25 cm2 flasks, and the culture medium was changed every other day.
Cell Seeding and Continuous Culture
Seeding Preparation
Before seeding, PDMS rods cast out of 27-gauge needles, which have a diameter of 210 μm, were positioned in the functionalized culture chambers via the four ports and then baked in a 90 °C oven for 1 h. During the device baking, the HUVECs and NHLFs were harvested with 0.25% trypsin solution (Gibco) and pelleted with centrifugation. Both pelleted HUVECs and NHLFs were resuspended with EGM-2 MV medium to reach the desired concentration. Lyophilized bovine fibrinogen (341573, Sigma) and bovine thrombin (605157, Sigma) were dissolved in sterile DPBS to prepare hydrogel stock solutions, which have a concentration of 20 mg/mL and 100 U/mL, respectively.
Cell Seeding
The baked devices were removed from the oven and cooled down to room temperature in a biosafety hood for the following seeding. To construct a hierarchical vessel network, 26 μL of cell-laden gel containing 4 mg/mL fibrinogen, 4 U/mL thrombin, 1 × 105 cells/mL NHLFs, and 3 × 106 to 7 × 106 cells/mL HUVECs was prepared from the stock solutions. The freshly prepared cell-laden gel was immediately injected into the chamber via one of the side ports throughout the entire chamber so that the inserted PDMS rods can be completely embedded in the gel.
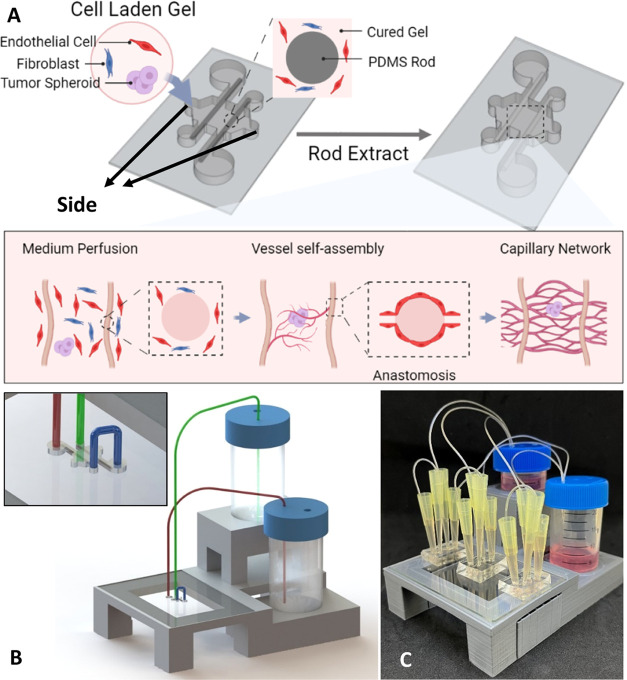
To construct the hierarchical vessel-supported tumor model, HCT116 cell spheroids were prepared and mixed with the cell-laden gel described above for seeding. HCT116 cell spheroids were prepared with the method developed by Shi et al.43 In brief, HCT116 cells harvested with 025% trypsin were pelleted and resuspended to 5 × 102 cells/mL. The cell suspension (2 mL) was seeded into a 35 mm Petri dish and cultured for 6 days. Then, the DMEM culture medium in the Petri dish was replaced with DMEM containing 0.16 mg/mL dispase II (EMD Millipore), and the HCT116 cells were cultured for another 3 days under shaking (60 rpm on an orbital shaker). During the shaking, dispase slowly detached the HCT116 cell colonies from the dish, thus, allowing them to form spheroids. The prepared spheroids were pre-stained with CellTracker green (C34251, Invitrogen). To get the tumor spheroid of uniform size, HCT116 spheroids suspending in DMEM were first filtered with a 70 μm cell strainer (431751, Corning) to remove the large spheroids, then the smaller spheroids suspending in medium were filtered with a 40 μm cell strainer (431750, Corning) to get rid of the individual cells and clusters. The spheroids caught on a 40 μm cell strainer were selected under the microscope with a pipette and mixed into cell-laden gel. The cell seeding workflow is shown in Figure 1A.
Figure 1.
Method for constructing hierarchical vessel-supported tumor model. (A) Schematic diagram of cell seeding and culture with continuous medium flow. (B) Siphon flow system model; inset shows the connections between tubing and inlet/outlet ports on the device. (C) Photo of the culture system.
To construct the nonhierarchical vessel-supported tumor model, the tumor spheroids prepared above were mixed with uncured fibrin gel and seeded to the devices. Specifically, 26 μL of fibrin gel containing 4 mg/mL fibrinogen, 4 U/mL thrombin, and three to five tumor spheroids were injected into the devices via one of the side ports. No HUVECs were encapsulated in the fibrin gel; thus, no capillary network was formed in nonhierarchical vessel-supported tumor model.
All seeded devices were left in an incubator for 20 min to cure the fibrin gel before PDMS rod removal. After the rod removal, for the nonhierarchical vessel-supported models, 10 μL of 6 × 106 cells/mL HUVECs was seeded into the channels cast by PDMS rods to construct the endothelialized lumens.
Continuous Medium Flow Setup
A siphon flow system44 was adapted to provide continuous EGM-2 MV medium flow to the devices (Figure 1B). To stabilize the hydraulic pressure which drives the medium flow, trimmed 200 μL pipette tips were inserted into the ports of the devices. Thin tubing with a 0.79 mm inner diameter was used to introduce medium from the conical tubes to trimmed pipette tips. The image of the continuous culture setup is shown in Figure 1C. During the incubation, the continuous culture setup was placed in a humidity chamber.25 The medium level in conical tubes was adjusted three times per day to achieve an average flow rate of around 417 μL/h. Cultured samples were discarded if the hydrogel collapsed or vascular formation was not observed.
Drug Testing
Paclitaxel (P-9600, LC labs) was administrated to the constructed tumor models on-chip to test their response to the anticancer drug. Paclitaxel was dissolved in DMSO at 5 mM to prepare the stock solution. To treat the tumor models with paclitaxel, the stock solution was diluted to 5 μM with EGM-2MV medium and perfused into the tumor model through the endothelialized lumens. The tumor models were incubated with paclitaxel-containing medium for 1 h followed by complete medium change.45 After incubating the treated models for another 2 days, the samples were stained with Live/Dead staining and imaged with a confocal microscope.
Sample Staining and Imaging
To image the cultured samples, the samples were stained with CellTracker Red (C34552, Invitrogen) diluted in EGM-2 MV medium for 15 min. Then, the culture medium was removed and 4% paraformaldehyde in PBS was applied to the sample for overnight fixation at 4 °C. The fixed samples were washed three times and permeabilized with 0.1% Triton X-100 before staining with 50 μg/mL DAPI in PBS at 4 °C overnight. The stained samples were washed thrice before imaging to reduce the background signal.
