Abstract
Introduction:
Platelet sequestration, inflammation, and inappropriate coagulation cascade activation are prominent in liver xenotransplant models and are associated with poor outcomes. Here, we evaluate a cassette of six additional genetic modifications to reduce anti-pig antibody binding (α-1,3-galactosyl transferase knockout [GalTKO]) and target coagulation dysregulation (human endothelial protein C receptor [hEPRC] and thrombomodulin [hTBM]), complement pathway regulation (human membrane cofactor protein, hCD46), inflammation heme oxygenase 1 [hHO-1]), and a self-recognition receptor (integrin-associated protein [hCD47]), as well as donor pharmacologic treatments designed to blunt these phenomena.
Methods:
Livers from GaltKO.hCD46 pigs (“2-gene,” n = 3) and GalTKO.hCD46 pigs also transgenic for hEPRC, hTBM, hCD47, and hHO-1 (“6-gene,” n = 4) were perfused ex vivo with whole human blood. Six-gene pigs were additionally pretreated with desmopressin (DDAVP) and clodronate liposomes to deplete vWF and kupffer cells, respectively.
Results:
The average perfusion times increased from 304 (±148) min in the 2-gene group to 856 (±61) min in the 6-gene group (p= .010). The average heparin administration was decreased from 8837 U/h in the 2-gene to 1354 U/h in the 6-gene group (p= .047). Platelet sequestration tended to be delayed in the 6-gene group (p= .070), while thromboxane B2 (TXB2, a platelet activation marker) levels were lower over the first hour (p= .044) (401 ± 124 vs. 2048 ± 712 at 60 min). Thrombin production as measured by F1+2 levels tended to be lower in the 6-gene group (p= .058).
Conclusions:
The combination of the hEPCR.hTBM.hCD47.hHO-1 cassette along with donor pig DDAVP and clodronate liposome pretreatment was associated with prolonged function of xenoperfused livers, reduced coagulation pathway perturbations, and decreased TXB2 elaboration, and reflects significant progress to modulate liver xenograft injury in a pig to human model.
Keywords: ex-vivo perfusion, liver xenotransplantation, transgenic pigs
1 |. INTRODUCTION
Liver xenotransplantation is a possible solution to the shortage of human organs available. However, in addition to the well- recognized immunologic incompatibilities between humans and pigs, liver xenotransplantation suffers from other unique molecular challenges. Exvivo perfusion studies utilizing porcine livers and human blood show rapid marked decreases in circulating platelets.1 In large animal models of in-vivo liver xenotransplant, profound thrombocytopenia, physiologically inappropriate activation of the coagulation cascade and net consumption of coagulation pathway components are associated with a high incidence of hemorrhagic complications and short recipient survival in experiments using both wild-type2 and transgenic pig livers.3 Notably, administration of exogenous coagulation factors yielded a significant survival benefit with GalTKO or GalTKO.hCD46 genetics, further supporting a role for coagulation pathway dysregulation as a critical barrier to clinical translation.4,5
Thrombocytopenia in xenoliver models results from both platelet sequestration in the xenograft and a consumptive coagulopathy that includes circulating platelet activation and injury, leading to platelet scavenging in the recipient. Porcine Kupffer cells (liver-resident macrophages) and liver sinusoidal endothelial cells (LSECs) are both shown to mediate physiologically inappropriate phagocytosis of human or baboon platelets.6,7 In addition, unlike human vWF, porcine vWF interacts constitutively with quiescent human platelets via glycoprotein Ib (GPIb) binding, leading to nonphysiologic platelet aggregation and coagulation cascade potentiation.8 Finally, porcine thrombomodulin, while capable of binding human thrombin, does not efficiently mediate activation of human protein C, resulting in a procoagulant phenotype.9
Recent advances in heart and kidney xenotransplant models were associated with genetic modifications additional to GalTKO.hCD46, including expression of humanized thrombomodulin (hTBM) and endothelial protein C receptor (hEPCR),10–12 and with several different pharmacologic interventions, including ε-aminocaproic acid,13 DDAVP,14,15 and platelet GPIb blockade.16 hEPCR amplifies the production of activated protein C (APC) by hTBM17 and provides protection against thrombodysregulation and inflammatory injury18 that are associated with xenotransplantation. Human CD47 (hCD47) is a self-recognition receptor that confers a “don’t eat me” signal to human leukocytes. Heme oxygenase 1 (HO-1) is an antiinflammatory and antioxidative protein with numerous effects, including inhibiting platelet aggregation and inhibiting apoptosis, that has been shown to be beneficial in xenotransplant models.19 The hEPCR.hTBM.hCD47.hHO-1 gene cassette was constructed to explore its utility to promote clinical translation of GalTKO.hCD46 pig-to-human organ xenotransplantation, with each gene included to simultaneously address one of the multiple known xenogeneic injury pathways.
Here, we describe the use of a strategy incorporating these genetic manipulations along with additional mechanism-directed pharmacologic interventions. Novel 6-gene transgenic pigs containing α-1,3-galactosyl transferase knockout (GalTKO) and human membrane cofactor protein (hCD46) were engineered to additionally express hEPCR, hTBM, hCD47, and hHO-1 (GalTKO.hCD46.hEPCR.hTBM.hCD47.hHO-1). Six-gene liver donors were pretreated with DDAVP to deplete vWF stores and clodronate liposomes to deplete Kupffer cells. The effects associated with these combined interventions on coagulation cascade activation, platelet activation and sequestration, and liver viability were evaluated by ex vivo liver perfusion with human blood.
2 |. MATERIALS AND METHODS
2.1 |. Animals
GalTKO.hCD46 (“2-gene”) pigs and GalTKO.hCD46.hEPCR.hTBM.hCD47.hHO1 (“6-gene”) pigs were provided by Revivicor Inc. (Blacks-burg, VA). hTBM and hCD47 expressions were driven the by porcine endothelial-specific ICAM2 promoter, whereas hEPCR and hHO1 expressions were driven by the constitutive CAG promoter. All animal protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee (IACUC).
2.2 |. Protein expression via western blotting
hCD46, hEPCR, hTBM, hCD47, and hHO-1 protein detection in liver tissue lysates was carried out on automated capillary western blotting system Simple WES (Protein simple). Liver tissue lysates were obtained using TPER buffer (Thermo Fisher Scientific, Waltham, MA, USA) with protease inhibitors (Thermo Fisher Scientific). Total protein concentrations were quantified using BCA kit (Thermo Fisher Scientific). A total of 3 μg of total protein was loaded into each well of 12–230 kDa iess/Wes separation module (Thermo Fisher Scientific). Anti-mouse detection module was used for mouse anti-human antibodies (hTBM, hEPCR, actin) and anti-rabbit detection was used for rabbit anti-human antibodies (hCD46, hHO-1, actin). Anti-sheep secondary antibodies were used for sheep anti-hCD47 antibody (Supporting information Table S1). All assays were run on manufacture’s recommended default program. Signal quantification was done by compass software and actin as a loading control. Signal intensity was represented by arbitrary units.
2.3 |. Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissues. Samples were sectioned at 4 μm, allowed to air-dry, deparaffinized in xylene, and rehydrated through graded alcohols to water. Endogenous peroxidases were quenched using Dual Endogenous Enzyme Block (Agilent Technologies, Santa Clara, CA, USA) for 10 min. After washing with TBS-Tween buffer (Sigma-Aldrich, St. Louis, MO, USA) sections were blocked with serum-free protein block (Agilent Technologies) for 5 min. Sections were gently drained and 100 μL of antibody (Supporting information Table S1) was dispensed onto each slide and allowed to incubate at room temperature for 30 min. After incubation, slides were rinsed in wash buffer then incubated in 100 μL of EnVision + Dual HRP secondary with 0.5% pig serum (Agilent Technologies) for 30 min. Slides were thoroughly rinsed in wash buffer. Antibody binding was then detected using a 5-min incubation of 100 μL of diaminobenzidine tetrahydrochloride (Agilent Technologies). Slides were rinsed in running tap water, counterstained with hematoxylin, dehydrated through graded alcohols and xylene, mounted on coverslips, and imaged. Positive staining was demonstrated by a deposition of brown pigment at the site of antibody binding.
2.4 |. Activated protein C (APC) assay
Porcine aortic endothelial cells (pAEC) were plated in 48-well tissue culture plates and grown to confluency. After washing once with TBS, 50 μL of thrombin (0.1 U/mL) and 50 μL of Protein C (PC, 0.1 U/mL) were added to each well of cells and the plate was incubated for 1 h at 38.5°C. The reaction was stopped by adding 5 μL of 40 IU/mL of hirudin to each well (Sigma-Aldrich). After 5 min, 100 μL of the resulting supernatant from each well was transferred to a new well on a 96-well plate containing a preprepared APC standard curve (Haematologic Technologies, Essex Junction, VT, USA). S-2366 substrate (Diapharma, West Chester, OH, USA) was added to each well of the 96-well plate (100 μL, 4 mM) and the plate was immediately read at 405 nm kinetically every minute for 1 h using a Biotek plate reader (Winooski, VT, USA). Quantitation of APC in each cell culture well was determined kinetically using the APC standard curve and Biotek GEN5 software.
2.5 |. Apoptosis assay
pAEC were plated in black-walled 96-well plates and grown overnight. Wells were washed once with PBS and 100 μL of medium containing 1 μM of STS or medium alone was added. In addition, the medium in all wells contained 4 μM of Cell Event Caspase-3/7 Green Detection Reagent (Thermo Fisher Scientific). The plate was then placed in a Biotek Cytation 5 multimode reader and cell imaging was performed every 30 min for 16 h. Each well was imaged sequentially at 4× magnification with both high-contrast bright field and fluorescent optics (GFP, 469/525). The plate was maintained in the reader at 37°C and 5% CO2 throughout the experiment. Images were preprocessed and analyzed using Biotek GEN5 software. Total cell count per field and # of green cells per field, within each well, was determined. The ratio: # green cells (Caspase 3/7+)/total cells per field was calculated (percent apoptosis). Due to a change in cell morphology that precluded bright-field cell counting after STS treatment, the total number of cells per field before treatment was used to calculate the ratio at each time point.
The animals used for pAEC isolation and assays were the animals used in this study or their reclones (identical genetics). pAECs were kept for no more than six passages prior to use. In-vitro assays were performed using cells from seven (APC assay) or four (apoptosis assay) of the 6-gene animals with three replicate assay wells per animal. Two gene control and human assays were done with single animals and three replicate assay wells.
2.6 |. Experimental groups
Anhepatic control experiments consisting of human blood perfused through the system without a liver (n = 2) and allogenic control experiments of GalTKO.hCD46 pig livers perfused with GalTKO.hCD46 porcine blood (n = 3) were performed. Seven xenogeneic experiments with GalTKO.hCD46 pig livers perfused with human blood (n = 3), and GalTKO.hCD46.hEPCR.hTBM.hCD47.hHO-1 pig livers perfused with human blood (n = 4) were performed.