To differentiate HUVECs from cocultured NHLFs, immunostaining was performed on some samples. For these samples, NHLFs were per-stained with CellTracker Green. After the vessel network was formed, the samples were fixed with 4% paraformaldehyde in PBS and washed thrice. Then, the samples were stained with 1:50 diluted PE/Cyanine5 labeled anti-human PECAM antibody (303149, BioLegend) and 50 μg/mL DAPI in 3% BSA at 4 °C overnight. The samples were washed thrice before imaging.
To confirm the growth status of the spheroids, HCT116 spheroids with similar size (40–70 μm) were prepared and cultured in fibrin gel for 2 days, the spheroids were then fixed and stained with 1:100 diluted anti-Ki67 (151211, BioLegend) and 50 μg/mL DAPI in 3% BSA at 4 °C overnight. The samples were washed thrice before imaging.
The stained samples were imaged with Nikon confocal microscope C2+. The collected images were processed with ImageJ and AngioTool.46
Simulation
To analyze the mass flow rate in a representative hierarchical vessel network, the z-stack scan image of the constructed hierarchical vessel was used to reconstruct its geometry. The projection of the z-stack scan was imported to SOLIDWORKS 2021 Education edition to reconstruct the 2D vessel geometry. Then, the 2D vessel geometry was introduced to Ansys Fluent 2021 R2 to predict the mass flow rate between the two endothelialized lumens. As a comparison, the mass flow rate between the two endothelialized lumens, which were not connected by the capillary network, was also analyzed. In such a case, the area between the two endothelialized lumens was treated as a porous hydrogel media with a permeability47 of 1.78 × 10–14 m2.
For the simulation, the geometries were discretized using standard meshing in Ansys, which generated 22625 nodes for the hierarchical vessel network and 10 366 nodes for the endothelialized lumens with porous area. A constant pressure difference of 50 Pa is applied between the inlet and outlet as indicated in Figure 2D for both scenarios.
Figure 2.
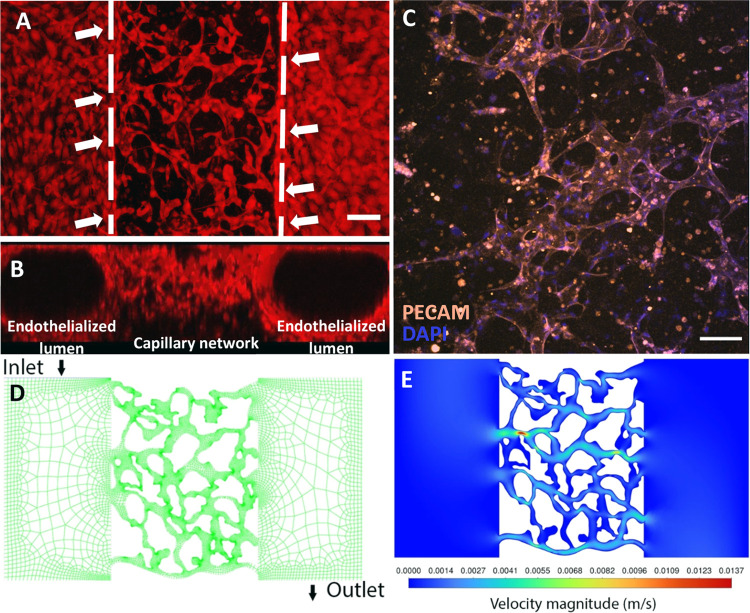
Constructed hierarchical vessel network. (A) Hierarchical vessel network achieved by inducing anastomosis between endothelialized lumens and self-assembled capillary network. Dashed lines label the boundaries of the endothelialized lumen and capillary network. White arrows indicate the anastomosis sites. The scale bar is 100 μm. (B) Side view of the hierarchical network formed by coculturing HUVECs with NHLFs under continuous medium flow. (C) Representative images of the capillary network formed by coculturing HUVECs with NHLFs under continuous medium flow. The scale bar is 100 μm. (D) Meshed geometry of the hierarchical network with 22 625 nodes. (E) Predicted velocities in the reconstructed vessel geometry.
A computational model is developed to provide insights into nutrient availability within the vessel network and the expedited tumor growth, following our prior work.48 The HCT116 tumor cell growth can be modeled using a simplified governing equation:49−55Np = Noexp(rNt), where Np is the number of cancer cells after a growth time t, No is the initial number of cells in the population, and rN is the growth rate that depends on cell type and nutrition availability. The change in nutrient supply over time is defined as Δnutr_avail = (Convin + Diffin – Consupop) × t, which is the balance of supply and consumption, Consupop, (g/h), where nutrients are driven into the tumor by both active convective flow, Convin, and passive diffusion, Diffin. The nutrient consumption rate for the entire population of cancer cells changes with time as the number of cells in the population increases due to growth. Nutrient availability for tumor spheroids includes additional factors such as interstitial fluid pressure, nutrient diffusion, and nutrient convection.49−55 However, factors such as hydrogel encapsulation and glucose consumption rate of different cell types were not considered in this adopted model for simplicity.
Statistical Analysis
All results are shown as mean ± standard derivation. Comparisons between two samples were performed with t-test, and multiple comparisons were performed with one-way ANOVA. Tukey test was used to correct the result of ANOVA. Statistical significance was set at p < 0.05.
Results and Discussion
Hierarchical Network Construction
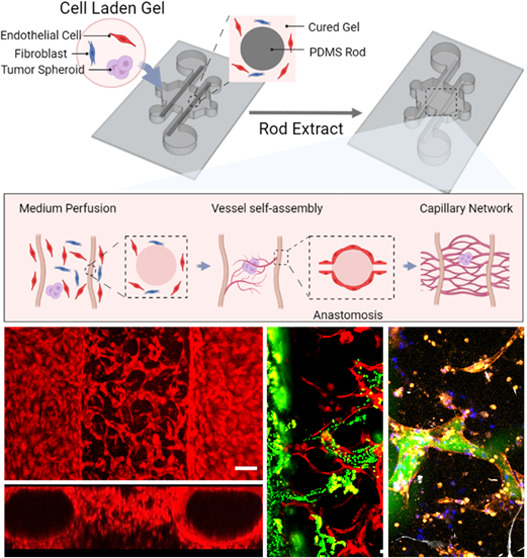
Before building a hierarchical vessel-supported tumor model, a hierarchical vessel network was first constructed. Microfluidic culture chambers were prepared by following the described method. By seeding the culture chamber with fibrin gel containing 7 × 106 cells/mL HUVECs and 1 × 105 cells/mL NHLFs, a hierarchical vessel network with a total gel thickness of 500 μm can be constructed after 5-day culture under continuous medium flow. As shown in the reconstructed images shown in Figure 2A,B, HUVECs in gel endothelialized the PDMS rods cast channels and formed the vessel lumen. In the meanwhile, HUVECs embedded in fibrin gel between the two endothelialized lumens self-assembled into a capillary network. The reconstructed 3D structure of the hierarchical vessel network is shown in Figure S2A. After the vessel network is constructed, the network is stained with anti-PECAM (see Figure 2C). Figure S2B shows the reconstructed 3D capillary network stained by anti-PECAM and DAPI.
Using the CFD simulation, the geometry of the hierarchical network was extracted and meshed (Figure 2D). When 50 Pa was applied between the inlet and outlet, the medium velocities in the network were predicted and shown in Figure 2E. The medium mass flow rate was found as 3.35 × 10–4 kg/s for the hierarchical vessel network. For the endothelialized lumens with only hydrogel (modeled as porous media) in between, the meshed geometry and velocity prediction are shown in Figure S3, and the medium mass flow rate was calculated as 1.20 × 10–6 kg/s, which is 2 orders of magnitude smaller than the hierarchical vessel network.
Vessel Network Perfusability Test
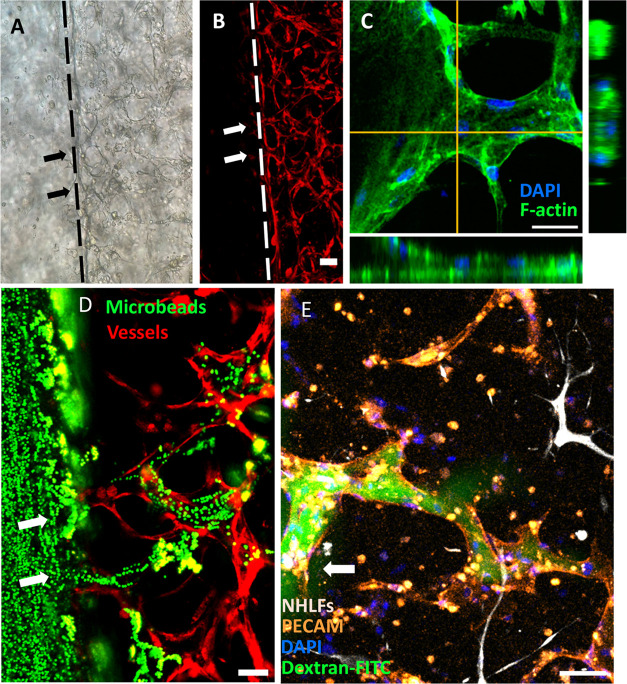
To determine if the hierarchical network was perfusable, the anastomosis sites between endothelialized lumens and capillary network were further examined. Representative image of anastomosis sites on a focal plane (Figure 3A,B) shows the structure of such connection, and the orthogonal views of an anastomosis site are shown in Figure 3C, in which the tubular shape can be observed. The reconstructed 3D structure of anastomosis is shown in Figure S4. To further test the perfusability, 2 μm fluorescent beads and dextran-FITC solution were introduced into the vessel network via one of the endothelialized lumens. As shown in Figure 3D,E, both microbeads and dye solution entered the capillary network through anastomosis sites, thus validating their perfusability. It is worth mentioning that some of the dextran-FITC solutions leaked into the gel from the vessel, which indicates the vessels formed with the current formula are leaky. As reported in the literature, the leakage can be avoided by including other stromal cells, such as pericytes, in the formula.56,57 The process of the 2 μm beads flow into the capillaries and the reconstructed 3D structure of dye-filled capillary vessels are shown in Figures S5 and S6. Furthermore, the distribution of the anastomosis sites along the endothelialized lumens is shown in Figure S7. It was observed that the anastomosis sites distributed evenly along the lumen direction, while along the height of the sample, more anastomosis sites formed closer to the bottom of the lumens. The unevenly distributed anastomosis sites along the height are presumably caused by the cell sinking during the gel polymerization, which makes the initial cell concentration higher around the chamber bottom.58
Figure 3.
Anastomosis between endothelialized lumens and capillaries. (A, B) Anastomosis sites between the endothelialized lumens and self-assembled capillary network. Dashed lines indicate the boundary between the vessel lumens and the capillary network. The scale bar is 100 μm. (C) Orthogonal view of an anastomosis site. The scale bar is 50 μm. (D) 2 μm beads flow from endothelialized lumens into the capillaries via anastomosis sites. The scale bar is 100 μm. (E) 500 kDa dextran-FITC solution perfused into a capillary vessel connecting with the endothelialized lumens. The scale bar is 100 μm. Arrows indicate anastomosis sites.
Flow Stimuli Promote Network Formation
Next, the effect of continuous flow on network formation was investigated. The hypothesis is that the flow stimuli will facilitate the vessel network self-assembly and anastomosis site formation. After 6-day culture, the samples were fixed and stained for imaging, the resultant networks are shown in Figure 4A,C. Samples cultured with the medium flow formed an interconnected perfusable network (Figure 4A), while the samples cultured under static conditions only formed broken fiber-like structures (Figure 4C). The morphological properties of the formed network, such as percentage of the vessel covered area, effective diameter, and junction density, were quantified from the collected images with the method utilized by Whisler et al.31 The effective diameters were calculated as the ratio of vessel covered area to total vessel length. The total vessel length was measured with AngioTool. Figure 4B,D shows the AngioTool processed images of the vessel network. The vessel networks cultured under flow covered 41.5 ± 2.0% of the total area on average and have an effective average diameter of 38.1 ± 4.6 μm, the average junction density of such networks is 106.1 ± 12.4 mm–2. Compared with flow cases, vessel networks formed under static culture cover less area 23.0 ± 2.9% (t-test, n = 3, p = 0.0004, Figure 4E), have a lower average effective diameter 21.3 ± 3.9 μm (t-test, n = 3, p = 0.004, Figure 4F), and the junction density of the networks is also smaller, 32.6 ± 8.0 mm–2 (t-test, n = 3, p = 0.008, Figure 4G). Additionally, the perfusable anastomosis sites formed under flow have a normalized density of 8.6 ± 3.3 mm–1 along the lumens, which is significantly more than the anastomosis sites formed under static culture (1.8 ± 0.4 mm–1, t-test, n = 3, p = 0.005, Figure S7C). The results suggest that flow stimuli facilitate the formation of vessel networks and anastomosis, which is consistent with the previous works.26,28,31
Figure 4.
Vessel network formed by HUVECs with and without flow stimuli. (A, C) Projection of a confocal z-stack scan of the vessel networks formed by 7 × 106 cells/mL under continuous medium flow and static culture. Scale bars are 100 μm. (B, D) Calculation of the vessel covered area and total vessel length for each group with AngioTool. (E)–(G) Comparison of the percentage of vessel covered area, effective vessel diameter, and junction density measured from the two culture conditions. Error bars show the standard derivation. t-test, **p < 0.01, ***p < 0.001, n = 3.