2.7 |. Donor animal preparation and liver procurement
All 6-gene experimental animals were treated with clodronate liposomes in the two consecutive days prior to liver donation. Liposomes were either made in-house as previously described with modifications,20,21 or ordered from clodronateliposomes.org (Vrije Universiteit, Netherlands). A dose of 2.5 mg/kg was given via peripheral IV. Additionally, these 6-gene animals received 3 μg/kg of desmopressin (DDAVP) (Diamondback, Scottsdale, AZ, USA) intramuscularly on the day prior to liver donation, and again on the day of donation prior to surgery. Our surgical procedure has been previously described.22 Briefly, animals were placed under inhalation anesthesia (isoflurane 1– 4%). A midline laparotomy was made and the infrarenal aorta cannulated. The liver was flushed in situ with cold (4°C) preservation solution (Organ Recovery Systems, Chicago, IL, USA) and then flushed via the portal vein on the back table. Cannulas were secured in the portal vein, suprahepatic inferior vena cava (IVC), celiac artery, and common bile duct. Prior to blood perfusion, the liver was flushed with saline to remove preservation solution.
2.8 |. Blood donation and drug regimen
Fresh whole human or porcine blood was collected into citrated blood bags in compliance with University of Maryland School of Medicine Institutional Review Board or IACUC approved protocols. Freshly collected blood was stored at room temperature and used within 4 h of donation. Fresh frozen human plasma was received from the Indiana Blood Center (Indianapolis, IN, USA). Porcine plasma was obtained from fresh whole pig blood collected in citrated blood bags, then centrifuged to obtain platelet poor plasma and frozen immediately. For perfusion experiments, whole blood was diluted with blood type compatible plasma in a 1:1 ratio, heparinized (7000 U/L) (Midwest Veterinary Supply, Lakeville, MN, USA), and recalcified with calcium chloride (8.8 mmol) (Midwest Veterinary Supply). Blood was also treated with famotidine (12 mg/unit of blood, Midwest Veterinary Supply), 1-benzylimidazole (BIA, 120 mg/unit of blood, Sigma-Aldrich), and a murine anti-human GPIb-blocking antibody (α-GPIb [10 μg/mL] courtesy of H. Deckmyn, Laboratory for Thrombosis Research, University of Leuven, Belgium). One anhepatic control experiment was not treated with α-GPIb due to temporary drug unavailability. During perfusion, all liver perfusion experiments received a continuous heparin infusion (Midwest Veterinary Supply) titrated to maintain a target activated clotting time (ACT) of >400 s. Average heparin dose (U/h) was calculated for each experiment.
2.9 |. Liver perfusion and blood sampling
Our liver perfusion system has been previously described.22 Blood was pumped from a reservoir through an oxygenator and the outflow split to perfuse the hepatic artery as well as shunted back to the reservoir at a rate of approximately 100 mL/min above the hepatic arterial flow rate which was measured by flow probe (Transmedics, Andover, MA, USA). Blood in the reservoir, partially oxygenated by the arterial shunt, was pumped into the portal vein and flow measured by probe. IVC outflow was returned to the reservoir. Blood samples were obtained from the IVC at timed intervals throughout the perfusion, and at termination of the experiment. All anhepatic and allogenic experiments were electively terminated. Xenogeneic experiments were terminated secondary to elevated portal and/or arterial perfusion pressure reflecting increased vascular resistance and loss of blood flow through the graft. Physiologic liver “failure” was usually preceded by progressive metabolic derangements, including acidosis and hyperkalemia, which became refractory to active remediation efforts (glucose and insulin boluses; bicarbonate boluses).
2.10 |. Blood testing
Complete blood counts, electrolytes, and liver function tests were processed by commercial laboratory (Antech, Rockville, MD, USA). In some experiments, platelets were enumerated by flow cytometry23 and analyzed using FlowJo software. Thromboxane B2 (TXB2) (thromboxane B2 EIA Kit, Cayman Chemical Company, Ann Arbor, MI; Catalog No. 519031) and β-thromboglobulin (β-TG) (Asserachrom β-TG; Diagnostica Stago, Asnieres, France) levels were measured as previously described.22 Thrombin generation, as measured by prothrombin fragment (F1 + 2) (enzygnost F1 + 2, Dade Behring/Siemens, Deerfield, IL, USA), was assayed via ELISA according to kit instructions. TXB2, β-TG, and F1 + 2 levels were not measured in allogeneic experiments due to incompatibility of the assay with porcine samples.
2.11 |. Statistical analysis
All data are presented as mean and standard error of the mean unless otherwise noted. Log-rank tests were used to assess liver perfusion duration (“viability”). Area under the curve analysis was used to assess changes over time. ANOVA was used to compare groups and two-tailed t-tests were used to compare individual time points between groups (GraphPad Prism software; GraphPad, Inc., La Jolla, CA, USA). A p value <.05 was considered significant, while a p value <.08 trended toward significance.