Effect of HUVEC Concentration on Network Formation
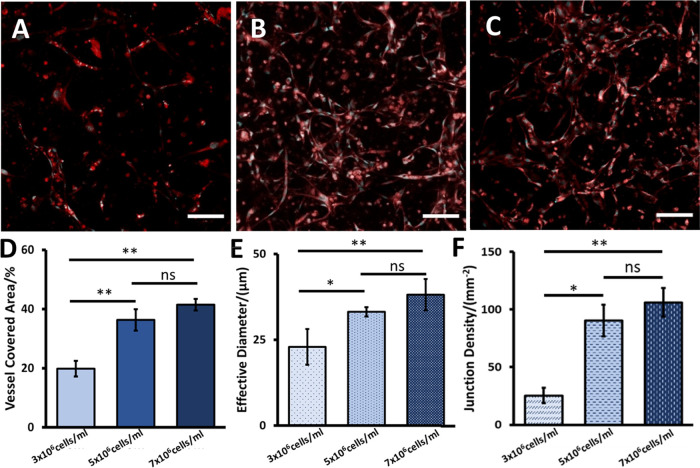
After the effect of flow stimuli was investigated, the networks formed by HUVECs at different concentrations were also evaluated. Fibrin gels containing HUVECs at different concentrations were seeded into the chambers with 1 × 105 cells/mL NHLFs and cultured for 6 days under flow. The vessel networks formed by HUVECs at different concentrations are shown in Figure 5A–C; 5 × 106 and 7 × 106 cells/mL HUVEC formed vessel networks in gel, while no interconnected vessel network was observed when the HUVEC concentration was at 3 × 106 cells/mL. The network morphological properties were quantified from the collected images. The percentage of the vessel coverage area, effective diameter, and junction density measured from the 3 × 106 cells/mL HUVEC group were significantly lower than the values measured from the other two groups. Comparing the 5 × 106 cells/mL HUVEC group to the 7 × 106 cells/mL HUVEC group, the evaluated vessel network properties are not significantly different from each other (ANOVA, n = 3; coverage percentages, p = 0.14; effective diameters, p = 0.32; junction density, p = 0.62. As shown in Figure 5D–F). The quantified network properties are shown in Table 1. Although the vessel networks formed by 5 × 106 and 7 × 106 cells/mL HUVECs are similar, it was observed that the higher HUVEC concentration allowed the network to form in a shorter time (3 days for 7 × 106 cells/mL HUVECs and 5 days for 5 × 106 cells/mL HUVECs, respectively). Additionally, in the current work, no interconnected vessel network was observed when the 3 × 106 cells/mL HUVECs were seeded and cultured for 6 days. Previously, a few groups have reported that the interconnected vessel networks can be built with low initial HUVEC concentrations after a longer culture time.35,36 Therefore, the culture time may need to be further investigated to determine if the perfusable and interconnected capillary network can be built with 3 × 106 cells/mL HUVECs on this platform. To construct the vessel network in a fast manner, HUVECs at 7 × 106 cells/mL were used to construct the hierarchical vessel-supported tumor model.
Figure 5.
Vessel network formed by HUVECs at different concentrations. (A)–(C) Vessel network formed by 3 × 106, 5 × 106, and 7 × 106 cells/mL HUVECs in fibrin gel, respectively. Scale bars are 100 μm. (D)–(F) Comparison of vessel covered area percentage, effective vessel diameter, and junction density measured from the vessel networks formed by HUVECs at different concentrations. Error bars show the standard derivation. *p < 0.05, **p < 0.01, ns is not significant, n = 3.
Table 1. Morphological Property Comparison of Vessel Networks Formed by HUVECs at Different Concentrations.
| 3 × 106 cells/mL | 5 × 106 cells/mL | 7 × 106 cells/mL | |
|---|---|---|---|
| vessel covered area (%) | 19.9 ± 2.7 | 36.3 ± 3.6 | 41.5 ± 2.0 |
| effective diameter (μm) | 22.9 ± 5.2 | 33.2 ± 1.4 | 38.1 ± 4.6 |
| junction density (mm–2) | 25.3 ± 6.6 | 90.4 ± 13.6 | 106.1± 12.4 |
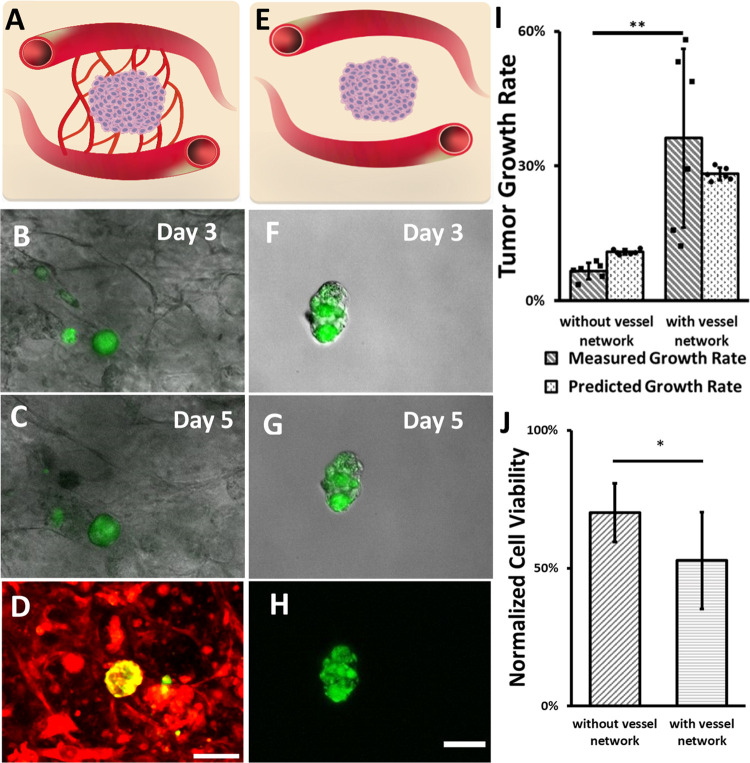
Hierarchical Vessel-Supported Tumor Model
To explore
the potential applications of the constructed hierarchical vessel
network, the hierarchical vessel-supported tumor model was constructed
using the described method. After 3-day culture, the growth of the
tumor spheroids was monitored in both models from day 3 to day 5 by
measuring the cross-sessional area of the tumor spheroids (Atumor). In this work, Atumor was monitored and used to calculate tumor growth rate
instead of volume, as scanning the tumor spheroids with the z-stack
function of a confocal microscope caused significant tumor cells and
HUVECs death, which was possible because of fluorescent phototoxicity.59,60 The growth rate of the tumor spheroids was calculated by the following
equation: 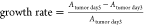 . As shown in Figure 6A–H, the growth of hierarchical vessel-supported
tumors was observed, while the tumors without capillary support had
neglectable growth in nonhierarchical vessel-supported tumor models.
The growth rate of tumors in the hierarchical vessel-supported tumor
model is 36 ± 20%. The measured tumor growth rate from the hierarchical
vessel-supported models has a large variance, which might be introduced
by the different distances between the tumor and the perfusable vessels.
As the location of the tumors was not controlled and the vessel perfusability
was not uniform, the availability of the medium flow and local environment
may vary for each spheroid. Another variance source was the proliferation
capability of the spheroids; since the growth rate was measured from
independent cultures, the proliferation capability of the spheroids
may vary from batch to batch. To confirm the growth capability of
the spheroids, HCT116 spheroids were cultured in fibrin gel for 2
days and fixed for anti-Ki67 staining. The staining result suggests
that the spheroids are proliferating (see Figure S8). Additionally, it is worth mentioning that cell migration
also possibly contributed to the spheroid expansion; thus, the contribution
of cell proliferation and migration to the spheroid expansion will
be analyzed in the future. Despite the variance, the measured growth
rate is significantly higher than the 7 ± 2% growth rate measured
from the nonhierarchical vessel-supported tumor model (t-test, n = 6; p = 0.0075, see Figure 6I).
. As shown in Figure 6A–H, the growth of hierarchical vessel-supported
tumors was observed, while the tumors without capillary support had
neglectable growth in nonhierarchical vessel-supported tumor models.
The growth rate of tumors in the hierarchical vessel-supported tumor
model is 36 ± 20%. The measured tumor growth rate from the hierarchical
vessel-supported models has a large variance, which might be introduced
by the different distances between the tumor and the perfusable vessels.
As the location of the tumors was not controlled and the vessel perfusability
was not uniform, the availability of the medium flow and local environment
may vary for each spheroid. Another variance source was the proliferation
capability of the spheroids; since the growth rate was measured from
independent cultures, the proliferation capability of the spheroids
may vary from batch to batch. To confirm the growth capability of
the spheroids, HCT116 spheroids were cultured in fibrin gel for 2
days and fixed for anti-Ki67 staining. The staining result suggests
that the spheroids are proliferating (see Figure S8). Additionally, it is worth mentioning that cell migration
also possibly contributed to the spheroid expansion; thus, the contribution
of cell proliferation and migration to the spheroid expansion will
be analyzed in the future. Despite the variance, the measured growth
rate is significantly higher than the 7 ± 2% growth rate measured
from the nonhierarchical vessel-supported tumor model (t-test, n = 6; p = 0.0075, see Figure 6I).
Figure 6.
Comparison of hierarchical vessel-supported and nonhierarchical vessel-supported tumor models. (A, E) Schematics of hierarchical vessel-supported and nonhierarchical vessel-supported tumor models. (B, C, F, G) Growth of capillary vessel network-supported and nonhierarchical vessel-supported tumor, respectively. (D, H) Confocal images of hierarchical vessel-supported and nonhierarchical vessel-supported tumor, respectively. Vessel cells are labeled in red, and tumors are in green. (I) Experimentally measured and simulation predicted tumor growth rate from day 3 to day 5 for both the nonhierarchical vessel-supported and hierarchical vessel-supported groups, n = 6. (J) Comparison of tumor cell viability two days after drug exposure, n = 9 for the nonhierarchical vessel-supported group and n = 8 for the hierarchical vessel-supported group. Scale bars are 100. Error bars show the standard derivation. t-test, *p < 0.05, **p < 0.01.
Assuming the spheroids expansion is only caused by cell proliferation, the tumor growth rate of both models was predicted with consideration of initial tumor initial size and medium mass flow rate by utilizing the tumor growth model previously developed in our lab.48 The predicted growth rate is shown in Figure 6I. The current tumor growth modeling within the vessel network based on convective and diffusive nutrient transport has shown good agreement with growth data observed experimentally. The results show that the measured growth rates are consistent with the predicted growth rates, which implies the expedited tumor growth in the hierarchical vessel-supported tumor model is mostly caused by increased medium flow provided to tumor tissue via the vessel network. To predict tumor growth in specific environments with higher accuracy, a more comprehensive model, which involves tumor spheroids size, tumor cell migration, nutrient consumption and interactions of various cell types, and hydrogel properties, needs to be developed in the future.
Next, to test the responses of both tumor models to paclitaxel, more samples were prepared. After 5-day incubation, both tumor models were subjected to drug testing. As shown in Figure 6J, cancer cell viability in the hierarchical vessel-supported tumor model (53 ± 18%) was significantly lower than cell viability measured in the nonhierarchical vessel-supported tumor model (70 ± 11%; t-test, n = 9 for the nonhierarchical vessel-supported group, n = 8 for the hierarchical vessel-supported group; p = 0.017). The results indicate that, in addition to enhanced nutrient transport, the hierarchical vascular network also facilitated the transport of paclitaxel to the tumor, therefore reducing the tumor resistance to anticancer drugs.
Discussion
The method reported in this work can be readily adapted to build other hierarchical vessel-supported tissue models, such as liver-on-a-chip and islet-on-a-chip platforms.61,62 However, to gain better control for the hierarchical vessel construction, other factors which may influence the anastomosis between the endothelialized lumens and self-assembled capillary network are worth further investigation in future. For instance, investigating the effects of hydrogels’ biochemical and mechanical properties may provide more controllability on hierarchical vessel construction.63 Ideally, the hydrogel should not only support the endothelial cell migration and transformation to form the vessel networks but also have enough mechanical strength to prevent the cast channels in the gel from collapsing. Furthermore, constructing the hierarchical vessels with endothelial and stromal cells56,64,65 of proper phenotypes should allow more functions of the vascular system to be recapitulated in vitro. In the present work, only HUVECs and lung fibroblasts were used to form the hierarchical vessel network, while a hierarchical vessel model mimicking microcirculation can be possibly built by seeding aortic and microvascular endothelial cells, as well as stromal cells, to proper regions of the chamber via different ports.
Conclusions
In this work, a hierarchical vessel network was constructed in vitro by combining two vessel construction approaches in fibrin gel without micropost support. With a one-step seeding procedure, both endothelialized lumens and self-assembled capillaries can be prepared. By providing the proper conditions, the spontaneous anastomosis between the endothelialized lumens and capillaries was induced, which allows the fluid to flow from the endothelialized lumens to the capillary vessels within 5 days. The effect of flow stimuli and HUVEC concentration was quantified for the hierarchical vessel network construction. To demonstrate the potential applications of this method, a hierarchical vessel-supported tumor model was constructed and subjected to tumor growth monitoring and anticancer drug testing. The results show that both tumor growth rate and drug resistance of the hierarchical vessel-supported tumor are significantly different from the nonhierarchical vessel-supported tumor, which indicates that the constructed hierarchical and perfusable vessels promote nutrients and drug transport. This method should inspire future vascularized organ-on-a-chip model development.
Acknowledgments
This work was supported by National Institute of Health grant R01HL131750, R21EB033102, National Science Foundation grant CBET 2039310, NSF OAC 2215789, Pennsylvania Department of Health Commonwealth Universal Research Enhancement Program (CURE), and Pennsylvania Infrastructure Technology Alliance (PITA).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.2c19453.