3 |. RESULTS
3.1 |. Transgene expression
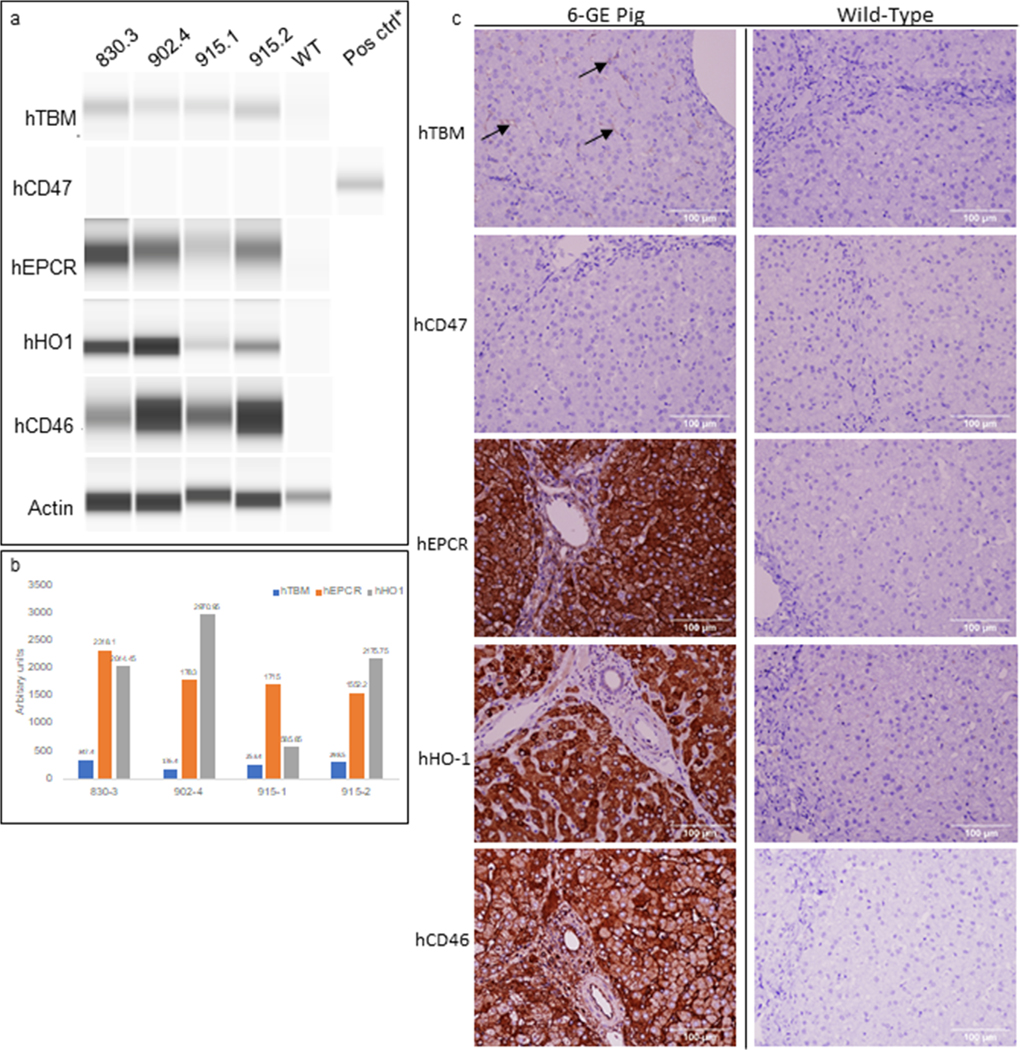
Gene expression of all six transgenes was confirmed using RT-PCR (data not shown). Protein expression as shown by western blotting (Figure 1A and B) and immunohistochemistry (Figure 1C) confirmed expression of hCD46, hEPCR, hTBM, and hHO-1. hCD47 expression was unable to be detected by western blotting or immunohistochemistry.
FIGURE 1.
Protein expression by capillary western and immunohistochemistry. (A) hTBM, hEPCR, hHO-1, and hCD46 expressed at detectable levels in preperfusion liver samples. hCD47 expression is below the assay detection limit. (B) Protein expression is normalized to actin and mean values from two different experiments are represented in arbitrary units. (C) Representative IHC shows detection of hTBM (black arrows), hEPCR, hHO-1, and hCD46. Expression of hCD47 falls below the level of detection. *indicated positive control for hCD47 detection
3.2 |. Coagulation protein function
APC assay was used to determine the ability of hEPCR and hTBM to generate APC and assess whether the transgenic expressed proteins lead to increased production. The 2-gene group had significantly lower levels of APC production compared to the 6-gene group and human samples (p = .023, .002 respectively) (Figure 2A). The difference in APC production between the 6-gene group and human samples was not statistically significant.
FIGURE 2.
Function of transgene protein expression. (A) The 2-gene group had significantly lower levels of APC production compared to the 6-gene group and human samples (p = .023, .002, respectively). The difference in APC production between the 6-gene group and human samples was not statistically significant. (B) There was significantly less apoptosis in the 6-gene group compared to the 2-gene group (p = .007) (*p < .05, **p < .005)
3.2.1 |. hHO-1 function
Apoptosis assays were used to assess the ability of transgenic HO-1 expression to inhibit apoptosis. There was significantly less apoptosis in the 6-gene group compared to the 2-gene group (p = .007) (Figure 2B).
3.3 |. Graft perfusion
Physiologic hepatic arterial and portal vein pressures and flows were routinely achieved after liver reperfusion, and bile was produced (but not quantified) for the duration of perfusion. Successful graft perfusion duration was 304 (±148, n = 3) min for the 2-gene reference group compared to 856 (±61, n = 4) min for the 6-gene experimental group (p = .010) (Table 1, Figure 3).
TABLE 1.
Experimental groups, perfusion duration, and mode of failure
| Group type | Experiment | Duration (min) | Reason for failure |
|---|---|---|---|
| Anhepatic | C1 | 480 | Elective |
| C2 | 900 | Elective | |
| Allogenic | A1 | 660 | Elective |
| A2 | 480 | Elective | |
| A3 | 480 | Elective | |
| Xenogeneic GalTKO.hCD46 “2-gene” | 816–11 | 115 | Increasing vascular pressures, loss of hepatic artery flow |
| 850–12 | 597 | Increasing vascular pressures, loss of hepatic artery flow | |
| 850–6 | 202 | Increasing vascular pressures, loss of hepatic artery flow | |
| Xenogeneic GalTKO.hCD46.hEPCR.hTBM.hCD47.hHO-1 “6-gene” | 830–3 | 785 | Increasing vascular pressures, loss of hepatic artery flow |
| 902–4 | 720 | Increasing vascular pressures. Loss of hepatic artery flow | |
| 915–1 | 960 | Increasing vascular pressures, metabolic derangements | |
| 915–2 | 960 | Increasing vascular pressures, loss of hepatic artery flow |
FIGURE 3.