Supplementary characterizations and results (PDF)
Reconstructed hierarchical network (S2A.avi) (AVI)
Reconstructed structure of the capillary network (S2B.avi) (AVI)
Reconstructed structure of anastomosis sites (S4A.avi) (AVI)
Reconstructed a single anastomosis site (S4B.avi) (AVI)
Microbeads perfusing through anastomosis sites (S5A.avi) (AVI)
Microbeads filled anastomosis site (S5B.avi) (AVI)
Dye-filled capillary vessel (S6.avi) (AVI)
Author Contributions
Y.L. conceived and supervised the study. Y.Z. and Y.W. designed and performed the experiments. Y.Z. collected and analyzed the data. Y.W. assisted with data analysis. R.P. performed the simulation. X.Q. assisted with the figure edition. Y.Z. wrote the manuscript. Y.W., R.P., X.Q., and Y.L. revised the manuscript. All authors discussed the results and approved the submission.
The authors declare no competing financial interest.
Supplementary Material
References
- Wu Q.; Liu J.; Wang X.; Feng L.; Wu J.; Zhu X.; Wen W.; Gong X. Organ-on-a-Chip: Recent Breakthroughs and Future Prospects. BioMed. Eng. OnLine 2020, 19, 9. 10.1186/S12938-020-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. M.; de Haan P.; Ronaldson-Bouchard K.; Kim G. A.; Ko J.; Rho H. S.; Chen Z.; Habibovic P.; Jeon N. L.; Takayama S.; Shuler M. L.; Vunjak-Novakovic G.; Frey O.; Verpoorte E.; Toh Y. C. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. 10.1038/s43586-022-00118-6. [DOI] [Google Scholar]
- Huh D.; Hamilton G. A.; Ingber D. E. From 3D Cell Culture to Organs-on-Chips. Trends Cell Biol. 2011, 21, 745–754. 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses S. R.; Adorno J. J.; Palmer A. F.; Song J. W. Vessel-on-a-Chip Models for Studying Microvascular Physiology, Transport, and Function in Vitro. Am. J. Physiol.: Cell Physiol. 2021, 320, C92–C105. 10.1152/AJPCELL.00355.2020/ASSET/IMAGES/LARGE/AJ-ACEL200042F003.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajal C.; Shin Y.; Li L.; Serrano J. C.; Jacks T.; Kamm R. D. The CCL2-CCR2 Astrocyte-Cancer Cell Axis in Tumor Extravasation at the Brain. Sci. Adv. 2021, 7, 8139–8162. 10.1126/sciadv.abg8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S.; Jeon J. S.; Dubini G.; Arrigoni C.; Chung S.; Charest J. L.; Moretti M.; Kamm R. D. A Microfluidic 3D in Vitro Model for Specificity of Breast Cancer Metastasis to Bone. Biomaterials 2014, 35, 2454–2461. 10.1016/J.BIOMATERIALS.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Phan D. T. T.; Sobrino A.; George S. C.; Hughes C. C. W.; Lee A. P. Engineering Anastomosis between Living Capillary Networks and Endothelial Cell-Lined Microfluidic Channels. Lab Chip 2016, 16, 282–290. 10.1039/C5LC01050K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D.; Matthews B. D.; Mammoto A.; Montoya-Zavala M.; Yuan Hsin H.; Ingber D. E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Valderrama A.; Messina A.; Mitjavila-Garcia M. T.; Guenou H. The Endothelium, a Key Actor in Organ Development and HPSC-Derived Organoid Vascularization. J. Biomed. Sci. 2020, 27, 67. 10.1186/s12929-020-00661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti G.; Prabhakarpandian B.; Garson C.; Smith A.; Pant K.; Wang B.; Kiani M. F. Bioinspired Microfluidic Assay for in Vitro Modeling of Leukocyte-Endothelium Interactions. Anal. Chem. 2014, 86, 8344–8351. 10.1021/ac5018716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Arozamena C.; Otero-Cacho A.; Carnero B.; Almenglo C.; Aymerich M.; Alonso-Alconada L.; Ferreiros A.; Abalo A.; Bao-Varela C.; Flores-Arias M. T.; Alvarez E.; Munuzuri A. P.; Abal M. Haemodynamic-Dependent Arrest of Circulating Tumour Cells at Large Blood Vessel Bifurcations as New Model for Metastasis. Sci. Rep. 2021, 11, 23231. 10.1038/s41598-021-02482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi S.; Wang S.; Tan J.; Xu J.; Yang J.; Liu Y. Nanoparticle Transport and Delivery in a Heterogeneous Pulmonary Vasculature. J. Biomech. 2017, 50, 240–247. 10.1016/j.jbiomech.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl C. G.; Gao Y.; Zhou S.; Liu Y. The Shape Effect on Polymer Nanoparticle Transport in a Blood Vessel. RSC Adv. 2018, 8, 8089–8100. 10.1039/C8RA00033F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki T.; Sivathanu V.; Kamm R. D. Vascularized Microfluidic Organ-Chips for Drug Screening, Disease Models and Tissue Engineering. Curr. Opin. Biotechnol. 2018, 52, 116–123. 10.1016/J.COPBIO.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Zhou Y.; Qin X.; Liu Y. From Cell Spheroids to Vascularized Cancer Organoids: Microfluidic Tumor-on-a-Chip Models for Preclinical Drug Evaluations. Biomicrofluidics 2021, 15, 061503 10.1063/5.0062697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virumbrales-Muñoz M.; Chen J.; Ayuso J.; Lee M.; Abel E. J.; Beebe D. J. Organotypic Primary Blood Vessel Models of Clear Cell Renal Cell Carcinoma for Single-Patient Clinical Trials. Lab Chip 2020, 20, 4420–4432. 10.1039/D0LC00252F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun M.; Ayuso J. M.; Brenneke R. A.; Virumbrales-Muñoz M.; Lugo-Cintrón K.; Kerr S.; Ponik S. M.; Beebe D. J. Elucidating Cancer-Vascular Paracrine Signaling Using a Human Organotypic Breast Cancer Cell Extravasation Model. Biomaterials 2021, 270, 120640 10.1016/J.BIOMATERIALS.2020.120640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso J. M.; Gong M. M.; Skala M. C.; Harari P. M.; Beebe D. J. Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells. Adv. Healthc. Mater. 2020, 9, 1900925 10.1002/adhm.201900925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Zhou Y.; Paul R.; Qin X.; Islam K.; Liu Y. Adaptable Microfluidic Vessel-on-a-Chip Platform for Investigating Tumor Metastatic Transport in Bloodstream. Anal. Chem. 2022, 94, 12159. 10.1021/ACS.ANALCHEM.2C02556. [DOI] [PubMed] [Google Scholar]