Graft viability. Kaplan–Meier curves showing significantly longer perfusion duration of the 6-gene group compared to the 2-gene group (p = .010)
3.4 |. Liver injury biomarkers
In all experiments, alanine transaminase (ALT) levels remained within the normal human range, albeit with an upward trend during liver perfusion (Figure 4A). In contrast, although aspartate transaminase (AST) levels remained within normal limits in the anhepatic experiments, it quickly rose to supraphysiologic levels in the allogenic, 2-gene, and 6-gene groups (Figure 4B). There was no difference between the 2- and 6-gene groups.
FIGURE 4.
Transaminase levels. Measured ALT (A) and AST (B) values comparing anhepatic, allogenic, and xenogeneic groups. ALT levels in all experiments remained within physiologic ranges. AST levels rose to supraphysiologic levels in the allogenic and the 2- and 6-gene xenogeneic groups. There was no significant difference between the 2- and 6-gene groups
3.5 |. Hematologic parameters
Platelet counts as measured by commercial lab were stable throughout perfusion in the anhepatic experiments and remained above 80% of initial values in allogenic experiments (Figure 5A). In contrast, at 15 min of perfusion, platelet counts by hemocytometry had fallen to 67% of the initial value in the 6-gene experiments, not significantly lower than in the allogeneic livers (p = .250), versus 33% for the 2-gene livers (p = .097 vs. 6-gene; p = .021 vs. allogeneic). Platelet counts measured by flow cytometry showed a decrease in values over time in all xenogeneic experiments with a trend toward reduced sequestration in the 6-gene experiments (n = 2, p = .070) (Figure 5B). Hematocrit was preserved above 75% of its initial value over the duration of perfusion in all experiments, with the lowest values for the xenogeneic 2-gene group.
FIGURE 5.
Platelet conservation. (A) Platelet levels measured by commercial lab (hemocytometer) during the first 15 min of perfusion in anhepatic, allogenic, and xenogeneic groups. Platelet counts were significantly different between the allogenic and 2-gene groups (p = .021). There was no significant difference between the allogeneic and 6-gene groups (p = .25) or the 2- and 6-gene groups (p = .097). (B) Platelet counts measured by flow cytometry over 4 h of perfusion. Platelet levels were lower in the 2-gene compared to 6-gene group with a trend toward significance (p = .07)
3.6 |. Platelet activation
TXB2 rose with similar kinetics during perfusion of the anhepatic circuit and 6-gene livers (Figure 6). In contrast, TXB2 levels rose sharply in the 2-gene group within 30 min of perfusion, peaked at 1 h, and then declined. TXB2 levels were significantly lower in the 6-gene group relative to the 2-gene group at 1 h (401 ± 124 vs. 2048 ± 712, p = .044). β-TG elaboration increased over time in the anhepatic, 2-gene, and 6-gene groups, though there were no significant differences (Figure 7).
FIGURE 6.
Thromboxane generation. Both the anhepatic and 6-gene experiments saw a steady rise in TXB2 over the course of perfusion. The 2-gene group had an initial sharp rise in TXB2 levels within 30 min of perfusion which peaked at 1 h and then declined. The difference in TXB2 levels between the 2- and 6-gene groups at 1 h was statistically significant (p = .044)
FIGURE 7.
β-TG elaboration. Β-TG elaboration increased over time in the anhepatic, 2-gene, and 6-gene groups, though there were no significant differences
3.7 |. Coagulation parameters
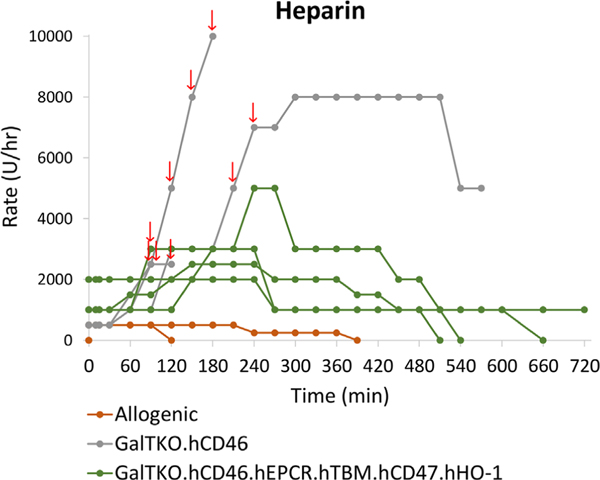
The anhepatic experiments did not exhibit any decline in ACT, and thus, did not receive any additional heparin after the initial bolus during blood preparation. For allogenic livers, after initial blood pretreatment, a low rate continuous heparin infusion was started by protocol but was quickly titrated-off based on therapeutic (>400) ACT values (Figure 8). In contrast, the xenogeneic experiments required continued heparin administration to maintain a therapeutic ACT, with the 2-gene group requiring escalating and bolus dosing (arrows). The cumulative heparin dose administered over the course of each xenogenic experiment was significantly greater for 2-gene (average 8837 U/h) than 6-gene livers (1354 U/h) (p = .047), with notable increases at the time of perfusion failure. Thrombin production, as F1 + 2 generation, was low and stable in the anhepatic experiments throughout the duration of the perfusion (Figure 9). F1 + 2 production was similar in the 2- and 6-gene groups through the first hour of perfusion but tended to be lower in the 6-gene group at subsequent intervals (p = .058).
FIGURE 8.
Heparin requirements. Heparin rate and bolus dosing (arrows) for allogenic and xenogeneic experiments. After initial blood pretreatment, a low rate continuous heparin infusion was started by protocol but was quickly titrated off based on therapeutic (>400) ACT values for allogenic experiments. In contrast, the xenogeneic experiments required continued heparin administration to maintain a therapeutic ACT, with the 2-gene group requiring escalating rates and boluses particularly around the time of graft failure. The cumulative heparin dose administered over the course of each xenogeneic experiment was significantly greater for 2-gene than 6-gene livers (p = .047)
FIGURE 9.
Thrombin generation as measured by F1 + 2 levels. The anhepatic experiments had stable very low levels of F1 + 2 throughout the duration of perfusion. The 2-gene and 6-gene groups had similar levels of F1 + 2 production through the first hour of perfusion and then diverged with the levels in the 2-gene group being approximately double that of the 6-gene group. These results trended toward statistical significance (p = .058)
3.8 |. Histology
Biopsies taken during perfusion demonstrated preserved liver architecture (Figure 10). Liver tissues appeared viable without evidence of arterial thrombi. Increasing sinusoidal congestion was noted over time.
FIGURE 10.
Histology of perfusion samples. Biopsies taken after 1, 4, and 8 hours of perfusion demonstrated preserved liver architecture (Figure 10). Liver tissues appeared viable without evidence of arterial thrombi. Increasing sinusoidal congestion was noted over time
4 |. DISCUSSION
Progress toward clinical liver xenotransplantation in pig to nonhuman primate models has been limited by profound thrombocytopenia and coagulation pathway dysregulation, which were in turn closely associated with short recipient survivals.2,3 The recent reports of prolonged survival of livers in vivo with the supplementation of exogenous coagulation factors4,24 highlight the importance of coagulation issues as a barrier to success, and motivates our efforts to target genetic and pharmacologic interventions to address the multiple specific known obstacles.
Here, we show that the addition of human coagulation regulatory genes in novel 6-transgene pigs combined with DDAVP and clodronate liposome treatment resulted in reduced platelet sequestration and activation, decreased thrombin generation, and longer ex vivo graft perfusion duration. The importance of incompatibilities between porcine and human thrombomodulin9 and the effect of hTBM on PC activation25 have led to the inclusion of hTBM and hEPCR in xenograft models, and prolong xenograft survival for both heart10,12 and lungs.26,27 Our data support the hypothesis that an additional transgenic cassette containing hTBM and hEPCR improves xenogeneic liver performance at least in part by increased APC generation thereby restoring balance between procoagulant and anticoagulant pathways.
The significantly lower heparin requirements and the trend toward decreased thrombin production imply that these modifications were at least partially successful at countering inappropriate coagulation. We believe that the lower heparin requirements in the 6-gene group indicate the ability of these livers to maintain a more physiologic coagulation balance, thus, resulting in a drastically lower heparin requirement.
While the difference in thrombin production between the 2- and 6-gene livers did not reach significance, this was likely impacted by small sample sizes at later time points. The observed trend toward lower F1 + 2 in associations with 6-gene livers supports the hypothesis that this genotype has a mechanism-based protective effect, although small experimental numbers prevent us from drawing firm conclusions in this regard. Importantly, the difference in perfusion times between the 2- and 6-gene groups was significant, and the 2-gene experiments were terminated earlier due to lack of flow in the hepatic artery while still histologically showing viability. In our estimation, this constellation of findings is likely attributable to dysregulated coagulation and vascular injury manifesting as increased vascular resistance and inflammation in association with the 2-gene pigs that appear to be attenuated in association with the 6-gene livers.
Based on prior work demonstrating platelet phagocytosis by Kupffer cells, primarily via a CD18 integrin-mediated pathway7,28 and data from 6-gene lungs that these added gene modifications did not alter platelet sequestration (unpublished observations), in this series, we elected to induce apoptosis of liver Kupffer cells with clodronate liposome pretreatment. Platelet loss in the 6-gene group with clodronate liposomes was not significantly different compared to the allogenic groups, whereas platelet loss in the 2-gene group without liposomes was significantly greater compared to the allogenic group. In addition, Kupffer cell clodronate pretreatment was associated with lower thromboxane elaboration. Lack of reference groups (2-gene with clodronate pretreatment; 6-gene without clodronate) prevent us from discerning whether the additional transgenes’ putative effect on coagulation parameters might also have contributed to decreased platelet consumption seen in the 6-gene group. Although we were not able to document whether clodronate liposome treatment depleted Kupffer cells and/or other monocytoid cell populations from the livers studied here, the approach has been thoroughly validated in pigs20,21 and in many other models.29,30 Multiple mechanisms for platelet loss in liver xenotransplant models in addition to Kupfer cells have been described, including aggregation,31,32 recognition of human sialic acid profiles,33 and phagocytosis by LSECs,34–36 likely via ASGR-1-mediated recognition6,28 and nonphysiologic pvWF/human GPIb interactions.37 These mechanisms likely contribute to the residual thrombocytopenia observed in these experiments.
When examining platelet counts in xenogeneic models, there are technical limitations. Most commercial platelet counting methods rely on hemocytometer counting which is dependent on particle size. As a result, cell fragments, resulting from hemolysis and graft destruction during perfusion, can be counted as platelets and contribute to artificially elevated platelet counts noted at later stages in xenoperfusions.38 For this reason, we have limited our presentation of hemocytometer platelet results to the initial time points up to 15 min, when hemocytometer data are highly concordant with flow data.22 Alternative methods of platelet counting using flow cytometry are promising as they rely on recognition of surface markers rather than size for identification. While we have shown these methods to be effective in xenotransplant models,39 they have not been routinely used by us explaining why these data are not available for all experiments. The trend toward decreased platelet sequestration observed in the 6-gene liver group in the limited set of flow, platelet data at later time points support a sustained protective effect associated with this study group and warrant further investigation.