- Kinstlinger I. S.; Saxton S. H.; Calderon G. A.; Ruiz K. V.; Yalacki D. R.; Deme P. R.; Rosenkrantz J. E.; Louis-Rosenberg J. D.; Johansson F.; Janson K. D.; Sazer D. W.; Panchavati S. S.; Bissig K. D.; Stevens K. R.; Miller J. S. Generation of Model Tissues with Dendritic Vascular Networks via Sacrificial Laser-Sintered Carbohydrate Templates. Nat. Biomed. Eng. 2020, 4, 916–932. 10.1038/s41551-020-0566-1. [DOI] [PubMed] [Google Scholar]
- Vila Cuenca M.; Cochrane A.; van den Hil F. E.; de Vries A. A. F.; Lesnik Oberstein S. A. J.; Mummery C. L.; Orlova Vv. Engineered 3D Vessel-on-Chip Using HiPSC-Derived Endothelial- and Vascular Smooth Muscle Cells. Stem Cell Rep. 2021, 16, 2159–2168. 10.1016/J.STEMCR.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S.; Schulte R.; Huang L.; Tsai H.; Hetzer M. W. Direct Reprogramming of Human Smooth Muscle and Vascular Endothelial Cells Reveals Defects Associated with Aging and Hutchinson-Gilford Progeria Syndrome. eLife 2020, 9, e54383. 10.7554/ELIFE.54383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. C. W. Endothelial-Stromal Interactions in Angiogenesis. Curr. Opin. Hematol. 2008, 15, 204. 10.1097/MOH.0B013E3282F97DBC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram P. N.; Hind L. E.; Jiminez-Torres J. A.; Huttenlocher A.; Beebe D. J. An Accessible Organotypic Microvessel Model Using IPSC-Derived Endothelium. Adv. Healthc. Mater. 2018, 7, 1700497 10.1002/ADHM.201700497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. B.; Whisler J. A.; Fröse J.; Yu C.; Shin Y.; Kamm R. D. On-Chip Human Microvasculature Assay for Visualization and Quantification of Tumor Cell Extravasation Dynamics. Nat. Protoc. 2017, 12, 865–880. 10.1038/nprot.2017.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Chung M.; Ahn J.; Lee S.; Jeon N. L. Interstitial Flow Regulates the Angiogenic Response and Phenotype of Endothelial Cells in a 3D Culture Model. Lab Chip 2016, 16, 4189–4199. 10.1039/C6LC00910G. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Cao Y.; Galan E. A.; Hao L.; Zhao H.; Tang J.; Sang G.; Wang H.; Xu B.; Ma S. Vascularized Tumor Spheroid-on-a-Chip Model Verifies Synergistic Vasoprotective and Chemotherapeutic Effects. ACS Biomater. Sci. Eng. 2022, 8, 1215–1225. 10.1021/acsbiomaterials.1c01099. [DOI] [PubMed] [Google Scholar]
- Kim S.; Lee H.; Chung M.; Jeon N. L. Engineering of Functional, Perfusable 3D Microvascular Networks on a Chip. Lab Chip 2013, 13, 1489–1500. 10.1039/C3LC41320A. [DOI] [PubMed] [Google Scholar]
- Offeddu G. S.; Hajal C.; Foley C. R.; Wan Z.; Ibrahim L.; Coughlin M. F.; Kamm R. D. The Cancer Glycocalyx Mediates Intravascular Adhesion and Extravasation during Metastatic Dissemination. Communications Biology 2021, 4, 255. 10.1038/s42003-021-01774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto Y.; Okada R.; Hanada S.; Arima Y.; Nishiyama K.; Miura T.; Yokokawa R. Vascularized Cancer on a Chip: The Effect of Perfusion on Growth and Drug Delivery of Tumor Spheroid. Biomaterials 2020, 229, 119547 10.1016/j.biomaterials.2019.119547. [DOI] [PubMed] [Google Scholar]
- Whisler J. A.; Chen M. B.; Kamm R. D. Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System. Tissue Eng., Part C 2014, 20, 543. 10.1089/TEN.TEC.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauty J.; Usuba R.; Cheng I. G.; Hespel L.; Takahashi H.; Kato K.; Kobayashi M.; Nakajima H.; Lee E.; Yger F.; Soncin F.; Matsunaga Y. T. A Vascular Endothelial Growth Factor-Dependent Sprouting Angiogenesis Assay Based on an In Vitro Human Blood Vessel Model for the Study of Anti-Angiogenic Drugs. EBioMedicine 2018, 27, 225–236. 10.1016/j.ebiom.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. A. V.; le Noble F.; Eichmann A. What Determines Blood Vessel Structure? Genetic Prespecification vs. Hemodynamics. Physiology 2006, 21, 388–395. 10.1152/physiol.00020.2006. [DOI] [PubMed] [Google Scholar]
- Sakariassen K. S.; Orning L.; Turitto V. T. The Impact of Blood Shear Rate on Arterial Thrombus Formation. Future Sci. OA 2015, 1, 30. 10.4155/FSO.15.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar B.; Debbi L.; Machour M.; Nachum N.; Redenski I.; Epshtein M.; Korin N.; Levenberg S.. A Micro-Channel Array in a Tissue Engineered Vessel Graft Guides Vascular Morphogenesis for Anastomosis with Self-Assembled Vascular Networks Acta Biomater 2022, 10.1016/J.ACTBIO.2022.05.026. [DOI] [PubMed]
- Szklanny A. A.; Machour M.; Redenski I.; Chochola V.; Goldfracht I.; Kaplan B.; Epshtein M.; Simaan Yameen H.; Merdler U.; Feinberg A.; Seliktar D.; Korin N.; Jaroš J.; Levenberg S. 3D Bioprinting of Engineered Tissue Flaps with Hierarchical Vessel Networks (VesselNet) for Direct Host-To-Implant Perfusion. Adv. Mater. 2021, 33, 2102661 10.1002/ADMA.202102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh S.; Tissot N.; Cache K.; Lima J.; Suzuki I.; Marinho P. A.; Rielland M.; Soeur J.; Takeuchi S.; Germain S.; Breton L. A Perfusable Vascularized Full-Thickness Skin Model for Potential Topical and Systemic Applications. Biofabrication 2021, 13, 035042 10.1088/1758-5090/ABFCA8. [DOI] [PubMed] [Google Scholar]
- Weaver B. A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. 10.1091/mbc.e14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman M.; Mekhail T. M. Paclitaxel in Cancer Therapy. Expert Opin. Pharmacother. 2005, 3, 755–766. 10.1517/14656566.3.6.755. [DOI] [PubMed] [Google Scholar]
- SU-8 2000 Data Sheet (2100-2150) - MicroChem, 2022, https://www.yumpu.com/en/document/read/18246422/su-8-2000-data-sheet-2100-2150-microchem (accessed 15 Oct, 2022).