In addition to Kupffer cell depletion, we targeted the nonphysiologic interaction between human platelet GPIb and porcine vWF by using DDAVP to deplete vWF stores prior to perfusion with the expectation that this intervention may reduce platelet activation, as previously reported.16 We did see significant decreases in the amount of TXB2 produced in the 6-gene group in the first hour of perfusion. The slow steady increase in TXB2 levels in the 6-gene group likely represents continued platelet and/or macrophage activation over time. In contrast, the decline in TXB2 in the 2-gene group after 1 h likely does not represent an actual decrease in platelet activation, but rather reflects an exhaustion of platelet degranulation as well as a decrease in remaining platelets with subsequent TXB2 metabolism. Additionally, the increase in TXB2 in the anhepatic experiments illustrates the platelet-associated activation caused by interaction of the blood with the circuit components, platelet activation inherent in our system, likely from mechanical stress/shear forces from the pumps as well as activation by the tubing. The rise in β-TG levels over time in both xenogeneic groups and the anhepatic group points to increasing platelet activation in part inherent to our system.
Previous work by our group has shown that targeting platelet binding pathways, such as GPIb and GPIIb/IIIa, can be effective.16,37 As such, both the 2- and 6-gene groups received α-GPIb based on our previous work. It is possible that if the 2-gene group had not received this drug, the difference between the 2- and 6-gene livers in terms of platelet activation (TXB2 levels) would have been more profound. Reduced platelet activation and subsequent adhesion in the 2-gene group due to α-GPIb could also have attenuated platelet consumption and contributed to the lack of significant difference in platelet numbers observed between the 2- and 6-gene groups.
AST and ALT are used clinically to measure liver injury. We interpret ALT levels that remained within physiologically normal ranges to indicate that minimal hepatocellular injury occurs during perfusion of transgenic pig livers with human blood. In contrast, the disproportionate AST elevations noted by our group,22,37,40 as well as others41 probably reflect hemolysis. This is further supported by functional data showing a protective effect of hHO-1 from apoptosis and preserved liver histology during perfusion.
CD47 is a self-recognition receptor that confers a “don’t eat me” signal to human leukocytes. Expression of CD47 in xenotransplant models has shown in-vitro and in-vivo benefits, including prolonged graft survival,19,42,43 and it has been argued that the inclusion of hCD47 will be necessary going forward.19 Specifically, the recent study by Nomura et al. demonstrated that expression of hCD47 on porcine podocytes and endothelial cells resulted in decreased phagocytosis by human macrophages. This effect was diminished when CD47 was masked.40 While we were not able to detect CD47 expression in tissues, as assayed via western blotting or immunohistochemistry, gene expression verified by RT-qPCR (data not shown) leads us to believe that CD47 was expressed at levels below detection in our assays.
In seminal in-vivo liver transplant studies, clodronate liposomes were associated with primary nonfunction (PNF) of liver xenografts.3 In the study by Ekser et al., two GalTKO.hCD46 donor pigs were treated with clodronate liposomes prior to baboon xenoliver transplants. It is unclear if the clodronate liposome treatment was the cause of the PNF. We are aware of no other reports using clodronate liposomes in xenoliver models. In our experiments, we noted successful perfusion of all livers based on physiologic vascular flows, correction of electrolytes, and bile production. Our results are inconsistent with linking clodronate liposomes with liver PNF, but the purported risk of this treatment will need to be further investigated by future studies.
Our study has some limitations. First, small group sizes, necessitated by pig availability and the nature of resource intensive large animal models, limit our ability to compare groups, particularly at later time points. Additionally, we have combined three interventions in our experimental group—the addition of a 4-gene cassette, clodronate liposome, and DDAVP pretreatment. Without separate groups utilizing just additional genetic modifications or individual drug pretreatments, we are unable to differentiate the individual effects of these interventions or appreciate potential synergistic effects.
Xenotransplantation will likely require incremental advances with both genetic and pharmacologic interventions to target the myriad interconnected pathways of coagulation, platelet function, and complement activation within xenografts. We have shown here that the combination of thromboregulatory and anti-inflammatory genetic modifications and drug treatments to deplete liver macrophages and vWF confers significant prolongation of liver viability. Prolongation on this scale could be clinically meaningful for ex-vivo perfusion in bridgeto-recovery and bridge-to-transplant applications.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by U19 AI090959 as well as unrestricted gifts from Revivicor and United Therapeutics. Thank you to Christopher Avon for assistance in the production of liposomes, Veronica Ritchie for completing β-TG assays, and Cinthia Drachenberg for histology review.