- Leivo J.; Virjula S.; Vanhatupa S.; Kartasalo K.; Kreutzer J.; Miettinen S.; Kallio P. A Durable and Biocompatible Ascorbic Acid-Based Covalent Coating Method of Polydimethylsiloxane for Dynamic Cell Culture. J. R. Soc., Interface 2017, 14, 20170318. 10.1098/RSIF.2017.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Wan Z.; Pavlou G.; Zhong A. X.; Xu L.; Kamm R. D.; Zhang S.; Wan Z.; Pavlou G.; Zhong A. X.; Kamm R. D.; Xu L. Interstitial Flow Promotes the Formation of Functional Microvascular Networks In Vitro through Upregulation of Matrix Metalloproteinase-2. Adv. Funct. Mater. 2022, 32, 2206767 10.1002/ADFM.202206767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W.; Kwon J.; Huang Y.; Tan J.; Uhl C. G.; He R.; Zhou C.; Liu Y. Facile Tumor Spheroids Formation in Large Quantity with Controllable Size and High Uniformity. Sci. Rep. 2018, 8, 6837 10.1038/s41598-018-25203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Zhao D.; Phan D. T. T.; Liu J.; Chen X.; Yang B.; Hughes C. C. W.; Zhang W.; Lee A. P. A Hydrostatic Pressure-Driven Passive Micropump Enhanced with Siphon-Based Autofill Function. Lab Chip 2018, 18, 2167–2177. 10.1039/C8LC00236C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase K.; Offeddu G. S.; Gillrie M. R.; Kamm R. D.; Haase K.; Kamm R. D.; Offeddu G. S.; Gillrie M. R. Endothelial Regulation of Drug Transport in a 3D Vascularized Tumor Model. Adv. Funct. Mater. 2020, 30, 2002444 10.1002/ADFM.202002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zudaire E.; Gambardella L.; Kurcz C.; Vermeren S. A Computational Tool for Quantitative Analysis of Vascular Networks. PLoS One 2011, 6, e27385. 10.1371/JOURNAL.PONE.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. H.; Huang S. A.; Aw W. Y.; Rathod M. L.; Cho C.; Ligler F. S.; Polacheck W. J. Multilayer Microfluidic Platform for the Study of Luminal, Transmural, and Interstitial Flow. Biofabrication 2022, 14, 025007 10.1088/1758-5090/AC48E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl C. G.; Liu Y. Microfluidic Device for Expedited Tumor Growth towards Drug Evaluation †. Lab Chip 2019, 19, 1458. 10.1039/c8lc01250d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P.; Kallinowski F.; Okunieff P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [PubMed] [Google Scholar]
- Ferreira S. C.; Martins M. L.; Vilela M. J. Reaction-Diffusion Model for the Growth of Avascular Tumor. Phys. Rev. E 2002, 65, 21907. 10.1103/PhysRevE.65.021907. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L.; Thomson S. P.; Moon T. E. Quantitation of the Number of Cells within Tumor Colonies in Semisolid Medium and Their Growth as Oblate Spheroids. Cancer Res. 1984, 44, 271–277. [PubMed] [Google Scholar]
- Baxter L. T.; Jain R. K. Transport of Fluid and Macromolecules in Tumors. I. Role of Interstitial Pressure and Convection. Microvasc. Res. 1989, 37, 77–104. 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Jain R. K.; Baxter L. T. Mechanisms of Heterogeneous Distribution of Monoclonal Antibodies and Other Macromolecules in Tumors: Significance of Elevated Interstitial Pressure. Cancer Res. 1988, 48, 7022–7032. [PubMed] [Google Scholar]
- Thurber G. M.; Schmidt M. M.; Wittrup K. D. Antibody Tumor Penetration: Transport Opposed by Systemic and Antigen-Mediated Clearance. Adv. Drug Delivery Rev. 2008, 60, 1421–1434. 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluen A.; Boucher Y.; Ramanujan S.; McKee T. D.; Gohongi T.; di Tomaso E.; Brown E. B.; Izumi Y.; Campbell R. B.; Berk D. A.; Jain R. K. Role of Tumor-Host Interactions in Interstitial Diffusion of Macromolecules: Cranial vs. Subcutaneous Tumors. Proc. Natl. Acad. Sci., U.S.A. 2001, 98, 4628–4633. 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi M.; Shin Y.; Osaki T.; Hajal C.; Chiono V.; Kamm R. D. 3D Self-Organized Microvascular Model of the Human Blood-Brain Barrier with Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. 10.1016/J.BIOMATERIALS.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K.; Yamaguchi Y.; Usui S.; Nashimoto Y.; Hanada S.; Kiyokawa E.; Uemura A.; Yokokawa R.; Nishiyama K.; Miura T. A New Perfusion Culture Method with a Self-Organized Capillary Network. PLoS One 2020, 15, e0240552 10.1371/JOURNAL.PONE.0240552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z.; Zhong A. X.; Zhang S.; Pavlou G.; Coughlin M. F.; Shelton S. E.; Nguyen T.; Lorch J. H.; Barbie D. A.; Kamm R. D.; Wan Z.; Zhong A. X.; Zhang S.; Pavlou G.; Coughlin M. F.; Shelton S. E.; Nguyen H. T.; Kamm R. D.; Lorch J. H.; Barbie D. A. A Robust Method for Perfusable Microvascular Network Formation In Vitro. Small Methods 2022, 6, 2200143 10.1002/SMTD.202200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau C.; Wee T. L.; Duh Y. R.; Couto M. P.; Ardakani K. H.; Brown C. M. Excitation Light Dose Engineering to Reduce Photo-Bleaching and Photo-Toxicity. Sci. Rep. 2016, 6, 30892 10.1038/srep30892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue P. P.; Alghamdi R. A.; Tomancak P.; Reynaud E. G.; Shroff H. Assessing Phototoxicity in Live Fluorescence Imaging. Nat. Methods 2017, 14, 657–661. 10.1038/nmeth.4344. [DOI] [PubMed] [Google Scholar]
- Tao T.; Wang Y.; Chen W.; Li Z.; Su W.; Guo Y.; Deng P.; Qin J. Engineering Human Islet Organoids from IPSCs Using an Organ-on-Chip Platform. Lab Chip 2019, 19, 948–958. 10.1039/C8LC01298A. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang H.; Deng P.; Chen W.; Guo Y.; Tao T.; Qin J. In Situ Differentiation and Generation of Functional Liver Organoids from Human IPSCs in a 3D Perfusable Chip System. Lab Chip 2018, 18, 3606–3616. 10.1039/C8LC00869H. [DOI] [PubMed] [Google Scholar]
- Cassel De Camps C.; Aslani S.; Stylianesis N.; Nami H.; Mohamed N. V.; Durcan T. M.; Moraes C. Hydrogel Mechanics Influence the Growth and Development of Embedded Brain Organoids. ACS Appl. Bio Mater. 2022, 5, 214–224. 10.1021/acsabm.1c01047. [DOI] [PubMed] [Google Scholar]
- Margolis E. A.; Cleveland D. S.; Kong Y. P.; Beamish J. A.; Wang W. Y.; Baker B. M.; Putnam A. J. Stromal Cell Identity Modulates Vascular Morphogenesis in a Microvasculature-on-a-Chip Platform. Lab Chip 2021, 21, 1150–1163. 10.1039/d0lc01092h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosyakova N.; Kao D. D.; Figetakis M.; López-Giráldez F.; Spindler S.; Graham M.; James K. J.; Won Shin J.; Liu X.; Tietjen G. T.; Pober J. S.; Chang W. G. Differential Functional Roles of Fibroblasts and Pericytes in the Formation of Tissue-Engineered Microvascular Networks in Vitro. npj Regener. Med. 2020, 5, 1–12. 10.1038/s41536-019-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.