Funding information
Revivicor and United Therapeutics, Grant/Award Number: U19AI090959
Footnotes
CONFLICT OF INTEREST
RNP has served without compensation on Revivicor’s Scientific Advisory Board. DLA is Executive VP and CSO of Revivicor, Inc. KK, LS, AD, WE, and CP are employees of Revivicor, Inc. Revivicor, Inc. is a wholly owned subsidiary of United Therapeutics Corporation.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Rees MA, Butler AJ, Chavez-Cartaya G, et al. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood.Transplantation. 2002;73:1194–1202. [DOI] [PubMed] [Google Scholar]
- 2.Powelson J, Cosimi AB, Austen W, et al. Porcine-to-primate orthotopic liver transplantation. Transplant Proc. 1994;26:1353–1354. [PubMed] [Google Scholar]
- 3.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. [DOI] [PubMed] [Google Scholar]
- 4.Shah JA, Navarro-Alvarez N, DeFazio M, et al. A bridge to some-where: 25-day survival after pig-to-baboon liver xenotransplantation. Ann Surg. 2016;263:1069–1071. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Alvarez N, Shah JA, Zhu A, et al. The effects of exogenous administration of human coagulation factors following pig-tobaboon liver xenotransplantation. Am J Transplant. 2016;16:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paris LL, Chihara RK, Reyes LM, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18:245–251. [DOI] [PubMed] [Google Scholar]
- 7.Chihara RK, Paris LL, Reyes LM, et al. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation. 2011;92:739–744. [DOI] [PubMed] [Google Scholar]
- 8.Schulte am Esch J, Cruz MA, Siegel JB, Anrather J, Robson SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90:4425–4437. [PubMed] [Google Scholar]
- 9.Roussel JC, Moran CJ, Salvaris EJ, et al. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008;8:1101–1112. [DOI] [PubMed] [Google Scholar]
- 10.Singh AK, Chan JL, DiChiacchio L, et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation. 2019;26:e12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramackers W, Rataj D, Werwitzke S, et al. Expression of human thrombomodulin on porcine endothelial cells can reduce platelet aggregation but did not reduce activation of complement or endothelium - an experimental study. Transpl Int. 2020;33:437–449. [DOI] [PubMed] [Google Scholar]
- 12.Chan JL, Singh AK, Corcoran PC, et al. Encouraging experience using multi-transgenic xenografts in a pig-to-baboon cardiac xenotransplantation model. Xenotransplantation. 2017;24. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Schuetz C, Elias N, et al. Up to 9-day survival and control of thrombocytopenia following alpha 1, 3-galactosyl transferase knock-out swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HJ, Lee G, Kim JY, et al. Pre-treatment of donor with 1-deamino-8-D-arginine vasopressin could alleviate early failure of porcine xenograft in a cobra venom factor treated canine recipient. Eur J Cardiothorac Surg. 2005;28:149–156. [DOI] [PubMed] [Google Scholar]
- 15.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:27–35. [DOI] [PubMed] [Google Scholar]
- 16.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplantation. 2016;23:222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvaris EJ, Moran CJ, Roussel JC, et al. Pig endothelial protein C receptor is functionally compatible with the human protein C pathway. Xenotransplantation. 2020;27:e12557. [DOI] [PubMed] [Google Scholar]
- 18.Lee KFE, Lu B, Roussel JC, et al. Protective effects of transgenic human endothelial protein C receptor expression in murine models of transplantation. Am J Transplant. 2012;12:2363–2372. [DOI] [PubMed] [Google Scholar]
- 19.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation. 2019;26:e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins BJ, Blum MG, Parker RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol. 2001;90:2257–2268. [DOI] [PubMed] [Google Scholar]
- 21.Gaca JG, Palestrant D, Lukes DJ, et al. Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res. 2003;112:19–25. [DOI] [PubMed] [Google Scholar]
- 22.Cimeno A, Hassanein W, French BM, et al. N-glycolylneuraminic acid knockout reduces erythrocyte sequestration and thromboxane elaboration in an ex vivo pig-to-human xenoperfusion model. Xenotransplantation. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Meer PF, Karssing-van Leeuwen W, Kurtz J, et al. A flow cytometric method for platelet counting in platelet concentrates. Transfusion. 2012;52:173–180. [DOI] [PubMed] [Google Scholar]
- 24.Shah JA, Patel MS, Elias N, et al. Prolonged survival following pigto-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. Am J Transplant. 2017;17:2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen B, Ramackers W, Tiede A, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009;16:486–495. [DOI] [PubMed] [Google Scholar]
- 26.Harris DG, Quinn KJ, French BM, et al. Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015;22:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdorf L, Azimzadeh AM, Pierson RN. Progress and challenges in lung xenotransplantation: an update. Curr Opin Organ Transplant. 2018;23:621–627. [DOI] [PubMed] [Google Scholar]
- 28.Paris LL, Estrada JL, Li P, et al. Reduced human platelet uptake by pig livers deficient in the asialoglycoprotein receptor 1 protein. Xenotransplantation. 2015;22:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno SG. Depleting macrophages in vivo with clodronate-liposomes. Methods Mol Biol. 2018;1784:259–262. [DOI] [PubMed] [Google Scholar]
- 30.Schiedner G, Hertel S, Johnston M, et al. Selective depletion or block-ade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. [DOI] [PubMed] [Google Scholar]
- 31.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ezzelarab M, Ekser B, Gridelli B, et al. Thrombocytopenia after pig-to-baboon liver xenotransplantation: where do platelets go? Xenotransplantation. 2011;18:320–327. [DOI] [PubMed] [Google Scholar]
- 33.Butler JR, Paris LL, Blankenship RL, et al. Silencing porcine CMAH and GGTA1 genes significantly reduces xenogeneic consumption of human platelets by porcine livers. Transplantation. 2016;100:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rees MA, Butler AJ, Brons IGM, et al. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12:13–19. [DOI] [PubMed] [Google Scholar]
- 35.Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010;17:350–361. [DOI] [PubMed] [Google Scholar]
- 36.Peng Q, Yeh H, Wei L, et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS One. 2012;7:e47273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaMattina JC, Burdorf L, Zhang T, et al. Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein Ib blockade. Xenotransplantation. 2014;21:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burdorf L, Harris D, Dahi S, et al. Thromboxane and histamine mediate PVR elevation during xenogeneic pig lung perfusion with human blood. Xenotransplantation. 2019;26:e12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sendil S, Braileanu G, Sun W, et al. An improved flow cytometry based platelet counting in xeno perfusion models. Am J Transplant: Off J Am Soc Transplant Am Soc Transplant Surgeons. 2016;16:643. [Google Scholar]
- 40.Cimeno A, French BM, Powell JM, et al. Synthetic liver function is detectable in transgenic porcine livers perfused with human blood. Xenotransplantation. 2018;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekser B, Gridelli B, Cooper DKC. Porcine alanine transaminase after liver allo-and xenotransplantation. Xenotransplantation. 2012;19:52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nomura S, Ariyoshi Y, Watanabe H, et al. Transgenic expression of human CD47 reduces phagocytosis of porcine endothelial cells and podocytes by baboon and human macrophages. Xenotransplantation. 2020;27:e12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe H, Sahara H, Nomura S, et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplantation. 2018;25:e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.